Note: Descriptions are shown in the official language in which they were submitted.
CA 02416410 2003-O1-14
VENTILATION INTERFACE FOR SLEEP APNEA THERAPY
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to ventilation devices, and
particularly to a ventilation device having nasal inserts which are
inserted into the nostrils and seal against the nostrils without the
aid of harnesses, head straps, adhesive tape or other external devices,
and having exhalation ports designed to eliminate whistling noises, the
ventilation interface having particular utility in various modes of
therapy for obstructive sleep apnea. The invention may include a valve
used in lieu of the exhalation port:, and may include nasal inserts
used with filters for eliminating allergens and irritants from inhaled
air but used without positive airw,sy pressure.
2. DESCRIPTION OF THE RELATED ART
i5 Sleep apnea is a potentially lethal affliction in which breathing
stops recurrently during sleep. Sl.eE:p apnea may be of the obstructive
type (sometimes known as the pickwickian syndrome) in which the upper
airway is blocked in spite of airflow drive; the central type with
decreased respiratory drive; or a mixed type. Breathing may cease for
periods long enough to cause or to exacerbate cardiac conditions, and
may be accompanied by swallowing of the tongue. Sleep apnea frequently
results in fitful periods of both day and night sleeping with
drowsiness and exhaustion, leaving the patient physically and mentally
debilitated.
1
CA 02416410 2003-O1-14
In recent years it has been found that various forms of positive
airway pressure during sleep can be an effective form of therapy for
the apnea sufferer. Ventilation ~~an be applied in the form of
Continuous Positive Airway Pressure (C: PAP) in which a positive pressure
is maintained in the airway throughout the respiratory cycle, Bilevel
Positive Airway Pressure (BiPAP) in which positive pressure is
maintained during inspiration but reduced during expiration, and
Intermittent Mechanical Positive Pressure Ventilation in which pressure
is applied when an episode c:>f apnea is sensed. Positive airway
pressure devices have traditionally employed either a face mask which
only covers the patient's nose, or nasal pillows as the interface
between the ventilation device and the patient's airway. However,
there are problems with both of these interfaces.
The face mask requires a harness, headband, or other headgear to
keep the mask in position, which many patients find uncomfortable,
particularly when sleeping. The face mask must seal the mask against
the patient's face, and may cause irritation and facial sores,
particularly if the patient moves his head while sleeping, causing the
mask to rub against the skim. Face masks are also position dependent,
and may leak if the mask changes position with movement of the
patient' s head. The face mask applies pressure to the sinus area of
the face adjacent to the nose, causing the airways to narrow, thereby
increasing the velocity of flow through the airway, but decreasing the
pressure against the nasal mucosal. walls. This strips moisture from
the mucosal wall during inspiration, thereby causing drying and a
burning sensation. These factors will often result in the patient' s
removal of the mask and discontinuance of positive airway pressure
therapy.
Nasal pillows are pillowed style nasal seals which are pressed
against the inferior portion of the nares to close the nostril
2
CA 02416410 2003-O1-14
openings. Nasal pillows require a headband or harness to maintain the
pressure, resulting in the same patient discomfort noted with face
masks. Nasal pillows have about a 0.2.'5" internal diameter at the nasal
entry port where the seal is made. 'therefore, pressurized air must
pass through a constricted port, increasing the velocity of airflow,
with resultant drying and burning of the nasal airways . The narrowed
interface diameter of the nasal pillows causes a pressure drop, which
is directly proportional to the drop in the number of available air
molecules within the closed system. :Ct is the volume of air molecules
at the area in the patient' s throat where the apneic events appear that
is needed to correct apnea. The narrower the airways or the internal
diameter of the nasal interface, the :Lower the volume of air molecules
that will be available and th~~ great.er the driving pressure that is
required to meet the volume demand. An increase in driving pressure
does not fully compensate for the l.o:~s in the number of air molecules
available.
A further problem with existing ventilation devices is that the
carbon dioxide bleed ports f:or venting exhaled gases are noisy on both
nasal face masks and nasal pillows. The whistling noise that occurs
while utilizing such devices can prove quite annoying to the patient,
awakening the patient and causing the patient to discontinue use of the
ventilation device.
A number of devices have been proposed which include a ventilation
interface for supplying gases to be inhaled, for collecting exhaled
gases, or for mounting sensors for measuring or monitoring respiratory
function.
U. S. Patent Nos. 5, 335, 654 and 5, 535, 739, issued on August 9, 1994
to Rapoport and July 16, 1996 to Rapoport et al., respectively,
describe a CPAP system using a conventional nasal mask, the innovation
comprising a flow sensor in the input line connected to a signal
3
CA 02416410 2003-O1-14
processor to determine the waveform of airflow, which is connected to
a flow controller to adjust the press°are of airflow as required. U.S.
