Note: Descriptions are shown in the official language in which they were submitted.
CA 02419700 2003-02-19
PARALLEL REACTOR WITH INTERNAL SENSING AND METHOD OF USING
SAME
s
to BACKGROUND OF THE IIWENTION
Technical Field
The present invention relates to methods, devices, and computer programs for
rapidly
making, screening, and characterizing an array of materials in which process
conditions are
controlled and monitored.
is
Discussion
In combinatorial chemistry, a large number of candidate materials are created
from a
relatively small set ofprecursors and subsequently evaluated for suitability
for a particular
application. As currently practiced, combinatorial chemistry permits
scientists to
2o systematically explore the influence of structural variations in candidates
by dramatically
accelerating the rates at which they are created and evaluated. Compared to
traditional
discovery methods, combinatorial methods sharply reduce the costs associated
with preparing
and screening each candidate.
Combinatorial chemistry has revolutionized the process of drug discovery. One
can
25 view drug discovery as a twn=step process: acquiring candidate compounds
through
laboratory synthesis or through natural products collection, faIlowed by
evaluation ar
screening for eff cacy. Pharmaceutical researchers have long used high-
throughput screening
(HTS) protocols to rapidly evaluate the therapeutic value of natural products
and libraries of
compounds synthesized and cataloged over many years. However, compared to HTS
30 protocols, chemical synthesis has historically been a slow, arduous
process. With the advent
CA 02419700 2003-02-19
of combinatorial methods, scientists can now create large libraries of organic
molecules at a
pace on par with HTS protocols.
Recently, combinatorial approaches have been used for discovery programs
unrelated
to drugs. For example, some researchers have recognized that combinatorial
strategies also
offer promise for the discovery of inorganic compounds such as high-
temperature
superconductors, magnetoresistive materials, luminescent materials, and
catalytic materials.
See, for example, U.S. patent 5,985,356 entitled "The Combinatorial
Synthesis of Novel Materials" (published as WO 96/11878) and ,U.S. patent
6,030,917 entitled "Combinatorial Synthesis and Analysis of Organometallic
Compounds and Catalysts" (published, in part, as WO 98/03251),
Because of its success in eliminating the synthesis bottleneck in drug
discovery, many
researchers have come to narrowly view combinatorial methods as tools for
creating
structural diversity_ Few researchers have emphasized that, during synthesis,
variations in
' temperature, pressure, ionic strength, and other process conditions can
strongly influence the
properties of library members. For instance, reaction conditions are
particularly important in
formulation chemistry, where one combines a set of components under different
reaction
coizditions or concentrations to determine their influence on product
properties.
Moreover, because xhe performance criteria inwmaterials science is often
different than
_ in pharmaceutical research, many workers have failed to realize that process
variables often
can be used to distinguish among library members both during and after
synthesis. For
example, the viscosity of reaction mixtures can be used to distinguish library
members based
on their ability to catalyze a solution-phase polymerization-at constant
polymer
concentration, the higher the viscosity of the solution, the greater the
molecular weight of the
polymer formed. Furthermore, total heat liberated and/or peak temperature
observed during
an exothermic reaction can be cased to rank catalysts.
Therefore, a need exists for an apparatus to prepare and screen combinatorial
libraries
in which one can monitor and control process conditions during synthesis and
screening.
-2-
CA 02419700 2003-02-19
SUMMARY OF THE INVENTION
The present invention generally provides an apparatus for parallel processing
of
reaction mixtures. The apparatus includes vessels for containing the reaction
mixtures, a
stirring system, and a temperature control system that is adapted to maintain
individual
vessels or groups of vessels at different temperatures. The apparatus may
consist of a
monolithic reactor block, which contains the vessels, or an assemblage of
reactor block
modules. A robotic material handling system can be used to automatically load
the vessels
with starting materials. In addition to heating or cooling individual vessels,
the entire reactor
block can be maintained at a nearly uniform temperature by circulating a
temperature-
controlled thermal fluid through channels formed in the reactor block. The
stirring system
generally includes stirnng members-blades, bars, and the like-placed in each
of the
vessels, and a mechanical or magnetic drive mechanism. Torque and rotation
rate can be
controlled and monitored through strain gages, phase lag measurements, and
speed sensors.
The apparatus may optionally include a system for evaluating material
properties of
the reaction mixtures. The system includes mechanical oscillators located
within the vessels.
When stimulated with a variable-frequency signal, the mechanical oscillators
generate
response signals that depend on properties of the reaction mixture. Through
calibration,
mechanical oscillators can be used to monitor molecular weight, specific
gravity, elasticity,
dielectric constant, conductivity, and other material properties of the
reaction mixtures.
2o The present invention also provides an apparatus for monitoring rates of
production or
consumption of a gas-phase component of a reaction mixture. The apparatus
generally
comprises a closed vessel for containing the reaction mixture, a stirnng
system, a temperature
control system and a pressure control system. The pressure control system
includes a
pressure sensor that communicates with the vessel, as well as a valve that
provides venting of
a gaseous product from the vessel. In addition, in cases where a gas-phase
reactant is
consumed during reaction, the valve provides access to a source of the
reactant. Pressure
monitoring of the vessel, coupled with venting of product or filling with
reactant allows the
investigator to determine rates of production or consumption, respectively.
One aspect of the present invention provides an apparatus for monitoring rates
of
consumption of a gas-phase reactant. The apparatus generally comprises a
closed vessel for
containing the reaction mixture, a stirring system, a temperature control
system and a
3-
CA 02419700 2003-02-19
pressure control system. The pressure control system includes a pressure
sensor that
communicates with the vessel, as well as a flow sensor that monitors the flow
rate of reactant
entering the vessel. Rates of consumption of the reactant can be determined
from the reactant
flow rate and filling time.
The present invention also provides a method of making and characterizing a
plurality
of materials. The method includes the steps of providing vessels with starting
materials to
form reaction mixtures, confining the reaction mixtures in the vessels to
allow the reaction to
occur, and stirnng the reaction mixtures for at least a portion of the
confining step. The
method further includes the step of evaluating the reaction mixtures by
tracking at least one
1 o characteristic of the reaction mixtures for at least a portion of the
confining step. Various
characteristics or properties can be monitored during the evaluating step,
including
temperature, rate of heat transfer, conversion of starting materials, rate of
conversion, torque
at a given stirring rate, stall frequency, viscosity, molecular weight,
specific gravity,
elasticity, dielectric constant, and conductivity.
t5 One aspect of the present invention provides a method of monitoring the
rate of
consumption of a gas-phase reactant. The method comprises the steps of
providing a vessel
with starting materials to form the reaction mixture, confining the reaction
mixtures in the
vessel to allow reaction to occur; and stirring the reaction mixture for at
least a portion of the
confining step. The method further includes filling the vessel with the gas-
phase reactant
2o until gas pressure in the vessel exceeds an upper-pressure limit, PH, and
allowing gas pressure
in the vessel to decay below a lower-pressure limit, Pi. Gas pressure in the
vessel is
monitored and recorded during the addition and consumption of the reactant.
This process is
repeated at least once, and rates of consumption of the gas-phase reactant in
the reaction
mixture are determined from the pressure versus time record.
25 Another aspect of the present invention provides a method of monitoring the
rate of
production of a gas-phase product. The method comprises the steps of providing
a vessel
with starting materials to form the reaction mixture, confining the reaction
mixtures in the
vessel to allow reaction to occur, and stirring the reaction mixture fox at
least a portion of the
confining step. The method also comprises the steps of allowing gas pressure
in the vessel to
3o rise above an upper-pressure limit, PH, and venting the vessel until gas
pressure in the vessel
falls below a lower-pressure limit, PL. The gas pressure in the vessel is
monitored and
CA 02419700 2003-02-19
recorded during the production of the gas-phase component and subsequent
venting of the
vessel. The process is repeated at Least once, so rates of production of the
gas-phase product
can be calculated 'from the pressure versus time record.
The present invention provides an apparatus for parallel processing of
reaction
mixtures comprising vessels for containing the reaction mixtures, a stirring
system for
agitating the reaction mixtures, a temperature control system for regulating
the temperature of
the reaction mixtures, and a fluid injection system. The vessels are sealed to
minimize
unintentional gas flow into or out of the vessels, and the fluid injection
system allows
introduction of a liquid into the vessels at a pressure different than ambient
pressure. The
fluid injection system includes fill ports that are adapted to receive a
liquid delivery probe,
such as a syringe or pipette, and also includes conduits, valves, and tubular
inj ectors. The
conduits provide fluid communication between the fill ports and the valves and
between the
valves and the injectors. The injectors are located in the vessels, and can
have varying
lengths, depending on whether fluid injection is to occur in the reaction
mixtures or in the
I5 vessel headspace above the reaction mixtures. Generally, a robotic material
handling system
manipulates the fluid delivery probe and controls the valves. The injection
system can be
used to deliver gases, liquids, and slurries, e.g., catalysts on solid
supports.
One aspect of the present invention provides an apparatus for parallel
processing of
reaction mixtures comprising sealed vessels, a temperature control system, and
a stirring
system having a magnetic feed through device for coupling an external drive
mechanism with
a spindle that is completely Contained within one of the vessels. The magnetic
feed through
device includes a rigid pressure barrier having a cylindrical interior surface
that is open along
the base of the pressure barrier. The base of the pressure barrier is attached
to the vessel so
that the interior surface of the pressure barner and the vessel define a
closed chamber. The
magnetic feed through device further includes a magnetic driver that is
rotatably mounted on
the rigid pressure barrier and a magnetic follower that is rotatably mounted
within the
pressure barrier. The drive mechanism is mechanically coupled to the magnetic
driver, and
one end of the spindle is attached to a leg portion of the magnetic follower
that extends into
the vessel headspace. Since the magnetic driver and follower are magnetically
coupled,
rotation of the magnetic driver induces rotation of the magnetic follower and
spindle.
-5-
CA 02419700 2003-02-19
Another aspect of the present invention provides an apparatus for parallel
processing
of reaction mixtures comprising sealed vessels, a temperature control system,
and a stirring
system that includes mufti-piece spindles that are partially contained in the
vessels. Each of
the spindles includes an upper spindle portion that is mechanically coupled to
a drive
mechanism, a removable stirrer contained in one of the vessels, and a coupler
for reversibly
attaching the removable stirrer to the upper spindle portion. The removable
stirrer is made of
a chemically resistant plastic material, such as polyethylethylketone or
polytetrafluoroethylene, and is typically discarded after use.
The exact combination of parallel processing features depends on the
embodiment of
1o the invention being practiced. In some aspects, the present invention
provides an apparatus
for parallel processing of reaction mixtures comprising sealed vessels and an
injection
system. The present invention also provides an apparatus for parallel
processing of reaction
mixtures comprising sealed vessels, an injection system and a stirring system.
The present
invention also provides an apparatus for parallel processing of reaction
mixtures comprising
is vessels having a temperature control system and a stirring system. The
present invention also
provides an apparatus for parallel processing of reaction mixtures comprising
sealed vessels
and a pressure control system. The present invention also provides an
apparatus for parallel
processing ofreaction mixtures comprising sealed vessels, an injection system
and a system
for property or characteristic monitoring.
2o The present invention also provides computer programs and computer-
implemented
methods for monitoring the progress and properties of parallel chemical
reactions. In one
aspect, the invention features a method of monitoring a combinatorial chemical
reaction. The
method includes {a) receiving a measured value associated with the contents of
each of a
plurality of reactor vessels; {b) displaying the measured values; and (c)
repeating steps (a) and
25 (b) multiple times over the course of the combinatorial chemical reaction.
Implementations of the invention can include one or more of the following
advantageous features. The measured values include a set of values for a
number of reaction
conditions associated with each of the reactor vessels. Step (c) is performed
at a
predetermined sampling rate. The method also includes changing a reaction
parameter
3o associated with one of the reactor vessels in response to the measured
value to maintain the
reactor vessel at a predetermined set point. Reaction parameters include
temperature,
CA 02419700 2003-02-19
pressure, and motor (stirnng) speed. The method also includes quenching a
reaction in one of
the reactor vessels in response to the measured value associated with the
contents of the
reactor vessel. The method also includes using the measured value to calculate
an
experimental variable or value for one of the reactor vessels. Examples of
experimental
variables include rates of change of temperature or pressure, percent
conversion of a starting
material, and viscosity. The method also includes displaying the experimental
variable.
In general, in another aspect, the invention features a method for controlling
a
combinatorial chemical reactor including multiple reactor vessels, each
containing a reaction
environment. The method includes receiving a set point for a property
associated with each
vessel's reaction environment; measuring a set of experimental values for the
property for
each vessel; displaying the set of experimental values; and changing the
reaction environment
in one or more of the plurality of reactor vessels in response to the set
point and a change in
one or more of the set of experimental values. For example, the method may
terminate a
reaction (change the reaction environment) in response to reactant conversion
(experimental
1 5 value) indicating that a target conversion (set point) has been reached.
During reaction, a
graphical representation of the set of experimental values is displayed, often
as a histogram.
In general, in another aspect, the invention features a computer program on a
computer-readable medium for monitoring a combinatorial chemical reaction. The
program
includes instructions to (a) receive a measured value associated with the
contents of each of a
2o plurality of reactor vessels, instructions to (b) display the measured
values, and instructions to
(c) repeat steps (a) and (b) multiple times during the course of the
combinatorial chemical
reaction. The computer program includes instructions to change a reaction
parameter
associated with one of the reactor vessels in response to the measured value
to maintain the
reactor vessel at a predetermined set point.
25, In general, in another aspect, the invention features a reactor control
system for
monitoring and controlling parallel chemical reactions. The reactor system
includes a system
control module for providing control signals to a parallel cl-~emical reactor
including multiple
reactor vessels; a mixing monitoring and control system, a temperature
monitoring and
control system, and a pressuxe monitoring and control system. The reactor
system also
3o includes a data analysis module for receiving a set of measured values from
the parallel
chemical xeactor and for calculating one or more calculated values for each of
the reactor
CA 02419700 2003-02-19
vessels. In addition, the reactor control system includes a user interface
module for receiving
reaction parameters and for displaying the set of measured values and
calculated values.
Advantages that can be seen in implementations of the invention include one or
more
of the following. Process variables can be monitored and controlled for
multiple elements in
a combinatorial library as a chemical reaction progresses. Data can be
extracted for each
library element repeatedly and in parallel over the course of the reaction,
instead of extracting
only a limited number of data points for selected library elements.
Calculations and
corrections can be applied automatically to every available data point for
every library
element over the course of the reaction. A single experimental value can be
calculated from
to the entire data set for each library element.
A further understanding of the nature and advantages of the present invention
may be
realized by reference to the remaining portions of the specification,
drawings, and claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig: 1 illustrates a parallel reactor system in accordance with the present
invention.
15 Fig. 2 shows a perspective view of a modular reactor block with a robotic
liquid
handling system.
Fig. 3 shows a temperature monitoring system.
Fig. 4 shows a cross-sectional view of an integral temperature sensor-vessel
assembly.
Fig. 5 shows a side view of an infrared temperature measurement system.
20 ' Fig. 6 shows a temperature monitoring and control system for a reactor
vessel.
Fig. 7 illustrates another temperature control system, which includes liquid
cooling
and heating of the reactor block.
Fig. 8 is a cross-sectional view of thermoelectric devices sandwiched between
a
reactor block and heat transfer plate.
25 Fig. 9 is a cross-sectional view of a portion of a reactor block useful for
obtaining
calorimetric data.
Fig. 10 is an exploded perspective view of a stirring system for a single
module of a
modular reactor block of the type shown in Fig. 2.
Fig. 11 is a schematic representation of an electromagnetic stirring system.
~.g-
CA 02419700 2003-02-19
Fig. 12-13 are schematic representations of portions of electromagnet stirnng
arrays in
which the ratios of electromagnets to vessel sites approach I:1 and 2:1,
respectively, as the
number of vessel sites becomes large.
Fig. 14 is a schematic representation of an electromagnet stirring array in
which the
ratio of electromagnets to vessei sites is 4:1.
Fig. 15 shows additional elements of an electromagnetic stirring system,
including
drive circuit and processor.
Fig. 16 illustrates the magnetic field direction of a 2 x 2 electromagnet
array at four
different times during one rotation of a magnetic stirnng bar.
to Fig. 17 illustrates the magnetic field direction of a 4 x 4 electromagnet
array at five
different times during one full rotation of a 3 x 3 array of magnetic stirring
bars.
Fig. 18 illustrates the rotation direction of the 3 x 3 array of magnetic
stirring bars
shown in Fig. 17.
Fig. 19 shows a wiring configuration for an electromagnetic stirring system.
15 Fig. 20 shows an alternate wiring configuration for an electromagnetic
stirring system.
Fig. 21 shows the phase relationship between sinusoidal source currents, I~(t)
and I,(t),
which drive two series of electromagnets shown in Fig. 19 and 20.
Fig. 22 is a block diagram of a power supply for an electromagnetic stirring
system.
Fig. 23 illustrates an apparatus for directly measuring the applied torque of
a stirring
20 system.
Fig. 24 shows placement of a strain gauge in a portion of a base plate that is
similar to
the tower plate of the reactor module shown in Fig. 10.
Fig. 25 shows an inductive sensing coil system for detecting rotation and
measuring
phase angle of a magnetic stirring blade or bar.
25 Fig. 26 shows typical outputs from inductive sensing coils, which
illustrate phase lag
associated with magnetic stirring for low and high viscosity solutions,
respectively.
Fig. 27 illustrates how amplitude and phase angle will vary during a reaction
as the
viscosity increases from a low value to a value sufficient to stall the
stirring bar.
Fig. 28-29 show bending modes of tuning forks and bimorph/unimorph resonators,
3o respectively.
~g-
CA 02419700 2003-02-19
Fig. 30 schematically shows a system for measuring the properties of reaction
mixtures using mechanical oscillators.
Fig. 31 shows an apparatus for assessing reaction kinetics based on monitoring
pressure changes due to production or consumption various gases during
reaction.
Fig. 32 shows results ofcalibration runs for polystyrene-toluene solutions
using
mechanical oscillators.
Fig. 33 shows a calibration curve obtained by correlating M. of the
polystyrene
standards with the distance between the frequency response curve for toluene
and each of the
polystyrene solutions of Fig. 32.
1o Fig: 34 depicts the pressure recorded during solution polymerization of
ethylene to
polyethylene.
Fig. 35-36 show ethylene consumption rate as a function of time, and the mass
of
polyethylene formed as a function of ethylene consumed, respectively.
Fig. 37 shows a perspective view of an eight-vessel reactor module, of the
type shown
15 in Fig. I0, which is fitted with an optional liquid injection system.
Fig. 38 shows a cross sectional view of a first embodiment of a fill port
having an o-
ring seal to minimize liquid leaks.
Fig. 39 shows a second' embodiment of a fill port.
Fig. 40 shows a phantom firont view of an injector manifold.
