Note: Descriptions are shown in the official language in which they were submitted.
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
6-O-SUBSTITUTED ERYTHROMYCIN DERIVATIVES HAVING IMPROVED
GASTROINTESTINAL TOLERANCE
Technical Field
This invention relates to novel semi-synthetic erythromycin derivatives having
antibacterial activity, compositions containing the compounds, and methods of
treatment
using the compounds. These compounds have a lower incidence of GI irritation
than the
erythromycin derivatives of the prior art.
Background of The Invention
The escalation of resistance to antibiotics once useful for treatment of
bacterial
infections resulting from pathogens such as Staphylococcus aureus is
problematic in the
United States and Europe (Drugs Exp. Clin. Res., XX, 215-224 (1994); Am. J.
Sung., SA
(Suppl.), 8S-12S (1995); Df°ugs, 48, 678-688 (1994); and Current
Pharmaceutical Design,
2(2), 175-194 (1996)). Thus, the development of new broad spectrum synthetic
and semi-
synthetic antibacterial compounds is the subject of constant current research.
Reference is made to commonly owned United States patent No. 5,866,549 and PCT
application WO/21871, published May 6, 1999, each of which teachs 6-O-
substituted
ketolide antibacterial compounds; although neither reference teaches by
specific example
combinations wherein the 6-O-substituted group is lower alkenyl or lower
alkynyl wherein
the lower alkynyl or lower alkenyl is substituted with an isoxazole, oxazole,
or isothiazole
substituent. While the compounds described in these applications represent an
advance in
antibacterial therapy, they suffer from the side effect of gastrointestinal
intolerance typically
associated with erythromycins (cf. Pilot and Williams, Mac~olides: Chemistfy,
Pharmacology and Clinical Uses; Briskier, Neu, and Tulkens, Eds.; Blackwell:
Paris, 1993;
pp. 659-673; and Itoh et al., Antimic~ob. Agents ChernotlZer., 26, 863-869
(1984)).
Thus, erythromycin derivatives which produce a lower incidence of
gastrointestinal
intolerance and the side effects associated therewith, such as nausea and
vomiting, would
represent an important contribution to the art.
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Summary of the Invention
In its principle embodiment, therefore, the instant invention provides a
series of 6-O-
substituted ketolides with an unexpectedly improved gastrointestinal
tolerability profile, the
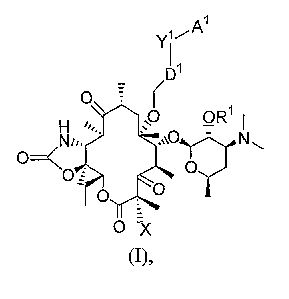
ketolides having structural formula (I)
1
Y1~A
D1
O
. ;~O OR1I
Nu, ,vO~N~
O X
(I),
or a therapeutically acceptable salt or prodrug thereof, wherein
to X is selected from hydrogen and fluoride;
D1 is selected from CH=CH or C=C;
YI is selected from isoxazole, oxazole, isothiazole, dihydroisoxazole, and
dihydro-oxazole;
Al is selected from aryl and heteroaryl; and
Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting group.
In another embodiment, the invention provides any compound, including
metabolic
precursors of the inhibitor compounds, which contain an essential inhibitory
group as
disclosed herein. These inhibitory groups can be in masked form or in
therapeutically
effective prodrug form and can be converted or released by metabolic or other
processes after
administration to a patient.
In yet another embodiment, the invention provides compositions comprising the
compounds in combination with a therapeutically acceptable excipient.
In still yet another embodiment, the invention provides a method of treating
bacterial
infections the method comprising administering a therapeutically effective
amount of a
compound
having structural formula (I)
-2-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
O
(I),
or therapeutically acceptable salt or prodrug thereof, wherein
X is selected from hydrogen and fluoride;
D1 is selected from CH=CH or C--__C;
Yl is selected from isoxazole, oxazole, isothiazole, dihydroisoxazole, and
dihydro-oxazole;
A1 is selected from aryl and heteroaryl; and
to Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting
group.
In still yet another embodiment, the invention provides a method for the
preparation
of the compounds of formula (Ia)
A1
1~
Y
O
,O OR1 I
Nu. ~ .,v0 N w
~O
X
O
1 s (Ia),
or a therapeutically acceptable salt or prodrug thereof, wherein
X is selected from hydrogen and fluoride;
Yl is selected from isoxazole, oxazole, isothiazole, and isothiazole;
2o A1 is selected from aryl and heteroaryl; and
Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting group,
the method comprising:
(a) reacting a compound of formula (vi)
-3-
1
Y1~A
p1
_ /
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
O
1!
,~O OR
,~0~ N ~
O
O X
(vi),
wherein
X is selected from hydrogen and fluoride, and
Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting
group,
with a compound of formula (vii)
1-Y1-A1
(vii),
wherein
Yl is selected from isoxazole, oxazole, and isothiazole, and
A1 is selected from aryl and heteroaryl,
a base, a coupling catalyst, and, optionally, an additive;
and
(b) optionally deprotecting the product of step (a). .
In still yet another embodiment, the invention provides a method for the
preparation
of the compounds of formula (Ib)
p-N
w
O X
(Ib),
or a therapeutically acceptable salt or prodrug thereof, wherein
X is selected from hydrogen and fluoride;
-4-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
A1 is selected from aryl and heteroaryl; and
Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting group,
the method comprising:
(a) reacting a compound of formula (x)
O
,O ORS I
Nn,~~~,.vO~N ~
X
(x),
wherein
to X is selected from hydrogen and fluoride, and
Rl is selected from hydrogen and Rp wherein Rp is a hydroxyl protecting
group,
with a compound of formula (ii)
NON
\/ A1
CI
(ii),
wherein
A1 is selected from aryl and heteroaryl,
2o and a base;
and
(b) optionally deprotecting the product of step (a).
In a preferred embodiment of the compound of formula (x),
X and Rl are hydrogen;
X is hydrogen and Rl is Rp wherein Rp is acetyl;
X is hydrogen and Rl is Rp wherein Rp is benzoyl;
X is fluoride and Rl is hydrogen;
X is fluoride and Rl is Rp wherein Rp is acetyl; and
X is fluoride and Rl is Rp wherein Rp is benzoyl.
-5-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Detailed Description of The Invention
The instant compounds are substituted ketolide antibiotics of formula (I)
Y~iA
O
ORS
6.
., ,aO~N~
>r
O X
s (I),
which have been numbered at the C-2 and C-6 positions for illustrative
purposes. The
compounds contain a number of asymmetric centers and optional substitution of
the
hydrogen atom at C-2 by a fluorine atom. Each variable substituent at C-2 is
represented by
l0 X. In a preferred embodiment for the practice of the invention, X is
hydrogen or fluoride. In
a particularly preferred embodiment, X is fluoride.
D1 can also vary without departing from the intent of the invention and can be
C2-
alkynylene or C2-alkenylene, the latter of which provides geometric isomers of
the
compounds. The invention contemplates the various geometric isomers and
mixtures thereof
15 which result from the disposal of substituents around a carbon-carbon
double bond.
Substituents around a carbon-carbon double bond are designated as being of Z
or E
configuration, wherein the term "Z" refers to higher order substituents on the
same side of the
carbon-carbon double bond, and the term "E" refers to higher order
substituents on opposite
sides of the carbon-carbon double bond. A thorough discussion of E and Z
isomerism is
20 provided in J. March, Advanced Organic Chemistry. Reactions, Mechanisms,
and Structure,
4th ed., John Wiley & Sons, New York, 1992, pp. 109-I 12. Accordingly, it will
be
appreciated by a skilled practioner that compounds of formula (Ia)
A~
- ~Y
N~
25 (Ia),
-6-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compounds of formula Z-(Ic)
A~-Y~
ORS I .
> N~
O
px
Z-(Ic),
and compounds of formula E-(Ic)
~Y~_A~
O
;O ORS
N~~, ,vO~N~
X
1 o E-(Ic),
and therapeutically acceptable salts or prodrugs thereof, are contemplated as
being within the
scope of the invention. In a preferred embodiment for the practice of the
invention, Di is C2-
alkynylene, as exemplified by compounds of formula (Ia).
The compounds further comprise substituted heteroarylene or heterocyclene
rings,
represented by Yl, connected to the parent molecular group through groups
represented by D1
and substituted by groups represented by Al. The groups represented by Yl are
stable, 5-
membered, diradical rings containing one nitrogen atom, one atom selected from
oxygen and
sulfur, and the remaining atoms are carbon. The rings are connected to D1 and
are substituted
2o by A1 through substitutable carbons. For combinations within Yl which
contain nitrogen and
oxygen atoms, the heteroatoms, i.e. non-carbon atoms, can be in adjacent or
non-adjacent
positions. For combinations within Yl which contain nitrogen and sulfur atoms,
the
heteroatoms are in adjacent positions. In each of the aformentioned cases, the
rings can
contain one or two double bonds. In a preferred embodiment for the practice of
the
invention, Yl is a five membered ring with two double bonds and a nitrogen and
oxygen
atom in adjacent positions, i. e. isoxazole, the structure and atom numbering
of which is
shown directly below for illustrative purposes.
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
1
~O~N 2
4 3
In a particularly preferred embodiment, the isoxazole ring is substituted by
A1 and D1
on the C-3 and C-5 positions, respectively, to provide a isoxazol-3,5-diyl.
Accordingly,
taking the listing of preferred substituents and combinations thereof, it will
be appreciated by
a skilled practioner that compounds of formula (Ib)
O-N
O
,O ORS I
,,v0 N ~
O~ O
O
O
O X
(Ib),
and therapeutically acceptable salts or prodrugs thereof, are contemplated as
being within the
scope of the invention.
A1 can also vary considerably without departing from the intent of the
invention and
can be aryl or heteroaryl. Preferred embodiments of A1 include unsubstituted
or substituted
monocyclic, aromatic groups such as phenyl, pyridyl, pyrimidinyl, thienyl,
thiazolyl,
tetrazolyl, and the like, and unsubstituted or substituted bicyclic, aromatic
groups such as
quinolinyl, benzothienyl, imidazo(2,1-b)thiazolyl, and the like. Each of the
aformentioned
groups, represented by Al, are connected to Yl through substitutable carbon
atoms in the
2o ring. Thus, Yl substituents such as, for instance, and by way of example
only, pyrid-2-yl,
pyrid-3-yl, pyrid-4-yl, pyrirnidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, and
the like, are
contemplated as being within the scope of the invention. A preferred
embodiment for the
practice of the invention is unsubstituted pyridyl, and a particularly
preferred embodiment is
unsubstituted pyrid-2-yl. Accordingly, taking the listing of preferred
substituents and
combinations thereof, it will be appreciated by a skilled practioner that
compounds of
formula (Ii)
-g-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
O-N T--U
~V
.-.
O
;~O ORS I
Nn. ,vO~N~
X
(Ii),
and therapeutically acceptable salts or prodrugs thereof, wherein one of T, U,
and V is
nitrogen and the remainder are carbon; and, more specifically, compounds of
formula (Ii)
wherein T is nitrogen and U and V are each carbon, are contemplated as being
within the
scope of the invention.
It is believed that when the compounds have attached thereto a hydroxyl,
amino, or
carboxylic acid group, prodrugs can be prepared from the compounds by
attaching thereto a
prodrug-forming group such as, but not limited to, include. carboxyl,
hydroxyl, and amino
protecting groups. These prodrugs can then be rapidly transformed in vivo to
the parent
compound, such as, for example, by hydrolysis in blood. The term
"therapeutically
acceptable prodrug," means those prodrugs of the compounds which are suitable
for use in
contact with the tissues of humans and lower animals with undue toxicity,
irritation, allergic
i5 response, and the like, commensurate with a reasonable benefit/risk ratio,
and effective for
their intended use, as well as the zwitterionic forms, wherein possible, of
the compounds.
As used in the specification, the following terms have the meanings assigned:
The term "additive," means monodentate phosphorus-containing ligands of
formulas
P(R°)3 (phosphines), P(ORd)3 (phosphites) and As(R°)3 (arsines),
wherein each R° is
independently hydrogen; alkyl such as methyl, ethyl, and tert-butyl;
cycloalkyl such as
cyclopropyl and cyclohexyl; optionally substituted aryl such as phenyl,
naphthyl, and ortho-
tolyl; and optionally substiuted heteroaryl such as furyl and pyridyl; and
wherein each Rd is
independently alkyl such as methyl, ethyl, and tent-butyl; cycloalkyl such as
cyclopropyl and
cyclohexyl; optionally substituted aryl such as phenyl, naphthyl, and ortho-
tolyl; and
optionally substituted heteroaryl such as furyl and pyridyl. Specific examples
of these
additives include tri(alkyl)phosphines such as trimethylphosphine,
triethylphosphine,
tributylphosphine, and the like; tri(cycloalkyl)phosphines such as
tricyclopropylphosphine,
tricyclohexylphosphine, and the like; tri(aryl)phosphines such as
triphenylphosphine,
trinaphthylphosphine, and the like; tri(heteroaryl)phosphines such as tri(fury-
2-yI)phosphine,
3o tri(pyrid-3-yl)phosphine, and the like; tri(alkyl)phosphites such as
trimethylphosphite,
triethylphosphite, tributylphosphite, and the like; tri(cycloalkyl)-phosphites
such as
-9-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
tricyclopropylphosphite, tricyclohexylphosphite, and the like;
tri(aryl)phosphites such as
triphenylphosphite, trinaphthylphosphite, and the like;
tri(heteroaryl)phosphites such as
tri(fury-2-yl)phosphite, tri(pyrid-3-yl)phosphite, and the like; and
triphenylarsine, and the
like. The term "additive," also means bidentate phosphines such as 1,4-
bis(diphenylphosphino)butane (dppb), 1,2-bis(diphenyl-phosphino)ethane (dppe),
1,1-
bis(diphenylphosphino)methane (dppm), 1,2-bis(dimethyl-phosphino)ethane
(dmpe), 1,1'-
bis(diphenylphosphino)ferrocene (dppfJ, and the like. The term "additive,"
also means
copper salts such as copper(I) iodide and copper(I) chloride.
The term "alkanoyl," means an alkyl group attached to the parent molecular
group
1o through a carbonyl group.
The term "alkanoyloxy," means an alkanoyl group attached to the paxent
molecular
group through an oxygen atom.
The term "alkoxy," means an alkyl group attached to the parent molecular group
through an oxygen atom.
15 The term "alkoxycarbonyl," means an alkoxy group attached to the parent
molecular
group through a carbonyl group.
The term "alkoxyalkoxy," means an alkoxy group to which attached at least one
other
alkoxy group.
The term "alkyl," means a straight or branched chain saturated hydrocarbon
radical
2o having from one to six carbon atoms.
The term "alkenyl," means a straight or branched chain hydrocarbon radical
having
from two to six carbon atoms and at least one carbon-carbon double bond.
The teen "C2-alkenylene," means a diradical formed by the removal of a
hydrogen
atom from each carbon atom of ethylene.
