Note: Descriptions are shown in the official language in which they were submitted.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
METHODS AND MATERIALS RELATING TO
NOVEL STEM CELL GROWTH FACTOR-LIKE POLYPEPTIDES
AND POLYNUCLEOTIDES
1. CROSS REFERENCE TO RELATED APPLICATIONS
This application claims priority of U.S. Provisional Application Serial No.
60/215,733, filed June 28, 2000, and U.S. Provisional Application Serial No.
60/266,614 filed February 5, 2001, Attorney Docket No. 21272-039, is a
continuation-in-part application of U.S. Application Serial No. 09/757,562
filed
January 09, 2001 entitled "Methods and Materials Relating to Novel Stem Cell
Growth Factor-Like Polypeptid~s, and..Polynucleotides", Attorney Docket No.
HYS-
4CON; which in turn is a conti~nt~ati~n application of LT.S. Application
Serial No.
09/543,774 filed April 5, 2000 entitled "Methods and Materials Relating to
Novel
Stem Cell Growth Factor-Like Polypeptides and Polynucleotides", Attorney
Docket
No. HYS-4; which in turn is a continuation-in-part application of U.S.
Application
Serial No. 09/496,914 filed February 03, 2000, entitled "Novel Contigs
Obtained
from Various Libraries", Attorney Docket No. 787; all of which are
incorporated
herein by reference in their entirety.
2. 'TECHNICAL FIELD
The present invention provides novel polynucleotides and proteins encoded
by such polynucleotides, along with uses for these polynucleotides and
proteins, for
example in therapeutic, diagnostic and research methods. In particular, the
invention relates to a novel human stem cell growth factor-like protein.
2.1 BACKGROUND ART
Technology aimed at the discovery of protein factors (including e. g. ,
cytokines, such as lymphokines, interferons, circulating soluble factors,
chemokines, and interleukins) has matured rapidly over the past decade. The
now
routine hybridization cloning and expression cloning techniques clone novel
polynucleotides "directly" in the sense that they rely on information directly
related
to the discovered protein (i. e. , partial DNA/amino acid sequence of the
protein in
1
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
the case of hybridization cloning; activity of the protein in the case of
expression
cloning). More recent "indirect" cloning techniques such as signal sequence
cloning, which isolates DNA sequences based on the presence of a now
well-recognized secretory leader sequence motif, as well as various PCR-based
or
low stringency hybridization-based cloning techniques, have advanced the state
of
the art by making available large numbers of DNA/amino acid sequences for
proteins that are known to have biological activity, for example, by virtue of
their
secreted nature in the case of leader sequence cloning, by virtue of their
cell or
tissue source in the case of PCR-based techniques, or by virtue of structural
similarity to other genes of known biological activity.
Identified polynucleotide and polypeptide sequences have numerous
applications in, for example, diagnostics, forensics, gene mapping;
identification of
mutations responsible for genetic disorders or other traits, to assess
biodiversity,
and to produce many other types of data and products dependent on DNA and
amino
acid sequences.
3. SUI~IARY OF THE INVENTION
The present invention provides an isolated polynucleotide encoding a
polypeptide having stem cell growth factor activity, said polynucleotide
comprising
the nucleotide sequence of SEQ ID NO: 9, 11, 12, 31 or 33 or the mature
protein
coding portion thereof, or a fragment, analog, variant or derivative thereof
that
encodes a polypeptide retaining stem cell growth factor activity. These
polypeptides
include those which hybridize to the complement of the nucleotide sequence of
SEQ
ID NO: 9, 11, 12, 31 or 33 under stringent hybridization conditions, those
which
comprise a nucleotide sequence having greater than about 85 % sequence
identity
with the nucleotide sequence of SEQ ID N0:9, 11, 12, 31 or 33, those which
comprise a nucleotide sequence having greater than about 90 % sequence
identity
with the nucleotide sequence of SEQ ID N0:9, 11, 12, 31 or 33 and those
polypeptides which comprise a nucleotide sequence having greater than about 92
%
sequence identity with the nucleotide sequence of SEQ ID N0:9, 11, 12, 31 or
33.
14. The polynucleotides may be a DNA. The present invention also encompasses
polynucleotides which comprise the complement of these polynucleotides.
2
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The present invention provides for isolated polynucleotide encoding a
polypeptide having stem cell growth factor activity, said polynucleotide
comprising
the nucleotide sequence of SEQ ID NO: 9, 11, 12, 31 or 33 or the mature
protein
coding portion thereof, or a fragment, analog, variant or derivative thereof
that
encodes a polypeptide retaining stem cell growth factor activity with the
proviso that
said polynucleotide sequence does not consist of the nucleotide sequence of
SEQ ID
NO: 47. These polypeptides include those which hybridize to the complement of
the nucleotide sequence of SEQ ID NO: 9, 11, 12, 31 or 33 under stringent
hybridization conditions and with the proviso that said polynucleotide
sequence does
not consist of the nucleotide sequence of SEQ ID NO: 47, those which comprise
a
nucleotide sequence having greater than about 85 % sequence identity with the
nucleotide sequence of SEQ ID N0:9, 11, 12, 31 or 33 and with the proviso that
said polynucleotide sequence does consist of the nucleotide sequence of SEQ ID
NO: 47, those which comprise a nucleotide sequence having greater than about
90
sequence identity with the nucleotide sequence of SEQ ID N0:9, 11, 12, 31 or
33
and with the proviso that said polynucleotide sequence does not consist of the
nucleotide sequence of SEQ ID NO: 47, and those polypeptides which comprise a
nucleotide sequence having greater than about 92 % sequence identity with the
nucleotide sequence of SEQ ID N0:9, I1, 12, 31 or 33 and with the proviso that
said polynucleotide sequence does not consist of the nucleotide sequence of
SEQ ID
NO: 47.
The present invention provides for an isolated polynucleotide that comprises
the mature protein coding sequence of SEQ ID NO: 9. 11, 12, 31 or 33. Th
invention also provides for an isolated polynucleotide that comprises the
nucleotide
sequence of SEQ ID NO: 9, 11, 12, 31 or 33.
The invention provides for a DNA encoding a polypeptide having stem cell
growth factor activity, said polynucleotide comprising the nucleotide sequence
of
SEQ ID NO: 9, I I, 12, 31 or 33 or the mature protein coding portion thereof,
or a
fragment, analog, variant or derivative thereof that encodes a polypeptide
retaining
stem cell growth factor activity wherein the encoded polypeptide has an amino
acid
sequence comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32,
or
an amino acid sequence comprising at least amino acid residues 22 to 272 of
SEQ
3
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
ID NO: 34; or the encoded polypeptide has an amino acid sequence including
deletion, substitution or insertion of one or several amino acids in the amino
acid
sequence comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32,
or
an amino acid sequence comprising at least amino acid residues 22 to 272 of
SEQ
ID NO: 34, and which.has an activity to support proliferation or survival of
hematopoietic stem cell or hernatopoietic progenitor cell, with a proviso that
C-
terminal amino acid sequence does not comprise the amino acid sequence of SEQ
ID
NO: 46.
The invention provides for a DNA encoding a polypeptide having stem cell
growth factor activity, said polynucleotide comprising the nucleotide sequence
of
SEQ ID NO: 9, 11, 12, 31 or 33 or the mature protein coding portion thereof,
or a
fragment, analog, variant or derivative thereof that encodes a polypeptide
retaining
stem cell growth factor activity, which is a DNA which comprises at least
nucleotides 574 to 1347 of SEQ ID NO: 31; or a DNA which is hybridizable with
the nucleotide sequence of SEQ ID NO: 31 or a probe prepared from said
sequence,
under stringent conditions, and which has an activity to support proliferation
or
survival of hematopoietic stem cell or hematopoietic progenitor cell. These
include
DNAs which hybridize under the following stringent conditions: 6 x SSClS x
Denhardt, 0.5% SDS and 68°C (SSC 3M NaCI, 0.3M sodium citrate, 50
x
Denhardt/1 % BSA/1 % polyvinyl pyrrolidone, 1 % Ficoll 400/, or 6 x SSC, 5 x
Denhardt, 0.5% SDS, 50% formamide and 42°C.
The invention provides for a DNA encoding a polynucleotide encoding a
polypeptide having stem cell growth factor activity, said polynucleotide
comprising
the nucleotide sequence of SEQ ID NO: 9, 11, 12, 31 or 33 or the mature
protein
coding portion thereof, or a fragment, analog, variant or derivative thereof
that
encodes a polypeptide retaining stem cell growth factor activity, which is a
DNA
which comprises at least nucleotides 321 to 1074 of SEQ ID NO: 33; or DNA
which is hybridizable with the nucleotide sequence of SEQ ID NO: 33 or a prove
prepared from said sequence, under stringent conditions, and which has an
activity
to support proliferation or survival of hematopoietic stem cell or
hematopoietic
progenitor cell. These include DNAs which hybridize under the following
stringent
conditions: 6 x SSC/5 x Denhardt, 0.5% SDS and 68°C (SSC 3M NaCI, 0.3M
4
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
sodium citrate, 50 x Denhardt/1 % BSA/1 % polyvinyl pyrrolidone, 1 % Ficoll
400,
or 6 x SSC, 5 x Deanhardt, 0.5% SDS, 50% Formamide and 42°C.
The invention also provides for vectors, including expression vectors,
comprising the polynucleotide of the present invention. The invention futher
provides for host cells genetically engineered to express a polynucleotide of
the
present invention. The invention provides for host cells genetically
engineered to
contain a polynucleotide of the present invention in operative association
with a
regulatory sequence that controls expression of the polynucleotide in the host
cell.
These host cells include those which have been genetically engineered to
contain a
heterologous regulatory sequence that increases expression of an endogenous
polynucleotide.
The invention provides for a method of producing a polypeptide having stem
cell growth factor activity comprising growing these host cells in a culture
medium
under conditions that permit expression of said polypeptide and isolating said
polypeptide from said host cell or said culture medium The invention also
encompasses a polypeptide produced by this method.
The invention provides for an isolated polypeptide comprising the amino acid
. sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity. These polypeptides include polypeptides which are
encoded
by an isolated polynucleotide encoding a polypeptide having stem cell growth
factor
activity, said polynucleotide comprising the nucleotide sequence of SEQ ID NO:
9,
11, 12, 31 or 33 or the mature protein coding portion thereof, or a fragment,
analog, variant or derivative thereof that encodes a polypeptide retaining
stem cell
growth factor activity and which hybridizes to the complement of the
nucleotide
sequence of SEQ ID NO: 9, 11, 12, 31 or 33 under stringent hybridization
conditions.
The invention provides for an isolated polypeptide comprising the amino acid
sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity which comprises an amino acid sequence having greater
than
about 85 % sequence identity with the nucleotide sequence of SEQ ID NO: 10,
13,
5
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
16, 32 or 34, an amino acid sequence having greater than about 92 % sequence
identity with the nucleotide sequence of SEQ ID NO: 10, 13, 16, 32 or 34, with
the
proviso that said polypeptide sequence does not consist of the amino acid
sequence
of SEQ ID NO: 48.
The invention also provides for an isolated polypeptide comprising the
mature protein portion of SEQ ID NO: 10, 13, 16, 32 or 34.
The invention provides for an isolated polypeptide comprising the amino acid
sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity, wherein the polypeptide comprises one or more motifs
selected from the group of a laminin-type EGF-like domain, a membrane attack
comple~c component/perforin domain, and neurohypophysial hormone signature.
The invention provides for polypeptides which are an expression product of a
DNA of the present invention, where these polypeptide which have an activity
to
support proliferation or survival of hematopoietic stem cell or hematopoietic
progenitor cell, with the proviso that the C-terminal amino acid sequence does
not
comprise the amino acid sequence of SEQ ID NO: 46.
The invention provides for an isolated polynucleotide that comprises the
nucleotide sequence of SEQ ID NO: 9, 11, 12, 31 or 33, which has an amino acid
sequence comprising at Least amino acid residues 22 to 279 of SEQ ID NO: 32,
or
an amino acid sequence including deletion, substitution or insertion of one or
several
amino acids in the amino acid sequence comprising at least amino acid residues
22
to 279 of SEQ ID NO: 32
The invention provides an isolated polypeptide comprising the amino acid
sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity polypeptide, which has an amino acid sequence
comprising at
least amino acid residues 22 to 272 of SEQ ID NO: 34, or an amino acid
sequence
including deletion, substitution or insertion of one or several amino acids in
the
amino acid sequence comprising at least amino acid residues 22 to 272 of SEQ
ID
NO: 34.
6
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The invention also provides for an isolated polypeptide comprising the amino
acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein
portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity, which is modified with one or more modifying agent
selected
from the group consisting of polyethylene glycol (PEG), dextran; poly(N-vinyl-
pyrrolidone), polypropylene glycol homopolymer, copolymer of polypropylene
oxide/ethylene oxide, polyoxyethylated polyol and polyvinyl alcohol.
The invention provides for an isolated polypeptide comprising the amino acid
sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein portion
. 10 thereof, or a fragment, analog, variant or derivative thereof that
retains stem cell
growth factor activity which comprises at least ten consecutive amino acids
from
SEQ ID NO: 10 or 13. The invention also provides for an isolated polypeptide
comprising the amino acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the
mature protein portion thereof, or a fragment, analog, variant or derivative
thereof
thaf retains stem cell growth factor activity, which comprises at least ten
consecutive
amino acids from the C-terminal seventeen amino acids of SEQ ID NO: 10 or 13.
The invention provides for a polypeptide with biological activity, said
polypeptide comprising at least 272 amino acids and having at least 98 %
identity
with SEQ ID NO: 10. The invention also provides for an isolated polypeptide
with
stem cell growth factor activity having at least 90% identity with SEQ ID NO:
IU,
13, or 16 and lacking amino acid sequence
GIEVTLAEGLTSVSQRTQPTPCRRRYL (SEQ ID NO: 29) wherein A=Alanine,
C=Cysteine, D=Aspartic Acid, E= Glutamic Acid, F=Phenylalanine,
G=Glycine, H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, M=Methionine,
N=Asparagine, P=Proline, Q=Glutamine, R=Arginine, S=Serine,
T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine.
The invention also provides for an isolated polypeptide with stem cell growth
factor activity having at least 90% identity with SEQ ID NO: 10, 13, or 16 and
lacking any 10 consecutive amino acids from a amino acid sequence
GIEVTLAEGLTSVSQRTQPTPCRRRYL (SEQ ID NO: 29), wherein A=Alanine,
C=Cysteine, D=Aspartic Acid, E= Glutamic Acid, F=Phenylalanine,
G=Glycine, H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, M=Methionine,
7
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
N=Asparagine, P=Proline, Q=Glutamine, R=Arginine, S=Serine,
T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine.
The invention provides for an isolated polypeptide with stem cell growth
factor activity having at least an amino acid sequence SVSVSTVH (SEQ ID NO:
27) or VSVSTVH (SEQ ID NO: 28), wherein A=Alanine, C=Cysteine,
D=Aspartic Acid, E= Glutamic Acid, F=Phenylalanine, G=Glycine,
H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, M=Methionine,
N=Asparagine, P=Proline, Q=Glutamine, R=Arginine, S=Serine,
T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine.
The invention encompasses a polynucleotide which encodes any of the
polypeptides of the present invention.
The invention provides for a lit comprising an isolated polypeptide
comprising the amino acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the
mature protein portion thereof, or a fragment, analog, variant or derivative
thereof
that retains stem cell growth factor activity.
The invention further provides for a culture medium comprising an amount
of an isolated polypeptide comprising the amino acid sequence of SEQ ID NO:
10,
13, 16, 32 or 34, or the mature protein portion thereof, or a fragment,
analog,
variant or derivative thereof that retains stem cell growth factor activity
polypeptide,
wherein the amount is effective to maintain survival of or promote
proliferation of a
stem cell or germ cell.
The composition comprising an isolated polypeptide comprising the amino
acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein
portion
thereof, or a fragment, analog, variant or derivative thereof that retains
stem cell
growth factor activity and a pharmaceutically acceptable carrier or diluent.
These
compositions can be pharmaceutical compositions including those having an
effect
to support proliferation or survival of hematopoietic stem cell or
hematopoietic
progenitor cell, which comprises:a polypeptide which has an amino acid
sequence
comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32, or an
amino
acid sequence comprising at least amino acid residues 22 to 272 of SEQ ID NO:
34;
or a polypeptide which has an amino acid sequence including deletion,
substitution
or insertion of one or several amino acids in the amino acid sequence
comprising at
8
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
least amino acid residues 22 to 279 of SEQ ID NO: 32, or an amino acid
sequence
comprising at least amino acid residues 22 to 272 of SEQ ID NO: 34, and which
has
an activity to support proliferation or survival of hematopoietic stem cell or
hematopoietic progenitor cell.
The invention provides for an antibody that binds to an isolated polypeptide
comprising the amino acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the
mature protein portion thereof, or a fragment, analog, variant or derivative
thereof
that retains stem cell growth factor activity. The antibodies of the present
invention
may specifically binds to a polypeptide having the amino acid sequence of SEQ
ID
NO: 10, 13, 16, 32 or 34 including those which do not bind to a polypeptide
having
the amino acid sequence of SEQ ID NO: 48. The antibodies of the present
invention include polyclonal antibodies, monoclonal antibodies, antibody
fragments,
chimeric antibodies, and humanized antibodies. Further, the invention
encompasses
kits comprising the antibodies of the present invention.
The invention provides for a method for detecting a polynucleotide of the
present invention in a sample, comprising: a) contacting the sample with a
compound that binds to and forms a complex with the polynucleotide for a
period
sufficient to form the complex; and b) detecting the complex, so that if a
complex is
detected, the polynucleotide is detected. The invention also provides for
methods
for detecting a polynucleotide of the present invention in a sample,
comprising: a)
contacting the sample under stringent hybridization conditions with nucleic
acid
primers that anneal to the polynucleotide under such conditions; b) amplifying
a
product comprising at least a portion of the polynucleotide; and c) detecting
said
product and thereby the polynucleotide in the sample. These methods include a
method wherein the polynucleotide detected encodes a polypeptide comprising
the
amino acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or the mature protein
portion thereof, or a fragment, analog, variant or derivative thereof that
retains stem
cell growth factor activitya polypeptide of claim 23, and the method further
comprises reverse transcribing an annealed RNA molecule into a cDNA
polynucleotide.
The invention also provides for a method for detecting a polypeptide of the
present invnetion in a sample, comprising: a) contacting the sample with a
9
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
compound that binds to and forms a complex with the polypeptide under
conditions
and for a period sufficient to form the complex; and b) detecting formation of
the
complex, so that if a complex formation is detected, the polypeptide is
detected.
The invention also provides for a method for identifying a compound that
binds to a polypeptide of the invention, comprising: a) contacting' the
compound
with the polypeptide under conditions and for a time sufficient to form a
polypeptide/cornpound complex; and b) detecting the complex, so that if the
polypeptide/compound complex is detected, a compound that binds to the
polypeptide is identified.
The invention also provides for a method for identifying a compound that
binds to the polypeptide of the present invention, comprising: a) contacting
the
compound with the polypeptide, in a cell, for a time sufficient to form a
polypeptide/compound complex, wherein the complex drives expression of a
reporter gene sequence in the cell; and b) detecting the complex by detecting
reporter gene sequence expression, so that if the polypeptide/compound complex
is
detected, a compound that binds to the polypeptide is identified.
The invention provides for a nucleic acid array comprising a polynucleotide
of the present invention or a unique segment of a polynucleotide of the
present
invnetion attached to a surface. These arrays include those which full-matches
to
the polynucleotide or a unique segment of the polynucleotide of the present
inventions, those which detect mismatches to the polynucleotide or a unique
segment
of the polynucleotide of the present invention.
The invention provides for a method of treatment of a subject in need of
enhanced activity or expression of stem cell growth factor-like polypeptide of
the
present invention comprising administering to the subject: (a) a composition
comprising a therapeutic amount of an agonist of said polypeptide; (b) a
composition
comprising a therapeutic amount of the polypeptide; or (c) a composition
comprising
a therapeutic amount of a polynucleotide encoding the polypeptide in form and
under conditions such that the polypeptide is produced; said composition
comprising
a pharmaceutically acceptable carrier or diluent.
The invention also provdies for a method of treatment of a subject having
need of decreased activity or expression of stem cell growth factor-like
polypeptide
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
of the present invention comprising administering to the subject: (a) a
composition
comprising a therapeutic amount of an antagonist of said polypeptide; (b) a
composition comprising a therapeutic amount of the polynucleotide that
inhibits the
expression of the nucleotide sequence encoding said polypeptide; and (c) a
composition comprising a therapeutic amount of a polypeptide that competes
with
the stem cell growth factor-like polypeptide for its ligand; said composition
comprising a pharmaceutically acceptable carrier or diluent.
The invention also provides for a method of supporting proliferation or
survival of a stem cell or germ cell comprising contacting said cell with an
amount
of a polypeptide of the present invention effective to maintain survival of or
promote
proliferation of said cell. These methods include those wherein said cell is a
primordial germ cell, germ line stem cell, embryonic: stem cell, hematopoietic
stem
cell, hematopoietic progenitor cell, pluripotent cell, or totipotent cell.
These
methods also include those wherein the polypeptide comprises an amino acid
sequence of SEQ ID NO: 10, 13, or 16, or comprises an amino acid sequence 90%
identical to SEQ ID NO. 10, 13, or 16. These methods further include those
wherein the stem cell growth factor-like polypeptide is encoded by a
polynucleotide
that hybridizes to the complement of a polynucleotide encoding SEQ ID NO: 10,
13, or 16 under stringent hybridization conditions.
The invention encompasses a stromal cell genetically engineered to express a
polypeptide of the invention in an amount effective to support proliferation
or
survival of a stem cell or germ cell. These cells include primordial germ
cells germ
line stem cells embryonic stem cells hematopoietic stem cells hematopoietic
progenitor cells pluripotent cells or totipotent cells
The invention provides for an implant comprising a cell genetically
engineered to express a polypeptide of the present invention in an amount
effective
to support proliferation or survival of a stem cell or germ cell. These
implantsof the
present invention include those wherein the cell is a primordial germ cell,
germ line
stem cell, embryonic stem cell, hematopoietic stem cell, hematopoietic
progenitor
cell, pluripotent cell, or totipotent cell.
The invention provides for an isolated polynucleotide comprising the protein
coding cDNA insert of the plasmid deposited with the National Institute of
11
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Bioscience and Human-Technology, Agency of Industrial Science and Technology
(Zip code 305-8566; Higashi 1-1-3, Tsukuba, Ibaraki, Japan) on June 26,2000
under accession number FERM BP-7198 and the mature polypeptide expressed by
this polynucleotide of in a suitable host cell.
The invention also provides for an isolated polynucleotide comprising the
protein coding cDNA insert of the plasmid deposited with the National
Institute of
Bioscience and Human-Technology, Agency of Industrial Science and Technology
(Zip code 305-8566; Higashi 1-1-3, Tsukuba, Ibaraki, Japan) on June 26,2000
under accession number FERM BP-7197 and the mature polypeptide expression
product expressed by this polynucleotide in a suitable host cell.
Optionally preferred are polynucleotides and polypeptides other than the
nucleotide sequence set forth as SEQ ID NO: 3284 (and the polypeptide sequence
encoded therein) in U.S. application serial no. 09/496,914 filed February 3,
2000
and the protein set out in Genbank Accession No. BAB28811.
The compositions of the present invention include novel isolated
polypeptides, novel isolated polynucleotides encoding such polypeptides,
including
recombinant DNA molecules, cloned genes or degenerate variants thereof,
especially naturally occurring variants such as allelic variants, antisense
polynucleotide molecules, and antibodies that specifically recognize one or
more
epitopes present on such polypeptides, as well as hybridomas producing such
antibodies. Specifically, the polynucleotides of the present invention are
based on
polynucleotides isolated from cDNA libraries prepared from human testis cells
(Hyseq clone identification numbers 2880984 and 2881695), from human fetal
skin
(Hyseq clone identification number 15375176), adult spleen (Hyseq clone
identification number 14856094), and human endothelial cells (Hyseq clone
identification numbers 13804756, 13687487, 13804756).
In one aspect, the invention involves an isolated polynucleotide with stem
cell growth factor activity comprising a nucleotide sequence of SEQ ID NO: 9,
11,
12, 31 or 30, the mature protein coding portion thereof, the extracellular
coding
portion thereof, or the active domain coding portion thereof.
In one embodiment, the invention involves an isolated polynucleotide
encoding a polypeptide with biological activity, and said polynucleotide
hybridizes
12
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
to the complement of the polynucleotide with stem cell growth factor activity
under
stringent hybridization conditions.
In a further embodiment, the invention involves an isolated polynucleotide
encoding a polypeptide with biological activity, said polynucleotide having at
least
about 92 % sequence identity with the polynucleotide with stem cell growth
factor
activity.
In a further embodiment, the invention involves an isolated polynucleotide
encoding a polypeptide with biological activity, said polypeptide having
greater than
about 95 % sequence identity with the polynucleotide with stem cell growth
factor
activity.
In a still further embodiment, the polynucleotide with stem cell growth factor
activity is a DNA.
In another embodiment, the invention involves an isolated polynucleotide
which comprises the complement of the polynucleotide with stem cell growth
factor
activity.
The invention also involves a vector comprising the polynucleotide with stem
cell growth factor activity. Alternatively, the invention involves an
expression
vector comprising the polynucleotide with stem cell growth factor activity. A
host
cell genetically engineered to express the polynucleotide with. stem cell
growth
factor activity is also provided. The host cell genetically engineered to
contain the
polynucleotide with stem cell growth factor activity in operative association
with a
regulatory sequence that controls expression of the polynucleotide in the host
cell.
In another aspect, the invention involves an isolated polypeptide comprising
an amino acid sequence consisting of SEQ ID NO: 10, 13, 16, 32 or 34, the
mature
protein portion thereof, the extracellular portion thereof, or active domain
thereof.
Also provided is a composition comprising the polypeptide and a carrier. In
another embodiment, the invention involves an antibody directed against the
polypeptide.
In another aspect, the invention involves a method for detecting the
polynucleotide with stem cell growth factor activity in a sample, comprising
contacting the sample with a compound that binds to and forms a complex with
the
13
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
polynucleotide for a period sufficient to form the complex; and detecting the
complex, so that if a complex is detected, the polynucleotide is detected.
'The invention also involves a method for detecting the polynucleotide with
stem cell growth factor activity in a sample, comprising contacting the sample
under
stringent hybridization conditions with nucleic acid primers that anneal to
the
polynucleotide under such conditions; amplifying a product comprising at least
a
portion of the polynucleotide; and detecting said product and thereby the
polynucleotide in the sample.
In a further embodiment, the polynucleotide is an RNA molecule that
encodes the polypeptide, and the method fiirther comprises reverse
transcribing an
annealed RNA molecule into a cDNA polynucleotide.
Also provided is a method for detecting the polypeptide in a sample,
comprising contacting the sample with a compound that binds to and forms a
complex with the polypeptide under conditions and for a period sufficient to
form
the complex; and detecting formation of the complex, so that if a complex
formation
is detected, the polypeptide is detected.
In another embodiment, the invention provides a method for identifying a
compound that binds to the polypeptide, comprising contacting the compound
with
the polypeptide of under conditions and for a time sufficient to form a
polypeptide/compound complex; and detecting the complex, so that if the
polypeptide/compound complex is detected, a compound that binds to the
polypeptide is identified.
In a further embodiment, the invention involves a method for identifying a
compound that binds to the polypeptide, comprising contacting the compound
with
the polypeptide in a cell, for a time sufficient to form a
polypeptide/compound
complex, wherein the complex drives expression of a reporter gene sequence in
the
cell; and detecting the complex by detecting reporter gene sequence
expression, so
that if the polypeptide/compound complex is detected, a compound that binds to
the
polypeptide is identified.
In another embodiment, the invention involves a method of producing the
polypeptide, comprising, culturing the host cell for a period of time
sufficient to
14
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
express the polypeptide in said cell; and isolating the polypeptide from the
cell
culture or cells.
In another aspect, the invention involves a kit comprising the polypeptide.
Also provided is a nucleic acid array comprising the polynucleotide or a
segment of
the polynucleotide attached to a surface. In a further embodiment, the array
detects
full-matches to the polynucleotide or a unique segment of the polynucleotide.
In
another embodiment, the array detects mismatches to the polynucleotide or a
unique
segment of the polynucleotide.
The invention also provides for a method of treatment of a subject in need
thereof enhanced activity or expression of stem cell growth factor-like
polypeptide
comprising administering to the subject a composition selected from the group
consisting of a) therapeutic amount of an agonist of said polypeptide; b) a
therapeutic amount of the polypeptide; and c) a therapeutic amount of a
polynucleotide encoding the polypeptide in form and under conditions such that
the
polypeptide is produced,
and a pharmaceutically acceptable carrier. The invention also provides for a
method
. . of treatment of a subject having need to inhibit activity or expression of
stem cell
growth factor-like polypeptide comprising administering to the subject a
composition
selected from the group consisting of a) a therapeutic amount of an antagonist
of
said polypeptide; b) a therapeutic amount of the polynucleotide that inhibits
the
expression of the nucleotide sequence encoding said polypeptide; and c) a
therapeutic amount of a polypeptide that competes with the stem cell growth
factor-
like polypeptide for its ligand, and a pharmaceutically acceptable carrier.
In another embodiment, the invention involves a polypeptide having stem
cell growth factor activity comprising at least ten consecutive amino acids
from SEQ
ID NO: 10, 13, 16, 32 or 34. In still another embodiment, the invention
involves
this polypeptide comprising at least ten consecutive amino acids from the C-
terminal
seventeen amino acids of SEQ ID NO: 10, 13, 16, 32 or 34.
Also provided is a polypeptide with biological activity, said polypeptide
comprising at least 272 amino acids and having at least 98 % identity with SEQ
ID
NO: 10 or 34 or said polypeptide comprising at least 273 amino acids and
having at
least 98 % identity with SEQ ID NO: 13 .
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
In a further embodiment, the invention involves an isolated polypeptide with
stem cell growth factor activity having at least 90 % identity with SEQ ID NO:
10,
13, 16, 32 or 34 and lacking amino acid sequence
GIEVTLAEGLTSVSQRTQPTPCRRRYL (SEQ ID NO: 29) wherein A=Alanine,
C=Cysteine, D=Aspartic Acid, E= Glutamic Acid, F=Phenylalanine,
G=Glycine, H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, M=Methionine,
N=Asparagine, P=Proline, Q=Glutamine, R=Arginine, S=Serine,
T=Threonine, V=Valine, W=Tryptophan, ~'=Tyrosine.
In yet another embodiment, the invention involves an isolated polypeptide
with stem cell growth factor activity having at least 90 % identity with SEQ
ID NO:
10, 13, 16, 32 or 34 and lacking any 10 consecutive amino acids from amino
acid
sequence GIEVTLAEGLTSVSQRTQPTPCRRRYL (SEQ ID NO: 29), wherein
A = Alanine, C = Cysteine, D =Aspartic Acid, E = Glutamic Acid,
F=Phenylalanine, G=Glycine; H=Histidine, I=Isoleucine, K=Lysine,
L=Leucine, M=Methionine, N=Asparagine, P=Proline, Q=Glutamine,
R= Arginine, S = Serine, T = Threonine, V = Valine, W = Tryptophan, Y =
Tyrosine.
In another embodiment, the invention concerns a method of maintaining or
promoting proliferation of a cell selected from the group consisting of
primordial
germ cells, germ line stem cells, embryonic stem cells, pluripotent cell, and
totipotent cells, comprising contacting the cell with an effective amount of a
stem
cell growth factor-like polypeptide. In a further embodiment, the polypeptide
comprises an amino acid sequence of SEQ ID NO: 10, 13, 16, 32 or 34, or
comprises an amino acid sequence 90% identical to SEQ ID NO. 10, 13, 16, 32 or
34. In still a further embodiment, the stem cell growth factor-like
polypeptide is
encoded by a polynucleotide that hybridizes to the complement of a
polynucleotide
encoding SEQ ID NO: 10, 13, 16, 32 or 34 under stringent hybridization
conditions.
The invention also involves an isolated polypeptide with stem cell growth
factor~activity having at least an amino acid sequence SVSVSTVH (SEQ ID NO:
27) or VSVSTVH (SEQ ID NO: 28), wherein A=Alanine, C=Cysteine,
D=Aspartic Acid, E= Glutamic Acid, F=Phenylalanine, G=Glycine,
H=Histidine, I=Isoleucine, K=Lysine, L=Leucine, M=Methionine,
16
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
N=Asparagine, P=Proline, Q=Glutamine, R=Arginine, S=Serine,
T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine.
In an additional embodiment, the invention concerns the polypeptide
according to this invention., wherein the polypeptide comprises one or more
motifs
selected from the group of a laminin-type EGF-like domain, a membrane attack
complex component/perforin domain, and neurohypophysial hormone signature.
The invention also encompasses any polynucleotides encoding a polypeptide
according to this invention.
The compositions of the present invention additionally include vectors,
including expression vectors, containing the polynucieotides of the invention,
cells
genetically engineered to contain such polynucleotides and cells genetically
engineered to express such polynucleotides.
The isolated polynucleotides of the invention include, but are not limited to,
a
polynucleotide comprising any one of the nucleotide sequences set forth in the
SEQ ID
NO: 9, 11, 12, 31 or 33; a polynucleotide comprising any of the full length
protein
coding sequences of the SEQ ID NO: 9, 11, 12, 31 or 33and a polynucleotide
comprising any of the nucleotide sequences of the mature protein coding
sequences of
the SEQ ID NO: 9, 11, 12, 31 or 33. The polynucleotides of the present
invention
also include, but are not limited to, a polynucleotide that hybridizes under
stringent
hybridization conditions to (a) the complement of any one of the nucleotide
sequences
set forth in the SEQ ID NO: 9, 11, 12, 31 or 33; (b) a nucleotide sequence
encoding
SEQ ID NO: 10, 13- 24, 32 or 34; a polynucleotide which is an allelic variant
of any
polynucleotides recited above; a polynucleotide which encodes a species
homolog
(e.g. orthologs) of any of the proteins recited above; or a polynucleotide
that encodes a
polypeptide comprising a specific domain or truncation of any of the
polypeptides
comprising SEQ ID NO: 10, 13- 24, 32 or 34.
The nucleic acid sequences of the present invention also include the sequence
information from the nucleic acid sequences of SEQ ID NO: 11, 12, 31 or 33.
The
sequence information can be a segment of any one of SEQ ID NO: 1-7 that
uniquely
identifies or represents the sequence information of SEQ ID NO: 11, l2, 31 or
33.
One such segment can be a twenty-mer nucleic acid sequence because the
probability
that a twenty-mer is fully matched in the human genome is 1 in 300. In the
human
17
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
genome, there are three billion base pairs in one set of chromosome. Because
there
are 42° possible twenty-mers exist, there are 300 times more twenty-
mers than there are
base pairs in a set of human chromosome. Using the same analysis, the
probability for
a seventeen-mer to be fully matched in the human genome is approximately 1 in
5.
When these segments are used in arrays for expression studies, fifteen-mer
segment
can be used. The probability that the fifteen-mer is fully matched in the
expressed
sequences is also approximately one in five because expressed sequences in one
tissue
comprise approximately 5 % of the entire genome sequence. Preferably , the
nucleic
acid fragment or subsequence comprise the twenty-one 3' coding nucleotides.
Similarly, when using sequence information for detecting' a single mismatch, a
segment can be a twenty-five mer. The probability that the twenty-five mer
would
appear in a human genome with a single mismatch is calculated by multiplying
the
probability for a full match (1-425) times the increased probability for
mismatch at each
nucleotide position (3 x 25). The probability that an eighteen mer with a
single
mismatch can be detected in an array for expression studies is approximately
one in
five. The probability that a twenty-mer with a single mismatch can be detected
in a
human genome is approximately one in five.
A collection as used in this application can be a collection of only one
polynucleotide. The collection of sequence information or unique identifying
information of each sequence can be provided on a nucleic acid array. In one
embodiment, segments of sequence information are provided on a nucleic acid
array to
detect the polynucleotide that contains the segment. The array can be designed
to
detect full-match or mismatch to the polynucleotide that contains the segment.
The
collection can also be provided in a computer-readable formatz
This invention also includes the reverse or direct complement of any of the
nucleic acid sequences recited above; cloning or expression vectors containing
the
nucleic acid sequences; and host cells or organisms transformed with these
expression
vectors.
Human stem cell growth factor-like polypeptide (SEQ ID NO: 10 or 34) is
approximately a 272-amino acid protein with a predicted molecular mass of
approximately 30 kDa unglycosylated. The mouse homolog is set out in SEQ ID
N0:32., Protein database searches with the BLAST algorithm indicate that SEQ
ID
18
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
NO: 10 is homologous to Mus musculus thrombospondin type 1 domain. Figure 1
shows the alignment of polynucleotide SEQ ID NO: 9 and EST sequences SEQ ID
NO: 1-7. The sequences of the present invention (SEQ ID NO: 1-12) are expected
to
have stem cell growth factor activity, including hematopoietic stem cell
growth factor
activity, as described herein.
Stem cell growth factor-like polypeptide (SEQ ID NO: 10) also has the
following motifs at the designated amino acid sequence corresponding to SEQ ID
NO: 10 wherein A = Alanine, C = Cysteine, D =Aspartic Acid, E = Glutamic Acid,
F=Phenylalanine, G=Glycine, H=Histidine, I=Isoleucine, K=Lysine;
L=Leucine, M=Methionine, N=Asparagine, P=Proline, Q=Glutamine,
R=Arginine, S=Serine, T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine:
Laminin-type EGF-like (LE) domain proteins at
100 ADCDTCFNKNFCTKCKSGFYLHL 122 (SEQ ID NO: 17)
Vertebrate metallothioneins proteins at
92 INKCTKCKADCDTCFNKNFCTKCKSGFYLHLGKCLDNc~PEGLEA.NN
137 (SEQ ID NO: I8)
Endogenous opioids neuropeptides precursors proteins at
33 MHPNVSQGCQGGCATCSDYN 52 (SEQ ID NO: 19)
Membrane attack complex components / perform proteins at
145 IVHCEVSEWNPWSPCTKKGKTCGFKRGTETRVREIIQ 18i (SEQ ID
NO: 20)
HMG-I and HMG-Y DNA-binding domain proteins (Ahook) at
213 KKGRERKRKK 222 (SEQ ID NO: 21)
HMGl/2 proteins at
198 KCTVQRKKCQKGERGKKGRERKRKKPNKGESKEAIPDSKSLE 239
(SEQ ID NO: 22)
19
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
VERTEBRATE METALLOTHIONEIN SIGNATURE at
104 TCFNKNFCTKCKSG 117 (SEQ ID NO: 23)
NEUROHYPOPHYSIAL HORMONE SIGNATURE at
148 CEVSEWNPWSPCTKKGKTCG 167 (SEQ ID NO: 24)
Motif 100-122, a laminin-type EGF-like domain, is a component of
extracellular matrix which promotes cell growth. The membrane attack complex
componentlperforin domain (145 - 185) is postulated to mediate cell-cell
interaction
and thus cell growth and differentiation. Neurohypophysial hormone is itself
regulated by many other factors including Interleukin-1 beta and Interleukin-
6. The
presence of these motifs are expected in stem cell growth factor activity.
Stem cell growth factor-Iike protein andlor fragments or derivatives would
have similar activity to stem cell growth factors and anabolic growth factors
and
receptors.
The isolated polypeptides of the invention include, but are not limited to, a
polypeptide comprising SEQ ID NO: 10, 13- 24, 32 or 34; or the corresponding
full
length or mature protein. Polypeptides of the invention also include
polypeptides with
biological activity that are encoded by (a) any of the polynucleotides having
a
nucleotide sequence set forth in the SEQ ID NO: 1-9; 11, I2, 31 or 33 or (b)
polynucleotides that hybridize to the complement of the polynucleotides of (a)
under
stringent hybridization conditions. Biologically or immunologically active
variants of
any of the protein sequences listed as SEQ ID NO: 10, 13- 24, 32 or 34, and
"substantial equivalents" thereof (e. g. , with at least about 65 % , 70 % ,
75 % , 80 % .,
85 % , 90 % , 91 % , 92 % , 93 % , 94 % , 95 % , 96 % , 97 % , 98 % or 99 %
amino acid
sequence identity) that preferably retain biological activity are also
contemplated. The
polypeptides of the invention may be wholly or partially chemically
synthesized but are
preferably produced by recombinant means using the genetically engineered
cells (e.g.
host cells) of the invention.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The invention also provides compositions comprising a polypeptide of the
invention. Polypeptide compositions of the invention may further comprise an
acceptable carrier, such as a hydrophilic, e. g. , pharmaceutically
acceptable, carrier.
The invention also. provides host cells transformed or transfected with a
polynucleotide of the invention.
The invention also relates to methods fox producing a polypeptide of the
invention comprising growing a culture of the host cells of the invention in a
suitable culture medium under conditions permitting expression of the desired
polypeptide, and purifying the protein from the culture or from the host
cells.
Preferred embodiments include those in which the protein produced by such
process
is a mature form of the protein.
Polynucleotides according to the invention have numerous applications in a
variety of techniques known to those skilled in the art of molecular biology.
These
techniques include use as hybridization probes, use as oligomers, or primers,
for
PCR, use in an array, use in computer-readable media, use for chromosome and
gene mapping, use in the recombinant production of protein., and use in
generation
of anti-sense DNA or RNA, their chemical analogs and the like. For example,
when the expression of an mRNA is largely restricted to a particular cell or
tissue
type, polynucleotides of the invention can be used as hybridization probes to
detect
the presence of the particular cell or tissue mRNA in a sample using, e.g., in
situ
hybridization.
In other exemplary embodiments, the polynucleotides are used in diagnostics
as expressed sequence tags for identifying expressed genes or, as well known
in the
art and exemplified by Vollrath et al., Science 258:52-59 (1992), as expressed
sequence tags fox physical mapping of the human genome.
A polynucleotide according to the invention can be joined to any of a variety
of other nucleotide sequences by well-established recombinant DNA techniques
(see
Sambrook, J., et al. (1989) Molecular Cloning: A Laboratory Manual, Cold
Spring
Harbor Laboratory, NY). Useful nucleotide sequences for joining to
polypeptides
include an assortment of vectors, e.g., plasmids, cosmids, lambda phage
derivatives, phagemids, and the like, that are well known in the art.
Accordingly,
the invention also provides a vector including a polynucleotide of the
invention and
21
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
a host cell containing the polynucleotide. In general, the vector contains an
origin
of replication functional in at least one organism, convenient restriction
endonuclease sites, and a selectable marker for the host cell. Vectors
according to
the invention include expression vectors, replication vectors, probe
generation
vectors, and sequencing vectors. A host cell according to the invention can be
a
prokaryotic or eukaryotic cell and can be a unicellular organism or part of a
multicellular organism.
The polypeptides according to the invention can be used in a variety of
conventional procedures and methods that are currently applied to other
proteins.
For example, a polypeptide of the invention can be used to generate an
antibody that
specifically binds the polypeptide. Such antibodies, particularly monoclonal
antibodies, are useful for detecting or quantitating the polypeptide in
tissue. The
polypeptides of the invention can also be used as molecular weight markers,
and as
a food supplement.
Methods are also provided for preventing, treating, or ameliorating a
medical condition which comprises the step of administering to a mammalian
subject
a therapeutically effective amount of a composition comprising a protein of
the
present invention and a pharmaceutically acceptable carrier:
In particular, the polypeptides and polynucleotides of the invention can be
utilized; for example, as part of methods for the prevention and/or treatment
of
disorders involving aberrant protein expression or biological activity.
The methods of the invention also provides methods for the treatment of
disorders as recited herein which may involve the administration of the ,
polynucleotides or polypeptides of the invention to individuals exhibiting
symptoms
or tendencies related to disorders as recited herein. In addition, the
invention
encompasses methods for treating diseases or disorders as recited herein
comprising
the step of administering compounds and other substances that modulate the
overall
activity of the target gene products. Compounds and other substances can
effect
such modulation either on the level of target gene/protein expression or
target
protein activity. Specifically, methods are provided for preventing, treating
or
ameliorating a medical condition, including neurological diseases, which
comprises
administering to a mammalian subject, including but not limited to humans, a
22
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
therapeutically effective amount of a composition comprising a polypeptide of
the
invention or a therapeutically effective amount of a composition comprising a
binding partner of (e.g., antibody specifically reactive for) stem cell growth
factor-
like polypeptides of the invention. The mechanics of the particular condition
or
pathology will dictate whether the polypeptides of the invention or binding
partners
(or inhibitors) of these would be beneficial to the individual in need of
treatment.
The invention also provides a method of promoting wound healing
comprising administering a stem cell growth factor-like polypeptide of the
present
invention to the site of a wound or injury. The invention provides a method of
promoting cell growth and morphogenesis comprising administering a stem cell
growth factor-like polypeptide of the present invention to a medium of nerve
cells.
According to this method, polypeptides of the invention can be administered to
produce an in vitro or in vivo promotion of cellular function. A polypeptide
of the
invention can be administered in vivo as a stem cell growth factor alone or as
an
adjunct to other therapies.
The invention further provides methods for manufacturing medicaments
. useful in the above described methods.
The present invention further relates to methods for detecting the presence of
,
the polynucleotides or polypeptides of the invention in a sample (e. g. ,
tissue or
sample). Such methods can, for example, be utilized as part of prognostic and
diagnostic evaluation of disorders as recited herein and for the
identification of
subjects exhibiting a predisposition to such conditions. The invention also
provides
kits comprising polynucleotide probes andlor monoclonal antibodies, and
optionally
quantitative standaxds, for carrying out methods of the invention.
Furthermore, the
invention provides methods for evaluating the efficacy of drugs, and
monitoring the
progress of patients, involved in clinical trials for the treatment of
disorders as
recited above.
The invention also provides methods for the identification of compounds that
modulate (i.e., increase or decrease) the expression or activity of the
polynucleotides andlor polypeptides of the invention. Such methods can be
utilized,
for example, for the identification of compounds that can ameliorate symptoms
of
disorders as recited herein. Such methods can include, but are not limited to,
assays
23
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
for identifying compounds and other substances that interact with (e. g. ,
bind to) the
polypeptides of the invention.
4. BRIEF DESCRIPTION OF THE DRAWINGS .
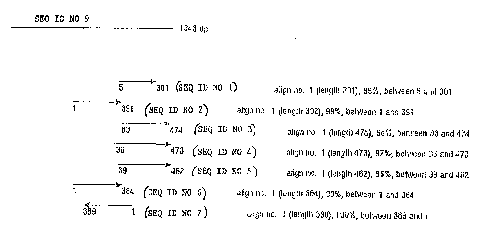
Fig. 1 shows the alignment of SEQ ID NO. 9 with SEQ ID NO. 1-7.
Figure 2 shows the BLASTP amino acid sequence alignment between the
SEQ ID NO: 10, stem cell growth factor-like polypeptide and mouse
thrombospondin type 1 domain protein SEQ ID NO: 25, indicating that the two
sequences share 64 % similarity over amino acid residues 19-254 of SEQ ID NO:
10
and 47 % identity over the same amino acid residues 19-254 of SEQ ID NO: 10,
wherein A=Alanine, C=Cysteine, D=Aspartic Acid, E= Glutamic Acid,
F=Phenylalanine, G=Glycine, H=Histidine, I=Isoleucine, K=Lysine,
L=Leucine, M=Methionine, N=Asparagine, P=Proline, Q=Glutamine,
R=Arginine, S=Serine, T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine.
Gaps are presented as dashes.
Figure 3 shows the BLASTP amino acid sequence alignment between the
SEQ ID NO: 10, stem cell growth factor-like polypeptide and human secreted
protein clone da228 6 protein (Patent Application No. W098./49302), SEQ ID NO:
26, indicating that the two sequences share 100 % similarity over amino acid
20, residues 1-265 of SEQ ID NO: 10 and 100% identity over the same amino acid
residues 1-265 of SEQ ID NO: 10, wherein A=Alanine, C=Cysteine, D=Aspartic
Acid, E= Glutamic Acid, F=Phenylalan'ine, G=Glycine, H=Histidine,
I=Isoleucine, K=Lysine, L=Leucine, M=Methionine, N=Asparagine,
P=Proline, Q=Glutamine, R=Arginine, S=Serine, T=Threonine, V=Valine,
W=Tryptophan, Y=Tyrosine. Gaps are presented as dashes.
Fig. 4 shows proliferation statuses of hematopoietic stem cells and
hematopoietic progenitor cells determined by a clonogenic assay after co-
culture of
CD34 positive hematopoietic stem cells with AGM-s3 subclone A9, A7, or D11
cells for two weeks;
Fig. 5 shows proliferation statuses of hematopoietic stem cells and
hematopoietic progenitor cells determined ~by a clonogenic assay after co-
culture of
24
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
CD34 positive hematopoietic stem cells with AGM-s3 subclone A9, A7, or OP9
cells for two weeks;
Fig. 6 shows time course of donor derived lymphoid lineage cells or myeloid
lineage cells reconstitution in irradiated recipient mice that received the
hematopoietic stem cells co-cultured with stromal cells; and
Fig. 7 shows time course of donor derived lymphoid lineage cells or myeloid
lineage cells reconstitution in irradiated recipient mice that received the
hematopoietic stem cells co-cultured with AGM-s3-A7 cell lines (A7lpMXIG-SCR-1
and A7lpMXIG) transfected with a vector including SCAR-1 (pMXIG-SCR-1) or a
vector which does not include SCR-1 (pMXIG).
5. DETAILED DESCRIPTION OF THE INVENTION
5.1 DEFINITIONS
The term "primordial germ cells (PGCs)" refers to a small population of
cells set aside from other cell lineages particularly from the yolk sac,
mesenteries,.
or gonadal ridges during embryogenesis that have the potential to
differentiate in to
germ cells and other cells. PGCs are the source from which USCs and ES cells
are
derived
The term "germ line stem cells (GSCs)" refers to stem cells derived from
primordial stem cells that provide a steady and continuous source of germ
cells for
the production of gametes.
The term "embryonic stem cells (ES)" refers to a cell which can give rise to
many differentiated cell types in an embryo or an adult, including the germ
cells.
The PGCs, the GSCs and the ES cells are capable of self renewal. Thus these
cells
not only populate the germ line and give rise to a plurality of terminally
differentiated cells which comprise the adult specialized organs, but are able
to
regenerate themselves.
The term "totipotent" refers to the capability of a cell to differentiate into
all
of the cell types of an adult organism.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The term "pluripotent" refers to the capability of a cell to differentiate
into a
number of differentiated cell types that are present in an adult organism. A
pluripotent cell is restricted in its differentiation capability in comparison
to a
totipotent cell.
The term "nucleotide sequence" refers to a heteropolymer of nucleotides or
the sequence of these nucleotides. The terms "nucleic acid" and
"polynucleotide"
are also used interchangeably herein to refer to a heteropolymer of
nucleotides.
Generally, nucleic acid segments provided by this invention may be assembled
from
fragments of the genome and short oligonucleotide linkers, or from a series of
oligonucleotides, or from individual nucleotides, to provide a synthetic
nucleic acid
which is capable of being expressed in a recombinant transcriptional unit
comprising
regulatory elements derived from a microbial or viral operon, or a eukaryotic
gene.
The terms "oligonucleotide fragment" or a "polynucleotide fragment",
"portion," or "segment" is a sequence of nucleotide residues which is long
enough
to use in polymerase chain reaction (PCR) or various hybridization procedures
to
identify or amplify identical or related parts of mRNA or DNA molecules. A
fragment or segment may uniquely identify each polynucleotide sequence of the
present invention.
The terms "oligonucleotides" or "nucleic acid probes" are prepared based on
the polynucleotide sequences provided in the present invention.
Oligonucleotides
comprise portions of such a polynucleotide sequence having at least about 15
nucleotides and usually at least about 20 nucleotides. Nucleic acid probes
comprise
portions of such a polynucleotide sequence having fewer nucleotides than about
6
kb, usually fewer than about 1 kb. After appropriate testing to eliminate
false
positives, these probes may, for example, be used to determine whether
specific
mRNA molecules are present in a cell or tissue or to isolate similar nucleic
acid
sequences from chromosomal DNA as described by Walsh et al. (Walsh, P.S. et
al.,
1992, PCR Methods Appl 1:241-250).
The term "probes" includes naturally occurring or recombinant or chemically
synthesized single- or double-stranded nucleic acids. They may be labeled by
nick
translation, Klenow fill-in reaction, PCR, or other methods well known in the
art.
Probes of the present invention, their preparation and/or labeling are
elaborated in
26
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Sambrook, J. et al., 1989, Molecular Cloning: A Laboratory Manual, Cold Spring
Harbor Laboratory, NY; or Ausubel, F.M. et al., 1989, Current Protocols in
Molecular Biology, John Wiley & Sons, New York NY, both of which are
incorporated herein by reference in their entirety.
The term "stringent" is used to refer to conditions that are commonly
understood in the art as stringent. Stringent conditions can include highly
stringent
conditions (i.e., hybridization to filter-bound DNA in 0.5 M NaHPO~, 7% sodium
dodecyl sulfate (SDS), 1 mM EDTA at 65°C, and washing in 0.1X SSC/0.1 %
SDS
at 68°C), and moderately stringent conditions (i.e., washing in 0.2X
SSC/0.1 % SDS
IO at 42°C). Other exemplary hybridization conditions are described
herein in the
examples.
In instances of hybridization of deoxyoligonucleotides, additional exemplary
stringent hybridization conditions include washing in 6X SSC/0.05 % sodium
pyrophosphate at 37°C (for 14-base oligos), 48°C (for 17-base
oligos), 55°C (for
20-base oligos), and 60°C (for 23-base oligos).
The term "recombinant, " when used herein to refer to a polypeptide or
protein, means that a polypeptide or protein is derived from recombinant
(e.g.,
microbial, insect, or mammalian) expression systems. "Microbial" refers to
recombinant polypeptides or proteins made in bacterial or fungal (e.g., yeast)
'20 expression systems. As a product, "recombinant microbial" defines a
polypeptide
or protein essentially free of native endogenous substances and unaccompanied
by
associated native glycosylation. Polypeptides or proteins expressed in most
bacterial
cultures, e.g., E. coli, will be free of glycosylation modifications;
polypeptides or
proteins expressed in yeast will have a glycosylation pattern in general
different
from those expressed in mammalian cells.
The term "recombinant expression vehicle or vector" refers to a plasmid or
phage or virus or vector, for expressing a polypeptide from a DNA (RNA)
sequence. An expression vehicle can comprise a transcriptional unit comprising
an
assembly of (1) a genetic element or elements having a regulatory role in gene
expression, for example, promoters or enhancers, (2) a structural or coding
sequence which is transcribed into mRNA and translated into protein, and (3)
appropriate transcription initiation and termination sequences. Structural
units
27
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
intended for use in yeast or eukaryotic expression systems preferably include
a
leader sequence enabling extracellular secretion of translated protein by a
host cell.
Alternatively, where recombinant protein is expressed without a leader or
transport
sequence, it may include an amino terminal methionine residue. This residue
may
or may not be subsequently cleaved from the expressed recombinant protein to
provide a final product.
The term "recombinant expression system" means host cells which have
stably integrated a recombinant transcriptional unit into chromosomal DNA or
carry
the recombinant transcriptional unit extrachromosomally. Recombinant
expression
systems as defined herein will express heterologous polypeptides or proteins
upon
induction of the regulatory elements linked to the DNA segment or synthetic
gene to
be expressed. This term also means host cells which have stably integrated a
recombinant genetic element or elements having a regulatory role in gene
expression, for example, promoters or enhancers. Recombinant expression
systems
as defined herein will express polypeptides or proteins endogenous to the cell
upon
induction of the regulatory elements linked to the endogenous DNA segment or
gene
to be expressed. The cells can be prokaryotic or eukaryotic.
. The term "open reading frame," ORF, means a series of nucleotide triplets
coding for amino acids without any termination codons and is a sequence
translatable into protein.
