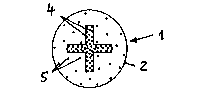Note: Descriptions are shown in the official language in which they were submitted.
CA 02401840 2002-09-03
1
BRISTLE IMPREGNATED WITH AN ANTI-MICROBIAL AGENT, BRUSHWARE COMPRISING
BRISTLES OF THIS TYPE AND PACKAGING FOR BRISTLES, OR BRUSHWARE OF THIS TYPE
The invention relates to a bristle with an antimicrobial finish, Which is
mainly of thermoplastic material and which is doped With an antimicrobial
substance which during use diffuses to the bristle surface. The invention. is
also directed at brushware having such bristles and to a pack for such
bristles or brushware.
Bristles having an antimicrobial finish have long been known, but have
scarcely been used in practice. Thus, at a very early date the known
antimicrobial action of silver in colloidal form and its possibility of use
in toothbrushes was recognized (GB 446 303), in that oligodynamic, colloidal
silver was embedded in bristle monofilaments or applied to the bristle
carrier. It is also known to embed in the bristle material or apply to the
bristle monovalent or polyvalent ion forming agents, including silver and
silver compounds (EP 678 548), silver being proposed in particle form < 10 dun
with a content of 100 ppm to 10 wt.%. It is finally known (DE 195 08 539) to
fill a bristle with particles of different size, the larger particles being
of silver with the oxidation number 0 and having a size of 1 to 50 ym.
it is also known (EP 413 B33) to coat bristle monofilaments with a solution
or emulsion of a polymer with free acid radicals and a mixed in, cationic
antibactericide, e.g. chlorohexidine and to then dry the coating. Production
is very complicated and the adhesion of the coating to the bristle core is
inadequate. The antibactericide also diffuses out too rapidly.
It is also known in textile fibre technology to bind silver to carrier
materials, e.g. zeolite (US 4,525,410, EP 275 047) and to disperse the thus
doped zeolite in particle form in the polymer melt for the monofilament and
extrude it with the latter. The fibre comprises a polymer core with a higher
melting point and an outer layer of a polymer with a low melting point, which
contains the silver-doped zeolite particles. It is also known (EP 116 865)
to coextrude a fibre having a Nylon core and outer segments or layers, which
contain silver-doped zeolite particles.
In all known systems the antimicrobial action is based on the fact that in a
moist environment antimicrobially active rations diffuse out of the bristle
or fibre and penetrate the thin cell wall of microorganisms, particularly
bacteria and block their protein metabolism.
NThen used as bristles or in brushware monofilaments of the aforementioned
type suffer from the disadvantage that if pure silver is used a~-the
antimicrobial substance this necessarily leads to a correspondingly high
filling of the bristle with the particulate silver. This leads to a
CA 02401840 2002-09-03
2
reduction in the stability of the bristles and consequently to a
deterioration of the use characteristics (flexibility, recovery capacity,
etc.). Thus, such monofilaments have not been adopted in practice.
Other microbial substances, particularly in the form of metal salts or
silver-doped zeolite, due to the high ballast material percentage, also lead
to a weakening of the bristle. This weakening can only be partly compensated
by a diameter increase, which is highly undesired in the case of much
brushware, particularly toothbrushes. This is often accompanied by an
undesired, high bristle roughness. All the known proposals suffer from the
further disadvantage that, even prior to use, particularly in a moist
atmosphere, the antimicrobial substance is given off, so that the substance
is used up prematurely.
On the basis of the prior art of EP 413 833, the problem of the present
invention is to propose a bristle for brushware, e.g. for toothbrushes, body
care, cosmetic and hygienic brushes or the like, which has an adequate
antimicrobial action and at the same time unchanged, highly satisfactory use
characteristics. The invention must also ensure that the antimicrobial
action is not prematurely used up.
According to the invention this problem is solved in that the bristle is
produced in a multicomponent extrusion process and has at least one first
cross-sectional area determining the mechanical use characteristics of the
bristle of a plastic component and at least one further cross-sectional area
of the other plastic component, and that the further cross-sectional area or
the sum of all further cross-sectional areas controls the diffusion rate of
the antimicrobial substance.
As a result of the construction according to the invention the use
characteristics of the bristle are guaranteed by the first cross-sectional
area, whereas the further cross-sectional area or areas are of minor
significance for the use characteristics of the bristle. The doping with the
antimicrobial substance takes place exclusively or preponderantly in one of
the cross-sectional areas, whereas the other cross-sectional area or areas
act in diffusion-controlling manner for the antimicrobial substance. The
control of the diffusion rate of the microbial substance can take place by
different doping of the cross-sectional areas With the substance or in that a
non-doped cross-sectional area acts as a diffusion brake or as a reflector
for the ionized atoms or molecules. This makes it possible to simultaneously
control the action period of the antimicrobial substance.
