Note: Descriptions are shown in the official language in which they were submitted.
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
VAPOR COLLECTION METHOD AND APPARATUS
Field of the Invention
This application is claiming priority to U.S. Provisional Application Serial
Nos.
60/235,214, filed September 24, 2000, 60/235,221, filed on September 24, 2000,
and
60/274,050, filed on March 7, 2001, all of which are hereby incorporated by
reference in
their entirety. The present invention relates to a vapor collection method,
and more
particularly to a method that enables the collection of gas phase components
without
substantial dilution.
Sack~round of the Invention
Conventional practices for the removal and recovery of components during
drying
of coated materials generally utilize drying units or ovens. Collection hoods
or ports are
utilized in both closed and open drying systems to collect the solvent vapors
emitted from
the substrate or material. Conventional open vapor collection systems
generally utilize air
handling systems that are incapable of selectively drawing only the desired
gas phase
components without drawing the ambient atmosphere. Closed vapor collection
systems
typically introduce an inert gas circulation system to assist in purging the
enclosed
volume. In either system, the introduction of ambient air or inert gas dilutes
the
concentration of the gas phase components. Thus the subsequent separation of
vapors
from the diluted vapor stream can be difficult and inefficient.
Additionally, the thermodynamics associated with the conventional vapor
collection systems often permit the undesirable condensation of the vapor at
or near the
substrate or material. The condensate can then fall onto the substrate or
material and
adversely affect either the appearance or functional aspects of the material.
In industrial
settings, the ambient conditions surrounding the process and processing
equipment may
include extraneous matter. In large volume drying units, the extraneous matter
may be
drawn into the collection system by the large volumetric flows of the
conventional drying
systems.
-1-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
It would be desirable to collect gas phase components without substantially
diluting the gas phase components with ambient air or inert gases.
Additionally, it would
be an advantage to collect gas phase components at relatively low volumetric
flows in an
industrial setting in order to prevent the entrainment of extraneous matter.
Summary of the Invention
The present invention provides a method and apparatus for transporting and
capturing gas phase components without substantial dilution. The method and
apparatus
utilize a chamber in close proximity to the surface of a substrate to enable
collection of gas
phase components near the surface of the substrate.
In the method of the present invention, at least one material is provided that
has at
least one major surface with an adjacent gas phase. A chamber is then
positioned in close
proximity to the surface of the material to define a gap between the chamber
and the
material. The gap is preferably no greater than 3 cm. The adjacent gas phase
between the
chamber and the surface and the material defines a region possessing an amount
of mass.
At least a portion of the mass from the adjacent gas phase is transported
through the
chamber by inducing a flow through the region. The flow of the gas phase is
represented
by the equation:
M1 + M2 + M3 = M4. (Equation I)
wherein Ml is the total net time-average mass flow per unit width through the
gap
into the region and through the chamber resulting from pressure gradients, M2
is the time-
average mass flow per unit width from the at least one major surface of the
material into
said region and through the chamber, M3 is the total net time-average mass
flow per unit
width through the gap into the region and through the chamber resulting from
motion of
the material, M4 is the time-average rate of mass transported per unit width
through the
chamber. For purposes of the invention the dimensions defining the width is
the length of
the gap in the direction perpendicular to the motion of the material. and in
the plane of the
material.
The present method and apparatus is designed to substantially reduce the
amount
of dilution gas transported through the chamber. The use of a chamber in close
proximity
- 2-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
of the surface of the material and small negative pressure gradients enables
the substantial
reduction of dilution gas, namely M1. The pressure gradient, Op, is defined as
the
difference between the pressure at the chambers lower periphery, pc, and the
pressure
outside the chamber, po, wherein dp=pc-po. The value of M1 is generally
greater than
zero but not greater than 0.25 kg/second/meter: Preferably, M1 is greater than
zero but not
greater than 0.1 kg/second/meter, and most preferably, greater than zero but
not greater
than 0.01 kg/second/meter.
In an alternative expression, the average velocity resulting from M1 may be
utilized to express the flow of dilution gas phase components entering the
chamber. The
use of a chamber in close proximity of the surface of the material, and small
negative
pressure gradients, enables the substantial reduction of the average total net
gas phase
velocity, <v>, through the gap. For the present invention, the value of <v> is
generally
greater than zero but not greater than 0.5 meters/second.
The present method attempts to significantly reduce dilution of the gas phase
component in the adjacent gas phase by substantially reducing M1 in Equation
I. M1
represents the total net gas phase dilution flow into the region caused by a
pressure
gradient. The dilution of the mass in the adjacent gas phase may adversely
affect the
efficiency of gas phase collection systems and subsequent separation
practices.. For the
present method, Ml is greater than zero but no greater than 0.25
kglsecond/meter.
Additionally, due to the relatively. small gap between the chamber and the
surface of the
material, the volumetric flow rate of gas phase components through the gap
caused by
induced flow is generally no greater than 0.5 meterslsecond.
