Note: Descriptions are shown in the official language in which they were submitted.
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 WATER SEPARATION FROM SOLVENT
2 CROSS REFERENCE TO RELATED APPLICATIONS
3 This application claims the benefit of copending U.S. provisional patent
application
4 serial No. 60/215,055 filed June 29, 2000, the teachings of which are
incorporated herein by
reference.
6 FIELD OF THE INVENTION
This invention generally relates to the field of chemical laboratory equipment
for
8 sample preparation and particularly to the use of a hydrophobic membrane to
separate water
9 from an organic solvent, and more particularly to an apparatus and method
for increasing the
l0 flow rate of the solvent through the membrane without adversely affecting
the performance
11 of the membrane.
12 BACKGROUND OF THE INVENTION
13 When samples are to be analyzed for organic and/or inorganic trace
compounds, the
14 samples are typically extracted with an organic solvent. The solvent
extracts the compounds
from the sample, due to selective chemistry.
16 Before the extract can be analyzed, all residual water should preferably be
removed
17 from the extracting solvent. This is due to the adverse effect residual
water can have on
18 subsequent sample preparation steps which are required to prepare and
analyze the samples.
19 Current practice embodies the use of a drying agent called sodium sulfate
and has
2o been the standard technique to remove the residual water from solvent
extracts. Sodium
21 sulfate is a granular material that has a high binding capacity for
residual water. The sodium
22 sulfate is first heated to. drive off any water that has been adsorbed into
the material. This
23 typically requires heating overnight at 400C. The sodium sulfate is then
placed into a glass
24 funnel containing filter paper, or a chromatography column. The funnel or
column is then
washed with extracting solvent to wash off any impurities, The extracting
solvent is then
26 discarded. Once the sodium sulfate is clean, the solvent extract is poured
on top of the
27 sodium sulfate. As the solvent drains slowly through, the residual water
becomes bound to
28 the surface of the sodium sulfate. The collected solvent passing through is
now dry and ready
29 for analysis.
3o The use of sodium sulfate, even though easy to use, requires many physical
31 manipulations. Sodium sulfate requires the use of glassware that must be
subsequently
32 washed so as not to introduce contaminants into the samples and requires
the purchase of, and
1
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 the disposal of, the used sodium sulfate. The labor time and the materials
costs, add
2 significantly to the total cost of performing sample extractions.
3 U.S. Patent 5,268,150 assigned to Corning Incorporated, discloses the use of
a
4 hydrophobic membrane in an extraction device which allows a solvent to pass
therethrough,
yet will not allow a significant amount of water from the sample liquid to
pass therethrough.
6 The patent discloses that hydrophobic membranes incorporating
polytetrafluoroethylene
7 (PTFE) have been found to be very effective in achieving the desired results
of letting solvent
8 pass, while retaining the sample usually consisting of a relatively large
portion of water or an
9 aqueous solution. The patent goes on to state that the typical dimensions of
the membrane
1o range from 10 to 50 millimeters in diameter with a thickness ranging from
0.1 to 5.0 microns
11 with a pore size ranging from 0.2 to 5.0 microns, depending upon the sample
being
12 processed.
13 Accordingly, it is an object of the invention to improve on the above
referenced
14 designs and provide a more efficient technique for separation water from a
given solvent.
More specifically, it is an object of the present invention to provide a
method and apparatus
16 and improved membrane design to improve the purification flow rate of a
solvent/water
17 mixture or emulsion through said membrane, to remove water, without
adversely effecting
18 membrane performance.
19 SUMMARY OF THE INVENTION
2o A method/apparatus for separating residual water from a solvent, comprising
the steps
21 of providing a reservoir containing a solution comprising solvent
containing residual water,
22 the reservoir having an opening to allow the solution to drain from the
reservoir, and passing
23 the solution in the reservoir through a fluoropolymer membrane supported on
a
24 fluoropolymer screen. The supported membrane is positioned in series with
the reservoir
opening, the membrane having a first side in contact with the solution and an
opposing
26 second side. Pressure is decreased on the second side of the supported
membrane relative to
27 the first side of said supported membrane to thereby increase the flow rate
of the solvent
28 through the membrane, wherein the fluoropolymer membrane operates to remove
water from
29 the solvent.
3o BRIEF DESCRIPTION OF THE DRAWINGS
31 Figure 1 is a sectional view of a first separator apparatus in accordance
with the
32 present invention, and
2
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 Figure 2 is a sectional view of a second separator apparatus in accordance
with the
2 present invention.
