Note: Descriptions are shown in the official language in which they were submitted.
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
LINEAR PROBE CARRIER
RELATED APPLICATIONS
This application claims the benefit of U.S. Application Ser. No. 60/175,225,
filed Jan.
I0, 2000, 601190,495, filed Mar. 20, 2000, and 60/244,418, filed Oct. 30,
2000, which
applications are incorporated by reference herein in their entirety as if
fully set forth below.
TECHNICAL FIELD
This invention relates generally to the field of target analysis by binding to
probes, as
is commonly found in DNA sequence identification. This invention also relates
to
arrangements of immobilized nucleic acid probes on a solid substrate. More
particularly, the
invention relates to packaging of probe carrier threads wherein probes are
immobilized in an
array alone a flexible carrier.
BACKGROUND OF THE INVENTION
Identification of molecular structure has become very important in research
and in
many industries, and the analysis of biological molecules such as nucleic
acids and proteins
forms the basis of clinical diagnostic assays. The procedures utilized often
involve large
1 S numbers of repetitive steps which consume Iarge amounts of time. (see,
e.g., Sarnbrook, J., et
al., Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory
Press, Cold
Spring Haxbor, NY (2nd ed. 1989)). Simpler and quicker analysis of molecules
has been
provided by the development of arrays of test sites formed on a planar
substrate. Each of the
test sites includes probes which bind with samples applied to the device. Such
probes may be
oligonucleotides, proteins, antibodies, or cell-binding molecules and the
choice of probes is
theoretically limited only by the possibilities of specific binding to or
reaction with sample.
The binding of a sample to a probe is detected, and the probe identified,
thereby identifying
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
the sample. Technology has primarily developed around the use of these two-
dimensional,
planar arrays, especially in the area of arrays of oligonucleotides, which
have become small
and dense enough to be termed microarrays.
The ability to manufacture microarrays in an efficient and cost-effective
manner is of
considerable interest to researchers worldwide and of significant commercial
value. The
importance of the microarray technology to the biotechnology industry and to
the entire
health care sector cannot be overstated. A microarray is capable of
dramatically boosting the
efficiency of traditional biochemical experiments. Tests that would have taken
years can now
be completed in hours or even minutes. The applications of this technology
affect more than
the healthcare sector including gene profiling, disease diagnostics, drug
discovery, forensics,
agronomics, biowarfare and even biocornputers. Various types of microarray
manufacturing
devices and technologies have been described.
The current direction of technical development continues to be toward ever-
denser
two dimensional arrays of probes on rigid substrates. This approach presents a
number of
problems. First, as the number of.test sites in an array is increased, the
complexity of
fabricating the array or pluralities of arrays is greatly increased. Second,
the conventional
methods of placing bio-molecules as probes on specific test sites--
photolithography,
mechanical spotting, and ink jetting--are time-consuming, expensive, often
lack the desired
accuracy and do not meet the desired size constraints. Photolithographic
synthesis of probes
ih situ is a labor intensive technique that may not provide satisfactory
accuracy and has a
limited range of probe lengths. Mechanical spotting is a slow process in which
the smallest
test site size is limited by the nature of the process. Chemical ink jetting
has an inaccuracy
similar to in-situ synthesis and test site size limits similar to mechanical
spotting. Third,
because of the complexity and extreme precision required in manufacturing
individual arrays,
and the low throughput, the fabrication cost of each array is very high, often
thousands of
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
dollars for arrays containing enough probes to evaluate complex biological
samples. Fourth,
the expense and complexity of the reading devices for detecting probe-sample
hybrids, which
is already extremely high, increases with each increase in array density, and
because the
reader has to carry out a two-dimensional scan with a very high spatial
precision (in the order
of 10~,m), processing time for each scan also increases with increasing
density of the two-
dimensional probe array.
In addition, the basis operating principle of microarray involves a probe
immobilized
on a substrate to react with specific molecules in sample fluid. Hybridization
requires
providing probes with sufficient chances to meet their complementary
molecules. In existing
systems, this is achieved through diffusion or driving the sample fluid across
the microarray..
The former is a random process and the later requires complex microfluidic
systems.
Hence, there is a need for an easily- and rapidly-constructed, inexpensive
probe
carrier which can accommodate thousands or hundreds of thousands of probes,
which is
capable of compact storage and use, can be manufactured at a high rate of
throughput; can
facilitate probe/target interaction with a high efficiency and does not
require expensive and
highly precise reading devices, can carry detailed information about
individual probes or
groups of probes on the substrate along with the probes themselves, can
accommodate probes
of varying lengths and degrees of complexity in customized groups, and which
is compact,
easy to use, and inexpensive enough to allow one-time use with resulting high
accuracy.
There is also a need for improved packaging of such probe Garners whereby the
required amount of hybridization fluids is minimized and laxge numbers of
probes can be
immobilized on a substrate without the concomitant increase in size as a
standard two-
dimensional gene chip matrix would necessitate.
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
BRIEF SUMMARY OF ASPECTS OF THE INVENTION
The present invention provides a new direction and approach in making a probe
carrier or probe configuration that does not require dense two-dimensional
symmetrical
arrays built upon a rigid substrate and also does not inherently limit the
size of the probes that
can be attached to a substrate. In addition, the present invention can be
relatively easily
fabricated through use of assembly-line-like techniques.
The invention provides a probe carrier in which a plurality of probes are
immobilized
in discrete areas, one probe per area, on an elongated flexible substrate with
a length:width
ratio of at least about 5:1, at least 50:1, at least 500:1, at least 10,000:1,
or at least 100,000:1.
In one embodiment, the length of each probe-containing area does not exceed
1000
micrometers, in another embodiment the length of each probe-containing area
does not
exceed 500 micrometers, in another embodiment the length of each probe-
containing area
does not exceed 100 micrometers, in still another embodiment the length of
each probe-
containing area does not exceed 50 micrometers, and in yet a further
embodiment the length
of each probe-containing area does not exceed 20 micrometers.
The invention also provides a probe carrier in which a plurality of probes are
immobilized in discrete areas, one probe per area, on a flexible substrate
with a length:width
ratio of at least about 5:1, where the substrate has layer on its surface, and
where the probes
axe immobilized on the surface of the layer. In a further aspect, the
invention also has a
second layer between the first layer and the substrate. In one embodiment, the
first layer
comprises silica and the second layer comprises a metallic material.
The invention also provides a linear one-dimensional arrangement of probes
immobilized in a single file on the surface of a flexible substrate, in which
the linear density
of the probes exceeds 10 probes per linear cm, or preferably 50 probes per
linear cm. In
another aspect of the invention, the linear density of the probes exceeds 100
probes per linear
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
cm, in a further aspect of the invention, the linear density of the probes
exceeds 200 probes
per linear cm, and in yet a further aspect of the invention, the linear
density of the probes
exceeds 500 probes per linear cm.
In addition, the invention provides a plurality of probes immobilized on
discrete areas
of the surface of a flexible tape substrate, one probe per area, where the
tape has a thickness
not exceeding 500 micrometers. In another aspect of the invention, the tape
does not exceed
100 micrometers in thickness, and in yet another aspect the tape does not
exceed 20
micrometers in thickness.
The invention also provides a plurality of probes immobilized on discrete
areas of the
surface of a flexible fiber substrate, one probe per area, where the fiber has
a diameter not
exceeding S00 micrometers. In another aspect of the invention, the fiber does
not exceed 200
micrometers in diameter, in yet another aspect the fiber does not exceed 100
micrometers in
diameter, and in still another aspect the fiber does not exceed 20 micrometers
in diameter:
All of the above aspects of the invention may further include a first marker
which
conveys information about a first set of probes, and a second marker which
conveys
information about a second set of probes. In some embodiments, the markers may
be optical
markers, such as optical bar codes or fluorescent markers, in another
embodiment the
markers may be magnetic. In one embodiment which includes markers, the probes
are
polynucleotides, in another embodiment which includes markers, the probes are
polypeptides,
in yet another embodiment which includes markers, the probes are antibodies,
and in still
another embodiment which includes markers, the probes are selected from the
group
consisting of cell surface receptors, oligosaccharides, polysaccharides, and
lipids.
Also in all of the above embodiments, whether or not they include markers, the
apparatus also includes a first layer, with the probes immobilized on that
layer; the layer may
be composed of silica. Alternatively, the apparatus includes both a first
layer and a second
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
layer; the first layer may be silica and the second layer may be a metallic
material. If the
second layer is metallic, it may also be magnetizable.
In all of the above embodiments the probes may be arranged as a linear
configuration
of spots, or as a linear configuration of stripes with the stripes being at an
angle to the long
axis of the substrate.
Also, in all the above embodiments, the probes may be polynucleotides, or
polypeptides, or antibodies, or ligands, or be selected from the group
consisting of cell
surface receptors, oligosaccharides, polysaccharides, and lipids. If
the,probes are
polynucleotides, they may be DNA, and if DNA, they may be single-stranded DNA.
The substrate for the invention may be silica glass, or plastic, or a metallic
material, :or
a polymer, and if a polymer, the polymer may be selected from the group
consisting of
polyimide and polytetrafluoroethylene. A preferred substrate is an optical
fiber. If the
substrate is a metallic material, it may also have a layer between the
substrate and the probes,
so that the probes are immobilized on the layer; the lay may be silica.
Furthermore, the
metallic substrate may be magnetizable.
The invention may be wound about a drum or a plurality of drums. It may also
be
wound upon itself in a flat spiral, with or without a flat backing, and it may
be fiu ther
attached to a spool at the center of the flat spiral. In addition, the
outermost end of the
substrate in the spiral may be extended and attached to a second spool.
In a different aspect, the invention comprises an apparatus for transporting a
plurality
of probe fluids to a substrate to print a probe array. Typically, the
apparatus includes a
reservoir with a plurality of wells, and a set of capillaries, where the
capillaries are arranged
so that one end of each capillary is connected to a well in the reservoir,
such that the contents
of the well may enter the capillary, and the second end of the capillaries are
arranged in a flat
single file row. In one embodiment of this apparatus, the reservoirs are wells
in a microtiter
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
plate. When using the apparatus of the invention, probe fluids may be moved
from the wells
in the reservoir into the capillary tubing by applying a pressure differential
between the
reservoir and the tubing, andlor by providing a voltage between the reservoir
and the
substrate. The capillary may be positioned parallel to and may move across the
longitudinal
axis of the elongated probe substrate to deposit a set of probes on the
substrate. Other
methods of probe deposition are described below in further detail.
A second probe transport apparatus has a row of probe containers configured in
a
fashion similar to a conveyer belt. The row of containers is moved at one
speed and direction
to intersect with the substrate, which is moving at another speed and
direction, to deposit
probes, one by one, onto the substrate. In an alternative configuration, the
row of probe
containers is moved to intersect a moving row of spotters, which are made of a
flexible strand
of material, such that each spotter intersects a container to transfer probe
from the container
to the spotter. A conveyor, for instance, also moves a substrate so that it
intersects the row of
probe-carrying spotters such that each spotter deposits its probe onto the
substrate after. it has
picked up the probe from a container. The row of spotters may be configured as
a loop, and
further the spotters may be washed in a washing station after they have
deposited probe on
the substrate and before they return to the containers.
The invention also includes methods of depositing probes from a fluid
transportation
apparatus onto the substrate surface. In one method, probes are painted as
strips on the
substrate. The probe may be carried on a thin, flexible and elastic spotter,
which contacts the
substrate surface in a brushing action to paint the strip. In one embodiment,
the spotter can be
a silica capillary or fiber. Alternatively, the capillary or fiber can be made
of other materials ,~
such as metal, ceramics, polymer, or other material that is capable of
transporting the probe-
containing fluid. In other methods, the probes rnay be deposited in a non-
contact fashion
either as strips or dots. These methods include magnetic, electric, thermal,
acoustic and inkjet
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
deposition. In a magnetic deposition method, the probe is attached to magnetic
beads. An
electromagnet placed underneath the substrate is activated as the spotter
carrying a probe
immobilized on magnetic beads intersects the substrate. The magnetic field
generated by.the
electromagnet pulls the probe from the fluid transportation apparatus to
deposit the probe
onto the surface of the substrate. In an electric deposition method, a voltage
of appropriate
polarity is applied between the substrate and the delivery device to establish
an electric field
to push the electrically charged probes (such as oligonucleotides) onto the
substrate surface.
In a thermal deposition method, rapid, localized heating is introduced into
the path of fluid,
producing a rapid local volume expansion (a bubble) that propels probe fluid
onto substrate.
Rapid heating can be introduced either electrically by a resistance heating
wire or optically
using suitable laser light. In acoustic deposition, an ultrasonic pulse is
introduced into probe
fluid which propels a droplet out onto the substrate surface. In inkjet
deposition, a
piezoelectric actuator is built in the probe fluid container. Activated by a
voltage signal, the
piezoelectric actuator rapidly reduces the volume of the container thus
pushing out the probe
fluid onto the substrate surface.
In another alternative method, painting the probes in strips on the substrate
may be
accomplished by a probe deposition apparatus in which a matrix of fibers is
dipped into a
corresponding matrix of reservoirs, each reservoir containing probe, then the
matrix of fibers
is moved across a first section of substrate, with the fibers and the
substrate positioned so that
each fiber deposits a separate line of probe across the substrate with the
desired substrate
between lines. In this method, the fiber matrix may be washed and dipped into
another matrix
of reservoirs then moved across another section of substrate to deposit
another set of strips of
probes.
In all of the above methods, the substrate may be a plurality of fibers
arranged in
parallel, so that several fibers receive probe with one pass of the.probe-
deposition instrument,
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
or the substrate may be a tape, where, after probe deposition, the tape is
optically cut along its
long axis to produce a plurality of probe-carrying tapes.
