Note: Descriptions are shown in the official language in which they were submitted.
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
1
METHOD AND DEVICE FOR THE PRODUCTION OF ALKYLATES
INVENTION BACKGROUND
(a) Field of the Invention
The present invention relates to a method for the production of alkylates by
sulfuric acid alkylation of isoparaffins with olefins. This method is
particularly
well adapted for use in the petroleum refining industry, using isobutanea as
isoparaffin, and butylenes as olefin.
The invention also relates to a device for mixing and reacting at least two
and
preferably three liquid components. This device is particularly well adapted
for
carrying out the above method even though it can be used for carrying out
many other methods.
(b) Brief description of the prior art
Alkylates are main components of high-octane motor fuels. They are produced
by alkylation of isoparaffins (mainly isobutane) by olefins (such as
propylene,
butylenes or amylenes) in the presence of sulfuric or hydrofluoric acid that
serves as a process catalyst. The most widely known method for the
production of alkylates in the petroleum refining industry consists of
carrying out
a sulfuric acid alkylation of isobutane by olefin.
Numerous methods for carrying out sulfuric acid alkylation of isobutane by
olefins are known. The method according to the invention distinguishes over
most of these known method in that the reaction is carried out in a compact
reactor which does not contain moving parts and in which jet mixing of the
3 0 reagents is achieved.
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
2
U.S. patent No. 3,544,652 issued on December 15t, 1970 discloses a method
for the alkylation of isoparaffin by olefins in the presence of sulfuric acid,
where
the olefin is reacted with an alkylating hydrocarbon-in-acid emulsion formed
by
thoroughly mixing isoparaffin with sulfuric acid before contact with the
olefin. In
this patent, the isoparaffin-to-olefin volume ratio is disclosed as being
equal to
about 12:1. The acid-to-hydrocarbons volume ratio is disclosed as being within
the range of 2.5:1 to 15:1 but it is mainly maintained at about 6:1. The
reaction
is carried out adiabatically, mainly in a continuous manner, in a reactor
called
"alkylation contactor", which is provided with a mixer that is devised for
forming
the isoparaffin-in-sulfuric acid emulsion and for thoroughly and homogeneously
mixing the so-formed emulsion with the olefin at the points of delivery of the
latter into the reactor.
As the liquid flows through the reactor, the temperature of the alkylating
mixture
rises continuously by 5 to 15 C, thereby reducing viscosity of the mixture and
increasing its turbulence. The method is carried out at a temperature of 5 to
60 C under a pressure sufficient for keeping the reagents in a liquid state
(from
2 to 10 ATMs). Prior to being mixed with the isoparaffin, sulfuric acid at a
concentration of 88 to 99% is cooled down to a temperature of about 4 C.
The emulsion preparation and the olefin injection and distribution inside the
reaction area are not disclosed in detail in this U.S. patent.
The method disclosed in US patent No. 3,544,652 is efficient but it requires a
substantial amount of power for circulating the acid due to the very high acid-
to-
hydrocarbons ratio. It also requires a settling equipment of a very large
size.
Moreover, the method disclosed in this patent cannot guarantee a low
consumption of sulfuric acid and a reasonably high quality of the final
product.
Russian patent No. 2,131,861 granted on July 25, 1994 (corresponding to US
patent Nos. 5,443,799 and 5,777,189) discloses a method for sulfuric acid
alkylation of isoparaffins by olefins and a device for carrying out this
method.
At the initial stage of the method disclosed in this patent, a thin
isoparaffin-in-
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
3
sulfuric acid emulsion is made by injecting isoparaffin into an acid medium
through a set of nozzles. Then the emulsion is delivered into a reaction area
where olefin is fed, through a number of points normal to the emulsion flow.
In
this method, the alkylation is carried out under isobaric and isothermal
conditions.
Russian patent No. 2,131,861 also discloses that the emulsion should
preferably flow in the emulsion area at a rate of 0.2 to 2 m/s - and within
the
reaction area at a rate of 0.04 to 0.27 m/s. Depending on the selected flow
rate, the contact between the reagents may last from a few to 60 seconds,
thereby reducing to a minimum the possibility of not-wanted side reactions
such
as oligomerization of olefins and autoalkylation of isoparaffins. Tests have
shown that this method permits to prepare a thin unstable emulsion.
Separation of the reaction mixture into a hydrocarbon phase and an acid phase
takes 5 to 8 seconds, thereby allowing reduction in the setting time.
Since the method described in Russian patent No. 2,131,861 does not require
rotary mixers, the equipment required for carrying it out is rather cheap and
of
easy control and maintenance.
Russian patent No. 2,131,861 further discloses a device for carrying out the
above method. This device comprises a tank for preparing the emulsion. A
special appliance is provided for isoparaffin injection within the tank. Such
an
appliance essentially consists of a set of axially arranged nozzles. An
appliance
is also provided for sulfuric acid injection within the tank. The device also
comprises a mixing chamber that is part of the tank, with an outlet throat,
and
a cylindrical reactor which is connected in line to the throat of the emulsion
preparation tank. To provide olefin injection, the device comprises a
perforated
branch pipe extending along the axis of the reactor.
The method and device described in the above Russian patent No. 2,131,861
and its foreign counterparts have rather acceptable technical and economic
parameters of operations, as proved by industrial tests. However, those
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
4
parameters could be improved if use is made of a higher level of flow
turbulence in the reaction zone. In practice, such a higher level of flow
turbulence could be obtained if the flow rate is increased in the reaction
zone
and the mixing conditions of olefin and emulsion flows are improved by using
a more efficient olefin feed unit instead of using a perforated branch pipe
extending along the axis of the reactor.
