Note: Descriptions are shown in the official language in which they were submitted.
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
REAGENT SYSTEMS FOR DETECTING THE PRESENCE OF A REDUCED
COFACTOR IN A SAMPLE AND METHODS FOR USING THE SAME
INTRODUCTION
Field of the Invention
The field of this invention is analyte detection, particularly reagent systems
for use in
analyte detection.
Back round of the Invention
1o Analyte detection in physiological fluids, e.g. blood or blood derived
products, is of
ever increasing importance to today's society. Analyte detection assays find
use in a variety
of applications, including clinical laboratory testing, home testing, etc.,
where the results of
such testing play a prominent role in diagnosis and management in a variety of
disease
conditions. Analytes of interest include alcohol, formaldehyde, glucose,
glutamic acid,
15 glycerol, beta-hydroxybutyrate, L-lactate, leucine, malic acid, pyruvic
acid, steroids, etc. In
response to this growing importance of analyte detection, a variety of analyte
detection
protocols and devices for both clinical and home use have been developed. Many
of the
protocols and devices that have been developed to date employ a signal
producing system to
identify the presence of the analyte of interest in a physiological sample,
such as blood.
2o One type of signal producing system that finds use in the detection of a
variety of
different analytes is one in which a dehydrogenase oxidizes the analyte of
interest and
concomitantly reduces an enzyme cofactor, such as NAD(P)+. The reduced form of
the
cofactor, e.g. NAD(P)H, is then detected through subsequent reaction with a
cofactor
oxidizing agent, e.g. phenazine methosulfate or a diaphorase, that transfers
an electron to a
25 redox indicator, such as a tetrazolium salt, to produce a detectable
product.
While a variety of such signal producing systems have been developed to date
for use
in the detection of a wide variety of different analytes, there continues to
be a need for the
fi~rther development of such systems. For example, a signal producing system
which
provided for an enhanced reaction rate and a lower cost would be of great
interest.
3o Relevant Literature
U.S. Patents of interest include: 4,629,697; 5,126,247 and 5,902,731. See also
Raap
et al., Histochem. J. (1983) 15:881-893.
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
SUMMARY OF THE INVENTION
Signal producing systems, reagent compositions, test strips and kits of the
same, as
well as methods for their use in the detection of an analyte in a sample, are
provided. The
subject signal producing systems are characterized by having at least a first
and second
electron transfer agent and a redox indicator, where in many preferred
embodiments the
systems include a proteinaceous and non-proteinaceous electron transfer agent,
e.g. a
phenazine compound and a diaphorase. In many preferred embodiments, the
subject systems
and kits further include both an enzyme cofactor and an enzyme having an
analyte oxidizing
activity, e.g. an analyte dehydrogenase. The subject systems, reagent
compositions, test
1o strips and kits find use in the detection of a wide variety of analytes in
a sample, such as a
physiological sample, e.g. blood or a fraction thereof.
BRIEF DESCRIPTION OF THE FIGURES
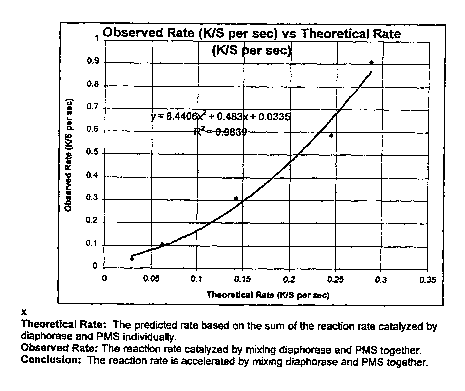
Fig. 1 provides a graphical representation of the observed rate of reaction in
a test
strip according to the subject invention vs. the theoretical expected rate of
reaction for a test
strip that includes both PMS and a diaphorase, clearly demonstrating that the
use of both a
non-proteinaceous and proteinaceous electron transfer agent, e.g. PMS and a
diaphorase,
provides for an unexpected increase in the rate of reaction.