The term "expression modulating fragment," EMF, means a series of
nucleotides which modulates the expression of an operably linked ORF or
another
EMF.
As used herein, a sequence is said to "modulate the expression of an
operably linked sequence" when the expression of the sequence is altered by
the
presence of the EMF. EMFs include, but are not limited to, promoters, and
promoter modulating sequences (inducible elements). One class of EMFs are
fragments which induce the expression or an operably linked ORF in response to
a
specific regulatory factor or physiological event.
As used herein, an "uptake modulating fragment," UMF, means a series of
nucleotides which mediate the uptake of a linked DNA fragment into a cell.
UMFs
28
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
can be readily identified using known UMFs as a target sequence or target
motif
with the computer-based systems described below.
The presence and activity of a UMF can be confirmed by attaching the
suspected UMF to a marker sequence. The resulting nucleic acid molecule is
then
incubated with an appropriate host under appropriate conditions and the uptake
of
the marker sequence is determined. As described above, a UMF will increase the
frequency of uptake of a linked marker sequence.
The term "active" refers to those forms of the polypeptide which retain the
biologic and/or immunologic activities of any naturally occurring polypeptide.
According to the invention, the term "biologically active" means that the
polypeptide retains at least one of the biological activities of the
polypeptide of the
invention. The term "stem cell growth factor activity" or "stem cell growth
factor-
like activity" refers to biological activity that is similar to the biological
activity of
stem cell growth factor polypeptide, such as cell growth or morphogenesis
activity.
The term "naturally occurring polypeptide" refers to polypeptides produced
by cells that have not been genetically engineered and specifically
contemplates
various polypeptides arising from post-translational modifications of the
polypeptide
including, but not limited to, acetylation, carboxylation, glycosylation,
phosphorylation, lipidation and acylation.
The term "derivative" refers to polypeptides chemically modified by such
techniques as ubiquitination, labeling (e.g., with radionuclides or various
enzymes),
covalent polymer attachment such as pegylation (derivatization with
polyethylene
glycol) and insertion or substitution by chemical synthesis of amino acids
such as
ornithine, which do not normally occur in human proteins.
The term "variant"(or "analog") refers to any polypeptide differing from
naturally occurring polypeptides by amino acid insertions, deletions, and
substitutions, created using, a g., recombinant DNA techniques. Guidance in
determining which amino acid residues may be replaced, added or deleted
without
abolishing activities of interest, may be found by comparing the sequence of
the
particular polypeptide with that of homologous peptides and minimizing the
number
of amino acid sequence changes made in regions of high homology (conserved
regions) or by replacing amino acids with consensus sequence.
29
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Preferably, amino acid "substitutions" are the result of replacing one amino
acid with another amino acid having similar structural and/or chemical
properties,
i. e. , conservative amino acid replacements. "Conservative" amino acid
substitutions may be made on the basis of similarity in polarity, charge,
solubility,
hydrophobicity, hydrophilicity, and/or the amphipathic nature of the residues
involved. For example, nonpolar (hydrophobic) amino acids include alanine,
leucine, isoleucine, valine, proline, phenylalanine, tryptophan, and
methionine;
polar neutral amino acids include glycine, serine, threonine, cysteine,
tyrosine,
asparagine, and glutamine; positively charged (basic) amino acids include
arginine,
lysine, and histidine; and negatively charged (acidic) amino acids include
aspartic
acid and glutamic acid. "Insertions" or "deletions" are typically in the range
of
' about 1 to 5 amino acids. The variation allowed may be experimentally
determined
by systematically making insertions, deletions, or substitutions of amino
acids in a
polypeptide molecule using recombinant DNA techniques and assaying the
resulting
recombinant variants for activity.
Alternatively, where alteration of function is desired, insertions, deletions
or
non-conservative alterations can be engineered to produce altered
polypeptides.
Such alterations can, for example, alter one or more of the biological
functions or
biochemical characteristics of the polypeptides of the invention. For example,
such
alterations may change polypeptide characteristics such as ligand-binding
affinities,
interchain affinities, or degradation/turnover rate. Further, such alterations
can be
selected so as to generate polypeptides that are better suited for expression,
scale up
and the like in the host cells chosen for expression. For example, cysteine
residues
can be deleted or substituted with another amino acid residue in order to
eliminate
disulfide bridges.
As used herein, "substantially equivalent" or "substantially similar" can
refer
both to nucleotide and amino acid sequences, for example a mutant sequence,
that
varies from a reference sequence by one or more substitutions, deletions, or
additions, the net effect of which does not result in an adverse functional
dissimilarity between the reference and subject sequences. Typically, such a
substantially equivalent sequence varies from one of those listed herein by no
more
than about 35 % (i. e. , the number of individual residue substitutions,
additions,
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
and/or deletions in a substantially equivalent sequence, as compared to the
corresponding reference sequence, divided by the total number of residues in
the
substantially equivalent sequence is about 0.35 or less). Such a sequence is
said to
have 65 % sequence identity to the listed sequence. In one embodiment, a
substantially equivalent, e.g., mutant, sequence of the invention varies from
a listed
sequence by no more than 30 % (70 % sequence identity); in a variation of this
embodiment, by no more than 25 % (75 % sequence identity); and in a further
variation of this embodiment, by no more than 20 % (80 % sequence identity)
and in
a further variation of this embodiment, by no more than 10 % (90 % sequence
identity) and in a further variation of this embodiment, by no more that 5 %
(95
sequence identity). Substantially equivalent, e.g., mutant, amino acid
sequences
according to the invention preferably have at least 80 % sequence identity
with a
listed amino acid sequence, more preferably at least 85 % sequence identity,
more
preferably at least 90 % sequence identity, more preferably at least 95 %
sequence
identity, more preferably at least 98 % sequence identity, and most preferably
at
least 99% sequence identity. Substantially equivalent nucleotide sequence of
the
invention can have lower percent sequence identities, taking into account, for
example, the redundancy or degeneracy of the genetic code. Preferably, the
nucleotide sequence has at least about 65 % identity, more preferably at least
about
75 % identity, more preferably at least about 80 % sequence identity, more
preferably at least 8S % sequence identity, more preferably at least 90 %
sequence
identity, more preferably at least about 95 % sequence identity, more
preferably at
least 98 % sequence identity, and most preferably at least 99 % sequence
identity.
For the purposes of the present invention, sequences having substantially
equivalent
biological activity and substantially equivalent expression characteristics
are
considered substantially equivalent. For the purposes of determining
equivalence,
truncation of the mature sequence (e. g. , via a mutation which creates a
spurious
stop colon) should be disregarded. Sequence identity may be determined, e.g.,
using the Jotun Hein method (Heir, J. (1990) Methods Enzymol. 183:626-645).
Identity between sequences can also be determined by other methods known in
the
art, e.g. by varying hybridization conditions.
31
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Nucleic acid sequences encoding such substantially equivalent sequences,
e.g., sequences of the recited percent identities can routinely be isolated
and
identified via standard hybridization procedures well known to those of skill
in the
art.
Where desired, an expression vector may be designed to contain a "signal or
leader sequence" which will direct the polypeptide through the membrane of a
cell.
Such a sequence may be naturally present on the polypeptides of the present
invention or provided from heterologous protein sources by recombinant DNA
techniques.
A polypeptide "fragment," "portion," or "segment" is a stretch of amino acid
residues of at least about 5 amino acids, often at least about 7 amino acids,
typically
at least about 9 to 13 amino acids, and, in various embodiments, at least
about 17 or
more amino acids. To be active, any polypeptide must have sufficient length to
display biological and/or immunological activity.
Alternatively, recombinant variants encoding these same or similar
polypeptides may be synthesized or selected by making use of the "redundancy"
in
the genetic code. Various codon substitutions, such as the silent changes
which
produce various restriction sites, may be introduced to optimize cloning into
a
plasmid or viral vector or expression in a particular prokaryotic or
eukaryotic
system. Mutations in the polynucleotide sequence may be reflected in the
polypeptide or domains of other peptides added to the polypeptide to modify
the
properties of any part of the polypeptide, to change characteristics such as
ligand-binding affinities, interchain affinities, or degradation/turnover
rate.
The term "activated" cells as used in this application are those which are
engaged in extracellular or intracellular membrane trafficking, including the
export
of secretory or enzymatic molecules as part of a normal or disease process.
The term "purified" as used herein denotes that the indicated nucleic acid or
polypeptide is present in the substantial absence of other biological
macromolecules,
e.g., polynucleotides, proteins, and the like. In one embodiment, the
polynucleotide
or polypeptide is purified such that it constitutes at least 95 % by weight,
more
preferably at least 99.8 % by weight, of the indicated biological
macromolecules
32
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
present (but water, buffers, and other small molecules, especially molecules
having
a molecular weight of less than 1000 daltons, can be present).
The term "isolated" as used herein refers to a nucleic acid or polypeptide
separated from at least one other component (e.g., nucleic acid or
polypeptide)
present with the nucleic acid or polypeptide in its natural source. In one
embodiment, the nucleic acid or polypeptide is found in the presence of (if
anything)
only a solvent, buffer, ion, or other component normally present in a solution
of the
same. The terms "isolated" and "purified" do not encompass nucleic acids or
polypeptides present in their natural source.
The term "infection" refers to the introduction of nucleic acids into a
suitable
host cell by use of a virus or viral vector.
The term "transformation" means introducing DNA into a suitable host cell
so that the DNA is replicable, either as an extrachromosomal element, or by
chromosomal integration.
The term "transfection" refers to the taking up of an expression vector by a
suitable host cell, whether or not any coding sequences are in fact expressed.
The term "intermediate fragment" means a nucleic acid between 5 and 1000
bases in length, and preferably between 10 and 40 by in length.
The term "secreted" includes a protein that is transported across or through a
membrane, including transport as a result of signal sequences in its amino
acid
sequence when it is expressed in a suitable host cell. "Secreted" proteins
include
without limitation proteins secreted wholly (e. g. , soluble proteins) or
partially (e. g. ,
receptors) from the cell in which they are expressed. "Secreted" proteins also
include without limitation proteins which are transported across the membrane
of the
endoplasmic reticulum. "Secreted" proteins are also intended to include
proteins
containing non-typical signal sequences (e.g. Interleukin-1 Beta, see Krasney,
P.A.
and Young, P.R. (1992) Cytokine 4(2):134 -143) and factors released from
damaged cells (e.g. Interleukin-1 Receptor Antagonist, see Arend, W.P. et. al.
(1998) Annu. Rev. Immunol. 16:27-55)
Each of the above terms is meant to encompasses all that is described for
each, unless the context dictates otherwise.
33
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.1.1 Description of the Invention
Since stromal cells can support the proliferation or the survival of
hematopoietic stem cells or hematopoietic progenitor cells ex vivo, stromal
cells are
expected to produce factors mediating support proliferation or survival of
hematopoietic stem cells or hematopoietic progenitor cells, as defined herein.
An object of the present invention is to provide a factors supporting
proliferation or survival of hematopoietic stem cells or hematopoietic
progenitor
cells and these factors are/can be derived from the stromal cells.
Mouse stromal cells produce factors supporting the proliferation or the
survival of hematopoietic stem cells or hematopoietic progenitor cells, as
mentioned
above. Attention is given that there are two kinds of stromal cells. One has
an
ability to support the proliferation or survival of hematopoietic stem cells
or
hematopoietic progenitor cells (hereafter sometimes referred to as "activity
to
support hematopoietic stem cells"). The other does not have the activity to
support
hematopoietic stem cells. This difference in the abilities rnaybe.due to
differential
expression of the factors that facilitate supporting hematopoietic stem cells
or
progenitor cells at the transcription level. That is to say it is speculated
that the
supportive stromal cells express at high levels of mRNAs coding the factors
and that
non-supportive stromal cells express less mRNAs. Thus mRNAs that code for the
factors maybe among the genes expressed higher in the supportive cells
compared to
in the non-supportive cells. In this context, the inventors confirmed the
hematopoietic stem and/or progenitor cell supporting ability of AGM-s3-A9, AGM-
s3-D11, OP9, and SWISS3T3 cell lines and the non-supportive ability of AGM-s3-
A7, AGM-s3-G1, and NIH3T3 cell lines (AGM-s3-A9, AGM-s3-D11, AGM-s3-
A7, and AGM-s3-G1 cell lines are obtained by subcloning the stromal cell
strain
AGM-s3 derived from AGM disclosed in the prior application W099/039~0).
Next, the genes that are highly expressed in AGM-s3-A9, AGM-s3-D11, OP9, and
3T3Swiss cell lines and show low expression or are undetected in AGM-s3-A7,
AGM-s3-G1, and NIH3T3 cell lines were identified. After the assessment of the
abilities of supporting the proliferation or the survival of the hematopoietic
stem
34
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
cells or the hematopoietic progenitor cells of these gene groups and careful
examinations, the present invention has been completed.
That is, the present invention provides the followings.
(1) A DNA coding for a polypeptide as defined in the following (A) or (B):
(A) a polypeptide which has an amino acid sequence comprising at least
amino acid residues 22 to 279 of SEQ ID NO: 32, or an amino acid sequence
comprising at least amino acid residues 22 to 272 of SEQ ID NO: 34; or
(B) a polypeptide which has an amino acid sequence including deletion,
substitution or insertion of one or several amino acids in the amino acid
sequence
comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32, or an
amino
acid sequence comprising at least amino acid residues 22 to 272 of SEQ ID NO:
34,
and which has an activity to support proliferation or survival of
hematopoietic stem
cell or hematopoietic progenitor cell, with a proviso that C-terminal amino
acid
sequence dose not comprise the amino acid sequence of SEQ ID NO: 45.
(2) The DNA according to (1), which is a DNA as defined in the following (a)
or (b)
(a) a DNA which comprises at least nucleotides 574 to 1347 of SEQ ID
NO: 31; or
(b) a DNA which is hybridizable with the nucleotide sequence of SEQ ID NO:
31 or a probe or fragment prepared from the sequence, under the stringent
condition, and which has an activity to support proliferation or survival of
hematopoietic stem cell or hematopoietic progenitor cell.
(3) The DNA according to (2), the stringent condition is 6 x SSC 5 x Denhardt,
0.5 % SDS and 68°C (SSC 3M NaCI, 0.3M sodium citrate, 50 x Denhardt 1
BSA 1 % polyvinyl pyrrolidone, 1 % Ficoll 400 , or 6 x SSC, 5 x Denhardt, 0.5
%
SDS, 50% formamide and 42°C.
(4) The DNA according to (1), which is a DNA as defined in the following (a)
or (b)
(a) a DNA which comprises at least nucleotides 321 to 1074 of SEQ ID
NO: 33; or
(b) a DNA which is hybridizable with the nucleotide sequence of SEQ ID NO:
33 or a prove prepared from the sequence, under the stringent condition, and
which
3S
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
has an activity to support proliferation or survival of hematopoietic stem
cell or
hematopoietic progenitor cell.
(5) The DNA according to (4), the stringent condition is 6 x SSC 5 x
Denhardt 0.5 % SDS and 68 °C (SSC 3M NaCI, 0.3M sodium citrate, 50
x
Denhardt 1 % BSA 1 % polyvinyl pyrrolidone, 1 % Ficoll 400 , or 6 x SSC, 5 x
Deanhardt, 0.5% SDS, 50% Formamide and 42°C.
(6) A expression vector which comprises a DNA of any one of (1) to (5) or
other polynucleotides of the invention in such a manner that the DNA can be
expressed.
(7) A cell which is introduced (i.e., transformed or transfected) with a DNA
of
any one of (1) to (5) or other polynucleotides of the invention in such a
manner that
the DNA can be expressed.
(8) A polypeptide (An isolated polypeptide) which is an expression product of
a
DNA according to any one of (1) to (5) or other polynucleotides of the
invention,
the polypeptide having an activity to support proliferation or survival of
hematopoietic stem cells or hematopoietic progenitor cells, with a proviso
that C-
terminal amino acid sequence dose not comprise the amino acid sequence of SEQ
ID
NO: 14.
(9) The polypeptide according to (8), which has an amino acid sequence
comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32, or an
amino
acid sequence including deletion, substitution or insertion of one or several
amino
acids in the amino acid sequence comprising at least amino acid residues 22 to
279
of SEQ ID NO: 32.
(10) The polypeptide according to (8), which has an amino acid sequence
comprising at least amino acid residues 22 to 272 of SEQ ID NO: 34, or an
amino
acid sequence including deletion, substitution or insertion of one or several
amino
acids in the amino acid sequence comprising at least amino acid residues 22 to
272
of SEQ ID NO: 34.
(11) The polypeptide according to (8) or other polypeptides of the invention,
which is modified with one or more modifying agent selected from the group
consisting of polyethylene glycol (PEG), dextran, poly(N-vinyl-pyrrolidone),
36
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
polypropylene glycol homopoymer, copolymer of polypropylene oxide/ethylene
oxide, polyoxyethylated polyol and polyvinyl alcohol.
(12) Pharmaceutical composition having an effect to support proliferation or
survival of hematopoietic stem cells or hematopoietic progenitor cells, which
comprises the polypeptide as defined in the following (A) (B) or (C): (A) a
polypeptide which has an amino acid sequence comprising at least amino acid
residues 22 to 279 of SEQ ID NO: 32, or an amino acid sequence comprising at
least amino acid residues 22 to 272 of SEQ ID NO: 34; or
(B) a polypeptide which has an amino acid sequence including deletion,
substitution or insertion of one or several amino acids in the amino acid
sequence
comprising at least amino acid residues 22 to 279 of SEQ ID NO: 32, or an
amino
acid sequence comprising at least amino acid residues 22 to 272 of SEQ ID NO:
34,
and which has an activity to support proliferation or survival of
hematopoietic stem
cell or hematopoietic progenitor cell, or
(C) any of the other polypeptides of the invention described therein.
(13) A monoclonal antibody which binds to the polypeptide of (9) to (11).
Terms used in this specification are defined as follows.
A hematopoietic stem cell is defined as a cell having totipotency, that is, a
capacity to differentiate into all the cell lineages of the hematopoietic
cells, and
simultaneously having a potency of self renew with retaining the totipotency.
Erythrocyte precursor cells hardly survive and proliferate in vitro culture
circumstances and rapidly disappear. If the survival and the proliferation of
the
erythrocyte precursor cells are confirmed, continuous production of the
erythrocyte
precursor cells seems to occur due to the survival and/or the proliferation of
the
more immature hematopoietic stem cells or the hematopoietic progenitor cells.
Therefore, to assess the survival and/or proliferation of the human
hematopoietic
stem cells, to enumerate the erythrocyte precursor cells ((BFU-E, CFU-E, and
CFU-Emix) in cultures is an appropriate way.
A hematopoietic progenitor cell is defined as a cell which can differentiate a
single cell lineage of the hematopoietic lineage or a plural cell lineages but
cannot
differentiate into all of the cell lineages. A stromal cell is defined as a
cell which
can be co-cultured together with the hematopoietic stem cells in vitro to
simulate in
37
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
vivo hematopoietic environment. Cells derived from any origin can be used as
long
as the cells can be co-cultured with the hematopoietic cells ira vitro.
Polypeptides in accordance with the present invention have an activity to
support proliferation or survival of hematopoietic stem cells or hematopoietic
progenitor cells. The concrete embodiment of the polypeptides in accordance
with
the present invention are an expressed product (hereafter sometimes referred
to as a
mouse "supporting factor for the proliferation of stem cells") of a gene named
SCR-
1 isolated from a mouse stromal cell (hereafter sometimes referred to as
"mouse
SCR-1 ") and an expressed product (hereafter sometimes referred to as a human
"supporting factor for the proliferation of stem cells") of a human
orthologous gene
thereof (hereafter sometimes referred to as "human SCR-1 "). The term SCR-1
may
be used herein to refer to the polypeptide sequences set out in SEQ ID NO: 10,
13,
16, 32 and 34 which are respectively encoded by the polnucleotide sequences
set out
in SEQ ID NOS: 9, 11, 12, 31 and 33.
Although an amino acid sequence of the expressed product of human SCR-1
(SEQ ID NO: 34) has homology at 97.4 % with the known polypeptide
(W098/49302) whose function has not been clear, the amino acid sequence at the
C-
terminal region thereof differs, so that it is a novel polypeptide. A part of
the amino
acid sequence in the above described polypeptide having unknown functions
which
is different from that in SEQ ID NO: 34 is shown in SEQ ID NO: 45. On the
other
hand, mouse SCR-1 has homology at 84.6 % with the above-described polypeptide.
The above described homologies are calculated as percentage of the number of
same amino. acids to the total number of amino acids using a comparison
manually
(266/273 and 237/280, respectively).
The supporting factor for the proliferation of stem cells, that is, the
polypeptides in accordance with the present invention can be produced by
preparing
transformed cells by transducing mouse or human SCR-1 into appropriate host
cells
and by expressing the DNAs in the transformed cells. When DNA including a
nucleotide sequence shown in SEQ ID NO: 31 is used as SCR-1, a mouse
supporting factor for the proliferation of stem cells is obtained. When DNA
including a nucleotide sequence shown in SEQ ID NO: 33 is used as SCR-1, a
human supporting factor for the proliferation of stem cells is obtained. The
mouse
38
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
supporting factor for the proliferation of stem cells and the human supporting
factor
for the proliferation of stem cells comprise amino acid sequences represented
by
SEQ ID NO: 32 and SEQ ID NO: 34., respectively. These supporting factors for
the proliferation of stem cells are precursors including signal peptides, and
are
assumed to be processed to mature supporting factors for the proliferation of
stem
cells in mouse or human cells. As based on the results of Signal P test which
searches breakage sites of the signal peptides in these amino acid sequences
(Nielsen
H. , protein Engineering, 10: 1-6, 1997; Nielsen H. , Int. J. Ner~ral Sys. ,
8: 5~ 1-599,
1997), the breakage or cleavage sites seem to exist between the amino acid 21
and
the amino acid 22 in the amino acid sequences of SEQ ID N0:.32 and SEQ ID NO:
34.
The mouse mature supporting factor for the proliferation of stem cells
comprises the amino acid sequence represented by amino acids 22 to 279 of SEQ
ID
NO: 32. The human mature supporting factor for the proliferation of stem cells
comprises the amino acid sequence represented by amino acids 22 to 272 of SEQ
ID
NO: 34.
When supporting factors for the proliferation of stem cells are prepared, SGI~-
1 which is transferred into host cells may be DNA coding precursor polypeptide
or
DNA coding mature polypeptide. An example of the DNA coding the mouse
mature supporting factor for the proliferation of stem cells comprises the DNA
comprising at least a nucleotide sequence consisting of nucleotide numbers 574
to
1347 of SEQ ID NO: 31. An example of the DNA coding the human mature
supporting factor for the proliferation of stem cells comprises the DNA
comprising
at least a nucleotide sequence consisting of nucleotide numbers 321 to 1074 of
SEQ
ID NO: 33.
DNA in accordance with the present invention may code the above described
factors which have amino acid sequences including substitution, deletion or
insertion
of one or several amino acids, as long as the activity of the supporting
factor for the
proliferation of stem cells to be coded is not lost. DNAs coding substantially
identical polypeptides to this supporting factor for the proliferation of stem
cells are
obtained by modifying the nucleotide sequences so as to include substitution,
39
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
deletion, insertion, addition, or inversion of amino acid residues in a
specific region
using site-directed mutagenesis.
The DNAs including the above described mutation can be expressed in
appropriate cells and the activity to support the hematopoietic stem cells of
the
expressed products can be examined, so that the DNAs coding the polypeptide
having functions which are substantially identical to this supporting factor
for the
proliferation of stem cells are obtained. In addition, the DNAs coding
substantially
identically active protein as this supporting factor for the proliferation of
stem cells
can be obtained by hybridization with DNAs including, for example, the
nucleotide
sequence as described in SEQ ID NO: 1 or SEQ ID NO: 3 from the cells having
thereof, or probes prepared from these DNAs under the stringent condition; and
by
isolating the DNAs coding the protein possessing the activity to support the
hematopoietic stem cells. The stringent condition is, for example, one in
which
DNAs having homology at not less than 70 % , preferably at not less than. 8Q %
, are
hybridized each other and DNAs having less homology than those are not
hybridized each other. The above described stringent condition is 6 x SSC, 5 x
Denhardt, 0.5 % SDS, 68°C (SSC; 3M NaCI, 0.3M sodium citrate) (50 x
Denhardt;
1 % BSA, 1 % polyvinyl pyrrolidone, 1 % Ficoll 400) or 6 x SSC, 5 .x
Deanhardt,
0.5% SDS, 50% Formamide, 42°C, or the like. Strategy of hybridization
is further
defined by final wash conditions as set out herein. ,
Microorganisms such as Escherichia coli and yeast, culture cells derived from
animals or plants, and the like are used for host cells for expressing SCR-1.
Preferably, culture cells derived from mammals are used as the host cells. In
the
case that prokaryotic cells are used as the host cells, the expression is
preferably
performed in a condition in which a signal peptide region is replaced with a
leader
sequence suitable for the prokaryotic cells such as -lactamase (bla), alkaline
phosphatase (phoA), and outer membrane protein A (ompA) and the like, or in a
form in which a methionine residue is added to the N-terminal site of the
mature
protein.
The supporting factor for the proliferation of stem cells obtained as above
may
be added with sugar chains at any of positions 23, 36 and 137, alone, or a
plurality
of positions thereof in mouse SCR-1. The supporting factor for the
proliferation of
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
stem cells obtained as above may be added with sugar chains at any of
positions 23,
36, 137 or 194, alone, or a plurality of positions thereof in human SCR-1.
For example, SCR-I is integrated into a vector corresponding to the host in a
form capable of expression and the obtained recombinant vector is transferred
into
the host cells, so that the transfer of SCR-1 into the host cells is
completed.
Examples of the culture cells derived from mammals are CHO cells, 293 cells,
COS7 cells, and the like. Gene expression regulatory sequence such as a
promoter
to express SCR-1 may be originated from SCR-1 itself, or may be derived from
other genes such as cytomegalovirus promoter and elongation factor 1 promoter
and
the like.
Examples of a vector for animal culture cells are plasmid vectors, retrovirus
vectors, adenovirus vectors (Neering, S.J., Blood, 88: 1147, 1996), herpes
virus
vectors (Dilloo, D., Blood, 89: 119, 1997), HIV vectors, and the like.
In order to transfer the recombinant vector into culture cells, the
conventional
methods which are usually employed for transformation of culture cells such as
calcium phosphate transfection, liposome method, DEAE dextran method,
electroporation and microinjection method are employed.
The polypeptides in accordance with the present invention also comprise
polypeptides having amino acid sequences in which one or several amino acids
are
substituted, deleted or inserted in the amino acid sequence represented in SEQ
ID
NO: 32 or SEQ ID NO: 34 or other polynucleotides of the invention, and having
activity to support the hematopoietic stem cells in addition to the
polypeptides
having the amino acid sequence represented in SEQ ID NO: 32 or SEQ ID NO: 34
or other polynucleotides of the invention. That is, even if a mouse and a
human
supporting factor for the proliferation of stem cells is modified by
substitution,
deletion, insertion or the like, polypeptides holding essential functions as a
supporting factor for the proliferation of stem cells can be considered to be
substantially identical with the supporting factor for the proliferation of
stem cells.
The above described "several" denotes ranging from two to 110, and preferably
ranging from two to 55 as a total number depending on the region of
polypeptide in
accordance with the present invention.
41
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
These modified supporting factors for the proliferation of stem cells can be
obtained by treating DNA coding the supporting factors for the proliferation
of stem
cells or host cells including the above mentioned DNA with mutagens, or by
mutating the above mentioned DNA so as to substitute, delete, or insert an
amino
acid at a specific site using site-directed mutagenesis. The residual of the
activity to
support hematopoietic stem cells in the obtained mutant polypeptides is
confirmed
by the examples described below. That is, after the cultured hematopoietic
stem
cells which express the mutant polypeptides are transferred into irradiated
mice,
peripheral hematological cellularity after the transfer may be observed over
time.
Since the nucleotide sequences of the invention have been described, the
modified supporting factor for the proliferation of stem cells can be also
obtained by
isolating the corresponding DNAs from mouse or human cDNA or chromosome
DNA libraries using PCR in which the oligonucleotides prepared based on these
nucleotide sequences are used as primers or using hybridization in which the
oligonucleotides prepared based on these nucleotide sequences are used as
probes.
In one aspect, the DNAs in accordance with the present invention was isolated
from cDNA library of AGM-s3-A9 cells which are a mouse stromal cell strain
having the activity to support hematopoietic stem cells using SBH (Sequencing
By
Hybridization) method (Drmanac, S. , Nat. Biotechhol. , 16. 54, 1998; Drmanac,
R. ,
Methods. Enzymol. , 303, 165, 1999) as described below. The mouse stromal cell
lines having the activity to support hematopoietic stem cells can be obtained
using
the method disclosed in W099/03980 or from Cell Development Bank of Institute
of
Physical and Chemical Research (RIKEN) or ATCC.
An outline of SBH method will be described below. Probes including eight or
nine nucleotides whose sequences are different from each other are prepared.
When
the nucleotide sequences corresponding to those of the probe exist in targeted
gene,
the probes can hybridize with the gene. The hybridization can be easily
detected
with utilization of radio isotope or fluorescence conjugated probes. Each
clone in
the library is picked up, and blotted on a membrane. Then, repeated
hybridizations
are performed with the above described probes, so that one can identify the
combination of probes that hybridize to each clone. Since the combination of
probes that hybridize to each gene depend on the sequences of clones,
identical
42
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
genes have identical signature hybridization patterns with the probes. That
is, the
same gene can be identified as a one group (cluster) according to the
signature of the
hybridized probes. The number of clones derived from each gene in the library
can
be determined by clustering and counting the members of the clusters based on
the
hybridization profiles of the probes. Thus, incidence of expression of each
gene in
the library can be determined.
Clustering analysis was performed for cDNA libraries derived from supportive
and non-supportive stromal cell lines. Thus, incidences of expressed genes
among
cells were compared, so that the genes specifically highly expressed in the
supportive stromal cell lines were selected. The incidences of these genes in
each
cell were further examined by Northern blot analysis, so that genes which
highly
expressed in the cells having activity to support the hematopoietic stem cells
were
obtained.
SCR-1 is one of the genes which was highly expressed with specificity in the
supporting cells obtained through the above described process. After
clustering and
analyzing using Northern blot analysis, the gene comprising nucleotide numbers
1032 to 1484 of SEQ ID NO; 31 was identified. The complete gene encoding SCR-
1 was cloned from the cDNA library derived from AGM-s3-A9 cells.
Further, in order to assess supporting ability for hematopoiesis of SCR-1, a
gene fragment including ORF (nucleotide numbers 511 to 1350 of SEQ ID NO: 31)
in SCR-1 gene was transferred into stromal cells (AGM-s3-A7 cell) which cannot
support the hematopoietic stem cells using a retrovirus vector, and assessed
the
change in the activity to support the hematopoietic stem cells of the stromal
cells.
Substantially, after the stromal cells which were not transferred with the
gene and
those which were transferred with the gene were independently co-cultured with
the
mouse hematopoietic stem cells, the hematopoietic cells were transplanted into
irradiated mice. Engraftment of the co-cultured hematopoietic cells in
recipient
mice were examined. As a result, the mice transplanted with the hematopoietic
stem cells which were co-cultured with the AGM-s3-A7 cell line transferred
with
SCR-1 showed increased chimerism after the transplantation compared with the
AGM-s3-A7 cell line which were not transferred with SCR-1 gene. This result
shows that the AGM-s3-A7 stromal cells that express SCR-1 have obtained
43
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
supporting activity for the proliferation or survival of the hematopoietic
stem cells
or the hematopoietic progenitor cells. As a result, it has become evident that
SCR-1
has a function to add the above described activity to the stromal cells that
originally
do not posses the activities for supporting proliferation or survival of
hematopoietic
stem cells or hematopoietic progenitor cells. Therefore, it was revealed that
SCR-1
has an activity to support the survival or the proliferation of the
hematopoietic stem
cell or the hematopoietic progenitor cell, or has an activity to add an
activity to
support the hematopoietic stem cells to stromal cells.
The polypeptides in accordance with the present invention can be used as a
medicine to proliferate or support human hematopoietic stem cell or human
hematopoietic progenitor cell. This pharmaceutical composition can be used for
supporting proliferation or survival of human hematopoietic stem cells or
human
hematopoietic progenitor cells ex vivo. It is of problem for hematopoietic
stem cell
transplantation therapies such as peripheral blood stem cell transplantation
and cord
blood stem cell transplantation that sometimes sufficient amount of the
hematopoietic stem cells cannot be collected and the transplantation may not
be
performed. Even if enough stem cells could not be collected; a sufficient
amount of
the hematopoietic stem cells could be obtained (and transplanted) by
amplification
of
the hematopoietic stem cells in vitro using polypeptides of the invention.
That is,
hematopoietic stem cells can be amplified without differentiation by culturing
the
hematopoietic stem cells in culture medium including polypeptides of the
invention.
It may be considered the hematopoietic stem cells are able to be amplified
more
efficiently with addition of a variety of cytokines to the medium.
When hematopoietic stem cells or hematopoietic progenitor cells are cultured
in the medium including the polypeptides in accordance with the present
invention,
the hematopoietic stem cells or the hematopoietic progenitor cells that will
be used
may be one of these cell types alone or may be both of the cell types. In
addition,
the cells should include at least the hematopoietic stem cells or the
hematopoietic
progenitor cells, and may include other hematopoietic cells. Further,
polypeptides
of the invention can be used for hematopoietic stem or progenitor expansion of
44
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
purified hematopoietic stem cell fraction or progenitor cell fractions from
the cell
populations that contain the hematopoietic stem cells or progenitor cells.
Examples of sources of hematopoietic stem cells and hematopoietic progenitor
cells in the methods in accordance with the present invention are fetal liver,
bone
marrow, fetal bone marrow, peripheral blood, peripheral blood from persons
from
whom stem cells are mobilized by cytokines and/or dosing of antitumor drugs,
cord
blood, and the like of mammals such as human and mouse and the like. Any
sources may be used as long as the tissue includes the hematopoietic stem
cells.
A culture method using petri dishes and flasks for culture may be employed to
culture the hematopoietic stem cells or the hematopoietic progenitor cells.
The
cultivation of the hematopoietic stem cells and/or progenitor cells may be
improved
by mechanically controlling medium composition, pH, and the like, and
employing
a bioreactor capable of high density cultivation (Schwartz, Proc. Natl. Acad.
Sci.
U.S.A., 88: 6760, 1991; Koller, M.R., BiolTechnology, 11: 358, 1993; IKoller,
M.R., Blood, 82: 378, 1993; Palsson, B.O.; BiolTechnology, Il: 368, 1993).
Since SCR-1 can increase activities of stromal cells to support the
hematopoietic stem cells under the conditions of co-culture of stromal cells
and
hematopoietic cells, the hematopoietic stem cells and/or progenitor cells can
be
efficiently expanded when whole bone marrow cells are cultured in the presence
of
SCR-1. This type of co-culture of the stromal cells and the hematopoietic
cells can
be performed simply after the collection of the bone marrow cells without
complicated cell separation. Furthermore, one can perform co-culture with
separate
components such as hematopoietic stem cells, progenitor cells and stromal
cells
from collected bone marrow cells and combine the hematopoietic cells and
stromal
cells from different individuals. Furthermore, one can grow stromal cells and
establish stromal cell culture prior to co-culture with the hematopoietic stem
cells
for the hematopoietic stem cells or progenitor cell expansion. At this time,
one can
utilize cell stimulating factors to promote growth and survival of stromal
cells to
establish stromal cell culture. Examples of cell stimulating factors includes
growth
factors which are typically a cytokine such as SCF (stem cell factor), IL-3
(interleukin-3), GM-CSF (granulocyte/macrophage colony-stimulating factor), IL-
6
(interleukin-6), TPO (thrombopoietin), G-CSF (granulocyte colony-stimulating
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
factor), TGF-b (transforming growth factor-b), MIP-la (Davatelis, G., J. Exp.
Med. 167: 1939, 1988); differentiation and proliferation control factors such
as
hematopoietic hormones such as EPO (erythropoietin), chemokine, Wnt gene
product, and notch ligand; and development control factors.
In addition, the proliferation and the survival of hematopoietic stem cells or
hematopoietic progenitor cells can be retained by culturing the hematopoietic
stem
and/or progenitor cells with recombinant SCR-1 alone or combination with the
cell
stimulating factors without stromal cells. Examples of the cell stimulating
factors
used in this case are above described cell stimulating factors and the like.
Medium used for culture is not specially restricted as long as the
proliferation
or the survival of the hematopoietic stem cells or the hematopoietic
progenitor cells
is not perturbed. Preferable media are, for example, MEM-a. medium (GIBCO
BRL), SF-02 medium (Sanko Junyaku), Opti-MEM medium (GIBCO BRL), IMDM
medium (GIBCO BRL), and PRMI1640 medium (GIBCO BRL). A culture
~ temperature is usually ranging from 25 to 39°C, and preferably
ranging from 33 to
39°C. Examples of additives to the medium are fetal bovine serum, human
serum,
horse serum, insulin, transferrin, lactoferrin, ethanolamine, sodium selenite,
monothiolglycerol, 2-mercaptoethanol, bovine serum albumin, sodium pyruvate,
polyethylene glycol, a variety of vitamins, and a variety of amino acids. A
concentration of COz is usually ranging from four to six percent, and
preferably five
percent.
Since hematopoietic stem cells can differentiate into all hematopoietic cell
lineages, hematopoietic stem cells can be manipulated to be differentiated
into a
specific cell type in vitro, and then the specific cells can be transplanted.
For
example, when erythrocytes are necessary, after cultivation and expansion of
the
patient's stem cells, hemopoietic cells whose main component is the
erythrocyte can
be artificially produced using an erythrocyte differentiation induction or
promoting
factors such as EPO. '
The hematopoietic stem cells or the hematopoietic progenitor cells cultured
using the polypeptides in accordance with the present invention can replace as
a
graft for the conventional bone marrow transplantation or cord blood
transplantation. Transplantation of the hematopoietic stem cells is superior
to the
46
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
conventional hematopoietic cell transplantation therapy, since the graft can
take
semipermanently.
The transplantation of the hematopoietic stem cells can be employed as
therapy for a variety of diseases in addition to as combination therapy for
total body
X-ray irradiation therapy or advanced chemotherapy for leukemia. For example,
when therapy accompanied with myelosuppression as an adverse reaction such as
chemotherapy, radiation therapy, and the like is performed for the patient
with solid
cancer, hematological disorder, hematological failure can be early improved as
follows. The bone marrow is collected before the therapy and the hematopoietic
stem cells or the hematopoietic progenitor cells are allowed to expand in
vitro.
Then, the expanded cells are infused to the patient after the therapy, so that
the
patient can get benefit of early recovery and stronger chemotherapy than the
conventional one can be performed to improve the therapeutic effect of the
chemotherapy. In addition, the hematopoietic stem cells or the hematopoietic
progenitor cells obtained according to the present invention are
differentiated into a
variety of hematopoietic cells. The transplantation of these cells into a
patient with
hypoplasia of a given hematopoietic cells can improve the patient's deficient
status.
In addition, this therapy can improve dyshemopoietic anemia to develop.anemia
such as aplastic anemia caused by bone marrow hypoplasia. Furthermore,
examples
of diseases in which the transplantation of the hematopoietic stem cells
according to
the present invention is effective are immunodeficiency syndrome such as
chronic
granulomatous disease, duplicated immunodeficiency syndrome,
agammaglobulinemia, Wiskott-Aldrich syndrome, acquired immunodeficiency
syndrome (AIDS), and the Like, thalassemia, hemolytic anemia due to enzyme
defect, congenital anemia such as sicklemia, Gaucher's disease, lysosomal
storage
disease such as mucopolysaccharidosis, adrenal white matter degeneration, a
variety
of cancers and tumors, and the like.
Transplantation of hematopoietic stem cells may be performed in the same
manner as conventional bone marrow transplantation or cord blood
transplantation
other than the differences of the cells used.
The hematopoietic stem cells which may be used for the above described
hematopoietic stem cell transplantation are derived from not only bone marrow
but
47
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
also the above described fetal liver, fetal bone marrow, peripheral blood,
peripheral
blood with stem cells induced by cytokines and/or dosing of antitumor drugs,
cord
blood, and the like.
The graft may be a composition including buffer solution and the like in
addition to the hematopoietic stem cells and the hematopoietic progenitor
cells
produced by the method according to the present invention.
The hematopoietic stem cells or the hematopoietic progenitor cells produced
according to the present invention may be used for ex vivo gene therapy. Since
the
incidence of recombination of target genes to the chromosome is low due to
dormancy of the stem cells, differentiation of stem cells during the culture
period,
and the like, gene therapy to the hematopoietic stem cells has been hard to
established. However, the present invention can amplify stem cells without
differentiation, so that efficacy of gene transfer is expected to be
remarkably
improved. In gene therapy, a foreign gene (a gene for therapy) is transferred
into
the hematopoietic stem cells or the hematopoietic progenitor cells, and then
the
obtained gene-transferred cells are used. The foreign gene to be transferred
is
appropriately selected according to disease. Examples of diseases in which the
target cells of gene therapy is the hematopoietic cells include
immunodeficiency
syndrome such as chronic granulomatous disease, duplicated immunodeficiency
syndrome, agammaglobulinemia, Wiskott-Aldrich syndrome, acquired
immunodeficiency syndrome (AIDS), and the like, thalassemia, hemolytic anemia
due to enzyme defect, congenital anemia such as sicklemia, Gaucher's disease,
lysosomal storage disease such as mucopolysaccharidosis, adrenal white matter
degeneration, a variety of cancers and tumors, and the like.
Usual method used for transfer of a gene into animal cells is employed for the
transfer of the gene for the therapy into hematopoietic stem cells or
hematopoietic
progenitor cells. Examples are a method using a vector for animal cells
derived
from virus utilized for gene therapy such as retrovirus vector such as Moloney
mouse leukemia virus, adenovirus vector, adeno-associated virus (AAV) vector,
herpes simplex virus vector, and HIV vector (with respect to a vector for gene
therapy, see Verma, LM., Nature, 389: 239, 1997); calcium phosphate
transfection,
DEAE-dextran transfection, electroporation, liposome method, lipofection
method,
48
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
microinjection method, and the like. Among them, methods using retrovirus
vector,
adeno-associated virus vector, or HIV vector are preferable, since expression
of a
gene is permanently expected due to insertion into the chromosome DNA of a
target
cell.
For example, adeno-associated virus (AAV) vector can be prepared as
follows. First, a vector plasmid inserted a gene for therapy into ITR
(inverted
terminal repeat) at both ends of wild-type adeno-associated virus DNA and a
helper
plasmid for supplementing virus protein are transfected into 293 cell strain.
Next,
adenovirus as helper virus is infected, so that virus particles including the
AAV
vector are produced. Alternatively, instead of adenovirus, a plasmid which
expresses adenovirus gene coding helper function may be transfected. The
obtained
virus particles are infected to the hematopoietic stem cells or the
hematopoietic
progenitor cells. Preferably, appropriate promoter and enhancer are inserted
into
upstream region of the target gene in the vector DNA, so that the expression
of the
gene is regulated. When marker gene such as a drug resistant gene is used in
addition to the gene for therapy, cells transferred with the gene for therapy
are
easily selected. The gene for therapy may be sense gene or antisense gene.
A composition for gene therapy may include buffer solution and a novel active
ingredient and the like in addition to the hematopoietic stem cells or the
hematopoietic progenitor cells by the method according to the present
invention.
A vector for gene therapy can be produced by transferring SCR-1 in
expression vector using a usual method. This vector for gene therapy is useful
to
treat diseases which need survival and proliferation.of the human
hematopoietic
stem cells. That is, a vector producing SCR-1 is transferred into the
hematopoietic
stem cells and the cells are cultured in vitro, so that the hematopoietic stem
cells or
the hematopoietic progenitor cells can proliferate dominatingly. The
hematopoietic
stem cells can proliferate irz vivo caused by returning these hematopoietic
stem cells
thus treated. The hematopoietic stem cells can significantly proliferate in
vivo by
introducing this vector for gene therapy into the body. Alternatively, the
bone
marrow cells derived from a patient are cultured and transferred with this
vector for
gene therapy, so that the hematopoietic stem cells or the hematopoietic
progenitor
cells can be proliferated in culture system. Alternatively, this vector for
gene
49
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
therapy is transferred into stromal cell derived from bone marrow and
cultivated and
mesenchaymal stem cell, so that the activity to support hematopoietic stem
cells can
be added or increased.
As shown in Examples, since it is possible that the stromal cells without the
activity to support the hematopoietic stem cells can be modified to include
this
activity using SCR-1, stromal cells derived from human or mouse can have the
activity to support the hematopoietic stem cells by gene transferring SCR-1.
The
stromal cells expressing SCR-1 and hematopoietic stem cells or hematopoietic
progenitor cells are co-cultured, so that the hematopoietic stem cells or the
hematopoietic progenitor cells can exist and proliferate so as to be useful
for a
variety treatment.
Since the hematopoietic stem cells or the hematopoietic progenitor cells can
survive and proliferate by expression of SCR-1 in the stromal cell, an
activity to
support the hematopoietic stem cells of the stromal cells can be assessed
using the
expression of SCR-1 as an index. The expression of SCR-1 in the stromal cells
can
be confirmed using antibody to SCR-1. PCR primers can be prepared from genes
included in SEQ ID NO: 31, SEQ ID NO: 33 or other polynucleotides of the
invention and RNA is prepared from the stromal cells of interest, and RT-PCR
is
performed, so that the expression of SCR-1 can be confirmed. Antibody to SCR-1
will be described below.
The pharmaceutical composition in accordance with the present invention can
be administered to human. The pharmaceutical composition having an activity to
proliferate or to support the human hematopoietic stem cells or the
hematopoietic
progenitor cells can be produced by mixing medically acceptable diluent,
stabilizer,
carrier, and/or other additives with the polypeptides in accordance with the
present
invention. At this time, in order to increase the stability of the protein ih
vivo the
polypeptides in accordance with the present invention may be modified by a
modifying agent. Examples of the modifying agent are polyethylene glycol
(PEG),
dextran, poly(N-vinyl-pyrrolidone), polypropylene glycol homopolymer,
polypropylene oxide/ethylene oxide copolymer, polyoxyethylated polyol,
polyvinyl
alcohol, and the like. Examples of modification of protein with PEG are a,
method
in which activated ester derivatives of PEG is reacted with the protein, a
method in
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
which aldehyde derivatives at end portion of PEG is reacted with protein under
the
presence of a reducing agent, and the like. Japanese Unexamined Patent
Application No. 10-510980 discloses modification of such protein in detail.
When the pharmaceutical composition in accordance with the present invention
is administered to human, recovery from hematological suppression due to an
adverse drug reaction of carcinostatics; early recovery of hematopoietic cells
at bone
marrow transplantation, peripheral blood stem cell transplantation, or cord
blood
transplantation; and recovery of hematopoietic function at pancytopenia such
as
aplastic anemia (AA) and myelodysplastic syndrome (MDS) are expected.
The antibody in accordance with the present invention reacts specifically to
the
above described polypeptides in accordance with the present invention. This
antibody may be monoclonal antibodies or polyclonal antibodies as long as they
react specifically to the above described polypeptides.
The antibody in accordance with the present invention can be prepared
according to usual methods. For example, the antibody can be prepared either
in
vivo method in which animals are additionally immunized by antigen together
with
adjuvant once or several times at an interval of several weeks or in vitro
method in
which immune cells are isolated and sensitized in an appropriate culture
system.
Examples of immune cells which. can produce the antibody in accordance with
the
present invention are splenic cells, tonsillar cells, lymph gland cells, and
the like.
The whole polypeptide according to the present invention is not necessarily
used as an antigen. A part of a polypeptide of the invention may be used as an
antigen. When the antigen is a short peptide, particularly, a peptide made of
about
20 amino acid residues, it may be used by binding it to a carrier protein
having high
antigenicity such as keyhole lympet hemocyanin or bovine serum albumin using
chemical modification and the like. Alternatively, the antigen may be used by
covalently binding it to a peptide having branching skeleton such as lysine
core
MAP peptide instead of the carrier protein (Posnett et al. , J. Biol. Chem. ,
263,
1719-1725, 1988; Lu et al. , Mol. Immunol. , 28, 623-630, 1991; Briand et al.
, J.
Immunol. Methods, 156, 255-265, 1992).
Examples of adjuvants are Freund's complete adjuvant, Freund's incomplete
adjuvant, aluminum hydroxide gel, and the like. Animals given the antigen are,
for
51
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
example, mouse, rat, rabbit, sheep, goat, chicken, bovine, horse, guinea pig,
hamster, and the like. The blood is collected from these animals and the serum
is
separated. Then, immunoglobulin is purified from the serum using an ammonium
sulfate precipitation method, anion exchange chromatography, protein A
chromatography, or protein G chromatography to obtain polyclonal antibodies.
With respect to chicken, antibodies can be purified from an egg. Monoclonal
antibodies can be purified and prepared from supernatant of culture of
hybridoma
cells or ascites from animals which received intrapertoneal administration of
hybridoma cells. Hybridomas are made by fusion of the immune cells sensitized
in
vitro, or immune cells from the above described animals with parent cells
capable of
cultivation. Examples of parent cells are X63, NS-1, P3U1, X63.653, SP210, Y3,
SKO-007, GM1500, UC729-6, HM2.0, NP4-1 cell strains, and the like.
Preparation may be performed by cultivating the immortalized antibody-forming
cells obtained by sensitization in vitro, or infection of a proper virus such
as EB
virus to the immune cells of the above described animals.
In addition to these cell engineering methods; antibodies can be obtained
using
gene engineering methods. For example, the antibody gene obtained from the in
vitro sensitized cells or immune cells derived from the above described
animals is
amplified by PCR (polymerase chain reaciion) and isolated, and the amplified
genes
are transferred into microorganisms such as E. coli to produce the antibodies.
Alternatively, the antibodies may be expressed on surfaces of phages as fused
protein. Antibodies of the invention are also addressed herein, infra.
SCR-1 can be measured in vivo using antibodies in accordance with the
present invention. Thus, the relationship between SCR-1 and pathological
status of
a variety of diseases can be clarified. Moreover, the antibodies can be used
for
diagnosis and treatment of diseases, and efficient affinity purification of
SCR-1.
The present invention provides polypeptides having an activity to support
survival or proliferation of hematopoietic stem cells or hematopoietic
progenitor
cells by effecting or acting thereon, or an activity to give an activity to
support the
hematopoietic stem cells to stromal cells by effecting thereon, and also
provides
DNA coding thereof. The polypeptides in accordance with the present invention
52
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
can efficiently maintain the proliferation or the survival of the
hematopoietic stem
cells or the hematopoietic progenitor cells.
In addition, the polypeptides in accordance with the present invention can be
used as a medicine to proliferate or to support human hematopoietic stem cells
or
human hematopoietic progenitor cells.
Alternatively, the invention is described as set out below.
5.2 NUCLEIC ACIDS AND POLYPEPTIDES OF THE INVENTION
Nucleotide and amino acid sequences of the invention are set forth as SEQ
ID NO: 1-24, and 31-34. Fragments of the proteins of the present invention
which
are capable of exhibiting biological activity are also encompassed by the
present
invention. Fragments of the protein may be in linear form or they may be
cyclized
using known methods, for example, as described in H. U. Saragovi, et al.,
Bio/Technology 10, 773-778 (1992) and in R. S. McDowell, et al., J. Amer.
Chem.
Soc. 114, 9245-9253 (1992), both of which are incorporated herein by
reference.
Such fragments may be fused to carrier molecules such as immunoglobulins for
many purposes, including increasing the valency of protein binding sites. For
example, fragments of the protein may be fused through "linker" sequences to
the
Fc portion of an immunoglobulin. For a bivalent form of the protein, such a
fusion
could be to the Fc portion of an IgG molecule. Other immunoglobulin isotypes
may
also be used to generate such fusions. For example, a protein-IgM fusion would
generate a decavalent form of the protein of the invention.
The present invention also provides both full-length and mature forms (for
example, without a signal sequence or precursor sequence) of the disclosed
proteins.
The protein coding sequence is identified in the sequence listing by
translation of the
disclosed nucleotide sequences. The mature form of such protein may be
obtained
by expression of a full-length polynucleotide in a suitable mammalian cell or
other
host cell. The sequence of the mature form of the protein is also determinable
from
the amino acid sequence of the full-length form. Where proteins of the present
invention are membrane bound, soluble forms of the pxoteins are also provided.
In
53
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
such forms, part or alI of the regions causing the proteins to be membrane
bound are
deleted so that the proteins are fully secreted from the cell in which it is
expressed.
The polynucleotides of the invention include naturally occurring or wholly or
partially synthetic DNA, e.g., cDNA and genomic DNA, and RNA, e.g., mRNA.
SEQ ID NO: 1-9, 11, 12, 31 or 33 may include the entire coding region of the
cDNA or may represent a portion of the coding region of the cDNA. Further 5'
and 3' sequence can be obtained using methods known in the art. For example,
full
length cDNA or genomic DNA that corresponds to any of the polynucleotides of
the
SEQ ID NO: 1-9, 11, 12, 31 or 33 can be obtained by screening appropriate cDNA
or genomic DNA libraries under suitable hybridization conditions using any of
the
polynucleotides of the SEQ ID NO: 1-9, 11, 12, 31 or 33 or a portion thereof
as a
probe. Alternatively, the polynucleotides of the SEQ ID NO: 1-9, 11, 12, 31 or
33
may be used as the basis for suitable primers) that allow identification
and/or
amplification of genes in appropriate genomic DNA or cDNA libraries.
The nucleic acid sequences of the invention can be assembled ESTs and
sequences (including cDNA and genomic sequences) obtained from one or more
public
databases, such as dbEST, gbpri, and UniGene. ~ The sequences falling within
the
scope of the present invention are not limited to these specific sequences,
but also
include allelic and species variations thereof. Allelic and species variations
can be
routinely determined by comparing the sequence provided in SEQ ID NO: 8-9, 11-
12,
31 or 33 a representative fragment thereof, or a nucleotide sequence at least
90
identical, preferably 99.9 % identical, to SEQ ID NO: 8-9, 11-12, 31 or 33
with a
sequence from another isolate of the same species. Furthermore, to accommodate
codon variability, the invention includes nucleic acid molecules coding for
the same
amino acid sequences as do the specific ORFs disclosed herein. In other words,
in the
coding region of an ORF, substitution of one codon for another which encodes
the
same amino acid is expressly contemplated.
The nucleic acids of the present invention, designated as SEQ ID NO. 8 and 9
were assembled using an EST sequence as a seed. The EST sequence can be
extended
into a full-length nucleic acid sequence by programs or algorithms known in
the art.
Preferably, a recursive algorithm is used to extend the seed EST into an
extended
assemblage, by pulling additional sequences from different databases (e.g.,
Hyseq's
54 -
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
database containing EST sequences, dbEST version 114, gb pri 114, and UniGene
version 101) that belong to this assemblage. The algorithm terminates when
there was
no additional sequences from the databases that will extend the assemblage.
Further,
the inclusion of component sequences into the assemblage is preferably based
on a
BLASTN hit to the extending assemblage with BLAST score greater than 300 and
percent identity greater than 95 % . BLAST, which stands for Basic Local
Alignment
Search Tool, is used to search for local sequence alignments (Altschul, S.F.,
J. Mol.
Evol. 36: 290 - 300 (1993) and Altschul S.F. et al., J. Mol. Biol. 21: 403-10
(1990)).
BLAST produces alignments of both nucleotide and amino acid sequences to
determine
sequence similarity. Because of the local nature of the alignments, BLAST is
especially useful in determining exact matches.
The EST sequences (SEQ ID NO. 1-7) can provide identifying sequence
information, representative fragment or segment information, or novel segment
information for the full-length gene.
The nearest neighbor result for the nucleic acids of the present invention,
including SEQ ID NO. 9, can be obtained by searching a database using an
algorithm
or a program. Preferably, a FASTA version. 3 search against Genpept, using
Fastxy
algorithm. The nearest neighbor result shows the closest homologue for each
assemblage from Genpept (and contains the translated amino acid sequences for
which
the assemblage encodes).
