Note: Descriptions are shown in the official language in which they were submitted.
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
1
6-PHENYLPYRROLOPYRIMIDINEDIONE DERIVATIVES
This invention relates to new therapeutically useful pyrrolopyrimidinedione
derivatives, to processes for their preparation and to pharmaceutical
compositions
containing them.
EP 0 480 659 relates to compounds of general formula
Z~ Z2
X1~ ~Z3
i
N
I
Y
wherein each of Z1, Zz and Z3. independently represents: a nitrogen atom, a
group
represented by general formula: =C(Xz)- or a group represented by general
formula:
=C(X3)-. When Zz and Z' represent a group of general formula: =C(Xz)- or a
group of
general formula: =C(X3)-, XZ and X3 may be combined together to form a group
represented by general formula:
O X24 O X25
II I II I
-C-N-G-N
or a group represented by general formula:
X26 O X27 O
1 II I II
-N-C-N-C-
wherein Xz~, XzS, Xzs and Xz' independently represent hydrogen or alkyl of 1
to 4
carbons atomsand Y does not represent'hydrogen; which possess angiotensin-II
receptor- antagonizing activity for the prevention or treatment of
hyperuricemia.
We have now found that certain 6-(disubstituted)phenyl-1,5-dihydropyrrolo[3,2-
d]pyrimidine-2,4-dione and 6-(disubstituted)phenyl-1,7-dihydropyrrolo[2,3-
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
7
d]pyrimidine-2,4-dione derivatives are potent and selective inhibitors of
phosphodiesterase 5 (PDE 5), and have efficacy in the treatment of angina,
hypertension, congestive heart failure, stroke, asthma, male erectile
dysfunction, female
sexual dysfunction, premature labour, dysmenorrhea, BPH, incontinence,
glaucoma and
irritable bowel syndrome.
Accordingly, the present invention provides compounds which are 6-
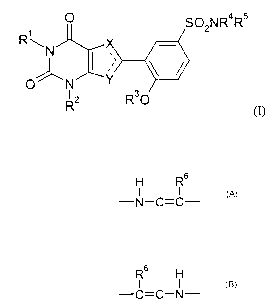
phenylpyrrolopyrimidine derivatives of formula (I):
O SO2N R4R~
R~.N X
\ f
O N
RZ R30
(I)
wherein: -X-C-Y- represents
H R6
I I
-N-C=C-
as in formula (II)
R1 O H S02NRøR5
~N N
I/ \ /
l
O R2 Rs R30
(II)
or -X-C-Y- represents
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
3
R6 H
I I
-C=C-N-
as in formula (III)
O R6 S02NR4R5
R~
\N
\/
O N N
R2 H R30
(III)
R', RZ and R3 each independently represent: a hydrogen atom; an alkyl group
which is unsubstituted or substituted by one or more hydroxy, alkoxy,
alkylthio, amino,
mono- or di-alkylamino, hydroxycarbonyl, alkoxycarbonyl, acylamino, carbamoyl
or
alkylcarbamoyl groups; or a group of formula
-(CH~)n R'
wherein n is an integer from 0 to 4 and R' represents: a cycloalkyl group; a
phenyl
group which may be unsubstituted or substituted by one or more halogen atoms
or alkyl,
hydroxy, alkylenedioxy, alkoxy, amino, mono- or di-alkylamino, nitro, cyano or
trifluoromethyl groups; or a 3 to 7-membered ring comprising from 1 to 4
heteroatoms
selected from nitrogen, oxygen and sulphur, which ring may be unsubstituted or
substituted by one or more halogen atoms or hydroxy, phenyl, alkoxycarbonyl,
amino,
mono-alkylamino, di-alkylamino or hydroxycarbonyl groups or one or more alkyl
groups which may in turn be unsubstituted or substituted by one or more
halogen atoms
or hydroxy, alkoxy, hydroxyalkoxy, phenyl, alkoxycarbonyl, amino, mono- or di-
alkylamino or.hydroxycarbonyl groups,
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
either R~ and R' together with the nitrogen atom to which they are attached
form
a 3 to 7-membered ring comprising a total of from 1 to 4 heteroatoms selected
from
nitrogen, oxygen and sulphur, which ring may be unsubstituted or substituted
by one or
two substituents selected from (a) halogen atoms and hydroxy, oxoalkyl,
carbamoyl,
hydroxycarbonyl, alkoxycarbonyl, amino, mono- and di-alkylamino groups and (b)
alkyl, alkenyl and divalent alkylene groups which may in turn be unsubstituted
or
substituted by one or more hydroxy, alkoxy, hydroxyalkoxy, amino or mono- or
di-
alkylamino groups, or
R4 and RS independently represent a hydrogen atom or an alkyl group which may
be unsubstituted or substituted by one or more hydroxy, alkoxy, alkylthio,
amino,
1 S ' mono- or di-alkylamino groups, or
R'' represents a hydrogen atom or an allryl group and RS represents a group of
formula -(CHz)n R' as defined above,
R6 represents a hydrogen or halogen atom, or a nitro or alkoxycarbonyl group,
or
an alkyl group which is unsubstituted or substituted by one or more hydroxy,
alkoxy,
alkylthio, amino, mono- or di-alkylamino, hydroxycarbonyl, alkoxycarbonyl,
acylamino, carbamoyl or allcylcarbamoyl groups,
or an N-oxide or a pharmaceutically acceptable salt thereof.
The alkyl groups and moieties such as.those present in the alkoxy,
alkylcarbamoyl, mono- or di-alkylamino, carb~amoyl, alkyl, alkylthio,
oxoalkyl,
alkylenedioxy and alkoxycarbamoyl groups mentioned herein unless otherwise
stated
are usually "lower" alkyl, that is containing from 1 to 6 particularly from 1
to 4 carbon
atoms, the hydrocarbon chain being branched or straight. Preferred alkyl
groups, and
where relevant alkyl moieties, include methyl, ethyl, n-propyl, i-propyl, n-
butyl, sec-
butyl and t-butyl.
A said divalent alkylene group is typically a C1-C6 alkylene group.
Preferably, it
is a C1-C~ alkylene group, for example a methylene, ethylene or propylene
group.
The alkenyl groups and moieties are typically C,-C6 alkenyl groups and
moieties, for example CZ-C~ alkenyl groups and moieties.
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 The halogen atoms mentioned in relation to the groups Rø to R' are selected
from
fluorine, chlorine, bromine and iodine and most preferably from bromine,
chlorine and
fluorine atoms.
In substituent groups of formula
-(CHZ)nR'
n may represent 0, l, 2, 3, or 4, preferably 0, l, 2 or 3.
The cycloalkyl group mentioned in relation to the group R' is preferably a
C3_~o
cycloalkyl group, more preferably a C3_, cycloalkyl group such as cyclopropyl,
cyclobutyl, cyclopentyl and cyclohexyl group. The cycloalkyl-alkyl groups
within the
definition -(CH~)n R' preferably include cyclopropylmethylene, cyclopropyi-
ethylene,
cyclopentylmethylene, cyclopentylethylene, cyclohexylmethylene and
cyclohexylethylene.
When R' represents a phenyl group substituted by one or more halogen atoms or
alkyl, hydroxy, alkoxy, amino, mono- or dialkyl amino, nitro, cyano or
trifluoroalkyl
groups, the phenyl ring may be substituted by 1, 2, 3, 4. or S substittients,
preferably 1, 2
or 3 substituents, each being independently selected from the possible
substituents set
out above. That is to say, the phenyl group (attached through its 1-position)
may be
substituted at any of the remaining positions, that is to say the 2, 3, 4, 5
or 6-positions.
A phenyl group having more than one substituent may be substituted at any
combination of positions. For example a phenyl group having two substituents
may be
substituted at the 2 and 3, 2 and 4, 2 and S, 2 and 6, 3 and 4 or 3 and 5
positions. If the
phenyl group is substituted by one or more alkylene dioxy groups then they may
be
present on any adjacent pair of substitutable positions.
When R'represents a 3-7 membered ring in accordance with formula (I), the
ring may be unsaturated or saturated and may represent for example a
piperidyl,
pyrrolidyl, azetidinyl, aziridyl, piperazinyl, morpholinyl, thiomorpholinyl,
pyrrolyl,
imidazolyl, imidazolidinyl, pyrazolinyl, indolinyl, isoindolinyl, pyridyl,
pyrazinyl,
pyrimidinyl, pyridazinyl, indolizinyl, isoindolyl, indolyl, indazolyl,
purinyl,
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
6
quinolizinyl, isoquinolyl, quinolyl, phthalazinyl, naphthyridinyl,
quinoxalinyl,
quinazolinyl, cinnolinyl, pteridinyl, quinuclidinyl, triazolyl, pyrazolyl,
triazolyl,
tetrazolyl or thienyl group, which group may be substituted or unsubstituted.
Typically,
the group is unsubstituted or substituted by one or more, for example 1, 2, 3
or 4,
substituents selected from C,-Ca alkyl groups, for example methyl groups.
In preferred compounds of the invention R', R' and,R3 each independently
represent hydrogen or an unsubstituted alkyl group selected from methyl,
ethyl, n-
propyl, i-propyl, n-butyl, sec-butyl and t-butyl. In the most preferred
compounds of the
invention R' is a methyl group, RZ is an n-propyl or i-butyl group (preferably
an n-
propyl group), and R3 is an ethyl or n-propyl group.
For compounds of the invention wherein R4 and R' together with the nitrogen
atom to which they axe attached form a ring, the ring may be saturated or
unsaturated for
example a piperidyl, pyrrolidyl, azetidinyl, aziridyl, piperazinyl;
morpholinyl,
thiomorpholinyl, pyrrolyl, pyrazolyl, imadazolyl, imadazolidinyl, pyrazolinyl,
diazacycloheptyl (diazapanyl), indolinyl or isoindolinyl group, said group
being
substituted or unsubstituted. Typically, when R4 and R5, together with the
nitrogen
atom to which they are attached, form a said 3- to 7- membered ring, the ring
is
unsubstituted or substituted by one or two halogen atoms or hydroxy, oxoalkyl,
carbamoyl, hydroxycarbonyl, alkoxycarbonyl, amino, mono- or di-alkylamino
groups or
one or two alkyl groups which may in ttun be unsubstituted or substituted by
one or
more hydroxy, alkoxy, hydroxyalkoxy, amino or mono- or di-alkylamino groups.
In one group of preferred compounds of the invention the ring formed by Rø, RS
and the nitrogen atom to which they are attached is a substituted or
unsubstituted 4, 5, 6
or 7 membered ring such as a piperidyl, piperazinyl, morpholinyl,
diazacycloheptyl,
azacyclobutyl, pyrrolidinyl or pyrazolyl group. More typically, in this group
of
preferred compounds, the ring is a substituted or unsubstituted 5, 6 or 7
membered ring
SllCh as a piperidyl, piperazinyl, moipholinyl, diazacycloheptyl, pyrrolidinyl
or
pyrazolyl group. If the ring is substituted then the substituents axe
preferably selected
from (a) hydroxy, carboxy, alkoxy, carbamoyl, carbaldehyde, alkoxycarbonyl,
amino,
mono- and di-alkylamino groups and (b) alkyl, alkenyl and divalent alkylene
groups
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
7
having up to 6 C atoms (preferably up to 3 C atoms), which groups may be
unsubstituted or substituted by hydroxy, methoxy, hydroxymethoxy or di-
alkylamino
groups. More typically, the substituents are selected from hydroxy, carboxy,
alkoxy,
carbamoyl, carbaldehyde, alkoxycarbonyl, amino, mono- or di-allcylamino groups
and
C1_6, preferably C,_,, alkyl groups which may be unsubstituted or substituted
by
hydroxy, methoxy, hydroxymethoxy or di-alkylamino groups.