The method is well suited for applications requiring the desired collection of
vaporous components in an efficient manner. Organic and inorganic solvents are
examples of components that are often utilized as carriers to permit the
deposition of a
desired composition onto a substrate or material. The components are generally
removed
from the substrate or material by supplying a sufficient amount of energy to
permit the
vaporization of the solvent. It is desirable, and often necessary for health,
safety, and
environmental reasons, to recover the vaporous components after they have been
removed
from the substrate or material. The present invention is capable of collecting
and
transporting vapor components without introducing a substantial volume of a
dilution
stream.
- 3-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
In a preferred embodiment, the method of the present invention includes the
use of
material that contains at least one evaporative component. The chamber is
positioned in
close proximity to a surface of the material. Energy is then directed at the
material to
vaporize the at least one evaporative component to form a vapor component. At
least a
portion of the vapor component is captured in the chamber. The vapor component
is
generally captured at a high concentration that allows subsequent processing,
such as
separation, to become more efficient.
The apparatus of the present invention includes a support mechanism for
supporting material. The material has at least one major surface with an
adjacent gas
phase. A chamber is placed in close proximity to a surface of the material to
define a gap
between the surface and the collection chamber. The adjacent gas phase between
the
chamber and the material defines a region containing an amount of mass. A
mechanism in
communication with the chamber induces the transport of at least a portion of
the mass in
the adjacent gas phase through the region. The transport of mass through the
region into
the chamber is represented by Equation I. The vapor in the chamber may
optionally be
conveyed to a separating mechanism for additional processing.
The method and apparatus of the present invention are preferably suited for
use in
transporting and collecting solvents from a moving web. In operation, the
chamber is
placed above the continuously moving web to collect vapors at a high
concentration. The
. low volumetric flows and high concentrations of the vapor improve the
efficiency of the
solvent recovery and substantially eliminate contamination problems associated
with
conventional component collection devices.
The method and apparatus of the present invention are preferably used in
combination with conventional gap drying systems. Gap drying systems generally
convey
a material through a narrow gap between hot plate and a condensing plate for
the
evaporation and subsequent condensation of evaporative components in the
material. The
configuration of the present apparatus, in various locations of a gap drying
system, enables
further capture of gas phase components which generally can be present in the
adj acent
gas phase on the surface of the material either prior to entering, or exiting
a gap drying
unit.
For purposes of the present invention, the following terms used in this
application
are defined as follows:
- 4-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
"time-average mass flow" is represented by the equation MI = I f J»zidt ,
wherein
t o
MI is the time-average mass flow in kg/second, t is time in seconds, and mi is
the
instantaneous mass flow in kg/second;
"pressure gradient" means a pressure differential between the chamber and the
external environment; and
"induced flow" means a flow generally created by a pressure gradient.
Other features and advantages will be apparent from the following
description of the embodiments thereof, and from the claims.
Brief Description of the Drawings
The above, as well as other advantages of the present invention will become
readily apparent to those skilled in the art from the following detailed
description when
considered in the light of the accompanying drawings in which:
FIG. 1 is a schematic view of the present invention;
FIG. 2 is a schematic view of a preferred embodiment of a gas phase collection
apparatus of the present invention;
FIG. 3 is a cross-sectional view of a preferred embodiment of a gas phase
collection apparatus of the present invention;
FIG. 4 is an isometric view of preferred embodiment of a gas phase collection
apparatus of the present invention;
FIG. 5a is a schematic view of one preferred embodiment of the present
invention
in combination with a gap drying system;
FIG. 5b is a schematic view of one preferred embodiment in combination with an
optional mechanical seal;
FIG. 6 is a schematic view of one preferred embodiment in combination with an
optional retractable mechanical seal; and
FIG. 7 is a schematic view of another preferred embodiment. of a gas phase
collection system and apparatus as described in the Example provided herein.
- 5-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
Detailed Descriution
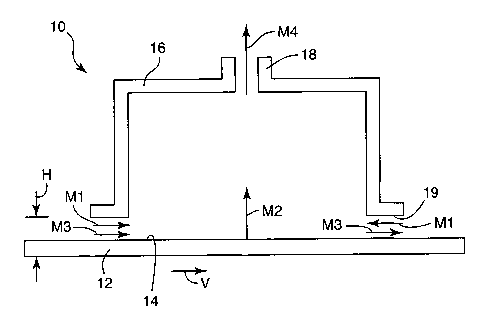
The method and apparatus 10 of the present invention axe generally described
in
FIG. 1. The method includes providing a material 12 having at least one major
surface 14
with an adjacent gas phase (not shown). A chamber 16, having an exhaust port
1~ is
positioned in close proximity to define a gap between the lower periphery 19
of the
chamber 16 and the surface 14 of the material 12. The gap has a height H,
which is
preferably 3 cm or less. The adjacent gas phase between the lower periphery 19
of the
chamber 16 and the surfacel4 of the material 12 define a region possessing an
amount of
mass. The mass in the region is generally in a gas phase. However, those
skilled in the art
recognize that the region may also contain mass that is in either the liquid
or solid phase,
or combinations of all three phases.
