Note: Descriptions are shown in the official language in which they were submitted.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
1
ENCAPSULATED FLOAT AND METHOD FOR MAKING SAME
REFERENCE TO RELATED APPLICATION
This application claims benefit of U.S. Provisional Patent Application Serial
No. 60/201.022 filed May 1, 2000, and hereby claims the benefit of the
embodiments
therein and of the filing date thereof.
FIELD OF THE INVENTION
This invention is in the field of rubber-based floats for measuring liquid
levels.
BACKGROUND OF THE INVENTION
Virtually every industry utilizes floats for liquid-level sensing in various
applications, such as fuel tanks and liquid reservoirs, where the accurate and
reliable
measurement of the liquid level is important. Many floats are designed to
include
magnets, inserts, or guiding devices, all of which aid the float in performing
its
function. Generally, these guiding devices, inserts or magnets are molded
within the
float and actually become a part of a float assembly.
Floats made of rubber materials, such as acrylonitrile rubber, are very
popular
because they are comparatively inexpensive, easy to manufacture into a variety
of
shapes and sizes and can be designed to meet a wide range of densities.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
2
Additionally, rubber-based floats are readily capable of being molded to
include
magnets, guiding devices, inserts, and the like, all of which aid the float in
performing
its function.
In spite of the aforementioned benefits of rubber-based floats, there is one
significant disadvantage to their use. Rubber-based floats have a tendency to
fail or
degrade in the presence of various chemicals, such as acids, certain
hydrocarbons,
chlorinated solvents, and alcohols. Failure or degradation of rubber-based
floats in
these environments is the result of either or both actual chemical attack of
the rubber
material or absorption of the chemical by the rubber-based float. Absorption
can
cause the float to fail by reason of an increase in weight or by fragmentation
and/or
cracking of the float.
Today, many Federal, State and Municipal governments have offered
financial incentives to individuals, businesses, and governmental agencies
that
utilize a fuel which is less polluting. One type of fuel which has gained
popularity is
an alcohol-gasoline blend. It has been found that use of such blends may lower
a
vehicle's emissions. With this key benefit in mind, many fuel manufacturers
have
spent considerable sums of money in developing and improving the alcohol-
gasoline
blends and have added alcohol-blended fuels to their product line.
As mentioned earlier, rubber-based floats are subject to partial or complete
failure when utilized in conjunction with alcohols or products that are
blended with
alcohols. In cases where a non-alcohol compatible rubber-based float is used
in
conjunction with an alcohol or alcohol-blended product, the rubber material
either
absorbs or is attacked by the alcohol. Ultimately such absorption or attack
will lead
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
3
to the failure of the rubber-based float. Consequently, rubber-based floats
have
limited utility in these type chemical environments.
Exposure to other chemicals, such as chlorinated solvents, acids, and certain
hydrocarbons, may also lead to the failure of a rubber-based float. Failure
under
these circumstances also occurs by attack or degradation of the float by the
chemicals or by absorption by the rubber material of the chemical. Again, the
inadvertent introduction of an incompatible float into a fuel tank or
reservoir
containing chlorinated solvents, certain hydrocarbons, or acids could lead to
the
failure of the rubber-based float, which in turn could result in serious,
catastrophic, or
possibly tragic consequences.
To date, the only means known to overcome the chemical incompatibility
problems of rubber-based floats was to simply avoid their use in certain
chemical
environments.
As a result of the aforementioned problems in using rubber-based floats, it is
the object of this invention to develop rubber-based floats which:
- can be easily molded into various sizes and shapes;
- can be molded to accept internal magnets, sensors, guiding devices,
inserts, and the like;
- are relatively easy and inexpensive to manufacture;
- have use within a wide range of applications;
- are resistant to attack or absorption from most chemicals, including
most chlorinated solvents, hydrocarbons, alcohols, and acids;
- are safe from catastrophic failure; and
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
4
- effectively prevents float deterioration.
