Note: Descriptions are shown in the official language in which they were submitted.
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
MICRO-FLUIDIC SYSTEM
BACKGROUND OF THE INVENTION
1. TECHNICAL FIELD
The present invention relates to micro-fluidic systems for use in
determining the presence, absence, and quantity -of various chemical and
biological substances in microscopic amounts of biological or other fluid
to samples and moving microscopic amounts of biological or other fluids.
2. BACKGROUND ART
In various mechanical, electrical, chemical, biochemical, and biological
arts, sampling and monitoring of fluids occur to determine various fluid
25 components and ofiher associated fluid characteristics. Such sampling and
monitoring occur through various passive and active sampling devices and
systems known to those of skill in the art. These devices often are
miniaturized instruments that monitor and sample minute or micro amounts of
fluids. Often, miniaturization of the instrumentation occurs in order to
2o significantly reduce reagent amounts, increase efficient throughput,
improve
data collection, and decrease the need for invasive sample withdrawal.
Currently, most real-time biological monitoring systems designed for
application in individuals employ implanted sensors. The major drawbacks to
these systems include, but are not limited to, a need for surgical
implantation,
25 periodic calibration, occurrence of protein adsorption onto the sensor
surface,
and capsule formation around the sensor. Both protein contamination and
capsule formation affect the performance and functionality of the sensor.
Although several new biocompatible materials have been found and utilized in
vivo to make ion-selective sensors, minimal protein adsorption on the surface
30 of the sensor affects sensor accuracy and response time. Additionally,
implantation trauma, such as edema, swelling, capsule formation, and
antigenic rejection skew the concentrations of certain molecules at the
implantation site.
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
In the biological arts, minimally invasive monitoring and sampling of
micro amounts of fluids can occur through transdermal collection (i.e.,
transdermal patch). Due to the high concentration of capillaries in the dermis
of a body, interstitial fluid concentrations are proportional to blood
concentrations of hundreds of relevant molecules, including blood
electrolytes, stress hormones, medical and recreational drugs, pesticides, and
chemical warfare agents. A critical factor however, affects the utility of
transdermal micro-fluidic systems. These transdermal systems must maintain
a very high surface area to volume ratio.
to Due to the large amount of required sample to detect molecules of
interest utilizing external assay systems, it is required to perform
transdermal
sampling for a long duration of time. The duration of sampling can be
significantly reduced by increasing the surface area to volume ratio, but
large
patches that cover the entire abdomen are impractical.
1~ Another way to improve the surface area to volume ratio is to decrease
the volume of the sampling system. Through the use of integrated,
microscopic sensors, capable of monitoring nanoliter quantities of sample,
this can be realized.
Transdermal techniques may utilize iontophoresis, osmosis,
2 o electroporation, and electro-osmosis. For instance, these transdermal
techniques can be used for introducing drugs into the bloodstream and to
withdraw fluids from the body. lontophoresis utilizes either a constant
current
or a pulsed current to aid in the transport of charged particles across the
stratum corneum (the outer layer of the epidermis, which creates a major
25 barrier to the loss of water by the body). Direct current has been reported
to
cause skin irritation due to the polarization of the skin surface, while
pulsed
current allows this layer to have time to repolarize, maintaining natural skin
permeability. When using iontophoresis for drug delivery, surfactants have
been employed to increase the flow of neutral molecules across the
3 o epidermis.
Osmotic methods take advantage of concentration gradients to draw
2
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
small, lipophilic ions across the skin barrier. In humans, the stratum corneum
is negatively charged and, therefore, allows cationic particles to diffuse
across
the barrier at a much higher rate than anionic particles. Often, salt
solutions
are utilized to provide the osmotic gradient to draw the interstitial fluids
from
the body. Unfortunately, salt acts as an irritant hence reducing the amount of
time that the patch may be used. However, when a sugar solution is used to
provide the primary osmotic driving force, the skin does not become irritated.
Electro-osmosis is a process by which an externally applied potential is
used to mobilize cations such as sodium, which freely cross the stratum
so corneum, to transfer their momentum to neutral molecules around them. This
technique has been used to measure glucose minimally invasively, utilizing
large electrodes and transdermal patches with excessively large surface
areas and volumes. Researchers employing electro-osmosis on the macro-
scale have successfully monitored interstitial glucose concentrations off-
line,
which have been demonstrated to correlate to blood glucose concentrations,
at 20-minute intervals with a temporal delay of approximately 20 minutes.
In order to sample and transport small volumes of biological or other
fluids, it is necessary to have micro-fluidic devices such as micro-fluidic
pumps, valves, and actuators that work to control micro-fluid flow. Typically,
~ o the actuators are the driving mechanism of these devices.
An actuator that produces out of plane movement is necessary for
many chip-scale (1 mm2 to 1 cm2) applications. Some of these applications
include: movement of small volumes of liquid using a micro-fluidic peristaltic
pump, valuing of solutions to deliver different chemicals to an area on a
chip,
mixing of solutions in a microscopic chamber, as well as through the
attachment to other devices like cilia, fans, or other devices to produce out
of
plane motion for a silicon micro-machined chip.
As previously stated, actuators are the driving mechanism behind
pumps that force fluid through a passageway, channel, port, or the like, and
3 o can possibly function as valves in micro-fluidic devices. These actuators
work
by various types of actuation forces applied to a flexible mechanism, valve or
3
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
other similar device. Actuation occurs through methods using various forces
such as electrostatic, piezoresistive, pneumatic, electrophoretic, magnetic,
acoustic, and thermal gas expansion.
Electrostatic actuation of a membrane is one of the fastest methods for
s pumping solutions through a system. Piezoresistive actuation is also very
fast, utilizing hybrids of thick and thin films to produce a resonant
structure
affecting pumping of solutions. While these devices exhibit very fast
actuation rates, they require very high voltages, from 100V to 200V, and 50V
to 500V respectively. Additionally, electrostatic and piezoresistive actuation
to require specialized valves that direct fluid flow in a particular
direction. As a
result, these valves require three chips to be separately machined and
bonded together to produce the device.
Pneumatic actuation requires an external pressurized gas source to
actuate the membranes that cause fluid flow. While this method is feasible in
15 a laboratory setting where pressurized gas is available, it is impractical
for in
the-field utilization.
Electrophoretic actuation utilizes electrodes within a solution to impart
a motive force to charged molecules within the solution. Neutral molecules
are then 'dragged' along with the charged particles. This method is amenable
2 o to size reduction; however, it does have critical side effects such as the
chromatographic phenomenon that causes a separation of molecules based
upon charge. Additionally the high voltages necessary to induce fluid
transport are incompatible with standard CMOS circuitry.
Ultrasonic actuation occurs through flexural plate waves. This
25 methodology, however, is inefficient and causes mixing due to enhanced
diffusion.
Thermal gas expansion relies on the expansion of trapped air in the
system to move fluid through the conduits. This is accomplished by
selectively producing hydrophobic and hydrophilic regions on the chip.
3 o The devices from these previous bodies of work lack the ability to cost-
effectively add integrated sensors or circuitry to the devices. Integrating
4
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
circuitry incorporated into the micro-fluidic devices reduces: (1) the need
for
costly instrumentation, (2) the overall power consumption of the system, and
(3) the complexity of the control signals and mechanisms. Additionally,
integrated circuitry allows for the addition of chemical and physical sensor
arrays, and for connection to telemetry systems for remote communication
with external devices.
Most, if not all, of the micro-fluidic actuators are produced on structures
that are not planar. (See, U.S. Patents 5,962,081 and 5,726,404).. Various
other efforts are also underway to build miniature valves and pumps in silicon
2o for micro-fluidics. It has been difficult to produce good sealing surfaces
in
silicon, and it turns out that these valves, although in principle can be mass-
produced on a silicon wafer, require expensive packaging to be utilized.