Russian patent No. 2,092,475 granted on December 6, 1995 is the closest prior
art known to the Applicant. It discloses a method for the production of
alkylates
in a tubular reactor, which comprises mixing sulfuric acid with isobutane
previously cooled down to a temperature of not over - 2 C; mixing the obtained
emulsion with olefins also previously cooled to a temperature of not over - 2
C,
in a plurality of stages; separating the sulfuric acid from the obtained
reaction
mass; and recycling it. This method requests that the sulfuric acid be mixed
with the isobutane and the obtained emulsion be mixed with the olefin in an
injector-type mixer, with an isobutane-to-sulfuric acid injection ratio of 3.3
to 5.2
and an isobutane-to-olefin volume ratio of 3000-5000:1. In this method,
sulfuric
acid is separated from the reaction mass in a hydrocyclone.
Russian patent No. 2,092,475 also discloses a device for carrying out the
above
method, which consists of a reactor provided with three concatenated injection
mixers. Each mixer is provided with an olefin injection appliance that
distributes
the feed in a helical fashion along the length of a device.
With the method and device disclosed in Russian patent No. 2,092,475, one
may carry out sulfuric acid alkylation of isoparaffins by olefins in a compact
reactor that has no moving parts. One may also obtain a high quality alkylate,
as proved by industrial tests. However, in practice, an isobutane-to-olefin
ratio
of 3,000-5,000:1 can be obtained in the reaction area only with very high
power
consumption. Alkylate quality and specific sulfuric acid consumption could
actually be improved by using a more efficient olefin feed unit than the
perforated tube with helical openings as described in this patent. Besides,
alkylate quality and specific sulfuric acid consumption could be improved by
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
optimization of the alkylation process conditions, by proper selection of
different
size ratios of the reactor elements and by an improved process of separation
of the reaction mixture and a device to carry out this process.
5 OBJECTS AND SUMMARY OF THE INVENTION
A first object of the present invention is to provide a method for the
production
of alkylates by sulfuric acid alkylation of isoparaffins by olefins, which has
the
following advantages:
- reduction in power consumption;
- reduction in sulfuric acid consumption; and
- improvement of the alkylate quality.
In accordance with the invention, this fist object is achieved with a method
for
the production of alkylate(s) by sulfuric acid alkylation of at least one
isoparaffin
with at least one olefin, comprising the steps of:
(a) preparing a mixture of said at least one isoparaffin with recycled
reaction
products by mixing said at least one isoparaffin previously cooled down to
a temperature lower than +12 C with recycled reaction products separated
from sulfuric acid and cooled down to a temperature lower than +12 C;
(b) making a hydrocarbons-in-sulfuric acid emulsion by mixing sulfuric acid
with
the mixture obtained in step (a);
(c) preparing another emulsion by injecting a given portion of said at least
one
olefin in jet streams through nozzles into the hydrocarbons-in-sulfuric acid
emulsion obtained in step (b);
(d) injecting the other emulsion obtained in step (c) through nozzles into a
reaction chamber of given height -and cross-section, where said other
emulsion is circulated in a closed circuit and a corresponding amount of
reaction mixture is continuously discharged;
(e) injecting another portion of the said at least one olefin in jet streams
into the
other emulsion through a system of injectors distributed in the reaction
chamber all over the cross-section and height of said reaction chamber;
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
6
(f) processing the reaction mixture discharged from the reaction chamber
through at least one hydrocyclone in order to separate said reaction mixture
into an acid-containing phase and an hydrocarbon-containing phase, and
subjecting each of said phases to a pressure reduction and a gas
separation;
(g) recycling to step (a) one part of the hydrocarbon-containing phase that is
in
a liquid form after said gas separation, said recycled part acting as said
recycled reaction products, recovering the remaining part of the
hydrocarbon-containing phase and subjecting said recovered part to
deacidification, purification and separation to extract the requested
alkylate(s); and
(h) recycling to step (b) the acid-containing phase after said gas separation
and
a cooling, said recycled acid-containing phase acting as said sulfuric acid
composition, part of said acid-containing phase being withdrawn to
regeneration prior to being recycled and being replaced by fresh acid.
In accordance with a preferred embodiment of the invention, the preparation of
the emulsion and the alkylation process carried out in steps (a) to (d) are
run
in vertical flows.
In accordance with another preferred embodiment of the invention, steps (b)
and (h) are controlled in such a manner that the amount of sulfuric acid
circulating through the reaction chamber and processed in step (f) ranges from
40 to 80 m3 per ton of commercial grade alkylate.
Preferably also, steps (b) and (d) are controlled in such a manner that in
step
(b), the first emulsion flows at a rate of 1.5 to 3.5 m/s and in step (d), the
second emulsion flows in the reaction chamber at a rate of 2 to 4 m/s.
3 o Further preferably, steps (c) and (d) are controlled in such a manner that
said
at least one olefin be injected with a pressure drop higher than 1 kg/cm2.
CA 02408037 2007-01-22
7
The above method for the production of alkylate(s) by sulfuric acid alkylation
of
isoparaffin(s) by olefin(s) Is quite efficient and ecologically safe. The
number of
pieces of equipment as well as the quantity of explosive, toxic and corrosive
substances needed to operate the unit are dramatically reduced, such reduction
being achieved not only by a lower time of reaction and a reaction chamber of
smaller volume, but also by a lower time of separation of the reaction mixture
in the hydrocyclone(s). Also reduced are the electric power consumption, the
size required for the unit site, the man-hours, etc... Furthermore, the
overhaul
life of the device is dramatically increased, thereby resulting in a reduction
in
lo production losses. Leakage of various products in the environment is also
dramatically reduced.
The method according to the invention also permits to obtain a substantial
reduction in power and sulfuric acid consumption. It further permits to
improve
the alkylate quality.
A second object of the present invention is to provide a device that is
designed,
in particular, for running the process of sulfuric acid alkylation of
isoparaffin by
olefins. This device can also be used for carrying out a great number of other
processes that require thorough mixing of several liquid components and
creation of suitable conditions for their interaction.
