Note: Descriptions are shown in the official language in which they were submitted.
CA 02418332 2003-02-04
1
Ca-Mm-Ni BASED HYDROGEN STORAGE ALLOYS
Field of the invention
The present invention relates to new Ca-Mm-Ni based alloys of the ABS type.
The invention also relates to a process for preparing these new alloys and to
the use
of such new alloys for hydrogen storage.
Background of the invention
The use of hydrogen gas as a fuel for PEM fuel cells has received considerable
attention
in recent years in view of the fact that PEM fuel cells using pure hydrogen
can provide
high efficiency and ultra clean power. Unfortunately, the widespread use of
hydrogen
energy is limited by economic and technological barriers. One of the important
barriers
is the lack of cost effective, safe hydrogen storage method.
Hydrogen gas is very light. It can be compressed under high pressure and
stored in
pressurized vessels. It can also be liquefied and stored in liquid form.
Hydrogen also
reacts with metal or non-metals to form hydrides. Some metal hydrides are
reversible at
ambient temperature and pressures. From a safety point of view, metal hydrides
are
intrinsically safe since the hydrogen must be released from the hydrides by an
endothermic process before it can burn or be oxidized.
The volumetric density of hydrogen storage in metal hydrides is usually high.
The most
serious shortcomings of the reversible low temperature metal hydrides are
their low
gravimetric storage density and their high cost. For stationary and some
mobile
applications, the weight of the hydrogen storage tank is not a problem.
However, the
high cost of conventional low temperature metal hydrides results in too
expensive
storage devices.
CA 02418332 2003-02-04
2
CaNis intermetallic compound represents a category of low cost hydrogen
storage
materials with a maximum storage capacity up to l.9wt.% (see reference 1).
However,
little attention has been paid to this system, probably due to its well-known
bad cycling
stability (see reference 2). Improvement ofthe hydrogen storage properties of
CaNis by
substitution of Ca or Ni with other elements has been tried (see references 3
to 5).
Ternary CaXMm,_XNiS and quaternary CaxMmi_XNis_yCuy alloys have been produced
by
melt casting and patented 20 years ago (see reference 6). Substitution of Mm
(mish
metal) for Ca can raise the plateau pressure of CaNiS. However, the plateau
slope is big
for the as-cast ternary alloys due to segregation. Annealing at elevated
temperatures
(> 1 OOOC) can reduce the slope to some extent. The inventor's previous work
show that
CaNis and Mm and/or Zn-substituted CaNiS type alloys with flat plateau can be
successfully produced (see reference 7).
Substitution of Ni by Mm and Al in the CaNiS type alloys can improve the
cycling
stability as disclosed in US 4,631,170 (see reference 8). However, the long
term cycling
stability of the alloys according to this patent is still not good enough.
Typically more
than 20% of the capacity is lost upon 200 times of hydrogen
absorption/desorption
cycling.
Further improvement has been achieved by concomitant substitution of Mm for Ca
and
Zn and Al for Ni, as reported by the present inventors of record (see
reference 9). The
capacity loss after 500 cycles has proved to be less than 20% for
Cao,BMmo.2Ni4,xZno.,Alo., and less than 10% for Cao_~Mmo,3Ni4_~AIo,~Zno.,.
However, the
maximum storage capacity is significantly reduced by substitution of Zn and A1
for Ni.
Summary of the invention
In accordance with the present invention, it has now been found that
substitution of Si,
Ge and some other metalloid elements (also called "semi-metals") for Ni in a
ternary
Ca-Mm-Ni alloy of the ABS type can substantially improve the long term
stability of
such an alloy without causing much reduction of the storage capacity.
Essentially, no
capacity loss has been observed after S00 hydrogen absorption and desorption
cycles.
CA 02418332 2003-02-04
3
Thus, a first object of the present invention is to provide new Ca-Mm-Ni based
alloys of
the ABS type, which are capable of absorbing and desorbing hydrogen from a gas
phase
at ambient temperature with a relative flat plateau pressure and a storage
capacity larger
than l.2wt.%. These new alloys are of the formula (I):
(CaXM~.x)c(Ni i-yTy)s (I)
where M is one or several metals selected from the group consisting of misch
metal
(Mm), Y and other rare earth metals;
T is one or several semi-metals such as Si, Ge and Ga;
0<x<1
0<y <_ 0.5 and
0.8 <_ t 5 1.2.
Another object of the invention is to provide a process for the preparation of
the above
mentioned alloys of formula (I), which comprises the following steps:
a) preparing a powder by milling a mixture of elemental powders and/or pre-
alloyed
substances of the elemental ingredients of the alloy to be prepared (such as,
for
example, Ca, Ni, Mm, CaNi2, CaNis, MmNis and so on) in adequate proportions to
obtain the required alloy; and
b) annealing and/or sintering the so prepared powder at elevated temperatures
in a
crucible for a short period of time m an inert or reactive atmosphere.