Des. Pat. No. 333, 015, issued February 2, 1993 to Farmer et al. . shows
an ornamental design for a nasal mask. U.S. Des. Patent No. 262,322,
issued December 15, 1981 to Mizerak, shows an ornamental design for a
nasal cannula with a mouth mask.
U. S. Patent No. 4, 782, 832, issued November 8, 1988 to Trimble et
al . , discloses nasal pillows held in the patient' s nose by a harness
arrangement, the device having a p:Lenum with two accordion or bellows
shaped nipples for fitting against the nostril openings. U.S. Patent
Nos. 4, 774, 946, issued October 4, 1988 to Ackerman et al., teaches a
nasal and endotracheal tube apparatus for administering CPAP to
infants, the nose tubes having a bulbous portion for seating in the
nares of an infant and a headband with a Velcro0 closure for supporting
the cannula and supply tubes.
U. S. Patent Nos . 5, 269, 296, issued to Landis on December 14, 1993,
and 5, 477, 852 and 5, 687, 715, issued to Landis et al. on December 26,
1995, and November 18, 1997, respectively, describe CPAP devices for
the treatment of sleep apnea with relatively stiff or rigid nasal
cannulae or prongs surrounded b;y inflatable cuffs to retain the
cannulae in the nares, but which also may be supplemented by an
inflatable head harness to position the cannulae and hold them in
place, the two cannulae being joined by a conduit having vent holes to
vent exhaled air. U. S. Patent No. 5, 533, 506, issued ,Tuly 9, 1996 to
the present inventor, discloses <~ nasal tube assembly in which the
tubes are tapered, frustro-conical assemblies with a soft membrane over
the distal tip and a washer at the k>ase of the nasal tube to prevent
the tubes from falling through a support bar connected to a harness,
the nasal tubes forming a positive seal with the inside of the nostrils
to prevent the escape of gases.
4
CA 02416410 2003-O1-14
U. S. Patent No. 5, 682, 881, issued November 4, 1997 to Winthrop et
al., shows a nasal cannula for CPAP therapy with cone shaped nasal
prongs in which the cannuia is secured to the patient' s upper lip by
adhesive tape strips. U. S. Patent. No. 4, 915, 105, issued April 10, 1990
to Lee, teaches a miniature respiratory breather apparatus in which
relatively stiff or rigid nasal tubes have elastomeric packings for
sealing the tubes in the nares.
Several patents describe improvements to nasal cannulae, but
without sealing the nose tubes against the nostrils to prevent leakage
of gas, including: U. S. Patents No. 3, 513, 844, issued May 26, 1970 to
Smith (metal strip in cannula cross-tube to retain configuration
matching patient's lip) ; U.S. Patent No. 4, 106, 505, issued August 15,
1978 to Salter et al. (cannula body with ends extending upward and
rearward) ; U.S. Patent No. 4, 915, 109, issued April 10, 1990 to Marcy
( clasp with lanyard supporting supply tubes t=o ease pressure on ears ) ;
U. S. Patent No. 5, 025, 805, issued June 25, 1991 to Nutter (cylindrical
soft sponge cuff around supply tubes to ease pressure and prevent skin
injuries); U.S. Patent No. 5,096,491, issued September 10, 1991 to
Derrick (device for collecting gases exhaled from both nose and mouth) ;
U.S. Patent No. 5, 335, 659, issued August 9, 1994 to Pologe (device for
mounting optical sensor on nasal septum) ; U. S. Patent No. 5, 509, 409,
issued April 23, 1996 to Weatherholt (nasal cannula with face guards) ;
U. S. Patent No. 5, 572, 994, issued NovE:mber 12, 1996 to Smith (rotatable
coupling in supply tubing) ; U. S. Patent No. 5, 636, 630, issued June 10,
1997 to Miller et al. (support for supply tubes); U.S. Patent No.
5, 704, 916, issued January 6, 1998 to Byrd (novel head strap for nasal
cannula); and U.S. Patent No. 5,'704,799, issued April 21, 1998 to
Nielsen (device with one-way flow through cannula and flow restrictor
to equalize flow into two nose members).
5
CA 02416410 2003-O1-14
None of the above inventions and patents, taken either singly or
in combination, is seen to describe the instant invention as claimed.
Thus a ventilation interface for s:Leep apnea therapy solving the
aforementioned problems is desired.
SUMMARY OF THE INVENTION
The present invention is a ventilation interface. The ventilation
interface includes a pair of rlasa~_ inserts . Each nasal insert is a
hollow body made from a flexible, resilient, soft, biocompatible
material. Each nasal insert has a :base end adapted for connection to
a ventilator air flow and an open distal tip end. Each nasal insert
is substantially oval in cross-section at the base end and the distal
end and continuously oval in cross-section between the base end and the
distal end. A flange is formed as a bead disposed about the distal tip
end of each nasal insert. The flange is adapted for forming a seal
against a naris of a patient's nose. Each nasal insert is capable of
being compressed and inserted into the patient' s naris to a patient' s
mucosal membrane and being retained therein solely by the flange, by
the resilience of the nasal insert, and by lateral pressure against the
naris from ventilator air flow through each nasal insert.