The use characteristics of the bristle on the one hand and the diffusion rate
on the other can also be influenced in that the cross-sectional areas are
made from different or differently finished plastics or have different cross-
CA 02401840 2002-09-03
3
sectional surfaces and/or different cross-sectional contours.
Preferably the further and appropriately smaller cross-sectional area of the
bristle is doped with the substance in high concentration, so that an
adequate charge carrier quantity diffuses into the moist atmosphere. In this
embodiment this can be further assisted in that the larger cross-sectional
area acts as a diffusion brake, so that the substance mainly diffuses out at
the surface of the highly doped cross-sectional area and only With a
significant time lag at the surface of the large cross-sectional area.
The large cross-sectional area of the monofilament can also be doped with the
substance in a concentration not impairing its mechanical use
characteristics, the further, smaller cross-sectional area acting exclusively
as a microbially inert diffusion brake and for regulating the active
substance delivery from the larger cross-section. However, the smaller
cross-section can also be highly doped With the antimicrobial substance and
then there is also a diffusion into the larger cross-sectional area, so that
the ions diffusing out there on the surface are constantly replaced from the
other cross-sectional area. However, at the same time the large cross-
sectional area forms a diffusion brake, because the ions preferably pass out
at the free surface of the smaller cross-sectional area, where the lower
diffusion resistance exists.
According to a preferred development, the further cross-sectional area is
located on the circumference of the large cross-sectional area and either
forms part of the bristle surface or completely embraces the bristle. This
on the one hand ensures a comparatively rapid delivery of the ions at the
free surface of the smaller cross-sectional area and on the other hand the
cross-sectional area decisive for the stability and bending behaviour of the
bristle is only slightly weakened, so that even very thin bristles can be
adequately doped with antimicrobial substance. In the case of thin bristles,
particularly With diameters smaller than 0.75 mm, the bristle can be
completely surrounded by a thin layer acting as a diffusion brake.
The further, smaller cross-sectional area can form a sector in the larger
cross-sectional area or also a layer on the surface thereof. In the first
case said further cross-sectional area can be highly doped, whereas when
constructed in the form of a layer it acts exclusively as a diffusion brake
through a corresponding material choice. This layer can be extruded with the
monofilament or can be subsequently applied.
In another embodiment the further, smaller cross-sectional area can be
located in the interior of the large cross-sectional area, e.g. in the form
of coextruded, thin monofilaments with a random cross-sectional shape
(circular, polygonal, cruciform, etc.). The further cross-sectional area can
CA 02401840 2002-09-03
4
also subdivide the large cross-sectional area into sectors, e.g. can be in
the form of a star-shaped layer of limited thickness. In this embodiment the
diffusion of the antimicrobial substance also takes place into the larger
cross-sectional area. To the extent that the surface of the smaller cross-
sectional area is exposed, an increased diffusing out takes place.
Preferably at least one further cross-sectional area has silver of oxidation
level 0 as the antimicrobial substance. Instead of this or in addition
thereto the large cross-sectional area can contain silver of oxidation level
0 and then preferably the further, smaller cross-sectional area has the same
substance in a higher concentration.
An advantageous embodiment is characterized in that the large cross-sectional
area has silver of oxidation level 0 with a content of 0<c<50,000 ppm and the
further cross-sectional area a content of c< 100.
In a preferred development the further, smaller cross-sectional area has
silver of oxidation level 0 with a content of 500<c<100,000 ppm. Practical
tests With such a bristle have shown that the combination of a high
concentration in the further, smaller cross-sectional area and a lower
concentration in the large cross-sectional area ensures a diffusion of the
antimicrobial substance at an adequate speed and over an adequate period of
time roughly corresponding to the use period of a toothbrush.
Another embodiment of the invention is characterized in that at least one
cross-sectional area has silver of oxidation level 1 and it can be in the
form of silver halides, sulphates, carbonates or organic silver salts, in
Which cation formation takes place as a function of the solubility product to
a greater extent.
The aforementioned antimicrobial substances can also be combined with one
another in such a way that one cross-sectional area has silver of oxidation
level 0 and at least one further cross-sectional area silver of oxidation
level 1.