In use, step a) may consist of a ball milling or of a mechanical alloying and
can be
carried out at room temperature or at high temperatures with or without anti-
sticking
agents.
Step b) is essential to the above process. This step must actually be carried
out to
achieve high reversible capacity and a flat plateau. In use, the annealing can
be carried
in a crucible made of stainless steel at a temperature higher than
600°C but not higher
than 1100°C.
CA 02418332 2003-02-04
4
Alternatively, the new compounds according to the invention can be produced by
conventional melt casting methods or powder sintering methods.
The compounds according to the invention are useful for hydrogen storage in a
gaseous
form and such is a further object of the invention.
DETAILED DESCRIPTION OF THE INVENTION
As aforesaid, the invention is directed to new alloys of the ABS type, which
are of the
formula:
(CaXMt-x)t~l-YTy)s
where M is one or several metals selected from the group consisting of misch
metal
Mm, Y and other rare earth metals
T is one or several semi-metals such as Si, Ge and Ga;
0<x< 1 (x~0), preferably 0.4<_x<_l;
0< y <_ 0.5 (y~0), preferably 0<y<_0.3; and
0.8<_t<_1.2.
As aforesaid, the invention is actually based on the discovery that
significant further
improvements have been achieved by substituting Si, Ge and/or other semi-
metals for
Ni in the above mentioned Ca-Mm-Ni alloy of the ABS type (see the definition
of T in
the formula given hereinabove). This substitution has significant effect of
improving the
long-term stability while keeping predominantly the ABS structure and hydrogen
storage capacity according to the invention that are particularly useful.
The new Ca-Mm-Ni alloys of the ABS type according to the invention with
improved
properties can be made by mechanical alloying of elemental powders (such as
Ca, Mm,
Ni5) and/or mixtures of intermetallic compounds (such as CaNiS, MmNis)
corresponding
to the required composition, followed by an thermal annealing treatment at
temperatures
CA 02418332 2003-02-04
higher than 600°C for short period of time, typically at 1000°C
or slightly higher for
O.Sh-lh in a steel crucible. Annealing at temperatures lower than 600C does
not improve
the hydrogen storage properties very much.
5 The invention and its advantages will be better understood upon reading the
following
description made with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
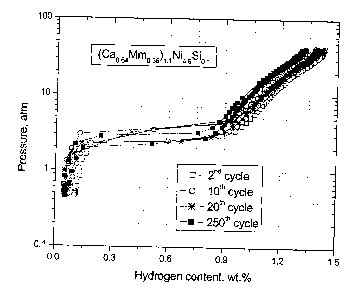
Fig. 1 is a curve giving the hydrogen storage capacity of an alloy of formula
(Cao.~
Mmo.36)i.l Nis as a function of the pressure after 3 cycles and 250 cycles;
Fig. 2 is a curve similar to the one of Fig. 1, but with an alloy of formula
(Cao.b4
Mmp.36)1.1 N14.9 S~O.I~
Fig. 3 is a curve similar to the one of Fig. 1, but with an alloy of formula
(Cao,b4
Mmo.36)1.1 Ni4.s Sio.a
Fig. 4a is a X-ray analysis of the alloy of formula (Cao.64 Mmo.s6) 1.1 Ni4.g
Sio.z mentioned
hereinabove (see Fig. 3);
Fig. 4b is an X-ray analysis of the alloy of formula (Cao.6a Mmo.ab)1.1 Nis
mentioned
hereinabove (see Fig. 1 ); and
Fig. 5 is a curve similar to the one of Fig. l, but with an alloy of formula
(Cao.sa
Mmo.36)1.1 Nia.as Geo.ls.
EXAMPLE 1 (Preparation of a known compound by the process of the invention)
(Cao.~Mmo.36)I.INis was synthesized by mechanical alloys in a SPEX high energy
ball
mill under argon. A MmNis powder (>99%, + 100mesh), Ca granules (>99.5, ~2mm
in
size) and Ni powders (<99.9%, -325mesh) were used as starting materials.
CA 02418332 2003-02-04
6
After alloying, an isothermal annealing was performed in a tubular furnace
under argon.
The mechanically alloyed powder was sealed in a stainless steel crucible
before
annealing. The powder was heated to 1050°C at a heating rate of
30C/min, and held at
1050°C for 1 hour, then cooled down to room temperature in the furnace.
The hydrogen absorption/desorption properties were measured by using an
automatic
Sievert's type apparatus. The annealed powder normally needs mild activation
treatment, such as heated to 200°C under vacuum and then cooled down.