In accordance with the invention, this second object is achieved with a device
for mixing and reacting at least two liquid components, comprising:
(a) a mixing chamber for preparing a mixture of two of said components;
(b) an emulsion chamber for preparing a first emulsion, where the mixture
prepared in the mixing chamber (a) is injected in multiple parallel jets;
(c) a pre-reaction chamber for preparing a second emulsion, where a given
portion of one of said components is injected In jet streams into the first
emulsion coming from the emulsion chamber (b); and
(d) a reaction chamber of given height and cross-section where the second
emulsion coming from the pre-reaction chamber (c) is injected through
nozzles and another portion of said one of the com-
ponents is injected in jet streams all over the
CA 02408037 2005-05-02
8
cross-section and height of said reaction chamber, said reaction chamber '
being devised so that said second emulsion is circulated In a ciosed circuit
and comprising an ou#iet through which a balanced amount of reaction
mixture Is continuously discharged;
wherein the mixing chamber (a), emulsion chamber (b), pre-reaction chamber
(c) and reaction chamber (d) are coaxially arranged one above the other in
vertical position and altogether form a reactor with the mixing chamber (a)
being located at the bottom of the reactor and the reaction chamber (d) on top
thereof.
When used for the production of alkylate(s), the above device more
specifically
comprises:
(a) mixing chamber for preparing a mixture of said at least one isoparaffin
with
recycied reaction products;
(b) an emulsion chamber for preparing a first hydrocarbon-in-sulfuric acid
emulsion by mixing sulfuric acid with the mixture prepared in the mixing
chamber (a);
(c) a pre-reaction chamber for preparing a second emulsion, where a given
porEion of said at least one olefin is injected in jet streams Into the first
hydrocarbons-in-sulfuric acid emulsion coming from the emulsion chamber
(b); and
(d) a reaction chamber of given height and cross-section where the second
emulsion coming from the pre-reaction chamber (c) is injected through
nozzles and another portion of the at least one olefin is Injected in jet
streams all over the cross-secdon and height of the reaction chamber,
wherein the mixing chamber (a), emulsion chamber (b), pre-reaction chamber
(c) and reaction chamber (d) are coaxially arranged one above the other. in
vertical position and altogether form a reactor with the mixing chamber (a)
being located at the bottom of the reactor and the reaction chamber (d) on top
thereof.
If desired, the above device may further comprises:
CA 02408037 2005-05-02
8a
(e) a device for separating the reaction mixture recovered from the reaction
chamber (d) to obtain a liquid acid-containing phase, a hydrocarbon-containing
phase and a vapour phase,
wherein said device comprises a horizontal vessel incorporating:
(a') at least one vertical overflow baffle plate that extends within said
vessel
and divides it into one settling tank and at least one accumulation tank;
(b') supply connecting pipes for introducing the reaction mixture into the
settling tank;
(c') outlet connecting pipes for discharging the acid-containing phase form a
bottom portion of the settling tank;
(d') other outlet connecting pipes for discharging the liquid hydrocarbon
phase in the form of two separate flows, one of said flows being directed back
to
the unit for use as recycled reaction products, the other one of said flows
consisting of commercial grade reaction products and being subjected to
rectification and fractionation to obtain commercial grade alkylates, wherein
the
other outlet connecting pipe through which the other one of said flows exits
from
the vessel are located in a bottom portion of said at least one accumulation
tank;
and
(f) at least one further outlet connecting pipe for discharging the vapor
phase
from an upper part of the vessel.
A third object of the present invention is to provide a method for separating
into
phases the reaction mixture exiting from the above unit of sulphuric acid
CA 02408037 2002-11-01
WO 01/94283 PCT/IB01/00962
9
alkylation of at least one isoparaffin by at least one olefin, the phases
including
a liquid hydrocarbon-containing phase and a liquid acid-containing phase. This
method has for the following advantages:
- alkylate quality improvement;
- reduction of sulfuric acid consumption;
- reduction of ethers other acid compounds in liquid reaction products; and
- reduction of acid compounds in vapor phase.
In accordance with the invention, this third object is achieved by a method
which comprises the steps of:
- injecting the reaction mixture at a speed of 4 to 10 m/s into a hydrocyclone
in order to separate it into a liquid hydrocarbon-containing phase and a
liquid acid-containing phase; and
- further processing the phases by subjecting each of them to a pressure
reduction, injecting the phase having been subjected to said pressure
reduction into a gas separator, recovering liquids from said gas separator
by means of a pump for further utilization, and extracting vapors from the
separator by means of a compressor.
In accordance with a first preferred embodiment of the invention, each of the
phases coming from the hydrocyclone and having been subjected to pressure
reduction, is fed into its own separator. Part of the liquid extracted from
the
hydrocarbon-containing phase separator is recycled for mixing with isoparaffin
in the process of alkylation of isoparaffin with olefins, as described above.
The
remaining part of the liquid is subjected to rectification and fractionation
to
extract the required alkylate. On the other hand, the main part of the liquid
extracted from the acid containing phase separator is recycled and mixed with
isoparaffin and the recycled reaction products to prepare the acid-to-
hydrocarbon emulsion, as described above. The remaining part of the sulfuric
3 o acid-containing phase flow is withdrawn for regeneration and replaced with
an
appropriate amount of make-up acid.
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
In accordance with another preferred embodiment of the invention, the phases
withdrawn from the hydrocyclone and subjected to pressure reduction are fed
to a common settling vessel separated by an overflow baffle plate into a
settling
tank and an accumulation tank. Commercial grade products are withdrawn from
5 the accumulation tank for rectification and extraction of the requested
alkylates.
Acid is withdrawn for recycling and regeneration from a lower part of the
settling
tank, and the reaction products to be recycled are extracted from settling
tank
at a level that is lower than the upper end of the overflow baffle plate of
the
settling vessel.
Advantageously, the hydrocarbon-containing phase can be subjected to
another separation in additional hydrocylone. After reduction and vapor
extraction, the upper lightweight flow from the additional hydrocyclone can be
subjected to rectification and fractionation to extract the requested
commercial
grade alkylate while the lower heavy weight flow after vapor extraction can be
recycled to the reactor.