BRIEF DESCRIPTION OF THE INVENTION
In light of the state of the art, the inventor has set out to 'produce a
rubber
float, particularly of closed cell foam acrylonitrile rubber which is
resistant to attack
from, or absorption of, most chemicals, such as chlorinated solvents, acids,
alcohols,
and certain hydrocarbons. Such a float must be relatively inexpensive to
manufacture, able to be molded into various sizes and shapes, and capable of
being
molded to include magnets, inserts, guiding devices, and the like. The float
must.
also have an application within a wide range of temperatures and pressures.
These objects, and others, which will become apparent upon consideration of
the following disclosure, are achieved by this invention, which briefly stated
comprises the encapsulation of acrylonitrile rubber-based float material
within a
container, such as polyethylene or some other thermoplastic, wherein the two
are
bonded together to create a closed-cell acrylonitrile rubber float, which is
completely
encapsulated within a precisely dimensional protective plastic skin. A float
of this
invention is virtually impervious to attack from or the absorption of most
chemicals,
while at the same time maintaining its low cost, broad application, and
flexibility of
design. The inventive float is also capable of being molded to include
magnets,
inserts, guiding devices, and the like.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
BRIEF DESCRIPTION OF THE DRAWINGS
This invention may be more clearly understood with the following detailed
description and by reference to the drawings in which:
Fig. 1, including Figs. 1 a and 1 b, are transverse sectional views of two
common prior art rubber floats;
Fig. 2, including Figs. 2a and 2b, are transverse sectional views of two
floats
similar to those of Fig. 1 modified in accordance with this invention;
Fig. 3 is an enlarged fragmentary sectional view of the bond region between
the acrylonitrile rubber core material and the thermoplastic protective skin;
Fig. 4, including Figs. 4a and 4b, 4a is a perspective view of a cylindrical
float
just prior to the encapsulation step, and Fig. 4b is a cross section of the
float of Fig.
4a;
Fig. 5 is a flow diagram for step 1, stage 1 of the process of this invention;
Fig. 6 is a flow diagram for step 1, stage 2 of the process of this invention;
Fig. 7 is a flow diagram of step 1, stage 3 of the process of this invention;
and
Fig. 8 is a flow diagram of step 2 the encapsulation.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
6
DETAILED DESCRIPTION OF THE INVENTION
Acrylonitrile rubber floats have been used for years for liquid level sensing;
however, their use has been limited to those situations where the environment
in
which the float was utilized did not also include chemicals, which would
either attack
or be absorbed by the acrylonitrile rubber. Such attack or absorption could
eventually result in the partial or complete failure of the float, and
possibly culminate
in human tragedy.
The present invention is concerned with rubber-based floats, particularly an
acrylonitrile rubber float, which is encapsulated in a protective skin, and
method for
making same. The skin protects the float from attack by various chemicals,
which
are aggressive towards and degrade acrylonitrile rubber, e.g., chlorinated
solvents,
acids, and certain hydrocarbons. In still other cases, the skin acts to
eliminate
absorption by the acrylonitrile rubber of various chemicals, such as alcohols,
chlorinated solvents, acids, and certain hydrocarbons.
The encapsulated acrylonitrile rubber float of this invention is manufactured
in
basically a two-step process. The first step is the manufacture of the
acrylonitrile
rubber float material or preform, with the second step being the encapsulation
of the
acrylonitrile rubber float material within a protective skin. Encapsulation
does not
alter the float's functionality or performance; however, it does allow the
float to resist
attack by or absorption of most other chemicals.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
7
THE PRODUCT
Fig. 1 is comprised of Figs. 1 a and 1 b, both of which depict two types of
commercially available prior art acrylonitrile rubber floats, neither of which
is
encapsulated. The floats of Fig. 1 are both comprised of acrylonitrile rubber
material
A. Fig. 1 a reveals an insert I, and Fig. 1 b reveals a carburetor pivot arm
PA, both of
which are imbedded within and held in place by the acrylonitrile rubber
material.
