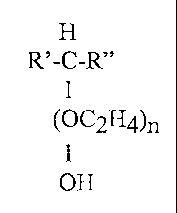Note: Descriptions are shown in the official language in which they were submitted.
CA 02418743 2003-02-12
EMULSIFIERS AND METHODS FOR CREATING IMPROVED EMULSIONS
FIELD OF THE INVENTION
The invention generally relates to the field of errmlsions. More specifically,
this
invention provides a non-terminal alcohol ethoxylate (NTAE) for use as an
emulsifier. In one
embodiment, the NTAE comprises the formula:
H
R'-C-R"
1
~OC2H4)n
1
OH
where R' is a Cl to C48 alkyl group, R" is a Cl to C48 alkyl group and n is 1
to 100. The
NTAE is particularly effective in forming an emulsion of water and a
hydrocarbon such as
bitumen. Emulsions created using the NTAE have a decreased droplet size to
facilitate
transportation and storage of the hydrocarbon as well as improving combustion
efficiency
when the emulsions are used as a fuel.
BACKGROUND OF THE INVENTION
The emulsification of whole bitumen with water and one or more emulsifiers is
well
known. Water/bitumen emulsions effectively reduce the viscosity of bitumen,
thereby
enabling its efficient transportation through pipelines and enabling its use
as a burnable fuel.
In particular, water/bitumen emulsions having a small particle size are
desirable because of
the increased stability of the emulsion as well as improved combustion
efficiency and
decreased stack emissions.
Many emulsifiers have been proposed and utilized in creating stable emulsions.
Of
these, phenol- or alcohol-based ethoxylates are the most common. In phenol- or
alcohol-based
ethoxylate emulsifiers, the base phenol or alcohol molecule is modified to
increase its
hydrophilicity by the addition of ethoxy groups to create phenol or alcohol
ethoxylates.
Environmental concerns have arisen with the use of phenols resulting in a
shift in recent years
-1-
CA 02418743 2003-02-12
towards the use of alcohol ethoxylates. As a result, the recent prior art in
particular teaches the
use of terminal alcohol ethoxylate (TAE) emulsifiers with the formula
R(OC2H4)"OH that are
derived from terminal alcohols (R-OH) in which the OH group is at the last or
terminal
carbon in the hydrocarbon chain.
While such compounds are effective as emulsifiers, there continues to be a
need for
emulsifiers having superior interfacial properties to enhance the creation,
handling and use of
bitumen emulsions. For example, there is a need for emulsifiers that improve
the quality of
the emulsion by enabling the creation of finer emulsions that will enhance
combustion
efficiency. Furthermore, there is a need for emulsifiers that do not require
other additives to
simplify the emulsification process and avoid problems associated with certain
additives.
Whereas additives such as amine or sodium have been used for the activation or
creation of
surfactants which may be naturally present in the bitumen, these additives may
otherwise
increase NOx emissions or increase the propensity for equipment corrosion.
There is also a need for emulsifiers having an appropriate hydrophile-
lipophile balance
for creating a water-external emulsion wherein the emulsifiers maximize
surface coverage at
the hydrocarbon/water interface in order to minimise the amount of surfactant
required and/or
reduce the size of the emulsion droplets. More specifically, there is a need
for an amphipathic
ethoxylate emulsifier with the above interfacial properties and one in which
the attachment of
the ethoxy groups to a non-terminal alcohol group creates a T-shaped molecule,
where the
head of the T is oleophilic and the stem of the T is hydrophilic. In addition,
there is a need for
emulsions that can utilize a variety of water sources for preparing the
emulsion including
municipal drinking water, water produced with bitumen, lake water, river
water, canal water,
ground water, brackish water or sea water. Finally, there is also a need for
an effective method
of preparing emulsions utilizing improved emulsifiers.
A review of the prior art has revealed that an emulsifier having this T-
structure and
these properties has not been previously disclosed.
For example, Canadian Patent 1117568 discloses an amphipathic terminal alcohol
exthoxylate (TAE} of the general formula R(CH2CH20)"OH, where R is an alkyl
group
containing 10 to 20 carbon atoms (the oleophilic moiety) and n is the number
of moles of
-2-
CA 02418743 2003-02-12
ethylene oxide (E0) that is between 5 and 40 (the hydrophilic moiety). Because
of the
essentially linear structure of this molecule at the interface, both the
oleophilic alkyl chain and
the hydrophilic ethoxy alcohol penetrate their respective phases leading to a
stick-like packing
of the molecule at the oil-water interface. This results in both a larger oil
droplet size in the
emulsion and an increased amount of surfactant to prepare a stable, fine
emulsion.