2o Fig. 40A shows a perspective view of an injector manifold 1006
Fig. 40B shows a cross sectional view of the injector manifold shown in Fig.
40A.
Fig. 41-42 show a cross sectional view ofan injector manifold along first and
second
section lines shown in Fig. 40, respectively.
Fig. 43 shows a phantom top view of an injector adapter plate, which serves as
an
25 interface between an injector manifold and a block of a reactor module
shown in Fig. 37.
Fig. 44 shows a cross sectional side view of an injector adapter plate along a
section
line shown in Fig. 43.
Fig. 45 shows an embodiment of a well injector.
Fig. 46 shows a top view of a reactor module.
30 Fig. 47 shows a "closed" state of an injector system valve prior to fluid
injection.
-10-
CA 02419700 2003-02-19
Fig. 48 shows an "open" state of an injector system valve prior during flezid
injection,
and shows a'stirring mechanism and associated seals for maintaining above-
ambient pressure
in reactor vessels.
Fig. 49 shows a cross sectional view of a magnetic feed through stirring
mechanism
that helps minimize gas leaks associatesi with dynamic seals.
Fig. 50 shows a perspective view of a stirring mechanism sfiown in Fig. 48,
and
provides details of a rnulti-piece spindle:
Fig. SOA shows an alternative design for a mufti-piece spindle.
Fig. SOB shows details of the alternative design for a mufti-piece spindle
shown in
1o Fig.50B.
Fig. 51 Shows details of a couplar portion of a rnuTti-piece spindle.
Fig. 52 is a cross sectional view of the coupler shown in Fig. 51 taken along
the line 1472_
Fig. 53 is a block diagram of a data processing system sfiowing an
implementation of
the invention.
:5 Fig. 54-57 are schemafic diagrams of a parallel reactor suitable for use
with the
invention.
Rig. 58 is a flow diagtaue of a method of controlling and analyzing a parallel
chemical
reaction.
dig. 59 is an illustration of a dialog window for user iizput of systan
configuration
2o information.
Fig. 60 is an illustration. of a dialgg window for user input of data display
information.
Fig. 6I is an illustration of a dialog window-for user input of parallel
reactor
parameters.
Fig. 62 is an illustration of a dialog window for user input of a temperature
gradient
z5 for reactor blocks in a parallel reactor.
Fig. 63-64 are illustrations ofvviridov~is displaying system status and
experimental
results for a parallel reactor.
Fig. 65 is an illustration of a window displaying experimental results for a
single
reactor vessel.
3o Fig. 66 is an illustration of a dialog window for user input of color
scaling parameters.
-.-i 1-
CA 02419700 2003-02-19
Fig. 67 is a schematic diagram of a computer platform suitable for
implementing the
data processing system of the invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention provides an apparatus, methods, and computer programs
for
carrying out and monitoring the progress and properties of multiple reactions
in situ. It is
especially useful for synthesizing, screening, and characterizing
combinatorial libraries, but
offers significant advantages over conventional experimental reactors as well.
For example,
in situ monitoring of individual reaction mixtures not only provides feedback
for process
controllers, but also provides data for determining reaction rates, product
yields, and various
properties of the reaction products, including viscosity and molecular weight
during an
experiment. Moreover, in situ monitoring coupled with tight process control
can improve
product selectivity, provide opportunities for process and product
optimization, allow
processing of temperature-sensitive materials, and decrease experimental
variability.
Other advantages result from using small mixture volumes. In addition to
conserving
valuable reactants, decreasing sample size increases surface area relative to
volume within
individual reactor vessels. This improves the uniformity of reaction mixtures,
aids gas-liquid
exchange in multiphase reactions, and increases heat transfer between the
samples and the
reactor vessels. Because large samples respond much slower to changes in
system
conditions, the use of small samples, along with in situ monitoring and
process control, also
allows for time-dependent processing and characterization.
The parallel reactor of this invention is useful for the research and
development of
chenucal reactions, catalysts and processes. The same type of reaction may be
preformed in
each vessel or different reactions may be performed in each vessel. Thus, each
reaction
vessel may vary with regard to its contents during an experiment. Each
reaction vessel can
vary by a process condition, including catalyst amounts (volume, moles or
mass), ratios of
starting components, time for reaction, reaction temperature, reaction
pressure, rate of
reactant addition to the reaction, reaction atmosphere, reaction stir rate,
injection of a catalyst
or reactant or other component (e.g., a reaction quencher) and other
conditions that those of
skill in the art will recognize. Each reaction vessel can also vary by the
chemicals present,
such as by using different reactants or catalysts in two or more vessels.
-12-
CA 02419700 2003-02-19
For example, the parallel reactor of this invention may have reaction vessels
that are
of different volume. The reactor vessel volume may vary from about 0.1
milliliter (ml) to
about 500 ml, more particularly from about 1 ml to about 100 ml and even more
particularly
from about 5 ml to about 20 ml. These reactor vessel sizes allow for reactant
volumes in a
range that functionally allow fox proper stirring (e.g., a 1 ~ ml reactor
vessel allows for
reactant volumes of between about 2-10 ml). Also, the parallel reactor of this
invention
allows the reactor pressure to vary from vessel to vessel or module to module
or cell to cell,
with each vessel being at a pressure in the range of from about atmospheric
pressure to about
500 psi and more particularly in the range of from atmospheric to about 300
psi. In still other
to embodiments, the reactor temperature may vary from vessel to vessel or
module to module or
cell to cell, with each vessel being at a temperature in the range of from
about -150°C to
about 250°C and more particularly in the range of from -100°C to
about 200°C. The stirring
rates may also vary from vessel to vessel or module to module or cell to cell,
with each vessel
being stirred by mechanical stirnng at a rate of from about 0 to about 3000
revolutions per
is minute (rpm) and more particularly at a rate of from about 10 to about 2000
rpm and even
more particularly at a rate of from about 100 to about 1000 rpm. In other
embodiments, the
parallel reactor of this invention allows for the injection of reactants or
other components
(such as catalysts) while a reactor vessel is at reaction pressure (as
discussed in detail below).
Generally, the injection of reactants or components allows for the reaction
conditions to be
20 varied from vessel to vessel, such as by adding a reaction quencher at a
timed frequency or a
conversion frequency. Reaction times can vary depending on the experiment
being
performed, but may be in the range from less than one minute to about 48
hours, more
particularly in the range of from about one minute to about 24 hours and even
more
particularly in the range of from about s minutes to about 12 hours.
25 Overview of Parallel Reactor
The parallel reactor system of the present invention is an integrated platform
for
effecting combinatorial research in chemistry and materials science
applications. An
integrated parallel reactor system comprises a plurality of reactors that can
be operated in
parallel on a scale suitable for research applications - typically bench scale
or smaller scale
3U (e.g., mini-reactors and micro-reactors). The reactors of such an
integrated system can
---i 3--
CA 02419700 2003-02-19
typically, but not necessarily, be formed in, be integral with or be linked by
a common
substrate, be arranged in a common plane, preferably with spatial uniformity,
and/or can
share a common support structure or housing. The integrated parallel reactor
system can also
include one or more control and monitoring systems that are fully or partially
integral
therewith.
Fig. 1 shows one embodiment of a parallel reactor system 100. The reactor
system
100 includes removable vessels 102 for receiving reactants. Wells 104 formed
into a reactor
block 106 contain the vessels 102. Although the wells 104 can serve as reactor
vessels,
removable vessels 102 or liners provide several advantages. For example,
following reaction
to and preliminary testing (screening), one can remove a subset of vessels 102
from the reactor
block 106 for further in-depth characterization. When using removable vessels
102, one can
also select vessels 102 made of material appropriate for a given set of
reactants, products, and
reaction conditions. Unlike the reactor block 106, which represents a
significant investment,
the vessels 102 can be discarded if damaged after use. Finally; one can lower
system 100
15 costs and ensure compatibility with standardized sample preparation and
testing equipment
by designing the reactor block 106 to accommodate commercially available
vessels.
As shown in Fig. 1, each of the vessels 102 contains a stirring blade 108. In
one
embodiment, each stirring blade I08 rotates at about the same speed, so that
each of the
reaction mixtures within the vessels 102 experience similar mixing. Because
reaction
2o products can be influenced by mixing intensity, a uniform rotation rate
ensures that any
differences in products does not result from mixing variations. In another
embodiment, the
rotation rate of each stirring blade 108 can be varied independently, which as
discussed
below, can be used to characterize the viscosity and molecular weight of the
reaction products
or can be used to study the influence of mixing speed on reaction.
25 Depending on the nature of the starting materials, the types of reactions,
and the
method used to characterize reaction products and rates of reaction, it may be
desirable to
enclose the reactor block I06 in a chamber 110. The chamber I 10 may be
evacuated or filled
with a suitable gas. In some cases, the chamber I 10 may be used only during
the loading of
starting materials into the vessels 102 to minimize contamination during
sample preparation,
30 for example, to prevent poisoning of oxygen sensitive catalysts. In other
cases, the chamber
1 I O may be used during the reaction process or the characterization phase,
providing a
14-
CA 02419700 2003-02-19
convenient method of supplying one or more gases to all of the vessels 102
simultaneously.
In this way, a gaseous reactant can be added to all of the vessels 102 at one
time. Note,
however, it is often necessary to monitor the rate of disappearance of a
gaseous reactant for
example, when determining rates of conversion-and in such cases the vessels
102 are each
sealed and individually connected to a gas source, as discussed below.
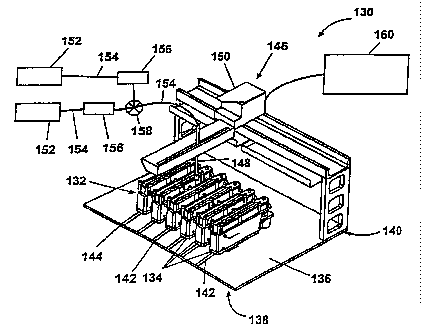
Fig. 2 shows a perspective view of a parallel reactor system 130 comprised of
a
modular reactor block 132. The modular reactor block 132 shown in Fig. 2
consists of six
modules 134, and each module 134 contains eight vessels (not shown). Note,
however, the
number of modules I 34 and the number of vessels within each of the modules
134 can vary.
FO In some embodiments, a module 134 maybe broken down into component cells
(not shown),
for example with each cell containing one well 104 holding a reaction vessel
102. Thus, if a
module is to contain eight reaction vessels, there may be eight cells, which
facilitates tower
cost manufacturing as well as replacement of damaged or worn cells. There may
any number
of cells per module, such as cell that contains two reaction vessels per cell,
etc.
15 The use of modules I 34 offers several advantages over a monolithic reactor
block.
For example, the size of the reactor block I32 can be easily adjusted
depending on the
number of reactants or the size of the combinatorial library. Also, relatively
small modules
134 are easier to handle, transport, and fabricate than a single, large
reactor block. A
damaged module can be quickly replaced by a spare module, which minimizes
repair costs
20 and downtime. Finally, the use of modules I 34 improves control over
reaction parameters.
For instance, stirring speed, temperature, and pressure of each of the vessels
can be varied
between modules.
In the embodiment shown in Fig. 2, each of the modules 134 is mounted on a
base
plate I36 having a front 138 and a rear 140. The modules 134 are coupled to
the base plate
25 136 using guides (not shown) that mate with channels 142 located on the
surface of the base
plate 136. The guides prevent lateral movement of the modules 134, but allow
linear travel
along the channels 142 that extend from the front 138 toward the rear 140 of
the base plate
136. Stops 144 located in the channels 142 near the front 138 of the base
plate 136 limit the
travel of the modules 134. Thus, one or more of the modules 134 can be moved
towards the
3o front 138 of the base plate I36 to gain access to individual vessels while
the other modules
-I S-
CA 02419700 2003-02-19
134 undergo robotic filling. In another embodiment, the modules 134 are
rigidly mounted to
the base plate 136 using bolts, clips, or other fasteners.
As illustrated in Fig. 2, a conventional robotic material handling system 146
is
ordinarily used to load vessels with starting materials. The robotic system
146 includes a
pipette or probe 148 that dispenses measured amounts of liquids into each of
the vessels. The
robotic system 146 manipulates the probe 148 using a 3-axis translation system
150. The
probe 148 is connected to sources 152 of liquid reagents through flexible
tubing 154. Pumps
156, which are located along the flexible tubing 154, are used to transfer
liquid reagents from
the sources 152 to the probe 148. Suitable pumps 156 include peristaltic pumps
and syringe
to pumps. A mufti-port valve 158 located downstream of the pumps 156 selects
which liquid
reagent from the sources 152 is sent to the probe 148 for dispensing in the
vessels.
The robotic fluid handling system 146 is controlled by a processor 160. In the
embodiment shown in Fig. 2, the user first supplies the processor 160 with
operating
parameters using a software interface. Typical operating parameters include
the coordinates
15 of each of the vessels and the initial compositions of the reaction
mixtures in individual
vessels. The initial compositions can be specified as lists of liquid reagents
from each of the
sources 152, or as incremental additions of various liquid reagents relative
to particular
vessels.
Temperature Control and Monitorine
2o The ability to monitor and control the temperature of individual reactor
vessels is an
important aspect of the present invention. During synthesis, temperature can
have a profound
effect on structure and properties of reaction products. For example, in the
synthesis of
organic molecules, yield and selectivity often depend strongly on temperature.
Similarly, in
polymerization reactions, polymer structure and properties-molecular weight,
particle size,
25 monomer conversion, microstructure-can be influenced by reaction
temperature. During
screening or characterization of combinatorial libraries, temperature control
and monitoring
of library members is often essential to making meaningful comparisons among
members.
Finally, temperature can be used as a screening criteria or can be used to
calculate useful
process and product variables. For instance, catalysts of exothermic reactions
can be ranked
--16-
CA 02419700 2003-02-19
based on peak reaction temperature and/or total heat released over the course
of reaction, and
temperature measurements can be used to compute rates of reaction and
conversion.
Fig. 3 illustrates one embodiment of a temperature monitoring system 180,
which
includes temperature sensors 182 that are in thermal contact with individual
vessels 102. For
clarity, we describe the temperature monitoring system 180 with reference to
the monolithic
reactor block 106 of Fig. 1, but this disclosure applies equally well to the
modular reactor
block I32 of Fig. 2. Suitable temperature sensors 182 include jacketed or non
jacketed
thermocouples (TC), resistance thermometric devices (RTD), and thermistors.
The
temperature sensors 182 communicate with a temperature monitor 184, which
converts
to signals received from the temperature sensors 182 to a standard temperature
scale. An
optional processor 186 receives temperature data from the temperature monitor
184. The
processor 186 performs calculations on the data, which may include wall
corrections and
simple comparisons between different vessels 102, as well as more involved
processing such
as calorimetry calculations discussed below. During an experimental run,
temperature data is
r 5 typically sent to storage l 88 so that it can be retrieved at a later time
for analysis.
Fig. 4 shows a cross-sectional view of an integral temperature sensor-vessel
assembly
200. The temperature sensor 202 is embedded in the wall 204 of a reactor
vessel 206. The
surface 208 of the temperature sensor 202 is located adjacent to the inner
wall 210 of the
vessel to ensure good thermal contact between the contents of the vessel 206
and the
2o temperature sensor 202. The sensor arrangement shown in Fig. 3 is usefut
when it is
necessary to keep the contents of the reactor vessel 206 free of obstructions.
Such a need
might arise, for example, when using a freestanding mixing device, such as a
magnetic
stirring bar. Note, however, that fabricating an integral temperature sensor
such as the one
shown in Fig. 4 can be expensive and time consuming, especially when using
glass reactor
25 vessels.
Thus, in another embodiment, the temperature sensor is immersed in the
reaction
mixture. Because the reaction environment within the vessel may rapidly damage
the
temperature sensor, it is usually jacketed with an inert material, such as a
fluorinated
thermoplastic. In addition to low cost, direct immersion offers other
advantages, including
3o rapid response and improved accuracy. In still another embodiment, the
temperature sensor is
placed on the outer surface 212 of the reactor vessel of Fig: 4. As long as
the thermal
-1 ~-
CA 02419700 2003-02-19
conductivity of the reactor vessel is known, relatively accurate and rapid
temperature
measurements can be made.
One can also remotely monitor temperature using an infrared system illustrated
in Fig.
5. The infrared monitoring system 230 comprises an optional isolation chamber
232, which
contains the reactor block 234 and vessels 236. The top 238 of the chamber 232
is fitted with
a window 240 that is transparent to infrared radiation. An infrared-sensitive
camera 242
positioned outside the isolation chamber 232, detects and records the
intensity of infrared
radiation passing through the window 240. Since infrared emission intensity
depends on
source temperature, it can be used to distinguish high temperature vessels
from low
1o temperature vessels. With suitable calibration, infrared intensity can be
converted to
temperature, so that at any given time, the camera 242 provides "snapshots" of
temperature
along the surface 244 of the reactor block 234. In the embodiment shown in
Fig. 5, the tops
246 of the vessels 236 are open. In an alternate embodiment, the tops 246 of
the vessels 236
are fitted with infrared transparent caps (not shown). Note that, with
stirring, the temperature
15 is uniform within a particular vessel, and therefore the surface
temperature of the vessel
measured by infrared emission will agree with the bulk temperature measured by
a TC or
RTD immersed in the vessel.
The temperature of the reactor vessels and block can be controlled as well as
monitored. Depending on the application, each of the vessels can be maintained
at the same
2o temperature or at different temperatures during an experiment. For example,
one may screen
compounds for catalytic activity by first combining, in separate vessels, each
of the
compounds with common starting materials; these mixtures are then allowed to
react at
uniform temperature. One may then further characterize a promising catalyst by
combining it
in numerous vessels with the same starting materials used in the screening
step. The mixtures
25 then react at different temperatures to gauge the influence oftemperature
on catalyst
performance (speed, selectivity). In many instances, it may be necessary to
change the
temperature of the vessels during processing. For example, one may decrease
the temperature
of a mixture undergoing a reversible exothermic reaction to maximize
conversion. Or, during
a characterization step, one may ramp the temperature of a reaction product to
detect phase
3o transitions (melting range, glass transition temperature). Finally, one may
maintain the
-1 s-
CA 02419700 2003-02-19
reactor block at a constant temperature, while monitoring temperature changes
in the vessels
during reaction to obtain calorimetric data as described below.
Fig. 6 shows a useful temperature control system 260, which comprises separate
heating 262 and temperature sensing 264 elements. The heating element 262
shown in Fig. 6
is a conventional thin (lament resistance heater whose heat output is
proportional to the
product of the filament resistance and the square of the current passing
through the filament.
The heating element 262 is shown coiled around a reactor vessel 266 to ensure
uniform radial
and axial heating of the vessel 266 contents. The temperature sensing element
264 can be a
TC, RTD, and the like. The heating element 262 communicates with a processor
268, which
l0 based on information received from the temperature sensor 264 through a
temperature
monitoring system 270, increases or decreases heat output of the heating
element 262. A
heater control system 272, located in the communication path between the
heating element
262 and the processor 268, converts a processor 268 signal for an increase
(decrease) in
heating into an increase (decrease) in electrical current through the heating
element 262.
