Note: Descriptions are shown in the official language in which they were submitted.
CA 02397674 2002-05-O1
WO 01/32955 PCT/US99/25894
-1-
COATING AN ALUMINUM ALLOY SUBSTRATE
The present invention relates to a process for coating an aluminum
alloy substrate with a polymer. More particularly, the invention relates to a
process
for pretreating an aluminum alloy substrate with a vinyl phosphonic acid-
acrylic acid
copolymer before polymer coating the substrate.
Although aluminum protects itself against corrosion by forming a
natural oxide coating, the protection is not complete. In the presence of
moisture and
electrolytes, aluminum alloys corrode much more rapidly than pure aluminum.
Accordingly, there is a need to treat aluminum alloy substrates with
pretreatments or other chemicals that provide improved corrosion resistance as
well as
good adhesion for polymers.
In the prior art, chemical conversion coatings have been formed on
aluminum alloys by "converting" a surface of the metal into a tightly adherent
coating, part of which consists of an oxidized form of aluminum. Chemical
conversion coatings provide high corrosion resistance and improved adhesion
for
polymer coatings. A chromium-phosphate conversion coating is typically
provided
by contacting aluminum with an aqueous solution containing hexavalent chromium
ions, phosphate ions and fluoride ions. In recent years, concerns have arisen
regarding the pollution effects of chromates and phosphates discharged into
waterways by such processes. Because of the high solubility and strongly
oxidizing
character of hexavalent chromium ions, expensive waste treatment procedures
must be
employed to reduce the hexavalent chromium ions to trivalent chromium ions for
waste disposal.
Attempts have been made in the prior art to produce acceptable
chromate-free conversion coatings for aluminum. For example, some chromate-
free
conversion coatings contain zirconium, titanium, hafnium and/or silicon,
sometimes
combined with fluorides, surfactants and polymers such as polyacrylic acid. In
spite
of the extensive efforts that have been made previously, there is still no
entirely
satisfactory non-chromate conversion coating or primer for improving the
adhesion
and corrosion resistance of polymer coated aluminum alloy substrates. Polymer
adhesion and corrosion resistance are important characteristics in aluminum
alloy
sheet used for making food container bodies and ends and beverage container
ends.
CA 02397674 2004-07-29
50989-21
-2-
Attempts have also been made in the prior art to pretreat substrates
with various organophosphorus compounds before coating them with a polymer. As
used herein, the term "organophosphorus compounds" includes organophosphoric
acids, organophosphinic acids, organophosphonic acids, as well as various
salts,
esters, partial salts, and partial esters of such acids. For example, Dutch
Patent
Application No. 263,668, filed April 14, 1961, discloses a process wherein
steel
sheets are treated with a vinylphosphonic acidlacrylic acid copolymer before
coating
with an alkyd resin enamel. Although some organophosphorus pretreatments may
perform adequately, they are expensive to implement. Accordingly, there still
remains a need to provide an. efficient and economical process for pretreating
an
aluminum alloy substrate with an organophosphorus compound before applying a
polymer coating.
A principal objective of the present invention is to provide an efficient
and economical process for pretreating an aluminum alloy substrate with an
organophosphorus compound before applying a polymer coating.
To accomplish this principal objective our process provides for
removing aluminum and other rations from pretreatment solutions, thereby
avoiding
costly disposal of such solutions.
Additional objectives and advantages of our invention will become
apparent to persons skilled in the art from the following detailed
description.
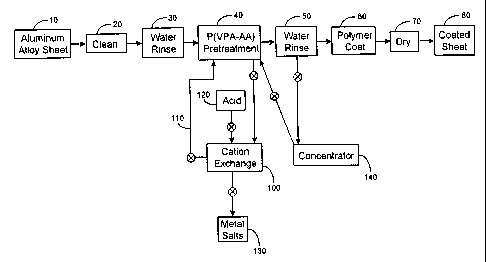
The sole Figure is a flowsheet diagram of the process of the present
invention.
