Note: Descriptions are shown in the official language in which they were submitted.
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
INJECTION SYSTEM FOR GENE DELIVERY
FIELD OF THE INVENTION
This invention relates to catheters, and to medical diagnostic and
therapeutic systems utilizing catheters. More specifically, it relates to
methods
of diagnosing and treating disorders of internal organs of mammalian patients,
and catheter apparatus specifically designed for use in such methods.
BACKGROUND OF THE INVENTION AND PRIOR ART.
ZO Injection catheters are known, for delivery of therapeutic
substances to internal body organs, by insertion of the catheter through an
artery in the patient's body to the vicinity of the organ which it is desired
to treat.
For example, injection catheters are known for administering treatment to the
heart. Such a catheter has a relatively long, flexible tube equipped at its
distal
end with an injection needle, and at its proximal end with an operating means
to
operate the injection needle. The catheter is introduced through a puncture in
the patient's artery and advanced, with the injection needle in a retracted
position, until the vicinity of the organ to be treated, e.g. the myocardium,
is
reached by its distal end. Then the operating means, outside the patient's
body,
is actuated so that the injection needle is made to extend beyond the distal
end
of the catheter tube and info the organ. A further actuation of the operating
means may cause discharge of therapeutic fluid, e.g. from a reservoir thereof
contained in the catheter tube, or from a syringe attached to the external
port of
the needle assembly, to be discharged through the needle and into the organ,
at the location of tissue penetration. An example of such a catheter is
described
and illustrated in United States patent 6,004,295 Langer and Stewart, issued
December 21,1999, the entire disclosure of which is incorporated herein by
reference.
One application for injection catheters of the above type is in the
delivery of extremely small quantities of therapeutic substances to precise
locations of an organ or vessel. This can arise, for example, in treatment of
a
SUBSTITUTE SHEET (RULE 26)
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-2-
patient's endocardium with a therapeutic fluid such as a DNA solution, in gene
therapy. Localized treatment of the endocardium, or other portions of the
heart
such as the myocardium, to repair focal damage, requires very precise control
over the location and delivery of the therapeutic DNA fluid, and knowledge on
the part of the operator of the precise location at which the therapeutic
fluid
delivery is being made.
Mukherjee, Debabrata et. al.. " Ten-fold Augmentation of
Endothelial Uptake of Vascular Endothelial Growth Factor with Ultrasound After
Systemic Administration", Journal of the American College of Cardiology,
Vo1.25, No. 6, May 2000, pp1678-86, describe perfluorocarbon-exposed
sonicated dextrose albumin (PESDA) and its use as ultrasound contrast
microbubbles to enhance the uptake of VEGF by the myocardium. PESDA is a
solution of microbubbles containing perfluorocarbon (-~6pm in diameter)
enveloped in an albumin shell, and is produced by sonicating a solution of
dextrose containing alkiumin and perfluorocarbon gas. The microbubbles act as
an ultrasound reflector, so that on application to the vicinity of the
microbubble
injection, of ultrasound of an appropriate energy level, a reflection of
ultrasound
from the microbubbles can be detected e.g. with a transducer, and the
reflection
analyzed to determine the location and distribution of the microbubbles. At
higher acoustic energies, the microbubbles burst in situ, and release their
contents to their environment.
SUMMARY OF THE INVENTION
The present invention, from one aspect, provides a catheter
having a catheter tube and equipped with means for delivering echocontrast
medium and, at its distal end, not only with an injection needle but also with
a
piezoelectric ultrasound device, capable of emitting ultrasound at two or more
energy levels. Other aspects of the invention are various processes,
diagnostic
and therapeutic, in which such a catheter may be used. The catheter can be
introduced into the patient's body e.g. advanced through an artery, to abut
the
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-3-
internal organ to be treated, e.g. to abut the myocardium. Then, with the
injection needle eifiher adjacenfi to or extending into the tissue of the
organ, low
energy ultrasound is delivered to the tissue by the ultrasound crystal. An
ultrasound contrast agent such as PESDA is delivered to the tissue by the
needle. The low energy ultrasound is reflected and imaged by use of an
appropriate transducer, so that the exacfi location of the injection needle's
penetration can be determined by the operator. Subsequently, e.g. when the
location of penetration has been verified, the ultrasound energy is raised to
a
second level, at which it causes focal tissue perturbation or even disruption.
This can, for example, be focal myocardial disruption so as to stimulate
angiogenesis at the location (e.g. direct myocardial revascularization). As
another example, it may be used to ablate conduction tissue during an
electrophysiology procedure to lock conduction in an accessory pathway.
Thus according to a first aspect of the invention, there is provided
an injection catheter system comprising:
an extended flexible catheter tube for insertion and extension
along a patient's artery, said tube having a distal end and a proximal end;
an injection needle at the distal end of the catheter tube capable
of being extended beyond the distal end of the catheter tube;
a piezoelectric ultrasound emitting device at the distal end of the
catheter tube, said device being capable of emitting ultrasound at a first,
lower
energy for detection of reflections thereof, and at a second, higher energy
for
localized disruption of adjacent tissue;
means for delivering ultrasound contrast material through the
injection needle;
and means for analyzing reflections of the ultrasound emitted by
the ultrasound emitting device and reflected by the ultrasound contrast
material.