The present invention also provides genes corresponding to the cDNA
sequences disclosed herein. The corresponding genes can be isolated in
accordance
with known methods using the sequence information disclosed herein. Such
methods
include the preparation of probes or primers from the disclosed sequence
information
for identification and/or amplification of genes in appropriate genomic
libraries or
other sources of genomic materials.
Species homologs (or orthologs) of the disclosed polynucleotides and
proteins are also provided by the present invention. Species homologs may be
isolated and identified by making suitable probes or primers from the
sequences
provided herein and screening a suitable nucleic acid source from the desired
species.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The invention also encompasses allelic variants of the disclosed
polynucleotides or proteins; that is, naturally-occurring alternative forms of
the
isolated polynucleotide which also encode proteins which are identical,
homologous
or related to that encoded by the polynucleotides.
5.3 NUCLEIC ACIDS OF THE INVENTION
The isolated polynucleotides of the invention include, but are not limited to,
a polynucleotide encoding a polypeptide comprising SEQ ID NO: 10, 13- 24, 32
and 34, or the mature protein portion thereof. Preferred nucleic acid
sequences are
set forth as SEQ ID NO: 9, 11, 12, 31 or 33.
The isolated polynucleotides of the invention further include, but are not
limited to a polynucleotide comprising any of the nucleotide sequence of the
SEQ ID
NO: 1-9, 11, 12, 31 or 33; a polynucleotide comprising the full length protein
coding sequence of the polynucleotides of the SEQ ID NO: 1-9, 11, 12, 31 or
33;
and a polynucleotide comprising the nucleotide sequence encoding the mature
protein coding sequence of the polynucleotides of the SEQ ID NO: 1-9, 11, 12,
31
or 33. The polynucleotides of the present invention also include, but are not
limited
to, a polynucleotide that preferably has stem cell growth factor activity and
that
hybridizes under stringent conditions (a) to the complement of any of the
nucleotides
sequences of the SEQ ID NO: 1-9, 11, 12, 31 or 33 (b) to a polynucleotide
encoding the polypeptide of SEQ ID NO: 10, 13- 24, 32 or 34, a polynucleotide
which is an allelic variant of any polynucleotide recited above; a
polynucleotide
which encodes a species homolog of any of the proteins recited above; or a
polynucleotide that encodes a polypeptide comprising a specific domain or
truncation of the polypeptide of SEQ ID NO: 10, 13- 24, 32 or 34. Domains of
interest may depend on the nature of the encoded polypeptide; e.g., domains in
receptor-like polypeptides include ligand-binding, extracellular,
transmembrane, or'
cytoplasmic domains, or combinations thereof; domains in immunoglobulin-like
proteins include the variable immunoglobulin-like domains; domains in enzyme-
Like
polypeptides include catalytic and substrate binding domains; and domains in
ligand
polypeptides include receptor-binding domains.
56
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Polynucleotides encoding preferred polypeptide truncations of the invention
can be used to generate polynucleotides encoding chimeric or fusion proteins
comprising one or more domains of the invention and heterologous protein
sequences.
The polynucleotides of the invention additionally include the complement of
any of the polynucleotides recited above.
The polynucleotides of the invention also provide polynucleotides including
nucleotide sequences that are substantially equivalent to the polynucleotides
recited
above. Polynucleotides according to the invention can have, e. g. , at least
about
65 % , at least about 70 % , at least about 75 % , at least about 80 % , 81 %
, 82 % , 83 % ,
84 % , more typically at least about 85 % , 86 % , 87 % , 88 % , 89 % , more
typically at
least about 90 % , 91 % , 92 % , 93 % , 94 % , , and even more typically at
least about
95 % , 96 % , 97 % , 98 % , 99 % sequence identity to a polynucleotide recited
above.
The invention also provides the complement of such polynucleotides. The
polynucleotide can be DNA (genomic, cDNA, amplified, or synthetic) or RNA.
Methods and algorithms for obtaining such polynucleotides are well known to
those
of skill in the art and can include, for example, methods for determining
hybridization conditions which can routinely isolate polynucleotides of the
desired
sequence identities.
A polynucleotide according to the invention can be joined to any of a variety
of other nucleotide sequences by well-established recombinant DNA techniques
(see
Sambrook J et al. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring
Harbor Laboratory, NY). Useful nucleotide sequences for joining to
polynucleotides include an assortment of vectors, e.g., plasmids, cosmids,
lambda
phage derivatives, phagemids, and the like, that are well known in the art.
Accordingly, the invention also provides a vector including a polynucleotide
of the
invention and a host cell containing the polynucleotide. In general, the
vector
contains an origin of replication functional in at least one organism,
convenient
restriction endonuclease sites, and a selectable marker for the host cell.
Vectors
according to the invention include expression vectors, replication vectors,
probe
generation vectors, and sequencing vectors. A host cell according to the
invention
57
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
can be a prokaryotic or eukaryotic cell and can be a unicellular organism or
part of
a multicellular organism.
The sequences falling within the scope of the present invention are not
limited to the specific sequences herein described, but also include allelic
variations
thereof. Allelic variations can be routinely determined by comparing the
nucleotide
sequences pxovided in the SEQ ID NO: 1-9, 11, 12, 31 or 33, a representative
fragment thereof, or a nucleotide sequence at least 99.9 % identical to any of
the
nucleotide sequences of the SEQ ID NO: 1-9, 11, 12, 31 or 33 with a sequence
from another isolate of the same species. To accommodate codon variability,
the
invention includes nucleic acid molecules coding for the same amino acid
sequences
as do the specific ORFs disclosed herein. In other words, in the coding region
of an
ORF, substitution of one codon for another which encodes the same amino acid
is
expressly contemplated. Any specific sequence disclosed herein can be readily
screened for errors by resequencing a particular fragment, such as an ORF, in
both
directions (i.e., sequence both strands).
The present invention further provides recombinant constructs comprising a
nucleic acid having any of the nucleotide sequences of the SEQ ID NO: 1-~9,
11, l2,
31 or 33 or a fragment thereof or any other polynucleotides of the invention.
In one
embodiment, the recombinant constructs of the present invention comprise a
vector,
such as a plasmid or viral vector, into which a nucleic acid having any of the
nucleotide sequences of the SEQ ID NO: 1-9, 11, 12, 31 or 33 or a fragment
thereof is inserted, in a forward or reverse orientation. In the case of a
vector
comprising one of the ORFs of the present invention, the vector may further
comprise regulatory sequences, including for example, a promoter, operably
linked
to the ORF. For vectors comprising the EMFs and UMFs of the present invention,
the vector may further comprise a marker sequence or heterologous ORF operably
linked to the EMF or UMF. Large numbers of suitable vectors and promoters are
known to those of skill in the art and are commercially available for
generating the
recombinant constructs of the present invention. The following vectors are
provided
by way of example. Bacterial: pBs, phagescript, PsiX174, pBluescript SK, pBs
KS, pNHBa, pNHl6a, pNHl8a, pNH46a (Stratagene); pTrc99A, pKK223-3,
58
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
pKK233-3, pDR540, pRITS (Pharmacia). Eukaryotic: pWLneo, pSV2cat, pOG44,
PXTI, pSG (Stratagene) pSVK3, pBPV, pMSG, pSVL (Pharmacia).
The isolated polynucleotide of the invention may be operably linked to an
expression control sequence such as the pMT2 or pED expression vectors
disclosed
in Kaufinan et al., Nucleic Acids Res. 19, 4485-4490 (1991), in order to
produce
the protein recombinantly. Many suitable expression control sequences are
known
in the art. General methods of expressing recombinant proteins are also known
and
are exemplified in R. Kaufinan, Methods in Enzymology 185,'537-566 (1990). As
defined herein "operably linked" means that the isolated polynucleotide of
the'
invention and an expression control sequence are situated within a vector or
cell in
such a way that the protein is expressed by a host cell which has been
transformed
(transfected) with the ligated polynucleotidelexpression control sequence.
Promoter regions can be selected from any desired gene using CAT
(chloramphenicol transferase) vectors or other vectors with selectable
markers.
Two appropriate vectors are pKK232-8 and pCM7. Particular named bacterial
promoters include lacI, lacZ, T3, T7, gpt, lambda PR, and trc. li;ukaryotic
promoters include CMV immediate early, HSV thymidine kinase, early and late
SV40, LTRs from retrovirus, and mouse metallothionein-I. Selection of the
appropriate vector and promoter is well within the level of ordinary skill in
the art.
Generally, recombinant expression vectors will include origins of replication
and
selectable markers permitting transformation of the host cell, e.g., the
ampicillin
resistance gene of E. coli and S. cerevisiae TRP1 gene, and a promoter derived
from a highly-expressed gene to direct transcription of a downstream
structural
sequence. Such promoters can be derived from operons encoding glycolytic
enzymes such as 3-phosphoglycerate kinase (PGK), a-factor, acid phosphatase,
or
heat shock proteins, among others. The heterologous structural sequence is
assembled in appropriate phase with translation initiation and termination
sequences,
and preferably, a leader sequence capable of directing secretion of translated
protein
into the periplasmic space or extracellular medium. Optionally, the
heterologous
sequence can encode a fusion protein including an amino terminal
identification
peptide imparting desired characteristics, e.g., stabilization or simplified
purification
of expressed recombinant product. Useful expression vectors for bacterial use
are
59
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
constructed by inserting a structural DNA sequence encoding a desired protein
together with suitable translation initiation and termination signals in
operable
reading phase with a functional promoter. The vector will comprise one or more
phenotypic selectable markers and an origin of replication to ensure
maintenance of
S the vector and to, if desirable, provide amplification within the host.
Suitable
prokaryotic hosts for transformation include E. coli, Bacillus subtilis,
Salmonella
typhimurium and various species within the.genera Pseudomonas, Streptomyces,
and
Staphylococcus, although others may also be employed as a matter of choice.
As a representative but non-limiting example, useful expression vectors for
bacterial use can comprise a selectable marker and bacterial origin of
replication
derived from commercially available plasmids comprising genetic elements of
the
well known cloning vector pBR322 (ATCC 37017). Such commercial vectors
include, for example, pKK223-3 (Pharmacia Fine Chemicals, Uppsala, Sweden) and
GEM 1 (Promega Biotech, Madison, WI, USA). These pBR322 "backbone"
1 S sections are combined with an appropriate promoter and the structural
sequence to
be expressed. Following transformation of a suitable host strain and growth of
the
host strain to an appropriate cell density, the selected promoter is induced
or
derepressed by appropriate means (e.g., temperature shift or chemical
induction)
and cells are cultured for an additional period. Cells axe typically harvested
by
centrifugation, disrupted by physical or chemical means, and the resulting
crude
extract retained for further purification.
Included within the scope of the nucleic acid sequences of the invention are
nucleic acid sequence fragments that hybridize under stringent conditions to
any of
the nucleotide sequences of the SEQ ID NO: 1-9, 11, 12, 31 or 33 or
complements
2S thereof, which fragment is greater than about 10 bp, preferably 20 to SO
bp, and
even greater than 100 bp, greater than 300 bp, or greater than S00 bp.
Fragments
of, e.g. 1S, 16, or 20 by or more that are selective for (i.e. specifically
hybridize to
any one of the polynucleotides of the invention) are contemplated. Probes
capable
of specifically hybridizing to a polynucleotide can differentiate
polynucleotide
sequences of the invention from other polynucleotide sequences in the same
family
of genes or can differentiate human genes from genes of other species, and are
preferably based on unique nucleotide sequences.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
In accordance with the invention, polynucleotide sequences comprising the
mature protein coding sequences corresponding to the SEQ ID NO: 10, 13- 24, 32
or 34 or functional equivalents thereof, may be used to generate recombinant
DNA
molecules that direct the expression of that nucleic acid, or a functional
equivalent
thereof, in appropriate host cells. Also included are the cDNA inserts of any
of the
clones identified herein.
The nucleic acid sequences of the invention are further directed to sequences
which encode variants of the described nucleic acids. These amino acid
sequence
variants may be prepared by methods known in the art by introducing
appropriate
nucleotide changes into a native or variant polynucleotide. There are two
variables
in the construction of amino acid sequence variants: the location of the
mutation
and the nature of the mutation. Nucleic acids encoding the amino acid sequence
variants are preferably constructed by mutating the polynucleotide to encode
an
amino acid sequence that does not occur in nature. These nucleic acid
alterations
can be made at sites that differ in the nucleic acids from different species
(variable
positions) or in highly conserved regions (constant regions). Sites at such
locations
will typically be modified in series, e.g., by substituting first with
conservative
choices (e.g., hydrophobic amino acid to a different hydrophobic amino acid)
and
then with more distant choices (e. g. , hydrophobic amino acid to a charged
amino
acid), and then deletions or insertions may be made at the target site. Amino
acid
sequence deletions generally range from about 1 to 30 residues, preferably
about 1
to 10 residues, and are typically contiguous. Amino acid insertions include
amino-
and/or carboxyl-terminal fusions ranging in length from one to one hundred or
more
residues, as well as inxrasequence insertions of single or multiple amino acid
residues. Intrasequence insertions may range generally from about 1 to 10
amino
residues, preferably from 1 to 5 residues. Examples of terminal insertions
include
the heterologous signal sequences necessary for secretion or for intracellular
targeting in different host cells and sequences such as FLAG or poly-histidine
sequences useful for purifying the expressed protein.
In a preferred method, polynucleotides encoding the novel amino acid
sequences are changed via site-directed mutagenesis. This method uses
oligonucleotide sequences to alter a polynucleotide to encode the desired
amino acid
61
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
variant, as well as a sufficient adjacent nucleotides on both sides of the
changed
amino acid to form a stable duplex on either side of the site of being
changed. . In
general, the techniques of site-directed mutagenesis are well known to those
of skill
in the art and this technique is exemplified by publications such as, Edelman
et al.,
DNA 2:183 (I983). A versatile and efficient method for producing site-specific
changes in a polynucleotide sequence was published by Zoller and Smith,
Nucleic
Acids Res. 10:6487-6500 (1982). PCR may also be used to create amino acid
sequence variants of the novel nucleic acids. When small amounts of template
DNA
are used as starting material, primers) that differs slightly in sequence from
the
corresponding region in the template DNA can generate the desired amino acid
variant. PCR amplification results in a population of product DNA fragments
that
differ from the polynucleotide template encoding the polypeptide at the
position
specified by the primer. The product DNA fragments replace the corresponding
region in the plasmid and this gives a polynucleotide encoding the desired
amino
' acid variant.
A further technique for generating amino acid variants is the cassette
mutagenesis technique described in Wells et aL, Gene ~4:3I5 (1985); and other
mutagenesis techniques well known in the art, such as, for example, the
techniques
in Sambrook et al. , supra, and Current Protocols in Molecular Biology,
Ausubel et
al. Due to the inherent degeneracy of the genetic code, other DNA sequences
which
encode substantially the same or a functionally equivalent amino acid sequence
may
be used in the practice of the invention for the cloning and expression of
these novel
nucleic acids. Such DNA sequences include those which are capable of
hybridizing
to the appropriate novel nucleic acid sequence under stringent conditions.
Polynucleotides of the invention can also be used to induce immune
responses. For example, as described in Fan et al., Nat. Biotech. 17:870-872
(1999), incorporated herein by reference, nucleic acid sequences encoding a
polypeptide may be used to generate antibodies against the encoded polypeptide
following topical administration of naked plasmid DNA or following injection,
and
preferably intramuscular injection of the DNA. The nucleic acid sequences are
preferably inserted in a recombinant expression vector and may be in the form
of
naked DNA.
62
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.3.1 ANTISENSE NUCLEIC ACIDS
Another aspect of the invention pertains to isolated antisense nucleic acid
molecules that can hybridize to, or are complementary to, the nucleic acid
molecule
comprising the stem cell growth factor-like nucleotide sequence, or fragments,
analogs or derivatives thereof. An "antisense" nucleic acid comprises a
nucleotide
sequence that is complementary to a "sense" nucleic acid encoding a protein
(e.g.,
complementary to the coding strand of a double-stranded cDNA molecule or
complementary to an mRNA sequence). In specific aspects, antisense nucleic
acid
molecules are provided that comprise a sequence complementary to at least
about
10, 25, 50, 100, 250 or 500 nucleotides or an entire stem cell growth factor-
like
coding strand, or to only a portion thereof. Nucleic acid molecules encoding
fragments, homologs, derivatives, and analogs of a stem cell growth factor-
like or
antisense nucleic acids complementary to a stem cell growth factor-like
nucleic acid
sequence of are additionally provided.
In one embodiment, an antisense nucleic acid molecule is antisense to a
"coding region" of the coding strand of a nucleotide sequence encoding a stem
cell
growth factor-like protein. The term "coding region" refexs to the region of
the
nucleotide sequence comprising codons which are translated into amino acid
residues. In another embodiment, the antisense nucleic acid molecule is
antisense to
a "conceding region" of the coding strand of a nucleotide sequence encoding
the
stem cell growth factor-like protein. The term "conceding region" refers to 5'
and
3' sequences which flank the coding region that are not translated into amino
acids
(i.e., also referred to as 5' and 3' untranslated regions).
Given the coding strand sequences encoding the stem cell growth factor-like
protein disclosed herein, antisense nucleic acids of the invention can be
designed
according to. the rules of Watson and Crick or Hoogsteen base pairing. The
antisense nucleic acid molecule can be complementary to the entire coding
region of
stem cell growth factor-like mRNA, but more preferably is an oligonucleotide
that is
antisense to only a portion of the coding or noncoding region of stem cell
growth
factor-like mRNA. For example, the antisense oligonucleotide can be
63
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
complementary to the region surrounding the translation start site of stem
cell
growth factor-like mRNA. An antisense oligonucleotide can be, fox example,
about
5, 10, 15, 20, 25, 30, 35, 40, 45, or 50 nucleotides in length. An antisense
nucleic
acid of the invention can be constructed using chemical synthesis or enzymatic
ligation reactions using procedures known in the art. For example, an
antisense
nucleic acid (e.g., an antisense oligonucleotide) can be chemically
synthesized using
naturally occurring nucleotides or variously modified nucleotides designed to
increase the biological stability of the molecules or to increase the physical
stability
of the duplex formed between the antisense and sense nucleic acids (e.g.,
phosphorothioate derivatives and acridine substituted nucleotides can be
used).
Examples of modified nucleotides that can be used to generate the antisense
nucleic acid include: 5-fluorouracil, 5-bromouracil, 5-chlorouracil, 5-
iodouracil,
hypoxanthine, xanthine, 4-acetylcytosine, 5-(carboxyhydroxylmethyl) uracil, 5-
carboxymethylaminomethyl-2-thiouridine, 5-carboxymethylaminomethyluracil,
dihydrouracil, beta-D-galactosylqueosine, inosine, N6-isopentenyladenine, 1-
methylguanine, 1-methylinosine, 2,2-dimethylguanine, 2-methyladenine, 2-
methylguanine, 3-methylcytosine, 5-methylcytosine, N6-adenine, 7-
methylguanine,
5-methylaminomethyluracil, 5-methoxyaminomethyl-2-thiouracil, beta-D-
mannosylqueosine, 5'-methoxycarboxymethyluracil, 5-methoxyuracil, 2-methylthio-
' N6-isopentenyladenine, uracil-5-oxyacetic acid (v), wybutoxosine,
pseudouracil,
queosine, 2-thiocytosine, 5-methyl-2-thiouracil, 2-thiouracil, 4-thiouracil, 5-
methyluracil, uracil-5-oxyacetic acid methylester, uracil-5-oxyacetic acid
(v), 5-
methyl-2-thiouracil, 3-(3-amino-3-N-2-carboxypropyl) uracil, (acp3)w, and 2,6-
diaminopurine. Alternatively, the antisense nucleic acid can be produced
biologically using an expression vector into which a nucleic acid has been
subcloned
in an antisense orientation (i.e., RNA transcribed from the inserted nucleic
acid will
be of an antisense orientation to a target nucleic acid of interest, described
further in
the following section).
The antisense nucleic acid molecules of the invention are typically
administered to a subject or generated in situ such that they hybridize with
or bind
to cellular mRNA and/or genomic DNA encoding a stem cell growth factor-like
protein thereby inhibit expression of the protein (e.g., by inhibiting
transcription and
64
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
translation). The hybridization can be by conventional nucleotide
complementarity
to form a stable duplex, or, for example, in the case of an antisense nucleic
acid
molecule that binds to DNA duplexes, through specific interactions in the
major
groove of the double helix. An example of a route of administration of
antisense
nucleic acid molecules of the invention includes direct injection at a tissue
site.
Alternatively, antisense nucleic acid molecules can be modified to target
selected
cells and then administered systemically. For example, for systemic
administration,
antisense molecules can be modified such that they specifically bind to
receptors or
antigens expressed on a selected cell surface (e.g., by linking the antisense
nucleic
acid molecules to peptides or antibodies that bind to cell surface receptors
or
antigens). The antisense nucleic acid molecules can also be delivered to cells
using
the vectors described herein. To achieve sufficient nucleic acid molecules,
vector
constructs in which the antisense nucleic acid molecule is placed under the
control
of a strong pol II or pol III promoter are preferred.
In yet another embodiment, the antisense nucleic acid molecule of the
invention is an alpha-anomeric nucleic acid molecule. An alpha-anomeric
nucleic
acid molecule forms specific double-stranded hybrids with complementary RNA in
which, contrary to the usual alpha-units, the strands run parallel to each
other. See,
e.g., Gaultier, et al., 1987. Nucl. Acids Res. 15, 6625-6641. 'The antisense
nucleic
acid molecule can also comprise a 2'-o-methylribonucleotide (see, e.g., moue,
et al.
1987. Nucl. Acids Res. 15, 6131-6148) or a chimeric RNA-DNA analogue (see,
e.g., Inoue, et al., 1987. FEBS Lett. 215, 327-330).
5.3.2 RIBOZYMES AND PNA MOIETIES
Nucleic acid modifications include, by way of non-limiting example,
modified bases, and nucleic acids whose sugar phosphate backbones are modified
or
derivatized. These modifications are carried out at least in part to enhance
the
chemical stability of the modified nucleic acid, such that they can be used,
for
example, as antisense binding nucleic acids in therapeutic applications in a
subject.
In one embodiment, an antisense nucleic acid of the invention is a ribozyme.
Ribozymes are catalytic RNA molecules with ribonuclease activity that are
capable
of cleaving a single-stranded nucleic acid, such as an mRNA, to which they
have a
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
complementary region. Thus, xibozymes (e.g., hammerhead ribozymes as
described in Haselhoff and Gerlach 1988. Nature 334, 585-591) can be used to
catalytically cleave stem cell growth factor-like mRN.A transcripts to thereby
inhibit
translation of stem cell growth factor-like mRNA. A ribozyme having
specificity
for a stem cell growth factor-like-encoding nucleic acid can be designed based
upon
the nucleotide sequence of a stem cell growth factor-like cDNA disclosed
herein.
For example, a derivative of a Tetrahymena L-19 IVS RNA can be constructed in
which the nucleotide sequence of the active site is complementary to the
nucleotide
sequence to be cleaved in a stem cell growth factor-like-encoding mRNA. See,
e.g., U.S. Patent 4,987,071 to Cech, et al. and U.S. Patent 5,116,742 to Cech,
et
al. Stem cell growth factor-like mRNA can also be used to select a catalytic
RNA
having a specific ribonuclease activity from a pool of RNA molecules. See,
e.g.,
Bartel et al., (1993) Science 261, 1411-1418.
Alternatively, stem cell growth factor-like gene expression can be inhibited
by targeting nucleotide sequences complementary to the regulatory region of
the
stem cell growth factor-like nucleic acid (e.g., the stem cell growth factor-
like
promoter and/or enhancers) to form triple~helical structures that prevent
transcription of the stem cell growth factor-like gene in target cells. See,
e.g.,
Helene, 1991. Anticancer Drug Des. 6, 569-84; Helene, et al. 1992. Ann. N.Y.
Acad. Sci. 660, 27-36; Maher, 1992. Bioassays 14, 807-15.
In various embodiments, the stem cell growth factor-like nucleic acids can be
modified at the base moiety, sugar moiety or phosphate backbone to improve,
e.g.,
the stability, hybridization, or solubility of the molecule. For example, the
deoxyribose phosphate backbone of the nucleic acids can be modified to
generate
peptide nucleic acids. See, e.g., Hyrup, et al., 1996. Bioorg Med Chem 4, 5-
23.
As used herein, the terms "peptide nucleic acids" or "PNAs" refer to nucleic
acid
mimics (e.g., DNA mimics) in which the deoxyribose phosphate backbone is
replaced by a pseudopeptide backbone and only the four natural nucleobases are
retained. The neutral backbone of PNAs has been shown to allow for specific
hybridization to DNA and RNA under conditions of low ionic strength. The
synthesis of PNA oligomers can be performed using standard solid phase peptide
66
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
synthesis protocols as described in Hyrup, et al., 1996. supra; Perry-O'Keefe,
et
al., 1996. Proc. Natl. Acad. Sci. USA 93, 14670-14675.
PN.As of stem cell growth factor-like can be used in therapeutic and
diagnostic applications. For example, PNAs can be used as antisense or
antigene
S agents for sequence-specific modulation of gene expression by, e. g. ,
inducing
transcription or translation arrest or inhibiting replication. PNAs of stem
cell
growth factor-like can also be used, for example, in the analysis of single
base pair
mutations in a gene (e.g., PNA directed PCR clamping; as artificial
restriction
enzymes when used in combination with other enzymes, e.g., S1 nucleases (see,
Hyrup, et al. , 1996. supra); or as probes or primers for DNA sequence and
hybridization (see, Hyrup, et al. , 1996, supra; Perry-O' Keefe, et al. ,
1996. supra) .
In another embodiment, PNAs of stem cell growth factor-like can be
modified, e.g., to enhance their stability or cellular uptake, by attaching
lipophilic
or other helper groups to PNA, by the formation of PNA-DNA chimeras, or by the
use of liposomes or other techniques of drug delivery known in the art. For
example, PNA-DNA chimeras of stem cell growth factor-like can be generated
that
may combine the advantageous properties of PNA and DNA_ Such chimeras allow
DNA recognition enzymes (e.g., RNase H and DNA polymerases) to interact with
the DNA portion while the PNA portion would provide high binding affinity and
specificity. PNA-DNA chimeras can be linked using linkers of appropriate
lengths
selected in terms of base stacking, number of bonds between the nucleobases,
and
orientation (see, Hyrup, et al., 1996. supra). The synthesis of PNA-DNA
chimeras
can be performed as described in Hyrup, et al. , 1996. supra and Finn, et al.
, 1996.
Nucl Acids Res 24, 3357-3363. For example, a DNA chain can be synthesized on a
solid support using standard phosphoramidite coupling chemistry, and modified
nucleoside analogs; e.g., 5'-(4-methoxytrityl)amino-5'-deoxy-thymidine
phosphoramidite, can be used between the PNA and the 5' end of DNA. See, e.g.,
Mag, et al. , 1989. Nucl Acid Res 17, 5973-5988. PNA monomers are then coupled
in a stepwise manner to produce a chimeric molecule with a 5' PNA segment and
a
3' DNA segment. See, e.g., Finn, et al., 1996. supra. Alternatively, chimeric
molecules can be synthesized with a 5' DNA segment and a 3' PNA segment. See,
e.g., Petersen, et al., 1975. Bioorg. Med. Chem. Lett. 5, 1119-11124.
67
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
In other embodiments, the oligonucleotide may include other appended
groups such as peptides (e.g., for targeting host cell receptors in vivo), or
agents
facilitating transport across the cell membrane (see, e.g., Letsinger, et al.,
1989.
Proc. Natl. Acad. Sci. U.S.A. 86, 6553-6556; Lemaitre, et al., 1987. Proc.
Natl.
Acad. Sci. 84, 648-652; PCT Publication No. W088/09810) or the blood-brain
barrier (see, e.g., PCT Publication No. W089/10134). In addition,
oligonucleotides can be modified with hybridization-triggered cleavage agents
(see,
e.g., Krol, et al., 1988. BioTechniques 6, 958-976) or intercalating agents
(see,
e.g., Zon, 1988. Pharm. Res. 5, 539-549). To this end, the oligonucleotide can
be
conjugated to another molecule, e.g., a peptide, a hybridization triggered
cross-
linking agent, a transport agent, a hybridization-triggered cleavage agent,
and the
like.
5.4 HOSTS
The present invention further provides host cells genetically engineered to
contain the polynucleotides of the invention. For example, such host cells may
contain nucleic acids of the invention introduced into the host cell using
known
transformation, transfection or infection methods. The present invention still
further
provides host cells genetically engineered to express the polynucleotides of
the
invention, wherein such polynucleotides are in operative association with a
regulatory sequence heterologous to the host cell which drives expression of
the
polynucleotides in the cell.
Knowledge of stem cell growth factor-like DNA sequences allows for
modification of cells to permit, or increase, expression of stem cell growth
factor-
like polypeptide. Cells can be modified (e.g., by homologous recombination) to
provide increased stem cell growth factor-like polypeptide expression by
replacing.,
in whole or in part, the naturally occurring stem cell growth factor-like
promoter
with all or part of a heterologous promoter so that the cells stem cell growth
factor-
like polypeptide is expressed at higher levels. The heterologous promoter is
inserted in such a manner that it is operatively linked to stem cell growth
factor-like
encoding sequences. See, for example, PCT International Publication No.
W094/12650, PCT International Publication No. W092/20808, and PCT
68
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
International Publication No. W091/09955. It is also contemplated that, in
addition
to heterologous promoter DNA, amplifiable marker DNA (e.g., ada, dhfr, and the
multifunctional CAD gene which encodes carbamyl phosphate synthase, aspartate
transcarbamylase, and dihydroorotase) and/or intron DNA may be inserted along
with the heterologous promoter DNA. If linked to the stem cell growth factor-
like
coding sequence, amplification of the marker DNA by standard' selection
methods
results in co-amplification of the stem cell growth factor-like coding
sequences in
the cells.
The host cell can, be a higher eukaryotic host cell, such as a mammalian cell,
a lower eukaxyotic host cell, such as a yeast cell, or the host cell can be a
prokaryotic cell, such as a bacterial cell. Introduction of the recombinant
construct
into the host cell can be effected by calcium phosphate transfection, DEAE,
dextran
mediated transfection, or electroporation (Davis, L. et al., Basic Methods in
Molecular Biology (1986)). The host cells containing one of polynucleotides of
the
invention, can be used in conventional manners to produce the gene product
encoded by the isolated fragment (in the case of an ORF) or can be used to
produce
a heterologous protein under the control of the EMF.
Any host/vector system can be used to express one or more of the Ol~Fs of
the present invention. These include, but are not limited to, eukaryotic hosts
such
as HeLa cells, Cv-1 cell, COS cells, and Sf9 cells, as well as prokaryotic
host such
as E. coli and B. subtilis. The most preferred cells are those which do not
normally
express the particular polypeptide or protein or which expresses the
polypeptide or
protein at low natural level. Mature proteins can be expressed in mammalian
cells,
yeast, bacteria, or other cells under the control of appropriate promoters.
Cell-free
translation systems can also be employed to produce such proteins using RNAs
derived from the DNA constructs of the present invention. Appropriate cloning
and
expression vectors for use with prokaryotic and eukaryotic hosts are described
by
Sambrook, et al., in Molecular Cloning: A Laboratory Manual, Second Edition,
Cold Spring Harbor, New York (1989), the disclosure of which is hereby
incorporated by reference.
Various mammalian cell culture systems can also be employed to express
recombinant protein. Examples of mammalian expression systems include the COS-
69
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
7 lines of monkey kidney fibroblasts, described by Gluzman, Cell 23:175
(1981),
and other cell lines capable of expressing a compatible vector, for example,
the
C127, 3T3, CHO, HeLa and BHK cell tines. Mammalian expression vectors will
comprise an origin of replication, a suitable promoter, and also any necessary
ribosome binding sites, polyadenylation site, splice donor and acceptor sites,
transcriptional termination sequences, and 5' flanking nontranscribed
sequences.
DNA sequences derived from the SV40 viral genome, for example, SV40 origin,
early promoter, enhancer, splice, and polyadenylation sites may be used to
provide
the required nontranscribed genetic elements. Recombinant polypeptides and
proteins produced in bacterial culture are usually isolated by initial
extraction from
cell pellets, followed by one or more salting-out, aqueous ion exchange or
size
exclusion chromatography steps. Protein refolding steps can be used, as
necessary,
in completing configuration of the mature protein. Finally, high performance
liquid
chromatography (HPLC) can be employed for final purification steps. Microbial
cells employed in expression of proteins can be disrupted by any convenient
method, including freeze-thaw cycling, sonication, mechanical disruption, or
use of
cell Iysing agents.
. A number of types of cells may act as suitable host cells for expression of
the protein. Mammalian host cells include, for example, monkey COS cells,
Chinese Hamster Ovary (CHO) cells, human kidney 293 cells, human epidermal
A431 cells, human Co1o205 cells, 3T3 cells, CV-1 cells, other transformed
primate
cell lines, normal diploid cells, cell strains derived from in vitro culture
of primary
tissue, primary~explants, HeLa cells, mouse L cells, BHK, HL-60, U937, HaK or
Jurkat cells.
Alternatively. it may be possible to produce the protein in Iower eukaryotes
such as yeast or in prokaryotes such as bacteria. Potentially suitable yeast
strains
include Saccharomyces cerevisiae, Schizosaccharomyces pombe, Kluyveromyces
strains, Candida, or any yeast strain capable of expressing heterologous
proteins.
Potentially suitable bacterial strains include Escherichia coli, Bacillus
subtilis,
Salmonella typhimurium, or any bacterial strain capable of expressing
heterologous
proteins. If the protein is made in yeast or bacteria, it may be necessary to
modify
the protein produced therein, for example by phosphorylation or glycosylation
of the
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
appropriate sites, in order to obtain the functional protein. Such covalent
attachments may be accomplished using known chemical or enzymatic methods.
In another embodiment of the present invention, cells and tissues may be
engineered to express an endogenous gene comprising the polynucleotides of the
invention under the control of inducible regulatory elements, in which case
the
regulatory sequences of the endogenous gene may be replaced by homologous
recombination. As described herein, gene targeting can be used to replace a
gene's
existing regulatory region with a regulatory sequence isolated from a
different gene
or a novel regulatory sequence synthesized by genetic engineering methods.
Such
regulatory sequences may be comprised of promoters, enhancers, scaffold-
attachment regions, negative regulatory elements, transcriptional initiation
sites, and
regulatory protein binding sites or combinations of said sequences.
Alternatively,
sequences which affect the structure or stability of the RNA or protein
produced
may be replaced, removed, added, or otherwise modified by targeting, including
polyadenylation signals, mRNA stability elements, splice sites, leader
sequences for
enhancing or modifying transport or secretion properties of the protein, or
other
sequences which alter or improve the function or stability of protein ox RNA
molecules .
The targeting event may be a simple insertion of the regulatory sequence,
placing the gene under the control of the new regulatory sequence, e.g.,
inserting a
new promoter or enhancer or both upstream of a gene. Alternatively, the
targeting
event may be a simple deletion of a regulatory element, such as the deletion
of a
tissue-specific negative regulatory element. Alternatively, the targeting
event may
replace an existing element; for example, a tissue-specific enhancer can be
replaced
by an enhancer that has broader or different cell-type specificity than the
naturally
occurring elements. Here, the naturally occurring sequences are deleted and
new
sequences are added. In all cases, the identification of the targeting event
may be
facilitated by the use of one or more selectable marker genes that are
contiguous
with the targeting DNA, allowing for the selection of cells in 'which the
exogenous
DNA has integrated into the host cell genome. The identification of the
targeting
event may also be facilitated by the use of one or more marker genes
exhibiting the
property of negative selection, such that the negatively selectable marker is
linked to
71
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
the exogenous DNA, but configured such that the negatively selectable marker
flanks the targeting sequence, and such that a correct homologous
recombination
event with sequences in the host cell genome does not result in the stable
integration
of the negatively selectable marker. Markers useful for this purpose include
the
Herpes Simplex Virus thymidine kinase (TK) gene or the bacterial xanthine-
guanine
phosphoribosyl-transferase (gpt) gene.
The gene targeting or gene activation techniques which can be used in
accordance with this aspect of the invention are more particularly described
in U.S.
Patent No. 5,272,071 to Chappel; U.S. Patent No. 5,578,461 to Sherwin et al.;
International Application No. PCT/US92/09627 (WO93109222) by Selden et al.;
and International Application No. PCTlUS90/06436 (W09I/06667) by Skoultchi et
al., each of which is incorporated by reference herein in its entirety.
5.5 POLYPEPTIDES OF THE INVENTION
The isolated polypeptides of the invention include, but are not limited to, a
polypeptide comprising: the amino acid sequence set forth as SEQ ID NO: 10, 13-
24 , 32 or 34 or an amino acid sequence encoded by any one of the nucleotide
sequences SEQ ID NO: 1-9, 11, 12, 31 or 33 or the corresponding full length or
mature protein. Polypeptides of the invention also include polypeptides
preferably
with biological or immunological activity that are encoded by: (a) a
polynucleotide
having any one of the nucleotide sequences set forth in the SEQ ID NO: 1-9,
11,
12, 31 or 33 or (b) polynucleotides encoding the amino acid sequence set forth
as
SEQ ID NO: 10, 13- 24, 32 or 34 or (c) polynucleotides that hybridize to the
complement of the polynucleotides of either (a) or (b) under stringent
hybridization
conditions. The invention also provides biologically active or immunologically
active variants of any of the polypeptide amino acid sequences set forth as
SEQ ID
NO: 10, 13- 24, 32 or 32 or the corresponding full length or mature protein;
and
"substantial equivalents" thereof (e. g. , with at least about 65 % , at least
about 70 % ,
at least about 75 % , at least about 80 % , at. least about 85 % , 86 % , 87 %
, 88 % , 89 % ,
at least about 90 % , 91 % , 92 % , 93 % , 94 % , typically at least about 95
% , 96 % ,
97 % , more typically at least about 98 % , or most typically at least about
99 % amino
72
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
acid identity) that retain biological activity. Polypeptides encoded by
allelic variants
may have a similar, increased, or decreased activity compared to polypeptides
comprising SEQ ID NO: 10, 13- 24, 32 or 34.
Protein compositions of the present invention may further comprise an
acceptable carrier, such as a hydrophilic, e.g., pharmaceutically acceptable,
carrier.
The invention also relates to methods for producing a polypeptide comprising
growing a culture of host cells of the invention in a suitable culture medium,
and
purifying the protein from the cells or the culture in which the cells are
grown. For
example, the methods of the invention include a process for producing a
polypeptide
in which a host cell containing a suitable expression vector that includes a
polynucleotide of the invention is cultured under conditions that allow
expression of
the encoded polypeptide. The polypeptide can be recovered from the culture,
conveniently from the culture medium, or from a lysate prepared from the host
cells
and further purified. Preferred embodiments include those in which the protein
produced by such process is a full length or mature form of the protein.
The present invention further provides isolated polypeptides encoded by the
nucleic acid fragments of the present invention or by degenerate variants of
the
nucleic acid fragments of the present invention. By "degenerate variant" is
intended
nucleotide fragments which differ from a nucleic acid fragment of the present
invention (e.g., an ORF) by nucleotide sequence but, due to the degeneracy of
the
genetic code, encode an identical polypeptide sequence. Preferred nucleic acid
fragments of the present invention are the ORFs that encode proteins. A
variety of
methodologies known in the art can be utilized to obtain any one of the
isolated
polypeptides or proteins of the present invention. At the simplest level, the
amino
acid sequence can be synthesized using commercially available peptide
synthesizers.
This technique is particularly useful in producing small peptides and
'fragments of
' larger polypeptides. Fragments are useful, for example, in generating
antibodies
against the native polypeptide. In an alternative method, the polypeptide or
protein
is purified from bacterial cells which naturally produce the polypeptide or
protein.
One skilled in the art can readily follow known methods for isolating
polypeptides
and proteins in order to obtain one of the isolated polypeptides or proteins
of the
present invention. These include, but are not limited to,
immunochromatography,
73
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
HPLC, size-exclusion chromatography, ion-exchange chromatography, and
immuno-affinity chromatography. See, e.g., Scopes, Protein Purification:
Principles and Practice, Springer-Verlag (1994); Sambrook, et al., in
Molecular
Cloning: A Laboratory Manual; Ausubel et al. , Current Protocols in Molecular
Biology. Polypeptide fragments that retain biological/immunological activity
include
fragments encoding greater than about 100 amino acids, or greater than about
200
amino acids, and fragments that encode specific protein domains.
The polypeptides and proteins of the present invention can alternatively be
purified from cells which have been altered to express the desired polypeptide
or
protein. As used herein, a cell is said to be altered to express a desired
polypeptide
or protein when the cell, through genetic manipulation, is made to produce a
polypeptide or protein which it normally does not produce or which the cell
normally produces at a lower level. One skilled in the art can readily adapt
procedures for introducing and expressing either recombinant or synthetic
sequences
into eukaryotic or prokaryotic cells in order to generate a cell which
produces one
of the polypeptides or proteins of the present invention.
The protein of the invention may also be expressed as a product of transgenic
animals, e.g., as a component of the milk of transgenic cows, goats, pigs, or
sheep
which are characterized by somatic or germ cells containing a nucleotide
sequence
encoding the protein.
The protein may also be produced by known conventional chemical
synthesis. Methods for constructing the proteins of the present invention by
synthetic means are known to those skilled in the art. The synthetically-
constructed
protein sequences, by virtue of sharing primary, secondary or tertiary
structural
and/or conformational characteristics with proteins may possess biological
properties in common therewith, including protein activity. Thus, they may be
employed as biologically active or immunological substitutes for natural,
purified
proteins in screening of therapeutic compounds and in immunological processes
for
the development of antibodies.
The proteins provided herein also include proteins characterized by amino,
acid sequences similar to those of purified proteins but into which
modification are
naturally provided or deliberately engineered. For example, modifications in
the
74
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
peptide or DNA sequences can be made by those skilled in the art using known
techniques. Modifications of interest in the protein sequences may include the
alteration, substitution, replacement, insertion or deletion of a selected
amino acid
residue in the coding sequence. For example, one or more of the cysteine
residues
may be deleted or replaced with another amino acid to alter the conformation
of the
molecule. Techniques for such alteration, substitution, replacement, insertion
or
deletion are well known to those skilled in the art (see, e.g., U.S. Pat. No.
4,518,584). Preferably, such alteration, substitution, replacement, insertion
or
deletion retains the desired activity of the protein. Regions of the protein
that are
important for the protein function can be determined by various methods known
in
the art including the alanine-scanning method which involved systematic
substitution
of single or strings of amino acids with alanine, followed by testing the
resulting
alanine-containing variant for biological activity. This type of analysis
determines
the importance of the substituted amino acids) in biological activity.
Other fragments and derivatives of the sequences of proteins which would be
expected to retain protein activity in whole or in part and are useful for
screening or
other immunological methodologies may also be easily made by those skilled in
the
art given the disclosures herein. .Such modifications are encompassed by the
present
invention.
The protein may also be produced by operably linking the isolated
polynucleotide of the invention to suitable control sequences in one or more
insect
expression vectors, and employing an insect expression system. Materials and
methods for baculovirus/insect cell expression systems are commercially
available in
kit form from, e.g., Invitrogen, San Diego, Calif., U.S.A. (the MaxBat'~ kit),
and
such methods are well known in the art, as described in Summers and Smith,
Texas
Agricultural Experiment Station Bulletin No. 1555 (1987), incorporated herein
by
reference. As used herein, an insect cell capable of expressing a
polynucleotide of
the present invention is "transformed. "
The protein of the invention may be prepared by culturing transformed host
cells under culture conditions suitable to express the recombinant protein.
The
resulting expressed protein may then be purified from such culture (i. e. ,
from
culture medium or cell extracts) using known purification processes, such as
gel
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
filtration and ion exchange chromatography. The purification of the protein
may
also include an affinity column containing'agents which will bind to the
protein; one
or more column steps over such affinity resins as concanavalin A-agarose,
heparin-toyopearf~ or Cibacrom blue 3GA Sepharose~M; one or more steps
involving
hydrophobic interaction chromatography using such resins as phenyl ether,
butyl
ether, or propyl ether; or immunoaffinity chromatography.
Alternatively, the protein of the invention may also be expressed in a form
which will facilitate purification. For example, it may be expressed as a
fusion
protein, such as those of maltose binding protein (MBP), glutathione-S-
transferase
(GST) or thioredoxin (TRX), or as a His tag. Kits for expression and
purification
of such fusion proteins are commercially available from New England BioLab
(Beverly, Mass.), Pharmacia (Piscataway, N.J.) and Invitrogen, respectively.
The
protein can also be tagged with an epitope and subsequently purified by using
a
specific antibody directed to such epitope. One such epitope ("FLAG ") is
commercially available from Kodak (New Haven, Cone.).
. Finally, one or more reverse-phase 'nigh performance liquid chromatography
(RP- HPLC) steps employing hydrophobic RP-HPLC media, e, g. , silica gel
having
pendant methyl or other aliphatic groups, can be employed to further purify
the
protein. Some or all of the foregoing purification steps, in various
combinations,
can also be employed to provide a substantially homogeneous isolated
recombinant
protein, The protein thus purified is substantially free of other mammalian
proteins
and is defined in accordance with the present invention as an "isolated
protein. "
The polypeptides of the invention include analogs (variants). Analogs
embrace fragments, as well as antagonists which comprise ,one or more amino
acids
deleted, inserted, or substituted. Analogs of the invention also embrace
fusions of
the polypeptide of the invention or modifications of the polypeptide of the
invention
or analog is fused to another moiety or moieties, e.g., targeting moiety,
imaging
moiety or another therapeutic agent. Such analogs may exhibit improved
properties
such as activity and/or stability. Examples of moieties which may be fused to
polypeptides of the invention or analogs thereof include, for example,
targeting
moieties which provide for the delivery of polypeptide to desired cell types.
Other
76
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
moieties which may be fused to the polypeptides of the invention include
therapeutic
agents which are used for treatment of disorders described herein.
5.5.1 DETERMINING POLYPEPTIDE AND POLYNUCLEOTIDE IDENTITY
AND SIMILARITY .
Preferred identity and/or similarity are designed to give the largest match.
between the sequences tested. Methods to determine identity and similarity are
codified in computer programs including, but are not limited to, the GCCT
program
package, including GAP (Devereux, J., et al., Nuclei; Acids Research 1?(1):
387
(1984); Genetics Computer Group, University of Wisconsin, Madison, WT),
BLASTP, BLASTN, BLASTX, FASTA (Altschul, S.F. et al., J. Molec. Biol.
215:403-410 (1990), PSI-BLAST (Altschul S.F. et al., Nucleic Acids Res. vol.
25,
pp. 3389-3402, herein incorporated by reference), the eMatrix software (Wu et
al.,
J. Comp. Biol., vol. 6, pp. 219-235 (1999), herein in corporated by
reference),
eMotif software (Nevill-Manning et al, ISMB-97, vol ~., pp. 2~~2 z09, herein
il-~corporated by reference), the C~eneAtlas .software (.Molecula_r
Simulations Inc.
(MSI), San Diego, CA) (Sanchez and Sali (1998) Proc. Natl. Acad. Sci., 95,
13597-"13602; Kitson DPI et al, (2000) "R~xnote homology detection using
s~:ructural
modeling - an evaluation" Submitted; Fischer and Eisenberg (1:996) Protein
Sci. 5,
947-955), Neural Network SignalP V 1.1 program (from Center for Biological
Sequence Analysis, The Technical University of Denmark).and the Kyte-Doolittle
hydrophobocity prediction algorithm (J. Mol Biol, 157, pp. 105-31 (1982),
incorporated herein by reference). The BLAST programs are publicly available
from the National Center for Biotechnology Information (NCBI) and other
sources
(BLAST Manual, Altschul, S., et al. NCB NLM NIH Bethesda, MD 20894;
Altschul, S., et al., J. MoI. Biol. 215:403-410 (1990).
5.6 CHIMERIC AND FUSION PROTE1NS
The invention also provides stem cell growth factor-like chimeric or fusion
proteins, As used herein, a stem cell growth factor-like "chimeric protein" or
"fusion protein" comprises a stem cell growth factor-dike polypeptide
operatively
linked to either a different stem cell growth factor-like polypeptide or a non-
stem
77
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
cell growth factor-like polypeptide. An "stem cell growth factor-like
polypeptide"
refers to a polypeptide having an amino acid sequence corresponding to a stem
cell
growth factor-like protein, whereas a "non-stem cell growth factor-like
polypeptide"
refers to a polypeptide having an amino acid sequence corresponding to a
protein
that is not substantially homologous to the stem cell growth factor-like
protein, e.g.,
a protein that is different from the stem cell growth factor-like protein and
that is
derived from the same or a different organism. Within a stem cell growth
factor-
like fusion protein the stem cell growth factor-like polypeptide can
correspond to all
or a portion of a stem cell growth factor-Iike protein. In one embodiment, a
stem
cell growth factor-like fusion protein comprises at least one biologically
active
portion of a stem cell growth factor-like protein. In another embodiment, a
stem
cell growth factor-like fusion protein comprises at least two biologically
active
portions of a stem cell growth factor-like protein. In yet another embodiment,
a
stem cell growth factor-like fusion protein comprises at least three
biologically
active portions of a stem cell growth factor-like protein. Within the fusion
protein,
the term "operatively-linked'' is intended to indicate that the stem cell
growth factor-
like polypeptide(s) and/or the non-stem cell growth factor-like polypeptide
are fused
in-frame with one another. The non-stem cell growth factor-like polypeptide
can be
fused to the N-terminus or C-terminus of the stem cell growth factor-like
polypeptide.
In one embodiment, the fusion protein is a GST-stem cell growth factor-like
fusion protein in which the stem cell growth factor-like sequences are fused
to the
C-terminus of the GST (glutathione S-transferase) sequences. Such fusion
proteins
can facilitate the purification of recombinant stem cell growth factor-like
polypeptides.
In another embodiment, the fusion protein is a stem cell growth factor-like
protein containing a heterologous signal sequence at its N-terminus. In
certain host
cells (e.g., mammalian host cells), expression and/or secretion of stem cell
growth
factor-like can be increased through use of a heterologous signal sequence.
In yet another embodiment, the fusion protein is a stem cell growth factor-
like-immunoglobulin fusion protein in which the stem cell growth factor-like
sequences are fused to sequences derived from a member of the immunoglobulin
78
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
protein family. The stem cell growth factor-like-immunoglobulin fusion
proteins of
the invention can be incorporated into pharmaceutical compositions and
administered to a subject to inhibit an interaction between a stem cell growth
factor-
like ligand and a stem cell growth factor-like protein on the surface of a
cell, to
S thereby suppress stem cell growth factor-like-mediated signal transduction
in vivo.
The stem cell growth factor-like-immunoglobulin fusion proteins can be used to
affect the bioavailability of a stem cell growth factor-like cognate ligand.
Inhibition
of the stem cell growth factor-like ligandlstem cell growth factor-like
interaction can
be useful therapeutically for both the treatment of proliferative and
differentiative
disorders, as well as modulating (e.g. promoting or inhibiting) cell survival.
Moreover, the stem cell growth factor-like-immunoglobulin fusion proteins of
the
invention can be used as immunogens to produce anti-stem cell growth factor-
like
antibodies in a subject, to purify stem cell growth factor-like ligands, and
in
screening assays to identify molecules that inhibit the interaction of stem
cell growth
factor-like with a stem cell growth factor-like ligand.
Stem cell growth factor-like chimeric or fusion protein of the invention can
be produced by standard recombinant DNA techniques. For example, DNA
fragments coding for the different polypeptide sequences are ligated together
in-
frame in accordance with conventional techniques, e.g., by employing blunt-
ended
or stagger-ended termini for ligation, restriction enzyme digestion to provide
for
appropriate termini, filling-in of cohesive ends as appropriate, alkaline
phosphatase
treatment to avoid undesirable joining, and enzymatic ligation. In another
embodiment, the fusion gene can be synthesized by conventional techniques
including automated DNA synthesizers. Alternatively, PCR amplification of gene
fragments can be carried out using anchor primers that give rise to
complementary
overhangs between two consecutive gene fragments that can subsequently be
annealed and reamplified to generate a chimeric gene sequence (see, e:g.,
Ausubel,
et al. (eds.) Current Protocols in Molecular Biology, John Wiley & Sons,
1992).
Moreover, many expression vectors are commercially available that already
encode
a fusion moiety (e.g., a GST polypeptide). Stem cell growth factor-like-
encoding
nucleic acid can be cloned into such an expression vector such that the fusion
moiety
is linked in-frame to the stem cell growth factor-like protein.
79
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.7 GENE THERAPY
Mutations in the polynucleotides of the invention gene may result in loss of
normal function of the encoded protein. The invention thus provides gene
therapy to
restore normal activity of the polypeptides of the invention; or to treat
disease states
involving polypeptides of the invention. Delivery of a functional genes
encoding
polypeptides of the invention to appropriate cells is effected ex vivo, in
situ, or in
vivo by use of vectors, and more particularly viral vectors (e.g., adenovirus,
adeno-associated virus, or a retrovirus), or ex vivo by use of physical DNA
transfer
methods (e.g., liposomes or chemical treatments). See, for example, Anderson,
Nature, supplement to vol. 392, no. 6679, pp.25-20 (1998). For additional
reviews
of gene therapy technology see Friedmann, Science, 244: 1275-1281 (1989);
Verma, Scientific American: 68-.84 (1990); and Miller, Nature, 357: 455-460
(1992). Introduction of any one of the nucleotides of the present invention or
a gene
encoding the polypeptides of the present invention can also be accomplished
with
extrachromosomal substrates (transient expression) or artificial chromosomes
(stable
expression). Cells may also be cultured ex vivo in the presence of proteins of
the
present invention in order to proliferate or to produce a desired effect on or
activity
in such cells. Treated cells can then be introduced in vivo for therapeutic
purposes.
Alternatively, it is contemplated that in other human disease states,
preventing the
expression of or inhibiting the activity of polypeptides of the invention will
be useful
in treating the disease states. It is contemplated that antisense therapy or
gene
therapy could be applied to negatively regulate the expression of polypeptides
of the
invention.
Other methods inhibiting expression of a protein include the introduction of
antisense molecules to the nucleic acids of the present invention, their
complements, or
their translated RNA sequences, by methods known in the art, the removal of
the
nucleic acids of the present invention such as using targeted deletion
methods, or the
insertion of a negative regulatory element such as a silencer, which is tissue
specific.
Further, the polypeptides of the present invention can be inhibited by the
introduction
of antisense molecules that hybridize to nucleic acids that encode for the
polypeptides
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
of the present invention and by the removal of a gene that encode for the
polypeptides
of the present invention.
The present invention still further provides cells genetically engineered ira
vivo
to express the polynucleotides of the invention, wherein such polynucleotides
are in
operative association with a regulatory sequence heterologous to the host cell
which
drives expression of the polynucleotides in the cell. These methods can be
used to
increase or decrease the expression of the polynucleotides of the present
invention.
Knowledge of DNA sequences provided by the invention allows for
modification of cells to permit, increase, or decrease, expression of
endogenous
polypeptide. Cells can be modified (e.g., by homologous recombination) to
provide
increased polypeptide expression by replacing, in whole or in part, the
naturally
occurring promoter with all or part of a heterologous promoter so that the
cells express
the protein at higher levels. The heterologous promoter is inserted in such a
manner
that it is operatively linked to the desired protein encoding sequences. See,
for
example, PCT International Publication No. WO 94/12650, PCT International
Publication No. WO 92/20808, and PCT International Publication No. WO
91109955.
It is also contemplated that, in addition to heterologous promoter DNA,
amplifiable
marker DNA (e.g., ada, dhfr, and the multifunctional CAD gene which encodes
carbamyl phosphate synthase, aspartate transcarbamylase, and dihydroorotase)
and/or
intron DNA may be inserted along with the heterologous promoter DNA. If linked
to
the desired protein coding sequence, amplification of the marker DNA by
standard
selection methods results in co-amplification of the desired protein coding
sequences in
the cells.