2o DESCRIPTION OF THE SPECIFIC EMBODIMENTS
Signal producing systems, reagent compositions, test strips and kits of the
same, as
well as methods for their use in the detection of an analyte in a sample, are
provided. The
subject signal producing systems are characterized by having at least a first
and second
electron transfer agent and a redox indicator, where in many preferred
embodiments the
systems include a proteinaceous and non-proteinaceous electron transfer agent,
e.g. a
phenazine compound and a diaphorase. In many preferred embodiments, the
subject systems
and kits further include both an enzyme cofactor and an enzyme having an
analyte oxidizing
activity, e.g. an analyte dehydrogenase. The subject systems, reagent
compositions, test
strips and kits find use in the detection of a wide variety of analytes in a
sample, such as a
3o physiological sample, e.g. blood or a fraction thereof.
Before the subject invention is described further, it is to be understood that
the
invention is not limited to the particular embodiments of the invention
described below, as
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
variations of the particular embodiments may be made and still fall within the
scope of the
appended claims. It is also to be understood that the terminology employed is
for the purpose
of describing particular embodiments, and is not intended to be limiting.
Instead, the scope
of the present invention will be established by the appended claims.
In this specification and the appended claims, singular references include the
plural,
unless the context clearly dictates otherwise. Unless defined otherwise, all
technical and
scientific terms used herein have the same meaning as commonly understood to
one of
ordinary skill in the art to which this invention belongs.
/o
SIGNAL PRODUCING SYSTEMS
As summarized above, the subject invention provides a signal producing system
that
is capable of detecting the presence of a reduced enzyme cofactor in a sample.
By signal
producing system is meant a collection of two or more compounds or molecules
which are
15 capable of acting in concert, when combined, to produce a detectable signal
that is indicative
of the presence of, and often amount of, a particular analyte in a given
sample. The term
signal producing system is used broadly to encompass both a mixture of all of
the reagent
constituents of the signal producing system as well as a system in which one
or more of the
reagent constituents are separated from the remainder of the reagent
constituents, e.g. as is
2o present in a kit.
A feature of the subject signal producing systems is the presence of two
distinct
electron transfer agents. By electron transfer agent is meant a compound or
molecule that
can transfer an electron, in the form of a hydride ion, from a reduced enzyme
cofactor to a
redox indicator. In the subject signal producing systems, the first of the
distinct electron
25 transfer agents is a low molecular weight molecule, while the second
electron transfer agent
is a high molecular weight molecule. In this specification, low molecular
weight means a
molecular weight that does not exceed about 2000 daltons, usually about 1000
daltons and in
many embodiments about 500 daltons. High molecular weight means a molecular
weight of
at least about 5000 daltons and in many embodiments 10,000 or 20,000 daltons
or higher.
3o The molecular weight of the high molecular weight electron transfer agent
often will not
exceed about 100,000 daltons. In many embodiments, the low molecular weight
electron
transfer agent is a non-proteinaceous compound while the high molecular weight
electron
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
transfer agent is a proteinaceous compound. By proteinaceous is meant a
polypeptide or
polymeric mimetic thereof.
A variety of low molecular weight non-proteinaceous electron transfer agents
are of
interest. These agents include: flavins such as riboflavin (RBF), alloxazine
s (ALL) and lumichrome (LC); phenazines such as phenazine, phenazine
methosulfate (PMS),
phenazine ethosulfate, methoxyphenazine methosulfate and safranine; methyl-1,
4-naphthol
(menadione), phenothiazines such as PT and its radical cation, PT+, thionin
(TH), azure A
(AA), azure B (AB), azure C (AC), methylene blue (MB), methylene green (MG)
and
toluidine blue O (TOL); phenoxazines such as phenoxazine (POA), basic blue 3
(BB3), and
to brilliant cresyl blue ALD (BCBA), benzo-oc-phenazoxonium chloride (Medola's
blue);
Indophenols such as 2,6-dichlorophenol indophenol (DCIP); and Indamines such
as
Bindschedler's green and phenylene blue; and the like.: Of particular interest
in many
embodiments are phenazine compounds, e.g. PMS, phenazine ethosulfate,
methoxyphenazine methosulfate and safranine, where PMS is the low molecular
weight,
is non-proteinaceous electron transfer agent in many embodiments.
In many embodiments, the high molecular weight proteinaceous electron transfer
agent is an enzyme that is capable of oxidizing a reduced cofactor, e.g.