Canadian Patent 2232490 (Intevep) discloses the use of additives in addition
to a TAE.
Specifically, this patent teaches the use of an amine and an electrolyte
(NaOH) in addition to
the TAE to form an emulsion having an average droplet size of 13 to 24 Vim.
However, the
use of nitrogen-containing amine in an emulsion fuel leads to higher NOx
emissions. Further,
the use of NaOH will also result in the undesirable side-reaction of sodium
reacting with
vanadium in the bitumen which will form a stubborn sodium-vanadate scale known
to cause
corrosion of boiler tubes.
Other prior art includes US Patent 4,666,457, US Patent 4,725,287, US Patent
5,000,872, US Patent 5,024,676, US Patent 4,618,348, US Patent 4,976,745, US
Patent
5,263,848, US Patent 5,283,001, US Patent 5,437,693, US Patent 5,851,245, US
Patent
5,879,419, US Patent 5,902,227, US Patent 5,964,906, US Patent 5,976,240, US
Patent
5,993,495, US Patent 6,069,178, US Patent 6,194,472, US Reissue Patent 36,983,
US Patent
6,113,659 and US Patent 6,384,091, none of which discloses the structure and
use of a non-
terminal alcohol ethoxylate as an emulsifier. Accordingly, there is a need for
a method to
create and utilise a non-terminal alcohol ethoxylate emulsifier for use as an
emulsifier.
-3-
CA 02418743 2003-02-12
SUMMARY OF INVENTION
In accordance with the invention, there is provided a non-terminal alcohol
ethoxylate
(NTAE) for use as an emulsifier, the NTAE preferably having the formula:
H
R'-C-R"
I
(OC2H4)n
1
OH
where R' is a C1 to C48 alkyl group, R" is a C1 to C48 alkyl group and n is 1
to 100.
More specific embodiments of the emulsifier include those wherein R' and R"
are C1
to C30, R' and R" are C1 to C20, R' is C5 to C10, R" is C4 to C7, and n is 6
to 40. In a
specific embodiment, R' is C~His, R" is CSHlI, and n is 15.
The NTAE is particularly effective in preparing an emulsion comprising water,
bitumen and the NTAE wherein the bitumen/water ratio is 5:95 to 95:5 (w/w). In
more
specific embodiments, the bitumen/water ratio is 20:80 to 80:20 (w/w), the
bitumen is
selected from any one of or a combination of Cold Lake bitumen, Athabasca
bitumen, Cerro
Negro bitumen or a resid fraction thereof and the emulsifier concentration is
0.03 to 2 % by
weight and more preferably 0.08 to 1.25% by weight.
In accordance with the invention, the emulsion may he further characterised in
terms
of the mean droplet size and bulk droplet size. In various embodiments, the
mean droplet size
of the emulsion is less than 2.6 p,m, less than 7.9 p,m or less than 31 pm. In
other
embodiments, the emulsion is characterised by having bulk droplet size wherein
90% by
volume of the droplets have a diameter less than 6 ~.m, less than 13 ~,m or
less than 65 pm.
In accordance with another embodiment of the invention, a method of preparing
a
bitumen/water emulsion comprising the step of mixing water, bitumen and an
NTAE
emulsifier in a mixing device to form the emulsion is provided. In a preferred
embodiment,
bitumen is introduced into the mixing device at an elevated temperature with
respect to the
water.
-4-
CA 02418743 2003-02-12
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the present invention will now be described, by way of example,
with
reference to the attached figures, wherein:
Figure 1 is a schematic diagram representation of a non-terminal alcohol
ethoxylate
(NTAE) in accordance with the invention and a ternzinal alcohol ethoxylate
(TAE) as
determined by computational chemistry;
Figure 2 is a comparison of actual and regression model droplet sizes; and,
Figure 3 is a comparison of actual and power-law model droplet sizes.
-5-
CA 02418743 2003-02-12
DETAILED DESCRIPTIOhl
The present invention provides a non-terminal alcohol ethoxylate (NT'AE) for
use as
an emulsifier and a method of creating a fine emulsion of water and
hydrocarbon utilizing the
NTAE.