15 Generally, each of the vessels I04 of the parallel reactor system 100 shown
in Fig. 1 or Fig. 3
are equipped with a heating element 262 and one or more temperature sensors
264, which
communicate with a central heater control system 272, temperature monitoring
system 270,
and processor 268, so that the temperature of the vessels 104 can be
controlled independently.
Other embodiments include placing the heating element 262 and temperature
sensor
20 264 within the vessel 266, which results in more accurate temperature
monitoring and control
of the vessel 266 contents, and combining the temperature sensor and heating
element in a
single package. A thermistor is an example of a combined temperature sensor
and heater,
which can be used for both temperature monitoring and control because its
resistance depends
on temperature.
25 Fig. 7 illustrates another temperature control system, which includes
liquid cooling
arid heating of the reactor block 106. Regulating the temperature of the
reactor block 106
provides many advantages. For example, it is a simple way of maintaining
nearly uniform
temperature in all of the reactor vessels 102. Because of the large surface
area of the vessels
102 relative to the volume of the reaction mixture, cooling the reactor block
106 also allows
30 one to carryout highly exothermic reactions. When accompanied by
temperature control of
individual vessels 102, active cooling of the reactor block 106 allows for
processing at sub-
-19-
CA 02419700 2003-02-19
ambient temperatures. Moreover, active heating or cooling of the reactor block
106
combined with temperature control of individual vessels 102 or groups of
vessels 102 also
decreases response time of the temperature control feedback. One may control
the
temperature of individual vessels 102 or groups of vessels 102 using compact
heat transfer
devices, which include electric resistance heating elements or thermoelectric
devices, as
shown in Fig. 6 and Fig. 8, respectively. Although we describe reactor block
cooling with
reference to the monolithic reactor block 106, one may, in a like manner,
independently heat
or cool individual modules 134 of the modular reactor block 132 shown in Fig.
2.
Returning to Fig. 7, a thermal fluid 290, such as water, steam, a silicone
fluid, a
1o fluorocarbon, and the like, is transported from a uniform temperature
reservoir 292 to the
reactor block 106 using a constant or variable speed pump 294. The thermal
fluid 290 enters
the reactor block 106 from a pump outlet conduit 296 through an inlet port
298. From the
inlet port 298, the thermal fluid 290 flows through a passageway 300 formed in
the reactor
block I06. The passageway may comprise single or multiple channels. The
passageway 300
shown in Fig. 7, consists of a single channel that winds its way between rows
of vessels 102,
eventually exiting the reactor block 106 at an outlet port 302. The thermal
fluid 290 returns
to the reservoir 292 through a reactor block outlet conduit 304. A heat pump
306 regulates
the temperature of the thermal fluid 290 in the reservoir 292 by adding or
removing heat
through a heat transfer coil 308. In response to signals from temperature
sensors (nat shown)
located in the reactor block 106 and the reservoir 292, a processor 310
adjusts the amount of
heat added to or removed from the thermal fluid 290 through the coil 308. To
adjust the flow
rate of thermal fluid 290 through the passageway 300, the processor 310
communicates with
a valve 3I2 located in a reservoir outlet conduit 314. The reactor block 106,
reservoir 292,
pump 294, and conduits 296, 304, 314 can be insulated to improve temperature
control in the
reactor block 106.
Because the reactor block 106 is typically made of a metal or other material
possessing high thermal conductivity, the single channel passageway 300 is
usually sufficient
for maintaining the temperature of the block 106 a few degrees above or below
room
temperature. To improve temperature uniformity within the reactor block 106,
the
3o passageway can be split into parallel channels (not shown) immediately
downstream of the
inlet port 298. In contrast to the single channel passageway 300 depicted in
Fig. 7, each of
-20-
CA 02419700 2003-02-19
the parallel channels passes between a single row of vessels 102 before
exiting the reactor
block 106. This parallel flow arrangement decreases the temperature gradient
between the
inlet 298 and outlet 302 ports. To further improve temperature uniformity and
heat exchange
between the vessels 102 and the block 106, the passageway 300 can be enlarged
so that the
wells I04 essentially project into a cavity containing the thermal fluid 290.
Additionally, one
may eliminate the reactor block 106 entirely, and suspend or immerse the
vessels I02 in a
bath containing the thermal fluid 290.
Fig. 8 illustrates the use of thermoelectric devices for heating and cooling
individual
vessels. Thermoelectric devices can function as both heaters and coolers by
reversing the
to current flow through the device. Unlike resistive heaters, which convert
electric power to
heat, thermoelectric devices are heat pumps that exploit the Pettier effect to
transfer heat from
one face of the device to the other. A typical thermoelectric assembly has the
appearance of a
sandwich, in which the front face of the thermoelectric device is in thermal
contact with the
object to be cooled {heated), and the back face of the device is in thermal
contact with a heat
15 sink (source). When the heat sink or source is ambient air, the back face
of the device
typically has an array of thermally conductive fins to increase the heat
transfer area.
Preferably, the heat sink or source is a liquid. Compared to air, liquids have
higher thermal
conductivity and heat capacity, and therefore should provide better heat
transfer through the
back face of the device. But, because thermoelectric devices are usually made
with bare
2o metal connections, they often must be physically isolated from the liquid
heat sink or source.
For example, Fig. 8 illustrates one way of using thermoelectric devices 330 to
heat
and cool reactor vessels 338 using a liquid heat sink or source. In the
configuration shown in
Fig. 8, thermoelectric devices 330 are sandwiched between a reactor block 334
and a heat
transfer plate 336. Reactor vessels 338 sit within wells 340 formed in the
reactor block 334.
25 Thin walls 342 at the bottom of the wells 340, separate the vessels 338
from the
thermoelectric devices 330, ensuring good thermal contact. As shown in Fig. 8,
each of the
vessels 338 thermally contacts a single thermoelectric device 330, although in
general, a
thermoelectric device can heat or cool more than one of the vessels 338. The
thermoelectric
devices 330 either obtain heat from, or dump heat into; a thermal fluid that
circulates through
3o an interior cavity 344 of the heat transfer plate 336. The thermal fluid
enters and leaves the
heat transfer plate 336 through inlet 346 and outlet 348 ports, and its
temperature is controlled
-21-
CA 02419700 2003-02-19
in a manner similar to that shown in Fig. 7. During an experiment, the
temperature of the
thermal fluid is typically held constant, while the temperature of the vessels
338 is controlled
by adjusting the electrical current, and hence, the heat transport through the
thermoelectric
devices 330. Though not shown in Fig. 8, the temperature of the vessels 338
are controlled in
a manner similar to the scheme depicted in Fig. 6. Temperature sensors located
adjacent to
the vessels 338 and within the heat transfer plate cavity 344 communicate with
a processor
via a temperature monitor. In response to temperature data from the
temperature monitor, the
processor increases or decrease heat flow to or from the thermoelectric
devices 330. A
thermoelectric device control system, located in the communication path
between the
thermoelectric devices 330 and the processor, adjusts the magnitude and
direction of the flow
of electrical current through each of the themnoelectric devices 330 in
response to signals
from the processor.
Calorimetric Data Measurement and Use
Temperature measurements often provide a qualitative picture of reaction
kinetics and
conversion and therefore can be used to screen library members. For example,
rates of
change of temperature with respect to time, as well as peak temperatures
reached within each
of the vessels can be used to rank catalysts. Typically, the best catalysts of
an exothermic
reaction are those that, when combined with a set of reactants, result in the
greatest heat
production in the shortest amount of time.
In addition to its use as a screening tool, temperature measurement-combined
with
proper thermal management and design of the reactor system-can also be used to
obtain
quantitative calorimetric data. From such data, scientists can, for example,
compute
instantaneous conversion and reaction rate, locate phase transitions (melting
point, glass
transition temperature) of reaction products, or measure latent heats to
deduce structural
information of polymeric materials, including degree of crystallinity and
branching.
Fig. 9 shows a cross-sectional view of a portion of a reactor block 360 that
can be
used to obtain accurate calorimetric data. Each of the vessels 362 contain
stirring blades 364
to ensure that the contents 366 of the vessels 362 are well mixed and that the
temperature
within any one of the vessels 362, T" is uniform. Each of the vessels 362
contains a
3o thermistor 368, which measures T, and heats the vessel contents 366. The
walls 370 of the
-z2-
CA 02419700 2003-02-19
vessels 362 are made of glass, although one may use any material having
relatively low
thermal conductivity, and similar mechanical strength and chemical resistance.
The vessels
362 are held within wells 372 formed in the reactor block 36U, and each of the
wells 372 is
lined with an insulating material 374 to further decrease heat transfer to or
from the vessels
362. Useful insulating materials 374 include glass wool, silicone rubber, and
the like. The
insulating material 374 can be eliminated or replaced by a thermal paste when
better thermal
contact between that reactor block 360 and the vessels 362 is desired-good
thermal contact
is needed, for example, when investigating exothermic reactions under
isothermal conditions.
The reactor block 360 is made of a material having high thermal conductivity,
such as
1o aluminum, stainless steel, brass, and so on. High thermal conductivity,
accompanied by
active heating or cooling using any of the methods described above, help
maintain uniform
temperature, T,; throughout the reactor block 360. One can account for non-
uniform
temperatures within the reactor block 360 by measuring Tw, the temperature of
the block 360
in the vicinity of each of the vessels 362, using block temperature sensors
376. In such cases,
15 TN, instead of T, is used in the. calorimetric calculations described next.
An energy balance around the contents 366 of one of the vessels 362 (jth
vessel)
yields an expression for fractional conversion, X" of a key reactant at any
time, t, assuming
that the heat of reaction, G~H., and the specific heat of the vessel contents
366, Cr,, are known
and are constant over the temperature range of interest:
dT! dX~
20 MfcP.i dt - mo.i~~.i dt + ~".; - Q°"r,;
In expression I, M, is the mass of the contents 366 of the jth vessel; m~ is
the initial mass of
the key reactant; Q,~ is the rate of heat transfer into the jth vessel by
processes other than
reaction, as for example, by resistance heating of the thermistor 368. Q"", is
the rate of heat
transfer out of the jth vessel, which can be determined from the expression:
25 ~ouf.J. ~IlJAa(T -To~=UfA;~T~ II
where A, is the heat transfer area-the surface area ofthe j~h, vessel-and U,
is the heat
transfer coefficient, which depends on the properties of the vessel 362 and
its contents 366, as
-23-
CA 02419700 2003-02-19
well as the stirring rate. U, can be determined by measuring the temperature
rise, DT" in
response to a known heat input.
Equations I and II can be used to determine conversion from calorimetric data
in at
least two ways. In a $rst method, the temperature of the reactor block 360 is
held constant,
and sufficient heat is added to each of the vessels 362 through the thermistor
368 to maintain
a constant value of 4T, . Under such conditions, and after combining equations
I and II, the
conversion can be calculated from the expression
tf
l
Xj - ,~~ UiAitf~Ti - ,~Q~n.jdt ~ III
mo~j ~r.j
where the integral can be determined by numerically integrating the power
consumption of
Io the thermistor 368 over the length of the experiment, t,. This method can
be used to measure
the heat output of a reaction under isothermal conditions.
In a second method, the temperature of the reactor block 360 is again held
constant,
but T, increases or decreases in response to heat produced or consumed in the
reaction.
Equation I and II become under such circumstances
tf
1
15 Xj = m ~ Mj c,,,j ~Tf,j - T,.,j ) + U j Aj ~QT dt . IV
o.! r.j
In equation IV, the integral can be determined numerically, and T" and T" are
temperatures of
the reaction mixture within the jth vessel at the beginning and end of
reaction, respectively.
tf
Thus, if T" equals T,,, the total heat liberated is proportional to ~OT dt .
This method is
simpler to implement than the isothermal method since it does not require
temperature control
20 of individual vessels. But, it can be used only when the temperature change
in each of the
reaction vessels 362 due to reaction does not significantly influence the
reaction under study.
One may also calculate the instantaneous rate of disappearance of the key
reactant in
the jth vessel, -r" using equation I, III or N since -r, is related to
conversion through the
relationship
-24-
CA 02419700 2003-02-19
''J Co.l dt ' V
which is valid for constant volume reactions. The constant C,,; is the initial
concentration of
the key reactant.
Stirring S sty ems
Mixing variables such as stirring blade torque, rotation rate, and geometry,
may
influence the course of a reaction and therefore affect the properties of the
xeaction products.
For example, the overall heat transfer coefficient and the rate of viscous
dissipation within the
reaction mixture may depend on the stirring blade rate of rotation. Thus, in
many instances it
is important that one monitor and control the rate of stirring of each
reaction mixture to
to ensure uniform mixing. Alternatively, the applied torque may be monitored
in order to
measure thewiscosity of the reaction mixture. As described in the next
section,
measurements of solution viscosity can be used to calculate the average
molecular weight of
polymeric reaction products.
Fig. 10 shows an exploded, perspective view of a stirring system for a single
module
390 of a modular reactor block of the type shown in Fig. 2. The module 390
comprises a
block 392 having eight wells 394 for containing removable reaction vessels
396. The number
of wells 394 and reaction vessels 396 can vary. The top surface 398 of a
removable lower
plate 400 serves as the base for each of the wells 394 and permits removal of
the reaction
vessels 396 through the bottom 402 of the block 392. Screws 404 secure the
lower plate 400
2o to the bottom 402 of the block 392. An upper plate 406, which rests on the
top 408 of the
block 392, supports and directs elongated stirrers 410 into the interior of
the vessels 396.
Each of the stirrers 410 comprises a spindle 412 and a rotatable stirnng
member or stirring
blade 414 which is attached to the lower end of each spindle 412. A gear 416
is attached to
the upper end of each of each spindle 412. When assembled, each gear 4i6
meshes with an
adjacent gear 416 forming a gear train (not shown) so that each stirrer 410
rotates at the same
speed. A DC stepper motor 418 provides torque for rotating the stirrers 410,
although an air-
driven motor, a constant-speed AC motor, or a variable-speed AC motor can be
used instead.
A pair of driver gears 420 couple the motor 418 to the gear train. A removable
cover 422
provides access to the gear train, which is secured to the block 392 using
threaded fasteners
-25-
CA 02419700 2003-02-19
424. In addition to the gear train, one may employ belts, chains and
sprockets, or other drive
mechanisms. In alternate embodiments, each of the stirrers 4I0 are coupled to
separate
motors so that the speed or torque of each of the stirrers 410 can be
independently varied and
monitored. Furthermore, the drive mechanism whether employing a single motor
and gear
train or individual motors-can be mounted below the vessels 362. In such
cases, magnetic
stirring blades placed in the vessels 362 are coupled to the drive mechanism
using permanent
magnets attached to gear train spindles or motor shafts.
In addition to the stirring system, other elements shown in Fig. 10 merit
discussion.
For example, the upper plate 406 may contain vessel seals 426 that allow
processing at
pressures different than atmospheric pressure. Moreover, the seals 426 permit
one to monitor
pressure in the vessels 396 ever time. As discussed below, such information
can be used to
calculate conversion of a gaseous reactant to a condensed species. Note that
each spindle 412
may penetrate the seals 426, or may be magnetically coupled to an upper
spindle member (not
shown) attached to the gear 416. Fig. 10 also shows temperature sensors 428
embedded in
the block 392 adjacent to each of the wells 394. The sensors 428 are part of
the temperature
monitoring and control system described previously.
In another embodiment, an array of electromagnets rotate freestanding stirring
members ar magnetic stirring bars, which obviates the need for the mechanical
drive system
shown in Fig. 10. Electromagnets are electrical conductors that produce a
magnetic field
2o when an electric current passes through them. Typically, the electrical
conductor is a wire
coil wrapped around a solid care made of material having relatively high
permeability, such
as soft iron or mild steel.
Fig. 11 is a schematic representation of one embodiment of an electromagnet
stirring
array 440. The electromagnets 442 or coils belonging to the array 440 are
mounted in the
lower plate 400 of the reactor module 390 of Fig. 10 so that their axes are
about parallel to the
centerlines of the vessels 396. Although greater magnetic field strength can
be achieved by
mounting the electromagnets with their axes perpendicular to the centerlines
of the vessels
396, such a design is more difficult to implement since it requires placing
electromagnets
between the vessels 396. The eight crosses or vessel sites 444 in Fig. 11 mark
the
3o approximate locations of the respective centers of each of the vessels 396
of Fig. 10 and
denote the approximate position of the rotation axes of the magnetic stirring
bars (not shown).
26--
CA 02419700 2003-02-19
In the array 440 shown in Fig. 11, four electromagnets 442 surround each
vessel site 444,
though one may use fewer or greater numbers of electromagnets 442. The minimum
number
of electromagnets per vessel site is two, but in such a system it is difficult
to initiate stirring,
and it is common to stall the stirring bar. Electromagnet size and available
packing density
primarily limit the maximum number of electromagnets.
As illustrated in Fig. 11, each vessel site 444, except those at the ends 446
of the array
440, shares its four electromagnets 442 with two adjacent vessel sites.
Because of this
sharing, magnetic stirnng bars at adjacent vessel sites rotate in opposite
directions, as
indicated by the curved arrows 448 in Fig. 11, which may Lead to stalling.
Other array
to configurations are possible. For example, Fig. I2 shows a portion of an
array 460 in which
the ratio of electromagnets 462 to vessel sites 464 approaches 1:1 as the
number of vessel
sites 464 becomes large. Because each of the vessel sites 464 shares its
electromagnets 462
with its neighbors, magnetic stirring bars at adjacent vessel sites rotate in
opposite directions,
as shown by curved arrows 466. In contrast, Fig. 13 shows a portion of an
array 470 in which
15 the ratio of electromagnets 472 to vessel sites 4?4 approaches 2:1 as the
number of vessel
sites becomes large. Because ofthe comparatively large number of
electromagnets 472 to
vessel sites 474, all of the magnetic stirring bars can be made to rotate in
the same direction
476, which minimizes stalling. Similarly, Fig. 14 shows an array 480 in which
the number of
electromagnets 482 to vessel sites 484 is 4:1. Each magnetic stirring bar
rotates in the same
20 direction 486.
Fig. 15 illustrates additional elements of an electromagnetic stirring system
500. For
clarity, Fig. i5 shows a square electromagnet array 502 comprised of four
electromagnets
504, although larger arrays, such as those shown in Fig. 12-14, can be used.
Each of the
electromagnets S04 comprises a wire 506 wrapped around a high permeability
solid core 508.
