Note: Descriptions are shown in the official language in which they were submitted.
CA 02421473 2003-03-10
1
Title: A CONTINUOUS PROCESS FOR MAKING AN AQUEOUS
HYDROCARBON FUEL EMULSION
Technical Field
The invention relates to a process for making aqueous hydrocarbon fuel
emulsions from a continuous or batch process with good stability. More
particularly,
the invention relates to a process for making an aqueous hydrocarbon fuel
emulsion
by employing an initial emulsion as one of the reactants in the process.
Background of the Invention
Internal combustion engines, especially diesel engines, that employ water
mixed with the fuel in the combustion chamber can produce lower nitrogen
oxides
(NOx), hydrocarbons and particulate emissions per unit of power output. The
reduction of nitrogen oxides is an environmental issue because they contribute
to
smog and air pollution. Governmental regulations and environmental concerns
have
driven the need to reduce NOx emissions from engines.
Diesel-fueled engines produce NOx due to the relatively high flame
temperatures reached during combustion. The reduction of NOx production
conventionally includes the use of catalytic converters, using "clean" fuels,
recirculation of exhaust and engine timing changes. These methods are
typically
expensive or complicated to be readily commercially available.
Water is inert toward combustion, but lowers the peak combustion
temperature resulting in reduced particulates and NOx formation. When water is
added to the fuel it forms an emulsion and these emulsions are generally
unstable.
Stable water in fuel emulsions of small particle size is difficult to reach
and maintain.
Stable water in fuel macroemulsions of small particles size are difficult to
make. It would be advantageous to develop a process to make water in fuel
macroemulsions in which a batch process did not need a statistical number of
tank
turnovers to produce a 1.0 micron or less water in fuel emulsion. Further, it
would be
advantageous to produce submicron mean average particles in a water in fuel
macroemulsion by a continuous process.
It has been found that including an emulsion as an initial component with the
water fuel and emulsifier in a batch or continuous process produces an
improved
stable water in fuel macroemulsion with a mean average particle size
distribution of 1
micron or less.
CA 02421473 2003-03-10
2
The term "NOx" is used herein to r efer to any o f the n itrogen o xides, NO,
NOz, NZO, or mixtures of two or more thereof. The terms "aqueous hydrocarbon
fuel
emulsion" and "water fuel emulsion" are interchangeable. The terms "aqueous
hydrocarbon fuel" and "water fuel blend" are interchangeable.
Summary of the Invention
The invention relates to a batch or continuous process for making an aqueous
hydrocarbon fuel a mulsion comprising: a mulsifying ( a) a 1 iquid hydrocarbon
fuel,
water and at least one emulsifier, (b) a reactant emulsion of the liquid
hydrocarbon
fuel, water and at least one emulsifier, and (c) water, under emulsification
conditions
to form an aqueous hydrocarbon fuel emulsion.
The aqueous hydrocarbon fuel is an emulsion comprised of water, fuel and an
emulsifier. The emulsifier comprises:
(i) at least one fuel-soluble product made by reacting at least one
hydrocarbyl-
substituted carboxylic acid acylating agent with ammonia or an amine, the
hydrocarbyl substituent of said acylating agent having about 50 to about 500
carbon
atoms;
(ii) at least one of an ionic or a nonionic compound having a hydrophilic-
lipophilic balance (HLB) of about 1 to about 40;
(iii) a mixture of (ii) with (i);
(iv) a w ater-soluble c ompound s elected f rom t he g roup c onsisting o f a
mine
salts, ammonium salts, azide compounds, nitrate esters, nitramine,
nitrocompounds,
alkali metal salts, alkaline earth metal salts, in combination with (i), (ii),
(iii), (v), (vii)
or combinations thereof;
(v) the reaction product of polyacidic polymer with at least one fuel soluble
product made by reacting at least one hydrocarbyl-substituted carboxylic acid
acylating agent with ammonia, an amine, a polyamine, alkanol amine, or hydroxy
amines;
(vi) an amino alkylphenol which is made by reacting an alkylphenol, an
aldehyde and an amine resulting in an amino alkylphenol, or
(vii) the combination of (vi) with (i), (ii), (iii), (iv), (v) or combinations
thereof.
The aqueous hydrocarbon fuel emulsion includes a discontinuous aqueous
phase in a continuous fuel phase. The discontinuous aqueous phase comprises
aqueous droplets having a mean diameter of 1.0 micron or less. Furthermore,
the use
CA 02421473 2003-03-10
3
of an emulsion as an initial component in the batch or continuous process has
improved the efficiency of the process and the stability of aqueous
hydrocarbon
emulsions for use as aqueous hydrocarbon fuel emulsion.
The Process
The invention provides for a batch or continuous process for making an
aqueous hydrocarbon fuel by forming a stable emulsion in which the water is
suspended in a continuous phase of fuel and wherein the water droplets have a
mean
diameter of 1.0 micron or less. The droplet size is in volume.
In the practice of the present invention the aqueous hydrocarbon fuel emulsion
is made by a batch or a continuous process capable of monitoring and adjusting
the
flow rates of the reactant emulsion, fuel, emulsifier, additives and/or water
to form a
stable emulsion with the desired water droplet size.
The batch process as described herein depicts one embodiment of the
invention. T he h ydrocarbon fuel, a rnulsifier, a nd r eactant emulsion are
added to a
vessel. The water is added to the vessel or, in the alternative is added close
to the
entry portal of the emulsification device, which is external to the vessel. In
the batch
process the following components are emulsified:
(1) about 10% to about 90% by weight of the fuel and about at least 0.1% to
about 25'% by weight of emulsifier,
(2) about 1 % to about 90% by weight of a reactant emulsion, and
(3) about 1 % to about 50% by weight of water, wherein the water contains
about 0% to about 30% by weight of the aqueous hydrocarbon emulsion.
The ratio of fuel, water and emulsifier to reactant emulsion is about 1 to
about
99, in another embodiment about 15 to about 85, in another embodiment about 40
to
about 60, in another embodiment about 99 to about 1, in another embodiment
about
85 to about 15, in another embodiment about 60 to about 40, and in another
embodiment 50 to about 50.
The mixture is emulsified using an emulsification device in the vessel, or
alternatively the mixture flows from the vessel via a circular line to the
emulsification
device which is external to the vessel, for about 1 to about 20 tank
turnovers, at a
temperature in the range of about ambient temperature to about 212°F,
and in another
embodiment in the range of about 40°F to about 150°F, and at a
pressure in the range
of about atmospheric pressure to about 10 atmospheres, in another embodiment
about
atmosphere pressure to about 60 psi, in another embodiment in the range of
about 10
CA 02421473 2003-03-10
4
psi to about 40 psi, resulting in stable aqueous hydrocarbon fuel emulsion
with a
mean droplet size of less than 1.0 micron, and in another embodiment in the
range of
about 1.0 micron to about 0.1 micron.
Examples 1-4
These examples are illustrations of making the hydrocarbon fuel emulsion
product by
a batch process. The blending equipment consists of a five-million-gallon-per-
year
batch blender.
Batch No. 1, using a 3:1 volume/volume ratio of raw material components to
reactant
emulsion:
1. A 25 gallon batch of hydrocarbon fuel emulsion was prepared using about
19.9 gallons of diesel fuel, about 4.4 gallons of water, and about 0.7 gallons
of
emulsifier A, which is the following:
Concentrate
by weight
Emulsifier 1 40.00
Emulsifier 2 7.14
Emulsifier 3 19.80
2-ethylhexylnitrate 23.80
Ammonium Nitrate 9.26
(54% by weight in water)
Emulsifier 1: Reaction product of dimethylethanolamine and PIBSA (Mn-2000)
Emulsifier 2: Reaction product of dimethylethanolamine and hexadeclysuccinnic
anhydride
Emulsifier 3: Reaction product of an ethylene polyamine and PIBSA (Mn-1000)
This 25 gallons was left in the processing tank to serve as the reactant
emulsion for the next batch.