The above-mentioned inserts aid the float in performing its function. Floats,
such as these, are subject to attack by or absorption of various chemicals, as
shown
later in Tables 1-8, resulting in change in weight, volume or durability of
the float.
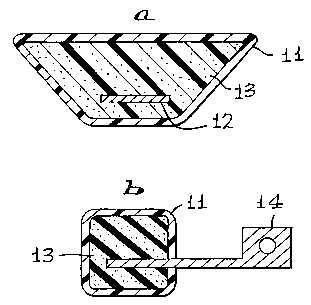
Fig. 2, comprised of Figs. 2a and 2b, illustrates an encapsulated version of
the same type floats of Fig. 1. Fig. 2 depicts the protective skin 11, which
encapsulates and protects the entire float. The protective skin does not alter
the
float's appearance or volume nor does it alter the float's functionality. This
protective
skin does eliminate all of the problems associated with the embodiment of
Figs. 1 a
and 1 b as disclosed above.
Fig. 2b reveals an insert 12 within acrylonitrile rubber material 13, while
Fig.
2a shows an encapsulated float with a carburetor pivot arm insert 14 within
the
acrylonitrile rubber material.
A comparison of the floats of Fig. 1 with those of Fig. 2 reveals that the
encapsulated floats are virtually identical to the non-encapsulated floats in
both size,
shape and weight, except that the encapsulated floats are more resistant to
chemical
attack or absorption, then a non-encapsulated float. A comparison of the
performance of the non-encapsulated float and encapsulated floats is set forth
in
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
8
detail in Tables 1-8 below.
Turning now to Fig. 3, which illustrates a closed cell acrylonitrile rubber
core
material 13 in bonding engagement 20 with the protective plastic skin 11.
Thermoplastics, such as high and iow-density polyethylene or high and low-
density
polypropylene may be used to encapsulate the acrylonitrile rubber float
material 13.
As can be seen in Fig. 3, the acrylonitrile rubber core material 13 and the
thermoplastic 11 form a strong bond at their interface 20. The bond is
sufficiently
strong to withstand attempts to separate the core material from the skin, and
may be
mechanical, thermal or chemical.
Attempts to separate the thermoplastic skin 11 from the acrylonitrile rubber
core material 13 by hand have been unsuccessful. The encapsulated float of
this
invention will actually tear before the bond 20 between the thermoplastic skin
11 and
acrylonitrile rubber core 13 will break. The bond 20 between the acrylonitrile
rubber
core material 13 and the thermoplastic skin 11 results in a unitary float
body.
The skin 11 is approximately 0.02 to 0.06 of an inch thick; however, wall
thickness can be designed to meet the demands of the environment. The skin
also
produces a precise weight change factor, which is designed into the float.
Fig. 4a illustrates a cylindrical float just before the encapsulation step,
wherein
the acrylonitrile rubber core material 13 is placed into the preformed skin 11
c with lid
11 L. Fig. 4b illustrates a cross section of Fig. 4a just prior to the bonding
of the
acrylonitrile rubber core material 13 to the protective skin 11 (11 c and 11
L).
The significance of the inventive float is that the acrylonitrile rubber core
material 13 enters into complete bonding engagement at the interface 20 of the
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
9
thermoplastic skin 11, providing uniform protection of the core material 13
from the
outside chemical environment.
SHE PROCESS
Turning now to step one, as shown in Figs. 5, 6, 7, and 8 of the manufacture
of the acrylonitrile rubber-based float material. Step one is comprised of
three
separate stages:
- Stage 1, the Banbury mix
- Stage 2, the roller mix
- Stage 3, the compression stage.
In both the Banbury stage, Fig. 5, and the roller mill stage, Fig. 6, below,
the
inventor has set forth a wide range of the additives to be mixed. This
represents the
broad range of acrylonitrile rubber formulations presently utilized within the
industry.