25 The pairs of electromagnets 504 located on the two diagonals of the square
array 502 are
connected in series to form a first circuit SI O and a second Circuit 512. The
first 510 and
second S 12 circuits are connected to a drive circuit 514, which is controlled
by a processor
516. Electrical current, whether pulsed or sinusoidal, can be varied
independently in the two
circuits 510, 512 by the drive circuit S 14 and processor 516. Note that
within each circuit
30 510, 512, the current flows in apposite directions in the wire 506 around
the core 508. In this
way, each of the electromagnets 504 within a particular circuit 510, 512 have
opposite
27-
CA 02419700 2003-02-19
magnetic polarities. The axes 518 of the electromagnets 504 are about parallel
to the
centerline 520 of the reactor vessel 522. A magnetic stirring bar 524 rests on
the bottom of
the vessel 522 prior to operation. Although the electromagnets 504 can also be
oriented with
their axes 518 perpendicular to the vessel centerline 520, the parallel
alignment provides
higher packing density.
Fig. Iti shows the magnetic field direction of a 2 x 2 electromagnet array at
four
different times during one full rotation of the magnetic stirring bar S24 of
Fig. 1 S, which is
rotating at a steady frequency of rvradians~s': In Fig. I6, a circle with a
plus sign 532
indicates that the electromagnet produces a magnetic field in a first
direction; a circle with a
l o minus sign 534 indicates that the electromagnet produces a magnetic field
in a direction
opposite to the first direction; and a circle with no sign 536 indicates that
the electromagnet
produces no magnetic field. At time t = 0, the electromagnets 530 produce an
overall
magnetic field with a direction represented by a first arrow 538 at the vessel
site. At time
t = ~~ , the electromagnets 540 produce an overall magnetic field with a
direction
represented by a second arrow 542. Since the magnetic stirring bar 524 (Fig.
15) attempts to
align itself with the direction of the overall magnetic fetd, it rotates
clockwise ninety degrees
from the first direction 538 to the second direction 542. At time t = n , the
electromagnets
544 produce an overall magnetic field with a direction represented by a third
arrow 546.
Again; the magnetic stirnng bar 524 aligns itself with the direction of the
overall magnetic
field, and rotates clockwise an additional ninety degrees. At time t = 2~ ,
the electromagnets
548 produce an overall magnetic field with a direction represented by a fourth
arrow 550,
which rotates the magnetic stirnng bar S24 clockwise another ninety degrees.
Finally, at time
t = 2~, the electromagnets 530 produce an overall magnetic field with
direction represented
by the first arrow 538, which rotates the magnetic stirring bar 524 back to
its position at time
t = 0 .
Fig. 17 illustrates magnetic field direction of a 4 x 4 electromagnetic array
at five
different times during one full rotation of a 3 x 3 array of magnetic stirring
bars. As in Fig.
15, a circle with a plus sign 570, a minus sign 572, or no sign 574 represents
the magnetic
-28-
CA 02419700 2003-02-19
field direction of an individual electromagnet, while an arrow 576 represents
the direction of
the overall magnetic field at a vessel site. As shown, sixteen electromagnets
are needed to
rotate nine magnetic stirring bars. But; as indicated in Fig. 18, due to
sharing of -
electromagnets by multiple magnetic stirring bars, the rotational direction of
the magnetic
fields is non-uniform. Thus, five of the fields rotate in a clockwise
direction 590 while the
remaining four fields rotate in a counter-clockwise direction 592.
Fig. 19 and Fig. 20 illustrate wiring configurations for electromagnet arrays
in which
each vessel site is located between four electromagnets defining four corners
of a
quadrilateral sub-array. For each vessel site, both wiring configurations
result in an electrical
1 o connection between electromagnets located on the diagonals of a given sub-
array. In the
wiring configuration 610 shown in Fig. 19, electromagnets 6I2 in alternating
diagonal rows
are wired together to form two series of electromagnets 612. Dashed and solid
lines represent
electrical connections between electromagnets 612 in a first series 614 and a
second series
616, respectively. Plus signs 618 and minus signs 620 indicate polarity
(magnetic field
15 direction) of individual electromagnets 6I2 at any time, t, when current in
the first series 614
and the second series 616 of electromagnets 612 are in phase. Fig. 20
illustrates an alternate
wiring configuration 630 of electromagnets 632, where again, dashed and solid
lines
represent electrical connections between the first 634 and second series 636
of electromagnets
632, and plus signs 638 and minus signs 640 indicate magnetic polarity.
2o Note that for both wiring configurations 6I0, 630, the polarities of the
electromagnets
612, 632 of the first series 614, 634 are not the same, though amplitudes of
the current
passing through the connections between the electromagnets 612, 632 of the
first series 614,
634 are equivalent. The same is true for the second series 616, 636 of
electromagnets 612,
632. One can achieve opposite polarities within the first series 614, 634 or
second series 616,
25 636 of electromagnets 612, 632 by reversing the direction of electrical
current around the core
of the electromagnet 612, 632. See, for example, Fig. 15. In the two wiring
configurations
610, 630 of Fig. 19 and 20, every quadrilateral array of four adjacent
electromagnets 612, 632
defines a site for rotating a magnetic stirring bar, and the diagonal members
of each of the
four adjacent electromagnets 6I2, 632 belong to the first series 614, 634 and
the second 616,
30 636 series of electromagnets 612, 632. Moreover, within any set of four
adjacent
electromagnets 612, 632, each pair of electromagnets 612, 632 belonging to the
same series
29-
CA 02419700 2003-02-19
have opposite polarities. The two wiring configurations 610, 630 of Fig. I9
and 20 can be
used with any of the arrays 460, 470, 480 shown in Fig. I 2-14.
The complex wiring configurations 610, 630 of Fig. 19 and 20 can be placed on
a
printed circuit board, which serves as both a mechanical support and alignment
fixture for the
electromagnets 612, 632. The use of a printed circuit board allows for rapid
interconnection
of the electromagnets 6I2, 632, greatly reducing assembly time and cost, and
eliminating
wiring errors associated with manual soldering of hundreds of individual
connections.
Switches can be used to tum stirring on and off for individual rows of
vessels. A separate
drive circuit may be used for each row of vessels, which allows stirring speed
to be used as a
1o variable during an experiment.
Fig. 21 is a plot 650 of current versus time and shows the phase relationship
between
sinusoidal source currents, I,(t) 652 and I,(t) 654, which drive,
respectively, the f rst series
614, 634 and the second series 616, 636 of electromagnets 612, 632 shown in
Fig. 19 and 20.
The two source currents 652, 654 have equivalent peak amplitude and frequency,
cy, though
15 I (t) 652 lags 1,(t) 654 by 2 radians. Because of this phase relationship,
magnetic stirnng
bars placed at rotation sites defined by any four adjacent electromagnets 612,
632 of Fig. 19
and 20 will each rotate at an angular frequency of cy, though adjacent
stirring bars will rotate
in opposite directions when the electromagnet array 460 depicted in Fig. 12 is
used. If,
however, the arrays 470, 480 shown in Fig. 13 and 14 are used, adjacent
stirring bars will
2o rotate in the same direction. In an alternate embodiment, a digital
approximation to a sine
wave can be used.
Fig. 22 is a block diagram of a power supply 670 for an electromagnet array
672.
Individual electromagnets 674 are wired together in a first and second series
as, for example,
shown in Fig. 19 or 20. The first and second series of electromagnets 674 are
connected to a
25 power source 676, which provides the two series with sinusoidal driving
currents that are 2
radians out ofphase. Normally, the amplitudes of the two driving currents are
the same and
do not depend on frequency. A processor 678 controls both the amplitude and
the frequency
of the driving currents.
Viscosity and Related Measurements
-30-
CA 02419700 2003-02-19
The present invention provides for in situ measurement of viscosity and
related
properties. As discussed below, such data can be used, for example, to monitor
reactant
conversion, and to rank or characterize materials based on molecular weight or
particle size_
The viscosity of a polymer solution depends on the molecular weight of the
polymer
and its concentration in solution. For polymer concentrations well below the
"semidilute
limit"-the concentration at which the solvated polymers begin to overlap one
another-the
solution viscosity, r~, is related to the polymer concentration, C, in the
Iirnit as C approaches
zero by the expression
~7= ~1-~- ~~l~~rls VI
l0 where r~= is the viscosity of the solvent. Essentially, adding polymer to a
solvent increases the
solvent's viscosity by an amount proportional to the polymer concentration.
The
proportionality constant C~~ , is known as the intrinsic viscosity, and is
related to the polymer
molecular weight, M, through the expression
~~7~=~rlo~Ma~ VII
z5 where ~r~a~ and care empirical constants. Equation VII is known as~the Mark-
Houwink-
Sakurda (MHS) relation, arid it, along with equation VI, can be used to
determine molecular
weight from viscosity measurements.
Equation VI requires concentration data from another source; with
polymerization
reactions, polymer concentration is directly related to monomer conversion. In
the present
20 invention, such data can be obtained by measuring heat evolved during
reaction {see equation
III and IV) or, as described below, by measuring the amount of a gaseous
reactant consumed
during reaction. The constants in the MHS relation are functions of
temperature, polymer
composition, polymer conformation, and the quality of the polymer-solvent
interaction. The
empirical constants, ~r~o ~ and c~ have been measured for a variety of polymer-
solvent pairs,
25 and are tabulated in the literature.
Although equations VI and VII can be used to approximate molecular weight, in
situ
measurements of viscosity in the present invention are used mainly to rank
reaction products
-31-
CA 02419700 2003-02-19
as a function of molecular weight. Under most circumstances, the amount of
solvent
necessary to satisfy the concentration requirement of equation VI would slow
the rate of
reaction to an unacceptable level. Therefore, most polymerizations are carried
out at polymer
concentrations above the semidilute limit, where the use of equations VI and
VII to calculate
molecular weight would lead to large error. Nevertheless, viscosity can be
used to rank
reaction products even at concentrations above the semidilute limit since a
rise in viscosity
during reaction generally reflects an increase in polymer concentration,
molecular weight or
both. If necessary, one can accurately determine molecular weight from
viscosity
measurements at relatively high polymer concentration by frst preparing
ternperature-
1o dependent calibration curves that relate viscosity to molecular weight. But
the curves would
have to be obtained for every polymer-solvent pair produced, which weighs
against their use
for screening new polymeric materials.
In addition to ranking reactions, viscosity measurements can also be used to
screen or
characterize dilute suspensions of insoluble particles-polymer emulsions or
porous supports
i5 for heterogeneous catalysts-in which viscosity increases with particle size
at a fixed number
concentration. In the case of polymer emulsions, viscosity can serve as a
measure of
emulsion quality. For example, solution viscosity that is constant over long
periods of time
may indicate superior emulsion stability, or viscosity within a particular
range may correlate
with a desired emulsion particle size. With porous supports, viscosity
measurements can be
2o used to~identify active catalysts: in many cases, the catalyst support will
swell during reaction
due to the formation of insoluble products within the porous support.
In accordance with the present invention, viscosity or related properties of
the reactant
mixtures are monitored by measuring the effect of viscous forces on stirring
blade rotation.
Viscosity is a measure of a fluid's resistance to a shear farce. This shear
force is equal to the
25 applied torque, T, needed to maintain a constant angular velocity of the
stirring blade. The
relationship between the viscosity of the reaction mixture and the applied
torque can be
expressed as
r = K~, ~c~, T~rJ, VIII
-32-
CA 02419700 2003-02-19
where KN is a proportionality constant that depends on the angular frequency,
try of the
stirring bar, the temperature of the reaction mixture, and the geometries of
the reaction vessel
and the stirring blade. K~ can be obtained through calibration with solutions
of known
viscosity.
During a polymerization, the viscosity of the reaction mixture increases over
time due
to the increase in molecular weight of the reaction product or polymer
concentration or both.
This change in viscosity can be monitored by measuring the applied torque and
using
equation VIII to convert the measured data to viscosity. In many instances,
actual values for
the viscosity are unnecessary, and one can dispense with the conversion step.
For example, in
l0 situ measurements of applied torque can be used to rank reaction products
based on molecular
weight or conversion, as long as stirring rate, temperature, vessel geometry
and stirring blade
geometry are about the same for each reaction mixture.
Fig. 23 illustrates an apparatus 700 for directly measuring the applied
torque. The
apparatus 700 comprises a stirring blade 702 coupled to a drive motor 704 via
a rigid drive
spindle 706. The stirring blade 702 is immersed in a reaction mixture 708
contained within a
reactor vessel 710. Upper 712 and lower 714 supports prevent the drive motor
704 and vessel
7I0 from rotating during operation of the stirring blade 702. For simplicity,
the lower
support 714 can be a permanent magnet. A torque or strain gauge 716 shown
mounted
between the upper support 71 Z and the drive motor 704 measures the average
torque exerted
by the motor 704 on the stirring blade 702. In alternate embodiments, the
strain gauge 716 is
inserted within the drive spindle 706 or is placed between the vessel 710 and
the lower
support 714. If located within the drive spindle 706, a system of brushes or
commutators (not
shown) are provided to allow communication with the rotating strain gauge.
Often,
placement of the strain gauge 716 between the vessel 710 and the lower support
714 is the
best option since many stirring systems, such as the one shown in Fig. 10, use
a single motor
to drive multiple stirring blades.
Fig. 24 shows placement of a strain gauge 730 in a portion of a base plate 732
that is
similar to the lower plate 400 of the reactor module 390 shown in Fig. 10. The
lower end 734
of the strain gauge 730 is rigidly attached to the base plate 732. A first
permanent magnet
736 is mounted on the top end 738 of the strain gauge 730, and a second
permanent magnet
740 is attached to the bottom 742 of a reactor vessel 744. When the vessel 744
is inserted in
-33-
CA 02419700 2003-02-19
the base plate 732, the magnetic coupling between the first magnet 736 and the
second
magnet 740 prevents the vessel 744 from rotating and transmits torque to the
strain gauge
730.
Besides using a strain gauge, one can also monitor drive motor power
consumption,
which is related to the applied torque. Refernng again to Fig. 23, the method
requires
monitoring and control of the stirring blade 702 rotational speed, which can
be accomplished
by mounting a sensor 718 adjacent to the drive spindle 706. Suitable sensors
718 include
optical detectors, which register the passage of a spot on the drive spindle
706 by a
reflectance measurement, or which note the interruption of a tight beam by an
obstruction
1o mounted on the drive spindle 706, or which discern the passage of a light
beam through a slot
on the drive spindle 706 or on a co-rotating obstruction. Other suitable
sensors 718 include
magnetic field detectors that sense the rotation of a permanent magnet affixed
to the spindle
706. Operational details of magnetic field sensors are described below in the
discussion of
phase lag detection. Sensors such as encoders, resolvers, Hall effect sensors,
and the like, are
IS comrnonIy integrated into the motor 704. An external processor 720 adjusts
the power
supplied to the drive motor 704 to maintain a constant spindle 706 rotational
speed. By
calibrating the required power against a series of liquids of known viscosity,
the viscosity of
an unknown reaction mixture can be determined.
In addition to direct measurement, torque can be determined indirectly by
measuring
2o the phase angle or phase lag between the stirring blade and the driving
force or torque.
Indirect measurement requires that the coupling between the driving torque and
the stirnng
blade is "soft," so that significant and measurable phase lag occurs.
With magnetic stirring, "soft" coupling occurs automatically. The torque on
the
stirring bar is related to the magnetic moment of the stirring bar, ,u, and
the amplitude of the
25 magnetic field that drives the rotation of the stirring bar, H, through the
expression
r = ,uH sin B,
where B is the angle between the axis of the stirring bar (magnetic moment)
and the direction
of the magnetic f eld. At a given angular frequency, and for known fr and H,
the phase angle,
B, will automatically adjust itself to the value necessary to provide the
amount of torque
-34-
CA 02419700 2003-02-19
needed at that frequency. If the torque required to stir at frequency m is
proportional to the
solution viscosity and the stirring frequency-an approximation useful for
discussion-then
the viscosity can be calculated from measurements of the phase angle using the
equation
r = fcH sin 8 = a ~rv X
where a is a proportionality constant that depends on temperature; and the
geometry of the
vessel and the stirring blade. In practice, one may use equation VIII or a
similar empirical
expression for the right hand side of equation X if the torque does not depend
linearly on the
viscosity-frequency product.
Fig. 25 shows an inductive sensing coil system 760 for measuring phase angle
or
1o phase lag, B. The system 760 comprises four electromagnets 762, which drive
the magnetic
stirnng bar 764, and a phase-sensitive detector, such as a standard lock-in
amplifier (not
shown). A gradient coil 766 configuration is used to sense motion of the
stirnng bar 764,
though many other well known inductive sensing coil configurations can be
used. The
gradient coil 766 is comprised of a first sensing coil 768 and a second
sensing coil '770 that
15 are connected in series and are wrapped in opposite directions around a
first electromagnet
772. Because of their opposite polarities, any difference in voltages induced
in the two
sensing coils 768, 770 will appear as a voltage difference across the
terminals 774, which is
detected by the lock-in amplifier. If no stirring bar 764 is present, then the
alternating
magnetic field of the first electromagnet 772 will induce approximately equal
voltages in
2o each of the two coils 768, 770-assuming they are mounted symmetrically with
respect to the
first electromagnet 772-and the net voltage across the terminals 774 will be
about zero.
When a magnetic stirnng bar 764 is present, the motion of the rotating magnet
764 will
induce a voltage in each of the two sensing coils 768, 770. But, the voltage
induced in the
first coil 768, which is closer to the stirnng bar 764, will be much larger
than the voltage
25 induced in the second coil 770, so that the voltage across the terminals
774 will he nonzero.
A periodic signal will thus be induced in the sensing coils 768, 770, which is
measured by the
lock-in amplifier.
Fig. 26 and Fig. 27 show typical outputs 790, 810 from the inductive sensing
coil
system 760 of Fig. 25, which illustrate phase lag associated with magnetic
stirring for low
30 and high viscosity solutions, respectively. Periodic signals 792, 812 from
the sensing coils
-35-
CA 02419700 2003-02-19
768, 770 are plotted with sinusoidal reference signals 794, 814 used to drive
the
electromagnets. Time delay, ~t 796, 816, between the periodic signals 792, 812
and the
reference signals 794, 814 is related to the phase angle by B= u~ ~ Ot .
Visually comparing the
two outputs 790, 810 indicates that the phase angle associated with the high
viscosity solution
is larger than the phase angle associated with the low viscosity solution.
Fig. 27 illustrates how amplitude and phase angle will vary during a reaction
as the
viscosity increases from a low value to a value sufficient to stall the
stirring bar. A waveform
or signal 820 from the sensing coils is input to a lock-in amplifier 822,
using the drive circuit
sinusoidal current as a phase and frequency reference signal 824. The lock-in
amplifier 822
t o outputs the amplitude 826 of the sensing coil signal 820, and phase angle
828 or phase lag
relative to the reference signal 824. The maximum phase angle is 2 radians,
since, as shown
by equation X, torque decreases with further increases in BIeading to slip of
the stirring bar
764 of Fig. 25. Thus, as viscosity increases during reaction, the phase angle
828 or phase lag
also increases until the stirring bar stalls, and the amplitude 826 abruptly
drops to zero. This
can be seen graphically in Fig. 27; which shows plots of A 830 and B 832, the
amplitude of
the reference signal and phase angle, respectively, averaged over many stirnng
bar rotations.