Also part of the invention is a ventilation interface which
includes a nasal insert. The nasal insert is a hollow body made from
a flexible, resilient, soft, biocompatible material, having a base end
and a tip end. The nasal insert is substantially oval in cross-section
at the base end and the tip end arid continuously oval in cross-section
between the base end and the tip end. A flange is formed as a bead
disposed about the tip end of the nasal insert. The flange is adapted
for forming a seal against a naris of a patient's nose. A valve is
included having a hollow valve body.. The hollow valve body includes
6
CA 02416410 2003-O1-14
a superior end attached to the base end of the nasal insert and an
inferior end adapted for attachment to supply tubing from a ventilator.
The valve has an exit port: defined in the valve body. A gate is
pivotally attached to the valve body. The gate has a rigid rim and a
one-way gas permeable diaphragm extending across the rim. The valve
has a flexible, tubular bladder having a first end attached to the rim
of the gate and an open second end attached to the valve body. The
nasal insert is capable of being compressed and inserted into the
patient' s naris to a patient' s mucosal membrane and being retained
therein solely by the flange and by the resilience of the nasal insert.
The gate pivots between a first position during inspiration and a
second position during expiration. In the first position the rim of
the gate is above the exit port, and the bladder inflates to form a
seal over the exit port. In the second position the rim of the gate
is below the exit port, opening the exit port for release of exhaled
air to the atmosphere.
Also part of the invention is a ventilation interface including
a nasal insert. The nasal insert is a hollow body made from a
flexible, resilient, soft, biocompai=ible material, having a base end
and a tip end. The nasal insert is substantially oval in cross-section
at the base end and the tip end and continuously oval in cross-section
between the base end and the tip end. A flange is formed as a bead
disposed about the tip end of the nasal insert. The flange is adapted
for forming a seal against a nari.s of a patient's nose. A one-way
expiratory diaphragm is disposed across the base end of the nasal
insert. The expiratory diaphragm permits passage of exhaled air from
the tip end through the base end, bui: prevents passage of inhaled air
through the base end towards the t:ip end. A one-way inspiratory
diaphragm is disposed in the nasal. insert adjacent the base end. The
inspiratory diaphragm permits passage of inhaled air from outside the
7
CA 02416410 2003-O1-14
nasal insert, through the inspiratoz:y diaphragm, and into the nasal
insert, but prevents passage of exhaled air through the inspiratory
diaphragm and out of the nasal insert. A removable filter is disposed
over the inspiratory diaphragm. Means are provided for retaining the
filter. The nasal insert is capable of being compressed and inserted
into the patient's naris to a pat:ient's mucosal membrane and being
retained therein solely by the flange and by the resilience of the
nasal insert. The inspiratory diaphragm is disposed below the
patient's naris.
BRIEF DESCRIPTION OF THE DRAWINGS
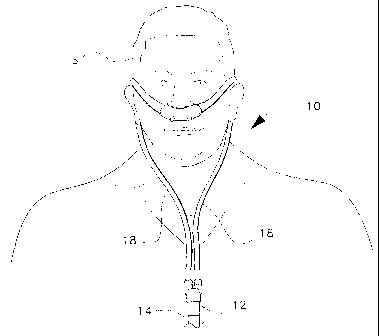
Fig. 1 is a front environmental view of a ventilation interface
for sleep apnea therapy according to the present invention.
Fig. 2A is an exploded elevational of a ventilation interface
according to the present invention.
Fig. 2B is a perspective view of a ventilation interface embodied
in a nasal cannula body according to the present invention.
Fig.3 is a section view along the lines 3-3 of Fig.
2A.
Fig.4 is a section view along the lines 4-4 of Fig.
2A.
Fig.5 is a section view along the lines 5-5 of Fig.
2A.
Fig. 6 is a perspective view of an embodiment of the ventilation
interface with the nasal inserts incorporated into independent supply
tubes.
Fig. 7 is a perspective view of an embodiment of the ventilation
interface with the nasal inserts incorporated into independent supply
tubes, and having valves disposed between the nasal inserts and supply
tubes.
8
CA 02416410 2003-O1-14
Fig. 8 is a longitudinal sectional view through the valve assembly
of Fig. 7 showing the position of the valve during the inspiratory
cycle.
Fig. 9 is a longitudinal sectional view through the valve assembly
of Fig. 7 showing the position of the valve during the expiratory
cycle.
