Note: Descriptions are shown in the official language in which they were submitted.
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
TITLE:
Polymer fuel cell structure
TECHNICAL FIELD
The present invention generally relates to fuel cells, and
in particular to improvements in performance of polymer
fuel cells.
BACKGROUND OF THE INVENTION
Polymer fuel cells are on the fringe of commercialization.
The progress made in catalyst and membrane research in the
last few years has enabled very high power densities
(>lTn1/cm~) with moderate efficiencies for the fuel cell
(400). The catalyst loading of electrodes has been reduced
to 0.1 mg Pt/cm2 while maintaining a high performance. The
price of the perfluorinated sulfonic acid membranes such as
Nafion (R) is expected to decrease, with increasing
production, whilst other proton conducting membrane
candidates have been discovered.
However, serious problems are encountered when polymer fuel
cell technology is scaled up to larger cells and stacks.
One of the main problems, in the stacks themselves, is the
.water management, since the proton conducting membrane must
be kept well humidified under operating conditions.
The dominating component, at the internal resistance loss
in the stack, is due to the limiting proton conductivity of
the membrane. Membranes tend to dry out, especially on the
anode side, at high current densities, since proton
migration drags water molecules away from the anode.
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
2
Drying of anode does not only affect resistance but also
the kinetics of hydrogen reduction reaction (HRR) at the
anode.
Therefore, in attempts to remedy this problem the anode
side is often humidified more intensively than the cathode
side. The cathode side of the cell can also be pressurized
to use the pressure gradient over the membrane to press the
water back to the anode. However, it is important that the
water management does not impede the gas flow inside the
cells.
One solution for this problem would be to use thinner
membranes, but this approach has limitations since
mechanical rigidity of the membrane must be sufficient.
Another solution is to have a direct water contact with the
membrane at the anode side, since the water content and
conductivity of the membrane are much higher when membrane
'is in equilibrium with water. Also, when liquid is
evaporated inside the fuel cell a considerable amount
(40-500) of the heat can be removed from the cell with the
produced water vapor.
In US-5,958,613 (Hamada et al) relates to such. direct water
humidification of fuel cell membranes. Therein is disclosed
a polymer fuel cell system with a capability to moisten the
solid-polymer film without providing a special humidifier
which humidifies the fuel gas or the oxidizer gas, and that
cools down the main cell body without providing cooling
channels. In this patent there is no disclosure of
specified operation principles for a fuel cell stack, when
the direct humidification is applied.
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
3
In US-5,935,726 (Chow et al) there is disclosed a method
and apparatus for improved humidification of membranes in
polymer fuel cells, by periodically reversing the flow
direction of the oxidant stream through a fuel cell flow
field. However, this patent is not concerned with cooling
of the fuel cell.
SUMMARY OF THE INVENTION
Despite the numerous attempts to improve the water
management in polymer fuel cells, there is still room for
improvements.
Thus, the object of the present invention is to provide
means for achieving better humidification, at low cost and
low cell complexity. The trade off between performance and
cost should be acceptable.
In the cell structure of the present invention, an aqueous
phase, preferably water, is used for direct humidification
of the membrane. The polymer electrolyte fuel cell
structure according to the invention comprises a proton
exchange membrane, an anode catalyst layer on one side of
the proton exchange membrane, a cathode catalyst layer on
the opposite side of the proton exchange membrane and a gas
distribution layer on each side of the proton exchange
membrane. It is characterized in that the anode side gas
distribution layer is a flat, porous structure having water
channels formed in the surface facing the membrane, and
that the anode side gas distribution layer is enclosed by a
coplanar, sealing plate with water inlet channels coupled
to said water channels in the gas distribution layer.
Thereby, it is possible to maintain a direct water contact
with the membrane at the anode side. This is beneficial for
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
4
the operation of the cell, since the water content and the
conductivity of the membrane are much higher when the
membrane is in equilibrium with water. Also, when water is
evaporated inside the fuel cell, a considerable amount of
the heat, produced in the cell, can be removed from the
cell by means of the produced water vapor.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention
will
now
be
further
described
in
the
follow ing, in a non-limiting way with reference to the
accomp anying drawings in which:
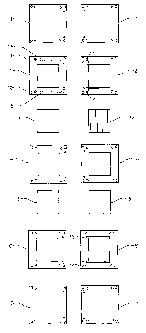
Fig. 1 is a perspective explosion view showing the
elements of a polymer fuel cell structure in
accordance with the invention,
Fig. 2 shows in plane view from above and below, all
elements of the structure in Fig. 1,
Fig. 3 is a section in larger scale, through a gas
distribution layer which is included in the
structure of Fig. 1 and 2,
Fig. 4 shows a second embodiment of the gas distribution
layer,
Fig. 5 shows a third embodiment of the gas distribution
layer,
Fig. 6 shows, in the same manner as Fig. 2, an
alternative structure layout, and
Fig. 7 shows still another embodiment of the gas
distribution layer.
DETAILED DESCRIPTION OF THE INVENTION
A preferred embodiment of the fuel cell structure according
to the invention is shown in figure 1 and 2. The fuel cell
comprises a conductive anode plate 1. An anode sealing
frame 2 is provided adjacent the bipolar plate 1. This
frame is provided with a central, rectangular opening for
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
5
an anode gas distribution layer 3. The frame 2 is also
provided with an anode gas inlet 9 and an outlet 10 and
distribution channels are formed as well as water inlets
and outlets 11, 12 respectively. The anode gas distribution
layer 3 is provided with a plurality of narrow water
channels 3a on the opposite side of the layer 3, with
reference to the anode plate 1. A proton exchange membrane
4 is arranged for cooperation with the plate 1 for
sandwiching the frame 2 and the diffusion layer 3 between
themselves.