When the ring is substituted by a divalent alkylene group, the divalent
alkylene
substiW ent may be attached to adjacent atoms on the ring or to atoms on the
ring which
are not adjacent. Preferably, when the ring is substituted by a divalent
alkylene group it
is a piperazinyl group.
Preferably, R~, RS and the nitrogen atom to which they are attached form a 5,
6 or
7 membered ring comprising a total of from 1 to 3 heteroatoms selected from
nitrogen,
oxygen or sulphur, which ring may be unsubstituted or substituted by one or
more
groups selected from hydroxy, carboxy, alkoxy, carbamoyl, carbaldehyde,
alkoxycarbonyl, amino, mono- or di-alkylamino groups and alkyl groups which
may be
unsubstituted or substituted by one or more hydroxy, methoxy, hydroxymethoxy
or di-
alkylamino groups.
Most preferably the ring is a 4-hydroxypiperidyl, 3-carbamoylpiperidyl,
4-carbamoylpiperidyl, 3-carboxypiperidyl, ~-carboxypiperidyl,
3-ethoxycarbonylpiperidyl, 4-ethoxycarbonylpiperidyl, 4-
dimethylaminopiperidyl, 4-(2-
dimethylaminoethyl)-4-methylpiperidyl, piperazinyl, 3-methylpiperazinyl,
4-methylpiperazinyl, 2,5-dimethylpiperazinyl, 3,5-dimethylpiperazinyl,
4-ethylpiperazinyl, 4-propylpiperazinyl, 4-hydroxyethylpiperazinyl, 4-methoxy-
ethylpiperazinyl, 4-ethoxyethylpiperazinyl, 4-hydroxypropylpiperazinyl,
4-ethoxycarbonylpiperazinyl, 4-ethoxycarbonyl-methylpiperazinyl,
4-propenylpiperazinyl, 4-(2-hydroxyethoxy)ethylpiperazinyl,
hexahydropyrrolo[1,2-
a]pyrazinyl, 3-methylhexahydropyrrolo[1,2-a]pyrazinyl,
7-hydroxyhexahydropyrrolo[1,2-a]pyrazinyl, 2,5-diazabicyclo[2.2.1].heptanyl,
morpholinyl, 4-methyl-1,4-diazacycloheptyl, 4-(2-hydroxyethyl)-1,4-
diazacycloheptyl,
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
8
3-(dimethylamino)-1-azacyclobutyl, 2-hydroxy-carbonylpyrrolidinyl, 4-
piperazinecarbaldehyde, 2-methoxycarbonylpyrrolidinyl or aminopyrazolyl group.
Especially preferred are a piperazinyl, 4-methylpiperazinyl, 4-ethyl-
piperazinyl,
morpholinyl, 4-(2-hydroxyethyl)piperazinyl, 4-(3-hydroxypropyl)piperazinyl, 4-
ethoxycarbonyl piperazinyl, 4-methyl-1,4-diazacycloheptyl, 4-(2-methoxy-
ethyl)piperazinyl or 4-piperazinecarbaldehyde group.
In one preferred group of compounds of the invention R' is a group of formula
-{CH~)nR'
wherein n is 0, l, 2 or 3 and R' is a group R8 which represents a substituted
or
unsubstituted morpholinyl, pyridyl, piperidyl, piperazinyl, quinuclidinyl,
triazolyl or
tetrazolyl group. Substituents can be, for example, alkyl, hydroxyallcyl,
alkoxyalkyl,
oxoalkyl, alkoxyalkyl, carbamoyl and alkylcarbamoyl groups.
Most preferably R$ represents an unsubstituted morpholinyl, pyridyl or
piperidyl
group, a piperidyl group substituted by 1, 2, 3 or 4 methyl groups, or a
piperazinyl or
quinuclidinyl group substituted at a nitrogen atom by a methyl, ethyl, 2-
hydroxyethyl,
2-methoxyethyl, propyl, 3-hydroxypropyl, 3-methoxypropyl, carbaldehyde or
ethoxycarbonyl group. More typically, in this preferred embodiment, R8
represents an
unsubstituted morpholinyl, pyridyl, piperidyl group or a piperazinyl or
quinuclidinyl
group substituted at a nitrogen atom by a methyl, ethyl, 2-hydroxyethyl, 2-
methoxyethyl, propyl, 3-hydroxypropyl, 3-methoxypropyl, carbaldehyde or
ethoxycarbonyl group.
In compounds of the invention wherein R4 and RS do not form a ring and RS is
not a group of formula
-(CHz)nR~
R4 and RS are preferably independently selected from a hydrogen atom, methyl
group, ethyl group or a C,_6 alkyl group substituted by one or more halogen
atoms or
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
9
hydroxy, alkoxy, alkylthio, oxo, hydroxycarbonyl, alkoxycarbonyl, carbamoyl,
alkylcarbamoyl or mono- or di-alkylamino groups.
In the most preferred compounds of the invention wherein R4 and RS together do
not form a ring, Ra represents a hydrogen atom or a methyl or 2-hydroxyethyl
group.
In the most preferred compounds of the invention wherein Ra and RS together do
not form a ring, RS represents a 2-hydroxyethyl, 2-dimethylaminoethyl, 2-
pyridylethyl,
N-piperidylethyl, 2,2,6,6-tetramethylpiperidin-4-yl, N-morpholinylethyl, N-
morpholinylpropyl or N-methyl-N-piperazinyl group. More typically, in this
preferred
embodiment, R' represents a 2-hydroxyethyl, 2-dimethylaminoethyl, 2-
pyridylethyl, N-
piperidylethyl, N-morpholinylethyl, N-morpholinylpropyl or N-methyl-N-
piperazinyl
group.
In preferred compounds of the invention R6 represents a hydrogen atom,
fluorine
atom, chlorine atom, bromine atom, iodine atom or a methyl group which is
unsubstituted or substituted by a dimethylamino or methoxy group. More
typically, in
these preferred compounds, R6 represents a hydrogen atom, fluorine atom,
chlorine
atom or a methyl group.
In compounds of the invention wherein -X-C-Y- represents
R6 H
I I
-C-C-N-
(as in formula (ITI) above) R6 preferably represents a hydrogen atom or a
dialkylaminoalkyl group.
Particular individual compounds of the invention include:
6-[2-Ethoxy-5-(4-methylpiperazine-1-sulphonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
6-[2-Ethoxy-5-(piperazine-1-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-(2-Ethoxy-5-(morpholine-4-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
N-{2-Dirnethylaminoethyl)-4-ethoxy-3-(methyldioxopropyl-2,3,4,5-tetrahydro-
1H-pyrrolo[3,2-d]pyrimidin-6-yl)benzene sulfonamide
6-[2-Ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-propyl-1, 5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
6-[2-Ethoxy-5-(4-methyl-[ 1,4]diazepane-1-sulfonyl)phenyl]-3-methyl-1-propyl-
10 1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
4-Ethoxy-3-(3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1 H-pyrrolo [3,2-
d]pyrimidin-6-yl)-N-(2-piperidin-1-yl-ethyl)benzenesulfonamide
4-Ethoxy-3-{3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-C-yl)-N-(2-morpholin-4-yl-ethyl)benzenesulfonamide
6-{2-Ethoxy-5-[4-{2-hydroxyethyl)piperazine-1-sulfonyl]phenyl}-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
4-Ethoxy-3-(3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-N-(3-morpholin-4-yl-propyl)benzenesulfonamide
6- ~2-Ethoxy-5-[4-(3-hydroxypropyl)piperazine-1-sulfonyl]phenyl}-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-Methyl-6-[5-(4-methylpiperazine-1-sulphonyl)-2-propoxyphenyl]-1-propyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[5-(4-Ethylpiperazine-1-sulphonyl)-2-propoxyphenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
6-{5-[4-{2-Hydroxyethyl)piperazine-1-sulphonyl]-2-propoxyphenyl}-3-methyl-
1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-Methyl-6-[5-(piperazine-1-sulfonyl)-2-propoxyphenyl]-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-Nlethyl-6-[5-(morpholine-4-sulfonyl)-2-propoxyphenyl]-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
N-(2-Dimethylaminoethyl)-3-(methyldioxopropyl-2,3,4,5-tetrahydro-1H-
pyrrolo[3,2-d]pyrimidin-6-yl)-4-propoxybenzenesulfonamide
N,N-B is-{2-hydroxyethyl)-3-(3 -methyl-2, 4-di oxo-1-propyl-2, 3 , 4, 5-
tetrahydro-
1H-pyrrolo[3,2-d]pyrimidin-6-yl)-4-propoxybenzenesuifonamide
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
11
3-Methyl-5-[5-(4-methyl-[1,4]diazepane-1-sulfonyl)-2-propoxypheny1]-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-{3-Methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-
6-yl)-N-(4-methylpiperazin-1-yl)-4-propoxybenzenesulfonamide
3-(3-Methyl-2,4-dioxo-1-propyl-2,3,4, 5-tetrahydro-1H-pyrrolo [3,2-d]pyrimidin-
6-yl)-4-propoxy-N-(2-pyridin-2-yl-ethyl)benzenesulfonamide
3-(3-Methyl-2,4-dioxo-1-propyl-2,3,4, 5-tetrahydro-1 H-pyrrolo [3,2-
d]pyrimidin-
6-yl)-N-(2-piperidin-1-ylethyl-4- propoxybenzenesulfonamide
3-(3-Methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-
6-yl)-N-(2-morpholin-4-yl-ethyl)-4-propoxybenzenesulfonamide
3-{3-Methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-
6-yl)-N-(3-morpholin-4-ylpropyl)-4-propoxybenzenesulfonamide
6- ~5-[4-(3-Hydroxypropyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-3-methyl-
1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-(piperazine-1-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-5-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6- f 2-ethoxy-5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]phenyl}-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-(Chloromethyldioxopropyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-
yl)-N-(2-dimethylaminoethyl)-4-ethoxybenzenesulfonamide
7-Chloro-6-[2-ethoxy-5-(4-methyl-[1,4]diazepane-1-sulfonyl)phenyl]-3-methyl-
1-propyl-1,5-dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
3-{7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-(2-morpholin-4-ylethyl)benzenesulfonamide
7-Chloro-3-methyl-6-[5-(4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
12
7-Chloro-6-[5-{4-ethylpiperazine-1-sulfonyl)-2-propoxyphenyl]-3-methyl-1-
propyl-1, 5-dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
7-Chloro-6- ~ 5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6-[2-ethoxy-5-(morpholine-4-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
4-[3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxybenzenesulfonyl]piperazine-1-carbaldehyde
7-Bromo-6-[2-ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6-[2-ethoxy-5-(4-methyl-[1,4]diazepane-1-sulfonyl)phenyl]-3-methyl-
1-propyl-1, 5-dihydropyrro to [3,2-d] pyrimi dine-2, 4-dione
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-(4-methylpiperazin-1-yl)benzenesulfonamide
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-(2-pyridin-2-ylethyl)benzenesulfonamide
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1 H-pyrrolo[3,2-
d]pyrimidin-b-yl)-4-ethoxy-N-(2-piperidin-1-ylethyl)benzenesulfonamide
7-Bromo-6-{2-ethoxy-5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]phenyl}-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo [3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-{2.-morpholin-4-ylethyl)benzenesulfonamide
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-(3-morpholin-4-ylpropyl)benzenesulfonamide
7-Bromo-6- {2-ethoxy-5-[4-(3-hydroxypropyl)piperazine-1-sulfonyl]phenyl -3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6- {2-ethoxy-5-[4-(2-methoxyethyl)piperazine-1-sulfonyl]phenyl'-3-
methyl-1-propyl-1, 5-dihydropyrro to [3,2-d]pyrimidine-2,4-dione
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
13
7-Bromo-3-methyl-b-[5-{morpholine-4-sulfonyl)-2-propoxyphenyl]-1-propyl-
1,5-dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
7-Bromo-3-methyl-6-[5-{4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
4-[3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-propoxybenzenesulfonyl]piperazine-1-carbaldehyde
7-.Bromo-6-[5-(4-ethylpiperazine-1-sulfonyl)-2-propoxyphenyl]-3-methyl-1-
propyl-1,5-dihydro-pyrrolo [3,2-d]pyrimidine-2,4-dione