Fig. 10 is a front perspective view of a left nostril nasal insert
fitted with a filter for therapeutic treatment of asthma and other
respiratory ailments, the right nostril nasal insert being a mirror
image.
Fig. 11 is a top view of the nasal insert of Fig. 10.
Fig. 12 is a section view along the lines 10-10 of Fig. 12.
Similar reference characters denote corresponding features
consistently throughout the attached drawings.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The ventilation interface for .sleep apnea therapy interfaces a
ventilation device which provides positive airway pressure (either
continuous, bilevel, or intermittent) with the patient's airways. The
ventilation interface includes a pair of nasal inserts made from
flexible, resilient silicone which are oval shaped in cross-section and
slightly tapered from a base proximal the ventilation supply to the
distal tip end. A bead flange is disposed about the exterior of each
insert at the distal end of the insert . A bleed port for release of
exhaled air is defined through a conical vent projecting normally to
the path of the incoming air flow, and continues through a nipple
extending to the exterior of the air conduit . In one embodiment, a
pair of nasal inserts are integral with a nasal cannula body, with
bleed ports axially aligned with each insert. In another embodiment,
9
CA 02416410 2003-O1-14
each insert is independently connected to a separate, thin-walled,
flexible supply line.
Advantageously, the construction, of the nasal inserts permits the
ventilation interface to be retained in the patient' s pares without
requiring a harness, head strap, or other retaining device. The nasal
inserts do not merely seal the base of the nostrils, but are inserted
into the nostrils farther than nasal pillows, as far as the nasal
mucosal membrane, and are retained by resilient expansion of the
inserts, the flanges engaging notches in the pares, together with the
pressure of incoming air, which forms a positive seal to prevent the
leakage of air past the inserts . The nasal inserts are constructed
according to specifications which permit the inserts to be relatively
thin-walled, and are oval-shaped in cross-section to conform to the
shape of the nostrils. This construction permits the nasal inserts to
have a large internal diameter in order to pass a greater volume of air
than nasal pillows or prongs, without significant narrowing of the air
passages, thereby maintaining lateral pressure, and avoiding drying and
burning of the patient' s nasal passages, as well as supplying a
sufficient number of air molecules at. the desired pressure to keep the
patient's airways patent. Consequently, the ventilation device is more
comfortable for the patient: to wear while sleeping than conventional
positive airway pressure devices, but at the same time is more
effective in treating the patien't's apnea.
The bleed ports are specially designed to avoid the whistling
~5 noises commonly experienced with conventional nasal masks and nasal
pillows . By proj ecting the vent structure into the air passage normal
to the direction of the air flow from the supply tubes, incoming air
must turn ninety degrees and exit through a long, restricted diameter
bleed port to vent to the atmosphere, eliminating whistling noises to
increase patient comfort. In the embodiment having a nasal cannula
CA 02416410 2003-O1-14
body, the bleed ports are axially aligned with the nasal inserts,
providing COZ with a direct path to exit the cannula body. When the
nasal inserts are attached to independent supply tubes, the bleed ports
are at the base of the nostrils, providing essentially normal
exhalation.
When the nasal inserts are directly connected to the supply tubes,
the nasal inserts may be even more train-walled than when attached to
a cannula body, resulting in an even greater volume of air supplied
through the cannula body, up to a 20o increase in volume. In this case
the supply tubes may be similar to he~it-shrink tubing, being made from
a very thin-walled thermoplastic material that is lightweight and
flexible so that the supply tubing may collapse when not in use, but
will expand to a predetermined diameter under pressure applied by a
ventilator.
Under some circumstances it may prove advantageous to insert a
valve between the nasal inserts and the supply lines to control the
flow of air through the inserts . The valve may serve as an alternative
to the bleed ports, providing isolation between inhaled and exhaled
air, or may be connected to an electrical or mechanical control device
for BiPAP or Intermittent Mechanical Positive Pressure Ventilation.
One valve which may be used includes a valve body having a gate with
a rim attached to one wall by a hinge and disposed to pivot between an
inspiratory position in which the rim extends transversely across the
inside perimeter of the nasal insert, and an expiratory position in
which the rim swings downward against a stop. A one-way diaphragm
extends across the rim which only permits inspiratory air to pass
through the diaphragm. An ex>it port is defined in a sidewall of the
valve body opposite the hinge. A flexible, inflatable bladder depends
from the rim and is attached to true sidewalk of the valve body below
the exit port. During inspiration incoming air inflates the bladder
11
CA 02416410 2003-O1-14
and raises the rim against a stop positioned above the exit port, the
bladder inflating against the e::it port and blocking the passage of air
through the exit port. On expiration, the pressure of expired air
against the one-way diaphragm opens the valve, expired air leaving the
valve body through the exit port.
