Note: Descriptions are shown in the official language in which they were submitted.
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97/29716 PCTIUS97/02377
-;-
ENDOVASCULAR APPARATUS
Background of the Invention
Field of the Invention
The present invention relates to the percutaneous treatment of vessels by
an apparatus and method wherein the apparatus is delivered via catheter and
comprises
a surgical graft which is fixated in a vessel by means of a chemical or
mechanical
hardening-filler material system.,
Geneml Backaround
Previous methods of treating aortic aneurysms include treatment via
surgical procedure in which an incision is made in the abdomen or chest of the
patient,
the diseased area is cleaned by the surgeon and an artificial graft is sutured
in place.
Tliis highly invasive proeedure usually results in long izospital stays and
lengthy
recoveries. Further, mortality and morbidity complications often result as a
consequence of this surgical procedure.
Other percutaneous methods have been attempted, such as are disclosed
in U.S. Patent No. 4,577,631 (utilizing occlusion catheters with pressure
sensitive
adhesives), U.S. Patent No. 4,740,207 (self-expanding stent-type materials)
and U.S.
Patents No. 4,271,839, 4,776,337 and 4,762,132 (other stent derived devices).
There still exists a need, however, for a simple method of repairing a
vessel with an intravascular graft which allows normal tissue ingrowth to
occur at the
repair site. There exists a specific need for a percutaneous approach in which
a
catheter could be loaded with a surgical graft that can be fixated in a vessel
such as the
aorta.
Summarv of the Invention
The present invention provides devices for repairing aortic aneurysms and
the like. The intraiuminal graft of the present invention in one embodiment
comprises
a flexible linear or bifurcated tubular sleeve delivered to a repair site in a
body by
suitable means such as a catheter. The sleeve is suitably made of woven or
cast
material, and has peripheral conduits or tubes at each end. Each conduit has
at least
a single port that is connected to an elongated introduction means associated
with the
catheter delivery ineans. The introduction means may be attached to the outer
surface
of the sleeve. The collapsed sleeve may be made rigid and circular by the
introduction
through the introduction means of a chemical or mechanical hardenin, means.
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97/29716 PCTIUS97/02377
-2-
The chemical hardening means may be a polymeric material introduced
through the introduction means through an external source, sucli as a catheter
or
syringe. Alternatively, the mechanical hardening means may comprise a single
wire
or multiple wires inserted into the conduits to support the ends, or any
portion of the
sleeve. The wires are not attached to the sleeve but reside in the conduits to
provide
a constant spring tension. The wires may be of any suitable material which
retains its
tension, such as spring wire or memory wire.
The introduction means may be detached from the sleeve after
introduction of the chemical or mechanical hardening means.
The sleeve may alternatively be associated with a fixation means
comprising either a series of cylindrical tubules or an enclosure which fits
over the
sleeve, with a hardening-filler system enclosed therein. The hardening-fillcr
system
includes an activatable hardening material which may be provided in the form
of
microspheres that upon external agitation may be disrupted, allowing the
contents to
react together and form a hardened material that fills the tubules or
enclosure, thereby
expanding and rigidifying the fixation means, and fixing the sleeve in place
in the site
of repair. Polymeric materials which are activatable include thioisocyanates,
aldehydes,
isocyanates, divinyl compounds, epoxides or acrylates. In addition to the
aforementioned, photoactivatable crosslinkable groups as succinimidyl azido
salicylate,
succinimidyl-azidobenzoate, succinimidyl dithio acetatc, azidoiodobenzene,
fluoro
nitrophenylazide, salicylate azides, benzophenone-maieimide, and the iike may
be used
as photoactivatable crosslinking reagents. The material may also consist of a
thin
coating which can be activated by external forces such as laser, radio
frequency,
ultrasound or the like, with the same hardening result taking place. These
materials
would allow for normal tissue ingrowth to take place,
Brief Description of the Fieures
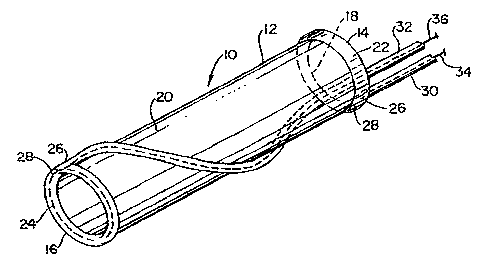
Figure 1 shows a perspective view of a vascular graft according to the
present invention in a folded state prior to placement and expansion thereof;
Figure 2 shows a perspective view of the vascular graft in an expanded
state by means of wires;
Figure 3 is a perspective view of the device as in Figurc 2 showing the
introduction of chemical hardening material via syringe;
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97129716 PCT/US97102377
-3-
Figure 4 is a perspective view of an alternate embodiment comprising a
series of cylindrical tubules;.