In all of the above methods, the probes may be covalently linked to the
substrate.
In all of the above methods, the further step of adding markers to the
substrate may be
included.
Among other factors, the invention is based in the technical finding that a
probe
carrier having a one-dimensional configuration of probes on a flexible
substrate provides a
simple, economical, reliable, and classification-specific Way to identify the
presence of target
molecules in a sample. Further, probes on a probe carrier of this invention
are not limited in
size. These technical findings and advantages and others are apparent from the
discussion
herein.
This invention encompasses a new way of improving the efficiency of
hybridization
to target or reaction with target. This method involves moving the probe
carrier through
sample fluid to enhance the chance for the probes immobilized on the carrier
to mix with
their target molecules in the sample fluid. The carrier may take a variety of
forms including
thread, tape, slide, coil, drum or pin. The movement can involve translation,
rotation or
vibration of the probe earner, either alone or in combination.
This invention also includes several designs of hybridization device, where
the probe
earner is inserted into a chamber containing the sample fluid. The gap between
the probe
carrier and the inner wall of the chamber is minimized to reduce the volume of
sample fluid.
The probe carrier is driven to move within the chamber to improve the
efficiency of
probe/target interaction. An additional method of hybridization enhancement
involves
applying a voltage between the probe carrier and the wall of the hybridization
chamber. At
one polarity, the electric field pulls target molecules towards the probes on
the carrier, which
increases the local concentration of target molecules and improves the
likelihood of
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
hybridization. At the opposite polarity, the electric field repels target
molecules away from
the probes, which can help to increase the specificity of hybridization. By
alternating the
polarities at suitable frequencies, the hybridization efficiency between the
target and probe
can be improved.
The invention may be used in the analysis of known point mutations, expression
analysis, genomic fingerprinting, polymorphism analysis, linkage analysis,
characterization
of mRNAs and mRNA populations, sequence determination, sequence confirmation,
disease
diagnosis, and other uses which will be apparent to those of skill in the art.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates one embodiment of the probe carrier, in which probes are
immobilized as spots on a substrate, which also carries markers in the form of
optical bar
codes. Probes may also be immobilized as stripes or as rows .of spots, and
markers may be
optical, magnetic, or any other identifiable marking;
Figure 2a is a cross=sectional view of a probe-carrier, in which the probes
are
immobilized in a notch in the Garner; and Figure 2b is a cross-sectional view
of two layers of
a probe-carrier in which the probes are immobilized in a notch on the Garner,
and illustrates
how the position of the immobilized probes in the notch protects them from
friction with the
next layer.
Figure 3 illustrates an apparatus and method of fabricating probe Garners in
which a
plurality of tubes transports probes from reservoirs to the substrate and
"paints" the probes in
stripes on the substrate.
Figure 4 schematically represents a method of fabricating probe Garners in
which
individual probe containers pass across a substrate or plurality of substrates
and deposit probe
on the substrate as each probe container passes over the substrate.
to
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
Figure 5 illustrates various types of probe containers which may be used in
the
preceding fabrication technique, and the means that each employs to deposit
probe from the
carrier onto the substrate.
Figures 6a - 6e illustrate methods of fabricating probe Garners. Figure 6a
illustrates a
S method of fabricating probe carriers in which probe is contained in liquid
in individual ,
reservoirs. Figure 6b illustrates a method of fabricating probe Garners in
which a moving belt
of spotters intersects the reservoirs so that each spotter picks up a separate
probe. Figure 6c
illustrates a method of fabricating probe Garners in which the moving belt of
spotters, with
probe associated, intersects a substrate or plurality of substrates and
deposits the probe
thereon. Figure 6d illustrates a method of fabricating probe Garners in which
the spotters can
be arranged in a continuous loop in which individual spotters are washed and
reused for
spotting new probes which are provided from a reservoir array. Figure 6e
depicts a method of
transferring probe from a reservoir to a spotter. In this configuration, the
spotter moves under
the substrate and the substrate surface is positioned face down to allow the
spotter to deposit
the probe from underneath the substrate.
Figure 7a and 7b illustrate another method of constructing probe Garners, in
which a ~.
matrix of spotters dips into a corresponding matrix of wells, each well of
which contains a
probe, then the spotter matrix is brushed across a substrate or plurality of
substrates at such
an angle that each spotter deposits a separate line, then the spotter array is
washed and moves
to a new matrix of probe containing wells, and repeats the dipping-brushing-
washing cycle on
a new section of substrate.
Figure ~ illustrates configurations of a probe Garner pin and a probe carrier
rod.
Figure 9 illustrates fabrication methods for a probe carrier pin and a probe
carrier rod.
Figure I Oa illustrates a top view of a flexible probe carrier in a coil
configuration.
Figure l Ob illustrates a side view of a flexible probe carrier in a coil
configuration. Figure
11
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
l Oc is a cross-sectional view of two adjacent turns of a probe-carrier thread
in which the
probes are immobilized within notches on the carrier.
Figure 11 a illustrates a flexible probe carrier in a spool configuration
packaged in a
mini cassette. Figure 1 1b is a cross-sectional view of two layers of a probe-
carrier in the
spool in which the probes are immobilized in a notch on the Garner, and
illustrates how the
position of the immobilized probes in the notch protects them from friction
with the next
layer.
Figure 12 illustrates a method of using an electric field to control
hybridization to a
probe Garner.
Figure 13 illustrates a method of hybridization to a probe carrier pin.
Figure 14 illustrates a method of parallel hybridization of multiple target
samples in
standard microtiter plate format using probe carrier pins.
Figure 15 is a view of hybridization equipment for a probe carrier rod as
viewed along
the axis of the rod.
Figure 16 is a side view of hybridization equipment for a probe carrier coil.
Figure 17a and 17b illustrate hybridization equipment for a probe carrier
spool.
Figure 18 illustrates a reader for scanning a probe Garner pin or a probe
carrier rod.
Figure 19 illustrates a reader for scanning a probe carrier coil.
Figure 20 illustrates a reader for scanning a probe carrier spool.
DETAILED DESCRIPTION OF THE INVENTION
1. The probe carrier apparatus
A. General description
Scanning and imaging of microarrays can be facilitated by one-dimensional
arrays of
probes because such arrays do not require the high degree of precision
necessary for imaging
12
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
in two dimensions. A number of apparatuses which utilize polynucleotides bound
to optical
fibers may be found in the following: "Nucleic Acid Biosensor Diagnostics,"
Krull, et al.;
WO # 98/58079 and WO # 95/26416; "Fiber optic biosensor for selectively
detecting
oligonucleotide species in a mixed fluid sample," Walt et al., WO # 98/50782;
"Analytical
method for detecting and measuring specifically sequenced nucleic acid,"
Sutherland, et al.,
EP # 0245206; "Gene probe biosensor method," Squirrel, WO # 93/06241; "Nucleic
acid
assay method," Hirschfield, US 5,242,797; Piunno et al., Fiber-optic DNA
sensor for
fluorometric nucleic acid determination, Anal. Chem. 67:2635-2643, 1995; Uddin
et al, A
fiber optic biosensor for fluorimetric detection of triple-helical DNA,
Nucleic Acids. Res.
25:4139-4146, 1997; Abel et al., Fiber-optic evanescent wave biosensor for the
detection of
oligonucleotides, Ahal. Chem. 68: 2905-2912, 1996; Kleinjung et al, Fibre-
optic genosensor
for specific determination of femtomolar DNA oligomers, Anal. Chim. Acta
150:51-58, 1997;
Zhang et al., A chemilluminescence fiber-optic biosensor for detection of DNA
hybridization,
Anal. Lett. 32:2725-2736, 1999; Ferguson et al., A fiber-optic DNA biosensor
microarray for
the analysis of gene expression, Nature Biotech., 14:1681-1684, 1996.
However, these apparatuses typically involve attachment of only one probe
molecule
sequence on the glass surface of single optical fibers. Krull, et al. (WO #
98/58079) have w
theorized the use of an undifferentiated mixture of more than one type of
probe, however, the
number of different probe sequences is sharply limited in these techniques by
the
unorganized distribution of probe molecules, which necessitates that each
individual probe
molecule be tagged by, for example, fluorescent labels (as suggested by Krull
et al.), in order
to identify it and distinguish it from its local neighbors, which may be
probes with different
sequences. In addition, previous approaches have used only short sections of
fiber, on the
order of a few centimeters or less, limiting the number and kinds of probes
that can be
immobilized. Finally, the previous techniques utilize the optical fiber on
which probes are
13
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
immobilized to conduct light both to and from the markers of hybridization,
which are
typically fluorophores. This detection technique relies on evanescent
illumination from the
optical fiber, which is inherently limited to the area immediately adjacent to
the fiber surface,
does not provide discrimination among groups of probes, and is limited in
sensitivity.
S Furthermore, the use of the optical fiber itself to conduct the excitation
and emission light
limits one to the use of optical fibers alone as substrates on which to
immobilize probes and
precludes the use of other substrates, such as metal wire or polymer, which
may offer other
advantages such as the ability to carry information about individual probes or
groups of
probes, as well as advantages in hybridization.
Unlike the established "gene-chip" technology where DNA probes form a two
dimensional matrix of spots on a planar slide, a "probe carrier thread"
immobilizes the probes
in an one dimensional array along a single length of thin, flexible thread. A
probe carrier
thread system is comprised of three essential elements: probe carrier thread
configuration and
fabrication, hybridization and readout. Improved packaging of a probe carrier
thread'by use
1 S of probe carrier pin, probe carrier rod, probe carrier coil and probe
carrier spool technologies
increases the density of probes and enhances the inherent advantages of the
probe tamer
thread technology. "Flexible," as used herein, means capable of being bent,
wound,~coiled or
otherwise flexed to the degree necessary for the operation of the invention
without breaking.
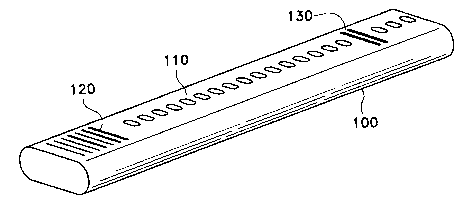
As illustrated in Fig. 1, in one embodiment of the invention probes are
immobilized as
spots (110) at the center or as narrow stripes (see Fig. 4, 404) across the
width of a long, thin
and flexible substrate (100). Alternatively, probes can be immobilized as
successive rows of
spots, said rows being at an angle to the long axis of the substrate. The
length:width ratio of
the substrate is at least about 5:1, preferably at least 50:1, more preferably
at least 500:1, and
most preferably at least 10,000:1. The length:width ratio of the probe-
containing portion of
2S the substrate is at least about 5:1, preferably at least 50:1, more
preferably at least 500:1, and
14
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
most preferably at least 10,000:1. The length:width ratios, the flexibility of
the substrate; and
the positioning of the probes in a one-dimensional or nearly one-dimensional
arrangement,
allow for new and simplified methods of manufacturing, using, and analyzing
the probe
carrier.
A "probe," as used herein, is a set of copies of one type of molecule or one
type of
molecular structure which is capable of specific binding to or specific
reaction with a
particular sample or portion of a sample. The set may contain any number of
copies of the
molecule or multimolecular structure. "Probes," as used herein, refers to more
than one such
set of molecules or multimolecular structures. The molecules or multimolecular
structures
may be polynucleotides, polypeptides, oligosaccharides, polysaccharides,
antibodies, cell
receptors, ligands, lipids, cells, small molecules as are used to e.g. screen
drugs as are used in'
screening pharmaceuticals, or combinations of these structures, or any other
structures to
which samples of interest or portions of samples of interest will bind or
react with specificity.
The probes may be immobilized on the substrate by either covalent or
noncovalent
attachment. "Flexible," as used herein, means capable of being bent, wound,
coiled or
otherwise flexed to the degree necessary for the operation of the invention
without breaking.
"Width" of the substrate is defined as the length of the longest perpendicular
to the long axis
of the substrate which is entirely contained within the substrate. "Width" of
the probe-
containing portion of a cylindrical such as a thread substrate is defined as
the linear distance
of the longest arc, contained within the probe-containing portion of the
substrate, which is
perpendicular to the long axis of the probe-containing portion of the
substrate. Length of the
probe-containing portion of the substrate is the linear distance along the
long axis of the
substrate from the first probe to the Iast probe of the probes on the
substrate or, if there is a
substantially larger gap between probes that form groups of probes, the length
is the linear
distance from the first to last probe in the group.
is
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
As with conventional two-dimensional microarrays arranged on a planar surface,
the
present apparatus is used to analyze samples by 1) distinguishing the probes
which have
bound or react with sample or sample fragment from those that have not bound
sample, then
2) establishing the identity of the probes) which have bound sample.
A further way to identify probes is by rnaxkers which serve to identify
individual
probes or sets of probes. Such markers may be used to convey more information
than simply
the identity of the probe.
The long, thin, and flexible nature of the probe carrier lends itself to
numerous novel
means of containment and use.'The probe carrier may be packaged in a number of
formats
including but not limited to a pin, a rod, a coil and a spool. Hybridization
methods are
considerably enhanced by requiring less hybridization fluid and enhanced
mixing. A flexible
probe carrier packaged in a spool is especially advantageous in applications
that require high
volume, low to medium scale microarrays, such as those involved in disease
diagnostics and
management in maj or hospitals. In these applications, the required number of
probes in the
array is small (in the range of several hundreds to several thousands) but a
very large number
of the same type of arrays may be consumed every day. With the flexible probe
carrier
format, tens thousands of copies of identical sets of probes axe arrayed
repetitively along a
continuous length of thread and sealed in a large coil or spool. A fully
automated system
integrates equipment for every stage of the analytical process, such as a
hybridization station
and a scanner. The machine takes in patients' DNA samples and feed the
flexible probe
Garner through the entire process and produces analysis and results in a fully
automated
manner without human intervention.