NTAE ChenzistYy
The starting alcohol for the NTAE emulsifier is a non-terminal alcohol:
H
R'-C-R"
1
OH
where the alcohol group, OH, is at a non-terminal carbon in the hydrocarbon
chain. This
starting non-terminal alcohol is ethoxylated to create an NTAE molecule in
which a number
of ethylene oxide or ethoxy groups (E0) are placed in between the non-terminal
carbon and
the OH group. This is contrasted with a terminal alcohol wherein the alcohol
group is attached
to a carbon atom that is at the end of the hydrocarbon chain according to the
general formula
R-OH, where R is an alkyl group that is unbranched or branched at a carbon
position not
adjacent to the alcohol group.
The NTAE emulsifier has the general formula:
H
-~-R99
1
(OC2H4)n
1
OH
where R' is an alkyl group with 1 to 48 carbon atoms (C 1 to C48) and R" is an
alkyl group
with 1 to 48 carbon atoms (C 1 to C48). The number, n, of ethoxy groups
(OC2H4) varies
from 1 to 100. The preferred range for R' and R" is from 1 to 30 carbon atoms
and for n from
3 to 50 and more preferably from 6 to 40. Both R' and R" can be linear or
branched with an
alkyl side chain.
-6-
CA 02418743 2003-02-12
For the emulsification of specific bitumen and/or fractions thereof, R' is C 1
to C20
and more preferably CS to C10, R" is Cl to C20 and more preferably C4 to C7.
In one embodiment of the NTAE, R' is C~HIS, R" is CSHlI, and the OH group is
at the
end of a chain of 15 EO groups.
With reference to Figure 1, an NTAE 2 and a terminal alcohol ethoxylate (TAE)
1 are
shown schematically in a configuration as drawn by computational chemistry
software (Titan
by Wavefunction, Inc.). The amphipathic NTAE 2 has a T-shaped structure having
hydrophobic 4 and hydrophilic moieties 5. As shown, the hydrophilic moiety 5,
including the
ethoxy chain 6, represents one arm of the 'T extending into the water phase 7
of a
water/hydrocarbon interface 8. The hydrophobic moiety 4 includes two alkyl
chains 9, 10
representing the top of the T extending across the water/hydrocarbon interface
8 on the
hydrocarbon side 11 of the interface. In this example, R' is C7 alkyl (C~H15),
R" is CS alkyl
(CSH~ 1), and the OH group 15 is at the end of a chain of 15 EO groups 6. R'
and R" may also
be branched alkyl groups.
In comparison, the TAE 1 has a generally linear structure having the general
formula
R-(EO)"-OH (R is Cl2Hza in this example) wherein the hydrophobic moiety 12
extends deeply
into the hydrocarbon phase 11. In other TAEs, R may be branched at carbon
positions not
adjacent the interface carbon position 14.
As a result, and as shown by computational chemistry and the following
examples, the
NTAE 2 covers more interfacial area than the TAE 1 and is, therefore, a better
surface-active
agent than the TAE 1.
NTAE Synthesis
The NTAE is synthesized in accordance with known techniques having
consideration
to the properties of bitumen or bitumen fractions being emulsified and the
desired properties
of the emulsion wherein the above ranges of R', R" and EO represent practical
ranges for the
synthesis of useable emulsifiers.
The NTAE is made conventionally by the reaction of ethylene oxide with a non-
terminal alcohol, in proportions set by the target moles of ethylene oxide.
The ability to obtain
CA 02418743 2003-02-12
a favourable reaction of ethylene oxide with a non-terminal alcohol by setting
the proportions
based on the target moles of ethylene oxide is known to persons skilled in the
art. In one such
conventional method, the reaction is carried out in the presence of an
alkaline catalyst
(sodium or potassium hydroxide) at about 120° to 150° C. Reactor
cooling is needed as the
reaction is exothermic. At the end of reaction, the alkaline catalyst is
neutralized with an acid.
The feed stock for ethylene oxide is natural gas or petroleum naphtha that is
thermally
cracked to produce ethylene. Reaction of ethylene with oxygen in the presence
of a silver
catalyst produces ethylene oxide, is well known to persons skilled in the art.
The non-terminal alcohol is made from naturally occurring oleochemical sources
(vegetable or animal oils) or synthesized from petrochemical sources. Starting
alcohols are
available in which the total number of carbon atoms can range from i to 50, as
is known to
persons skilled in the art.
As indicated above, in the NTAE structure, the head part (the two alkyl groups
attached to a carbon atom) is oleophilic and the stem part (OCZH4 groups) is
hydrophilic. The
ratio of these two parts determines the hydrophile-lipophile balance (HLB) of
the emulsifier
molecule. To emulsify a given oil, the surfactant requires the proper HLB. The
HLB of a
surfactant also correlates with its water solubility - the higher the HLB, the
more water-
soluble the surfactant is.