2. About 59.6 gallons of diesel fuel was added to the processing tank followed
by
about 2.1 gallons of emulsifier A.
3. The reactant emulsion, diesel fuel, and emulsifier A were circulated
through
an IKA high shear mixer for about 30 seconds and back to the processing tank.
4. Following about 30 second mix and while continuing to circulate through the
mixer, a total of about 13.3 gallons of water were added through a charging
line immediately upstream of the mixer. The water feed time was about 85
seconds.
5. Once all water was added, the mixture continued to circulate through the
IKA
mixer for about an additional 12 minutes and 36 seconds.
6. Samples of emulsion were taken from the processing tank at various time
intervals during this mix period representing 1, 2, 4, 7 and 9 tank turnovers.
A
tank turnover is defined as the duration to pump 100 gallons through the
CA 02421473 2003-03-10
mixer.
7. The results are found in Table I.
5 Batch No. 2, using a 1:1 volume/volume ratio of raw material components to
reactant
emulsion.
I . A 50.1 gallon batch of hydrocarbon fuel emulsion was prepared using about
39.8 gallons of diesel fuel, about 8.9 gallons of water, and about 1.4 gallons
of
emulsifier A. This 50.1 gallons were left in the processing tank to serve as
the
reactant emulsion for the next batch.
2. About 39.7 gallons of diesel fuel was added to the processing tank followed
by
about 1.4 gallons of emulsifier A.
3. The reactant emulsion, diesel fuel and emulsifier A were circulated through
the I KA high-shear m fixer f or about 30 seconds and back to t he p rocessing
tank.
4. Following about 30 second mix and while continuing to circulate through the
mixer, a total of about 8.8 gallons of water was added through a charging line
immediately upstream of the mixer. The water feed time was about 56
seconds.
5. Once all water was added, the mixture continued to circulate through the
IKA
mixer for about an additional 12 minutes and 36 seconds.
6. Samples of emulsion were taken from the processing tank at various time
intervals during this mix period representing 1, 2, 4, 7 and 9 tank turnovers.
A
tank turnover is defined as the duration to pump 100 gallons through the
mixer.
7. The results are found in Table I.
Batch No. 3, using a 3:1 volume/volume ratio of raw material components to
reactant
emulsion. A concentrated emulsion formula was used for this example whereby
approximately 85% volume of the formula amount of diesel fuel was omitted
during
the processing.
1. A 25.1 gallon batch of concentrated aqueous hydrocarbon fuel emulsion was
prepared using about 8.6 gallons of GARB diesel fuel, about 14.2 gallons of
water and about 2.3 gallons of emulsifier A. About 25.1 gallons were left in
the processing tank to serve as the reactant emulsion for the next batch.
2. About 25.6 gallons of CARB diesel fuel were added to the processing tank
followed by about 6.6 gallons of emulsifier A.
3. The reactant emulsion, diesel fuel and emulsifier A were circulated through
the IKA h igh s hear mixer for a bout 3 0 s econds a nd b ack t o t he
processing
tank.
CA 02421473 2003-03-10
6
4. Following about 30 second mix and while continuing to circulate through the
mixer, a total of about 42.7 gallons of water was added through a charging
line
immediately upstream of the mixer.
5. Once all water was added, the mixture continued to circulate through the
IKA
mixer for about an additional 16 minutes.
6. Samples of concentrated emulsion were taken from the processing tank at
various time intervals during this mix period representing 1, 2, 4, 7 and 10.4
tank turnovers. A tank turnover is defined as the duration to pump 100 gallons
through the mixer.
7. The concentrated emulsion was pumped to the diluter tank and diluted with
about 229.3 gallons of GARB diesel fuel.
8. The diluter tank was circulated with a centrifugal pump for about 9
minutes.
9. A sample of the aqueous hydrocarbon fuel emulsion was taken from the
processingtank.
10. The results are found in Table I.
Batch No. 4, using a 1:1 volume/volume ratio of raw material components to
reactant
emulsion. A concentrated emulsion formula was used for this example whereby
approximately 85% volume of the formula amount of diesel fuel was omitted
during
processing.
About 50.1 gallon batch of concentrated aqueous hydrocarbon fuel emulsion
was prepared using about 17.1 gallons of CARB diesel fuel, about 28.5
gallons of water, and about 4.5 gallons of emulsifier A. About 50.1 gallons
were left in the processing tank to serve as the reactant emulsion for the
next
batch.
2. About 17.1 gallons of CARB diesel fuel were added to the processing tank
followed by about 4.4 gallons of emulsifier A.
3. The reactant emulsion, diesel fuel, and emulsifier A were circulated
through
the IKA h igh s hear mixer for a bout 3 0 s econds a nd b ack t o t he
processing
tank.
4. Following about 30 second mix and while continuing to circulate through the
mixer, a total of about 28.4 gallons of water were added through a charging
line immediately upstream of the mixer.
5. Once all water was added, the mixture continued to circulate through the
IKA
mixer for about an additional 16 minutes.
6. Samples of concentrated emulsion were taken from the processing tank at
various time intervals during this mix period representing 1, 2, 4, 7 and 10.4
CA 02421473 2003-03-10
7
tank turnovers. A tank turnover is defined as the duration to pump 100 gallons
through the mixer.
7. The concentrated emulsion was pumped to a diluter tank and diluted with
about 229.3 gallons of GARB diesel fuel.
8. The diluter tank was circulated with a centrifugal pump for about 9
minutes.
9. A sample of the anal aqueous hydrocarbon fuel emulsion was taken from the
diluter tank.
10. The results are found in Table I.
Table I
b - -''
Particle O o o ~ ~ o o
Size o ,~ R = 0 s
Distribution 3 ~ 3
Sample desCrlptlonMean Mode 7
m m day 7
static da
storage static
(room stora
tem a
erature 43C
Example 1: 1:24 0.78 0.47 6 94 3 9 _ 7
mn:sec _0.7_70.47 6 94 4 9 91 7
Example 1: 2:48 0.77 0.52 4 96 4 9 91 7
mn: sec 0.79 0.52 4 96 4 9 91 7
Example 1: 5:36 91
mn:sec
Example 1: 9:48
mn:sec
Example 1: 12:360.78 0.52 6 94 4 9 91 7
mnaec
Exam le 2: 1:24 0.63 0.43 6 94 4 6 94 6
mnaec
Example 2: 2:48 0.64 0_.436 94 4 7 93 6
mnaec
Example 2: 5:36 0.6_8 0_.474 96 4 7 93 6
mnaec_ 0.63 0.47 6 94 3 7 93 6
Example 2: 9:48
mnaec
Example 2: 12:360.63 0.47 4 96 3 7 93 6
mn:sec
Exam le 3: 1:32 1.31 1.45
mnaec
xample 3: 3:04 1.40 1.59
mnaec 1.30 0.91
Example 3: 6:06
mnaec
Example 3: 10:4_4_1.05_1.20_
mnaec
Example 3: 16:000.92 1.24
mnaec
Example 3: final0.89 0.63 3 97 7 3 6 91 12
sam le
Example 4: 1:32 1.12 1.32
mn:sec
Exam le 4: 3:04 1.05 1.00
mn:sec
Example 4: 6:06 0.98 1.00
mnaec
xample 4: 10:44 0.90 0.83
mnaec 0.83 0.76
xample 4: 16:00
mnaec
Example 4: final0.86 0.83 3 97 7 4 4 91 10
sample
The continuous process described herein depicts another embodiment of the
invention. The feeds of the hydrocarbon fuel, emulsifier, reactant emulsion
and water
are introduced as discreet feeds or in the alternative combinations of the
discreet
CA 02421473 2003-03-10
8
feeds, to form a homogeneous hydrocarbon fuel emulsion. It is preferable that
the
processing streams of the fuel, emulsifier, water and emulsion reactant, are
introduced
as close to the inlet of the emulsification device as possible. It is
preferable that the
emulsifier is added to the fuel as a hydrocarbon fuel emulsifier stream prior
to the
discreet feeds combining together.