According to another aspect of the invention, there is provided a
process for the diagnosis and/or treatment of localized internal body organ
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-4-
disorders in a mammalian patient, which comprises:
introducing a catheter into the vicinity of the internal body organ
surface so that the distal end thereof is adjacent to the surface of the
organ;
projecting an injection needle from the distal end of the catheter to
penetrate the organ surface;
delivering ultrasound contrast material through the injection
needle into the organ surface at the location of penetration;
transmitting ultrasound signals of a first, energy from the distal
end of the catheter to the location of penetration of the organ surface and
collecting reflected ultrasound signals from said ultrasound contrast
material;
analyzing said reflected signals to determine the precise location
of penetration of the organ surface by the injection needle;
and transmitting ultrasound signals of a second, tissue-perturbing
energy level from the distal end of the catheter following verification of the
location of penetration.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In addition, in a further embodiment in which a catheter as
defined above may, if desired, be used, the invention provides a treatment
process whereby a therapeutic substance such as DNA is delivered along with
the ultrasound contrast material. The ultrasound, at the same or at a
different
energy level, causes perturbation, possibly disruption, of the tissue to
promote
action of the therapeutic substance on the tissue and perhaps to separate it
from the contrast material, but at the same time allows the operator to
visualize
the therapeutic biologics! and the contrast material as it enters the tissue.
Accordingly its location within the tissue can be confirmed. This is a major
advantage, especially when treating the myocardium, for example, since it
allows the operator to know that indeed intramyocardial agent delivery was
accomplished, a difficult determination with other myocardial injection
procedures and apparatus. This significantly reduces the risk that injectate
might leak back, or even be delivered directly into the circulation.
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-5-
Another embodiment of the invention contemplates the delivery in
this manner of echo contrast material with or without a tissue-affecting
substance to the location of desired tissue perturbation or disruption. Once
the
correct location of the contrast material has been confirmed by ultrasonic
imaging, a graduated increase in ultrasound energy can be delivered to cause
a focal disruption of tissue at carefully predetermined locations of a body
organ
or vessel such as the heart. Energy levels can be chosen to result in
reversible
damage, for example to an accessory electrical pathway, to confirm that a
desired therapeutic result can be achieved, and then permanent ablation of the
offending tissue can be accomplished with high energy ultrasound.
Another embodiment of the invention, in which the device
defined above can also, if desired, be used, combines the benefits of
therapeutic substance delivery in combination with echocontrast material,
allowing visualization of the focal delivery of the therapeutic material as
described above, with the benefits of focal tissue perturbation by ultrasound
emission. Once the location of penetration of the organ by the injection
needle
has been verified by analysis of the ultrasound reflections at the first,
lower
energy level, the ultrasound energy level from the ultrasound emitting device
can be adjusted if necessary to a second level at which it disrupts any
combination of the therapeutic material and the echocontrast material, and
then
adjusted again, if necessary, to raise it to a level at which it causes focal
tissue
disruption. In this way, the therapeutic substance is delivered to the tissue
and
transferred to the myocardium in the precise location required to be treated.
The ultrasound and the penetration of the injection needle combine to render
the tissue and cells at the treatment location physically more receptive to
accept
the therapeutic substance, e.g. by tissue perturbation or even tissue
disruption,
for a gene therapy process of enhanced efficiency, and at the same time
augment the angiogenic response by eliciting a trigger mechanism for
angiogenesis, e.g. tissue injury.
Another preferred application of the catheter and process of the
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-6-
invention is in the diagnosis and treatment of vascular disorders such as
stenosis, for example in combination with balloon angioplasty. The delivery of
echocontrast material and the imaging of ultrasound reflections into the
precise
location can be accomplished using modifications of angioplasty balloon
catheters to incorporate the ability to inject this material directly into the
media
of the arterial vesel. As before, the localization of the echo contrast
material can
be confirmed using standard intravascular ultrasound imaging approaches.
Perturbation or even disruption of the tissue at that locafiion can beachieved
by
the delivery of ultrasound of an appropriate energy level can be used to
assist
in the repair of the damage. Therapeutic material to counteract tendency to re-
stenosis may be administered to the tissue along with this perturbation-
causing
ultrasound, which can result in increased gene transfer efficiency as
described
above,
The preferred echocontrast material is the aforementioned
PESDA in microbubble form, although it is by no means limited thereto. Other
ultrasound echocontrast materials used for internal imaging in medical
applications may be used as well. When a microbubble form of echocontast
material is used, the therapeutic material is preferably delivered while
enclosed
within the microbubbles. The ultrasound, at a higher energy level, causes
disruption of the microbubbles to release the therapeutic material, at the
precise, accurately visualized delivery location. The disruption of the
microbubbles by the ultrasound may cause transient perturbation of myocyte
cell membranes, when the process is, as is preferred, applied to treatment of
ZS the myocardium with gene therapy, opening pores and allowing genetic
material
to enter the cells. This may result in increased transfection efficiency.