B. Specific descri tp ion
1.1 Substrate.
16
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
The substrate of the invention can be made of various materials. The
requirements of
the substrate are that it have sufficient flexibility to withstand the
conformational changes
necessary to the manufacture and use of the apparatus, and that it be capable
of immobilizing
the particular probes to be used, or be capable of modification (for example,
by coating) so
that it is capable of such immobilization. The substrate may also comprise
various layers
made of different materials, each of which has a function in the apparatus.
Specific embodiments will require differing degrees of flexibility.
Flexibility may be
measured by the ability to withstand winding to a certain diameter, for
example a diameter of
cm, 5 cm, 2 cm, 1 cm, 0.5. cm, or 0.1 cm. Preferred materials for the
substrate of the
10 present invention are silica glass, metallic materials, plastics, and
polymers of sufficient
strength to withstand the processes of manufacture and use.
For immobilizing polynucleotides and polypeptides, silica, i.e. pure glass, is
a
preferred material because polynucleotides and polypeptides can be covalently
attached to a
treated glass surface and silica gives out a minimum fluorescent noise signal.
The silica may
be a layer on another material, or it may be the substrate, core or base
material of the
apparatus, or both. One embodiment of the present invention comprises a metal
wire as the
core substrate, with a coating of silica on it for probe immobilization.
Another embodiment
comprises a plastic or polymer tape as a base substrate, with a coating of
silica for probe
embodiment. In this embodiment, a further layer of metallic material may be
added, either on
the opposite side of the tape from the silica layer, or sandwiched between the
silica layer and
the polymer or plastic. Yet another embodiment of the invention is a silica
fiber with a layer
of metallic material on the silica core and another layer of silica on the
metallic material;
probes are immobilized on this outer silica layer.
Optical fibers. The probe carrier thread can be made of different materials. A
preferred material is silica because DNA can be covalently attached onto a
treated glass
17
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
surface and silica emits minimum fluorescent noise. Contrary to common
perception that
glass is a rigid and easily breakable material, fibers made of silica are
flexible and have great
elastic strength. For example, the optical fiber currently mass-produced for
the
telecommunication industry is made of silica. Optical fiber is a substrate
material which is
made of primarily of silica and provides the necessary requirements. Although
such fibers are
manufactured for the purpose of transmitting light, the present invention does
not require this
feature of the fibers (although it may be used in some embodiments). Rather,
it is other
features of the optical fiber which make it particularly advantageous for the
present invention.
The mechanical strength of optical fibers has been measured at 7 GPa, about 4
times that of
the strongest steel while only 1l6 of its weight. Optical fibers are also
highly flexible.
Standard 125 ~,m diameter fibers can be coiled in loops down to Smm in
diameter without
breakage.
Also, the process of making optical fibers lends itself to customization,
especially in
terms of the cross-sectional shape of the fiber. Optical fibers are made from
preforms,
typically 1 meter long and 3 crn in diameter, fabricated using silica. The
center portion of the
preform is doped with Germanium to create a core with higher refractive index
to guide light
through. Then the perform is installed on a fiber draw tower in clean room
environment,
which heats it to the melting point and pulls out the fiber on to a Iarge
drum. The cross-
section shape of the fiber generally resembles that of the preform and the
diameter of the
fiber can be controlled through the pulling speed. Most optical fibers on he
market have
circular cross-sections and an outer diameter of 125~,m. However, other
diameters and
shapes, particularly "D" cross-sectional shapes are also available. This is
especially useful if
the final probe carrier is to be wound upon itself fox storage and ease of
use. As shown in Fig.
2a, the cross-section of the fiber can be adjusted using a notched, D-shaped
preform so that
the fiber (200) has a notch, or groove (202) , in which probes (110) are
immobilized. This
is
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
design protects the probes of one layer from friction with the substrate of a
succeeding layer,
as shown in Fig. 2b, where the cross-sections of two successive layers are
shown one on top
of the other.
In addition, owing to the high purity of the material and careful control of
the
fabrication process, optical fibers have very few structural defects. Also,
optical fibers have
excellent dimensional precision. Diameters are controlled to within ~1 ~,m.
Finally, the cost of
optical fibers is very low, at about 1~2ø per meter. This is because the
fabrication process of
fibers is fairly straightforward and a single preform can produce up to 100 km
of standard
telecommunication fibers.
A number of apparatuses which utilize polynucleotides bound to optical fibers
have
been described in the following: "Nucleic Acid Biosensor Diagnostics," Krull,
et al., WO #
98/58079 and WO # 95/26416; "Fiber optic biosensor for selectively detecting
oligonucleotide species in a mixed fluid sample," Walt et al., WO # 98/50782;
"Analytical
method for detecting and measuring specifically sequenced nucleic acid,"
Sutherland, et al.,
EP # 0245206; "Gene probe biosensor method," Squirrel, WO # 93/06241; "Nucleic
acid
assay method," Hirschfield, US 5,242,797; Piunno et al., Fiber-optic DNA
sensor for
fluorometric nucleic acid determination, Anal. Chem. 67:2635-2643, 1995; Uddin
et al, A
fiber optic biosensor for fluorimetric detection of triple-helical DNA,
Nucleic Acids Res.
25:4139-4146, 1997; Abel et al., Fiber-optic evanescent wave biosensor for the
detection of
oligonucleotides, Anal. Chem. 68: 2905-2912, 1996; Kleinjung et al, Fiber-
optic genosensor
for specific determination of femtomolar DNA oligomers, Anal. Chem. Acta
150:51-58,
1997; Zhang et al., A chemilluminescence fiber-optic biosensor for detection
of DNA
hybridization, Anal. Lett. 32:2725-2736, 1999; Ferguson et al., A fiber-optic
DNA biosensor
microarray for the analysis of gene expression, Nature Biotech., 14:1681-1684,
1996.
19
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
These apparatuses typically involve attachment of only one probe molecule
sequence
on the glass surface of single optical fibers, greatly limiting their
usefulness. Previous
approaches have used only short sections of fiber, on the order of a few
centimeters or less,
limiting the number and kinds of probes that can be immobilized. Finally, the
previous
techniques utilize the optical fiber on which probes are immobilized to
conduct light both to
and from the markers of hybridization, which are typically fluorophores. This
detection
technique relies on evanescent illumination from the optical fiber, which is
inherently limited
to the area immediately adjacent to the fiber surface, does not provide
discrimination among
groups of probes, and is limited in sensitivity. Furthermore, the use of the
optical fiber itself
to conduct the excitation and emission light limits one to the use of optical
fibers as substrates
on which to immobilize probes and precludes the use of other substrates, such
as metal wire
or polymer, which may offer other advantages such as the ability to carry
information about
individual probes or groups of probes, as well as advantages in hybridization,
as discussed
below.
Commercial telecom fibers are coated with a layer of non-porous polymer, which
is
not optimal for probe immobilization. The coating can be removed by techniques
known in
the art, such as those described in U.S. Patent #5,948,202, which is
incorporated by reference
herein in its entirety. However, bare fiber without this coating is prone to
attack by water
vapor, which generates micro-cracks on the fiber surface and degrades its
strength. As a
result, the bare silica fiber may not survive the very tough environment
during the
hybridization stage. There are several approaches to solving this problem.
One approach is to wind the fiber into a spiral coil along an elongated
cylinder or
drum after probe immobilization. The fibers sit side-by-side on the drum and
are attached to
its solid surface. The probes are aligned along a side of the fiber that is
distal to the side of
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
the fiber attached to the drum. The drum provides mechanical support to the
fiber during
hybridization of sample and detection of the hybridization pattern.
A second approach is to strengthen the fiber substrate by applying one or
several
layers of coating to the silica fiber, which protect the fiber from the
onslaught of water vapor
and at the same time maintain good binding to probes. The strengthened fiber
can then be
wound, for example, on a specially designed spool and assembled in a sealed
cassette for
transportation and handling. An example of a substrate strengthening method is
to coat the
fiber with a metallic material, then an additional layer of silica. To protect
the bare silica fiber
from moisture absorption, one or several layers of hermetic coating can be
applied. Suitable
coating materials including gold, silver and titanium due to their relative
inertness in
chemical solutions. Carbon coating is also widely used in the fiber optic
telecommunications
industry for hermetic sealing. This invention in one embodiment further
provides an
additional layer of silica coating over the hermetic layers) to provide
covalent binding with
DNA probes. Such a coating can be implemented through low cost sol-gel process
and
provides a surface for immobilization of probes, especially by covalent
binding.
In addition to silica, other materials can also be used as the main body of
the
substrate. These include thin metalwires or strong polymer (polyimide or
polytetrafluoroethylene (PTFE), for example) tapes. Again a sol-gel silica
coating can be
applied to the substrate to facilitate probe binding. For the polymer tape
substrate, one can
add a layer of metallic material sandwiched between the tape and the silica.
The metallic material element in all the substrate designs described above not
only
protects and/or strengthens the substrate, it may provide additional benefits
during fabrication
of the probe carrier and during binding of samples which carry a charge, which
are described
below.
21
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
The substrate is elongated. "Elongated," as used herein, means that the
length:width
ratio of the substrate exceeds about 5:1, preferably exceeds 100:1, more
preferably exceeds
1000:1, and most preferably exceeds 10,000:1. It is contemplated that the
length: width ratio
can be even greater, such as at Ieast 100,000:1 or at least 1,000,000:1. As
defined above,
S "width" of the substrate is defined as the length of the longest
perpendicular to the long axis
of the substrate which is entirely contained within the substrate. If the
substrate is of varying
widths, the width to be used to calculate the length:width ratio is the
longest width. "Width"
of the probe-containing portion of the substrate is defined as the longest arc
(for an arc
shaped probe-containing area, as is typically found on a cylindrical thread-
like substrate) or
the large lineal distance for a flat substrate, contained within the probe-
containing portion of
the substrate, which is perpendicular to the long axis of the probe-containing
portion of the
substrate. "Length" of the substrate is defined as the length of the long axis
of the substrate.
If the substrate has more than one length, the shortest of the lengths is used
to calculate the
length: width ratio.
1 S The cross-section of the substrate can be of any shape. The "cross-
section," as used
herein, is defined as the planar section through the substrate perpendicular
to the long axis of
the substrate. Although the cross-section can be any shape, two particular
shapes represent
different embodiments of the invention. First, as used herein, a "tape" refers
to an
embodiment utilizing a tape- , ribbon-, or strip-like substrate, whose cross-
section is
rectangular or nearly rectangular, or in the shape of a parallelogram. Such a
tape will have a
thickness, corresponding to the width of the cross-sectional area. In various
embodiments of
the invention, this thickness does not exceed S00 micrometers, or 100
micrometers, or SO
micrometers, or 20 micrometers. Second, as used herein, a "fiber" is an
embodiment which
utilizes a fiber-, thread-, or wire-like substrate, whose cross-section is
rounded. The cross-
2S section may be circular, elliptical, or partially circular, for instance as
with a fiber with a D-
22
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
shaped cross-section. The cross-section has a diameter, defined herein as the
longest linear
dimension of the cross section. In various embodiments of the invention, the
diameter of the
fiber does not exceed 500 micrometers, or 200 micrometers, or 100 micrometers,
or 50
micrometers, or 20 micrometers. The terms "tape" and "fiber" are intended to
represent two
parts of a spectrum of cross-sectional shapes. The invention can, however,
have a cross-
section of any shape. The substrate may incorporate a groove or grooves
running
approximately parallel to the long axis of the fiber, in which probes are
immobilized, as
illustrated in Figure 2a. Such a groove can be seen as an indentation in the
cross-section. The
use of such a groove or indentation reduces or eliminates friction between
probes
immobilized in the groove and other surfaces; for example, when the substrate
is wound on
itself in a spiral, probes immobilized on one winding would be protected from
the substrate
on the next winding due to being recessed in the groove. A D-shaped cross
section
incorporating such an indentation facilitates stacking of one layer of a
winding on the next, as
well as protection of probes. Other embodiments may utilize different cross-
sections, which
will be useful in the use of the apparatus, and will be apparent to one of
skill in the art.
1.2 Probes
A "probe," as used herein, is a set of copies of one type of molecule or one
type of
multimolecular structure which is capable of specific binding to a particular
sample or
portion of a sample. "Probes," as used herein, refers to more than one such
set of molecules.
A probe may be immobilized on the substrate by either covalent or noncovalent
attachment.
Probes may be polynucleotides, polypeptides, oligosaccharides,
polysaccharides, antibodies,
cell receptors, ligands, lipids, cells, or combinations of these structures,
or any other
structures to which samples of interest or portions of samples of interest
will bind with
specificity. The set of probes chosen depends on the use of the apparatus. For
example, if the
apparatus uses polynucleotides as probes, if one is performing sequence
analysis, one would
23
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
prefer a complete or nearly complete set of n-mers; the use of such sets is
more fully
described in U.S. Patents # 5,700,637 and 6,054,270, which are hereby
incorporated herein
by reference in their entirety. On the other hand, if a device is to be used
to analyze mutations
or polymorphisms in a gene or set of genes, polynucleotides representing a
complete or
chosen set of mutations, such as substitution, deletion, and insertion
mutations, for sections of
the particular gene or genes of interest may be preferred. As a further
example, in diagnostics
such as for cancer-related mutations, particular mutational "hot spots" in a
set of genes
known to be associated with a particular cancer or cancers would be the areas
to which
complementary polynucleotides would serve as the set of probes. These examples
are merely .~
illustrative of the various custom sets of probes that might be selected for a
particular
apparatus and focus on polynucleotides because these are the types of probes
now most
commonly in use; it is to be understood that other types of probes and other
sets of
polynucleotides will be readily apparent to the skilled worker in the field.