Advantageously also, the acid-containing phase can be subjected to another
separation in another additional hydrocyclone. The upper lightweight flow
extracted from 'the additional hydrocyclone can be recycled while the lower
heavy weight flow can be separated in yet another additional hydrocyclone. The
lower heavy weight flow from the last hydrocyclone can be used as a second
portion of acid to be recycled to the reactor while the upper lightweight flow
can
be withdrawn for regeneration.
A fourth and last object of the present invention is to provide a device that
is
designed, in particular, for separation of the reaction mixture of the unit of
sulfuric acid alkylation of isoparaffins by olefins into liquid acid-
containing and
hydrocarbon-containing phases and a vapor phase. This device can be also
used as a 3- or 4-phase separator in processes where vapor phase is
withdrawn by a single flow while liquid phases are withdrawn in three separate
flows. This device can be used, for example, as a 3-phase separator for the
treatment of gas-saturated and water containing oil where casing-head gas is
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
11
withdrawn by a single flow while oil and water are withdrawn by three separate
flows. For example, dry oil can be withdrawn by a single flow while water can
be withdrawn by two separate flows. One flow is directed to after purification
and subsequent pumping in an oil bed or pond discharge wherein another flow
is directed to the head of the process for system recycling as a heat carrier
or
demineralizing agent.
In accordance with the invention, this fourth object is achieved with a device
for
separating a reaction mixture containing immiscible liquids of different
densities
and free gas or vapor, in such a manner as to obtain no less than three liquid
flows and at least one gas or vapor flow. This device comprises a horizontal
vessel incorporating:
(a) at least one vertical overflow baffle plate that extends within said
vessel and
divides it into one settling tank and at least one accumulation tank;
(b) supply connecting pipes for introducing the reaction mixture into the
settling
tank;
(c) outlet connecting pipes for discharging the acid-containing phase from a
bottom portion of the settling tank;
(d) other outlet connecting pipes for discharging the liquid hydrocarbon phase
in the form of two separate flows, one of said flows being directed back to
the unit for use as recycled reaction products, the other one of said flows
consisting of commercial grade reaction products and being subjected to
rectification and fractionation in order to obtain commercial grade alkylates,
wherein the other outlet connecting pipes through which-the other one of the
flows exits from the vessel is located in a bottom portion of said at least
one
accumulation tank; and
(e) at least one further outlet connecting pipes for discharging the vapor
phase
from an upper part of the vessel.
In accordance with a first preferred embodiment of the invention, the
connecting
pipes used for withdrawal of the reaction products to be recycled is in the
form
of a perforated pipe header extending in the settling tank at a given distance
from the baffle plate, said pipe header having an axis parallel to the
overflow
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
12
baffle plate and extending upwards at a given height from the bottom portion
of said settling tank.
In accordance with another preferred embodiment of the invention, the vessel
comprises two overflow baffle plates that divide the vessel into one settling
tank
and two accumulating tanks. One of the accumulating tanks is used for
collecting the commercial grade reaction products and the other accumulating
tank is used for collecting the recycled reaction products. The settling tank
can
also be divided by another baffle plate of a given height into two settling
sections for collecting commercial grade products and recycled reaction
products, respectively.
The invention and its advantages will be better understood upon reading the
following non-restrictive description of preferred embodiments thereof made
with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. I is a schematic representation of a device for the production of
alkylate(s)
2o according to a preferred embodiment of the invention;
Fig. 2 is a schematic representation of an alkylation unit incorporating the
device shown in Fig. 1;
Fig. 3 is a schematic representation of another alkylation unit incorporating
the device shown in Fig. 1 and a device for separating the obtained products;
Fig. 4 is schematic representation of a further alkylation unit incorporating
the
device shown in Fig. 1 and another device for separating the obtained
products.
It is worth noting that the dimensions and relative proportions of each of the
components of the device and units shown in the accompanying drawings do
CA 02408037 2002-11-01
WO 01/94283 PCT/IB01/00962
13
not reflect the invention as it can be reduced to practice. By way of example,
the- hydrocyclone(s) shown in Figs. 2 and 3 may be, in practice, 2 to 4 times
larger in size than the device per se. Also shown in a simplified way are the
elements of the emulsion chamber of the reactor and of a unit for the
introduction of sulfuric acid into said emulsion chamber. However, such
dimensions and proportions are not essential and are actually obvious for any
one who would manufacture the device or alkylation unit according to the
invention.
1 o It is worth noting also that the same reference numerals have been used
throughout the following description to identify the same structural elements,
whatever be the illustrated embodiment.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The device according to the preferred embodiment of the invention as shown
in Fig. 1 includes the following basic units:
- a mixing chamber 1 for mixing isoparaffin with recycled reaction products;
- an emulsion chamber 2 comprising a peripheral annular space 4 with a pipe
connection 5 for introduction of a sulfuric acid composition, a mixing area 6,
and a system of inlet branch pipes 3 that are parallel to the chamber axis
and are designed for injection of the mixture coming from the mixing
chamber, each pipe 3 being provided with a bell mouth at the outlet;
- a pre-reaction chamber 7; and
- a reaction chamber 8.
The units are concatenated as shown in Fig. 1, and they altogether form an
3 0 adiabatic reactor that can be used for sulfuric acid alkylation of
isoparaffin by
olefins with jet mixing of the components.
As is shown, all the chambers of the device are coaxial and installed
vertically
in their operative position with the mixing chamber 1 in the lower part of the
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
14
device and the reaction chamber 8 on top of it.
The emulsion chamber 2 includes a centrally positioned cylindrical socket 9
which defines the peripheral annular space 4. The emulsion chamber has a
height equal to 20 to 60 times the internal diameter of the inlet branch pipes
3
used for the hydrocarbon mixture injection. An enlargement fitting 10 is
joined
to the outlet of the chamber 2.
The pre-reaction chamber 7 extends in line on top of the enlargement fitting
10.