The HLB of a surfactant made from a given starting alcohol can be modified by
changing the number of moles of OCZH4. As the number of moles of OCZH4
increases, the
surfactant's hydrophile-lipophile balance increases along with its solubility
in water. The
physical state of the surfactant changes from liquid to solid as the OCZH4
number increases.
The melting point of the surfactant also increases with the OCZH4 number.
Thus, the number of moles of OC2H4 relative to the carbon chain numbers in the
two
alkyl groups in R' and R" is important in determining a favourable surfactant
chemistry to
emulsify a given hydrocarbon.
_g_
CA 02418743 2003-02-12
Water and Bitumen Sources
The water and bitumen sources for an emulsion prepared with the NTAE may vary.
Unlike emulsions made with an ionic surfactant that is known to be sensitive
to the water
salinity and the presence of divalent ions, such as calcium and magnesium in
the water, an
emulsion using the non-ionic NTAE can be prepared with water from different
sources with
variable salinity and divalent ion concentrations.
Suitable sources of water include municipal drinking water, water produced
with
bitumen, lake water, river water, canal water, ground water, brackish (saline)
water or sea
water all of which may be used either in whole or in part to make the
emulsion.
The bitumen for making the emulsion may be held-produced bitumen that may
contain emulsified produced water.
In one embodiment, the field-produced, oil-externall emulsion is not dewatered
if it
contains less than the target water content of the emulsion fuel. Additional
water from any of
the above sources is added to meet the water concentration target of the water-
external
emulsion.
In a further embodiment, the produced bitumen contai~vng emulsified water is
dewatered conventionally to less than a predetermined amount of water and then
additional
water from any of the above sources is added to make the target water content
of the final
water-external emulsion.
Further still, the bitumen may be completely dewatered and the water from any
of the
above sources added to make a water-external emulsion with a target water
content.
In still yet another embodiment, the dewatered bitumen may contain an amount
of
diluent used in typical dewatering processes. The bitumen and the water may
also contain oil-
field demulsifier(s).
Typical hydrocarbons that can be emulsified include but are not limited to
Cold Lake
Bitumen, Athabasca Bitumen, and Cerro Negro Bitumen whose typical properties
are shown
in Table 1. Other hydrocarbons include Fischer-Tropsch liquids and waxes.
-9-
CA 02418743 2003-02-12
Table 1. Typical Bitumen Properties
Properties Bitumen
Cold Lake Cerro Negro Athabasca
API Gravity 11 7.9 8.8
Viscosity @ 15C 84184 7584702 251706
Viscosity @ 40C 4112 133371 12311
Basic Nitrogen, 4014 6188 4100
ppm
Sulfur, wt% 4.3 3.7 4.7
CCR, wt% 13.8 17.2 13.3
Neutralization 1.3 3.0 2 - 4
No.
(mg KOH/g)
Vandium, ppm 178 367 210
Nickel, ppm 85 96 83
Method for Preparing Emulsions
An emulsion can be prepared in accordance with the following general
methodology.
A hydrocarbon, such as bitumen, water and emulsifier are introduced at
elevated
temperature into a mixing device and vigorously mixed to form an emulsion.
Upon exiting the
mixing device, the resulting emulsion may be cooled for subsequent downstream
handling.
The process may be batch or continuous and may be practised using a variety of
mixing
devices, including a rotor-stator assembly having an adjustable clearance,
where both rotor
and stator have circular grooves intersected by radial grooves.
Other embodiments of mixing devices include on-line, non-moving mixing
devices.
Stable emulsions may be prepared by forcing bitumen, water and emulsifier
through mixing
devices such as sintered porous metal or perforated disks.
The bitumen to water ratio is 5:95 to 95:5 and preferably 20:80 to 90:10
(w/w). The
range of emulsifier concentrations is preferably 0.03 to 2% by weight and more
preferably
0.08 to 1.25% by weight, all based on emulsion. The pre-mixing temperature of
the bitumen
may be 40° C to 300° C and preferably 60° C to
100° C. The pre-mixing temperature of the
water may be 4° C to 150° C and preferably 30° C to
90° C. The temperature of the bitumen
- 10-
CA 02418743 2003-02-12
is generally higher than the water in order to promote pumping of the bitumen
into the mixing
device. The temperature of the water is preferably less than 100° C to
eliminate the need for
vessel pressurization.
ZTse of Emulsions
The emulsions prepared in accordance with the invention can be used in a
variety of
applications principally either to facilitate transportation of hydrocarbon
through pipelines, or
by tankers (land and sea) and/or as a fuel.