The nasal inserts may also be used without a mechanical
ventilation supply, or positive airway pressure, in certain
applications. For example, a one-way expiratory diaphragm may be
placed across the base of the nasal inserts . A one-way inspiratory
diaphragm is disposed in the sidewall of the nasal insert adjacent the
base, so that the inspiratory diaphragm is disposed below the bottom
of the nostril when the nasal inserts are worn. The inspiratory
diaphragm may include a removable filter which is retained against the
diaphragm by an elastic mesh, spring clips, hooks, or other retainer
means. The filter may be of the type used to filter out dust, pollen,
bacteria, allergens, and other nasal irritants. Use of the nasal
inserts fitted with the filter while sleeping may be of therapeutic
value in the treatment of asthma and other respiratory ailments.
The ventilation interface for sleep apnea therapy is designated
generally as 10 in the drawings. The ventilation interface 10 provides
an interface for connecting a ventilation device which provides
positive airway pressure (either continuous, bilevel, or intermittent)
with the patient's airways. As shown in Figs. 1 and 2A, the
ventilation interface 10 includes a conventional adapter or Y-connector
12 having a first end adapted to receive a supply hose 14 from a
mechanical ventilator (not shown) .and a second end having a pair of
ports 16 with barbed connectors far attachment to two supply tubes 18.
Supply tubes 18 may be, e. g. , 0. 312 5" ID (inside diameter) flexchem
tubing, made of polyvinyl chloride or other conventional gas supply
tubing. For sleep apnea therapy, the mechanical ventilator will
12
CA 02416410 2003-O1-14
usually supply room air at a pressure of between five and fifteen
centimeters of water. The room air may be supplemented with oxygen if
desired by splicing an oxygen supply line into supply hose 14 or using
a triple port connector in lieu o.f '~-connector 12. It is normally
unnecessary to humidify or add moisture to the air supplied by the
mechanical ventilator in using the ventilation interface 10 of the
present invention, as the interface 10 is designed to avoid stripping
moisture from the nares, so that moi~~ture does not have to be added to
relieve patient discomfort from drying or burning sensation in the
nasal airways.
In the embodiment shown in Figs . 1 and 2A, the ends of the supply
tubes distal from the Y-connector 12 are attached to opposite ends of
a nasal cannula body 22 by barbed connectors 20. Barbed connectors 20
preferably have an inside diameter substantially equal to the inside
diameter of supply tubes 18 in order to prevent any constriction or
narrowing of the air passage which may cause increased velocity in air
flow. Nasal cannula body 22, described more fully below, has a pair
of nasal inserts 30 which are inser~;.ed into the nares of the patient
P. The supply tubes may be looped over the patient's ears and joined
to the Y-connector 12, which may be suspended at about the patient' s
chest level when the patient :is star,,ding, as shown in Fig. 1. For Bi-
level Positive Airway Pressure (BiPAP) or Intermittent Mechanical
Positive Pressure Ventilation l.herapy, a suitable valve may be
connected between the supply tubes 18 and the cannula body 22. An
exemplary valve is described in the Applicant's prior application,
Serial Number 09/524,371, filed March 13, 2000.
The nasal cannula body 22 is s:~own in greater detail in Fig. 2B.
The cannula body 22 is an arcuate, hollow, body having substantially
flat top wall 22a and flat sidewalls 22b merging with a semi-
13
CA 02416410 2003-O1-14
cylindrical bottom wall 22c defining an air chamber 22d (seen more
clearly in Fig. 3) for the passage of air and other gases, and having
cylindrical tubes 24 at opposite ends which receive one end of the
barbed connectors 20. A notch 26 is defined transversely across the
top wall 22a of the cannula body 22, defining a pair of mounting pads
28. A pair of nasal inserts 30 are formed integral with the mounting
pads 28. The nasal inserts 30 are hallow and form a continuous flow
path or conduit for the passage of inhaled and exhaled gases between
the patient's nasal air passages and the air chamber 22d.
The nasal inserts are shown in greater detail in Figs . 3, 4, and
5. The nasal inserts 30 are substantially oval in cross-section, with
the major axis substantially parallel with the notch and the minor axis
normal to the notch. The nasal inserts 30 taper slightly from a wide
base 32 proximal the cannula body 22 to the open distal tip ends 39.
The nasal inserts 30 have a flange 36 about the distal tip ends 34 on
the exterior surface of the inserts 30, which may be formed as a semi-
cylindrical bead.
The cannula body 22, including the nasal inserts 30, are
preferably made from silicone elastomer. The cannula body 22 or air
chamber 22d has an internal diameter of at least 0.3125 inches
throughout its length. The walls of the nasal inserts 30 may be
thinner than the top wall 22a. The thickness of the walls of the nasal
inserts 30 are preferably between about 1/32 and 1/20 inches. The
thickness of the walls at the flange 36 may be about 1/16 inches. The
hardness of the walls of the nasal insert 30, as tested on a type A
Shore durometer, may range between about 15 and 40, preferably about
30. If the walls of the nasal inserts 30 are made any thinner, they
will fail to have sufficient integrity, and if made any thicker, they
will have insufficient flexibility to form a seal against the nares.