The exact formulation will vary with the consumer needs and specifications.
The preferred embodiment, which is the most common of these formulations,
is set forth in parenthesis beside each ingredienfi additive range.
The first stage, or Banbury mix, Fig. 5, occurs as follows: Blending in a
Banbury Mill or the like, by weight, add between 30.0% to 55.0% (37.04% to
37.50%) acrylonitrile rubber, 0.5% to 4.2% (0.84% to 1.01 %) zinc oxide, 1.0%
to
15.0% (1.67% to 2.02%) stearic acid, 0.1 % to 2.0% (0.21 to 0.51 %) retarder
SAX
salicylic acid (0-hydroxybenzoic acid) C6H4 (OH)(COOH), 3.0% to 22.0% (14.14%
to
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
15.83%) mistron vapor Mg3Si4010 (0H)2, 0.3% to 2.75% (0.83% to 1.35%)
Coumarone Indene Resin absorbed on Synthetic Calcium Silicate (72% Cumar P-
25), and 8.0% to 15.0% (10.10% to 10.83%) sulfur, while maintaining the
temperature
of the mixture to below 250°F.
The inventor has determined that, for best results, the addition of the
compounds in the Banbury stage should occur in the sequence set forth above.
Blending is completed after a period of 60 to 180 minutes or when the mixture
turns a pale yellow-brown in color.
Upon completion of the Banbury mixture stage, confirm that the Shore A
hardness of the mixture is between 15-90. For the preferred embodiment, the
Shore
A hardness is between 65 and 75.
Transfer the completed stage one mixture to a suitably sized roller mill to
begin stage 2, as shown in Fig. 6. To the stage one mixture, add by weight,
between
12.0% to 40.0% (22.22% to 22.50%) phenolic resin (C6H60.CH20)X, 2.5% to 10.0%
(3.33% to 4.03%) carbon black, 0.25% to 1.5% (0.63% to 0.84%) Altax C~4HgN2S4,
1.75% to 15% (2.50% to 2.70%) epoxy-resin, and finally 1.0% to 5.0% (3.33% to
4.04%) blowing agent (NO)2C5H~pN4, while controlling the temperature of the
mixture
throughout this stage to less than 200°F. The inventor has determined
that for best
results, the addition of compounds in roller mix stage should also occur in
the
sequence listed above.
Mixing is completed in approximately 15 to 50 minutes or when the mixture is
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
11
uniform and turns to a black, smooth sheet.
A Shore A hardness test is conducted to confirm hardness of between 15 and
90. For the preferred embodiment, the Shore A hardness is between 65 and 75.
The compression stage of Fig. 6 is the third and final stage of step 1. The
pre-cured acrylonitrile rubber-based mixture of completed step 2 is placed
into a
conventional compression tool where, depending upon the formation, the
pressure is
increased to between 20-900 Ibs/cubic inch for between 5 to 45 minutes at a
temperature of between 200°F. to 350°F., Fig. 7. In the
preferred embodiment, the
pressure in this stage is between 350 and 410 Ibs/cubic inch for 10 to 18
minutes at
a temperature of between 265°F. and 275°F.
The acrylonitrile rubber foam is removed from the compression tool and
allowed to cool. Cooling may be achieved in air or by any convention manner.
After the acrylonitrile rubber foam has cooled, it is inspected for visual
defects
in preparation for the encapsulation step. The foam material is tested again
to
confirm that its Shore A hardness is between 15-90. For the preferred
embodiment,
the Shore A hardness is between 65 and 75.
The acrylonitrile rubber foam material is weighed and trimmed of any excess
material, if necessary, to meet the desired unit shape and weight. Stage Three
and
Step One are now complete.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
12
iv
The acrylonitrile rubber foam is now ready for the encapsulation step Fig. 8.
However, prior to the encapsulation step, any magnets, inserts, guides, or the
like
are placed into the acrylonitrile rubber foam by conventional means, such as
drilling
an opening and inserting the inset in place.