The ratio of the hydrocarbon fuel, emulsifier and water to reactant emulsion
in
one embodiment is about 1% hydrocarbon fuel, emulsifier and water to about 99%
reactant emulsion, in another embodiment a bout 99% hydrocarbon fuel,
emulsifier
and water to about 1% reactant emulsion, in another embodiment about 15%
hydrocarbon fuel, emulsifier a nd w ater t o a bout 8 5% r eactant emulsion, i
n another
embodiment about 40% hydrocarbon fuel, emulsifier and water to about 60%
reactant
emulsion, in another embodiment about 60% hydrocarbon fuel, emulsifier and
water
to about 40% reactant emulsion, and in another embodiment 50% hydrocarbon
fuel,
emulsifier and water to about 50% reactant emulsion. The hydrocarbon fuel
emulsifier stream during startup and shutdown is such that the ratio of water
to
hydrocarbon fuel emulsion mixture is never greater than the steady state
condition.
The continuous process generally occurs under ambient conditions. The
continuous process is generally done at atmospheric pressure to about 500 psi,
in
another embodiment in the range of about atmospheric pressure to about 120
psi, and
in another embodiment in the range of about atmospheric pressure to about 50
psi.
The continuous process generally occurs at ambient temperature. In one
embodiment
the temperature is in the range of about ambient temperature to about
212°F, and in
another embodiment in the range of about 40°F to about 150°F.
The emulsification provides for the desired particle size and a uniform
dispersion of water in the fuel and occurs at a shear rate in the range of
greater than 0
s-~ to about 500,000 s-~, preferably about 20,000 s-~ to about 200,000 s-1,
more
preferably of about 2 5,000 s~~ to about 1 25,000 s~ ~ o f shearing. If more t
han one
emulsification step is used, the shear rates of the emulsification steps can
be the
same, similar or different, depending on the emulsifier used and the ratio of
reactant
emulsion to fuel additive and/or water.
In another embodiment the emulsion flows through at least one to several
emulsification devices. In another embodiment, the emulsion flows through the
next
one to five emulsification devices. The emulsion flows through the emulsion
devices
CA 02421473 2003-03-10
9
in series, directly from one emulsification device to the next emulsification
device in
the series.
In one embodiment there is no intermediate holding tank between the
emulsification steps. The emulsion is not aged between the emulsification
steps.
S Generally the time the emulsion flows from one emulsification device to
another
emulsification device in less than 5 minutes, in another embodiment less than
4
minutes, in another embodiment less than 3 minutes, in another embodiment less
than
2 minutes, in another embodiment less than 1 minute, and in another embodiment
less
than 30 seconds.
The other emulsification steps, in series, are a high-shear process and occur
under ambient conditions as described in the emulsification step above. The
shear
rate temperature and pressure can be the same, similar or different than the
other
emulsification steps so long as the conditions are such to provide the desired
mean
droplet particle size.
The emulsification step is a high shear process and results in a uniform
dispersion of the hydrocarbon fuel emulsion having a mean particle droplet
size in the
range of about 0.1 micron to about 1 micron, in one embodiment in the range of
about
0.1 micron to about .95 micron, in one embodiment in the range of about 0.1
micron
to about 0.8 micron, and in one embodiment in the range of about 0.1 micron to
about
0.7 m icron, and i n o ne embodiment in t he r ange o f about 0.2 m icron t o
a bout 0 .5
micron. A critical feature of the invention is that the water phase of the
aqueous fuel
emulsion product is comprised of water droplets having a mean diameter of less
than
one micron, in another embodiment one micron to about 0.1 micron, and in
another
embodiment 1.0 micron to about 0.2 micron.
The emulsification occurs by any method known in the industry including but
not limited to mixing, mechanical mixer agitation, static mixers, shear
mixers, sonic
mixers, high-pressure homogenizers, and the like. Examples of the
emulsification
devices include but are not limited to an Aquashear, pipeline static mixers
and the
like. The Aquashear is a low-pressure hydraulic shear device. The material is
forced
through two facing plates with drilled holes into the mixing chamber. The two
plates
cause counter rotational flow and allow the material to mix. The Aquashear
mixers
are available from Flow Process Technologies Inc.
Additional emulsification devices include high-shear devices such as IKA
Works, Inc. Dispax Reactor. The IKA shear mixers use a DR3-6 with three stages
of
CA 02421473 2003-03-10
1~
rotor/stator combinations. The tip speed of the rotor/stator generators may be
varied
by a variable frequency drive that controls the motor. The Silverson mixer is
a two-
stage mixer, w hich incorporates a r otor/stator d esign. The m fixer h as
high-volume
pumping characteristics similar to centrifugal pump. Inline shear mixers by
Silverson
Corporation (a rotor-stator emulsification approach); Jet Mixers (venturi-
style/cavitation shear mixers), Ultrasonolator made by the Sonic Corp.
(ultrasonic
emulsification approach), Microfluidizer shear mixers available by
Microfluidics Inc.
(high-pressure homogenization shear mixers), ultrasonic mixers, and any other
available high-shear mixer.
These emulsification devices have to have the ability to reduce the mean
particles size of the water droplet in the range of less than one micron to
about 0.1
micron or even less.
The hydrocarbon fuel, at least one emulsifier and water are emulsified to form
a reactant emulsion. The reactant emulsion is formed from recycling the
aqueous
hydrocarbon fuel emulsion or separately by emulsifying the hydrocarbon fuel
with at
least one emulsifier in a separate vessel. The water may optionally contain
water-
soluble a dditives. The reactant a mulsion is g enerally recycled in t he
process. T he
reactant emulsion may contain the same, similar or different hydrocarbon fuel
and/or
emulsifier than the aqueous hydrocarbon fuel. 'the reactant emulsion may be
the
same, similar or different composition as the desired aqueous hydrocarbon fuel
emulsion. By using a reactant emulsion as an initial component, the overall
particle
size decreases and the aqueous hydrocarbon fuel emulsion stability is
increased.
The hydrocarbon fuel and emulsifier contains about 50% to about 99% by
weight, p referably about 8 5% t o a bout 9 8% b y w eight, a nd m ore p
referably about
95% to about 98% by weight hydrocarbon fuel, and it further contains about
0.05% to
about 25%, preferably about 1% to about 15%, and more preferably about 1% to
about 5% by weight of at least one emulsifier.
Optionally, additives may be added to the reactant emulsion, hydrocarbon
fuel, emulsifier, water or combinations thereof. The additives include but are
not
limited to cetane improvers, organic solvents, antifreeze agents, surfactants,
other
additives k nown f or their use in fuel and the 1 ike. T he additives a re a
dded to the
reaction emulsion, hydrocarbon fuel, emulsifier or the water, prior to and in
the
alternative at the first emulsification step dependent upon the solubility of
the
additive. The additives are generally in the range of about 1 % to about 40%
by
CA 02421473 2003-03-10
11
weight, preferably about 5% to about 30% by weight, and more preferably about
7%
to about 25% by weight of the additive emulsifier.
The water can optionally include but is not limited to the water-soluble
additives such as antifreeze, ammonium nitrate, ammonium salts or mixtures
thereof.
Ammonium nitrate is generally added to the water mixture as an aqueous
solution. In
one embodiment the water, the antifreeze and/or the ammonium nitrate are mixed
dynamically and fed continuously to the process. In another embodiment the
water,
antifreeze, ammonium nitrate or mixtures thereof flow out of separate tanks
and/or
combinations thereof into or mixed prior to the first emulsification step. In
one
embodiment the water, water alcohol, water-ammonium nitrate, or water-alcohol
ammonium nitrate mixture meets the hydrocarbon fuel additives mixture
immediately
prior to or in the emulsification step.