In one use, the emulsion is burned as a fuel in a dual-fuel burner almost
immediately
after synthesis, thereby eliminating or reducing the need for storage and/or
transportation of
the emulsion. A dual-fuel burner can burn either gaseous fuel when the gaseous
fuel price is
low and burn the emulsion fuel when the gaseous fuel price is high. In another
embodiments
where an emulsion is synthesized almost immediately before; use as a fuel, and
where longer-
term emulsion stability may not be required, the emulsion may be synthesized
using less
water while maintaining a small droplet size, thereby improving the combustion
efficiency.
In another embodiment, the water-soluble NTAE emulsifier is used alone or in
combination with other conventional water-soluble surfactants (non-ionic,
cationic and
anionic) or chemicals that generate surfactants in situ by forming soaps of
carboxylic and
napthenic acids present in the bitumen. Such chemicals are alkali (sodium
hydroxide, NH3,
ammonium hydroxide, potassium hydroxide and sodium carbonate). In addition,
other low-
molecular weight alcohols, such as methyl, ethyl, propyl, iso-propyl, butyl,
iso-butyl, pentyl,
iso-pentyl to decyl and iso-decyl alcohol can be used as a co-surfactant.
Examples
Example 1
Produced Cold Lake Bitumen was de-watered to 0.7% water and subsequently
emulsified using the NTAE of Figure 1 by adding additional water. Bitumen at
85° C was
pumped at 6900 L/h and a water solution containing the emulsifier at
53° C was pumped at
3300 L/h through a commercial-size rotor-stator assembly rotating at 4200 rpm.
Upon exiting
-11-
CA 02418743 2003-02-12
the rotor-stator assembly, the emulsion was rapidly cooled from 70° C
to 48° C to maintain
emulsion quality and reduce possible coalescence that may occur at higher
temperatures. The
resulting emulsion had a water volume of 33.6% and an emulsifier concentration
of 0.75°/~ by
weight. The average droplet size was 2.5 ~,m, the median droplet size was 2.3
~.m and 90% by
volume of the droplets were smaller than 4.7 p,m. The resulting emulsion was
stable for
longer than one month and had a viscosity of 125 cp at 50° C.
Example 2
The conditions for this example were the same as those for Example 1 except
that the
rotor speed was reduced from 4200 rpm to 3600 rpm. The lower rotor speed
resulted in
slightly larger particles with a mean droplet size of 2.6 pxn, a median of 2.2
l.un and 90% by
volume of the droplets being smaller than 5.0 pm.
Example 3
The conditions for this example were the same as those for Examples 1 and 2
except
that the rotor speed was further reduced to 3000 rpm. Further lowering of the
rotor speed
resulted in a further increase in the droplet size with a mean of 2.9 p,m, a
median of 2.3 p,m
and 90% by volume of the droplets being smaller than 5.7 pm.
Example 4
The conditions for this example were the same as those in Example 3 except
that the
emulsifier concentration was increased to 1.1 wt%. The increased emulsifier
concentration
resulted in a finer emulsion with a mean droplet size of 2.3 yn, a median of
1.8 pm and 90%
by volume of the droplets being smaller than 4.4 pm.
Example 5
The conditions for this example were the same as those for Example 4 except
that the
rotor speed was increased from 3000 rpm to 3600 rpm. This resulted in a
slightly finer
-12-
CA 02418743 2003-02-12
emulsion with a mean droplet size of 2.1 p,m, a medium draplet size of 1.8 ~.m
and 90% by
volume of the droplets being smaller than 3.8 pm.
Example 6
The conditions for this example were the same as those for Example 5 except
that the
rotor speed was increased from 3600 rpm to 4200 rpm. This resulted in a
slightly finer
emulsion with a mean droplet size of 2.0 prn, a medium droplet size of 1.8 pm
and 90% by
volume of the droplets being smaller than 3.6 pm.
Example 7
This example repeated E~cample 2 to confirm reproducibility and resulted in a
mean
droplet size of 2.4 pm, a medium droplet size of 2.0 and 90% by volume of the
droplets being
smaller than 4.4 pxn. The statistical significance of the variations between
Examples 2 and 7
is explained in the description of example 9 below.