25 The term "alkynyl," means a straight or branched chain hydrocarbon radical
having
from two to six caxbon atoms and at least one carbon-carbon triple bond.
The term "C2-alkynylene," means a diradical formed by the removal of both
hydrogen
atoms from acetylene.
The term "amino," means -NH2 or derivatives thereof formed by independent
3o replacement of one or both hydrogen atoms thereon with a substituent or
substituents
independently selected from alkyl, alkanoyl, aryl, arylalkyl, cycloalkyl,
cycloalkylalkyl,
heteroaxyl, heteroarylalkyl, and an amino protecting group.
The term "aminoalkyl," means an alkyl group, as defined herein, to which is
attached
at least one amino substituent.
35 The terms "amino protecting group," or "nitrogen protecting group," mean
selectively
introducible and removable groups which protect amino groups against
undesirable side
reactions during synthetic procedures. Examples of amino protecting groups
include
-10-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
methoxycarbonyl, ethoxycarbonyl, trichloroethoxycarbonyl, benzyloxycarbonyl
(Cbz),
chloroacetyl, trifluoroacetyl, phenylacetyl, benzoyl (Bn), benzyl (Bz),
dimethoxybenzyl, tert-
butoxycarbonyl (Boc), para-methoxybenzyloxycarbonyl, isopropoxycarbonyl,
phthaloyl,
succinyl, diphenylmethyl, triphenylmethyl (trityl), methanesulfonyl, para-
toluenesulfonyl,
trimethylsilyl, triethylsilyl, triphenylsilyl, and the like. Amino protecting
groups can also be
used as prodrug-forming groups.
The term "aryl," means an aromatic, carbocyclic ring containing six carbon
atoms.
The aryl group can be optionally fused to another aryl group, a cycloalkyl
group, or a
cycloalkenyl group. Aryl groups of the invention are exemplified by phenyl,
naphthyl,
l0 indenyl, indanyl, dihydronaphthyl, tetrahydronaphthyl, and the like. The
aryl groups are
connected to the parent molecular group through a substitutable carbon. The
aryl groups of
the invention can be optionally substituted with 1-5 substituents
independently selected from
alkyl, alkenyl, alkynyl, alkoxyalkoxy, amino, aminoalkyl, cyano, cyanoalkyl,
halo, haloalkyl,
vitro, perfluoroalkyl, perfluoroalkoxy, oxo, -(CH2)aC(O)R5, -(CH2)aC(O)ORS,
-(CH2)aN(RS)C(O)R5~ -(CH2)aC(O)N(R5)2~ -(CH2)aN(RS)C(O)N(RS)2~ -(CH2)aORS~
-(CH2)as02R5~ -(CH2)aSR6~ ~d -(CH2)aR7~
wherein a is zero to six;
RS is selected from hydxogen, unsubstituted or substituted alkyl,
unsubstituted or
substituted cycloalkyl, unsubstituted or substituted aryl, unsubstituted or
substituted
2o heteroaryl, and unsubstituted or substituted heterocyclyl; R6 is selected
fxom unsubstituted or
substituted alkyl, unsubstituted or substituted cycloalkyl, unsubstituted or
substituted aryl,
unsubstituted or substituted heteroaryl, and unsubstituted or substituted
heterocyclyl; and R~
is selected from unsubstituted or substituted aryl, unsubstituted or
substituted heteroaryl, and
unsubstituted or substituted heterocyclyl;
the term "substituted alkyl" means an alkyl group substituted with 1-3
substituents
independently selected from alkoxy, alkanoyloxy, alkoxycarbonyl, amino,
unsubstituted
phenyl, carboxamido, carboxy, cyano, unsubstituted cycloalkyl, halo,
unsubstituted
heteroaryl, hydroxy, vitro, perfluoroalkyl, oxo, and thioalkoxy.
the term "substituted aryl" means an aryl substituted with 1-5 substituents
3o independently selected from unsubstituted alkyl, alkenyl, alkynyl, alkoxy,
alkanoyloxy,
alkoxycarbonyl, amino, carboxamido, carboxy, cyano, cycloalkyl, halo, hydroxy,
vitro,
perfluoroalkyl, oxo, and thioalkoxy.
the term "substituted cycloalkyl" means a cycloalkyl group substituted with
one to
four substituents independently selected from unsubstituted alkyl, alkoxy,
alkanoyloxy,
alkoxycarbonyl, amino, unsubstituted phenyl, carboxamido, carboxy, cyano,
halo, hydroxy,
vitro, perfluoroalkyl, oxo, and thioalkoxy.
-11-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
the term "substituted heteroaryl" means a heteroaryl substituted with one to
four
substituents independently selected from unsubstituted alkyl, alkenyl, alkoxy,
alkanoyloxy,
alkoxycaxbonyl, amino, carboxamido, carboxy, cyano, cycloalkyl, halo, hydroxy,
nitro,
perfluoroalkyl, oxo, and thioalkoxy.
the term "substituted heterocyclyl" means a heterocyclyl group substituted
with one to
four substituents independently selected from unsubstituted alkyl, alkenyl,
alkoxy,
alkanoyloxy, alkoxycarbonyl, amino, unsubstituted phenyl, caxboxamido,
carboxy, cyano,
halo, hydroxy, nitro, perfluoroalkyl, oxo, and thioalkoxy.
The term "arylalkyl," means an alkyl group to which is attached at least one
aryl
to group.
The term "base," means reagents capable of accepting protons during the course
of a
chemical reaction. Examples of bases include carbonates such as lithium
carbonate, lithium
bicarbonate, sodium carbonate, sodium bicarbonate, potassium carbonate,
potassium
bicarbonate, cesium carbonate, and the like; phosphates such as potassium
phosphate,
15 potassium hydrogen phosphate, potassium dihydrogen phosphate, and the like;
trialkylaxnines
such as triethylamine, diisopropylethylamine, N,N,N,N-tetramethyl-1,8-
naphthalenediamine
(Proton-Sponge~), and the like; heterocyclic amines such as imidazole,
pyridine, pyridazine,
pyrimidine, pyrazine, and the like; and bicyclic amines such as 1,5-
diazabicyclo[4.3.0]non-5-
ene (DBN), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and the like. The base
chosen for a
2o particular conversion depends on the nature of the starting materials, the
solvent or solvents
in which the reaction is conducted, and the temperature at which the reaction
is conducted.
The term "carbonyl," means -C(O)-.
The term "carboxy," means -C02H.
The term "carboxy protecting group," means selectively introducible and
removable
25 groups which protect carboxy groups against undesirable side reactions
during synthetic
procedures and includes all conventional carboxy protecting groups. Examples
of carboxy
protecting groups include methyl, ethyl, n-propyl, isopropyl, l,l-
dimethylpropyl, n-butyl,
tent-butyl, phenyl, naphthyl, benzyl, diphenylmethyl, triphenylmethyl
(trityl), para-
nitrobenzyl, para-methoxybenzyl, acetylmethyl, benzoylmethyl, para-
nitrobenzoylrnethyl,
30 paxa-bromobenzoylmethyl, 2-tetrahydropyranyl 2-tetrahydrofuranyl, 2,2,2-
trichloroethyl
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, methoxymethyl,
methoxyethoxymethyl,
arylalkoxyalkyl benzyloxymethyl 1,1-dimethyl-2-propenyl, 3-methyl-3-butenyl,
allyl, and the
like. Carboxy protecting groups can also be used as prodrug-forming groups.
The term "coupling catalyst" means palladium(0) complexes such as
35 tetrakis(triphenylphosphine)palladium(0),
Iris(dibenzylideneacetone)dipalladium(0),
allylpalladium chloride dimer, dipalladium tris(dibenzylidine acetone), and
the like;
palladium(II) salts such as palladium acetate, palladium chloride, and the
like; palladium(II)
-12-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
complexes such as dichlorobis(triphenylphosphine)palladium(II), (l,l'-
bis(diphenylphosphino)ferrocene)dichloropalladium(II),
bis(acetato)bis(triphenylphosphine)palladium(II),
bis(acetonitrile)dichloropalladium(II), and
the like; nickel(0) complexes such as tetrakis(triphenylphosphine)nickel(0)
and the like; and
nickel(II) complexes such as dichlorobis(triphenylphosphine)nickel(II) and the
like.
The term "cyano," means -CN.
The term "cyanoalkyl," means an alkyl group to which is attached at least one
cyano
substituent.
The term "cycloalkyl," means a monovalent saturated cyclic or bicyclic
hydrocarbon
to of three to fifteen carbons.
The term "cycloalkylalkyl," means an alkyl group to which is attached at least
one
cycloalkyl group.
The term "halo," means F (fluoride), Cl (chloride), Br (bromide), and I
(iodide).
The term "haloalkyl," means means an alkyl group to which is attached at least
one
15 halo substituent.
The term "heteroaryl," means cyclic, aromatic five- and six-membered groups,
wherein at least one atom is selected from the group consisting of nitrogen,
oxygen, and
sulfur, and the remaining atoms are carbon. The five-membered rings have two
double
bonds, and the six-membered rings have three double bonds. Heteroaryls are
exemplified by
2o furanyl, thienyl, pyrrolyl, oxazolyl, thiazolyl, imidazolyl, isoxazolyl,
isothiazolyl,
oxadiazolyl, oxadiazolyl, triazolyl, tetrazolyl, thiadiazolyl, pyridyl,
pyridazinyl, pyrimidinyl,
pyrazinyl, pyrazolyl, pyrrolyl, triazinyl, and the like. The heteroaryl groups
of the invention
are connected through a substitutable carbon or nitrogen (for imidazolyl or
pyrrolyl) in the
ring. The heteroaryl groups of the invention can be fused to an aryl group, a
heterocyclyl, or
25 another heteroaryl. Fused heteroaryls are exemplified by quinolinyl,
isoquinolinyl,
benzofuranyl, benzothienyl, indolyl, imidazo(2,1-b)(1,3)thiazolyl, and the
like. The
heteroaryl groups of the invention can be optionally substituted with 1-4
substituents
independently selected from alkyl, alkenyl, alkynyl, alkoxyalkoxy, amino,
aminoalkyl,
alkylsulfanyl, alkylsulfonyl, cyano, cyanoalkyl, halo, haloalkyl, vitro,
perfluoroalkyl,
3o perfluoroalkoxy, oxo, -(CH.,)aC(O)R5, -(CH2)aC(O)ORS,
-(CH2)aN(RS)C(O)R5~ -(CH2)aC(O)N(RS)2~ -(CH2)aN(RS)C(O)N(R5)2~ -(CH2)aORS~
-(CH2)aS02R5~ -(CH2)aSR6~ ~d -(CHz)aR~~
The term "heteroarylalkyl," means an alkyl group to which is attached at least
one
heteroaryl group.
35 The term "heteroarylene," means a diradical formed by the removal of two
hydrogen
atoms from a heteroaryl, as defined directly above. Heteroarylenes are
exemplified by
-13-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
isoxazol-3,4-diyl, isoxazol-3,5-diyl, isothiazol-3,4-diyl, isothiazol-3,5-
diyl, oxazol-2,4-diyl,
oxazol-2,5-diyl, oxazol-4,5-diyl, and the like.
The term "heterocyclyl," means cyclic or bicyclic, non-aromatic, four-, five-,
six-, or
seven-membered rings containing at least one atom selected from the group
consisting of
oxygen, nitrogen, and sulfur. The four-membered rings have zero double bonds,
the five-
membered rings have zero or one double bonds, the six- and seven-membered
rings have
zero, one, or two double bonds; and the bicyclic heterocyclyls have zero to
two double bonds.
Heterocyclyls of the invention are exemplified by dihydropyranyl,
dihydropyridinyl, 1,3-
dioxolanyl, 1,4-dioxanyl, morpholinyl, piperazinyl, piperidinyl, pyrrolidinyl,
l0 tetrahydropyridinyl, thiomorpholinyl, and the like. The heterocyclyl groups
of the invention
can be fused to an aryl group, a heteroaryl, or another heterocyclyl. Fused
heterocyclyls are
exemplified by 1,3-benzodioxole, 2,3-dihydro-1,4-benzodioxine, and the like.
The
heterocyclyl groups of the invention are connected through a substitutable
caxbon or nitrogen
atom in the ring. The heterocyclyl groups of the invention can be optionally
substituted with
1-5 substituents independently selected from alkyl, alkenyl, alkynyl,
alkoxyalkoxy, amino,
aminoalkyl, alkylsulfanyl, alkylsulfonyl, cyano, cyanoalkyl, halo, haloalkyl,
nitro,
perfluoroalkyl, perfluoroalkoxy, oxo,
-(CH2)aC(~)RSa -(CHZ)aC(~)~RS~ -(CH2)aN(RS)~(~)R5~ -(CH2)a~(~)N(RS)2~
-(CH2)aN(RS)C(0)N(RS)2~ -(CH2)a~Rs~ -(CH2)as~2R5~ '(~H2)asR6~ ~d -(CHz)aR~~
2o The term "heterocyclene," means a diradical formed by the removal of two
hydrogen
atoms from a heterocyclyl, as defined directly above. Heterocyclenes are
exemplified by
pyrrolidin-2,4-diyl, 1,3-dioxolan-2,4-diyl, and the like.
The term "hydroxy protecting group," means selectively introducible and
removable
groups which protect hydroxy groups against undesirable side reactions during
synthetic
procedures. Examples of hydroxy protecting groups include benzyloxycarbonyl, 4-
nitrobenzyloxycarbonyl, 4-bromobenzyloxycarbonyl, 4-methoxybenzyloxycarbonyl,
methoxycarbonyl, tent-butoxycarbonyl, isopropoxycarbonyl,
diphenylmethoxycarbonyl,
2,2,2-trichloroethoxycaxbonyl, trimethylsilyl (TMS), triethylsilyl, 2-
(trimethylsilyl)-
ethoxycarbonyl, 2-furfuryloxycarbonyl, allyloxycarbonyl, acetyl, formyl,
chloroacetyl,
3o trifluoroacetyl, methoxyacetyl, phenoxyacetyl, benzoyl, methyl, tent-butyl,
2,2,2-
trichloroethyl, 2-trimethylsilylethyl, 1,1-dimethyl-2-propenyl, 3-methyl-3-
butenyl, allyl,
benzyl, para-methoxybenzyldiphenylmethyl, triphenylmethyl (trityl),
tetrahydrofuryl
methoxymethyl, methylthiomethyl, benzyloxymethyl, 2,2,2-trichloroethoxymethyl,
2-
(trimethylsilyl)ethoxymethyl, methanesulfonyl, para-toluenesulfonyl,
trimethylsilyl,
triethylsilyl, triisopropylsilyl, and the like. Hydroxy protecting group can
also be used as
prodrug-forming groups. Preferred hydroxy protecting groups for the practice
of the
invention are acetyl and benzoyl.
-14-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
The term "vitro," means -N02.
The term "oxo," means a group formed by the replacement of two hydrogen atoms
on
the same carbon atom with a single oxygen atom.
The term "perfluoroalkyl," means an alkyl group in which all of the hydrogen
atoms
have been replaced by fluoride atoms.