At least a portion of the mass from the region is transported through the
chamber
16 by induced flow. Flow may be induced by conventional mechanisms generally
recognized by those skilled in the art. The flow of mass per unit width into
and through
the chamber are represented by Equation I:
M1 + M2 + M3 = M4 (Equation I)
FIG. 1 depicts the various flow streams encountered in practicing the method
of
the present invention. M1 is the total net time-average mass flow per unit
width through
the gap into the region and through the chamber resulting from pressure
gradients. For
purposes of the present invention, M1 essentially represents a dilution
stream. M2 is the
time-average mass flow per unit width from the at least one major surface of
the material
into said region and through the chamber. M3 is the total net time-average
mass flow per
unit width through the gap into the region and through the chamber resulting
from motion
of the material. M3 is generally recognized as mechanical drag and covers
both.the mass
pulled in by the motion of the material under the chamber and the mass exiting
from
underneath the chamber as the material passes. In cases where the material is
static under
the chamber, M3 would be zero. In case where the gap H is uniform (i.e., the
gap at the
entrance and exit of the chamber are equal) M3 is zero. M3 is non zero when
the entrance
and exit gaps are non uniform (i.e., not equal). M4 is the time-average rate
of mass
transported per unit width through the chamber. It is understood that mass can
be
- 6-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
transported through the gap and into the region without being transported
through the
chamber. Such flows are not included in the total net flows included in
Equation 1. For
purposes of the invention the dimension defining the width is the length of
the gap in the
direction perpendicular to the motion of the material and in the plane of the
material.
The present method and apparatus is designed to substantially reduce the
amount
dilution gas transported through the chamber. The use of a chamber in close
proximity of
the surface of the material and extremely small negative pressure gradients
enables the
substantial reduction of dilution gas, namely M1. The pressure gradient, Op,
is defined as
the difference between the pressure at the chambers lower periphery, pc, and
the pressure
outside the chamber, po, wherein 0p=pc-po. The value of M1 is generally
greater than
zero but not greater than 0.25 kg/second/meter. Preferably, M1 is greater than
zero but not
greater than 0.1 kg/second/meter, and most preferably, greater than zero but
not greater
than 0.01 kg/second/meter.
In an alternative expression, the average velocity resulting from M1 may be
utilized to express the flow of dilution gas phase components through the
chamber. The
use of a chamber in close proximity of the surface of the material, and small
negative
pressure gradients, enables the substantial reduction of the total net average
gas phase
velocity, <v>, through the gap. The average gas phase velocity resulting from
Ml is
defined as; <v>=M1/pA. Wherein M1 is defined above, p is the gas stream
density in
kg/cubic meter and A is the cross sectional area available for flow into the
region in square
meters. Wherein, A=H(2w+21) where H is defined above, w is the length of the
gap in the
direction perpendicular to the motion of the material, and 1 is the length of
the gap in the
direction of material motion. For the present invention, the value of <v> is
generally
greater than zero but not greater than 0.5 meters/second.
The close proximity of the chamber to the surface, and the relatively small
pressure
gradient, enable the transport of the mass in the adjacent gas phase through
the chamber
with minimal dilution. Thus lower flow rates at higher concentrations may be
transported
and collected. The present method is also suitable for transporting and
collecting
relatively small amounts of mass located in the adjacent gas phase. The gap
height is
generally 3 cm or less, preferably 1.5 cm or less , and most preferably 0.75
cm or less.
Additionally, in a preferred embodiment, the gap is substantially uniform
around the
periphery of the chamber. However, the gap may be varied, or non-uniform for
specific
_ 7_
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
applications. In a preferred embodiment, the chamber may have a periphery
wider than
the material, or web conveyed under the chamber. In such cases, the chamber
can be
designed to seal the sides to further reduce time-average mass flow per unit
width from
pressure gradients (Ml). The chamber can also be designed to conform to
different
geometry material surfaces. For example, the chamber can have a radiused lower
periphery to conform to the surface of a cylinder.
The material utilized may include any material that is capable of being
positioned
in close proximity of the chamber. The preferred material is a web. The web
may include
one or more layers of material or coatings applied onto a substrate.
The chamber is sized and operated appropriately to provide the sufficient
collection of gas phase components without substantial dilution or without
excessive loss
of gas phase components for failure to draw them into the chamber. Those
skilled in the
art are capable of designing and operating a chamber to address both the
evaporation rate
of given materials and the needed fluid flow rate for proper recovery of the
gas phase
components. With flammable gas phase components, it is preferred to capture
the vapors
at concentrations above the upper flammability limit for safety reasons.
Additionally, the
gap may be maintained over a substantial portion of the web. Several chambers
may also
be placed in operation at various points along the web processing path. Each
individual
chamber may be operated at different pressures, temperatures and gaps to
address process
and material variants.