One can optimize the sensitivity of the phase angle 828 measurement by proper
choice of the
magnetic field amplitude and frequency.
To minimize interference from neighboring stirring bars-ideally, each set of
gradient
z0 coils should sense the motion of a single stirring bar-each vessel should
be provided with
electromagnets that are not shared with adjacent vessels. For example, a 4:1
magnet array
shown in Fig. 14 should be used instead of the 2:1 or the 1:1 magnet arrays
Shawn in Fig. 13
and 12, respectively. In order to take readings from all of the vessels in an
array, a
multiplexer can be used to sequentially route signals from each vessel to the
lock-in
amplifier. Normally, an accurate measurement of the phase angle can be
obtained after
several tens of rotations of the stirnng bars. For rotation frequencies of 10-
20 Hz, this time
will be on the order of a few seconds per vessel. Thus, phase angle
measurements for an
entire array of vessels can be typically made once every few minutes,
depending on the
number of vessels, the stirring bar frequency, and the desired accuracy. In
order to speed up
3o the measurement process, one may employ multiple-channel signal detection
to measure the
-36-
CA 02419700 2003-02-19
phase angle of stirxing bars in more than one vessel at a time. Alternate
detection methods
include direct digitization of the coil output waveforms using a high-speed
multiplexer and/or
an analog-to-digital converter, followed by analysis of stored waveforms to
determine
amplitude and phase angle.
Phase angle measurements can also be made with non-magnetic, mechanical
stirring
drives, using the inductive coil system 760 of Fig. 25. For example, one may
achieve
sufficient phase lag between the stirring blade and the drive motor by joining
them with a
torsionally soft, flexible connector. Alternatively, the drive mechanism may
use a resilient
belt drive rather than a rigid gear drive to produce measurable phase lag. The
stirring blade
l0 must include a permanent magnet oriented such that its magnetic moment is
not parallel to
the axis of rotation. For maximum sensitivity, the magnetic moment of the
stirring blade
should lie in the plane of rotation. Note that one advantage to using a non-
magnetic stirnng
drive is that there is no upper Limit on the phase angle.
In addition to directly or indirectly measuring torque, one may sense
viscosity by
increasing the driving frequency, rvo, or decreasing the magnetic field
strength until, in either
case, the stirring bar stalls because of insufficient torque. The point at
which the stirring bar
stops rotating can be detected using the same setup depicted in Fig. 25 for
measuring phase
angle. During a ramp up (down) of the driving frequency (field strength); the
magnitude of
the lock-in amplifier output will abruptly fail by a large amount when the
stirring bar stalls.
2o The frequency or field strength at which the stirnng bar stalls can be
correlated with
viscosity: the lower the frequency or the higher the field strength at which
stalling occurs, the
greater the viscosity of the reaction mixture.
With appropriate calibration, the method can yield absolute viscosity data,
but
generally the method is used to rank reactions. For example, when screening
multiple
reaction mixtures, one may subject all of the vessels to. a series of step
changes in either
frequency or field strength, while noting which stirring bars stall after each
of the step
changes. The order in which the stirring bars stall indicates the relative
viscosity of the
reaction mixtures since stirnng bars immersed in mixtures having higher
viscosity will stall
early. Note that, in addition to providing data on torque and stall frequency,
the inductive
3o sensing coil system 760 of Fig. 25 and similar devices can be used as
diagnostic tools to
indicate whether a magnetic stirring bar has stopped rotating during a
reaction.
37-
CA 02419700 2003-02-19
Mechanical Oscillators
Piezoelectric quartz resonators or mechanical oscillators can be used to
evacuate the
viscosity of reaction mixtures, as well as a host of other material
properties, including
molecular weight, specific gravity, elasticity, dielectric constant, and
conductivity. In a
s ~ typical application, the mechanical oscillator, which can be as small as a
few mzn in length, is
immersed in the reaction mixture. The response ofthe oscillator to-an
excitation signal is
obtained for a range of input signal frequencies, and depends on the
composition and
properties of the reaction mixture. By calibrating the resonator with a set of
well
characteri2ed liquid standards, the properties of the, reaction mixture can be
determined from
1 o _ the response of the mechanical oscillator. Further details on the use of
piezoelectric quartz
oscillators to measure material properties are described in U.S. Patent
6,336,353
entitled ' "Method arid Apparatus for Chara~;terizing Materials by Using a
Mechanical
Resonator," which issued on January 8, 2002
Although many different kinds of mechanical oscillators currently exist, some
are less
1s useful for measuring properties of liquid solutions. For example,
ultrasonic transducers or
oscillators cannot be used in act liquids due to diffraction e#feets and
steady acoustic
(compressive) waves generated within, the reactor vessel. These effects
usually occur when
the size of the oscillator and the vessel are not much greater than the
characteristic
wavelength of the acoustic waves. Thus, for reactor vessel diameters .on the
order of a few
2o centimeters, the frequency of the mechanical oscillator should be above I
MMlE3z.
Unfortunately, complex liquids and mixtures, including polymer solutions,
often behave Like
elastic gels at these high frequencies, which results in inaccurate resonator
r_.esportse.
Often, shear-mode transducers as well as various surface-wave transducers can
be .
used to avoid some of the problems associated with typical ultrasonic
transducers. Because .
25 of the manner in which they vibrate, shear mode transducers generate
viscous shear waves
instead of acoustic waves. Since viscous shear waves decay exponentially with
distance from
the sensor surface, such sensors tend to be insensitive to the geometry of the
measurement
volume, thus eliminating most diffraction and reflection problems.
Unfortunately, the
operating frequency of these sensors is also high, which, as mentioned~above,
restricts their
3o use to simple fluids. Moreover, at high vibration frequencies, most of the
interaction between
the sensor and the fluid is confined to a thin layer of liquid near the sensor
surface, Any
..-3g..~ .
CA 02419700 2003-02-19
modification of the sensor surface through adsorption of solution components
will often
result in dramatic changes in the resonator response.
Tuning forks 840 and bimorph/unimorph resonators 850 shown in Fig. 28 and Fig.
29,
respectively, overcome many of the drawbacks associated with ultrasonic
transducers.
Because of their small size, tuning forks 840 and bimorph/unimorph resonators
850 have
difficulty exciting acoustic waves, which typically have wavelengths many
times their size.
Furthermore, though one might conclude otherwise based on the vibration mode
shown in
Fig. 28, tuning forks 840 generate virtually no acoustic waves: when excited,
each of the tines
832 of the tuning fork 840 acts as a separate acoustic wave generator, but
because the tines
832 oscillate in opposite directions and phases, the waves generated by each
of the tines 832
cancel one another. Like the shear mode transducers described above, the
bimorph/unimorph
850 resonators produce predominantly viscous waves and therefore tend to be
insensitive to
the geometry of the measurement volume. But unlike the shear mode transducers,
bimorph/unimorph 850 resonators operate at much lower frequencies, and
therefore can be
t 5 used to measure properties of polymeric solutions.
Fig. 30 schematically shows a system 870 for measuring the properties of
reaction
mixtures using mechanical oscillators 872. An important advantage of the
system 870 is that
it can be used to monitor the progress ~f a reaction. The oscillators 872 are
mounted on the
interior walls 874 ofthe reaction vessels 876. Alternatively, the oscillators
872 can be
mounted along the bottom 878 of the vessels 876 or can be freestanding within
the reaction
mixtures 880. Each oscillator 872 communicates with a network analyzer 882
(for example,
an HP8751A analyzer), which generates a variable frequency excitation signal.
Each of the
oscillators 872 also serve as receivers, transmitting their response signals
back to the network
analyzer 882 for processing. The network analyzer 882 records the responses of
the
oscillators 872 as functions of frequency, and sends the data to storage 884.
The output
signals of the oscillators 872 pass through a high impedance buffer amplifier
886 prior to
measurement by the wide band receiver 888 of the network analyzer 882.
Other resonator designs may be used. For example, to improve the suppression
of
acoustic waves, a tuning fork resonator with four tines can be used. It is
also possible to
3o excite resonator oscillations through the use of voltage spikes instead of
a frequency
sweeping AC source. With voltage spike excitation, decaying free oscillations
of the
-39-
CA 02419700 2003-02-19
resonator are recorded instead of the frequency response. A variety of signal
processing
techniques well known to those of skill in the art can be used to distinguish
resonator
responses.
Alternate embodiments can be described with reference to the parallel reactor
system
130 shown in Fig. 2. A single resonator (not shown) is attached to the 3-axis
translation
system I50. The translation system 150, at the direction of the processor 160,
places the
resonator within a reactor vessel of interest. A reading of resonator response
is taken and
compared to calibration curves, which relate the response to viscosity,
molecular weight,
specific gravity, or other properties. In another embodiment, a portion of the
reaction mixture
is withdrawn from a reactor vessel, using, for example, the liquid handling
system 146, and is
placed in a separate vessel containing a resonator. The response of the
resonator is measured
and compared to calibration data. Although the system 870 shown in Fig. 30 is
better suited
to monitor solution properties in situ, the two alternate embodiments can be
used as post-
characterization tools and are much simpler to implement.
15 In addition to mechanical oscillators, other types of sensors can he used
to evaluate
material properties. For example, interdigitated electrodes can be used to
measure dielectric
properties of the reaction mixtures.
Pressure Control S,~stem
Another technique for assessing reaction kinetics is to monitor pressure
changes due
2o to production or consumption of various gases during reaction. One
embodiment of this
technique is shown in Fig. 3I. A parallel reactor 910 comprises a group of
reactor vessels
912. A gas-tight cap 914 seals each of the vessels 912 and prevents
unintentional gas flow to
or from the vessels 91 Z. Prior to placement of the cap 914, each of the
vessels 912 is loaded
with liquid reactants, solvents, catalysts, and other condensed-phase reaction
components
zs using the liquid handling system 146 shown in Fig. 2. Gaseous reactants
from source 916 are
introduced into each of the vessels 912 through a gas inlet 918. Valves 920,
which
communicate with a controller 922, are used to fill the reaction vessels 912
with the requisite
amount of gaseous reactants prior to reaction. A pressure sensor 924
communicates with the
vessel head space-the volume within each of the vessels 912 that separates the
cap 914 from
3o the liquid components-through a port 926 located in the cap 914. The
pressure sensors 924
CA 02419700 2003-02-19
are coupled to a processor 928, which manzpulates.and stores data. During
reaction;. any
changes in the head space pressure, at constant temperature, reflect changes
in the amount of
gas present in the head space, This pressure data can be used to determine the
molar
production or consumption rate, r,, of a gaseous component since, for an ideal
gas at constant
temperature,
_ _1 dp;
RT dt
where R is the universal gas constant and p, is the partial pressure of the
ith gaseous
component. Temperature sensors 930; which communicate with the processor 928
through
monitor 932, provide data that can be used to account for changes in pressure
resulting from
1o variations in head space temperature. The ideal gas law or similar equation
of state can be
used to calculate the pressure correction.
In an alternate embodiment, the valves 920 are used to compensate for the
consumption of a gaseous reactant, in a reaction where there is a net loss in
moles of gas-
phase components. The valves 920 are regulated by the valve controller 922,
which
15 communicates with the processor 928. At the beginning of the reaction, the
valves 920 open
to allow gas from the high pressure source 916 to enter each of the vessels
912. Once the
pressure within each of the vessels 912, as read by the sensor 924, reaches a
predetermined
value, P", the processor 928 closes the valves 920. As the reaction consumes
the source 916
gas, the total pressure within each of the vessels 912 decreases. Once the
pressure in a
2o particular vessel 912 falls below a predetermined value, Pt, the processor
928 opens the valve
920 associated with the particular vessel 912, repressurizing it to P". This
process-filling
each of the vessels 912 with source 916 gas to P", allowing the head space
pressure to drop
below P~, and then refilling the vessels 912 with source 916 gas to PH is
usually repeated
many times during the course of the reaction. Furthermore, the total pressure
in the head
25 space of each of the vessels 912 is continuously monitored and recorded
during the gas fill-
pressure decay cycle.
An analogous method can be used to investigate reactions where there is a net
gain of
gas-phase components. At the beginning of a reaction, ail reaction materials
are introduced
into the vessels 912 and the valves 920 are closed. As the reaction proceeds,
gas production
'-41-
CA 02419700 2003-02-19
results in a rise in head space pressure, which sensors 924 and processor 928
monitor and
record. Once the pressure within a particular vessel 912 reaches P", the
processor 928 directs
the controller 922 to open the appropriate valve 920 to depressurize the
vessel 912. The valve
920, which is a mufti-port valve, vents the gas from the head space through an
exhaust line
934. Once the head space pressure falls below Py, the processor 928 instructs
the controller
922 to close the valve 920. The total pressure is continuously monitored and
recorded during
the gas rise-vent cycle.
The gas consumption (production) rates can be estimated from the total
pressure data
by a variety of methods. For simplicity; these methods are described in terms
of a single
reactor vessel 912 and valve 920, but they apply equally well to a parallel
reactor 910
comprising multiple vessels 912 and valves 920. One estimate of gas
consumption
(production) can be made from the slope of the pressure decay (growth) curves
obtained when
the valve is closed. These data, after converting total pressure to partial
pressure based on
reaction stoichiometry, can be inserted into equation XI to calculate r" the
molar consumption
is (production) rate. A second estimate can be made by assuming that a fixed
quantity of gas
enters (exits) the vessel during each valve cycle. The frequency at which the
reactor is
repressurized (depressurized) is therefore proportional to the gas consumption
(production)
rate. A third, more accurate estimate can be obtained by assuming a known gas
flow rate
through the valve. Multiplying this value by the time during which the valve
remains open
2o yields an estimate for the quantity of gas that enters or leaves the vessel
during a particular
cycle. Dividing this product by the time between the next valve cycle-that is,
the time it
takes for the pressure in the vessel head space to fall from P" to P~ yields
an average value
for the volumetric gas consumption (production) rate for the particular valve
cycle. Summing
the quantity of gas added during all of the cycles equals the total volume of
gas consumed
25 (produced) during the reaction.
The most accurate results are obtained by directly measuring the quantity of
gas that
flows through the valve. This can be done by noting the change in pressure
that occurs
during the time the valve is open-the ideal gas law can be used to convert
this change to the
volume of gas that enters or leaves the vessel. Dividing this quantity by the
time between a
3o particular valve cycle yields an average volumetric gas consumption
(production) rate for that
-42-
CA 02419700 2003-02-19
cycle. Summing the volume changes for each cycle yields the total volume of
gas consumed
(produced) in the reaction.
In an alternate embodiment shown in Fig. 31, the gas consumption rate is
directly
measured by inserting flow sensors 936 downstream of the valves 920 or by
replacing the
valves 920 with flow sensors 936. The flow sensors 936 allow continuous
monitoring of the
mass flow rate of gas entering each of the vessels 912 through the gas inlet
918. To ensure
meaningful comparisons between experiments, the pressure of the source 916 gas
should
remain about constant during an experiment. Although the flow sensors 936
eliminate the
need for cycling the valves 920, the minimum detectable flow rates of this
embodiment are
i o less than those employing pressure cycling. But, the use of flow sensors
936 is generally
preferred for fast reactions where the reactant flow rates into the vessels
912 are greater than
the threshold sensitivity of the flow sensors 936.
Illustrative Example of Calibration of Mechanical Oscillators for Measurinu
Molecular
i5 Mechanical oscillators were used to characterize reaction mixtures
comprising
polystyrene and toluene. To relate resonator response to the molecular weight
of polystyrene,
the system 870 illustrated in Fig. 30 was calibrated using polystyrene
standards of known
molecular weight dissolved in toluene. Each of the standard polystyrene-
toluene solutions
had the same concentration, and were run in separate (identical) vessels using
tuning fork
2o piezoelectric quartz resonators similar to the one shown in Fig. 28.
Frequency response
curves for each resonator were recorded at intervals. between about 10 and 30
seconds.
The calibration runs produced a set of resonator responses that could be used
to relate
the output from the oscillators 872 immersed in reaction mixtures to
polystyrene molecular
weight. Fig. 32 shows results of calibration runs 970 far the polystyrene-
toluene solutions.
25 The curves are plots of oscillator response for polystyrene-toluene
solutions comprising no
polystyrene 952, and polystyrene standards having weight average molecular
weights (M.) of
2.36x103 954, 13.7x103 956, 114.2x10' 958, and 1.88x106 960.
Fig. 33 shows a calibration curve 970 obtained by correlating M of the
polystyrene
standards with the distance between the frequency response curve for toluene
952 and each of
-43-
CA 02419700 2003-02-19
the polystyrene solutions 954, 956, 958, 960 of Fig. 32. This distance was
calculated using
the expression:
f~( 1
.fa l .fo '' tRo ~ R' ~ Z ff
XII
where f and f are the Lower and upper frequencies of the response curve,
respectively; R, is
the frequency response of the resonator in toluene, and R, is the resonator
response in a
particular polystyrene-toluene solution. Given response curves for an unknown
polystyrene-
toluene mixture and pure toluene 952 (Fig. 32), the distance between the two
curves can be
determined from equation XII. The resulting d, can be Located along the
calibration curve 970
of Fig. 33 to determine M for the unknown polystyrene-toluene solution.
lo Illustrative Example of Measurement of Gas-Phase Reactant Consumption by
Pressure
Monitoring and Control
Figure 34 depicts the pressure recorded during solution polymerization of
ethylene to
polyethylene. The reaction was carried out in an apparatus similar to that
shown in Fig. 3I .
An ethylene gas source was used to compensate for ethylene consumed in the
reaction. A
valve, under control of a processor, admitted ethylene gas into the reaction
vessel when the
vessel head space pressure dropped below P~ ~ 16.1 psig due to consumption of
ethylene.
During the gas filling portion of the cycle, the valve remained open until the
head space
pressure exceeded PN ~ 20.3 psig.
Fig. 35 and Fig. 36 show ethylene consumption rate as a function of time, and
the
2o mass of polyethylene formed as a function of ethylene consumed,
respectively. The average
ethylene consumption rate, - r~2,k (atm~miri'), was determined from the
expression
_\Px_Pc~k
rC2.k - Qt XIII
k
where subscript k refers to a particular valve cycle, and Otk is the time
interval between the
valve closing during the present cycle and the valve opening at the beginning
of the next
-44-
CA 02419700 2003-02-19
cycle. As shown in Fig. 35, the constant ethylene consumption rate at later
times results from
catalyzed polymerization of ethylene. The high ethylene consumption rate early
in the
process results primarily from transport of ethylene into the catalyst
solution prior to
establishing an equilibrium ethylene concentration in the liquid phase. Fig.