The piezoelectric ultrasound emitting device which is used in the
process and apparatus of the invention is suitably one more piezoelectric
crystals, e.g. arranged in an array. The same or different ones of the
crystals
may both emit ultrasound and receive the reflected ultrasound. Different
crystals may be used to transmit the ultrasound of different energy levels, or
a
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
_7_
single crystal my be arranged to emit a variable ultrasound energy level. The
ultrasound emitting and receiving crystals) are connected to a stand
ultrasound
machine for analsis of reflected signals and supply of apptopriate power.
BRIEF REFERENCE TO THE DRAWINGS
Figure 1 of the accompanying drawings is a diagrammatic
illustration, with parts cut away, of a form of catheter according to the
present
invention, and useful in the processes of the invention;
Figures 2, 3 and 4 are diagrammatic illustrations of the operation
of the distal end of a catheter as generally illustrated in Figure 1, in
conducting
a process according to a preferred embodiment of the present invention.
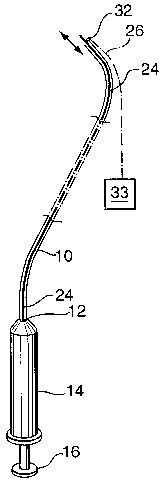
One form of catheter for use in a system according to the
invention is diagrammatically illustrated in Figure 1 of the accompanying
drawings. It comprises an elongated flexible catheter tube 10 having at its
proximal end 12 a syringe 14 with a plunger 16. A "nitinol" type long
injection
needle 24, in fluid communication with the syringe 14, extends the length of
the
catheter 10.
A piezoelectric ultrasound emitting and receiving device 32 is
provided at the distal end of the catheter tube 10. The device 32 may comprise
a plurality or array of piezoelectric crystals, of known type, and is
connected via
connector 26 to a standard ultrasound machine 33 for supply of power and for
reception and analysis of reflected ultrasound signals.
In operation to treat the myocardium of a patient, the catheter 10
is moved within the artery to the position shown diagrammatically in Figure 2,
with its distal end against the myocardial wall 34 of the patient, and the
injection
needle 24 extending beyond the distal end of the catheter 10 to penetrate the
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
_g_
myocardial wall 34. This causes some degree of disturbance and perturbation
of the tissue of the myocardial wall, as indicated at 36.
Next, the syringe 14, 16 on the end of the catheter, is operated so
that microbubbles 38 of ultrasound contrast material containing therapeutic
DNA are delivered from the syringe 14 and discharged from the injection needle
24 into the tissue of the myocardial wall 34. This is the position shown in
Figure
3 of the accompanying drawings. Ultrasound is now emitted, at a first energy
level, from piezoelectric device 32. The frequency of the ultrasound may be
adjusted to improve the image received - higher frequencies tend to give
shallow penetrations of the ultrasound (which is all that is normally required
in
the process of the present invention). The reflections of the ultrasound are
detected and analyzed, by ultrasound machine 33, to crest an image and to
determine the exact location of penetration of the injection needle 24 into
the
myocardial wall 34. '
When this location has been verified, the power of the ultrasound
emitted by the piezoelectric device 32 is increased, as indicated in Figure 4,
so
that the microbubbles 38 are disrupted and release their therapeutic DNA
contents, to the location of penetration. This increased ultrasound power also
causes additional tissue and cell perturbation and disruption of the location,
for
easier acceptance of the DNA material therein, and for triggering angiogenesis
in the myocardium at the location of treatment.
The process and apparatus according to the invention provides a
means not only for accurate location and positioning of an injection catheter
for
delivery of therapeutics such as DNA material in gene therapy, but also a
process and means for enhancing the uptake of the therapeutic material, by
ultrasound perturbation or disruption of cells and tissues at the location to
be
treated. Very small amounts of therapeutic material, e.g. volumes of the order
of 100 microliters can be delivered this way. The material is both visualized
and
delivered in an advantageous manner by the process and apparatus of the
CA 02413269 2002-12-18
WO 01/97698 PCT/CA01/00953
-9-
present invention. The process and apparatus allows the operator to know
exactly where the gene delivery is taking place, to improve the gene transfer
process, and to verify that the delivery and transfer has taken place.
It will be understood that the apparatus and process described
herein is by way of example only, and that variations of the apparatus and
technique can be made_within the scope of the present invention. It is of
general application to diagnosis and treatment of internal body organs,
vessels
and the like, where precise knowledge of the location to be treated is
required
to be established, and where precise control of the internal location of
tissue
perturbation or disruption, for imitation of angiogenesis, subsequent delivery
of
therapeutics such as gene therapy, or subsequent application during
electrophysiology to produce ablation of electrical or the like is to be
undertaken.
20
30