NAD(P)H, and
concomitantly reducing a redox indicator. In many embodiments, this electron
transfer
enzyme is a diaphorase, such as lipoic dehydrogenase, ferredoxin-NADP
reductase,
20 lipoamide dehydrogenase, NADPH dehydrogenase, etc. A variety of diaphoreses
are
available and may be employed, where representative commercially available
diaphoreses
that may be present in the subject signal producing systems include bacillus
diaphorase,
clostridium diaphorase, vibrio diaphorase, porcine diaphorase, and the like.
In the subject signal producing systems, the ratio of the first to the second
electron
25 transfer agent is chosen to provide for an accelerated reaction rate as
compared to a control,
e.g. a comparable signal producing system with a single electron transfer
agent, e.g. only
PMS or a diaphorase. Typically, the ratio of the first to the second electron
transfer agent in
the subject systems ranges from about 0.001 to 10, usually from about 0.01 to
1.0 and more
usually from about 0.05 to 0.5 (nmole/U), respectively.
3o In addition to the above described first and second electron transfer
agents, the
subject signal producing systems also include a redox indicator. By redox
indicator is meant
a compound that is capable of being reduced by the electron transfer agents to
produce a
detectable, e.g. chromogenic, product. Where the redox indicator produces a
chromogenic
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
product, i.e. the redox indicator is a chromogen, suitable chromogens are any
compound
capable of changing color upon reduction by one or more electrons, where
suitable
chromogens are generally ones that accept electrons from the electron transfer
agents,
described above.
A variety of different redox indicator compounds are of interest. Compounds of
interest include: oxazines, thiazines, and tetrazolium salts. Of particular
interest in many
embodiments are tetrazolium salts which are capable of accepting the captured
hydride from
the electron transfer agents to form a colored formazan product. In many
embodiments, these
salts have the advantageous feature of being faint yellow in the oxidized
form, but turn
to bright visible colors upon electron reduction and conversion to formazan
dyes. Tetrazolium
compounds or salts that are of particular interest include: 2-(2'
benzothiazolyl)-5-styryl-3-
(4'-phthalhydrazidyl) tetrazolium (BSPT); 2-benzothiazolyl-(2)-3,5-diphenyl
tetrazolium
(BTDP); 2,3-di(4-nitrophenyl) tetrazolium (DNP); 2,5-diphenyl-3-(4-
styrylphenyl)
tetrazolium (DPSP); distyryl nitroblue tetrazolium (DS-NBT); 3,3'-[3,3'-
dimethoxy-(1,1'-
15 biphenyl)-4,4'-diyl]-bis[2-(4-nitrophenyl)-5- phenyl(-2H tetrazolium (NBT);
3-(4,5-
dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium (MTT); 2-phenyl-3-(4-
carboxyphenyl)-5-
methyl tetrazolium (PCPM); tetrazolium blue (TB); thiocarbamyl nitroblue
tetrazolium
(TCNBT); tetranitroblue tetrazolium (TNBT); tetrazolium violet (T~; 2-
benzothiazothiazolyl-3-(4-carboxy-2-methoxyphenyl)-5-[4-(2-
sulfoethylcarbamoyl)phenyl]-
20 2H-tetrazolium (WST-4); 2,2'-dibenzothiazolyl-S,5'-bis[4-di(2-
sulfoethyl)carbamoylphenyl]-3,3'-(3,3'-dimethoxy- 4,4'-
biphenylene)ditetrazolium,
disodium salt (WST-S); 2-(p-nitrophenyl)-3-(p-iodophenyl)-5-phenyltetrazolium
chloride
(INT); and the like. WST-5 is preferred in many embodiments because it readily
dissolves in
an aqueous medium, which is most compatible with biological samples.
Furthermore, the
25 resulting formazan compound exhibits strong spectral absorption at the
purple-blue region,
thus reducing the need for correcting the background signal from hemoglobin.
Other useful
tetrazolium salts are disclosed in U.S. Pat. Nos. 4,490,465; 4,491,631;
4,598,042; 4,351,899;
4,271,265; 4,247,633; 4,223,090; 4,215,917; 4,142,938; 4,024,021; 3,867,259;
3,867,257;
3,791,931; and 4,254,222; the disclosures of which are herein incorporated by
reference.