An olefin feed injection unit 11 is provided at the bottom of the pre-reaction
chamber 7. This injection unit comprises injectors 12 having their axes
running
upward at an angle of 0 to 300 with respect to the vertical.
The reaction chamber 8 is separated from the pre-reaction chamber 7 by
means of a baffle 13 having nozzles 14 mounted therein for providing passage
to the reaction mixture. It comprises a vertical housing 15 and a circulation
pipe
16 which is coaxial with the housing and installed with side clearances
relative
to the housing to allow recirculation of the injected emulsion in a closed
circuit.
Several connecting pipes 17 are tied into the bottom of the housing 15 of the
reaction chamber 8 for withdrawal of the reaction mixture.
Injectors 19 connected to supply pipes 18 are provided for injecting an olefin
feed near the top and bottom ends of the circulation pipe 16 and in one or
several tiers over the pipe height. These injectors 19 have their axes running
upward at an angle of 0 to 30 with respect to the vertical. Preferably, each
tier
provided along the height of the circulation pipe 16 comprises at least three
injectors 19. '
The nozzles 14 mounted in the baffle 13 separating the pre-reaction chamber
from the reaction chamber are arranged along a circle whose diameter is equal
to 0.6 to 0.75 times the inner diameter of the circulation pipe 16. The inner
diameter of the circulation pipe 16 is equal to 0.55 to 0.75 times the inner
diameter of the housing 15 of the reaction chamber.
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
The height of the circulation pipe is preferably equal to 3 to 9 times its
inner
diameter. Preferably also, the sum of the cross-sections of the nozzles 14 of
the
baffle 13 is equal to 0.04 to 0.2 times the cross-section of the circulation
pipe.
5
Advantageously, the reaction chamber 8 is also provided with an additional
axial connecting outlet 20 on its top to allow liquid or vapor emission
directly
from the reaction chamber whenever required during regular operation of the
reactor and/or during preparatory and final operations for reactor start-up
and
10 shut-down.
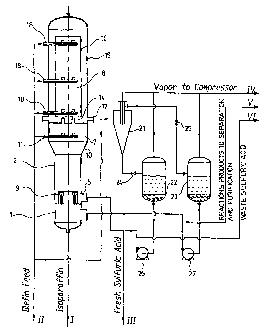
Fig. 2 is a simplified flow chart of an example of alkylation unit in which
incorporating the device according to the preferred embodiment invention as
shown in Fig. 1 can be incorporated.
As can be seen, the unit shown in Fig. 2 incorporates the device shown in Fig.
1, a hydrocyclone 21 connected to the connecting pipes 17 and injectors 19 of
the reaction chamber 8, an acid gas separator 22 and a hydrocarbon gas
separator 23 connected to the hydrocyclone 21, a set of pressure reducing
valve 24 and 25 respectively connected to the gas separators 22 and 23
upstream of the same, and a set of pumps 26 and 27 respectively connected
to the gas separators downstream of the same.
In use, the isoparaffin used as starting material is cooled down to a
temperature
lower than '-12 C. The so called isoparaffin is fed via a line I into the
mixing
chamber 1 of the device where recycled reaction products cooled down at a
temperature lower than +12 C are simultaneously injected by the pump 27. The
mixture obtained in the chamber 1 is fed in multiple parallel jets through the
inlet
branch pipes 3 into the emulsion chamber 2 where sulfuric acid is also fed.
The
jets are directed through the pipe connection 5 into the peripheral annular
space 4 of the emulsion chamber 2. The fine-dispersed emulsion formed in the
emulsion chamber 2, exits from the same through the enlargement fitting 10.
A given portion of the olefin supplied by the olefin feed injection unit 11 is
fed
CA 02408037 2002-11-01
WO 01/94283 PCT/IB01/00962
16
through the nozzles 12 in the emulsion to react with the isoparaffin contained
in it. Such a reaction occurs in the pre-reaction chamber 7. The second
emulsion which is so formed, is fed through the nozzles 14 into the reaction
chamber 8 where it is circulated in a closed circuit by means of the
circulating
pipe 16, the housing 15, the baffle 13 and the upper end of the reactor. A
balanced amount of the reaction mixture formed within the reaction chamber
8 is withdrawn through the connecting pipe 17. In several tiers over the
height
of the reaction chamber, near the inlet and outlet ends of the circulation
pipe 16
as well as near the middle part of its height, another portion of the olefin
feed
is injected in jet streams into the emulsion through the supply pipes 18 and
injectors 19. A further portion of the olefin feed also can be injected into
the
reaction chamber at the outlet of the same.
The reaction mixture exiting the reaction chamber 8 is fed into the
hydrocyclone
21 where it is separated into a heavy weight, acid-containing phase and a
light
weight, hydrocarbon-containing phase. Instead of one hydrocyclone as shown
in Fig. 2, use could be made of a set of hydrocyclones that would include
several concatenated hydrocyclones per each phase to be extracted in order
to provide a required level of rectification of every such phase. Such will be
better disclosed hereinafter with reference to Fig. 3 and Fig. 4.
In use, vapor may be liberated in the hydrocyclone. Therefore, the
hydrocyclone is preferably designed in order to provide a vapor exit via a
separate line IV leading to a compressor (not shown). The separated acid-
containing phase is withdrawn from the boL"Lorii of the hydrocyclone 21 and
fed
into the gas separator 22 via a pressure reducing valve 24. As a result of
throttling in the valve, a given amount of hydrocarbons contained in the acid
is
boiled away, thereby cooling off the acid. Vapor formed by boiling is
withdrawn
from the separator 22 and directed to a compressor (not shown) via the line IV
while the pump 26 recycles the cooled sulfuric acid composition into the
emulsion chamber 2. A given amount of waste acid may be withdrawn for
regeneration via a line VI. A*corresponding amount of fresh acid may then be
fed via a line I I I to compensate it
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
17
The light weight, hydrocarbon-containing phase is fed from the hydrocyclone
through a reducing valve 25 into another gas separator 23 where vapor
separated as a result of throttling is also directed to the compressor (not
shown)
via the line . The reaction products cooled due to evaporation of their most
easily boiling components, are separated into two parts. One part is recycled
into the mixing chamber 1 by the pump 27 (as recirculated reaction products),
while the other part is fed via a line V to an adjacent neutralization,
rectification
and separation unit (not shown) for the purpose of obtaining the requested
base
product - alkylate.