As used herein, "polynucleotide" means a polymeric form of nucleotides of any
length, which contain deoxyribonucleotides, ribonucleotides, and/or their
analogs. The terms
"polynucleotide" and "nucleotide" as used herein are used interchangeably.
Polynucleotides
may have any three-dimensional structure, and may perform any function, known
or
unknown. The term "polynucleotide" includes double- or single-stranded, and
triple-helical
molecules. Unless otherwise specified or required, any embodiment of the
invention
described herein that includes a polynucleotide encompasses both the double-
stranded form
and each of two complementary single-stranded forms known or predicted to make
up the
double stranded form. Relatively shorter lengths of polynucleotides (less than
about 100
nucleotides) are also referred to, as oligonucleotides.
The following are non-limiting examples of polynucleotides: a gene or gene
fragment,
exons, introns, mRNA, tRNA, rRNA, ribozymes, cDNA, recombinant
polynucleotides,
24
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
branched polynucleotides, plasmids, vectors, isolated DNA of any sequence,
isolated RNA of
any sequence, nucleic acid probes, and primers. A polynucleotide may comprise
modified
nucleotides, such as methylated nucleotides and nucleotide analogs. Analogs of
purines and
pyrimidines are known in the art, and include, but are not limited to,
aziridinylcytosine, 4-
acetylcytosine, 5-fluorouracil, 5-bromouracil, 5-carboxymethylaminomethyl-2-
thiouracil, 5-
carboxymethyl-aminomethyluracil, inosine, N6-isopentenyladenine, 1-
methyladenine, 1-
methylpseudouracil, 1-methylguanine, 1-methylinosine, 2,2-dimethylguanine, 2-
methyladenine, 2-methylguanine, 3-methylcytosine, 5-methylcytosine, pseudo-
uracil, S-
pentynyl-uracil and 2,6-diaminopurine. The use of uracil as a substitute for
thymine in a
deoxyribonucleic acid is also considered an analogous form of pyrimidine.
If present, modification to the nucleotide structure may be impaxted before or
after
assembly of the polymer. The sequence of nucleotides may be interrupted by non-
nucleotide
components. A polynucleotide may be further modified after polymerization,
such as by
conjugation with a labeling component. Other types of modif cations included
in this
1 S definition axe, for example, "caps", substitution of one or more of the
naturally occurring
nucleotides with an analog, internucleotide modifications such as, for
example, those with
uncharged linkages (e.g., methyl phosphonates, phosphotriesters,
phosphoamidates,
carbamates, etc.) and with charged linkages (e.g., phosphorothioates,
phosphorodithioates,
etc.), those with intercalators (e.g., acridine, psoralen, etc.), those
containing chelators (e.g.,
metals, radioactive metals, boron, oxidative metals, etc.), those containing
alkylators, those
with modified linkages (e.g., alpha anomeric nucleic acids, etc.), as well as
unmodified forms
of the polynucleotide(s).
Further, any of the hydroxyl groups ordinarily present in the sugars may be
replaced
by phosphonate groups, phosphate groups, protected by standard protecting
groups, or
activated to prepaxe additional linkages to additional nucleotides or to solid
supports. The 5'
2s
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
and 3' terminal OH groups can be phosphorylated or substituted with amines or
organic
capping groups moieties of from 1 to 20 carbon atoms. Other hydroxyls may also
be
derivatized to standard protecting groups.
Polynucleotides can also contain analogous forms of ribose or deoxyribose
sugars that
are generally known in the art, including, but not limited to, 2'-O-methyl-,
2'-O-allyl, 2'-
fluoro- or 2'-azido-ribose, carbocyclic sugar analogs, a-anomeric sugars,
epimeric sugars
such as arabinose, xyloses or lyxoses, pyranose sugars, furanose sugars,
sedoheptuloses,
acyclic analogs and abasic nucleoside analogs such as methyl riboside. As
noted above, one
or more phosphodiester linkages may be replaced by alternative linking groups.
These
alternative linking groups include, but are not limited to, embodiments
wherein phosphate is
replaced by P(O)S ("thioate"), P(S)S ("dithioate"), "(O)NR2 ("amidate"),
P(O)R, P(O)OR',
CO or CH2 ("formacetal"), in which each R or R' is independently H or
substituted or
unsubstituted allcyl (1-20 C) optionally containing and ether (-O-) linkage,
aryl, alkenyl,
cycloalkyl, cycloalkenyl or araldyl. Not all linkages in a polynucleotide need
be identical,
Substitution of analogous forms of sugars, purines and pyrimidines can be
advantageous in'
designing a final product, as can alternative backbone structures like a
polyamide backbone.
The terms "polypeptide", "oligopeptide", "peptide" and "protein" are used
interchangeably herein to refer to polymers of amino acids of any length. The
polymer may
be linear or branched, it may comprise modified amino acids, and it may be
interrupted by
non-amino acids. The terms also encompass an amino acid polymer that has been
modified
naturally or by intervention; for example, disulfide bond formation,
glycosylation, Iipidation,
acetylation, phosphorylation, or any other manipulation or modification, such
as conjugation
with a labeling component. Also included within the definition are, for
example, polypeptides
containing one or more analogs of an amino acid (including, for example,
unnatural amino
26
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
acids, etc.), as well as other modifications known in the art. Polypeptides
can occur as single
chains or associated chains.
A "ligand," as used herein, is a molecule which binds to a particular
receptor. The
receptor may be a cell receptor or it may be a portion of another molecule,
for example, a
receptor for an allosteric modifier of an enzyme. Examples of ligands include,
but are not
limited to, enzyme cofactors, substrates and inhibitors, allosteric modifiers
of enzymes,
agonists and antagonists for cell membrane receptors, toxins and venoms, viral
epitopes,
haptens, hormones, lectins, and drugs such as opiates and steroids.
A "cell receptor," as used herein, is a cellular molecule, which may be
normally
located either intracellularly or in association with the cell membrane, which
has an affinity
for a given ligand. Examples include, but are not limited to, hormone
receptors, cellular
transporters, cytokine receptors, and neurotransmitter receptors.
1.3 hnmobilization of Probes
Oligonucleotide probes of the invention are affixed, immobilized, provided,
and/or
applied to the surface of the solid support using any available means to fix,
immobilize;
provide and/or apply oligonucleotides at a particular location on the solid
support. The
various species may be placed at specific sites using ink jet printing (LJ.S.
Pat. No.
4,877,745), photolithography (See, U.S. Pat. Nos. 5,919,523, 5,837,832,
5,831,070,
5,770,722 and 5,593,839), silk printing, offset printing, stamping, mechanical
application
with micropipets using an x-y stage or other rastering technique, or any other
method which
provides for the desired degree of accuracy and spatial separation in placing
the bound
component.
Combinatorial array approaches, such as described by Southern et al. (LT.S.
Pat. Nos.
5,770,367, 5,700,637, and 5,436,327), Pirrung et al. (U.S. Pat. No.
5,143,854), Fodor et al.
(U.S. Pat. Nos. 5,744,305 and 5,800,992), and Winkler et al. (U.S. Pat. No.
5,384,261), have
27
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
been used with success in cases in which polymers of short sequences are
required. In these
"GeneChips," oligonucleotide probes (20-25-mers) or peptide nucleic acids
(PNAs) are
produced either in situ during microarray fabrication, or offline using
traditional methods and
spotted on the microarrays. U.5. Pat. Nos. 5,445,934 and 5,744,305 to Fodor et
al. describe
the manufacture of substrates containing multiple sequences at density of 400
different
probes per square centimeter or higher. These chip are synthesized using solid-
phase
chemistry and photolithographic technology. The combinatorial approaches
generate
significant biological and chemical diversity but are unable to construct
microarrays of large
macromolecules and can also be expensive and difficult to implement.
Ink jet dispenser devices are used to deposit small drops of liquid on a solid
substrate.
The fabrication of biological and chemical arrays by such technology has been
shown by
Brennan (U.5. Pat. No. 5,474,796), Tisone (ILS. Pat. No. 5,741,554), and Hayes
et al. (IJ.S.
Pat. No. 5,658,802). These non-contact technologies are unable to array large
numbers of
samples easily and to control the quality of the resultant microaxrays.
IS A.third category of arraying devices work by direct surface contact
printing as
described by Augenlicht (ILS. Pat. No. 4,981,783), Drmanac et al. (U.5. Pat.
No. 5,525,464),
Roach et al. (U.5. Pat. No. 5,770,151), Brown et al. (CT.S. Pat. No.
5,807,522) and Shalon et
al. (LT.S. Pat. No. 6,110,426). In this format, the probes are long
complementary DNAs
(cDNAs) 500-5000 bases Long, synthesized by traditional methods before
immobilization.
Deficiencies of such technologies as quill-based spotters include imprecise
sample uptake
and delivery as well as lack of durability.
Martinsky et al. (U.5. Pat. No. 6,101,946) describe the use of an electronic
discharge
machine (EDM) which can be attached to a motion control system for precise and
automated
movement in three dimensions. The oligonucleotide primers may also be applied
to a solid
support as described in Brown and Shalom U.S. Pat. No. 5,807,522 (1998).
Additionally, the
2s
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
primers may be applied to a solid support using a robotic system, such as one
manufactured
by Genetic Microsystems (Woburn, MA), GeneMachines (San Carlos, CA) or
Cartesian
Technologies (Irvine, CA).
One may use a variety of approaches to bind an oligonucleotide to the solid
substrate.
By using chemically reactive solid substrates, one may provide for a
chemically reactive
group to be present on the nucleic acid, which will react with the chemically
active solid
substrate surface. One may form silicon esters for covalent bonding of the
nucleic acid to the
surface. Instead of silicon functionalities, one may use organic addition
polymers, e.g.
styrene, acrylates and methacrylates, vinyl ethers and esters, and the like,
where
functionalities are present which can react with a functionality present on
the nucleic acid.
Amino groups, activated halides, carboxyl groups, mercaptan groups, epoxides,
and the like,
may also be provided in accordance with conventional ways. The linkages may be
amides,
amidines, amines, esters, ethers, thioethers, dithioethers, and the like.
Methods for forming
these covalent linkages may be found in U.S. Pat. No. 5,565,324 and references
cited therein.
One may prepare nucleic acids with ligands for binding and sequence tags by
primer
extension, where the primer may have the ligand and/or the sequence tag, or
modified NTPs
may be employed, where the modified dNTPs have the ligand and/or sequence tag.
For RNA,
one may use in vitro transcription, using a bacteriophage promoter, e.g. T7,
T3 or SP6, and a
sequence tag encoded by the DNA, and transcribe using T7, T3 or SP6
polymerase,
respectively, in the presence of NTPs including a labeled NTP, e.g. biotin-16-
UTP, where the
resulting RNA will have the oligonucleotide sequence tag at a predetermined
site and the
binding ligand relatively randomly distributed in the chain.
In the present invention, probes may be synthesized in situ on the substrate
or may be
manufactured then immobilized on the substrate. This technique has been
described for
polynucleotides in U.S. Patent No. 5,419,966, incorporated herein by
reference.
29
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
Alternatively, polymeric probe's, such as polynucleotides, may be synthesized
in a stepwise
fashion from individual monomers or from smaller polynucleotides or other
subunits.
Preferably, probes are immobilized in discrete areas of the substrate.
Alternatively, more than
one probe can be immobilized in a particular area, with individual probe
molecules of a
particular type being distinguishable from other probe molecules by
differential labeling, for
example, with differently colored fluorescent tags. "Immobilize," as used
herein, means to
attach a probe to the substrate by covalent or non-covalent means, with
sufficient affinity to
withstand manufacturing, sample-binding, sample analysis steps, and, if
necessary, re-use.
Methods and materials for derivatization of solid phase supports for the
purpose of
immobilizing polynucleotides and polypeptides are well-known in the art
and~are described
in, fox example, U.S. Patent Nos. 5,744,305 and 5,919,523, which are hereby
incorporated by
reference in their entirety. For non-covalent attachment, the preferred method
is by biotin-
streptavidin attachment, but any method of non-covalent attachment that
provides the
necessary affinity is possible with the present invention.
In addition to oligonucleotides or any other organic entity, assemblages of
molecules
may also be used as in the case of organelles, e.g. nuclei, mitochondria,
plastids, liposomes,
etc., or cells, both prokaryotic and eukaryotic. The bound component may be
directly bound
to a solid substrate or indirectly bound, using one or more intermediates,
which serve as
bridges between the bound component and the solid substrate. In general, where
a molecule is
to be covalently bonded to the solid substrate surface, the surface may be
activated using a
variety of functionalities for reaction, depending on the nature of the bound
component and
the nature of the surface of the solid substrate.
For example, one may use a variety of approaches to bind the oligonucleotide
to the
solid substrate. By using chemically reactive solid substrates, one may
provide for a
chemically reactive group to be present on the nucleic acid, which will react
with the
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
chemically active solid substrate surface. One may form silicon esters for
covalent bonding of
the nucleic acid to the surface. Instead of silicon functionalities, one may
use organic addition
polymers,=e.g, styrene, acrylates and methacrylates, vinyl ethers and esters,
and the like,
where functionalities are present which can react with a functionality present
on the nucleic
acid. Amino groups,~activated halides, carboxyl groups, mercaptan groups,
epoxides, and the
like, may also be provided in accordance with conventional ways. The Linkages
rnay be
amides, amidines, amines, esters, ethers, thioethers, dithioethers, and the
like. Methods for
forming these covalent linkages may be found in U.S. Pat. No. 5,565,324 and
references cited
therein.