In another embodiment of the present invention, cells and tissues may be
engineered to express an endogenous gene comprising the polynucleotides of the
invention under the control of inducible regulatory elements, in which case
the
regulatory sequences of the endogenous gene may be replaced by homologous
recombination. As described herein, gene targeting can be used to replace a
gene's
existing regulatory region with a regulatory sequence isolated from a
different gene or
a novel regulatory sequence synthesized by genetic engineering methods. Such
regulatory sequences may be comprised of promoters, enhancers, scaffold-
attachment
regions, negative regulatory elements, transcriptional initiation sites,
regulatory protein
81
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
binding sites or combinations of said sequences. Alternatively, sequences
which affect
the structure or stability of the RNA or protein produced may be replaced,
removed,
added, or otherwise modified by targeting. These sequence include
polyadenylation
signals, mRNA stability elements, splice sites, leader sequences for enhancing
or
modifying transport or secretion properties of the protein, or other sequences
which
alter or improve the function or stability of protein or RNA molecules.
The targeting event may be a simple insertion of the regulatory sequence,
placing the gene under the control of the new regulatory sequence, e.g.,
inserting a
new promoter or enhancer or both upstream of a gene. Alternatively, the
targeting
event may be a simple deletion of a regulatory element, such as the deletion
of a
tissue-specific negative regulatory element. Alternatively, the targeting
event may
replace an existing element; for example, a tissue-specific enhancer can be
replaced by
an enhancer that has broader or different cell-type specificity than the
naturally
occurring elements. Here, the naturally occurring sequences are deleted and
new
sequences are added. In all cases, the identification of the targeting event
may be
facilitated by the use of one or more selectable marker genes that are
contiguous with
the targeting DNA, allowing for the selection of cells in which the exogenous
DNA
has iiltegrated into the cell genome. The identification of the targeting
event may also
be facilitated by the use of one or more marker genes exhibiting the property
of
negative selection, such that the negatively selectable marker is linked to
the exogenous
DNA, but configured such that the negatively selectable marker flanks the
targeting
sequence, and such that a correct homologous recombination event with
sequences in
the host cell genome does not result in the stable integration of the
negatively selectable
marker. Markers useful for this purpose include the Herpes Simplex Virus
thymidine
kinase (TK) gene or the bacterial xanthine-guanine phosphoribosyl-transferase
(gpt)
gene.
The gene targeting or gene activation techniques which can be used in
accordance with this aspect of the invention are more particularly described
in U.S.
Patent No. 5,272,071 to Chappel; U.S. Patent No. 5,578,461 to Sherwin et al.;
International Application No. PCTlUS92/09627 (W093/09222) by Selden et al.;
and
International Application No. PCT/US90/06436 (W091106667) by Skoultchi et al.
,
each of which is incorporated by reference herein in its entirety.
82
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
.5.8 TRANSGENIC ANIMALS
In pr eferred methods to determine biological functions of the polypeptides of
the invention in vivo, one or more genes provided by the invention are either
over
expressed or inactivated in the germ line of animals using homologous
recombination [Capecchi, Science 244:1288-1292 (1989)]. Animals in which the
gene is over expressed, under the regulatory control of exogenous or
endogenous
promoter elements, are known as transgenic animals. Animals in which an
endogenous gene has been inactivated by homologous recombination are referred
to
as "knockout" animals. Knockout animals, preferably non-human mammals, can be
prepared as described in U.S. Patent No. 5,557,032, incorporated herein by
reference. Transgenic animals are useful to determine the roles polypeptides
of the
invention play in biological processes, and preferably in disease states.
Transgenic
animals are useful as model systems to identify compounds that modulate lipid
metabolism. 'Transgenic animals, preferably non-human mammals, are produced
using methods as described in U.S. Patent No 5,489,743 and PCT Publication No.
W094/28I22, incorporated herein by reference.
Transgenic animals can be prepared wherein all or part of a polynucleotides
of the invention promoter is either activated or inactivated to alter the
level of
expression of the polypeptides of the invention. Inactivation can be carried
out using
homologous recombination methods described above. Activation can be achieved
by
supplementing or even replacing the homologous promoter to provide for
increased
protein expression. The homologous promoter can be supplemented by insertion
of
one or more heterologous enhancer elements known to confer promoter activation
in
a particular tissue.
5.9 USES AND BIOLOGICAL ACTIVITY OF STEM CELL GROWTH
FACTOR-LIKE POLYPEPTIDE
Stem cell growth factor-like polypeptide is based on polynucleotides isolated
from cDNA libraries prepared from human testis cells (Hyseq clone
identification
numbers 2880984 and 2881695), from human fetal skin (Hyseq clone
identification
number 15375176), adult spleen (Hyseq clone identification number 14856094),
and
83
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
human endothelial cells (Hyseq clone identification numbers 13804756,
13687487,
13804756).
Figure 1 shows the alignment of polynucleotide SEQ ID NO: 9 and EST
sequences SEQ ID NO: 1-7. The nucleic acid sequences of the present invention
(SEQ ID NO: 1-9) are expected encode polypeptides having stem cell growth
factor
activity, including hematopoietic stem cell growth factor activity, as
described herein.
The polypeptide of SEQ ID NO: 10, fragments thereof, sequences having at least
90
homology, are also expected to have stem cell growth factor activity,
including
hematopoietic stem cell growth factor activity , as described herein.
The stem cell growth factor=like polypeptide of SEQ ID NO: 10 is an
approximately 272-amino acid protein with a predicted molecular mass of
approximately 30 kDa unglycosylated. Protein database searches with the BLASTX
algorithm (Altschul S.F. et al., J. Mol. Evol. 36:290-300 (1993) and Altschul
S.F.
et al., J. Mol. Biol. 21:403-10 (1990), herein incorporated by reference)
indicate
that SEQ ID NO: 10 is homologous to thrombospondin type I domain and a human
secreted protein clone da 228 6. Protein database search with eMATRIX software
(Stanford University, Stanford CA) further show that a portion of SEQ ID NO:
10
has a laminin-type EGF-like (LE) domain, a vertebrate metallothioneins domain,
an
endogenous opioids neuropeptides precursors proteins domain, a membrane attack
complex components/perforin proteins domain, an HMG-I and HMG-Y DNA-
binding domain proteins (Ahook), an HMGl/2 protein domain, a vertebrate
metallothionein signature domain, and a neurohypophysial hormone signature
domain.
A predicted approximately twenty-one residue signal peptide is encoded from
approximately residue 1 to residue 21 of SEQ ID NO: 10 (SEQ ID NO: 15). The
extracellular portion is useful on its own. This can be confirmed by
expression in
mammalian cells and sequencing of the cleaved product. The signal peptide
region
was predicted using Neural Network SignalP V 1.1 program (Nielsen et al,
(1997)
Int. J. Neural Syst. 8, 581-599). One of skill in the art will recognize that
the actual
cleavage site may be different than that predicted by the computer program.
SEQ
ID NO: 16 is the peptide resulting when the predicted signal peptide is
removed
from SEQ ID NO: 10.
84.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Using eMATRIX software package (Stanford University, Stanford, CA) (Wu
et al., J. Comp. Biol., vol. 6, pp. 219-235 (1999), herein incorporated by
reference), Siglec-like polypeptide of SEQ ID NO: 10 is expected to have
following
domains, wherein A=Alanine, C=Cysteine, D=Aspartic Acid, E= Glutamic Acid,
F=Phenylalanine, G=Glycine, H=Histidine, I=Isoleucine, K=Lysine,
L=Leucine, M=Methionine, N=Asparagine, P=Proline, Q=Glutamine,
R=Arginine, S=Serine, T=Threonine, V=Valine, W=Tryptophan, Y=Tyrosine:
Laminin-type EGF-like (LE) domain proteins at
100 ADCDTCFNKNFCTKCKSGFYLHL 122 (SEQ ID NO: 17)
Vertebrate metallothioneins proteins at
92INKCTKCKADCDTCFNKNFCTKCKSGFYLHLGKCLDNCPEGLEANN
137 (SEQ ID NO: 18)
Endogenous opioids neuropeptides precursors proteins at
33 MHPNVSQGCQGGCATCSDYN 52 (SEQ ID NO: 19)
Membrane attack complex components / perform proteins at
145 IVHCEVSEWNPWSPCTKKGKTCGFKRGTETRVRE1TQ 181 (SEQ ID
NO: 20)
HMG-I and HMG-Y DNA-binding domain proteins (Ahook) at
213 KKGRERKRKK 222 (SEQ ID NO: 21)
HMG1/2 proteins at
198 KCTV QRKKCQKGERGKKGRERKRKKPNKGESKEAIPDSKSLE 239
(SEQ ID NO: 22)
VERTEBRATE METALLOTHIONETN SIGNATURE at
104 TCFNKNFCTKCKSG 117 (SEQ ID NO: 23)
NEUROHYPOPHYSIAL HORMONE SIGNATURE at
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
148 CEVSEWNPWSPCTKKGKTCG 167 (SEQ ID NO: 24)
Motif 100-122, a laminin-type EGF-like domain, is a component of
extracellular matrix which promotes cell growth. The membrane attack complex
S component/perforin domain (145 - 185) is postulated to mediate cell-cell
interaction
and thus cell growth and differentiation. Neurohypophysial hormone is itself
regulated by many other factors including Interleukin-1 beta and Interleukin-
6. The
presence of these motifs are expected in stem cell growth factor activity.
Stem cell growth factor-like protein and/or fragments or derivatives would
have similar activity to stem cell growth factors and anabolic growth factors
and
receptors.
Polypeptides of the invention having stem cell growth factor-like activity are
useful for but not limited to cell growth and morphogenesis, including
hematopoietic
stem cell growth and/or growth of a particular hematopoietic cell type (such
as B or T
cells), tissue specific stem cell growth, epithelial cell growth and
regulation, ovarian
follicle development, promoting nerve cell growth, sustaining neuronal
populations,
cartilage remodeling, wound repair, bone gxowth, immunosuppression, immune
response modulation, modulating antibody and cell mediated immunity and
vascular
remodeling. The polypeptides of the invention can therefore be employed in but
not
limited to the prophylaxis or treatment of disorders and diseases caused by or
involving
wound healing, growth and development, regulation of cartilage growth and
development, vascular remodeling (angiogenesis), immunosuppression, follicle
growth
and development and neurite growth and development. Polypeptides of the
invention
can also be used in the production of and maintenance of transplants or
epidermal
2S grafts.
The polynuclebtides and proteins of the present invention are expected to
exhibit one or more of the uses or biological activities (including those
associated
with assays cited herein) identified herein. Uses or activities described for
proteins
of the present invention may be provided by administration or use of such
proteins
or of polynucleotides encoding such proteins (such as, for example, in gene
therapies or vectors suitable for introduction of DNA.). The mechanism
underlying
the particular condition or pathology will dictate whether the polypeptides of
the
86
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
invention, the polynucleotides of the invention or modulators (activators or
inhibitors) thereof would be beneficial to the subject in need of treatment.
Thus,
"therapeutic compositions of the invention" include compositions comprising
isolated polynucleotides (including recombinant DNA molecules, cloned genes
and
degenerate variants thereof) or polypeptides of the invention (including full
length
protein, mature protein and truncations or domains thereof), or compounds and
other substances that modulate the overall activity of the target gene
products, either
at the level of target gene/protein expression or target protein activity.
Such
modulators include polypeptides, analogs, (variants), including fragments and
fusion
proteins, antibodies and other binding proteins; chemical compounds that
directly or
indirectly activate or inhibit the polypeptides of the invention (identified,
e.g., via
drug screening assays as described herein); antisense polynucleotides and
polynucleotides suitable for triple helix formation; and in particular
antibodies or
other binding partners that specifically recognize one or more epitopes of the
polypeptides of the invention.
The protein of the present invention may likewise be involved in cellular
activation or in one of the other physiological pathway s described herein.
5.9.1 RESEARCH USES AND UTILITIES
The polynucleotides provided by the present invention can be used by the
research community for various purposes. The polynucleotides can be used to
express recombinant protein for analysis, characterization or therapeutic use;
as
markers for tissues in which the corresponding protein is preferentially
expressed
(either constitutively or at a particular stage of tissue differentiation or
development
or in disease states); as molecular weight markers on gels; as chromosome
markers
or tags (when labeled) to identify chromosomes or to map related gene
positions; to
compare with endogenous DNA sequences in patients to identify potential
genetic
disorders; as probes to hybridize and thus discover novel, related DNA
sequences;
as a source of information to derive PCR primers for genetic fingerprinting;
as a
probe to "subtract-out" known sequences in the process of discovering other
novel
polynucleotides; for selecting and making oligomers for attachment to a "gene
chip"
or other support, including for examination of expression patterns; to raise
87
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
anti-protein antibodies using DNA immunization techniques; and as an antigen
to
raise anti-DNA antibodies or elicit another immune response. Where the
polynucleotide encodes a protein which binds or potentially binds to another
protein
(such as, for example, in a receptor-ligand interaction), the polynucleotide
can also
be used in interaction trap assays (such as, for example, that described in
Gyuris et
al., Cell 75:791-803 (1993)) to identify polynucleotides encoding the other
protein
with which binding occurs or to identify inhibitors of the binding
interaction.
The proteins provided by the present invention can similarly be used in
assays to determine biological activity, including in a panel of multiple
proteins for
high-throughput screening; to raise antibodies or to elicit another immune
response;
as a reagent (including the labeled reagent) in assays designed to
quantitatively
determine levels of the protein (or its receptor) in biological fluids; as
markers for
tissues in which the corresponding protein is preferentially expressed (either
constitutively or at a particular stage of tissue differentiation or
development or in a
disease state); and, of course, to isolate correlative receptors or ligands.
Where the
protein binds or potentially binds to another protein (such as, for example,
in a
receptor-ligand interaction), the protein can be used to identify the other
protein
with which binding occurs or to identify inhibitors of the binding
interaction.
Proteins involved in these binding interactions can also be used to screen for
peptide
or small molecule inhibitors or agonists of the binding interaction.
The polypeptides of the invention are also useful for making antibody
substances that axe specifically immunoreactive with stem cell growth factor-
like
proteins. Antibodies and portions thereof (e.g., Fab fragments) which bind to
the
polypeptides of the invention can be used to identify the presence of such
polypeptides in a sample. For example, the level of the native protein
corresponding to SEQ ID NO: 10 in a tissue sample can be determined as an
indication of chrondrocyte differentiation or embryonic status. Such
determinations
are.carried out using any suitable immunoassay format, and any polypeptide of
the
invention that is specifically bound by the antibody can be employed as a
positive
control.
Any or all of these research utilities are capable of being developed into
reagent grade or kit format for commercialization as research products.
88
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Methods for performing the uses listed above are well known to those skilled
in the art. References disclosing such methods include without limitation
"Molecular Cloning: A Laboratory Manual", 2d ed., Cold Spring Harbor
Laboratory Press, Sambrook, J., E. F. Fritsch and T. Maniatis eds., 1989, and
"Methods in Enzymology: Guide to Molecular Cloning Techniques", Academic
Press, Berger, S. L. and A. R. Kimmel eds., 1987.
5.9.2 NUTRITIONAL USES
Polynucleotides and proteins of the present invention can also be used as
nutritional sources or supplements. Such uses include without limitation use
as a
protein or amino acid supplement, use as a carbon source, use as a nitrogen
source and
use as a source of carbohydrate. In such cases the protein or polynucleotide
of the
invention can be added to the feed of a particular organism or can be
administered as a
separate solid or liquid preparation, such as in the form of powder, pills,
solutions,
suspensions or capsules. In the case of microorganisms, the protein or
polynucleotide
of the invention can be added to the medium in or on which the microorganism
is
cultured.
Additionally, the polypeptides of the invention can be used as molecular
weight
markers, and as a food supplement. A polypeptide consisting of SEQ ID NO: 10,
for
example, has a molecular mass of approximately 30 kDa in its unprocessed and
unglycosylated state. Protein food supplements are well known and the
formulation of
suitable food supplements including polypeptides of the invention is within
the level of
skill in the food preparation art.
5.9.3 CYTOKINE AND CELL
PROLIFERATION/DIFFERENTIATION ACTIVITY
A protein of the present invention may exhibit activity relating to cytokine,
cell proliferation (either inducing or inhibiting) or cell differentiation
(either
inducing or inhibiting) activity or may induce production of other cytokines
in
certain cell populations. A polynucleotide of the invention can encode a
polypeptide
exhibiting such attributes. Many protein factors discovered to date, including
all
known cytokines, have exhibited .activity in one or more factor-dependent cell
89
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
proliferation assays, and hence the assays serve as a convenient confirmation
of
cytokine activity. The activity of therapeutic compositions of the present
invention i,s
evidenced by any one of a number of routine factor dependent cell
proliferation
assays for cell lines including, without limitation, 32D, DA2, DA 1 G, T10,
B9,
B9/11, BaF3, MC9/G, M+(preB M+), 2E8, RBS, DA1, 123, T1165, HT2,
CTLL2, TF-1, Mo7e, CMK, HUVEC, and Caco. Therapeutic compositions of the
invention can be used in the following:
Assays for T-cell or thymocyte proliferation include without limitation those
described in: Current Protocols in Immunology, Ed by J. E. Coligan, A. M.
. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, Pub. Greene
Publishing
Associates and Wiley-Interscience (Chapter 3, In Vitro assays for Mouse
Lymphocyte Function 3.1-3.19; Chapter 7, Immunologic studies in Humans); Takai
et al., J. Immunol. 137:3494-3500, 1986; Bertagnolli et al., J. Immunol.
145:1706-1712, 1990; Bertagnolli et al., Cellular Immunology 133:327-341,
1991;
Bertagnolli, et al. , I. Immunol. 149:3778-3783, 1992; Bowman et al. , I.
Immunol.
152:1756-1761,1994.
Assays for cytokine production and/or proliferation of spleen cells, lymph
node cells or thymocytes include, without limitation, those described in:
Polyclonal
T cell stimulation, Kruisbeek, A. M. and Shevach, E. M. In Current Protocols
in
Immunology. J. E. e.a. Coligan eds. Vol 1 pp. 3.12.1-3.12.14, John Wiley and
Sons, Toronto. 1994; and Measurement of mouse and human interleukin- ,
Schreiber, R. D. In Current Protocols in Immunology. J. E. e.a. Coligan eds.
Vol 1
pp. 6.8.1-6.8.8, John Wiley and Sons, Toronto. 1994.
Assays for proliferation and differentiation of hematopoietic and
lymphopoietic cells include, without limitation, those described in:
Measurement of
Human and Murine Interleukin 2 and Interleukin 4, Bottomly, K., Davis, L. S.
and
Lipsky, P. E. In Current Protocols in Immunology. J. E. e.a: Coligan eds. Vol
1
pp. 6.3.1-6.3.12, John Wiley and Sons, Toronto. 1991; deVries et al., J. Exp.
Med. 173:1205-1211, 1991; Moreau et al., Nature 336:690-692, 1988; Greenberger
et al., Proc. Natl. Acad. Sci. U.S.A. 80:2931-2938, 1983; Measurement of mouse
and human interleukin 6--Nordan, R. In Current Protocols in Immunology. J. E.
Coligan eds. Vol 1 pp. 6.6.1-6.6.5, John Wiley and Sons, Toronto. 1991; Smith
et
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
al., Proc. Natl. Aced. Sci. U.S.A. 83:1857-1861, 1986; Measurement of human
Interleukin 11--Bennett, F., Giannotti, J., Clark, S. C. and Turner, K.,J. In
Current
Protocols in Immunology. J. E. Coligan eds. Vol 1 pp. 6.15.1 John Wiley and
Sons, Toronto. 1991; Measurement of mouse and human Interleukin 9--Ciarletta,
A., Giannotti, J., Clark, S. C. and Turner, K. J. In Current Protocols in
Immunology. J. E. Coligan eds. Vol 1 pp. 6.13.1, John Wiley and Sons, Toronto.
1991.
Assays for T-cell clone responses to antigens (which will identify, among
others, proteins that affect APC-T cell interactions as well as direct T-cell
effects by
measuring proliferation and cytokine production) include, without limitation,
those
described in: Current Protocols in Immunology, Ed by J. E. Coligan, A. M.
Kruisbeek, D. H. Margulies, E. M. Shevach, W Strober, Pub. Greene Publishing
Associates and Wiley-Interscience (Chapter 3, In Vitro assays for Mouse
Lymphocyte Function: Chapter 6, Cytokines and their cellular receptors;
Chapter 7,
Immunologic studies in Humans); Weinberger et al., Proc. Natl. Acad. Sci. USA
77:6091-6095, 1980; Weinberger et al., Eur. J. Immun. 11:405-411. 1981; Takai
et
aL, J. Immunol. 137:3494-3500, 1986; Takai et al., J. Inununol. 140:508-512,
1988.
5.9.4 STEM CELL GROWTH FACTOR ACTIVITY
Polypeptides of the present invention have been shown to exhibit stem cell
growth factor activity and to be involved in the proliferation,
differentiation and
survival of pluripotent and totipotent stem cells including primordial germ
cells,
embryonic stem cells, neural stem cells, skeletal muscle stem cells,
mesencymal
stem cells, hematopoietic stem cells and/or germ line stem cells.
Administration of
the polypeptide of the invention to stem cells in vivo or ex vivo maintains
and
expands cell populations in a totipotential or pluripotential state which
would be
useful for re-engineering damaged or diseased tissues, transplantation
including '
solid organs and bone marrow transplants, manufacture of bio-pharmaceuticals
and
the development of bio-sensors. The ability to produce large quantities of
human
cells has important working applications for the production of human proteins
which
91
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
currently must be obtained from non-human sources or donors, implantation of
cells
to treat diseases such as Parkinson's, Alzheimer's and other neurodegenerative
diseases; tissues for grafting such as bone marrow, skin, cartilage, tendons,
bone,
muscle (including cardiac muscle), blood vessels, cornea, neural cells,
gastrointestinal cells and others; and organs for transplantation such as
kidney, liver,
pancreas (including islet cells), heart and lung.
It is contemplated that multiple different exogenous growth factors and/or
cytokines may be administered in combination with the polypeptide of the
invention
to achieve the desired effect, including any of the growth factors listed
herein, other
stem cell maintenance factors, and specifically including stem cell factor
(SCF),
leukemia inhibitory factor (LIF), Flt-3 ligand (Flt-3L), any of the
interleukins,
recombinant soluble IL-6 receptor fused to IL-6, macrophage inflammatory
protein
1-alpha (MIP-1-alpha), G-CSF, GM-CSF, thrombopoietin (TPO), platelet factor 4
(PF-4), platelet-derived growth factor (PDGF), neural growth factors and basic
fibroblast growth factor (bFGF).
Since totipotent stem cells can give rise to virtually any mature cell type,
expansion of these cells in culture will facilitate the production of large
quantities of
mature cells. Techniques for culturing stem cells are known in the art and
administration of polypeptides of the invention, optionally with other growth
factors
and/or cytokines, is expected to enhance the survival and proliferation of the
stem
cell populations. This can be accomplished by direct administration of the
polypeptide of the invention to the culture medium. Alternatively, stroma
cells
transfected with a polynucleotide that encodes for the polypeptide of the
invention
can be used as a feeder layer for the stem cell populations in culture or in
vivo.
Stromal support cells for feeder layers may include embryonic bone marrow
fibroblasts, bone marrow stromal cells, fetal liver cells, or cultured
embryonic
fibroblasts (see U.S. Patent No.; 5,690,926). '
Stem cells themselves can be transfected with a polynucleotide of the
invention to induce autocrine expression of the polypeptide of the invention.
This
will allow for generation of undifferentiated totipotential/pluripotential
stem cell
Iines that are useful as is or that can then be differentiated into the
desired mature
cell types. These stable cell lines can also serve as a source of
undifferentiated
92
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
totipotential/pluripotential mRNA to create cDNA libraries and templates for
polymerase chain reaction experiments. These studies would allow for the
isolation
and identification of differentially expressed genes in stem cell populations
that
regulate stem cell proliferation and/or maintenance.
Expansion and maintenance of totipotent stem cell populations is useful in
the treatment of many pathological conditions. For example, polypeptides of
the
present invention may be used to manipulate stem cells in culture to give rise
to
neuroepithelial cells that can be used to augment or replace cells damaged by
illness,
autoimmune disease, accidental damage or genetic disorders, inflammatory
disease,
immunodeficiency, leukemia and neoplastic myeloid disorders. The polypeptide
of
the invention can be useful for inducing the proliferation of neural cells and
for the
regeneration of nerve and brain tissue, i.e. for the treatment of central and
peripheral nervous system diseases and neuropathies, as well as mechanical and
traumatic disorders which involve degeneration, death or trauma to neural
cells or
nerve tissue. In addition, the expanded stem cell populations can also be
genetically
altered for gene therapy purposes and to decrease host rejection of
replacement
tissues after grafting or implantation. T'he polypeptide of the invention can
also be
useful for inducing the proliferation of cardiac stern cells and for
regenerating
functional heart tissue following cardiac damage induced by cardiac disorders
such
as myocardial infarctions and artery blockage. In addition, the polypeptides
of the
invention may strengthen cardiac muscle cells and prevent and/or repair the
heart
tissue damage due to heart failure. See Weismann, Science, 287: 1442-1446,
2001; Vogel, Science, 290: 1672-1674, 2000 I~ajstura et al., Nature, 410: 701-
705,
2001.
Expression of the polypeptide of the invention and its effect on stem cells
can
also be manipulated to achieve controlled differentiation of the stem cells
into more
differentiated cell types. A broadly applicable method of obtaining pure
populations
of a specific differentiated cell type from undifferentiated stem cell
populations
involves the use of a cell-type specific promoter driving a selectable marker.
The
selectable marker allows only cells of the desired type to survive. For
example,
stem cells can be induced to differentiate into cardiomyocytes (Wobus et al. ,
93
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Differentiation, 48: 173-182 , (1991); Klug et al., J. Clin. Invest., 98(1):
216-224,
(1998)) or skeletal muscle cells (Browder, L. W. In: Principles of Tissue
Engineering eds. Lanza et al., Academic Press (1997)). Alternatively, directed
differentiation of stem cells can be accomplished by culturing the stem cells
in the
presence of a differentiation factor such as retinoic acid and an antagonist
of the
polypeptide of the invention which would inhibit the effects of endogenous
stem cell
factor activity and allow differentiation to proceed.
In vitro cultures of stem cells can be used to determine if the polypeptide of
the invention exhibits stem cell growth factor activity. Stem cells are
isolated from
any one of various cell sources (including hematopoietic stem cells and
embryonic
stem cells) and cultured on a feeder layer, as described by Thompson et al.
Proc.
Natl. Acad. Sci, U.S.A., 92: 7844-7848 (1995), in the presence of the
polypeptide
of the invention alone or in combination with other growth factors or
cytokines. The
ability bf the polypeptide of the invention to induce stem cells proliferation
is
determined by colony formation on semi-solid support e.g. as described by
Bernstein et al., Blood, 77: 2316-2321 (1991).
S.9.S HEMATOPOIESIS REGULATING ACTIVITY
A protein of the present invention rrray be involved in regulation of
hematopoiesis and, consequently, in the treatment of myeloid or lymphoid cell
disorders. Even marginal biological activity in support of colony forming
cells or of
factor-dependent cell lines indicates involvement in regulating hematopoiesis,
e.g. in
supporting the growth and proliferation of erythroid progenitor cells alone or
in
combination with other cytokines, thereby indicating utility, for example, in
treating
various anemias or for use in conjunction with irradiation/chemotherapy to
stimulate
the production of erythroid precursors and/or erythroid cells; in supporting
the
growth and proliferation of myeloid cells such as granulocytes and
monocytes/macrophages (i. e. , traditional circulating soluble factor
activity) useful,
fox example, in conjunction with chemotherapy to prevent or treat consequent
myelo-suppression; in supporting the growth and proliferation of
megakaryocytes
and consequently of platelets thereby allowing prevention or treatment of
various
94
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
platelet disorders such as thrombocytopenia, and generally for use in place of
or
complimentary to platelet transfusions; and/or in supporting the growth and
proliferation of hematopoietic stem cells which are capable of maturing to any
and
all of the above-mentioned hematopoietic cells and therefore find therapeutic
utility
in various stem cell disorders (such as those usually treated with
transplantation,
including, without limitation, aplastic anemia and paroxysmal nocturnal
hemoglobinuria), as well as in repopulating the stem cell compartment post
irradiation/chemotherapy, either in-.vivo or ex-wivo (i.e., in conjunction
with bone
marrow transplantation or with peripheral progenitor cell transplantation
(homologous or heterologous)) as normal cells or genetically manipulated for
gene
therapy.
Therapeutic compositions of the invention can be used in the following:
Suitable assays for proliferation and differentiation of various hematopoietic
lines are cited above.
Assays for embryonic stem cell differentiation (which will identify, among
others, proteins that influence embryonic differentiation hematopoiesis)
include.
without limitation, those described in: Johansson et al. Cellular Biology
15:141-151,
1995; Keller et aL, Molecular and Cellular Biology 13:473-486, 1993;
McClanahan
et al., Blood 81:2903-2915, 1993.
Assays for stem cell survival and differentiation (which will identify, among
others, proteins that regulate Iympho-hematopoiesis) include, without
limitation,
those described in: Methylcellulose colony forming assays, Freshney, M. G. In
Culture of Hematopoietic Cells. R. I. Freshney, et al. eds. Vol pp. 265-268,
Wiley-Liss, Inc., New York, N.Y. 1994; Hirayama et al., Proc. Natl. Acad. Sci.
USA 89:5907-5911, 1992; Primitive hematopoietic colony forming cells with high
proliferative potential, McNiece, I. K. and Briddell, R. A. In Culture of
Hematopoietic Cells. R. I. Freshney, et al. eds. Vol pp. 23-39, Wiley-Liss,
Inc.,
New York, N.Y. 1994; Neben et al., Experimental Hematology 22:353-359, 1994;
Cobblestone area forming cell assay, Ploemacher, R. E. In Culture of
Hematopoietic Cells. R. I. Freshney, et al. eds. Vol pp. 1-21, Wiley-Liss,
Inc_,
New York, N.Y. 1994; Long term bone marrow cultures in the presence of stromal
cells, Spooncer, E., Dexter, M. and Allen, T. In Culture of Hematopoietic
Cells.
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
R. I. Freshney, et al. eds. Vol pp. 163-179, Wiley-Liss, Inc., New York, N.Y.
1994; Long term culture initiating cell assay, Sutherland, H. J. In Culture of
Hematopoietic Cells. R. I. Freshney, et aI. eds. Vol pp. 139-162, Wiley-Liss,
Inc.,
New York, N.Y. 1994.
5.9.6 TISSUE GROWTH ACTIVITY
A protein of the present invention also may be involved in bone, cartilage,
tendon, ligament and/or nerve tissue growth or regeneration, as well as in
wound
healing and tissue repair and replacement, and in healing of burns, incisions
and
ulcers.
For example, induction of cartilage and/or bone growth in circumstances
where bone is not normally formed, has application in the healing of bone
fractures
and cartilage damage or defects in humans and other animals. Compositions of a
protein, antibody, binding partner, or other modulator of the invention may
have
prophylactic use in closed as well as open fracture reduction and also in the
improved fixation of artificial points. De novo bone formation induced by an
osteogenic agent contributes to the repair of congenital, trauma induced, or
oncologic resection induced craniofacial defects, and also is useful in
cosmetic
plastic surgery.
A protein of this invention may also be involved in attracting bone-forming
cells, stimulating growth of bone-forming cells, or inducing differentiation
of
progenitors of bone-forming cells. Treatment of osteoporosis, osteoarthritis,
bone
degenerative disorders, or periodontal disease, such as through stimulation of
bone
andlor cartilage repair or by blocking inflammation or processes of tissue
destruction (collagenase activity, osteoclast activity, etc.) mediated by
inflammatory
processes may also be possible using the composition of the invention.
Another category of tissue regeneration activity that may involve the protein
of the present invention is tendon/ligament formation. Induction of
tendon/ligament-like tissue or other tissue formation in circumstances where
such
tissue is not normally formed, has application in the healing of tendon or
ligament
tears, deformities and other tendon or ligament defects in humans and other
animals.
Such a preparation employing a tendon/ligament-like tissue inducing protein
may
96
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
have prophylactic use in preventing damage to tendon or ligament tissue, as
well as
use in the improved fixation of tendon or ligament to bone or other tissues,
and in
repairing defects to tendon or ligament tissue. De nova tendonlligament-like
tissue
formation induced by a composition of the present invention contributes to the
repair
of congenital, trauma induced, or other tendon or ligament defects of other
origin,
and is also useful in cosmetic plastic surgery for attachment or repair of
tendons or
ligaments. The compositions of the present invention may provide environment
to
attract tendon- or ligament-forming cells, stimulate growth of tendon- or
ligament-forming cells, induce differentiation of progenitors of tendon- or
ligament-forming cells. or induce growth of tendon/ligament cells or
progenitors ex
vivo for return in vivo to effect tissue repair. The compositions of the
invention may
also be useful in the treatment of tendinitis, carpal tunnel syndrome and
other
tendon or ligament defects. The compositions may also include an appropriate
matrix andlor sequestering agent as a carrier as is well known in the art.
The compositions of the present invention may also be useful for
proliferation of neural cells and for regeneration of nerve and brain tissue,
i.e. for
the treatment of central and peripheral nervous system diseases and
neuropathies, as
well as mechanical and traumatic disorders, which involve degeneration, death
or
trauma to neural cells or nerve tissue. More specifically, a composition rnay
be used
in the treatment of diseases of the peripheral nervous system, such as
peripheral
nerve injuries, peripheral neuropathy and localized neuropathies, and central
nervous system diseases, such as Alzheimer's, Parkinson's disease,
Huntington's
disease, amyotrophic lateral sclerosis, and Shy-Drager syndrome. Further
conditions
which may be treated in accordance with the present invention include
mechanical
and traumatic disorders, such as spinal cord disorders, head trauma and
cerebrovascular diseases such as stroke. Peripheral neuropathies resulting
from
chemotherapy or other medical therapies may also be treatable using a
composition
of the invention.
Compositions of the invention may also be useful to promote better or faster
closure of non-healing wounds, including without limitation pressure ulcers,
ulcers
associated with vascular insufficiency, surgical and traumatic wounds, and the
like.
97
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Compositions of the present invention may also be involved in the generation
or regeneration of other tissues, such as organs (including, for example,
pancreas,
liver, intestine, kidney, skin, endothelium), muscle (smooth, skeletal or
cardiac) and
vascular (including vascular endothelium) tissue, or for promoting the growth
of
cells comprising such tissues. Inhibition or modulation of fibrotic scarring
may
allow normal tissue to regenerate
A composition of the present invention may also be useful for gut protection
or regeneration and treatment of lung or liver fibrosis; reperfusion injury in
various
tissues, and conditions resulting from systemic cytokine damage.
A composition of the present invention may also be useful for promoting or
inhibiting differentiation of tissues described above from precursor tissues
or cells;
or for inhibiting the growth of tissues described above.
Therapeutic compositions of the invention can be used in the following:
Assays for tissue generation activity include, without limitation, those
described in: International Patent Publication No. W095/16035 (bone,
cartilage,
tendon); International Patent Publication No. W095/OS846 (nerve, neuronal);
International Patent Publication No. W091/0749I (skin, endothelium).
Assays for wound healing activity include, without limitation, those
described in: Winter, Epidermal Wound Healing, pps. 71-112 (Maibach, H. I. and
Rovee, D. T., eds.), Year Book Medical Publishers, Inc., Chicago, as modified
by
Eaglstein and Mertz, J. Invest. Dermato1~71:382-84 (I978).
5.9.7 IMMUNE STIMULATING OR SUPPRESSING ACTIVITY
Compositions of the present invention may also exhibit immune stimulating
or immune suppressing activity, including without limitation the activities
for which
assays are described herein. A polynucleotide of the invention can encode a
polypeptide involved in such activities. A protein or antibody, other binding
partner, or other modulator of the invention may be useful in the treatment of
various immune deficiencies and disorders (including severe combined
immunodeficiency (SCID)), e.g., in regulating (up or down) growth and
proliferation of T and/or B lymphocytes,, as well as effecting the cytolytic
activity of
NK cells and other cell populations. These immune deficiencies may be genetic
or
98
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
be caused by viral (e.g., HIV) as well as bacterial or fungal infections, or
may
result from autoimmune disorders. More specifically, infectious diseases
caused by
viral, bacterial, fungal or other infection may be treatable using a protein,
antibody,
binding partner, or other modulator of the invention, including infections by
HIV,
hepatitis viruses, herpesviruses, mycobacteria, Leishmania spp., malaria spp.
and
various fungal infections such as candidiasis, as well as other conditions
where a
boost to the immune system generally may be desirable, e.g., in the treatment
of
cancer.
Autoimmune disorders which may involve a protein of the present invention
include, for example, connective tissue disease, multiple sclerosis, systemic
lupus
erythematosus, rheumatoid arthritis, autoimmune pulmonary inflammation,
Guillain-Barre syndrome, autoimmune thyroiditis, insulin dependent diabetes
mellitis, myasthenia gravis, graft-versus-host disease and autoimmune
inflammatory
eye disease. Such a protein of the present invention may also to be involved
in
allergic reactions and conditions, such as asthma (particularly allergic
asthma) or
other respiratory problems.
Using the proteins, antibody, binding partners. or other modulators of the
invention it may also be possible to modulate immune responses, in a number of
ways. The immune response may be enhanced or suppressed. Down regulation may
be in the form of inhibiting or blocking an immune response already in
progress or
may involve preventing the induction of an immune response. The functions of
activated T cells may be inhibited by suppressing T cell responses or by
inducing
specific tolerance in T cells, or both. Immunosuppression of T cell responses
is
generally an active, non-antigen-specific, process which requires continuous
exposure of the T cells to the suppressive agent. Tolerance, which involves
inducing
non-responsiveness or anergy in T cells, is distinguishable from
immunosuppression
in that it is generally antigen-specific and persists after exposure to the
tolerizing
agent has ceased. Operationally, tolerance can be demonstrated by the lack of
a T
cell response upon reexposure to specific antigen in the absence of the
tolerizing
agent.,
Down regulating or preventing the immune response, e.g., preventing high
level lymphokine synthesis by activated T cells, will be useful in situations
of tissue,
99
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
skin and organ transplantation and in graft-versus-host disease (GVHD). Fox
example, blockage of T cell function should result in reduced tissue
destruction in
tissue transplantation. Typically, in tissue transplants, rejection of the
transplant is
initiated through its recognition as foreign by T cells, followed by an immune
reaction that destroys the transplant. The administration of a molecule which
inhibits
or blocks the immune response (e.g. a receptor fragment, binding partner, or
other
modulator such as antisense polynucleotides) may act as an immunosuppressant.
The efficacy of particular immune response modulators in preventing organ
transplant rejection or GVHD can be assessed using animal models that are
predictive of efficacy in humans. Examples of appropriate systerrxs which can
be
used include allogeneic cardiac grafts in rats and xenogeneic pancreatic islet
cell
grafts in mice, both of which have been used to examine the immunosuppressive
effects of CTLA4Ig fusion proteins in vivo as described in Lenschow et al.,
Science
257:789-792 (1992) and Turka et al.; Proc. Natl. Acad. Sci USA, 89:11102-11105
(1992). In addition, marine models of GVHD (see Paul ed., Fundamental
Immunology, Raven Press, New York, 198°, pp. 846-847) can be used to
determine
the effect of blocking B lymphocyte antigen function in vivo on the
development of
that disease.
Blocking the inflammatory response may also be therapeutically useful for
treating autoimmune diseases. Many autoimmune disorders are the result of
inappropriate activation of T cells that are reactive against self tissue and
which
promote the production of cytokines and autoantibodies involved in the
pathology of
the diseases. Preventing the activation of autoreactive 'T cells may reduce or
eliminate disease symptoms. Administration of reagents which block
costimulation
of T cells can be used to inhibit T cell activation and prevent production of
autoantibodies or T cell-derived cytokines which may be involved in the
disease
process. Additionally, blocking reagents may induce antigen-specific tolerance
of
autoreactive T' cells which could lead to long-term relief from the disease.
The
efficacy of blocking reagents in preventing or alleviating autoimmune
disorders can
be determined using a number of well-characterized animal models of human
autoimmune diseases. Examples include marine experimental autoimmune
encephalitis, systemic lupus erythematosus in MRL/lpr/lpr mice or NZB hybrid
100
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
mice, murine autoimmune collagen arthritis, diabetes mellitus in NOD mice and
BB
rats, and murine experimental myasthenia gravis (see Paul ed., Fundamental
Immunology. Raven Press, New York, 1989, pp. 840-856).
Upregulation of immune responses, may also be useful in therapy.
Upregulation of immune responses may be in the form of enhancing an existing
immune response or eliciting an initial immune response. For example,
enhancing
an immune response may be useful in cases of viral infection such as
influenza, the
common cold, and encephalitis.
Alternatively, anti-viral immune responses may be enhanced in an infected
patient by removing T cells from the patient, costimulating the T cells in
vitro and
reintroducing the in vitro activated T cells into the patient.
The activity of therapeutic compositions of the invention may, among other
means, be measured by the following methods:
Suitable assays for thymocyte or splenocyte cytotoxicity include, without
limitation, those described in: Current Protocols in Immunology, Ed by J. E.
Coligan, A. M. Kruisbeek, D. H. Margulies, E.. M: Shevach, W. Strober, Pub.
Greene Publishing Associates and Wiley-Interscience (Chapter 3, In Vitro
assays for
Mouse Lymphocyte Function 3.1-3.19; Chapter 7, hnmunologic studies in
Humans); Herrmann et al., Proc. Natl. Acad. Sci. USA 78:2488-2492, 1981;
Herrmann et al., J. Immunol. 128:1968-1974, 1982; Handa et al., J. Immunol.
135:1564-1572, 1985; Takai et al., I. Immunol. 137:3494-3500, 1986; Takai et
al.,
J. Immunol. 140:508-512, 1988; Herrmann et al., Proc. Natl. Acad. Sci. USA
78:2488-2492, 1981; Herrmann et al., J. Immunol. 128:1968-1974, 1982; Handa et
al., J. Immunol. 135:1564-1572, 1985; Takai et al., J. Immunol. 137:3494-3500,
1986; Bowmanet al., J. Virology 61:1992-1998; Takai et al., J, Immunol.
140:508-512, 1988; Bertagnolli et al., Cellular Immunology 133:327-341, 1991;
Brown et al., J. Immunol. 153:3079-3092, 1994.
Assays for T-cell-dependent immunoglobulin responses and isotype
switching (which will identify, among others, proteins that modulate T-cell
dependent antibody responses and that affect Th1/Th2 profiles) include,
without
limitation, those described in: Maliszewski, J. Immunol. 144:3028-3033, 1990;
and
Assays for B cell function: In vitro antibody production, Mond, J. J. and
101
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Brunswick, M. In Current Protocols in Immunology. J. E. e.a. Coligan eds. VoI
1
pp. 3.8.1-3.8.16, John Wiley and Sons, Toronto. 1994.
Mixed lymphocyte reaction (MLR) assays (which will identify, among
others, proteins that generate predominantly Thl and CTL responses) include,
S without limitation, those described in: Current Protocols in Immunology, Ed
by J.
E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, Pub.
Greene Publishing Associates and Wiley-Interscience (Chapter 3, In Vitro
assays for
Mouse Lymphocyte Function 3.1-3.19; Chapter 7, Immunologic studies in
Humans); Takai et al., J. Immunol. 137:3494-3500, 1986; Takai et al., J.
Immunol.
140:508-512, 1988; Bertagnolli et al., J. Irnmunol. 149:3778-3783, 1992.
Dendritic cell-dependent assays (which will identify, among others, proteins
expressed by dendritic cells that activate naive T-cells) include, without
limitation,
those described in: Guery et al., J. Immunol. 134:536-544, 1995; Inaba et al.,
Journal of Experimental Medicine 173:549-559, 1991; Macatonia et al., Journal
of
Immunology 154:5071-5079, 1995; Porgador et al., Journal of Experimental
Medicine 182:255-260, 1995; Nair et al., Journal of Virology 67:4062-4069,
1993;
Huang et al., Science 264:961-965, 1994; Macatonia et al., Journal of
Experimental
Medicine 169:1255-1264, 1989; Bhardwaj et al., Journal of Clinical
Investigation
94:797-807, 1994; and Inaba et al., Journal of Experimental Medicine 172:631-
640,
1990.
Assays for lymphocyte survivallapoptosis (which will identify, among
others, proteins that prevent apoptosis after superantigen induction and
proteins that
regulate lymphocyte homeostasis) include, without limitation, those described
in:
Datzynkiewicz et al. , Cytometry 13: 795-808, 1992; Gorczyca et al. , Leukemia
7:659-670, 1993; Gorczyca et al., Cancer Research 53:1945-1951, 1993; Itoh et
al.,
Cell 66:233-243, 1991; Zacharchuk, Journal of Immunology 145:4037-4045, 1990;
Zamai et al., Cytometry 14:891-897, 1993; Gorczyca et al, , International
Journal of
Oncology 1:639-648, 1992.
Assays for proteins that influence early steps of T-cell commitment. and
development include, without limitation, those described in: Antica et al.,
Blood
84:111-117, 1994; Fine et al., Cellular Immunology 155:111-122, 1994; Galy et
102
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
al., Blood 85:2770-2778, 1995; Toki et al., Proc. Nat. Acad Sci. USA
88:7548-7551, 1991.
5.9.8 ACTIVIN/INHIBIN ACTIVITY
A protein of the present invention may also exhibit activin- or inhibin-
related
activities. A polynucleotide of the invention rnay encode a polypeptide
exhibiting
such characteristics. Inhibins are characterized by their ability to inhibit
the release
of follicle stimulating hormone (FSH), while activins and are characterized by
their
ability to stimulate the release of follicle stimulating hormone (FSH). Thus,
a
protein of the present invention, alone or in heterodimers with a member of
the
inhibin family, may be useful as a contraceptive based on the ability of
inhibins to
decrease fertility in female mammals and decrease spermatogenesis in male
mammals. Administration of sufficient amounts of other inhibins can induce
infertility in these mammals. Alternatively, the protein of the invention, as
a
homodimer or as a heterodimer with other protein subunits of the inhibin
group,
may be useful as a fertility inducing therapeutic, based upon the ability of
actimin
molecules in stimulating FSH release from cells of the anterior pituitary.
See, for
example, U,S. Pat. No. 4,798,885. A protein of the invention may also be
useful
for advancement of the onset of fertility in sexually immature mammals, so as
to
increase the lifetime reproductive performance of domestic animals such as,
but not
limited to, cows, sheep and pigs.
The activity of a protein of the invention may, among other means, be
measured by the following methods.
Assays for activin/inhibin activity include, without limitation, those
described in: Vale et al. , Endocrinology 91: 562-572, 1972; Ling et al. ,
Nature
321:779-782, 1986; Vale et aL, Nature 321:776-779, 1986; Mason et al., Nature
318:659-663, 1985; Forage et al., Proc. Natl. Acad. Sci. USA 83:3091-3095,
1986.
5.9.9 CHEMOTACTICICHEMOKINETIC ACTIVITY
A protein of the present invention may be involved in chemotactic or
chemokinetic activity for mammalian cells, including, for example, monocytes,
fibroblasts, neutrophils, T-cells, mast cells, eosinophils, epithelial and/or
endothelial
103
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
cells. A polynucleotide of the invention can encode a polypeptide .exhibiting
such
attributes. Chemotactic and chemokinetic receptor activation can be used to
mobilize or attract a desired cell population to a desired site of action.
Chemotactic
or chemokinetic compositions (e.g. proteins, antibodies, binding partners, or
modulators of the invention) provide particular advantages in treatment of
wounds
and other trauma to tissues, as well as in treatment of localized infections.
For
example, attraction of lymphocytes, monocytes or neutrophils to tumors or
sites of
infection may result in improved immune responses against the tumor or
infecting
agent.
A protein or peptide has chemotactic activity for a particular cell population
if it can stimulate, directly or indirectly, the directed orientation or
movement of
such cell population. Preferably, the protein or peptide has the ability to
directly
stimulate directed movement of cells. Whether a particular protein has
chemotactic
activity fox a population of cells can be readily determined by employing such
protein or peptide in any known assay for cell chemotaxis.
Therapeutic compositions of the invention can be used in the following:
Assays for chemotactic activity (which will identify proteins that induce or
prevent chemotaxis) consist of assays that measure the ability of a protein to
induce
the migration of cells across a membrane as well as the ability of a protein
to induce
the adhesion of one cell population to another cell population. Suitable
assays for
movement and adhesion include, without limitation, those described in: Current
Protocols in Immunology, Ed by J. E. Coligan, A. M. Kruisbeek, D. H.
Marguiles,
E. M. Shevach, W. Strober, Pub. Greene Publishing Associates and
Wiley-Interscience (Chapter 6.12, Measurement of alpha and beta Chemokines
6.12.1-6.12.28; Taub et al. J. Clin. Invest. 95;,1370-1376, 1995; Lind et al.
APMIS
103:140-146, 1995; Muller et al Eur. J. Immunol. 25:1744-1748; Gruber et al.
J. of
Immunol. 152:5860-5867, 1994; Johnston et al. J. of Immunol. 153:1762-1768,
1994.
5.9.10 HEMOSTATIC AIrTD THROMBOLYTIC ACTIVITY
A protein of the invention may also be involved in hemostatis or
thrombolysis or thrombosis. A polynucleotide of the invention can encode a
104
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
polypeptide exhibiting such attributes. Compositions may be useful in
treatment of
various coagulation disorders (including hereditary disorders, such as
hemophiliac)
or to enhance coagulation and other hemostatic events in treating wounds
resulting
from trauma, surgery or other causes. A composition of the invention may also
be
useful for dissolving or inhibiting formation of thromboses and for treatment
and
prevention of conditions resulting therefrom (such as, for example, infarction
of
cardiac and central nervous system vessels (e.g., stroke).
Therapeutic compositions of the invention can be used in the following:
Assay for hemostatic and thrombolytic activity include, without limitation,
those described in: Linet et al., J. Clin. Pharmacol. 26:131-140, 1986;
Burdick et
al. , Thrombosis Res. 45:413-419, 1987; Humphrey et al. , Fibrinolysis 5:71-79
(1991); Schaub, Prostaglandins 35:467-474, 1988.
5.9.11 CANCER DIAGNOSIS AND THERAPY
Polypeptides of the invention may be involved in cancer cell generation,
proliferation or metastasis. Detection of the presence or amount of
polynucleotides
or polypeptides of the invention may be useful for the diagnosis and/or
prognosis of
one or more types of cancer. For example, the presence or increased expression
of
a polynucleotide/polypeptide of the invention may indicate a hereditary risk
of
cancer; a precancerous condition, or an ongoing malignancy. Conversely, a
defect
in the gene or absence of the polypeptide may be associated with a cancer
condition.
Identification of single nucleotide polymorphisms associated with cancer or a
predisposition to cancer may also be useful for diagnosis br prognosis.
Cancer treatments promote tumor regression by inhibiting tumor cell
proliferation, inhibiting angiogenesis (growth of new blood vessels that is
necessary
to support tumor growth) and/or prohibiting metastasis by reducing tumor cell
motility or invasiveness. Therapeutic compositions of the invention may be
effective in adult and pediatric oncology including in solid phase
tumors/malignancies, locally advanced tumors, human soft tissue sarcomas,
metastatic cancer, including lymphatic metastases, blood cell malignancies
including
multiple myeloma, acute and chronic leukemias, and lymphomas, head and neck
cancers including mouth cancer, larynx cancer and thyroid cancer, lung cancers
105
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
including small cell carcinoma and non-small cell cancers, breast cancers
including
small cell carcinoma and ductal carcinoma, gastrointestinal cancers including
esophageal cancer, stomach cancer, colon cancer, colorectal cancer and polyps
associated with colorectal neoplasia, pancreatic cancers, liver cancer,
urologic
cancers including bladder cancer and prostate cancer, malignancies of the
female
genital tract including ovarian carcinoma, uterine (including endometrial)
cancers,
and solid tumor in the ovarian follicle, kidney cancers including renal cell
carcinoma, brain cancers including intrinsic brain tumors, neuroblastoma,
astrocytic
brain tumors, gliomas, metastatic tumor cell invasion in the central nervous
system,
bone cancers including osteomas, skin cancers including malignant melanoma,
tumor progression of human skin keratinocytes, squamous cell carcinoma, basal
cell
carcinoma, hemangiopericytoma and Karposi's sarcoma.
Polypeptides, polynucleotides, or modulators of polypeptides of the invention
(including inhibitors and stimulators of the biological activity of the
polypeptide of
the invention) may be administered to treat cancer. . Therapeutic compositions
can
be administered in therapeutically effective dosages alone or in combination
with
adjuvant cancer therapy such as surgery, chemotherapy, radiotherapy,
thermotherapy, and laser therapy, and may provide a, beneficial effect, e. g,
reducing
tumor size, slowing rate of tumor growth, inhibiting metastasis, or otherwise
improving overall clinical condition, without necessarily eradicating the
cancer.
The composition can also be administered in therapeutically effective
amounts as a portion of an anti-cancer cocktail. An anti-cancer cocktail is a
mixture
of the polypeptide or modulator of the invention with one or more anti-cancer
drugs
in addition to a pharmaceutically acceptable carrier for delivery. The use of
anti-
cancer cocktails as a cancer treatment is routine. Anti-cancer drugs that are
well
known in the art and can be used as a treatment in combination with the
polypeptide
or modulator of the invention include: Actinomycin D, Aminoglutethimide,
Asparaginase, Bleomycin, Busulfan, Carboplatin, Carmustine, Chlorambucil,
Cisplatin (cis-DDP), Cyclophosphamide, Cytarabine HCl (Cytosine arabinoside),
Dacarbazine, Dactinomycin, Daunorubicin HCI, Doxorubicin HCI, Estramustine
phosphate sodium, Etoposide (V 16-213), Floxuridine, 5-Fluorouracil (5-Fu),
Flutamide, Hydroxyurea (hydroxycarbamide), Ifosfamide, Interferon Alpha-2a,
106
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Interferon Alpha-2b, Leuprolide acetate (LHRH-releasing factor analog),
Lomustine, Mechlorethamine HCl (nitrogen mustard), Melphalan, Mercaptopurine,
Mesna, Methotrexate (1VITX), Mitomycin, Mitoxantrone HCl, Octreotide,
Plicamycin, Procarbazine HCl, Streptozocin, Tamoxifen citrate, Thioguanine,
Thiotepa, Vinblastine sulfate, Vincristine sulfate, Amsacrine, Azacitidine,
Hexamethylmelamine, Interleukin-2, Mitoguazone, Pentostatin, Semustine,
Teniposide, and Vindesine sulfate.
In addition, therapeutic compositions of the invention may be used for
prophylactic treatment of cancer. There are hereditary conditions and/or
environmental situations (e.g. exposure to carcinogens) known in the art that
predispose an individual to developing cancers. Under these circumstances, it
may
be beneficial to treat these individuals with therapeutically effective doses
of the
polypeptide of the invention to reduce the risk of developing cancers.
In vitro models can be used to determine the effective doses of the
polypeptide of the invention as a potential cancer treatment. These in vitro
models
include proliferation assays of cultured tumor cells, growth of cultured tumor
cells
in soft agar (see Freshney, (1987) Culture of Animal Cells: A Manual of Basic
Technique, Wily-Liss, New York, NY Ch 18 and Ch 21), tumor systems in. nude
mice as described in Giovanella et aL, J. Natl. Can. Inst., 52: 921-30 (1974,
mobility and invasive potential of tumor cells in Boyden Chamber assays as
described in Pilkington et al., Anticancer Res., 17: 4107-9 (1997), and
angiogenesis
assays such as induction of vascularization of the chick chorioallantoic
membrane
or induction of vascular endothelial cell migration as described in Ribatta et
al. , Intl.
