Note: Descriptions are shown in the official language in which they were submitted.
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
MODULAR MOULD TOOLS
This invention relates to a novel tooling method and novel process for use of
such
tools.
Traditionally, the only option available to designers and engineers when
designing
plastics components has been to work from fabricated components produced from
bulk material supplied in sheet or bar stock. However, there are many problems
associated with fabricating plastics parts, which make this route less than
ideal. For
example, not all materials are available in sheet stocle, flexible hinges and
springs can
be difficult to reproduce and small features can be di~cult to fabricate.
Therefore
fabricated parts have been typically used to produce proof of concept models
only.
Designers have then had to of design and manufacture pre-production mould
tooling
before being able to complete the design and proof of concept stages. These
pre-
production mould tools are normally scaled-down versions of the final
production
tools and can take up to 20 weeks to produce.
More recently, with the advent of techniques such as stereolithography, it has
been
possible to produce one-off models which can be used as "masters" for resin
moulds
which can allow small volumes of parts to be produced. However, the number of
materials which can be produced in this way is limited and this technique is
best
suited to development of proof of concept models only.
Conventionally, production mould tools can normally be divided into three
sections:
(i) a cavity/cavities which represent the form of the component to be
produced;
(ii) a fixed half which includes the feed system; and
(iii) a moving half which includes the ejection system.
The purpose of these moulds is to allow for the production of high volume, low-
cost
components on automated injection mould machines. However, these tools are
often
1
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
complex and modifications can be difficult to make if further optimisation of
the
design is required.
With the ever-increasing need to reduce the time taken to commercialise new
products, it is essential that a multifunctional approach is taken to the
development of
medical devices, such as inhalers. This means that teams of designers,
engineers and
scientists must work together from the early stages of product development to
ensure
that the final inhaler design is suitable for and available for use in pivotal
clinical
trials.
Traditional tooling is considered an obstacle to rapid development of devices,
owing
to the long lead times in production and the di~culties associated with
easy°
modification of these types of tools.
The development of new devices demands a multifunctional approach from
designers, engineers, scientists and clinicians. Design and rapid engineering
of
prototypes represents the first critical step in this process. We have now
found a
novel tooling technique based on the manufacture of hard tools composed of
plates or
segmental parts permits prototyping to be conducted quickly and economically,
using
material relevant to the potential unique interplay, such as electrostatic
interactions,
between specific drug substances/excipients and specific plastic materials. A
further
advantage of this technique is the ability to easily modify tools as device
prototypes
axe refined.
We have developed a novel solution to the problems involved in a rapid
production
of hardened mould tooling.
Thus according to the invention we provide an injection moulding system
comprising
a modular bolster set provided with at least a pair of plates which can be
stacked
together to form a cavity, the plates include a feed and ejection system; and
a cavity
moulding set adapted for moulding of an article.
2
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
The injection moulding system of the invention is especially suitable for the
manufacture of small plastics devices e.g. medical devices, such as an
inhalation
device. The inhalation device manufactured by the system of the invention may
comprise any conventionally known inhalation device, for example, a metered
dose
inhaler (MDI) and especially, a dry powder inhaler (DPI). Examples of DPIs
which
may be mentioned are those currently available from Innovate Biomed in the UK
under the Trade Marks CLICKHALER~ and TECHNOHALER~. CLICKHALER is
described in International Patent application No. WO 92/00771, which is
incorporated herein by reference. TECHNOHALER is described in International
Patent application No. W093/16748 which is incorporated herein by reference.
The cavity moulding set used in the system of the invention preferably
comprises an
assembly of plates and segments. The plates are preferably wire eroded plates.
According to a further feature of the invention we provide a method of
manufacturing
a plastics device which comprises injecting a molten plastics material into an
injection moulding system as hereinbefore described.
We further provide a plastics device manufactured by the method described
above.
The plastics device may be a medical device, such as an inhalation device e.g.
a DPI.
In one embodiment of the invention the method comprises the manufacture of a
CLICKHALER, for example, an inhaler comprising a body defining a storage
chamber for a substance to be delivered and further defining an inhalation
passage
through which air is drawn via the mouthpiece; a metering member operable to
transfer a volumetric dose of the substance from the storage chamber to the
inhalation passage, the metering member having a metering surface which is
indented
to provide at Ieast one dispensing cup and being moveable between a first
position in
which a dispensing cup is presented to the inhalation passage wherein in the
second
position the dispensing cup is upwardly open , the inhaler comprising cup
clearing
3
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
means which comprises means for moving each dispensing cup into a position
form
which any of the substance remaining in the dispensing cup would tend to fall,
under
the influence of gravity, out of the cup, after a dose of the substance has
been
presented in the cup to the inhalation passage and before the cup id again
presented
to the storage chamber.