Transport of the mass from the region through the chamber is accomplished by
inducing a pressure gradient. A pressure gradient is generally created by
mechanical
devices, for example, pumps, blowers, and fans. The mechanical device that
induces the
pressure gradient is in communication with the chamber. Therefore, the
pressure gradient
will initiate mass flow through the chamber and through an exhaust port in the
chamber.
Those skilled in the art also recognize that pressure gradients may also be
derived from
density gradients of gas phase components.
The chamber may also include one or more mechanisms to control the phase of
the
mass transported through the chamber thereby controlling phase change of the
components
in the mass. For example, conventional temperature control devices may be
incorporated
into the chamber to prevent condensation from forming on the internal portions
of the
chamber. Non-limiting examples of conventional temperature control devices
include
_ g_
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
heating coils, electrical heaters, and external heat sources. A heating coil
provides
sufficient heat in the chamber to prevent the condensation of the vapor
component.
Conventional heating coils and heat transfer fluids are suitable for use with
the present
invention.
Depending on the specific gas phase composition, the chamber may optionally
include flame arresting capabilities. A flame arresting device placed
internally within the
chamber allows gases to pass through but stops flames in order to prevent a
fire or
explosion. A flame is a volume of gas in which a self -sustaining exothermic
(heat
producing) chemical reaction occurs. Flame arresting devices are generally
needed when
the operating environment includes oxygen, high temperatures and a flammable
gas mixed
with the oxygen in suitable proportions to create a combustible mixture. A
flame arresting
device works by removing one of the noted elements. In a preferred embodiment,
the gas
phase components pass through a narrow gap bordered by heat absorbing
materials. The
size of both the gap and the material are dependent upon the specific vapor
composition.
For example, the chamber may be filled with expanded metallic heat-absorbing
material,
such as for example aluminum, contained at the bottom by a fine mesh metallic
screen
with mesh openings sized according to the National Fire Protection Association
Standards.
Optional separation devices and conveying equipment utilized in the present
invention may also possess flame arresting capabilities. Conventional
techniques
recognized by those skilled in the art are suitable for use with the present
invention. The
flame arresting devices are utilized in the chamber and the subsequent
processing
equipment without the introduction of an inert gas. Thus the concentration of
the vapor
stream is generally maintained to enable efficient separation practices.
The present method is suitable for the continuous collection of a gas phase
composition. The gas phase composition generally flows from the chamber to a
subsequent processing step, preferably without dilution. The subsequent
processing steps
may include such optional steps as, for example, separation or destruction of
one or more
components in the gas phase. The separation processing step may occur
internally within
the chamber in a controlled manner, or it may occur externally. Preferably,
the vapor
stream is separated using conventional separation processes such as for
example
absorption, adsorption, membrane separation or condensation. The high
concentration and
low volumetric flows of the vapor composition enhance the overall efficiency
of
- 9-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
conventional separation practices. Most preferably, at least a portion of the
vapor
component is captured at concentrations high enough to permit subsequent
separation of
the vapor component at a temperature of 0° C or higher. This
temperature prevents the
formation of frost during the separation process which has both equipment and
process
advantages.
The vapor stream from the chamber may contain either the vapor, or vapor and
liquid phase mixture. The vapor stream may also include particulate matter
which can be
filtered prior to the separation process. Suitable separation process may
include, for
example, conventional separation practices such as: concentration of the vapor
composition in the gaseous stream; direct condensation of the dilute vapor
composition in
the gaseous stream; direct condensation of the concentrated vapor composition
in the
gaseous stream; direct two stage condensation; adsorption of the dilute vapor
composition
in the gaseous stream using activated carbon or synthetic adsorption media;
adsorption of
the concentrated vapor composition in the gaseous stream using activated
carbon or
synthetic adsorption media; absorption of the dilute vapor phase component in
the gaseous o
stream using media with high absorbing properties; and absorption of the
concentrated
vapor phase component in the gaseous stream using media with high absorbing
properties.
Destruction devices would include conventional devices such as thermal
oxidizers.
Optionally, depending upon the composition of the gas phase component, the
stream may
be vented or filtered and vented after exiting the chamber.
One preferred embodiment of the present invention is described in FIGS. 2-4.
The
inventive apparatus 20 includes a web 22 conveyed by a web conveying system
(not
shown) between a heating element 24 and a chamber 26. The web 22 comprises a
material
containing at least one evaporative component (not shown). The chamber 26
includes a
lower periphery 28. The chamber 26 is positioned in close proximity to the web
22 such
that the lower periphery 28 of the chamber 26 defines a gap H between the
chamber and
the web 22. The chamber 26 optionally includes a heating coil 30, flame
arresting
elements 32 and a head space 39 above flame arresting elements 32. A manifold
34
provides a connection to a pressure control mechanism (not shown). The
manifold 34
ultimately provides an outlet 36 to convey the vapors to subsequent processing
steps.
In operation, the heating element 24 provides primarily conductive thermal
energy
to the bottom side of the web material 22 to vaporize the evaporative
component in the
-1Q
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
web material. The chamber 26 is operated with a pressure gradient so that as
the vapors
evolve from the web material 22 at least a portion are conveyed across the
vertical gap H
and into the chamber 26. The vapors drawn into the chamber 26 are conveyed
through the
manifold 34 and the outlet 36 for further processing. The gap H and the
pressure gradient
permit the capture of the vapors in the chamber 26 without substantial
dilution.