Z. ~1
CA 02416410 2003-O1-14
The thinness and softness of the nasal inserts 30 make them virtually
unnoticeable while in the nostrils. For an adult patient, the nasal
inserts may have a height of between <about 0.25 and 0.75 inches. The
internal diameter of the nasal inserts 30 may measure about 0.75" on
the major axis and 0.5" on the minor axis, allowing for generous
laminar air flow and delivering pressure more by volume of air
molecules than velocity of air flow, and deliver about double the
volume of nasal pillows, which have a round internal diameter of, for
example, about 0.25 inches. Nasal pillows cannot be made with such
large internal diameters, because it. becomes difficult to create a seal
under the bottom of the nose, as the pillows would have an internal
diameter larger than the internal diameter of the nares, and the
pillows are not as flexible as the nasal inserts 30 of the present
invention.
/ In use, the nasal inserts 30 are inserted up the patient' s
nostrils until the flanges 36 lodge against the mucous membranes. As
such, the nasal inserts 30 are considered an invasive device. Testing
has confirmed that the nasal inserts 30 are biocompatible and meet
regulatory requirements. The nasal inserts are retained in the
patient's nares by the flanges 36, by the flexibility and resiliency
of the silicone elastomer, and by lateral pressure of the room air,
which is maintained at between five and fifteen centimeters of water.
The oval cross-section of the nasal inserts 30 is shaped to conform to
the normally oval shape of the nares. The relative large internal
diameter of the nasal inserts 30 permits air to be supplied to the
patient's airways in sufficient volume at the driving pressure without
accelerating the rate of airflow that the patient has sufficient
positive airway pressure to be of therapeutic value in maintaining the
patient's airways patent during an episode of obstructive apnea without
drying the nasal passages. The notch 26 in the top wall 22a of the
CA 02416410 2003-O1-14
cannula body 22 lends additional fl.e~;ibility to the cannula body 22,
so that the nasal cannula 22 can be adjusted for deviated septums,
thick septums, and other anatomical variations in the configuration of
the nostrils.
The cannula body 22 has a pair of bleeder ports 38 disposed in the
bottom wall 22c directly below and axially aligned with the nasal
inserts 30. The bleeder ports are formed by an upper conically shaped
nipple 40 extending upward into the air chamber 22d, and a lower
conically shaped nipple 42 extending below the bottom wall 22c. The
bleeder port has an internal diameter_ if about three millimeters and
extends for a length of abc>ut 0.25 inches. The upper nipple 40 extends
about 0.125 inches into the air chamber 22d. The internal diameter of
the bleeder port 38 is ample to permit venting of carbon dioxide
exhaled by the patient while not being so large as to cause a
significant pressure drop in the cannula body 22, and axial alignment
of the bleeder port 38 with the nasal inserts 22 creates a direct path
for venting of the expired gases. Ate the same time, laminar flow of
air supplied by the supply tubes is normal to the bleeder ports 38, so
that air supplied by the ventilator must bend ninety degrees to exit
through the elongated bleeder port 38. The effect of this construction
is that the bleeder port 38 is virtually silent in operation,
eliminating the whistle associated with bleeder holes in conventional
ventilation interfaces.
Fig. 6 is a general~yy diagrammatic view of an alternative
embodiment of the ventilation interface, designated 50 in the drawing.
In this embodiment, each nasal insert 52 is connected to a separate
supply tube 54, the supply tubes 54 b~°ing connected to the mechanical
ventilator supply hose 56 by a suitab:Le Y-connector 58 or adapter, the
cannula body 22 and common air chamber 22d being omitted. The nasal
16
CA 02416410 2003-O1-14
inserts 52 have substantial:Ly the same construction as nasal inserts
30, being oval in cross-section and having a similar height and an
annular flange 60 about the distal t.ip for lodging the nasal insert 52
in a naris. The nasal insert 52 is al:~o made from silicone elastomer,
and has the same softness, thickness, flexibility and resilience as the
nasal insert 30. In this configuration, since the inserts are not
connected to the cannula body 22, the angle at which the inserts 52
enter the nostrils is not restricted by the cannula body 22, and
therefore the nares can accept a greater displacement, and may
accommodate a 20% greater volume of air molecules through the insert
52 than the insert 30.
