Note: Descriptions are shown in the official language in which they were submitted.
CA 02416606 2003-02-03
METHOD AND APPARATUS FOR REDUCTION OF BIAS IN
AMPEROI~IETRIC SENSORS
Field of the Invention
The present invention generally relates to a biosen-
sor, and, more particularly, to a~ new and improved method
and apparatus :for reducing bias in amperometric sensors.
Background of the Invention
The quantitative determination of analytes in body
fluids is of great importance in the diagnoses and main-
tenance of certain physiological abnormalities. For ex-
ample lactate, cholesterol and bilirubin should be moni-
toyed in certain individuals . 7tn particular, the deter-
mination of glucose in body fluids is of great importance
to diabetic individuals who must frequently check the
level of glucose in their body fluids as a means of regu-
lating the glucose intake in their diets. While the re-
mainder of the disclosure herein will be directed towards
the determination of glucose, it is to be understood that
the procedure and apparatus of this invention can be used
for the determination of other analytes upon selection of
the appropriate enzyme. The ideal diagnostic device for
the detection of glucose in fluids must be simple, so as
not to require a high degree of technical skill on the
part of the technician administering the test. In many
cases, these tests are administered by the patient which
lends further emphasis to the need for a test which is
easy to carry out. Additionally, such a device should be
based upon elements which are sufficiently stable to meet
situations of prolonged storage.
MSE #1899
CA 02416606 2003-02-03
2
Methods for determining analyte concentration in
fluids can be based on the electrochemical reaction be-
tween the analyte and an enzyme specific to the analyte
and a mediator which maintains the enzyme in its initial
oxidation state. Suitable redox enzymes include oxi
dases, dehydrogenases, catalase and peroxidase. For ex
ample, in the case where glucose is the analyte, the re
action with glucose oxidase and oxygen is represented by
ZO equation (AD.
glucOSe -h ~2 glucose oxidase (GO) > gl~COnOlaCtOne -H
(A~
In a colorimetric assay, the released hydrogen per-
oxide, in the presence of a peroxidase, causes a color
change in a redox indicator which. color change is. propor-
tional to the :Level of glucose in the test fluid. While
colorimetric ts~sts can be made semi-quantitative by the
use of color charts for comparison of the color change of
the redox indicator with the color change obtained using
test fluids.of' known glucose concentration, and can be
rendered more highly quantitative by reading the result
with a spectrophotometric instrument, the results are
generally not as accurate nor are they obtained as
quickly as those obtained using a biosensor. As used
herein, the term biosensor is intended to refer to wn
analytical device that responds selectively to analytes
in an appropriate sample and converts their concentration
into an electrical signal via a combination of a biologi-
cal recognition signal and a physico-chemical transducer.
Aside from its greater accuracy,. a biosensor is an in-
MSE #1899
CA 02416606 2003-02-03
3
strument whicri generates an electrical. signal directly
thereby facilitating a simplified design. In principle,
all the biosensor needs to do is measure the time and
read the current. Furthermore, a biosensor offers the
advantage of low material cost since a thin layer of
chemicals is deposited on the elc=ctrodes and little mate-
rial is wasted.
Referring to the above equation (A), a suitable
electrode can measure the formation HZOz due tc> its in-
troduction of electrons into the test fluid according to
equation B:
Hz~Z >~2 + 2H~ + 2e
(B)
The electron flow is then converted to the electrical
signal which directly correlates to the glucose concen-
tration.