Thermoplastic containers, which have been pre-molded to meet the desired
size and shape specifications, are visually inspected for defects. The
inventor has
determined that both high and low-density polyethylene, and high and low-
density
polypropylene, bond equally as well with the acrylonitrile rubber core
material. The
pre-molded thermoplastic is in a container shape and is preferably formed to
have
only one open end or lid and thus only one seam, although multi-seam
containers
may be used.
The inventor has determined that the physical characteristics of the
encapsulation material, e.g., wall thickness, is more easily controlled when
it is
premolded. By contrast, dipping, painting or spraying the acrylonitrile rubber
foam
material with the encapsulation material would likely result in a non-uniform
skin, and
thus an improper float.
The desired weight of pre-cured acrylonitrile rubber foam unit, taking into
account the container and lid weight, is placed info the thermoplastic
container, to fill
the space. The container lid is loosely placed onto the container, to close
the
container and meet final products dimensional requirements. Thereafter, the
plastic
container, with acrylonitrile rubber core material and/or insert, are placed
into a finish
mold tool. The finish mold tool is designed to conform to the size and shape
of the
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
13
thermoplastic container. The inventor has, for purposes of this application,
elected
to describe the encapsulation process wherein high-density polyethylene is
utilized
as the encapsulation material.
Thereafter, the finish mold tool is, depending on formulation, heated by any
conventional means to between 250°F, to 550°F. for between 20 to
180 minutes to
allow the acrylonitrile rubber material to expand into intimate contact with
the entire
interior surface of the thermoplastic container and enter into bonding
engagement
thereto. During this step, the container and lid are also bonded to seal the
float .
For the preferred embodiment, the finish mold tool is heated to between
250°F. to 500°F. for between 30 to 70 minutes. The temperature
and time may vary
where high-density polyethylene, or high and low-density polypropylene, are
used as
the encapsulation material. After the allotted time period, the finished mold
tool is
allowed to cool by any conventional means until it is ambient temperature.
Upon
completion of this step, the acrylonitrile rubber foam material with insert,
if any, is
bonded to and encapsulated within the protective polyethylene skin, Figs. 2
and 3.
The resultant float is now a unitary body.
In the description of the process above, the temperatures, times and
quantities have been shown to be successful in manufacturing said float;
however, it
is believed that minor variations in time, temperature and quantity would also
yield
an acceptable product.
Table 1 is a summary of results of a Shore A hardness test comparing
the acrylonitrile rubber encapsulated float of the invention (denoted U-2000)
with two
non-encapsulated, but commercially available acrylonitrile rubber floats
denoted "A"
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
14
and "B" after a methanol soak. The three floats were submerged in a 100%
methanol bath and allowed to soak for 28 days at a temperature of between
about
64° to 78°F.
After removal from the methanol soak each day, the floats were each given
identical shore hardness tests and then returned to the bath.
As shown in Table 1, the acrylonitrile rubber float of this invention did not
lose
any of its Shore A hardness, while the two competitive but non-encapsulated
acrylonitrile rubber floats lost 17.5 points of hardness (A float) and 11.5
points of
hardness (B float), respectively.
The conclusion drawn from this test is that the encapsulated float of this
invention did not absorb any methanol and thus lose hardness, as compared with
non-encapsulated floats.
TABLE 1
Methanol Soak - Shore A Hardness
Test Parameter - Temperature 64-78 Deg. F,
Commercial Grade Methanol (CAS 67-56-1 )
A B U-2000
Start 94.5 95.5 93
Day 1 92 94.5 93
Day 2 89 93 93
Day 3 87 93 93
Day 4 87 92 93
Day 8 86 92 93
Day 9 86 90 93
Day 10 85 90 93
Day 14 82 87 93
Day 17 80 87 93
Day 22 78 85 93
Day 28 77 84 93
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
Turning now to Table 2, which presents the results of a methanol immersion
test comparing the same three types of floats, as utilized in Table 1. Here,
the three
floats were again immersed into a 100% methanol bath for 28 days. This test
measured the amount of methanol absorbed by each float as an increase in each
float's weight. Each day the floats were removed from the methanol bath,
weighed
and returned to the methanol bath.