3o The above described signal producing systems are capable of detecting the
presence
of a reduced enzyme cofactor in a sample, particularly an aqueous sample and
more
particularly a physiological sample, e.g. whole blood or a fraction or
derivative thereof. A
variety of different reduced enzyme cofactors may be detected using the
subject signal
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
producing systems, where representative reduced enzyme cofactors include the
reduced
forms of the following cofactors: beta-nicotinamide adenine dinucleotide (beta-
NAD), beta-
nicotinamide adenine dinucleotide phosphate (beta-NADP), thionicotinamide
adenine
dinucleotide, thionicotinamide adenine dinucleotide phosphate, nicotinamide
1,N6-
ethenoadenine dinucleotide, nicotinamide 1,N6-ethenoadenine dinucleotide
phosphate, and
pyrrolo-quinoline quinone (PQQ). The subject signal producing systems are
particularly
suited for use in the detection of NADH or NAD(P)H.
In many applications in which the subject signal producing systems find use,
the
reduced enzyme cofactor is one that is produced following the oxidation of an
analyte of
to interest in a sample. In many embodiments, therefore, the subject signal
producing systems
also include the enzyme cofactor and an analyte oxidizing enzyme that is
capable of
oxidizing the analyte of interest and concomitantly reducing the enzyme
cofactor. Enzyme
cofactors of interest include those described above, i.e. beta-nicotinamide
adenine
dinucleotide (beta-NAD), beta-nicotinamide adenine dinucleotide phosphate
(beta-NADP),
thionicotinamide adenine dinucleotide, thionicotinamide adenine dinucleotide
phosphate,
nicotinamide I,N6-ethenoadenine dinucleotide, nicotinamide 1,N6-ethenoadenine
dinucleotide phosphate, and pyrrolo-quinoline quinone (PQQ). Enzyme cofactors
of
particular interest that may be included in the subject signal producing
systems include:
NADH or NAD(P)H.
2o The analyte oxidizing enzyme present in the signal producing system
necessarily
depends on the nature of the analyte to be detected with the system.
Representative analyte
oxidizing enzymes of interest include: alcohol dehydrogenase for alcohol,
formaldehyde
dehydrogenase for formaldehyde, glucose dehydrogenase for glucose, glucose-6-
phosphate
dehydrogenase for glucose-6-phosphate, glutamate dehydrogenase for glutamic
acid,
glycerol dehydrogenase for glycerol, beta-hydroxybutyrate dehydrogenase for
beta-
hydroxybutyrate, hydroxysteroid dehydrogenase for steroid, L-lactate
dehydrogenase for L-
lactate, leucine dehydrogenase for leucine, malate dehydrogenase for malic
acid, and
pyruvate dehydrogenase for pyruvic acid. As can be seen from the above
representative list,
the analyte oxidizing enzyme is typically a dehydrogenase.
REAGENT COMPOSITIONS
Also provided by the subject invention are reagent compositions for use in
detecting
at least a reduced enzyme cofactor, and in many embodiments and analyte, in a
sample. The
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
reagent compositions may be fluid, e.g. aqueous, or dry compositions, where in
many
embodiments the reagent compositions are dry compositions. At a minimum, the
subject
reagent compositions are ones that include the first and second electron
transfer agent and
the redox indicator, where these components are described above. Such reagent
compositions are suitable for use in the detection of reduced enzyme
cofactors, e.g.
NAD(P)H, in a sample. In many embodiments, however, the reagent compositions
further
include an enzyme cofactor and an analyte oxidizing enzyme, where these
components are
described above.
REAGENT TEST STRIPS
Also provided by the subject invention are reagent test strips for use in
detecting the
presence of an analyte in a sample. In particular, the invention provides dry
strips for
assaying for a particular analyte in whole blood, e.g. in beta-
hydroxybutyrate, glucose, etc.
In the broadest sense, the reagent test strip includes a solid support and a
dry reagent
composition present thereon, where the dry reagent composition is made up of
all of the
reagent compounds necessary to produce a detectable signal in the presence of
the analyte of
interest. In most embodiments of the subject invention, the dry reagent
composition present
on the subject test strip is one that includes the following members: an
analyte oxidizing
enzyme, an enzyme cofactor, first and second electron transfer agents and a
redox indicator,
2o where each of these constituent members are described in greater detail
supra.
In many embodiments, the subject test strips include a membrane test pad that
is
affixed to a solid support. The support may be a plastic - e.g., polystyrene,
nylon, or
polyester - or metallic sheet or any other suitable material known in the art.