The preferred embodiment is directed to transporting and collecting
evaporative
components from materials. The evaporative component may be included within
the
material, on the surface of the material, or in the adjacent gas phase.
Materials include,
for example, coated substrates, polymers, pigments, ceramics, pastes, wovens,
non-
wovens, fibers, powders, paper, food products, pharmaceutical products or
combinations
thereof. Preferably, the material is provided as a web. However, either
discrete sections
or sheets of materials may be utilized.
The material includes at least one evaporative component. The evaporative
component is any liquid or solid composition that is capable of vaporizing and
separating
from a material. Non-limiting examples would include organic compounds and
inorganic
compounds or combinations thereof, such as water or ethanol. In general, the
evaporative
component may have originally been used as a solvent for the initial
manufacturing of the
material. The present invention is well suited for the subsequent removal of
the solvent.
In accordance with the present invention, a sufficient amount of energy is
applied
to the material to vaporize at least one evaporative component. The energy
needed to
vaporize the evaporative component may be applied through radiation,
conduction,
convection or combinations thereof. Conductive heating, for example could
include
passing the material in close proximity to a flat heated plate, curved heated
plate or
partially wrapping the material around a heated cylinder. Examples of
convective heating
may include directing hot air by nozzle, jet or plenum at the material.
Electromagnetic
radiation such as radio frequency, microwave energy, or infrared energy, may
be directed
at the material and absorbed by the material causing internal heating of the
material.
Energy may be applied to any or all surfaces of the material. Additionally,
the material
may be supplied with sufficient internal energy, for example a pre-heated
material or an
exothermic chemical reaction occurring in the material. The energy application
techniques
may be used individually or in combination.
-11-
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
Those skilled in the art recognize that the energy for heating may be supplied
from
conventional sources. For example, sufficient energy may be provided by
electricity, the
combustion of fuels, or other thermal sources. The energy may be converted to
heat
directly at the.application point, or indirectly through heated liquids such
as water or oil,
heated gasses such as air or inert gas or heated vapors such as steam or
conventional heat
transfer fluids.
The chamber of the present invention is positioned in close proximity to the
material in order to form a gap between the lower periphery of the chamber and
the
material. The gap is preferably a substantially uniform spatial distance
between the
surface of the material and the bottom of the chamber. The gap distance is
preferably 3
centimeters or less, most preferably 1.5 centimeters or less, and even more
preferably 0.75
centimeters or less. The chamber is operated at a pressure gradient so that
the vapors are
pulled into the chamber. The close proximity of the chamber to the material
minimizes
the dilution of the vapors as the vapors are pulled into the chamber. In
addition to the gap,
the dilution of the vapor component may also be minimized by using mechanical
features,
such as extensions 35, 37 in FIGS. 2-4, added to the chamber. The extension
may also
provide side seals when extending beyond the web and contacting against the
hot platen
24.
In accordance with the present invention, it is preferred that the total mass
flow is
selected to closely match the generation rate of gas phase components from the
material.
This will assist in preventing either the dilution or loss of vapor
components. The total
volumetric flow rate from the chamber is preferably at least 100% of the
volumetric flow
of the vapor component. Additionally, the present invention is capable of
achieving
substantially uniform flow across the inlet surface of the chamber. This may
be achieved
when a head space is present in the chamber above a layer of porous media. In
the noted
case, the pressure drop laterally in the head space is negligible with respect
to the pressure
drop through the porous media. One skilled in the art will recognize that the
head space
and pore size of porous media may be adjusted to adjust the flow rate across
the inlet
surface of the chamber.
In another preferred embodiment, the chamber of the present invention may be
incorporated with a conventional gap drying system. Gap drying is a system
which uses
direct solvent condensation in combination with conduction dominant heat
transfer and
-12~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
therefore does not require the use of applied forced convection to evaporate
and carry
away the solvent vapors. A gap dryer, consists of a hot plate and a cold plate
separated by
a small gap. The hot plate is located adjacent to the uncoated side of the
web, supplying
energy to evaporate the coating solvents. The cold plate, located adjacent to
the coated
side, provides a driving force for condensation and solvent vapor transport
across the gap.
The cold plate is provided with a surface geometry which prevents the liquid
from
dripping back onto the coated surface. The drying and simultaneous solvent
recovery
occurs as the coated substrate is transported through the gap between the two
plates. Gap
drying systems are fully described in U.S. Patent Nos. 6,047,151, 4,980,697,
5,813,133,
5,694,701, 6,134,808 and 5,581,905 herein incorporated by reference in their
entirety.
The chamber may be positioned at several optional points in the gap drying
system. For example, a chamber may be placed at either opposing ends of the
gap dryer,
internally within the gap dryer or combinations thereof. FIG. 5a shows the
chamber 40
positioned at the trailing edge 44 of the gap drying system 42.