The emulsion from Example 7 was pumped through a gear pump to a 26.4 GJ
commercial-size demonstration boiler capable of burning both a gaseous fuel
and a liquid
emulsion fuel. This capability allows switching between a gaseous fuel and a
liquid emulsion
fuel depending on the gaseous feel price. Droplet analysis before and after
the pump showed
no sign of emulsion degradation by the pump. The combustion of the emulsion
exhibited
excellent flame characteristics with long, slender and stable flames with a
few sparklers. Very
fine oil droplets in the emulsion led to high carbon conversion efficiency,
low particulates,
low carbon monoxide and low NGx emissions. The emulsion fuel was comparable to
burning
No. 6 fuel oil. The switching between gaseous and liquid emulsion fuel and
vice versa also
went very smoothly.
Example 8
The conditions for this example were the same as those in Example 7 except
that the
emulsifier concentration was reduced from 0.75% to 0.25% by weight. The rotor
speed was
-13-
CA 02418743 2003-02-12
set at 3600 rpm. The resulting emulsion had a mean droplet size of 7.9 hum, a
median droplet
size of 7.5 ~m with 90% by volume of the droplets being smaller than 12.7 Vim.
Example 9
This example repeated Examples 2 and 7 to confirm reproducibility in emulsion
quality. The mean droplet size was 2.2 ~m and the median was 1.8 ~m with 90%
by volume
of the droplets being smaller than 4.0 ~.Lm. This emulsion was also burnt
successfully in the
26.4 GJ dual-fuel burner. This example again demonstrates the excellent
reproducibility of the
emulsion quality using the emulsifier and method of this invention. The mean
particle size
from three repeat runs was 2.3 ~.m with a very tight 95% confidence interval
of 2.1 tc~ 2.5 Vim.
As in Example 7, the switching between the gaseous and liquid emulsion fuel
went very
smoothly, demonstrating that the same burner can be used for both fuels.
Example 10 (Comparison of NT'AE with a Commercial Asphalt Emulsifier)
Examples 1 to 9 relate to whole bitumen emulsification. In Example 10, a
heavier
fraction of bitumen was used to compare the effectiveness of the NTAE
emulsifier with a
commercial asphalt emulsifier consisting of an amine and hydrochloric acid.
A 520° C+ fraction (resid) of Cold Lake bitumen was blended with water
containing
the commercial asphalt emulsifier in a laboratory blender. The hydrocarbon to
water ratio was
68:32 (w/w). The resid was preheated to 160° C and the water containing
the amine (0.2% by
weight based on emulsion) and enough hydrochloric acid to lower its pH to 3
was heated to
80° C. In the first test, all of the resid was poured into the water in
the blender. The blending
of this mixture was very difficult and noisy as the unemulsified resid got
stuck in the blender's
blades. No emulsion was formed. A repeat attempt to make the emulsion by
slowly pouring
the resid to the emulsifier solution while blending was also unsuccessful.
A similar emulsification test was conducted with the NTAE emulsifier of this
invention and the same resid. Blending in this case was smooth and after
blending for 30
seconds an oil-in-water emulsion containing 31 % by volume water was formed.
The
- 14-
CA 02418743 2003-02-12
emulsifier concentration in the emulsion was 0.24% by weight. An oil-in-water
emulsion was
also formed in another repeat test where the resid was added i.n two stages.
This example shows that under conditions where a commercial asphalt emulsifier
cannot emulsify a heavy fraction of bitumen, the emulsifier of the present
invention creates an
emulsion without requiring additional compounds including amine or
hydrochloric acid.
Discussion of Examples 1 to 9
The oil droplet sizes for emulsions prepared with the NTAE are much lower than
that
disclosed in CA 2232490. In Tables 2, 3, 4, 5 and 6, and in Figures 1, 9, 10,
13 and l.4 of CA
2232490, the mean droplet size is described as ranging from 13 to 24 pm. This
was achieved
by a combination of three additives. Eight out of nine examples using this
invention show
mean particle size in the range of 2 to 2.9 Vim. These are lower than those in
CA 2232490 by a
factor of 4.5 to 12Ø In particular., one example of the subject invention
with 0.25% by weight
surfactant shows mean particle size of 7.5 pm with 90% by volume of the
particles being
lower than 12.7 ~,m, which is still smaller than the lowest mean particle size
obtained by CA
2232490.
Further Examples Relating to mater Content, Surfactant Concentration and
Preparation
Further tests using NTAE reveal that the water content in a whole bitumen
emulsion
can be reduced by at least half from the industry standard of 30% by volume
water based on
the total volume of the emulsion. In addition, further testing has
demonstrated that the
concentration of NTAE surfactant can be reduced from those used in Examples 1
to 9.