In many
embodiments, the test pad preferably comprises a bibulous, such as filter
paper or polymer
membrane. Associated with the test pad, e.g. coated onto the test pad,
incorporated into the
test pad, etc., is the reagent composition. The strip may also be configured
in more complex
arrangements, e.g. where the test pad is present between the support and a
surface layer,
where one or more reagents employed in sample processing may be present on the
surface
layer. In addition, flow paths or channels may be present on the test strip,
as is known in the
3o art. Of interest in many embodiments are the test strip configurations
disclosed in U.S.
Patent No. 5,902,731, the disclosure of which is herein incorporated by
reference.
The subject test strips may be fabricated employing any convenient protocol.
One
convenient protocol is to contact at least the test pad portion of the strip
with an aqueous
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
composition that includes all of the members of the reagent composition that
is to be
associated with the test pad in the final reagent test strip. Conveniently,
the test pad may be
immersed in the aqueous composition, maintained therein for a sufficient
period of time and
then dried, whereby the test pad of the reagent test strip which has
associated therewith the
s reagent composition is produced. As stated above, the aqueous composition
will include the
various members of the reagent composition to be associated with the test pad
of the reagent
test strip, where the various members are present in amounts sufficient to
provide for the
desired amounts in the reagent composition that is produced on the test pad.
As such, the
concentration of non-proteinaceous electron transfer agent present in this
aqueous
l0 composition typically ranges from about 1 gM to 1000 p,M, usually from
about 10 ~tM to
500 ~M; while the concentration of proteinaceous electron transfer agent in
the aqueous
composition ranges from about 50U to 3000 U, usually from about 100 U to 1000
U. The
concentration of redox reagent present in the aqueous composition ranges from
about 3 mM
to 36 mM, usually from about 6 mM to 24mM. When present, the enzyme cofactor
ranges in
15 concentration from about 1.5 mM to 28 mM, usually from about 3.5mM to 14
mM.
Similarly, the analyte oxidizing agent enzyme ranges in concentration from
about 100 U to
2000 U, and usually from about 200 U to 1000 U when present. Other components
that may
be present in this aqueous composition employed to prepare the reagent test
strip include
sodium chloride, magnesium chloride, Tris, PSSA, Tetronic 1307, Crotein-SPA,
sucrose,
20 oxamic acid, sodium salt, and the like. See the experimental section,
infra, for a more
detailed description of a representative method for preparing the subject
reagent test strips.
METHODS OF ANALYTE DETECTION
The above described signal producing systems, reagent compositions and test
strips
25 find use in methods of detecting the presence of, and often the amount of,
an analyte in a
sample. A variety of different analytes may be detected using the subject
methods, where
representative analytes include those described above, e.g. alcohol,
formaldehyde, glucose,
glutamic acid, glycerol, beta-hydroxybutyrate, L-lactate, leucine, malic acid,
pyruvic acid,
steroids, etc. While in principle, the subject methods may be used to
determine the presence,
3o and often concentration, of an analyte in a variety of different
physiological samples, such as
urine, tears, saliva, and the like, they are particularly suited for use in
determining the
concentration of an analyte in blood or blood fractions, e.g. blood derived
samples, and more
particularly in whole blood.
s
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
An important feature of the subject methods is that use of the subject signal
producing systems that include both first and second electron transfer agents
provides for an
accelerated reaction time as compared to a control system, e.g. a system that
includes a
single electron transfer agent. Generally, the reaction rate is accelerated or
enhanced by a
factor of 1 to 3 depending on the ratio of PMS and diaphorase. Furthermore,
the reaction rate
is greater than the theoretical or predicted rate which would be expected
based on the
summation of the rates provided by the individual electron transfer agents by
a factor of 1
to3. Where the reaction rate is measured in terms of K/S per sec (see the
experimental
section infra) the K/S per sec for a reaction in which the subject signal
producing systems
to are employed typically ranges from about 0.01 to 10, usually from about
0.05 to 5 and more
usually from about 0.1 to 2.
In the subject methods, the sample and the signal producing system are
combined
into a reaction mixture, the reaction is allowed to proceed for a sufficient
period to time to
generate a signal indicative of the presence of (and often amount of) analyte
in the sample,
15 and the resultant signal is detected and related to the presence of (and
often amount of)
analyte in the sample. In the broadest sense, the reaction mixture may be
produced in any
convenient environment, such as a cuvette or other fluid containment means.