In accordance with our invention there is provided a process for
coating an aluminum alloy substrate with an organic polymer. The aluminum
alloy
substrate may be provided in the form of a sheet plate, extrusion or casting
and is
preferably a sheet.
According to one aspect of the present invention,
there is provided a process for pretreating an aluminum
alloy sheet having a surface portion in order to improve
adhesion of a polymer coating to said surface portion,
comprising: (a) in a first container, pretreating an
aluminum alloy sheet having a surface portion comprising
aluminum oxide or aluminum hydroxide with a pretreatment
n, *.....
CA 02397674 2005-06-15
50989-21
-2a-
solution consisting essentially of water and an
organophosphorus compound, thereby forming a layer
comprising a reaction product of said compound and said
oxide or hydroxide, and contaminating said solution with
aluminum ions; (b) transferring at least a portion of said
solution to a second container containing a cation exchange
resin comprising a polymer and therein adsorbing aluminum
ions onto said resin, thereby producing a treated solution
containing said compound and having a reduced concentration
of aluminum ions; and (c) returning said treated solution to
said first container.
Various aluminum alloys available in sheet form
are suitable for practice of the present invention,
including alloys belonging to the AA2000, 3000, 5000, 6000
and 7000 series. Aluminum-magnesium alloys of the AA5000
series and particularly the AA5042 and AA5182 alloys are
preferred. Sheet made from these alloys is useful for
shaping into polymer coated food container bodies or ends,
and beverage container ends.
r L. o ~i~i~i . "I ii i~~~l~~ ". l. ~ ,~
CA 02397674 2005-06-15
50989-21
-3-
Aluminum alloys suitable for container end panels such as AA5182 are
provided as an ingot or billet or slab by casting techniques known in the art.
Before
working, the ingot or billet is subjected to elevated temperature
homogenization. The
alloy stock is then hot rolled to provide an intermediate gauge sheet. For
example,
the material may be hot rolled at a metal entry temperature of about
700°-975°F to
provide an intermediate product having a thickness of about 0.100 inch to 0.1
SO inch.
This material is cold rolled to provide a sheet ranging in thickness from
about 0.006
to 0.015 inch. We prefer AA5182 aluminum alloy sheet in the H19 temper.
Aluminum alloy 5042 sheet for end panels is preferably in the H19 temper:
Aluminum alloys such as AA5042 are provided as an ingot that is
homogenized. This is followed by hot rolling to an intermediate gauge of about
0.100 inch to 0.150 inch. Typically, the intermediate gauge product is
annealed,
followed by hot rolling and then cold rolling to a final gauge product having
a
thickness of about 0.006 to 0.015 inch. The sheet is coated with a polymer and
then
drawn and redrawn into food container bodies. We prefer AA5042 aluminum alloy
sheet in the H2x temper.
The natural oxide coating on an aluminum alloy sheet surface is
generally sufficient for practice of our invention. The natural oxide coating
ordinarily has a thickness of approximately 30-50 angstroms. For better
protection
against corrosion, the oxide coating can be grown by treatments such as anodic
oxidation or hydrothermal treatment in water, water vapor or aqueous
solutions.
Aluminum alloy sheet of the invention is generally cleaned with an
alkaline surface cleaner to remove any residual lubricant adhering to the
surface, and
then rinsed with water. Cleaning can be avoided if the residual lubricant
content is
negligible.
The cleaned sheet surface is then pretreated in a first container with a
composition consisting essentially of water and an organophosphorus compound.
The
solution preferably contains about 1-20 g/L of a vinyl phosphoric acid-acrylic
acid
copolymer (VPA-AA copolymer). Solutions containing about 4-10 g!h of the
copolymer are preferred. The copolymer usually comprises about 5-50 mole
vinylphosphonic acid, preferably about 20-40 mole %. The VPA-AA copolymer may
have a molecular weight of about 20,000 to 100,000, preferably about 50,000 to
CA 02397674 2002-05-O1
WO 01/32955 PCT/US99/25894
-4-
80,000. A particularly preferred VPA-AA copolymer contains about 30 mole % VPA
and about 70 mole % AA. The solution has a temperature of about 100°-
200°F,
more preferably about 120°-180°F. A particularly preferred
solution has a
temperature of about 170°F.