In conventional gap drying type configurations, some gas phase components are
transported by drag from a moving web. The gas phase components in the gap
between
the web and the top plate can be a concern because it may be nominally
saturated with the
evaporative component. This component (solvent or other component) can be of
concern
because of environmental, health or safety considerations. When the gap is
small enough,
the volume of this Exhaust Flow Q can be readily calculated from the web speed
Vweb,
the top gap height, hu, and the film/web width W:
Q=( 1/2)(Vweb)(W)(hu)
For example, for a 0.508 meters/second web speed, with 1.53 meters width and a
0.0492
cm gap, this means a flow of 0.00123 cubic meters per second. This is a small
and much
more manageable flow to consider than with other more conventional drying
means with
gas phase flows of several orders of magnitude higher than the present
invention.
Thus the chamber of the present invention is a suitable means for transporting
and
collecting the relatively small volume of mass in the adjacent gas phase of
the web
material. The basic embodiment is illustrated in FIG. 5a. A gap drying system
42
includes a web 46 positioned between a condensing plate 48 and a hot plate 50.
A gap, of
-13
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
distance H, is formed between the upper surface of the web 46 and the
condensing plate
48. The condensing plate 48 includes a capillary surface 52 to convey
condensed material
away from the condensing surface 54. A chamber 40 is provided at the point
where the
web 46 exits the gap to collect the gas phase components exiting the gap
drying system 42.
The mass flow through the chamber may be assisted by applying a seal to a
trailing
edge of the chamber. The seal functions as a sweep to prevent gas from exiting
the
trailing edge of the chamber, thus forcing it into the chamber. The seal could
include
either a forced gas or mechanical seal. FIG. 5a depicts an optional forced gas
air flow F in
the direction of the downward arrow on the outer portion 41 of the chamber.
The forced
gas blocks any gas phase components carried by the moving web 46. The gas
could be
clean air, nitrogen, carbon dioxide or other inert gas systems. .
A mechanical seal may also be utilized for forcing gas phase components into
the
chamber. FIG. 5b illustrates the utilization of a flexible seal element 56 at
the outer
portion 41 of the chamber 40 to reduce the amount of dilution transported
through the
chamber 40. The flexible seal 56 could drag on the web 46 or be spaced at a
small gap to
the web 46. In this case, the gap is non-uniform, with H at the exit near the
seal
approaching zero.
The mechanical seal may also comprise a retractable sealing mechanism as
depicted in FIG. 6. The retractable sealing mechanism 76 is shown in an
engaged position
for normal continuous operation with a chamber 60 and a gap drying system 62,
including
condensing plate 68 and hot plate 70. In this arrangement, the retractable
sealing
mechanism 76 may be set at a smaller gap to the surface of the web 66 than
with other
forms of mechanical seals. The smaller gap is more effective in removing the
boundary
layer of gas phase components from the moving web 66 for capture without
possible
scratching or damaging the coating or web surface. This gap to the surface of
the web 66
could be 0.00508 cm to .0508 cm or more. The smaller the gap, the more
effective in
removing the boundary layer of gas phase components. The effectiveness of the
retractable sealing mechanism 76 is improved by increasing the thickness of
the seal while
maintaining a sealing face 78 that corresponds to the web at the sealing
point. With an
idler roll 80 as shown in the FIG. 6, the retractable sealing mechanism 76 has
a radiused
shape corresponding to the radius of the idler roll 80. The thickness of the
retractable
sealing mechanism could be 1.5 cm to more than 3 cm. The thicker the plate,
the greater
-14
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
the sealing area and thus more effective. The practical thicl~ness will depend
on factors
such as idler radius and idler wrap angle. The seal may be moved to a
retracted position
through use of an actuator 82 or other mechanical means. The raised
arrangement
prevents contamination to the sealing mechanism 76, damage to the web 66,
allows
passage of overthick coatings, or allows passage of a splice or other upset
condition.
Those skilled in the art recognize that the retraction of the retractable
sealing mechanism
76 could be automated and controlled for known upsets such as splices or
coating
overthicknesses, or even connected to a sensor (not shown) for upsets (such as
a tip bar,
laser inspection device etc.) to allow retraction for unanticipated events.
The apparatus of the present invention utilizes a material supporting
mechanism
for securing the material in close proximity to the chamber to ensure an
appropriate gap.
Conventional material handling systems and devices are suitable for use with
the present
invention.
The apparatus includes a chamber, as described above, which is then placed
over
the material to define a gap between a surface of the material and the lower
periphery of
the chamber. The chamber is constructed of conventional materials and may be
designed
to meet specific application standards. The chamber may exist as a stand-alone
device or
it may be placed in an enclosed environment, such as, for example, an oven
enclosure.
Additionally, the flame arresting devices and heating coils optionally placed
in the
chamber may include conventional recognized equipment and materials.
An energy source, as described above, is used to provide sufficient energy to
the
material in order to vaporize the at least one evaporative component in the
material.