Figure 5 is a perspective view of an alternative embodiment of the
device, where the vascular graft includes an enclosure which fits over the
sleeve;
.5 Figure 6 is an alternative embodiment of the present invention having a
fluid track comprising a continuous cylindrical tubule which is helically
wound around
the proximal and distal ends of the sleeve;
Figures 7a and 7b represent an alternative embodiment comprising a
bifurcated vascular graft including a dual guide wire delivery system;
Figures 8a through 8d show placement of a bifurcated vascular graft
according to the present invention;
Figure 9 shows a further alternative embod'sment of a vascular graft
according to the present invention;
Figures l0a through 10c show filling of the cylindrical tubules after
placement of the graft;
Figures 11a through .11d are fragmentary views of vascular grafts
according to the present invention; and
Figures 12a and 12b are cross sectionai vicws of a vascular graft
according to the present invention.
Detailed Description of the Invention
The present invention provides a device and method for repairing an
aneurysm or the like in a vessel, such as the aorta.
Referring to Figures 1 and 2, a vascular graft comprising a sieeve is
shown generally at 10. Sleeve 10 is shown in a folded conformation in Figure 1
and
in an expanded state in Figure 2. Sleeve 10 is either a flexible linear or
bifurcated (as
shown in Figures 7-12) tubular sleeve made of woven or extruded cast material.
Sleeve
10 is made of a biocompatible polymeric material. Fabrics from which sleeve 10
may
be made are polyamides, such as nylon 6, nylon 6,6, and the like, DacronO,
polyesters,
such as PET, polyethers, fluorinated polymers, such as polytetrafluoroethylene
(PTFE),
or biodegradable or nonbiodegradable fibers derived from natural sources such
as
carbohydrates, collagens, and proteins. The fabric may be of a woven knit, or
solid
structure. The most preferred materials are Dacronm and PTFE. Sleeve 10 is
suitably
CA 02420638 2007-03-13
-4-
delivered by a catheter. Catheters of polyurethane, PTFE, PVC silicone or the
like with
internal diameters of 1 to about 3mm are suitable for polymer injection.
Sleeve 10 has a proximal end 14, a distal end 16, an interior portion 18, an
exterior
portion 20 and peripheral circular conduits or tubes 22, 24 located one at
each end 14, 16
respectively. Each conduit 22, 24 has at least one inlet port 26 and at least
one outlet or
exhaust port 28, inlet(s) 26 being connected to elongated introduction means
30, 32
respectively. Introduction means 30, 32 may be attached to exterior portion 20
of sleeve 10.
Referring to Figure 2, collapsed sleeve 10 is expanded and made rigid by the
insertion of a
spring wire or wires 34, 36 inserted through introduction means 30, 32. A
single wire or
multiple wires may be inserted to support ends 14, 16, the center body or any
portion of
sleeve 10. Wires 34, 36 are not attached to sleeve 10 but reside in
introduction means 30,
32 or conduits 22, 24, providing a constant spring tension. The entrance
tubing may be
detached from the sleeve after placement of supporting wires 34, 36 in end
tubes 22, 24.
The supporting wire may be made of stainless steel, spring steel, memory shape
metals (such as nitinol, for example), titanium, or metal alloys of any kind,
not limited to the
aforementioned. Furthermore, the configuration of the supporting wire maybe
solid, braided
or woven.