In a further embodiment of the invention the method comprises the manufacture
of a
TECHNOHALER, for example, apparatus for dispensing a plurality of desired
volumetric doses of a flowable substance comprising a storage chamber for the
substance and an outlet conduit communicating with the chamber, characterised
by a
plurality of metering devices, each metering device having first and second
end
elements adapted to seal against the inner walls of the conduit so as to
define an
intermediate dosing space of the desired dose volume and means fox advancing
the
metering devices in series from within the storage chamber into the outlet
conduit so
as to cause substance surrounding each device in the storage chamber to pass
with the
device into the conduit in the respective dosing space.
The novel method of the invention resolves the difficulties involved with
fabrication
and the lead-times in producing traditional mould tools is to strip away the
complex
ejection and feed systems used in hardened mould tools. These are replaced
with a
modular bolster set which contains a common feed system and takes a cavity set
which is assembled from wire eroded plates and segments.
In use the cavity moulding set may be assembled by hand and loaded into the
modular bolster set whilst in the press. Material e.g. plastics can then be
then
injected into the tool. When cooled the entire cavity moulding set removed by
hand.
The plates can then be dismantled, the component removed, the cavity
reassembled
and the process repeated. The use of wire eroding allows plate mould tools to
be
produced rapidly with simple parts taking less than one day and even very
complex
forms taking only a week.
4
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
The main advantages of this system are that parts can be moulded in the
relevant
production materials, designers can test and modify the moulded parts very
quickly
and finished devices can be assembled rapidly for early trials. Furthermore
the pre-
production tools can be produced with confidence as the moulded part has been
tested, reducing the tooling development time.
The method of invention is especially suited to the manufacture of small
plastics
medical devices, such as an inhalation device.
The method of the invention is advantageous in that;
it allows complex models to be made using injection moulded parts;
it allows devices to be tested and refined without the need for producing pre-
production moulds;
it permits early testing to be conducted using the final materials avoiding
problems with material compatibility; and
it offers considerable cost and time saving over traditional methods.
The invention will now be described by way of example only and with reference
to
the accompanying drawings in which Figure 1 is a moulding tool of the prior
art; and
Figure 2 is a perspective view of a cavity injection moulding system of the
invention.
Referring to Figure 1, an injection moulding system (1) of the prior art
comprises a
moving half (2) and a fixed half (3). The fixed half (3) is provided with an
injection
point and runner (4) and a component cavity (5). The moving half (2) is
provided
with an ejection point (6). The moving half (2) is also provided with a female
connector (7) at each corner (8) adapted to engage with corresponding male
connections (9) situated at each corner (10) of the fixed half (3). A plastics
component (11) is also shown.
5
CA 02401085 2002-08-22
WO 01/66334 PCT/GBO1/00893
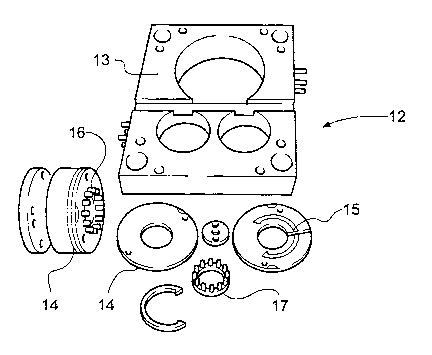
Referring to Figure 2, a modular bolster set (12) comprises a tool block (13)
and a
plurality of stackable cavity plates (14) provided with an injection point and
runner
(15) and ejection pins (16). A plastics component (17) is also shown.
Example I CLICKHALER
An initial design was prepared on CAD and plate mould tools were produced of
the
three new components required in the new design. From these parts minor
changes
were made to the positions of the gear teeth and flexible ratchet fingers.
Within one
month working models of the new design had been prepaxed which were suitable
for
laboratory testing to confirm shot weight performance and dose consistency of
the
new inhaler design. The component designs were then produced in fully hardened
cavities for use in a modulax bolster system allowing for the production of
devices for
clinical trials; the whole programme taking approximately three months.
Example 2 TECHNOHALER
TECHNOHALER is a new mufti-unit dose powder inhaler currently under
development by Tnnovata Biomed. The TECHNOHALER has a unique system for
dispensing the powder, which allows a strip of spools to be pre-filled at the
factory
and then dispensed from a carousel in the device. This ensures a high accuracy
in
dosing as well as adding moisture protection to the individual dose. Proof of
concept
models were produced using the plate moulding technique described eaxlier. The
availability of working prototypes of the device mechanism and casing allowed
the
engineering team to evaluate the design without the need for production of
expensive
pre-production mould tools.
6