3 Figure 3 is an exploded view of a preferred separator apparatus in
accordance with the
4 presentinvention.
The above and other objects, feature, and advantages of the present invention
will be
6 apparent in the following detailed description thereof when read in
conjunction with the
'7 appended drawings wherein the same reference numerals denote the same or
similar parts
8 throughout the several views.
9 DETAILED DESRIPTION OF THE DRAWINGS
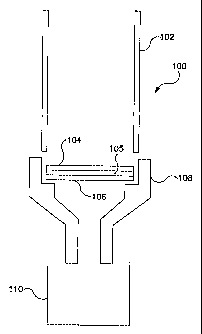
1o Referring to the drawings, there is illustrated generally a first
concentrator/extractor
11 apparatus 100. The concentrator/extractor apparatus 100 comprises a column
102 and
12 fluoropolymer material layers 104 and 105. Preferably, fluoropolymer layer
104 is laminated
13 to fluoropolymer layer 105 to provide a membrane type construction. A
preferred
14 fluoropolymer for layer 104 is PTFE and a preferred fluoropolymer for layer
105 is ethylene-
chlorotrifluroethylene (ECTFE).
16 A screen support layer is shown at 106, in addition to a base assembly 108,
and a
17 collection vessel 110. The column 102 forms a reservoir to hold a solvent.
The column 102,
18 which may be pressed down on top of the membrane (fluoropolymer layer 104
laminated to
19 fluoropolymer layer 105) may be used to hold the membrane in place. The
column 102 may
2o seal the membrane and prevent any solvent from passing around the edge of
the membrane.
21 The column 102 and the collection vessel 110 are preferably made of glass.
The screen
22 support member 106 is preferably an ECTFE or ETFE fluoropolymer fabric
screen with 0.5- .
23 1.0 mm openings, 0.5 -1.0 mm thick, and a 0.25-0.50 mm thread.
24 The membrane comprises layers 104 and 105 are preferably characterized as
follows:
Pore Size: 0.05 to 0.2 micron;
26 Bubble Point: Individual between 24.0 psi and 34.0 psi (47 mm membrane;
27 isopropanol at 21°C)
28 WEP: 50.0 psi minimum individual
29 Gurley Number: Mean < 30.0 seconds (100 cc air through 1 in2 orifice, 4.88"
water
pressure drop)
3
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 Thickness: Preferably 1.0 mils to 20 mils.
2 The following definitions apply to the above:
3 Gurley number: A measure of the air permeability of the fluoropolymer. The
Gurley
4 number is the time in second required for 100cc of air to pass through a one
square inch area
of membrane, when a constant pressure of 4.88 inches of water is applied.
6 Bubble point: The minimum pressure in KG/CMZ required to force air through
the
7 fluoropolymer that has been prewetted with water, isopropanol, or methanol.
8 Water entry pressure: The pressure at which water permeates through the
membrane.
9 This is a visual test.
In a preferred embodiment, the PTFE layer 104 has usable diameters in the
range of
11 40-100 mm. The fluoropolymer layer 104 and fabric support member 105 are
positioned in
12 series between the column 102 and the collection vessel 110. In a most
preferred
13 embodiment, a 3 mil thick PTFE layer 104 with a 0.1 micron pore size is
supported on a 10
14 mil thick non-woven layer 105, comprised of ECTFE polymer, which ECTFE
polymer is
preferably obtained from Ausimont and sold under the tradename "HALAR".
16 It is worth noting that in a preferred embodiment, a 3.0 mil PTFE layer is
laminated to
17 a 10 mil ECTFE layer, and. a resulting thickness of 3-7 mils is produced
for the laminate as a
18 result of the heat setting laminating process.
19 In accordance with the present invention, the screen layer 106 is
preferably ethylene-
trifluroethylene copolymer (ETFE). The screen layer serves to gap or space
laminated layers
21 104 and 105 on the funnel surface such that it is possible to distribute
the pressure differential
22 across the entire cross-sectional area of the funnel surface to achieve
more efficient
23 performance. However, while it can be appreciated that screen layer 106 is
a separate
24 components, it can be appreciated that screen layer 106 may actually be
incorporated directly
into the surface of the funnel upon which the laminated layers 104 and 106
rest. This would
26 provide the equivalent effect of spacing laminated layers 104 and 106 to
evenly distribute the
27 pressure differential created~by vacuum.