The sheet surface may be dipped into the composition or the
composition may be roll coated or sprayed onto the sheet surface. A preferred
continuous cleaning and pretreating line is operated at about 500-1500 feet
per
minute. A contact time of about 6 seconds between the sheet surface and the
composition is sufficient when the line is operated at 1000 feet per minute.
The
VPA-AA copolymer reacts with the oxide or hydroxide coating to form a layer on
the sheet surface.
Aluminum alloy sheet passing through the pretreatment solution
contaminates the solution with ions of various elements, including aluminum,
magnesium, iron, chromium and manganese. The pretreatment solution loses
effectiveness when the aluminum concentration rises above about 150-200 ppm.
Accordingly, we provide a process for removing ions of aluminum and other
metals
from the pretreatment solution.
At least a portion of the pretreatment solution is transferred to a second
container containing a cation exchange resin. The resin may be provided as
pellets,
beads, fibers, or particles and preferably is a hard, spherical gel type bead.
The resin
has a minimum total capacity in the hydrogen form, wet, of 1.9 meq/m.L. A
preferred resin has an average particle size of about 650 microns, a specific
gravity of
about 1.22-1.23, and a bulk density of about 49.9 lb/ft3.
The resin is preferably a gel comprising a styrene-divinylbenzene
copolymer functionalized with acid groups, preferably sulfonate groups.
Alternatively, the copolymer may be functionalized with phosphonic acid or
arsonic
acid groups. A particularly preferred cation exchange resin is sold by The Dow
Chemical Company of Midland, Michigan under the trademark DOWER G-26(H).
Less preferably, the cation exchange resin may comprise ethylene
copolymerized with an unsaturated carboxylic acid such as acrylic acid.
After the pretreatment solution passes through the second container, it
contains a reduced concentration of aluminum. The aluminum concentration in
the
CA 02397674 2002-05-O1
WO 01/32955 PCT/US99/25894
-5-
treated solution is less than about 75 ppm, more preferably less than about 25
ppm,
and optimally about 10 ppm or less. The treated solution, containing the
organophosphorus compound and a reduced concentration of aluminum, is returned
to
the first container.
Optionally, the pretreated sheet may be rinsed with water to remove
excess VPA-AA copolymer. The rinse water preferably has a temperature of about
170°-180°F. The rinse water is concentrated by removing excess
water so that the
VPA-AA copolymer can be recycled. Some preferred concentrating techniques
include reverse osmosis and membrane filtration. After concentration, the
rinse water
may be transferred to the first container in order to recover VPA-AA copolymer
values.
The primed sheet is coated with a polymer composition that preferably
includes an organic polymer dispersed in an organic solvent. Three preferred
coating
polymers are the epoxies, polyvinyl chloride and polyesters. The suitable
epoxies
include phenolic-modified epoxies, polyester-modified epoxies, epoxy-modified
polyvinyl chloride, and cross linkable epoxies. The polymer composition may be
clear or it may contain pigment particles. The pigment particles are
preferably
titanium dioxide, alumina or silica. We prefer titanium dioxide particles in
the 0.5 to
10 microns median particle size range.
Alternatively, the primed sheet may be coated by electrocoating, slot
coating, extrusion coating, flow coating, spray coating, or other continuous
coating
processes.
The polymer coated sheet is dried, coiled, and then finally shaped into
container bodies or container end panels.
As shown schematically in the Figure, there is provided a coil of
AA5182-H19 aluminum-magnesium alloy sheet 10 having a thickness of about 8.8
mils (224 microns). The sheet 10 is cleaned with an alkaline surface cleaner
in a vat
20 to remove any residual lubricant on the sheet surface. The cleaned sheet is
then
rinsed in a deionized water bath 30.