Consequently, such micro-fluidic components cannot be considered
inexpensive andlor disposable. In addition, these micro-fluidic pumps and
s5 valves must be interconnected into systems including sensors, electronic
controls, telemetric circuitry, etc. such that the interconnection becomes
expensive.
Accordingly, it would therefore be useful to develop a micro-fluidic
sensor system that is integrated, low power, planar, and overcomes all of the
2 o problems of the prior art.
5
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
SUMMARY OF THE INVENTION
According to the present invention, there is provided a micro-fluidic
sensor system including a micro-conduit for carrying fluid therethrough having
a flexible wall portion, at least one micro-fluidic actuator having a closed
cavity, flexible mechanism defining a wall of the cavity and flexible wall
portion of the micro-conduit for deflecting upon an application of pressure
thereto, and expanding mechanism disposed in the cavity for selectively
expanding the cavity and thereby selectively flexing . said expanding
mechanism, and sensor mechanism in fluid communication with the micro-
so conduit for sensing the presence or absence of molecules. The present
invention further provides for a micro-fluidic system for moving microscopic
amounts of fluid including a micro-conduit and at least -one micro-fluidic
actuator in fluid communication with the micro-conduit.
20
30
6
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
DESCRIPTION OF THE DRAWINGS
Other advantages of the present invention are readily appreciated as
the same becomes better understood by reference to the following detailed
description when considered in connection with the accompanying drawings
wherein:
Figure 1 is a schematic CAD layout of an embodiment of a micro-fluitlic
chip of the present invention including pumps, mono-stable valves, sensor
chambers, and buffer/calibration/wash reservoirs (size = 8 mm X 4mm);
Figure 2 is a CAD layout of an embodiment of a micro-fluitlic chip
to utilizing a bi-stable valve design (size = 8 mm X 4 mm);
Figure 3 is a schematic layout of an embodiment of a micro actuator.;
Figure 4 is a CAD layout of another embodiment of a micro actuator;
Figure 5 is a schematic layout of an embodiment of a micro-fluidic-
pump;
Figure 6 is a picture of an embodiment of a flexible mechanism of the
present invention in an expanded position;
Figure 7 is a schematic diagram of an embodiment of the present
invention of a sensor array of the present invention with rectangular
electrode
geometry;
2 o Figure 8 A and B are schematic views of an embodiment of a bi-stable
valve, wherein 8A is a top view of an embodiment of the bi-stable valve and
8B is a cross-sectional view of an embodiment of the bi-stable valve;
Figure 9A and B illustrate an embodiment of a mono-stable valve in a
normally open and actuated closed state, respectively;
Figure 10 is a side, elevational cross section view of an embodiment of
the micro-fluitlic system, wherein arrows indicate fluid flow;
Figure 11 a side, elevational cross section view of another embodiment
of the micro-fluitlic system; and
Figure 12 is a top view of a layout of an embodiment of a sampling
3 o chamber of the present invention with teardrop-shaped standoff posts.
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
DETAILED DESCRIPTION OF THE INVENTION
Generally, the present inventionprovidesan automated micro-fluidic
sensor system,generally shown 6, whichis capable of numerous
at
applications uses. The presentinventioncan be passive and
and be
connected to external circuitry or can be active and use integrated circuitry.
Additionally, the present invention can be connected to various accessory
devices such as telemetric transmitters, GPS systems to monitor location,
audible alarm devices triggered by presence or absence of materials in fluids,
solid-state sensors for analysis of fuel cell effluent or biological samples,
and
Zo any other similar accessory devices known to those of skill in the art.
The present invention can be a micro-fluidic system that monitors
minute samples such as tears, saliva, urine, interstitial fluids, and the
like.
The present invention can also be used in devices that detect toxic materials
such as engine fuels, methanol, chemical warfare weapons, and neurotoxins,
15 biological markers such as blood electrolytes, blood glucose, therapeutic
drugs, drugs of abuse, pesticides, herbicides, and hormones, and any other
similar compound or substance known to those of skill in the art.
Additionally,
the present invention can be utilized in micro hydraulic systems, lubrication
device systems, fuel cell systems, microvilli systems, micro-fan systems, and
20 other similar systems known to those of skill in the art.
The present invention is aimed to work under a variety environmental
of conditions. For instance, they can function at an extremely wide
temperature range, but typically work in ranges of 10° C to 90°
C.
Additionally, the present invention functions in various atmospheric pressures
2 s such as 0.1 ATM to 3.00 ATM.
The term "actuator" as used herein is meant to include, but is not
limited to, a device that causes something to occur. The actuator 10 activates
the operation of a valve, pump, villi, fan, blade, or other microscopic
device.
Typically, the actuator 10 of the present invention affects fluid flow rates
3 o within a chamber.
s
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
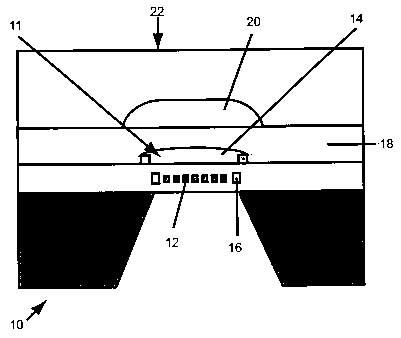
The term "closed cavity" 11 as used herein is meant to include, but is
not limited to, a sealed cavity that contains a liquid or solid expanding
mechanism 14 that is expanded or vaporized to generate expansion or
actuation of a flexible mechanism 18. The closed cavity 11 must be
completely sealed in order to contain the expansion therein, and must be
flexible on at least one side.
The term "expanding mechanism" 14 as used herein is meant to
include, but is not limited to, a fluid 14 capable of being vaporized and
condensed within the closed cavity 11 enclosed by the flexible mechanism
20 18. The expanding mechanism 14 operates upon being actuated or heated.
The expanding mechanism 14 includes, but is not limited to, water, wax,
hydrogei (solid or non-solid), hydrocarbon, and any other similar substance
known to those of skill in the art. Condensation of the expanding mechanism
14 occurs when the heat, which is generated to induce expansion of the
15 expanding mechanism, is removed by a surrounding medium such as a gas,
liquid or solid. Then, once condensation occurs, contraction of the flexible
mechanism takes place.
The term "flexible mechanism" 18 as used herein is meant to include,
but is not limited to, any flexible mechanism 18 that is capable of expanding
2 o and contracting with the vaporization and condensation of the expanding
mechanism 14. The flexible mechanism 18 must be able to stretch without
breaking when the expanding mechanism 14 is vaporized. The flexible
mechanism 18 is made of any material including, but not limited to, silicone
rubber, rubber, polyurethane, PVC, polymers, combinations thereof, and any
25 other similar flexible mechanism known to those skilled in the art.
The term "heating mechanism" 12 as used herein is meant to include,
but is not limited to, a heating device 12 that is incorporated with the
actuator
of the present invention. The heating mechanism 12 generates heat to
induce expansion of the expanding mechanism 14. The heating mechanism
3 0 12 is disposed adjacently to the flexible mechanism 18 in order to turn on
and
off and maintaining on and off selective expansion of the expanding
9
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
mechanism 14. The heating mechanism 12 can be powered using any power
source known to those of skill in the art. In an .embodiment, the heating
mechanism 12 is powered by a battery. However, both AC and DC
mechanisms are used to minimize power requirements. Generally, the
s heating mechanism 12 is formed of materials including, but not limited to,
polysilicon, elemental metal, silicide, or any other similar heating elements
known to those of skill of the art. Moreover, the heating mechanism 12 is
disposed within a medium such as SiO~ or other solid medium known to those
of skill in the art.
2o The term "temperature sensor" as used herein, is meant to include, but
is not limited to, a device designed to determine temperature. A resistive
temperature sensor 16 is made from material including, but is not limited to,
polysilicon, elemental metal, silicide, and any other similar material known
to
those of skill in the art. Thermocouple temperature sensors can also be used.