It is intended that the definition of any substituent or variable at a
particular part in a
molecule be independent of its definition elsewhere in the molecule. Thus, for
example,
substituents such as -(CH2)aC(O)RS represent -CHZC(O)H, and -CHZC(O)CH3; and
substituents such as -(CH2)aN(RS)C(O)N(RS)2 represent
CH2CH2N(H)C(O)N(CH3)(C3H7)
1o and -CH2N(CH3)C(O)NH(CH3), and the like.
The compounds of the invention can exist as therapeutically acceptable salts.
The
term "therapeutically acceptable salt," means salts or zwitterionic forms of
the compounds of
the invention which are water or oil-soluble or dispersible, which are
suitable for treatment of
diseases without undue toxicity, irritation, and allergic response, which are
commensurate
15 with a reasonable benefit/risk ratio, and which are effective for their
intended use. The salts
can be prepared during the final isolation and purification of the compounds
or separately by
reacting an amino group with a suitable acid. Representative acid addition
salts include
acetate, adipate, alginate, citrate, aspartate, benzoate, benzenesulfonate,
bisulfate, butyrate,
camphorate, camphorsulfonate, digluconate, glycerophosphate, hemisulfate,
heptanoate,
20 hexanoate, formate, fumarate, hydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethansulfonate (isethiovate), lactate, maleate, mesitylenesulfonate,
methanesulfonate,
naphthylenesulfonate, nicotinate, 2-naphthalenesulfonate, oxalate, palmoate,
pectinate,
persulfate, 3-phenylpropionate, picrate, pivalate, propionate, succinate,
tartrate,
trichloroacetic, trifluoroacetic, phosphate, glutamate, bicarbonate, para-
toluenesulfonate, and
25 undecanoate. Also, amino groups in the compounds of the invention can be
quaternized with
as methyl, ethyl, propyl, and butyl chlorides, bromides and iodides; dimethyl,
diethyl,
dibutyl, and diamyl sulfates; decyl, lauryl, myristyl, and stearyl chlorides,
bromides, and
iodides; benzyl and phenethyl bromides. Examples of acids which can be
employed to form
therapeutically acceptable acid addition salts include inorganic acids such as
hydrochloric,
3o hydrobromic, sulphuric, and phosphoric and oxganic acids such as oxalic,
malefic, succinic,
and citric.
Basic addition salts can be prepared during the final isolation and
purification of the
compounds by reacting a carboxy group with a suitable base such as the
hydroxide,
carbonate, or bicarbonate of a metal cation or with ammonia or an organic
primary, secondary
35 or tertiary amine. Therapeutically acceptable salts cations based on
lithium, sodium,
potassium, calcium, magnesium, and aluminum and nontoxic quaternary ammonia
and amine
cations such as ammonium, tetramethylammonium, tetraethylammonium,
methylamine,
-15-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
dimethylamine, trimethylamine, triethylamine, diethylamine, ethylamine,
tributylamine,
pyridine, N,N-dimethylaniline, N-methylpiperidine, N-methylmorpholine,
dicyclohexylamine, procaine, dibenzylamine, N,N-dibenzylphenethylamine, 1-
ephenamine,
and N,N'-dibenzylethylenediamine. Other representative organic amines useful
for the
formation of base addition salts include ethylenediamine, ethanolamine,
diethanolamine,
piperidine, and piperazine.
The compounds of this invention can exist as therapeutically acceptable
prodrugs.
The term "therapeutically acceptable prodrug," as used herein, represents
those prodrugs of
the compounds of this invention which are, within the scope of sound medical
judgment,
l0 suitable for use in contact with the tissues of humans and lower animals
without undue
toxicity, irritation, allergic response, and the like, commensurate with a
reasonable
benefit/risk ratio, and effective for their intended use, as well as the
zwitterionic forms, where
possible, of the compounds of this invention.
The teen "prodrug," as used herein, represents compounds, which are rapidly
15 transformed in vivo to the parent compound of the above formula, for
example, by hydrolysis
in blood. A thorough discussion is provided in T. Higuchi and V. Stella, Pro-
drugs as Novel
Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, and in Edward B.
Roche, ed.,
Bioreversible Carriers iu Drug Desigh, American Pharmaceutical Association and
Pergamon
Press, 1987.
Examples of compounds encompassed by Formula I include
a) a compound of formula (I) wherein Dl is C---C, Y' is isoxazol-3,5-diyl,
A' is pyrid-2-yl, X is fluoride, and R' is hydrogen;
b) a compound of formula (I) wherein D' is C---C, Y' is isoxazol-3,5-diyl,
A' is quinol-3-yI, X is hydrogen, and R' is hydrogen;
c) a compound of formula (I) wherein D' is C---C, Y' is isoxazol-3,5-diyl,
A1 is quinol-2-yl, X is hydrogen, and R' is hydrogen;
d) a compound of formula (I) wherein D' is C---C, Y' is isoxazol-3,5-diyl,
3o A' is quinol-4-yl, X is hydrogen, and R' is hydrogen;
e) a compound of formula (I) wherein Dl is C---C, Y' is isoxazol-3,5-diyl,
A' is 4-fluorophenyl, X is hydrogen, and Rl is hydrogen;
f) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
A' is pyrid-4-yl, X is hydrogen, and R' is hydrogen;
g) a compound of formula (I) wherein D' is C---C, Yl is isoxazol-3,5-diyl,
A' is 4-cyanophenyl, X is hydrogen, and R' is hydrogen;
h) a compound of formula (I) wherein D' is C---C, Yl is isoxazol-3,5-diyl,
A' is pyrid-3-yl, X is hydrogen, and R' is hydrogen;
-16-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
i) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
A' is thien-2-yl, X is hydrogen, and R' is hydrogen;
j) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
A' is thiazol-2-yl, X is hydrogen, and R' is hydrogen;
k) a compound of formula (I) wherein D' is C--_C, Y' is isoxazol-3,5-diyl,
A' is 3,4-difluorophenyl, X is hydrogen, and R' is hydrogen;
1) a compound of formula (I) wherein D' is C---C, Y' is isoxazol-3,5-diyl,
A' is 3-(trifluoromethyl)phenyl, X is hydrogen, and R' is hydrogen;
m) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 3,4-dichlorophenyl, X is hydrogen, and R' is hydrogen;
n) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
A' is 3-cyanophenyl, X is hydrogen, and R' is hydrogen;
o) a compound of formula (I) wherein D' is C-___C, Y' is isoxazol-3,5-diyl,
A' is 4-cyano-3-(methylsulfanyl)pyrid-2-yl, X is hydrogen, and R' is hydrogen;
p) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl, '
A' is thiazol-5-yl, X is hydrogen, and R' is hydrogen;
q) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 6-chloroimidazo(2,1-b)thiazol-5-yl, X is fluoride, and R' is hydrogen;
r) a compound of formula (I) wherein D' is CSC, Y' is isoxazol-3,5-diyl,
2o A' is thiazol-5-yl, X is fluoride, and R' is hydrogen;
s) a compound of formula (I) wherein D' is C--_C, Y' is isoxazol-3,5-diyl,
A' is thiazol-2-yl, X is fluoride, and R' is hydrogen;
t) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 3,4-dichlorophenyl, X is fluoride, and R' is hydrogen;
u) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
A' is pyrimidin-5-yl, X is fluoride, and R' is hydrogen;
v) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 2-methyl-2H-tetrazol-5-yl, X is hydrogen, and R' is hydrogen;
w) a compound of formula (I) wherein D' is C=C, Y' is isoxazol-3,5-diyl,
3o A' is 2-methyl-2H-tetrazol-5-yI; X is fluoride; and R' is hydrogen;
x) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 2-chloroquinol-3-yl, X is hydrogen, and R' is hydrogen;
y) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
A' is 3-methylbenzo(b)thien-2-yl, X is hydrogen, and R' is hydrogen;
z) a compound of formula (I) wherein D' is C--_C, Y' is isoxazol-3,5-diyl,
A' is pyrid-2-yl, X is hydrogen, and R' is hydrogen;
aa) a compound of formula (I) wherein D' is C--__C, Y' is isoxazol-3,5-diyl,
-17-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
A' is quinol-3-yl, X is fluoride, and R' is hydrogen.
One particular embodiment of this invention is a subgenus of formula I which
may be
represented by the following formula:
O-N
A~
O
,O ORS J
N~~, ~ ,,~0 N ~
,O . O
O 'O
O X
in which X is hydrogen or fluoride;R' is hydrogen, and A' is represented by
aryl or
heteroaryl. More specifically A' is phenyl, substituted phenyl, pyridyl,
pyrimidinyl, thienyl,
l0 thiazolyl, quinolyl, benzothienyl, or imidazo (2,1-b) thiazolyl, in which
any of said
heterocycles may be fiuther substituted. Even more particuslarly, A' is pyrid-
2-yl, pyrid-3-yl,
pyrid-4-yl, pyrimidin-2-yI, pyrimidin-4-yl or pyrimidin-5-yI.
In accordance with pharmaceutical compositions, methods of treatment, use as
medicaments and as medicaments, the compounds can be administered alone to
achieve an
15 antibacterial effect or in combination with other antibacterial agents. The
therapeutically
effective dose level depends on factors such as the disorder being treated and
the severity of
the disorder; the activity of the compound used; the composition employed; the
age, body
weight, general health, sex, and diet of the patient; the time of
administration; the route of
administration; the rate of excretion of the compound; the duration of
treatment; and drugs
20 used in combination with or coincidentally with the compounds. The
compounds can be
administered orally, parenterally, nasally, rectally, vaginally, or topically
in unit dosage
formulations containing therapeutically acceptable excipients such as
carriers, adjuvants,
diluents, vehicles, or combinations thereof. The term "parenteral" includes
infusion,
subcutaneous, intravenous, intramuscular, and intrasternal injection.
25 The antibacterial effect of parenterally administered compounds can be
controlled by
slowing their absorption, such as, for example, by administration of
injectable suspensions of
crystalline, amorphous, or otherwise water-insoluble forms of the compounds;
administration
of the compounds as oleaginous solutions or suspensions; or administration of
microencapsulated matrices of the compounds trapped within liposomes,
microemulsions, or
3o biodegradable polymers. In each case, the ratio of compound to excipient
and the nature of
the excipient influences the rate of release of the compound. Transdermal
patches also
-18-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
provide controlled delivery of compounds using rate-controlling membranes.
Conversely,
absorption enhancers can be used to increase absorption of the compounds.
Solid dosage forms for oral administration of the compounds include capsules,
tablets,
pills, powders, and granules. These compositions can contain diluents,
lubricants, and
buffering agents. Tablets and pills can be prepared with release-controlling
coatings, and
sprays can optionally contain propellants.
Liquid dosage forms for oral administration of the compounds include
emulsions,
microemulsions, solutions, suspensions, syrups, and elixirs. These
compositions can also
contain adjuvants such as wetting, emulsifying, suspending, sweetening,
flavoring, and
perfuming agents.
Topical dosage forms of the compounds include ointments, pastes, creams,
lotions,
gels, powders, solutions, sprays, and inhalants. Suppositories for rectal or
vaginal
administration comprise compounds with a suitable nonirritating excipient.
Ophthalmic
formulations such as eye drops and eye are also contemplated as being within
the scope of
this invention.
The total daily dose of the compounds administered to a patient in single or
divided
doses can be in amounts from about 0.1 to about 200 mg/kg body weight or
preferably from
about 0.25 to about 100 mg/kg body weight. Single dose compositions contain
these
amounts or submultiples thereof to make up the daily dose.
Determination of Biological Activity
Ih l~itro Assay of Antibacterial Actiyity
Representative compounds were assayed ih vitro for antibacterial activity as
follows:
twelve petri dishes containing successive aqueous dilutions of the test
compound and 10 mL
of sterilized Brain Heart Infusion (BHI) agar (Difco 0418-Ol-5) were prepared.
Each plate
was inoculated with 1:100 (or 1:10 for slow-growing Streptococcus strains)
dilutions of the
nine microorganisms shown in Table 1 using a Steers replicator block. The
inoculated plates
were incubated at about 35-37 °C for 20-24 hours. A control plate,
using BHI agar
containing no test compound, was also prepared and incubated at the beginning
and end of
3o each test. Finally, a plate containing Erythromycin A was prepared and
incubated as another
control and to provide test-to-test comparability.
After incubation, each plate was inspected. The minimum inhibitory
concentration
(MIC) was defined as the lowest concentration of drug yielding no growth, a
slight haze, or
sparsely isolated colonies on the inoculum spot as compared to the growth
control. The
compounds inhibited the growth of these bacteria with MIC's in a range of
about 0.004
~g/mL to about 128 ~.g/mL; in a more preferred range, the compounds inhibited
the growth
of bacteria with MIC's in a range of about 0.004 ~,g/mL to about 2 ~,g/mL; and
in a most
-19-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
preferred range, the compounds inhibited the growth of bacteria with MIC's in
a range of
about 0.004 ~,g/mL to about 4 ~g/mL.
The results of this assay demonstrate the antibacterial activity of the
compounds of
the invention.
Table 1
Microorganism
Staphylococcus aureus ATCC 6538P
Staphylococcus aureus A5177
Streptococcus pyogenes EES61
Streptococcus pyogenes 930
Streptococcus pyogehes PIU 2548
Streptococcus pueumonfae ATCC 630
Streptococcus pneumouiae 5737
Streptococcus p~ceumoniae 5649
Haemophilus influenzae DILL AMP R
Gastrointestinal Tolerability Study
Example 3 and three reference compounds were investigated for their ability to
produce nausea and emesis in conscious ferrets using the method as described
in Drugs,
53(2), 206-234 (1997) and Cancer Treat. Rep., 66(1), 187-189 (1982). Each
compound was
administered to 6 ferrets by oral gavage at 30 mg/kg in 2 mL of ethanol and 4
mL of water.
Following administration, the ferrets were observed for 90 minutes for signs
of nausea and
vomiting. Nausea was preceded by up to five of the following behaviors in the
ferret: licking,
2o down, flop, backing, and gag. From these behaviors a nausea score is
determined for each
compound, one point assigned for each behavior exhibited. The mean nausea
score for a
compound is the total number of nausea behaviors divided by the number of
animals given
the compound. Percent emesis is the total number of vomiting ferrets divided
by the number
of animals administered the compound. The results, shown in Table 2,
demonstrate the
unexpected gastrointestinal tolerance of Example 3.
Table 2
-20-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Compound Dose (mg/kg)No. Animals% Emesis Nausea Score
Erythromycin 30 6 67 % 1.2
Clarithromycin30 6 83 % 1.4
Telithromycin30 6 50 % 1.8
Example 3 30 6 0 % 0.17
This enhanced gastrointestinal tolerability represents a significant advantage
for the
compounds of this invention. These compounds will have an improved side effect
profile
when compared with the erythromycin derivatives of the prior art. Patients
consuming these
compounds will experience a reduced incidence of nausea, vomiting,
gastrointestinal
discomfort, cramping, and other GI side effects typically associated with
erythromycin
therapy. As used in this application, "enhanced gastrointestinal tolerance"
refers to a
reduced incidence of GI side effects in a patient population, and not to a
total absence of GI
l0 side effects. As is well known to those skilled in the art, even placebo
dosage forms made of
sugar produce some measurable incidence of side effects. Thus an enhanced
profile must be
interpreted in light of the relevant art.