In the initial step of the reaction represented by
equation (A), glucose present in the test sample converts
the oxidized f:lavin adenine dinu.cleotide (FAD) center of
the enzyme into its reduced form, (FADHZ). Because these
redox centers are essentially electrically insulated
within the en::yme molecule, direct electron transfer to
the surface of a conventional electrode does not occur to
any measurable degree in the absence of an unacceptably
high cell voltage. An improvement to this system in-
wolves the use of a nonphysiological redox coupling be-
tween the elecarode and the enzyme to shuttle electrons
between the (~:ADHZ) and the electrode. This is repre-
MSE #1E99
CA 02416606 2003-02-03
4
sented by the following scheme in which the redox cou-
pler, typica115y referred to as a mediator, is represented
by M:
Glucose + GO(FAD) ---> gluconolactone + GO(FADHZ)
GO ( FADHZ ) + 2M~ > G~ ( FAD ) + 2Mr~e + 2Hø
2Mg~ > 2M~ + 2e- ( at the electrode
In the scheme, GO(FAD) represents the oxidized form
of glucose ox.idase and GO(FADH~) indicates its reduced
form. The mediating species M"X/M=~ shuttles electrons
from the reduced enzyme to the electrode thereby oxidiz-
ing the enzyme causing its regeneration in situ which, of
course, is desirable for reasons of economy. The main
purpose for using a mediator is to reduce the working po
tential of the sensor. An ideal mediator would be re
oxidized at the electrode at a low potential under which
impurity in the chemical layer and interfering substances
in the sample would not be oxidized thereby minimizing
interference.
Many compounds are useful as mediators due to their
ability to accept electrons front the reduced enzyme and
transfer them to the electrode. Among the mediators
known to be useful as electron transfer agents in ana-
lytical determinations are the substituted benzo- and
naphthoquinonee> disclosed in U.S. Patent 4,746,607; the
N-oxides, nitr~oso compounds, hydroxylamines and oxines
specifically disclosed in EP 0 354 441; the flavins,
phenazines, phE:nothiazines, indophenols, substituted 1,4-
benzoquinones and indamins disclosed in EP 0 330 517 and
the phenazinium/phenoxazinium salts described in U.S.
MSE #1899
CA 02416606 2003-02-03
Patent 3,791,988. A comprehensive review of electro-
chemical mediators of biological redox systems can be
found in Analyt:ica Clinica Acta. I40 (1982), Pp 1-18.
5 Among the more venerable mediators is hexac:yanofer-
rate, also known as ferricyanide~, which is discussed by
Schlapfer et a.1 in Clinica Chimica Acta. , 57 ( 1974 ) , Pp.
283-289. In U.S. Patent 4,929,545 there is disclosed the
use of a soluble ferricyanide compound in combination
with a soluble ferric compound in a composition f:or enzy-
matically determining an analyte in -a sample. Substitut-
ing the iron salt of ferricyanide for oxygen in equation
(A) provides~
Glucose + 2 ~'e'++ ( CN) ~-6 G° > gluconolactone + 2 Fe'+ ( CN ) 4
s
since the ferricyanide is reduced to ferrocyanide by its
acceptance of electrons from the glucose oxidase enzyme.
Another way of expressing this reaction is by use of
the following E=.quation (C)°
Glucose + GO(FAD) > Gluconalactone + GO(FADHz)
GogFADH2) + 2 Fe(crr3)3'6 -> co(FAU) + 2 Fe'cN)se- + 2H;
2 5 2 Fe ( CN ) 64- - > 2 Fe ( CN ) 63- + 2 e- ( at the electrode )
(C)
The electrons released are directly equivalent to the
amount of glucose in the test fluid and can be related
thereto by measurement of the current which is produced
through the fluid upon the app:Lication of a potential
MSE #1899
CA 02416606 2003-02-03
6
thereto. Oxidation of the ferrocyanide at the anode re-
news the cycle.
As is apparent from the above description, a neces-
sary attribute of a mediator is the ability to remain in
the oxidized state under the conditions present on the
electrode surface prior to the use of the sensor. Any
reduction of the mediator will increase the background
current resulting in the biosensor reading being biased.
It has been discovered that these mediators do tend to be
reduced over time, especially under conditions of stress,
thereby dimin:Lshing the usefulness of the sensors to
which they are applied.
