Note: Descriptions are shown in the official language in which they were submitted.
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
AN ELECTROLYTIC REDUCTION CELL AND COLLECTOR BAR
The present invention relates to an electrolytic
reduction cell for the production of a metal, such as
aluminium.
The present invention relates particularly to a
collector bar construction for use in such cells.
Aluminium metal is generally produced in an
electrolytic reduction cell by the Hall-Heroult process in
which electrical current is passed through an electrolytic
bath comprising alumina dissolved in molten cryolite to
cause the electrodeposition of molten aluminium as a metal
pad on the cell cathode. An electrolytic reduction cell
comprises an outer steel shell that is lined with a layer
of insulating material, such as refractory bricks. Blocks
of carbonaceous material are placed on top of the
insulating layer on the base of the cell and these blocks
form the cathode of the cell. The blocks are hereinafter
referred to as "cathode blocks". The cathode must last
for the expected operating life of the cell, which is
typically 1000 to 2000 days. A number of consumable
anodes are located a short distance above the metal pad
that forms above the cathode. In an operating cell, an
electrolytic bath is located between the metal pad and the
anodes, and the passage of electrical current through the
electrolytic bath breaks down the dissolved alumina in the
electrolytic bath into aluminium and oxygen and the molten
aluminium collects in the metal pad on the cathode. The
molten aluminium is periodically drained from the metal
pad, typically on a daily basis.
Electrolytic reduction cells are arranged in
potlines in which a large number of cells are connected in
series. Electrical current enters a cell through the
anodes, passes through the electrolytic bath and pad of
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 2 -
molten metal and into the cathode. The current in the
cathode is collected and passes to an external current
carrier, such as an external bus bar, and then along to
the next cell.
In conventional aluminium reduction cell
technology, collector bars that are embedded in the
cathode blocks are used to collect electrical current from
the cathode and conduct it to an external ring bus. In
conventional embedded collector bar technology, the bar is
made from steel and is either cast or glued into a channel
formed in the underside of a cathode block.
In an operating cell, the cathode current
density distribution along the length of cathode blocks is
uneven with the outermost portions of the blocks drawing
current at up to three to four times higher density
compared to the inner portions of the blocks. Current
travels unevenly through the cathode blocks as it finds
the least resistance path from the cell. Specifically,
current tends to travel through the cathode blocks towards
the ends of the collector bars rather than directly down
through the cathode into the collector bars, thus
increasing the average current path length in the cathode.
Poor conductivity of steel collector bars and the use of
high conductivity cathode material contribute to the
uneven current density.
One consequence of the uneven current density is
an uneven current distribution on the surface of the
cathode blocks. It is highest near to the outer edge of
the anode shadow or ledge toe. The uneven cathode current
distribution has a dual effect on cell operation: on the
one hand it increases the rate of erosion of carbonaceous
material by increasing the chemical activity of sodium
(this drives the aluminium carbide-forming reaction) in
the affected region; and on the other hand it increases
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 3 -
the rate of transport of dissolved aluminium carbide by
inducing circulation of metal and catholyte. This
increased circulation can result either from increased
metal pad heave due to interaction in the metal pad of
horizontal currents with vertical magnetic fields or from
the Marangonni effect (i.e. circulation induced by uneven
interfacial tension between catholyte and aluminium due to
uneven cathode current density distribution at the
interface). The rate of erosion of carbon is therefore
directly related to the current density and the rate of
circulation of metal and catholyte.
As neither the horizontal currents in the metal
pad nor the vertical magnetic fields are even, balanced,
or static, their coupling can lead to hydrodynamic
instability of the metal-bath interface. The circulation
of the metal, the deformation of its surface and the
instability of the metal-bath interface are the three most
significant limitations of the current technology
aluminium reduction cells which affect potlife (cathode
and sidewall erosion) and operating efficiency. Moreover,
these limitations make it difficult to reduce the
anode/cathode spacing. This spacing has a major impact on
the power requirements of aluminium reduction cells.
In conventional aluminium reduction cell
technology it is difficult to have a completely uniform
cathode current density distribution throughout the cell.
The best outcome which can be achieved to date is to
reduce the variation of current density distribution by
constructing relatively narrow but long cells having
relatively deep, high resistivity, anthracitic cathode
blocks and large steel collector bars. The problem of
metal heave and metal pad stability (product of field
current interaction) is then addressed through the
modification of bus bars to control the vertical magnetic
field. Modern magnetically compensated cells are a good
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 4 -
example of this type of engineering within the limitations
of the system.
