Note: Descriptions are shown in the official language in which they were submitted.
CA 02398226 2002-08-15
INCREASED-DOSAGE NELFINAVIR TABLET
AND METHOD OF MAKING SAME
FIELD OF THE INVENTION
The present invention is directed to a high-dosage form of nelfinavir tablets
and a method of making such tablets. More particularly, the present invention
is
directed to nelfinavir tablets having a dosage significantly greater than
presently
available tablets to reduce the patient pill burden.
BACKGROUND OF THE INVENTION
It has been shown that individuals infected with HIV may be treated with
HIV-protease inhibitors to prevent or inhibit the rapid proliferation of the
virus in
the patient. HIV-protease inhibitors block a key enzymatic pathway in the
virus,
substantially reducing viral loads, and slowing the steady decay of the immune
system. Nelfinavir compounds, in particular, nelfmavir mesylate (Viracept~),
have been shown to be effective protease inhibitors in the treatment of HIV-
infected patients. Nelfinavir mesylate and methods of making nelflnavir are
disclosed in U.S. Patent No. 5,484,926 to Dressman et al., the disclosure of
which
is incorporated by reference herein. Intermediates for making HIV-protease
inhibitors, such as nelfinavir mesylate, are disclosed in U.S. Patent No.
5,705,647
to Babu et al., the disclosure of which is incorporated by reference herein.
Nelfinavir mesylate is presently available in tablets that provide a dosage
equivalent to 250 mg of the nelfinavir free base. Therefore, as the twice-
daily
standard dosage is 1250 mg, a patient is required to take five tablets twice a
day.
As ten tablets a day is a significant pill burden for the patient, it would be
advantageous to reduce this high pill burden, and promote compliance.
Therefore,
a need exists for a nelfinavir tablet providing a higher dosage of nelfmavir
than the
presently available 250 mg tablet.
1
CA 02398226 2002-08-15
SUMMARY OF THE INVENTION
The high-dosage nelfinavir tablet of the invention comprises a nelfinavir
compound and a binder. The tablets of the invention have a nelfinavir binder
weight ratio of from about 3:1 to about 5:1. Preferably, the nelfinavir to
binder
weight ratio is greater than about 3.5 to 1 and less than about 5 to l, and,
more
preferably, is 4 to 1. The nelfinavir compound is preferably present in an
amount
sufficient to provide a nelfinavir dosage equivalent to about 625 mg of
nelfinavir
free base. The nelfinavir compound is preferably nelfinavir mesylate, and the
preferred binder is calcium silicate. Where the nelfinavir compound is
nelfinavir
mesylate, the compound is preferably present in an amount of about 730 mg per
tablet. The tablet of the invention may further comprise at least one
excipient,
which is preferably selected from the group consisting of magnesium stearate,
crospovidone, and colloidal silicon dioxide, and is preferably coated. Each
tablet
preferably comprises at least one of from about 15 to about 20 weight percent
crospovidone, from about 0.1 to about 0.5 weight percent silicon dioxide, and
from
about 0.5 to about 1.5 weight percent magnesium stearate, based on total
tablet
weight. A preferred tablet comprises about 17.5 weight percent crospovidone,
0.25
weight percent, silicon dioxide and about 1 percent by weight magnesium
stearate
based on total tablet weight.
The coating is preferably hydroxypropyl methycellulose, which is
preferably present in an amount of from about 1 to about 3 percent by weight
of
the tablet, more preferably in an amount of about 2 percent by weight of the
tablet.
The method of the invention comprises forming a granulated mixture of a
nelfmavir compound and a binder in a granulating process, and processing the
granulated mixture to form a high-dosage nelfinavir tablet, where the
granulating
process comprises adding moisture and applying mechanical energy to the
mixture,
and curing the mixture in at least one curing step in which no moisture is
added
and no mechanical energy is applied to the mixture. Preferably, each curing
step is
for a cure time of at least about 1 minute, more preferably for a cure time of
from
about 1 minute to about 30 minutes, and even more preferably for a cure time
of
from about 10 minutes to about 15 minutes. The nelfinavir compound and the
binder may be dry blended before granulating the mixture.
2
CA 02398226 2002-08-15
After granulating, the wet granulated mixture may be dried at a temperature
of from about 60°C to up to 80°C, preferably to a moisture
content of from about 4
to about 8 percent LOD (Loss On Drying). The dried granulate is then
preferably
milled, and may be blended with at least one excipient, such as, e.g.,
magnesium
stearate, crospovidone, and colloidal silicon dioxide. Preferably, the
granulation
mixture is milled with a round-hole screen having a hole size of from about
0.024
inch to about 0.045 inch.