A programmable logic controller (plc) is optionally employed for governing
the continuous flow of the components, thereby controlling the flow rates and
mixing
ratio in accordance with the Erescribed blending rates. The plc stores
component
percentages input by the operator. The plc then uses these percentages to
define
volumes/flow of each component required. Continuous flow sequence is
programmed
into the plc. The plc electronically monitors all level switches, valve
positions and
fluid meters.
Example 5
This example is illustrative of making a hydrocarbon fuel emulsion product by
a continuous process. A mixture having the following components was prepared.
Concentrate Emulsion
Diesel --- 78.12
Water --- 20.00
Emulsifier 1 40.00 0.5
Emulsifier 2 7.14 0.214
Emulsifier 3 19.80 0.297
2-ethylhexylnitrate23.80 0.714
Ammonium Nitrate 9.26 0.15
(54% by weight in
water
Emulsifier 1: Reaction product ofdimethylethanolamine and YIBSA (Mn-2000)
Emulsitier 2: Reaction product of dimethylethanolamine and hexadecylsuccinnic
anhydride
Emulsifier 3: Reaction product of an ethylene polyamine and PIBSA (Mn-1000)
CA 02421473 2003-03-10
12
About 2.88% weight of an emulsifier reactant is added to about 97.12%
weight o f d iesel fuel a nd m fixed t o p roduce a h ydrocarbon fuel a
mulsifier m fixture.
The hydrocarbon fuel and emulsifier, at a flow rate of 4.95 gallons per minute
(F1),
was emulsified with water that had a flow rate of about 1.05 gallons per
minute (F2)
at room temperature along with the emulsion reactant that had a flow rate of
about 6.0
gallons per minute (F4). 'The processing streams were introduced close to the
entry
portal of the shear mixer as possible. The shear mixer was a 12 GPM IKA Works
Dispax mixer with three superfine mixing elements operating at about 9600 rpm
(revolutions p er m mute). T he output from t his mixer ( F3) w as t hen split
into t wo
streams where about 6.0 gallons per minute was diverted through a conduit to
the inlet
of the IKA Works Dispax mixer (F4) and about 6.0 gallons per minute was pumped
through a conduit to storage (FS). This process has been shown to produce a
water in
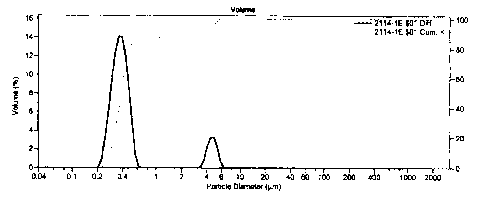
oil emulsion with the following particle size distribution (see Figure 1).
The p article s ize of the r esulting a mulsion m ade by t he continuous
process
with an identical formulation made via a process without the emulsion co-feed
(i.e. F4
= 0 gallons per minute) is shown in Figure 2.
The example showed that a continuous process using an emulsion co-feed
unexpectedly consistently produced higher quality results.
The aqueous hydrocarbon fuel emulsion product produced by the continuous
process involves less processing time than by batch processing. Thus, the
inventive
process to make the same water-blended fuel product is an improvement over
other
processes because of the increased throughput and efficiency.
The Hydrocarbon Fuel
The liquid hydrocarbon fuel comprises hydrocarbonaceous petroleum distillate
fuel, non-hydrocarbonaceous materials that include but are not limited to
water, oils,
liquid fuels derived from vegetables, liquid fuels derived from minerals and
mixtures
thereof. The liquid hydrocarbon fuel may be any and all hydrocarbonaceous
petroleum distillate fuels including not limited to motor gasoline as defined
by ASTM
Specification D439 or diesel fuel or fuel oil as defined by ASTM Specification
D396
or t he 1 ike (kerosene, n aphtha, aliphatics a nd p araffinics). T he 1 iquid
hydrocarbon
fuels comprising non-hydrocarbonaceous materials include but are not limited
to
alcohols such as methanol, ethanol and the like, ethers such as diethyl ether,
methyl
ethyl ether and the like, organo-nitro compounds and the like; liquid fuels
derived
from vegetable or mineral sources such as corn, alfalfa, shale, coal and the
like. The
CA 02421473 2003-03-10
13
liquid hydrocarbon fuels also include mixtures of one or more
hydrocarbonaceous
fuels and one or more non-hydrocarbonaceous materials. Examples of such
mixtures
are combinations of gasoline and ethanol and of diesel fuel and ether. In one
embodiment, the liquid h ydrocarbon fuel i s any g asoline. Generally,
gasoline i s a
mixture of hydrocarbons having an ASTM distillation range from about
60°C at the
10% distillation point to about 205°(~ at the 90% distillation point.
In one
embodiment, the gasoline is a chlorine-free or low-chlorine gasoline
characterized by
a chlorine content of no more than about 10 ppm.
In one embodiment, the liquid hydrocarbon fuel is any diesel fuel. Diesel
fuels typically have a 90% point distillation temperature in the range of
about 300°C
to about 390°C, and in one embodiment about 330°C to about
350°C. The viscosity
for these fuels typically ranges from about 1.3 to about 24 centistokes at
40°C. The
diesel fuels can be classified as any of Grade Nos. 1-D, 2-D or 4-D as
specified in
ASTM D975. The diesel fuels may contain alcohols and esters. In one embodiment
the diesel fuel has a sulfur content of up to about 0.05% by weight (low-
sulfur diesel
fuel) as determined by the test method specif ed in ASTM D2622-87. In one
embodiment, the diesel fuel is a chlorine-free or low-chlorine diesel fuel
characterized
by chlorine content of no more than about 10 ppm.
The liquid hydrocarbon fuel is present in the aqueous hydrocarbon fuel
emulsion at a concentration of about 50% to about 95% by weight, and in one
embodiment about 60% to about 95°/> by weight, and in one embodiment
about 65%
to about 85% by weight, and in one embodiment about 80% to about 90% by weight
of the aqueous hydrocarbon fuel emulsion.
The Water
The water used in the aqueous hydrocarbon fuel emulsion may be taken from
any source. The water includes but is not limited to tap, deionized,
demineralized,
purified, for example, using reverse osmosis or distillation, and the like.
The water
includes water mixtures that further includes antifreeze such as alcohols and
glycols,
ammonium salts such as ammonium nitrate, ammonium maleate, ammonium acetate
and the like, and combinations thereof.
The water may be present in the aqueous hydrocarbon fuel emulsions at a
concentration of about 1% to about 50% by weight, and in one embodiment about
5%
to about 50% by weight, and in one embodiment about 5% to about 40% being
CA 02421473 2003-03-10
14
weight, and in one embodiment about 5% to about 25% by weight, and in one
embodiment about 10% to about 20% water.
The Reactant Emulsion Component
The reactant emulsion is at least one of the ingredients in the process and
not
necessarily a reactant in a chemical reaction. The reactant emulsion comprises
the
hydrocarbon fuel, water and at least one emulsifier. The reactant emulsion may
be
prepared by the steps of (1) mixing the fuel and an emulsifying amount of at
least one
emulsifier using standard mixing techniques to form the initial emulsion
component
or, in the alternative, under emulsification mixing conditions to form the
reactant
emulsion. The reactant emulsion can be prepared from any emulsion process
including the process of this invention. It is in effect a recycling of a
finished
emulsified fuel into the process for making more emulsifier fuels.
The initial emulsion component contains about 50% to about 99% by weight,
in another embodiment about 85% to about 98% by weight, and in another
embodiment about 95% to about 98% by weight hydrocarbon fuel, and it further
contains about 0.05% to about 25%, in another embodiment about 1% to about
15%,
and in another embodiment about 1 % to about 5'% by weight of the emulsifier.