Heating and heat transfer equipment generally recognized in the art are
suitable for use
with the present invention.
The concentrated vapor stream collected in the chamber may be further
separated
utilizing conventional separation equipment and processes generally described
as
absorption, adsorption, membrane separation or condensation. Those skilled in
the art are
capable of selecting specific separation practices and equipment based on the
vapor
composition and desired separation efficiency.
In operation, the present invention captures at least a portion of the vapor
component without substantial dilution and without condensation of the vapor
component
in the drying system. The collection of the vapor component at high
concentrations
-1~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
permits efficient recovery of the material. The absence of condensation in the
drying
system reduces product quality issues involved with condensate falling onto
the product.
The present invention also utilizes relatively low air flow which
significantly reduces the
introduction of extraneous material into the drying system and thus prevents
product
quality problems with the finished product.
EXAMPLES
Example 1
With reference to FIG. 7, an oven 100, with a direct fired heater box 102 was
utilized in
the present Example. The oven 100 had a supply air plenum 104 with multiple
high
velocity nozzles 106. These high velocity convection nozzles 106 were placed
within 2.5
cm from the substrate material 108. The material 108 was a web of plastic film
having a
semi-rigid vinyl dispersion coated on the surface. The high velocity nozzles
106, provided
high heat transfer to the material 108. The discharge air velocity at the
nozzle exit was 20-
30 meters per second' at the oven temperature. The heater box had a
recirculation fan 110,
and a modulating direct fired burner 112. The heater box mixed the
recirculation air 114,
and fresh make up air 116, and passed this through the heater box 102. The
direct fired
burner 112, was modulated to control discharge air temperature at 150°
to 200° C. The
desired operating pressure of the oven is maintained by controlling oven
exhaust 118, and
the make up air 116. Chamber 120, is a 10 cm by 10 cm by 200 cm long structure
made
out of stainless steel. Multiple chambers (not shown), were mounted within 1.5
cm from
the material 108 throughout the oven 100. Each chamber 120 had three 1.2 cm
outlets at
the top. The three outlets are joined in a 2 cm in diameter manifold 122. The
manifold
122, was 2 cm in diameter and penetrated through the oven casing to outside
the oven 100.
The manifold 122, outside the oven body was connected to a condenser 124. The
condenser 124 was a tube within a tube design and was made out of stainless
steel. The
inner tube was 2 cm in diameter and the outer tube was 3.S cm in diameter. The
condenser 124, had 2 cm in diameter plant chilled water inlet 126, and a 2 cm
in diameter
chilled water outlet 128. The plant chilled water was at 5°-10°
C at the chilled water inlet
126. A vapor component from the material 108 was collected within chamber 120;
subsequently condensed in condenser 124, and then collected in a separator
130. Clean
gaseous flow from the separator 130, was routed to a vacuum pump 132 through a
2 cm in
-1 C~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
diameter PVC pipe. The vacuum pump 132 was controlled to maintain chamber 120,
at a
pressure gradient with respect to the oven operating pressure. The discharge
of the
vacuum pump 132 was routed back to the oven body. This method collects
substantial
amount of vaporized components from the material 108 without substantial
dilution.
Material build up was observed in the internal area of the oven 100 after 4000
hours of
operation. This corresponds to an approximate 100% improvement from the
conventional
system.
Examples 2-5
The comparison table below, Table l, provides example calculations for
different
systems at typical equipment configurations and operating conditions. The
definitions for
M1, M2, M3, and M4 are the same as described above. M5 represents the time-
average
mass flow per unit width of any additional dilution stream provided to the
chamber (for
example the makeup air stream in convection ovens) in kglsecond/meter. The
width ("w")
of the material, in centimeters, is the measurement (of the gap) in the
direction
perpendicular to the motion of the material. The time-average gas phase
velocity ("<v>")
was defined above and has units of meters per second. The pressure
difference("DP") is
the pressure gradient between the lower periphery of the chamber and outside
the chamber
in Pascals. The material velocity ("V") is measured in meters per second.
