Note: Descriptions are shown in the official language in which they were submitted.
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
NON-INVASIVE CAROTID COOLER BRAIN HYPOTHERMIA MEDICAL
DEVICE
BACKGROUND OF THE INVENTION
This disclosure relates to a medical device and more specifically to a non-
invasive
medical device used to lower human brain temperature.
The medical device industry produces a wide variety of electronic and
mechanical
devices for treating patient medical conditions. Non-invasive medical devices
are used to
treat medical conditions without the need for invasive procedures such as
insertion of a
catheter into a blood vessel. Non-invasive medical devices are particularly
suitable for the
early responders to a medical condition such as emergency medical technicians
and
persons having limited training. The early application of a medical device
designed to
reduce injury to a patient can greatly enhance restoration of an individual to
a more
healthful condition and a fuller life. One type of non-invasive medical device
designed to
reduce injury is a brain cooler hypothermic medical device.
Cooling the brain can significantly reduce brain injury caused by lack of
blood
flow to the brain (ischemic) or lack of oxygen to the brain (anoxic). Ischemic
and anoxic
brain injury can be caused by conditions such as strokes, cardiac arrest,
transient ischemia
attacks (TIA), brain injury, toxic poisoning, respiratory arrest, suffocation,
electrocution,
edema, and head trauma. Under ischemic or anoxic conditions, reversible brain
damage
can start as early as four minutes after the condition has begun and
irreversible brain
damage can start as early as six minutes after the condition has begun.
Lowering brain
temperature, hypothermia, slows brain metabolic activity to slow or reduce
brain injury.
Early hypothermia of the brain in the initial critical minutes after injury
can significantly
reduce further injury compared with hypothermia of the brain performed once
the patient
reaches a facility such as a hospital.
Previous topical brain cooling medical devices do not efficiently target heat
removal from the patient's carotid arteries and can require application of an
encircling
collar to the patient. The cooling source is typically applied to the entire
collar, and the
collar is not shaped to apply the cooling source to the close proximity of the
patient's
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
carotid arteries. Additionally, the collar may be difficult to apply and risk
further injury to
patient's with neck and head.
Previous endotracheal brain cooling medical devices also do not efficiently
target
heat removal from the patient's carotid arteries. The cooling source is placed
in the
patient's oral cavity which is a considerable distance from the patient's
carotid arteries.
An example of a non-invasive brain cooling medical device is disclosed in U.S.
Patent No.
5,916,242 "Apparatus For Rapid Cooling Of The Brain And Method Of Performing
Same" issued to Schwartz (June 29, 1999).
For the foregoing reasons, there is a need for a non-invasive brain cooling
medical
device that more efficiently targets the patient's carotid arteries and
provides additional
improvements.
SUMMARY OF THE INVENTION
The non-invasive carotid cooler brain hypothermia medical device applies
cooling
efficiently to carotid arteries to rapid cool the brain to decrease brain
injury. In one
embodiment, at least one cooling element is configured to remove heat from an
area
substantially proximate the patient's carotid arteries. The cooling element is
carried on a
patient side of a topical carotid cooler. The topical carotid cooler is
configured for
placement on a patient's neck proximate the patient's carotid arteries and is
coupled to a
cooling source. In another embodiment, the cooling element is carned on an
endotracheal
carotid cooler. Tn other embodiments, methods for creating brain hypothermia
with a non-
invasive carotid cooler are provided.
BRIEF DESCRIPTTON OF THE DRAWINGS
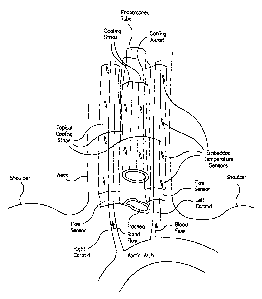
FIG. 1 shows an environment view of a patient anatomy;
FIG. 2 shows another environment view of the patient anatomy;
FIG. 3 shows a topical carotid cooler applied to a patient embodiment;
FIG. 4 shows a brain hypothermia system embodiment;
FIG. 5 shows a flow diagram of a method for brain hypothermia embodiment;
FIG. 6 shows an endotracheal carotid cooler applied to a patient embodiment;
FIG. 7 shows a cross-section view of both a topical carotid cooler and an
endotracheal
carotid cooler applied to a patient embodiment; and,
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
FIG. 8 shows front view of both a topical carotid cooler and an endotracheal
carotid cooler
applied to a patient embodiment.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
FIGS. 1 and 2 show environmental views of a patient; FIG. 3 shows a topical
carotid cooler applied to a patient embodiment; and, FIG. 4 shows a brain
hypothermia
system embodiment. One embodiment of the medical device for non-invasive brain
hypothermia comprises a topical carotid cooler, a cooling source, and a
cooling element.
This medical device embodiment can also include a cooling controller, a
patient
thermometer, a carotid flow sensor, a temperature sensor. The topical carotid
cooler is
configured for application on a patient's neck proximate the patient's carotid
arteries. The
topical carotid cooler has a patient side that is placed against the patient's
neck when the
topical carotid cooler is positioned on the patient. The cooling source is
coupled to the
topical carotid cooler and can be a wide variety of cooling sources capable of
cooling in
the range from about 37° Celsius (98.6° Fahrenheit) to about
0° Celsius (32° Fahrenheit).