BRIEF DESCRIPTION OF THE DRAWINGS
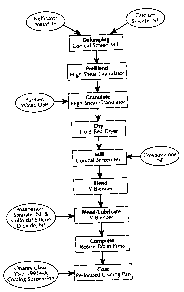
10 Fig. 1 is a flow diagram of the process used in example 1; and
Fig. 2 is a flow diagram of the process used in example 2.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to a high-dosage nelfinavir tablet and to a
15 process of making such a tablet. As used herein, the term "nelflnavir
compound"
means nelfinavir free base (1,1-dimethylethyl) decahydro-2-[2-hydroxy-3-[(3-
hydroxy-2-methylbenzoyl)amino]-4- (phenyl-thin-buty)]-3-
isoquinolinecarboxamide) or a pharmaceutically acceptable salt, such as
nelfinavir
mesylate (1,1-dimentylentyl) decahydro-2-[2-hydroxy-3-[(3-hydroxy-2-
20 methylbenzoyl)amino]-4- (phenyl-thio-buty)]-3-isoquinolinecarboxamide
mono-methane sulfone). The term "high-dosage" refers to nelfinavir tablets
that
provide a therapeutic dosage effectively greater than that provided in a 250
mg
tablet. Generally, the dosage of nelfinavir compound in a tablet will be
equivalent
to about 531 mg to about 719 mg, and, preferably, from about 593 mg to about
25 656 mg of the nelfinavir free base. More preferably, the dosage of
nelfinavir
compound is equivalent to 625 mg of nelfinavir free base. That is, the tablets
of
the invention provide a nelfinavir compound in an amount sufficient to provide
the
same therapeutic effect as a 625 mg dose of nelfinavir free base. For example,
for
nelfinavir mesylate, the preferred nelfinavir compound, the amount of drug
30 required to provide the same therapeutic effect as a 625-mg dose of
nelfmavir free
base is about 730 mg. It should be noted that, unless otherwise stated, all
weights
given herein are for anhydrous material. Those of ordinary skill in the art
will
3
CA 02398226 2002-08-15
understand how to adjust the weight of each ingredient used in the tablet to
account
for any residual moisture or solvent that may be present.
Tablets of the invention comprise a nelfinavir compound ("the drug") and a
binder in a drug/binder weight ratio of from about 3 to 1 to about 5 to 1.
Preferably, the drug/binder weight ratio is between about 3.5 to 1 and about 5
to 1,
more preferably in a range of from about 3.5 to 1 to about 4.5 to 1, and, even
more
preferably, is about 4 to 1. Preferably, the nelfinavir compound is nelfinavir
mesylate, and the binder is calcium silicate (CaSi03). The use of calcium
silicate
as a binder provides optimal dissolution and crystallinity in high-dosage
nelfinavir
tablets.
Lower-dosage (250 mg) forms of nelfinavir tablets that are commercially
available having a druglbinder ratio of about 2 to 1. The higher drug/binder
weight
ratio of the tablets of the invention makes the absorption of water
more_difficult
during processing. As a result, to obtain an adequate absorption of moisture
into
the drug-binder matrix, at least one curing step is required during
granulation.
During each curing step of the granulation process, no moisture is added, and
no
mechanical energy is applied for a period of time referred to herein as a
"cure
time". The at least one curing step during granulation is of a time adequate
for
absorption of the moisture required to allow the product of the granulation to
be
further processed into tablets. A cure time is not required in the presently
available
250-mg nelfinavir tablets, as the larger proportion of binder in the 250-mg
tablets
allows absorption of moisture without curing. Thus, in contrast to the process
of
the present invention, the addition of water and the use of a mechanical
energy
source, e.g., a chopper and/or impeller, is continuous in the production of
the
250 mg tablets.
In one embodiment, the high-dosage nelfinavir tablets of the invention are
produced in the following process. The amounts of the nelfinavir compound and
binder required for a given batch size and drug/binder weight ratio are first
determined, accounting for any residual moisture and/or solvent. Preferably
the
nelfinavir compound and the binder are first screened to remove any lumps.
The nelfinavir compound and binder are then mixed and wet granulated.
The drug and binder may be dry blended prior to granulation, or may be mixed
in
4
CA 02398226 2002-08-15
the granulation apparatus. Preferably, the granulation is performed in a high-
shear
granulator.
During granulation, water is added to the drug/binder mixture under the
action of the granulator, e.g., by an impeller and/or a chopper. The
granulation
process also comprises at least one curing step to allow for the adequate
absorption
of water by the nelfinavir compound/binder mixture. During the curing step, no
water or mechanical energy is added to the mixture, i.e., the addition of
water and
any impeller and/or chopper action is stopped. The time for a curing step,
i.e., the
cure time, is typically at least about 1 minute, preferably from about 1 to
about 30
minutes, and more preferably from about 10 to 15 minutes. The granulation
process may comprise for more cure steps. Preferably, the granulation process
comprises from 1 to about 6 cure steps, and more preferably comprises from I
to
about 3 cure steps. The granulation process may further comprise one or more
wet
massing steps in which mechanical energy is added without the addition of
moisture.