However, relatively narrow, but long reduction
cells are a disadvantage as they have a high external
surface to production volume ratio and hence have a high
heat loss. Nevertheless, in conventional cell
construction methods, the limitations resulting from
embedded collector bar technology have been accepted as
inherent to the nature of the aluminium reduction cells
cathode and its negative impact has been minimised by
focussing on improving the magnetic field aspect of the
current/field interaction. Modern aluminium reduction
cells are designed with magnetic compensation in order to
improve the hydrodynamic stability of the cells, and
therefore achieve reductions in anode/cathode spacing.
However, this requires relatively expensive external bus
bars.
An objective of the present invention is to
improve the efficiency of electrolytic reduction cells by
improving the spacial current density distribution in the
cells cathode and metal pad.
According to the present invention there is
provided an electrolytic reduction cell for the production
of a metal, which cell includes: an outer shell and an
inner lining of insulating material which form a base,
side walls and end walls for containing an electrolytic
bath; an anode; a cathode located on the base of the cell;
and a plurality of collector bars which electrically
connect the cathode to an electrical current carrier that
is external to the cell, wherein each collector bar
includes an elongated first section that contacts the
cathode and at least one end section that extends through
one of the side walls and is electrically connected to the
electrical current carrier, and wherein the cell is
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 5 -
characterised in that, for the purpose of controlling
current distribution, the first section of each collector
bar includes a core of relatively high electrical
conductivity material and an outer housing of a more
mechanically stable and chemically resistant material than
the core material and the end section of each collector
bar is formed from relatively low thermal conductivity
material.
The applicant has made the following findings in
computer modelling studies and in operation of several
test cells.
1. The use of collector bars having a highly
electrically conductive core improves the
spatial current density and therefore the
stability of an electrolytic reduction
cell.
2. The use of collector bars having a
relatively low thermal conductivity end
section avoids excessive heat loss from the
cell via the collector bars.
3. Construction of collector bars with the
conductive core enclosed in a more
mechanically and chemical resistant
material than the core material achieves
collector bar durability at least
equivalent to conventional steel collector
bars.
More particularly, the applicant has found that
the use of relatively high electrical conductivity
material, such as copper, as the cores of collector bars
does not have the disadvantages that were found with prior
art proposals, such as US patent 3,551,319 of Elliot and
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 6 -
are likely to arise with the proposal disclosed in the US
patent 5,976,333 of Pate.
In the Elliot proposal, copper cored bars were
originally used to improve voltage losses but were not
applied for commercial production purposes. The copper
extended all the way to the ends of the collector bars, ie
outside the cell, and the high thermal conductivity copper
extracted much more heat than conventional steel bars and
resulted in an overall increased cell heat loss, excessive
cell instability and long term thermal cycling. The
result was a reduced performance and overall higher
voltages. The applicant has realised that a significant
proportion of the voltage savings that were thought to be
possible with copper cored collector bars can be achieved
without having to form the collector bars with highly
electrically (and thermally) conductive end sections
outside the cathode. As a consequence, with the present
invention the applicant has been able to achieve reduced
cell overall heat loss and maintain correct cathode heat
balance to permit stable operation. The net effect has
been a greater overall energy saving through lower voltage
requirements and lower energy consumption due to required
current efficiency and controlled current distribution.
Preferably the core material is copper or a
copper alloy.
Preferably the outer housing material is a
relatively low electrical conductivity material compared
to the core material.
Preferably the outer housing material is steel.
Preferably the end section material is steel.
Preferably the cathode is in the form of a
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 7 -
plurality of blocks that are positioned side by side on
the base of the cell.
More preferably the cathode blocks extend side by
side along the length of the cell with the ends of the
blocks contiguous with the side walls of the cell.
In one embodiment there is one collector bar per
cathode block, with the first section extending along the
length of the block and the end sections of the bar being
formed from relatively low thermal conductivity material
and extending through opposite side walls.
In another, although not the only other,
embodiment there are two collector bars per block, with
the first section of one bar extending substantially half
way along the length of the block with an end section
extending through one side wall and the first section of
the other bar extending substantially half way along the
length of the block with an end section extending through
the other side wall.
Preferably the undersurface of the block includes
a channel which receives the first section of the
collector bar.
Preferably the first section of the collector bar
is cast or glued in the channel.