The cooling source can include technologies such as a Peltier device, a
cryogenic fluid,
ice, salinated ice, cold water, active refrigerant systems, Joule-Thompson
cryostat, and the
like. The cooling source delivers cooling to the cooling element.
'The cooling element is at least one cooling element carried on the topical
carotid
cooler patient side, The cooling element is coupled to the cooling source, and
the cooling
element is configured to remove heat largely from an area substantially
proximate the
patient's carotid arteries. The cooling element is positionable over both
patient carotid
arteries. The cooling element can be at least a first cooling element
positionable over one
carotid artery and a second cooling element positionable over the other
carotid artery.
When a first and second cooling element are used, the cooling elements can be
positionable laterally to a patient's trachea. The temperature of the cooling
element is
regulated by the cooling controller.
The cooling controller is coupled to the cooling source for regulating the
amount of
cooling delivered by the cooling element. The cooling controller regulates the
cooling
source, so the temperature of the cooling element cools blood flowing through
the carotid
arteries to rapidly reduce brain core temperature down to no lower than about
30.0°
Celsius (86.0° F). The cooling controller regulates the cooling source
so the temperature
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
4
of the cooling element is not lower than about minus 2.0° Celsius
(35.6° F). The cooling
controller is also configured to accept a variety of sensory inputs such as
patient
temperature, cooling element surface temperature, carotid blood flow, and the
like. The
cooling controller can use these sensor inputs in its cooling control
algorithm to regulate
brain temperature to a predetermined value and otherwise regulate cooling. The
cooling
controller can also use sensor inputs to alert the medical device operator of
conditions
such as inadequate carotid blood flow. The cooling controller can also include
communication capabilities to access telephone, radio, and Internet networks
as desired.
For a patient temperature input to the cooling controller, patient temperature
can be
measured with a patient therometer.
The patient thermometer is coupleable to patient for measuring patient
temperature. The thermometer is can be any type of thermometer that measures a
patient's
temperature that can be correlated to brain temperature such as an infrared
thermometer
positionable in a patient's ear, bimetallic thermometers, thermistors,
resistive temperature
devices (RTDs), and the like. An ear thermometer which measures tympanic
membrane
temperature can be configured similar to a stereo headphone set for ease of
application to
the patient. The thermometer is coupled to the cooling controller to provide
an input of
patient temperature correlateable to patient brain temperature. Another sensor
that the
cooling controller can use for adjusting cooling or identifying conditions to
the operator is
a carotid flow sensor.
The carotid flow sensor is carned on the topical carotid cooler patient side
to
determine if there is adequate carotid arterial blood flow to the brain.
Patient carotid
blood flow to the brain is a critical parameter that should be monitored to
avoid conditions
that could adversely affect blood flow. The carotid flow sensor can use a wide
variety of
sensors technologies capable of sensing carotid arterial flow such as Doppler
ultrasound,
Doppler microwave, impedance plethysmography, accelerometry, Doppler laser
interferometry, and the like. The carotid flow sensor is coupled to the
cooling controller to
provide an input of patient carotid blood flow. Another sensor that the
cooling controller
can use for adjusting cooling or identifying conditions to the operator is a
temperature
sensor.
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
The temperature sensor is carried on the cooling element in a manner to
measure
cooling element surface temperature. The temperature sensor provides a cooling
element
temperature input to a cooling controller. The cooling controller will
typically regulate the
cooling element to prevent cooling below -2.2° Celsius (28° F)
because these
temperatures can induce tissue damage due to freezing also known as frostbite.
The
temperature sensor can be coupled to the cooling controller to assist in
regulating cooling.
'The topical carotid cooler can be used to perform a method for non-invasive
brain
hypothermia.
FIG. 5 shows a flaw diagram of a method for non-invasive brain hypothermia
embodiment. The medical device embodiment can be used to perform a method of
creating bxain hypothermia which may be beneficial in cases of stoke, cardiac
arrest,
transient ischemia attack (TIA), brain injury, toxic poisoning, respiratory
arrest,
suffocation, edema, head trauma, and the like. The method begins by applying a
topical
carotid cooler topically to a patient's neck. The topical carotid cooler is
operationally
positionable on a patient neck without the requirement of circumscribing the
patient's
neck. After application of the topical carotid cooler, the topical carotid
cooler is
positioned so that the cooling element is in close proximity to the patient's
carotid arteries.
Cooling the topical carotid cooler also cools blood flowing through carotid
arteries to the
patient's brain. Cooling blood destined for the patient's brain in turn cools
the patient's
brain. The cooled brain reduces tissue metabolism in the brain. By reducing
tissue
metabolism in the brain, the rate of irreversible brain damage caused by toxic
metabolic
byproducts can also be reduced. Toxic metabolic byproducts, including
neurochemical
such as glutamate, are produced by brain tissue from conditions such as stoke,
cardiac
arrest, transient ischemia attack (TIA), brain injury, toxic poisoning,
respiratory arrest,
suffocation, edema, head trauma, and the like. Additionally, the cooled brain
reduces
inflammation and swelling (edema) that often occurs with head trauma. Other
embodiments of the carotid cooler include an endotracheal carotid cooler.