Abbreviations
Abbreviations which have been used in the descriptions of the schemes and the
examples that follow are: THF for tetrahydrofuran, DMF for N,N-
dimethylformamide, DME
for 1,2-dimethoxyethane, LDA for lithium diisopropylamide and DDQ for 2,3-
dichloro-5,6-
dicyanobenzoquinone.
Synthetic Methods
The compounds can be prepared by employing reactions shown in Schemes 1-10. It
will be readily apparent to one of ordinary skill in the art that the
compounds can be
synthesized by substitution of the appropriate reactants in these syntheses,
and that the steps
themselves can be conducted in varying order. It will also be appaxent that
protection and
deprotection steps can be performed to successfully complete the syntheses of
the
compounds. A thorough discussion of protecting groups is provided in T.W.
Greene and
P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd edition, John Wiley &
Sons, New
York (1999). The groups X, Al, D1, Yl, Rl, and Rp are defined hereinabove and
the groups
R, Wl, and Xl are defined hereinbelow.
_21 _
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 1
HON HO-N - SnR3 -N
\~q~ ~q~
H CI (iii) R3Sn~J~/\
(i) (ii) (iv)
Iz
O-N
I
(v)
As shown in Scheme l, conversion of compounds of formula (i) to compounds of
formula (ii) can be achieved by treatment of the former with chlorinating
agents. Examples
of chlorinating agents include N-chlorosuccinimide and chlorine gas. Solvents
useful for the
reaction include DMF, THF, ethyl acetate, and mixtures thereof. The
temperatures at which
the reactions are conducted typically range from about 25 °C to about
40 °C.-
Conversion of compounds of formula (i) to compounds of formula (iv) can be
l0 achieved by ih situ treatment of compounds of formula (ii), prepared as
described above, with
compounds of formula (iii) (R is Cl-C4 alkyl) and a base. Examples of bases
include sodium
bicarbonate, sodium carbonate, triethylamine, and N,N-diisopropylethylamine.
Solvents
useful for the reaction include DMF, THF, ethyl acetate, and mixtures thereof.
The
temperatures at which the reactions are conducted typically range from about
25 °C to about
40 °C; and reaction times are typically from about 12 hours to about 48
hours.
Conversion of compounds of formula (iv) to compounds of formula (v) can be
achieved by treatment of the former with iodine. Solvents useful for the
reaction include
THF, 1~,4-dioxane, toluene, and mixtures thereof. The temperature at which the
reactions are
conducted is typically ambient; and reaction times are typically from about 2
hours to about 4
2o hours.
-22-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 2
O
oBz ~
N\ n~Nn, ~ ,vO~N~
O X O X
(vi) (vi)
(X is hydrogen) (X is F)
As shown in Scheme 2, compounds of formula (vi), wherein X is hydrogen, can be
intraconverted to compounds of formula (vi), wherein X is fluoride, by
treatment of the
former with a fluorinating agent and, optionally, a base. Examples of
fluorinating agents
include 3,5-dichloro-1-fluoropyridinium tetrafluoroborate, N-
fluorobenzenesulfonimide, 3,5-
dichloro-1-fluoropyridinium triflate, and N-fluorobenzenesulfonimide, N-fluoro-
N-methyl-
para-toluenesulfonamide, N-fluoropyridinium triflate, or N-
fluoroperfluoropiperidine and a
to base. Examples of bases include sodium hydride, potassium hydride, LDA,
triethylamine,
and N,N-diisopropylethylamine. Solvents useful for the reaction include THF,
diethylether,
and mixtures thereof. The temperatures at which the reactions are conducted
typically range
from about -78 °C to about 0 °C; and reaction times are
typically from about 2 hours to about
24 hours.
-23-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 3
A1
y~ ~
O -
,O ORS
Nn. ~ ,a0 N~ I-y~-A~
O
(vii)
O X O x
(vi) (Ia)
O-N
~A~
I
(v)
O-N
A~
I
N~
(Ib)
As shown in Scheme 3, compounds of formula (vi) can be converted Ito compounds
of
formula (Ia) by treatment of the former with compounds of formula (vii), a
base, a coupling
catalyst, and, optionally, an additive. Examples of bases include
triethylamine and N,N-
diisopropylethylamine. Examples of coupling catalysts include
dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
l0 tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II).
Examples of additives include triphenylphosphine, triphenylarsine, copper(I)
iodide, and
mixtures thereof. Solvents useful for the reaction include acetonitrile, THF,
triethylaxnine,
and mixtures thereof. The temperatures at which the reactions are conducted
typically range
-24-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
from about 50 °C to about 80 °C; and reaction times are
typically from about 12 hours to
about 48 hours.
In a particular embodiment of this reaction, the compounds of formula (vi) can
be
treated with compounds of formula (v) to provide compounds of formula (Ib).
Scheme 4
Y~-A~ A~_.Y~
,O ORS ~ ~ O, ,O ORS I
.. ~,,v0~ N ~ Nn, ~ ~,,v0~ N ~
O I [~
X I n X
(Ia) Z_(Ic)
As shown in Scheme 4, compounds of formula (Ia), wherein D1 is C---C, can be
to converted to compounds of formula Z-(Ic), wherein D1 is CH=CH in the Z
configuration, by
treatment of the former with hydrogen gas, a reduction catalyst, and,
optionally, an additive.
Examples of reduction catalysts include Lindlar catalyst and palladium on
barium sulfate. An
example of an additive is quinoline. Solvents useful for the reaction include
C1-C4 alcohols
such as methanol, ethanol, propanol, butanol, iso-propanol, tart-butanol, and
the like
acetonitrile, THF, ethyl acetate, and mixtures thereof. The temperature at
which the reactions
are conducted is typically ambient; and reaction times are typically from
about 1 hour to
about 6 hours.
-25-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 5
_ ~ ~g~pH)2
~R1 ~
N~ ~ N~
O\ J
O x
(vi) (viii)
O-N
~A~
I
O_N I-Y~-A~
_ \ ~ \ A~ (v) (vii)
ORS ~
N~
O ~Y~_A~
-O
O x H ,,,,. ' ,,O ORS
,Nn. vO~N~
E-(Id)
O
O X
E-(Ic)
As shown in Scheme 5, compounds of formula (vi) can be converted to compounds
of
formula (viii) by treatment of the former with borane~THF. Solvents useful for
the reaction
include THF, dioxane, diethylether, and mixtures thereof. The temperatures at
which the
reactions are conducted is typically about -20 °C to about 25
°C; and reaction times are
typically from about 1 hour to about 6 hours.
Compounds of formula (viii) can be converted to compounds of formula E-(Ic),
Io wherein D1 is CH=CH in the E configuration, by treatment of the former with
compounds of
formula (vii), a coupling catalyst, a base, and, optionally, an additive.
Examples of coupling
catalysts include dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II). Examples of bases include sodium
carbonate,
potassium carbonate, cesium carbonate, triethylamine, and N,N-
diisopropylethylamine.
Examples of additives include triphenylphosphine, tributylphosphine, and
triphenylarsine.
Solvents useful for the reaction include acetonitrile, THF, DMF, DME, and
mixtures thereof.
-26-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
The temperatures at which the reactions are conducted typically range from
about 50 °C to
about ~0 °C; and reaction times are typically from about 12 hours to
about 4~ hours.
In a particular embodiment of this reaction, the compounds of formula (viii)
can be
treated with compounds of formula (v) to provide compounds of formula E-(Id).
Scheme 6
H3~3
O
;O ORS I
Nn. ,a0 N~
~O
(i X
(vi)
(ix)
O-N
HON
- ~~A~ _ /
CI I
(ii) N
(Ib)
(x)
As shown in Scheme 6, conversion of compounds of formula (vi) to compounds of
l0 formula (ix) can be achieved by treatment of the former with 1-iodo-2-
. (trimethylsilyl)acetylene, a base, a coupling catalyst, and, optionally, an
additive. Examples
of bases include triethylamine and N,N-diisopropylethylamine. Examples of
coupling
catalysts include dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II). Examples of additives include
copper(I) iodide,
triphenylphosphine, and triphenylarsine. Solvents useful for the reaction
include acetonitrile,
triethylamine, THF, and mixtures thereof. The temperatures at which the
reactions are
-27-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
conducted typically range from about 25 °C to about 80 °C; and
reaction times are typically
from about 6 hours to about 24 hours.
Conversion of compounds of formula (ix) to compounds of formula (x) can be
achieved by treatment of the former with a base. Examples of bases include
potassium
carbonate and sodium carbonate. Solvents useful for the reaction include
methanol or
ethanol, and mixtures thereof. The temperature at which the reactions are
conducted is
typically ambient; and the reaction times are typically about 5-15 minutes.
Conversion of compounds of formula (x) to compounds of formula (Ib) can be
achieved by treatment of the former with compounds of formula (ii) and a base.
Examples of
to bases include sodium bicarbonate, sodium carbonate, triethylamine, and N,N-
diisopropylethylamine. Solvents useful for the reaction include DMF, THF,
ethyl acetate,
and mixtures thereof. The temperatures at which the reactions are conducted
typically range
from about 25 °C to about 40 °C; and reaction times are
typically from about 12 hours to
about 48 hours.
-28-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 7
s
O O
O ORS
i~., : 1
,a0 N H ~,, ,.O OR
Nn, .v0 Nw
O
O O
w - w
O O _~O O
O X n X
(xi)
HO-N
\~A~
CI
(ii)
-N
O N
r ~ a
O -
,O OR
,, ,,v0 1 N ~ ~ OR
O O
O O
n X
(Ib) (Ie)
As shown in Scheme 7, conversion of compounds of formula (vi) to compounds of
formula (xi) can be achieved by treatment of the former with vinyl bromide, a
base, a
coupling catalyst, and, optionally, an additive. Examples of bases include
triethylamine and
N,N-diisopropylethylamine. Examples of coupling catalysts include
dichlorobis(triphenylphosphine)palladium(II),
tris(dibenzylideneacetone)dipalladium(0),
tetrakis(triphenylphosphine)palladium(0), and
dichlorobis(triphenylphosphine)nickel(II).
1o Examples of additives include triphenylphosphine, triphenylarsine, and
copper(I) iodide.
Solvents useful for the reaction include acetonitrile, THF, triethylamine, and
mixtures
thereof The temperatures at which the reactions are conducted typically range
from about 25
°C to about 80 °C; and reaction times are typically from about
12 hours to about 48 hours.
Conversion of compounds of formula (xi) to compounds of formula (Ie) can be
achieved by treatment of the former with compounds of formula (ii) and a base.
Examples of
-29-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
bases include sodium bicarbonate, sodium carbonate, triethylamine, and N,N-
diisopropylethylarnine. Solvents useful for the reaction include DMF, THF,
ethyl acetate,
and mixtures thereof. The temperatures at which the reactions are conducted
typically range
from about 25 °C to about 40 °C; and reaction times axe
typically from about 12 hours to
about 48 hours.
Conversion of compounds of formula (Ie) to compounds of formula (Ib) can be
achieved by treatment of the former with oxidizing agents. Examples of
oxidizing agents
include manganese dioxide, barium manganate, and DDQ. Solvents useful for the
reaction
include THF, 1,4-dioxane, and mixtures thereof. The temperatures at which the
reactions are
to conducted typically range from about 50 °C to about 100 °C;
and reaction times are typically
from about 12 hours to about 96 hours.
Crhama R
O
A1
O /
1 O
O OR
H .,,.
Nn, ,,v0 N~ H ,,~. ~O OR1 I
,Nn ~ ~~~,v0~ N ~
,r
O X I O
(x)
(xii)
g-N
A1
/ r
O
1
,, ,. . ;~O OR I
Nn. ,,v0~ N ~
X
1 s (I~
As shown in Scheme 8, compounds of formula (x) can be converted to compounds
of
formula (xii) by treatment of the former with comopunds of formula Xl-C(O)-A',
wherein Xl
is Br or Cl, a coupling catalyst, a base, and, optionally, an additive.
Examples of coupling
-30-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
catalysts include allylpalladium chloride dimer,
tetrakis(triphenylphosphine)palladium(0),
dichlorobis(triphenylphosphine)palladium(II), and
dichlorobis(triphenylphosphine)nickel(II).
Examples of bases include N,N,N,N-tetramethyl-1,8-naphthalenediamine (Proton-
Sponge~),
triethylamine and N,N-diisopropylethylamine. Examples of additives include
triphenylphosphine, triphenylarsine, and copper(I) iodide. Solvents useful for
the reaction
include acetonitrile, THF, 1,4-dioxane, DME, triethylamine, and mixtures
thereof. The
temperatures at which the reactions are conducted typically range from about
25 °C to about
100 °C; and reaction times are typically from about 6 hours to about 24
hours.
Alternatively, compounds of formula (x) can be converted to compounds of
formula
l0 (xii) by treatment of the former with compounds of formula Wl-A', wherein
WI is halogen, -
OS02CF3, or -SnR3 (R is CI-C4 alkyl), carbon monoxide, a coupling catalyst,
and, optionally,
a base and an additive. Examples of coupling catalysts include allylpalladium
chloride
dimer, tetrakis(triphenylphosphine)palladium(0),
dichlorobis(triphenylphosphine)palladium(II), and
dichlorobis(triphenylphosphine)nickel(II).
15 Examples of bases include N,N,N,N-tetramethyl-1,8-naphthalenediamine
(Proton-Sponge~),
triethylamine and N,N-diisopropylethylamine. Examples of additives include
triphenylphosphine, triphenylarsine, and copper(I) iodide. Solvents useful for
the reaction
include acetonitrile, THF, 1,4-dioxane, DME, triethylamine, and mixtures
thereof. The
temperatures at which the reactions are conducted typically range from about
25 °C to about
20 100 °C; and reaction times are typically from about 6 hours to about
24 hours.
Conversion of compounds of formula (xii) to compounds of fornula (If) can be
achieved by treatment of the former with N-hydroxylamine-O-sulfonic acid,
sodium
hydrosulfide, and a base. Examples of bases include sodium bicarbonate, sodium
carbonate,
and potassium carbonate. Solvents useful for the reaction include C1-C4
alcohols, water,
25 acetonitrile, THF, 1,4-dioxane, DME, and mixtures thereof. The temperatures
at which the
reactions are conducted typically range from about 0 °C to about 50
°C; and reaction times
are typically from about 6 hours to about 24 hours.