Tests carried out by the Applicant have shown that best results are achieved
when the amount of sulfuric acid circulating through the device according to
the
invention and of the other components of the alkylation unit that are used for
separation of the recycled reaction mixture ranges between 40 to 80 m3/t of
commercial grade alkylate.
Best results are also achieved when the first emulsion flows at a speed of 1.5
to 3.5 m/s and the second emulsion at a speed of 2 to 4 m/s within the device.
Best results are further achieved when the pressure drop at the level of the
nozzles 12 and 19 connected to the olefin feed injection unit 11 and to the
supply pipes 18 is higher than 1 kg/cm2, and preferably between 1 and 4
kg/cm2.
Best results are still further achieved when the reaction mixture is
introduced
into the hydrocyclone 21 at a speed of 4 to 10 m/s.
Advantageously, sensors (not shown) connected to a control panel may be
provided to check all these parameters and ensure that they fall within the
above mentioned ranges.
CA 02408037 2002-11-01
WO 01/94283 PCT/IB01/00962
18
Fig. 3 is a flow chart of another example of an alkylation unit incorporating
the device according to the preferred embodiment of the invention as shown
in Fig. 1.
The unit shown in Fig. 3 comprises the device shown in Fig. 1. The unit also
comprises a hydrocyclone 21 connected to the outlet connecting pipe 17 of the
reaction chamber 8 to separate the reaction mixture into 'a hydrocarbon-
containing phase and an acid-containing phase. The unit further comprises a
gas separator 28 for removing gas and vapor from the hydrocarbon-containing
and acid-containing phases after the same have been subjected to a pressure
reduction through a set of valves 24 and 25. The unit still further comprises
pumps 26 and 27 for recycling the reaction products and acid.
The gas separator 28 consists of a horizontal vessel that is divided by a
vertical
baffle plate 29 into a settling tank 30 and an accumulation tank 31. The
setting
tank 30 is provided with supply connecting pipes 32 and 33 connected to the
valves 24 and 25, respectively, through which the acid-containing and
hydrocarbon-containing phases are separately fed. The settling tank is also
provided with outlet connecting pipes 34 and 35 through which the settied acid
and reaction products to be recycled may be extracted. These connecting pipes
are connected to the pumps 26 and 27 for recirculation of the reaction
products
and acid. The gas separator 28 also comprises a separate connecting pipe 36
for discharge of spent acid for regeneration. This connecting pipe 36 opens
into
a compartment formed at the bottom of the settling tank between the overflow
baffle plate 29 and an additional baffle 37 provided at the bottom of the
settling
tank upstream the baffle plate 29. Such an arrangement allows discharge of a
lighter acid-containing phase.
3 o Another outlet connecting pipe 38 is provided in the lower part of the
accumulating tank 31 for allowing withdrawal of liquid reaction products in
liquid
form. This connecting pipe 38 is connected to a pump 41 which discharges the
reaction products for rectification and deisobutanization in order to extract
the
CA 02408037 2002-11-01
WO 01/94283 PCT/IB01/00962
19
requested commercial grade alkylate. As shown, the upper part of the
accumulation tank 31 is provided with a knockout drum 39 and a further outlet
socket 40 for vapor withdrawal to a compressor.
Advantageously, the unit shown in Fig. 3 may also comprise another outlet
connecting pipe positioned to allow withdrawal of the reaction products for
recycling purpose from a level that is lower than the height of overflow
baffle
plate. This connecting pipe 42 can be in the form of an horizontal perforated
pipe header or collector having an axis parallel to the overflow baffle plate
29
and a height measured from a bottom portion of the vessel 28 that is equal to
H,. H, is preferably 0.5 to 0.8 time the height H of the overflow baffle
plate. The
header or collector 42 is located at a distance L from the baffle plate, that
is
preferably equal to 0.25 to 1.0 time the height H of said baffle plate.
Fig. 4 is a flow chart of a further example of an alkylation unit
incorporating the
device according to the preferred embodiment of the invention as shown in Fig.
1.
The unit shown in Fig. 4 comprises the device shown in Fig. 1. It also
comprises
2 o a hydrocyclone 21 connected to the outlet connecting pipe 17 of the
reaction
chamber 8. It further comprises two other hydrocyclones 43 and 44 for sharp
separation of the acid-containing phase separated within the hydrocylone 21,
and one further hydrocyclone 45 for sharp separation of the hydrocarbon-
containing phase separated within the hydrocyclone 21. The unit also
comprises a cyclone'gas separator 46 for processing the heavy weight lower
flow discharged from the hydrocyclone 45, a cyclone gas separator 47 for
processing the lightweight upper flow discharged from the hydrocyclone 45, and
a separator 48.
3 o The separator 48 consists of an horizontal vessel that is separated by a
set of
baffle plates 49, 50 into two opposite liquid hydrocarbons accumulating tanks
51 and 52 and a central settling tank. The settling tank that is formed
between
the baffles 49 and 50, is also divided by a vertical baffle 53 into a settling
CA 02408037 2005-05-02
section 54 for the reaction products to be recycled, and a set#ling section 55
for
the commercial grade reaction products to be recovered. The lower end of the
baffle 53 extends at a distance away from the bottom of the vessel 48 while
its
upper end extends at 30 to 100 mm above the upper ends of the baffles 49 and
50. The bottom portion of the accumulating tank 51 Is provided with a
connecting pipe 56 for discharge of the reaction products to be recycled while
the bottom portion of the accumulating tank 52 is provided with a connecting
pipe 38 for discharge of the commercial grade reaciion products.