FIG. 6 shows an endotracheal carotid cooler embodiment applied to a patient;
FIG.
7 shows a cross-section view of both a topical carotid cooler and an
endotracheal carotid
cooler applied to a patient; and, FIG. 8 shows a front view of both a topical
carotid cooler
and an endotracheal carotid cooler applied to a patient. The endotracheal
carotid cooler
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
embodiment of the medical device for non-invasive brain hypothermia comprises
an
endotracheal carotid cooler, a cooling source, and a cooling element. This
medical device
embodiment can also include a cooling controller, a patient thermometer, a
carotid flow
sensor, a temperature sensor, and a positioning balloon. The elements that are
common
between the previously described topical carotid cooler embodiment and the
endotracheal
carotid cooler embodiment are generally further described to the extent the
common
elements differ.
The endotracheal carotid cooler is configured for placement in a patient's
trachea.
The endotracheal carotid cooler forms an air passage having an inner surface,
an outer
surface, a distal end, and a proximal end. The cooling source is coupled to
the
endotracheal carotid cooler and serves as a means for removing heat from an
area
substantially proximate the patient's carotid arteries. At least one conduit
extending from
the proximal end of the endotracheal carotid cooler couples the cooling source
to the
cooling element. The cooling element is at least one cooling element and can
be more
than one cooling element carned on the endotracheal carotid cooler near the
distal end.
The cooling element is positionable distal to a patient's oral cavity to cool
blood flowing
through the patient's carotid arteries. The cooling source is regulated by a
cooling
controller.
The cooling controller is coupled to the cooling source for regulating the
amount of
cooling delivered by the cooling source. The cooling controller is
configurable to couple
to both the topical carotid cooler embodiment and the endotracheal cooler
embodiment to
regulate the cooling source either independently or in conjunction with one
another. The
carotid flow sensor carried on the endotracheal carotid cooler determines if
there is
adequate carotid arterial blood flow. The carotid flow sensor can be used as
an input for
adjusting the positioning balloon to ensure adequate blood flow. Once the
medical device
operator becomes aware that the carotid flow rate is inadequate, the medical
device
operator can change the position of the topical carotid cooler and
endotracheal carotid
cooler as necessary to achieve adequate carotid arterial blood flow. The
endotracheal
carotid cooler can also include a positioning balloon to assist in positioning
the cooling
element proximate the patient's carotid arteries.
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
The positioning balloon urges the cooling element toward the patient's carotid
arteries upon inflation of the positioning balloon. The positioning balloon
will typically
have an inflation conduit extending out of the endotracheal tube proximal end.
The
positioning balloon can be inflated manually by the medical device operator or
automatically by the cooling controller. When inflated manually, the medical
device
operator typically measures the inflation pressure by tactile feel or with an
instrument such
as a pressure gauge. When inflated automatically, the cooling controller
inflates the
positioning balloon to a predetermined inflation pressure or an inflation
pressure
dependent upon a sensed parameter such as the force the endotracheal carotid
cooler is
exerting near the patient's carotid arteries, or the sensed patient carotid
blood flow. The
endotracheal carotid cooler can be used to perform a method for non-invasive
brain
hypothermia.
FIG. 5 shows a flow diagram of a method for non-invasive brain hypothermia
embodiment. The medical device embodiment can be use to perform a method of
creating
brain hypothermia. The method begins by inserting the endotracheal carotid
cool into the
patient's trachea. The endotracheal carotid cooler is positioned distal to the
patient's oral
cavity at this time. After positioning of the endotracheal carotid cooler such
that the
cooling element is urged proximate to the patent's carotid arteries, the
endotracheal carotid
cooler is cooled. Cooling the endotracheal carotid cooler also cools blood
flowing through
carotid arteries to the patient's brain. Cooling blood destined for the
patient's brain, cools
the patient's brain. The cooled brain reduces tissue metabolism in the brain.
By reducing
tissue metabolism in the brain, the rate of irreversible brain damage caused
by toxic
metabolic byproducts is also reduced. Toxic metabolic byproducts, including
neurochemical such as glutamate, are produced by brain tissue from conditions
such as
stoke, cardiac arrest, transient ischemia attack (TIA), brain injury, toxic
poisoning,
respiratory arrest, suffocation, edema, head trauma, and the like. Also the
cooled patient's
brain also appears to reduce inflammation and swelling that often occurs with
head
trauma.
Thus, embodiments of a non-invasive brain hypothermia medical device are
disclosed to rapid cool the brain to decrease brain injury. One skilled in the
art will
appreciate that the present invention can be practiced with embodiments other
than those
CA 02415878 2003-O1-13
WO 02/05736 PCT/USO1/22036
disclosed. The disclosed embodiments are presented for purposes of
illustration and not
limitation, and the present invention is limited only by the claims that
follow.