15 Typically, the temperature sensor 16 is situated within or near the heating
element of the heating mechanism 12.
The terms "chamber," "micro chamber," "pulsating micro chamber,"
"micro-conduit," and "conduit" as used herein are meant to include, but not
limited to, any type of tube, pipe, planar channel, conduit, or any other
similar .
2o chamber known to those of skill in the art. The conduit has a wall
mechanism
made from material including, but not limited to, silicon, glass, rubber,
silicone, plastics, polymers, metal, and any other similar material known to
those of skill in the art. In one embodiment of the micro-fluidic valve, the
chamber encompassing the micro-actuator is etched out of glass in a nearly
25 hemispherical shape. A variety of conformations of spherically cut patterns
(i.e. 1/3 of a sphere, '/ of a sphere, etc.) with differing radii and
footprints are
employed to provide different valuing characteristics.
The micro-fluidic system can be incorporated into a "dermal patch" that
contains the sensor system, interstitial fluid sampling system, calibration
3 o system, pumping system, and electronics for device control, sensor
monitoring, and incorporation into a telemetry system to name a few
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
functions. The resulting micro-fluidic sensor system has the capability to
continuously monitor .the concentrations of a large number . of relevant
biological molecules continuously from an ambulatory patient and has the
ability to trigger an audible alarm in the case of dangerous exposure to
hazardous materials or out-of-therapeutic range for medicinal drugs, or
provide closed-loop injection of therapeutic drugs. . .
With regard to patches, a key feature is that the present invention is
well suited in being able to obtain micro-amounts of fluids from a smaller
surface area. Smaller area electrodes (less than 1 cm~) with an equivalent
to current density do not produce as significant physiological "side-effects,"
compared to large electrodes; however, the reduced surface area results in a
significantly reduced volume of drawn interstitial fluid. By reducing the test
volume required for analysis by three orders of magnitude, the surface area of
the transdermal patch can be significantly reduced by utilizing microscopic
15 semiconductor sensors.
In one embodiment, the present invention includes test chambers
designed for a microscopic volume (50nL), therefore, minimal calibration
solution is required. Additionally, very stable amperometric and
pofientiometric sensors that require calibration only 2-4 times/day to
maintain
2 0 - accuracy are utilized. The transdermal buffer sampling solution consists
of a
combination of enough salt to provide electrical connectivity and a high
concentration of sugar to provide the osmotic gradient to induce osmotic flow
of interstitial fluids. In addition to buffer solutions, calibration solutions
and
washing solutions are employed within the system.
2s The actuators 10 of the present invention are the driving mechanism
behind various devices of the present invention. The micro-fluidic valves
have various pressures and temperatures required for their actuation. The
peristaltic pump is selectively controlled and actuated through an integrated
CMOS circuit or computer control, which controls actuation timing, electrical
3 o current, and heat generation/dissipation requirements for actuation.
Integration of control circuitry is important for the reduced power
requirements
11
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
of the present invention. In one particular embodiment for example, sensors
and circuitry responsible for monitoring the effluent of a fuel cell with
concomitant control of the micro-fluidic fuel delivery system to increase or
..
decrease the flow rate of fuel is designed. This ensures optimal. fuel
s utilization in the device. Closed loop feedback provides the basis of
automated adjustment of circuitry within the micro actuator.
The actuator 10 includes a closed cavity 11, flexible mechanism 18,
and expanding mechanism 14. Fabrication of actuators 10 is accomplished
by generating electron-beam and/or optical masks from CAD designs of the
to micro-fluidic system. Then, using solid-state mass production techniques,
silicon wafers are fabricated and the flexible mechanisms 18 for the actuators
are subsequentlyplaced on the chips.
In the device without integrated circuitry, the control circuitry is
produced on external breadboards and/or printed circuit boards. In this
15 manner, the circuitry is easily, quickly, and inexpensively optimized prior
to
miniaturization and incorporation as CMOS circuitry on-chip that can be
controlled manually, or through the use of a computer with digital and analog
output. Optimized CMOS circuitry, modeled utilizing CAD solid state MEMS
and CMOS design and simulation tools, is integrated into the.vactive device
2 o making it a stand-alone functional unit.
Using an arbitrary wave-form generator, and/or computer controlled
digital-to-analog (d/a) and analog-to-digital (a/d) PCI computer cards (for
example, the PCIMlO16XH, National Instruments) the optimal operating
parameters (i.e., stimulatory waveform patterns) are configured to generate
25 peristaltic pumping action. Electronic control of the actuators 10 is
optimized
to maximize flow rates, maximize pressure head, and minimize power
utilization and heat generation. Another parameter that is evaluated includes
the temperature profile of the medium being pumped. To minimize power
consumption and heat generation, a resistor-capacitor circuit is utilized to
3 o exponentially decrease the voltage of the sustained pulse. Further,
12
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
integrated circuitry initiation and clocking of the circuitry ,provide control
of the
second-generation actuators.
An e-prom is also included on-chip to provide digital compensation of
resistors and capacitors to compensate .for process variations and, therefore,
s improve the process yield. Electrical access/test pads are designed into the
chips to allow for tl~e testing of internal nodes of the circuits.
The flexible mechanism 18 deflects upon the application of pressure
thereto. In one embodiment, he flexible mechanism 18 is screen-printed
over the expanding mechanism 14 utilizing an automated screen-printing
Zo device, a New ~ Long LS-15TV screen printing system. ~ The flexible
mechanism 18 is very elastic and expands many times its initial. volume as
the expanding mechanism 14 under the flexible mechanism is vaporized.
Due to the large deflection, it is possible to completely occlude"a micro-
channel with this flexible mechanism 18, hence providing the functionality of
15 an electrically actuated microscopic valve. The present invention can also
apply flexible mechanism 18 with syringe or pipette devices or spin coat it on
the entire wafer. Photo curable membrane can also be used to pattern the
flexible mechanism 18 on the wafer. T ~ ~.
A wide variety' of commercially available polymers can be utilized as
2 o the flexible mechanism 18, including, but not limited to: Polyurethane,
PVC,
and silicone rubber. The actuator flexible mechanism 18 must possess
elastomeric properties, and must adhere well to the silicon or other substrate
surface. A material with excellent adhesion to the surface, as well as
appropriate physical properties, is silicone rubber.
25 In an embodiment of the present invention, the flexible mechanism 18
is made of silicone rubber. The silicone rubber can be dispensed utilizing
automated dispensing equipment, or can be screen-printed directly upon the
silicon wafer. Screen-printing methods have the advantage that the entire
wafer, containing hundreds of pump and valve actuators 10, can be produced
3 o at once. By varying the amount of solvent in the polymer, such as silicone
13
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
rubber, the flexible mechanism 18 thickness and its resulting physical .force
characteristics can be precisely controlled.
The flexible mechanism 18 can serve the dual purpose of actuation as
well as serving as the bonding material used to attach the liquid flow
channels
to the silicon chip containing the actuators 10. By covering the entire area
of
the chip with the flexible mechanism 18, with the exception of the sensing.r.~
regions and the bonding pads, the glass or plastic channels can be "glued" to
the actuator 10 containing silicon chip. This method provides additional ~.
anchoring and strength to the actuation flexible mechanism 18, and allows
Zo the actuation area to encompass the entire actuation chamber 20. The only
drawback to this method is potential protein and/or steroid adsorption onto
the micro-fluidic conduits 56. :However, with proper flexible mechanism 18
selection and chemical treatment, molecular adsorption can be minimized, or
a second, thin, inert layer can be. used to coat the flexible mechanism 18.
i5 The expanding mechanism 14 selectively expands the cavity 11.
defined by the flexible mechanism 18 thereof and thereby selectively flexes.
the flexible mechanism 14. The expanding mechanism can be made of
various materials. In one embodiment, the expanding mechanism is a
hydrogel material, which contains a .large amount of water or other
20. hydrocarbon medium, which is vaporized by the underlying heating
mechanism. In this embodiment, the volume of hydrogel needed to produce
the desired actuation and pressure for the flexible mechanism 18 is
approximately 33 pL. With this design, approximately 97% of the energy
generated by the heating mechanism 12 is transferred into the hydrogel for
25 vaporization.