The setting section 54 for the reaction products to be recycled is connected
by
means of outlet connecting pipes 57, 58 to the cyclone gas separator 46. As
10 shown, the outlet connecting pipe 57 that Is used for vapor discharge from
the
separator 46 extends higher than the upper end of the baffle 49. As also
shown,
the connecting pipe 58 used for extraction of the liquids from cyclone gas
separator 46, extends In the lower part of the separator 48.
The settling section 55 for the commercial grade reaction products to be
recovered is connected by means of outlet connecting pipes to the cyclone gas
separator 47. As shown, the outlet connecting pipe 59 that is used for vapor
discharge from the separator 47 extends higher than the upper end of the
baffle
50. As also shown, the connecting pipe 60 used for extraction of the liquids
20 from the cyclone gas separator 47 extends In the lower part of the settier
48.
As may be seen, the separators 46 and 47 are connected to the corresponding
outlets of the hydrocyclone 45 through a pair of pressure reduction valves 61
and 62.
A connecting pipe 34 is provided at the bottom of the setting section 55 for
discharging the acid to be recycled. A vortex breaker or hood 63 is mounted
above the connecting pipe 34 to exclude funnel formation and hydrocarbon
suction into the acid to be recycled. The upper part of each accumulating tank
is
also provided with a knockout drum 39 and a connecting pipe 40 for vapour
discharge to a compressor. Each knockout drum 39 is provided with a bottom
39' from
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
21
which projects a downcomer leg 64, 65 having a lower open end dropping into
the lower part of the corresponding accumulating tank.
The method that may be carried out with the unit shown in Fig. 3 is generally
similar to the one described earlier. The main difference is that after
discharge
from the hydrocyclone 21 and pressure reduction through the valves 24 and 25,
the acid-containing and hydrocarbon-containing phases obtained from the
reaction mixture are fed in the gas separator 28 for complete mixture
separation. The separated vapor is discharged to a compressor through the
knockout drum 39 and connecting pipe 40. A downcomer leg projecting beneath
the liquid level of the settling tank trickles down the liquid separated in
the
knockout drum. In this settling tank, parallel flows of acid and liquid
hydrocarbons provides further rectification due to their density difference.
An
acid cooling is also achieved since the layer of reaction products which is
cooler
than the layer of acid, is located above the layer of acid. As proved by
results
of tests carried out by the Applicant, the acid temperature at the inlet of
the gas
separator is 2-3 C higher than the temperature of the hydrocarbons that have
been cooled down. Such is due to partly evaporation of isoparaffins by
pressure
reduction. To sum up, heat exchange efficiency is very high due to following
reasons:
- direct contact of mediums with different temperatures;
- constant renewal of contact surface due to emergence and evaporation of
reaction products obtaining heat from a warmer acid located beneath;
- sinking of acid globules cooled at the contact surface and replacement
thereof with warmer globules emerging from the bottom.
The reaction products to be recycled are directed by the pump 26 back to the
mixing chamber 1 through the perforated collector 42 and the connecting pipe
3 o 35. The remaining amount of reaction products flows over the baffle plate
into
the accumulation tank 31, from which they are discharged by the pump 41
through the connecting pipe 38 towards a rectification and deisobutanization
unit (not shown) in order to obtain the requested commercial grade alkylate.
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
22
The sulfuric acid to be recycled is withdrawn from the bottom part of the
vessel
28 via the connecting pipe 34 and is pumped back for recycling by the pump 27
as it was described earlier.
As shown in Fig. 3, withdrawal of spent acid for regeneration is provided via
the
connecting pipe 36 connected to the compartment formed between the overflow
baffle plate 29 and the additional baffle 37. Such allows withdrawal of a
lighter
phase of the reaction mixture to regeneration.
The alkylation unit shown in Fig. 3 permits to produce a high quality,
commercial grade alkylate and to reduce the sulfuric acid consumption due to
a rapid emulsion separation in the hydrocyclone, an efficient cooling of the
acid
in the.gas separator and an efficient after-settling process of the commercial
grade reaction products that provides minimum carry-over of acid particles to
the accumulating and roll-out areas.
2 o However, the way of carrying out the method according to the invention is
not
limited to the flow chart given as example only in Fig. 3. As a matter of
fact, Fig.
4 shows another possible way of carrying out the method according to the
invention for sulfuric acid alkylation of isoparaffins by olefins.
Like in the examples shown in Figs. 2 and 3, the hydrocyclone 21 of the unit
shown in Fig. 4 is used to separate the reaction mixture into a hydrocarbon-
containing phase and an acid-containing phase. Such a separation suspends
further chemical conversion in the emulsion and sets the composition of the
reaction products. However, unlike the earlier examples, the hydrocarbon-
containing phase separated within the hydrocyclone 21 is subjected to another
separation into an upper lightweight flow and a bottom heavy weight flow
within
the other hydrocyclone 45 that is located downstream. The upper lightweight
flow exiting from the upper outlet of the hydrocyclone 45 qualifies as
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
23
commercial grade reaction products and is fed via the pressure reduction valve
62 and the gas separator 47, to the settling section 55 of the vessel 48. The
vapor phase at the upper outlet of the gas separator 47 exits above the level
of the overflow baffle plate 50 that determines the liquid level in the
vessel. The
liquid phase separated within the gas separator 47 exits from the same below
that level, e.g. beneath the liquid layer. The bottom heavy weight flow
exiting
from the bottom outlet of the hydrocyclone 45 forms the reaction products to
be
recycled and is fed via the pressure reduction valve 61 and the gas separator
46 to the other settling section 54 of the vessel 48. Vapor and liquid phases
exiting the gas separator 46 are fed into the section 54 at heights similar to
what has been described earlier.