The reactant emulsifier can have the same, similar or a different emulsifier
and
the same, similar or different fuel then is used to form the aqueous
hydrocarbon fuel
emulsion. The emulsifier includes but is not limited to:
(i) at least one fuel-soluble product made by reacting at least one
hydrocarbyl-
substituted carboxylic acid acylating agent with ammonia or an amine including
but
not limited to alkanol amine, hydroxy amine, and the like, the hydrocarbyl
substituent
of said acylating agent having about 50 to about 500 carbon atoms;
(ii) at least one of an ionic or a nonionic compound having a hydrophilic-
lipophilic balance (HLB) of about 1 to about 40;
(iii) mixture of (ii) with (i);
(iv) a w ater-soluble c ompound s elected from t he g roup c onsisting o f a
mine
salts, ammonium salts, azide compounds, nitrate esters, nitramine,
nitrocompounds,
alkali metal salts, alkaline earth metal salts, in combination with (i), (ii),
(iii), (v), (vii)
or combinations thereof;
(v) the reaction product of polyacidic polymer with at least one fuel soluble
product made by reacting at least one hydrocarbyl-substituted carboxylic acid
CA 02421473 2003-03-10
acylating agent with ammonia, an amine, a polyamine, an alkanol amine or
hydroxy
amines;
(vi) an amino alkylphenol which is made by reacting an alkylphenol, an
aldehyde and an amine resulting in an amino alkylphenol, or
5 (vii) the combination of (vi) with (i), (ii), (iii), (iv), (v) or
combinations
thereof.
The Emulsifier
Fuel Soluble Product (i)
10 The fuel-soluble product (i) may be at least one fuel-soluble product made
by
reacting at least one hydrocarbyl-substituted carboxylic acid acylating agent
with
ammonia or an amine including but not limited to alkanol amines, hydroxy
amines,
and the like, the hydrocarbyl substituent of said acylating agent having about
50 to
about 500 carbon atoms, and is described in greater detail in USSN 09/761,482,
An
15 Emulsifier For An Aqueous Hydrocarbon Fuel, incorporated by reference
herein.
The hydrocarbyl-substituted carboxylic acid acylating agents may be
carboxylic acids or reactive equivalents of such acids. The reactive
equivalents may
be acid halides, anhydrides, or esters, including partial esters and the like.
The
hydrocarbyl substituents for these carboxylic acid acylating agents may
contain from
about 50 to about 500 carbon atoms, and in one embodiment about 50 to about
300
carbon atoms, and in one embodiment about 60 to about 200 carbon atoms. In one
embodiment, the hydrocarbyl substituents of these acylating agents have number
average molecular weights of about 700 to about 3000, and in one embodiment
about
900 to about 2300.
The hydrocarbyl-substituted carboxylic acid acylating agents may be made by
reacting one or more alpha-beta olefinically unsaturated carboxylic acid
reagents
containing 2 to about 20 carbon atoms, exclusive of the carboxyl groups, with
one or
more olefin polymers as described more fully hereinafter.
In one embodiment, the hydrocarbyl-substituted carboxylic acid acylating
agent is a polyisobutene-substituted succinic anhydride, the polyisobutene
substituent
having a number average molecular weight of about 1,500 to about 3,000, in one
embodiment about 1,800 to about 2,300, in one embodiment about 700 to about
1300,
in one embodiment about 800 to about 1000, said first polyisobutene-
substituted
succinic anhydride being characterized by about 1.3 to about 2.5, and in one
CA 02421473 2003-03-10
16
embodiment about 1.7 to about 2.1 In one embodiment, the hydrocarbyl-
substituted
carboxylic acid acylating agent is a polyisobutene-substituted succinic
anhydride, the
polyisobutene substituent having a number average molecular weight of about
1,500
to about 3,000, and in one embodiment about 1,800 to about 2,300, said first
polyisobutene-substituted succinic anhydride being characterized by about 1.3
to
about 2.5, and in one embodiment about 1.7 to about 2.1, in one embodiment
about
1.0 to about 1.3, and in one embodiment about 1.0 to about 1.2 succinic groups
per
equivalent weight of the polyisobutene substituent.
The fuel-soluble product (i) may be formed using ammonia, an amine and/or
metals such as Na, K, Ca, and the like. The amines useful for reacting with
the
acylating agent to form the product (i) including but are not limited to,
monoamines,
polyamines, alkanol amines, hydroxy amines, and mixtures thereof, and amines
may
be primary, secondary or tertiary amines.
Examples of primary and secondary monoamines include ethylamine,
diethylamine, n-butylamine, di-n-butylamine, allylamine, isobutylamine,
cocoamine,
stearylamine, laurylamine, methyllaurylamine, oleylamine, N-methyloctylamine,
dodecylamine, and octadecylamine. Suitable examples of tertiary monoamines
include trimethylamine, triethylamine, tripropylamine, tributylamine,
monoethyldimethylamine, dimethylpropylamine, dimethylbutylamine,
dimethylpentylamine, dimethylhexylamine, dimethylheptylamine, and
dimethyloctylamine.
The amines include but are not limited to hydroxyamines, such as mono-, di-,
and triethanolamine, dimethylethanol amine, diethylethanol amine, di-(3-
hydroxy
propyl) amine, N-(3-hydroxybutyl) amine, N-(4-hydroxy butyl) amine, and N,N-di-
(2-hydroxypropyl) amine; alkylene polyamines such as methylene polyamines,
ethylene polyamines, butylene polyamines, propylene polyamines, pentylene
polyamines, and the like. Specific examples of such polyamines include
ethylene
diamine, diethylene triamine, triethylene tetramine, propylene diamine,
trimethylene
diamine, tripropylene tetramine, tetraethylene pentamine, hexaethylene
heptamine,
pentaethylene hexamine, or a mixture of two or more thereof; ethylene
polyamine; is
a polyamine bottoms or a heavy polyamine. The fuel-soluble product (i) may be
a salt,
an ester, an ester/salt, an amide, an imide, or a combination of two or more
thereof.
The fuel-soluble p roduct ( i) may b a p resent in t he water-fuel a mulsion
at a
concentration of up to about 15% by weight based on the overall weight of the
CA 02421473 2003-03-10
17
emulsion, and in one embodiment about 0.1 to about 15% by weight, and an one
embodiment about 0.1 to about 10% by weight, and in one embodiment about 0.1
to
about 5% by weight, and in one embodiment about 0.1 to about 2% by weight, and
in
one embodiment about 0.1 to about 1 % by weight, and in one embodiment about
0.1
to about 0.7% by weight.
The Ionic or Nonionic Compound (ii)
The ionic or nonionic compound (ii) has a hydrophilic-lipophilic balance
(HLB, which refers to the size and strength of the polar (hydrophilic) and non-
polar
(lipophilic) groups on the surfactant molecule) in the range of about 1 to
about 40, and
in one embodiment about 4 to about 15 and is described in greater detail in
USSN
09/761,482, An Emulsifier For An Aqueous Hydrocarbon Fuel, incorporated by
reference herein. Examples of these compounds are disclosed in McCutcheon's
Emulsifiers and Detergents, 1998, North American & International Edition.