In this embodiment, the supply tubes 54 may be made from a
flexible, lightweight, but relatively inelastic thermoplastic material,
similar to heat shrink tubing, so that the supply tubes 54 may be at
least partially collapsed in the absence of pressure from the
mechanical ventilator, but expand to their maximum diameter under a
pressure of between five to fifteen centimeters of water. The light
weight of the supply tubes 54 decreases any pressure on the patient' s
ears resulting from the weight of the supply tubes, increasing patient
comfort. The bleeder ports 62 have a similar construction to the
bleeder ports 38, having an internal nipple 65 normal to the axis of
the nasal insert 52 and an external nipple 64, the bleeder ports 62
being j ust above the base of the inserts 52 and normal to the f low of
supply air through the inserts 52.
It will be understood by those skilled in the art that the
dimensions of the nasal inserts 30 and 52, and of the bleeder ports 38
and 62, are representative dimensions for a ventilation interface 10
or 50 designed for adults, and that the ventilation interface 10 or 50
may be made with correspondingly reduced dimensions for teenage
17
CA 02416410 2003-O1-14
children, preteens, and infants . It will also be understood that the
nasal inserts 30 and 52 may be made from thermoplastic elastomers other
than silicone, providing that the material has similar softness,
resilience, flexibility, and biocompatibility. It will also be
understood by those skilled in the art that the nasal inserts 30 and
52, although illustrated in conjunction with ventilation devices for
the treatment of sleep apnea, may be used in any other application
where it is desirable to have an interface forming a seal between a
person's nasal airways and a ventilation or gas collection device,
including, but not limited to, rescue breathing apparatus used by
firefighters and other emergency personnel., scuba diving tanks, etc.
In lieu of bleeder ports, the ventilation interface may use a
valve for providing an exit port fo:r exhaled air, and for providing
isolation between inhaled and exhaled air. Figs. 7-9 show the
apparatus of Fig. 6 modified by a flapper type valve inserted inline
between the nasal inserts 70 and the supply tubes 54. The valve
includes a valve body 72 having an exit port 74 defined by a mesh grid
in a sidewall of the valve body 74. In Fig. 7 the components shown
below the valve body 72 are identical to those shown in Fig. 6, and
will not be described further. Nasal inserts 70 are identical in
construction to inserts 30 and 52, and will not be described further.
Valve body 72 may be constructed from the same material as nasal
inserts 70. Although shown as generally oval in cross-section in Figs.
7-9, the shape of the valve body is not critical and it will be
understood that the valve body 72 may have any suitable shape in
transverse cross-section, including oval, circular, square, etc.
Fig. 8 is a sectional view showing the position of the valve
components during the inspiratory cycle. The valve body 72 is hollow
and defines an air conduit extending between its inferior end 76 and
18
CA 02416410 2003-O1-14
superior end 78. Disposed within the valve body 72 is a flapper type
disk or gate 80, having a relatively rigid rim 82 defining the
perimeter of the gate 80, and a one-way diaphragm 84 stretched across
and supported by the rim 82. The perimeter of the rim 82 is slightly
smaller than the inside perimeter of t:he valve body 72 so that the gate
80 closes the air conduit when dispo~>ed in the position shown in Fig.
8. The one-way diaphragm 84 permits air from the supply tubes 54 to
pass through the diaphragm in the direction shown by the arrows in Fig.
8, but does not permit expired air tc travel through the diaphragm 84
in the opposite direction. The gate 80 is pivotally attached to a
sidewall of the valve body 72 by a hinge 86. A flexible,
inflatable/deflatable tubular bladder 88 extends between the inferior
end 76 of the valve body 72 and the rim 82 of the gate 80. The bladder
88 is open at the inferior end of the valve body 72 and is closed by
the rim 82 and diaphragm 84 at the opposite end of the bladder 88.
During inspiration, inspired air travels from the supply tubes 54
and enters the valve body 72 at the inferior end 76. The inspired air
inflates the bladder 88, causing the rim 82 of the gate 80 to pivot
upward against a stop 90 disposed on a sidewall of the valve body 72
which limits travel of the gate 80. The stop 90 may be a post or
protrusion extending into the hollow valve body 72, or the stop 90 may
be an internal flange disposed about the entire inner circumference of
the valve body 72 which defines a valve seat and which forms a seal
with the rim 82 during inspiration. As shown in Fig. 8, the bladder
88 inflates against the exit port 74, sealing the exit port 74 so that
air does not escape through the exit port 74 during inspiration.
Inspired air continues through the one-way diaphragm 84 and exits the
superior end of the valve body 72, thence passing through the nasal
inserts 70 and into the patient's nasal air passages.
79
CA 02416410 2003-O1-14
Fig. 9 shows the posit=ion of tree valve during expiration. The
patient exhales air through the nasal inserts 70 and the air enters the
superior end of the valve body 72. 'The pressure of the expired air
against the one-way diaphragm causes the gate 80 to pivot on the hinge
86 until the rim 82 engages a stop post 92 disposed on a sidewall of
the valve body 72, which limits downward travel of the gate 80. The
flexible bladder 88 is drawn down by the rim 82, uncovering the exit
port 74. Expired air is then released to the atmosphere through the
exit port 74, as shown by the direction of the arrows in Fig. 9.