Preferably the cell includes a means for
increasing the effective surface area of electrical
contact between the cathode and the relatively high
electrical conductivity material core of each collector
bar.
Preferably the cell also includes a means for
improving both the longitudinal and transverse
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 8 -
distribution of current in the cathode.
In one embodiment the electrical contact means
includes a plurality of electrical contact plugs mounted
in electrical contact to the cathode and to the collector
bars.
Preferably the collector bar is cylindrical and
the diameter of the core is 60-80%, more preferably 70%,
of the diameter of the collector bar.
The present invention is based on thermal,
electrical and stress modelling studies on a proposed
aluminium reduction cell design and on the results of
operation of test cells based on the cell design at the
smelter of the applicant situated at Bell Bay, Tasmania,
Australia. The cell design is based on the use of
collector bars having a copper core housed in an outer
steel sleeve. The cell design is described in more detail
in section D in relation to the figures.
A. Thermal Modelling of Cell Design
Thermal modelling of the cell design with a
preferred form of copper-cored collector bars in
accordance with the present invention predicted the
following:
1. The cell design would not incur any thermal
penalty because of the use of the low thermal
conductivity end design of the bars.
2. when operated at standard Bell Bay operating
conditions with a metal level of around 150 mm
there could be a small voltage benefit.
3. At a lower metal level higher voltage savings
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 9 -
could be achieved.
B. Electrical Modelling
Electric modelling of current distribution in the
test and conventional cells established that significant
improvements in current density distribution can be
achieved through the use of copper-cored collector bars.
Table 1 contains a compilation of the expected
current distribution data obtained through electrical (3-
D) modelling which shows that the cell design (-the Test
Cell") had a significantly more uniform cathode current
density distribution and significantly reduced horizontal
currents compared to two standard cells ("Std" and
"Graphitic Std").
Table 1: Vertical and Horizontal Current Distribution in
Cells
Cell Design Metal Vertical Horizontal
Height Current Current
(mm) Distribution Distribution
( amp / cm2 ) ( amp / cm2 )
Ave. S.D. Ave. S.D.
Std 180 0.756 0.245 0.320 0.166
Graphitic 180 0.744 0.296 0.804 0.188
Std.
Test Cell 180 0.796 0.106 0.166 0.071
Std 60 0.757 0.229 1.121 0.550
Graphitic 60 0.746 0.295 1.329 0.682
Std
Test Cell 60 0.795 0.106 0.547 0.212
C. Stress Modelling
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 10 -
The expansion coefficient of copper is higher
than that of steel, leading to differential expansion of
copper.
Consideration of a situation where the copper-
core perfectly fits the steel tubing indicated the
possibility of high hoop stresses developing on the outer
surface of the steel hollow. However, the modelling
showed that, even under the worst case assumptions, the
stresses which could be generated would not exceed the
tensile strength of mild steel. Hence, the modelling
showed that cracking of steel is unlikely to be a problem.
Under operating conditions and temperature of 900 C both
copper and steel are ductile and would easily deform to
relieve these stresses.
Physical modelling of this worst-case scenario -
using a sample in the form of a 150mm long copper core
tightly-fitted into a steel tube and heated to 1000 C and
held at temperature for 2 weeks - showed that cracking is
not a problem. Electrical resistance testing of the
interface between the copper and steel of the sample
indicate a low contact resistance of about 0.05S2mm2 (<lmV).
The sample was cut open and the interface between
the copper and the steel was examined using SEM and
Microprobe analysis. The examination showed the
following:
1. The interface between copper and steel was
subject to oxide penetration to a distance of 10-
20mm from the ends;
2. The oxide combined with the alloying elements in
steel (Si and Mn) to cause precipitation of oxide
particles and grain boundary embrittlement in
steel;
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 11 -
3. There was mutual migration of copper and iron
across the copper/steel interface and a
metallurgical bond formed in the regions of
interface which were not affected by oxide
penetration;
4. Regions affected by oxygen penetration did not
form this metallurgical bond.
The work established the need for care to exclude
the possibility of air access to the copper/steel
interface to avoid deterioration of contact resistance.
Also, the work also showed that, if exclusion of air is
successful, there is a likelihood that a metallurgical
bond may form between the copper and steel to make this
interface more resistant to any attack by sodium in
subsequent service.
D. Cell Design
The test cells were constructed with collector
bars of half-cell length. It is noted that the present
invention is not restricted to such arrangements and
extends to full cell collector bars.