-31-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Scheme 9
O
- / ~ / OH
' O
I H ,~, ;O ORS I
Nw ~Nn, .W N~
O, n O
O
O x . cj X
(xi) (xiii)
H2N1 A~
HoJ
(xiv)
N--~~ A N--~~ A
_ / O / O
O
H ,,, ,O ORS I
Nn, ,ap N ~
E
.O . O
n O
O x
(Ih)
(Ig)
As Shown in Scheme 9, compounds of formula (xi) can be converted to compounds
of formula (xiii) by treatment of the former with an oxidizing agent, and,
optionally, au
additive. Examples of oxidizing agents include potassium permanganate, sodium
periodate,
and ozone. Examples of additives include osmium tetroxide, N-methylmorpholine
N-oxide,
and hydrogen peroxide. Solvents useful for the reaction include acetonitrile,
acetone, water,
THF, and mixtures thereof. The temperatures at which the reactions are
conducted typically
to range from about 0 °C to about 50 °C; and reaction times are
typically from about 1 hour to
about 4 hours.
Conversion of compounds of formula (xiii) to compounds of formula (Ig) can be
achieved by treatment of the former with compounds of formula (xiv),
triphenylphosphine,
and an additive. Examples of additives include carbon tetrachloride, carbon
tetrabromide,
-32-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
and diethyl azodicarboxylate. Solvents useful for the reaction include
acetonitrile, THF, 1,4-
dioxane, and mixtures thereof. The temperatures at which the reactions are
conducted
typically range from about 0 °C to about 50 °C; and reaction
times are typically from about 1
hour to about 24 hours.
Conversion of compounds of formula (Ig) to compounds of formula (Ih) can be
achieved by treatment of the former with oxidizing agents. Examples of
oxidizing agents
include manganese dioxide, barium manganate, and DDQ. Solvents useful for the
reaction
include THF, 1,4-dioxane, and mixtures thereof. The temperatures at which the
reactions are
conducted typically range from about 50 °C to about 100 °C; and
reaction times are typically
1 o from about 12 hours to about 96 hours.
Scheme 10
i,
O
P
~~O OR
Nn, .,v0 N ~
O O
,Of 'n O
X
(I) (I)
As shown in Scheme 10, compounds of formula (I), wherein Rl is Rp, can be
intraconverted to compounds of formula (I), wherein Rl is hydrogen, by
treatment of the
former with methanol. The temperatures at which the reactions are conducted
typically range
from about 25 °C to about 65 °C; and reaction times axe
typically from about 2 hours to about
60 hours.
The invention will now be described in connection with other particularly
preferred
embodiments of Schemes 1-10, which are not intended to limit its scope. On the
contrary,
the invention covers all alternatives, modifications, and equivalents which
are included
within the scope of the claims. Thus, the following examples will illustrate
an especially
preferred practice of the invention, it being understood that the examples are
for the purposes
of illustration of certain preferred embodiments and are presented to provide
what is believed
to be the most useful and readily understood description of its procedures and
conceptual
aspects.
-33-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 1 A
compound of formula (vi) in Scheme 2: X is hydrogen; Rl is C6HSC O
A solution of Example 246 of commonly owned U.S. Patent 5,866,549 in
dichloromethane can be treated with 90% technical grade benzoic anhydride and
triethylamine over 10 minutes, stirred for 48 hours, treated with saturated
NaHC03, and
stirred for 30 minutes. The layers can be separated, and the organic layer can
be washed with
water and brine, dried (Na2S04), filtered, and concentrated. The concentrate
can be triturated
with a warm mixture of hexane and ethyl acetate and dried in a vacuum oven at
ambient
temperature to provide the desired product.
to
Example 1 B
compound of formula (ix) in Scheme 6: X is hydrogen; R~ is C6H5C~
A solution of Example 1A (15 g, 0.02 mol) in acetonitrile (150 mL) and
triethylamine
(75 mL) at room temperature was treated with
dichlorobis(triphenylphosphine)palladium(II)
15 (0.994 g, 1.4 mmol), copper(I) iodide (0.115 g, 0.6 mmol), and 1-iodo-2-
(trimethylsilyl)acetylene (5.9 mL, 0.0385 mol), stirred at room temperature
for 14 hours, and
concentrated. The concentrate was suspended in ether and filtered through
diatomaceous
earth (Celite~). The filtrate was washed with water and brine, dried (NaZSO4),
filtered, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
2o with 85:15 hexanes/acetone to provide the desired product.
Example I C
compound of formula (x) in Scheme 6: X is hydrogen; Rl is C6HSC~
A solution of Example 1B (7.07g, 8.44 mmol) in methanol (80 mL) at room
25 temperature was treated with potassium carbonate (0.514 g, 4.22 mmol),
stirred for 10
minutes, treated with ethyl acetate, washed with water and brine, dried
(Na2SO4), filtered, and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 80:20 hexanes/acetone to provide the desired product. MS (ESI(+)) m/z 765
(M+H)+.
3o Example 1D
compound of formula (x) in Scheme 6: X is hydrogen; Rl is hydrogen
A solution of Example 1 C (3.5 g, 4.57 mmol) in methanol (40 mL) at room
temperature was stirred for 60 hours and concentrated. The concentrate was
purified by flash
column chromatography on silica gel with 98:1.5:1
dichloromethane/methanol/concentrated
35 ammonium hydroxide to provide the desired product.
-34-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 1E
compound of formula (x) in Scheme 6: X is hydrogen; RI is CH3C~0~
A solution of Example 1D (I.4 g, 2.12 mmol) and triethylamine (428 mg, 4.24
mmol)
in dichloromethane (10 ml) at room temperature was treated with acetic
anhydride (432 mg,
4.24 mmol), stirred for 3 hours, treated with dichloromethane (65 mL), washed
with 5%
NaHC03, brine, dried (Na2S04), filtered, and concentrated. The concentrate was
purified by
flash column chromatography on silica gel with 70:30.hexanes/acetone to
provide the desired
product.
l0
Example 2A
compound of formula (vi) from Scheme 2: X is fluoride; Rl is C6HSC O
The desired compound was prepared as described in Example 1 of WO 99/21871,
and
substituting the instant Example 1A for the compound of formula (I) wherein Rp
is benzoyl,
15 Rl is methyl, and X is F.
Example 2B
compound of formula (ix) in Scheme 6: X is fluoride; Rl is C6H5C~
The desired product was prepared by substituting Example 2A for Example 1A in
2o Example 1B.
Example 2C
compound of formula (x) in Scheme 6: X is fluoride; Rl is C6HSC~0~
The desired product was prepared by substituting Example 2B for Example 1 B in
25 Example 1 C.
Example 3
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is pyrid-2-
yl,
X is fluoride, Rl is hydrogen
Example 3A
2-(S-(tributylstannyl)-3-isoxazolyl)pyridine
or
compound of formula (iv) in Scheme 1: A1 is pyrid-2-yl; R is n-butyl
A solution of 2-pyridinecarbaldehyde oxime (5.81 g, 47.6 mmol),
tributyl(ethynyl)stannane (10.0 g, 31.7 mmol), and sodium bicarbonate (9.6 g,
114 mmol) in
ethyl acetate (65 mL) and water (5 mL) was treated with N-chlorosuccinimide
(6.36 g, 47.6
-35-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
mmol), stirred for 18 hours at room temperature, treated with ethyl acetate,
washed with S%
NaHC03, water, and brine, dried (MgSO4), filtered, and concentrated. The
concentrate was
purified by flash column chromatography on silica gel with 95:5 hexanes/ethyl
acetate to
provide the desired product. MS m/z 435 (M+H)+.
Example 3B
2-(S-iodo-3-isoxazolyl)pyridine
or
compound of formula (v) in Scheme 1: A1 is pyrid-2-yl
Io A solution of Example 3A (11.8 g, 27.3 mmol) in THF (200 mL) at room
temperature
was treated with iodine (7 g, 27.6 mmol), stirred for 2 hours, treated with
diethyl ether,
washed with saturated NaHC03 and saturated NaZS203, dried (MgS04), filtered,
and
concentrated. The concentrate was purified by flash column chromatography on
silica gel
with 10:1 to 5:1 hexanes/ethyl acetate to provide the desired product. MS m/z
272 (M+H)~.
Example 3 C
compound of formula (Ib) in Scheme 3: A1 is pyrid-2-yl; X is fluoride; Rl is
C6H5
A solution of Example 2A (1.715 g, 2.26 mmol) and Example 3B (737 mg, 2.71
mmol) in acetonitrile (10 mL) and triethylamine (2 mL) at room temperature was
degassed,
2o treated with dichlorobis(triphenylphosphine)palladium(II) (S mole %),
degassed again, stirred
for 30 minutes, heated at 65 °C for 18 hours, concentrated to remove
most of the solvent,
treated with isopropyl acetate (500 mL), washed with saturated NaHC03, water,
and brine,
dried (Na2S04), filtered, and concentrated. The concentrate was purified by
flash column
chromatography on silica gel with 2:1 hexanes/acetone to provide the desired
product. MS
mlz 903 (M+H)+.
Example 3D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrid-2-
yl,
X is fluoride, Rl is hydrogen;
3o A solution of Example 3C (42.3 g, 46.8 mmol) in methanol (400 mL) was
heated at
reflux for 6 hours and concentrated. The concentrate was recrystallized from
hexanes/acetone
to provide the desired product. MS m/z 799 (M+H)+; i3C NMR (7S MHz, CDC13) 8
216.5, d
(204.2 and 203.8), d (166.2 and 165.9), 163.3, 157.3, 153.7, 149.7, 148.7,
136.8, 124.5,
121.8, 107.1, 104.2, d (99.0 and 96.3), 96.1, 83.4, 80.7, 80.1, 78.7, 70.3,
69.8, 65.8, 58.1,
50.8, 44.0, 40.5, 40.2, 38.3, 37.5, 28.1, d (25.4 and 25.1), 22.2, 21.1, 20.3,
17.6, 15.3, 13.7,
13.2, 10.5; HRMS m/z calcd (M+H)+ for C4lHssFN40in 799.3924. Found: 799.3924.
-3 6-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 4
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
yl,
X is hydrogen, Rl is hydrogen;
Example 4A
3-quinolinecarbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is quinol-3-yl
The desired product was prepared by substituting 3-quinolinecarbaldehyde for 4-
l0 formylbenzonitrile in Example 9A.
Example 4B
3-(5-(tributylstannyl)-3-isoxazolyl)quinoline
or
15 compound of formula (iv) in Scheme 1: A1 is quinol-3-yl; R is n-butyl
The desired product was prepared by substituting Example 4A for Example 11A in
Example 11B and purified by flash column chromatography on silica gel with
95:5
hexanes/acetone.
2o Example 4C
3-(5-iodo-3-isoxazolyl)quinoline
or
compound of formula (v) in Scheme 1: A1 is quinol-3-yl
The desired product was prepared by substituting Example 4B fox Example 11 B
in
25 Example 11 C.
Example 4D
compound of formula (I): D1 is C=_C, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
1;
X is hydrogen; RI is C6H5
30 , The desired product was prepared by substituting Example 4C fox Example
11 C in
Example 11D and purified by flash column chromatography on silica gel with
90:10
hexanes/acetone.
Example 4E
35 compound of formula (I): D1 is CSC, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
yl,
X is hydrogen, Rl is hydrogen
-37-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
A solution of Example 4D (198 mg, 0.212 mmol) in methanol (10 mL) was heated
at
55 °C for 16 hours and concentrated. The concentrate was purified by
flash column
chromatography on silica gel with 98:1:1 dichloromethane/methanol/concentrated
ammonium hydroxide to provide the desired product. 13C NMR (CDC13) ~ 217.0 (C-
9),
205.2 (C-3), 169.6 (C-1), 160.3, 157.9, 154.1, 148.8, 148.4, 134.2, 130.5,
129.5, 128.4, 127.5,
127.3, 121.8, 106.3, 103.1, 96.1, 83.6, 80.I, 77.4, 77.3, 72.4, 70.2, 69.5,
66.1, 58.I, 51.3,
51.1, 47.0, 44.7, 40.2, 38.6, 37.4, 28.5, 22.4, 21.1, 19.9, 18.0, 15.0, 14.5,
13.6, 13.6, 10.6;
HRMS m/z calcd (M+H)+ calcd for C45H59N4011' 831.4175. Found 831.4173.
to Example 5
compound of formula L): DI is C---C, Yl is isoxazol-3,5-diyl, A1 is quinol-2-
yl; X is
hydrogen; Rl is hydrogen
Example 5A
2-quinolinecarbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is quinol-2-yl
The desired product was prepared by substituting 2-quinolinecarbaldehyde for 4-
formylbenzonitrile in Example 9A.
Example 5B
2-(5-(tributylstannyl)-3-isoxazolyl)quinoline
or
compound of formula (iv) in Scheme 1: A1 is quinol-2-yl; R is n-butyl
The desired product was prepared by substituting Example 5A for Example 11A in
Example 11B'and purified by flash column chromatography on silica gel with
95:5
hexanes/acetone.
Example 5C
2-(5-iodo-3-isoxazolyl)quinoline
or
compound of formula (v) in Scheme 1: A1 is quinol-2-yl
The desired product was prepared by substituting Example 5B for Example 11B in
Example 11 C and purified by flash column chromatography on silica gel with
95:5
hexanes/acetone.
-3 8-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 5D
compound of formula (I): D1 is C-_-C, Yl is isoxazol-3,5-diyl, A1 is quinol-2-
yl;
X is hydrogen; RI is C6H5
The desired product was prepared by substituting Example 5C for Example 11 C
in
Example 11D and purified by flash column chromatography on silica gel with
75:25
hexanes/acetone.
Example 5E
compound of formula (I): D1 is C;C, Yl is isoxazol-3,5-diyl, A1 is quinol-2-
yl;
to X is hydrogen; RI is hydrogen
The desired product was prepared by substituting Example 5D for Example 4D in
Example 4E. 13C NMR (CDC13) 8 216.8 (C-9), 205.1 (C-3), 169.6 (C-1), 163.8,
157.8,
154.8, 148.2, 148.0, 136.8, 129.9, 129.8, 128.4, 127.6, 127.3, 119.2, 107.6,
103.3, 95.8, 83.5,
80.1, 77.5, 77.3, 72.7, 70.3, 69.7, 65.9, 58.1,51.3, 51.1, 46.9, 44.7, 40.2,
38.6, 37.4, 28.2,
22.4, 21.2, 19.8, 18.0, 15.0, 14.5, 13.6, 13.6, 10.6; HRMS m/z (M+H)+ calcd
for
C45H59N4~11' 831.4175. Found 831.4175.
Example 6
compound of formula (I): D1 is C-C, Yl is isoxazol-3,5-diyl, A1 is quinol-4-
yl;
2o X is hydrogen; Rl is hydrogen
Example 6A
4-quinolinecarbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is quinol-4-yl
The desired product was prepared by substituting 4-quinolinecarbaldehyde for 4-
formylbenzonitrile in Example 9A.
Example 6B
4-(5-(tributylstannyl)-3-isoxazolyl)quinoline
or
compound of formula (iv) in Scheme 1: A1 is quinol-4-yl; R is n-butyl
The desired product was prepared by substituting Example 6A for Example 1 1A
in
Example 11B and purified by flash column chromatography on silica gel with
95:5
hexanes/acetone.
-39-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 6C
4-(5-iodo-3-isoxazolyl)quinoline
or
compound of formula (v) in Scheme 1: A1 is quinol-4-yl
The desired product was prepared by substituting Example 6B for Example 11B in
Example 11 C.