One may prepare nucleic acids with ligands for binding and sequence tags by
primer
extension, where the primer may have the Iigand and/or the sequence tag, or
modified NTPs
may be employed, where the modified dNTPs have the Iigand and/or sequence tag.
For RNA,
one may use in vitro transcription, using a bacteriophage promoter, e.g. T7,
T3 or SP6~ and a
sequence tag encoded by the DNA, and transcribe using T7, T3 or SP6
polymerase,
respectively, in the presence of NTPs including a labeled NTP, e.g. biotin-16-
UTP, where the
resulting RNA will have the oligonucleotide sequence tag at a predetermined
site and the
binding ligand relatively randomly distributed in the chain.
1.4 Markers
The position of each probe on the substrate, as well as other information
about the
probe andlor the probe-sample complex, can be determined by using markers for
probes or
sets of probes. Such maxkers may be used with conventional two-dimensional
arrays as well
was with the present, one-dimensional configurations. "Markers," as used
herein, are any
type of identifiable marking, arrangement, or other structure or pattern on,
in, or associated
with the substrate andlor probes which conveys information about a particular
probe or set of
probes. One type of marker can be optical. These can be space markers (i.e.,
breaks in the
31
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
row of probes on the substrate, as described above) and/or bar codes ,
fluorescent markers,
chemilluminescent markers, or any other marker capable of being detected with
light. A
further type of marker is magnetic markers. The present invention lends itself
to such markers
because the substrate may contain metallic elements which are magnetizable.
They may be
located on the same side of the substrate as the probes are, on the other side
of the substrate
from the probes, or may be sandwiched or otherwise incorporated into the
interior of the
substrate. One method for space marking and/or additional information is to
coat the reverse
side of the substrate from the probes with a layer of magnetic thin film. Then
spatial or probe
identification can be recorded during the fabrication process, by magnetic
means. An
important advantage of this approach is that additional information regarding
the target can
be written on to the substrate itself during the hybridization stage. And at
the scanning stage,
scanning parameters and other digital outputs may also be written on to the
same tape for
further reference. Other types of markers will be apparent to one of ordinary
skill in the art.
1.5 Sets of probes.
Probes may be immobilized in sets on the substrate, each set sharing some
common
characteristic. For example, if probes are nucleotides, a group of nucleotides
requiring
common hybridization conditions may be immobilized along a certain length of
substrate,
and another group requiring a different set of hybridization conditions may be
immobilized
along another length of substrate. In this manner, each set of probes may be
exposed to
sample under a different set of conditions, optimizing sample binding.
Alternatively, a single probe carrier can carry different sets of probes for
diagnosing
different diseases. For example, one set of probes located along one stretch
of a carrier might
be used to diagnose H1V, which another set could be used to diagnose herpes,
etc. As another
example, a carrier or portion of a carrier could be devoted to the HER-2/neu
gene. The HER-
2 gene, also known as HER-2/neu and c-erbB2, plays a key role in the
regulation of normal
32
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
cell growth, but during the development of cancer, it becomes amplified. The
amplified HER-
2 gene results in the over-production of protein receptors found on the
surface of tumor cells.
These special proteins bind with other circulating growth factors to cause
uncontrolled tumor
growth. The probe Garner could contain probes for the HER-2/neu genes) and
variations.
In another embodiment, the sets of probes may be redundant, allowing a single
carrier
to be used repeatedly for the same assay, with a new set ofprobes used for
each successive
assay.
1.6 Configuration of the apparatus.
Probes may be immobilized on the substrate in any configuration that allows
one to
distinguish and identify probes which have bound sample from those which have
not. The
simplest way to do this is by placing probes in discrete areas, one probe per
area. The areas
may be spots, as shown in Fig. 1, or lines, as shown in Fig. 4, 404. The areas
may be
configured as a single row running along or parallel to the long axis of the
substrate. The
probes may be directly attached to the substrate, or, in an alternative
embodiment, the probes
may be attached to beads which are then attached to the substrate. Methods of
attaching
probes to beads of various materials are well-known in the art and are
described in, for
example, WO 99/60170, which is incorporated herein by reference in its
entirety. Another
alternative possible configuration is to have rows of spots, so that a
plurality of probes is
contained in a given row.
As illustrated in Figure 1, DNA probes 100 axe immobilized as spots at the
center or
as narrow stripes across the width of a long, thin and flexible thread
substrate 100. Probe
identification is achieved through space markers and/or bar codes 120 printed
in the space
130 between groups of probes. Alternatively, these markers can be printed on
the other side
of the thread substrate. If necessary, the thread 100 can have a special cross-
sectional shape.
As shown in Fig. 2a, the cross-section of the fiber can be adjusted so that
the fiber 200 has a
33
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
notch or groove 202 in which probes 110 are immobilized. This design protects
the probes of
one layer from friction with the substrate of a succeeding layer, as shown in
Fig. l 1b, where
the cross-sections of two successive layers are shown one on top of the other.
The long, thin, and flexible nature of the probe carrier lends itself to
numerous novel
means of containment, arrangement and use. The probe carrier may be packaged
in a number
of formats including but not limited to a pin, a rod, a coil and a spool.
Hybridization methods
are considerably enhanced by requiring less hybridization fluid and enhanced
mixing. A
flexible probe carrier packaged in a spool is especially advantageous in
applications that
require high volume, low to medium scale microarrays, such as those involved
in disease
diagnostics. In these applications, the required number of probes in the array
may be small (in
the range of several hundreds to several thousands) but a very large number of
the same type
of arrays may be available for consumption every day. With the flexible probe
carrier format,
tens of thousands of copies of identical sets of probes are arrayed
repetitively along a
continuous length of thread and sealed in a large coil or spool.
A pin or rod package is made by spirally winding a certain length of
fabricated
flexible probe Garner thread around a section of solid cylinder or tube. The
thread sits tightly
side-by-side on the outer surface of the supporting cylinder in the preferred
embodiment and
may be permanently attached to it by glue, cement or other means. The
difference between
the probe carrier pin and the probe Garner rod is the size. A probe carrier
pin normally has a
diameter less than 1 Omm while a probe carrier rod is larger and can have a
much larger
diameter thus accommodating many more probes. For example, a 1.5 meter long,
SO~,m
diameter thread occupies only a short Smm section after being wound on a Smm
diameter
probe carrier pin, which may carry approximately 15,000 probes, presuming a
100~m probe
space along the thread. On the other hand; a probe carrier rod of 30mm wide
and 40mm in
34
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
diameter can accommodate as many as 700,000 probes along a 70 meter long,
SO~,m diameter
thread.
In a probe Garner coil, the fabricated flexible probe carrier thread is wound
into a flat,
disc shape coil. In one preferred embodiment of the invention, the probes on
the thread are
exposed on one side of the disc while the other side is permanently attached
to a solid disc-
shaped planar support by epoxy, cement or other suitable means. Optionally,
probes are
deposited in a notch on the surface of the probe carrier thread. The planar
support can be pre-
coated with a conductive layer to facilitate hybridization control. Assuming a
SO~,m diameter
thread, a probe carrier coil of 40mm in diameter can accommodate up to 24
meters of probe
carrier thread, carrying 240,000 probes.
The configuration of a probe carrier spool can be very similar to that of the
probe
carrier coil. However, unlike the probe carrier coil, the probe carrier thread
is not
permanently attached to a supporting surface, thus allowing the thread to
unwind from the
spool for hybridization, reading and other purposes. In addition, since each
turn of the thread
stacks on top of each other in the spool, the cross-section shape of the probe
carrier thread
can be designed to avoid friction between DNA probes and the probe carrier
thread in an
adjacent turn. Also shown in Figure 17, a cassette can be constructed to
protect the probe
carrier spool and facilitate its winding and unwinding. In addition, multiple
spools can be
stacked up in one cassette.
2. Fabrication of the Probe Carrier
In certain embodiments of the probe deposition technology discussed herein, a
fiber-
or tape-like substrate is intrinsically suitable for continuous, high speed,
mass production.
Figures 3 through 7 show examples of fabrication systems designs. Although
conventional
spotting techniques may be used to produce discrete areas containing one probe
each, one
aspect of the present invention is the use of brushing or painting of probes
on substrates. Such
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
techniques, coupled with the essentially one-dimensional nature of the
substrate, lend
themselves to fabrication systems in which multiple copies of the same tape or
fiber may be
manufactured at once at high speed and with great precision.
2.1 Multi-stranded brush
An exemplary embodiment of this apparatus and a method of manufacture is
presented in Fig. 3. One method of manufacture, shown in Fig. 3, comprises
transporting
probes, which are either in a suitable liquid or are liquid themselves (for
example, some lipids
which are liquid at room temperature or can be liquefied at suitable
temperatures), into tubing
and depositing the probe from the tubing onto the substrate by moving the
tubing relative to
the substrate while at the same time driving the probe-liquid from the tube
onto the substrate.
It will be understood that such movements are relative and can be accomplished
by moving
the tubing assembly, moving the substrate, or both. During deposition, the tip
of the capillary
tube may contact the substrate surface. Alternatively, the tip may move a
short distance above
or underneath the substrate surface. The probe fluids are deposited onto the
substrate using
one of the non-contact deposition methods. These include attaching probes to
magnetic beads
suspended in the probe fluid and placing an electromagnet under the substrate.
The magnet is
activated during when the capillary and substrate intersect (i.e. the
capillary passes in
proximity to the substrate), which pulls the magnetic beads and their
associated probe onto
the substrate surface. Another non-contact deposition method is to coat a
metal layer on both
the end facet of the capillary tubing and either the substrate surface or the
support under the
substrate, then apply a high voltage between the capillary and the substrate
or substrate
support. The electric field will pull the electrically charged probes (such as
oligonucleotides)
onto the substrate surface. Using either of the above two methods, if the
electric activation
signal is a very short pulse, probe will be deposited on the substrate as a
dot. If the signal is
on for much or all of the time that the capillary and substrate intersect, the
result will be a
36
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
stripe of probe on the substrate. Conditions may be selected to ensure
immobilization of the
probe on the substrate. A plurality of tubes may be joined together to create
a "brush" capable
of depositing multiple probe stripes simultaneously. Further, a plurality of
such brushes may
be arranged to multiply the number of probes which may be deposited, either
simultaneously
or sequentially as different brushes move relative to the substrate and
deposit probe stripes. In
addition, if the substrate is a fiber, several fiber substrates may be
positioned so that one
stroke of a brush deposits probes stripes on all of the substrates.
Alternatively, a wide tape
substrate may be used to receive the probe stripes, then the tape may be cut
lengthwise into a
plurality of individual, thinner, tapes. It can be appreciated that such a
method of manufacture
greatly multiplies the number of probe carriers which may be produced
simultaneously,
increasing throughput and reducing cost. It can also be appreciated that
standard mass
production methods, such as the use of conveyor belts, can be readily adapted
to automate
and control this and other methods of manufacture presented herein.
In Figure 3, a set of flexible capillaries 300 are glued into a hole under
each well of a
standard microtitre plate 302. A capillary 300 can also be inserted into the
well from the top.
The capillary 300 is then lined up into a linear array to form a "brush" 304.
Each individual
DNA probe is stored in a separate well in the plate and is driven into the
capillary 300 linked
to the well by pressure differentiation or by applying a voltage between the
well and the tip of
the brush 304. Because DNA molecules are negatively charged, a negative
polarity should be
applied to the well end. Multiple such capillary brushes can be constructed.
After the
capillaries are filled, the capillary array is moved to "brush" across a
stationary probe caxrier
tape substrate 306 and deposit an array of DNA probes I I0, then the tape
substrate 306 will
move forward to a new position to enable a second capillary "brush" 304 to
deposit more
probes 110 on subsequent positions. Alternatively, the same brush can be used
to deposit
more copies of the same probe array along the tape substrate 306. In
additional, a large
37
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
number of thread substrates can be laid in parallel under the brush so that
each "brushing"
action can produce multiple copies of the 1-dimensional probe array on
different threads or
tapes.
A further refinement of this technique, and of all the techniques presented,
is to also
deposit or establish markers for the probes 110 (see, for example, Fig. 1).
Such markers may
be spaces between or around probes, or they may be optical bar codes (Fig. 1,
120), or
fluorescent markers, or magnetic markers encoded on a metallic element in the
substrate, or
any other means that would serve to identify a particular probe or group of
probes. They may
be established on the same side of the substrate that the probes are deposited
on, on the
opposite side, or both. It will be understood that a substrate may have only
one surface (for
example, a fiber with a circular cross-section), and that the term "side" in
this context refers
to the particular area of the surface on which probe is deposited. In the case
of a tape, with a
more defined top surface and bottom surface, "side" means one of these top or
bottom
surfaces.
I5 A variety of means may be used to provide the force to drive probe from the
reservoir,
into the tubing, and onto the substrate. For example, a pressure differential
may be
established. Alternatively, if the probe is charged--as is the case with, for
example, DNA--a
voltage may be established between the reservoir and the substrate, such that
the probe moves
from the reservoir and tubing onto the substrate. The substrate may contain a
metallic
element such as a metal layer which forms an electrode.
2.2 Probe printin hg-earls
Figure 4 shows a second design of a probe carrier thread fabrication system,
where
each probe is stored in its own "printing head" 4I0 of a print system 408. A
large number of
such printing heads 410 are arranged into a one-dimensional array on a
conveyer belt 400
moving at a constant speed Vh. The belt can wind across pulleys or capstans
412 into a spools
38
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
406 to conserve space. The spacing between the heads can be as large as
several millimeters
and be sufficient to accommodate a reservoir for each probe. A probe earner
tape substrate
402 is placed under the printing heads array, also moving at a constant,
albeit slower speed
Vt. When a printing head intersects the probe carrier tape substrate 402, it
"prints" a spot or a
stripe on the probe earner tape substrate 402. Presuming the spacing between
two adjacent
printing heads on conveyer belt is L~, and the desired spacing between two
adjacent probes on
the probe carrier tape substrate 402 is Lp, the speeds of the printing head
belt Vp and that of
the probe carrier tape substrate 402, Vt, can be precisely controlled to
satisfy Vp/Vt = Lp/Lr .