Pages 1-
235 of the North American Edition and pages 1-199 of the International Edition
are
incorporated herein by reference for their disclosure of such ionic and
nonionic
compounds having an HLB in the range of about 1 to about 40, in one embodiment
about 1 to about 30, in one embodiment about 1 to 20, and in another
embodiment
about 1 to about 10. Examples include low molecular weight variants of (i) or
(vii)
such as those having a hydrocarbon group in the range of Cg or C2~. Useful
compounds include alkanolamides, carboxylates including amine salts, metallic
salts
and the like, alkylarylsulfonates, amine oxides, poly(oxyalkylene) compounds,
including block copolymers comprising alkylene oxide repeat units,
carboxylated
alcohol ethoxylates, ethoxylated alcohols, ethoxylated alkylphenols,
ethoxylated
amines and amides, ethoxylated fatty acids, ethoxylated fatty esters and oils,
fatty
esters, fatty acid amides, including but not limited to amides from tall oil
fatty acids
and polyamides, glycerol esters, glycol esters, sorbitan esters, imidazoline
derivatives,
lecithin and derivatives, lignin and derivatives, monoglycerides and
derivatives, olefin
sulfonates, phosphate esters and derivatives, propoxylated and ethoxylated
fatty acids
or alcohols or alkylphenols, sorbitan derivatives, sucrose esters and
derivatives,
sulfates or alcohols or ethoxylated alcohols or fatty esters, sulfonates of
dodecyl and
tridecyl benzenes or condensed naphfhalenes or petroleum, sulfosuccinates and
derivatives, and tridecyl and dodecyl benzene sulfonic acids. In the preferred
embodiment of an amine salt, it is a Cg-Czo alkenyl succinic ester amine salts
such as
CA 02421473 2003-03-10
18
the reaction product of an alkenyl succinic anhydride with alkanol amine such
as N,N-
dimethylethanol amine, N,N-diethylethanol amine or the like.
Emulsifier Mixture (iii)
A mixture of (i) and (ii) is described in greater detail in USSN 09/761,482,
An
Emulsifier For An Aqueous Hydrocarbon Fuel, incorporated by reference herein.
The Water-Soluble Compound (in iv)
The water-soluble compound may be an amine salt, ammonium salt, azide
compound, nitro compound, alkali metal salt, alkaline earth metal salt, or
mixtures of
two or more thereof and is described in greater detail in USSN 09/761,482, An
Emulsifier For An Aqueous Hydrocarbon Fuel, incorporated by reference herein.
These compounds are distinct from the fuel-soluble product (i) and the ionic
or
nonionic compound (ii) discussed above. These water-soluble compounds include
organic amine nitrates, nitrate esters, azides, nitramines and nitro
compounds. Also
included are alkali and alkaline earth metal carbonates, sulfates, sulfides,
sulfonates,
and the like.
Particularly useful are the amine or ammonium salts such as ammonium
nitrate, ammonium acetate, methylammonium nitrate, methylammonium acetate,
ethylene diamine diacetate; urea nitrate; urea; guanidinium nitrate; and
combinations
thereof.
The water-soluble compound may be present in the water-fuel emulsion at a
concentration of about 0.001 to about 1 % by weight, and in one embodiment
from
about 0.01 to about 1 % by weight.
Emulsifier (v)
In one embodiment the emulsifier (v) is the reaction product of A) a
polyacidic polymer, B) at least one fuel soluble product made by reacting at
least one
hydrocarbyl-substituted carboxylic acid acylating agent, and C) a hydroxy
amine
and/or a polyamine and is described in greater detail in USSN 09/761,482, An
Emulsifier For An Aqueous Hydrocarbon Fuel, incorporated by reference herein.
The fuel soluble product is made by reacting at least one hydrocarbyl-
substituted carboxylic agent with a hydroxy amine and/or polyamine and is
described
earlier in the specification.
The polyacidic polymers used in the reaction include but are not limited to C4
to C3o, preferably C'.~ to CZO olefin/maleic anhydride copolymers. The alpha-
olefins
include 1-butene, 1 -pentene, 1-hexene, 1 -heptene, 1 -octene, 1-nonene, 1-
decene, 1-
CA 02421473 2003-03-10
19
undecene, 1-dodecene, I-tridecene, 1-tetradecene, 1-pentadecene, 1-hexadecene,
1-
heptadecene, 1-octadecene, 1-eicosene, 1-docosene, 1-triacontene, and the
like. the
alpha olefin fractions that are useful include C,5_ig alpha-olefins, C,z_~6
alpha-olefins,
C,4_,6 alpha-olefins, C~a-~a alpha-olefins, C»,_~g alpha-olefins, C1$_za alpha-
olefins, ~~8_30
alpha-olefins, and the like. Mixtures of two or more of any of the foregoing
alpha-
olefins or alpha-olefin fractions may be used.
Other polyacidic polymers suitable for reaction include but are not limited to
malefic anhydride/styrene copolymers; poly-malefic anhydride; acrylic and
methacrylic
acid containing polymers; poly-(alkyl)acrylates; reaction products of malefic
anhydride with polymers with multiple double bonds; and combinations thereof.
The
preferred is polyacidic polymer C~g [1-octadecene]/malefic anhydride
copolymer.
In another embodiment the polyacidic polymer is a copolymer of an olefin and
a monomer having the structure:
X-C-HC=CH-C-X ~
(I)
1 ~ wherein X and X1 are the same or different provided that at least one of X
and X, is
such that the copolymer can function as a carboxylic acylating agent.
The olefin includes a p olymerizable o lefin characterized b y the p resence
of
one or more ethylenically unsaturated groups. 'The olefin monomers include but
are
not limited to 1-hexene, octadecene-1 and diisobutylene. The olefin preferably
is a
Ca-C3o olefin.
The emulsifier produced from the reaction product of the polyacidic polymer
with the fuel soluble product (i) comprises about 25% to about 95% of fuel
soluble
product a nd about 0 .1 °/. t o a bout 50% of t he p olyacidic p
olymer; p referably about
SO% to about 92% fuel soluble product and about 1 % to about 20% of the
polyacidic
polymer, and most preferably about 70°ro to about 90% of fuel soluble
product and
about 5% to about 10% of the polyacidic polymer. In one embodiment the
emulsifier
is described as a polyalkenyl succinimide crosslinked with an olefin/maleic
anhydride
copolymer.
Amino Alkylphenol Emulsifier (vi) and (vii)
The amino alkyl emulsifier is comprised of the reaction product of an amino
alkylphenol, an aldehyde, and an amine resulting in amino alkylphenol. The
amino
CA 02421473 2003-03-10
alkylphenol can be made by (a) the reaction of alkylphenol directly with an
aldehyde
and an amine resulting in an alkylphenol monomer connected by a methylene
group
to an amine, (b) the reaction of an alkylphenol with an aldehyde resulting in
an
oligomer wherein the alkylphenols are bridged with methylene groups, the
oligomer is
S then reacted with more aldehyde and an amine to give a Mannich product, or
(c) a
mixture of (a) and (b)
The alkylphenols have an alkyl group selected from C I to Czoo~ preferably C6
to C~7o wherein the alkyl group is either linear, branched or a combination
thereof.
The alkylphenols include, but are not limited to, polypropylphenol,
polybutylphenol,
10 poly(isobutenyl)phenol, polyamylphenol, tetrapropylphenol, similarly
substituted
phenols and the like. The preferred alkylphenols are tetrapropenylphenol and
poly(isobutenyl)phenol. For example, in place of the phenol, alkyl-substituted
compounds of resorcinol, hydroquinone, catechol, cresol, xylenol, amyl phenol,
hydroxydiphenyl, benzylphenol, phenylethylphenol, methylhydroxydiphenyl, alpha
15 and beta naphthol, alpha and beta methylnaphthol, tolylinaphthol,
xylylnaphthol,
benzylnaphthol, anthranol, phenylmethylnaphtol, phenanthrol, m onomethyl ether
of
catechol, phenoxyphenol, chlorophenol, hydroxyphenyl sulfides and the like may
be
used.
The aldehydes include, but are not limited to, aliphatic aldehydes, such as
20 formaldehyde; acetaldehyde; aldol ((3-hydroxy butyraldehyde); aromatic
aldehydes,
such as benzaldehyde; heterocyclic aldehydes, such as furfural, and the like.
The
aldehyde may contain a substituent group such a s hydroxyl, halogen, nitro and
the
like; in which the substituent does not take a major part in the reaction. The
preferred
aldehyde is formaldehyde.