7-Bromo-3-methyl-6-[5-{4-methyl-[1,4]diazepane-1-sulfonyl)-2-
propoxyphenyl]-1-propyl-1,5-dihydro-pyrrolo[3,2-d]pyrimidine-2,4-dione
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-N-(2-morpholin-4-ylethyl)-4-propoxybenzenesulfonamide
7-Bromo-b- {5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-3-
methyl-1-propyl-1,5-dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
3-{7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo [3,2-
d]pyrimidin-6-yl)-N-(3-morpholin-4-ylpropyl)-4-propoxybenzenesulfonamide
7-Bromo-6- {5-[4-(3-hydroxypropyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-
3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-b-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-1-isobutyl-3-
methyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-{4-ethylpiperazine-1-sulfonyl)phenyl]-1-isobutyl-3-
methyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6- {2-ethoxy-5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]phenyl} -1-
isobutyl-3-methyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-(piperazine-1-sulfonyl)phenyl]-1-isobutyl-3-methyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-{4-methyl-4-oxypiperazine-1-sulfonyl)pheny1]-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-{7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1 H-pyrrolo [3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-(2-methylaminoethyl)benzenesulfonamide
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
14
7-Chloro-6- f2-ethoxy-5-[4-(3-hydroxypropyl)piperazine-1-sulfonyl]phenyl}-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
3-(7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-{2-pyridin-2-ylethyl)benzenesulfonamide
7-Chloro-b- ~2-ethoxy-5-[4-(2-ethoxyethyl)piperazine-1-sulfonyl]phenyl }-3-
' methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[5-(4-Allylpiperazine-1-sulfonyl)-2-ethoxyphenyl]-7-chloro-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-(4-isopropylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-~2-ethoxy-5-[(S)-{hexahydropyrrolo[1,2-a]pyrazin-2-yl)sulfonyl]-
phenyl } -3-methyl-1-propyl-1, 5-dihydropyrro to [3 , 2-d]pyrimi dine-2, 4-
dione
7-Chloro-6-~2-ethoxy-5-[(R)-(hexahydropyrrolo[1,2-a]pyrazin-2-yl)sulfonyl]-
phenyl}-3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-({3R,8aS)-3-methylhexahydro pyrrolo[1,2-a]pyrazine-2-
sulfonyl)phenyl]-3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-
dione
7-Chloro-b-[2-ethoxy-5-((7R,8aS)-7-hydroxyhexahydro pyrrolo[1,2-a]pyrazine-
2-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-
dione
7-Chloro-6-[2-ethoxy-5-({ 1 S,4S)-5-methyl-2,5-diazabicyclo[2.2.1 ]heptane-2-
sulfonyl)phenyl]-3-methyl-1-prop y1-1, 5-dihydropyrrolo [3,2-d]p yrimidine-2,
4-dione
6-[2-Ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-7-iodo-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[5-(3-Dimethylaminoazetidine-1-sulfonyl)-2-ethoxy phenyl]-7-iodo-3-methyl-
1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[2-Ethoxy-5-(4-methyl-[1,4]diazepane-1-sulfonyl) phenyl]-7-iodo-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[2 -Ethoxy-5-(4-ethylpip erazine-1-sulfonyl)phenyl]-7-io do-3 -methyl-1-
propyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[5-(4-Allylpiperazine-1-sulfonyl)-2-ethoxyphenyl]-7-iodo-3-methyl-1-propyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 6-[2-Ethoxy-5-{4-isopropylpiperazine-1-sulfonyl)phenyl]-7-iodo-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6- f 2-Ethoxy-5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl] phenyl]-7-iodo-3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-{2-Ethoxy-5-[4-(2-methoxyethyl)piperazine-1-sulfonyl] phenyl}-7-iodo-3-
10 methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[2-Ethoxy-5-(piperazine-1-sulfonyl)phenyl]-7-iodo-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
4-Ethoxy-3-(7-iodo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-
pyrrolo[3,2-d]pyrimidin-6-yl)-N-(2,2,6,6-tetramethylpiperidin-4-
yl)benzenesulfonamide
15 6-~2-Ethoxy-5-[4-(2-hydroxyethyl)[1,4]diazepane-1-sulfonyl]phenyl}-7-iodo-3-
methyl-1-propyl-1,5-dihydro pyrrolo[3,2-d]pyrimidine-2,4-dione
6-[2-Ethoxy-5-((15,45)-5-methyl-2,5-diazabicyclo [2.2.1]heptane-2-
sulfonyl)phenyl]-7-iodo-3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-
2,4-
dione
4-[3-(3-Methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-1H-pyrrolo[2,3-
d]pyrimidin-6-yl)-4-propoxybenzenesulfonyl] piperazine-1-carboxylic acid ethyl
ester
3-Methyl-6-[5-(piperazine-1-sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
3-Methyl-6-[5-(morpholine-4-sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
3-lVlethyl-6-[5-(4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
5-Dimethylaminomethyl-3-methyl-b-[5-{4-methylpiperazine-1-
sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-dihydropyrrolo [2,3-d]pyrimidine-2,4-
dione
3-Methyl-6-[2-propoxy-5-(4-propylpiperazine-1-sulfonyl) phenyl]-1-propyl-1,7-
dihydropyrrolo [2, 3 -d]pyrimidine-2, 4-dione
4-[3-(5-Dimethylaminomethyl-3-methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-
1H-pyrrolo[2,3-d]pyrimidin-6-yl)-4-propoxy benzenesulfonyl]piperazine-1-
carboxylic
acid ethyl ester
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
16
5-Dimethylaminomethyl-3-methyl-6-[S-(morpholine-4-sulfonyl)-2-
propoxyphenyl]-1-propyl-1,7-dihydropyrrolo[2,3-d] pyrimidine-2,4-dione
4-[3-{5-Methoxymethyl-3-methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-1H-
pyrrolo[2,3-d]pyrirnidin-6-yl)-4-propoxy benzenesulfonyl]piperazine-1-
carboxylic acid
ethyl ester
Of outstanding interest are:
3-Methyl-6-[5-(4-methylpiperazine-1-sulphonyl)-2-propoxyphenyl]-1-propyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
6-[5-(4-Ethylpiperazine-1-sulphonyl)-2-propoxyphenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-3-methyl-6-[5-(4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-
propyl-1, 5 -dihydropyrro to [3,2-d]pyrimidine-2 ,4-dione
7-Chloro-6-[5-(4-ethylpiperazine-1-sulfonyl)-2-propoxyphenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Chloro-6- ~5-[4-{2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-3-
methyl-1-propyl-1, 5-dihydropyrrolo [3, 2 -d]pyrimidine-2, 4-dione
7-Bromo-6- ~2-ethoxy-5-[4-(3-hydroxypropyl)piperazine-1-sulfonyl]phenyl}-3-
methyl-1-propyl-1, 5-dihydropyrro to [ 3,2-d]pyrimidine-2, 4-dione
7-Bromo-3-methyl-6-[5-{4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6- { 5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl } -3-
methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
7-Bromo-6- {5-[4-{3-hydroxypropyl)piperazine-1-sulfonyl]-2-propoxyphenyl} -
3-methyl-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione.
According to a further feature of the present invention, the 6-phenyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione derivatives of general formula (II)
are
prepared by reaction of the corresponding sulphonyl chloride of formula (1V):
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
17
'-' H S02C!
RAN N
\/
0 RZ Rs Ra
(IV)
(wherein R', R'-, R' and R6 are as hereinbefore defined) and the corresponding
amine
(V):
R~
HN
5
R (V)
(wherein R'' and R' are as hereinbefore defined). The reaction is carried out
in an
organic solvent, preferably a polar aprotic organic solvent such as dioxane,
methylene
chloride or tetrahydroW ran, at a temperature from 10°C to 40°C
and in the presence of
an organic base, preferably an amine base such as triethylamine or polymer
supported
morpholine. The thus obtained 6-phenyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-
dione
derivative is then isolated by the usual method known in the ark.
In the case that R6 is hydrogen, the sulphonyl chloride (IV) is obtained from
the
corresponding compound of formula (VI):
O H
R~
N N
~/ \ /
O N
R2 Rs
(VI)
(wherein R', Rz and R3 are as hereinbefore defined), by reaction with an
excess of
chlorosulphvnic acid and optionally thionyl chloride, preferably under a
nitrogen
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
18
atmosphere and at a temperature from -5°C to 10°C and where the
solvent is the same
chlorosulphonic acid.
In the case that R6 is a chlorine atom, the corresponding sulphonyl chloride
(IV)
is obtained from the corresponding compound of formula (VI) by reaction with
an
mixture of chlorosulphonic acid and sulphuryl chloride, preferably under a
nitrogen
atmosphere and at a temperature from -5°C to 10°C and where the
solvent is the same
chlorosulphonic acid.
In the case that R6 is a bromine atom, the desired sulphonyl chloride (IV) is
obtained from the corresponding sulphonyl chloride (IV) where R6 is a hydrogen
atom
by reaction with bromine in glacial acetic acid at room temperature.
Other substitutions at R6 can be introduced by reaction of the corresponding
compound of general formula (VI), (IV) or (II, R6 = H) or a protected version
of them,
with an appropiate electrophile.
The 6-phenyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione derivatives of
general formula (VI) can be prepared by reaction of the corresponding 6-methyl-
5-
nitrouracils (VII):
O O
II+
RAN N~O_
O N CH3
R2
(VII)
(wherein R' and R' are as hereinbefore defined), and the corresponding
benzaldehydes
(VIII):
O H
O R3
(VIZI~
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
19
{wherein R' is as hereinbefore defined), followed by reductive cyclization of
the
resulting 5-nitro-6-styryluracils by methods known per se, e.g. C. E. Muller
et al., J.
Med. Chem. 1994, 37, 1526-1534 and references cited therein.
a
According to a further feature of the present invention, the 6-phenyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione derivatives of general formula (III)
are
prepared by condensation of the corresponding 6-aminouracil of formula (IX):
O
R'
\'N
O N NH2
R2
(IX)
(wherein R' and RZ are as hereinbefore defined), with the corresponding
bromoacetophenones of formula (X):
O
Br ~ S02NR4R5
Rs0 (X)
(wherein R', Rø and RS are as hereinbefore defined), by methods known per se,
e.g. C. W. Noell et al., J. Heterocycl. Chem. 1964, l, 34-41, and H. Ogura et
al., Chem.
Pharm. Bull. 1972, 6, 404-40~.
Other substitutions at R6 can be introduced by subsequent reaction of the
corresponding compound of general formula (III) or a protected version of it,
with the
corresponding electrophile, as in Example 90. When R6 is a dimethylaminomethyl
group, substitution of the dimethylamino moiety by nucleophiles can take place
by
reaction in the presence of methyl iodide.
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 The 6-alninouracils of general formula (IX) can be prepared from the
corresponding N,N'-disubstituted ureas by methods known per se, e.g. V.
Papesch et
al., J. Org. Chem. 1951, 16, 1879-90.