The activated
(Cao.baMmo.36)i.iNis alloy exhibits a relative flat plateau and a maximum
storage
capacity of 1.44 wt.% under 4.OMPa of charging pressure.
A hydrogen absorption/desorption cycling experiment was performed at
30°C under an
absorption pressure of 3.SMPa and a desorption pressure of O.OIMPa. The
absorption
and desorption time was 12 minutes respectively. Under these conditions, the
alloys
could be fully hydrided and dehydrided. The hydrogen purity was 99.999%. As
shown
in Fig. l , the maximum storage capacity was reduced to 1.23 wt.% after 250
cycles (20%
loss). The reduction of the effective reversible storage capacity is even
bigger.
EXAMPLE 2 (Compound according to the invention made by the process of the
invention)
(Cao.6aMmo.36O.~Nia..ySio,, was synthesized by mechanical alloying of
elemental Ca, Si
and MmNis powder blends. The alloy was annealed in the same manner as in
Example
1. This alloy had a maximum hydrogen storage capacity of l.4wt.%. The maximum
hydrogen storage capacity are slightly reduced by 8% after 250 cycles as shown
in Fig.2
in contrast to the 20% loss in the (Cao.6aMmo.36O.~Nis.
EXAMPLE 3 (Compound according to the invention made by the process of the
invention)
(Cao.6aMlno.s6O. ~Nia.sSio.z was synthesized by mechanical alloying of
elemental Ca, Si
and MmNis powder blends. The alloy was annealed in the same manner as in
Example
CA 02418332 2003-02-04
7
(Cao.6aMmo.s6)~.~Nia.sSio,z was synthesized by mechanical alloying of
elemental Ca, Si
and MmNiS powder blends. The alloy was annealed in the same manner as in
Example
1. This alloy had a hydrogen storage capacity of 1.3wt.%. The maximum and
reversible
hydrogen storage capacities are slightly improved upon hydrogen absorption and
desorption cycling as shown in Fig.3.
X-ray analyses show that Si-substituted alloys have very high resistance to
peak
broadening upon cycling as shown in Fig.4a. While the (Caq.64Mmo.36O.~Nis
alloy
without Si substitution shows obvious peak broadening after cycling as shown
in Fig.
4b. It was believed that hydrogen absorption/desorption cycling introduces
defects, such
as microstrain, chemical disorders and grain boundaries (reduced grain size),
therefore
leads to reduced storage capacity. The peak broadening reflects the defects
introduced
during cycling experiments.
EXAMPLE 4 (Compound according to the invention made by the process of the
invention)
Cao,~ Mmo,4Ni4.85Geo.is was synthesized by mechanical alloying of elemental
powder
blends. The alloy was annealed in the same manner as in Example 1. This alloy
had a
maximum hydrogen storage capacity of 1.3wt.% in the as-synthesized state.
Substantial
improvement in the maximum and reversibly storage capacity is observed after
500
cycles as shown in Fig.S.
CA 02418332 2003-02-04
8
REFERENCES
( 1 ) "A new family of hydrogen storage alloys based on the system nickel-
misch
metal-calcium)" by G. D. Sandrock, proc. 12'" intersociety energy conversion
Engineering Conference, 12'" IECEC, Am. Nuclear Society, 1 ( 1977) 95 l .
(2) "Stability of Rechargeable hydriding alloys during extended cycling" by P.
D. Goodell, J Less-Common Met., 99 ( 1984) 1.
(3) "Systematic B-metal substitution in CaNiS" by J.O. Jensen and N.J.
Bjerrum:
J. of Alloys and Compounds 293-295 (1999) 185
(4) "Hydriding behavior in Ca-Mg-Ni-B" by H. Oesterreicher, K. Ensslen, A.
Kerlin and E. Bucher": Mat. Res. Bull. 15 (1980) 275.
(5) "Mechanical alloying and hydrogen storage properties of CaNiS-based
alloys", by G. Liang, J. Huot and R. Schulz, J. Alloys & Compounds, 321
(2001 ) 146.
(6) "Nickel-misch metal-Calcium alloys for hydrogen storage" by G.D.
Sandrock, US patent Nos. 4,096,639 and 4,161,402.
(7) "Synthesis of nanocrystalline CaNiS-based alloys and use for metal hydride
electrode", by G. Liang, S. Ruggeri, C. Lenain, H. Alamdari, J. Huot, L.
Roue and R. Schulz", J. Metastable and Nanocrystalline Materials 11 (2001)
71.
(8) "Calcium-Nickel-misch metal-Aluminium quaternary alloy for hydrogen
storage", by K. Ohnishi, T. Ogawa, US patent, 4631170.
(9) "Synthesis of low cost metal hydrides by mechanical alloying" by G. Liang
and R. Schulz, Report for the CRADA project (CR-99-004), 2001.