If silver of oxidation level 0 is used, it can be contained in the further,
smaller cross-sectional area in the form of at least one thread which, during
the extrusion of the monofilament, also follows. Preferably the silver of
oxidation level 0 or 1 is present in disperse form in the plastic matrix and
is extruded together with the plastic melt. For this purpose it is possible
to use a granulate, which already contains the particles. Instead of this
silver-containing polymer particles can be extruded together With the
polymer. Since as a result of the silver content the polymer particles have
a better dimensional stability, they largely maintain their particle form.
Bristles of this type Without an antimicrobial finish and their production
CA 02401840 2002-09-03
are e.g. described in WO 17/09906, whose content is made into the subject
matter of the present application.
Instead of this it is possible for the silver of oxidation level 0 to be
applied to one of the cross-sectional areas in layer form.
All the cross-sectional areas of the bristle can comprise the same
thermoplastic material. However, it is also possible to form the further,
smaller cross-sectional area from a thermoplastic elastomer, which has a
lower diffusion resistance to the antimicrobial substance.
In addition, the cross-sectional areas can be differently surface or through-
dyed, in order to provide a use indication, in that increasing wear on the
free bristle end or bristle jacket is indicated by a corresponding colour
change.
The preferred thermoplastic materials are those having a water absorptivity
of at least 0.1 wt.% and more particularly polyamides, polyesters and
polyurethanes.
The diffusion rate can also be controlled in that the cross-sectional areas
of the bristle or the polymers forming them have a different water
absorptivity, so that the cations are more rapidly formed in the cross-
sectional area with the higher water absorptivity and more rapidly diffuse
out than in the other cross-sectional area.
The cross-sectional areas of the bristle can also be formed by two or more
combined monofilaments, whereof each forms one of the cross-sectional areas.
The invention also relates to brushware having a plastic bristle carrier and
bristles constructed according to the invention. Such brushware is
characterized in that also the bristle carrier is at least zonally
antimicrobially finished. Thus, in the case of toothbrushes, the invention
takes account of the scientifically proven finding that as a result of the
permanently moist atmosphere, as well as the cavities which have evolved in
the bristle configuration area, a relatively high bacterial attack occurs on
the bristle carrier.
If, as is usually the case, the bristle carrier is made from plastic, it
preferably has particulate silver of oxidation level 0 or 1.
If, as is also known, the hristle carrier is made from two different or
different types of plastic, which are produced by multicomponent injection
moulding, at least one of the components is filled With particulate silver of
oxidation level 0 or 1.
CA 02401840 2002-09-03
6
In a preferred development the bristle carrier is solely or preponderantly
filled in the vicinity of the fastening of the bristles with particulate
silver of oxidation level 0 or 1, i.e. in the area which is particularly
endangered by bacterial attack and the establishment of bacteria.
Finally, in the case of such brushware, the bristles are preferably
individually fastened to or in the bristle carrier, which compared with a
bundle-like arrangement of the bristles aids free diffusion, because on the
one hand moisture has uniformly rapid access to all the bristles and on the
other the bristle configuration dries more rapidly after use, which prevents
bacterial attack and blocks diffusion.
Moreover, either individually or in bundle form, the bristles are joined to
the bristle carrier in gap-free manner by thermal processes in order to
prevent the establishment of bacteria and in order to prevent excessively
rapid consumption of the antimicrobial substance. Such processes more
particularly include injecting in, welding or thermal bonding.
Bristles and brushware, particularly when used for hygienic purposes, such as
toothbrushes, cosmetic brushes, etc., reach the processor or final consumer
in packs. Frequently they are in the form of plastic packs or blister packs
with a cardboard support and a transparent plastic blister. As packing
cannot take place under sterile clean room conditions, it is also not
possible to exclude that during packing germs can enter the pack. Despite
the air-tight seal germ growth can occur if moisture is present in the pack,
particularly if as a result of temperature changes the moisture condenses on
the inside of the pack and which reacts more rapidly to temperature changes
than the actual packed article.
To prevent contamination of the packed bristles or brushware With germs,
according to the invention the pack is characterized in that it is finished
with an antimicrobial substance. Said substance is preferably silver or
silver salts and can be incorporated in disperse form into the material of
the pack or can be applied in layer form to the inside of the pack.
The invention is described in greater detail hereinafter relative to
embodiments represented in the drawings.