36 shows the
amount of polyethylene produced as a function of the amount of ethylene
consumed by
reaction. The amount of polyethylene produced was determined by weighing the
reaction
products, and the amount of ethylene consumed by reaction was estimated by
multiplying the
constant average consumption rate by the total reaction time. A linear least-
squares fit to
these data yields a slope which matches the value predicted from the ideal gas
law and from
1o knowledge of the reaction temperature and the total volume occupied by the
gas (the product
of vessel head space and number of valve cycles during the reaction).
Automated. High Pressure Infection Systern
Fig. 37 shows a perspective view of an eight-vessel reactor module 1000, of
the type
shown in Fig. 10, which is fitted with an optional liquid injection system
1002. The liquid
~ 5 injection system 1002 allows addition of liquids to pressurized vessels,
which, as described
below, alleviates problems associated with pre-loading vessels with catalysts.
In addition, the
liquid injection system 1002 improves concurrent analysis of catalysts by
permitting
screening reactions to be selectively quenched through the addition of a
liquid-phase catalyst
poison.
20 The liquid injection system 1002 helps solve problems concerning liquid-
phase
catalytic polymerization of a gaseous monomer. When using the reactor module
390 shown
in Fig. I0 to screen or characterize polymerization catalysts, each vessel is
normally loaded
with a catalyst and a solvent prior to reaction. After sealing, gaseous
monomer is introduced
into each vessel at a specified pressure to initiate polymerization. As
discussed in Example 1,
25 during the early stages of reaction, the monomer concentration in the
solvent increases as
gaseous monomer dissolves in the solvent. Although the monomer eventually
reaches an
equilibrium concentration in the solvent, catalyst activity may be affected by
the changing
monomer concentration prior to equilibrium. Moreover, as the monomer dissolves
in the
solvent early in the reaction, additional gaseous monomer is added to maintain
the pressure in
30 the vessel headspace. This makes it diffcult to distinguish between
pressure changes in the
--45-
CA 02419700 2003-02-19
vessels due to polymerization in the liquid phase and pressure changes due to
monomer
transport into the solvent.to establish an equilibrium concentration. These
analytical
diffculties can be avoided using the liquid injection system 1002, since the
catalyst can be
introduced into the vessels after the monomer has attained an equilibrium
concentration in the
Liquid phase.
The liquid injection system 1002 of Fig. 37 also helps solve problems that
arise when
using the reactor module 390 shown in Fig. 10 to investigate catalytic co-
polymerization of
gaseous and liquid co-monomers. Prior to reaction, each vessel is loaded with
a catalyst and
the liquid co-monomer. After sealing the vessels, gaseous co-monomer is
introduced into
1o each vessel to initiate co-polymerization. However, because appreciable
time may elapse
between loading of liquid components and contact with the gaseous co-monomer,
the catalyst
may homo-polymerize a significant fraction of the liquid co-monomer. In
addition, the
relative concentration of the co-monomers in the liquid-phase changes during
the early stages
of reaction as the gaseous ca-monomer dissolves in the liquid phase. Both
effects lead to
15 analytical difficulties that can be avoided using the liquid injection
system 1002, since
catalysts can be introduced into the vessels after establishing an equilibrium
concentration of
the gaseous and liquid co-monomers in the vessels. In this way, the catalyst
contacts the two
co-monomers simultaneously.
The liquid injector system 1002 shown in Fig. 37 also allows users to quench
20 reactions at different times by adding a liquid phase catalyst poison,
which improves
screening of materials exhibiting a broad range of catalytic activity. When
using the reactor
module 390 of Fig. 10 to concurrently evaluate library members for catalytic
performance,
the user may have little information about the relative activity of library
members. If every
reaction is allowed to proceed for the same amount of time, the most active
catalysts may
25 generate an excessive amount of product, which can hinder post reaction
analysis and reactor
clean up. Conversely, the least active catalysts may generate an amount of
product
insufficient for characterization. By monitoring the amount of product in each
of the
vessels-through the use of mechanical oscillators or phase lag measurements,
for instance-
the user can stop a particular reaction by injecting the catalyst poison into
the vessels once a
3o predetermined conversion is achieved. Thus, within the same reactor and in
the same
experiment, low and high activity catalysts may undergo reaction for
relatively long and short
-,q6--
CA 02419700 2003-02-19
time periods, respectively, with both sets of catalysts generating about the
same amount of
product.
Referring again to Fig. 37, the liquid injection system 1002 comprises fill
ports 1004
attached to an injector manifold 1006. An injector adapter plate 1008,
sandwiched between
an upper plate 1010 and block 1 OI2 of the reactor module 1000, provides
conduits for liquid
flow between the injector manifold 1006 and each of the wells or vessels (not
shown) within
the block 1012. Chemically inert valves 1014 attached to the injector manifold
1006 and
located along flow paths connecting the fill ports 104 and the conduits within
the adapter
plate 1008, are used to establish or prevent fluid communication between the
fill ports 1004
to and the vessels or wells. Normally, the liquid injection system 1002 is
accessed through the
fill ports 1004 using a probe 1016, which is part of an automated liquid
delivery system such
as the robotic material handling system 146 shown in Fig. 2. However, liquids
can be
manually injected into the vessels through the fill ports 1004 using a
pipette, syringe, or
similar liquid delivery device. Conventional high-pressure liquid
chromatography Loop
injectors can be used as fill ports 1004. Other useful fill ports 1004 are
shown in Fig. 38 and
Fig. 39.
Fig. 38 shows a cross sectional view of a first embodiment of a fill port
1004' having
an o-ring seal to minimize liquid leaks. The fill port 1004' comprises a
generally cylindrical
fill port body 1040 having a frst end I042 and a second end 1044. An axial
bore 1046 runs
2o the length of the fill port body 1040. An elastomeric o-ring 1048 is seated
within the axial
bore 1046 at a point where there is an abrupt narrowing 1050, and is held in
place with a
sleeve 1052 that is threaded into the first end 1042 of the fill part body
1040. The sleeve
1052 has a center hole 1054 that is sized to accommodate the widest part of
the probe.1016.
The sleeve 1052 is typically made from a chemically resistant plastic, such as
polyethylethylketone (PEEK), polytetrafIuoroethylene (PTFE), and the Like,
which minimizes
damage to the probe 1016 and fill port 1004' during liquid injection. To aid
in installation
and removal, the fill port 1004' has a knurled first outer surface 1056
located adjacent to the
first end 1042 of the fill port 1004', and a threaded second outer surface
1058, located
adjacent to the second end 1044 of the fill port 1004'.
3o Fig. 38 also shows the position ofthe probe 1016 during liquid injection.
Like a
conventional pipette, the probe 2016 is a cylindrical tube having an outer
diameter (~D) at
-47-
CA 02419700 2003-02-19
the point of liquid delivery that is smaller than the OD over the majority of
the probe 101 b
length. As a result, near the probe tip 1060, there is a transition zone 1062
where the probe
1016 OD narrows. Because the inner diameter (ID) of the o-ring 1048 is about
the same as
the OD of the probe tip 1060, a liquid-tight seal is formed along the probe
transition zone.
1060 during liquid injection.
Fig. 39 shows a second embodiment of a fill port 1004". Like the first
embodiment
1004' shown in Fig. 38, the second embodiment 1004" comprises a generally
cylindrical fill
port body 1040' having a first end 1042' and a second end 1044'. But instead
ofan o-ring,
the fill port 1004" shown in Fig. 39 employs an insert 1080 having a tapered
axial hole 1082
l0 that results an interference fit, and hence a seal, beriveen the probe tip
1060 and the ID of the
tapered axial hole I082 during liquid injection. The insert 1080 can be
threaded into the first
end 1042' of the fill port 1004". Typically, the insert I 080 is made from a
chemically
resistant plastic, such as PEEK, PTFE, and the like, which minimizes damage to
the probe
1016 and fll port 1004" during liquid injection. To aid in removal and
installation, the fill
IS port' has a knurled first outer surface 1056' located adjacent to the first
end 1042' of the fill
port 1004", and a threaded second outer surface 1058' located adjacent to the
second end
1044' of the fill port 1004".
Fig. 40 shows a phantom front view of the injector manifold 1006. The injector
manifold 1006 includes a series of fill port seats 1100 located along a top
surface 1 I02 of the
20 injector manifold 1006. The fill port seats 1100 are dimensioned to receive
the second ends
1044, 1044' of the fill ports 1004', 1004" shown in Fig. 38 and Fig. 39.
~,ocating holes 1104,
which extend through the injector manifold 1006, locate the valves 1014 of
Fig. 37 along the
front of the injector manifold I 006.
An alternative design far the valve 1014, which is used with the injection
ports is
25 shown is Fig. 40A and Fig. 40B. Fig. 40A shows the injector manifold 1006,
which is shown
in a cross sectional view in Fig. 408. The alternative valve design is
essentially a check
valve that has a spring 2005 under a'poppet 2006. When not injecting, the
spring 2005
assisted by the pressure of the reaction vessel pushes the poppet 2006 against
a seal 2007 to
seal the reaction vessel. The seal may be of a type known to those of skill in
the art, such as
30 an o-ring seal. When injecting, a pump associated with the probe 1016
forces the material to
be injected against the poppet 2006 overcoming the pressure in the chamber and
the spring
--48-
CA 02419700 2003-02-19
2005 force to allow the material being injected to flow past the poppet into
the reaction vessel
via the channel in the module.
Fig. 41 shows a cross sectional view of the injector manifold 1006 along a
first
section line I 106 of Fig. 40. The cmss section illustrates one of a group of
first flaw paths
1130. The first flow paths 1130 extend from the fill port seats 1100, through
the injector
manifold 1006, to valve inlet seats 1132. Each of the valve inlet seats 1132
is dimensioned to
receive an inlet port (not shown) of one of the valves 1014 depicted in Fig.
37. The first flow
paths 1 I30 thus provide fluid communication between the fill ports 1004 and
the valves 1014
of Fig. 37.
to Fig. 42 shows a cross sectional view of the injector manifold 1006 along a
second
section line 1108 of Fig. 40. The cross section iilustrates one of a group of
second flow paths
1150. The second flow paths I I50 extend from valve autiet seats 1152, through
the injector
manifold 1006, to manifold outlets 1154 located along a back surface 1156 of
the injector
manifold 1006. Each of the valve outlet seats 1152 is dimensioned to receive
an outlet port
15 (not shown) of one of the valves 1 OI4 depicted in Fig. 37. The manifold
outlets 1 I 54 mate
with fluid conduits on the injector adapter plate 1008. Annular grooves I 158,
which surround
the manifold outlets 1154, are sized to receive o-rings (not shown) that seat
the fluid
connection between the manifold outlets I I54 and the fluid conduits on the
injector adapter
plate 1008. The second flow paths 1150 thus provide fluid communication
between the
20 valves 1014 and the injector adapter plate 1008.
Fig. 43 shows a phantom top view of the injector adapter plate 1008, which
serves as
an interface between the injector manifold 1006 and the block l 012 of the
reactor module
1000 shown in Fig. 37. The injector adapter plate 1008 comprises holes 1180
that provide
access to the vessels and wells within the block 1012. The injector adapter
plate 1008 also
25 comprises conduits 1182 extending from a front edge 1 I84 to the bottom
surface of the
adapter plate 1008. When the adapter plate 1008 is assembled in the reactor
module 1000,
inlets 1186 of the conduits I I82 make fluid connection with the manifold
outlets 1154 shown
in Fig. 42.
As shown in Fig. 44, which is a cross sectional side view of the injector
adapter plate
3o 1008 along a section line I 188 of Fig. 43, the conduits I 182 terminate on
a bottom surface
1210 of the injector plate 1008 at conduit outlets 1212. The bottom surface
1210 of the
-~ 9--
CA 02419700 2003-02-19
adapter plate 1008 forms an upper surface of each of the wells in the reactor
module 1000
block 1012 of Fig. 37. To ensure that liquid is properly delivered into the
reaction vessels,
elongated well injectors, as shown in Fig. 45 and Fig. 48 below, are connected
to the conduit
outlets 1212.
Fig. 45 shows an embodiment of a well injector 1230. The well injector 1230 is
a
generally cylindrical tube having a frst end I232 and a second end 1234. The
well injector
1230 has a threaded outer surface 1236 near the first end 1232 so that it can
be attached to
threaded conduit outlets I2I2 shown in Fig. 44. Flats 1238 located adjacent to
the threaded
outer surface 1236 assist in twisting the first end 1232 of the well injector
1230 into the
1o conduit outlets 1212. The length of the well injector 1230 can be varied.
For example, the
second end 1234 of the well injector 1230 may extend into the liquid mixture;
alternatively,
the second end 1234 of the injector 1230 may extend a portion of the way into
the vessel
headspace. Typicaily, the well injector 1230 is made from a chemically
resistant plastic, such
PEEK, PTFE, and the like.
15 Liquid injection can be understood by referring to Fig. 46-48. Fig. 46
shows a top
view of the reactor module 1000, and Fig. 47 and Fig. 48 show, respectively,
cross sectional
side views of the reactor module 1000 along first and second section lines
1260, 1262 shown
in Fig. 46. Prior to injection of a catalyst or other liquid reagent, the
probe I OI6, which
initially contains a first solvent, withdraws a predetemnined amount of the
liquid reagent from
z0 a reagent source. Next, the probe 1016 withdraws a predetermined amount of
a second
solvent from a second solvent source, resulting in a slug of liquid reagent
suspended between
the first and second solvents within the probe 1016. Generally, probe
manipulations are
carried out using a robotic material handling system of the type shown in Fig.
2, and the
second solvent is the same as the first solvent.
25 Fig. 47 and 48 show the inlet and outlet paths of the valve 1014 prior to,
and during,
liquid injection, respectively. Once the probe 1016 contains the requisite
amount of liquid
reagent and solvents, the probe tip 1058 is inserted in the fill port 1004,
creating a seal as
shown, for exampIe,,in Fig. 38 and Fig. 39. The valve 1014 is then opened, and
the second
solvent, liquid reagent, and a portion of the first solvent are injected into
the reactor module
30 1000 under pressure. From the fill port 1004, the liquid flows into the
injector manifold 1006
through one of the first flow paths 1130 that extend from the fill port seats
1100 to the valve
-50--
CA 02419700 2003-02-19
inlet seats 1132. The liquid enters the valve I014 through an inlet port 1280,
flows through a
valve flow path 1282, and eXitS the valve 1014 through an outlet port 1284.
After leaving the
valve 1014, the liquid flows through one of the second flow paths 1150 to a
manifold outlet
1154. From the manifold outlet 1 I54, the liquid flows through the injector
adapter plate 1008
within one of the fluid conduits 1182, and is injected into a reactor vessel
1286 or well 1288
through the well injector 1230. In the embodiment shown in Fig. 48, the second
end 1234 of
the well injector 1230 extends only a fraction ofthe way into the vessel
headspace 1290. In
other cases, the second end 1234 may extend into the reaction mixture 1292.
Liquid injection continues until the slug of liquid reagent is injected into
the reactor
to vesset 1286 and the flow path from the fill port 1004 to the second end
1234 of the well
injector 1230 is filled with the first solvent. At that point, the valve 1014
is closed, and the
probe 1016 is withdrawn from the fll port 1004.
Reactor Vessel Pressure Seal and Maenetic Feed-Through Stirnn~ Mechanism
Fig. 48 shows a stirring mechanism and associated seals for maintaining above-
15 ambient pressure in the reactor vessels 1286. The direct-drive stirring
mechanism 1310 is
similar to the one shown in Fig. 10, and comprises a gear 1312 attached to a
spindle 1314 that
rotates a blade or paddle 1316. A dynamic lip seal 1318; which is secured to
the upper plate
1010 prevents gas leaks between the rotating spindle 1314 and the upper plate
1010. When
newly installed, the lip seal is capable of maintaining pressures of about 100
psig. However,
2o with use, the lip seal 1318, like o--rings and other dynamic seals, will
leak due to frictional
wear. High service temperatures, pressures, and stirring speeds hasten dynamic
seal wear.
Fig. 49 shows a cross sectional view of a magnetic feed through 1340 stirring
mechanism that helps minimize gas leaks associated with dynamic seals. The
magnetic feed-
through 1340 comprises a gear 1342 that is attached to a magnetic driver
assembly 1344
25 using cap screws 1346 or similar fasteners. The magnetic driver assembly
1344 has a
cylindrical inner wall 1348 arid is rotatably mounted on a rigid cylindrical
pressure barrier
1350 using one or more bearings 1352. The bearings 1352 are located within an
annular gap
1354 between a narrow head portion 1356 of the pressure barner I350 and the
inner wall
1348 of the magnetic driver assembly 1344. A base portion 1358 of the pressure
barrier 1350
3o is affixed to the upper plate 1010 of the reactor module 1000 shown in Fig.
48 so that the axis
-51-
CA 02419700 2003-02-19
of the pressure barrier 1350 is about coincident with the centerline of the
reactor vessel 1286
or well 1288. The pressure barrier 1350 has a cylindrical interior surface
1360 that is open
only along the base portion 1358 of the pressure barrier 1350. Thus, the
interior surface 1360
of the pressure homer 1350 and the reactor vessel 1286 or well 1288 define a
closed chamber.
s . As can be seen in Fig. 49, the magnetic feed through 1340 further
comprises a
cylindrical magnetic follower 1362 rotatably mounted within the pressure
barrier 1350 using
first 1364 and second 1366 flanged bearings. The first 1364 and second 1366
flanged
bearings are located in first 1368 and second 1370 annular regions 1368
delimited by the
interior surface 1360 of the pressure barrier 1350 and relatively narrow head
1372 and leg
1374 portions of the magnetic follower 1362, respectively. A keeper 1376 and
retaining clip
1378 located within the second annular region 1370 adjacent to the second
flanged bearing
1366 help minimize axial motion of the magnetic follower 1362. A spindle (not
shown)
attached to the free end 1380 of the leg 1374 of the magnetic follower 1362,
transmits torque
to the paddle 1316 immersed in the reaction mixture 1292 shown in Fig. 48.
During operation, the rotating gear 1342 and magnetic driver assembly 1344
transmit
torque through the rigid pressure barrier 1350 to the cylindrical magnetic
follower 1362.
Permanent magnets (not shown) embedded in the magnetic driver assembly 1344
have force
vectors lying in planes about perpendicular to the axis of rotation 1382 of
the magnetic driver
assembly 1344 and follower 1362. These magnets are coupled to permanent
magnets (not
shown) that are similarly aligned and embedded in the magnetic follower 1362.
Because of
the magnetic coupling, rotation of the driver assembly 1344 induces rotation
of the follower
1362 and stirring blade or paddle 1316 of Fig. 48. The follower I 362 and
paddle 1316 rotate
at the same frequency as the magnetic driver assembly, though, perhaps, with a
measurable
phase lag.