However, in
many embodiments, the above steps take place on a reagent test strip as
described supra. As
such, the subject methods are now discussed further in terms of methods in
which a reagent
2o test strip is employed.
In practicing the subject methods, the first step is to apply a quantity of
the
physiological sample to the test strip, where the test strip is described
supra. The amount of
physiological sample, e.g. blood, that is applied to the test strip may vary,
but generally
ranges from about 2~L to 40~.L, usually from about Sp.L to 20gL. Because of
the nature
25 ofthe subject test strip, the blood sample size that is applied to the test
strip may be
relatively small, ranging in size from about 2p.L to 40~t.L,, usually from
about Sp.L, to 20p.L,.
Where blood is the physiological sample, blood samples of a variety of
different hematocrits
may be assayed with the subject methods, where the hematocrit may range from
about 20%
to 65%, usually from about 25% to 60%.
3o Following application of the sample to the test strip, the sample is
allowed to react
with the members of the signal producing system to produce a detectable
product that is
present in an amount proportional to the initial amount of the analyte of
interest present in
the sample. The amount of detectable product, i.e. signal produced by the
signal producing
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
system, is then determined and related to the amount of analyte in the initial
sample. In
certain embodiments, automated instruments that perform the above mentioned
detection
and relation steps are employed. The above described reaction, detection and
relating steps,
as well as instruments for performing the same, are further described in U. S.
Patent Nos.
4,734,360; 4,900,666; 4,935,346; 5,059,394; 5,304,468; 5,306,623; 5,418,142;
5,426,032;
5,515,170; 5,526,120; 5,563,042; 5,620,863; 5,753,429; 5,573,452; 5,780,304;
5,789,255;
5,843,691; 5,846,486; 5,902,731; 5,968,836 and 5,972,294; the disclosures of
which are
herein incorporated by reference. In the relation step, the derived analyte
concentration
takes into account the constant contribution of competing reactions to the
observed signal,
1o e.g. by calibrating the instrument accordingly.
KITS
Also provided by the subject invention are kits for use in practicing the
subject
methods. The kits of the subject invention at least include a signal producing
system as
described above, where the signal producing system components may be combined
into a
single reagent composition or separated, e.g. present in separate containers.
In certain
embodiments, the signal producing system will be present in the kits in the
form of a reagent
test strip, as described supra. The subject kits may further include a means
for obtaining a
physiological sample. For example, where the physiological sample is blood,
the subject kits
2o may further include a means for obtaining a blood sample, such as a lance
for sticking a
finger, a lance actuation means, and the like. In addition, the subject kits
may include a
control solution or standard, e.g. an analyte control solution that contains a
standardized
concentration of analyte. In certain embodiments, the kits also include an
automated
instrument, as described above, for detecting the amount of product produced
on the strip
following sample application and relating the detected product to the amount
of analyte in
the sample. Finally, the kits include instructions for using the subject kit
components in the
determination of an analyte concentration in a physiological sample. These
instructions may
be present on one or more of the packaging, a label insert, containers present
in the kits, and
the like.
The following examples are offered by way of illustration and not by way of
limitation.
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
EXPERIMENTAL
A. Preparation of Ketone Test Strip
An 0.8 ~tm nylon membrane obtained from Cuno (Meridien, CT) was dipped into
the
reagent of Table 1, until saturated.
Table 1
Com onent _ Quantity
Water 100 ml
Tris(hydroxvmeth 1)aminomethane 1.2
sodium chloride (MW 56.44, Si ma St. Louis, 560 m
MO)
Ma esium Chloride (MW 203, Si , St. Louis, MO) 2.5
PSSA, of s enesulfonic acid, sodium salt (MW 3
70,000)
Crotein-SPA (Croda Inc., Parsi an , N.n 3
Oxamic acid, sodium salt 250 m
Tetronic 1307 (BASF Co oration, Mount Olive, 2 m
N.n
Sucrose (MW 342.30, Aldrich Chemicals, Milwaukee5 gm
WI)
NAD (MW 663.4, N7004, Si a, St. Louis, MO) 450 m
D-3-hvdroxvbutvrate dehvdrogenase 50.000 U
WST-5 (MW 1331.37, Dojindo, 1a an) 1.8
Dia horase 0-15000 U
Phenazine Methosulfate (PMS) 0-3 m
The excess reagent was scraped off gently with a glass rod. The resulting
membrane was
hung to dry in a 56 °C oven for 10 minutes. Porex (0.6 mm thick) was
soaked in the 5%
nitrite solution and then hung to dry in a 100 °C oven for ten hours.