J. Dev. Biol., 40: 1189-97 (1999) and Li et al., Clin. Exp. Metastasis, 17:423-
9
(1999) respectively. Suitable tumor cells lines are available, e.g. from
American
Type Tissue Culture Collection catalogs.
5.9.12 RECEPTOR/LIGAND ACTIVITY
A protein of the present invention may also demonstrate activity as receptor,
receptor ligand or inhibitor or agonist of receptor/ligand interactions. A
. polynucleotide of the invention can encode a polypeptide exhibiting such
characteristics. Examples of such receptors and ligands include, without
limitation,
107
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
cytokine receptors and their ligands, receptor kinases and their ligands,
receptor
phosphatases and their ligands, receptors involved in cell-cell interactions
and their
ligands (including without limitation, cellular adhesion molecules (such as
selectins,
integrins and their ligands) and receptor/ligand pairs involved in antigen
S presentation, antigen recognition and development of cellular and humoral
immune
responses. Receptors and ligands are also useful for screening of potential
peptide
or small molecule inhibitors of the relevant receptor/ligand interaction. A
protein of
the present invention (including, without limitation, fragments of receptors
and
ligands) rnay themselves be useful as inhibitors of receptor/ligand
interactions.
The activity of a protein of the invention may, among other means, be
measured by the following methods:
Suitable assays for receptor-ligand activity include without limitation those
described in: Current Protocols in Immunology, Ed by J. E. Coligan, A. M.
Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, Pub. Greene Publishing
1 S Associates and Wiley- Interscience (Chapter 7.28, Measurement of Cellular
Adhesion under static conditions 7.28.1- 7.28.22), Takai et al., Proc. Natl.
Acad.
Sci. USA 84:6864-6868, 1987; Bierer et al. , J. Exp. Med. 168:1145-1156, 1988;
. Rosenstein et al., J. Exp. Med. 169:149-160 1989; Stoltenborg et al., J.
Immunol.
Methods 17S:S9-68, 1994; Stitt et al., Cell 80:661-670, 1995.
By way of example, the polypeptides of the invention may be used as a
receptor for a ligand(s) thereby transmitting the biological activity of that
ligand(s).
Ligands may be identified through binding assays, affinity chromatography,
dihybrid screening assays, BIAcore assays, gel overlay assays, or other
methods
known in the art.
2S Studies characterizing drugs or proteins as agonist or antagonist or
partial
agonists or a partial antagonist require the use of other proteins as
competing
ligands. The polypeptides of the present invention or ligand(s) thereof may be
labeled by being coupled to radioisotopes, colorimetric molecules or a toxin
molecules by conventional methods. ("Guide to Protein Purification" Murray P.
Deutscher (ed) Methods in Enzymology VoI. 182 (I990) Academic Press, Inc. San
Diego). Examples of radioisotopes include, but are not limited to, tritium and
carbon-14 . Examples of colorimetric molecules include, but are not limited
to,
108
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
fluorescent molecules such as fluorescamine, or rhodamine or other
colorimetric
molecules. Examples of toxins include, but are not limited, to ricin.
5.9.13 DRUG SCREENING
This invention is particularly useful for screening chemical compounds by
using the novel polypeptides or binding fragments thereof in any of a variety
of drug
screening techniques. The polypeptides or fragments employed in such a test
may
either be free in solution, affixed to a solid support, borne on a cell
surface or
located intracellularly. One method of drug screening utilizes eukaryotic or
prokaryotic host cells which are stably transformed with recombinant nucleic
acids
expressing the polypeptide or fragment. Drugs are screened against such
transformed cells in competitive binding assays. Such cells, either in viable
or fixed
form, can be used for standard binding assays. One may measure, for example,
the
formation of complexes between polypeptides. of the invention or fragments and
the
agent being tested or examine the diminution in complex formation between the
novel polypeptides and an appropriate cell Line, which are well known in the
art.
Sources for test compounds that may be screened for ability to bind to or
modulate (i.e., increase or decrease) the activity of polypeptides of the
invention
include (1) inorganic and organic chemical libraries, (2) natural product
libraries,
and (3) combinatorial libraries comprised of either random or mimetic
peptides,
oligonucleotides or organic molecules.
Chemical libraries may be readily synthesized or purchased from a number
of commercial sources, and may include structural analogs of known compounds
or
compounds that are identified as "hits" or "leads" via natural product
screening.
The sources of natural product libraries are microorganisms (including
bacteria and fungi), animals, plants or other vegetation, or marine organisms,
and
libraries of mixtures for screening may be created by: (1) fermentation and
extraction of broths from soil, plant or marine microorganisms or (2)
extraction of
the organisms themselves. Natural product libraries include polyketides, non-
ribosomal peptides, and (non-naturally occurring) variants thereof. For a
review,
see Science 282:63-68 (1998).
109
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Combinatorial libraries are composed of large numbers of peptides,
oligonucleotides or organic compounds and can be readily prepared by
traditional
automated synthesis methods, PCR, cloning or proprietary synthetic methods. Of
particular interest are peptide and oligonucleotide combinatorial libraries.
Still other
libraries of interest include peptide, protein, peptidomimetic, multiparallel
synthetic
collection, recombinatorial, and polypeptide libraries. For a review of
combinatorial chemistry and libraries created therefrom, see Myers, Curr.
Opin.
Biotechnol. 8:701-707 (1997). For reviews and examples of peptidomimetic
libraries, see AI-Obeidi et al., Mol. Biotechnol, 9(3):205-23 (1998j; Hruby et
al.,
IO Curr Opin Chem Biol, 1(1):114-I9 (1997); Dorner et al., Bioorg Med Chem,
4(5):709-15 (1996) (alkylated dipeptides).
Identification of modulators through use of the various libraries described
herein permits modification of the candidate "hit" (or "lead") to optimize the
capacity of the "hit" to bind a polypeptide of the invention. The molecules
identified
in the binding assay are then tested for antagonist or agonist activity in in
vivo tissue
culture or animal models that are well known in the art. In brief, the
molecules are
titrated into a plurality of cell cultures or animals and then tested for
either
' cell/animal death or prolonged survival of the animal/cells.
The binding molecules thus identified may be complexed with toxins, e.g.,
ricin or cholera, or with other compounds that axe toxic to cells such as
radioisotopes. The toxin-binding molecule complex is then targeted to a tumor
or
other cell by the specificity of the binding molecule for a polypeptide of the
invention. Alternatively, the binding molecules may be complexed with imaging
agents for targeting and imaging purposes.
5.9.14 ASSAY FOR RECEPTOR ACTIVITY
The invention also provides methods to detect specific binding of a
polypeptide e.g. a ligand or a receptor. The art provides numerous assays
particularly useful for identifying previously unknown binding partners for
receptor
polypeptides of the invention. For example, expression cloning using mammalian
or
bacterial cells, or dihybrid screening assays can be used to identify
polynucleotides
encoding binding partners. As another example, affinity chromatography with
the
110
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
appropriate immobilized polypeptide of the invention can be used to isolate
polypeptides that recognize and bind polypeptides of the invention. There are
a
number of different libraries used for the identification of compounds, and in
particular small molecule, that modulate (i.e., increase or decrease)
biological
activity of a polypeptide of the invention. Ligands for receptor polypeptides
of the
invention can also be identified by adding exogenous ligands, or cocktails of
Iigands
to tvo cells populations that are genetically identical except for the
expression of the
receptor of the invention: one cell population expresses the receptor of the
invention
whereas the other does not. The response of the two cell populations to the
addition
of ligands(s) are then compared. Alternatively, an expression library can be
co-expressed with the polypeptide of the invention in cells and assayed for an
autocrine response to identify potential ligand(s). As still another example,
BIAcore
assays, gel overlay assays, or other methods known in the art can be used to
identify
binding partner polypeptides, including, (1) organic and inorganic chemical
libraries, (2) natural product libraries, and (3) combinatorial libraries
comprised of
random peptides, oligonucleotides or organic molecules.
The role of downstream intracellular signaling molecules in the signaling
cascade of the polypeptide of the invention can be determined. For example, a
chimeric protein in which the cytoplasmic domain of the polypeptide of the
invention is fused to the extracellular portion of a protein, whose ligand has
been
identified, is produced in a host cell. The cell is then incubated with the
ligand
specific for the extracellular portion of the chimeric protein, thereby
activating the
chimeric receptor. Known downstream proteins involved in intracellular
signaling
can then be assayed for expected modifications i.e. phosphorylation. Other
methods
known to those in the art can also be used to identify signaling molecules
involved
in receptor activity.
5.9.15 ANTI-INFLAMMATORY ACTIVITY
Compositions of the present invention may also exhibit anti-inflammatory
activity. The anti-inflammatory activity rnay be achieved by providing a
stimulus to
cells involved in the inflammatory response, by inhibiting or promoting cell-
cell
interactions (such as, for example, cell adhesion), by inhibiting or promoting
111
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
chemotaxis of cells involved in the inflammatory process, inhibiting or
promoting
cell extravasation, or by stimulating or suppressing production of other
factors
which more directly inhibit or promote an inflammatory response. Compositions
with such activities can be used to treat inflammatory conditions including
chronic
or acute conditions), including without limitation intimation associated with
infection
(such as septic shock, sepsis or systemic inflammatory response syndrome
(SIRS)),
ischemia-reperfusion injury, endotoxin lethality, arthritis, complement-
mediated
hyperacute rejection, nephritis, cytokine or chemokine-induced lung injury,
inflammatory bowel disease, Crohn's disease or resulting from over production
of
cytokines such as TNF or IL-1. Compositions of the invention may also be
useful to
treat anaphylaxis and hypersensitivity to an antigenic substance or material.
Compositions of this invention may be utilized to prevent or treat condition
such as,
but not limited to, utilized, for example, as part of methods for the
prevention
and/or treatment of disorders involving sepsis, acute pancreatitis, endotoxin
shock,
cytokine induced shock, rheumatoid arthritis, chronic inflammatory arthritis,
pancreatic cell damage from diabetes mellitus type 1, graft verses host
disease,
inflammatory bowel disease, inflamation associated with pulmonary disease,
other
autoimmune disease or inflammatory disease, an antiproliferative agent such as
for
acute or chronic mylegenous leukemia or in the prevention of premature labor
secondary to intrauterine infections.
5.9.16 LEUKEMIAS
Leukemias and related disorders may be treated or prevented by
administration of a therapeutic that promotes or inhibits function of the
polynucleotides and/or polypeptides of the invention. Such leukemias and
related
disorders include but are not limited to acute leukemia, acute lymphocytic
leukemia,
acute myelocytic leukemia, myeloblastic, promyelocytic, myelomonocytic,
monocytic, erythroleukemia, chronic leukemia, chronic myelocytic
(granulocytic)
leukemia and chronic lymphocytic leukemia (for a review of such disorders, see
Fishman et al., 1985, Medicine, 2d Ed., J.B. Lippincott Co., Philadelphia).
112
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.9.17 NERVOUS SYSTEM DISORDERS
Nervous system disorders, involving cell types which can be tested for
efficacy of intervention with compounds that modulate the activity of the
polynucleotides and/or polypeptides of the invention, and which can be treated
upon
thus observing an indication of therapeutic utility, include but are not
limited to
nervous system injuries, and diseases or disorders which result in either a
disconnection of axons, a diminution or degeneration of neurons, or
demyelination.
Nervous system lesions which may be treated in a patient (including human and
non-human mammalian patients) according to the invention include but are not
limited to the following lesions of either the central (including spinal cord,
brain) or
peripheral nervous systems:
(i) traumatic lesions, including lesions caused by physical injury or
associated with surgery, for example, lesions which sever a portion of the
nervous
system, or compression injuries;
(ii) ischemic lesions, in which a lack of oxygen in a portion of the
nervous system results in neuronal injury or death, including cerebral
infarction or
ischemia, or spinal cord infarction or ischemia;
(iii) infectious lesions, in which a portion of the nervous system is
destroyed or injured as a result of infection, for example, by an abscess or
associated with infection by human immunodeficiency virus, herpes zoster, or
herpes simplex virus or with Lyme disease, tuberculosis, syphilis;
(iv) degenerative lesions, in which a portion of the nervous system is
destroyed or injured as a result of a degenerative process including but not
limited to
degeneration associated with Parkinson's disease, Alzheimer's disease,
Huntington's
2S chorea, or amyotrophic lateral sclerosis;
(v) lesions associated with nutritional diseases or disorders, in which a
portion of the nervous system is destroyed or injured by a nutritional
disorder or
disorder of metabolism including but not limited to, vitamin B12 deficiency,
folic
acid deficiency, Wernicke disease, tobacco-alcohol amblyopia,
Marchiafava-Bignami disease (primary degeneration of the corpus callosum), and
alcoholic cerebellar degeneration;
11~
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
(vi) neurological lesions associated with systemic diseases including but
not limited to diabetes (diabetic neuropathy, Bell's palsy), systemic lupus
erythematosus, carcinoma, or sarcoidosis;
(vii) lesions caused by toxic substances including alcohol, lead, or
particular neurotoxins; and
(viii) demyelinated lesions in which a portion of the nervous system is
destroyed or injured by a demyelinating disease including but not limited to
multiple
sclerosis, human immunodeficiency virus-associated myelopathy, transverse
myelopathy or various etiologies, progressive rriultifocal
leukoencephalopathy, and
central pontine myelinolysis.
Therapeutics which are useful according to the invention for treatment of a
nervous system disorder may be selected by testing for biological activity in
promoting the survival or differentiation of neurons. For example, and not by
way
of limitation, therapeutics which elicit any of the following effects may be
useful
according to the invention:
(i) increased survival time of neurons in culture;
(ii) increased sprouting of neurons in culture or in vivo;
(iii) increased production of a neuron-associated molecule in culture or irz
vivo, e. g. , choline acetyltransferase or acetylcholinesterase with respect
to motor
neurons; or
(iv) decreased symptoms of neuron dysfunction in vivo.
Such effects may be measured by any method known in the art. Tn
preferred, non-limiting embodiments, increased survival of neurons may be
measured by the method set forth in Arakawa et al. (1990, J. Neurosci.
10:3507-3515); increased sprouting of neurons may be detected by methods set
forth
in Pestronk et al. (1980, Exp. Neurol. 70:65-82) or Brown et al. (1981, Ann.
Rev.
Neurosci. 4:17-42); increased production of neuron-associated molecules may be
measured by bioassay, enzymatic assay, antibody binding, Northern blot assay,
etc.,
depending on the molecule to be measured; and motor neuron dysfunction may be
measured by assessing the physical manifestation of motor neuron disorder,
e.g.,
weakness, motor neuron conduction velocity, or functional disability.
114
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
in specific embodiments, motor neuron disorders that may be treated
according to the invention include but are not limited to disorders such as
infarction,
infection, exposure to toxin, trauma, surgical damage, degenerative disease or
malignancy that may affect motor neurons as well as other components of the
nervous system, as well as disorders that selectively affect neurons such as
amyotrophic lateral sclerosis, and including but not limited to progressive
spinal
muscular atrophy, progressive bulbar palsy, primary lateral sclerosis,
infantile and
juvenile muscular atrophy, progressive bulbar paralysis of childhood (Fazio-
Londe
syndrome), poliomyelitis and the post polio syndrome, and Hereditary
Motorsensory
Neuropathy (Charcot-Marie-Tooth Disease).
5.9.18 ARTHRITIS ANII INFLAMMATION
The imrnunosuppressive effects of the compositions of the invention against
rheumatoid arthritis is determined in an experimental animal model system, The
experimental model system is adjuvant induced arthritis in rats, and the
protocol is
described by J. Holoshitz, et at., 1983, Science, 219:56, or by B. Waksman et
al.,
1963, Int. Arch. Allergy Appl. Irnmunol., 23:129. Induction of the disease can
be
caused by a single injection, generally intradermally, of a suspension of
killed
Mycobacterium tuberculosis in complete Freund's adjuvant (CFA). The route of
injection can vary, but rats may be injected at the base of the tail with an
adjuvant
mixture. The inhibitor is administered in phosphate buffered solution (PBS) at
a
dose of about 1-5 mg/kg. The control consists of administering PBS only.
The procedure for testing the effects of the test compound would consist of
intradermally injecting killed Mycobacterium tuberculosis in CFA followed by
immediately administering the inhibitor and subsequent treatment every other
day
until day 24. At 14, 15, 18, 20, 22, and 24 days after injection of
Mycobacterium
CFA, an overall arthritis score may be obtained as described by J. Holoskitz
above.
An analysis of the data would reveal that the test compound would have. a
dramatic
affect on the swelling of the joints as measured by a decrease of the
arthritis score.
115
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.9.19 OTHER ACTIVITIES
A protein of the invention may also exhibit one or more of the following
additional activities or effects: inhibiting the growth, infection or function
of, or
killing, infectious agents, including, without limitation, bacteria, viruses,
fungi and
other parasites; effecting (suppressing or enhancing) bodily characteristics,
including, without limitation, height, weight, hair color, eye color, skin,
fat to lean
ratio or other tissue pigmentation, or organ or body part size or shape (such
as, for
example, breast augmentation or diminution, change in bone form or shape);
effecting biorhythms or circadian cycles or rhythms; effecting the fertility
of male or
female subjects; effecting the metabolism, catabolism, anabolism, processing,
utilization, storage or elimination of dietary fat, lipid, protein,
carbohydrate,
vitamins, minerals, co-factors or other nutritional factors or component(s);
effecting
behavioral characteristics, including, without limitation, appetite, libido,
stress,
cognition (including cognitive disorders), depression (including depressive
1 S disorders) and violent behaviors; providing analgesic effects or other
pain reducing
effects; promoting differentiation and growth of embryonic stem cells in
lineages
other than hematopoietic lineages; hormonal or endocrine acrivity; in the case
of
enzymes, correcting deficiencies of the enzyme and treating deficiency-related
diseases; treatment of hyperproliferative disorders (such as, for example,
psoriasis);
immunoglobulin-like activity (such as, fox example, the ability to Bind
antigens or
complement); and the ability to act as an antigen in a vaccine composition to
raise
an immune response against such protein or another material or entity which is
cross-reactive with such protein.
. 5.9.20 IDENTIFICATION OF POLYMORPHISMS
The demonstration of polymorphisms makes possible the identification of
such polymorphisms in human subjects and the pharmacogenetic use of this
information for diagnosis and treatment. Such polymorphisms may be associated
with, e.g., differential predisposition or susceptibility to various disease
states (such
as disorders involving inflammation or immune response) or a differential
response
to drug administration, and this genetic information can be used to tailor
preventive
or therapeutic treatment appropriately. For example, the existence of a
116
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
polymorphism associated with a predisposition to inflammation or autoimmune
disease makes possible the diagnosis of this condition in humans by
identifying the
presence of the polymorphism.
Polymorphisms can be identified in a variety of ways known in the art which
all generally involve obtaining a sample from a patient, analyzing DNA from
the
sample, optionally involving isolation or amplification of the DNA, and
identifying
the presence of the polymorphism in the DNA. For example, PCR may be used to
amplify an appropriate fragment of genomic DNA which may then be sequenced.
Alternatively, the DNA may be subjected to allele-specific oligonucleotide
hybridization (in which appropriate oligonucleotides are hybridized to the DNA
under conditions permitting detection of a single base mismatch) or to a
single
nucleotide extension assay (in which an oligonucleotide that hybridizes
immediately
adjacent to the position of the polymorphism is extended with one or more
labeled
nucleotides). In addition, traditional restriction fragment length
polymorphism
analysis (using restriction enzymes that provide differential digestion of the
genomic
DNA depending on the presence or absence of the polymorphism) may be
performed. Arrays with nucleotide sequences of the present invention can be
used
to detect polymorphisms. The array can comprise modified nucleotide sequences
of
the present invention in order to detect the nucleotide sequences of the
present
invention. In the alternative, any one of the nucleotide sequences of the
present
invention can be placed on the array to detect changes from those sequences.
Alternatively a polymorphism resulting in a change in the amino acid
sequence could also be detected by detecting a corresponding change in amino
acid
sequence of the protein, e.g., by an antibody specific to the variant
sequence.
5.10 THERAPEUTIC METHODS
The compositions (including polypeptide fragments, analogs, variants and
antibodies or other binding partners or modulators including antisense
polynucleotides) of the invention have numerous applications in a variety of
therapeutic methods. Examples of therapeutic applications include, but are not
limited to, those exemplified herein.
117
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.10.1 EXAMPLES
Another embodiment of the invention is the administration of an effective
amount of the polypeptide or other composition of the invention to individuals
affected by a disease or disorder which can be modulated by regulating the
IgSF
member of the invention. While the mode of administration is not particularly
important, parenteral administration is preferred. An exemplary mode of
administration is to deliver an intravenous bolus. The dosage of the
polypeptide or
composition of the invention will normally be determined by the prescribing
physician. It is to be expected that the dosage will vary according to the
age,
weight, condition and response of the individual patient. Typically, the
amount of
protein or other active ingredient administered per dose will be in the range
of about
0.1 to 25 mg/kg of body weight, with the preferred dose being about 0.1 to 10
mg/kg of patient body weight. For parenteral administration, the polypeptides
or
other active ingredient of the invention will be formulated in an injectable
form that
includes a pharmaceutically acceptable parenteral vehicle. Such vehicles are
well
known in the art and examples include water, saline, Ringer's solution,
dextrose
solution, and solutions consisting of small amounts of the human serum
albumin.
The vehicle may contain minor amounts of additives that maintain the
isotonicity
and stability of the polypeptide or other active ingredient. The preparation
of such
solutions is within the skill of the art. Typically, the cytokine inhibitor
will be
formulated in such vehicles at a concentration of about 1-8 mg/ml to about 10
mg/ml.
2S 5.11 PHARMACEUTICAL FORMULATIONS AND ROUTES OF
ADMINISTRATION
A protein or other composition of the present invention (from whatever
source derived, including without limitation from recombinant and non-
recombinant
sources and including antibodies and other binding partners of the
polypeptides of
the invention) may be administered to a patient in need, by itself, or in
pharmaceutical compositions where it is mixed with suitable carriers or
excipient(s)
at doses to treat or ameliorate a variety of disorders. Such a composition may
118
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
optionally contain (in addition to protein or other active ingredient and a
carrier)
diluents, fillers, salts, buffers, stabilizers, solubilizers, and other
materials well
known in the art. The term "pharmaceutically acceptable" means a non-toxic
material that does not interfere with the effectiveness of the biological
activity of the
active ingredient(s). The characteristics of the carrier will depend on the
route of
administration. The pharmaceutical composition of the invention may also
contain
cytokines, lymphokines, or other hematopoietic factors such as M-CSF, GM-CSF,
TNF, IL-l, IL-2, IL-3, IL-4, IL-S, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-
12.
IL-13, IL-14, IL-15, IFN, TNF- , TNF1, TNF2, G-CSF, Meg-CSF,
thrombopoietin, stem cell factor, and erythropoietin. In further compositions,
proteins of the invention may be combined with other agents beneficial to the
treatment of the bone and/or cartilage defect, wound, or tissue in questions.
These
agents include various growth factors such as epidermal growth factor (EGF),
platelet-derived growth factor (PDGF), transforming growth factors (TGF- and
TGF- ), insulin-like growth factor (IGF), as well as cytokines described
herein.
The pharmaceutical composition may further contain other agents which
either enhance the activity of the protein or other active ingredient or
compliment its
activity or use in treatment. Such additional factors and/or agents may be
included
in the pharmaceutical composition to produce a synergistic effect with protein
or
other active ingredient of the invention, or to minimize side effects.
Conversely,
protein or other active ingredient of the present invention may be included in
formulations of the particular cytokine, lymphokine, other hematopoietic
factor,
thrombolytic or anti-thrombotic factor, or anti- inflammatory agent to
minimize side
effects of the cytokine, lymphokine, other hematopoietic factor, thrombolytic
or
anti-thrombotic factor, or anti-inflammatory agent. A protein of the present
invention may be active in multimers (e.g., heterodimers or homodimers) or
complexes with itself or other proteins. As a result, pharmaceutical
compositions of
the invention may comprise a protein of the invention in such multimeric or
complexed form.
As an alternative to being included in a pharmaceutical composition of the
invention including a first protein, a second protein or a therapeutic agent
may be
concurrently administered with the first protein (e.g., at the same time, or
at
119
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
differing times provided that therapeutic concentrations of the combination of
agents
is achieved at the treatment site). Techniques for formulation and
administration of
the compounds of the instant application may be found in "Remington's
Pharmaceutical Sciences," Mack Publishing Co., Easton, PA, latest edition. A
therapeutically effective dose further refers to that amount of the compound
sufficient to result in amelioration of symptoms, e.g., treatment, healing,
prevention
or amelioration of the relevant medical condition, or an increase in rate of
a treatment, healing, prevention or amelioration of such conditions: When
applied to
an individual active ingredient, administered alone, a therapeutically
effective dose
refers to that ingredient alone. When applied to a combination, a
therapeutically
effective dose refers to combined amounts of the active ingredients that
result in the
therapeutic effect, whether administered in combination, serially or
simultaneously.
In practicing the method of treatment or use of the present invention, a
therapeutically effective amount of protein or other active ingredient of the
present
invention is administered to a mammal having a condition to be treated.
Protein or
other active ingredient of the present invention may be administered in
accordance
with the method of the invention either alone or in combination w ith other
therapies
such as treatments employing cytokines, lymphokines or other hematopoietic
factors. When co- administered with one or more cytokines, lymphokines or
other
hematopoietic factors, protein or other active ingredient of the present
invention
may be administered either simultaneously with the cytokine(s), lymphokine(s),
other hematopoietic factor(s), thrombolytic or anti-thrombotic factors, or
sequentially. If administered sequentially, the attending physician will
decide on the
appropriate sequence of administering protein or other active ingredient of
the
present invention in combination with cytokine(s), lymphokine(s), other
hematopoietic factor(s), thrombalytic or anti-thrombotic factors.
5.11.1 ROUTES OF ADMINISTRATION
Suitable routes of administration may, for example, include oral, rectal,
transmucosal, or intestinal administration; parenteral delivery, including
intramuscular, subcutaneous, intramedullary injections, as well as
intrathecal, direct
intraventricular, intravenous, intraperitoneal, intranasal, or intraocular
injections.
120
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Administration of protein or other active ingredient of the present invention
used in
the pharmaceutical composition or to practice the method of the present
invention
can be carried out in a variety of conventional ways, such as oral ingestion,
inhalation, topical application or cutaneous, subcutaneous, intraperitoneal,
parenteral or intravenous injection. Intravenous administration to the patient
is
preferred.
Alternately, one may administer the compound in a local rather than
systemic manner, for example, via injection of the compound directly into a
arthritic
joints or in fibrotic tissue, often in a depot or sustained release
formulation. In
order to prevent the scarring process frequently occurring as complication of
glaucoma surgery, the compounds may be administered topically, for example, as
eye drops. Furthermore, one may administer the drug in a targeted drug
delivery
system, for example, in a liposome coated with a specific antibody, targeting,
for
example, arthritic or fibrotic tissue. The liposomes will be targeted to and
taken up
1~5 selectively by the afflicted tissue.
The polypeptides of the invention are administered by any route that delivers
an effective dosage to the desired site of action. The determination of a
suitable
route of administration and an effective dosage for a particular indication is
within
the level of skill in the art. Preferably for wound treatment, one administers
the
therapeutic compound directly to the site. Suitable dosage ranges for the
polypeptides of the invention can be extrapolated from these dosages or from
similar
studies in appropriate animal models. Dosages can then be adjusted as
necessary by
the clinician to provide maximal therapeutic benefit.
5.11.2 COMPOSITIONSIFORMULATIONS
Pharmaceutical compositions for use in accordance with the present
invention thus may be formulated in a conventional manner using one or more
physiologically acceptable carriers comprising excipients and auxiliaries
which
facilitate processing of the active compounds into preparations which can be
used
pharmaceutically. These pharmaceutical compositions may be manufactured in a
manner that is itself known, e.g., by means of conventional mixing,
dissolving,
granulating, dragee-making, levigating, emulsifying, encapsulating, entrapping
or
121
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
lyophilizing processes. Proper formulation is dependent upon the route of
administration chosen. When a therapeutically effective amount of protein or
other
active ingredient of the present invention is administered orally, protein or
other
active ingredient of the present invention will be in the form of a tablet,
capsule,
powder, solution or elixir. When administered in tablet form, the
pharmaceutical
composition of the invention may additionally contain a solid carrier such as
a
gelatin or an adjuvant. The tablet, capsule, and powder contain from about 5
to 95
J'~rein or other active ingredient of the present invention, and preferably
from
about 25 to 90 % protein or other active ingredient of the present invention.
When
administered in liquid form, a liquid carrier such as water, petroleum, oils
of animal
or plant origin such as peanut oil, mineral oil, soybean oil, or sesame oil,
or
synthetic oils may be added. The liquid form of the pharmaceutical composition
may further contain physiological saline solution, dextrose or other
saccharide
solution, or glycols such as ethylene glycol, propylene glycol or polyethylene
glycol. When administered in liquid form, the pharmaceutical composition
contains
from about 0.5 to 90 % by weight of protein or other active ingredient of the
present
invention, and preferably from about 1 to 50 % protein or other active
ingredient of
the present invention. '
When a therapeutically effective amount of protein or other active ingredient
of the present invention is administered by intravenous,' cutaneous or
subcutaneous
injection, protein or other active ingredient of the present invention will be
in the
form of a pyrogen-free, parenterally acceptable aqueous solution. The
preparation
of such parenterally acceptable protein or other active ingredient solutions,
having
due regard to pH, isotonicity, stability, and the like, is within the skill in
the art. A
preferred pharmaceutical composition for intravenous, cutaneous, or
subcutaneous
injection should contain, in addition to protein or other active ingredient of
the
present invention, an isotonic vehicle such as Sodium Chloride Injection,
Ringer's
Injection, Dextrose Injection, Dextrose and Sodium Chloride Injection,
Lactated
Ringer's Injection, or other vehicle as known in the art. The pharmaceutical
composition of the present invention may also contain stabilizers,
preservatives,
buffers, antioxidants, or other additives known to those of skill in the art.
For
injection, the agents of the invention may be formulated in aqueous solutions,
122
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
preferably in physiologically compatible buffers such as Hanks' solution,
Ringer's
solution, or physiological saline buffer. For transmucosal administration,
penetrants
appropriate to the barrier to be permeated are used in the formulation. Such
penetrants are generally known in the art.
For oral administration, the compounds can be formulated readily by
combining the active compounds with pharmaceutically acceptable carriers well
known in the art. Such carriers enable the compounds of the invention to be
formulated as tablets, pills, dragees, capsules, liquids, gels, syrups,
slurries,
suspensions and the like, for oral ingestion by a patient to be treated.
Pharmaceutical preparations for oral use can be obtained solid excipient,
optionally
grinding a resulting mixture, and processing the mixture of granules, after
adding
suitable auxiliaries, if desired, to obtain tablets or dragee cores. Suitable
excipients
are, in particular, fillers such as sugars, including lactose, sucrose,
mannitol, or
sorbitola cellulose preparations such as, for example, maize starch, wheat
starch,
rice starch, potato starch, gelatin, gum tragacanth, methyl cellulose,
hydroxypropylinethyl-cellulose, sodium carboxymethylcellulose, and/or
polyvinylpyrrolidone (PVP). If desired, disintegrating agents may be added,
such
as the cross-linked polyvinyl pyrrolidone, agar, or alginic acid or a salt
thereof such
as sodium alginate. Dragee cores are provided with suitable coatings. For this
purpose, concentrated sugar solutions may be used, which may optionally
contain
gum arabic, talc, polyvinyl pyrrolidone, carbopol gel, polyethylene glycol,
and/or
titanium dioxide, lacquer solutions, and suitable organic solvents or solvent
mixtures. Dyestuffs or pigments may be added to the tablets or dragee coatings
for
identification or to characterize different combinations of active compound
doses.
Pharmaceutical preparations which can be used orally include push-fit
capsules made of gelatin, as well as soft, sealed capsules made of gelatin and
a
plasticizer, such as glycerol or sorbitol. The push-fit capsules can contain
the active
ingredients in admixture with filler such as lactose, binders such as
starches, and/or
lubricants such as talc or magnesium stearate and, optionally, stabilizers. In
soft
capsules, the active compounds may be dissolved or suspended in suitable
liquids,
such as fatty oils, liquid paraffin, or liquid polyethylene glycols. In
addition,
stabilizers may be added. AlI formulations for oral administration should be
in
123
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
dosages suitable for such administration. For buccal administration, the
compositions may take the form of tablets or lozenges formulated in
conventional
manner.
For administration by inhalation, the compounds for use according to the
present invention are conveniently delivered in the form of an aerosol spray
presentation from pressurized packs or a nebuliser, with the use of a suitable
propellant, e. g. , dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In the case
of a
pressurized aerosol the dosage unit may be determined by providing a valve to
deliver a metered amount. Capsules and cartridges of, e.g., gelatin for use in
an
inhaler or insufflator may be formulated containing a powder mix of the
compound
and a suitable powder base such as lactose or starch,. The compounds may be
formulated for parenteral administration by inaection, e.g., by bolus
injection or
continuous infusion. Formulations for injection may be presented in unit
dosage
form, e.g., in ampules or in multi-dose containers, with an added
preservative. The
compositions may take such forms as suspensions, solutions or emulsions in
oily or
aqueous vehicles, and may contain formulatory agents such as suspending,
stabilizing and/or dispersing agents.
Pharmaceutical formulations. for par=enteral administration include aqueous
solutions of the active compounds in water-soluble form Additionally,
suspensions
of the active compounds may be prepared as appropriate oily injection
suspensions.
Suitable lipophilic solvents or vehicles include fatty oils such as sesame
oil, or
synthetic fatty acid esters, such as ethyl oleate or triglycerides, or
liposomes.
Aqueous injection suspensions may contain substances which increase the
viscosity
of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or
dextran..
Optionally, the suspension may also contain suitable stabilizers or agents
which
increase the solubility of the compounds to allow for the preparation of
highly
concentrated solutions. Alternatively, the active ingredient may be in powder
form
for constitution with a suitable vehicle, e. g. , sterile pyrogen-free water,
before use.
The compounds may also be formulated in rectal compositions such as
suppositories or retention enemas, e.g., containing conventional suppository
bases
such as cocoa butter or other glycerides. In addition to the formulations
described
124
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
previously, the compounds may also be formulated as a depot preparation. Such
long acting formulations may be administered by implantation (for example
subcutaneously or intramuscularly) or by intramuscular injection. Thus, for
example, the compounds may be formulated with suitable polymeric or
hydrophobic
materials (for example as an emulsion in an acceptable oil) or ion exchange
resins,
or as sparingly soluble derivatives, for example, as a sparingly soluble salt.
A pharmaceutical carrier for the hydrophobic compounds of the invention is
a cosolvent system comprising benzyl alcohol, a nonpolar surfactant, a
water-miscible organic polymer, and an aqueous phase. The cosolvent system may
be the VPD co-solvent system. VPD is a solution of 3 % w/v benzyl alcohol, 8 %
w/v of the nonpolar surfactant polysorbate 80, and 65 % w/v polyethylene
glycol
300, made up to volume in absolute ethanol. The VPD co-solvent system
(VPD:SW) consists of VPD diluted 1:1 with a 5% dextrose in water solution.
This
co-solvent system dissolves hydrophobic compounds well, and itself produces
low
toxicity upon systemic administration. Naturally, the proportions of a co-
solvent
system may be varied considerably without destroying its solubility and
toxicity
characteristics. Furthermore, the identity of the co-seilvent components may
be
varied: for example, other low-toxicity nonpolar surfactants may be used
instead of
polysorbate 80; the fraction size of polyethylene glycol may be varied; other
biocompatible polymers may replace polyethylene glycol, e.g. polyvinyl
pyrrolidone; and other sugars or polysaccharides may substitute for dextrose.
Alternatively, other delivery systems for hydrophobic pharmaceutical compounds
may be employed. Liposomes and emulsions are well known examples of delivery
vehicles or carriers for hydrophobic drugs. Certain organic solvents such as
dimethylsulfoxide also may be employed, although usually at the cost of
greater
toxicity. Additionally, the compounds may be delivered using a sustained-
release
system, such as semipermeable matrices of solid hydrophobic polymers
containing
the therapeutic agent. Various types of sustained-release materials have been
established and are well known by those skilled in the art. Sustained-release
capsules
may, depending on their chemical nature, release the compounds for a few weeks
up
to over I00 days. Depending on the chemical nature and the biological
stability of
125
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
the therapeutic reagent, additional strategies for protein or other active
ingredient
stabilization may be employed.
The pharmaceutical compositions also may comprise suitable solid or gel
phase carriers or excipients. Examples of such carriers or excipients include
but are
not limited to calcium carbonate, calcium phosphate, various sugars, starches,
cellulose derivatives, gelatin, and polymers such as polyethylene glycols.
Many of
the active ingredients of the invention may be provided as salts with
pharmaceutically compatible counterions. Such pharmaceutically acceptable base
addition salts are those salts which retain the biological effectiveness and
properties
of the free acids and which are obtained by reaction with inorganic or organic
bases
such as sodium hydroxide, magnesium hydroxide, ammonia, trialkylamine,
dialkylamine, monoalkylamine, dibasic amino acids, sodium acetate, potassium
benzoate, triethanol amine and the like.
The pharmaceutical composition of the invention may be in the form of a
complex of the proteins) or other active ingredient of present invention along
with
protein or peptide antigens. The protein and/or peptide antigen will deliver a
stimulatory signal to both B and T lymphocytes. B lymphocytes will respond to
antigen through their surface immunoglobulin receptor. T lymphocytes will
respond
to antigen through the T cell receptor (TCR) following presentation of the
aniigen
by MHC proteins. MHC and structurally related proteins including those,
encoded
by class I and class II MHC genes on host~cells will serve to present the
peptide
antigens) to T lymphocytes. The antigen components could also be supplied as
purified MHC-peptide complexes alone or with co-stimulatory molecules that can
directly signal T cells. Alternatively antibodies able to bind surface
immunoglobulin and other molecules on B cells as well as antibodies able to
bind
the TCR and other molecules on T cells can be combined with the pharmaceutical
composition of the invention. The pharmaceutical composition of the invention
may
be in the form of a liposome in which protein of the present invention is
combined,
in addition to other pharmaceutically acceptable carriers, with amphipathic
agents
such as lipids which exist in aggregated form as micelles, insoluble
monolayers,
liquid crystals, or lamellar layers in aqueous solution. Suitable lipids for
liposomal
formulation include, without limitation, monoglycerides, diglycerides,
sulfatides,
126
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
lysolecithins, phospholipids, saponin, bile acids, and the like. Preparation
of such
liposomal formulations is within the level of skill in the art, as disclosed,
for
example, in U.S. Patent Nos. 4,235,871; 4,SOI,728; 4,837,028; and 4,737,323,
all
of which are incorporated herein by reference.
The amount of protein or other active ingredient of the present invention in
the pharmaceutical composition of the present invention will depend upon the
nature
and severity of the condition being treated, and on the nature of prior
treatments
which the patient has undergone. Ultimately, the attending physician will
decide the
amount of protein or other active ingredient of the present invention with
which to
treat each individual patient. Initially, the attending physician will
administer low
doses of protein or other active ingredient of the present invention and
observe the
patient's response. Larger doses of protein or other active ingredient of the
present
invention may be administered until the optimal therapeutic effect is obtained
for the
patient, and at that point the dosage is not increased further. It is
contemplated that
the various pharmaceutical compositions used to practice the method of the
present
invention should contain about 0.01 ~.g to about 100 rng (preferably about 0.1
~,g to
about 10 mg, more preferably about 0.1 ,ug ~o about 1 mg) of protein or other
active
ingredient of the present invention per kg body weight. For compositions of
the
present invention which are useful for bone, cartilage, tendon or ligament
regeneration, the therapeutic method includes administering the composition
topically, systematically, or locally as an implant or device. When
administered,
the therapeutic composition for use in this invention is, of course, in a
pyrogen-free,
physiologically acceptable form. Further, the composition may desirably be
encapsulated or injected in a viscous form for delivery to the site of bone,
cartilage
or tissue damage. Topical administration may be suitable for wound healing and
tissue repair. Therapeutically useful agents other than a protein or other
active
ingredient of the invention which may also optionally be included in the
composition
as described above, may alternatively or additionally, be administered
simultaneously or sequentially with the composition in the methods of the
invention.
Preferably for bone and/or cartilage formation, the composition would include
a
matrix capable of delivering the protein-containing or other active
ingredient-containing composition to the site of bone and/or cartilage damage,
127
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
providing a structure for the developing bone and cartilage and optimally
capable of
being resorbed into the body. Such matrices may be formed of materials
presently
in use for other implanted medical applications.
The choice of matrix material is based on biocompatibility, biodegradability,
mechanical properties, cosmetic appearance and interface properties. The
particular application of the compositions will define the appropriate
formulation.
Potential matrices for the compositions may be biodegradable and chemically
defined calcium sulfate, tricalcium phosphate, hydroxyapatite, polylactic
acid,
polyglycolic acid and polyanhydrides. Other potential materials are
biodegradable
and biologically well-defined, such as bone or dermal collagen. Further
matrices
are comprised of pure proteins or extracellular matrix components. Other
potential
matrices are nonbiodegradable and chemically defined, such as sintered
hydroxyapatite, bioglass, aluminates, or other ceramics. Matrices may be
comprised of combinations of any of the above mentioned types of material,
such as
polylactic acid and hydroxyapatite or collagen and tricalcium phosphate. The
bioceramics may be altered in composition, such as in calcium-aluminate-
phosphate
and processing to alter pore size, particle size, particle shape, and
biodegradability.
Presently preferred is a 50:50 (mole weight) copolymer of lactic acid and
glycolic
acid in the form of porous particles having diameters ranging from 150 to 80U
microns. In some applications, it will be useful to utilize a sequestering
agent, such
as carboxymethyl cellulose or autologous blood clot, to prevent the protein
compositions from disassociating from the matrix.
A preferred family of sequestering agents is cellulosic materials such as
alkylcelluloses (including hydroxyalkylcelluloses), including methylcellulose,
ethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose,
hydroxypropyl-methylcellulose, and carboxymethylcellulose, the most preferred
being cationic salts of carboxymethylcellulose (CMC). Other preferred
sequestering
agents include hyaluronic acid, sodium alginate, polyethylene glycol),
polyoxyethylene oxide, carboxyvinyl polymer and polyvinyl alcohol). The amount
of sequestering agent useful herein is 0.5-20 wt % , preferably 1-10 wt %
based on
total formulation weight, which represents the amount necessary to prevent
desorption of the protein from the polymer matrix and to provide appropriate
128
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
handling of the composition, yet not so much that the progenitor cells are
prevented
from infiltrating the matrix, thereby providing the protein the opportunity to
assist
the osteogenic activity of the progenitor cells. In further compositions,
proteins or
other active ingredient of the invention may be combined with other agents
beneficial to the treatment of the bone and/or cartilage defect, wound, or
tissue in
question. These agents include various growth factors such as epidermal growth
factor (EGF), platelet derived growth factor. (PDGF), transforming growth
factors
(TGF- and T'GF- ), and insulin-like growth factor (IGF).
The therapeutic compositions are also presently valuable for veterinary
applications. Particularly domestic animals and thoroughbred horses, in
addition to
humans, are desired patients for such treatment with proteins or other active
ingredient of the present invention. The dosage regimen of a protein-
containing
pharmaceutical composition to be used in tissue regeneration will be
determined by
the attending physician considering various factors which modify the action of
the
proteins, e.g., amount of tissue weight desired to be formed, the site of
damage, the
condition of the damaged tissue, the size of a wound, type of damaged tissue
(e.g.,
bone), the patient's age, sex, and diet, the severity of any infection, time
of
administration and other clinical factors. The dosage may vary with the type
of
matrix used in the reconstitution and with inclusion of other proteins in the
pharmaceutical composition. For example, the addition of other known growth
factors, such as IGF I (insulin like growth factor I), to the final
composition, may
also effect the dosage. Progress can be monitored by periodic assessment of
tissue/bone growth and/or repair, for example, X-rays, histomorphometric
determinations and tetracycline labeling.
5.11.3. EFFECTIVE DOSAGE
Pharmaceutical compositions suitable for use in the present invention include
compositions wherein the active ingredients are contained in an effective
amount to
achieve its intended purpose. More specifically, a therapeutically effective
amount
means an amount effective to prevent development of or to alleviate the
existing
symptoms of the subject being treated. Determination of the effective amount
is
well within the capability of those skilled in the art, especially in light of
the detailed
129
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
disclosure provided herein. For any compound used in the method of the
invention,
the therapeutically effective dose can be estimated initially from appropriate
in vitro
assays. For example, a dose can be formulated in animal models to achieve a
circulating concentration range that can be used to more accurately determine
useful
doses in humans. For example, a dose can be formulated in animal models to
achieve a circulating concentration range that includes the ICso as determined
in cell
culture (i. e. , the concentration of the test compound which achieves a half
maximal
inhibition of the IgSF protein's biological activity). Such information can be
used to
more accurately determine useful doses in humans.
A therapeutically effective dose refers to that amount of the compound that
results in amelioration of symptoms or a prolongation of survival in a
patient.
Toxicity and therapeutic efficacy of such compounds can be determined by
standard
pharmaceutical procedures in cell cultures or experimental animals, e. g. ,
for
determining the LDso (the dose lethal to 50 % of the population) and the EDSO
(the
dose therapeutically effective in 50% of the population). The dose ratio
between
toxic and therapeutic effects is the therapeutic index and it can be expressed
as the
ratio between LDSO and EDso. Compounds which exhibit high therapeutic indices
are preferred. The data obtained from these cell culture assays and animal
studies
can be used in formulating a range of dosage for use in human. The dosage of
such
compounds lies preferably within a range of circulating concentrations that
include
the EDso with little or no toxicity. The dosage may vary within this range
depending upon the dosage form employed and the route of administration
utilized.
The exact formulation, route of administration and dosage can be chosen by the
individual physician in view of the patient's condition. See, e.g., Fingl et
al., 1975,
in "The Pharmacological Basis of Therapeutics", Ch. 1 p.1. Dosage amount and
interval may be adjusted individually to provide plasma levels of the active
moiety
which are sufficient to maintain the desired effects, or minimal effective
concentration (MEC). The MEC will vary for each compound but can be estimated
from in vitro data. Dosages necessary to achieve the MEC will depend on
individual characteristics and route of administration. However, HPLC assays
or
bioassays can be used to determine plasma concentrations.
130
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Dosage intervals can alsa be determined using MEC value. Compounds
should be administered using a regimen which maintains plasma levels above the
MEC fox 10-90 ~ of the time, pr eferably between 30-90 % and most preferably
between 50-90 % . In cases of local administration or selective uptake, the
effective
local concentration of the drug may not be related to plasma concentration.
An exemplary dosage regimen for polypeptides or other compositions of the
invention will be in the range of about 0.01 to 100 mglkg of body weight
daily, with
the preferred dose being about 0.1 to 25 mg/kg of patient body weight daily,
varying in adults and children. Dosing may be once daily, or equivalent doses
may
be delivered at longer or shorter intervals.
The amount of composition administered will, of course, be dependent on
the subject being treated, on the subject's age and weight, the severity of
the
affliction, the manner of administration and the judgment of the prescribing
physician.
5.11.4. PACKAGING
The compositions may, if desired, be presented in a pack or dispenser device
which may contain one or more unit dosage forms containing the active
ingredient.
The pack may, for example, comprise metal or plastic foil, such as a blister
pack.
The pack or dispenser device may be accompanied by instructions for
administration. Compositions comprising a compound of the invention formulated
in a compatible pharmaceutical carrier may also be prepared, placed in an
appropriate container, and labeled for treatment of an indicated condition.
5.12 ANTIBODIES
Also included in the invention are antibodies to proteins, or fragments of
proteins of the invention. The term "antibody" as used herein refers to
immunoglobulin molecules and immunologically active portions of immunoglobulin
(Ig) molecules, i.e., molecules that contain an antigen-binding site that
specifically
binds (immunoreacts with) an antigen. Such antibodies include, but are not
limited
to, polyclonal, monoclonal, chimeric, single chain, Fab, Fab~ and F~ab~>a
fragments,
and an Fab expression library. In general, an antibody molecule obtained from
131
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
humans relates to any of the classes.IgG, IgM, IgA, IgE, and IgD, which differ
from one another by the nature of the heavy chain present in the molecule.
Certain
classes have subclasses as well, such as IgG~, IgGz, and others. Furthermore,
in
humans, the light chain may be a kappa chain or a lambda chain. Reference
herein
to antibodies includes a reference to all such classes, subclasses, and types
of human
antibody species.
An isolated related protein of the invention may be intended to serve as an
antigen, or a portion or fragment thereof, and additionally can be used as an
immunogen to generate antibodies that immunospecifically bind the antigen,
using
standard techniques for polyclonal and monoclonal antibody preparation. The
full-length protein can be used or, alternatively, the invention provides
antigenic
peptide fragments of the antigen for use as immunogens. An antigenic peptide
fragment comprises at least 6 amino acid residues of the amino acid sequence
of the
full length protein, such as an amino acid sequence shown in SEQ ID NO: 10, 13-
24, 32 or 34 and encompasses an epitope thereof such that an antibody raised
against the peptide forms a specific immune complex with the full length
protein or
with any fragment that contains the epitope. Preferably, the antigenic peptide
comprises at least 10 amino acid residues, or at least 15 amino acid residues,
or at
least 20 amino acid residues, or at least 30 amino acid residues. Preferred
epitopes
encompassed by the antigenic peptide are regions of the protein that are
located on
its surface; commonly these are hydrophilic regions.
In certain embodiments of the invention, at least one epitope encompassed by
the antigenic peptide is a region of TGF alpha-like protein that is located on
the
surface of the protein, e.g., a hydrophilic region. A hydrophobicity analysis
of the
human related protein sequence will indicate which regions of a related
protein are
particularly hydrophilic and, therefore, are likely to encode surface residues
useful
for targeting antibody production. As a means for targeting antibody
production,
hydropathy plots showing regions of hydrophilicity and hydrophobicity may be
generated by any method well known in the art, including, for example, the
Kyte
Doolittle or the Hopp Woods methods, either with or without Fourier
transformation. See, e.g., Hopp and Woods, 1981, PYOC. Nat. Acad. Sci. USA 78:
3824-3828; Kyte and Doolittle 1982, J. Mol. Biol. 157: 105-142, each of which
is
132
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
incorporated herein by reference in its entirety. Antibodies that are specific
fox one
or more domains within an antigenic protein, or derivatives, fragments,
analogs or
homologs thereof, are also provided herein.
A protein of the invention, or a derivative, fragment, analog, homolog or
ortholog thereof, may be utilized as an immunogen in the generation of
antibodies
that immunospecifically bind these protein components.
The term "specific for" indicates that the variable regions of the antibodies
of the invention recognize and bind polypeptides of the invention exclusively
(i. e. ,
able to distinguish the polypeptide of the invention from other similar
polypeptides
despite sequence identity, homology, or similarity found in the family of
polypeptides), but may also interact with other proteins (for example, S.
aureus
protein A or other antibodies in ELTSA techniques) through interactions with
sequences outside the variable region of the antibodies, and in particular, in
the
constant region of the molecule. Screening assays to determine binding
specificity
of an antibody of the invention are well known and routinely practiced in the
art.
For a comprehensive discussion of such assays, see Harlow et al. (Eds),
Antibodies
A Laboratory Manual; Cold Spring Harbor Laboratory; Cold Spring Harbor, NY
(1988), Chapter 6. Antibodies that recognize and bind fragments of the
polypeptides of the invention are also contemplated, provided that the
antibodies are
first and foremost specific for, as defined above, full-length polypeptides of
the
invention. As with antibodies that are specific for full length polypeptides
of the
invention, antibodies of the invention that recognize fragments are those
which can
distinguish polypeptides from the same family of polypeptides despite inherent
sequence identity, homology, or similarity found in the family of proteins.
Antibodies of the invention are useful for, for example, therapeutic purposes
(by modulating activity of a polypeptide of the invention), diagnostic
purposes to
detect or quantitate a polypeptide of the invention, as well as purification
of a
polypeptide of the invention. Kits comprising an antibody of the invention for
any
of the purposes described herein are also comprehended. In general, a kit of
the
invention also includes a control antigen for which the antibody is
immunospecific.
The invention further provides a hybridoma that produces an antibody according
to
133
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
the invention. Antibodies of the invention are useful fox detection and/or
purification of the polypeptides of the invention.
Monoclonal antibodies binding to the protein of the invention may be useful
diagnostic agents for the immunodetection of the protein. Neutralizing
monoclonal
antibodies binding to the protein may also be useful therapeutics for both
conditions
associated with the protein and also in the treatment of some forms of cancer
where
abnormal expression of the protein is involved. In the case of cancerous cells
or
leukemic cells, neutralizing monoclonal antibodies against the protein may be
useful
in detecting and preventing the metastatic spread of the cancerous cells,
which may
be mediated by the protein.
The labeled antibodies of the present invention can be used for iu vitro, ira
vivo, and ifa situ assays to identify cells or tissues in which a fragment of
the
polypeptide of interest is expressed. The antibodies may also be used directly
in
therapies or other diagnostics. The present invention further provides the
above-described antibodies immobilized 'on a solid support. Examples of such
solid
supports include plastics such as polycarbonate, complex carbohydrates such as
agarose and Sepharose~, acrylic resins and such as polyacrylamide and latex
beads.
Techniques for coupling antibodies to such solid supports are well known in
the art
(Weir, D.M. et al., "Handbook of Experimental Immunology" 4th Ed., Blackwell '
Scientific Publications, Oxford, England, Chapter 10 (1986); Jacoby, W.D. et
aL,
Meth. Enzym. 34 Academic Press, N.Y. (1974)). The immobilized antibodies of
the present invention can be used for ih vitro, in vivo, and iya situ assays
as well as
for immuno-affinity purification of the proteins of the present invention.
Various procedures known within the art may be used for the production of
polyclonal or monoclonal antibodies directed against a protein of the
invention, or
against derivatives, fragments, analogs homologs, or orthologs thereof (see,
for
example, Antibodies: A Laboratory Manual, Harlow E, and Lane D, 1988, Cold
Spring Harbor Laboratory Press, Cold Spring Harbor, NY, incorporated herein by
reference). Some of these antibodies are discussed below.
134
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.12.1 POLYCLONAL ANTIBODIES
For the production of polyclonal antibodies, various suitable host animals
(e.g., rabbit, goat, mouse or other mammal) may be immunized by one or more
injections with the native protein, a synthetic variant thereof, or a
derivative of the
foregoing. An appropriate immunogenic preparation can contain, fox example,
the
naturally occurring immunogenic protein, a chemically synthesized polypeptide
representing the immunogenic protein, or a recombinantly expressed immunogenic
protein. Furthermore, the protein may be conjugated to a second protein known
to
be immunogenic in the mammal being immunized. Examples of such immunogenic
proteins include but are not limited to keyhole limpet hemocyanin, serum
albumin,
bovine thyroglobulin, and soybean trypsin inhibitor. The preparation can
further
include an adjuvant. Various adjuvants used to increase the immunological
response
include, but are not limited to, Freund's (complete and incomplete), mineral
gels
(e. g. , aluminum hydroxide), surface-active substances (e. g. , lysolecithin,
pluronic
polyols, polyanions, peptides, oil emulsions, dinitrophenol, etc.), adjuvants
usable
in humans such as Bacille Calmette-Guerin and Corynebacterium parvum, or
similar
immunostimulatory agents. Additional examples of adjuvants that can be
employed
include MPL-TDM adjuvant (monophosphoryl Lipid A, synthetic trehalose
dicorynomycolate) .
The polyclonal antibody molecules directed against the immunogenic protein
can be isolated from the mammal (e.g., from the blood) and further purified by
well
known techniques, such as affinity chromatography using protein A or protein
G,
which provide primarily the IgG fraction of immune serum. Subsequently, or
alternatively, the specific antigen which is the target of the immunoglobulin
sought,
or an epitope thereof, may be immobilized on a column to purify the immune
specific antibody by immunoaffinity chromatography. Purification of
immunoglobulins is discussed, for example, by D. Wilkinson (The Scientist,
published by The Scientist, Inc., Philadelphia PA, Vol. 14, No. 8 (April 17,
2000),
pp. 25-28).