28 Furthermore, in the context of the present invention it should be
appreciated that the
29 removal of water from a given solvent containing, e.g., some analyte to be
evaluated by
4
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 techniques such as gas-chromatography/mass spectrometry (GC/MS), is such
that the
2 removal of water is highly efficient and allows for the generation of a
GC/MS analysis that is
3 not compromised by the presence of water. In that regard, it has been found
that the present
4 invention allows for removal of water down to a level at or below 1.0 ppm.
Expanding upon the above, it will be appreciated that with respect to the
removal of
6 water herein, it has been found that by reference to the generation of a
GC/MS analysis that is
7 not compromised by the presence of water, it should also be understood that
this is reference
8 to the fact that the water removal herein is sufficient to reduce the water
levels to that level
9 wherein the possibility of contamination of the GC column by a water soluble
inorganic acid
1o is removed or attenuated. In addition, the possibility of any degradation
of the GC column
11 due to the presence of water soluble inorganic salts is also equally
attenuated or removed, and
12 GC/MS can proceed without such problems.
13 Additionally, it is worth noting that the invention herein is preferably
applied to a
14 water/solvent mixture wherein the solvent is denser than water. However, in
broad context
the invention herein is not so limited.
16 As shown in Figure 2, there is illustrated generally a second
concentrator/extractor
17 apparatus 200. The concentrator/extractor apparatus 200 comprises a column
202, a
18 fluoropolymer layer 204 (PTFE) and a fluoropolymer layer 205 (ECTFE) that,
as noted
19 above, are preferably laminated to one another. In addition, a support
screen member 206 is
shown, a base assembly 20~, and a collection vessel 210. The apparatus 200 can
be coupled
21 to an external low-level vacuum 216. A low level vacuum is one that
preferably creates a
22 pressure drop of less than 6" Hg. Alternatively, the assembly 200 could
include a vacuum
23 generator device that uses a compressed gas source to create a pressure
differential. This
24 assembly 200 could be manufactured as a unit and could sit in a hood,
directly underneath a
separatory funnel. Once the gas source is set, the operator may select one of
a plurality of
26 vacuum levels on a vacuum level selector panel 214. The vacuum selector
panel 214 controls
27 the pressure drop across the membrane. These levels may include: off, low,
medium, and
28 high. Alternatively, the vacuum level may be continuously variable. Being
able to select
29 from a variety of different vacuum levels has shown to be useful, as
samples which create a
significant emulsion can be quite easily broken if no vacuum is used. Once the
emulsion has
31 broken, then the vacuum setting can be increased to significantly reduce
the sample process
5
CA 02413799 2002-12-30
WO 02/02211 PCT/USO1/20555
1 time. For example, lOml of methylene chloride may take about 4 minutes to
flow through
2 with a 5"Hg vacuum, but the same sample through the same membrane may only
take 15-20
3 second at 6"Hg. This is a significant time savings.
4 A controller 212 coupled to the vacuum 216 can be added that will vary the
pressure
drop across the membrane as a function of time. For example, the controller
212 can be
6 programmed to have an initial predetermined period of time during which no
vacuum or a
7 very low first predetermined vacuum level is applied and a second
predetermined period of
8 time during which an increased second predetermined vacuum level is applied.
The
9 controller 212 can also be programmed to turn off the vacuum after a third
predetermined
to period of time to prevent the apparatus from pulling residual water through
the membrane.
11 Given sufficient time, approximately 6 - 12 hours, any residual water on
the surface of the
12 membrane may "wet" the membrane and flow through with the organic solvent.
Therefore,
13 there is a limited time window for allowing water to reside on the
membrane, but this time is
14 not a problem for the application that this device will be used for.
In addition, testing has shown that draining the emulsion directly into the
membrane
16 , reservoir aids with the breaking of emulsions. Once the emulsion has
broken, if the analyst
17 desires, after each drying step, the retained water and emulsion can be
poured back into the
18 separatory funnel for additional extractions. This could possibly
significantly increase
19 recovery values.
As noted, Figure 3 is an exploded view of a preferred separator apparatus in
21 accordance with the present invention. More specifically, as shown therein
there can be seen
22 locking ring 310, wave spring 312, thrust ring 314, reservoir 316, base 318
for membrane and
23 screen (not shown), stopcock 322, shut-off connectors 324 and 326 (through
which vacuum
24 may be applied), bracket 328 and support rod 330.
It should be understood that, while the present invention has been described
in detail
26 herein, the invention can be embodied otherwise without departing from the
principles
27 thereof, and such other embodiments are meant to come within the scope of
the present
28 invention as def ned in the following claims.
29
6