A practical technique for the micro-fluidic pumping of moderate
volumes of liquid is through the use of peristaltic pumping utilizing
pneumatic
actuation. The integrated micro-fluidic pumping system of the present
invention is designed to sample small amounts of interstitial fluid from the
3 o body on a continuous basis. In order to analyze the microscopic volumes,
silicon micro-machining methods and recent improvements in membrane
14
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
deposition technologies are utilized to produce a microscopic test chamber 60
on the order of 50nL in volume, roughly 3-4 orders of magnitude less volume
than current systems. In addition to the improved response time, . the
reduction to microscopic volumes allows the use of very small amounts of
calibration solution to effect calibration and rinsing, hence reducing the
overall
size of the package. In some systems the calibration . solutions are a
significant portion of the entire package (Malinkrodt Medical/IL) where, even
..though miniature sensors are used, liters of calibration solutions are
necessary.
so In one embodiment, the micro-fluidic. pump design is based upon
. electrically activated pneumatic actuation of. a micro-screen printed
silicon
rubber membrane. Generally, the pump includes the.micro-fluidic actuator 10
including a closed cavity 11, flexible mechanism 18 defining a wall of the
. closed cavity 11, and expanding mechanism 14 disposed within the closed
cavity. The flexible mechanism 18 deflects upon the application of pressure
thereto and the expanding mechanism 14 selectively expands the cavity and
thus flexible mechanism 18 and thereby selectively flexes the expanding
mechanism :14.
The micro-fluidic actuator 10 is based upon electrically activated
2 o pneumatic actuation of a micro-screen-printed or casted flexible mechanism
18. The peristaltic pump generally includes three actuators 10 placed in
series wherein each actuator 10 creates a pulse once it is activated (See
Figure 5). By working in tandem, the actuators -10 peristaltically pump
fluids.
The optimal firing order and timing for each actuator 10 depends upon the
2s requirements for the system and are under digital control to create the
peristaltic pumping action.
The advantage of pneumatic actuation is that large deflections can be
achieved for the flexible mechanism 18 (See Figure 6). To actuate the
flexible mechanism 18, a vaporizable fluid 14 is heated and converted into
3 o vapor to provide the driving force. Utilizing an integrated heating
mechanism
12, the expanding mechanism 14 is vaporized under the flexible mechanism
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
18 to provide the pneumatic actuation. This actuation occurs without the
requirement of utilizing external pressurized gas.
The liquid or gaseous fluid being pumped serves the purpose of acting
as a heat sink to condense the vapor back to liquid and hence return the
s flexible mechanism 18 to is relaxed state when the heating mechanism 12 is
inactivated. A temperature sensor 16 is integrated adjacent to the actuator
to monitor the temperature of the micro-fluidic integrated heating
mechanism 1..2 and hence, expanding mechanism 14. ..
The heating mechanism 12 requires very low power to achieve
1o sufficient temperatures for fluid vaporization. As an example, miniature
inkjet
nozzles that require temperatures in excess of 330° C, utilize 20p,
second
pulses of 16mA to heat the fluid and fire an ink droplet. Considerably lower
power would be' required to vaporize the liquid in the present micro-fluidic
pump application. In the field, it is necessary to utilize low power devices
and
circuitry to conserve energy and allow the use of very small, lightweight,
film
or button batteries.
Once the heating mechanism 12 is activated, vaporization of the
expanding mechanism ~14 takes place. The expanding mechanism 14
component imposes a~ pressure upon the flexible mechanism 18 causing it to
2 o expand and be displaced above the heating mechanism 12 and reduce the
volume of the chamber 20. This methodology can be utilized to displace fluid
between the flexible mechanism 18 and the walls of the chamber 20
(pumping action), to occlude fluid flow through the chamber 20 (valuing
action), to provide direct contact to the glass substrate to effect heat
transfer,
or to provide the driving force fo'r locomotion of a physical device (i.e., as
in a
walking caterpillar and/or a swimming paramecium with a flapping flagella, in
which case the glass chamber 20 encompassing the micro-actuator 10 would
not be used).
The heat flux through each of the layers composing the device is
3 o calculated using existing boundary conditions. The temperature required to
vaporize the expanding mechanism 14 varies according to the physical and
16
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
chemical properties of the expanding mechanism 14 itself. Due to the
differences in heat transfer through liquid versus gas, approximately twice as
much heat flux travels through the device when the expanding mechanism 14
is all liquid compared to all vapor. In order to reduce heat dissipation into
the
medium being pumped, while the expanding mechanism 14 is in the liquid
state, the heating mechanism 12 is quickly ramped to the temperature
required to vaporize the liquid. Once the expanding mechanism 1~4 is
vaporized, heat transfer to the medium being pumped is minimized. . ,.
In one embodiment, the temperature of the saturated liquid hydrogel,
to at 1 ATM, is assumed to be 100°C. -The heat flux to the air, through
the back
of the heating mechanism .12,~ is calculated to be 1263 W/K-m2. The total
heat flux through the device is calculated to be 46,995 W/K-mz with a
total~.flux
from the heating mechanism '.12 of 47,218 W/K-m~ (i.e. 97% efficiency of
focused heat transfer). In this embodiment, the temperature of the inactive
. 15 state hydrogel varies between 86° C and 94° C.
The temperature of the activated, vapor state hydrogel is
approximately 120°C, which is the saturation temperature for steam at 2
ATM.
The heat transfer coefficient for convection can be calculated directly from
the thermal conductivity. The heat fluX to the air through the back of the
2 o heating mechanism 12 is 2818 W/K=m~. The heat flux through the device is
21,352 W/K-m~ with a total flux from the heating mechanism 12 of 24,170
W/K-m~. When the aqueous component of the hydrogel is completely in the
vapor state, there is no fluid 14 in the channel and the thin film of solution
between the flexible mechanism 18 and the glass is approximately at
60°C.
2~ These values and calculations vary according to the type of actuator,
valve,
pump, and micro device being used.
In an embodiment of the present invention, the volume of the
expanding mechanism 14, in this case, liquid hydrogel, is determined based
on the volume of vapor needed to expand the flexible mechanism 18
3 o completely at 2 ATM using the ideal gas law. This assumption is valid
because the temperatures and pressures are moderate. The volume of liquid
17
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
hydrogel necessary to achieve this volume of gas at this pressure, assuming
the hydrogel is 10% water and all of the water is completely evaporated, is
0.033 nL. Cylindrically shaped sections of hydrogel are utilized within the
actuator 10. This shape has been chosen to .optimize encapsulation by the
actuator flexible mechanism 18. The cylinders have either a diameter of
approximately 140 p,m and a height of 2.14 p,m, or a diameter of 280 p,m with
a height of 0.54 p,m (identical volumes, different orientation to the heating
element). Of course, the shapes and volumes vary according to the type of
expanding mechanism being used. For example, photocurable liquid
hydrogels have different parameters.
. For flexible mechanism 18 actuation= and hydrogel vaporization, it is
necessary to raise the temperature of the hydrogel from ambient temperature
' to the boiling point, 120°C at 2 ATM. Thermodynamic models indicate
that
approximately 8.03 x 10-' J of heat transfer is required to raise the
°~15 temperature of the hydrogel from 37°C to 120°C (1.08
x 10-' J) and vaporize
all of the water (6.95 x 10-' J). This is consistent with the sum of enthalpy
equation.