As shown in Figure 3, the graft may be expanded and made rigid and circular by
a
chemical hardening means introduced into a single spiral tube, or
alternatively, as shown in
Figure 4, a series of interconnected concentric cylindrical tubules 40
attached to and encasing
the sleeve 10. Tubules 40 are interconnected by means of connecting tubes 41
extending
between the tubules. The chemical hardening means may be introduced in the
form of an
injectable polymeric material comprised of a one part system, a two part
system, self
expanding systems, thermosets, thermoplastics and the like. These polymers or
polymeric
systems would fill tubes 32 or tubules 40, causing them to expand and
rigidify, thereby fixing
the sleeve at the site of repair. This embodiment is of particular use for
fusing such grafts in
large vessels such as the aorta or pulmonary arteries.
Two part activatable hardening material may be supplied in the form
ofmicrospheres
(not shown) that upon agitation by an external force may be disrupted. The
external energy
could originate from any suitable source including IR, visible or UV light
through optic fiber
on mechanical vibrational means from about 1 to 100,000
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97129716 PCTIUS97/02377
-5-
hertz supplied by mechanical or electrical transducers or by heat upon
disruption of the
microspheres, the activatable hardening material is liberated and allowed to
harden.
Disruption of the microspheres releases the separated components, allowing the
components to react together and form a hardened material that fills series of
tubules
40 thereby fixing sleeve 10 in place at the site of repair. Polymeric systems
may be
comprised of vinyl or divinyl compounds in which an initiator is contained in
the
microspheres, epoxies containing microencapsulated amine component, or
diisocyanates
with encapsulated amine or hydroxyl terminated prepolymers. Amino groups can
be
so isolated from methylacetimidate, ethyl acetimidate, dimethylglutarimidate,
dimetltyl,
adipidate, dimethyl sebaimidate, diisothionyl propionimidate, dimethyl
oxydipropionimidatesuccinate bis-esters, disuccinimidyl tartarate,
dieyanatobenzene,
dichlorodinitrobenzene, adipaldehyde, glutaraldchyde and the like.
These hardening-filler systems would allow for normal tissue ingrowth
in series of tubules 40 to take place. Because the tubules comprise only a
small fraction
of the total surface area of the sleeve, these hardening filling systems would
allow for
tissue ingrowth to take place into the sieeve material not impeded by the
tubules,
providing further reinforcement of the placement of the sleeve 10.
In a further embodiment shown in Figure 5, the material may be
introduced by means of a hardening-filler system comprising an enclosure 50
attached
to sleeve 10. Enclosure 50, like tubules 40, is filled with an activatable
hardening
material consisting of either a one-part polymer system, a two-part polymer
system or
a self-expanding monomer, which upon polymerization would fill enclosure 50,
causing
it to expand and rigidify, tliereby fixing sleeve 10 at the site of repair.
The activatable
hardening material is described above with reference to Figure 4.
Referring now to Figure 6, an alternative embodiment of sleeve 10 is
shown in place at a repair site 60. Sleeve 10 has a fluid track comprising a
continuous
cylindrical tubule 40 which is helically wound around proximal end 14 and
distal end
16 of sleeve 10. Tubule 40 can be filled witlt a curing polymer selected from
thermoset
polymers or two part polymers, as described hereinabove. Sleeve 10 may
optionally
include supplemental physical attachment means (not shown) such as spikes,
barbs or
the like at proximal and distal ends 14,16.
Figures 7-9 represent an alternative embodiment comprising a bifurcated
vascular graft 110 including a dual guide wire delivery system 112. Graft 110
has a
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97/29716 PCT/US97102377
-6-
proximal end 114 and at least two distal ends 116,118. Figures 8a through 8d
show
placement of bifurcated vascular graft 110 at a repair site 160 where the
vessel
bifurcates. Graft 110 and delivery system 112 are advanced through a vessel to
repair
site 160. Delivery system 112 includes guide wires 120,122 whereby ends
114,116,118
are placed at different branches of the vessel bifurcation. Figure 7b shows
graft 110
in place at site 160.