Finally, the membrane
1o was laminated between a polyester stock (0.4 mm Melenex~ polyester from ICI
America,
Wilmington DE) and the nitrite impregnated Porex.
B. Assays
Using the following protocol, 10 pL of aqueous samples comprising 40mg/dL (D)
(3-
hydroxybutyrate were tested on strips as described above, where the strips
varied in terms of
the amount of PMS and/or Diaphorase present on the strip. A 10 ml aqueous
sample was
applied onto a freshly prepared test strip. The strip was inserted into a
reflectometer and
data acquisition was commenced. The relectance of the reading strip was
monitored at 660
nm at one-second intervals for two minutes. Next, the data were uploaded from
the
2o reflectometer's memory buffer to a personal computer via a modified serial
cable. The
reaction rate was calculated based on the initial rate of change in K/S at the
range where the
reaction profile was linear. The results summarized in Table 2 were averages
of five
replicates.. In each individual assay, the reaction rate (in terms of K/S per
sec) was observed.
11
CA 02404421 2002-09-27
WO 01/73114 PCT/USO1/07619
(K/S is a measure of reflectance, discussed and defined in USP 4,935,346, col.
14, the
disclosure of which is herein incorporated by reference.) Table 2 provides the
results.
Table 2
DAD DAD and
and PMS
PMS Formulated
Formulation To ether
Separately
DAD Rate PMS Rate SeparatetDAD PMS Together
U/ml (K/S/sec)( m) (K/S/sec)(K/S/sec)U/ml ( m) (K/S/sec)
150 0.0166 10 0.013 0.0296 150 10 0.0385
300 0.0392 20 0.0225 0.0617 300 20 0.1054
600 0.0943 40 0.0484 0.1427 600 40 0.3095
900 0.1666 60 0.078 0.2446 900 60 0.5861
1200 0.1787 80 0.1085 0.2872 1200 80 0.9107
1500 0.2866 100 0.13 0.4166 1500 100 Too fast*
f Theoretical rate, i.e. the predicted rate based on the sum ot-the reaction
rate catalyzed by
s diaphorase and PMS individually.
* The reaction is too fast and difficult to calculate the rate precisely.
C. Observed Rate v. Theoretical Rate
Table 3 below provides a comparison of the observed rate and the expected or
to theoretical rate for the above described assays.
Table 3
Theoretical Rate (K/S er Observed Rate (K/5 er sec)
sec)
0.0296 0.0385
0.0617 0.1054
0.1427 0.3095
0.2446 0.5861
0.2872 0.9107
Fig. 1 provides a graph of the observed rate vs. the theoretical rate.
As can be seen by the graph of Figure 1, the reaction rate of the signal
producing
15 system of the test strip is accelerated by the presence of both PMS and
Diaphorase, where
the magnitude of the observed acceleration is unexpectedly greater than the
predicted
amount of acceleration based on the sum of the reaction rates of systems
having PMS or
Diaphorase individually.
2o It is evident from the above results and discussion that the subject
invention provides
for a significant and unexpected enhancement in the rate of reaction observed
in an analyte
detection protocol based on the oxidation of an analyte and the concomitant
reduction of a
redox indicator. In addition, the subject invention provides for a more
economical manner of
analyte detection, as compared to certain prior art methods, e.g. ones that
rely solely on
12
CA 02404421 2002-09-27
WO 01/73114 PCTNSO1/07619
diaphorase as the electron transfer agent. As such, the subject invention
represents a
significant contribution to the art.
All publications and patents cited in this specification are herein
incorporated by
reference as if each individual publication or patent were specifically and
individually
indicated to be incorporated by reference. The citation of any publication is
for its disclosure
prior to the filing date and should not be construed as an admission that the
present invention
is not entitled to antedate such publication by virtue of prior invention.
to Although the foregoing invention has been described in some detail by way
of
illustration and example for purposes of clarity of understanding, it is
readily apparent to
those of ordinary skill in the art in light of the teachings of this invention
that certain changes
and modifications may be made thereto without departing from the spirit or
scope of the
appended claims.
13