In published international patent application
PCTlUS92/01659 there is disclosed the use of potassium
dichromate as an oxidizing agent in a colorimet,ric rea-
gent strip. The purpose of the oxidizing agent is to
oxidize impurities in other reagent components to improve
the colorimetric sensorrs stability. This publication
mentions USS07/451,671 (now U.S. 5,288,636) and charac-
terizes it as describing a system in which a reduced me-
diator is re-oxidized by the application of a potential
and measuring the current after a specific time to deter-
mine the concentration of the analyte. More specifi-
cally, the '636 patent requires the complete oxidation of
the glucose by glucose oxidase. As the enzyme is reduced
by the glucose, the ferricyanide reacts with enzyme to
produce ferrocyanide. The ferrocyanide produced by this
enzymatic reaction is combined with ferrocyanide produced
during storage. This latter ferrocyanide is the result
of a reaction between ferricyanide and impurities found
MSE #1899
CA 02416606 2003-02-03
in materials deposited with the glucose oxidase and fer-
ricyanide. The '63~ patent snakes no distinction between
ferrocyanide pz-oduced between these two sources.
5 It would be desirable, and it is an object of the
present invention to provide a method whereby the unde
sired reduction of mediator compounds stored on an elec
trodes surface can be reversed to minimize its effect on
estimating the analyte values in fluid test samples with
IO very low analyt;e concentrations.
It is a further object to provide such a method in
which the accuracy of the analyte determination is en-
hanced.
It is a further object to provide such a method
wherein the ana~lyte is glucose.
An additi~anal object is to provide a mathematical
means for furtraer enhancement of the accuracy of the ana
lyte determination.
It is a further object to provide apparatus for ac-
curately determining analyte values.
It is a further object to provide such s.pparatus
that is simple and economical to manufacture.
MSE #1899
CA 02416606 2003-02-03
8
Summary of the Invention
The present invention involves a method for
determining the con<~entration of an analyte in a fluid
test sample by applying the test sample to the surface of
a working electrode. The electrode has on its surface: a
composition comprising an enzyme specific for i~he
analyte, a mediator which is reduced as a result of a
reaction between t:he analyte and the enzyme, which
mediator has undergone partial reduction to its reduced
state as a result of having been exposed to ambiernt
conditions. There is disclosed herei:r~ an apparatus i:or
analyte determination in a test sample comprising:
amperometric sensor means for receiving t:he
test sample;
voltage potential means for applying a first
burnoff voltage potential to said amperometric
sensor means and far applying a second read voltage
potential to sa~_d amperometric sensor means;
timer means for identifying a burnoff- time
interval and a read time interval;
means for measuring a first current i1 resulti.ng
from said applied burnoff voltage potential and a
second current :i2 resulting from said applied second
read voltage potential; and
means responsive to said current measuring
means for identifying an analyte value of the test
sample.
CA 02416606 2003-02-03
9
Brief Description of the Drawings
The present invention together with the above and
other objects and acwantages may best be understood f~_om
the following detailed description of the preferred
embodiments of the invention illustrated in the drawings,
wherein:
FIG. 1 is a chart illustrating potential and current
relative to time in. accordance with the method of t:he
invention;
FIG. 2 is a block diagram representation of a device
for determining analyte values employed to perform t:he
method of the invention; and
FIG. 3 is a flow chart illustrating the sequential
steps performed by <~ processor of FIG. 2 in accordance
with the method of the invention.
CA 02416606 2003-02-03
Description of the Invention
The present invention is a method that reduces the
5 background bias due to oxidizab:Le impurities in an am-
perometric sensor used for measuring a specific analyte,
such as glucose, in blood. The background current of
such a sensor will increase if it is stored over a long
period of times or under stress (heat, moisture, etc.)
10 due to the increased presence of reduced medj.ator or
other reduced impurity present in the sensor such as en-
zyme stabilizers, e.g. glutamate, and surfactants having
reducing equivalents. For example, in a ferricyanide
based amperometric sensor, the background bias is related
to the presence of ferrocyanide (from the reduction of
ferricyanide) near the electrode surface. This accumu-
lated ferrocyanide, as opposed to the ferrocyanide pro-
duced during use of the sensor (fresh ferrocyanide), is
oxidized back to ferricyanide to reduce the background
bias it causes and thereby extend the sensor shelf life.