Removable and Disposable Stirrer
The stirring mechanism 1310 shown in Fig: 48 includes a mufti-piece spindle
1314
comprising an upper spindle portion 1400, a coupler 1402, and a removable
stin:er 1404. The
mufti-piece spindle 1314 offers certain advantages over a one-piece spindle.
Typically, only
the upper drive shaft 1400 and the coupler 1402 are made of a high modules
material such as
stainless steel: the removable stirrer 1404 is made of a chemically resistant
and inexpensive
-52---
CA 02419700 2003-02-19
plastic, such as PEEK, PTFE, and the like. In contrast; one-piece spindles,
though perhaps
coated with PTFE, are generally made entirely of a relatively expensive high
modulus
material, and are therefore normally reused. However, one-piece spindles are
often difficult
to clean after use, especially following a polymerization reaction.
Furthermore, reaction
product may be lost during cleaning, which leads to errors in calculating
reaction yield. With
the mufti-piece spindle 1314, one discards the removable stirrer 1404 after a
single use,
eliminating the cleaning step. Because the removable stirrer 1404 is less
bulky than the one-
piece spindle, it can be included in certain post-reaction characterizations,
including product
weighing to determine reaction yield.
Fig. 50 shows a perspective view of the stirnng mechanism 13I 0 of Fig. 48,
and
provides details of the mufti-piece spindle 1314. A gear 1312 is attached to
the upper spindle
portion 1400 of the rnulti-piece spindle 1314. The upper spindle 1400 passes
through a
pressure seal assembly 1420 containing a dynamic lip seal, and is attached to
the removable
stirrer 1404 using the coupler 1402. Note that the removable stirrer 1404 can
also be used
with the magnetic feed through stirring mechanism 1340 illustrated in Fig. 49.
In such cases,
the upper spindle 1400 is eliminated and the leg 134 of the cylindrical
magnetic follower
1362 or the coupler 1402 or both are modified to attach the magnetic follower
1362 to the
removable stirrer 1404.
Fig. 51 shows details of the coupler 1402, which comprises a cylindrical body
having
2o first 1440 and second 1442 holes centered along an axis of rotation 1444 of
the coupler 1402.
The first hole 1440 is dimensioned to receive a cylindrical end 1446 ofthe
upper spindle
1400. A shoulder 1448 formed along the periphery of the upper spindle 1400
rests against an
annular seat 1450 located within the first hole 1440. A set screw {not shown)
threaded into a
locating hole 1452 prevents relative axial and rotational motion of the upper
spindle 1400 and
z5 the coupler 1402.
Refernng to Fig. 50 and 51, the second hole 1442 of the coupler 1402 is
dimensioned
to receive a first end 1454 of the removable stirrer 1404. A pin 1456, which
is embedded in
the first end 1454 of the removable stirrer, cooperates with a locking
mechanism 1458 located
on the coupler 1402, to prevent relative rotation of the coupler 1402 and the
removable stirrer
30 1404. The locking mechanism 1458 comprises an axial groove 1460 formed in
an inner
-53-
CA 02419700 2003-02-19
surface 1462 of the coupler. The groave 1460 extends from an entrance 1464 of
the second
hole 2 442 to a lateral portion 1466 of a slot 1468 cut through a wall 1470 of
the coupler 1402.
As shown in Fig. 52, which is a cross sectional view of the coupler 1402 along
a
section line 1472, the lateral portion 1466 of the slot I46$ extends about 60
degrees around
the circumference of the coupler 1402 to an axial portion 1474 of the slot
1468. To connect
the removable stirrer 1404 to the coupler 1402, the first end 1454 of the
removable stirrer
1404 is inserted into the second hole 2 442 and then rotated so that the pin
1456 travels in the
axial groove 1460 and lateral portion 1466 of the slot 1468. A spring I 476,
mounted between
the coupler 1402 and a shoulder 1478 formed on the periphery of the removable
stirrer i 404,
1o forces the pin 1456 into the axial portion 1474 of the slot 1468.
An alternative design for the multi-piece spindle 1314 is shown in Fig. 50A,
which
has an upper spindle portion 1400, a coupler 1402 and a removable stirrer
1404.. The details
of this alternative design are shown in Fig.. 50B. This alternative design is
essentially a
spring lock mechanism that allows for quick removal of the removable stirrer
1404. The
15 removable stirrer 1404 is locked in to the coupling mechanism by a series
ofballs 2001 that
are held into a groove in the removable stirrer 1404 by a collar 2002, which
is part of the
coupler 1402. The removable stirrer 1404 is released by pulling the collar
2002 back against
a spring 2003 and allowing the balls 2001 to fall into a pocket in the collar
2002 and releasing
the removable stirrer.
20 Parallel Pressure Reactor Control and Analysis
Fig. 53 shows one implementation of a computer-based system for monitoring the
progress and properties of multiple reactions in situ. Reactor control system
1500 sends
control data 1502 to and receives experimental data 1504 from reactor 1506. As
will be
described in more detail below, in one embodiment reactor 1506 is a parallel
polymerization
25 reactor and the control and experimental data 1502 and 1504 include set
point values for
temperature, pressure, time and stirring speed as well as measured
experimental values for
temperature and pressure. Alternatively, in other embodiments reactor I506 can
be any other
type of parallel reactor or conventional reactor, and data 1502, 1504 can
include other control
or experimental data. System control module 1508 provides reactor 1506 with
control data
30 1502 based on system parameters obtained from the user through user I/O
devices 1510, such
--54---
CA 02419700 2003-02-19
as a display monitor, keyboard or mouse. Alternatively, system control module
1508 can
retrieve control data 1502 from storage 1512.
Reactor control system 1500 acquires experimental data 1504 from reactor 1506
and
processes the experimental data in system control module 1508 and data
analysis module
1514 under user control through user interface module 1516. Reactor control
system 1500
displays the processed data both numerically and graphically through user
interface module
1 S 16 and user I/O devices 1510, and optionally through printer 1518.
Fig. 54 illustrates an embodiment of reactor 1506 in which pressure,
temperature, and
mixing intensity are automatically controlled and monitored. Reactor 1506
includes reactor
lo block 1540, which contains sealed reactor vessels I542 for receiving
reagents. In one
embodiment, reactor block 1540 is a single unit containing each of reactor
vessels 1542.
Alternatively, reactor block 1540 can include a number of reactor block
modules, each of
which contains a number of reactor vessels 1542. Reactor 1506 includes a
mixing control
and monitoring system 1544; a temperature control and monitoring system 1546
and a
15 pressure control and monitoring system 1548. These systems communicate with
reactor
control system 1500.
The details of mixing control and monitoring system 1544 are illustrated in
Fig. S5.
Each of reactor vessels 1542 contains a stirrer 1570 for mixing the vessel
contents. In one
embodiment, stirrers 1570 are stirring blades mounted on spindles 1572 and
driven by motors
20 1574. Separate motors 1574 can control each individual stirrer 1570;
alternatively, motors
1574 can control groups of stirrers 1570 associated with reactor vessels 1542
in separate
reactor blocks. In another embodiment, magnetic stirring bars or other known
stirring
mechanisms can be used. System control module 1508 provides mixing control
signals to
stirrers 1570 through interface 1576; 1578, and one or more motor cards 1580.
Interface
25 1576, 1578 can include a commercial motor driver 1576 and motor interface
software 1578
that provides additional high level motor control, such as the ability to
initialize motor cards
1580, to control specific motors or motor axes {where each motor 1580 controls
a separate
reactor block), to set motor speed and acceleration, and to change or stop a
specified motor or
motor axis.
3o Mixing control and monitoring system 1544 can also include torque monitors
1582,
which monitor the applied torque in each of reactor vessels 1542. Suitable
torque monitors
-55---
CA 02419700 2003-02-19
1582 can include optical sensors and magnetic field sensors mounted on
spindles 1572, or
strain gauges (not shown), which directly measure the applied torque and
transmit torque data
to system control module 1508 and data analysis module 1514. Monitors 1582 can
also
include encoders, resolvers; Hall effect sensors and the like, which may be
integrated into
motors 1574. These monitors measure the power required to maintain a constant
spindle
1572 rotational speed, which is related to applied torque.
Referring to Fig. 56, temperature control and monitoring system 1546 includes
a
temperature sensor I600 and a heating element 1602 associated with each
reactor vessel 1542
and controlled by temperature controller 1604. Suitable heating elements 1602
can include
to thin filament resistance heaters, thermoelectric devices, thennistors, or
other devices for
regulating vessel temperature. Heating elements can include devices for
cooling, as well as
heating, reactor vessels 1542. System control unit 1508 transmits temperature
control signals
to heating elements 1602 through interface 1606, 1608 and temperature
controller 1604.
Interface 1606, 1608 can include a commercial temperature device driver 1606
implemented
15 to use hardware such as an RS232 interface, and temperature interface
software 1608 that
provides additional nigh level communication with temperature controller 1604,
such as the
ability to control the appropriate communication port, to send temperature set
points to
temperature controller 1604, and to receive temperature data from temperature
controller
1604.
2o Suitable temperature sensors 1600 can include thermocouples, resistance
thermoelectric devices, thennistors, or other temperature sensing devices.
Temperature
controller 1604 receives signals from temperature sensors 1600 and transmits
temperature
data to reactor control system 1500. Upon determining that an increase or
decrease in reactor
vessel temperature is appropriate, system control module 1508 transmits
temperature control
25 signals to heating elements 1602 through heater controller 1604. This
determination can be
based on temperature parameters entered by the user through user interface
module 1516, ar
on parameters retrieved by system control module 1508 from storage. System
control module
1508 can also use information received from temperature sensors 1600 to
determine whether
an increase or decrease in reactor vessel temperature is necessary.
30 As shown in Fig. 57, pressure control and monitoring system 1548 includes a
pressure
sensor 1630 associated with each reactor vessel 1542. Each reactor vessel 1542
is famished
--56--
CA 02419700 2003-02-19
with a gas inlet/outlet 1632 that is controlled by valves 1634. System control
module 1508
controls reactor vessel pressure through pressure interface 1636, 1638 and
pressure controller
1640. Pressure interface 1636, 1638 can be implemented in hardware, software
or a
combination of both. Pressure controller 1640 transmits pressure control
signals to valves
1634 allowing gases to enter or exit reactor vessels 1542 through inlet/outlet
1632 as required
to maintain reactor vessel pressure at a level set by the user through user
interface 15I 6.
Pressure sensors 1630 obtain pressure readings from reactor vessels 1542 and
transmit
pressure data to system control module 1508 and data analysis module I 514
through pressure
controller 1640 and interface 1636, 1638. Data analysis module 1514 uses the
pressure data
l0 in calculations such as the determination of the rate of production of
gaseous reaction
products or the rate of consumption of gaseous reactants, discussed in more
detail below.
System control module 1508 uses the pressure data to determine when
adjustments to reactor
vessel pressure are required, as discussed above.
Fig. 58 is a flow diagram illustrating the operation of a reactor control
system 1500.
15 The user initializes reactor control system 1500 by setting the initial
reaction parameters, such
as set points for temperature, pressure and stirring speed and the duration of
the experiment,
as well as selecting the appropriate hardware configuration for the experiment
(step 1660).
The user can also set other reaction parameters that can include, for example,
a time at which
additional reagents, such as a liquid co-monomer in a co-polymerization
experiment, should
20 be added to reaction vessels 1542; or a target conversion percentage at
which a quenching
agent should be added to terminate a catalytic polymerization experiment.
Alternatively,
reactor control system 1500 can load initial parameters from storage I5I2. The
user starts the
experiment (step 1662). Reactor control system 1500 sends control signals to
reactor 1 I0,
causing motor, temperature and pressure control systems 1544, 1546 and 1548 to
bring
25 reactor vessels 1542 to set point levels (step 1664).
Reactor control system 1500 samples data through mixing monitoring system
1544,
temperature monitoring system 1546 and pressure monitoring system 1548 at
sampling rates,
which may be entered by the user (step 1666). Reactor control system 1500 can
provide
process control by testing the experimental data, including sampled
temperature, pressure or
30 torque values as well as elapsed time, against initial parameters (step
1668). Based on these
inputs, reactor control system 1500 sends new control signals to the mixing,
temperature
CA 02419700 2003-02-19
and/or pressure control and monitoring systems of reactor 1506 (steps 1670,
1664). These
control signals can also include instructions to a material handling robot to
add material, such
as a reagent or a catalyst quenching agent, to one or more reactor vessels
based upon
experimental data such as elapsed time or percent conversion calculated as
discussed below.
The user can also enter new parameters during the course of the experiment,
such as changes
in motor speed, set points for temperature or pressure, or termination
controlling parameters
such as experiment time or percent conversion target (step 1672), which may
also cause
reactor control system 1500 to send new control signals to reactor 1506 (steps
1672, 1670,
1664).
to Data analysis module 1514 performs appropriate calculations on the sampled
data
(step 1674), as will be discussed below, and the results are displayed on
monitor 1510 (step
I 676). Calculated results and/or sampled data can be stored in data storage 1
S 12 for later
display and analysis. Reactor control system 1500 determines whether the
experiment is
complete-for example, by determining whether the time for the experiment has
elapsed (step
is 1678). Reactor control system 1500 can also determine whether the reaction
occurring in one
or more of reactor vessels 1542 has reached a specified conversion target
based on results
calculated in step 1674; in that case, reactor control system 1500 causes the
addition of a
quenching agent to the relevant reactor vessel or vessels as discussed above,
terminating the
reaction in that vessel. For any remaining reactor vessels, reactor control
system 1500
20 samples additional data (step 1666) and the cycle begins anew. When all
reactor vessels 1542
in reactor block 1540 have reached a specified termination condition, the
experiment is
complete (step 1680). The user can also cause the reaction to terminate by
aborting the
experiment at any time. It should be recognized that the steps illustrated in
Fig. 58 are not
necessarily performed in the order shown; instead, the operation of reactor
control system
25 1500 can be event driven, responding, for example, to user events, such as
changes in reaction
parameters, or system generated periodic events.
Anaiysis of Experimental Data
The type of calculation performed by data analysis module I 514 (step 1674)
depends
on the nature of the experiment. As discussed above, while an experiment is in
progress,
3o reactor control system 1500 periodically receives temperature, pressure
and/or torque data
-58--
CA 02419700 2003-02-19
from reactor 1506 at sampling rates set by the user (step 1666). System
control module 1508
and data analysis module 1514 process the data for use in screening materials
or for
performing quantitative calculations and for display by user interface module
I5I6 in formats
such as those shown in Fig. 63-64 and 65.
Reactor control system I 500 uses temperature measurements from temperature
sensors 1600 as a screening criteria or to calculate useful process and
product variables. For
instance, in one implementation, catalysts of exothermic reactions are ranked
based on peak
reaction temperature reached within each reactor vessel, rates of change of
temperature with
respect to time, or total heat released over the course of reaction.
Typically, the best catalysts
to of an exothermic reaction are those that, when combined with a set of
reactants, result in the
greatest heat production in the shortest amount of time. In other
implementations, reactor
control system 1500 uses temperature measurements to compute rates of reaction
and
conversion.
In addition to processing temperature data as a screening tool, in another
implementation, reactor control system 1500 uses temperature measurement-
combined with
proper thermal management and design of the reactor system-to obtain
quantitative
calorimetric data. From such data, reactor control system 1500 can, for
example, compute
instantaneous conversion and reaction rate, locate phase transitions (e.g.,
melting point, glass
transition temperature) of reaction products, or measure latent heats to
deduce structural
2o information of polymeric materials, including degree of crystaIlinity and
branching. For
details of calorimetric data measurement and use, see description accompanying
Fig. 9 and
equations I-V.
Reactor control system 1500 can also monitor mixing variables such as applied
stirring blade torque in order to determine the viscosity of the reaction
mixture and related
properties. Reactor control system 1500 can use such data to monitor reactant
conversion and
to rank or characterize materials based on molecular weight or particle size.
See, for
example, the description of equations VI-VIII above.
Reactor control system 1500 can also assess reaction kinetics by monitoring
pressure
changes due to production or consumption of various gases during reaction.
Reactor control
3o system 1500 uses pressure sensors 1630 to measure changes in pressure in
each reactor vessel
headspace-the volume within each vessel that separates the liquid reagents
from the vessel's
-59--
CA 02419700 2003-02-19
sealed cap. During reaction, any changes in the head space pressure, at
constant temperature,
reflect changes in the amount of gas present in the head space. As described
above (equation
XI), reactor system 1500 uses this pressure data to determine the molar
production or
consumption rate, n, of a gaseous component:
s Operation of a Reactor Control Svstem
Refernng to Fig. 59, reactor control system 1500 receives system configuration
information from the user through system configuration window 1700, displayed
on monitor
1510. System configuration window l 700 allows the user to specify the
appropriate
hardware components for an experiment. For example, the user can choose the
number of
1 o motor cards l 580 and the set a number of motor axes per card in motor
pane 1702.
Temperature controller pane 1704 allows the user to select the number of
separate
temperature controllers 1604 and the number ofreactor vessels (the number
offeedback
control loops) per controller. In pressure sensor pane 1706, the user can set
the number of
pressure channels corresponding to the number of reactor vessels in reactor
1506. The user
15 can also view the preset safety limits for motor speed, temperature and
pressure through
system configuration window 1700.
As shown in Fig. 60, reactor control system I 500 receives data display
information
from the user through system option window l 730. Display interval dialog 1732
lets the user
set the refresh interval for data display. The user can set the number of
temperature and
2o pressure data points kept in memory in data point pane 1734.
At any time before or during an experiment, the user can enter or modify
reaction
parameters for each reactor vessel 1 S42 in reactor block 1540 using reactor
setup window
1760, shown in Fig. 61. In motor setup pane 1762, the user can set a motor
speed (subject to
any preset safety limits), and can also select single or dual direction motor
operation. The
2s user can specify temperature parameters in temperature setup pane 1764.
These parameters
include temperature set point 1766, tum off temperature 1768, sarnpIing rate I
770, as well as
the units for temperature measurement and temperature controller operation
modes. By
selecting gradient button 1772, the user can also set a temperature gradient,
as will be
discussed below. Pressure parameters; including a pressure set point and
sampling rate, can
30 be set in pressure setup pane I774. Panes 1762, 1764 and 17?4 can also
display safety limits
--do-
CA 02419700 2003-02-19
for motor speed, temperature and pressure, respectiveiy. The values
illustrated in Fig. 61 are
not intended to limit this invention and are illustrative only. Reactor setup
window 1760 also
lets the user set a time for the duration of the experiment. Reactor setup
window 1760 lets
the user save any settings as defaults for future use, and load previously
saved settings.
Fig. 62 illustrates the setting of a temperature gradient initiated by
selecting gradient
button 1772. In gradient setup window I 800, the user can set a temperature
gradient across
reactor 1506 by entering different temperature set points I 802 for each
reactor block module
of a.multi-block reactor 1506. As with other setup parameters, such
temperature gradients
can be saved in reactor setup window 1760.