135
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.12.2 MONOCLONAL ANTIBODIES
The term "monoclonal antibody" (MAb) or "monoclonal antibody
composition", as used herein, refers to a population of antibody molecules
that
contain only one molecular species of antibody molecule consisting of a unique
light
chain gene product and a unique heavy chain gene product. In particular, the
complementarity determining regions (CDRs) of the monoclonal antibody are
identical in all the molecules of the population. MAbs thus contain an antigen-
binding site capable of immunoreacting with a particular epitope of the
antigen
characterized by a unique binding affinity fox it.
Monoclonal antibodies can be prepared using hybridoma methods, such as
those described by Kohler and Milstein, Nature, 256:495 (1975). In a hybridoma
method, a mouse, hamster, or other appropriate host animal, is typically
immunized
with an immunizing agent to elicit lymphocytes that produce or are capable of
producing antibodies that will specifically bind to the immunizing agent.
Alternatively, the lymphocytes can be immunized in vitro.
The immunizing agent will typically include the protein antigen, a fragment
thereof, or a fusion protein thereof. Generally, either peripheral blood
lymphocytes
are used if cells of human origin are desired, or spleen cells or lymph node
cells are
used if non-human mammalian sources are desired. The lymphocytes are then
fused
with an immortalized cell line using a suitable fusing agent, such as
polyethylene
glycol, to form a hybridoma cell (coding, Monoclonal Antibodies: Principles
and
Practice, Academic Press, (1986) pp. 59-103). Immortalized cell lines are
usually
transformed mammalian cells, particularly myeloma cells of rodent, bovine, and
human origin. Usually, rat or mouse myeloma cell lines are employed. The
hybridoma cells can be cultured in a suitable culture medium that preferably
contains one or more substances that inhibit the growth or survival of the
unfused,
immortalized cells. For example, if the parental cells lack the enzyme
hypoxanthine
guanine phosphoribosyl transferase (HGPRT or HPRT), the culture medium for the
hybridomas typically will include hypoxanthine, aminopterin, and thymidine
("HAT
medium"), which substances prevent the growth of HGPRT-deficient cells.
Preferred immortalized cell lines are those that fuse efficiently, support
stable high level expression of antibody by the selected antibody-producing
cells,
136
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
and are sensitive to a medium such as HAT medium. More preferred immortalized
cell lines are murine myeloma lines, which can be obtained, for instance, from
the
Salk Institute Cell Distribution Center, San Diego, California and the
American
Type Culture Collection, Manassas, Virginia. Human myeloma and mouse-human
heteromyeloma cell lines also have been described for the production of human
monoclonal antibodies (Kozbor, J. Immunol. , 133:3001 (1984); Brodeur et al. ,
Monoclonal Antibody Production Techniques and Applications, Marcel Dekker,
Inc., New York, (1987) pp. 51-63).
The culture medium in which the hybridoma cells are cultured can then be
assayed for the presence of monoclonal antibodies directed against the
antigen.
Preferably, the binding specificity of monoclonal antibodies produced by the
hybridoma cells is determined by immunoprecipitation or by an in vitro binding
assay, such as radioimmunoassay (RIA) or enzyme-linked immunoabsorbent assay
(ELISA). Such techniques and assays are known in the art. The binding affinity
of
the monoclonal antibody can, for example, be determined by the Scatchard
analysis
of Munson and Pollard, Anal. Biochem., 107:220 (1980). Preferably, antibodies
having a high degree of specificity and a high binding affinity for the target
antigen
are isolated.
After the desired hybridoma cells are identified, the clones can be subcloned
by limiting dilution procedures and grown by standard methods. Suitable
culture
media for this purpose include, for example, Dulbecco's Modified Eagle's
Medium
and RPMI-1640 medium. Alternatively, the hybridoma cells can be grown in vivo
as ascites in a mammal.
The monoclonal antibodies secreted by the subclones can be isolated or
purified from the culture medium or ascites fluid by conventional
immunoglobulin
purification procedures such as, for example, protein A-Sepharose,
hydroxylapatite
chromatography, gel electrophoresis, dialysis, or affinity chromatography.
The monoclonal antibodies can also be made by recombinant DNA methods,
such as those described in U.S. Patent No. 4,816,567. DNA encoding the
monoclonal antibodies of the invention can be readily isolated and sequenced
using
conventional procedures (e.g., by using oligonucleotide probes that are
capable'of
binding specifically to genes encoding the heavy and light chains of murine
137
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
antibodies). The hybridoma cells of the invention serve as a preferred source
of
such DNA. Once isolated, the DNA can be placed into expression vectors, which
are then transfected into host cells such as simian COS cells, Chinese hamster
ovary
(CHO) cells, or myeloma cells that do not otherwise produce immunoglobulin
protein, to obtain the synthesis of monoclonal antibodies in the recombinant
host
cells. The DNA also can be modified, for example, by substituting the coding
sequence for human heavy and light chain constant domains in place of the
homologous murine sequences (U.S. Patent No. 4,816,567; Morrison, Nature 368,
812-13 (1994)) or by covalently joining to the immunoglobulin coding sequence
all
or part of the coding sequence for a non-immunoglobulin polypeptide. Such a
non-
immunoglobulin polypeptide can be substituted for the constant domains of an
antibody of the invention, or can be substituted for the variable domains of
one
antigen-combining site of an antibody of the invention to create a chimeric
bivalent
antibody.
5.12.3 HIJIVIANIZED ANTIBODIES
The antibodies directed against the protein antigens of the invention can
further comprise humanized antibodies or human antibodies. These antibodies
are
suitable for administration to humans without engendering an immune response
by
the human against the administered immunoglobulin. Humanized forms of
antibodies are chimeric immunoglobulins, immunoglobulin chains, or fragments
thereof (such as Fv, Fab, Fab' , Flab' )z or other antigen-binding
subsequences of
antibodies) that are principally comprised of the sequence of a human
immunoglobulin, and contain minimal sequence derived from a non-human
immunoglobulin. Humanization can be performed following the method of Winter
and co-workers (Jones et al., Nature, 321:522-525 (1986); Riechmann et al.,
Nature, 332:323-327 (1988); Verhoeyen et al., Science, 239:1534-1536 (1988)),
by
substituting rodent CDRs or CDR sequences for the corresponding sequences of a
human antibody. (See also U.S. Patent No. 5,225,539). In some instances, Fv
framework residues of the human immunoglobulin are replaced by corresponding
non-human residues. Humanized antibodies can also comprise residues that are
found neither in the recipient antibody nor in the imported CDR or framework
138
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
sequences. In general, the humanized antibody will comprise substantially all
of at
least one, and typically two; variable domains, in which all or substantially
all of the
CDR regions correspond to those of a non-human immunoglobulin and all or
substantially all of the framework regions are those of a human immunoglobulin
consensus sequence. The humanized antibody optimally also will comprise at
least
a portion of an immunoglobulin constant region (Fc), typically that of a human
immunoglobulin (Jones et al., 1986; Riechmann et al., 1988; and Presta, Curr.
Op.
Struct. Biol., 2:593-596 (1992)).
5.12.4 HUMAN ANTIBODIES
Fully human antibodies relate to antibody molecules in which essentially the
entire sequences of both the light chain and the heavy chain, including the
CDRs,
arise from human genes. Such antibodies are termed "human antibodies", or
"fully
human antibodies" herein. Human monoclonal antibodies can be prepared by the
trioma technique; the human B-cell hybridoma technique (see Kozbor, et al.,
1983
Immunol Today 4: 72) and the EBV hybridoma technique to produce human
monoclonal antibodies (see Cole, et al. , 1985 In: MONOCLONAL ANTIBODIES AND
CANCER THERAPY, Alan R. Liss, Inc., pp. 77-96). Human monoclonal antibodies
may be utilized in the practice of the present invention and may be produced
by
using human hybridomas (see Cote, et al. , 1983 . Proc Natl Acad Sci USA 80:
2026-2030) or by transforming human B-cells with Epstein Barr Virus in vitro
(see
Cole, et al. , 1985 In: MONOCLONAL ANTIBODIES AND CANCER THERAPY, Alan R.
Liss, Inc., pp. 77-96).
In addition, human antibodies can also be produced using additional
techniques, including phage display libraries (Hoogenboom and Winter, J. Mol.
Biol., 227:381 (1991); Marks et al., J. Mol. Biol., 222:581 (1991)).
Similarly,
human antibodies can be made by introducing human immunoglobulin loci into
transgenic animals, e.g., mice in which the endogenous immunoglobulin genes
have
been partially or completely inactivated. Upon challenge, human antibody
production is observed, which closely resembles that seen in humans in all
respects,
including gene rearrangement, assembly, and antibody repertoire. This approach
is
described, for example, in U.S. Patent Nos. 5,545,807; 5,545,806; 5,569,825;
139
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5,625,126; 5,633,425; 5,661,016, and in Marks et al. (Bio/Technology 10, 779-
783
(1992)); Lonberg et al. (Nature 368 856-859 (1994)); Morrison (Nature 368, 812-
13
(1994)); Fishwild et al, (Nature Biotechnology 14, 845-S1 (1996)); Neuberger
(Nature Biotechnology 14, 826 ( 1996)); and Lonberg and Huszar (Intern. Rev.
Immunol. 13 65-93 (1995)).
Human antibodies may additionally be produced using transgenic nonhuman
animals that are modified so as to produce fully human antibodies rather than
the
animal's endogenous antibodies in response to challenge by an antigen. (See
PCT
publication W094/02602). The endogenous genes encoding the heavy and light
immunoglobulin chains in the nonhuman host have been incapacitated, and active
loci encoding human heavy and light chain immunoglobulins are inserted into
the
host's genome. The human genes are incorporated, for example, using yeast
artificial chromosomes containing the requisite human DNA segments. An animal
which provides all the desired modifications is then obtained as progeny by
crossbreeding intermediate transgenic animals containing fewer than the full
complement of the modifications. The preferred embodiment of such a nonhuman
animal is a mouse, and is termed the XenomouseTM as disclosed in PCT
publications
WO 96/33735 and WO 96/34096. This animal produces B cells that secrete fully
human immunoglobulins. The antibodies can be obtained directly from the animal
after immunization with an immunogen of interest, as, for example, a
preparation of
a polyclonal antibody, or alternatively from immortalized B cells derived from
the
animal, such as hybridomas producing monoclonal antibodies. Additionally, the
genes encoding the immunoglobulins with human variable regions can be
recovered
and expressed to obtain the antibodies directly, or can be further modified to
obtain
analogs of antibodies such as, for example, single chain Fv molecules.
An example of a method of producing a nonhuman host, exemplified as a
mouse, lacking expression of an endogenous immunoglobulin heavy chain is
disclosed in U.S. Patent No. 5,939,598. It can be obtained by a method
including
deleting the J segment genes from at least one endogenous heavy chain locus in
an
embryonic stem cell to prevent rearrangement of the locus and to prevent
formation
of a transcript of a rearranged immunoglobulin heavy chain locus, the deletion
being
effected by a targeting vector containing a gene encoding a selectable marker;
and
140
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
producing from the embryonic stem cell a transgenic mouse whose somatic and
germ cells contain the gene encoding the selectable marker.
A method for producing an antibody of interest, such as a human antibody,
is disclosed in U.S. Patent No. 5,916,771. It includes introducing an
expression
vector that contains a nucleotide sequence encoding a heavy chain into one
mammalian host cell in culture, introducing an expression vector containing a
nucleotide sequence encoding a light chain into another mammalian host cell,
and
fusing the two cells to form a hybrid cell. The hybrid cell expresses an
antibody
containing the heavy chain and the light chain.
In a further improvement on this procedure, a method for identifying a
clinically relevant epitope on an immunogen, and a correlative method for
selecting
an antibody that binds immunospecifically to the relevant epitope with high
affinity,
are disclosed in PCT publication WO 99/53049.
5.12.5 FAB FRAGMENTS AND SINGLE CHAIN ANTIBODIES
' According to the invention, techniques can, be adapted fox the production of
single-chain antibodies specific to an antigenic protein of the invention (see
e.g.,
U.S. Patent No. 4,946,778). In addition, methods can be adapted for the
construction of Fabexpression libraries (see e.g., Huse, et al., 1989 Science
246:
1275-1281) to allow rapid and effective identification of monoclonal Fab
fragments
with the desired specificity for a protein or derivatives, fragments, analogs
or
homologs thereof. Antibody fragments that contain the idiotypes to a protein
antigen may be produced by techniques known in the art including, but not
limited
to: (i) an F~ab~>z fragment produced by pepsin digestion of an antibody
molecule; (ii)
an Fan fragment generated by reducing the disulfide bridges of an F~ab'>z
fragment;
(iii) an Fab fragment generated by the treatment of the antibody molecule with
papain
and a reducing agent and (iv) F~ fragments.
5.12.6 BISPECIFIC ANTIBODIES
Bispecific antibodies are monoclonal, preferably human or humanized,
antibodies that have binding specificities for at least two different
antigens. In the
present case, one of the binding specificities is for an antigenic protein of
the
141
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
invention. The second binding target is any other antigen, and advantageously
is a
cell-surface protein or receptor or receptor subunit.
Methods for making bispecific antibodies are known in the art.
Traditionally, the recombinant production of bispecific antibodies is based on
the
co-expression of two immunoglobulin heavy-chain/light-chain pairs, where the
two
heavy chains have different specificities (Milstein and Cuello, Nature,
305:537-539
(1983)). Because of the random assortment of immunoglobulin heavy and light
chains, these hybridomas (quadromas) produce a potential mixture of ten
different
antibody molecules, of which only one has the correct bispecific structure.
The
purification of the correct molecule is usually accomplished by affinity
chromatography steps. Similar procedures are disclosed in WO 93/08829,
published 13 May 1993, and in Traunecker et al., 1991 EMBO J., 10:3655-3659.
Antibody variable domains with the desired binding specificities (antibody-
antigen combining sites) can be fused to immunoglobulin constant domain
sequences. The fusion preferably is with an immunoglobulin heavy-chain
constant
domain, comprising at least part of the hinge, CH2, and CH3 regions. It is
preferred to have the first heavy-chain constant region (CH1) containing the
site
necessary for light-chain binding present in at least one of the fusions. DNAs
encoding the immunoglobulin heavy-chain fusions and, if desired, the
immunoglobulin light chain, are inserted into separate expression vectors, and
are
co-transfected into a suitable host organism. For further details of
generating
bispecific antibodies see, for example, Suresh et al., Methods in Enzymology,
121:210 (1986).
According to another approach described in WO 96/27011, the interface
between a pair of antibody molecules can be engineered to maximize the
percentage
of heterodimers that are recovered from recombinant cell culture. The
preferred
interface comprises at least a part .of the CH3 region of an antibody constant
domain. In this method, one or more small amino acid side chains from the
interface of the first antibody molecule are replaced with larger side chains
(e.g.
tyrosine or tryptophan). Compensatory "cavities" of identical or similar size
to the
large side chains) are created on the interface of the second antibody
molecule by
replacing large amino acid side chains with smaller ones (e.g. alanine or
threonine).
142
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
This provides a mechanism for increasing the yield of the heterodimer over
other
unwanted end-products such as homodimers.
Bispecific antibodies can be prepared as full length antibodies or antibody
fragments (e.g. F(ab')z bispecific antibodies). Techniques for generating
bispecific
antibodies from antibody fragments have been described in the literature. For
example, bispecific antibodies can be prepared using chemical linkage. Brennan
et
al., Science 229:81 (1985) describe a procedure wherein intact antibodies are
proteolytically cleaved to generate F(ab')z fragments. These fragments are
reduced
in the presence of the dithiol complexing agent sodium arsenite to stabilize
vicinal
dithiols and prevent intermolecular disulfide formation. The Fab' fragments
generated are then converted to thionitrobenzoate (TNB) derivatives. One of
the
Fab'-TNB derivatives is then reconverted to the Fab'-thiol by reduction with
mercaptoethylamine and is mixed with an equimolar amount of the other Fab'-TNB
derivative to form the bispecific antibody. The bispecific antibodies produced
can
be used as agents for the selective immobilization of enzymes.
Additionally, Fab' fragments can be directly recovered from E. coli and
chemically coupled to form bispecific antibodies. Shalaby et al., J. Exp. Med.
175:217-22S ( 1992) describe the production of a fully humanized bispecific
antibody
F(ab')z molecule. Each Fab' fragment was separately secreted from E. coli and
subjected to directed chemical coupling in vitro to form the bispecific
antibody. The
bispecific antibody thus formed was able to bind to cells overexpressing the
ErbB2
receptor and normal human T cells, as well as trigger the lytic activity of
human
cytotoxic lymphocytes against human breast tumor targets.
Various techniques for making and isolating bispecific antibody fragments
2S directly from recombinant cell culture have also been described. For
example,
bispecific antibodies have been produced using leucine zippers. Kostelny et
al., J.
Immunol. 148(S):1547-1553 (1992). The leucine zipper peptides from the Fos and
Jun proteins were linked to the Fab' portions of two different antibodies by
gene
fusion. The antibody homodimers were reduced at the hinge region to form
monomers and then re-oxidized to form the antibody heterodimers. This method
can also be utilized for the production of antibody homodimers. The "diabody"
technology described by Hollinger et al. , Proc. Natl. Acad. Sci. USA 90:6444-
6448
143
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
(1993) has provided an alternative. mechanism for making bispecific antibody
fragments. The fragments comprise a heavy-chain variable domain (Va) connected
to a light-chain variable domain (VL) by a linker which is too short to allow
pairing
between the two domains on the same chain. Accordingly, the VH and VL domains
of one fragment are forced to pair with the complementary VL and VH domains of
another fragment, thereby forming two antigen-binding sites. Another strategy
for
making bispecific antibody fragments by the use of single-chain Fv (sFv)
dimers has
also been reported. See, Gruber et al., J. Immunol. 152:5368 (1994).
Antibodies with more than two valencies are contemplated. For example,
trispecific antibodies can be prepared. Tutt et al., J. Immunol. 147:60
(1991).
Exemplary bispecific antibodies can bind to two different epitopes, at least
one of which originates in the protein antigen of the invention.
Alternatively, an
anti-antigenic arm of an immunoglobulin molecule can be combined with an arm
which binds to a triggering molecule on a leukocyte such as a T-cell receptor
molecule (e.g. CD2, CD3, CD28, or B7), or Fc receptors for IgG (Fc R), such as
Fc RI (CD64), Fc RII (CD32) and Fc R~II (CD16) so as to focus cellular defense
mechanisms to the cell expressing the particular antigen. Bispecific
antibodies can
also be used to direct cytotoxic agents to cells which express a particular
antigen.
These antibodies possess an antigen-binding arm and an arm which binds a
cytotoxic
agent or a radionuclide chelator, such as EOTUBE, DPTA, DOTA, or TETA.
Another bispecific antibody of interest binds the protein antigen described
herein
and further binds tissue factor (TF).
5.12.7 HETEROCONJUGATE ANTIBODIES
Heteroconjugate antibodies are also within the scope of the present invention.
Heteroconjugate antibodies are composed of two covalently joined antibodies.
Such
antibodies have, for example, been proposed to target immune system cells to
unwanted cells (U.S. Patent No. 4,676,980), and for treatment of HIV infection
(WO 91/00360; WO 92/200373; EP 03089). It is contemplated that the antibodies
can be prepared in vitro using known methods in synthetic protein chemistry,
including those involving crosslinking agents. For example, immunotoxins can
be
constructed using a disulfide exchange reaction or by forming a thioether
bond.
144
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Examples of suitable reagents for this purpose include iminothiolate and
methyl-4-
mercaptobutyrimidate and those disclosed, for example, in U.S. Patent No.
4,676,980.
5.12.8 EFFECTOR FUNCTION ENGINEERING
It can be desirable to modify the antibody of the invention with respect to
effector function, so as to enhance, e.g., the effectiveness of the antibody
in
treating cancer. For example, cysteine residues) can be introduced into the Fc
region, thereby allowing interchain disulfide bond formation in this region.
The
homodimeric antibody thus generated can have improved internalization
capability
and/or increased complement-mediated cell killing and antibody-dependent
cellular
cytotoxicity (ADCC). See Caron et al., J. Exp Med., 176: 1191-1195 (1992) and
Shopes, J. Immunol., 148: 2918-2922 (1992). Homodimeric antibodies with
enhanced anti-tumor activity can also.be prepared using heterobifunctional
cross-
linkers as described in Wolff et al. Cancer Research, 53: 2560-2565 (1993).
Alternatively, an antibody can be engineered that has dual Fc regions and can
thereby have enhanced complement Iysis and ADCC capabilities. See Stevenson et
al., Anti-Cancer Drug Design, 3: 219-230 (1989).
5.12.9 IMMLTNOCONJUGATES
The invention also pertains to immunoconjugates comprising an antibody
conjugated to a cytotoxic agent such as a chemotherapeutic agent, toxin (e. g.
, an
enzymatically active toxin of bacterial, fungal, plant, or animal origin, or
fragments
thereof), or a radioactive isotope (i.e., a radioconjugate).
Chemotherapeutic agents useful in the generation of such immunoconjugates
have been described above. Enzymatically active toxins and fragments thereof
that
can be used include diphtheria A chain, nonbinding active fragments of
diphtheria
toxin, exotoxin A chain (from Pseudomonas aeruginosa), ricin A chain, abrin A
chain, modeccin A chain, alpha-sarcin, Aleurites fordii proteins, dianthin
proteins,
Phytolaca americana proteins (PAPI, PAPA, and PAP-S), momordica charantia
inhibitor, curcin, crotin, sapaonaria officinalis inhibitor, gelonin,
mitogellin,
restrictocin, phenomycin, enomycin, and the tricothecenes. A variety of
145
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
radionuclides are available for the production of radioconjugated antibodies.
Examples include zlzBi, 1311, I'IIn, 9°Y, and j$°Re.
Conjugates of the antibody and cytotoxic agent are made using a variety of
bifunctional protein-coupling agents such as N-succinimidyl-3-(2-
pyridyldithiol)
propionate (SPDP), iminothiolane (IT), bifunctional derivatives of imidoesters
(such
as dimethyl adipimidate HCL), active esters (such as disuccinimidyl suberate),
aldehydes (such as glutareldehyde), bis-azido compounds (such as bis (p-
azidobenzoyl) hexanediamine), bis-diazonium derivatives (such as bis-(p-
diazoniumbenzoyl)-ethylenediamine), diisocyanates (such as tolyene 2,6-
diisocyanate), and bis-active fluorine compounds (such as 1,5-difluoro-2,4-
dinitrobenzene). For example, a ricin immunotoxin can be prepared as described
in
Vitetta et al., Science, 238: 1098 (1987). Carbon-14-labeled 1-
isothiocyanatobenzyl-3-methyldiethylene triaminepentaacetic acid (MX-DTPA) is
an
exemplary chelating agent for conjugation of radionucleotide to the antibody.
See
W094/I1026.
In another embodiment, the antibody can be conjugated to a "receptor" (such
streptavidin) for utilization in tumor pretargeting wherein the antibody-
receptor
conjugate is administered to the patient, followed by removal of unbound
conjugate
from the circulation using a clearing agent and then administration of a
"ligand"
(e.g., avidin) that is in turn conjugated to a cytotoxic agent.
5.13 COMPUTER READABLE SEQUENCES
In one application of this embodiment, a nucleotide sequence of the present
invention can be recorded on computer readable media. As used herein,
"computer
readable media" refers to any medium which can be read and accessed directly
by a
computer. Such media include, but are not limited to: magnetic storage media,
such
as floppy discs, hard disc storage medium, and magnetic tape; optical storage
media
such as CD-ROM; electrical storage media such as RAM and ROM; and hybrids of
these categories such as magnetic/optical storage media. A skilled artisan can
readily appreciate how any of the presently known computer readable mediums
can
be used to create a manufacture comprising computer readable medium having
recorded thereon a nucleotide sequence of the present invention. As used
herein,
146
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
"recorded" refers to a process for storing information on computer readable
medium. A skilled artisan can readily adopt any of the presently known methods
for recording information on computer readable medium to generate manufactures
comprising the nucleotide sequence information of the present invention.
A variety of data storage structures are available to a skilled artisan for
creating a computer readable medium having recorded thereon a nucleotide
sequence of the present invention. The choice of the data storage structure
will
generally be based on the means chosen to access the stored information. In
addition, a variety of data processor programs and formats can be used to
store the
nucleotide sequence information of the present invention on computer readable
medium. The sequence information can be represented in a word processing text
file, formatted in commercially-available software such as WordPerfect and
Microsoft Word, or represented in the form of an ASCII file, stored in a
database
application, such as DB2, Sybase, Oracle, or the like. A skilled artisan can
readily
adapt any number of data processor structuring formats (e.g.. text file or
database) in
order to obtain computer readable medium having recorded thereon the
nucleotide
sequence information of the present invention. '
By providing any of the nucleotide sequences SEQ ID NO: 1-9, 11, 12, 31
or 33 or a representative fragment thereof; or a nucleotide sequence at least
99.9 %
identical to any of the nucleotide sequences of the SEQ ID NO: 1-9, 11, 12, 31
or
33 in computer readable form, a skilled artisan can routinely access the
sequence
information for a variety of purposes. Computer software is publicly available
which allows a skilled artisan to access sequence information provided in a
computer readable medium. The examples which follow demonstrate how software
which implements the BLAST (Altschul et al., J. Mol. Biol. 215:403-410 (1990))
and BLAZE (Brutlag et al., Comp. Chem. 17:203-207 (1993)) search algorithms on
a Sybase system is used to identify open reading frames (ORFs) within a
nucleic
acid sequence. Such ORFs may be protein encoding fragments and may be useful
in
producing commercially important proteins such as enzymes used in fermentation
reactions and in the production of commercially useful metabolites.
As used herein, "a computer-based system" refers to the hardware means,
software means, and data storage means used to analyze the nucleotide sequence
147
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
information of the present invention. The minimum hardware means of the
computer-based systems of the present invention comprises a central processing
unit
(CPU), input means, output means, and data storage means. A skilled artisan
can
readily appreciate that any one of the currently available computer-based
systems
are suitable for use in the present invention. As stated above, the computer-
based
systems of the present invention comprise a data storage means having stored
therein a nucleotide sequence of the present invention and the necessary
hardware
means and software means for supporting and implementing a search means. As
used herein, "data storage means" refers to memory which can store nucleotide
1 U sequence information of the present invention, or a memory access means
which can
access manufactures having recorded thereon the nucleotide sequence
information of
the present invention.
As used herein, "search means" refers to one or more programs which are
implemented on the computer-based system to compare a tar get sequence or
target
structural motif with the sequence information stored within the data storage
means.
Search means are used to identify fragments or regions of a known sequence
which
match a particular target sequence or target motif. A variety of
knowmalgorithms
are disclosed publicly and a variety of commercially available software for
conducting search means are and can be used in the computer-based systems of
the
present invention. Examples of such software includes, but is not limited to,
Smith-
Waterman, MacPattern (EMBL), BLASTN and BLASTA (NPOLYPEPTIDEIA).
A skilled artisan can readily recognize that any one of the available
algorithms or
implementing software packages for conducting homology searches can be adapted
for use in the present computer-based systems. As used herein, a "target
sequence"
can be any nucleic acid or amino acid sequence of six or more nucleotides or
two or
more amino acids. A skilled artisan can readily recognize that the longer a
target
sequence is, the less likely a target sequence will be present as a random
occurrence
in the database. The most preferred sequence length of a target sequence is
from
about 10 to 100 amino acids or from about 30 to 300 nucleotide residues.
However,
it is well recognized that searches for commercially important fragments, such
as
sequence fragments involved in gene expression and protein processing, may be
of
shorter length.
148
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
As used herein, "a target structural motif, " or "target motif, " refers to
any
rationally selected sequence or combination of sequences in which the
sequences)
are chosen based on a three-dimensional configuration which is formed upon the
folding of the target motif. There are a variety of target motifs known in the
art.
Protein target motifs include, but are not limited to, enzyme active sites and
signal
sequences. Nucleic acid target motifs include, but are not limited to,
promoter
sequences, haixpin structures and inducible expression elements (protein
binding
sequences).
5.14 EYPRESSION MODULATING SEQUENCES
EMF sequences can be identified within a genome by theix proximity to the
ORFs. An intergenic segment, or a fragment of the intergenic segment, from
about
10 to 200 nucleotides in length, taken 5' from any ORF will modulate the
expression of an operably linked 3' ORF in a fashion similax to that found
with the
naturally linked ORF sequence. As used herein, an "intergenic segment" refers
to
the fragments of a genome which are between two ORF(S) herein described.
Alternatively, EMFs can be identified using known EMFs as a target sequence or
target motif in the computer-based systems of the present invention.
The presence and activity of an EMF can be confirmed using an EMF trap
vector. An EMF trap vector contains a cloning site 5' to a marker sequence. A
marker sequence encodes an identifiable phenotype, such as . antibiotic
resistance or
a complementing nutrition auxotrophic factor, which can be identified or
assayed
when the EMF trap vector is placed within an appropriate host under
appropriate
conditions. As described above, an EMF will modulate the expression of an
operably linked marker sequence. A more detailed discussion of various marker
sequences is provided below. A sequence which is suspected of being an EMF is
cloned in all three reading frames in one or more restriction sites upstream
from the
marker sequence in the EMF trap vector. The vector is then transformed into an
appropriate host using known procedures and the phenotype of the transformed
host
is examined under appropriate conditions. As described above, an EMF will
modulate the expression of an operably linked marker sequence.
149
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.15 TRIPLE HELIX FORMATION
In addition, the fragments of the present invention, as broadly described, can
be used to control gene expression through triple helix formation or antisense
DNA
or RNA, both of which methods are based on the binding of a polynucleotide
sequence to DNA or RNA. Polynucleotides suitable for use in these methods are
usually 20 to 40 bases in length and are designed to be complementary to a
region of
the gene involved in transcription (triple helix - see Lee et al., Nucl. Acids
Res.
6:3073 (1979); Cooney et al., Science 15241:456 (1988); and Dervan et al.,
Science
251:1360 (1991)) or to the mRNA itself (antisense - Olmno, J. Neurochem.
56:560
(1991); Oligodeoxynucleotides as Antisense Inhibitors of Gene Expression, CRC
Press, Boca Raton, FL (1988)). Triple helix-formation optimally results in a
shut-off of RNA transcription from DNA, while antisense RNA hybridization
blocks
translation of an mRNA molecule into polypeptide. Both techniques have been
demonstrated to be effective in model systems. Information~contained in the
1.5 sequences of the present invention is necessary for the design of an
antisense or
triple helix oligonucleotide.
5.16 DIAGNOSTIC ASSAYS AND KITS
The present invention further provides methods to identify the presence or
expression of one of the ORFs of the present invention, or homolog thereof, in
a
test sample, using a nucleic acid probe or antibodies of the present
invention,
optionally conjugated or otherwise associated with a suitable label.
In general, methods for detecting a polynucleotide of the invention can
comprise contacting a sample with a compound that binds to and forms a complex
with the polynucleotide for a period sufficient to form the complex, and
detecting
the complex, so that if a complex is detected, a polynucleotide of the
invention is
detected in the sample. Such methods can also comprise contacting a sample
under
stringent hybridization conditions with nucleic acid primers that anneal to a
polynucleotide of the invention under such conditions, and amplifying annealed
polynucleotides, so that if a polynucleotide is amplified, a polynucleotide of
the
invention is detected in the sample.
150
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
In general, methods for detecting a polypeptide of the invention can
comprise contacting a sample,with a compound that binds to and forms a complex
with the polypeptide for a period sufficient to form the complex, and
detecting the
complex, so that if a complex is detected, a polypeptide of the invention is
detected
S in the sample.
In detail, such methods comprise incubating a test sample with one or more
of the antibodies or one or more of the nucleic acid probes of the present
invention
and assaying for binding of the nucleic acid probes or antibodies to
components
within the test sample.
Conditions for incubating a nucleic acid probe or antibody with a test sample
vary. Incubation conditions depend on the format employed in the assay, the
detection methods employed, and the type and nature of the nucleic acid probe
or
antibody used in the assay. One skilled in the art will recognize that any one
of the
commonly available hybridization, amplification or immunological assay formats
can readily be adapted to employ the nucleic acid probes or antibodies of the
present
invention. Examples ofsuch assays can be found in Chard, T., An Introduction
to
Radioimmunoassay and Related Techniques, Elsevier Science Publishers,
Amsterdam, The Netherlands (1986); Bullock, G.R. et al., Techniques in
Immunocytochemistry, Academic Press, Orlando, FL Vol. 1 (1982), Vol. 2 (1983),
Vol. 3 (1985); Tijssen, P., Practice and Theory of immunoassays: Laboratory
Techniques in Biochemistry and Molecular Biology, Elsevier Science Publishers,
Amsterdam, The Netherlands (1985). The test samples of the present invention
include cells, protein or membrane extracts of cells, or biological fluids
such as
sputum, blood, serum, plasma, or urine. The test sample used in the
2S above-described method will vary based on the assay format, nature of the
detection
method and the tissues, cells or extracts used as the sample to be assayed.
Methods
for preparing protein extracts or membrane extracts of cells are well known in
the
art and can be readily be adapted in order to obtain a sample which is
compatible
with the system utilized.
In another embodiment of the present invention, kits are provided which
contain the necessary reagents to carry out the assays of the present
invention.
Specifically, the invention provides a compartment kit to receive, in close
151
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
confinement, one or more containers which comprises: (a) a first container
comprising one of the probes or antibodies of the present invention; and (b)
one or
more other containers comprising one or more of the following: wash reagents,
reagents capable of detecting presence of a bound probe or antibody.
In detail, a compartment kit includes any kit in which reagents are contained
in separate containers. Such containers include small glass containers,
plastic
containers or strips of plastic or paper. Such containers allows one to
efficiently
transfer reagents from one compartment to another compartment such that the
samples and reagents are not cross-contaminated, and the agents or solutions
of each
container can be added in a quantitative fashion from one compartment to
another.
Such containers will include a container which will accept the test sample, a
container which contains the antibodies used in the assay, containers which
contain
wash~reagents (such as phosphate buffered saline, Tris-buffers, etc.), and
containers
which contain the reagents used to detect the bound antibody or probe. Types
of
detection reagents include labeled nucleic acid probes, labeled secondary
antibodies,
or in the alternative, if the primary antibody is labeled, the enzymatic, or
antibody
a
binding reagents which axe capable of reacting with the labeled antibody. One
skilled in the art will readily recognize that the disclosed probes and
antibodies of
the present invention can be readily incorporated into one of the established
kit
formats which are well known in the art.
5.17 MEDICAL IMAGING
The novel polypeptides and binding partners of the invention are useful in
medical imaging of sites expressing the molecules of the invention (e.g.,
where the
polypeptide of the invention is involved in the immune response, for imaging
sites
of inflammation or infection). See, e.g., Kunkel et al., U.S. Pat. NO.
5,413,778.
Such methods involve chemical attachment of a labeling or imaging agent,
administration of the labeled polypeptide to a subject in a pharmaceutically
acceptable carrier, and imaging the labeled polypeptide ih vivo at the target
site.
152
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5.18 SCREENING ASSAYS
Using the isolated proteins and polynucleotides of the invention, the present
invention further provides methods of obtaining and identifying agents which
bind to
a polypeptide encoded by an ORF corresponding to any of the nucleotide
sequences
set forth in the SEQ ID NO: 1-9, 11, 12, 31 or 33 or bind to a specific domain
of
the polypeptide encoded by the nucleic acid. In detail, said method comprises
the
steps of:
(a) contacting an agent with an isolated protein encoded by an ORF of
the present invention, or nucleic acid of the invention; and
(b) determining whether the agent binds to said protein or said nucleic
acid.
In general, therefore, such methods for identifying compounds that bind to a
polynucleotide of the invention can comprise contacting a compound with a
polynucleotide of the invention for a time sufficient to form a
polynucleotidelcompound complex, and detecting the complex, so that if a
polynucleotide/compound complex is detected, a compound that binds to a
polynucleotide of the invention is identified.
Likewise, in general, therefore, such methods for identifying compounds that
bind to a polypeptide of the invention can comprise contacting a compound with
a
polypeptide of the invention for a time sufficient to form a
polypeptide/compound
complex, and detecting the complex, so that if a polypeptide/compound complex
is
detected, a compound that binds to a polynucleotide of the invention is
identified.
Methods for identifying compounds that bind to a polypeptide of the
invention can also comprise contacting a compound with a polypeptide of the
invention in a cell for a time sufficient to form a polypeptide/compound
complex,
wherein the complex drives expression of a receptor gene sequence in the cell,
and
detecting the complex by detecting reporter gene sequence expression, so that
if a
polypeptide/compound complex is detected, a compound that binds a polypeptide
of
the invention is identified.
Compounds identified via such methods can include compounds which
modulate the activity of a polypeptide of the invention (that is, increase or
decrease
its activity, relative to activity observed in~the absence of the compound).
153
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Alternatively, compounds identified via such methods can include compounds
which
modulate the expression of a polynucleotide of the invention (that is,
increase or
decrease expression relative to expression levels observed in the absence of
the
compound). Compounds, such as compounds identified via the methods of the
invention, can be tested using standard assays well known to those of skill in
the art
for their ability to modulate activity/expression.
The agents screened in the above assay can be, but are not limited to,
peptides, carbohydrates, vitamin derivatives, or other pharmaceutical agents.
The
agents can be selected and screened at random or rationally selected or
designed
using protein modeling techniques.
For random screening, agents such as peptides, carbohydrates,
pharmaceutical agents and the like are selected at random and are assayed for
their
ability to bind to the protein encoded by the ORF of the present invention.
Alternatively, agents may be rationally selected or designed. As used herein,
an
, agent is said to be "rationally selected or designed" when the agent is
chosen based
on the configuration of the particular protein. For example, one skilled in
the art
can readily adapt currently available procedures to generate peptides,
pharmaceutical agents and the like capable of binding to a specific peptide
sequence
in order to generate rationally designed antipeptide peptides, for example see
Hurby
et al., Application of Synthetic Peptides: Antisense Peptides," In Synthetic
Peptides, A User's Guide, W.H. Freeman, NY (1992), pp. 289-307, and Kaspczak
et al., Biochemistry 28:9230-8 (1989), or pharmaceutical agents, or the like.
In addition to the foregoing, one class of agents of the present invention, as
broadly described, can be used to control gene expression through binding to
one of
the ORFs or EMFs of the present invention. As described above, such agents can
be randomly screened or rationally designed/selected. Targeting the ORF or EMF
allows a skilled artisan to design sequence specific or element specific
agents,
modulating the expression of either a single ORF or multiple ORFs which rely
on
the same EMF for expression control. One class of DNA binding agents are
agents
which contain base residues which hybridize or form a triple helix formation
by
binding to DNA or RNA. Such agents can be based on the classic phosphodiester,
154
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
ribonucleic acid backbone, or can be a variety of sulfhydryl or polymeric
derivatives
which have base attachment capacity.
Agents suitable for use in these methods usually contain 20 to 40 bases and
are designed to be complementary to a region of the gene involved in
transcription
(triple helix - see Lee et al., Nucl. Acids Res. 6:3073 (1979); Cooney et al.,
Science 241:456 (1988); and Dervan et al., Science 251:1360 (1991)) or to the
mRNA itself (antisense - Okano, J. Neurochem. 56:560 (1991);
Oligodeoxynucleotides as Antisense Inhibitors of Gene Expression, CRC Press,
Boca Raton, FL (1988)). Triple helix-formation optimally results in a shut-off
of
RNA transcription from DNA, while antisense RNA hybridization blocks
translation
of an mRNA molecule into polypeptide. Both techniques have been demonstrated
to
be effective in model systems. Information contained in the sequences of the
present invention is necessary for the design of an antisense or triple helix
oligonucleotide and other DNA binding agents. Agents which bind to a protein
encoded by one of the ORFs of the present invention can be used as a
diagnostic
agent, in the control of bacterial infection by modulating the activity of the
protein
encoded by the ORF. Agents which bind to a protein encoded by one of the ORFs
of the present invention can be formulated using known techniques to generate
a
pharmaceutical composition.
5.19 USE OF NUCLEIC ACIDS AS PROBES
Another aspect of the subject invention is to provide for polypeptide-specific
nucleic acid hybridization probes capable of hybridizing with naturally
occurring
nucleotide sequences. The hybridization probes of the subject invention may be
derived from any of the nucleotide sequences SEQ ID NO: 1-9, 11, 12, 31 or 33.
Because the corresponding gene is only expressed in a limited number of
tissues, a
hybridization probe derived from of any of the nucleotide sequences SEQ ID NO:
1-9, 11, 12, 31 or 33 can be used as an indicator of the presence of RNA of
cell
type of such a tissue in a sample.
Any suitable hybridization technique can be employed, such as, for example,
in situ hybridization. PCR as described in US Patents Nos. 4,683,195 and
4,965,188 provides additional uses for oligonucleotides based upon the
nucleotide
155
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
sequences. Such probes used in PCR may be of recombinant origin, may be
chemically synthesized, or a mixture of both. The probe will comprise a
discrete
nucleotide sequence for the detection of identical sequences or a degenerate
pool of
possible sequences for identification of closely related genomic sequences.
Other means for producing specific hybridization probes for nucleic acids
include the cloning of nucleic acid sequences into vectors for the production
of
mRNA probes. Such vectors are known in the art and are commercially available
and may be used to synthesize RNA probes in vitro by means of the addition of
the
appropriate RNA polymerise as T7 or SP6 RNA polymerise and the appropriate
radioactively labeled nucleotides. The nucleotide sequences may be used to
construct hybridization probes for mapping their respective genomic sequences.
The nucleotide sequence provided herein may be mapped to a chromosome or
specific regions of a chromosome using well known genetic and/or chromosomal
mapping techniques. These techniques include in situ hybridization, linkage
analysis against known chromosomal markers, hybridization screening with
libraries
or flow-sorted chromosomal preparations specific to known chromosomes, and the
like. The technique of fluorescent in situ hybridization of cluomosome spreads
has
been described, among other places, in Verma et al (1988) Human Chromosomes:
A Manual of Basic Techniques, Pergamon Press, New York NY.
Fluorescent in situ hybridization of chromosomal preparations and other
physical chromosome mapping techniques may be correlated with additional
genetic
map data. Examples of genetic map data can be found in the 1994 Genome Issue
of
Science (265:1981fj. Correlation between the location of a nucleic acid on a
physical chromosomal map and a specific disease (or predisposition to a
specific
disease) may help delimit the region of DNA associated with that genetic
disease.
The nucleotide sequences of the subject invention may be used to detect
differences
in gene sequences between normal, carrier or affected individuals. The
nucleotide
sequence may be used to produce purified polypeptides using well known methods
of recombinant DNA technology. Among the many publications that teach methods
for the expression of genes after they have been isolated is Goeddel (1990)
Gene
Expression Technology, Methods and Enzymology, Vol 185, Academic Press, San
Diego. Polypeptides may be expressed in a variety of host cells, either
prokaryotic
156
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
or eukaryotic. Host cells may be from the same species from which a particular
polypeptide nucleotide sequence was isolated or from a different species.
Advantages of producing polypeptides by recombinant DNA technology include
obtaining adequate amounts of the protein for purification and the
availability of
simplified purification procedures.
5.20 PREPARATION OF SEQUENCING CIIIPS AND ARRAYS
A basic example is using 6-mers attached to 50 micron surfaces to give a chip
with dimensions of 3 x 3 mm v~hich can be combined to give an array of 20 x 20
cm.
Another example is using 9-mer oligonucleotides attached to 10 x 10 n ucrons
surface
to create a 9-mer chip, with dimensions of 5 x 5 mm. 4000 units of such chips
may be
used to create a 30 x 30 cm array. In an array in which 4,000 to 16,000
oligachips are
arranged info a square array. A plate, or collection of tubes., as also
depicted, mail be
packaged with the array as part of the sequencing kit. .
The arrays may be separated physically _from each other or by hydrophabic
surfaces. One possible way to utilize the hydrophobic strip separation i.s to
use
technology such as the Iso-Grid Microbi,:~logy ~~ystem produced by QA
:C.,aboratories,
Toronto, Canada.
Hydrophobic grid membrane filters (HGMF) have been in use in analytical ,
food microbiology for about a decade where they exhibit unique attractions of
extended
numerical range and automated counting of colonies. One commercially-available
grid
is ISO-Gh..IDTM from QA Laboratories Ltd. (Toronto, Canada) which consists of
a
square (60 x 60 cm) of polysulfone polymer (Gehnan Tuffryn HT-450, 0.45u pore
size) on which is printed a black hydrophobic ink grid consisting of 1600 (40
x 40)
square cells. HGMF have previously been inoculated with bacterial suspensions
by
vacuum filtration and incubated on the differential or selective media of
choice.
Because the microbial growth is confined to grid cells of known position and
size on the membrane, the HGMF functions more like an MPN apparat~.is than a
conventional plate or membrane filter. Peterl~in et crl. (1987) reported that
these
HGMFs can be used to propagate and store genomic libraries when used with a
HGMF replicator. One such instrument replicates growth from each of the 1600
cells
of the ISO-GRID and enables many copies of the master HGMF to be made
(Peterkin
et al., 1987).
157
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Sharpe et ~l. (1989) also used ISU-GRID HGMF form QA Laboratories and an
automated HGMF counter (MI-100 Interpreter) and RP-100 Replicator. They
reported
a technique for maintaining and screening many microbial cultures.
Peterkin and colleagues later described a method for screening DNA probes
using the hydrophobic grid-membrane filter (Peterkin et cal., 1989). These
authors
reported methods for' effective colony hybridization directly on HG.MFs.
Previously,
poor results had been obtained due to the low DNA binding capacity of the
epoxysulfone polymer on which the HGIVIFs are printed. However, Peterkin et
czl.
( 1989) reported that the binding of DNA to the suxface of the membrane was
improved
by treating the replic ated and incubated HCTMF with polyethyleneimine, 'a
polycation,
prior to contact with DNA. Although this early work uses cellular DNA
attachment,
and has a different objective to the present invention, tf~e methodology
described: may
be readily adapted for Format 3 SBH.
In order to identify useful sequences rapidly, Pete~kin'et ~zl. (1989) used
~ radiolabeled plasmid DNA from various clones and tested its specificity
against the
DNA on the prepared HGMFs. In this way, DNA from recombinant pltasmids was
rapidly screened by colony hybridization~against 100 orgarlisn?s on HGMF
replicates v
which can be easily and reproducibly prepared.
le!(anipulation with small (2-3 mm) chips, and parallel execution of thousands
of .
~ the reactions. The solution of the inventian is to keep the chips and the
probes in the
corresponding arrays. In one example, chips contai~.iing 250,00Q 9'-mers axe
synthesized on a silicon.wafer in the form of 8 x 8 niM plates (15
i'Vlioligonucleotide,
Pease et al., 1994) arrayed in 8 x 12 format (96 chips) with a 1 W 1V1 groove
in
between. Probes are added either by multichannel pipette ~r pin array, one
probe on
one chip. To score all 4000 6-mers, 42 chip arrays have to be used, either
using
different ones, or by reusing one set of chip arrays several times.
In the above case, using the earlier nomenclahire of the application, F=9;
P=6; and F + P = 15. Chips may have probes of formula BxNn, where x is a
number of specified bases B; and n is a number of non-specified bases, :o that
x = 4
to 10 and n = 1 to 4. To achieve more efficient hybridization, and to avoid
potential
influence of any support oligonucleotides, the specified bases can be
surrounded. by
unspecified bases, thus represented by a formula such as (N)nBx(N)m.
158
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
5,21 PREPARATION OF SUPPORT BOUND OLIGONUCLEOTIDES
Oligonucleotides, i.e., small nucleic acid segments, may be readily prepared
by, for example, directly syntr~esizing the oligonucleotide by chemical means,
as is
commonly practiced using an automated oligonucleotide synthesizer .
Support bound oligonucleotides may be prepared by any of the methods known
to those of skill in the art using. any suitable support such as glass,
polystyrene or
Teflon. One strategy is to precisely spot oligonucleotides synthesized by
standard
synthesizers. Immobilization can be achieved using passive adsorption (Inouye
& ~ '
Hondo, 1990); using LTV light (Nagata et al., 1985; Dahlen et ~.zl., 1987;
Morriey ~
IO Collies, 1989) or by covalent binding of base modified DNA (I~eller et al.,
1988;
. 1989); all. references being specifically incorporated herein.
Another strategy that may be employed is the use of the strong
biotin-streptavidin interaction as a linker. For example, Broude et al. (1994)
describe
the use of Biotinylated probes, although these are duplex probes, that are
inuncbilized
IS on streptavidin-coated magnetic beads. fStreptavidin~coated beads may be
purchased . .
.from Dynal, Oslo. Of course, this same linking chemistry is applicable zoo
coating any
aurface with streptavidin. Biotinylated probes may lie purchased from various
sources,
such as, e.g., Operon Technologies (Alameda, CA}:
Nunc Laboratories (Naperville, IL.) is also selling suitable material that
could
20 be used Nunc Laboratories have developed a method by which DNA can be
covalently bound to the microwell surface termed Covalink NH. CovaLi~k NH is -
a
polystyrene surface grafted with secondary amino groups ( > NH) that serve as
bridge-heads for further covalent coupling. CovaLink Modules may be purchased
.
from Nunc Laboratories. DNA molecules may be bound to CovaLink exclusively at
25 the 5'-end by a phosphoramidate bond, allowing immobilization of more than
1 pmol
of DNA (Rasmussen et' al., 1991).
The use of CovaLink NH strips for covalent binding of DNA molecules at the
5'-end has been described (Rasmussen et al., 1991). In this technology, a
phosphoramidate bond is employed (Chu et al., 1983). This is beneficial as
30 immobilization using only a single covalent bond is preferred. The
phosphoramidate
bond joins the DNA to the CovaLink NH secondary amino groups that are
positioned
at the end of spacer arms covalently grafted onto the polystyrene surface
through a 2
159
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
nm long spacer arm. To link an oligonucleotide to CovaLink NH via an
phosphoramidate bond, the oligonucleotide terminus must have a 5'-end
phosphate
group. It is, perhaps, even possible for biotin to be covalently bound to
CovaLink and
then streptavidin used to bind the probes.
More specifically, the linkage method includes dissolving DNA in water (7.5
.ng/ul) and denaturing for 10 m.in. at 95°C and cooling on ice for 10
min. Ice-cold 0.1
M 1-methylimidazole, pH 7Ø (1-Melm~), is then added to a final concentration
of 10
mM 1-MeIm~. A ss DNA solution is then dispensed into CovaLink NH strips (75 .
ul/well) standing on ice. ,
Carbodiiir~idv- 0.2 M. 1-ethyl-3-(3-:limethylaminopropyl.)-carbodiimide
(P;DC),
dissolved in 10 mM 1-Melim, is made fresh and 25 w1 added per well. The strips
are
incubated for 5 hours at 50°C. After incubation thevstrips are washed
using, e.g.,
Nunc-Immuno Wash; first the wells are washed 3 times, then they are soaked
with
washing solution for 5 min., and finally tlxey are washed 3 times (where .in
the washing
~ solution is 0.4 N NaOH, 0.25 % SDS heated to 50°C). .
f . It is contemplated That a further suitable mefhod for use' with the
presen, '
,invention is that described in PCT Patent Application W O 90/03382 (Southern
&
Maskos), incorporated herein by reference. This rrFethod of preparing an
aligonucleotide bound to a support involves attachh~g a nucleoside 3'-reagent
through ..
.the phosphate gr oup by a covalent phosphodiester link to aliphatic hydroxyl
groups
carried by the support. The oligonucleotide is then synthesized on the
supported
nucleoside and protecting groups removed from the synthetic oligonuclecatide
chain
under standard conditions that do not cleave the oligonucleotide from the
support. .
Suitable reagents include nucleoside phosphoramidite and nucleoside hydrogen
phosphorate.
An on-chip strategy for the preparation of DNA probe for the preparation of
DNA probe arrays may be employed. For example, addressable laser-activated
photodeprotection may be employed in the chemical synthesis of
oligonucleotides
directly on a glass surface, as described by Fodor et al. (1991), incorporated
herein by
reference. Probes may also be immobilized on nylon supports as described by
Van
Ness et al. (199I); or linked to Teflon using the method of Duncan & Cavalier
(1988);
all references being specifically incorporated herein.
160
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
To link an oligonucleotide to a nylon support, as described by Van Ness et al.
(199I), requires activation of the nylon surface via alkylation and selective
activation
of the 5'-amine of oligonucleotides with cyanuric chloride.
One particular way to prepare support bound oligonucleotides is to utilize the
light-generated synthesis described by Pease et cal., (1994, incorporated
herein by
reference). These authors used current photolithographic techniques to
generate arrays
of immobilized oligonucleotide probes (DNA chips). These methods, iwwhich
light is
used to direct the synthesis of oligonucleotide probes in high-density,
miniaturized
arrays, utilize photolabile 5'-protected N acyl-deoxynucleoside
phosphoramidites,
surface lil~ker chemistry and versatile combinatorial synthesis strategies. A
matrix of
256 spatially defined oligonucleotide~probes may be generated in this manner
and then
used in the advantageous Format 3 sequencing, as described herein.
5.22 PREPA1~.7CION OF 1VIICLEIC ACID FL2AGMENTS
1 ~ The nucleic acids to be sequenced may be obtained from any appropriate
sour.e, such as cDNAs, genomic DNA, chromoso~x~al DNA, microclissected ~ ~ '
,Y: . , , . .. . w)
chromosome bands, cosmid or YAC inse~ ts, and RNA, including mRNA without any
,, . ,
amplification steps. For example, Sambrook et czl. (19891 describes three
protocols for
the isolation of high molecular weight DNA from mammalian cells (p. 9.14-
9.23).
DNA fragments may be prepared as clones in M13, plasmid or Iainbda vectors
~andlor prepared dirECtly from genomic DNA or cD~NA by PCR or other
amplification
methods. ~8amples may be prepared ar dispensed in multiwell plates. About 100-
1000
ng of DNA samples may be prepared in 2-500 ml of final vohzme.
The nucleic acids would then be fragmented by any of the methods known to
those of skill in the art including, for example, using restriction enzymes as
described
at 9.24-9.28 of Sambrook et al. (1989), shearing by ultrasound and NaOH
treatment.
Low pressure shearing is also appropriate, as described by Schriefer et al.
(1990, incorporated herein by reference). In this method, DNA samples are
passed
through a small Fxench pressure cell at a variety of low to intermediate
pressures. A
lever device allows controlled application of low to intermediate pressures to
the cell.
The results of these studies indicate that low-pressure shearing is a useful
alternative to
sonic and enzymatic DNA fragmentation methods.
161
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
One particularly suitable way for fragmenting DNA is contemplated to be that
using the two base recognition endonuclease, CviJI, described by Fitzgerald et
al.
(1992). These authors described an approach for the rapid fragmentation and
fractionation of DNA into particular sizes that they contemplated to be
suitable for
shotgun cloning and sequencing. The present inventor envisions that this will_
also be
particularly useful for generating random, but relatively small, fragments of
DNA for
use in the present sequencing technology.
The restriction endonuclease CviJI normallycleaves the recognition sequence
PuGCPy between the G and C to leave blunt ends. Atypical reaction conditions,
which alter the specificity of this enzyzne (CviJI*'~), yield a quasi-random
distribution
of DNA fragments form the small molecule pUCl9 (268S~base pairs). Fitzgerald
et
al. (1992) quantitatively evaluated the randomness of this fragmentation
strategy, using
a CviJI'k* digest of pIJCl9 that was size fractionated by a rapid gel
filtration method
and directly ligated, without end repair, to a lac Z minus M13 cloning vector.
Sequence analysis of 76 clones showed that CvirI*=r~ restricts pyGCPy and
PuGCPu, . in
addition to PuGCPy sites, and that new sequence data is accur-~ulated at a
rate ,
consistent with random fragmentation.