The bromoacetophenones (~) can be prepared from the corresponding 2-
alkoxyacetophenones (XI):
O
H3C ~ \
Rs0
(XI)
(wherein R3 is as hereinbefore defined), by ~chlorosulphonyiation, reaction
with the
corresponding amine (V):
1S
R~
HN
.Rs (u)
and further bromination of the resulting compound by methods known per se.
The 2-alkoxyacetophenones of general formula (XI) can be prepared from 2-
hydroxyacetophenone by methods known per se, e.g. M. Covello et al., Farmaco,
Sci.
Ed. 1964, 19, 675.
When the defined groups R' to RS are susceptible to chemical reaction under
the
conditions of the hereinbefore described processes or are incompatible with
said
processes, alternative processes can be readily carried out utilising organic
synthetic
chemistry methods to, for example, protect functional groups and finally
eliminate
protecting groups.
The 6-phenyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione and 6-phenyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione derivatives of formula (I) can be
converted
by methods known per se into pharmaceutically acceptable salts or N-oxides.
Preferred
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
21
salts are acid addition salts obtainable by treatment with organic or
inorganic acids such
as fumaric, tartaric, succinic or hydrochloric acid. Also 6-phenyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione and 6-phenyl-1,7-dihydropyrrolo[2,3-
d]pyrimidine-2,4-dione derivatives of formula (I) in which there is the
presence of an
acidic group may be converted into pharmacologically acceptable salts by
reaction with
an alkali metal hydroxide or an organic base such as sodium or potassium
hydroxide.
The acid or alkali addition salts so formed may be interchanged with suitable
pharmaceutically acceptable counter ions using processes known per se.
When the compound of the invention is an N-oxide, the N-oxide group is
preferably in the moiety NR4R'. Preferably, when the compound of the invention
is an
N-oxide, the moiety NR4R5 is a piperazinyl group and the N-oxide is present at
the 4- N
atom.
The cyclic GMP specific phosphodiesterase (PDE 5) was isolated from human
platelet lysates by ion exchange chromatography using a Mono-Q column. The
enzyme
activity was determined using 0.25 mM [3H]-cyclic GMP as substrate. The
purification
of the enzyme and the assessment of the PDE 5 inhibitory activity of our
compounds
were performed essentially as described by Gristwood et al., Br. J. Pharmacol.
1992,
105, 985-991.
The results are shown in Table 1.
TABLE 1
Example ICSO PDES (nM)
12 14
13 8 .
27 3.4
3 3 4.2
34 3.6
3.4
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
22
47 9.2
50 4.5
55 6.6
57 4
It can be seen from Table 1 that the compounds of formula (I) are potent
inhibitors of cyclic GMP specific phosphodiesterase (PDE 5). Preferred 6-
phenyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione and 6-phenyl-1,7-dihydropyrrolo[2,3-
d]pyrimidine-2,4-dione derivatives of the invention possess an ICso value for
the
inhibition of PDE 5 (determined as defined above) of less than 30 nM,
preferably less
than 15 nM and most preferably less than 10 nM. The 6-phenyl-1,5-
dihydropyrrolo[3,2-
d]pyrimidine-2,4-dione and 6-phenyl-1,7-dihydropyrrolo[2,3-d]pyrimidine-2,4-
dione
derivatives of the invention are useful in the treatment, including
preventative treatment,
of any disease or condition which is mediated by or associated with cyclic GMP
specific
phosphodiesterase (PDE 5) activity including stable, unstable and variant
angina,
hypertension, pulmonary hypertension, congestive heart failure, renal failure,
atherosclerosis, conditions of reduced blood vessel potency, peripheral
vascular disease,
vascular disorders (e.g. Raynaud's disease), stroke, bronchitis, chronic
asthma, allergic
asthma, allergic rhinitis, glaucoma, male erectile dysfunction, female sexual
dysfunction
and diseases characterised by disorders of gut motility, e.g. irritable bowel
syndrome.
In addition to their principle activity (the inhibition of cyclic GMP specific
phosphodiesterase (PDE 5)) the compounds of the invention may also show
activity
in the inhibition of other phosphodiesterases, in particular
phosphodiesterases l and 2,
thus having a synergistic effect in raising cyclic nucleotide levels.
Accordingly, the 6-phenyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione and
6-phenyl-1,7-dihydropyrrolo[2,3-d]pyrimidine-2,4-dione derivatives of the
invention
and pharmaceutically acceptable salts thereof, and pharmaceutical compositions
comprising such compound andlor salts thereof, may be used in a method of
treatment
of disorders of the hmnan body which comprises administering to a patient
requiring
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
23
such treatment an effective amount of a 6-phenyl-1,5-dihydropyrrolo[3,2-
d]pyrimidine-
2,4-dione and 6-phenyl-1,7-dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
derivative of the
invention or a pharmaceutically acceptable salt thereof
The present invention also provides pharmaceutical compositions which
comprise, as an active ingredient, at least a 6-phenyl-1,5-dihydropyrrolo[3,2-
d]pyrimidine-2,4-dione and 6-phenyl-1,7-dihydropyrrolo[2,3-d]pyrimidine-2,4-
dione
derivative of formula (I) or a pharmaceutically acceptable salt thereof in
association
with a pharmaceutically acceptable excipient such as a carrier or diluent. The
active
ingredient may comprise 0.001% to 99% by weight, preferably 0.01% to 90% by
weight, of the composition depending upon the nature of the formulation and
whether
further dilution is to be made prior to application. Preferably the
compositions are made
up in a form suitable for oral, topical, nasal, rectal, percutaneous or inj
ectable
administration.
The pharmaceutically acceptable excipients which are admixed with the active
compound, or salts of such compound, to form the compositions of this
invention are
well-known per se and the actual excipients used depend inter alia on the
intended
method of administering the compositions.
Compositions of this invention are preferably adapted for injectable and per
os
administration. In this case, the compositions for oral administration may
take the form
of tablets, retard tablets, sublingual tablets, capsules, inhalation aerosols,
inhalation
solutions, dry powder inhalation, 6r liquid preparations, such as mixtures,
elixirs, syrups
or suspensions, all containing the compound of the invention; such
preparations may be
made by methods well-known in the art.
The diluents which may be used in the preparation of the compositions include
those liquid and solid diluents which are compatible with the active
ingredient, together
with colouring or flavouring agents, if desired. Tablets or capsules may
conveniently
contain between 2 and 500 mg of active ingredient or the equivalent amount of
a salt
thereof.
The liquid composition adapted for oral use may be in the form of solutions or
suspensions. The solutions may be aqueous solutions of a soluble salt or other
derivative
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
24
of the active compound in association with, for example, sucrose to form a
syrup. The
suspensions may comprise an insoluble active compound of the invention or a
pharmaceutically acceptable salt thereof in association with water, together
with a
suspending agent or flavouring agent.
Compositions for parenteral injection may be prepared from soluble salts,
which
may or may not be freeze-dried and which may be dissolved in pyrogen free
aqueous
media or other appropriate.parenteral injection fluid.
Effective doses are normally in the range of 10-600 mg of active ingredient
per
day. Daily dosage may be administered in,one or more treatments, preferably
from 1 to
4 treatments, per day.
The syntheses of the compounds of the invention and of the intermediates for
use therein are illustrated by the following Examples (including Preparation
Examples
(Preparations 1-16)) which do not limit the scope of the invention in any way.
'H Nuclear Magnetic Resonance Spectra were recorded on a Varian Gemini 300
spectrometer. Low Resolution Mass Spectra (m/z) were recorded on a Micromass
ZMD
mass spectrometer using ESI ionization. Melting points were recorded using a
Perkin
Elmer DSC-7 apparatus. The chromatographic separations were obtained using a
Waters
2690 system equipped with a Symmetry C18 (2.1 x 10 mm, 3.5 mM) column. The
mobile phase was formic acid (0.4 mL), ammonia (0.1 mL), methanol (500 mL) and
acetonitrile (500 mL) (B) and formic acid (0.46 mL), ammonia (0.115 mL) and
water
(1000 mL) (A): initially from 0% to 95% of B in 20 min, and then 4 min. with
95% of
B. The reequilibration time between two injections was 5 min. The flow rate
was 0.4
mL/min. The inj ection volume was 5 ~,L. Diode array chromatograms were
collected at
210 nM.
PREPARATION EXAMPLES
PREPARATION 1
6-(2 -Ethoxyphenyl)-3 -methyl-1-propyl-1, 5-dihydropyrro to
[3,2-d~pyrimidine-2,4-dione
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 a) A mixture of 3,6-dimethyl-5-nitro-1-propyl-1H-pyrimidine-2,4-dione (6.25
g,
27.5 mmol), 2-ethoxybenzaldehyde (4.24 mL, 30.3 mmol), piperidine (2.83 mL,
28.6
mmol) and 3~ molecular sieves (8.75 g) in ethanol (125 mL) was refluxed for 5
hours.
The resulting suspension was filtered and the filtrate was evaporated under
reduced
pressure. The residue was triturated with isopropyl ether and the precipitate
collected by
10 filtration and dried under vacuum to yield 6-[(E)-2-(2-ethoxyphenyl)vinyl]-
3-methyl-5-
nitro-1-propyl-1H-pyrimidine-2,4-dione (7.42 g, 75%) as a yellow solid.
b) To a stirred solution of the above compound (7.42 g, 20.6 mmol) in formic
acid (185 mL) was slowly added sodium dithionite (21.I g, 103 mmol) and the
mixture
was refluxed overnight. The resulting mixture was cooled to room temperature
and
IS water was added (500 mL). The precipitate was collected by filtration and
washed with
water, then dried under vacuum to yield 6-(2-ethoxyphenyl)-3-methyl-1-propyl-
1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione (4.82 g, 72%) as a white solid.
8(CDCl3): I.OS (t, 3H), 1.62 (t, 3H), 1.83 (m, 2H), 3.45 (s, 3H), 3.98 (t,
2H),
4.28 (q, 2H), 6.36 (s, 1H), 7.05 (m, 2H), 7.37 (t, 1H), 7.73 (d, 1H),10.41
(bs, 1H).
20 '
PREPARATION 2
4-Ethoxy-3-(3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)benzenesulphonyi chloride
The title compound ofPreparation 1 (2 g, 6.12 mmol) was added portionwise to
25 a mixture of chlorosulphonic acid (10 mL) and thionyl chloride (1 mL) and
stirred at
0° C for 45 minutes. The reaction mixture was carefully poured into
stirred ice-water
and the aqueous suspension was partitioned between dichloromethane and brine,
then
the organic phase was separated, washed with water, dried (MgSO~) and
evaporated
under reduced pressure to yield the title product (2.2 g, 87%) as a white
solid.
b(CDCl3): 1.05 (t, 3H), 1.65 (t, 3H), 1.86 (m, 2H), 3.47 (s, 3H), 3.98 (t,
2H),
4.43 (q, 2H), 6.50 (s, 1H), 7.20 (d, 1H), 8.01 (d, IH), 8.38 (s, IH), 10.30
(bs, 1.H).
PREPARATION 3
3-(7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
26
d]pyrimidin-6-yl)-4-ethoxybenzenesulphonyl chloride
The title compound of Preparation 1 (5 g, 15.3 mmol) was added portionwise to
a mixture of chlorosulphonic acid (25 mL) and sulphuryl chloride (12 mL) and
stirred at
0°C for 2 hours. The reaction mixture was carefully poured into stirred
ice-water and the
aqueous suspension was partitioned between dichloromethane and brine, then the
organic phase was separated, washed with water, dried (MgSO~) and evaporated
under
reduced pressure to yield the title compound (6.3 g, 90%) as a yellowish
solid.
8(CDCl3): 1.05 (t, 3H), 1.48 (t, 3H), 1.83 (m, 2H), 3.31 (s, 3H), 4.30 (m,
4H),
7.19 (d, 1H), 8.09 (d, 1H), 8.40 (s, 1H), 10.92 (bs, 1H).