To achieve this objective, the method uses an electro-
chemical approach. The background bias is further re-
duced when the electrochemical approach is augmewted with
an algorithmic correction.
Referring to FIG. 1, the method of our invewtion in-
volves first applying a positive potential pulse (called
the " burn-off'° pulse) which precedes the normal poten-
tial profile during use of the sensor. This is,tyypically
accomplished by applying a positive potential of :from 0.1
to 0.9 volt (;preferably 0.3 to 0.7 volt) between the
working and reference electrodes of the sensor for a pe-
MSE #1899
CA 02416606 2003-02-03
11
riod of from ~. to 15 seconds (preferably 5 to 10 sec-
onds). The burn-off pulse oxidizes the initial ferrocya-
nide (or other oxidizable impurity), so that the sensor
can begin the assay with a clean background. Typically,
the background is not perfectly clean since only a por-
tion of the oxidizable impurity is oxidized by the burn-
off pulse. This is the case because the chemical layer
covers both tyre working and the reference electrodes.
The initial ferrocyanide exists in the chemical layer
since it comes from ferricyanide. When sample fluid is
applied and they chemical layer re-hydrates, the ferrocya-
nide near the working electrode is re-oxidized. The rest
of the ferrocyanide diffuses into the sample fluid and is
mixed with the glucose. That portion of the initial fer-
rocyanide cannot be re-oxidized without affecting the
glucose. The _'Lnitial ferrocyanidle is near the electrode
for a very short time (a few seconds) after tie fluid
test sample is applied. The reason for this is that the
chemicals (enzyme and ferricyanide, etc.) are deposited
as a thin layer. on the working and reference electrodes .
The burn-off technique takes ad~~antage of this since a
significant amount of the initial ferrocyanide can be
burned off without noticeable reduction of the analyte
concentration in the fluid test sample most of which does
not come into direct contact with the electrode. Experi-
meets have demonstrated that thc~ background bias of a
stressed sensor can be reduced by 40~ with proper appli-
cation of the burn-off pulse.
The background bias can be further reduced by the
use of a background correction algorithm which works in
conjunction with the burn-off pulse. The algoi°ithm is
MSE #1899
CA 02416606 2003-02-03
12
based on the taking of two current readings. The first
reading (i1) is taken during the burn-off pulse and the
second (iz) at the end of the mead time, i.a. the time
elapsed from the moment when the second potential pulse
is applied to the moment when the current iZ is measured.
The length of the read time is t3-tZ, as shown in FIG. 1.
The analyte concentration is then calculated from the two
current readings, i1 and i2. Tests on sensors have shown
that the background correction algorithm is able to re-
move at least 80% of the remaining background bias, and,
as a result, the sensor stability can be improved to pro-
vide a significant extensi~n in shelf life.
An amperometric glucose sensor of the type useful in
the practice o.f the present invention is constructed as
followsa Two carbon electrodes are printed on a polymer
substrate. Next a layer of chemical components is depos-
ited on the electrodes and dried. A preferred chemical
composition is 5 pL of a medium containing 55 mM ferricy-
snide (potassi~xm salt), 8.5 units of glucose oxidase,
0.53% of polyethylene oxide), 0.40% of cremophor as sur-
factant and 83 mM phosphate buffea= at pH 7.2. During the
glucose assay, a potential profile consisting of three
consecutive time periods is applied to the sensor" These
time periods are, in sequence, the burn-off time
(typically 0.4 volt for 10 seconds); delay period (open
circuitry for 15 seconds) and read time (0.4 volts, 5
seconds). The exact time of the delay period is not
critical but is normally in the range of 10 to 40 sec-
onds. This decay period allows sufficient time for the
reaction to build up sufficient ferrocyanide to allow the
current resulting from the reoxidation of the fEarr~cya-
M5E #1899
CA 02416606 2003-02-03
13
nide to be measured without difficulty. These time peri-
ods are illustrated in FIG. 1 which plots potential and
current against time. Current measurements are taken at
the end of the burn-off period (i1) and read time (i2)
whereupon the corresponding glucose concentration is cal-
culated using equation 1: The constants in the equation,
e.g. slopes and intercepts are predetermined values.