Fig. 1 is a cross-section through a bristle 1, whose first, larger cross-
sectional area 2 comprises a thermoplastic material, e.g. polyamide,
polyester or polyurethane and which has a further cross-sectional area 3 with
a much smaller cross-section and Which forms a partly cylindrical sector of
the overall cross-section. The cross-sectional area 2 has the use
characteristics necessary for a bristle With respect to the bending behaviour
CA 02401840 2002-09-03
7
and recovery capacity and at least to a limited extent this is aided by
cross-sectional area 3. Cross-sectional area 3 can be made from a different
plastic, particularly a different thermoplastic or an elastomer.
As can be gathered from fig. 2, the smaller cross-sectional area 3 passes
over the entire length of the bristle 1 and production preferably takes place
by the extrusion of both material components for cross-sectional areas 2 and
3. The smaller cross-sectional area 3 is filled with an antimicrobial
substance in particle form, preferably silver of oxidation level 0 or 1. A
high doping of the antimicrobial substance takes place. The larger cross-
sectional area 2 can also be filled with an antimicrobial substance 5 in
particle form. The thermoplastic material of the larger cross-sectional area
2 forms in this embodiment a diffusion brake for the highly doped,
antimicrobial substance in the smaller cross-sectional area 3 and which
diffuses out more rapidly on its free surface, whereas the substance from the
larger cross-sectional area diffuses out at a lower speed and more slowly due
to the longer diffusion paths.
The bristle 1 according to fig. 3 once again has a large cross-sectional area
2, which determines the use characteristics thereof. In said cross-sectional
area 2 the small cross-sectional area 3 is present as a core and is produced,
optionally together With the larger crass-sectional area 2, by coextrusion.
It can once again comprise a different plastic and is in this case highly
doped with the antimicrobial substance 4. In this case the larger cross-
sectional area 2 acts as a diffusion brake or conversely the smaller cross-
sectional area 3 forms a slow and continuously flowing source of
antimicrobial substance.
In the embodiment according to fig. 4 the larger cross-sectional area 2
determining the bristle use characteristics contains three smaller cross-
sectional areas 3 in the form of strands and Which are once again highly
doped with the antimicrobial substance. Once again the larger cross-
sectional area 2 acts as a diffusion brake.
In the embodiment according to fig. 5 the further, smaller cross-sectional
area 3 is incorporated in the form of a cruciform cross-section in the larger
cross-sectional area 2. In this embodiment the cruciform cross-sectional
area 3 is highly doped and the larger cross-sectional area 2 less highly
doped With the antimicrobial substance.
Fig. 6 shows an embodiment in which the larger cross-sectional area 2 is
subdivided in star-like manner into sectors by the smaller cross-sectional
area 3 and only the smaller cross-sectional area 3 is highly doped with the
antimicrobial substance.
' CA 02401840 2002-09-03
~ 8
Fig. 7 shows a bristle 6 with a polygonal, namely square cross-section
leading to a higher mechanical cleaning action than with a circular bristle.
The bristle 6 once again has a larger cross-sectional area 2 of a
thermoplastic material and a smaller cross-sectional area 3 in the form of a
segment, which is more highly doped with the antimicrobial substance than the
large cross-sectional area 2. The bristle 7 according to fig. 8 differs from
that of fig. 7 only in that it has two segmental, smaller cross-sectional
areas 3 at diagonally positioned corners. In both cases the antimicrobially
acting cations are mainly delivered at the free surface of the smaller cross-
sectional area 3. However, part thereof is diffused into the larger cross-
sectional area 2 and to this extent a diffusion brake is formed, because the
cations must firstly migrate through this cross-sectional area in order to
arrive at the free surface thereof.
In the embodiment according to fig. 9 the smaller crass-sectional area 3
forms a layer on the larger cross-sectional area 2 only extending over a
small part of the circumference. The cross-sectional area 3 is preferably
diffusion-tight with respect to the antimicrobial substance 4 in the large
cross-sectional area 2, so that the inherently slow diffusion on the larger,
free surface of cross-sectional area 2 is intensified. According to fig. 10
this can optionally take place in locally oriented form, in that the outer
layer 3 forming the diffusion brake covers a larger part of the circumference
of the large cross-sectional area 2 or, as in the embodiment of fig. 11, the
entire circumference. In both cases the antimicrobial substance is
exclusively housed in the large cross-sectional area 2.
Fig. 12 shows a star-shaped bristle 8, whose bearing cross-sectional area 2
has a four-arm construction. At the ends of each arm are located the smaller
cross-sectional areas 3, which are highly doped with the antimicrobial
substance 4, whereas the cross-sectional area 2 is less highly doped.