PREPARATION 4
3-(7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxybenzenesulphonyl chloride
To a solution of the title compound of Preparation 2 (0.7 g, 1.65 mmol) in
glacial acetic acid (10 mL), was slowly added bromine (0.1 mL, 1.9 mmol) and
the
mixture was stirred at room temperature for 1 hour. The reaction mixture was
then
poured into ice-water and partitioned between dichloromethane and brine, the
organic
phase was separated, dried (MgSO~) and evaporated under reduced pressure to
yield the
title product (0.67 g, 88%).
~(CDCl3): 1.05 (t, 3H), 1.48 (t, 3H), 1.83 (m, 2H), 3.32 (s, 3H), 4.26 (t,
2H),
4.35 (q, 2H), 7.19 (d, 1H), 8.16 (d, 1H), 8.39 (s, 1H), 10.61 (bs, 1H).
PREPARATION 5
3-Methyl-6-(2-propoxyphenyl)-1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-
dione
Obtained as a white solid (60% overall) from 3,6-dimethyl-5-vitro-1-propyl-1H-
pyrimidine-2,4-dione and 2-propoxybenzaldehyde following the procedure
described in
Preparation 1.
b(CDCl3): 1.05 (t, 3H), 1.20 (t, 3H), 1.82 (m, 2H), 2.02 (m, 2H), 3.48 (s, 3H)
3.95 (t, 2H), 4.19 (t, 2H), 6.38 (s, 1H), 7.09 (m, 2H), 7.35 (t, 1H), 7.75 (d,
1H), 10.39
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
27
(bs, 1H).
PREPARATION 6
3-(3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-
yl)-4-
propoxybenzenesulphonyl chloride
Obtained as a white solid (75%) from the title compound of Preparation 5,
using
the procedure described in Preparation 2.
8(CDCl3): 1.05 (t, 3H), 1.20 (t, 3H), 1.82 (m, 2H), 2.05 (m, 2H), 3.45 (s,
3H),
3.97 (t, 2H), 4.36 (t, 2H), 6.50 (s, 1H), 7.20 (d, 1H), 7.98 (d, 1H), 8.38 {s,
1H), 10.3 {bs,
1 H).
PREPARATION 7
3-(7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-propoxybenzenesulphonyl chloride
Obtained as a yellowish solid (79%) from the title compound of Preparation 5,
using the procedure described in Preparation 3.
~(CDCl3): 1.05 (m, 6H), 1.82 (m, 4H), 3.31 (s, 3H), 4.15 (t, 2H), 4.30 (t,
2H),
7.18 (d, 1H), 8.16 (d, 1H) 8.21 (s, 1H), 10.9 (bs, 1H).
PREPARATION 8
3-{7-Bromo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-propoxybenzenesulphonyl chloride
Obtained as a white solid (83%) from the title compound of Preparation 6,
using
the procedure described in Preparation 4
8(CDCl3): 1.05 (m, 6H); 1.82 (m, 4H), 3.30 (s, 3H), 4.16 {t, 2H), 4.35 (t,
2H),
7.18 (d, 1H), 8.I6 (d, 1H), 8.36 (s, 1H), 11.3 (bs, 1H).
PREPARATION 9
6-(2-Ethoxyphenyl)-1-isobutyl-3-methyl-1,5-dihydropyrrolo [3,2-d]pyrimidine-
2,4-
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
28
dione
Obtained as a white solid (40% overall) from 1-isobutil-3,6-dimethyl-1H-
pyrimidine-2,4-dione and 2-ethoxybenzaldehyde following the procedure
described in
Preparation 1.
b{CDCl3): 1.02 (d, 6H), 1.61 (t, 3H), 2.25 {m, 1H), 3.44 (s, 3H), 3.80 (d,
2H),
4.26 (q, 2H), 6.36 (s, 1H), 7.02 (m, 2H), 7.38 (d, 1H), 7.75 (d, 1H), 10.38
(bs, 1H).
PREPARATION 10
3-{7-Chloro-1-isobutyl-3-methyl-2,4-dioxo-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxybenzenesulfonyl chloride
Obtained as a yellowish solid (82%) from the title compound of Preparation 9,
using the procedure described in Preparation 3.
8(CDCl3): 1.01 (d, 6H), 1.51 {t, 3H), 2.25 (m, 1H), 3.39 (s, 3H), 4.30 (m,
4H),
7.18 (d, 1H), 8.08 (dd, 1H), 8.50 (d, 1H), 10.30 (bs, 1H).
PREPARATION 11
4-Ethoxy-3-(7-iodo-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-
pyrrolo[3,2-
d]pyrimidin-6-yl)benzenesulfonyl chloride
Obtained as a yellow ochre solid (41 %) from the title compound of Preparation
2
and iodine monochloride following the procedure described in Preparation 4.
8(CDCl3): 1.03 (t, 3H), 1.42 (t, 3H), 1.83 (m, 2H), 3.88 (s, 3H), 4.25 {m,
4H),
7.18 (d, 1H), 8.11 (dd, 1H), 8.19 (d, 1H), 11.38 (bs, 1H).
PREPARATION 12
3-acetyl-4-propoxybenzenesulfonyl chloride
To a mixture of chlorosulphonic acid (27 mL) and thionyl chloride (3.6 mL) at
0 °C was added portionwise 1-(2-propoxyphenyl)ethanone {5.0 g, 28.1
mmol). The
resulting mixture was stirred at 0°C for 20 min. and at room
temperature overnight,
poured cautiously into ice-water and extracted with methylene chloride. The
organic
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
29
solution was washed sequentially with water, aqueous sodium bicarbonate and
brine,
dried (MgSOa), and the solvent removed under reduced pressure to yield the
title
compound (6.3 g, 81%) as an off white solid.
PREPARATION 13
4-{3-Acetyl-4-propoxybenzenesulfonyl)piperazine-1-carboxylic acid ethyl ester
To a mixW re of ethyl 1-piperazinecarboxylate {4.64 mL, 31.6 mmol) and
triethylamine (3.8 mL, 27.2 mmol) in dichloromethane (80 mL) was
added.dropwise the
title compound of Preparation 12 (6.26 g, 22.6 mmol) in dichloromethane (20
mL) and
the resulting mixture was stirred at room temperatz~re overnight. The reaction
mixture
was diluted with dichloromethane, washed with water, aqueous solution of
sodium
bicarbonate brine, dried (MgSO~) and evaporated under reduced pressure. The
resulting
crude residue was purified by flash column chromatography on silica-gel
(hexane-ethyl
acetate 1:1) to yield the title compound (7.60 g, 84%) as a white solid.
8(CDCl3): 1.12 {t, 3H), 1.20 (t, 3H), 1.92 (m, 2H), 2.63 (s, 3H), 2.95 (m,
4H),
3.56 (m, 4H), 4.12 (m, 4H), 7.08 (d, 1H), 7.82 (d, 1H), 8.10 {s, 1H).
PREPARATION 14
4-[3-(2-Bromoethanoyl)-4-propoxybenzenesulfonyl]piperazine-1-carboxylic acid
ethyl
ester
A solution of bromine (l .l mL, 20.9 mmol) in chloroform (15 mL) was added
dropwise to a solution of the title compound of Preparation 13 {7.60 g, 19.1
mmol) in
chloroform {40 mL). After the addition of bromine is complete, a rapid stream
of
nitrogen is bubbled through the solution which is then stirred for 1 hour at
room
temperature under these conditions. The resulting mixW re is diluted with
chloroform
and washed with aqueous sodium bicarbonate and brine, dried (MgSOø) and
evaporated
under reduced pressure to yield the title compound (6.20 g, 67%) as a white
solid.
8(CDCl3): 1.12 (t, 3H), '1.23 (t, 3H), 1.97 (m, 2H), 2.96 (m, 4H), 3.58 (m,
4H),
4.04 (q, 2H), 4.10 (q, 2H), 4.52 (s, 2H), 7.10 {d, 1H), 7.88 (d, 1H), 8.16 (s,
1H).
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 PREP AR_ATION 15
1-[5-(Morpholine-4-sulfonyl)-2-propoxyphenyl] ethanone
Obtained as a white solid (quantitative yield) from the title compound of
Preparation 12 and morpholine following the procedure of Preparation 13.
8(CDCl3): 1.14 (t, 3H), 1.92 (m, 2H), 2.64 (s, 3H), 2.98 (m, 4H), 3.72 (m,
4H),
10 4.14 (t, 2H), 7.10 (d, 1H), 7.84 (d, 1H), 8.11 (s, 1H).
PREPARATION 16
2-Bromo-1-[5-(morpholine-4-sulfonyl)-2-propoxyphenyl]ethanone
Obtained as a slightly orange solid (87%) from the title compound of
15 Preparation 15 following the procedure of Preparation 14.
8(CDC13): 1.07 (t, 3H), 1.98 (m, 2H), 2.92 (m, 4H), 3.76 (m, 4H), 4.12 (t,
2H),
4.52 (s, 2H), 7.12 (d, 1H), 7.88 (d, 1H), 8.18 (s, 1H).
wnTa~me
20 TABLE 2
O H S02NRøR5
~N N
\/
1
O R2 Rs R30
(II)
Example
R' RZ R' R6 NR~Rs
No
1 Me nPr Et H ~N-CH3
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
31
Example
R' R~ R' Rs NR''R'
No
2 Me nPr Et H N NH
3 Me nPr Et H N O
4 NIe nPr Et H NH~N(CH3)z
Me nPr Et H
N
N-~
~
CH3
~N.CH3
'
6 Me nPr Et H N
7 Me nPr Et H N
NHS
8 Me nPr Et H
N J
NHS
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
32
Example
R' RZ R3 Rs NR~RS
No
OH
M P E H ~
9 . e n t N
r N-
U
~ NHS
Me nPr Et H
0
11 Me nPr Et H
N
12 Me nPr nPr H N
N-CH3
O
13 Me nPr nPr H N N
C
H3
/--~ OH
14 Me nPr nPr H N N--
U
~
Me nPr nPr H N
NH
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
33
Example
R' R'' R3 R6 NR''Rs
No
16 Me nPr nPr H N O
17 Me nPr nPr H NH~N(CH3)z
18 Me nPr nPr H N(CH~CHzOH)~
~
~NoCH3
19 Me nPr nPr H N
~N~CHa
20 Me nPr nPr H
~N
NH
21 Me nPr nPr H
NH N
'~2 Me nPr nPr H NJ
NHS
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
34
Example
R' R' R3 Rs NR~R'
No
~o
23 Me nPr nPr H
~N
NH
NHS
24 Me nPr nPr H O
N~OH
25 Me rlPr nPr H ~
N
~
26 Me nPr Et Cl N
NH
O
27 Me nPr Et Cl N
N-CH3
U
28 Me nPr Et Cl N N
G
H3
~--OH
29 Et l O
Me nPr C N
N
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
Example
R' R'' R3 R6 NR4Rs
No
30 Me nPr Et Cl NH~N(CH3)2
~N.CH3
31 Me nPr Et Cl N
32 Me nPr Et Cl
~N
NH
33 Me nPr nPr Cl N
N-CH3
O
34 lVle nPr nPr Cl N N--~
_ ~
CH3
OH
l j
~
35 Me nPr nPr C N-
N
U
36 Me nPr Et Br N O
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
36
Example
R' R~ R3 R6 NR~R'
~to
37 Me nPr Et Br NON-CH3
O
38 Me nPr Et Br N-CHo
N
39 Me nPr Et Br N N-~
CH3
~N.CH3
40 Me nPr Et Br N
~N CHs
41 Me nPr Et Br
NN~N
42 Me nPr Et Br
NH N
43 Me nPr Et Br N
NHS
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
37
Example
R' R' R3 R6 NR.''Rs
No
OH
44 P ~ B
r Et r N N--
Me n
~O
45 Me nPr Et Br NJ
S
NH
46 Me nPr Et Br
0
H~oH
47 Me nPr Et Br ~
N
OCH3
48 Me nPr Et Br
49 Me nPr nPr Br N O
U
50 Me nP'r nPr Br ~N-CH3
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
38
Example
R' RZ R' R6 NR.'~Rs
No
O
51 Me nPr nPr Br N
N-CHo
U
52 Me riPr nPr Br ~N~
CH3
~N.CH3
53 Me nPr nPr Br
~o
54 Me nPr nPr Br
N
NH ~/
--~ ~- OH
55 M P B J
e n nPr r N N-
r
NH's
56 Me nPr nPr Br 0
N~OH
57 Me nPr nPr Br ~
N
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
39
Example
R' R'' R3 Rs NR~'R'
No
58 Me iBu Et Cl NON-CH3
59 Me iBu Et Cl N N-~
~
~
--
CH3
OH
Cl
60 Me iBu Et N N-
U
61 Me iBu Et Cl N NH
U
62 Me nPr Et Cl N
U
63 Me nP Et Cl H N ~ H ~
r
~N~OH
64 Me nPr Et Cl
NJ
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
Example
R' Rz R3 R6 NR''Rs
No
65 Me nPr Et Cl
NH N
66 Me nPr Et Cl ~o
/~
N
N
U
N
67 Me nPr Et Cl
68 Me nPr Et Cl N N
"",.