The following discussion relates to a fluid test
sample in which glucose is the analyte to be detected and
involves a sensor in which ferricyanide is the mediator.
However, the discussion is equally applicable to systems
for the determination of other analytes and in which the
oxidizable species is something other than ferrocyanide.
The burn-off technique, i.e.. application of a posi-
tive potential pulse to the electrode to oxidize at least
a gortion of the mediator back to its oxidized form, is
illustrated by FIG. 1. In FIG. 1, in which the potential
and current profiles are plotted, the timing is as fol-
lows:
to - sample is detected, burnoff period begins. Sam-
ple is detected by inserting the sensor into the in-
strument which causes the immediate application,of a
0.4 volt potential. The current is continuously
checked t:o see if a larger than predetermined
threshold (e. g. 250 nA) is measured. When a larger
current than the threshold value is detected, a sam-
ple has been detected to begin the burnoff time pe-
riod.
MSE #1899
CA 02416606 2003-02-03
14
t1 - end of burn-off period and current i1 is meas-
ured. The length of the burnoff period, tl-t~, is
usually 5 to 10 seconds . The potential is 0 . 4 volt
at t1 but switches to an open circuit or to a poten-
tial that substantially reduces the current to mini-
mize the rate of electrochemical reaction at the
working electrode for a set delay period after the
burnoff period.
t2 - end of set delay period. The length of the wait
period, tz-tl, is normally 1f7 to 40 seconds. A read
potential of 0.4 volt is applied at ta.
t3 - end of read time when current i2 is measured.
The length of the read time, t3-t2, is 5 to ZO sec
onds.
The burn-cuff pulse, i.e. application of the 0.4 volt
potential from t~ to t1, is designed to eliminate part of
initial ferrocyanide (accumulated ferro) or other oxidi-
zable interferents in the enzyme layer.
The burn-off algorithm calculates glucose ccencentra
tion from two current measuremewts i1 and i2 using equa
tion lm
= is-rnt xo~( iliZ)
slope (1)
where
3 0 ~(g' t= ) - _Im_t slope ° i, - s' ° iz
' slope ~ slope ° as _~~ - s, ° ii ,~ ( 2 )
MSE #1899
CA 02416606 2003-02-03
~. 5
Equation 1 is a partial correction algorithm which
is intended to achieve a compromise between reducing
stress-related background bias and preserving system pre-
y vision. The basic scheme is to use is as a glucose read-
ing
G = I2 - int
- s 1 ope
where int and slope are the intercept and slope saf i2 re-
spectively. The term ~(il,iz) is the estimated background
increase, due iro stress or other causes, derived from the
current i1 and i2. For fresh sensors, this term is close
to zero. The parameter k is selectively provided or set
to a value from 0 to 1. There will be no background cor-
rection if k is set at zero. On the other hand a full
correction can be achieved if k is 1. In the i:ollowing
examples k is set at 0.8 for partial correction because
it has been found that the variation of i1 is lax°ger than
that of i2 when multiple sensors are tested under the same
glucose concentration. Compared with the glucose value
calculated from i2 alone, k = 0 in equation (1), the glu-
cose value calculated from i1 and i2 jointly will be
slightly lower in precision (a larger standard deviation)
and, of course, a much smaller background bias. The
tradeoff between the precision and bias can be achieved
by choosing the proper k value. If k - 0, there is no
background correction and i1 is not used. In this case,
the highest prevision can be obtained, but it is accompa-
nied by a high background bias. If k = 1, the fall back-
ground correction is applied whereupon the lowest bias
can be achieved but at the cost of precision. The k
MSE #1899
CA 02416606 2003-02-03
16
value is set at 0.8 in the example to achieve k3 compro-
mise between precision and bias.