In another embodiment, for flexible mechanism 18 contraction and
expanding mechanism 14 condensation, it'is assumed that all heat dissipation
2 o from the activated, vaporized expanding mechanism 14, as it condenses, is
transferred into the solution being pumped. The calculation for this
condensation involves condensing all of the water in the hydrogel plus sub
cooling the hydrogel from 100°C to 90°C in order to completely
contract the
actuator 10. Modeling conduction through the actuator 10 flexible mechanism
25 18 using Fourier's equation provides a flux of 0.0015 J/s and a
condensation
time of 0.00473 seconds. This represents a worst case scenario, neglecting
thermal conduction to the silicon substrate.
In an embodiment of the present invention, based upon the geometry
of the 100 pm tall chamber 20, it is calculated that a circular actuator 10
with
3 o a diameter of 300 p,m is required to deliver 4.9 nL quantities of liquid
per
18
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
actuation of the flexible mechanism 18. The heating mechanism 12 is laid out
as a square that encompasses the majority of the circular expanding
mechanism 14 area without extending past the edge of the chamber 20.
Other shapes are also employed, such as circular and triangular layouts to
encompass as much of the expanding mechanism 14 as possible. In order to
provide::efficient micro-actuation in 150 p,s, requirements for the . heating
mechanism 12 power output and electrical resistance are calculated. To
provide the required 777 nJ of energy, the resistance of the poly-silicon
heating mechanism 12 is calculated to between 450 to 50052, based upon
1o utilizing a 5V power supply. Actuation requires a 150 ~.s pulse of
approximately 11 mA of current, providing the 777 nJ of energy required. In
order to.achieve a pumping rate of 10 ~.L/minute, approximately 677 ~,W of
power is required. In previous work, poly-silicon structures at a thickness of
6000 A, having a 'resistance of 15 s2/elemental square have been produced.
To provide the required resistance, 5 poly-silicon heafing mechanism 12 lines
are arranged in parallel (See figure 4). The poly-silicon heating mechanism
12 elements have a width of 5 ~,m. The total resistance of the heating
mechanism 12 is 450 S2.
In this case, the heating mechanism 12 is poly-silicon, but can be any
2 o similar material or mechanism, such as direct metals, known to those of
skill
in the art. Because of its high thermal conductivity, the silicon substrate
acts
as a heat sink. To reduce thermal conduction to the silicon 'substrate, a
window in the silicon, located beneath the heafiing mechanism 12, provides
the expanding mechanism 14 with an isolated platform. This window is only
2s slightly larger than the heating mechanism 12 to maintain some thermal
conduction to the substrate. After the actuator 10 is energized, thermal
conduction to the silicon provides decreased time to condense the liquid in
the expanding mechanism 14. This decreases constriction time and provides
improved pumping rates. If the window is significantly larger than the
actuator
19
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
10, there is no heat conduction path to the substrate, hence increasing
condensation time and decreasing the maximal flow rate.
In one embodiment, the expanding mechanism 14 hydrogel. ~ is
presented as a cylinder with diameter of 280 p,m and height of 0.5 - 1, pm.
The actuation chamber 20 encompasses the entire cavity etched in the glass
substrate. . -
Fabrication of this device is based upon the development of a process
flow. The fabrication process utilizes bulk silicon micro-machining techniques
to produce the isolation windows, and thick film screen printing techniques,
spin coating, mass dispensing, or mechanical dispensing of actuation°
membranes.
A polymeric hydrogel -(or hydrocarbon) can be utilized to provide a
physically supportive structure that withstands the application of flexible
mechanism 18 as well as to provide the aqueous component required for
actuation. Several commercially available materials meet these
requirements. A hydrogel is selected that contains approximately 30%
aqueous component that vaporizes near 100°C. Several materials have
been
identified, each of which is suitable in this application, including, but not
limited to, hydroxyethylmethacrylate (HEMA) and polyvinylpyrrolidone (PVP).
2 o Additionally, hydrocarbons can be used since they possess lower boiling
points than aqueous hydrogels, and therefore require less power to effect
pneumatic actuation.
Dispensing hydrogel (or hydrocarbon) into the desired location is
accomplished utilizing one of three methods. First, a promising method for
25 patterning the hydrogel is to utilize a photopatternable-crosslinking
hydrogel.
The hydrogel is cross-linked by incorporating an UV photo-initiator
polymerizing agent within the hydrogel that cross-links when exposed to UV
radiation. Using this technique, the hydrogel would be evenly spun on the
entire wafer using standard semiconductor processing techniques. A
3 o photographic mask is then placed over the wafer, followed by exposure to
UV
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
light. After the cross-linking reaction is completed, excess (non-cross-linked
hydrogel) is washed from the surface.
The second method involves dispensing liquid hydrogel into well-rings
created around the poly-silicon heating mechanism 12. These wells have the
ability to retain a liquid in a highly controlled manner. Two photopatternable
polymers have been utilized to create microscopic well-ring structures, SU-8
and a photopatternable polyimide. These well-rings can be produced in any
height from 2 pm to 50 ~.m, sufficient to contain the liquid hydrogel. Once
the ..
hydrogel solidifies, flexible mechanisms can be deposited over them. This
1o can be accomplished in an automated manner utilizing commercially available
dispensing equipment.
w In a third alternate method, a pre-solidified hydrogel is used that.. has
been cut into the desire size and shape. This is facilitated by extruding the
hydrogel in the desired radius and slicing it with a microtome to the desired
height, or by spinning the hydrogel ~to the desired thickness and cutting it
into
cylinders of the desired radius. Utilizing micro-manipulators, the patterned
gel
is placed in the desired area. This process can also be automated.
It is assumed that the temperature on both sides of the SiO~ that
encapsulates the heating mechanism is constant, and that heat flux in each
2o direction is dependant upon the heating mechanism 12 temperature and the
resistance to heat flow either through the device or to an air pocket on the
heating mechanism backside. A schematic of.a cross section of the actuator
device is provided in Figure 3. Steady-state heat flow through the entire
actuator, for the fully actuated state, the intermediate state, and the
resting
state are modeled. These data are calculated for the static case during which
time no fluid flow is occurring (i.e. steady-state; the system is poised at
100°
C, waiting to be initiated). The fluid temperature is greater for the
contracted
state since the liquid hydrogel conducts heat at a greater rate than vapor.
Once fluid flow is initiated, the temperature of the solution is raised by
only a
3 o few degrees Celsius.
21
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
A typical problem experienced with many micro-fluidic designs revolves
around the methodology for mixing of solutions and reagents. The micro-
fluidic peristaltic pump design of the present invention provides mixing
action..
in concert with the pumping action. To construct the micro-fluidic valves and
pumps in a manner compatible with the sensor technologies and to integrate
the entire system on a single silicon chip, the pump is preferably fabricated
using planar MEMS technologies that do not require special wafer bonding,
although other methods of fabrication can also be used as are known to those
of skill in the art.
so For encapsulating a liquid within a silicone rubber membrane, micro-
machining techniques, including wafer bonding of.multiple chips, are used by
others to create a cavity where the liquid is stored. This requires several
machining steps to produce the actuator, reducing the overall yield of
functional pumps and valves, and increasing the cost.
~5 By properly placing the planar actuators ,within the fluidic channels,
micro-pumps, fluidic multiplexers, and valves can be formed. CAD/CAM tools
are used to design the photo-masks. This can be accomplished in
conjunction with the design of the fluidic channels, ports, and test chambers.
The pneumatically actuated membrane is utilized to produce the micro
2 o fluidic valves. The micro-fluidic actuator's silicone rubber -membrane is
very
elastic and .expands many times its initial volume as the liquid under the
membrane is vaporized (See Figure 6). At least two techniques for the
valuing of solutions can be used.
The first utilizes the flexible mechanism 18 actuation to completely fill a
25 micro-fluidic channel when actuated, hence providing the functionality of
an
electrically actuated microscopic valve. The second utilizes the flexible
mechanism 18 to occlude an orifice to block fluid flow.