69 Me nPr Et Cl
N N
U
70 Me nPr Et Cl
N N
"",.
71 Me nPr Et Cl N"N
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
41
Example
R' R' R3 R6 NRR'
No
<~ O H
72 Me nPr Et Cl ~"~~~.
N
N
U
73 Me nPr Et Cl N N-
74 Me nPr Et I NON-CH3
75 Me nPr Et I N N/
~N~CH3
76 Me nPr Et I N
77 Me nPr Et I N N-\
CH3
N
78 Me nPr Et I
U
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
42
Example
R' Rz R3 Rs NRøRs
No
79 Me nPr Et I N N
U
OH
80 Me nPr Et I N~N-
f--~ OCN3
81 Me nPr Et I ~N-
82 Me nPr Et I N N H
83 Me nPr Et I H N \N H
w
OOH
84 Me nPr Et I N
N
85 NIe nPr Et I N N-
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
43
TABLE 3
O R6 S02NR~R5
R~
\N
\/
O N
R H R O (zzz)
Example
R' R' R3 Rs NR4R'
No
N
86 Me nPr nPr H -COZEt
U
~
87 Me nPr nPr H N
NH
88 Me nPr nPr H N O
U
89 Me nPr nPr H NON-CH3
(Me)~NC/
90 Me nPr nPr N-CH3
N
H O
9 P = O
1 Me r nPr z N
n I N
U
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
44
Example
R' Rz R3 R6 NR~Rs
No
(Me)zNC
92 Me nPr nPr N~ -COZEt
H
z
(Me)zNC/~
93 Me nPr nPr N O
H,
N
94 Me nPr nPr MeOCHz N
-CO2Et
~/
EXAMPLE 1
6-[2-Ethoxy-5-(4-methylpiperazine-1-sulphonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
To a mixture of the title compound of Preparation 2 (50 mg, 0.12 mmol) and
polymer bound morpholine ~(85 mg, 2.75 mmol/g based on nitrogen analysis) in
dichloromethane (3 mL) was added 1-methylpiperazine (0.015 mL, 0.13 mmol) and
the
resulting mixture was stirred at room temperature overnight. The reaction
mixture was
filtered and the filtrate was evaporated under reduced pressure. The residue
was
triturated with diethyl ether and the precipitate was collected by filtration
and dried
under vacuum to yield the title compound (22 mg, 39%) as a white solid.
ESI/MS mle: 490 ([M+H]~, C~3H3,NjO5S)
Retention Time (min.): 11.7
EXAMPLES 2-11
The compounds of this invention were synthesized from the title compound of
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 Preparation 2 following the procedure of example 1 and using the
corresponding
reactant respectively. The ESI/MS data, HPLC retention times and yields axe
summarised in Table 4.
TABLE 4
ESI/MS
ll~Iolecular Retention
xample mle ield
Formula Time (min.)
[M+H]+
2 ~ CzzHz9N50;S 476 11.7 72
3 CzzHz8N~06S 477 16.5 87
4 CzzH3,NsOsS 478 11.0 70
5 CzaHs3NsCsS 504 11.8 39
6 CzaHs3Ns~sS 504 11.5 73
7 CzsH3sNs~sS 518 11.6 71
8 Cz~H33Ns06S 520 11.3 48
9 Cz~H~3N506S 520 11.7 67
10 CzsH3sNs06S 534 11.2 57
11 CzsH3sN506S 534 11.7 84
EXAMPLE 12
3-Methyl-6-[5-(4-methylpiperazine-1-sulphonyl)-2-propoxyphenyl]-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
To a mixture of the title compound of Preparation 6 (0.7 g, 1.6 111mo1) and
triethylamine (0.26 mL, 1.9 mmol) in dichloromethane (30 mL) was added
dropwise 1-
methylpiperazine (0.21 mL, 1.9 mmol) and the resulting mixture was stirred at
room
temperature overnight. The reaction mixture was diluted with dichloromethane,
washed
with aqueous solution of sodium bicarbonate in water, dried (MgS04) and
evaporated
under reduced pressure. The resulting crude residue was purified by flash
column
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
46
chromatography on silica-gel (dichloromethane-methanol 9:1) to yield the title
compound (240 mg, 30%).
m.p.: 198°C
8(DMSO-d6): 0.93 (t, 3H), 1.06 (t, 3H), 1.70 (m, 2H), 1.88 (m, 2H), 2.14 (s,
3H), 2.37 (m, 4H), 2.93 {m, 4H), 3.28 (s, 3H), 3.87 {t, 2H), 4.18 (t, 2H),
6.77 (s, 1H),
7.36 (d, 1H), 7.67 (dd, 1H), 8.19 (d, 1H), 12.54 (bs, 1 H).
E~:AMPLE 13
6-[5-(4-Ethylpiperazine-1-sulphonyl)-2-propoxyphenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (32%) from the title compound of Preparation 6 and 1-
ethylpiperazine following the procedure of example 12.
m.p.: 194°C
8(D1V1SO-d6): 0.92 (m, 6H), 1.06 {t, 3H), 1.69 (m, 2H), 1.85 (m, 2H), 2.29 (q,
2H), 2.42 (m, 4H), 2.91 (m, 4H), 3.28 (s, 3H), 3.87 (t, 2H), 4.18 (t, 2H),
6.77 {s, 1H),
7.36 (d, 1H), 7.67 (dd, 1H), 8.19 (d, 1H), 12.55 (bs, 1H).
Elemental analysis calculated for Cz5H3sNsOsS: C, 58.01; H, 6.82; N, 13.53; S,
6.19. Found: C, 57.13; H, 6.87; N, 12.97; S, 6.08.
EXAMPLE 14
6-{5-[4-{2-Hydroxyethyl)piperazine-1-sulphonyl]-2-propoxyphenyl}-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid {35%) from the title compound of Preparation 6 and 1-
(2-hydroxyethyl)piperazine following the procedure of example 12.
m.p.: 237°C
8(DMSO-d6): 0.93 (t, 3H), 1.06 (t, 3H), 1.70 (m, 2H), 1.88 (m, 2H), 2.36 (t,
2H), 2.50 (m, 4H), 2.91 (m, 4H), 3.28 (s, 3H), 3.41 (q, 2H), 3.86 (t, 2H),
4.18 (t, 2H),
4.37 (t, 1H), 6.77 (s, 1H), 7.36 (d, 1H), 7.67 (dd, 1H), 8.20 (d, 1H), 12.55
(bs, 1H).
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
47
EXAMPLE 15
3-Methyl-6-[S-(piperazine-1-sulfonyl)-2-propoxyphenyl]-1- propyl-l,S-
dihydrop yrrolo [ 3,2-d]pyrimidine-2, 4-dione
Obtained as a white solid (40°l0) from the title compound of
Preparation 6 and
piperazine following the procedure of example 12.
m.p.:240°C
8(DMSO-d6): 0.94 (t, 3H), 1.07 (t, 3H), 1.70 (q, 2H), 1.88 (q, 2H), 2.74 (m,
4H),
2.82 (m, 4H), 3.36 (s, 3H), 3.88 (t, 2H), 4.18 (t, 2H), 6.78 (s, 1H), 7.36 (d,
1H), 7.66
(dd, 1H), 8.19 (d, 1H), 12.56 (bs, 1H). .
Elemental analysis calculated for Cz3H31N505S~ C, 56.43; H, 6.38; N, 14.31; S,
6.55. Found: C, 56.18; H, 6.50; N, 13.86; S, 6.60.
EXAMPLES 16-25
These compounds were synthesized from the title compound of Preparation 6
following the procedure of example l and using the corresponding reactant
respectively.
The ESIlMS data, HPLC retention times and yields are summarised in Table 5.
TABLE S
Molecular ESI/MS Retention
Example Time Yield
Formula m/e [M+H]+ %
(min.)
16 CzsH3oNa06S 491 17.5 ~ 58
17 Cz3H33NsOsS 492 11.8 S 1
18 C,3H32NaO,S 509 15.7 67
19 CZSH3;N505S 518 12.3 67
20 C~4H3~N605S S 19 12.1 57
21 CzsHsiNsOsS 526 15.0 29
22 C~6H,7NSOSS 532 12.3 60
23 CzsHssNsOeS 534 12.1 40
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
48
24 CZ6H~~N506S548 12.0 40
25 C26H~~N506S548 12.4 60
EXAMPLE 26
7-Chloro-6-[2-ethoxy-5-(piperazine-1-sulfonyl)phenyl]-3-methyl-1-propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (32%) from the title compound of Preparation 3 and
piperazine following the procedure of example 12.
m.p.: 257°C
8(DMSO-d6): 0.94 (t, 3H), 1.32 (t, 3H), 1.70 (m, 2H), 2.73 (m, 4H), 2.81 (m,
4H), 3.27 (s, 3H), 3.34 (bs, 1H), 4.18 (m, 4H), 7.38 (d, 1H), 7.63 (d, 1H),
7.78 (dd, 1H),
12.86 (bs, 1H).
Elemental analysis calculated for C~ZH~BCINSOSS: C, 5I.8I; H, 5.53; N, 13.73;
S,
6.29. Found: C, 51.26; H, 5.50; N, 13.39; S, 6.22.
EXAMPLE 27
7-Chloro-6-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (50%) from the title compound of Preparation 3 and 1-
methylpiperazine following the procedure of example 12.
m.p.: 256°C
8(DMSO-d6): 0.94 {t, 3H), 1.32 (t, 3H), 1.70 (m, 2H), 2.15 (s, 3H), 2.38 (m,
4H), 2.91 (m, 4H), 3.28 (s, 3H), 4.17 (m, 4H), 7.38 (d, 1H), 7.63 (d, 1H),
7.80 {dd, 1H),
12.87 (bs, 1H).
Elemental analysis calculated for C~3H3°C1NSOSS: C, 52.72; H, 5.77; N,
13.37; S,
6.I2. Found: C, 52.33; H, 5.93; N, 12.97; S, 6.25.