The parameters in these equations are:
Int - intercept of read current iz,nA.
s.tope - slope of read current .~a,nA°dLlmg.
i1_1~ - average burn-off current il,nA, at the low
glucose calibration level, i.e. 50 mg/dL.
iz_lo - avE~rage read time current iz,nA, at the low
glucose calibration level. Actually, izl~ is
not an independent parameter. It can be cal-
culated from Int and slope:
az to = Int + slope ° 5~.
sa - slope to burn-off current, nA°dL/mg:
k - set to 0.8 for partial correction.
Int, slope, i~ lo, and s1 are local parameters; each sensor
lot has its own parameter values which values are deter-
mined experimentally. The algorithm needs two known cur-
rent values, one for i1 and one for iz foy° normal
{unstressed) sensors. The illo and aalo are available
since they are used in determining the intercept (Int)
and slopes (s1 and slope). ~f course, current at other
glucose levels can be used in the algorithm. This would
however introduce the extra step of adding two additional
independent parameters. The procedure of the present in-
vention is demonstrated in the following examples:,
MBE #1899
CA 02416606 2003-02-03
17
Example I:
The follocaing steps are taken to determine the lot
parameter values necessary in the algorithm~
A. Test 16 sensors from the lot at the low cali
bration level, 50 mgldL, and. obtain the average cur
rents al 1~ and ~.z 1~ of the brrn-off current and read
time current, respectively. It is found that illo =
195'1.2 nA and .~z to = 1952.3 n~A.
B. Test 16 sensors at the high calibration level,
400 mgldL,, and obtain the .average current il_h$ and
iz n1. It is found that i1 n~ - 6003.3 nA and az_ni
8831.7 nA.
C. Calc~nlate the parameter valuesa
50 ~ (a2 ,~ ~ i2 to ) 50 ~ (8831.7 -1952.3)
Int = iZ s~ -' 350 =19523-~ 350 = 9695 nA
slope = az ~' - gz ro - 8831.7 -1952.3 =19.65 nA ~ dL l mg
350 350
s1 = tt a~r ° ~a r0 - 6003.3 -19512 =11 ~8 ~ . dL l mg
350 350
Therefore, equation (1) becomes:
MSE #1899
CA 02416606 2003-02-03
18
r, ~ 'z ---~.8. ~,~tI
19.65
969.5 19.65 ~ i, -1158 - i~ _
0~~,~.~) r 1
19.65 ~ ~ 19.65 ~ 195 L2 --11.58 ~ 195?..3
= 0 06162 ~ I, - 0.03631 ~ i= - 49.3
Example II
It has been discovered that the burn-off pulse alone
will significantly reduce the background bias a~aen with-
out the use of the background correction algorithm.
In this experiment, ten sensors were stressed under
30°C and 91% humidity for 3 hours. Aqueous glucose at 50
mg/dL was used as sample. Five stressed sens~oxs were
tested with a 10 second burn-off pulse and five without
the pulse. In addition, ten unstressed sensors were
tested as control (five with the 10 second burn-off and
five without) and the bias calculated using the following
equation (3)s
2 5 b~ _ . i,~,r - i.~.~.e.. X 10096
a~.....r C 3
It was found that the bias was 30.6% without the burn-off
pulse and l8.Cn with it which data demonstrate that the
burn-off pulse alone reduces the background bias by about
40~.
MSE #1899
CA 02416606 2003-02-03
19
Example III
This example explains how the algorithm corrects for
background bias:
Eight sensors were stored at below -20°C for two
weeks and another eight sensors were stressed at 50°C for
four weeks . A~_1 sixteen sensors were tested using whole
blood having a 100 mg/dL glucose concentration. The pa-
rameter values were determined from fresh sensors. The
glucose readings, G, were calculated as follows:
A. No bcdckground bias correction algorithrn: Equa-
tion 1 with k = 0.
B. Partial correction: Equation 1 with k = 0.8.
The bias in percent is calculated using Equation 4
with the results being listed in .'able 1.