Lower surfactant and water concentrations are expected to reduce the cost of
commercializing emulsions as an alternate fuel technology, particularly in a
scenario where
emulsions are burnt as they are made. In this scenario, the need for storage
and/or pumping
emulsions at low temperatures (that is, less than about 50° C) is
eliminated. Therefore,
emulsions can be made with a lower surfactant concentration and a lower water
content. In
addition to cost savings associal;ed with a lower surfactant concentration, a
lower water
-15-
CA 02418743 2003-02-12
content will improve the economics of the process by increasing the heat value
of the fuel per
unit volume of emulsion, and dec~~easing the water handling and recycling
costs.
Further evaluation of NT.AE was conducted in an in-line static mixing unit
wherein
emulsions were made by varying the water content from 6.6% to 36.6% by vohune
and
varying the surfactant concentrai:ion from 0.03% to 0.18% by weight, both
based on total
emulsion. In addition to these two variables, the effects of maximum liquid
superficial
velocity (MXLSV), bitumen and surfactant temperatures on mean oil droplet size
were also
studied. MXLSV is a measure of shear rate (or agitation) and is determined by
the measuring
the flow rate of both separate and combined components (bitumen and water)
through a static
mixing emulsification process (SMEP) apparatus. The MXLSV was varied from 3..6
to 4.3
m/s, the bitumen temperature from 74° to 98° C and the NTAE
solution temperature from 53°
to 68° C.
NTAE was dissolved in Calgary tap water and preheated from 53° to
68° (J. Field-
produced Cold Lake bitumen devratered to 0.7% water was preheated from
74° to 98° C in a
separate vessel. Each liquid wa.s pumped separately through the following
static mixer
combination: 30.5 cm x 1.27 cm; 30.5 cm x 0.95 cm; 30.5 cm x 0.64 cm. The
inside diameter
of 0.64 cm of the smallest static mixer set was used to calculate the maximum
superficial
velocity of the co-mingled bitumen and NTAE water. The pressure upstream of
tlhe static
mixer was monitored and the emulsion type checked using the water dispersion
test. In the
dispersion test, a small drop of emulsion is placed on top of water taken in a
small container.
If the drop easily disperses into tile water, the emulsion has a continuous
water phase that is
miscible with the water in the cantainer. On the other hand, if the emulsion
drop does not
disperse in the water but stays a.s a drop, the emulsion has a continuous oil
phasfs that is
immiscible with water. The water content of the emulsion was determined using
a Dean-Stark
analysis. A Beckman Coulter LS - 230 particle size analyzer (available from
E~eckman
Coulter, Miami, Florida) was used to determine particle size distribution. The
shelf stability
of the emulsion was assessed by observing the amount of water separation at
the bottom of
the bottle stored at room tempera~.ure. Stability was recorded. after 1 hour,
2 hours, 2 0 hours
and one month.
- 16-
CA 02418743 2003-02-12
Results
Table 1 shows the results of the SMEP tests.
Table 1
NTAE Bitumen Mean Mean Mean
% Water*Flow Emulsifier PressureSize, Size,
Rate Emulsion
MXLSV
Concn.* T T T um um Size,
um
wt% vol% cm3/minmis C C C KPag Exptl. Regressionpwer-
Law
Model Model
0.090 36.6 4680 4.26 74 59.0 69 958.3721.4 21.7 20.9
0.088 35.4 4680 4.2 75 59.0 70 923.9021.8 22.0 21.1
6
0.068 27.0 2410 2.2 75 59.0 73 379.2138.2 38.0 37.9
0
0.068 27.1 2410 2.20 76 59.0 73 406.7938.1 38.2 38.2
0.097 38.3 4500 4.10 95 53.0 70 786.0021.5 20.9 20.9
0.097 38.2 4500 4.10 96 53.0 72 730.8423.7 21.0 21.0
0.088 35.1 4320 3.94 97 53.0 79 613.6322.6 23.2 22.6
0.088 35.4 4320 3.94 98 53.0 79 634.3220.9 23.4 22.8
0.157 31.4 4320 3.94 75 58.0 67 923.9012.0 11.7 15.3
0.135 27.0 4140 3.77 76 58.0 70 854.9514.9 15.7 16.8
0.030 6.6 3815 3.48 77 58.0 78 875.6331.0 30.7 31.4
0.175 17.5 3960 3.61 75 67.0 76 854.9516.3 15.5 15.7
0.182 18.2 3960 3.61 76 67.0 77 827.3714.3 14.8 15.6
0.162 16.2 3960 3.C~1 76 67.0 78 820.4717.3 17.6 16.2
0.163 16.3 3960 3.61 76 68.0 79 799.7918.5 18.2 16.5
SOS 15.98 32.43
'based MSOS 2.00 4.05
on STD 1 2
41 01
emulsion DEVN. , .