EXAMPLE 28
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
49
7-Chloro-6-[2-ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-3-methyl-1-propyl-
1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (36%) from the title compound of Preparation 3 and 1-
ethylpiperazine following the procedure of example 12.
m.p.: 213°C
8(DMSO-d6): 0.94 (t, 3H), 1.32 (t, 3H), 1.70 {m, 2H), 2.31 (q, 2H), 2.43 (m,
4H), 2.92 (m, 4H), 3.28 (s, 3H), 4.19 (m, 4H), 7.39 (d, 1H), 7.63 {d, 1H),
7.80 (dd, 1H),
12.86 (bs, 1H).
EXAMPLE 29
7-Chloro-6-{2-ethoxy-5-[4-{2-hydroxyethyl)piperazine-1-sulfonyl]phenyl}-3-
methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (30%) from the title compound of Preparation 3 and 1-
(2-hydroxyethyl)piperazine following the procedure of example 12.
m.p.: 146°C
8(DMSO-d6): 4.94 (t, 3H), 1.32 (t, 3H), 1.70 (m, 2H), 2.38 (m, 2H), 2.50 {m,
4H), 2.90 (m, 4H), 3.28 (s, 3H), 3.43 (m, 2H), 4.18 (m, 4H), 4.41 (bs, 1H),
7.39 {d, 1H),
7.63 (d, 1H), 7.79 (dd, 1H), 12.87 (bs, 1H).
EXAMPLES 30, 31 and 32
, Obtained from the title compound of Preparation 3 following the procedure of
example 1 and using the corresponding reactant respectively. The ESI/MS data,
HPLC
retention times and yields are summarised in Table 6.
TABLE 6
ESI/MS
Molecular Retention
Example m/e Yield
Formula Time
[M+H]+
C~~H,C1N;OSS512 11.9 61
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
31 Cz~H3zC1N505S538 12.3 68
32 C.,~H3zC1N506S554 12.2 62
5
EXAMPLE 33
7-Chloro-3-methyl-6-[5-(4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-
propyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (45%) from the title compound of Preparation 7 and 1-
10 methylpiperazine following the procedure of example 12.
m.p.: 197°C
8(DMSO-d6): 0.93 (m, 6H), 1.72 {m, 4H), 2.15 {s, 3H), 2.38 (m, 4H), 2.91 (m,
4H), 3.27 (s, 3H), 4.09 (t, 2F3), 4.15 (t, 2H), 7.39 (d, 1H), 7.62 (d, 1H),
7.80 (dd, 1H),
12.84 (bs, 1H).
EXAMPLE 34
7-Chloro-6-[5-(4-ethylpiperazine-1-sulfonyl)-2-propoxyphenyl]-3-methyl-1-
propyl-1,5-
dihydropyrrolo [3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (42%) from the title compound of Preparation 7 and 1-
ethylpiperazine following the procedure of example 12.
m.p.: 215°C
8(DMSO-d6): 0.92 (m, 9H), 1.67 (m, 4H), 2.40 (m, 6H), 2.89 (m, 4H), 3.25 (s,
3H), 4.09 (m, 4H), 7.38 (d, 1H), 7.62 (d, 1H), 7.78 (dd, 1H), 12.83 (bs, 1H).
EXAMPLE 35
7-Chloro-6- ~5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]-2-propoxyphenyl}-3-
methyl-
1-propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (60%) from the title compound of Preparation 7 and 1-
(2-hydroxyethyl)piperazine following the procedure of example 12.
m.p.:140°C
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
51
8(DMSO-d6): 0.91 (m, 6H), 1.67 (m, 4H), 2.34 (t, 2H), 2.47 (m, 4H), 2.87 (m,
4H), 3.25 (s, 3H), 3.39 (q, 2H), 4.10 (m, 4H), 4.37 (t, 1H), 7.37 (d, 1H),
7.60 (d, 1H),
7.77 (dd, 1H), 12.81 (bs, 1H).
EXAMPLES 3 6-48
The compounds of this invention were synthesized from the title compound of
Preparation 4 following the procedure of example 1 and using the corresponding
reactants respectively. The ESI/MS data, HPLC retention times and yields are
summarised in Table 7.
TABLE 7
ESI/MS
Molecular Retention
xample m/e ield
Formula Time
[M+H]+
36 CZ~H?~BrNa06S555 17.4 84
37 CZSH3oBrN;O;S568 12.0 84
38 C23H28BrN;06S582 16.2 84
39 C,4H3~BrN;O;S582 12.2 50 .
40 Cz4H3zBrN;O;S582 12.0 85
41 C~3H31BrN60;S583 11.6 78
42 CZ;H~BBrN;O;S590 14.8 81
43 C~;H3aBrN;O;S596 12.1 59
44 C~aH3~BrN;06S598 12.1 57
45 CZ~H32BrN;06S598 11.9 80
46 CZ;H3~BrN;06S612 11.7 45
47 C~;H3,~BrN;06S612 12.1 78
48 C~;H3,~BrN;06S612 12.5 80
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
52
EXAIyIPLES 49-57
The compounds of this invention were synthesized from the title compound of
Preparation 8 following the procedure of example 1 and using the corresponding
reactants respectively. The ESI/MS data, HPLC retention times and yields are
summarised in Table 8.
TABLE 8
ESI/MS
Molecular Retention
xample ' m/e ield
Formula Time
[M+H]+
49 C~3Hz~BrN~06S569 18.2 80
50 C,H3~BrN;O;S 582 12.9 87
51 C~~H3BrN;O6S 596 17.0 96
52 C~;H~aBrN;O;S596 12.9 74
53 CzsHsaBrN;O;S596 12.8 78
54 CZ;H3~BrN;06S612 12.7 81
55 CZ;H3BrN;O6S 612 12.9 57
56 . C,6H36BrN;06S626 12.5 68
57 C26H36BrN;06S626 12.8 75
EXAMPLE 5 8
7-Chloro-6-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl] -1-isobutyl-3-
methyl-
1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (51%) from the title compound of Preparation 10 and
1-methylpiperazine following the procedure of example 12.
m.p.: 257°C
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
53
~(DMSO-d6): 0.92 (d, 6H), 1.31 (t, 3H), 2.13 (m, 1H), 2.15 (t, 3H), 2.38 (m,
4H), 2.92 (m, 4H), 3.28 (s, 3H), 4.05 (d, 2H), 4.19 (q, 2H), 7.37 (d, 1H),
7.63 (s, 1H),
7.80 (d, 1H), 12.89 (bs, 1H).
EXAMPLE 59
7-Chloro-6-[2-ethoxy-5-(4-ethylpiperazine-1-sulfonyl)phenyl]-1-isobutyl-3-
methyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (48%) from the title compound of Preparation 10 and
1-ethylpiperazine following the procedure of example 12.
m.p.: 239°C
8(DMSO-d6): 0.93 (d, 6H), 1.31 (t, 3H), 2.13 (m, 1H), 2.33 (m, 2H), 2.44 (m,
4H), 2.92 (m, 4H), 3.28 (s, 3H), 4.06 (d, 2H), 4.19 (q, 2H), 7.37 (d, 1H),
7.63 (s, 1H),
7.81 (d, 1H), 12.89 (bs, 1H).
EXAMPLE 60
7-Chloro-6-(2-ethoxy-5-[4-(2-hydroxyethyl)piperazine-1-sulfonyl]phenyl}-1-
isobutyl-
3-methyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (40%) from the title compomd of Preparation 10 and
1-(2-hydroxiethyl)piperazine following the procedure of example 12.
m.p.: 148°C
b (DMSO-d6): 0.92 (d, 6H), 1.31 (t, 3H), 2.14 (m, 1H), 2.37 (t, 2H), 2.50 (m,
4H), 2,91 (m, 4H), 3.28 (s, 3H), 3.43 (m, 2H), 4.06 (d, 2H), 4.19 (q, 2H),
4,39 (bs, 1H),
7.38 (d,,lH), 7.63 (s, 1H), 7.81 (d, 1H), 12.87 (bs, 1H).
EXAMPLE 61
7-Chloro-6-[2-ethoxy-5-(piperazine-1-sulfonyl)phenyl]-1-isobutyl-3-methyl-1,5-
dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
Obtained as a white solid (28%) from the title compound of Preparation 10 and
piperazine following the procedure of example 12.
m.p.: 246°C
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
54
8(DMSO-d6): 0.93 (d, 6H), 1.31 {t, 3H), 2.12 (m, 1H), 2.76 (m, 4H), 2.82 (m,
4H), 3.28 (s, 3H), 4.06 (d, 2H), 4.18 (q, 2H), 7.38 (d, 1H), 7.64 (s, 1H),
7.80 (d, 1H).
EXAMPLE 62
7-Chloro-6-[2-ethoxy-5-{4-methyl-4-oxypiperazine-1-sulfonyl)phenyl]-3-methyl-1-
propyl-1,5-dihydropyrrolo[3,2-d]pyrimidine-2,4-dione
To a solution of the title compound of example 27 (300 mg, 0.57 mmol) in
dichloromethane (20 mL) at 0°C, was slowly added 3-chloroperoxybenzoic
acid (136
mg, 0.63 mmol). The mixture was stirred at room temperature for 2 hours and
then
poured into an aqueous solution of sodium bicarbonate. The precipitate was
collected by
filtration, washed with water and dried to yield the title product (300 mg,
97%) as a
white solid.
m.p.: 227°C
8(DMSO-d6): 0.93 (t, 3H), 1.32 (t, 3H), 1.69 (m, 2H), 2.9-3.5 (m, 8H), 3.26
(s,
3H), 4.20 (m 4H), 7.38 (d, 1H), 7.69 (s, 1H), 7.80 {d, 1H).
EXAMPLE 63
3-(7-Chloro-3-methyl-2,4-dioxo-1-propyl-2,3,4,5-tetrahydro-1H-pyrrolo[3,2-
d]pyrimidin-6-yl)-4-ethoxy-N-{2-methylaminoethyl)benzenesulfonamide
a) Following the procedure of example 12, from the title compound of
Preparation 3 (1.64 g, 3.56 mmol) and N-benzyl-N-methylethylenediamine (0.67
g, 4.1
mmol), N-[2-{benzyl-methyl-amino)ethyl]-3-(7-chloro-3-methyl-2,4-dioxo-1-
propyl-
2,3,4,5-tetrahydro-1H-pyrrolo[3,2-d]pyrimidin-6-yl)-4-ethoxy-
benzenesulfonamide
(0.93 g, 44%) was obtained.
b) A mixture of the above compound (0.5 g, 0.85 mmol), ethanol (40 mL),
concentrated hydrochloric acid (0.1 mL) and palladium hydroxide (80 mg) was
hydrogenated for 4 hours at 30 p.s.i. The catalyst was removed by filtration
thxough
celite and the filtrate was evaporated under reduced pressure. The resulting
cmde
product was purified by flash column chromatography on silica gel
(dichloxomethane-
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 ethanol-ammonium hydroxyde 100:8:1) to yield the title compound (0.18 g,
43%) as an
o.ff white solid.
m.p.: 226°C
cS(DMSO-d6): 0.94 (t, 3H), 1.29 (t, 3H), 1.70 (m, 2H), 2.18 (s, 3H), 2.49 (t,
2H),
2.81 (t, 2H), 3.27 (s, 3H), 4.20 (m, 4H), 7.33 (d, 1H), 7.74 (s, 1H), 7.86 (d,
1H).
EXAMPLES 64-73
The-compounds of this invention were synthesized from the title compound of
Preparation 3 following the procedure of example 1 and using the corresponding
reactants respectively. The ESI/MS data, HPLC retention times and yields are
summarised in Table 9.
TABLE 9
Molecular ESI/MS Retention
Example Time Yield
Formula mle [M+H]+
(min.)