The encapsulated acrylonitrile rubber float of this invention exhibited only
0.01
grams weight gain after 28 days, while the two non-encapsulated, acrylonitrile
rubber
floats exhibited a significantly higher increase in weight. Acrylonitrile
rubber float A
increased 8.20 grams in weight after 28 days, while the B acrylonitrile rubber
float
increased 9.62 grams in weight' after 28 days.
The conclusion drawn from this test is that the encapsulated float of this
invention did not absorb significant amounts of methanol over the test period,
whereas non-encapsulated floats did absorb a substantial amount of methanol.
Applicant wishes to point out that in regards to Tables 2-7, applicant was
required to remove excess liquid from the floats by hand blotting prior to
weighing.
Hand blotting was used for measurement purposes during testing and did not
result
in completely dry samples.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
16
TABLE 2
Methanol Immersion Weight Change (gms) Testing
Temperature 64-78 Deg. F
Commercial Grade Methanol (CAS 67-56-1 )
A B U-2000
Start 48.48 53.96 8.02
Day 1 49.03 54.33 8.03
Day 2 49.55 54.83 8.02
Day 3 49.88 55.43 8.02
Day 4 50.20 55.96 8.08
Day 8 51.02 57.58 8.08
Day 9 51.60 57.85 8.09
Day 10 51.86 58.10 8.05
Day 11 51.93 58.36 8.04
Day 14 52.81 59.28 8.03
Day 17 53.73 60.08 8.06
Day 22 55.09 61.41 8.04
Day 28 56.28 62.58 8.03
Referring now to Table 3, which sets forth the results of the 1,1,1
Trichloroethane immersion test. The same three types of floats, as described
in
Table 1, were immersed in 100% 1,1,1 trichloroethane at a temperature of
between
150°F. to 170°F. The test measured the amount of 1,1,1
trichloroethane absorbed
by each float as an increase in each float's weight. Each day the floats were
removed from the bath and weighted then returned to the bath.
The acrylonitrile rubber float of this invention (U-2000) increased only 0.02
grams in weight over a 24-hour period, while acrylonitrile rubber float A
increased
30.83 grams in weight after only one hour, and acrylonitrile rubber float B
increased
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
17
29.32 grams weight after only one hour. Both the A and the B floats were
partially
dissolved, severely swollen, and severely cracked after only one hour of
immersion
in the 1,1,1 trichloroethane. The conclusion from this test is that a non-
encapsulated
float would fail in a short period of time in this chlorinated solvent,
whereas the
inventive float would not.
TABLE 3
1,1,1, Trichloroethane Absorption Immersion Test (gms) - Hot
A B U-2000
Start 48.22 54.05 8.17
Hour 79.05 3.37 8.21
1
Hour * * ~8.2
8
Hour * * 8.19
24
*- Sample failure after 1 hour - sample partially dissolved,
severely cracked, severely swollen.
Table 4 sets forth the results of an immersion test in an alcohol/gasoline
blend, whereby two commercially available acrylonitrile rubber-based floats, A
and C
as well as the encapsulated acrylonitrile rubber float (U-2000) of this
invention were
immersed in a blend of 15% methanol and 85% regular unleaded commercially
available gasoline by weight. The immersion test was conducted over 28 days at
a
temperature of between 68° to 77°F. The test measured the amount
of absorption of
the methanol/gasoline blend by each float as an increase in weight of each
float.
Each day the floats were removed from the bath, weighed and then returned to
the
bath.
The encapsulated acrylonitrile rubber float increased 0.01 grams in weight
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
18
after 28 days, while acrylonitrile rubber float A increased 0.8 grams in
weight, and
acrylonitrile rubber float C increased 0.9 grams in weight.