The pneumatically actuated membrane is also utilized to produce the
micro-fluidic pumps. The micro-fluidic actuator's flexible membrane is very
3 o elastic and expands many times its initial volume as the liquid under the
membrane is vaporized (See Figure 6). The micro-fluidic channels are
22
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
designed such that all media flow is in the laminar regime while minimizing
fluid volume, dead volume, and residence time. Further, the routing of the
micro-fluidic channels is designed such that the required calibration and wash
solutions can be routed into the sensing chamber. The channels and sensing
s chamber accommodate approximately 50nL volumes of solution.
Once modeled and optimized, photomasks are created.: for the fluidic
system. Valves at the various ports are optimally designed to start and stop
the flow of the various calibration and wash solutions.
In one embodiment, the integration of a sampling system to the device
Zo allows transdermal sampling techniques for the acquisition of interstitial
fluids.
This sampling chamber 60 has a maximized surface area within the confines
of the device and an extremely minute volume to reduce the required sample
volume and to decrease the sampling time. This chamber is micro=machined
into the backside.of the glass fluidic channel chip.
15 Due to the high surface. area to volume ratio required in order to effect
transdermal sampling, the sampling chamber 60 is designed to be very thin,
approximately 20 pm~ in height (Figure 12). The sampling chamber 60 include
stand-off posts 62, which serve two functions. First, they are required to
keep.
the skin from conforming to the chamber surface 64 thereby occluding the
2 o volume of the chamber. Second, they effect fluid flow within the sampling
chamber 60 and promote sampling mixing. The simplest design is to produce
the posts as cylinders perpendicular to the skin 66. Teardrop shaped posts
62 reduce dead volume and create eddies along the back side of the posts.
Teardrop shaped posts 62 are approximated by two connected cylinders,
25 one with a smaller diameter adjacent to one with a larger diameter and
filling
the space between the two. Since the posts are etched out of the glass, most
any continuous shape can be produced.
The posts 62 are staggered in a triangular pitch to support the skin 66
evenly. To improve mixing of the solution and reduce molecular diffusion
time, eddies can be forced in the chamber 60. If more eddies are desired, the
posts can be designed with a flat and wide profile in the direction of flow.
23
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
The posts 62 are shown in the CAD layout of the sampling chamber 60
(Figure 12). The chamber 60 utilizes a gradual expansion to eliminate dead
zones and eddy currents, as described for the sensor chamber 60. In one.
embodiment, the sampling chamber 60 approximately is 5 mm long (left to
right) and 2.4 mm wide at the widest region. The chamber is etched to 20 p,m
deep to provide the very high surface area to volume ratio required for
transdermal sample acquisition. The total area of the chamber 60 is 9.1 mm~,
and the area of the posts 62 is 2:07 mm~. The posts 62 constitute 22% of the.
,
total cross sectional area of the sampling chamber 60. Therefore, the total '
exposed skin area is 7 mm2 and the volume of the chamber 60 is 140 n1.
The most important factor for sampling interstitial fluids transdermally is
the surface to volume ratio. As the surface to volume ration increases, the:
efficiency of transdermal fluid sampling increases. In the prior art, the most
efficient transdermal sampling devices utilize a surface to volume ratio of 2
x
1s : 103 mm~/mL. The present invention possesses a ratio of at least of 5 x
104.
mm~/mL: effectively at least 25 times the surface area to volume ratio of the
best device reported in the literature. .
.. Figure 11 depicts a schematic cross-section of a portion of the chip
that contains the transdermal sampling chamber 60. The micro-fluidic pumps
'2 o are utilized as the driving force for the ~transdermal monitoring system
6. The
transdermal monitoring system includes an insulating air gap that improves
the thermodynamic and electrical efficiency of the micro-actuators, integrated
heater mechanisms 12, three micro-actuators 20 in series to effect peristaltic
pumping, integrated amperometric/potentiometric/optical sensor arrays 70,
2 s 72, and the waste fluid reservoir 74.
The reservoirs are 1 mm squares that have miniature, silicone
membrane based "pouches" attached. These can contain buffer, calibration,
and wash solutions for calibration of sensors, regeneration of sensor
reactions, and buffering of the interstitial fluid samples. The volumes of
fluids
3 o can be altered by attaching different sized "pouches".
24
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
There was developed a device for ambulatory measurement,
collection, wireless -firansmission, and electrodes res~ponsibie for
transdermal
sampling, and/or chemical and physical sensing. ..The reference electrode
included in each sensor site and the global reference electrode were coated
with silver (Ag) and electrolytically chloridized to provide reversible
Ag/AgCI
electrodes.
The fabrication process entails the use of a reactive ion etch (RIE)
plasma as a chloride source. This technique .allows wafer level chloridation
of
all reference electrodes within each sensor array at once, prior to separating
to the silicon wafer into individual chips. This methodology eliminates the
necessity to provide electrolysis current during chloridation and improves the
accuracy and precision of the silver .chloride fabrication process.
Through the use of integrated ion selective electrodes (ISEs), a wide
variety of important ions are detectable including electrolytes, stress
. hormones, C02, local anesthetics, a variety. of herbicides, heparin,
medicinal
drugs, lithium, etc. Additionally, amperometric sensors are utilized to defect
a
large variety of more complex molecules, including proteins. More complex
and/or non-oxidizable molecules, such as neurotoxins:and other molecules of
biological warfare, are detected by immobilizing antibodies and/or enzymes
2 0 on the surface of an ion-selective membrane and performing enzyme assays
or enzyme-linked immunosorbent assay (ELISA) for example.
The micro-fluidic system 6 described herein, including integrated
sensors, enables the system to deliver known quantities of samples, wash
solutions, enzymes, reagents, and chromophores to the sensor chambers,
allowing the processing and analysis of minute quantities of the sample fluid.
The small size and mass producibility of the assay system, including pumps
and valves, allows for low cost, disposable devices (laboratories-on-chip) to
be produced. The micro-fluidic system 6 described herein significantly
reduces the sample processing time periods as well as provides the ability to
3 o monitor dozens of other biological molecules on-line and in near real-
time.
lontophoretic and electro-osmotic methods are becoming more
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
acceptable for the delivery of therapeutic levels of drugs. These techniques
utilize electrodes to~deliver electrical current to the skin surface to
enhance
the delivery system. Operated in reverse, each of these techniques can be
used to remove small amounts of interstitial fluid from the body for
measurement. In the present invention, iontophoresis is used to both acquire
interstitial fluid samples as well as to deliver therapeutic levels of drugs
under
closed-loop control based upon integrated sensor analysis of the interstitial
fluid samples, however other transdermal sampling techniques, known to
those skilled in the art, can be utilized, such as osmosis, iontophoresis,
to electro-osmosis, and electro poration.
Capable of employing each of these methods, the integrated micro-
fluidic system 6 is designed to withdraw small amounts of interstitial
fluid.from
the body on a continuous basis. A minor temporal delay is incurred due to
the homeostatic relationship between blood and interstitial fluid as well as
mass transport. The emporal delay can be effectively reduced by. reducing
the volume of the testing chamber several orders of magnitude and by
developing analysis algorithms.
Additionally, very stable amperometric 72 and potentiometric 70
sensors have been developed that require calibration only.2-.4 times/day to
2 o maintain accuracy. The transdermal sampling buffer solution consists of a
combination of enough salt to provide electrical connectivity and a high
concentration of sugar to provide the osmotic gradient to induce osmotic flow
of interstitial fluids. In addition to buffer-solutions, calibration
solutions, and
washing solutions are employed, stored on-chip, and pumped and valued as
required for the intended operation.