64 . C2;H3~C1N506S568 12.4 62
65 CYsHasCIN;O;S546 15.1 60
66 CzsHs6C1N;O6S582 13.3 55
67 CzsH3zCIN;O;S550 13.2 68
68 C~;H,~C1N;O;S552 12.6 58
69 C2SHs2C1N50;S550 8.5 72
70 CzsHszCIN;O;S550 8.5 71
71 C~6H3~C1N;OSS564 8.4 56
72 C,,;H32C1N;06S566 8.5 25
73 C~aH3C1N;O;S536 8.1 66
EXAMPLES 74-85
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
56
The compounds of this invention were synthesized from the title compound of
Preparation 11 following the procedure of example 1 and using the
corresponding
reactants respectively. The ESI/MS data, HPLC retention times and yields are
summarised in Table 10.
TABLE 10
Molecular ESI/MS Retention
Example Time Yield
Formula m/e [M+H]~
(min.)
74 Cz3H3oINs~sS616 8.2 55
75 Cz3H3oINs~sS616 8.3 45
76 CzaH3z~s~ss630 8.2 62
77 C.,4H3~INspss630 8.3 65
78 C.,sH3z~spsS642 8.8 72
79 CzsHs4~s~sS644 8.3 75
80 C~4H ~sp6S 646 8.2 55
81 C~sH3~INsp6s660 8.5 42
82 CzzHzs~s~sS602 8.2 22
83 C~~H38~spsS672 8.2 35
84 CzsHsa~s~6S660 8.2 44
85 Cz4H3oINs~ss628 8.1 65
EXAMPLE 86
4-[3-(3-Methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-1H-pyrrolo[2,3-
d]pyrimidin-6-
yl)-4-propoxybenzenesulfonyl]piperazine-1-carboxylic acid ethyl ester
A mixture of 6-amino-3-methyl-1-propyl-1H-pyrimidine-2,4-dione (2.0 g, 10.8
mmol) and the title compound of Preparation 14 (6.2 g, 13.0 mmol) in N,N-
dimethylformamide (55 mL) was refluxed for 18 h. The resulting solution was
evaporated under reduced pressure and the resulting residue was partitioned
between
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
57
ethyl acetate and aqueous sodium bicarbonate solution, then the organic phase
separated, washed with water and brine, dried (MgSOa) and evaporated under
reduced
pressure. The resulting crude residue was purified by flash column
chromatography on
silica-gel (hexane-ethyl acetate 1:1 -~ 1:3) to yield the title compound (2.80
g, 46%) as
a white solid.
m.p.:181°C
8(DMSO-46):0.93 (t, 3H), 1.04 (t, 3H), 1.12 (t, 3H), 1.71 (m, 2H), 1.83 (m,
2H),
2.89 (m, 4H), 3.24 (s, 3H), 3.45 (m, 4H), 3.97 (q, 2H), 4.04 (t, 2H), 4.14 (t,
2H), 6.90 (s,
1I-I), 7.32 (d, 1H), 7.62 (d, 1H), 7.88 (s, 1H), 11.62 (s, 1H).
EXAMPLE 87
3-Methyl-6-[5-(piperazine-1-sulfonyl)-2-propoxyphenyl~-1-propyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
A mixture of the title compound of Example 86 (0.5 g, 0.89 mmol) and
potassium hydroxide (0.58 g, 8.90 mmol, 85%) in isopropanol (20 mL) was
refluxed for
18 h. The resulting mixture was evaporated under reduced pressure and the
resulting
residue was acidified with 6N hydrochloric acid. Then it was basified with
aqueous
sodium bicarbonate solution and extracted with dichloromethane. The organic
extracts
were dried (MgSOd) and evaporated under reduced pressure. The resulting.cmde
residue
was purified by flash column chromatography on silica-gel (dichloromethane-
ethanol-
ammonium hydroxide 180:8:1) to yield the title compound (0.24 g, 55%) as a
white
solid.
m.p.: 237°C
8(DMSO-d6): 0.95 (t, 3H), 1.04 (t, 3H), 1.69 (m, 2H), 1.82 (m, 2H), 2.71 (m,
4H), 2.77 (m, 4H), 3.24 (s, 3H), 4.04 (t, 2H), 4.14 (t, 2H), 6.89 (s, 1H),
7.33 (d, 1H),
7.60 (d, 1H), 7.90 (s, 1H).
EXAN»'LE 88
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
58
3-Methyl-6-[5-(morpholine-4-sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-
dihydropyrrolo[2,3-d]pyrimidine-2,4-dione
Obtained as a white solid (28%) from the title compound of Preparation 16 and
6-amino-3-methyl-1-propyl-1H-pyrimidine-2,4-dione following the procedure of
Example 86.
m.p.:271°C
8(DMSO-d6): 0.96 {t, 3H), 1.02 (t, 3H), 1.67 (m, 2H), 1.84 (m, 2H), 2.82 (m,
4H), 3.22 (s, 3H), 3.63 (m, 4H), 4.03 (t, 2H), 4.17 (t, 2H), 6.89 (s, 1H),
7.36 (d, 1H),
7.62 (d, 1H), 7.89 {s, 1H), 11.63 {s, 1H).
EXAMPLE 89
3-Methyl-6-[5-{4-methylpiperazine-1-sulfonyl)-2-propoxyphenyl]-1-propyl-1,7-
dihydropyrrolo [2,3-d]pyrimidine-2,4-dione
A mixture of the title compound of Example 87 (0.4 g, 0.82 mmol),
formaldehyde (0.14 mL, 37% in water,1.63 mmol) and palladium 10% on activated
carbon (40 mg) was hydrogenated for 2h. The catalyst was removed by filtration
through celite and the filtrate was evaporated under reduced pressure. The
resulting
crude product was purified by flash column chromatography on silica-gel
(dichloromethane-ethanol-ammonium hydroxide 180:8:1) to yield the title
compound
(0.07 g, 17%) as an off white solid.
m.p.:240°C
8(DMSO-d6): 0.93 (t, 3H), 1.05 (t, 3H9, 1.73 (m, 2H), 1.86 (m, 2H), 2.13 (s,
3H), 2.36 (m, 4H), 2.89 (m, 4H), 3.23 (s, 3H), 4.03 (t, 2H), 4.14 (t, 2H),
6.87 (s, 1H),
7.33 (d, 1H), 7.63 (d, 1H), 7.88 (s, 1H), 11.63 (bs, 1H).
EXAMPLE 90
5-Dimethylaminomethyl-3-methyl-6-[5-(4-methylpiperazine-1- sulfonyl)-2-
propoxyphenyl]-1-propyl-1,7-dihydropyrrolo [2,3-d]pyrimidine-2,4-dione
A mixture of the title compound of Example 89 (30 mg, 0.06 mmol) and N,N-
dimethylmethyleneiminium iodide (57 mg, 0.23 mmol) in anhydrous
dichloromethane
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
59
was stirred at room temperaW re overnight. The reaction mixture was purified
by solid
phase extraction using a Varian Bond Elut SCX cartridge (methanol for washing,
methaazol-ammonia for elution) to yield the title compound (32 mg, 95%).
ESI/MS m/e: 461 ([M+H]+, Cz,H~°N60;S)
Retention Time (min.): 7.6
EXAMPLE 91
3-Methyl-6-[2-propoxy-5-(4-propyl-piperazine-1-sulfonyl) phenyl]-1-propyl-1,7-
dihydropyrrolo [2,3-d]pyrimidine-2,4-dione
A mixture of the title compound of example 87 (50 mg, 0.1 mmol), propanal
(0.073 mL, 1 mmol) and 3~ molecular sieves {300 mg) in methanol (2 mL) and
acetic
acid (0.1 mL), was stirred at room temperature for 4 hours. Then sodium
cianoborohydride (32 mg, 0.51 mmol) was slowly added and the mixture was
stirred at
room temperature overnight. The reaction mixture was purified by solid phase
extraction using a Varian Bond Elut SCX cartridge (methanol for washing,
methanol-
ammonia for elution) to yield the title compound (40 mg, 72%).
ESI/MS m/e: 532 ([M+H]~, C~6H3~N;O;S)
Retention Time (min.): 11.9
EXAMPLE 92
4-[3-(5-Dimethylaminomethyl-3-methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-1H-
pyrrola[2,3-d]pyrimidin-6-yl)-4-propoxybenzenesulfonyl]-piperazine-1-
carboxylic acid
ethyl ester
Obtained as a white solid (89%) from the title compound of example 86
following the procedure of example 90.
ESI/MS mle: 619 ([M+H]~, Cz9H42N6~7~)
Retention Time (rnin.): 11.6
EXAMPLE 93
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
5 5-Dimethylaminomethyl-3-methyl-6-[5-(morpholine-4-sulfonyl)-2-
propoxyp.henyl]-1-
propyl-1,7-dihydropyrrolo[2,3-d] pyrimidine-2,4-dione
Obtained as a white solid (92%) from the title compound of example 88
following the procedure of example 90.
ESI/MS m/e: 548 ([M+H]~", CL6H,~N;O.6S)
10 Retention Time (min.): 10.4
EXAMPLE 94
4-[3-(5-Methoxymethyl-3-methyl-2,4-dioxo-1-propyl-2,3,4,7-tetrahydro-1H-
pyrrolo[2,3-d]pyrimidin-6-yl)-4-propoxybenzene sulfonyl]piperazine-1-
carboxylic acid
15 ethyl ester
To a solution of the title compound of example 92 (50 mg, 0.08 mmol) in
methanol (5 mL) was added methyl iodide (0.015 mL, 0.24 mmol) and the mixture
was
stirred for 3 hours at room temperature. Then sodium methoxide (0.03 mL from a
solution 5.3M in methanol, 0.16 mmol) was added and the mixture was stirred at
room
20 temperature overnight. The solvent was removed under reduced pressure and
the residue
partitioned between water and dichloromethane. The organic phase was washed
with
brine, dried (MgS04) and evaporated to yield the title compowd (34 mg, 71%) as
a
white solid.
ESI/MS m/e: 606 ([M+H]+, Ca8H39N50sS)
25 Retention Time (min.): 11.0
The following examples illustrate pharmaceutical compositions according to the
present invention and procedure for their preparation.
30 COMPOSITION EXAMPLE 1
50,000 capsules each containing 100 mg of 3-methyl-6- [5-(4-methylpiperazine-
1-sulphonyl)-2-propoxyphenyl]-1-propyl-1,5-dihydropyrrolo [3,2-d]pyrimidine-
2,4-
dione (active ingredient) were prepared according to the following
formulation:
CA 02411013 2002-12-06
WO 01/94350 PCT/EPO1/06306
61
Active ingredient 5 Kg
Lactose monohydrate 10 Kg
Colloidal silicone dioxide 0.1 Kg
Corn starch 1 Kg
Magnesium stearate ~ 0.2 Kg
Procedure
The above ingredients were sieved through a 60 mesh sieve, and were loaded
into a suitable mixer and filled into 50,000 gelatine capsules.
COMPOSITION EXAMPLE 2
50,000 tablets each containing 50 mg of 6-[5-(4-ethylpiperazine-1-sulphonyl)-2-
propoxyphenyl]-3-methyl-1-propyl-1,5-dihydropyrralo[3,2-d]pyrimidine-2,4-dione
(active ingredient) were prepared from the following formulation:
Active ingredient 2.5 Kg
Microcrystalline cellulose 1.95 Kg
Spray dried lactose 9.95 Kg
Carboxymethyl starch 0.4 Kg
Sodium stearyl fumarate 0.1 Kg
Colloidal silicon dioxide 0.1 Kg
Procedure
All the powders were passed through a screen with an aperture of 0.6 mm, then
mixed in a suitable mixer for 20 minutes and compressed into 300 mg tablets
using 9
mm disc and flat bevelled punches. The disintegration time of the tablets was
about 3
minutes.