The cathode side of the fuel cell is structured in a
similar manner as the anode side. Thus, the opposite side
of the membrane 4 is arranged for cooperation with a
conductive cathode plate 7 for sandwiching a cathode
sealing frame 6 and a cathode gas distribution layer 5
between themselves. The cathode diffusion layer 5 is not
provided with any water channels as the anode diffusion
layer 3. The cathode sealing frame 6 is provided with a
cathode gas inlet 13 and an outlet 14.
In figure 2 the detailed structure of water channels and
how the water distribution is organized in a stack is
shown. The left-hand side of the figure shows the upside
and the right-hand side of the figure shows the down side.
Each sealing frame 2 in a stack has a number of holes made
through it. The holes located in the corners are for
clamping bolts used when assembling a number of cell units
to a cell stack. The remaining holes, together with
corresponding holes in the other components of a stack,
form channels through the stack for water, fuel gas and
oxidant gas respectively.
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
6
Furthermore, the upper side (as defined above) of the
sealing 2 has gas channels 15 running along the inner edge
of the frame like structure. A number of distribution
apertures (in the figure there are five) are diverted from
each channel 15, so as to distribute incoming gas into the
diffusion/distribution material located in the frame. The
second hole from left (in the figure) in the upper array of
holes is the inlet channel 9 for incoming gas, and the
second hole from left in the lower array of holes is the
outlet channel 10 for gas exiting.from the cell on the
anode side. The anode sealing 2 has the same configuration
of gas channels regardless of position in the stack.
On the down side (as defined above) of each sealing 2 there
are provided channels for water, having a common water
inlet 11 and a common water outlet 12.
In the middle of the stack the membrane 4 is arranged,
separating the anode and cathode parts of the stack. On the
cathode side, a cathode gas distribution layer 5 is
provided, and then there is sealing 6 for cathode wherein
cathode gas inlet and outlet 13, 14 are formed, in a
similar way as in the anode sealing 2.
Figure 3 shows a more detailed structure of a gas
distribution layer. The layer 3 is provided with water
channels 3a adjacent the membrane 4. In a typical
embodiment of the invention, the water channels 3a may have
a width of about 50-100~m, a depth of about 100-300um and
the channels may be separated by a distance of about 200-
1000~Zm. By making the water channels narrow, blocking of the
channels due to membrane expansion is avoided. One possible
method of producing the channels 3a would be to press the
gas distribution layer against a template having a ridge
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
7
structure surface corresponding to the desired water channel
structure.
Figure 4 shows an embodiment of the invention, where the gas
distribution layer 3 is provided with a catalyst layer 16. A
non-porous or almost non-porous proton conducting polymer
layer 17 is arranged so that it lines the water channel.
In this embodiment, a hydrogen peroxide or other oxygen
evolving compounds may be added to the humidification and
cooling water, which is fed into the cell on the anode side.
Since the oxygen is released in the vicinity of the
catalyst, CO adsorption at the anode catalyst may be
avoided, in a manner which is effective and which leads to
less consumption of oxygen. Part of the hydrogen peroxide
will be decomposed at the electrode surface to generate
oxygen with the reaction H202->H20+1/202. In this system
possible benefits of hydrogen peroxide are achieved even if
the decomposition is not complete. The path of the hydrogen
peroxide and evolved oxygen is marked as arrows in Fig. 4.
However, this method can be applied to other direct water
humidification systems in polymer fuel cells.
Figure 5 shows a gas distribution layer 3, the edges of
which has been treated with a hydrophobous polymer to
prevent the water from entering the cell gas chamber. In
this structure there are no gas channels in the bipolar
plates 1, 7 or in the gas distribution layers 3, 5. The gas
distribution layers can have a porosity exceeding 90% and
they should be good electrical conductors and have proper
corrosion resistance against acid proton conducting
membrane.
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
8
The present invention may be combined with the
conventional serpentine channel structure. The principle
of this is illustrated in figure 6. The same reference
numbers has been used as in the embodiment according to
Fig. 2. The anode layer side of each bipolar plate 1, 7
may be provided with an anode gas channel 19 and at
least one water inlet 20. A water outlet 21 may also be
provided. The cathode layer side of each bipolar plate
is provided with at least one cathode gas channel 22.
An alternative structure for the water channels is presented
in Fig. 7. In this embodiment of the invention, the catalyst
layer 16 is located on top of the membrane 4. A hydrophobous
layer 18 is positioned between the membrane and the gas
distribution layer. The function of this layer 18 is to let
gas diffuse to the electrode (catalyst layer) but not let
the water to escape from the water channel 3a.
The embodiment according to Fig. 7 may be used for operation
of a liquid-gas direct methanol fuel cell. In such an
embodiment of the invention, the anode side of the cell is
fed with a liquid water-methanol mixture, which is totally
or partially evaporated in the cell. The liquid mixture is
fed in such a way that most part of the anode electrode is
in contact with a thin film of liquid methanol-water
mixture. The remaining area of the anode electrode is in
contact with the gas phase free from liquid. This in order
to enable both fast release of gaseous carbon dioxide as
well as for humidifying of membrane by water vapor reactant
to remaining part of the anode area. Water and methanol are
transferred from fuel feeding channels to the anode
electrode both directly and via gas phase. This is
illustrated by means of arrows in Figure 7. The above
CA 02412180 2002-12-09
WO 02/05373 PCT/SE01/01514
9
described method may also be applied to other types of fuel
cell structures which are direct liquid cooled.
The water channel structure is preferably applied to the
anode side. However, this structure can also be applied to
the cathode side or to both sides simultaneously.
The invention is not limited to the above described
embodiments, instead several modifications are possible
l0 within the scope of the following patent claims.