~-15 reported in the literature, advantages of this approach compared to
sonication and agarose gel fractionation ilnclude: smaller amounts of DNA are
~ required (0.2-0.5 ug instead of 2-5 ug); and fewer steps are involved (no
preligation,
end repair., chemical extraction, or agarose gel electrophoresis and elution
are needed)
'These advantages are also proposed to be of use when preparing DNA for
sequencing
by Format. 3.
Irrespective of the manner in which the nucleic acid fragments are obtained or
prepared, it is important to denature the DNA to give single stranded pieces
available
for hybridization. This is achieved by incubating the DNA solution for 2-5
minutes at
80-90°C. The solution is then cooled quickly to 2°C to prevent
renaturation of the
DNA fragments before they are contacted with the chip. Phosphate groups must
also
be removed from genomic DNA by methods known in the art.
5.23 PI'~EPARA7fION OF I)NA ARRAYS
Arrays may be prepared by spotting DNA samples on a support such as a
nylon membrane. Spotting may be performed by using arrays of metal pins (the
162
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
positions of which correspond to an array of wells in a microtiter plate) to
repeated by
transfer of about 20 n1 of a DNA solution to a nylon membrane. By offset
printing, a
density of dots higher than the density of the wells is achieved. One to 2S
dots may be
accommodated in I mm2, depending on the type of label used. By avoiding
spotting in
some preselected number of rows and columns, separate subsets (subarrays) may
be
formed. Samples in one subarray may be the same genomic segment of DNA (or the
same gene) from different individuals, or may be different, overlapped genomic
clones. Each of the subarrays may represent replica spotting of the same
samples. In
one example, a selected gene segment may be amplified from 64 patients.. For
each
patient, the amplified gene segment may be in one 96-well plate (all 96 wells
.
containing the same sample). A plate for~each of the 64 patients is prepared.
By using :.
a 96-pin device, all samples rnay be spotted on one.8 x l2~cm. membrane.
Subariays
may contain 64 samples, one from each patient: Where the 96 subarrays are
identical,
the dot span may be I mm' and there may be a. I mm space between subarrays.
w 15 Another approach is to use membranes or plates (a.vailable from 1'~UNC,
Naperville, Illinois);~vhich may be partitioned by physical spacers e.g. a
plastic grid
molded over the membrane, the grid being similar to the sort of membrane
applied to
the bottom of multiwell plates, or hydrophobic strips. A fixed ephysieal
spacer is net
preferred for imaging by exposure to flat phosphor-storage screens or x-ray
films.
~ . . .. , ,
. The present invention is illustrated in the following examples. Upon
consideration of the present disclosure, one of skill in the art will
appreciate that many
other embodiments and variations may be made in the scope of the present
invention.
Accordingly, it is intended that the broader aspects of the present invention
not be
limited to the disclosure of the following examples. The present invention is
not to be
limited in scope by the exemplified embodiments which are intended as
illustrations of
single aspects of the invention; and compositions and methods which are
functionally
equivalent are within the scope of the invention. Indeed, numerous
modifications and
variations in the practice of the invention are expected to occur to those
skilled i~1 the
art upon consideration of the present preferred embodiments. Consequently, the
only
limitations which should be placed upon the scope of the invention are those
which
appear in the appended claims.
163
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
All references cited within the body of the instant specification are hereby
incorporated by reference in their entirety.
6.0 EXAMPLES
EXAMPLE 1
Isolation of SEQ ID NO: 1-7 from a cDNA Libraries of Human CeIIs
A plurality of novel nucleic acids were obtained from a cDNA library
prepared from human testis cells (Hyseq clone identification numbers 2880984
and
2881695), from human fetal skin (Hyseq clone identification number 15375176).
vadult spleen (Hyseq clone identification number 14856094}, and human
endutheiial
cells (Hyseq clone identification numbers 13804756, 13687487, 13804756) using
standard PCR, seqLiencing by hybridization sequence signature analysis, and
Sanger
sequencing techniques. The inserts of the library: were amplified with PCR
using
primers specific for vector sequences flanking the~.inserts. These samples
were
r spotted onto nylon membranes and interrogated with oligonucleotide probes to
give
sequence signatures. The clones were clustered FntO groups: of similar or
identic;:tl
sequences, and single reps esentative clones were selected from each group for
gel
sequencing. The 5' sequence of the amplified inserts was then deduced using
the
reverse M13 sequencing primer in a typical Sanger sequencing protocol. PCR
products were purified and subjected to fluorescent dye terminator cycle
sequencing.
Single-pass gel sequencing was done using a 377 Applied Biosystems (ABI)
sequences. These inserts was identified as a novel sequence not previously
obtained
from this library and not previously reported in public databases. These
sequences
are designated as SEQ ID NO: 1-7 in the attached sequence listing.
EXAMPLE 2
Assemblage of SEQ ID NO: 8 and 9
The novel nucleic acids (SEQ ID NO: 8 and 9) of the invention were
assembled from sequences that were obtained from a cDNA library by methods
described in Example 1 above, and in some cases sequences obtained from one or
more public databases. The sequence was assembled using an EST sequence (SEQ
ID
NO: 2) as a seed. Then a recursive algorithm was used to extend the seed EST
into an
164
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
extended assemblage, by pulling additional sequences from different databases
(i.e.,
Hyseq's database containing EST sequences, dbEST version 114, gb pri 114, and
UniGene version 101) that belong to this assemblage. The algorithm terminated
when
there was no additional sequences from the above databases that would extend
the
S assemblage. Inclusion of component sequences into the assemblage was based
on a
BLASTN hit to the extending assemblage with BLAST score greater than 300 and
percent identity greater than 95 % . SEQ 1D NO: 8 was further manually edited
to
obtain SEQ ID NO: 9. Fig. 1 shows the alignment of SEQ ID NO. 9 with SEQ ID
NO. 1-7
~ Tlie nearest neighbor result for t?-~e assembled sequence (SEQ ID NO. 8)
w~~s
'. obtained by a FASTA version 3 search against Genpept release 114, using
Fastxy
~.~algori.thm. Fastxy is an improved version of FASTA alignment which allows
in-codon
frame shifts. The nearest neighbor result showed the closest homologue for
each
-assemblage from Genpept (and contains tire translated amino acid sequences
for which
> the assemblage encodes). The nearest neighbor results is set forth below:
Accession Description . ~ .~ Smith-~ ~~ % ~ .
No.:' ~~ w : Waterman Score Identity ~ .
AB016768 Mug musculus thrombospondin type 56 43.750
1 domain . . .,
The predicted amino acid sequence for SEQ ID NO: 8 was obtained by using
a software program called FASTY (available from ht~t ;//fasta:bioch.vir
inia.edu)
which selects a polypeptide based on a comparison of translated novel
polynucleotide to known polynucleotides (W.I~. Pearson, Methods in Enzymology,
183:63-98 (1990),. incorporated herein by reference).
For SEQ ID NO: 8:
Predicted Predicted end Amino acid segment containing signal peptide
beginning nucleotide (A=Alanine, C=Cysteine, D=Aspartic Acid,
nucleotide location E= Glutamic Acid, F=Phenylalanine,
location ~ ~ G=Glycine, H=Histidine, I=Isoleucine,
corresponding I K=Lysine, L=Leucine, M=Methionine,
165
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
correspondingto first N=Asparagine, P=Proline, Q=Glutamine,
amino
to first acid residueR=Arginine, S=Serine, T=Threonine,
amino
acid residueof amino V=Valine, W=Tryptophan, Y=Tyrosine,
acid
of amino sequence X=Unknown, ~=Stop Codon, /=possible
acid
sequence nucleotide deletion, \=possible
nucleotide
insertion)
575' 1054 CTK.CKADCDTCFNKNFCTKCKSG
FYLHLGKCLDNCPEGLEANNHTM
ECVSIVHCEVSEWNPWSPCTKKGK
TCGFKRGT:.~TRVIZEIIQHP;>AKGN
LCPPTNE7:'RKC'TVQRKKCQKGER
GKKGRER.KrRKKPNK GESKEAIPDS
KSLESSK.EI,PEQRENKQQQ
(SEQ ID NO: 14) .
EXAMPLE 3~,. .
Assemblage off' SEQ III NO: 1f3
A polypeptide (SEQ ID NO: 10) was predicted to bc: encoded by SEQ ID :r.
~ NO: ~~ as set forth below. The polypeptide was ~yedicted using a software
prograriz ~. .. ,
called BLAST~i which selects a polypeptide based on a comparison of translated
novel polynucleotide to known polynucleotides. 'Che initial rriethionine
starts at
position 291 of SEQ ID NO: 9 and the putative stop codon, TAG, begins at
position
1107 of the nucleotide sequence.
EXAMPLE 4
Cloning of Stem Cell Growth Factor-Like Gene; and Expression and Purification
of Stem Cell Growth Factor-Like Protein
Stem cell growth factor-like polynucleotide (SEQ ID NO: 11 or 12) was cloned
I S by PCR into pIBlVS-His TOPO TA cloning vector (Invitrogen) from Hyseq's
full-
length stem cell growth factor-like clone. Stem cell growth factor-like gene
was
further subcloned into pCDNA3.1-Myc-His vectors (Invitrogen) and expressed
with or
without VS-His tag. Insect cells (high Five, Invitrogen) were transfected with
stem
cell growth factor-like gene with the His-5 tag by using the InsectSelect
system
166
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
(Invitrogen) using manufacturer's suggested protocols. Stem cell growth factor-
like
protein was purified from the cell media by a combination of pH adjustment,
cation
exchange chromatography, and affinity chromatography as de;rcribed below.
Briefly,
the pI~ of the medium was adjusted to 7.0 and the protease inhibitors PMSF and
EDTA were added. Column chromatography purification was per formed on
Pharmacia Akta instrument system at room temperature using sequential removal
of
contaminants on appropriately sized columns of SP-Sepharose Fast Flow, Hitrap
heparin Sepharose, and Ni-NTA resins. Column elution fractions were analyzed
by
separation on 16% SDS-PAGE gels, transfer to Immobilin membranes (Millipore),
and detection of the tagged protein by anti-VS antibody using manutacturer's
,protocols Fractions containing stem cell growth factor-like activity eluted
from Ni-
NTA column were pooled and equilibrated with PBS and stored at -
$0°C until
analyzed for stem cell growth factor-like activity.
EXAMPLE 5
Expression of Stem Cell Growth Factor-Like ~'rotein~ fu _l'rin ~a~Human Cells
The product of the secondary nested PCR from Marathon spleen library
(SEQ ID NO: 11 or 12) or any other polynucleotide encodiri~ stem cell growth
factor-like polypeptide were cloned into MSCV retroviral vector (Clontech)
into
suitable cloning sites using appropriate forward and reverse PCR primers. This
retroviralwectorwas then transfected using FUGENE-6 transfection reagent into
packaging cell lines to produce suitably large quantities of retrovir us that
will have
the stem cell growth factor-like DNA cloned in it. Retrovirus containing
supernatants were prepared from packaged cell lines and mixed with stromal or
stem
cells. Upon retrovirus transduction these transduced cells may express the
stem cell
growth factor-like protein.
EXAMPLE 6
Assay for Growth and Differentiation of Stem cells using Coculture Assay
1 x 10ø mouse stem cells were co-cultured with 1 x 104 stem cell growth
factor-like polynucleotide-transduced stromal cells or vector-transduced
stromal
cells (produced by Example 5) in the serum-free medium. On day seven, IL-3 (10
167
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
ng/ml) and IL-6 (10 ng/ml) were added as additional growth factors. Cultures
were
monitored microscopically every day. After appropriate further incubation,
cells
were harvested, and counted using hemacytometer. Results from one experiment
are presented in the table below:
Conditions Day 15 (approximate Day 18 (approximate
number of cell/ml) number of cell/ml)
Vector-transduced stroma 30000 105000
cells + stem cells
Stem cell growth factor 405000 8? 5000
polynucleotide-transduced
stroma cells + stem cells
Sr
EXAMPLL 7
Assay lEor Proliferation And Differentiation of Stem Cells
CD34+ hematopoietic stem cells (HSC) were purified from mobilized
periphera? bloo~:l (purchased from ALLCeIIs). CD 34+ cells w:;~re puriti-r.~a
by pc~sita~rely .
selecting cells using Miltenyi breads (Miltenyi). Stem cells a-ere plated in
96-well
plates at 103/well. Purified stem cell growth factor-like protein and otPuer
hen~atopoietic cytokines (purchased f~orr~ R & D systems), and the
combinations ,
thereof were added to the cultures for assessing the stem cell growth factor
activity.
The gro~~th and differentiation of stem cells were examined 5 days after
culture by .
light microscope. The results of six experiments are shown in the table below,
wherein
positive effect of stem cell growth factor protein was observed in three out
of si.x
experiments .
Abbreviations: Stem cell growth factor-like protein = SCGF; Interleukin-3 = IL-
3;
thrombopoietin = TPO; Fms-like tyrosine kinase-3 ligand = flt-3 ligand
(+) indicates growth and/or differentiation of stem cells; (-) indicates no
growth or
differentiation and loss of viability of stem cells. '
Growth factors) added Growth and Growth and
morphological morphological
changes changes
168
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Growth factors) added Growth and Growth and
morphological morphological
changes changes
Experiment 1 Experiment 2
None (-) (-)
Stem cell growth factor (-) (-)
(50 ng/ml) ,
IL-3 (10 ng/ml) . (+/-) (+) .
SCGF (50 ng/ml)-I-.IL-3 (-+-) ~(+)
(10 ng/ml) ~
Growth factors) added ~ Growth and . Growth and Growth and
morphological morphological morphological
changes changes changes.
Experiment 3 Experiment 4 Experiment 5
'~ None , (_) , ,.(-) --- (-) .
p Stem cell growth factor (50 (-) ~ (-)' ~ (_f .
ng/ml) . _
TPO (I00 ng/ml) ~--_~._ -_ ~_~ -,. , ( ) _ ( ) __
. kit ligand (50 ng/ml) + fli-3 ' I ' ' - ~'_~ . (-) ___ _ _( ) __
y ligand (50 ng/ml)
kit ligantl (50 ng/ml) + flt-3 (+) (-) (-)
ligand (50 ng/ml) + SCGF . .
(50 ng/inl)
Growth factors) added Growth and
morphological
,
changes
! Experiment 6
None T (-)
Stem cell growth factor (50 ng/n~l) (-)
kit ligand (50 ng/ml) (-)
flt-3 ligand (50 ng/ml) (-)
169
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Growth factors) added Growth and
morphological
changes
lcit ligand (50 ng/n~l) + flt-3 ligand (-)
(50 ng/ml)
kit ligand (50 ng/ml) + flt-3 ligand (50 (+)
ng/ml) + SCGF (50
ng/ml)
EXAMPLE ~
Establishment of Stromal Cell Strain derived froan Mouse AGM
(1) Isolation of AGM regioaa from fetal mouse
, C3H/HeNSLc mouse of both genders (purchased from Japan SI C INC.) vas
bred under a SPF (specific pathogen-free) circumstance. One or two female mice
and one male mouse were reared in the same cage over a night. In the next
morning; the female mice in which the existence of a vaginal plug was
confirmed
were raiisferred to othei cages. arid breeded. The day when the existence of
the
vaginal plug was confirmed was defined io b~; thE: 0.5th day of pregnancy. On
~:he
T,,
10.5th day of the pyegnancy; after moi!se was sacrificed by cervical
dislocation,
fetuses W ere extirpated. Isolation of AGM regioxas was 'performed according
to the
method by Godin et al. (Godin, I., Pr-oc. Natl. Acad. Sci. U. S.A. , 92: 773-
777,
1995) arid the method by Medvins~y et,al. (Medvinsky, A.L., Blood; 87: 557-
565,
1996). The fetuses were placed in.a culture dishes to which PBS(-) (phosphate
buffered saline) (produced by Nissui Seiyaku) was added in a volume just
sufficient
to cover: them. After the ACvM regions were carefully excised so as not to
include
other regions under a stereoscopic microscope, they were put in another 24-
well
culture dish (Nuns) .
(2) Establishment of cell lines derived from AGM
One drop of MEM medium (Sigma) containing 10 % FCS (Hyclone) was
added to the AGM regions in the 24-well culture dish (Nunc), and AGM regions
were cultured in incubator overnight. The cultures wera performed in the MEM
medium (Sigma) including 10 % FCS (Hyclone) at 37°C, in an atmosphere
of 5 %o
COz, and at a humidity of 100 % . When the cells corresponding to the AGM
170
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
regions adhered to the culture dish due to overnight cultivation, two
milliliters of
MEM medium containing 10 % FCS was further added. Stromal cells began to
appear around the AGM region tissue fragment after the continuous cultivation.
After one-week cultivation, adhesive cells were trypsinized (0.05 % trypsin.in
PBS
S containing 0.53 mM EDTA (Gibco BILL) at 37°C for three to five
minutes) and
dispersed. The stromal cells were then washed twice with the medium, and
seeded
on 6-well culture dish (Nunc). On the next day, the cells which did not
adhered to
the culture dish and the medium were removed, and then, fresh medium were
added. 'awo weeks after transfer, the cells in the. 6-well culture dish were y-
ray
I0 irradiated at 900 l~.ad to eliminate endagenoLSS bematapoietic cell:y.
Although
attempts of the dir ect cell cloning by limiting dilution from ahis culture
system was
failed, so that no cell proliferation was: observed. Then, attempts were made
according to as follows: after adaptation of cells,so as to be.able to
proliferate from
a small number of cells by increasing the nuznber of seeded cells in one well,
the
1 S ~ cells were cloned by limiting dilutzon. .. '
Traat is, the ~1GM was extirpated~and c:ultur°d in the same manner
as . ,-
described above. The culture system two weeks:after the :y-ray radiation was
trypsinized (0.0S % trypsin in PBS containing 0.53 mM EDTA at 3'7°C for
'three to
five minutes) and the cells were suspended, so that the cells were seeded in a
2~1- -', , s~.
20 . well culture dish ranging from SO to 100 cellslwell. After the culture
was continued
. for three weeks, the cells were seeded.in a 9~-well culture dish (Nunc) by
means of
limiting dilution so as to be 0.3 cells/ well. The cells which were derived
from the
well seeded only one cell and. proliferated were allowed to enlarge culture.
As a
result, the cells were successfully cloned to obtain fibroblast like cells and
cobble
2S stone like cells.
CD34 positive cell fraction derived from the human cord blood was co-
cultured with the fibroblast like cells fer two weeks. Colony forming cells
could not
be found in the co-culture system with the fibroblast like cells. Then, the
similar
examination was performed for seven cell clones showing cobble stone like
30 morphotype. Three clones having activity to proliferate and support the
human
hematopoietic stem cells were obtained and were named AGM-s1, AGM-s2, and
AGM-s3 .
171
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
EXAMPLE 9
Pr eparation of Hematopoietic Stem Cells from Mouse Bon a Marrow
The bone marrow was collected from the femur of C57BL/6-Ly 5.1 pep (week
ages ranging from eight to ten, and male) (the gift from Professor K.
Nakauchi,
University of Tsukuba), and suspended in PBS. After the mouse bone marrow
mononuclear cells were concentrated by specific gravity centrifugation
according to
the usual method (S. Kouzu, Fundamental techniques for immunology, YODOSHA,
1995 j, the cells were suspended with staining buffer (PBS containing 5 % FCS
and
0.05 % NaNs).
The most immature hematopoietic stem cell fraction was obtained as follows
(Osawa, M. et al.,,Science 273: 242-245, 1996).
The mononuclear cells were incubated with biotylated anti-lineage monoclonal
antibodies (CD45R, CD4, CDB, Gr-1, Ter119, and CDl.ie, purchased from
Pharmingen), fluoxescein isathiocyanate (FITC)-anti-CDs-, phycoerythrin (PEl-
. anti-Sca-1, and allophyce.cyanin (APC;)-anti-~;-Ki:i for ~0 min on ice. ,
AI~Ler the.
stained ;:ells were washed twice with staining buffer, CD3~ negative, Sca-1
positive, -
c-Kit positive, and Lin negative cells were isolated on a F!~.CS Vantage
(Becton
Dickinson) . .
; EXAMPLE 19 '
Subcloning of Mouse Stxomal Cell Str ain and Assessment of an _
Activity to Support the klematopoietic Stem :ells of a_Variet of Cell Strains
(1) Subcloning of mouse stromal cell strain .
1) Isolation of AGM-s3 subclone
Stromal cell strain AGM-s3 derived form A GM which was subcultured in
MEMa medium (GIBCO BRL) including non-active 10 % FCS (bovine fetal serum,
Hyclone) was suspended in PBS containing 5 % FCS (PBS-FCS). Clone sorting was
performed in a 96-well culture dish (Falcon) at one cell/ well using a cell
sorter
(FACS Vantage; Becton Dickinson). Among cells in the 96 wells, cultures of the
cells which proliferated were expanded, so that thirteen kinds of AGM-s3
subclones
were obtained. The activity to support the hematopoietic cells of these AGM-s3
subclones were assessed.
172
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
2) Isolation of human cord blood CD34 positive stem cell
The human cord blood was collected at normal delivery according to the
criteria approved by Drug Discovery Institute, Ethics committee, Kirin Brewery
Co., LTD. The cord blood was collected using a syringe added with heparin so
as
not to coagulate. The heparin treated cord blood was overlaid on Lymphoprep
(NYCOMED PHARMA), and mononuclear cells were separated by centrifugation
(at 4006, at room temperature, and for 30 minutes). Erythrocytes contaminated
in
the mononuclear cell fraction were lyzed by treatment with ammonium chloride
buffer solution (0.83 % NHaCI-Tris HCI, 'Z0 mM, pH 6.8) at room temperature
for
two~minutes. After the mononuclear cells were washed with PBS-FCS, tem
milligrams of human IgG was added and allowed to stand on ice for ten minutes
Then, the cells were further washed with PBS-FCS, added~with biotiuylated
antibodies against the antigens specific'to the huf~ian differentiated blood
cells that
; is, CD2, CDllc (purified from ATCC hybridoma), CD19..,(Pharmingen), CD1~,
and CD41 (Leinco Technologies Inc.): and the ~i7.tibody against Glycophorin ~:
(Cosmo Bio), and. allowed to stand.on ice for 20.min. Altar washing with PBS-
. r'
FCS, the cells were suspended in one vnilliliter of PBS containing 5 % FCS, 10
mM
EDTA, and 0.05 °~o NaNs (PBS-FCS-EDTA-NaN3), added with magnetic
beads
bound with streptavidin (BioIVIag. Per Septive Diagnostics)., and allowed to
stand on
ice for 40 min,. The differentiated blood cells .which expressed
differentiation
antigens were removed using a magnetic separator (DynaI,MPC-1 Dynal). FITC
labeled CD34 antibody (Immunotech S.A., Marseilles, France) were added to the
remaining differentiated blood cell antigen negative cell fraction. After
incubation
on ice for 20 min., CD34 positive fraction was recovered using a cell sorter.
This
cell fraction was defined as a hematopoietic stem cell fraction derived from
the
human cord blood.
3) Co-culture of the human hematopoietic stem cells and AG1VI-s3 subclone
After 13 kinds of AGM-s3 subclones or stromal cell strain MS-5 derived from
the mouse bone marrow were seeded in a 24-well culture dish (Falcon) at 1 x
10ø
cells/well, and cells were cultured in one milliliter of MEMa medium
containing
173
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
10% FCS until the cells covered all over the bottom surfaces of the wells.
CD34
positive hematopoietic stem cells derived from the human cord blood were
sorted on
the above described stromal cells at 500 cells/ well, and co-cultured in one
milliliter
of MEPdIa medium containing 10% FCS. One week after the initiation of the co-
y culture, one milliliter of the same medium was further added. Two weeks
after the
initiation of the co-culture, the stromal cells and the human blood cells were
trypsinized (0.05 % trypsin in PBS containing 0.5 mM EDTA (GIBCO BRL) at
37°C and standing for two to five min.) and dispersed from the culW re
dish.
Activities to support the hematopoietic stem cells were assessed with a colony
assay
4) Assessment of proliferation statuses.of the hematopoietic stem cells and
hetr~opoietic precursor cells by clonogenic assay '
The cells which proliferated in the above described co-culture system were
appropriately diluted, added to one. milliliter of txxethylcellulose culture
systenx., and
1 ~ analyzecl in triplicate. The analysis using the m.~thylcellulose culture
system were
performed using a 6-well culture dish (Falc:on) in the preseirce of l0 nglml.
of
human' SCF, human IL=3, human IL-6, human ~-CSF; hunxan TPO, and I;PO at 2
IU/nxl to MethoCult H4230 (Stem ~CeI1 Technologies Iixc.).' All of a variety
of the
-above described hematopoietic factors were recombinants iand pure. Two weeks
- after the culture, developed colonies were observed under a microscope and
counted
numbers of CFU-GM (granulocyte-macrophage. differentiating series), BFU-E
(erythraid burst forming unit), and CF1J-E mix (erythrocyte mixed
differentiating
series).
Figure 4 shows the results from two-week co-culture' of the CD34 positive
hematopoietic stem cells and AGM-s3 subclones A9, A7, or D 11. As a result of
the
co-culture, A9 and D11 subclones among 13 kinds of AGM-s3 subclones supported
proliferation of all three lineages of CFU-GM, BFU-E~ and CFU-E mix.
Especially, although BFU-E and CFU-E mix, that is, the precursor cells of an
erythrocytes were hardly to be supported in usual, they proliferated in the co-
culture
system with A9 or D11 cells. The results showed that proliferation or
maintenance
of the hematopoietic stem cells or the hemopoietic precursor cells occurred in
the
co-culture with A9 or D 11 cells and the precursor cells of the erythrocyte
were
174
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
continuously supplied . In contrast, although cellular morphology of A7 was
similar
to that of A9, A7 did not support CFU-GM, BFU-E, and CFU-E mix.
5) Comparison of an activity to support the human hematopoietic stem cells
between
A9 and a stromal cell strain OP9 derived from mouse fetus
Comparison of activities to support the CD34 positive hematopoietic stem.
cells
derived: from the human cord blood between AGM-s3 subclones A9 and A7, and a
stromal cell line OP9 derived from mouse fetus were performed with CFU-G1VI, .
BF1J-E, CFU-E. and CFU-E mix as indexes using the above described method.,
Fig.
S shows the results from the two-week co-cul.t~.irc. In the A7 cell culture
syste~::n,
CFU-CTM, BFU-E, and CFLj-E were.significantly decreased and CFU-E mix was
completely disappeared. , In contrast., with OP9 yells, a variety of blood
cell
precursor cells including CFU-E mix were supported, although the supporting
ability was less than that of A9 cel~Is. Therefare, OP9 ceps were clear to
possess the.
activity to support. the hematopoietic stem cells. ; ~ , .
(2) Assessment c~l~ activities to suppo:~~t the henzatapoietif~.stem cells in
a vaP::iety . r ~:,
of cell strains .
The above described stromal cell lines(AGTVI-s3-A9; AGM-s3-AA7, and AGM-
s3-G1), ~3T3Swiss (ATCC), OP9 (RCB1124, RII~EN Cell Development Bank), and
NIH3T3 (ATCC) were seeded in a 24-well cultuxe dish. (Falcon) at 5 x 10ø
cells/well. The cell Lines were cultured in MEVicx rr~edium-(GIBCO 13RL)
containing non-active 10 % FCS (bovine fetal serum, Hyclone) for one day and
allowed to proliferated until the cells covered all over the bottom surfaces
of the
wells. Then, the medium was replaced to one milliliter of fresh medium, thirty
cells
of the mouse hematopoietic stem cells (derived from C57BL/6-Ly5.1) obtained in
Example 9 were sorted on this cell layer, and co-culture was initiated.
On seventh day of the cultivation, the cells were trypsinized (0.05 % trypsin
in
PBS containing 0.5 mM EDTA (GIBCO BRL) at 37°C for two to five
minutes) and
dispersed and all the cells on the culture dish were recovered. The recovered
whole
cells of each cell line and whole bone marrow cells at 200,000 cells (derived
from
C57BL/6-Ly5.2 mouse, Charles River) were transplanted into the C57BL/6-Ly5.2
175
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
mice (eight weeks age and male, Charles River) irradiated with X-ray at 8.5 Gy
through the tail vein. After the transplantation, the peripheral blood was
collected
from the retro-orbital sinus at intervals, and calculated the ratio of a cell
number
derived from the C57BL/6-LyS.1 prep mouse with FACS. The peripheral blood
was analyzed according to the usual method (S. Kouzu, Fundamental techniques
for
immunology, YODOSHA, 1995). Three hundreds and fifty yL of distilled water
was added to 50 yL of the peripheral blood, allowed to stand for 30 sec. so as
to
lyze the erythrocytes. Then, PBS at twice concentrations was added and
centrifuged, so that the white blood cells were recovered. After the cells
were
washed'. once using the staining buffer (PBS containing 5 % x CS and O .OS %:
NaNs),
anti-CD16 antibody, LyS.l (CD45.1) antibody labeled with FITC, Gr-1 and CDllc~
. antibodies labeled with phycoerythrin; and CD4SR (B220) .antibody and CD90
.. (Thyl) antibody labeled with allophycocyanin (all of these were purchased
from
Pharmingeri) 'were added. After these ,cells were allowed to stand far
reaction in the
ice bath for 30 min., they were washed with the' staining buffer and FACS
analysis
was pert ormed.
Expansion iii the number of cells capable of reconstitution durivg the
hematopoietic stem cell culture was assessed'by calculating the proportions of
E.,yS.I
positive cells in the Gr-1 or CDllc positive'cell's (myeloid cells) or LyS.l
positive
cells in the CD90 or CD45R positive cells (lymphoid cells)-in the peripheral
blood
at. intervals post transplantation. . ' - .
Fig. 6 shows the results. When the cells were co-cultured with ACJM-s3-A9,
OP9, and 3T3Swiss cells, high chimerism of donor cells were maintained after
the
transplantation. Therefore, these stromal cells were considered to have a high
activity.to support the hematopoietic stem cells. In contrast, when the cells
were co-
cultured with AGM-s3-A7, AGM-s3-G1, and NIH3T3 cells, high chimerism were
not observed in the transplanted cells. Therefore, these stromal cells had low
-
activity to support the hematopoietic stem cells or the hemopoietic progenitor
cells.
176
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
Example 11
Isolation of Mouse SCR-1 Fragment
Total RNA was prepared from AGM-s3-A9 cells at 1.4 x 10~ cells dissolved
in 20 mL of 1SOGEN (Nippon gene, Japan) according to the attachment.
Messenger RNA was purified from orie milligram of the total RNA according to
the
protocol of the mRNA purification kit (Amersham Pharmacia, U.S.A.). cDNA was
synthesized from this mRNA by oligo-dT primed with Superscript Plasmid System
(GIBCO Lifetech, U.S.A.) and inserted into pSPORTl (GIBCO Lifetech, U.S.A.).
. An AGM-s3-A9 cell specific cDNA clone was obtained from this library with
SBH
IO .' method (Hyseq, iJ.S.A.). A nucleotide sequence of the clone was
determined using
ABI377 DNA sequencer (Perkin Elmer, U.S.A.). The obtained sequence was
. ... analyzed by homology search, so that the gene was identified as a novel
gene SCR- '
1. The .nucleotide sequence obtained was nucleotide numbers 1032 to 1484 of
SEQ
. ID NO:'31.
~ E~!~MI'LE 12
V~l~ole Cloning of Mouse SCR-1
Total RNA 'was prepared from A GM-s3-A9 cells at 1.4 x 10~ ells dissolved
in 20 mL of .ISOGEN (Nippon gene, Japan) according to the attachment.
Messenger RI'VA was purified from one milligram of the total RNA atrcording to
the . . '
protocol of the mRNA purification kit (Amersham Pharmacia, U.S.A.). cDNI~
library vas constructed from 2 mg of p repared naRNA using SMART cDNA library
construction kit (CLON TECH, U.S.A.) according to the attachment. This library
included about 400,000 kinds of independent clones in total arid divided into
15
fractions. The fraction containing SCR-1 cDNA clone was identified by PCR
using
the following conditions.
The following primers were synthesized based on the gene fragment sequence
obtained in Example 11 PCR at 35 cycles was performed using each fraction of
AGM-s3-A9 cDN,~ library as a template, one cycle being a step performed at
94°C
for 30 seconds, at 55°C for 30 seconds, and 72°C for one minute.
SCR-1F1: AGTACAAAGAAAGAAGTGTTC (SEQ ID NO: 35)
SCR-1R1: TGAGTCTACAGTAACCTCGCA (SEQ ID NO: 36)
177
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
The PCR products were subjected to a 2 % agarose gel electrophoresis, and the
fraction in which a PCR product had an expected size was identified. Two
positive
fractions were seeded on petri dishes at a dian~eier of 15 cm at 50,000
plaque, each
fractions being seeded on two petri dishes. After incubating the dishes at
37°C for
S 10 hours, each plaque was transferred to a Biodyne nylon filter (Pall,
U.S.A.).
DNAs on the nylon filters wereimmobilized according to the attachment.
Screening was performed using. a 32P labeled DNA probe.
The probe was prepared as follows. PC'R ~a.t 35 cycles was performed using
SCR-1R1 and T7 primer (TAATACC'~ACTCACT'ATAGGG) (SEA ID NO: 37), and
. a plasmi.d including the gene: fragrr~ent obtained in Example 11 as a
template, ojue
cycle being a step performed at 9~°C for 30 seconds, at 55°C for
30 seconds, and
72°C for one minute. The PCR products were subjected to a 2% agarose
gtl '
electrophoresis, and the amplified fragment was purified using JETSORB
(GENOMED Ger.). 32P labeled DNA probe was prepared using Megaprime
labeling kit (Arnersham Pha~~macia U.S.A.) and 25 ng of purified PCR fragment
as , .
a template.
hybridization using Express.HybSolution (t.~,LONTEC:H, U.S.~..) and washing
v~~
were performed according to the attachment. ~i-:ray films (Fuji Photo Film Co.
Ltd. ~ Japan)were exposed to, the hybridized nyla?i filters for one day and
developed
. using a.~'uji film auto-developer apparatus. ~3asPd on the analyzed results,
the
plaque which coxresponded ~o the.~spct' strongly exposed was scratched from
the
petri dish. 'The plaque was :again seeded on a petri dish at a diameter of 10
cm so as
to generate about 200 piaquea. Screening was performed again according to the
method described above, so that a. single plaque was isolated. The obtained
phage
clone was introduced into E. coli BM2~.8 strain according to the attachment of
the
SMART cDNA library constructing kit, so that it was excised ifa vivo in E.
coli
BM25.8 strain. The infected E. coli was cultured on LB agar medium added with
50 mg/mL of ampicillin until colonies were formed. A single colony was seeded
in
three milliliters of LB medium containing 50 mglmL of ampicillin and cultured
overnight at 30°C. About 10 mg of plasmid was purified from the
cultured cells
using RPM Kit (BIO101, U.S.A.). The sequence of the both ends of the inserted
fragment was determined using ~,TriplExS'LD-Insert Screening Amplimer
178
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
(CTCGGGAAGCGCGCCATTGTGTTGGT: CLONTECH, U.S.A; SEQ ID NO:
30.) by ABI377 DNA sequencer. The clone was found to include cDNA which has
a nucleotide sequence beginning from 1 in SEQ ID NO: 31. After the whole
nucleotide sequence of the inserted cDNA was determined using ABI377 DNA
sequencer, the nucleotide sequence of SEQ ID NO: 31 was confirmed. Amino acid
sequences predicted from the above nucleotide sequence were shown in SEQ ID
NO: 31 and SEQ ID NO: 32.
The plasmid including DNA with the nucleotide sequence of SEQ ID NO: 31
has beet' internationally deposited in National Infititute of. Bioscience and
Huma.n-
~ Technology, Agency of Industrial Scii:;nce anti 1'echnelogy (Zip code 305-8
566;
Higashi.I-I-3, Tsukuba, Ibaraki, rapan) on June 26,2000, and the registered
number 'was given to be FERM BP-7198.
EXAMPLE 13 ' .
Glonirrg Of Htln~ale SCR-1.
. ~., : ~. . .,
Based on the nucleotide sequence of mouse. SCR-l, tl~e database of GenBank
(NCBI, r~J.S.A.) was searched using Blast. ~1 ac>n~c;logous rzuclec>tid~:
sequence: with
. ~ mouse SCR-1 was found (Accession Nos. AI872133 and A~~V316562). The
,. . , .
following primers were synthesized using this sequence derived from huma?~.
h782F1: TCGCGGGGATGCCAGCCACCCCAG (SEQ III NO: 38)
h782F2: AGCAGGCCTATCGGATGTGAGAGGAGA,AGT (SEQ ID NO: 39)
h782R1: CTATTAACAAATATATTTATTGTGGTGGC-~ (SEQ ID NO: 40)
h782R2: TGGTGGCTTTC'I'CCCCT~,CTAGATATACC T (SEQ ID NO: 41)
cDNA was synthesized from 3 yg of mRNA derived from the placenta and the
skeletal muscle (C.LONTECH, U.S.A..) using oligo-dT primer and reverse
transcriptase (SuperscriptII, GIBCO-BRL). PCR was performed using this cDNA
as a template; h782F1, h782F2, h782R1, or h782R2 as a primer; and Platinum Pfx
DNA Polymerase (GIBCO Lifetech, U.S.A.). As a result, an arriplified fragment
was obtained from. each organ. Among them, the PCR fragment derived from the
placenta was ligated to pCR-Blunt vector (Invitrogen, U.S.A.), and the gene
was
introduced into E. coli DHSa. Then, the transferred E. coli was seeded on LB
agar
medium containing 100 mg/ml of ampicillin, so that colonies were formed. Each
of
isolated 1.6 colonies was added to 10 ml of PCR reaction solution, and treated
at
179
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
94°C for five minutes. Then, ,PCR at 35 cycles was performed, one cycle
being a
step performed at 94°C for 30 seconds, at 55°C for 30 seconds,
and 72°C for one
minute. T7 primer or SP6 primer (GATTTTAGGTGACACTA'TAG) (SEQ ID NO:
42) was used as a primer at a final concentration of 0.2 mM. The PCR products
were subjected to a 2% agarose gel electrophoresis. After the amplified
fragment
was confirmed, sequences of the three confirmed fragments were determined
using
ABI377 DNA sequencer. A nucleotide sequence of the obtained cDNA (SEQ ID
N0: 33) was confirmed to be a human orthologous to that of mouse SCR-1.
The plasmid including DNA with the nucleotide sequence of SEQ ID NO: 33
20 has been internaticmally deposited in National Institute of Bioscience and
H.umar~-
Technology, Agency of Industrial Science and Technology (Zip code 305-8566;
Higashi 1-1-3, Tsukuba, Ibaraki, Japari) on June 26,2000, and the registered
number was given to be FERM BP-7197. a
With respect to mouse SCR-1 and human SCR-1.when the established
database was searched, a human gene sequence ~h~aving urilcilovn function was
found
(WO98149302). T he homology of coding regions of these genes is shown in Table
;~ ~ : a . ~ .
1. The comparison of the homology «~as performed using a homology search
. ~ .
function of DNAIS-Mac version 3.7 and calculating with the default settings of
the
software (nucleic acid Mode: Normal, Range: AlI (1-819 base), Cutoff: 45,
Ktup:
4, amino acid Range: All (1-273 a.a.), Cutoff: 45, Ktup: 2). In this method,
since
only high homologous regions are used for calculation, low homologous regions,
locating at an end, are excluded for calculation.
Table 1
Mouse SCR-1 Gene having unknown function
88.5 ! 98.5
Human SCR-1
(87.1) ( 100) .
87.2
Mouse SCR-1 y
(85.I)
The upper numbers show the homology in nucleotide level
The lower numbers (parenthesize) show the homology in amino acid level.
180
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
~Iuman SCR-1 and the gene having unknown function had the same .
nucleotides from the initiation codon to the first nucleotide of the 266th
codon
(nucleotide number 10S4 in SEQ ID NO: 33) in the coding region. I-lowever, the
down stream sequences thereof were not identical. The nucleotide sequence and
the
S amino acid sequence of this nonidentical region in this gene having unknown
function were shown in SEQ ID NO: 4S and SEQ ID NO: 46, respectively. The
first nucleotide in SEQ ID NO: 4S corresponded to the nucleotide number 1054
in ' v
SEQ ID NO: 33, and these were identical. One nucleotide was nonidentical at
the ' .
position corresponding to the S67th nucleotide in SEQ ID NO: 33 in both genes.
~ E~A11~I'LE .14
Study Of The Ex~r ession Rc:giou Of SCR-I
Northern blot analysis was performed using probes used in Example 13.
Hybridization was performed with respect to Northern blots of Human II~ITIir
Blot I, v
II, III, Immune System II, Mouse MTN Blot (CLONTECI4, U.S.A.). 'fhc
15~ hybridization was performed using ExpressHyb ~Iybridizarion Solution
(CI,ON l'EC~I. IJ.S.A.) according to the supplie.c's instruction. After
., ; . ..
prehybi.idization at 68°C for two hours, a lalieleil probe was added.
Hybridization
was further performed at 68°C for l.8 hours. Washing of the filter «~as
perforrrcea. at. '
room temperature in 2 x SSC, O.OS % SDS solutian for 30 .min and repeated
once.
Further washing was performed at SO°C, in 0.1 x SSC, 0.1 % SDS solution
for 30
min twir_e. Analysis of the hybridization was performed by exposure to an
imaging
plate: (Fuji Photo film Co. Ltd., Japan) for three hours using a bioimaging
analyzer '.
BAS2000 (Fuji Photo Film Co., Ltd., ~~apan). Northern blot technique analysis
was
performed using probes used in Example 13 and. MTN blot (CLONTECH, U.S.A.),
2S . so that the expression thereof in human was examined. As a result, the
expression
of mRNA at about 2.6 kb was confirmed in many organs including the liver, the
placenta, the skeletal muscle, and the uterus. In mouse, the expression of
mRNA at
about 2.6 kb was confirmed'in similar organs.
181
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
EXAMPLE 15
Expression Of Mouse SCR-1 In Stromal Cell
(1) Construction of retrovirus vector for expression of moose SCR-1
Only ORF sequence in SCR-1 gene (a nucleotide sequence from nucleotides
numbers 511 to 1350 in SEQ ID NO: 31) was inserted into a retrovirus vector,
so
that a vector for expression in stromal cells was constructed.
Messenger RNA was purified from one milligram of the total RNA in AGM-
s3-A9 cell according to the protocol of the mRNA purification kit (Amersharn
Pharmacia, U.S.A.). cDNA was synthesized from this mRNA according to the
conventional method. The following primers were synthesized and PCR a~ 30
cycles was performed using the above described~cDNA as a template, and
Platinum
~~
Pfx DNA Polymerase (GIBCO Lifetech, U.S.A.), one cycle being a~step performed
.. .. ~.. . ~ , . : . , . ,
. , at. 94°, C, for 2Q seconds, at 55°C for 30 seconds, and
68°C for one minute.
v : ~ . ~. : .
m782F2: CCGC'I'CGAGCCACCATGCACTTGCJ'GACTGATTTC (SEQ ID NO:
-. ~. ; . . , : . . .
,15 , 4.3) ~.
m782R2: ATTGAATTCCTAG'TGTACAGTGCTGAC'I'G (SEA? ID 1~t0: 41~)
An amplified fragment was digested with restriction enzyrr~es EcoRI and Xhol.
After electrophoresis, a DNA fragment,was purified using, JETSC3RB (Genomed,
,., .. ~ . .
Gexmany). The purified DNA fragment was ligated with pMX-IRES-GFP vector -;;
. digested with EcoRI and XhoI (gift form Professor T. I~itamura, TO~YO UNIV.
INST. OF MEDICAL SCIENCE, Japan). The BMX-IRES-GFP vector was a
plasmid in which IRES GFP was inserted into the retrovirus vector pMX. The
obtained recombinant vectox was transferred into E. coli DHSa, and was seeded
on
LB agar medium containing 100 mg/ml of ampicillin, so that independent
colonies
were formed. After the isolated colony was cultured in 200 mL of LB medium
containing 100 mg/ml of ampicillin, plasmid was purified using QIAGENtip200
(QIAGEN, U.S.A.). The sequence of the inserted gene was determined using
conventional method, so that the sequence was confirmed to be identical to the
corresponding region in SEQ ID NO: 31.
182
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
(2) Traalsfer of mouse SCR-1 into stromal cell
Initially, BOSC23 cells at 2 x lOG cells/dish were seeded on a collagen type I
coated 60 mm dislt (Asahi technoglass), and cultured in DMEM medium containing
% FCS at 37°C, under an atmosphere of S % COz, and a1: a humidity of
100 % .
5 Twelve to I8 hours after the start of the culture, the medium was replaced
by two
milliliters of OPTI MEM medium (GIBCO BRL).
About 3 yg of plasmid inserted with SCR-1 into the above described pMX-
IRES-GFP was added to 181.LL of LIPOFECTAMINE Reagent (GIBCO BRL)
diluted mith 100~.~L of OPTI MEM.medium, and .allowed to stand at room
10 temperature for 30 min. The prepared DNA solution was added to the above-
prepared,BOSC23 cell cultuxe solution. After about five hours, two milliliters
of
D1VIEM-medium containing 20 % FCS: (GIBCO BRL) was added
IRES (Internal Ribosome Entry Site) was determined ;.by an access of the .
. ribosonye to the internal site of the mRNA. Therefore, two: genes could be
expressed from one anRNA caused by ligation of upward and downward genes
.: separated by IRES in one transcription unit during the consta~uctic~2~ of
an expression q
v vector. .With respect to the above-described plas~nid, cI~N~~ of SCR-.~l was
inserted .
in upward site and GFP (Green Fluorescence Protein) was inserted iy dow~~waxd
r
site. Thus, the expression of SCR-,I could be monitored by~detecting. the
expression .
, of GFP using FACS.
After about 24 hours, the medium was replaced by 4 ml of DMEM containing
10% FC'S. Further, after about 48 hours, the culture medium was harvested.
After
the culture medium was filtrated through 0.45 ym filter, the filtrate was
centrifuged
at I,200g for I6 hours and the supernatant was removed, so that the virus
precipitation was obtained.
AGM-s3-A7 cells were cultured in one milliliter of MEMcc medium containing
10 % FCS (GIBCO BRL) on a 24-well culture dish (FALCON) at 1 x 104 cells/well.
After 1.2. to 18 hours, the virus precipitation was suspended in one
milliliter of
MEMa medium containing 10 % FCS, so that the stromal cell culture medium and
the virus suspension were replaced. Next, POLYBRENE (Sigma, SEQUA-
BRENE) was added to be 10 ~~g/mL. After the culture dish was centrifuged at
700g
far 45 min., the cells were cultured at 37°C, under an atmosphere of 5%
COz, and
183 '
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
at a humidity of 100 % . After 48 hours, the medium was replaced by one
milliliter
of MEM medium containing 10% FCS. After 24 hours, the cells were passaged
on a 6-well culture.dish (FALCON) and cultured in three milliliters of MEM
medium containing 10% FCS. Forty-eight hours after the passage, GFP expression
in the stromal cells was detected using a cell sorter (FACSVantage, Becton
Dickinson), so that it was indirectly confirmed that not less than 80% cells
expressed SCR-1.
EXAMPLE l6
Co-Culture Of Str omal Cells In Which SCR-I Gene Was Overe~ ressed With
, Mouse Plematopoietic ;item Cells '
AGM-s3-A~ cells, AGM-s3-A7 cells, or AGM-s3-A7.cells, transduced wifh
SCR-1 gene by retrovirus infections, were seeded in a 24-~~ell culture dish at
a
density of 1 x 10$, cells/well, and were cultured in MEMa medium containing 10
%
FCS for one day in order to allow the cells to proliferate to cover the whole
bottom
surface of the culture dish.
T~.ien, the medium wa,~ replaced by 1 nil of fresh modium and tl.~irt.y cells
~uf_
the.mo~;se hematopoietic stem cells (derived from C5713L/.r?-LyS. t) obtained
in .
Example 9 were sorted on this cell layer to initiate the co-cultures.
After 7 days of culture, all the cells in tl~e co-culture E~ere harvested by
trypsinization (0.05 % trypsin in PBS containing 0.5 mM EDTA at 37°C
for two to
.: five minutes), and the extent of the expansion. of hematopoietic stem
and/or
progenitor cells was analyzed in the following experiment.
EXAMPLE 17
Tr nsplantation Of Hematopoietic Cells Into Irradiated Recipient Mice
Thirty freshly isolated hematopoietic stem cells, obtained from C57BL/6-
LyS.1 mice by the procedure described above (CD 34 negative, Sca-1 positive, c-
Kit positive, Lin negative cells, or cells derived from C57BL/6-LyS. ? pep
mouse),
or whole the cells harvested on the 7th day of the co-culture:., which was
initiated
with 30 hematopoietic stem cells, were transplanted into the five C57BL/6-
Ly5.2
mice (eight weeks age and male, Charles River),which were irradiated with X-
ray at
184
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
8.5 Gy, through the tail vein together with the 200,000 whole bone marrow
cells
derived from C57BL/6-Ly5.2 mice (Charles River).
After the transplantation, the peripheral blood cells were collected from the
retro-orbital sinus over time, and analyzed for the proportion of the cells
that were
derived from Ly5.1 hematopoietic cells. The peripheral blood was analyzed
according to the usual method (S. Kouzu, Fundamental techniques for
immunology,
YODOSHA, 1995). In order to lyre erythrocytes, three hundreds and fifty ~,L of
distilled water was added to 50 yL of the peripheral blood and 30 seconds
after
addition c~f distilled water, ~ the sarrie amount of the twice concentr ated
PBS and
centrifuged, so that. white blood ~cell.s uvere recovered. Afttrr the cells
were washed
once,using the staining buffer (PB,S containing 5% FCS~and 0.05% NaNs), they
were stained with anti-CD16 antibody. FITG=anti-Ly5.l, PE-anti-myeloid cells
(Gr-
1 and CD 11 c) and APC-anti-lymphoid cells (B220 and Thy 1 )(purchased ~ from
v Pharmingen) and incubated for 30°ixiin on ice. Stained cells were
~Fashed using the
staining buffer and FRCS analysis:vas performed.. ~ '
' Expansions in the cumber of rhea cells, that 'were capable of
rcccrnstituting~..
'..~ hem~ttopoietic cells during the culture.uf the hematopoietic ;stem cells
were estir!~.aled
by calculating proportions of LyS.I positive cells in the Gr=1 or CDl lc
positive '
cells (myeloid cells), or LyS.1 positive cells in the CD90 0~ CD45R positive
cells;:
' (lymphoid cells) in the peripheral blood in the transplanted mice at
intervals.
, . . Fig. 7 shows the results. V~Ihen the cells co-cultured with AGM-s3-A.7
cells .
were tr ansplanted, .high chimerism derived from the cultured Ly 5. l
hematopoietic
cells was not observed. From this result, it was demonstrated that AGM-s3-A7
cells themselves showed low activity to support the hematopoietic stern cells
or
hemopoietic precursor cells. When cells co-cultured with AGM-s3-A7 cells in
which SCR-1 gene was overexpressed were transplanted, a significant high
proportion of cells derived from the cultured cells was detected in both
myeloid and
. lymphoid cells in the peripheral blood. Therefore, it was clear that the
hematopoietic stem cells and the hemopoietic precursor cells, which could
reconstitute the hemopoietic system of the irradiated mice, were supported and
amplified on the A7 stromal cells in which SCR-1 gene was overepressed.
185
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
As a result, it was evident that SCR-1 had a function to give the stromal
cells
without an activity to support the survival or the proliferation of the
hematopoietic
stem cells or the hematopoietic progenitor cells the above described activity.
Frorrz
these results, it was evident that SCR-1 had an activity to effect on support
the
proliferation or the survival of the hematopoietic stem cells or the
hemopoietic
precursor cells; or an activity to effect the strorr~al cells so as to give
them an
activity to support the hematopoietic stem cells. . '
EXAMPLE 18
. SCR-1 Tr~ns~~lliC l~~iice
'ihe activity of mouse and human SCR-I can be confirmed by, establishing
. a genetically modified mice, such a~.trarzsgenic mice. Appropriate promoters
ark.
selected for expression of SEQ ID NOS: 31 and 33 which allows their activity
for
.' hematopoietic stem cell growth or survival promotion to be confirmed. GATA-
2
promoter drives expression of genes in. very early hematopoietic stem or
progenitor
cell ~pop~.ilation. E~cpression of the~SCk;-1 gene (SEQ ILK NOSL 31 0~~ 33)
abider the
regulation of DATA-2 promoter in transgenic mice will cause the hernatopoietic
,Mc.
stem or progeyiitor cells to express the SCR-l~ gene. This SCR-1 gene
exprossic~n in
hematop,oietic stern progenitor cells will lead: to expansion..of
hematopoietic stem or ~:
progenitor cells which mill r esult in an increase of hematopoietic cells in
embr yos, w ' w
neonates or adult mutant mice. GATA-2 promoters described by Minegishi et al
may be tised for this purpose (J Biol Chem. l 998 Feb 6;273(6):3625-34).
The SCR-1 gene (SE:Q ID NO S: 31 or 33) may also be expressed under the
control of CAG or other promoters that work in ubiquitous tissues (Kiwaki et
al.
2S Gene Ther, 1996 May 1;7(7):821-30). This wild allow for determination of
the
effects of SCR-1 gene expression in other tissue cell types together with
hematopoietic cells. Transgenic mice can be established accroding to the
methods
described in "Manipulating the Mouse Embryo" (Brigid Hogan, Rosa Beddington,
Frank Costantini, Elizabeth Lacy, 1994, Cold Spring Harbor Laboratory Press.
186
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
SEQUENCE LISTING
<11G> Hyseq, Inc.
Kirin Brewery Co. Ltd.
<1.20:~ METHODS AND MATERIALS RELATING TO NOZTEL STEM CELL GROWTH FACTOR-LIKE
POLYPEPTIDES AND POLYNUCLEOTTDES
<130> 28110/37260
<140:~
<:~41> 2001-04-05
<150> 60/266,614
<151> 2001-U?-05
:I_50> 60'215, 733
<151v~ 2000-05-28
<150> 09/757,562
<:LS;L> 2001-01-09
<150:. 09%543,'774
<151: 2000-04-05
<J.6()> 4H
<I'70> Patertln version 3.0
<2i0> 1 ,
<21J.> 301
<212> DNA
<213> Homo sap.iens
<400>
I.
gca'~gagacgaggaaaaaaagga.agggagaggaaaagaaaaaaacctaataaaggagaaa 60
gl:aaagaagcaatacctgacagcaaaagtctggaatocagcaaagaaatcccagagcaac 120
gagaaaacaaacagcagcagaagaagcgaaaagtccaagataaacag~aaatcggtatcag 180
tcagcactgtacactagagggttccatgagattattgtagactcatgatgctgctatctc 240
aaccagatgcccaggacaggtgctctagccattaggaccacaaatggacatgtcagttat 300
t 301
<21.0> 2
<211> 392
< 212 > DILTA
<213> Homo Sapiens
<400> 2
tggaactcga tatccagata taaataagcg tacaaaatgc aaagctgact gtgatacctg 60
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
_7_
tttcaacaaagatttctgcacaaaatgtaaaagtggattttacttacaccttggaaagtg 120
ccttgacaattgcccagaagggttggaagccaacaaccatactatggagtgtgtcagtat 180
tgtgcactgtgaggtcagtgaatggaatccttggagtccatgcacgaagaagggaaaaac 240
atgtggcttcaaaagagggactgaaacacgggtccgagaaataatacagcatccttcagc 300
aaagggtaacctatgtcccccaacaaatgagacaagaaagtgtacagtgcaaaggaagaa 360
gtgtcagaagggagaacgaggaaaataaggag 392
<210> 3
<211> 475 .