Referring to Fig. 63, the user can monitor an experiment in reaction window
1830.
System status pane 1832 displays the current system status, as well as the
status of the
hardware components selected in system configuration window 2700. Setting pane
1834 and
time pane 1836 display the current parameter settings and time selected in
reactor setup
window 1760, as well as the elapsed time in the experiment. Experimental
results are
displayed in data display pane 1838, which includes two dimensional array 1840
for
numerical display of data points corresponding to each reactor vessel I 542 in
reactor 1506,
and graphical display 1842 for color display of the data points displayed in
array 1840. Color
display 1842 can take the form of a two dimensional array of reactor vessels
or three
dimensional color histogram I 870, shown in Fig. 64. The coior range for
graphical display
2o 1842 and histogram 1870 is displayed in legends 1872 and 1874,
respectively. Data display
pane 1838 can display either temperature data or conversion data calculated
from pressure
measurements as described above. In either case, the displayed data is
refreshed at the rate
set in the system options window I730.
By selecting an individual reactor vessel 1542 in data display pane 1838, the
user can
view a detailed data window 1900 for that vessel, as shown in Fig. 65. Data
window 1900
provides a graphica3 display of experimental results, including, for example,
temperature;
pressure, conversion and molecular weight data for that vessel for the
duration of the
experiment.
Referring again to Fig. 64, toolbar 1876 lets the user set reactor parameters
(by
3o entering reactor setup window 1760) and color scaling for color displays
1842 and 1870. The
user can also begin or end an experiment, save results and exit system 1500
using toolbar
CA 02419700 2003-02-19
1876. The user can enter any observations or comments in comment box 1878.
User
comments. and observations can be saved with experimental results.
Referring to Fig. 65, the user can set the color scaling for color displays
1842 and
1870 through color scaling window 1920: Color scaling window 1920 lets the
user select a
color range corresponding to temperature or conversion in color range pane
1922. The user
can also set a color gradient, either linear or exponential, through color
gradient pane 1924.
Color scaling window 1920 displays the selected scale in color legend 1926.
The invention can be implemented in digital electronic circuitry, or in
computer
hardware, firmware, sofrware, or in combinations of them. Apparatus of the
invention can be
to implemented in a computer program product tangibly embodied in a machine-
readable
storage device for execution by a programmable processor; and method steps of
the invention
can be performed by a programmable processor executing a program: of
instructions to
perform functions of the invention by operating on input data and generating
output. The
invention can be implemented advantageously in one or more computer programs
that are
15 executable on a programmable system including at least one programmable
processor coupled
to receive data and instructions from, and to transmit data and instructions
to, a data storage
system, at least one input device, and at least one output device. Each
computer program can
be implemented in a high-level procedural or object-oriented programming
language, or in
assembly or machine language if desired; and in any case, the language can be
a compiled or
2o interpreted language.
Suitable computer programs in modules 1508 and 1514 can be implemented in
classes
as set forth in the following tables. (The prefix "o" in a name indicates that
the corresponding
property is a user-defined object; the prefix "c" in a name indicates that the
corresponding
property is a collection.}
25 1. Application class
Property Table:
Category Name Access Description/ Comments
General ClsName Get Class name
AppName Get Application name
-62-
CA 02419700 2003-02-19
Category Name Access Description/ Comments
sRootDir Get/Let Root directory of all
system files
bDebugMode Get/Let System running mode. If
TRUE;
display message boxes
for errors in
addition to error logging.
If
FALSE, log the error to
the log f Ie
DBIsConnected Get/Let Whether database is connected
System SectionGeneral Get General section
Registry
SectivnSystemLimitsGet Section for System Limit
Values
SectionDefaultParamGet Section for system default
parameters
Coloi'ScalingoTempScale Get Color Scale object for
temperature
data
oViscosityScaleGet Color Scale object for
viscosity data
oConversionScaleGet Color Scale object for
conversion
data
oMWScale Get Color Scale object for
molecule
weight data
Method Table:
Name Argument List Return TypeDescription/Comments
SaveCnfg Boolean Save application configurations
to
the system registry
2. CoIorScale class
--63-
CA 02419700 2003-02-19
Parent Class: Application
Property Table:
Name Access Description/Comments
ClsName Get Class name
Highest Get/Let Highest value
GradientTypeGetfLet Type of the gradient between the lowest
and highest to the
log file
LegendValuesGet A collection of legend values
Method Table:
Name Argument Return Description/Comments
List Type
SetLegendValues Recalculate the legend
values
according to the current
property
values
GetLegendColor(Value long Get color of the specified
data
value
3. ColorLegend class
Parent Class: ~ColorScale
Property Table:
Name Access DescriptionlComments
CIsName Get Class Name
ColorCountGet Number of colors used in the legend
Method Table:
Narae Argument List Return Type Description/Comments
-_
CA 02419700 2003-02-19
Name Argument ListReturn TypeDescripteon/Commet~ts
GetColorValuefValue long Get color for the specified
data
value
---65-
CA 02419700 2003-02-19
4. System class
Property Table:
Category Name Access Description/Comments
General CIsName Get
ExpID
System Status Status Get/Let Status variable
STATUS OFF Get constant
STATUS RUN Get constant
STATUS IDLE Get constant
STATUS ERROR Get constant
System Timing oExpTiming Get Control and record the
experiment time
oDisplayTimingGet Control the data display
updating rate
System AlarmingoAlarnn Get Provide alarm when system
error
occurs
System ComponentsoMotors Get
oHeaters Get
oPressures Get
-...6G---
CA 02419700 2003-02-19
Method Table:
Name Argument ListReturn Type Description/Camments
Run
StopRunning
Archive
5. ExpTiming class
Parent Class: System
Property Table:
Name Access DescriptionlComments
ClsName Get Class Name
TimingByTime Get/Let Boolean type
TimingByPressureGetILet Boolean type
TimingByTemperatureGet/Let Boolean type
TargetTime Get/Let System will stop if specified
target value is
achieved
TargetPressure Get/Let System will stop if specified
target value is
achieved
TargetTemperatureGet/Let System will stop if specified
target value if
achieved
ExpDate Get/Let Date when experiment starts to
run
ExpStartTime Get/Let Time when experiment starts to
fun
ExpEndTime Get/Let Time when experiment stop running
ExpElapsedTime Get/Set The time passed during the experiment
~ ~
-b7-
CA 02419700 2003-02-19
Name Access DescriptionlComments
Timerinterval Let Timer used to update the elapsed
time
Method Table:
Name Argument Return Type Description
List
LoadDefaultExpTiming Boolean
SaveDefauItExpTiming Boolean
6. DisplayTiming class
Parent Class: System
Property Table:
Name Access Description/Comments
ClsName Get Class Name
DispIayTimerGet/Set Timer used to update the data
TimerIntercalGet/Let
Method Table:
Name Argument List Return Type Description
SaveDefaultParam Boolean
-6s-
CA 02419700 2003-02-19
7. Alarm class
Parent Class: System
Property Table:
Name Access Description/Comrnents
CIsName Get Class Name
BeepTimer Set Timer used to control beep
PauseTimer Set Timer used to pause the beep
BeepStatus Get A Boolean value: FALSE if paused, otherwise
TRUE
BeepPauseTimeLet Time duration for beep to pause
Method Table:
Name Argument.I,istReturn TypeDescription
TurnOnBeep Start to beep
TurnOffBeep Stop beeping
BeepPause Disable beep
BeepResume Enable beep
8. Motors class
Parent Class: System
Property Table:
Name Access DescriptionlComments
ClsName Get Class Name
SpeedLimit GetlLet Safety Limit
MotorIsOn GetlLet Status variable
---69-
CA 02419700 2003-02-19
Name Access DescriptionlComments
CardlAxesCountGet/Let Axes count in cardI
Card2AcesCountGetlL.et Axes count in card2
oMotorCardl Get Motor card object
oMotorCard2 Get Motor card object
oSpinTimer Get/Set Timer for dual spin
FoundDLL Get Motion DLL
ErrCade Get Error code
Method Table:
Category Name Argument Return Description
List Type
To/From system LoadDefaultParam Boolean
Registry
SaveDefaultParam Boolean
SaveCardAxesCount Boolean
SaveSystemLimit Boolean
CreatelDelete CardCreateCardl iAxesCount
Objects
CreateCard2 iAxesCount
DeleteCardl
DeleteCard2
-70-
CA 02419700 2003-02-19
Category Name Argument Return Description
List Type
Motor Control Init BooleanFor all
axes
Spin iAxis, Boolean
dSpeed
run BooleanFor aII
axes
StopRunning BooleanFor all
axes
Archive ArchiveParam iFileNo Boolean
MotorAxis class
Parent Class: Motors
Property Table:
Name Access Descrigtion/Comments
ClsName Get Class Name
Parent Set Reference to the parent object
MotorID Get/Let Motor Axis ID
oGurParam Get Reference to current parameter setting
Method Table:
Name Argument Return Type. Description
List
GetParamSettingjindexj MotorPararnReturn the last in the
parameter
collection
Run Boolean Add oCurParam to the
Param
-71-
CA 02419700 2003-02-19
Name Argument Return TypeDescription
Lisi
collection, and run
this motor axis
10. MotorParam class
Parent Class: Motors
Property Table:
Name Access Description/Comments
clsName Get Class Name
Parent Set Reference to the parent object
MotionType Get/Let Dual or single direction spin
DeltaT Get/Let Time duration before changing spin
direction
SpinRate GetlLet Spin rate in RPM
EffectiveTimeGetlLet Time the parameters take effect
Method Table:
Na~ue Argument ListReturn Type Description
PrintParamiFileNo Boolean Print the parameters
to file
I 1. Heaters class
Parent Class: System
Property Table:
Name Access Description/Comments
ClsName Get Class Name
oParent Get Reference to the parent object
TempLimit Get/Let Temperature Safety Limit
-72-
CA 02419700 2003-02-19
Name Access DescriptionlComments
SplRateLimit GetlLet Sample Rate Limit
CtIrLoopCount Get/Let Loop count in controllerl
CtlrLoopCount GetlLet Loop count in controller2
HeaterIsOn Get/Let Status variable
oHeaterCtlrl Get Heater controller object as clsHeaterCtlr
oHeaterCtlr2 Get Heater controller object as clsHeaterCtlr
oData Get Data object as clsHeaterData
lDataPointsInMemGet/Let Number of data paints kept in memory
FoundDLL Get RS232 DLL. If found, 1, otherwise
-1
ErrCode Get Error Code
Method Table:
Category Name Argument Return Descriptions
List Type
To/From system LoadDefaultParam Boolean
Registry
SaveDefaultParam Boolean
SaveCtlrLoopCount Boolean
SaveSystemLimit Boolean
Create/Delete Create Ctlr aLoopCount
Ctlr 1
Objects
-73-
CA 02419700 2003-02-19
Category Name Argument Retnrn Descriptions
List Type
Create Ctlr ~LoopCount
2
Delete CtIr
I
Delete Ctlr
2
Heater Control Init Boolean Open
COM I ,COM2
OutputHeat Boolean For all loops
Turnoff Boolean For all loops
GetTemp Boolean For all loops
SafetylVlonitorIcount,vData Check
Temperature
SafetyHandler
Archive ArchiveParam iFileNo Boolean
12. HeaterCtlr class
Parent Class: Heaters
Property Table:
Name Access Description/Comments
ClsName Get Class Name
Parent ~Set' ( Reference to the parent object
-74-
CA 02419700 2003-02-19
Name Access Description/Comments
oCurParamGet Reference to current parameter setting
Method Table:
Name Argument Return Description
List Type
AddParanlSettingoParam Boolean Add the parameter object
to the
parameter collection
GetParamSetting[index) HeaterParamReturn the last in the
parameter collection
13. HeaterParam class
Parent Class: HeaterCtlr
Property Table:
Name Access DescriptionlComments
clsName Get Class Name
Parent Set Reference to the parent object
Setpoint Get/Let Setpoint for temperature
SplRate GetILet Sampling Rate (Hz)
EffectiveTimeGet/Let Time the parameters take effect
-75-
CA 02419700 2003-02-19
Method Table:
Name Argument List Return Type Description
PrintParamiFileNo Boolean Print the parameters
to file
14. HeaterData class
Parent Class: Heaters
Property Table:
Name Access DescriptionlComments
clsName Get Class Name
Parent Set Reference to the parent object
DataPointsInMemLet
LoopCount Let Total Ioop count
DataCount Get Data point count
crime Get Get time data collection
cTemp Get Get teraperature data collection
Method Table:
Name Argument Return Description
List Type
GetData ByRef fTime,Boolean Get current data set ,
or the data set
ByRef vTemp with specified index
[,index)
AddData fTime, vTemp Add the data set to the
data collections
ClearData Clear the data collection
CA 02419700 2003-02-19
Name Argument Return Description
List ' Type
WriteToDisk Write the current data
to disk fle
1 S. Pressures class
Parent Class: System
Property Table:
Name Access Description/Comments
ClsName Get Class Name
oParent Get Reference to the parent object
PressureLimit GetlLet Pressure Safety Limit
SpiRateLimit Get/Let Sample Rate Limit
ChanneICount Get/Let Analog Input channel count
PressureIsOn GetlLet Status variable
oData Get Data object as clsPressureData
1 DataPointsInlVIemGet2et Number of data points kept in memory
oCWAOP Get Object of analog output ActiveX
control
oCWAIP Get Object of analog input ActiveX control
ErrCode Get Error code
CA 02419700 2003-02-19
Method Table:
Category Name ArgumentReturn Description
List Type
ToIFrom System LoadDefaultParam Boolean
Registry
SaveDefaultParam Boolean
SaveChannelCount Boolean
SaveDataPointsInMem
SaveSystemLimit Boolean
Pressure System AnalogOutput BooleanOutput Pset
Control ,
GetAIData BooleanAnalog Input
Archive ArchivePararn iFileNo Boolean
16. PressureParatn class
Parent Class': Pressures
Property Table:
Name Access Description/Comments
cIsName Get Class Name
Parent Set Reference to the parent object
Setpoint Get/Let Setpoint for pressure (psi)
SplRate Get/Let Sampling Rate (Hz}
-7s-
CA 02419700 2003-02-19
Name Access Description/Comments
Ef~ectiveTimeGetlLet Time the parameters take effect
Method Table:
Name Argument Return TypeDescription
List
PrintParam iFileNo Boolean Print the parameters
to the file
I7. PressureData class
Parent Class: Pressures
Property Table:
Name Argument Access Description/Comments
clsName Get Class Name
Parent Set Reference to the parent
object
DataPointsInMem Let
ChannelCount Let Total AI channel count
PresCount Get Pressure data point count
ConvCount Get Conversion data point count
cPresTime Get Get time collection for
pressure data
cPressure Get Get pressure data collection
cConvTime iChannelNoGet Get time collection for
conversion data
cConversion iChannelNoGet Get conversion data collection
-79-
CA 02419700 2003-02-19
Method Table:
Name Argument Return Description
List Type
GetCurPres ByRef vl'resBoolean Get current pressure
data set
GetCurConv ByRef vConvBoolean Get current conversion
data set
AddPres - fTime, vPres Add the pressure data
set to the
pressure data collections,
then
calculate conversions
ClearData Clear all the data collections
WritePresToDisk Boolean Write the current pressure
data
to disk file
WriteConvToDisk Boolean Write the current conversion
data to disk file
18. ErrorHandier class
Property Table:
Name Access Description/Comments
ClsName Get Class Name
LogFile GetJLet Log file for error messages
Method Table:
Name Argument Return TypeDescription
List
SaveConfg Boolean
OpenLogFileiFileNo Boolean Open Iog file with specified
file
number for APPEND, Lock
WRITE
-80-
CA 02419700 2003-02-19
Name Argument Return TypeDescription
List
OpenLogfileiFileNo Boolean Open log file with specified
file
number for .APPEND; lock
WRITE
CIoseLogFile
LogError sModName, Write error messages to
the log file,
sFuncName, also call DisplayF,rror
in debug mode'
iErrNo,
sErrText
DisplayErrorsModName, Show messageBox to display
the
sFuncName, error
iErrNo,
sErrText
Suitable processors include, by way of example, both general and special
purpose
microprocessors. Generally, a processor will receive instructions and data
from a read-only
memory andlor a random access memory. Storage devices suitable for tangibly
embodying
computer program instructions and data include all forms of non-volatile
memory, including
by way of example semiconductor memory devices, such as EPROM, EEPROM, and
flash
memory devices; magnetic disks such as internal hard disks and removable
disks;
magneto-optical disks; and CD-ROM disks. Any of the foregoing can be
supplemented by,
or incorporated in, ASICs application-specific integrated circuits).
To provide for interaction with a user, the invention can be implemented on a
to computer system having a display device such as a monitor or LCD screen for
displaying
information to the user and a keyboard and a pointing device such as a mouse
or a trackball
by which the user can provide input to the computer system. The computer
system can be
programmed to provide a graphical user interface through which computer
programs interact
with users.
An example of one such type of computer is shown in Fig. 67, which shows a
block
diagram of a programmable processing system 1950 suitable for implementing or
performing
-81-
CA 02419700 2003-02-19
the apparatus or methods of the invention. The system 1950 includes a
processor 1952, a
random access memory (RAM) 1954, a program memory 1956 (for example, a
veritable read-
only memory (ROM) such as a flash ROM), a hard.drive controller 1958, and an
input/output
(1/O) controller 1960 coupled by a processor (CPtJ) bus 1962. The system 1950
can be
preprogrammed, in ROM, for example, or it can be programmed (and
reprogt~mnmed) by
loading a program from another source (for example, from a floppy disk, a CD-
ROM; or
another computer).
The hard drive controller 1958 is coupled to a hard disk 1964 suitable for
storing
executable computer programs, including programs embodying the present
invention, and
t o data including the images, masks, reduced data values and calculated
results used in and
generated by the invention. The UO controller 1960 is coupled by means of an
UO bus 1966
to an UO interface 1968. The 1/O interface 1968 receives and transmits data in
analog or
digital form over communication links such as a serial link, local area
network, wireless link,
~d parallel link. Also coupled to the UO bus 1966 is a display 1970 and a
keyboard 1972.
Alternatively, separate connections (separate buses) can be used for the UO
interface 1966,
display 1970 and keyboard 1972.
The invention has been described in terms ofparticular embodiments. Other
embodiments are within the scope of the following claims. Although elements of
the
invention are described in terms of a software implementation, the invention
may be
2o implemented in software or hardware or. firmware, or any combination of the
three. In
addition, the steps of the invention can be performed in a different order and
still achieve
desirable results.
Moreover, the above description is intended to be illustrative and not
restrictive.
Many embodiments and many applications besides the examples provided will be
apparent to
those of skill in the art upon reading the above description. The scope of the
invention should
therefore be determined, not with reference to the above description, but
should instead be
determined with reference to the appended claims, along with the full scope of
equivalents to
which such claims are entitled. '
-82-