While several micro-fluidic systems 6 are small, the instrumentation
and circuitry required to control the micro-fluidics and to operate the sensor
systems to monitor the samples are complex, remain large, are not integrated
into the micro-fluidic system, and are often expensive. This is acceptable for
3 0 laboratory or hospital work, buff it is not practical for either
ambulatory
26
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
utilization or autonomous operation (i.e. laboratory-on-a-chip). The miniature
size of the micro-fluidic sensing system with integrated instrumentation
circuitry reported here is required for many applications, both medical, .
biological, and industrial (i.e. chemical process control).
For mobile applications, automated control of the pumps, valves, and
sensors is required to coiltinuously monitor and calibrate the microscopic
"lab-on-a-chip" devices. Using integrated electronics, the sensors can be
calibrated on a regular basis in an automated manor that is transparent to the
user, ensuring accuracy of the data obtained. The sensing system also
so requires integrated circuitry to buffer the signals, reduce noise,
transduce the
chemical concentrations into electronic signals, and analyze the signals,
allowing untrained personnel to utilize the device.
Another application for integrated circuitry is for the telemetric
communication of the device with ~ a base unit, which can then relay the
information to a remote location. Moreover, the circuitry can perform closed-
loop feedback control for biological applications. For example, closed-loop
feedback control can be used to inject insulin into an individual when the
transdermal sensor system detects hyperglycemic levels of glucose in the
transdermally sampled interstitial fluid; thereby maintaining euglycemia. '
2 o The sensor arrays are fabricated in a three-mask process with two
metal layers, silver and platinum. Since these metals are difficult to etch
using wet chemistry, a resist lift-off process was used to pattern them. This
provided an additional advantage in allowing the use of layered materials in a
metal strucfiure to modify electrode properties and still allowed for
patterning
25 to occur in one step.
Additionally, other sensor conformations can be produced in
accordance with the present invention, each with differing transduction and
membrane encapsulation properties. These designs incorporate rectangular,
circular, and concentric circle shaped electrodes.
27
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
The sensor arrays are ideal for use in a micro-fluidic transdermal patch
system in that they provide a large number of individual sensors, each of
which can be encapsulated by a different membrane using the automated
micro-screen printing device, the New Long LS-15TV, to confer sensitivity to
individual biological ions and molecules of interest. Multiple conformations
of
sensor arrays are constructed using electrode sizes of 2, 4, 8, 32, and
100pm.
Figures 1 and 2 show a top view. schematic layout of the micro-fluidic
pumping system. In this example, two micro-fluidic pumps are utilized as the
to driving force for a transdermal monitoring system able to minimally
invasively
monitor the concentration of circulating hormones, drugs, electrolytes,
toxins,
etc. in ambulatory human subjects, continuously and in real-time. This
includes two micro-fluidic bi-stable valves for the valuing of the
calibration/wash solutions; three micro-actuators in series to effect
peristaltic
;15 pumping, with two separate pumps on the same chip; the optional integrated
ampermetric/potentiometric/optical sensor array in the sensor chamber; the
waste fluid, calibration/wash solutions, and buffer solution reservoirs; and
bonding pads for interconnecting wires. Also, incorporated are two different
layouts of the transdermal sampling chamber 60 (Fig.ures 10-12). Not shown
2 o are thermistor/thermocouple regulator, sensor chamber heater for
accelerated
assay control, integrated power supply, and integrated control electronics
which can optionally be included.
In any embodiment, the valves of the present invention utilize an
actuating mechanism 10 to occlude a micro-conduit 20 and thereby
2s decreasing or preventing fluid flow. The ability to occlude is selective,
in that
the valve can effectively open and close a passageway of the micro-conduit.
The micro-fluidic actuators 10 are the driving mechanism behind the micro-
fluidic valves 22 of the present invention.
The micro-fluidic valve 22 has various pressures and temperatures
3 o required for their actuation. The valve 22 can be selectively controlled
and
actuated through an integrated CMOS circuit or computer control, which
28
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
controls actuation timing, electrical current, and heat'
generation/dissipation
requirements for actuation. Integration of control circuitry is important for
reduced power requirements of the present invention.. In. one particular
embodiment for example, sensors and circuitry responsible for monitoring the
effluent of a fuel cell, with concomitant control of the micro-fluidic fuel
delivery
system to increase o.r decrease the flow rate of fuel, is designed. This
ensures optimal fuel utilization in the device. Closed loop feedback provides
the basis of automated adjustment of circuitry and therefore, valuing, within
the micro actuator. In another embodiment, closed loop feedback control can
Zo be used to inject insulin into an individual when the transdermal sensor
system detects hyperglycemic levels of glucose in the transdermally sampled
interstitial fluid,-thereby maintaining euglycemia.
In one embodiment of the present invention, the actuator 10 includes a
closed .cavity 11, flexible mechanism 18, and expanding mechanism 14.
z5 Fabrication of actuators 10 is accomplished by generating optical and/or
electron-beam (e-beam) .masks from the CAD designs of the micro-fluidic
system. Then, using solid-state mass production techniques, silicon wafers
are fabricated and . the flexible mechanisms 18 for' the actuators 10
subsequently are placed on the chips.
2 o In the device without integrated circuitry, the control circuitry is
produced on external breadboards and/or printed circuit boards. In this
manner, the circuitry is easily, quickly, and inexpensively optimized prior to
miniaturization and incorporation as CMOS circuitry on-chip that can be
controlled manually, or through the use of a computer with digital and analog
25 output. Optimized CMOS circuitry, modeled utilizing solid state MEMS and
CMOS design and simulation tools, is integrated into the active device making
it a stand-alone functional unit.
Electronic control of the actuators 10 is optimized to maximize
pumping rates and valuing forces, and to minimize power utilization and heat
3 o generation. An e-prom is also included on-chip to provide digital
compensation of resistors and capacitors to compensate for process
29
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
variations and, therefore, improve the process yield. Electrical access/test
pads are designed into the chips to allow for the esting of infiernal. nodes
of
the circuits.
The liquid or gaseous fluid being va.lved serves the purpose of acting
as a heat sink to condense the gas back to liquid and hence return the
flexible mechanism 18 to is relaxed state when the heating mechanism 12 is
inactivated. A temperature sensor 16 is integrated adjacent to the actuator
to monitor the .. temperature of the micro-fluidic integrated heating .
mechanism 12 and hence, expanding mechanism 14.
so Once the heating mechanism 12 is activated, vaporization of the
expanding mechanism 14 takes place. The expanding mechanism 14
component imposes a pressure upon the flexible mechanism 18 causing it to
expand and be displaced above the heating mechanism 12 and reduce .the
volume of the chamber 20. This methodology can be utilized to occlude fluid
flow through the chamber 20 (valuing action, see Figure 3).
For the mono-stable valve, it is assumed that the temperature on both
sides of the SiOa that encapsulates the heating mechanism 12 is constant,
and that heat flux in each direction .is dependent upon the heating mechanism
12 temperature and the resistance. to heat flow either through the device or
to
2 o the air from the backside. In order to isolate the heater, a cavity is
etched: in
the backside of the wafer, providing thermal isolation.
in one embodiment, a mono-stable valve 22 requires continuous power
to maintain a closed-stated position. Utilizing the heating mechanism 12, an
expanding mechanism 14 is vaporized under the encapsulating flexible
25 mechanism 18 thereby providing the pneumatic driving force required to
expand the flexible mechanism 18 and hence occluding the micro-conduit 20.
The mono-stable, normally open valve utilizes a single actuator to
effectively actuate the valve. As the hydrogel is expanded, the silicone
rubber of the actuator completely occludes the micro-fluidic channel to effect
3 o valuing of the solution. Schematics of the mono-stable valves are
presented
in Figures 3 and 9 and are depicted in the layout of the entire micro-fluidic
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
system design presented in Figures 1,2, and 4. While the normally open
valve is less complicated to construct, it requires continuous power or pulsed
power to keep the valve closed.