Example 6D
to compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is quinol-
4-yl;
X is hydrogen; Rl is C6HSC O
The desired product was prepared by substituting Example 6C for Example 11 C
in
Example 11D and purified by flash column chromatography on silica gel with
70:30
hexanes/acetone.
Example 6E
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl, A1 is quinol-4-
yl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 6D for Example 4D in
2o Example 4E. 13C NMR (CDC13) ~ 217.0 (C-9), 205.2 (C-3), 169.6 (C-1), 160.8,
157.8,
153.8, 150.0, 148.8, 134.5, 130.0, 129.8, 127.7, 125.8, 125.4, 121.4, 109.1,
103.3, 96.4, 83.6,
80.1, 77.4, 77.3, 72.2, 70.2, 69.7, 65.8, 58.1, 51.3, 51.1, 47.0, 44.7, 40.2,
38.6, 37.4, 28.2,
22.4, 21.2, 19.9, 18.0, 15.0, 14.5, 13.6, 13.6, 10.5. HRMS m/z (M+H)+ calcd
for
~45H59N4~11 831.4175. Found 831.4174.
Example 7
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 4-
fluorophenyl;
X is hydrogen; Rl is hydrogen
3o Example 7A
4-fluoro-N-hydroxybenzenecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is 4-fluorophenyl
A solution of 4-fluorobenzaldoxime (1.0 g, 7.19mmo1) in DMF (6 mL) at room
temperature was treated with HCl gas, collected from the head space of a
bottle of 12M HCI,
(5 mL) and N-chlorosuccinimide (0.960 g, 7.19 mmol) such that the reaction
temperature was
-40-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
below 35 °C, cooled to room temperature, treated with ethyl acetate,
washed with water and
brine, dried (Na2S04), filtered, and concentrated to provide the desired
product.
Example 7B
compound of formula (I): D1 is CSC, Yl is isoxazol-3,5-diyl, A1 is 4-
fluorophenyl;
X is hydrogen; Rl is C6HSC O
A solution of Example 1C (0.12 g, 0.157 mmol) in ethyl acetate (1 mL) and
water
(one drop) was treated with Example 7A (37 mg, 0.212 mmol) and sodium
bicarbonate (26.3
mg, 0.314 mmol), stirred at room temperature for 16 hours, and concentrated.
The
concentrate was purified by flash column chromatography on silica gel with
80:20
hexanes/acetone to provide the desired product.
Example 7C
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl, A1 is 4-
fluorophenyl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 7B Example 11 D in
Example 11E and purified by flash column chromatography on silica gel with
50:50
hexanes/acetone. MS (ESI(+)) m/z 798 (M+H)+.
Example 8
compound of formula (I): DI is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrid-4-
ylz
X is hydrogen; Rl is hydrogen
Example 8A
4-(5-(tributylstannyl)-3-isoxazolyl)pyridine
or
compound of formula (iv) in Scheme 1: A1 is pyrid-4-yl; R is n-butyl
The desired product was prepared by substituting 4-pyridinecarbaldehyde oxime
for
Example 1 1A in Example 11B and purified by flash column chromatography on
silica gel
3o with 95:5 hexanes/acetone.
Example 8B
4-(5-iodo-3-isoxazolyl)pyridine
or
compound of formula (v) in Scheme 1: A1 is pyrid-4-yl
The desired product was prepared by substituting Example 8A for Example 11 B
in
Example 11 C.
-41-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 8C
compound of formula (I): Di is C--_C, Yl is isoxazol-3,5-diyl, A1 is pyrid-4-
yl;
X is hydrogen; Rl is C6H5C O
The desired product was prepared by substituting Example 8B fox Example 11 C
in
Example 11D.
Example 8D
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is pyrid-4-
yl;
to X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 8C fox Example 4D in
Example 4E. 13C NMR (CDCl3) 8 217.0 (C-9), 205.1 (C-3), 169.5 (C-1), 2-160.7,
157.8, 2-
155.5, 150.6, 136.0, 121.0, 106.3, 103.2, 96.3, 83.6, 80.1, 77.3, 77.2, 72.2,
70.2, 69.7, 65.9,
58.0, 51.2, 51.1, 47.0, 44.7, 40.2, 38.5, 37.4, 28.3, 22.4, 21.2, 19.9, 18.0,
15.1, 14.4, 13.6,
15 13.6, 10.6; HRMS m/z (M+H)+ calcd for C41HS~N4O11. 781.4018. Found
781.4019.
Example 9
co~ound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, AI is 4-
cyanophenyl;
X is hydrogen; Rl is hydrogen
Example 9A
4-((hydroxyimino)methyl)benzonitrile
or
compound of formula (i) in Scheme 1: A1 is 4-cyanophenyl
A solution of 4-formylbenzonitrile (2 g, 15.27 mmol) in methanol (6 mL) was
treated
with hydroxylamine hydrochloride (1.09g, 15.72 mmol), stirred at, room
temperature for 24
hours, and concentrated. The concentrate was treated with 5% Na2C03 and
extracted with
ethyl acetate. The extract was washed with brine, dried (Na2S04), filtered,
and concentrated
to provide the desired oxime.
Example 9B
4-cyano-N-hydroxybenzenecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is 4-cyanophenyl
A solution of Example 9A (0.3 g, 2.05 mmol) in DMF (1.5 mL) at room
temperature
was treated with HCl gas, collected from the head space of a bottle of 12M
HCI, (5 mL) and
N-chlorosuccinimide (0.273 g, 2.05 mmol) such that the reaction temperature
was below 30
-42-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
°C, cooled to room temperature, treated with ice/water (10 mL), and
filtered. The solid was
washed with water and dried to provide the desired product as a white solid.
Example 9C
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is 4-
cyanophen 1;
X is hydrogen; Rl is hydrogen
A solution of Example 1D (0.1g, 0.131 mmol) in benzene (1.5 mL) was treated
with
Example 9B (23.5 mg, 0.131 mmol) and triethylamine (19.8 mg, 0.196 mmol),
stirred at
room temperature for 18 hours, treated with additional Example 9B (19 mg,
0.105 mmol) and
l0 triethylamine (13.2 mg, 0.131 mmol), treated with ethyl acetate, washed
with water and brine,
dried (Na2S04), filtered, and concentrated. The concentrate was purified by
flash column
chromatography on silica gel with 98: I :1
dichloromethane/methanol/concentrated
ammonium hydroxide to provide the desired product. MS (ESI(+)) 805 (M+H)+.
15 Example 10
compound of formula (I): Di is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrid-3-
yl;
X is hydrogen; Rl is h drogen
Example 10A
2o N-hydroxy-3-pyridinecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is pyrid-3- 1
The desired product was prepared by substituting nicotinaldehyde oxime for
Example
9A in Example 9B.
Example 10B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrid-3-
yl;
X is hydrogen; Rl is h drogen
The desired product was prepared by substituting Example 10A for Example 9B in
3o Example 9C. 13C NMR (CDC13) 8 217.0 (C-9), 205.1 (C-3), 169.6 (C-1), 160.1,
157.8,
154.1, 151.1, 148.0, 134.2, 124.8, 123.8, 106.1, 103.2, 96.1, 83.6, 80.1,
77.3, 77.3, 72.3, 70.2,
69.7, 65.9, 58.0, 51.3, 51.1, 47.0, 44.7, 40.2, 38.6, 37.4, 28.2, 22.4, 21.2,
19.9, 18.0,
15.1,14.5, 13.6, 13.6, 10.6; HRMS m/~ (M+H)+ calcd for C41H57N4011: 781.4018.
Found
781.4015.
Example 11
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is thien-2-
yl;
X is hydrogen; Rl is hydrogen
-43-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 11 A
2-thiophenecarbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is thien-2-yl
The desired product was prepared by substituting 2-thiophenecarbaldehyde for 4-
formylbenzonitrile in Example 9A.
Example 11 B
3-(2-thienyl)-5-(tributylstannyl)isoxazole
or
compound of formula (iv) in Scheme 1: A1 is thien-2-yl; R is n-butyl
A solution of Example 11A (3 g, 23.6 mmol) in ethyl acetate (70 mL) and water
(100
~.L) was treated with tributyl(ethynyl)stannane (6.82 mL, 23.6 mmol), N-
chlorosuccinimide
(3.13g, 23.6 mmol) and sodium bicarbonate (4.75 g, 56.6 mmol), stirred at room
temperature
for 48 hours, treated with more ethyl acetate (100 mL), washed with water and
brine, dried
(Na2SO4), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 98:2 hexanes/ethyl acetate to provide the
desired product.
Example 11 C
5-iodo-3-(2-thienyl)isoxazole
or
compound of formula (v) in Scheme 1: A1 is thien-2-yl
A solution of Example 11B (1.05 g, 2.38 mmol) in THF (25 mL) at room
temperature
was treated with iodine (0.54 g, 2.14 mmol) in THF (15 mL) over 10 minutes,
stirred for 3
hours, treated with ether (75 mL), washed with 5% NaHC03, 5% Na2S203, and
brine, dried
(Na2S04), filtered, and concentrated. The concentrate was treated with hexanes
and filtered
to provide the desired product.
Example 11 D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thien-2-
yl;
X is hydrogen; Rl is C6HSC O
A solution of Example 1A (503 mg, 0.68 mmol) in degassed acetonitrile (6 mL)
and
triethylamine (3 mL) was treated with Example 11 C (216 mg, 0.782 mmol),
dichlorobis(triphenylphosphine)palladium(II) (47.6 mg, 0.068 mmol) and
copper(I) iodide
-44-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
(3.9 mg, 0.02 mmol), heated at 80 °C for 16 hours, and concentrated.
The concentrate was
purified by flash column chromatography on silica gel with 85:15
hexanes/acetone.
Example 11 E
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thien-2-
yl;
X is hydrogen; Rl is hydrogen
A solution of Example 11D (370 mg, 0.416 mmol) in methanol (15 mL) was stirred
at
room temperature for 60 hours and concentrated. The concentrate was purified
by flash
column chromatography on silica gel with 98:1:1
dichloromethane/methanol/concentrated
l0 ammonium hydroxide to provide the desired product. 13C NMR (CDC13) 8 216.9
(C-9),
205.0 (C-3), 169.6 (C-1), 157.8, 157.8, 153.5, 130.2, 127.8,127.7, 127.6,
106.4, 103.2, 95.7,
83.6, 80.1, 2-77.3, 72.4, 70.2, 69.7, 65.9, 58.0, 51.3, 51.1, 46.9, 44.7,
40.2, 38.6, 37.4, 28.2,
22.4, 21.2, 19.8, 18.0, 15.1, 14.5, 13.6, 13.6, 10.6; HRMS m/z (M+H)+ calcd
for
~40H56N3~llsv 786.3630. Found 786.3619.
Example 12
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-2-
yl;
X is hydrogen; Rl is hydrogen
2o Example 12A
thiazole-2-carbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is thiazol-2-yl
The desired product was prepared by substituting thiazole-2-carbaldehyde for 4-
formylbenzonitrile in Example 9A.
Example 12B
3-(thiazol-2-yl)-5-(tributylstannyl)isoxazole
or
compound of formula (iv) in Scheme 1: Al is thiazol-2-yl; R is n-butyl
3o The desired product was prepared by substituting Example 12A for Example
11A in
Example 11B and purified by flash column chromatography on silica gel with
98:2
hexanes/ethyl acetate.
Example 12C
5-iodo-3-(thiazol-2-yl)isoxazole
or
-45-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (v) in Scheme 1: A1 is thiazol-2-yl
The desired product was prepared by substituting Example 12B Example 11B in
Example 11 C.
Example 12D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-2-
yl;
X is hydrogen; Rl is C6H5C O
The desired product was prepared by substituting Example 12C for Example 11 C
in
Example 11D and purified by flash column chromatography on silica gel with
80:20
to hexanes/acetone.
Example 12E
compound of formula (I): D1 is C--__C, Yl is isoxazol-3,5-diyl, A1 is thiazol-
2-yl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 12D for Example 4D in
Example 4E. 13C NMR (CDC13) 8 216.9 (C-9), 205.0 (C-3), 169.7 (C-1), 158.6,
157.8,
155.0, 154.2, 143.8,121.0, 106.5, 103.2, 96.4, 83.5, 80.1, 77.4, 77.3, 72.3,
70.2, 69.7, 65.9,
58.0, 51.3, 51.1, 46.9, 44.7, 40.2, 38.6, 37.4, 28.2, 22.3, 21.2, 19.8, 18.0,
15.0,14.5,13.6,13.5,10.5; HRMS nalz (M+H)+ calcd for C39H5sN40nS~ 787.3583.
Found
787.3581.
Example 13
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
difluorophenyl;
X is hydrogen; Rl is hydrogen
Example 13A
3,4-difluorobenzaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is 3,4-difluorophenyl
3o The desired product was prepared by substituting 3,4-difluorobenzaldehyde
for 4-
formylbenzonitrile in Example 9A.
Example 13B
3,4-difluoro-N-hydroxybenzenecarboximidoyl chloride
or
-46-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (ii) in Scheme 1: A1 is 3,4-difluorophenyl
The desired product was prepared by substituting Example 13A for Example 9A in
Example 9B.
Example 13 C
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
difluorophenyl;
X is hydrogen; Rl is C6HSC(O)
The desired product was prepared by substituting Examples 1C and 13B for
Examples
1 D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
to silica gel with 85:15 hexanes/acetone.
Example 13D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
difluorophenyl;
X is hydrogen; Rl is hydrogen
15 The desired product was prepared by substituting Example 13C for Example 4D
in
Example 4E. 13C NMR (CDC13) 8 217.0 (C-9), 205.1 (C-3), 169.5 (C-1), 160.8,
157.8,
154.0, 152.7, 150.0 125.7, d (123.3 and 123.5), d (118.0 and 117.8), d (116.2
and 116.0),
106.2, 103.2, 95.9, 83.6, 80.1, 77.3; 77.3, 72.3, 70.2, 69.6, 66.0, 58.0,
51.3, 51.1, 47.0, 44.7,
40.2, 38.5, 37.4, 28.4, 22.4, 21.2, 19.9, 18.0, 15.1, 14.5, 13.6, 13.6, 10.6;
HRMS m/z (M+H)+
2o calcd for G42H56F2N3011 816.3877. Found 816.3886.
Example 14
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl,
A1 is 3-(trifluoromethyl)phenyl; X is hydrogen; Rl is hydrogen
Example 14A
N-hydroxy-3-(trifluoromethyl)benzenecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is 3-(trifluoromethyl)phenyl
The desired product was prepared by substituting 3-
(trifluoromethyl)benzaldehyde
oxime for Example 9A in Example 9B.
Example 14B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 3-(trifluoromethyl)phenyl; X is hydrogen; Rl is C~HS
The desired product was prepared by substituting Examples 1C and 14A for
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
silica gel with 85:15 hexanes/acetone.