Figures 9-12 show an alternative embodiment of a vascular graft
according to the present invention, indicated generally at 210. Graft 210 has
proximal
and distal ends 214,216 and cylindrical tubule 240. Tubule 240 has a first end
242 and
a second end 244, located near proximal end 214. After placement of graft'210,
tubule
240 is filled.
Referring to Figures 10a, lOb and IOc, FilIing means 250 is shown.
Although filling means 250 is shown in conjunction with a tubular vascular
graft, such
a filling means may be used with any vascular graft according to the present
invention.
Filling means 250 comprises casing 251, fIiing tubc 252 with distal infusion
inlet 254
and exhaust tube 256 with distal exhaust vent 258. Filling means 250 may be
incorporated into the vascular graft delivery means or may alternatively be
separate
from but associated with the detivery means. Figure 10b is an enlarged
fragmentary
view of filling tube 252 which shows the manner in vyhich infusion inlet 254
connects
to first end 242 of tubule 240, via pinch ring 262 locatcd near the distal end
of infusion
inlet 254. Distal end of infusion inlet 254 is advanced into end 242 of tubule
240 until
pinch ring 262 is inserted in tubule 240. As shown in Figure 10c, casing 251
of filling
means 250 is advanced over end 242 of tubule 240 wliereby pinch ring 262
creates an
interference fit between filling tube 252 and end 242 of tubule 240. Exhaust
vent 258
connects to end 244 of tubule 240 in the same manner.
Figures 11-12 show alternative embodiments of the inventive vascular
graft. Figure 11a shows a graft 310 having an outer layer 370 surrounding
tubules 340.
Figure Ilb shows graft 310 having two outer layers 370,372 surrounding tubules
340.
Figure 1 ic shows graft 410 having no outer layer over tubules 440, and
lacking
connection between tubule 440 and proximal coil 480. Figure IId shows a cross
section of graft 510, having an inner core 590. Figures 12a and 12b show a
longitudinal cross section of graft 610 in place in repair site 660, wherein
graft 610 has
CA 02420638 2003-03-03
CA 02246355 1998-08-11
WO 97/29716 PCT/US97/02377
-7
an enlarged proximal coil 680 located directly at proximal end 614 of graft
610, i.e. not
more than about 5mm from proximal end 614.
The unique features of the device are the manner of its delivery and
fixation at the site of repair, its low profile which may prevent interference
with normal
heart functions, and the non-invasive nature of the delivery which would
reduce costs
normally associated with closure of such a defect. The device and method of
fixation
provides a non-invasive treatment of aortic aneurysms and the like. The device
is made
of polymeric material and is delivered via catheter in a non-invasive
procedure. In one
embodiment, the device operates through chemicat means to repair an aneurysm.
Advantages of the apparatus and method of the prescnt invcntion are
many. No preformed stent is required and the apparatus has a smaller insertion
diametcr than previous vascular grafts. rurther, the vascular graft has a
lower cost of
production than previous graft materials and procedures.
The practice of the present invention achieves several objectives and
advantages. Currently, there are no percutaneous devices available to cure a
septal
defect or the like. The device and method of the present invention provides an
advantage over surgery in that the cost of the procedure is substantially
less, the risk
of infection is less, the hospital residency time is less and there is no
physically
deforming scar.
Further advantages include applicability to procedures such as repair of
PDA, patent ductus anomaly. The non-invasive mode of delivery would reduce
costs
associated with this type of procedure. In addition, the low profile of the
apparatus
may minimize or prevent interference with normal heart functions.
While this invcntion may be embodied in many different forms, there are
described in detail lierein specific prefcrred embodiments of the invention.
This
description is an exemplification of the principles of the invention and is
not intended
to limit the invention to the particular embodiments illustrated.
The above Examples and disclosure are intended to be illustrative and not
exhaustive. These examples and description will suggest many variations and
alternatives to one of ordinary skill in this art. All these alternatives and
variations are
intended to be included within the scope of the attaclled claims. Those
familiar with
the art may recognize other equivalents to the specific embodiments described
herein
which equivalents are also intended to be encompassed by the claims attached
hereto.