In another embodiment of the present invention, a bi-stable valve is
designed that utilizes lower power consumption and a wax material to provide
passively open and passively closed functionality, i.e. bi-stability. Thus, ,
power is only required to transition from one state to the other. The bi-
stable --
valve design is based upon the utilization of a moderate melting point solid,
such as paraffin wax, which possesses a melting point between 50° C and
so 70° C. Figure 8a shows a top view and 8b shows a cross-section of
the bi-
stable valve in the open state. The two actuators on the left, which contain
the paraffin wax, are connected to each other by~a fluid conduit.
The bi-stable valve 23 similarly utilizes actuating mechanisms 10 to
occlude the micro-conduit 20. The mono-stable valve can only provide the
25 functionality of a normally open valve. During ahe period that the valve 23
must be maintained in a closed position, continuous power must be applied.
In this embodiment, there is a bi-stable .valve 22 that utilizes micro-fluidic
actuators 10 to provide both zero-power open and closed functionality.
The bi-stable valve utilizes a .total : of three micro-fluidic actuating
2o v: mechanisms 10, 15. Although, any number~of actuating mechanisms 10, 15
can be used without departing from the spirit of the present invention. Two
actuating mechanisms 15 are physically.connected by a micro-fluid conduit
formed under the membrane and are filled with a low melting point solid such
as paraffin wax as opposed to an aqueous hydrogel 14 (see above for mono
2~ stable actuation). The third is a standard design micro-actuator 10 filled
with
an aqueous hydrogel connected by the expansion chamber to the middle wax
filled actuator 15. The first two micro-actuators 15 are activated causing the
wax to melt. The third, standard, micro-actuator 10 is then activated,
providing pneumatic force on the wax containing actuators 15, causing the
30 orifice containing chamber 20 to close. The wax is then allowed to
solidify.
31
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
Again, the advantage of this valve 22 is that it requires power only to
transform from the stable open to the stable closed state.
In the open state, medium in the channel readily flows. To switch from
the open state to the closed state, the wax is melted and the pneumatic
actuator 10 on the right is expanded. This creates pressure outside the
middle actuator 15, forcing the paraffin into ::the smaller left chamber,
expanding the membrane, thereby blocking fluid flow. The wax is allowed to
solidify, after which the power can be removed from the actuator providing the
driving force pressure, resulting in an electrically passive closed state. To
to ' transition from the closed state to the open state, the wax is melted and
membrane tension forces the wax from the small left chamber back into the
middle chamber. The micro-valve design provides bi-stable functionality,
which only requires power to switch between each state, but is completely
passive once in either the open or closed position.
The time to heat and cool the wax in the bi-stable valve is calculated
using Fick's equation for unsteady-state heat transfer. The partial
differential
equation is reduced to solving simultaneous ordinary differential equations
using numerical methods of lines with Polymath Software.
To calculate the unsteady-state heating and cooling, it is necessary to
2 o assume an insulated boundary at one side of the wax and either a
convective
(cooling) or a , conductive constant temperature (heating) boundary at the
other side. The assumption of the insulating boundary is appropriate for the
400 pm radius middle wax chamber since there is a pocket of air on the other
side of the membrane that is in contact with the wax. This can be
approximated as an insulated boundary.
In one embodiment, three actuators are needed for implementation of
the bi-stable valve. Wax is contained in the small actuator 15 in the left
chamber, which is in the shape of a hemisphere with radius of 140 pm and a
height of 20 pm when the valve is open and a height of 120 pm when the
3 o valve is closed. The middle chamber has a radius of 400 pm and a height of
pm when the valve is open, and when closed, the wax is be forced into the
32
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
small chamber leaving a height of 20.25 pm. Using these dimensions to
calculate the volume of wax in each chamber yields 1.23 nL of solid wax in
the small chamber and 15 nL of solid wax in the middle chamber. with the
valve open (i.e. membranes relaxed).
The insulating assumption used for the small 120 pm wax slab, in the
expanded valve,:_which blocks the fluid channel, is a conservative assumption
.
and provides a maximum cooling time using only a convective boundary on
one side of the wax. A more realistic estimate is similar to that.of the
constant
temperature boundary condition, with the flowing solution in the channel as
Zo the constant temperature sink. The speed at which the wax is-forced into
the
channel, thereby closing the valve, affects the cooling time of the wax. When
the valve is closed slowly, the flowing solution in the channel absorbs heat
from the wax, thereby reducing cooling time. If the valve is closed quickly,
heat from the wax is not able to be transferred to the solution, hence
15 increasing cooling time.
The time required to heat the wax is significantly shorter than that
required to cool the wax. This is true since heating uses a constant
temperature source at the boundary (an embedded poly-silicon or other type
of heater) without thermal resistance to the wax, and the cooling calculations
2 o utilized a high thermal convective resistance (air).
It is important to consider expansion and contraction of the wax during
heating and cooling: slower cooling rates combined with the use of a, lower
melting point wax can reduce shrinkage of the wax after it has occluded the
channel. To eliminate problems with shrinkage and thermal breakdown, the
25 wax should not be heated to a temperature greater than that necessary for
it
to liquefy. For a typical paraffin wax, the temperature should be kept below
65° C to prevent oxidation. Paraffin wax has a melting point of
60° C and a
congealing point of 59° C, therefore the temperature range of phase
transition
is narrow, thereby providing a uniform temperature distribution and uniform
3 o melting. Other types of waxes have a wider temperature range of phase
transition that can be used for other temperature range applications.
33
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
The thermal shrinkage of the wax is important because too much
shrinkage would allow the valve to open slightly, thereby allowing solution to
pass. Based upon the densities of melted and solidified wax, the contraction
of the wax in the device is calculated to be approximately 9 percent, and the
s device can be optimized by utilizing methods to force more wax into the
chamber to account for this shrinkage. A slower cooling rate applied to the
wax reduces shrinkage. Another method to compensate for shrinkage
involves cooling the left, valuing chamber while the middle and right
chambers.
remain heated. This forces more wax into the valuing chamber as the wax
wo cools. The power required to melt~the vwax is also important to consider
and
minimize. The calculated steady-state heat flux through each wax slab in the
device is calculated to be approximately 550 W/m2. .
In one embodiment, to calculate the pressure required to actuate the
valuing membrane, the overlap between the two chambers with wax-based
15 actuators is estimated to be approximately 200 pm wide. Using the thickness
of the wax in the small valuing chamber, the height is calculated to be 20 pm.
The pressure required to push melted wax through a 200 by 20 pm channel,
modeled as parallel plates, is 0.06 ~ A-TM .or 0.9 psi above atmosphere, a
v : readily obtainable pressure.
2 o The method of actuation is as follows. The heating mechanism 12 is
activated, thereby vaporizing the fluid component of the vaporizable fluid 14.
The vaporized fluid 14 component imposes a pressure upon the membrane
18 causing it to expand (be displaced above the heating mechanism 12) and
completely fill the chamber 20. This methodology can be utilized to occlude
25 fluid 14 flow through the chamber 20 (valuing action), or can be used for
other
purposes such as providing direct contact to the glass substrate to effect
heat
transfer or to provide the driving force for locomotion of a physical device
(i.e.
as in a walking caterpillar and/or a swimming paramecium with a flapping
flagella, in which case the glass chamber 20 encompassing the micro
3 o actuator 10 would not be used).
Throughout this application, various publications, including United
34
CA 02420682 2003-02-25
WO 02/18785 PCT/USO1/27340
States patents, are referenced by author and year and patents by number.
Full citations for the publications are listed below. The disclosures of these
publications and patents in their entireties . are hereby incorporated by
reference infio this application in order to more fully describe the state of
the
° art to which this invention pertains.
._ The invention has been described in an illustrative manner, and it is to
be understood that the terminology which has been used is intended to be in
~. . the nature of words of description rather than of limitation. .
Obviously, many modifications and variations of the present invention
to . are possible in light of the above teachings: It is, therefore, to be
understood
that within the scope of the appended claims, the invention can be practiced
otherwise than as specifically described.