The cleaned and rinsed sheet is pretreated in a first container 40 with a
solution comprising about 10 g/L of a VPA-AA copolymer containing about 30
mole
percent VPA and about 70 mole percent AA units, dissolved in water. The
solution
CA 02397674 2002-05-O1
WO 01/32955 PCT/US99/25894
-6-
has a temperature of about 170°F (77°C) and it initially
contains about 10 ppm
aluminum. The VPA-AA copolymer reacts with an aluminum oxide or hydroxide
coating on the sheet surface to form a layer comprising a reaction product of
the
copolymer and the oxide or hydroxide.
S The pretreated sheet is then rinsed with water 50 to remove excess
VPA-AA copolymer. The rinse water 50 preferably has a temperature of about
170°-180°F.
The rinsed sheet is roll coated with a polymer composition 60 that
preferably includes an organic polymer and pigment particles dispersed in an
organic
solvent. The organic polymer is preferably an epoxy resin. Some suitable
epoxies
include phenolic-modified epoxies, polyester-modified epoxies, epoxy-modified
polyvinyl chloride, and cross linkable epoxies.
The polymer coated sheet is dried in a hot air dryer 70 and then
recoiled as a coated sheet product 80.
In order to maintain a low concentration of metal ions in the
pretreatment solution, portions of the solution are periodically transferred
from the
first container 40 to a second container 100 holding a cation exchange resin.
A
particularly preferred resin is sold by Dow Chemical Company of Midland,
Michigan
under the trademark DOWEX G-26 (H) strong cation exchange resin. The strong
cation exchange resin is sold as hard spherical beads with a 650 micron dry
mesh
size. The strong cation exchange resin is a gel comprised of a styrene-divinyl
benzene copolymer functionalized with sulfonate groups. Treatment with the
resin
produces a treated solution having an aluminum concentration that is optimally
less
than about 10 ppm. The treated solution is returned through a pipe 110 from
the
second container 100 to the first container 40.
The cation exchange resin eventually becomes saturated with metal
salts. The resin is regenerated by washing with a strong acid solution 120,
such as
6-10 vol.% HCl or 6-12 vol.% sulfuric acid in water. Metal salts 130 washed
from
the second container 100 are discarded.
Used rinse water from the water rinse 50 is also recycled to recover
VPA-AA copolymer values. The used rinse water is first sent to a concentrator
140
where water is removed, for example by reverse osmosis or membrane
ultrafiltration.
CA 02397674 2002-05-O1
WO 01/32955 PCT/US99/25894
_7_
The concentrated rinse water is then returned to the first container 40.
The canon exchange process of our invention maintains aluminum
concentrations at acceptable levels in the pretreatment solution. A 200 mL
aliquot of
the pretreatment solution at 140°F containing 10 g/L VPA-AA copolymer,
350 ppm
aluminum, and other metals was placed in a 250 mL Ehrlenmeyer flask containing
40
mL wet volume of the DOWEX G-26(H) resin in the hydrogen form. The flask was
placed in a water bath and held at 140°F (60°C) for 16-20 hours.
The resin was
prepared by washing with 400-600 mL of 6 vol.% HCI, followed by rinsing with
600-800 mL of deionized water.
After 16 to 20 hours of contact time, the pretreatment solution was
filtered and the resin was rinsed with 25 mL of deionized water. The solution
was
analyzed and the results are presented below in the Table. All concentrations
were
corrected to reflect a volume of 200 mL, for comparison.
Table-Analysis of Pretreatment Solution
Initial After
Concentration G-26(H)
Element (ppm) (ppm)
Al 350 5.3
Na 14 0.5
Si 11 12
Fe 22 18
Ca 4.1 0.8
Mg 100 0.3
Mn 1.5 n.d
Ni 0.6 n.d
Zn 0.3 n.d
Cr 11 10
K 1.8 0.5
P 820 777
n.d. = non detectable
Having described the invention with reference to some presently
preferred embodiments, persons skilled in the art will understood that our
invention
CA 02397674 2002-05-O1
WO 01/32955 PCT/LTS99/25894
_g_
may be otherwise embodied without departing from the spirit and scope of the
appended claims.