In this way, a linear array of the DNA stripes 404 can be deposited on the
probe carrier tape
substrate 402 at high speed in a continuous fashion. The line may be diagonal
across the
substrate, since the substrate is moving, or the substrate and conveyor may
intersect at an
angle that results in a line of probe that is perpendicular to the long axis
of the substrate. The
substrate may instead be stopped while the print head prints, then advanced to
the next
printing position before the next probe is applied.
Each printing head in the system consists of a reservoir that holds a certain
quantity of
the probe sample and a means to transfer the probe onto probe carrier or tape
thread substrate:
Probes are dispersed in a liquid (or are themselves liquid) which provides the
necessary
conditions for transfer and immobilization of the probes on the substrate, the
exact nature of
which depends on the particular probe and the particular substrate.
Figure 5 shows some possible designs for the printing heads. In Figure 5a, a
very thin,
flexible fiber 500 is attached to a small opening 502 under a probe reservoir
504. The fiber
500 is hydrophilic so it draws the probe fluid onto its surface through
surface tension or
capillary effect. In addition, the fiber 500 has to be thin (<80~,m), flexible
and yet not deform
plastically. A solid or hollow silica fiber coated with metal or nylon is a
good candidate.
When intersected with the probe carrier tape substrate 402, it "draws" a
stripe on the surface
39
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
of the probe Garner tape substrate 402 using the probe as "inlc". In the case
of metal coated
fiber, a negative voltage can be applied to the fiber to push the DNA sample
on to the probe
Garner thread or tape.
Figures 5(b)-(h) show different designs based on the ink jet principle, where
a pin
hole is produced on the bottom of the probe reservoir. Pulse energy is
introduced into the
reservoir, which ejects droplets out of the pinhole on to the probe carrier
thread underneath.
In Figure Sb, a piezo ring 506 is glued on the wall ofthe reservoir tube,
which squeezes the
tube under a voltage and ejects a droplet. In Figure Sc, a piezo-film 506 is
coated on a
diaphragm 508 on top of the reservoir 504, which will have the same function
as the piezo
ring but could be less expensive when using a large number of reservoirs 504.
In Figure Sd, a
current pulse through a resister wire 510 generates a bubble through localized
heating, which
in turn pushes out the droplet. In Figure Se, the ejecting energy is
introduced through an
external ultrasound transducer 512. In Figure Sf, the reservoir tube is
transparent and the
heating is realized by focusing a laser 514 to a light absorption patch inside
the tube. In
Figure Sg, the reservoir 516 is made of metal. A high voltage is applied
between the reservoir
and the probe carrier tape substrate 402 (or object underneath the probe
carrier tape substrate
402) with the negative polarity on the reservoir. Since DNA carries negative
charge, the
electric field will eject the sample on to the probe carrier tape substrate
402 surface. In Figure
Sh, probe molecules are attached to magnetic beads suspended in fluid 520
within reservoir
518. A current pulse is applied to the electromagnet 519 underneath the
substrate, which
attracts the probe from the small opening under the reservoir onto the
substrate surface 402.
Note in design Se to Sh, the actuators are external and do not move with the
printing head.
Since each reservoir (504 or 516 or 518) only intersects the probe carrier
tape substrate 402
once at a fixed location, only one such actuator is needed in the system.
Presuming a
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
reservoir spacing of 2mm, a 150,000 reservoirs array is 300m long and can be
accommodated
in a spool less than 80cm in diameter.
2.3 Spotters and reservoirs
Fig. 6 illustrates another probe carrier fabrication system design, where the
printing
head configuration of the previous design is separated into a "spotter
configuration" and a
"reservoir configuration." Each probe has its own reservoir, the structure of
which is kept
simple to reduce cost. Figure 6a illustrates one of the possible reservoir
designs, where the
combination of liquid internal pressure and surface tension causes the liquid
600, which
contains the probe 110, to bulge up a little at the opening 612. A large
number of reservoirs
602 are assembled on a conveyer belt to form a linear array. As shown in Fig.
6b, the spotter
configuration 604 can be fabricated by, for example, shaping a thin metal tape
into a linear
configuration of miniature teeth or gluing short silica fibers 606 to a
flexible metal tape 608.
Any material or combination of materials which produces a row of fibers
suitable for
transferring probe may be used. The tip of the spotter is suspended a short
distance above the
reservoir opening that allow the tip to contact the probe fluid. When the
spotter is made of
highly elastic materials, such as silica fiber, the spotter tip can actually
slightly lower the
opening so that the spotter can tip into the opening to collect the probe
fluid. Both the spotter
and reservoir configurations are driven to travel at e.g. a constant speed in
different directions
as indicated by arrows 614 and 616. When a spotter intersects opening 612 of a
reservoir 602,
a droplet of probe liquid 600 will be transferred from the reservoir to form
droplet 610 on the
spotter. The amount of liquid in the droplet can be controlled by the duration
of the
intersection and the shape and size of the spotter. The movement pattern of
the spotter and
reservoir configurations is designed in such a way (to be described later)
that each
consecutive spotter will intersect with corresponding consecutive reservoir so
that each
spotter now carries a different probe droplet. Then, as illustrated in Fig.
6c, the spotter
41
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
configuration 604 moves on to intersect with a substrate 618 moving in a
direction such that
the spotter configuration and the substrate intersect. As with the reservoirs,
the tip of each
spotter 606 may physically contact the substrate or may be suspended a very
short distance
(several tens of micrometers) above the substrate, at a distance that allows
droplet 610 to
contact the substrate 618. The probe droplet will be transferred from the
spotter configuration
to the substrate to form a linear probe configuration 630 on the substrate. If
the probe is
charged, it is possible to charge a particular spotter by electronic means
when it intersects a
probe reservoir with a charge that will attract probe, then switch the charge
on the spotter to
the opposite charge when it later intersects the substrate, in order to repel
the probe from the
spotter and onto the substrate. This refinement allows the size of the droplet
on the spotter to
be controlled precisely with good consistency. Such a method to transfer probe
material to
the substrate is termed "spotting", and is widely used manually in
laboratories.
Alternatively, as shown in Figure. 6d, the spotter configuration 604 may move
in a
circle and the number of spotters in the configuration can be far fewer than
the total number
of probes. After the spotter leaves the substrate 618, it can be washed in a
washing area 620,
dried in a drying area 622 and reused by circling around to intersect 626 the
probe reservoir
configuration 624 again. Since the washing is carried out in parallel with
spotting, it does not
affect fabrication throughput. A spotter according to this invention is easy
to clean
In an alternative configuration shown in Figure 6e, the probe reservoir can be
a
straight tubing 632 or a well 634 with a small opening 636 at its bottom 638.
The probe fluid
600 bulges downwaxd at opening due to the combination of gravity and capillary
force. The
spotter 606 intersects the probe reservoir from underneath and collects some
probe fluid 600
at its tip. Then the spotter moves on to intersect the probe carrier substrate
610 and paints a
narrow stripe on the substrate in a configuration similar to Figure 6c, except
the spotter
carrier 406 now passes under the substrate instead of above it. The substrate
is positioned
42
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
face down to be painted by spotters. The configuration of the entire
fabrication system
including probe collection, spotting, washing and drying is similar to that
shown in Figure 6d.
In the two fabrication systems described above, every component can move at a
pre-
defined, constant speed. This reduces the complexity of the motion control and
precision
requirement. In addition, a large number of probe carriers 100 can be
manufactured
continuously without manual intervention. As a result, the manufacturing
throughput can be
very high. Furthermore, if silica fiber or thin wire is adapted as the probe
Garner substrate,
many fibers can be attached in parallel to a wide carrier tape for the
fabrication stage. So,
multiple copies of the same probe carrier thread can be manufactured at the
same time.
A wide tape substrate can be used on the fabrication station, with probe
droplets being
deposited in a line across the tape as illustrated in area 628 of Fig. 6d. The
wide tape can be
cut after the probe deposition to produce many copies of the same probe Garner
threads 100,
as illustrated in Fig. 1. In either case, the throughput can be further
dramatically increased.
These two system designs are therefore suitable for mass production at a
dedicated central
microarray fabrication facility. In one system of the invention, spacing
between probes on the
thread and that between reservoirs (and spotters) can be 100~,m and 5mm,
respectively.
Assuming the thread substrate moves at a speed of lcm/s, the reservoir and
spotter arrays
travel at 50cm/s, which is easily to achieve. Further, where the carrier tape
618 is separated
into 20 thread substrates, the above two system designs should be capable of
manufacturing a
150,000 probe array every 7.5 seconds.
2.4 Spotter matrix
Figure 7 shows a fourth fabrication system design, which has more flexibility
and is
particularly suitable for custom fabrication of smaller scale probe carriers.
Fig. 7a is an
overhead view and Fig. 7b is a frontal view of the system. Here, probes are
stored in standard
microtitre plates or similar matrix containers 700. A matching spotter matrix
702 has the
43
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
same spacing as the well matrix in the plate. Different from conventional
spotting pins, each
spotter is a thin, flexible hydrophilic fiber 704, as used in the preceding
system. The spotter
matrix is first dipped into the well matrix, then moved to intersect the probe
carrier tape
substrate 706, which is temporarily held stationary. The direction that the
spotter moves is
perpendicular to the probe Garner tape substrate 706, but the direction of the
matrix rows is
tilted at a small angle of a. Each spotting fiber will produce a separate line
708 across the
probe carrier tape substrate 706 (see enlarged view) for:
a = arc.sin[L~/(C+1)LR] (1)
where L~ and LR are the fiber spacing in column and row of the spotter matrix,
respectively and C is the number of columns in the spotter matrix.
The spotter matrix array is washed and cleaned in a cleaning area 710 and
dried at a
drying station 712. At the same time, the probe carrier tape substrate 706
advances to a fresh
section and a new well matrix 712 is loaded, ready for the next "dipping and
spotting" cycle.
In this design, the spacing between probes on the probe carrier tape substrate
706 is given by
Le/(R+I), and can be about 250~,m. Such a density is useful for smaller scale
custom arrays,
as a probe carrier tape substrate 706 carrying 10,000 probes at a 259 um probe
spacing is 2.5
meters long, which can be wound into a spool less than 3 cm in diameter.
3. Packaging of Probe Carriers
The flexibility of the probe carrier thread platform enables the fabricated
probe carrier
thread to be packaged into a wide variety of different formats, which
includes, but is not
limited to, probe Garner pin, probe carrier rod, probe carrier coil and probe
carrier spool. The
superior strength, precision and flexibility of the probe Garner thread
substrate are ideal for
the precise probe positioning and transportation required in the probe carrier
thread
fabrication and reading process. Assuming both the probe spacing and thread
thickness being
100~.m, the entire set of human genes (150,000) can be accommodated along a
15m thread,
44
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
which can be wound into a spiral coil I.Scm high and 3cm in diameter or a
spool of O.Imm
thick and less than 4cm in diameter. Thus probe carrier thread packaging is
preferred for
greater compaction of probes. Several modes of packaging a probe carrier
thread and tape are
described below.
3.1 Probe Carrier Pin and Probe Carrier Rod
As shown in Figure 8, probe carrier pin 810 and probe carrier rod 820 are made
by
spirally winding a certain length of fabricated probe carrier thread 100
around a section of an
elongated support member 804 such as a solid cylinder or tube. The tightly-
wound thread .100
sits side-by-side 806 on a section 802 of the outer surface of the supporting
cylinder 804 and
may be permanently attached to it by glue, cement or other means. The cylinder
804 maybe
coated with conductive material before the winding process for hybridization
control. The
probes 110 are located on a side of the probe carrier thread 100 distal from
the side of the
probe carrier thread 100 which is contact with the support member 804.
As discussed previously, the difference between the probe carrier pin 810 and
probe
carrier rod 820 is the relative size and shape. A probe carrier pin 810
normally has a diameter
less than l Omm while a probe carrier rod 820 is larger and thus accommodates
many more
probes. For example, a I .5 meter long, SOpm diameter thread occupies only a
short Smm
section after being wound on a Smm diameter pin 810, which may carry
approximately
15,000 probes, presuming a 100pm probe space along the thread 100. On the
other hand, a
probe carrier rod 820 of 30mm wide and 40mm in diameter can accommodate as
many as
700k probes along a 70 meter long, SO~,m diameter thread 100. In one
embodiment, the
flexible probe carrier may be a tape substrate carrying probes immobilized in
a 2-dimensional
array. Fabrication of such arrays is illustrated in Figs. 3 and 4 for
instance. Such a flexible
probe caxrier tape can be wrapped around a pin 810 or a rod 820 instead of
winding a probe
carrier thread 100.
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
The probe carrier pin 810 and probe carrier rod 820 described above can be
manufactured efficiently at a high throughput. As illustrated in Figure 9, a
certain length of
"blank" space 904 is introduced between any two sets of probe arrays along the
probe carrier
thread or tape 100 during thread fabrication and prior to placing the thread
or tape on the
supporting cylinder. Then the probe carrier thread 100 is wound continuously
along a long
supporting cylinder 804. The cylinder 804 is pre-coated with epoxy or other
adhesive at
certain positions, where sections of the probe carrier thread 100 carrying
probes 110 will be
attached. After the epoxy is cured, the Iong cylinder 804 with thread 100 on
can be cut at
appropriate intervals to produce multiple probe carrier pins 810 or probe
carrier rods 820 with
probe carrier thread 100, with probes 110 attached, wound around the cylinder'
and at certain
sections 902. Because the section 904 of the supporting cylinder 804, where
the blank thread
is attached to, is not pre-coated with epoxy, the blank thread will come loose
and break off
the cylinder after cutting, thus exposing a section 904 of the original
supporting cylinder,
which can be used to fit into adapters during the hybridization process.