R2 0.98 0.96
As shown in Table l, emulsions were made at or around the industry standard.
of 30%
water, with 16% to 18% water, and with as little as 6.6% water, all by volume.
The results in
Table 1 also show the ability to make an emulsion with less than 0.25% by
weight N7.'AE, the
lowest concentration tried previously in a commercial-scale rotor-stator
assembly. In the
SMEP unit, emulsions were made with O.IB% by weight NTAE or lower and with as
little as
0.03% by weight (300 ppm) NTAE in the emulsion.
The most significant observation from the tests in Table 1 is that an oil-in-
water
emulsion can be made with as little as 0.03% (or 300 ppm) by weight NTAE
concentration
-17-
CA 02418743 2003-02-12
and with as little as 6.6 % by volume of water, both based on emulsion. The
mean oil. particle
size at this combination is, however, 31 ~m with 90% by volume of the
particles being
smaller than 65 Vim. Using this combination of NTAE and water concentration,
finer
emulsions can be made using higher MXLSV and/or additional static mixers.
In all the tests, the emulsic>ns made were stable at least for a month showing
no sign of
water separation. One-month stability is sufficient for applications where the
emulsion is
burned as a fuel shortly after its s~mthesis.
An emulsion made at 3.6 m/s MXLSV, 0.18% by weight NTAE, 18% by volume
water, a bitumen temperature of 76° C and an NTAE solution temperature
of 67° C had a
mean particle size of 14.3 ~m and was stable for at least one month. In
addition, the pressure
upstream of the static mixer was :not much different from that of the 31.4%
water by volume
emulsion as shown in Table I. A.s mentioned earlier, finer emulsions can be
prepared using
higher MXLSV and/or additional static mixers.
Emulsion Droplet Size Prediction
Two types of models, a power-law and a linear regression model were used to
relate
the mean oil droplet size to the independent variables in the study described
as follow;a:
Regressiofz Model:
ODS = 0.0234 - (158.7547 * SC) - (6.9422 * MXLSV) + ( 0.1913 * WC)
+ (0.1669 ~' BT) + (0.7834 * sT)
where,
ODS = mean oil droplet size, ~,m
SC = surfactant concentration, % by weight based on emulsion
MXLSV = maximum liquid superficial velocity, m/s
WC = water content, % by volume based on emulsion
BT = bitumen temperature, °C
ST = NTAE soluticm temperature, °C
For this model, the mean sum of squares (Mean SOS), defined as the sum of
squares
of the differences between the experimental and model-predicted particle sizes
divided by the
-18-
CA 02418743 2003-02-12
degrees of freedom is 2.0 (p.m)2. The degrees of freedom, defined as the
number of
experimental runs (15 in this case) minus the number of parameters in the
model (~6 in this
case) minus l, is 8. The standard deviation (Std. Devn.), defined as the
square root of the
Mean SOS, is 1.4 pin, while the: correlation coefficient, R2, between the
experimental and
predicted mean oil droplet size is 0.98.
Table 1 and Figure 2 show that the experimental mean particle sizes compare
well
with the ones predicted by the regression model.
Power-Law Model:
ODS = (1.205 E - 02) * (S~')-°~s7°' * (MXLSV)'° 7430
* (WC)o.zaz4
* (BT)°.4802 * (ST)1.0478
Mean SOS = 4.05 (pm)Z
Std. Devn. = 2.01 pin
R2 = 0.96
Table 1 and Figure 3 show good agreement between the experimental and the;
power-
law model predicted mean particle sizes.
Both models allow the quantification of the effect of each pertinent variable
on the
mean droplet size. As expected, both models predict that the particle size
should decrease
with increased NTAE concentration and/or increased flow velocity.
An unexpected prediction from the models is that a reduction in water content
decreases oil droplet size. What this means is that an emulsion created with
less than the
industry standard of 30% by volume water has smaller oil droplets. While this
emulsion may
be less stable than conventional emulsions because of lower water, it may be
particularly
effective as a fuel that is burnt alrr~ost immediately after preparation.
The above-described embodiments of the present invention are intended to be
examples only. Alterations, modifications and variations may be effected to
the particular
embodiments by those of skill in the art without departing from the scope of
the invention,
which is defined solely by the claims appended hereto.
- 19-