The amines are those which contain an amino group characterized by the
presence of at least one active hydrogen atom. The amines may be primary amino
groups, secondary amino groups, or combinations of primary and secondary amino
groups.
The amines include, but are not limited to, alkanolamines such as
monoethanol amine, diethanolamine, N-(2-aminoethyl) ethanolamine and the like;
di-
and polyamine (polyalkyene amines) such as dimethylaminopropylamine, 3-
aminopropyl morpholine, ethylendiamine, diethylenetriamine, triethylene
tetramine,
tetraethylene pentamine and the like including distillation bottoms such as
HPAX
CA 02421473 2003-03-10
21
(commercially available from The Union Carbide Corporation), E-100
(commercially
available from Dow Chemical Co.), and the like; polyalkyl polyamines;
propylenediamine, the aromatic amines such as o-, m- and p-phenylene diamine,
diamino naphthalenes; the acid-substituted polyalkylpolyamines, such as N-
acetyl
tetraethylenepentamine, and the corresponding formyl-, propionyl-, butyryl-,
and the
like N-substituted compounds; and the corresponding cyclized compounds formed
therefrom, such as the N-alkyl amines of imidazolidine and pyrimidine.
(Secondary
heterocyclic amines that are suitable are those characterized by attachment of
a
hydrogen atom to a nitrogen atom in the heterocyclic group such as morpholine,
thiomorpholine, pyrrole, pyrroline, pyrrolidine, indole, pyrazole, pyrazoline,
pyrazolidine, imidazole, imidazoline, imidazolidine, piperidine, phenoxazine,
phenthiazine and their substituted analogs. Substituent groups attached to the
carbon
atoms of these amines are typified by alkyl, aryl, alkaryl, aralkyl,
cycloalkyl, and
amino compounds referred to above.)
The "amine" includes, but is not to be limited, to the product obtained
by reacting an alkenyl succinic anhydride such as succinic anhydride of the
formula
O
R--CH--
/O
CHZ--C
or alkenyl succinic acid such as succinic acids of the formula
R--~H-COOH
CHZ--COON
with the amines of the foregoing paragraph.
In the above formulae, R is an alkylene group. The alkenyl radical can be
straight-chain or branched-chain; and it can be saturated at the point of
unsaturation
by the addition of a substance that adds to olefinic double bonds, such as
hydrogen,
sulfur, bromine, chlorine, or iodine. There must be at least two carbon atoms
in the
alkenyl radical, but there is no real upper limit to the number of carbon
atoms therein.
The alkenyl succinic acid anhydrides and the alkenyl succinic acids are
CA 02421473 2003-03-10
22
interchangeable for the purposes of the present invention. Nonlimiting
examples of
the alkenyl succinic acid anhydride component are ethenyl succinic acid
anhydride;
ethenyl succinic acid; ethyl succinic acid anhydride; propenyl succinic acid
anhydride; sulfurized propenyl succinic acid anhydride; butenyl succinic acid;
2-
methylbutenyl succinic acid anhydride; 1,2-dichloropentyl succinic acid
anhydride;
hexenyl succinic acid anhydride; hexyl succinic acid; sulfurized 3-
methylpentyl
succinic acid anhydride; 2,3-dimethylbutenyl succinic acid anhydride; 3,3-
dimethylbutenyl succinic acid; 1,2-dibromo-2-ethylbutyl succinic acid;
heptenyl
succinic acid anhydride; 1,2-diiodooctyl succinic acid; octenyl succinic acid
anhydride; diisobutenyl succinic acid anhydride; 2-methylheptenyl succinic
acid
anhydride; 4-ethylhexenyl succinic acid; 2-isopropylpentenyl succinic acid
anhydride;
nonenyl succinic a cid a nhydride; 2 -propylhexenyl s uccinic a cid a
nhydride; d ecenyl
succinic acid; decenyl succinic acid anhydride; 5-methyl-2-isopropyl-hexenyl
succinic acid anhydride; 1,2-dibromo-2-ethyloctenyl succinic acid anhydride;
decyl
succinic acid anhydride; undecenyl succinic acid anhydride; 1,2-
dichloroundecyl
succinic acid; 3-ethyl-2-t-butylpentenyl succinic acid anhydride;
tetrapropenyl
succinic acid anhydride; tetrapropenyl succinic acid; triisobutenyl succinic
acid
anhydride, 2-propyl-nonyl succinic acid anhydride, 3-butyloctenyl succinic
acid
anhydride; tridecenyl succinic acid anhydride; tetradecenyl succinic acid
anhydride;
hexadecenyl succinic acid anhydride; sulfurized octadecenyl succinic acid;
octadecyl
succinic acid anhydride; 1,2-dibromo-2-methylpentadecenyl succinic acid
anhydride;
8-propylpentadecyl succinic acid anhydride; eicosenyl succinic acid anhydride;
1,2-
dichloro-2-methylnonadecenyl succinic acid anhydride; 2-octyldodecenyl
succinic
acid; 1,2-diiodotetracosenyl succinic acid anhydride; hexacosenyl succinic
acid;
hexacosenyl succinic acid anhydride; hentriacontenyl succinic acid anhydride
and
combinations thereof. In general, alkenyl succinic acid anhydrides having from
about
8 to about 35, and preferably, from about 9 to about 18 carbon atoms in the
alkenyl
group. Methods for preparing the alkenyl succinic acid anhydrides are known to
those familiar with the art, the most feasible method comprising the reaction
of an
olefin with malefic acid anhydride.
The reaction is prepared by any known method such as an emulsion, a
solution, a suspension, a continuous additive bulk process or the like. The
reaction is
carried out under conditions that provide for the formation of the desired
product.
The reaction temperature i s in the range o f a bout 40°C to a bout
200°C, p referably
CA 02421473 2003-03-10
23
about 5 0°C t o about 160°C, and m ore p referably about 6
0°C t o about 1 50°C. The
reaction may be carried out at elevated or reduced pressure, but is preferably
earned
out at atmospheric pressure. The reaction is generally carried out over a
period of
time in the range of about 15 minutes to about 8 hours, preferably about 1
hour to
about 6 hours, and more preferably about 2 hours to about 4 hours.
The amino alkylphenols emulsifier of this invention may be made by reacting
the alkylphenol:aldehyde:amine in a ratio range of 1:1:0.1 molar to 1:2:2
molar, in
one embodiment preferably 1:0.9:0.1 to 1:1.9:1.9, in one embodiment preferably
1:1.5:1.2 molar to 1:1.9:1.8 molar, and in one embodiment preferably 1:0.8:0.3
to
1:1.5:0.7, resulting in the amino alkylphenol emulsifier.
Ranges for the emulsifier treated in the water blend fuel are in the
concentration of about 0.05% to about 20% by weight, and in another embodiment
0.05% to about 10% by weight, and in another embodiment about 0.1% to about
5%,
and in another embodiment 0.1 % to about 3% by weight of the total emulsion.
In another embodiment of this invention the amino alkylphenol is made by the
reaction of an alkylphenol with an aldehyde, resulting in an oligomer wherein
the
alkylphenols a re b ridged w ith m ethylene g roups; then t he oligomer i s r
eacted w ith
more aldehyde and amine to give the emulsifier Mannich product of this
invention.
The r eaction i s prepared b y a ny k nown m ethod such a s an a mulsion, a
solution, a
suspension, and a continuous addition bulk process. The reaction is carried
out under
conditions that provide for the forniation of the desired product.
The reaction is carried out at a temperature in the range of about 0°C
to about
150°C, preferably to about 20"C to about 100°C, and more
preferably about 30°C to
about 70°C over a period of time in the range of about 15 minutes to
about 8 hours,
preferably about 1 hour to about 6 hours, and more preferably about 2.5 hours
to
about 5 hours, resulting in an oligomer wherein the alkylphenols a re bridged
with
methylene groups. This intermediate product is then reacted in the range of
about 1
mole oligomer:0.1 mole amine to about 1 mole oligomer:2 moles amine;
preferably
about 1 mole oligomer:0.2 mole amine to about 1 mole oligomer:l.5 moles
amines,
and more preferably about 1 mole oligomer:0.3 moles amine to about 1 mole
oligomer:0.9 moles amine, resulting in amino alkylphenol product.