MSE #1899
CA 02416606 2003-02-03
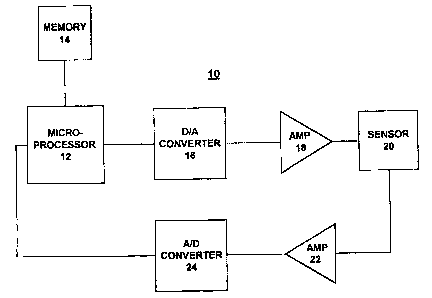
A device capable of carrying out the invention is
represented by FIG. 2. Referring to FIG. 2, there is
shown a block diagram representation of a device for ac-
s curately determining analyte values designated as a whole
by the reference character 10 and arranged in accordance
with principles of the present invention. Device 10 in-
cludes a microprocessor 12 together with a memory device
14. Microprocessor 12 is suitalcoly programmed to perform
10 the method of the invention as illustrated in FIG. 3.
Various commercially available devices, such as a DS5000
microcontrolle:r manufactured by Dallas Semiconducaor, can
be used for th,e microprocessor 12 and memory 14. Memory
14 can be included within the microprocessor 12 or sepa
15 rately provided as illustrated in. FIG. 2.
Digital data from the microprocessor 12 is applied
to a digital-to-analog (D/A) converter 16. D/A c:onverter
16 converts the digital data to an analog signal. An am-
20 plifier 18 coupled to the D/A converter 16 amplf.fies the
analog signal. The amplified analog signal output of am-
plifier 18 is applied to a sensor 20.
Sensor 20 is coupled to an amplifier 22. The ampli-
Pied sensed signal is applied to an analog-to-digital
(A/D) converter 24 that converts the amplified, analog
sensor signal 'to a digital signal.. The digital ~oignal is
applied to the microprocessor 12.
Various commercially available devices can be used
for D/A converter 16, amplifiers 18 and 20 and A/D con-
verter 24. For example, a device type PM-752F4FS manu-
MSE #1899
CA 02416606 2003-02-03
21
factured by PM:ITcan be used for D/A converter 16. Opera
tional amplifz.er device type TL074AC manufactured and
sold by Linear Technology can be used for amplifiers 18
and 22. A device type MAX 135 CWI manufactured and sold
'S by MaxumTM can be usedyfor the A/D converter 24.
Referring also to FIG. 3, there are shown the se-
quential steps for accurate analyte determination ~f the
invention. Initially microprocessor 12 applies a burnoff
pulse, for example a potential of 0.4 volts, to the sen-
sor 20 as indicated at a block 300. Then the m:Lcroproc-
essor checks to identify a sample corresponding to a de-
tected sensor thresh~ld current value as indicated at a
decision block 302. When a sample ~s detected at block
302, a. predetE~rmined burnoff time interval, sus~h as 10
seconds is identified at a decision block 304. Next the
current i1 is measured as indicated at a block 3CI6 and an
open circuit ins applied to the sensor 20 as indicated at
a block 308. 'then a set delay o~:- predetermined yJait time
interval, such as fifteen (15) seconds is identified at a
decision block 310. After the set delay, a read pulse or
potential of 0..4 volts is applied to the sensor 20 as in-
dicated at a block 312. Then a predetermined read time
interval for the read pulse, such as 5 seconds is identi-
fled at a decision block 314 and the current iZ is mess-
cared as indicated at a block 3160 Next microprocessor 12
gets the stored parameters for a particular sens~.r 20 in-
cluding Int, slope, i1 go, iz_lo, Si and k, as indicated at a
block 320 . The correction term Delta ( i1, i2 ) is calcu-
lated utilizing the stored parameters and measured burn-
off current i1 and read current i2 as indicated block 322.
Next the analyte value, such as glucose readir,~g G, is
CA 02416606 2003-02-03
22
calculated utilizing the read current i2 and the calcu-
lated correcticm term Delta ~ i1, :LZ ) multiplied by' the se-
lected scaling value k, as indicated at a block 3~4.
MSE #1899