The conclusion drawn from this test is that the encapsulated float of this
invention did not absorb significant amounts of the methanol/gasoline mixture
over
the test period, whereas non-encapsulated floats did absorb a substantial
amount of
the methanol/gasoline mixture.
TABLE 4
15% Methanol/85% Unleaded Gasoline by Volume
Immersion Weighfi Change Test (gms)
Methanol - (CAS 67-56-1 )
Gasoline (CAS 8006-61-9)
Ambient 68-77 Deg. F
A C U-2000
Start 48.0 48.2 8.11
7 Days 48.6 48.8 8.13
12 Days 48.7 48.8 8.14
21 Days 48.8 48.9 8.1
28 Days 48.8 49.1 8.12
Table 5 describes the results of an ethanol immersion test wherein the same
three types of floats as described in Table 4 were submerged in 100% ethanol
for 28
days. The temperature during the 28-day test was between 64° and
78°F. The test
measured the amount of ethanol absorbed by each float as an increase in weight
of
each float. Each day the floats were removed from the bath, weighed and
returned
to the bath.
Both the A acrylonitrile rubber float and the C acrylonitrile rubber float
increased 0.2 grams in weight, while the encapsulated acrylonitrile rubber
float of
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
19
this invention increased only 0.01 grams in weight.
The conclusion drawn from this test is that the encapsulated float of this
invention did not absorb significant amounts of ethanol over the test period,
whereas
non-encapsulated floats did absorb a substantial amount of ethanol.
TABLE 5
100% Ethanol Immersion Testing Weight Change (gms)
Temperature 64-78 Deg. F
CAS 64-17-5
A C U-2000
Start 48.3 48.0 8.24
Day 7 48.5 . 48.4 8.27
Day 12 48.5 ' 48.4 8.25
Day 21 48.5 48.4 8.26
Day 28 48.5 48.6 8.25
Turning to Table 6, which summarizes the results of a float immersion test in
a
15% ethanol and 85% commercially available Regular Unleaded gasoline blend.
The test measured the absorption of the ethanol gasoline blend by each float
as an
increase in the weight of each float over a 28-day period. This test was
conducted at
fiemperatures ranging from 64° to 78°F. The same three types of
floats, as disclosed
in Table 4, were utilized. Each day the floats were removed from the bath,
weighed
and returned to the bath.
The A non-encapsulated acrylonitrile rubber float increased 0.5 grams and the
C non-encapsulated acrylonitrile rubber float increased 0.6 grams in 28 days,
while
the encapsulated acrylonitrile rubber float of this invention (U-2000) only
increased
0.01 grams during the same time period.
The conclusion drawn from this test is that the encapsulated float of this
invention did not absorb significant amounts of the ethanol/gasoline mixture
over the
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
test period, whereas non-encapsulated floats did absorb a substantial amount
of the
ethanol/gasoline mixture.
TABLE 6
15% Ethanol/85% Unleaded Gasoline by Volume
Immersion Weight Change Test (gms)
Methanol- (CAS 64-15-5)
Gasoline (CAS 8006-61-9)
Ambient 68-77 Deg. F
A C U-2000
Start 48.4 48.1 8.17
7 Days 48.6 48.5 8.2
12 Days 48.6 48.5 8.19
21 Days 48.8 48.6 8.19
28 Days 48.9 48.7 8.18
Referring now to Table 7, which sets forth the results of the diesel fuel
immersion test. The same three types of floats, as described in Table 4, were
immersed in commercially available diesel fuel. The test was conducted at
temperatures of between 64° and 78°F. for 28 days and measured
the amount of
diesel fuel absorbed as an increase in weight of each float: Each day the
floats were
removed from the bath, weighed and returned to the bath. Both the A and C
acrylonitrile rubber floats increased 0.7 grams in weight while the
encapsulated
acrylonitrile rubber float of this invention increased 0.07 grams in weight
during the
28-day test.