-47-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 14C
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 3-(trifluoromethyl)phenyl; X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 14B for Example 4D in
Example 4E and purified by flash column chromatography on silica gel with
98.5:1:0.5
dichloromethane/methanol/concentrated ammonium hydroxide. 13C NMR (CDCl3) ~
216.9
(C-9), 205.1 (C-3), 169.5 (C-1), 161.5, 157.8, 154.1, 131.7, 130.1, 129.5,
126.7, d (126.6 and
128.6), 123.8, d (123.8 and 123.7), 106.3, 103.2, 96.0, 83.6, 80.1, 77.3,
72.3, 72.4, 70.2, 696,
66.0, 58.0, 51.3, 51.1, 47.0, 44.7, 40.2, 38.6, 37.4, 28.4, 22.4, 21.1, 20.0,
18.0, 15.1, 14.5, ,
13.6, 13.6, 10.6; HRMS m/z (M+H)+ calcd for C43HS~F3N3O11: 848.3940. Found
848.3948.
Example 15
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
dichlorophenyl;
X is hydrogen; Rl is hydrogen
Example 15A
3,4-dichloro-N-hydroxybenzenecarboximidoyl chloride
or
, compound of formula (ii) in Scheme 1: A1 is 3,4-dichlorophenyl
The desired product was prepared by substituting 3,4-dichlorobenzaldehyde
oxime for
Example 9A in Example 9B.
Example 15B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
dichlorophenyl;
X is hydrogen; Rl is C6H5
The desired product was prepared by substituting Examples 1 C and 1 SA for
Examples
1 D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
silica gel with 85:15 hexanes/acetone.
Example 15C
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
dichlorophenyl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 15B for Example 14B
in
Example 14C. 13C NMR (CDC13) 8 217.0 (C-9), 205.1 (C-3), 169.5 (C-1), 160.7,
157.9,
154.1, 134.3, 133.3, 130.9, 128.7, 128.6, 126.1, 106.1, 103.3, 96.1, 83.6,
80.1, 77.3, 72.3,
70.2, 69.7, 65.9, 58.0, 51.2, 51.1, 47.0, 44.7, 40.2, 38.5, 37.4, 28.2, 22.4,
21.2, 19.9, 18.0,
-48-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
15.1, 14.5, 13.6, 13.6, 10.6; HRMS m/z (M+H)+ calcd for C42Hs6C1aN3DW
848.3286. Found
848.3303.
Example 16
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3-
cyanophenyl;
X is hydrogen; Rl is hydrogen
Example 16A
3-((hydroxyimino)inethyl)benzonitrile
or
compound of formula (i) in Scheme 1: A1 is 3-cyanophenyl
The desired product was prepared by substituting 3-formylbenzonitrile for 4-
formylbenzonitrile in Example 9A.
Example 16B
3-cyano-N hydroxybenzenecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is 3-cyanophenyl
The desired product was prepared by substituting Example 16A for Example 9A in
Example 9B.
Example 16C
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 3-
cyanophenyl;
X is hydrogen; Rl is C6H5
The desired product was prepared by substituting Examples 1 C and 16B foi
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
silica gel with 85:15 hexanes/acetone.
Example.16D
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is 3-
cyanophenyl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 16C for Example 4D in
Example 4E. 13C NMR (CDCl3) 8 217.1 (C-9), 205.1 (C-3), 169.5 (C-1), 160.8,
157.8,
154.4, 133.3, 131.1, 130.3, 130.0, 129.9, 117.1,113.3, 106.1, 103.3, 96.2,
83.6, 80.1, 77.3,
3s 72.2, 70.2, 69.7, 65.9, 58.0, 51.2, 51.1, 47.1, 44.7, 40.2, 38.5, 37.4,
28.2, 22.4, 21.2, 19.9,
18.0, 15.2, 14.4, 13.6, 13.6, 10.6; HRMS ~z/z (M+H)+ calcd for C43HS~N4O11:
805.4018.
Found 805.4012.
-49-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 17
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 4-cyano-3-(methylsulfanyl)pyrid-2-yl; X is hydrogen; Rl is hydrogen
Example 17A
6-((hydroxyimino)methyl)-2-(methylsulfanyl)nicotinonitrile
or
compound of formula (i) in Scheme 1: A1 is 4-cyano-3-(methylsulfanyl)pyrid-2-
yl
The desired product was prepared by substituting 6-formyl-2-
(methylsulfanyl)nicotinonitrile for 4-formylbenzonitrile in Example 9A.
Example 17B
5-cyano-N-hydroxy-6-(methylsulfanyl)-2-pyridinecarboximidoyl chloride
or
compound of formula (ii) in Scheme 1: A1 is 4-cyano-3-(methylsulfanyl)pyrid-2-
yl
The desired product was prepared by substituting Example 17A for Example 9A in
Example 9B.
Example 17C
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 4-cyano-3-(methylsulfanyl)pyrid-2-yl; X is hydrogen; Rl is CH3C O
A solution of Example 1E (125 mg, 0.179 mmol) in ethyl acetate (2 mL) at room
temperature was treated with Example 17B (6lmg, 0.267mmol) and sodium
bicarbonate
(44.8 mg, 0.534 mmol), stirred for 2 hours, treated with additional Example
17B (202 mg,
0.89 mmol) and sodium bicarbonate (30.0 mg, 0.3576 rmnol) added simultaneously
in 4
portions over 7 hours, treated with ethyl acetate, washed with water and
brine, dried
(Na2S04), filtered, and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 60:40 hexanes/acetone to provide the desired
product.
Example 17D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 4-cyano-3-(methylsulfanyl)pyrid-2-yl; X is hydrogen; Rl is hydrogen
A solution of Example 17C (130 mg, 0.145 mmol) in methanol (10 mL) was stirred
at
room temperature for 16 hours, concentrated, and dried to constant weight to
provide the
desired product. MS (ESI(+)) 852 (M+H)+. .
-50-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 18
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is hydrogen; Rl is hydrogen
Example 18A
2,4-dibromothiazole-5-carbaldehyde
A mixture of thiazolidine-2,4-dione (4.54 g, 34.88 mmol) and phosphorus
oxybromide (50 g, 174.4 mmol) was treated with DMF (3.05 mL, 39.41 mmol),
stirred at
room temperature for 30 minutes, heated at 80 °C for 30 minutes, heated
at 108 °C until
l0 hydrogen bromide evolution ceased (approximately 7 hours), cooled to room
temperature,
treated with ice/water (300 mL), and extracted with dichloromethane. The
extract was
washed with 5% aqueous NaHC03 and brine, dried (MgS04), filtered, and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
98:2
hexanes/ethyl acetate to provide the desired product.
Example 18B
thiazole-5-carbaldehyde
The desired product was prepared from Example 18A as described in Syh. Comm.,
25(24), 4081-4086 (1995).
Example 18C
thiazole-5-carbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is thiazol-5-yl
The desired product was prepared by substituting Example 18B for 4-
formylbenzonitrile in Example 9A.
Example 18D
3-(thiazol-5-yl)-5-(tributylstannyl)isoxazole
or
3o compound of formula (iv) in Scheme 1: A1 is thiazol-5-yl; R is n-butyl
The desired product was prepared by substituting Example 18C for Example 1 1A
in
Example 11B.
Example 18E
5-iodo-3-(thiazol-5-yl)isoxazole
or
-51-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (v) in Scheme 1: A1 is thiazol-5-yl
The desired product was prepared by substituting Example 18D for Example 11B
in
Example 11 C.
Example 18F
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is hydrogen; Rl is C6H5
The desired product was prepared by substituting Example 18E for Example 11 C
in
Example 11D and purified by flash column chromatography on silica gel with
80:20
1o hexaneslacetone.
Example 18G
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is hydrogen; Rl is hydrogen
15 The desired product was prepared by substituting Example 18F for Example 4D
in
Example 4E. 13C NMR (CDCl3) 8 217.1 (C-9), 205.1 (C-3), 169.6 (C-1), 157.8,
155.4,
154.6, 154.1, 143.4 106.3, 103.2, 96.4, 83.6, 80.1, 77.3, 72.1, 70.2, 69.7,
65.9, 58.0, 53.4,
51.2, 51.1, 47.0, 44.7, 40.2, 38.6, 37.4, 28.2, 22.4, 21.1, 19.8, 18.0, 15.1,
14.5, 13.6, 13.6,
10.6; HRMS m/z (M+H)+ calcd for C39H55N4011S~ 787.3588. Found 787.3583.
Example 19
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 6-chloroimidazo(2,1-b)thiazol-5-yl; X is fluoride; Rl is hydrogen
Example 19A
6-chloroimidazo(2,1-b)thiazole-5-carbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is 6-chloroimidazo(2,1-b)thiazol-5-yl
The desired product was prepared by substituting 6-chloroimidazo(2,1-
b)thiazole-5-
carbaldehyde for 4-formylbenzonitrile in Example 9A.
Example 19B
6-chloro-5-(5-(tributylstannyl)-3-isoxazolyl)imidazo(2,1-b)thiazole
or
-52-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (iv) in Scheme 1: A1 is 6-chloroimidazo(2,1-b)thiazol-5-
yl; R is n
butyl .
The desired product was prepared by substituting Example 19A for Example 1 1A
in
Example 11B.
Example 19C
6-chloro-5-(5-iodo-3-isoxazolyl)imidazo(2,1-b)thiazole
or
compound of formula (v) in Scheme 1: A1 is 6-chloroimidazo(2,1-b)thiazol-5-yl
l0 The desired product was prepared by substituting Example 19B for Example
11B in
Example 11 C.
Example 19D
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl,
15 A1 is 6-chloroimidazo(2,1-b)thiazol-5-yl; X is fluoride; Rl is C6HSC(O)
The desired product was prepared by substituting Examples 2A and 19C for
Examples
1 A and 11 C, respectively, in Example 11 D and purified by flash column
chromatography on
silica gel with 80:20 hexanes/acetone.
2o Example 19E
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl,
A1 is 6-chloroimidazo(2,1-b)thiazol-5-yl; X is fluoride; Rl is hydrogen
The desired product was prepared by substituting Example 19D for Example 4D in
Example 4E and purified by flash column chromatography on silica gel with 98:2
25 dichloromethane/methanol. 13C NMR (CDC13) 8 216.7 (C-9), d (204.3 and
204.0) (C-3), d
(166.4 and 166.3) (C-1), 157.3, 153.4, 152.6, 149.5, 134.0,121.9, 112.8,
105.5, 104.4, 99.0,
96.6, 96.3, 83.4, 80.7, 80.0, 78.8, 72.1, 70.3, 69.3, 66.8, 58.0, 50.7, 44.1,
40.6, 40.2, 38.3,
37.5, 28.1, 25.4, 25.1, 22.3, 21.2, 20.4, 17.7, 15.3, 13.8, 13.3, 10.7; HRMS
m/z (M+H)+
calcd for C41H5aC1FN5011S: 878.3208. Found 878.3199.
Example 20
compound of formula (I): D1 is C~-_C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is fluoride; Rl is hydrogen
Example 20A
compound of formula (I): D1 is C-C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is fluoride; Rl is C6HSC(O)
-53-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
The desired product was prepared by substituting Examples 2A and 18E for
Examples
1A and 11C, respectively, in Example 11D and purified by flash column
chromatography on
silica gel with 80:20 hexanes/acetone.
Example 20B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-5-
yl;
X is fluoride; Rl is hydrogen
The desired product was prepared by substituting Example 20A for Example 4D in
1o Example 4E. 13C NMR (CDC13) ~ 216.9 (C-9), d (204.3 and 203.9) (C-3), d
(166.1 and
166.8) (C-1), 157.4, 155.5, 154.6, 154.0, 143.5, 125.8, 106.3, 104.2, d (99.0
and 96.2), 96.8,
83.5, 80.8, 80.0, 78.7, 72.0, 70.3, 69.8, 65.8, 58:0, 50.7, 44.1, 40.5, 40.2,
38.3, 37.5, 28.1, d
(25.4 and 25.1), 22.2, 21.2, 20.3, 17.7, 15.4, 13.7, 13.3, 10.6; HRMS m/z
(M+H)+ calcd for
C39H54N4011FS: 805.3494. Found 805.3466.
is
Example 21
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is thiazol-2-
yl;
X is fluoride; Rl is hydrogen
2o Example 21A
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-2-
yl;
X is fluoride; Rl is C6HSC O
The desired product was prepared by substituting Examples 2A and 12C for
Examples
1A and 11C, respectively, in Example 11D and purified by flash column
chrorriatography on
25 silica gel with 80:20 hexanes/acetone.
Example 21 B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is thiazol-2-
yl;
3o X is fluoride; Rl is hydrogen
The desired product was prepared by substituting Example 21A for Example 14B
in
Example 14C. 13C NMR (CDCl3) 8 216.6 (C-9), d (204.2 and 203.8) (C-3), d
(166.1 and
165.9) (C-1), 158.6, 157.3, 155.9, 154.2, 143.8, 121.0, 106.5, 104.2, d (98.8
and 96.2), 96.8,
83.4, 80.8, 80.0, 78.7, 72.2, 70.3, 69.8, 65.8, 58.1, 50.8, 44.1, 40.5,40.2,
38.3, 37.5, 28.1, d
35 (25.3 and 25.1), 22.2, 21.2, 20.2, 17.6, 15.3, 13.7, 13.2, 10.5; HRMS m/z
(M+H)+ calcd for
C39H54FN4011s~ 805.3488. Found 805.3484.
-54-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
Example 22
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
dichlorophenyl;
X is fluoride; Rl is hydrogen
Example 22A
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is 3,4-
dichlorophenyl;
X is fluoride; Rl is C6HSC O
The desired product was prepared by substituting Examples 2C and 15A for
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
1 o silica gel with 85:15 hexanes/acetone.
Example 22B
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl, AI is 3,4-
dichlorophenyl;
X is fluoride; Rl is hydrogen
15 The desired product was prepared by substituting Example 22A for Example 4D
in
Example 4E. 13C NMR (CDC13) 8 216.8 (C-9), d (204.3 and 203.9) (C-3), d (166.0
and
166.8) (C-1), 160.7, 157.4, 154.1, 134.3, 133.3, 131.0, 128.7, 128.5, 126.1,
106.2, 104.2, d
(98.8 and 96.3), 96.5, 83.5, 80.7, 80.0, 78.8, 72.2, 70.3, 69.8, 65.9, 58.1,
50.7, 44.1, 40.5,
40.2, 38.3, 37.4, 28.2, d (25.4 and 25.1), 22.3, 21.2, 20.4, 17.6, 15.4, 13.7,
13.2, 10.6; HRMS
2o mlz (M+H)+ calcd for C42H55C12FN3~ll 866.3192. Found 866.3196.
Example 23
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrimidin-
5-yl;
X is fluoride; Rl is hydrogen
Example 23A
5-pyrimidinecarbaldehyde
A solution of 5-bromopyrimidine (12 g, 0.075 mol) in THF (500 mL) at -100
°C was
treated with 2.5M n-butyllithium in hexanes (30.2 mL, 79 mmol) over 35
minutes, stirred for
15 minutes at -100 °C, treated with ethyl formate (6.7 mL, 0.0825 mol)
over 15 minutes,
stirred for 15 minutes at -95 °C, treated with 1M HCl in ether (79 mL,
0.0787 mol) over 10
minutes, warmed to room temperature over 1 hour, and concentrated. The
concentrate was
treated with dichloromethane, and the resulting solution was washed with water
and brine,
dried (MgS04), filtered, and concentrated to provide the desired product.