Figures 1 to 6 illustrate the construction of one
test cell.
Figure 1 is a vertical cross-section along the
length of the cell, Figure 2 is an enlargement of the
right hand end of the cell shown in Figure 1, Figure 3 is
a vertical cross-section across one half of the cell,
Figures 4 and 5 are longitudinal cross-sections of the
collector bar used in the cell, and Figure 6 is a
perspective view of the collector bar.
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 12 -
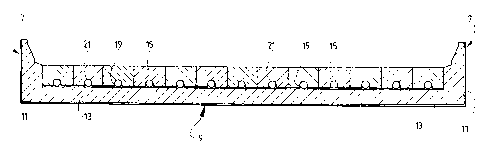
The cell has parallel side walls 5 (Figure 3),
parallel end walls 7 (Figures 1 and 2), and a base 9
(Figures 1 to 3). As with conventional aluminium
reduction cells, the test cell is relatively long and
narrow.
The side walls 5, end walls 7 and base 9 include
an outer steel shell 11 and an inner lining 13 of suitable
refractory material.
The cell also includes a plurality of cathode
blocks 15 located on the refractory lining 13 of the base
9 and arranged to extend across the cell to the side walls
5 and side-by-side along the length of the cell.
The cell also includes a plurality of anodes (not
shown).
Each cathode block 15 is formed with a channel 19
in the undersurface of the block 15. The channels 19
extend along the whole length of the blocks.
The cell further includes collector bars 21 which
electrically connect each cathode block 15 to an external
ring bus (not shown). Each collector bar 21 includes an
elongated section 27 that is cast or glued in one of the
channels 19 in a cathode block 15 and an end section 29
that extends through one of the side walls 5 and is
connected to the ring bus.
The elongated section 27 is generally cylindrical
and has a central core 31 of copper and an outer sleeve 33
of steel. The terminal end of the elongated section 27 is
closed by a steel disc 35. The end section 29 is
generally blocked-shaped and is formed from steel.
A preferred method of constructing the collector
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 13 -
bar 21 (of preferred dimensions) is described below.
1. Construction of end section 29
(i) Cut a 370mm long 100 x 100mm steel bar,
drill and prepare ends;
(ii) Centrally drill a 70mm die hole (37 in
Figures 4 and 5) to a depth of 55mm;
(iii)Cut a 45 external bevel (39 in Figures 4
and 5) to create a groove for welding;
2. Construction of elongate section 27
(i) Cut a 70mm diameter X 1150mm long copper
rod 31.
(ii) Slide fit the copper rod 31 into a 1045mm
long steel tubing 33 with 100mm OD and 70mm
ID. Bevel the edges at 45 at a depth of
10mm on one end.
3. Assembly of collector bar
(i) Insert the copper rod 31 into the hole in
the 370mm steel collector bar and weld
copper to steel.
(ii) Place the assembly into a 200 tonne press
and push the copper into the hollow steel
tube until the pressure increases reaches
the press maximum. The copper core 31
should end up being 30-70mm shorter than
the outer steel tube 33.
(iii)To this end of the assembly weld a steel
CA 02401204 2002-08-26
WO 01/63014 PCT/AU01/00199
- 14 -
disc 35, 10mm thick and 100mm diameter.
Appendix 9 contains the drawings which
describe the CCCB assemblies.
The first test cell was operated for 876 days
until it was deliberately cut out for autopsy. At the
completion of the autopsy the cell was reconstructed and
restarted successfully and operates as the second test
cell.
The autopsy results indicate that the performance
of the test cell was favourable when compared with
standard operating cells of the applicant. Specifically,
the test cell had a statistically lower voltage (100mV on
average for the majority of the operating period) than
that of the standard operating cell, a similar current
efficiency to the standard operating cell, and the noise
was lower or similar to that of the standard operating
cell.
Many modifications may be made to the preferred
embodiment without departing from the spirit and scope of
the present invention.
By way of example, whilst the preferred
embodiment of the collector bar 21 shown in the figures
includes a generally cylindrical copper-cored elongated
section 27 located within the cell and a generally block-
shaped steel end section 29 that extends through the side
walls 5 and from the cell, the present invention is not
limited to this construction. The collector bar 21 may be
of any suitable configuration. By way of example, the
collector bar may be generally flat rather than
cylindrical and block shaped. Moreover, the flat
collector bar may have a relatively wide section located
in the cell and a relatively narrow section extending
through and outwardly from the side walls of the cell.