The conclusion drawn from this test is fihat the encapsulated float of this
invention did not absorb significant amounts of diesel fuel over the test
period,
whereas non-encapsulated floats did absorb a significant amount of diesel
fuel.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
21
TABLE 7
Diesel Fuel Immersion Weight Change (gms) Test
Diesel (Ref. CAS 544-76-3)
Ambient Temperature 64-78 Deg. F
A C U-2000
Start 47.5 48.3 8.11
7 Days 48.2 49.0 8.21
12 Days 48.2 49.0 8.18
21 Days 48.2 49.0 8.22
28 Days 48.2 49.0 8.19
Table 8 sets forth the affects of a nitric acid and ammonium bifluoride
mixture
on encapsulated and non-encapsulated acrylonitrile rubber floats. Four
separate
floats were introduced into a nitric acid mixture comprised of 250 ml of 70%
commercial grade nitric acid and 27 grams ammonium bifluoride. Two of the
floats
were commercially available non-encapsulated acrylonitrile rubber. floats,
denoted A
and C. The other remaining floats were the encapsulated acrylonitrile rubber
float of
this invention, and a commercially available hollow, stainless steel float.
Each float
was allowed to float upon the nitric acid mixture, thus directly exposing the
bottom
and portions of the side of each float to the acid mixture.
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
22
TABLE 8
Acid Test
Mixture of Nitric Acid (CAS 7697-37-2) and
Ammonium Bifluoride (CAS 1341-49-7)
Mixed 250 ml 70% Commercial Grade Nitric Acid and 27 grams Ammonium
Bifluoride Ambient Temperature, Closed container, Sample Weights in Grams
Note - Test Materials Floated on SurFace of Mixture, Remaining
Surfaces of Materials Exposed to Vapor
A C U-2000 Stainless Steel*
Start 0.4 2.96 8.2 8.19
48 hours 0.28 2.42 8.21 8.19
96 hours 0.2 2.21 8.2 8.18
168 hours ** 1.18 8.22 8.19
336 hours ** ** 8.22 8.17
504 hours ** ** 8.22 8.16
672 hours ** ** 8.21 8.16
* - Stainless Steel Float is Innovative Fusion P/N 1012-LW, 303
stainless steel with weld. Later stage of test showed etch effect.
** - Samples failed Parts were greatly dissolved and crumbling
upon failure.
After 336 hours, both of the non-encapsulated acrylonitrile rubber floats, A
and C, were destroyed. The stainless steel float weighted slightly less (0.03
grams)
after 672 hours; however, there were visual signs of etching of the stainless
steel.
The encapsulated acrylonitrile rubber float (U-2000) exhibited no weight loss
after
672 hours.
The conclusion from this test is that fihe encapsulated float and the
stainless
steel float are able to withstand immersion in a nitric acid bath, whereas the
non-
encapsulated floats are incapable of use in this type of environment.
The above-described embodiments of the present invention are merely
CA 02407770 2002-10-29
WO 01/84092 PCT/USO1/13645
23
descriptive of its principles and are not to be considered limiting. The scope
of the
invention instead shall be determined from the scope of any claims in a
corresponding non-provisional application, including their equivalents.
LIST OF TABLES
Table 1 is a summary of results for Shore A hardness after a methanol soak;
Table 2 is a summary of absorption after a methanol immersion;
Table 3 is a summary of absorption after a 1,1,1 trichloroethane immersion;
Table 4 is a summary of absorption results after immersion in a 15%
methanol, 85% regular unleaded gasoline mixture;
Table 5 is a summary of absorption results after an ethanol immersion;
Table 6 is a summary of absorption results after immersion in a 15% ethanol,
85% regular unleaded gasoline mixture;
Table 7 is a summary of absorption results after a diesel fuel immersion.
Table 8 is a summary of float degradation after a nitric acid/ammonium
bifluoride mixture soak.