3.2 Probe Carrier Coil
In a probe carrier coil shown in Figure 10, the fabricated probe carrier
thread 100 is
wound into a flat, disc shape coil 1012. Fig. 10a shows a top view and Fig.
10b shows a side
view of a probe carrier coil 1012 assembly. The probes 110 on the thread 100
are exposed on
one side of the disc 1012 while the other side is permanently attached to a
solid planar
support disc 1010 by epoxy, cement or other suitable means. Note that in
Figure l Oc, which
illustrates an enlarged view of a cross-section 1000 of a probe carrier coil
1012, probes 110
are deposited in a notch 202 on the probe carrier thread 100 surface. This
feature is optional
in this packaging format. The planar support 1010 can be pre-coated with a
conductive layer
to facilitate hybridization control, which will be discussed in detail below.
Presuming a SO~.m
46
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
diameter thread, a probe carrier coil 1012 of 40mm in diameter can accommodate
up to 24
meters of thread, carrying 240,000 probes.
3.3 Probe Carrier Spool
The configuration of a probe carrier spool 1110 is very similar to that of the
probe
carrier coil. However, unlike probe carrier coil 1012, the probe carrier
thread 100 is not
permanently attached to a supporting surface 1010, thus allowing the thread to
unwind from
the spool for hybridization, reading and other purposes (although the end of
the thread may
be attached to the substrate). In addition, as shown in Figure 1 1b, since
each turn of the
thread 100 stacks on top of each other in the spool 1110, the cross-sectional
shape of the
probe carrier thread 100 can be designed to avoid friction between DNA probes
and the
thread in adjacent turns. The cross-section of the substrate used to
manufacture the probe
Garner thread 100 can be selected such that the fiber 200 has a notch or
groove 202 in which
probes 110 are immobilized. This design protects the probes of one layer from
friction with
the substrate of a succeeding layer. Also, as shown in the Fig. 11 a, a
cassette 1100 can be
1 S constructed to protect the spool 1110 and facilitate its winding and
unwinding. In addition,
multiple spools 1110 can be stacked up in a single cassette 1100.
4. Hybridization
The use of an apparatus according to the present invention involves: 1)
preparation of
the sample; 2) formation of a probe-sample complex; and 3) analysis of the
binding pattern in
order to identify the individual probes to which sample has bound.
The preparation of the sample varies, depending on the sample type. Sample
preparation protocols for analysis of polynucleotides, including labeling of
samples with
fluorescent tags in order to facilitate step 3), analyzing the binding
pattern, are well-known in
the art. See, for example, U.S. Patent No. 5,800,992, which is hereby
incorporated by ;
reference in its entirety. In the case of polynucleotides, the sample is
fragmented, using
47
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
Known techniques such as restriction endonuclease digestion, converted to
single-stranded
form, and the single-stranded fragments are labeled with an appropriate
fluorescent tag.
Upon contact of sample with the apparatus, sample or sample fragments which
have
an affinity for particular probes bind with those probes. Present microarrays
generally utilize
hybridization of complementary strands of DNA as the binding method. DNA
hybridization
is highly dependent upon hybridization conditions, which have been extensively
studied and
described; see, for example, U.S. Patent Nos. 6,054,270 and 5,700,637, which
are hereby
incorporated by reference in their entirety.
However, the present invention also encompasses any sort of sample-probe
binding
which will allow one to derive information from determining which probes have
bound to
sample or sample fragments. Examples include determining the identity of
antigens or
antibodies in a sample by using various antibodies or antigens, respectively,
as probes, or
identifying hormones in a sample by the receptors to which they bind, etc. The
list of
sample/probe pairs extends to any sets of pairs which bind with each other
with a sufficient
degree of affinity and specificity to be identified, and further examples of
sample/probe pairs
will be readily appaxent to those of skill in the art.
Nucleic acid hybridization generally involves the detection of small numbers
of target
nucleic acids (DNA and RNA) among a large amount of non-target nucleic acids
with a high
degree of specificity. Stringent hybridization conditions are necessary to
maintain the
required degree of specificity and various combinations of agents and
conditions such as salt,
temperature, solvents, denaturants and detergents are used for the purpose.
Nucleic acid
hybridization has been conducted on a variety of solid support formats. (see,
e.g., Beltz, G.A.,
et al., Methods in Enzymology, Vol. 100, part B, 19: 266-308, Academic Press,
NY (1985)).
Recent developments in DNA microarray technology make it possible to conduct a
large-scale assay of a plurality of target molecules on a single solid phase
support. Generally,
48
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
a DNA chip including an oligonucleotide array is comprised of a number of
individual
oligonucleotides linked to a solid support in a regular pattern such that each
oligonucleotide
is positioned at a known location. After generation of the array, samples
containing the target
sequences are exposed to the array, hybridized to the complementing
oligonucleotides bound
to the array, and detected using a wide variety of methods, most commonly
radioactive or
fluorescent labels. U.S. Pat. No. 5,837,832 (Chee et al.) and related patent
applications
describe immobilizing an array of oligonucleotide probes for hybridization and
detection of
specific nucleic acid sequences in a sample.
This invention also provides some specially designed equipment for the
hybridization
of probe Garner thread based microarrays. In existing systems, hybridization
is achieved by
either natural diffusion or forced fluid circulation. The format is slow and
the latter system .is
complicated to fabricate. In one embodiment of this invention, the
hybridization chambers are
designed to ensure that there is only a very thin layer of target fluid
between the probe carrier
thread or its packaged form and the inner wall of the hybridization chamber.
In this way, only
a very small volume of the target fluid is required for the hybridization,
improving contact
between probe molecules and target molecules. Hybridization acceleration is
thus achieved
by e.g. moving the probe Garner thread 100 or its packaged format through the
target fluid.
In addition, the hybridization process can be further controlled by applying a
voltage
between the support of probe carrier thread and the inner wall of the
hybridization chamber.
During the process, hybridizations may also be accelerated by adding cations,
volume
exclusion or chaotropic agents. When an array consists of dozens to hundreds
of addresses, it
is important that the correct ligation product sequences have an opportunity
to hybridize to
the appropriate address. This may be achieved by the thermal motion of
oligonucleotides at
the high temperatures used, by mechanical movement of the fluid in contact
with the array
surface, or by moving the oligonucleotides across the array by electric
fields. After
49
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
hybridization, the array is washed sequentially with a low stringency wash
buffer and then a
high stringency wash buffer.
As shown in Figure 12, when the probe carrier thread 100 has positive
polarity, the
DNA molecules in the target fluid are attracted towards the probe Garner
thread 100, creating
a temporary localized concentration near the thread surface to enhance
hybridization. If the
polarity is reversed, the electric field will repulse mismatched nucleic acid
molecules away ,
from the probes 110 while hybridized probes retain their target molecules,
thus increasing the
specificity df hybridization. Therefore, an AC oscillation voltage 1220 can be
applied
between the probe carrier thread 100 or its support 1200 and the wall of the
hybridization
chamber 1210 to improve the efficiency of the process. The support 1200 and
the wall of the
hybridization chamber 1210 have conductive coating 1212 in order to facilitate
the process.
As described below, all probe Garner thread formats may also rotate during
hybridization,
increasing agitation and mixing and thus improving contact between probes and
target
molecules. A brush slip ring can be used to conduct voltage on to the moving
electrode. The
design of such electric slip ring is well known in the art.
As shown in Figure 13, a probe Garner pin 810 can be hybridized by directly
plugging
into a well 1300 containing target fluid 1310. The diameter of the well 1300
is only slightly
larger than the outer diameter of the probe carrier pin 810. As there is only
a very thin layer
of target fluid 1310 between the probe carrier pin 810 and.the inner wall of
the fluid well
1300, the required volume for the target fluid is minimal. For example,
presuming the well is
8mm in diameter and the probe carrier pin is only SO~,m smaller in diameter
and the wound
section is Smm high, 3~,1 target fluid would be sufficient to cover the entire
effective section
of the probe carrier pin. The probe carrier pin can undergo an up-and-down
translational 1330
or back-and-forth 1332 rotational motion, or the combination of the two, in
order to increase
the hybridization speed. Because of the spiral winding pattern on probe
carrier pin 810, probe
so
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
carrier rod 820 and probe carrier coil 1012, rotational motion drives the
target fluid along not
only the circular direction but also the axial or radial directions of the
package. It efficiently
moves ''the molecules in the target over the entire surface area covered by
probe carrier thread
100.
Multiple probe carrier pins 810 can be plugged into an adapter frame 1400 to
form a
matrix that is compatible with the spatial pitch and pattern of a standard
microtiter plate 1420.
In this way, multiple hybridization processes can be carried out in parallel
directly in a
standard microtiter plate 1420 by dipping each probe earner pin 810 into a
corresponding
well 1410 of the standard microtiter plate 1420, as shown in Figure 14, and
optionally
translating the adapter plate up and down or rotating the individual probe
earner pins.
A probe earner rod 820 can be hybridized in a similar, albeit larger
hybridization
chamber as that of probe earner pin. Alternately, a chamber design 1500 shown
in Figure 15
can be used. A probe earner rod 820 is rotated 1520 to move the target fluid
1510 over the
probes. Because of the spiral winding pattern of the probe carrier thread 100
on the probe
1.5 carrier rod 820, target fluid 1510 can be moved not only along the
circular but also axial
direction of the rod 820, thus covering all probe positions on the probe
caxrier rod 820. An
AC oscillation voltage 1530 can be applied between the probe earner thread 100
and the wall
of the hybridization chamber 1500 to improve the efficiency of the process.
A hybridization chamber design 1600 for probe carrier coil 1012 is shown in
Figure
16. Again, a back and forth rotational motion 1620 is introduced to the coil
through a
mechanical drive or a magnetic drive and AC oscillation voltage alteration
1630 is applied
between the coil support and the chamber to enhance the hybridization
efficiency.
Figure 17a shows a chamber design 1700 for the hybridization of probe carrier
thread
100 that is unwound from a probe carrier spool 1110, in which a mostly water-
tight capillary
1760 is formed by closing a lid 1770 on a narrow slot produced on a substrate
1780. The
s1
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
cross-sectional size of the slot is slightly larger than the probe Garner
thread as shown in Fig.
17b. Target fluid 1750 is introduced into the middle section of slot before
closing the lid or it
is introduced through a small opening 1790 in the Iid after the lid is closed
onto the slot.. The
probe carrier thread 100 is moved back-and-forth through the chamber to
enhance the
efficiency of the hybridization. As the thread is hydrophobic, the target
fluid is retained inside
the slot by the capillary force. Again, the hybridization efficiency can be
further improved by
applying an alternating voltage 1730 between a metal layer on the probe
carrier thread 100
and the inner wall of the capillary 1760 of chamber 1700.
5. Reader
All probe Garner thread packaging formats described above can be read using a
scanning microscope with laser or broadband excitation. Scanning can be carned
out by
scanning electron microscopy, confocal microscopy, a charge-coupled device,
scanning
tunneling electron microscopy, infrared microscopy, atomic force microscopy,
electrical
conductance, and fluorescent or phosphor imaging. However, special scanning
motions may
preferably be provided in the readout instrument for various probe carrier
thread formats.
As illustrated schematically in Figure 18, both probe carrier pin 8I0 and
probe carrier
rod 820 can be plugged into an adapter in the readout instrument designed to
hold the ends of
the pin or rod and rotate 1810 andjor translate 1812 them along the
longitudinal axis at a pre-
determined ratio of speeds. This motion brings all probes distributed along
the probe carrier
thread 100 under the optical excitation and readout lens 1800. Alternatively,
the probe Garner
pin 810 or probe carrier rod 820 may rotate 1810 while the optical head 1800
translates along
the axis of the pin or rod to scan the length of the probe carrier thread 100
mounted on a
probe Garner pin 810 or a probe carrier rod 820.
s2
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
Similarly, as shown in Figure 19, probe carrier coil 1012 can be scanned by
introducing a rotation 1910 of the coil 1012 and a relative translation 1912
motion between
the coil 1012 and the optical read head 1900 along a radial direction of the
coil.
In probe carrier spool scanner illustrated in Figure 20, a probe carrier spool
1110
contained in a cassette 1100 unwinds a stretch of unwound probe carrier thread
100 which is
passed under an optical read head 2002 and a marker reader 2004. The unwound
probe carrier
thread 100 carries the entire set of probes moving under the optical read head
2002, which
can remain stationary. The unwound probe carrier thread 100 can be collected
in a second
spool 2012.
6. Methods of Using Probe Carriers
The apparatus lends itself to use in a number of fields. An apparatus which
uses
polynucleotides as probes may be used for analysis of known point mutations,
genomic
fingerprinting, linkage analysis, characterization of mRNAs and mRNA
populations,
sequence determination, disease diagnosis, and polymorphism analysis. An
apparatus which
uses antibodies as probes would be especially useful in diagnostics. Other
uses involving
other probes will be apparent to those of skill in the art.
The use of the apparatus involves: 1) Preparation of the sample, if necessary;
2)
Formation of probe-sample complex; 3) Analyzing the binding pattern in order
to identify the
individual probes to which sample has bound.