Following granulation, the granulated drug/binder mixture is processed into
tablets. Processing steps may include (but are not limited to) drying,
screening,
milling, blending with excipients, pressing, and coating. Useful excipients
include
(but are not limited to) crospovidone, colloidal silicon dioxide, and
magnesium
stearate.
EXAMPLES
The following non-limiting examples are illustrative of the preferred
embodiments of the invention and are not to be construed as limiting the
invention,
the scope of which is deEned by the appended claims.
Example 1
Batches of 117,490 tablets having a dosage equivalent to 625 mg of
nelfinavir free base and a total weight of 140.6 kg were produced using the
manufacturing process scheme shown in Fig.l. Each tablet contained, on
average,
730.6 mg U.S. Spec. nelBnavir mesylate, 182.7 mg calcium silicate, 196.7 mg
5
CA 02398226 2002-08-15
crospovidone, NF (Kollidon, CFA, 2.8 mg colloidal silicon dioxide, NF, and
11.2
mg magnesium stearate, NF, for a total core weight of 1173 mg, on average.
Each
tablet was coated with 23.5 mg Oradry Clear YS-2-19114-A for a total average
tablet weight of 1,196 mg. Any water used in the processing was essentially
removed during the process, such that no more than about four percent residual
moisture remained in a tablet.
After screening through a Quadro Comil having a round-edge impeller and
a 0.032-inch round-hole screen, 21.47 kg of calcium silicate was added to
88.60 kg
(equivalent to 85.85 kg anhydrous and solvent free) nelfinavir mesylate, which
had
been screened through a 20-mesh screen, in the granulation bowl of a Zanchetta
Model Roto F900L granulator. The drug and binder were mixed for about 5
minutes with the impeller at high velocity setting and the chopper at low
velocity
setting. The impeller velocity was then changed to low for 1 minute.
The mixture of nelfinavir mesylate and calcium silicate was then granulated
as follows. Over a period of 5 to 10 minutes, at a rate of 3 to 6 kg/minute,
30 kg of
water was added to the mixture with an impeller velocity of high and a chopper
velocity of low. The addition of water was then stopped, and the impeller
velocity
and chopper reduced to low for a period of 1 minute in a wet massing step. The
impeller and chopper were then turned off, and the wet mixture was allowed to
cure for a curing time of 10 to 15 minutes. The impeller was then turned back
to
low, and an additional 6 kg of water was added over a period of 1 to 2
minutes.
The chopper was then turned on, and an additional 3 kg of water was then added
with both the impeller and chopper velocities set to low. The addition of
water
was again stopped, and a wet massing step was performed for 30 seconds with
both
the impeller and chopper velocities set at low. The impeller and chopper were
turned off, and the wet mixture was cured for an additional curing time of 10
to 15
minutes. An additional 3.8 kg of water was added over a time of about 1 minute
with the impeller and chopper velocities set at low. This was followed by a
wet
massing step of about 1 minute with the impeller and chopper velocities
unchanged.
The granulated mixture was then dried at 60 t5°C until the
moisture
content reached 4 to 8 percent LOD with a target of 6 percent LOD. The dried
6
CA 02398226 2002-08-15
mixture was then milled with a Quadro Comil Model 196-S having a round-edge
impeller, a 0.032-inch round-hole screen, and a 0.2-inch spacer.
Approximately half of the total dried and milled granulations was then
placed into a type "V" blender, 30 cubic feet, using a vacuum loading, and
23.11 kg of Crospovidone, NF, (Kollidon CL) was added to the blender. The
remaining granulation mixture was then added to the blender, and the combined
materials were mixed for 30 minutes. A mixture of 1.32 kg magnesium stearate,
NF, and 0.32 kg colloidal silicon dioxide, NF, was mixed, and passed through a
20-mesh stainless steel screen before being added to other ingredients in the
blender, and mixed for 5 minutes to form a final blend.
The final blend was then compressed into white, oval-shaped, 0.750 x
0.425-inch (19.1 x 10.8 mm) tablet cores debossed on one side with a "V" and
"625" on the others. The weight of the tablets ranged from 1.144 g to 1.202 g,
with a target of 1.173 g. The thickness of the tablets ranged from 0.322 inch
(8.18mm) to 0.342 inch (8.69mm), with a target of 0.332 inch (8.43mm), and the
hardness ranged from 22 Kp to 34 Kp, with a target of 28 Kp. Friability was no
more than 1.0 percent, and disintegration was no more than 15 minutes.