This reaction occurs at a temperature of about 40°C to about
200°C, preferably
about SO°C to about 160°C, and more preferably about 60°C
to about 150°C. The
reaction may be earned out at elevated to reduced pressure, but is preferably
carried
CA 02421473 2003-03-10
24
out at atmospheric pressure. The reaction continues until the Mannich product
is
formed.
This embodiment is illustrated as follows:
H O
H~H K
SraLe l
Re.,in jormarion
Formaldehyde
CrzHzS Base or Acid catalyst
Dodecylphenol (tetrapropenyl I or
alkyl chain) RR nCH,
n
R
Amine
Sray22
Munnich reaclian R~ N
O and
Formaldehyde
Ff 'H
/R
~R/'
n
Wherein R, Rt and Rr ~ can be the same or difrerent or H
and are an alkyl group. Preferably R = CHZNR R' = H or
K = R'-- C'H,CH,OH
n=0- 10 - Y
x = H or CH_Ot~, CH~NRR'
The emulsifier may be a mixture of the amino alkylphenol with
(i) at least one fuel-soluble product made by reacting at least one
hydrocarbyl-
substituted carboxylic acid acylating agent with ammonia or an amine,
including, but
not limited to, alkanol amines, hydroxy amines, and the like, the hydrocarbyl
substituent of said acylating agent having about 50 to about 500 carbon atoms;
(ii) at least one of an ionic or a nonionic compound having a hydrophilic-
lipophilic balance (HLB) of about 1 to about 40;
CA 02421473 2003-03-10
(iii) mixture of (ii) with (i);
(iv) a w ater-soluble c ompound s elected f rom t he g roup c onsisting o f a
mine
salts, ammonium salts, azide compounds, nitrate esters, nitramine, nitro
compounds,
alkali metal salts, alkaline earth metal salts, in combination with at least
one of (i),
5 (ii), (iii), (v), (vii) or combinations thereof;
(v) the reaction product of polyacidic polymer with at least one fuel soluble
product made by reacting at least one hydrocarbyl-substituted carboxylic acid
acylating agent with ammonia, an amine, a polyamine, alkanol amines, or
hydroxy
amines; or
10 (vi) combinations thereof.
The emulsifier may be present in the water fuel emulsion at a concentration of
about 0.05% to about 20°/~ by weight, in another embodiment about 0.05%
to about
10% by weight, in another embodiment about 0.1 % to about 5% by weight, and in
a
further embodiment of about 0.01 % to about 3% by weight of the water fuel
15 emulsion.
Cetane Improver
In one embodiment, the water-fuel emulsion contains a cetane improver. The
cetane improvers that are useful include but are not limited to peroxides,
nitrates,
nitrites, nitrocarbamates, and the like. Useful cetane improvers include but
are not
20 limited to nitropropane, dinitropropane, tetranitromethane, 2-nitro-2-
methyl-1-
butanol, 2-methyl-2-nitro-1-propanol, and the like. Also included are nitrate
esters of
substituted or unsubstituted aliphatic or cycloaliphatic alcohols which may be
monohydric or polyhydric. These include substituted and unsubstituted alkyl or
cycloalkyl nitrates having up to about 10 carbon atoms, and in one embodiment
about
25 2 to about 10 carbon atoms. The alkyl group may be either linear or
branched, or a
mixture of linear or branched alkyl groups. Examples include methyl nitrate,
ethyl
nitrate, n-propyl nitrate, isopropyl nitrate, allyl nitrate, n-butyl nitrate,
isobutyl nitrate,
sec-butyl nitrate, tert-butyl nitrate, n-amyl nitrate, isoamyl nitrate, 2-amyl
nitrate, 3-
amyl nitrate, tert-amyl nitrate, n-hexyl nitrate, n-heptyl nitrate, n-octyl
nitrate, 2-
ethylhexyl nitrate, sec-octyl nitrate, n-nonyl nitrate, n-decyl nitrate,
cyclopentyl
nitrate, cyclohexyl nitrate, methylcyclohexyl nitrate, and isopropylcyclohexyl
nitrate.
Also useful are the nitrate esters of alkoxy-substituted aliphatic alcohols
such as 2-
ethoxyethyl n itrate, 2-(2-ethoxy-ethoxy) ethyl nitrate, 1 -methoxypropyl-2-
nitrate, 4 -
CA 02421473 2003-03-10
26
ethoxybutyl nitrate, etc., as well as diol nitrates such as 1,6-hexamethylene
dinitrate.
A useful cetane improver is 2-efhylhexyl nitrate.
The concentration of the cetane improver in the water-fuel emulsion may be at
any concentration sufficient to provide the emulsion with the desired cetane
number.
In one embodiment, the concentration of the cetane improver is at a level of
up to
about 10% by weight, and in one embodiment about 0.05 to about 10% by weight,
and in one embodiment about 0.05 to about 5% by weight, and in one embodiment
about 0.05 to about 1 % by weight.
Additional Additives
In addition to the foregoing materials, other fuel additives that are well
known
to those of skill in the art may be used in the water-fuel emulsions of the
invention.
These include but are not limited to dyes, rust inhibitors such as alkylated
succinic
acids and anhydrides, bacteriostatic agents, gum inhibitors, metal
deactivators, upper
cylinder lubricants, and the like.
The total concentration of chemical additives, including the foregoing
emulsifiers, in the water-fuel emulsions of the invention may range from about
0.05 to
about 30% by weight, and in one embodiment about 0.1 to about 20% by weight,
and
in one embodiment about 0.1 to about 15% by weight, and in one embodiment
about
0.1 to about 10% by weight, and in one embodiment about 0.1 to about 5% by
weight.
Organic Solvent
The additives, including the foregoing emulsifiers, may be diluted with a
substantially inert, normally liquid organic solvent such as naphtha, benzene,
toluene,
xylene or diesel fuel to form an additive concentrate which is then mixed with
the fuel
and water to form the water-fuel emulsion.
The water-fuel emulsions may contain up to about 60% by weight organic
solvent, and in one embodiment about 0.01 to about 50% by weight, and in one
embodiment about 0.01 to about 20% by weight, and in one embodiment about 0.1
to
about 5% by weight, and in one embodiment about 0.1 to about 3% by weight.
Antifreeze Agent
The water-fuel emulsions of the invention may additionally contain an
antifreeze agent. The antifreeze agent is typically an alcohol. Examples
include but
are not limited to ethylene glycol, propylene glycol, methanol, ethanol,
glycerol and
mixtures of two or more thereof. The antifreeze agent is typically used at a
concentration sufficient to prevent freezing of the water used in the water-
fuel
CA 02421473 2003-03-10
27
emulsions. The concentration is therefore dependent upon the temperature at
which
the fuel is stored or used. In one embodiment, the concentration is at a level
of up to
about 20% by weight based on the weight of the water-fuel emulsion, and in one
embodiment about 0.1 to about 20% by weight, and in one embodiment about I to
about 10% by weight.
The Engines
The engines that may be operated in accordance with the invention include all
compression-ignition (internal combustion) engines for both mobile (including
locomotive and marine) and stationary power plants. These include engines that
use
diesel, gasoline, and the like. The engines that can be used include but are
not limited
to those used in automobiles, trucks such as all classes of truck, buses such
as urban
buses, locomotives, light and heavy duty diesel engines, stationary engines
and the
like. Included are on- and off highway engines, including new engines as well
as m
use engines. These include diesel engines of the two-stroke-per-cycle and four
I 5 stroke-per-cycle types.