Example 23B
5-pyrimidinecarbaldehyde oxime
-55-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
or
compound of formula (i) in Scheme 1: A1 is pyrimidin-5-yl
The desired product was prepared by substituting Example 23A for 4-
formylbenzonitrile and substituting dichloromethane for ethyl acetate in the
work-up to
provide the desired product.
Example 23C
N-hydroxy-5-pyrimidinecarboximidoyl chloride
or
l0 compound of formula (ii) in Scheme 1: A1 is pyrimidin-5-yl
The desired product was prepared by substituting Example 23B for Example 9A in
Example 9B.
Example 23D
15 compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is
pyrimidin-5-yl;
X is fluoride; Rl is C6HSC O
The desired product was prepared by substituting Examples 2C and 23C for
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
silica gel with 80:20 hexanes/acetone.
Example 23E
compound of formula (I): Dl is C---C, Yl is isoxazol-3,5-diyl, A1 is pyrimidin-
5-yl;
X is fluoride; Rl is hydrogen
The desired product was prepared by substituting Example 23D for Example 14B
in
Example 14C. 13C NMR (CDC13) 8 216.9 (C-9), d (204.3 and 204.0) (C-3), d
(166.1 and
166.8) (C-1), 159.7, d (157.6 and 157.4), 154.8, 154.7, 154.6, 123.3, 105.8,
104.2, d (99.0
and 96.2), 97.2, 83.5, 80.8, 80.0, 78.7, 71.9, 70.2, 69.8, 65.9, 58.0, 50.7,
44.1, 40.5, 40.2,
38.3, 37.5, 28.2, d (25.4 and 25.1), 22.3, 21.2, 20.4, 17.6, 15.4, 13.7, 13.2,
10.6; HRMS m/z
(M+H)+ calcd for C4oH55FN5O11: 800.3879. Found 800.3876.
Example 24
_compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 2-methyl-2H-tetrazol-5-yl; X is hydrogen; Rl is hydrogen
Example 24A
2-methyl-2H-tetrazole
A solution of sodium hydroxide (14.2 g, 0.357 mol) in water (600 mL) at room
temperature was treated with 1H-tetrazole (25 g, 0.357 mol) until homogeneous,
treated with
-56-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
dichloromethane (600 mL), dimethyl sulfate (47.2 g, 0.375 mol), and N-
tetrabutylammonium
bromide (S.7 g, 0.0178 mol), and stirred for 14 hours. The organic layer was
separated and
concentrated, and the concentrate was distilled to provide the desired product
(143 °C/1 atm).
Example 24B
2-methyl-2H-tetrazole-5-carbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is 2-methyl-2H-tetrazol-S-yl
A solution of 2M lithium diisopropyl amide in heptane/THF/ethylbenzene (6.5S
mL,
l0 13.09 mmol) in THF (8.S mL) at -7S °C was treated with Example 24A
(1 g, 11.9 mmol) in
THF (10 mL) over 3 minutes, stirred for 30 minutes, treated with DMF (3.7 mL,
47.84 mmol)
over 10 minutes, stirred for S minutes, warmed to room temperature, treated
with
hydroxylamine hydrochloride (1.1 S g, 17.85 mmol) in methanol (6 mL) over 2
minutes,
stirred for 20 hours, and concentrated. The concentrate was treated with ethyl
acetate,
15 washed with S% NaHC03, water, and brine, dried (Na2S04), filtered, and
concentrated to
provide the desired product.
Example 24C
N-hydroxy-2-methyl-2H-tetrazole-S-carboximidoyl chloride
20 or
compound of formula (ii) in Scheme 1: A1 is 2-methyl-2H-tetrazol-S-yl
The desired product was prepared by substituting Example 24B for Example 9A in
Example 9B.
Example 24D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,S-diyl,
Al is 2-methyl-2H-tetrazol-5-yl; X is hydrogen; Rl is C HSC(O)
The desired product was prepared by substituting Examples 1 C and 24C for
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
silica gel with 80:20 hexanes/acetone.
Example 24E
compound of formula (I): Dl is C---C, Yl is isoxazol-3,S-diyl,
A1 is 2-methyl-2H-tetrazol-S-yl; X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting the product from Example 24D
for
Example 14B in Example 14C. 13C NMR (CDC13) 8 217.1 (C-9), 205.0 (C-3), 169.7
(C-1),
-S7-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
157.8, 155.8, 154.6, 153.1, 107.4, 103.2, 96.8, 83.5, 80.2, 77.4, 77.3, 72.1,
70.2, 69.6, 65.9,
58.0, 51.3, 51.1, 46.9, 44.7, 40.2, 39.8, 38.6, 37.4, 28.2, 22.3, 21.1, 19.7,
17.9, 14.9, 14.5,
13.6, 13.5, 10.5; HRMS m/z (M+H)+ calcd for C3gH56N~011: 786.4038. Found
786.4016.
Example 25
compound of formula (I): Di is C---C, YI is isoxazol-3,S-diyl,
A1 is 2-methyl-2H-tetrazol-S-yl; X is fluoride; Rl is hydrogen
Example 25A
l0 compound of formula (I): Dl is C---C, Yl is isoxazol-3,S-diyl,
A1 is 2-methyl-2H-tetrazol-5-yl; X is fluoride; Rl is C6HSC O
The desired product was prepared by substituting Example 2C for Example 1 C in
Example 24D.
Example 25B
compound of formula (I): D1 is C---C, Yl is isoxazol-3,S-diyl,
A1 is 2-methyl-2H-tetrazol-S-yl; X is fluoride; Rl is hydrogen
The desired product was prepared by substituting Example 25A for Example 4D in
Example 4E. 13C NMR (CDCl3) 8 217.1 (C-9), 205.0 (C-3), 169.7 (C-1), 157.8,
155.8,
154.6, 153.1, 107.4, 103.2, 96.8, 83.5, 80.2, 77.4, 77.3, 72.1, 70.2, 69.6,
65.9, 58.0, 51.3,
51.1, 46.9, 44.7, 40.2, 39.8, 38.6, 37.4, 28.2, 22.3, 21.1, 19.7, 17.9, 14.9,
14.5, 13.6, 13.5,
10.5; HRMS m/z (M+H)+ calcd for C38HSSN7011F: 804.3944. Found 804.3922.
Example 26
compound of formula (I): D1 is C---C, Y1 is isoxazol-3,5-diyl, A1 is 2-
chloroquinol-3-yl;
X is hydrogen; Ri is hydrogen
Example 26A
2-chloro-3-quinolinecarbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is 2-chloroguinol-3-yl
The desired product was prepared by substituting 2-chloro-3-
quinolinecarbaldehyde
for 4-formylbenzonitrile in Example 9A.
Example 26B
2-chloro-N-hydroxy-3-quinolinecarboximidoyl chloride
or
-S 8-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (ii) in Scheme 1: Al is 2-chloroquinol-3-yl
The desired product was prepared by substituting Example 26A for Example 9A in
Example 9B.
Example 26C
compound of formula (I): D1 is C---C, YI is isoxazol-3,5-diyl, A1 is 2-
chloroquinol-3-yl;
X is hydrogen; Rl is CH3C O
A solution of Example 1E (125 mg, 0.1788 mmol) in benzene (1.5 ml) was treated
with Example 26B (Slmg, 0.213mmo1) and triethylamine (30.5, 0.302 mmol), added
in 3
portions over 30 minutes, stirred at room temperature for 20 hours and
concentrated. The
concentrate was purified by flash column chromatography on silica gel with
99:1
dichloromethane/methanol to provide the desired product.
Example 26D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl, A1 is 2-
chloroquinol-3-yl;
X is hydrogen; Rl is hydrogen
The desired product was prepared by substituting Example 26C for Example 17C
in
Example 17D. 13C NMR (CDC13) 8 216.8 (C-9), d (204.2 and 203.8) (C-3), d
(166.2 and
165.0) (C-1), 157.3, 156.8, 154.5, 153.1, 107.3 104.2, d (99.0 and 96.3),
97.2, 83.4, 80.8,
80.0, 78.6, 72.0, 70.3, 69.8, 65.8, 58.0, 50.8, 44.0, 40.6, 40.2, 39.8, 38.4,
37.5, 28.1, d (25.3
and 25.0), 22.2, 21.2, 20.2, 17.6, 15.3, 13.7, 13.2, 10.5; HRMS m/z (M+H)+
calcd for
~39H53FN4~llsv 809.3973. Found 809.3966.
Example 27
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 3-methylbenzo(b)thien-2-yl; X is hydrogen; Rl is hydrogen
Example 27A
3-methylbenzo(b)thiophene-2-carbaldehyde oxime
or
compound of formula (i) in Scheme 1: A1 is 3-methylbenzo(b)thien-2-yl
The desired product was prepared by substituting 3-methylbenzo(b)thiophene-2-
carbaldehyde for 4-formylbenzonitrile in Example 9A.
Example 27B
N-hydroxy-3-methylbenzo(b)thiophene-2-carboximidoyl chloride
or
-59-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
compound of formula (ii) in Scheme 1: Al is 3-methylbenzo(b)thien-2-yl
The desired product was prepared by substituting Example 27A for Example 9A in
Example 9B.
Example 27C
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl,
A1 is 3-methylbenzo(b)thien-2-yl; X is hydrogen; Rl is C HSC O
The desired product was prepared by substituting Examples 1 C and 27B for
Examples
1D and 9B, respectively, in Example 9C and purified by flash column
chromatography on
to silica gel with 85:15 hexanes/acetone.
Example 27D
compound of formula (I): D1 is C---C, Yl is isoxazol-3,5-diyl,
A1 is 3-methylbenzo(b)thien-2-yl; X is hydrogen; Rl is hydrogen
15 The desired product was prepared by substituting Example 27C for Example
14B in
Example 14C. 13C NMR (CDC13) 8 217.0 (C-9), 205.2 (C-3), 173.3, 169.6 (C-1),
158.1,
157.8, 153.5, 140.7, 139.4, 133.0, 125.5, 124.3, 122.6, 122.3, 108.2, 103.1,
96.0, 83.6, 80.1,
77.3, 77.2, 72.5, 70.2, 69.6, 65.9, 58.1, 51.8, 51.3, 51.0, 46.8, 44.7, 40.2,
38.6, 37.4, 28.4,
22.4, 21.2, 19.7, 18.0, 14.9, 14.5, d (13.6 and 13.6), 13.2, 10.5; HRMS m/z
(M+H)~ calcd for
2o C45H60N3~11s~ 850.3943. Found 850.3947.
Example 28
compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl, A1 is pyrid-2-yl;
X is hydrogen; Rl is hydrogen
Example 28A
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is pyrid-2-
yl;
X is hydrogen; Rl is C HSC O
A solution of Example 1A (1.42 g, 2.0 mmol) and Example 3B (408 mg, 3 mmol) in
acetonitrile (6 mL) and triethylamine (1 mL) at room temperature was degassed,
treated with
tris(dibenzylideneacetone)dipalladium(0) (9I mg, O.I mmol) and triphenylarsine
(61 mg, 0.2
mmol), degassed again, heated at 80 °C for 24 hours, and concentrated.
The concentrate was
purified by flash column chromatography on silica gel with 2:1 hexanes/acetone
to provide
the desired product.
Example 28B
compound of formula (I): D1 is C--_C, Yl is isoxazol-3,5-diyl, A1 is pyrid-2-
yl;
-60-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
X is hydrogen; Rl is hydrogen
A solution of Example 28A (I.24 g, 1.4 mmol) in methanol (20 mL) was heated at
reflux for 6 hours and concentrated. The concentrate was purified by flash
column
chromatography on silica gel with 100:1:0.5
dichloromethane/methanol/concentrated
ammonium hydroxide to provide the desired product. MS m/z 781 (M+H)+; i3C NMR
(75
MHz, CDC13) ~ 216.7, 205.0, 169.6, 163.3, 157.8, 153.7, 149.7, 148.1, 136.7,
124.4, 121.7,
107.2, 103.2, 95.7, 83.5, 80.0, 77.4, 77.3, 70.2, 69.6, 65.8, 58.1, 51.3,
51.1, 46.9, 44.6, 40.2,
38.6, 37.4, 28.2, 22.3, 21.1, 19.8, 18.0, 15.0, 14.5, 13.6, 13.5, 10.5; HRMS
rnlz calcd (M+H)+
for C41H56N4~11~ 781.4018. Found: 781.4018.
l0
Example 29
compound of formula (I): D1 is CSC, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
yl;
X is fluoride; Rl is hydrogen
Example 29A
3-(5-(tributylstannyl)-3-isoxazolyl)quinoline
or
compound of formula (iv) in Scheme 1: A1 is quinol-3-yl; R is n-butyl
The desired product was prepared by substituting 3-quinolinecarbaldehyde oxime
for
2-pyridinecarbaldehyde oxime in Example 3A.
Example 29B
3-(5-iodo-3-isoxazolyl)quinoline
or
compound of formula (v) in Scheme 1: A1 is quinol-3-yl
The desired product was prepared by substituting Example 29A for Example 3A in
Example 3B.
Example 29C
3o compound of formula (I): D1 is C=C, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
yl;
X is fluoride; Rl is C6HSC~0~
The desired product was prepared by substituting Examples 2A and 29B for
Examples
1A and 3B, respectively, in Example 28A.
Example 29D
compound of formula (I): D1 is CSC, Yl is isoxazol-3,5-diyl, A1 is quinol-3-
yl;
X is fluoride; Rl is hydrogen
-61-
CA 02420012 2003-02-17
WO 02/32919 PCT/USO1/32278
The desired product was prepared by substituting Example 29C for Example 28A
in
Example 28B. MS m/z 849 (M+H)+; i3C NMR (75 MHz, CDCl3) 8 216.8, d (204.3 and
203.9), d (166.1 and 165.8), 106.4, 157.5, 154.0, 148.6, 148.4, 134.3, 130.5,
129.4, 128.4,
127.5, 127.3, 121.8, 106.3, 104.2, d (99.0 and 96.2), 96.6, 83.5, 80.7, 80.0,
78.7, 72.3, 70.3,
69.8, 65.8, 58.1, 50.7, 44.1, 40.5, 40.2, 38.3, 37.4, 28.1, d (25.4 and 25.1),
22.2, 21.2, 20.3,
17.6, 15.4, 13.7, 13.2, 10.6; HRMS ~/z calcd (M+H)+ for C45HS~FN4011:
849.4081. Found:
849.4087.
It will be evident to one skilled in the art that the invention is not limited
to the
forgoing examples, and that it can be embodied in other specific forms without
departing
to from the essential attributes thereof. Thus, it is desired that the
examples be considered as
illustrative and not restrictive, reference being made to the claims, and that
all changes which
come within the meaning and range of equivalency of the claims be embraced
therein.
w
-62-