6.1. Preparation of the sample.
The preparation of the sample will vary, depending on the sample type. Sample
preparation protocols for analysis of polynucleotides, including labeling of
samples with
fluorescent tags in order to facilitate step 3), analyzing the binding
pattern, are well-known in
the art. See, for example, U.S. Patent No. 5,800,992, which is hereby
incorporated by
53
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
reference in its entirety. In the case of polynucleotides, the sample is
fragmented, using
known techniques such as restriction endonuclease digestion, converted to
single-stranded
form, and the single-stranded fragments axe labeled with an appropriate
fluorescent tag.
6.2 Formation of the probe-sample complex.
S Upon contact of sample with the apparatus, sample or sample fragments which
have
an affinity for particular probes bind with those probes. Present microarrays
generally utilize
hybridization of complementary strands of DNA as the binding method. DNA
hybridization
is highly dependent upon hybridization conditions, which have been extensively
studied and
described; see, for example, U.S. Patent Nos. 6,054,270 and 5,700,637, which
are hereby
incorporated by reference in their entirety.
However, the present invention also encompasses any sort of sample-probe
binding
which will allow one to derive information from determining which probes have
bound to
sample or sample fragments. As an, example only, the sample may be composed of
a number
of molecules, some of which are enzymes. The probes of the apparatus to be
used to analyze
this sample could be substrates for various enzymes (here the word "substrate"
is used in the .
sense of a reactant upon which an enzyme works as a catalyst), and the
identity of the
enzymes in the samples may be obtained by determining which substrate=probes
have bound
enzymes after contact. Other examples include determining the identity of
antibodies in a
sample by using various antigens as probes, or identifying hormones in a
sample by the
receptors to which they bind, etc. The list of sample/probe pairs extends to
any sets of pairs
which bind with each other with a sufficient degree of affinity and
specificity to be identified,
and further examples of sample/probe pairs will be readily apparent to those
of skill in the art.
One aspect of the present invention can greatly enhance binding of charged
sample.
This is the ability to supply a voltage across the substrate, where the
substrate contains a
metallic element or is otherwise electrically conductive. For example, if DNA
is the sample
54
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
to be analyzed, an oscillating voltage across the substrate will alternately
attract the
negatively charged DNA to the probe Garner, then repel it. The attraction will
facilitate the
binding of complementary strands, while the repulsion cycle will expedite the
release of non-
specifically bound or incompletely hybridized sample. The same principle holds
true for any
type of charged sample, and increases the efficiency and fidelity of sample
binding.
6.3 Anal sis of the binding_pattern.
There are generally two steps in the analysis of the binding pattern: locating
the
probes which nave bound sample, and identifying what those probes are. It is
possible that for.
a particular sample/probe pair the two steps may reduce to one, if binding of
a particular
sample to its corresponding probe produces a change which is unique to that
sample/probe
pair.
Distinguishing probe-sample pairs from probes which have not bound sample may
be
done in any manner that allows localization. Many such techniques are well-
established in the
art. Detecting labeled sample polynucleotides, for example, can be conducted
by standard
methods used to detect the type of label used. Thus, for example fluorescent
labels or
radiolabels can be detected directly. Other labeling techniques may require
that a label such
as biotin or digoxigenin that is incorporated into the sample during
preparation of the sample
and detected by an antibody or other binding molecule (e.g. streptavidin) that
is either labeled
or which can bind a labeled molecule itself, for example, a labeled molecule
can be e.g. an
anti-streptavidin antibody or anti-digoxigenin antibody conjugated to either a
fluorescent
molecule (e.g. fluorescein isothiocyanate, Texas red and rhodamine), or
conjugated to an
enzymatically active molecule. Whatever the label on the newly synthesized
molecules, and
whether the label is directly in the sample or conjugated to a molecule that
binds the sample
(or binds a molecule that binds the sample), the labels (e.g. fluorescent,
enzymatic,
chemilluminescent, or colorimetric) can be detected by a laser scanner or a
CCD camera, or
ss
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
X-ray film, depending on the label, or other appropriate means for detecting a
particular
label. For example, in most uses of microarrays of polynucleotides for gene
analysis, sample
polynucleotides are fragmented, then each sample fragment is tagged with a
fluorescent label.
Following contact with the probe polynucleotide array, the sample fragments
which have
hybridized with complementary probe polynucleotides may be located by the
fluorescent tag
on the sample. Probes which have not bound sample fragments have no such
fluorescent
label. Similar fluorescent tagging may be done for other types of molecules,
such as
antibodies, enzymes, etc. Other types of tags, such as radioactive labels,
chemilluminescent
labels, phosphorescent labels, magnetic labels, etc., will be readily apparent
to one of skill in
the art.
For detection of probes that have bound sample, light detectable means are
preferred,
although other methods of detection may be employed, such as radioactivity,
atomic
spectrum, and the like. For light detectable means, one may use fluorescence,
phosphorescence, absorption, chemilluminescence, or the like. The most
convenient will be
1 S fluorescence, which may take many forms. One may use individual
fluorescers or pairs of
fluorescers, particularly where one wishes to have a plurality of emission
wavelengths with
large Stokes shifts (at least 20 nm). Illustrative fluorescers include
fluorescein, rhodamine,
Texas red, cyanine dyes, phycoerythrins, thiazole orange and blue, etc. When
using pairs of
dyes, one may have one dye on one molecule and the other dye on another
molecule which
binds to the first molecule. The important factor is that the two dyes when
the two
components are bound are close enough for efficient energy transfer.
The present invention provides opportunities for greatly streamlining and
expanding
the second step in the analysis, that is, the step of identifying the specific
probes to which
samples or sample fragments have bound. In conventional probe microarrays,
such as
polynucleotide arrays, the identity of a probe is established by determining
its x-y position in
56
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
the array; the x-y position of every probe is known. Determining the position
of the probe by
known techniques requires complex and expensive imaging equipment. Because the
probes in
the present invention are arranged in a one-dimensional row, positional
analysis is much
easier and requires much less complex equipment, because only one dimension
need be
tracked (as is the case for a spooled thread), rather than two.
The use of markers associated with probes or groups of probes provides a means
for
keeping track of probes in any of the embodiments of the invention. This has
been discussed
previously. Markers may be simple or complex, may be on the same side of the
substrate as
the probes or a different side, may be of more than one type, and may contain
more
information than just the identity of the probe or probes.
Probe carriers of the present invention can be used to construct very large
probe
arrays packaged in minimal volume which are subsequently hybridized with a
target nucleic
acid. Analysis of the hybridization pattern of the chip provides an immediate
fingezprint
identification of the target nucleotide sequence. Patterns can be manually or
computer
analyzed, but it is clear that positional sequencing by hybridization lends
itself to computer
analysis and automation. Algorithms and software have been developed for
sequence
reconstruction which are applicable to the methods described herein (R.
Drmanac et al., J.
Biomol. Struc. & Dyn. 5:105-1102, 1991; P. A. Pevzner, J. Biomol. Struc. &
Dyn. 7:63-73,
199, both of which are herein specifically incorporated by reference).
Flexible probe carriers containing immobilized nucleic acid sequences prepared
in
accordance with the invention can be used for large scale hybridization assays
in numerous
genetic applications, including analysis of known point mutations, genomic
fingerprinting,
linkage analysis, characterization of mRNAs and mRNA populations, sequence
determination, disease diagnosis, and polymorphism analysis. An apparatus
which uses
s7
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
antibodies as probes would be especially useful in diagnostics. Other uses
involving other
probes will be apparent to those of skill in the art.
For gene mapping, a gene or a cloned DNA fragment is hybridized to an ordered
array
of DNA fragments, and the identity of the DNA elements applied to the array is
unambiguously established by the pixel or pattern of pixels of the array that
are detected. One
application of such arrays for creating a genetic map is described by Nelson,
et al., Nature
Genetics 4:11-18 (1993). In constructing physical maps of the genome, arrays
of immobilized
cloned DNA fragments are hybridized with other cloned DNA fragments to
establish whether
the cloned fragments in the probe mixture overlap and are therefore contiguous
to the
immobilized clones on the array. For example, Lehrach et al., "Hybridization
Fingerprinting
in Genome Mapping and Sequencing," in Genome Analysis, vol. I: Genetic and
Physical
Mapping. (K.E. Davies & S.M. Tilghman, Eds.) Cold Spring Harbor Laboratory
Press, pp.
39-81 (1990), describe such a process.
Flexible probe carriers of immobilized DNA fragments may also be used for
genetic
diagnostics. To illustrate, a probe carrier containing multiple forms of a
mutated gene or
genes can.be probed with a labeled mixture of a patient's DNA which will
preferentially
interact with only one of the immobilized versions of the gene. The detection
of this
interaction can lead to a medical diagnosis. Also, detection of expression
levels of certain
genes are diagnostic of certain medical conditions. For example, amplification
of the HER-
2/neu (c-erbB-2) gene resulting in overexpression of the p185HER-2 growth
factor receptor
occurs in approximately 25% of early stage breast cancers. HER-2/neu has been
established
as an important independent prognostic factor in early stage breast cancer in
Iarge cohorts of
patients and in cohorts with very long (30 year) follow-up duration. New data
has emerged to
suggest that HER-2/neu may be useful not only as a prognostic factor but also
as a predictive
marker for projecting response to chemotherapeutics, antiestrogens, and
therapeutic anti-
ss
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
HER-2/neu monoclonal antibodies. HER-2/neu codes for a 185 kD transmembrane
oncoprotein which is amplified and/or over-expressed in some breast cancer
patients, a
feature generally associated with a poorer prognosis than that for women with
unamplified
HER-2/neu. While this locus has been studied for a number of years, technical
problems
S associated with the most commonly used methodologies (Southern blotting and
immunohistochemical staining) have led to some inconsistencies in the data.
Pegram M.D. et
al., HER-2/neu as a predictive marker of response to breast cancer therapy.
Beast Cancer
Research and Treatment 52(1-3): 65-77, 1998. The rapid processing of multiple
samples
enabled by the present invention, allow for rapidly testing multiple controls
to avoid
inconsistencies.
7. Utilities of Probe Carriers
Flexible probe carriers of immobilized DNA fragments can also be used in DNA
probe diagnostics. For example, the identity of a pathogenic microorganism can
be
established unambiguously by hybridizing a sample of the unknown pathogen's
DNA to a
probe carrier containing many types of known pathogenic DNA. A similar
technique can also
be used for unambiguous genotyping of any organism. Other molecules of genetic
interest,
such as cDNAs and RNAs can be immobilized on the probe Garner or alternately
used as the
labeled probe mixture that is applied to the probe carrier.
In one application, a probe carrier of cDNA clones representing genes is
hybridized
with total cDNA from an organism to monitor gene expression for research or
diagnostic
purposes. Labeling total cDNA from a normal cell with one color fluorophore
and total
cDNA from a diseased cell with another color fluorophore and simultaneously
hybridizing
the two cDNA samples to the same array of cDNA clones allows for differential
gene
expression to be measured as the ratio of the two fluorophore intensities.
This two-color
59
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
experiment can be used to monitor gene expression in different tissue types,
disease states,
response to drugs, or response to environmental factors.
By way of example and without implying a limitation of scope, such a procedure
could be used to simultaneously screen many patients against all known
mutations in a
disease gene. This invention could be used in the form of, for example, 96
identical probe
Garner pins in a matrix where each probe carrier pin could contain, for
example,1500 DNA
fragments representing all known mutations of a given gene. The region of
interest from each
of the DNA samples from 96 patients could be amplified, labeled, and
hybridized to the 96
individual arrays with each assay performed in 10 microliters of hybridization
solution. The
adapter matrix containing all 96 identical probe carrier pins assayed with the
96 patient
samples is incubated, rinsed, detected and analyzed as a single sheet of
material using
standard radioactive, fluorescent, or colorimetric detection means (Maniatis,
et al., 1989):
Previously, such a procedure would involve the handling, processing and
tracking of 96
separate membranes in 96 separate sealed chambers. By processing all 96
patient samples in
a single step with minimal hybridization liquid, significant time and cost
savings are possible.
The assay format can be reversed where the patient or organism's DNA is
immobilized as the probe elements and each probe Garner is hybridized with a
different
mutated allele or genetic marker. A probe Garner matrix can also be used for
parallel non-
DNA ELISA assays. Furthermore, the invention allows for the use of all
standard detection
methods.
One aspect of this invention involves the detection of nucleic acid sequence
differences using coupled ligase detection reaction (LDR) and polymerase chain
reaction
(PCR) as disclosed in U.S. Pat. No. 6,027,889 entitled "Detection of nucleic
acid sequence
differences using coupled ligase detection and polymerase chain reactions" to
Baranyi, et al.
which is incorporated herein by reference in its entirety.
SUBSTITUTE SHEET (RULE 26)
CA 02397069 2002-07-09
WO 01/51207 PCT/USO1/01026
In addition to the genetic applications listed above, arrays of whole cells,
peptides,
enzymes, antibodies, antigens, receptors, ligands, phospholipids, polymers,
drag cogener
preparations or chemical substances can be fabricated by the means described
in this
invention for large scale screening assays in medical diagnostics, drug
discovery, molecular
biology, immunology and toxicology.
All publications and patent applications mentioned in this specification are
incorporated herein by reference to the same extent as if each individual
publication or patent
application were specifically and individually indicated to be incorporated by
reference.
The foregoing description of preferred embodiments of the invention has been
presented by way of illustration and example for purposes of clarity and
understanding. It is
not intended to be exhaustive or to limit the invention to the precise forms
disclosed: It will
be readily apparent to those of ordinary skill in the art in light of the
teachings of this
invention that many changes and modifications may be made thereto without
departing from
the spirit of the invention. It is intended that the scope of the invention be
defined by the
appended claims and their equivalents.
61
SUBSTITUTE SHEET (RULE 26)