The acid-containing phase collected at the bottom of the hydrocyclone 21 is
separated within the hydrocyclone 43 into an upper lightweight flow and a
bottom heavy weight flow. The upper flow that is enriched by hydrocarbons is
fed to the settling tank of the vessel 48, preferably to the settling section
54, as
reaction products to be recycled. The bottom heavy weight flow exiting from
the
hydrocyclone 43 is fed to a further hydrocyclone, viz. the one numbered 44,
for
further separation. The heavy weight layer exiting from the bottom of
2 0 hydrocyclone 44 is fed to the settiing section 54 of the vessel 48 for
further
utilization as recycled acid, along with the upper lightweight flow exiting
from the
hydrocyclone 43. The upper lightweight flow exiting from the hydrocyclone 44
is discharged from the unit as waste acid.
Several settling areas are formed within the vessel 48. The vapor flows are
collected above the level of the overflow baffle plates 49 and 50. Some of
these
vapors come from the gas separators 46 and 47. Other vapors come from
isobutane evaporation in the settling sections 54 and 55 due to the heat
exchange between the hydrocarbons and the lower warmer layer of acid. All
these vapors are withdrawn via the knockout drums 39 and connecting pipes
located above each of the accumulating tanks 51 and 52. The vapors are
then directed to a compressor for compression, condensation, cooling and
return to recycling as one of the portions of recycled isobutane. Reaction
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
24
products to be recycled are collected in the settling tank 54 formed between
the
baffles 49 and 53. As the level of baffle 49 is 30 to 100 mm lower than the
level
of baffle 53, the reaction products that reach the top edge of the baffle 49,
spill
over it into the accumulating tank 51, from where they are pumped via the
connecting pipe 56 and the pump 27 back to the reactor. Prior to reaching the
settling section 55, the liquid reaction products fed therein are subjected to
a
two-stage acid separation in the hydrocyclones 21 and 45. Therefore, they
practically do not contain any more acid particles. Thus, upon spilling over
the
baffle 50 into the accumulating tank 52, they can be pumped via the connecting
pipe 38 and pump 41 for further treatment, where they require significantly
less
rectification efforts.
Sulfuric acid which forms the heaviest flow, is collected in the bottom part
of the
vessel 48. The so collected acid is completely decontaminated (degased) and
cooled. The acid may freely flow under the baffle 53 since the bottom end of
this baffle 53 is spaced apart from the bottom of the vessel. In the
embodiment
shown in Fig. 4, heat exchange between the acid and hydrocarbons is
intensified due to the introduction of the acid into the layer of hydrocarbons
and
vice versa. In the embodiment shown in Fig. 4, acid is also withdrawn via the
2 o hood 63 with a crosspiece mounted over the connecting pipe 34. This
feature
eliminates funnel formation and suction by acid flow, of hydrocarbons located
under the acid layer.
EXAMPLE
Tests to check the efficiency of the method according to the invention were
carried out in a pilot unit having a structure similar to the one shown in
Fig. 3
and the following particulars:
- diameter of the emulsion chamber 2: 300 mm
- diameter of the reaction chamber 15: 700 mm
- number of nozzles 12: 3
- number of nozzles 19: 9
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
- amount of H2SO4 circulated through the device: 50 m3/per ton of alkylate
- first emulsion flow rate: 2.55 m/s
- second emulsion flow rate: 3.6 m/s
- olefin injection pressure drop: 2.5 kg-force/cm2
5
During the tests, isobutane was fed into the device after having been cooled
down to a temperature of +3.8 C. Recycled reaction products were also fed into
the device after having been separated from the sulfuric acid and then cooled
down to a temperature of +3.2 C. A mixture of hydrocarbons was fed to the
10 emulsion chamber of the reactor in multiple parallel jets. Sulfuric acid
circulating
in the system and separated from the reaction products was fed in the
peripheral annular space of the reactor beneath the diffuser of the emulsion
chamber. Then, 27% of the total olefin feed was injected in wakes through the
nozzles 12 into so prepared emulsion of hydrocarbons in sulfuric acid. The
15 olefin feed represented a mixture of incoming butane-butylenes fraction and
some part of internally recycled isobutane. The composition of the olefin feed
was as follows:
components C3H8 C3H6 1-C4HIo n-C4Hio E C4H8 i-C5H,2 rest of the
components
% vol. 1.1 0.1 74.3 8.3 15.9 0.2 0.1
An interaction process of olefins (essentially butylenes) contained in the
feed
with isobutane took place in the pre-reaction chamber 7 above the nozzles 12.
Such a process was also continued through the nozzles 14 mounted on the
baffle that separates the pre-reaction chamber 7 from the reaction chamber 8.
The injectors 19 for the injection of the balance of the olefin feed were
arranged
below the low end of the circulation pipe 16 and in two tiers over its height.
All
the balance of the feed was injected through the injectors 19 into the
emulsion.
The so-obtained reaction mixture was removed from the reactor through three
connecting pipes located at the bottom part of the reaction chamber, and it
was
directed to the hydrocyclone 21 for separation. The reaction mixture
CA 02408037 2002-11-01
WO 01/94283 PCT/1B01/00962
26
temperature at the outlet of the reactor was +6.5 C. Initial separation of the
reaction mixture from the reactor was provided in the hydrocyclone at a rate
at
supply pipe of 4.7 m/s. Final separation after pressure reduction was obtained
in the horizontal separator vessel divided into one settling tank and one
accumulating tank.
After separation, part of the reaction products was directed for an acid and
alkali wash with a further isolation of alkylate while the other part was
recycled
through the device.
After separation from the hydrocarbons and cooling, the acid was recycled in
the unit with a discharge of part of the waste acid and a replenishment of a
make-up (fresh) acid, in such a way that the average strength of the recycled
acid mixture was maintained at 91-92%.
The so obtained alkylate has the following characteristics:
Results of ASTM D-86 single stage laboratory distillation
Initial boiling Boil-off End boiling MON
10% 50% 90%
Temperature 37 64 108 149 195 93*
in C
* octane number
Of course, numerous modifications could be made to the device and units
disclosed hereinafter without departing from the scope of the invention.