The flexible bladder 88 may be made from a thin layer of
biocompatible, gas impermeable material, e. g. , latex. The rim 82 of
the gate 80 may be made from any rigid plastic material. The one-way
diaphragm 84 may be any one-way gas permeable membrane. Such membranes
are well-known in the medical arts.
The nasal inserts may also be used without being connected to a
source of positive airway pressure. Figs. 10-12 show an embodiment of
the nasal inserts fitted with a filter that may be used for the
treatment and prevention of asthmatic attacks and other respiratory
impairments. A front view of a nasal insert adapted for the left
nostril is shown in Fig. 10, the nasal insert for the right nostril
being a mirror image. The nasal insert 100 has substantially the same
construction as the nasal inserts 30, 52, and 70, i.e., the nasal
inserts 100 are substantially oval in cross-section, tapering slightly
from a wide base 102 to the tip end 104. The nasal insert 100 has a
flange 106 about the tip end 104 on the exterior surface of the insert
100, which may be formed as a semi-cylindrical bead.
The nasal insert, 100 is preferably made from silicone elastomer.
The thickness of the walls of the nasal insert 100 is preferably
between about 1/32 and 1/20 inches. The thickness of the wall at the
~.0
CA 02416410 2003-O1-14
flange 106 may be about 1/16 inches. The hardness of the wall of the
nasal insert 100, as tested on a ty~>e A Shore durometer, may range
between about 15 and 40, preferably about 30. The thinness and
softness of the nasal insert 100 makes the insert virtually
unnoticeable while in the nostrils. For an adult patient, the nasal
insert 100 may have a height of bet=ween about 0 . 25 and 0 . 75 inches .
The internal diameter of the nasal insert 100 may measure about 0.75"
on the major axis and 0.5" on the minor axis, allowing for generous
laminar air flow.
As shown in Figs. 10-12, the nasal insert 100 has a one-way
expiratory diaphragm 108 disposed across the base 102 of the insert and
is adapted for receiving a filter insert in the sidewall which is
disposed laterally in the insert 100. The one-way expiratory diaphragm
108 is positioned directly below the patient's naris, and permits the
flow of exhaled air through the diaphragm 108 in the direction shown
by the solid arrows 110 in Fig. 12, but does not permit air flow
through the diaphragm in the opposite direction.
The nasal insert includes a one-way inspiratory diaphragm 112
disposed laterally in the sidewall of the insert 100. The inspiratory
diaphragm 112 permits the flow of air into the insert 100 in the
direction shown by the dashed arrows. 114 in Fig. 12, but not in the
opposite direction. The inserts 100 include a removable, disposable,
replaceable filter 116 and means for maintaining the filter 116 in the
sidewall of the insert 100. Fig. 12 shows an elastic mesh 118, the
elastic mesh 118, one-way diaphragm 112 and sidewall 120 defining an
envelope for retaining the filter 116, the mesh 118 and diaphragm
defining a slot 122. The filter 116 may be inserted through the slot
122 where it is retained against the one-way diaphragm 112 by the
elastic mesh 118, and may be removed by using a fingernail, toothpick,
21
CA 02416410 2003-O1-14
nail file, or other device for pulling the filter 116 out of the
envelope. Other devices may be used to retain the filter 116 against
the one-way diaphragm 112 if desired, e.g., spring clips, hooks, etc.
The filter 116 filters out any particles that may cause allergies
or asthmatic attacks, such as dust, pollen, allergens, and bacteria
from inspired air. Such filters are well known in the medical arts,
and will not be described further.
The preferred embodiments of t:he invention provide a ventilation
interface for sleep apnea therapy having nasal inserts which seal
against the hares and do not require a harness, head strap, or other
external devices to maintain pressure for retaining the inserts in or
against the patient's no~str:Lls. The nasal inserts are made of
flexible, resilient plastic with a bead flange for retaining the
inserts in the hares. The walls of the insert are thin-walled and
maintain lateral pressure in the hares in order to provide a greater
internal diameter for the delivery of a greater volume of air molecules
at a constant delivery pressure and without forcing ventilation gases
through restricted ports or passageways. Drying and burning of the
patient's nasal airways is avoided while delivering a therapeutic
volume of air to maintain the apnei.c patient's airways in a patent
condition. The ventilation interface may be equipped with a valve
disposed between the nasal inserts and the source of positive airway
pressure for controlling the flow of air through the nasal inserts.
The ventilation interface may be equipped with a removable filter for
filtering allergens from inspired air in order to prevent asthmatic and
allergic attacks.
It is to be understood that the present .invention is not limited
to the embodiments described above, but encompasses any and all
embodiments within the scope of th.e following claims.
p2