The tablet cores were then coated with 2.76 kg of Opadry Clear
YS-2-19114-A in gurified water on a Glatt PC 1500 60" coater and dried. The
tablets were found to be bioequivalent to commercially available 250-mg
nelfinavir tablets.
Example 2
Nefinavir mesylate tablets of the invention were produced using the
manufacturing process scheme shown in Fig.2. Nelfinavir mesylate and calcium
silicate were blended in a 500-liter bin-blender for 15 minutes to form a 60-
kg
blend of nelfinavir mesylate and calcium silicate having a drug/binder weight
ratio
of 4:1. A connection factor was applied to the nelfinavir mesylate to account
for
the presence of 3.1 percent volatiles. The height of the blended powder was
monitored for changes to determine whether aeration was occurnng, but no
significant change was observed. The blended powder was discharged into
polyethylene bag lined fiber drums, and showed a characteristic fluid-like
flow.
7
CA 02398226 2002-08-15
Within a few minutes of discharge, a decrease in volume of greater than
percent was observed, and a reduction of almost 50 percent was observed
following overnight storage. It is believed that the powder blend became
aerated
during discharge, causing an increase in volume. And, thus, as air in the
blend
5 slowly dissipated during storage, the volume decreased.
The powder blend was fed via a side stuffer into a twin-screw wet
granulator (34-mm Leistriz G2anulator). The components of a screw design of
the
type used are described below. The screw granulator comprised the following
sections, in the direction of flow: a spacer, a first convey section, a first
mixing
10 section, a second convey section, a second mixing section, a third convey
section, a
chopper section, a spacer section, and a torpedo. Water for granulation was
injected at the first convey section and the first mixing section. The
granulation
screw was run at 300 rpm and the side stuffer at 200 rpm. The powder blend had
a
feed rate of 7.8 kg/hour and water was injected at 47 ml/minute to provide a
consistent wet output of at least 10 kg/hour.
The bulk density dry powder blend had a moisture content of 2.2 percent
LOD, a bulk density of 0.15 g/ml, and a tapped density of 0.25 g/ml. The wet
granulation had a moisture content of 27.88 percent LOD, a bulk density of
0.45 g/ml, and a tapped density of 0.75 g/ml.
During granulation, the drug/binder blend was cured twice, once as the
blend passed through the second convey section and once as it passed through
the
third convey section.
The wet granulation was dried in two portions, A and B, in a fluid-bed
dryer with a 60° C inlet temperature at 750 cfm (ft 3/m) to a moisture
level of 4 to
8 percent LOD. The final moisture level of portion A was 4.81 percent LOD, and
that of portion B was below the target level at 3.7 percent LOD. Although both
dried portions were carried forward for processing, portion B was only used
for
compaction studies.
The dried granulations were passed through a Quadro Comil 197S
equipped with a 0.032-in. screen and a 0.150-in. screen with a 2300 rpm
impeller
speed.
8
CA 02398226 2002-08-15
The milled granulation was blended with external excipients in a 250-liter
bin blender, first with crospovidone for 15 minutes and then with a previously
blended and delumped mixture of colloidal silicon dioxide and magnesium
stearate
for an additional 10 minutes. The amounts of crospovidone, silicon dioxide,
and
magnesium stearate used were, respectively, 16.44 percent, 0.23 percent, and
0.94
percent based on the total weight of the final tablet formulations.
The final blend was compressed in a 19-station Kikusui Virgo tablet press
using 0.750 x 0.425 inch (19.1 x 10.8 mm) oval, deep concave punches. The
press
was run at the minimum press speed of 22 rpm and the paddle feeder at 55 rpm.
Fre-compression was 0.90 tons (818 kg), and the main compression was 1.0 ton
(909 kg).
Ten-kilogram batches of tablet cores were coated with Opadry clear film in
an Accela Cota coating system using a 24-in. perforated pan to a theoretical
weight
gain of 2 percent. Pan speed was maintained at 10 rpm with a spray rate of
between 20 and 40 ml/minute. As a result of a loss of moisture by the cores
during
coating, no weight gain was observed.
The dissolution profile of the 625-mg tablets obtained was compared with
that of 250-mg commercial tablets. The 17.1-minute disintegration time of the
625-mg tablets is several-fold longer than the 1.75-minute time of the 250-mg
tablets. However, within 15 minutes, the dissolution of the 625-mg tablets in
simulated gastric fluid without pepsin was 68 percent compared to 78 percent
for
the 250-mg tablets, and within 45 minutes, the 88 percent dissolution of the
625-mg tablets compared to the 89 percent dissolution of the 250-mg tablets.
This invention is not limited by the embodiments disclosed herein. It is
intended that the appended claims cover all such modifications and embodiments
that fall within the true spirit and scope of the present invention.
9