<212> DNA
<213> Homo Sapiens
<220>
<221> mist feature
<222> (1) . . (475)
<223> n = A, T, G, or C
<400>
3
gtnagtacccccagggatttcactgagngcctggactgaggacccgtcnaanngcnngan 60
ccacgcgtncgc:ccacgcgtccggagaggaaaagaaaaaaacctaatttaggagaaagta 120
aagaagcaatacctgacagcggaagtctggaatggagcaaagaaatcccagagcaacgag 180
aaaacaaacagcagcagaagaagcgaaaagtccaagataaacagaaatcggtatcagtca 240
gcactgtacactagagggttccatgagattattgtagactcatgatgctgctatctcaac 300
cagatgcccaggacaggtgctctagccattaggaccacaaatggacatgtcagt'tattgc360
tctgtctaaacaacattcccagtagttgctatattcttcatacaagcatagttaacaaca 420
aagagccaaaagatcaaagaagggatactttcagatggttgtcttgtgtgCttCTl 475
<2l0> 4
<211> 473
<2l2> DNA
<2l3> Homo Sapiens
<220>
<221> mist feature
<222> (1) . . (473)
<223> n = A, T, G, or C
<400> 4
tgggcannnn aaanttttga nattcgatcc gcgctgcagg aattcggcac gagacgagga 60
aaaaaaggaa gggagaggaa aagaaaaaaa cctaataaag gagaaagtaa agaagcaata I20
cctgacagca aaagtctgga atccagcaga gaaatcccag agcaacgaga aaacaaacag 180
cagcagaaga agcgaaaagt ccaagataaa cagaaatcgg tatcagtcag cactgtacac 24,0
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-3-
tagagggttc catgagatta ttgtagactc atgatgctgc tatctcaacc agatgcccag 300
gacaggtgct ctagccatta ggaccacaaa tggacatgtc agttattgct ctgtctaaac 360
aacattccca gtagttgcta tattcttcat acaagcatag ttaacaacaa agagccaaaa 420
gatcaaagaa gggatacttt cagatggttg tcttgtgtgc ttctctgcat ttt 473
<210> 5
<211> 462
<212> DNA
<213> Homo sapiens
<220>
<221> misc 'feature
<222> (1)..(462)
<223> n = A, T, G, or C
<400>
tgggagannnntttgaaactgagatcgtcgcanacncnacnangaataaaaggaagggag60
agggaaagaaaaaaacctaataaaggagaaagtaaagaatcaatttctgacagcaaaagt120
ctggaatccat caaagaaatcccatatcaacgagaaaacagacagcagcacaaaaagcga180
aaagtccaagata.aacagaaatcggtatcagtcagcactgtacactagagggttccatga'440
gattattgtagactcatgatgctgctatctcaaccagatgcccaggacaggtgctctatc3'00
cattacgaccacaaatggacatgtcagttattgctctgtctaaacaacattcccagtagt360
tgctatattcttcatacaagcatagttaacaacaaagagccaaaagatcaaagaagggat420
actttcagatggttgtcttgtgtgcttctctgcatttttaas 462
<210> 6
<211> 384
<212> DNA
<213> Homo Sapiens
<400>
6
aataatgtgtacaaaatgcaaagctgactgtgatacctgtttcaacaaaaatttctgcac 60
aaaatgtaaaagtggattttacttacaccttggaaagtgccttgacaattgcccagaagg 120
gttggaagccaacaaccatactatggagtgtgtcagtattgtgcactgtgaggtcagtga 180
atggaatccttggagtccatgcacgaagaagggaaaaacatgtggcttcaaaagagggac 240
tgaaacacgggtccgagaaataatacagcatccttcagcaaagggtaacctatgtccccc 300
aacaaatgagacaagaaagtgtacagtgcaaaggaagaagtgtcagaagggagaacgagg 360
aaaaaaagga agggagagga aaag 384
<210> 7
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
_q._
<211> 390
<212> DNA
<213> Homo sapiens
<220>
<221> mist feature
<222> (1) . . (390)
<223> n = A, T, G, or C
<400> 7
cgttgctctg ggatttcttt gctggattcc agacttttgc tgtcaggtat tgcttcttta 60
CtttCtCCtt tattaggttt ttttCttttC CtCtCCCttC CtttttttCC tCgttCtCCC 120
ttctgacact tcttcctttg cactgtacac tttcttgtct catttgttgg gggacatagg 180
ttaccctttg ctgaaggatg ctgtattatt tctcggaccc gtgtttcagt ccctctttt.-.g 240
aagccacatg tttttccctt cttcgtgcat ggactccaag gattCCattc actgacctca 300
cagtgcacaa tactgacaca ctccatagta tggttgttgg cttccaaccc ttctgggcaa 360
ttgtcaaggc actttccaag gtgtaagtan 390
<2l0> 8
<211> 1345
<212> DNA
<213> Homo sapiens
<220>
<221> misC feature
<222> (321)..(1235)
<223> similar to gi4519541 in the genpept database release 114, Run wit
h FASTXY3.3t00, default parameter
<400>
8
gcggccgccccggcggctcctggaaccccggttcgcggcgatgccagccaccccagcgaa 60
gccgccgcagttcagtgcttggataatttgaaagtacaatagttggtttccctgtccacc 120
cgccccacttcgcttgccatcacagcacgcctatcggatgtgagaggagaagtcccgctg 180
ctcgggcactgtctatatacgcctaacacctacatatattttaaaaacattaaatataat 240
taacaatcaaaagaaagaggagaaaggaagggaagcattactgggttactatgcacttgc 300
gactgatttcttggctttttatcattttgaactttatggaatacatcggcagccaaaacg 360
cctcccggggaaggcgccagcgaagaatgcatcctaacgttagtcaaggctgccaaggag 420
gctgtgcaacatgctcagattacaatggatgtttgtcatgtaagcccagactattttttg 480
ctctggaaagaattggcatgaagcagattggagtatgtctcatcttcatgtccaagtgga 540
tattatggaactcgatatccagatataaataatgtgtacaaaatgcaaagctgactgtga 600
tacctgtttcaacaaaaatttctgcacaaaatgtaaaagtggattttacttacaccttgg 660
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
_$_
aaagtgcctt gacaattgcccagaagggttggaagccaacaaccatactatggagtgtgt720
cagtattgtg cactgtgaggtcagtgaatggaatccttggagtccatgcacgaagaaggg780
aaaaacatgt ggcttcaaaagagggactgaaacacgggtccgagaaataatacagcatcc840
ttcagcaaag ggtaacctatgtcccccaacaaatgagacaagaaagtgtacagtgcaaag900
gaagaagtgt cagaagggagaacgaggaaaaaaaggaagggagaggaaaagaaaaaaacc960
taataaagga gaaagtaaagaagcaatacctgacagcaaaagtctggaatccagcaaaga1020
aatcccagag caacgagaaaacaaacagcagcagaagaagcgaaaagtccaagataaaca1080
gaaa.tcggtatcagtcagcactgtacactagagggttccatgagattattgtagactcat1140
gatgctgcta tctcaaccagatgcccaggacaggtgctctagccattaggaccacaaatg1200
gacatgtcag ttattgctctgtctaaacaacattcccagtagttgctatattcttcatac1260
aagcatagtt aacaacaaagagccaaaagatcaaagaagggatactttcagatggttgtc1320
ttgtgtgctt ctctgcatttttaaa 1345
<210> 9
<211> 1343
<212> DNA
<213> Homo Sapiens
<220>
<221> CDS
<222> (291) . . (1109)
<400>
9
gcggccgccccggcggctcc tggaaccccggttcgcggcgatgccagccaccccagcgaa60
gccgccgcagttcagtgctt ggataat.ttgaaagtacaatagttggtttccctgtccacc120
cgccccacttcgcttgccat cacagcacgcctat~cggatgtgagaggagaagtcccgctg180
ctcgggcactgtctatatac gcctaacacctacatatattttaaaaacattaaatataat240
taacaatcaaaagaaagagg agaaaggaagggaagcattactgggttactatg cac 296
Met His
1
ttg cga att tct tgg ctt atc att aac ttt gaa tac 344
ctg ttt ttg atg
Leu Arg Ile Ser Trp Leu Ile Ile Asn Phe Glu Tyr
Leu Phe Leu Met
10 15
atc ggc caa aac gcc tcc gga agg cag cga atg cat 392
agc cgg cgc aga
Ile Gly Gln Asn A1a Ser Gly Arg Gln Arg Met His
Ser Arg Arg Arg
20 25 30
cct aac agt caa ggc tgc gga ggc gca aca tca gat 440
gtt caa tgt tgc
Pro Asn Ser Gln Gly Cys Gly Gly A1a Thr Ser Asp
Val Gln Cys Cys
35 40 45 50
tac aat tgt ttg tca tgt ccc aga ttt ttt ctg gaa 488
gga aag cta get
Tyr Asn Cys Leu Ser Cys Pro Arg Phe Phe Leu Glu
G1y Lys Leu Ala
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
55 60 65
agaatt ggcatg aagcagatt ggagtatgt ctctcttca tgtcca agt 536
ArgIle GlyMet LysGlnIle GlyValCys LeuSerSer CysPro Ser
70 75 ' 80
ggatat tatgga actcgatat ccagatata aataagtgt acaaaa tgc 584
GlyTyr TyrGly ThrArgTyr ProAspIle AsnLysCys ThrLys Cys
85 90 95
aaaget gactgt gatacctgt ttcaacaaa aatttctgc acaaaa tgt 632
LysAla AspCys AspThrCys PheAsnLys AsnPheCys ThrLys Cys
100 105 110
aaaagt ggattt tacttacac cttggaaag tgccttgac aattgc cca 680
LysSer GlyPhe TyrLeuHis LeuGlyLys CysLeuAsp AsnCys Pro
115 120 ~ 125 l30
gaaggg ttggaa gccaacaac catactatg gag~tgtgtc agtatt gtg 728
GluGly LeuGlu AlaAsnAsn HisThrMet GluCysVal SerIle Val
135 140 145
cactgt gaggtc agtgaatgg aatCcttgg agtccatgc acgaag aag 776
HisCys GluVal SerGluTrp .AsnProTrp SerProCys ThrLys Lys
150 155 160
ggaaaa acatgt ggcttcaaa agagggact gaa.acacgg gtccga gaa 824
GlyLys ThrCys GlyPheLys ArgGlyThr G1uThrArg ValArg G1u
165 170 175
ataata cagcatsccttcagca aagggtaac ctat ccc ccaacZ aat 8'72
gt
IleIle GlnHis ProSerAla LysGlyAsn LeuCysPro ProThr Asn
180 185 190
gagaca agaaag tgtacagtg caaaggaag aagtgtcag aaggga gaa 920
GluThr ArgLys CysThrVal G1nArgLys LysCysGln LysGly Glu
195 200 205 210
cgagga aaaaaa ggaagggag aggaaaaga aaaaaacct aataaa gga 968
ArgGly LysLys GlyArgGlu ArgLysArg LysLysPro AsnLys Gly
215 220 225
gaaagt aaagaa gcaatacct gacagcaaa agtctggaa tccagc aaa 1016
GluSer LysGlu AlaIlePro AspSerLys SerLeuGlu SerSer Lys
230 235 240
gaaatc ccagag caacgagaa aacaaacag cagcagaag aagcga aaa 1064
GluIle ProGlu GlnArgGlu AsnLysGln GlnGlnLys LysArg Lys
245 250 255
gtccaa gataaa cagaaatcg gtatcagtc agcactgta cactag 1109
ValGln AspLys GlnLysSer ValSerVal SerThrVal His
260 265 270
agggttccat gagattattg tagactcatg atgctgctat ctcaaccaga tgc,ccaggac 1169
aggtgctcta gccattagga ccacaaatgg acatgtcagt tattgctctg tctaaacaac 1229
attcccagta gttgctatat tcttcataca agcatagtta acaacaaaga gccaaaagat 1289
caaagaaggg atactttcag atggttgtct tgtgtgcttc tctgcatttt taaa 1343
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
<210> 10
<211> 272
<212> PRT
<213> Homo sapiens
<400> 10
Met His Leu Arg Leu Ile Ser Trp Leu Phe Ile I1e Leu Asn Phe Met
1 5 10 15
G1u Tyr Ile Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln Arg Arg
20 25 30
Met His Pro Asn Va1 Ser Gln Gly Cys Gln Gly Gly Cys A7.a Thr Cys
35 ' 40 45
Ser Asp Tyr Asn Gly Cys Leu Sex Cys Lys Pro Arg Leu Phe Phe Ala
50 55 60
Leu Glu Arg Ile Gly Met Lys Gln I1e Gly Va1 Cys Leu Ser Ser Cys
65 70 75 80
Pro Ser Gly Tyr Tyr Gly Thr Arg Tyr Pro Asp Ile Asn L~js Cys Tlar
85 90 95
Lys Cys Lys Ala Asp Cys A.sp Thr Cys Phe Asn Lys Asn Phe Cys Thr
100 105 110
Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Asn
115 120 125
Cys Pro Glu Gly Leu Glu A1a Asn Asn His Thr Met G.lu Cr_~s Val Ser
130 135 140
Ile Val His Cys Glu Val Ser Glu Trp Asn Pro Trp Ser Pro Cys Thr
145 150 155 160
Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr G1u Thr Arg Val
165 170 175
Arg Glu Ile Ile Gln His Pro Ser Ala Lys Gly Asn Leu Cys Pro Pro
l80 185 190
Thr Asn G1u Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys
195 200 205
Gly Glu Arg Gly Lys Lys Gly Arg G1u Arg Lys Arg Lys Lys Pro Asn
210 215 220
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
_g_
Lys Gly Glu Ser Lys Glu Ala Ile Pro Asp Ser Lys Ser Leu Glu Ser
225 230 235 240
Ser Lys Glu Ile Pro Glu Gln Arg Glu Asn Lys Gln Gln Gln Lys Lys
245 250 255
Arg Lys Va1 G1n Asp Lys Gln Lys Ser Val Ser Val Ser Thr Val His
260 265 270
<210> 11
<211> 819
<212> DNA
<213> Homo Sapiens
<400> 11
atgcacttgc gactgatttcttggctttttatcattttgaactttatggaatacatcggc 60
agccaaaacg cctcccggggaaggcgccagogaagaatgcatcctaacgttagtcaaggc 120
tgccaaggag gctgtgcaacatgctcagattacaatggatgtttgtcatgtaagcccaga 180
ctattttttg ctctggaaagaattggcatgaagcagattggagtatgtctctcttcatgt 240
ccaagtggat ata atggaactcgatatccagatataaataagtgtacaaaatgcaaagct 30U
gactgtgata cctgtttcaacaaaaat:ttctgcacaaaatgtaaaagtggattttactta 360
caccttggaa agtgccttgacaattgcccagaagggttggaagccaacaaccataccatg 4'20
gagtgtgtca gtattgtgcactgtgaggtcagtgaatggaatccttggagtccatgcacg 480
aagaagggaa aaacatgtggcttcaaaagagggactgaaacacgggtccgagaaataata.540
cagcatcctt cagcaaagggtaacctatgtcccccaacaaatgagacaagaaagtgtaca 600
gtgcaaagga agaagtgtcagaagggagaacgaggaaaaaaaggaagggagaggaaaaga 660
aaaaaaccta ataaaggagaaagtaaagaagcaatacctgacagcaaaagtctggaatcc 720
agcaaagaaa tcccagagcaacgagaaaacaaacagcagcagaagaagcgaaaagtccaa 780
gataaacaga aatcggtatcagtcagcactgtacactag 819
<210> 12
<211> 822
<212> DNA
<213> Homo Sapiens
<220>
<221> CDS
<222> (1)..(822)
<400> 12
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
atg ggt cacttg cgactgatt tcttggctt tttatcatt ttgaac ttt 48
Met Gly HisLeu ArgLeuIle SerTrpLeu PheIleIle LeuAsn Phe
1 5 10 15
atg gaa tacatc ggcagccaa aacgcctcc cggggaagg cgccag cga 96
Met Glu TyrIle GlySerG1n AsnAlaSer ArgGlyArg ArgGln Arg
20 25 30
aga. atg catcct aacgttagt caaggctgc caaggaggc tgtgca aca 144
Arg Met HisPro AsnValSer GlnGlyCys GlnGlyGly CysAla Thr
35 40 45
tgc tca gattac aatggatgt ttgtcatgt aagcccaga ctattt ttt 192
Cys Ser AspTyr AsnGlyCys LeuSerCys LysProArg LeuPhe Phe
50 5S 60
get ctg gaaaga attggcatg aagcagatt ggagtatgt ctctct tca 260
A1a Leu GluArg IleGlyMet LysGlnIle GlyValCys LeuSer Ser
65 70 75 80
tgt cca agtgga tattatgga actcgatot ccagatata aataag tgt 288
Cys Pro SerGly TyrTyrGly ThrArgTyr ProAspIle AsnLys Cys
85 w- 90 95
aca aaa tgcaaa getgactgt gatacct-gtttcaacaaa aatttc tgc 336
Thr Lys CysLys AlaAspCys AspThrCys PheAsnLys AsnPhe Cys
100 l05 1.10
aca~ aaa tgtaaa agtggattt tacttacac cttggaaag tgcctt gac :384
Thr Lys CysLys SerGlyPhe 'ryrLeuHis LeuGlyLys C:ysLe~zAsp
115 l2C : 125
aat tgc ccagaa gggttggaa gccaac~.accatactatg gagtgt gtc 432
Asn Cys ProGlu GlyLeuGlu AlaAsnAsn HisThrMet GluCys Val
130 135 7.40
agt att gtgcac tgtgaggtc agtgaatgg aatccttgg agtcca tgc 480
Ser Ile ValHis CysGluVa1 SerGluTrp AsnProTrp SerPro Cys
145' 150 155 160
acg aag aaggga aaaacatgt ggcttcaaa agagggact gaaaca cgg 528
Thr Lys LysGly LysThrCys GlyPheLys ArgGlyThr GluThr Arg
165 170 175
gtc cga gaaata atacagcat ccttcagca aagggtaac ctatgt ccc 576
Val Arg GluIle TleG1nHis ProSerAla LysGlyAsn LeuCys Pro
180 185 190
cca aca aatgag acaagaaag tgtacagtg caaaggaag aagtgt cag 624
Pro Thr AsnG1u ThrArgLys CysThrVal GlnArgLys LysCys Gln
195 200 205
aag gga gaacga ggaaaaaaa ggaagggag aggaaaaga aaaaaa cct 672
Lys Gly GluArg GlyLysLys GlyArgGlu ArgLysArg LysLys Pro
210 215 220
aat aaa ggagaa agtaaagaa gcaatacct gacagcaaa agtctg gaa 720
Asn Lys GlyGlu SerLysGlu AlaIlePro AspSerLys SerLeu G1u
225 230 235 240
tcc agc aaagaa atcccagag caacgagaa aacaaacag cagcag aag 768
Ser Ser LysGlu IleProGlu GlnArgGlu AsnLysGln GlnGln Lys
245 250 255
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-10-
aag cga aaa gtc caa gat aaa cag aaa tcg gta tca gtc agc act gta 816
Lys Arg Lys Val G1n Asp Lys Gln Lys Ser Val Ser Val Ser Thr Val
260 265 270
cac tag 822
His
<210> 13
<211> 273
<212> PRT
<213> Homo sapiens
<400> 13
Met Gly His Leu Arg Leu Ile Ser Trp Leu Phe Ile Ile Leu Asn Phe
1 5 10 15
Met Glu Tyr Ile Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln Arg
20 25 ' 30
Arg Met His Pro Asn Val Ser Gln Gly Cys Gln Gly Gly Cys Ala 'I'hr
35 40 45
Cy;~ Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Fro Arg Leu Phe Phe
50 55 6U
Ala Leu Glu Arg Ile Gly Met Lys Gln Ile Gly V,al Cys Leu Ser Ser
65 70 75 80
Cys Pro Ser Gly Tyr Tyr Gly Thr Arg Tyr Pro Asp Ile Asn Lys Cys
85 90 95
Thr Lys Cys Lys Ala Asp Cys Asp Thr Cys Phe Asn Lys Asn Phe Cys
100 105 . 110
Thr Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp
115 120 125
Asn Cys Pro Glu Gly Leu Glu Ala Asn Asn His Thr Met Glu Cys Val
130 135 140
Ser Ile Val His Cys Glu Val Ser Glu Trp Asn Pro Trp Ser Pro Cys
145 150 155 l60
Thr Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Thr Arg
165 170 175
Val Arg Glu Ile Ile Gln His Pro Ser Ala Lys Gly Asn Leu Cys Pro
180 185 190
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-11-
Pro Thr Asn Glu Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys Gln
195 200 205
Lys Gly Glu Arg Gly Lys Lys Gly Arg Glu Arg Lys Arg Lys Lys Pro
2l0 215 220
Asn Lys Gly Glu Ser Lys Glu Ala Ile Pro Asp Ser Lys Ser Leu Glu
225 230 235 240
Ser Ser Lys G1u Ile Pro Glu G1n Arg Glu Asn Lys Gln G1n Gln Lys
245 250 255
Lys Arg Lys Val Gln Asp Lys G1n Lys Ser Val Ser Val Ser Thr Val
260 265 270
His
<210> 14
<211> 160
<212> PRT
<213> Homo.sapiens
<400> 14
Cys Thr Lys Cys Lys Ala Asp Cys Asp Thr Cys Phe Asn Lys Asn Phe
1 5 10 15
Cys Thr Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu
20 25 30
Asp Asn Cys Pro G7.u G1y Leu Glu Ala Asn Asn His Thr Met Glu Cys
35 40 45
Val Ser Ile Val His Cys Glu Val Ser Glu Trp Asn Pro 'rrp Ser Pro
50 55 60
Cys Thr Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Thr
65 70 75 80
Arg Val Arg Glu Ile Ile Gln His Pro Ser Ala Lys Gly Asn Leu Cys
85 90 95
Pro Pro Thr Asn Glu Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys
100 105 110
Gln Lys Gly Glu Arg Gly Lys Lys Gly Arg G1u Arg Lys Arg Lys Lys
115 120 125
Pro Asn Lys Gly Glu Sex Lys Glu Ala Ile Pro Asp Ser Lys Ser Leu
130 135 140
Glu Ser Ser Lys Glu I1e Pro Glu Gln Arg Glu Asn Lys Gln Gln Gln
145 150 155 160
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-12-
<210> 15
<211> 21
<212> PRT
<213> Homo Sapiens
<400> 15
Met His Leu Arg Leu Ile Ser Trp Leu Phe Ile Ile Leu Asn Phe Met
1 5 10 15
Glu Tyr Ile Gly Ser
<210> 16
<211> 251
<212> PRT
<213> Homo Sapiens
<400> 16
Gln Asn Ala Ser Arg Gly Arg Arg G1n Arg Arg Met His Pro Asn Val
1 5 10 15
Ser Gln G1y Cys Gln Gly Gly C'.ys Ala 'rhr Cys Ser Asp Tyr Asn Gl.y
20 25 30
Cys Leu Ser Cys Lys Pro Arg Leu Phe Phe Ala Leu Glu Arg Ile Gly
35 40 ' 45
Met Lys Gln Ile Gly Val Cys Leu Ser Ser Cys Pro Ser Gly Tyr Tyr
50 55 60
Gly Thr Arg Tyr Pro Asp Ile Asn Lys Cys Thr Lys Cys Lys Ala Asp
65 70 75 80
Cys Asp Thr Cys Phe Asn Lys Asn Phe Cys Thr Lys Cys Lys Ser Gly
85 90 95
Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Asn Cys Pro Glu Gly Leu
100 105 110
Glu Ala Asn Asn His Thr Met Glu Cys Val Ser Ile Val His Cys Glu
115 120 125
Val Ser Glu Trp Asn Pro Trp Ser Pro Cys Thr Lys Lys Gly Lys Thr
130 135 140
Cys Gly Phe Lys Arg Gly Thr G1u Thr Arg Val Arg Glu Ile Ile Gln
14S 150 155 160
His Pro Ser Ala Lys Gly Asn Leu.Cys Pro Pro Thr Asn Glu Thr Arg
165 170 175
Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys Gly Glu Arg Gly Lys
180 185 190
Cys Thr Lys Lys Gly Lys Thr Cys Gly
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-13-
Lys G1y Arg G1u Arg Lys Arg Lys Lys Pro Asn Lys Gly Glu Ser Lys
l95 200 205
G1u Ala Ile Pro Asp Ser Lys Ser Leu G1u Ser Ser Lys Glu Ile Pro
210 215 220
Glu Gln Arg Glu Asn Lys Gln Gln Gln Lys Lys Arg Lys Val Gln Asp
225 230 235 240
Lys Gln Lys Ser Val Ser Val Ser Thr Val His
245 250
<210> 17
<211> 23
<212> PRT
<213> Homo Sapiens
<400> 17
Ala Asp Cys Asp Thr Cys Phe Asn Lys Asn Phe Cys Thr Lys Cys Lys
1 5 10 15
Ser Gly Phe Tyr Leu His Leu
<210> 18
<211> 46
<2l2> PRT
<213> Homo Sapiens
<400> 18
Ile Asn Lys Cys Thr Lys Cys Lys Ala Asp Cys Asp Thr Cys Phe Asn
1 5 10 I5
Lys Asn Phe Cys Thr Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gl.y
20 25 30
Lys Cys Leu Asp Asn Cys Pro Glu Gly Leu Glu Ala Asn Asn
35 40 45
<2l0> l9
<211> 20
<212> PRT
<213> Homo Sapiens
<400> 19
Met His Pro Asn Val Ser,Gln Gl'y Cys G1n Gly Gly Cys Ala Thr Cys
1 5 10 15
Ser Asp Tyr Asn
<210> 20
<211> 37
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-1 ~-
<212> PRT
<213> Homo Sapiens
<400> 20
IleVal CysGlu Val Ser Glu Trp Asn Pro Trp Ser Pro
His Cys Thr
1 5 10 15
LysLys LysThr Cys Gly Phe Lys Arg Gly Thr Glu Thr
Gly Arg Vat.
20 25 30
ArgGlu IleGln
Ile
35
<210> 21
<211> 10
<212> PRT
<213> Homo Sapiens
<400> 21 -
Lys Lys Gly Arg Glu Arg Lys Arg Lys Lys
1 5 ZO
<210> 22
<211> 42
<212> PRT
<213> Homo Sapiens
<400> 22
Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys Gly Glu A.rg Gly Lys
1 5 10 15
Lys Gly Arg Glu Arg Lys Arg Lys Lys Pro Asn Lys Gly Glu Ser Lys
20 25 30
Glu Ala Ile Pro Asp Ser Lys Ser Leu Glu
35 40
<210> 23
<211> 14
<212> PRT
<2l3> Homo Sapiens
<400> 23
Thr Cys Phe Asn Lys Asn Phe Cys Thr Lys Cys Lys Ser Gly
1 5 10
<210> 24
<211> 20
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-15-
<212> PRT
<213> Homo Sapiens
<400> 24
Cys G1u Val Ser G1u Trp Asn Pro Trp Ser Pro Cys Thr Lys Lys Gly
Z 5 10 l5
Lys Thr Cys Gly
<210>25
<211>229
<212>PRT
<213>Mus musculus
<400> 25
Val Gly Ser Arg Gly Ile Lys G1y Lys Arg Gln Arg Arg Ile Ser Ala
1 5 10 ' Z5
Glu Gly Ser Gln Ala Cys Ala Lys Gly Cys Glu Leu Cys Ser Glu Val
20 25 30
Asn Gly Cys Leu Lys Cys Ser Pro Lys Leu Phe Ile Leu Lru Glu At:g
35 40 45
Asn Asp Tle Arg Gln Val Gly Val Cys Leu Pro Ser Cys Pro Pro Gl.y
50 55 60
Tyr Phe Asp A1a Arg Asn Pro Asp Met Asn Lys Cys Ile Lys Cys Lys
65 70 75 80
Ile Glu His Cys Glu Ala Cys Phe Ser His Asn Phe Cys Thr Lys Cys
85 90 95
Gln Glu Ala Leu Tyr Leu His Lys Gly Arg Cys Tyr Pro Ala Cys Pro
100 105 11U
Glu Gly Ser Thr Ala Ala Asn Ser Thr Met Glu Cys Gly Ser Pro Ala
115 120 125
Gln Cys Glu Met Ser Glu Trp Ser Pro Trp Gly Pro Cys Ser Lys Lys
130 135 140
Arg Lys Leu Cys Gly Phe Arg Lys Gly Ser Glu Glu Arg Thr Arg Arg
145 150 155 160
Val Leu His A1a Pro Gly Gly Asp His Thr Thr Cys Ser Asp Thr Lys
165 170 175
G1u Thr Arg Lys Cys Thr Val Arg Arg Thr Pro Cys Pro Glu Gly Gln
180 185 190
Lys Arg Arg Lys Gly Gly Gln Gly Arg Arg Glu Asn Ala Asn Arg His
195 200 205
Pro Ala Arg Lys Asn Ser Lys Glu Pro Arg Ser Asn Ser Arg Arg His
210 215 220
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-1G-
Lys Gly Gln Gln Gln
225
<2l0> 26
<211> 265
<212> PRT
<213> Homo sapiens
<400> 26
Met His Leu Arg Leu Ile Ser Trp Leu Phe Ile Tle Leu Asn Phe Met
1 5 10 15
Glu Tyr Ile Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln A~:g Arg
20 25 30
Met His Pro Asn Val Ser G1n Gly Cys Gln Gly Gly Cys Ala Thr Cys
35 40 45
Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Pro Arg Leu Phe Phe Ala
50 55 60
Leu Glu Arg Ile Gly Met Lys G1n Ile Gly Val Cys Leu Ser Ser Cys
.. 65 70 75 80
Pro. Ser GllT Tyr Tyr Gly Thr A:cg Tyr Pro Asp Ile Asn Lys Cys Thr
85 90 95
Lys .Cys Lys Ala Asp Cys Asp 7:'hr Cys Phe Asn Lys Asn Phe Cys
°rh:r
100 105 1.10
Lys-Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Asn
115 120 125
Cys Pro Glu Gly Leu Glu Ala Asn Asn His Thr Met Glu Cys Val Ser
130 135 140
Ile Val His Cys Glu Val Ser Glu Trp Asn Pro Trp Ser Pro Cv_s.Th:r.
145 150 155 150
Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Thr Arg Val
165 170 175
Arg Glu Ile Tle Gln His Pro Ser Ala Lys Gly Asn Leu Cys Pro Pro
180 185 190
Thr Asn Glu Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys
195 200 205
Gly Glu Arg.Gly Lys Lys Gly Arg Glu Arg Lys Arg Lys Lys Pro Asn
210 215 220
Lys Gly Glu Ser Lys G1u Ala Ile Pro Asp Ser Lys Ser Leu Glu Ser
225 230 235 240
Ser Lys Glu Ile Pro Glu Gln Arg Glu Asn Lys Gln Gln Gln Lys Lys
245 250 255
Arg Lys Val Gln Asp Lys Gln Lys Ser
260 265
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-17-
<210> 27
<211> 8
<212> PRT
<213> Homo Sapiens
<400> 27
Ser Val Ser Val Ser Thr Val His
1 5
<210> 28
<211> 7
<212> PRT
<213> Homo Sapiens
<400> 28
Val Ser Val Ser Thr Va1 His
1 5
<210> 29
<211>. 27
<212> PRT
<213> Homo Sapiens
<400> 29
Gly Ile Glu Val Thr heu Ala Glu Gly Leu Thr Ser Val Sex Gln Arg
1 5 1 0. 15
Thr Gln Pro Thr Pro Cys Arg Arg Arg Tyr Leu
20 25
<210> 30
<211> 30
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 30
ctcgggaaga agcgcgccat ttgtgttggt 30
<210> 31
<211> 2384
<212> DNA
<213> Mus musculus
<220>
<221> CDS
<222> (511)..(1347)
<400> 31
ggagcggctc ctgctcagaa cgccagaagc agctcgggtc tctccagcgc cccttgacca 60
tggctgcggt acccacggcg tccgcttccc tgcgctcccg gggtccctgc cacagccgca 120
gccgctgcag cctctgagcc ccaggggcca ctgctcgcct ggattccgcc cgcagccgcc 180
gctgctgtgc aaccgaggct aacctgcggc cagccaggag gctcctgcaa ccttcgctcg 240
cggcgatgac agccacccca gagcagccgg ctgtgttcgg acaatttgag aatgcaattg 300
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-18-
ttggtttccc ggtccacccg tcccgcttcg cttgccatca cagcacgcct gttggatctc 360
agtggagaag tcccgctgct ctggtttttc tactcttcgt atagactcgc ctaacaccta 420
catacatatt tttctttaaa aaaaaacatt aaatataact aacagtgaaa agaaaaagga 480
gagaaaaaag ggaaacatta cagggttact atg cac ttg cga ctg att tct tgt 534
Met His Leu Arg Leu Ile Ser Cys
1 5
ttt ttt atc att ttg aac ttt atg gaa tac att ggc agc caa aac gcc 582
Phe Phe Tle Ile Leu Asn Phe Met Glu Tyr Tle Gly Ser Gln Asn Ala
15 20
tcc cga gga agg cgc cag cga aga atg cat cct aat gtc agt caa ggc 630
Ser Arg Gly Arg Arg Gln Arg Arg Met His Pro Asn Val Ser Gln Gly
25 30 35 40
tgc caa gga ggc tgt gca acg tgt tca gat tac aat ggc tgt ttg tca 678
Cys Gln Gly Gly Cys Ala Thr Cys Ser Asp Tyr Asn Gly Cys Leu Ser
45 50 55
tgt aag ccc aga ctg ttt ttt gtt ctg gaa agg att ggc atg aag cag 726
Cys Lys Pro Arg Leu Phe Phe Val Leu Glu Arg Ile Gly Met Lys Gl.n
60 65 70
ata gga gtg tgt ctc tct t.cg tgt cca agt gga tat tac gga act cga 7'74
Ile Gly Val Cys Leu Ser Ser Cys Pro Ser Gly Tyr Tyr Gly Thr Arg
75 80 85
tat cca gat ata aat aaa tgt aca aaa tgc aaa gtt gac tgt gat acc 822
Tyr.Pro Asp Tle Asn Lys C-ys Thr Lys Cys Lys Val Asp Cys Asp Thr
90 95 100
tgt ttc aac aaa aat ttc tgc aca aag tgt aaa agt gga ttt tac tta 870
Cys Phe Asn Lys Asn Phe Cys Thr Lys Cys'Lys Ser G1y Phe Tyr Leu
105 110 115 120
cac ctt gga aag tgc ctt gac agt tgc cca gaa ggg tta gaa gcc aac 918
His Leu Gly Lys Cys Leu Asp Ser Cys Pro Glu G1y Leu Glu Ala Asn
125 130 135
aat cat act atg gaa tgt gtc agt att gta cac tgt gag gcc agt gaa 966
Asn His Thr Met Glu Cys Val Ser Ile Val His Cys Glu Ala Ser Glu
140 145 150
tgg agt cca tgg agt cca tgt atg aag aaa gga aaa aca tgt ggc ttc 1014
Trp Ser Pro Trp Ser Pro Cys Met Lys Lys Gly Lys Thr Cys Gly Phe
155 160 165
aaa agg ggg act gaa aca cgg gtc cga gat ata cta cag cat cct tca 1062
Lys Arg G1y Thr Glu Thr Arg Val Arg Asp Tle Leu Gln His Pro Ser
170 175 180
gcc aag ggt aag ggt aac ctg tgc ccc cca acc agc gag aca aga act 1110
Ala Lys Gly Lys Gly Asn Leu Cys Pro Pro Thr Ser Glu Thr_ Arg Thr
185 190 195 200
tgt ata gta caa aga aag aag tgt tca aag gga gag cga gga aaa aag 1158
Cys Ile Val Gln Arg Lys Lys Cys Ser Lys Gly Glu Arg Gly Lys Lys
205 210 215
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-19-
gga aga gag aga aaa cga aaa aaa ctg aat aaa gaa gaa aga aag gaa 1206
Gly Arg Glu Arg Lys Arg Lys Lys Leu Asn Lys Glu Glu Arg Lys Glu
220 225 230
aca agc tcc tcc tct gac agc aaa ggt ttg gag tcc agc att gag acc 1254
Thr Ser Ser Ser Ser Asp Ser Lys Gly Leu Glu Ser Ser Ile Glu Thr
235 240 245
cca gac cag cag gaa aac aaa gag agg cag cag cag cag aag aga aga 1302
Pro Asp G1n Gln Glu Asn Lys G1u Arg Gln G1n Gln Gln Lys Arg Arg
250 255 260
gcc cga gac aag caa cag aaa tcg gta tca gtc agc act gt'a cac 1347
Ala Arg Asp Lys G1n Gln Lys Ser Val Ser Val Ser Thr Val His
265 270 275
tagagggtcc tgcgaggtta ctgtagactc atgatgctgc tatctcaacc agatgtccag 1407
gacaggtgtt ctagccatta gaaccacaaa tggacaacac atcagttacc actctgtcta 1467
aacaacattc ctaatagttg ctatattctt catacaaaca tagtaaacag caaagagcca 1527
aatgttcaaa gaagggatac tttcagatgg ttatcttatg tgcttctgtg tatttttaaa 1587
agatgagaaa atttgtacat aattatcaat aagctataag atatcctcaa tgtaatgacg 1647
acagctggac aagaatcatc ttttctttat~.aaaaaaatta ttcttcgaat aattgtcttt 1707
aagaagcaaa aggtaattct gcaacttcaa aaatgcagtg tr_cctcaaaa ccaagatttg 1767
tcaggggaga gaatcatggc tccatgtaca gggtggattt gtcccggaga acta.gtgaat 182'7
gctcagaatt agggcctggc attttgaatc ctagagttaa tcatcacaga agcaagtggt 188'7
ttaggattgc ttcggttgc~~ ctcctctgca agaaactgaa catgcataat agagttaaat 194'7
atattgtgtg gagttggaat aaggcaagct gtggaagaaa tcatagagct ggagaccatc 2007
ttgtgctttc cagaaccgtg aggggttttg gtcacctgga acagggctcc aatctatatt 2067
agcactgtgt ggttgatctt ccactactcc ttggtttata taagtctgta aacatgtacc 2127
tgtacctttc ttccaaaagt aaaaccatac ttactagaag aaaattctaa ctttatggaa 2187
aacaaaagtg taagaagaat gtgacatgtt tgcaaagttg agtgttttct ttctgaaatg 2247.
aggggaaaac tattttatta cctgcctatg ggtccacctg gaactaaagg gatactactt 2307
tctaacaagg tgtatctagt aggagagaaa gccaccacaa taaatatatt tgttaatagn 2367
taaaaaaaaa aaaaaaa 2384
<210> 32
<211> 279
<212> PRT
<213> Mus musculus
<400> 32
Met His Leu Arg Leu Ile Ser Cys Phe Phe Ile Ile Leu Asn Phe Met
1 5 10 15
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-20-
Glu Tyr Ile Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln Arg Arg
20 25 30
Met His Pro Asn Val Ser G1n Gly Cys Gln Gly G1y Cys Ala Thr Cys
35 40 45
Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Pro Arg Leu Phe Phe Val
50 55 60
Leu Glu Arg Ile Gly Met Lys Gln Ile Gly Val Cys Leu Ser Ser Cys
65 70 75 80
Pro Ser Gly Tyr Tyr G7_y Th.r Arg Tyr Pro Asp Ile Asn Lys Cys Thr
85 90 95
Lys Cys Lys Val Asp Cys Asp Thr Cys Phe Asn Lys Asn Phe Cys Thr
100 105 110
Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Ser
125 120 125
Cys Pro Glu Gly Leu Glu Ala Asn Asn His Thr Met Glu Cys Val Ser
130 135 140
Ile Val His Cys Glu Ala Ser Glu Trp S~er Pro Trp Ser Pro Cys Me1=
145 150 155 160
'Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Thr Arg Val
165 . 170 175
Arg Asp I1e Leu Gln His Pro Ser Ala Lys Gl_y Lys Gly Asn Leu Cys
180 l85 190
Pro Pro Thr Ser Glu Thr Arg Thr Cys Ile Val Gln Arg Lys Lys Cys
195' 200 . 205
Ser Lys G1y Glu Arg Gly Lys Lys Gly Arg Gl.u Arg Lys Arg Lys Lys _
210 215 220
Leu Asn Lys Glu Glu Arg Lys Glu Thr Ser Ser Ser Ser Asp Ser Lys
225 230 2,35 240
Gly Leu Glu Ser Ser Ile Glu Thr Pro Asp Gln Gln Glu Asn Lys Glu
245 250 255
Arg Gln Gln Gln Gln Lys Arg Arg Ala Arg Asp Lys Gln Gln Lys Ser
260 265 270
val Ser Val Ser Thr Val His
275
<210> 33
<211> 2101
<212> DNA
<213> Homo Sapiens
<220>
<221> CDS
<222> (259)..(1074)
<400> 33
tcgcggcgat gccagccacc ccagcgaagc cgccgcagtt cagtgcttgg ataatttgaa 60
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-21-
agtacaatag ttggtttccc tgtccacccg ccccacttcg cttgccatca cagcacgcct 120
atcggatgtg agaggagaag tcccgctgct cgggcactgt ctatatacgc ctaacaccta 180
catatatttt aaaaacatta aatataatta acaatcaaaa gaaagaggag aaaggaaggg 240
aagcattact gggttact atg cac ttg cga ctg att tct tgg ctt ttt atc 291 "
Met His Leu Arg Leu Ile Ser Trp Leu Phe Ile
1 5 10
att ttg aac ttt atg gaa tac atc ggc. agc caa aac gcc tcc cgg gga 339
IIe Leu Asn Phe Met Glu Tyr Tle Gly Ser GIn Asn Ala Ser Arg Gly
15 20 25
agg cgc cag cga aga atg cat cct aac gtt agt caa ggc tgc caa gga 387
Arg Arg Gln Arg Arg Met His Pro Asn Val Ser Gln Gly Cys Gln Gly
30 35 40
ggc~tgt gca aca tgc tca gat tac aat gga tgt ttg tca tgt aag ccc 435
Gly Cys Ala Thr Cys Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Pro
45 50 55
aga cta ttt ttt get ctg gaa aga att ggc atg aag cag att gga gta 483
Arg Leu Phe Phe Ala Leu Glu Arg Ile Gly Met Lys Gln Tle Gly Val
60 65 70 7S
tgt ctc tct tca tgt cca agt gga tat tat gga act cga tat cca gat 531
Cys Leu Ser Ser Cys Pro Ser Gly Tyr Tyr Gly Thr Arg Tyr Pr_o Asp
80 85 9C
ata aat aag tgt aca aaa tgc aaa get sac tgt gat aoc tgt ttc aac 579
Ile Asn Lys Cys Thr Lys Cys Lys Ala Asp Cys Asp Thr. Cys Phe Asn
95 100 105
aaa aat ttc tgc aca aaa tgt aaa agt gga ttt tac tta cac ctt gga 627
Lys Asn Phe Cys Thr Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly
110 115 . 120
aag tgc ctt gac aat tgc cca gaa ggg ttg gaa gcc aac aac cat act 675
Lys Cys Leu Asp Asn Cys Pro G1u Gly Leu Glu Ala Asn Asn His Thr
125 130 135
atg gag tgt gtc agt att gtg cac tgt gag gtc agt gaa tgg aat cct 723
Met G1u Cys Val Ser Ile Val His Cys Glu Val Ser Giu Trp Asn Pro
140 145 150 155
tgg agt cca tgc acg aag aag gga aaa aca tgt ggc ttc aaa aga ggg 771
Trp Ser Pro Cys Thr Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg G1y
160 165 ~ 170
act gaa aca cgg gtc cga gaa ata ata cag cat cct tca gca aag ggt 819
Thr Glu Thr Arg Val Arg Glu Ile Ile Gln His Pro Ser Ala Lys Gly
175 . 180 185
aac cta tgt ccc cca aca aat gag aca aga aag tgt aca gtg caa agg 867
Asn Leu Cys Pro Pro Thr Asn Glu Thr Arg Lys Cys Thr Va1 Gln Arg
190 195 200
aag aag tgt cag aag gga gaa cga gga.aaa aaa gga agg gag agg aaa 915
Lys Lys Cys Gln Lys Gly Glu Arg Gly Lys Lys Gly Arg Glu Arg Lys
205 210 215
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
aga aaa aaa cct aat aaa gga gaa agt aaa gaa gca ata cct gac agc 963
Arg Lys Lys Pro Asn Lys Gly Glu Ser Lys Glu Ala Ile Pro Asp Ser
220 225 230 235
aaa agt ctg gaa tcc agc aaa gaa atc cca gag caa cga gaa aac aaa 1011
Lys Ser Leu Glu Ser Ser Lys Glu 21e Pro Glu Gln Arg Glu Asn Lys
240 245 250
cag cag cag aag aag cga aaa gtc caa ga.t aaa cag aaa tcg gta tca 1059
Gln G1n Gln Lys Lys Arg Lys Val Gln Asp Lys Gln Lys Ser Val Ser
255 260 265
gtc agc act gta cac tagagggttc catgagatta ttgtagactc atgatgctgc 1114
Val Ser Thr Val His
270
tatctcaacc agatgcccag gacaggtgct ctagcr_atta ggaccacaaa tggacatgtc 1174 ,
agttattgct ctgtctaaac aacattccca gtagttgcta tattcttcat acaagcatag 123,4
ttaacaacaa agagccaaaa gatcaaagaa gggatacttt cagatggttg tcttgtgtgc 2294
ttctctgcat ttttaaaaga caagacattc ttgtacatat tatcaatagg ctataagatg 1354
taacaacgaa atgatgacat ctggagaaga aacatctttt ccttataaaa atgtgttttc 1414
aagctgttgt tttaagaagc aaaagatagt tctgc~aaatt caaagataca gtatcccttc 1474
aaaacaaata ggagttcagg gaagagaaac atccttcaaa ggacagtgtt gttttgaccg 1534
ggagatctag agagtgctca gaattagggc ctggcatttg~ gaa.tcacagg atttatcatc 1594,;
acagaaacaa ctgttttaag attagttcca tcactetcat cctgtatttt tataagaaac 1654
acaagagtgc ataccagaat tgaatatacc atatgggatt ggagaaagac aaatgtggaa 1714 .
gaaatcatag agctggagac tacttttgtg ctttacaaaa ctgtgaagga ttgtggtcac 1774 ,.
ctggaacagg tctccaatct atgttagcac tatgtggctc agcctctgtt accccttgga 1834
ttatatatca acctgtaaac atgtgcctgt aacttacttc caaaaacaaa atcatactta 1894
ttagaagaaa attctgattt tatagaaaaa aaatagagca aggagaatat aacatgtttg 1954
caaagtcatg tgttttcttt ctcaatgagg gaaaaacaat tttattacct gcttaatggt 2014
ccacctggaa ctaaaaggga tactattttc taacaaggta tatctagtag gggagaaagc 2074
caccacaata aatatatttg ttaatag 2101
<210> 34
<21l> 272
<212> PRT
<213> Homo Sapiens
<400> 34
Met His Leu Arg Leu Ile Ser Trp Leu Phe Ile Ile Leu Asn Phe Met
1 5 10 15
Glu Tyr Ile Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln Arg Arg
20 25 30
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-23-
Met His Pro Asn Val Ser Gln Gly Cys Gln Gly Gly Cys Ala Thr Cys
35 40 45
Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Pro Arg Leu Phe Phe Ala
50 55 60
Leu Glu Arg Ile Gly Met Lys Gln Ile Gly Val Cys Leu Ser Ser Cys
65 70 75 80
Pro Ser Gly Tyr Tyr Gly Thr Arg Tyr Pro Asp Ile Asn Lys Cys Thr
85 . 90 95
Lys Cys Lys Ala Asp Cys Asp Thr Cys Phe Asn Lys Asn Phe Cys Thr
100 105 110
Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Asn
115 120 125
Cys Pro Glu Gly Leu Glu Ala Asn Asn His Thr Met Glu Cys Val Ser
l30 135 140
Tle Val His Cys Glu Val Ser Glu Trp Asn Pro Trp Ser Pro Cys Thr
145 150 ' 155 . 160
Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Th.r Arg Val
165 170 175
Arg Glu Ile Ile Gln His Pro Ser Ala Lys G1y Asn,Leu Cys Pro Pro
180 185 ' 1.,90
Thr Asn Glu Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys
195 200 205
Gly Glu Arg G1y Lys Lys Gly Arg Glu Arg Lys Arg Lys Lys Pro Asn
210 215 220 .
Lys Gly Glu Ser Lys Glu Ala Ile Pro Asp Ser Lys Ser Leu Glu Ser
225 230 235 240
Ser Lys Glu Ile Pro Glu Gln Arg Glu Asn Lys Gln Gln Gln Lys Lys
245 '~50 255
Arg Lys Val Gln Asp Lys Gln Lys Ser Val Ser Val Ser Thr Val His
260 265 270
<210> 35
<211> 21
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 35
agtacaaaga aagaagtgtt c 21
<210> 36
<21l> 21
<212> DNA
<213> Artificial Sequence
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-24-
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 36
tgagtctaca gtaacctcgc a 21
<210> 37
<2l1> 20
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 37
taatacgaca cactataggg 2U
<210> 38
<211> 24 _
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<40'0> 38 -
tcgcggcqat gccagccacc ccag 24
<210> 39
<211.> 30.
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 3J
agcacgcr.,ta tcggatgtga gaggagaagt 30
<210> 40
<211> 30
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 40
ctattaacaa atatatttat tgtggtggct 30
<210> 4l
<211> 30
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-25-
<400> 41
tggtggcttt ctcccctact agatatacct 30
<210> 42
<211> 20
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 42
gattttaggt gacactatag 20
<210> 43
<211> 34
<212> DNA
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 43
CCgCtCgagC CaCCatgCaC ttgcgactga tttc 34
<210> 44
<21i> 29
<212> DNA
<27.3> Artificial' Sequence
<220>
<223> Description of Artificial Sequence: PCR primer
<400> 44
attgaattcc tagtgtacag tgctgactg 29
<210> 45
<211> 84
<212> DNA
<213> Homo Sapiens
<220>
<223> Description of Artificial Sequence: PCR primer
<22U>
<221> CDS
<222> (1)..(81)
<400> 45
ggg att gaa gtc acc cta get gaa ggc ctc acc agt gtt tca cag agg 48
Gly I1e Glu Val Thr Leu Ala Glu Gly Leu Thr Ser Val Ser Gln Arg
1 5 10 15
aca cag ccc acc cct tgc agg agg agg tat ctc tga 84
Thr Gln Pro Thr Pro Cys Arg Arg Arg Tyr Leu
20 25
<2l0> 46
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-26-
<211> 27
<212> PRT
<213> Homo Sapiens
<400> 46
Gly Tle Glu Val Thr Leu Ala Glu Gly Leu Thr Ser Val Ser Gln Arg
1 5 10 15
Thr Gln Pro Thr Pro Cys Arg Arg Arg Tyr Leu
20 25
<210> 47
<211> 1436
<212> DNA
<2i3> Homo Sapiens
<400> 47
cccggcggct cctggaaccc cggttcgcgg cgatgccagc caccccagcg aagccgccgc 60
agttcagtgc ttggataatt tgaaagtaca atagttggtt tccctgtcca cccgccccac 120
ttcgcttgcc atcacagcac gcctatcgga tgtgagagga gaagtcccgc tgctcgggca 180
ctgtctatat acgcctaaca cctacatata ttttaaaaac attaaatata attaacaatc 240
aaaagaaaga ggagaaagga agggaagcat tactgggtta cta gcactt gcgactgatt 300
tcttggcttt ttatcatttt gaactttatg gaatacatcg gcagccaaaa egcctcccgg 360
ggaaggcgcc agcgaagaat gcatcctaac gttagtcaag gctgccaagg aggctgtgca 420.
acatgctcag attacaatgg atgtttgtca tgtaagccca gactattttt tgctctggaa 480
agaattggca tgaagcagat tggagtatgt ctctcttcat gtccaagtgg atattatgga 540,
actcgatatc cagatataaa taagtgtaca aaatgcaaag ctgactgtga tacctgtttc 600
aacaaaaatt tctgcacaaa atgtaaaagt ggattttact tacaccttgg aaagtgcctt 660
gacaattgcc cagaagggtt ggaagccaac aaccatacta tggagtgtgt cagtattgtg 720
cactgtgagg tcagtgaatg gaatccttgg agtccatgca cgaagaaggg aaaaacatgt 780
ggcttcaaaa gagggactga aacacgggtc cgagaaataa tacagcatcc ttcagcaaag 840
ggtaacctgt gtcccccaac aaatgagaca agaaagtgta cagtgcaaag gaagaagtgt 900
cagaagggag aacgaggaaa aaaaggaagg gagaggaaaa gaaaaaaacc taataaagga 960
gaaagtaaag aagcaatacc tgacagcaaa agtctggaat ccagcaaaga aatcccagag 1020
caacgagaaa acaaacagca gcagaagaag cgaaaagtcc aagataaaca gaaatcgggg 1080
attgaagtca ccctagctga aggcctcacc agtgtttcac agaggacaca gcccacccct 1140
tgcaggagga ggtatctctg agtgtgcagc acagaatcgc atgacccacc ttaaccttcc 1200
tgttgtcatg gaaggatgca cggctgctct gtccactgtg attcctagcc ctctcaagat 1260
cactgctttc tgaagaattt gcaatgactc tggcttctgg ctgcttatct ctggacaccc 1320
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-2 7-
gttctccacc agttgtacag ttcatgtaat ctacttggct taattgattt tccacttctc 1380
tcttcctctt ctaagatata aacattttaa atgatttaaa aaaaaaaaaa aaaaaa 1436
<210> 48
<211> 292
<212> PRT
<213> Homo Sapiens
<400> 48
Met His Leu Arg Leu Tle Ser Trp Leu Phe Ile Ile Leu Asn Phe Met
1 5 10 15
G1u Tyr IIe Gly Ser Gln Asn Ala Ser Arg Gly Arg Arg Gln Arg Arg
20 25 3U
Met His Pro Asn Val Ser Gln Gly Cys Gln Gly Gly Cys Ala Thr Cys
35 40 45
Ser Asp Tyr Asn Gly Cys Leu Ser Cys Lys Pro Arg Leu Phe Phe Ala
50 55 60
Leu Glu Arg Ile Gly Met Lys GIn Tle Gly Val Cys Leu Ser Ser Cys
65 70 75 80
Pro Ser Gly Tyr Tyr Gly Thr Arg Tyr Pro Asp Ile Asn Lys Cys Thr
85 . . 90 95
Lys Cys Lys Ala Asp .Cys Asp Thr Cys Phe Asn Lys Asn Ph<< Cys Thr
10U 105 1l0
Lys Cys Lys Ser Gly Phe Tyr Leu His Leu Gly Lys Cys Leu Asp Asn
17.5 120 125
Cys Pro Glu Gly Leu Glu Ala Asn Asn His Thr Met Glu Cys Val Sex
13 0 13 5 14 0
Ile Va1 His Cys Glu Val Ser Glu Trp Asn Pro Trp Ser Pro Cys Thr
145 150 . ~ 7.55 160
Lys Lys Gly Lys Thr Cys Gly Phe Lys Arg Gly Thr Glu Thr Arg Val
l65 170 175
Arg Glu Tle Ile Gln His Pro Ser Ala Lys Gly Asn Leu Cys Pro Pro
180 185 190
Thr Asn Glu Thr Arg Lys Cys Thr Val Gln Arg Lys Lys Cys Gln Lys
195 200 205
Gly Glu Arg Gly Lys Lys Gly Arg Glu Arg Lys Arg Lys Lys Pro Asn
210 215 220
Lys Gly Glu Ser Lys Glu Ala Ile Pro Asp Ser Lys Ser Leu Glu Ser
225 230 235 240
Ser Lys Glu Ile Pro Glu GIn Arg GIu Rsn Lys Gln G1n Gln Lys Lys
245 250 255
Arg Lys Val Gln Asp Lys GIn Lys Ser Gly Ile Glu Val Thr Leu Ala
260 265 270
CA 02405104 2002-10-04
WO 01/77169 PCT/USO1/11208
-2 ~-
G1u Gly Leu Thr Ser Val Ser Gln Arg Thr Gln Pro Thr Pro Cys Arg
275 280 285
Arg Arg Tyr Leu
290