The average velocity of gas phase components through the gap, <v>, can be
measured using a velocity meter such as a hot wire anemometer, calculated from
Equation
1 along with knowing the system gap cross sectional area, or estimated using
<v>=1.288 I ~ pl . (Equation 2)
The relationship between volumetric flow, Q, and mass flow, M, is M=pQ where p
is the
density of the gas phase components in kilograms per cubic meter. The gas
phase
temperature dependence can be incorporated by substitution of the Ideal Gas
Law
resulting in
M = Map , (Equation 3)
RT
wherein MW is the molecular weight of the gas phase, p is the pressure , R is
the gas
constant , and T is the gas phase temperature. The dilution flow M1 can be
computed
-1~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
using Equation 1, if it is the only unknown, or calculated from using the
following
equation
Ml=pH<v>. (Equation 4)
Comparative Example 2
A typical air convection drying system consisted of a large enclosure
containing high
velocity convection nozzles. The material, in web form, entered through an
entrance gap
having a width of 76.2 cm and a height of 10.2 cm. The material exited through
an exit
slot having the same dimensions as the entrance gap. The material was
transported through
the center of the gap at a velocity of about 1 meter/second. The material
consisted of a
polyester web with an organic solvent based coating and was dried as it passed
through the
enclosure. The dryer system operating conditions were as follows. The overall
recirculation flow within the chamber of 18.6 kg/second/meter and with the
enclosure
(chamber) pressure set to -5 Pa. The exhaust flow through the chamber M4 was
7.43
kg/second/meter. The flow through the entrance and exit gaps and into the
chamber, M1,
resulting from the -5 Pa pressure gradient, was 0.71 kg/second/meter. M1 was
calculated
using Equation 4. The flow resulting from the evaporation of the coating
solution
solvents, M2, (i.e., drying) was 0.022 kg/seconds/meter. The M2 value was
calculated
assuming the flow stream, M4, was maintained at 20% Lower Flammability Limit
(LFL)
for a solvent with LFL of 1.5 % by volume solvent concentration. The net flow
into the
gap resulting from the motion of the material through the chamber, M3, was 0.
The flow
of make up air M5 into the chamber was 6.7 kg/second/meter. The total net
average gas
phase velocity through the gap was calculated using Equation 2, <v> = 2.9
mlsec. The
calculated value was verified by measurements obtained using a hotwire
anemometer.
Comparative Example 3
A typical inert convection drying system consisted of a large enclosure
containing high
velocity convection nozzles. The material entered through an entrance gap
having a width
of 76.2 cm and a height of 2.54 cm. The material exited through an exit gap
having the
same dimensions as the entrance gap. The material was transported through the
center of
the gaps at a velocity of 1 meter/second. The material consisted of a
polyester web with a
organic solvent based coating and was dried as it passed through the
enclosure. The dryer
-1~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
system operating conditions were as follows. The overall recirculation flow
within the
chamber of 5.66 kg/second/meter and with the enclosure pressure set to 2.5 Pa.
The
exhaust flow through the chamber M4 was 1.48 kg/second/meter. The flow through
the
entrance and exit gaps out of the chamber, M1, resulting from the positive 2.5
Pa pressure
gradient was 0.12 kg/secondlmeter. M1 was calculated using Equation 4. The
flow
resulting from the evaporation of the coating solution solvents, M2, (i.e.,
drying) was 0.03
kg/second/meter. This was determined from the 2% by volume of solvent
recovered (at
the separation device) out of M4 prior to being returned to the dryer as part
of dilution
stream M5. The net flow into the gap resulting from the motion of the material
through
the chamber, M3, was 0. The additional dilution stream M5, was 1.57
kg/second/meter.
This was made up of return flow from the separation device and the inert gas
makeup
stream. The total net average gas phase velocity through the gap was
calculated using
Equation 2, <v> = 2 m/sec.
Example 4
Jn this example the vapor collection apparatus was integrated with a
conventional gap
drying system to capture and collect the gas phase components exiting the gap
dryer. The
web was conveyed by a conveying system through the apparatus of the present
invention.
The web was comprised of polyester film coated with inorganic material
dispersed in
ethanol and water. The web entered through an entrance gap having a width, w,
of 30.5
cm and a height, H, of 0.32 cm. The material exited through an exit gap having
the same
dimensions as the entrance gap. The web was transported through the gap and
underneath
the chamber at a velocity of 0.015 meter/second. The exhaust flow M4 was
measured to
be 0.0066 kg/second/meter. The flow through the entrance and exit gaps out of
the
chamber, M1, resulting from the induced pressure gradient was approximately
the same,
0.0066 kg/second/meter. M1 was calculated using Equation 1. The web and
coating were
for all practical purposes dry upon exiting the gap dryer, thus MZ was 0. This
was verified
using a standard redry measurement where a sample of the web and coating
displayed
virtually no weight loss while being redried at an elevated temperature. The
net flow into
the gap resulting from the motion of the material through the chamber, M3, was
0 and
there were no additional dilution streams M5. The average gas phase velocity
through the
-19~
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
gap was calculated from Equations 1 and 4, <v> = 0.086 m/sec. The pressure
gradient
was calculated to be 0.0045 Pa using Equation 2.
Example 5
In this example, a web was conveyed by a conveying system through apparatus
substantially similar to that disclosed in FIBS. 2-4. The web was comprised of
polyester
film coated with a material consisting of a 10% styrene butadiene copolymer
solution in
toluene. The web passed under a chamber thereby forming a gap between the
lower
periphery of the chamber and the exposed surface of the material. The gap had
a width, w,
of 15 cm and a height, H, of 0.32 cm. The material exited from underneath the
chamber at
a gap having the same dimensions as the entrance gap. The web was transported
through
the gap and underneath the chamber at a velocity of 0.0254 meter/second. The
dryer
system operating conditions were as follows. The heating element was
maintained at 87 C
and the chamber was maintained at 50 C. The exhaust flow (M4) was measured to
be
0.00155 kg/second/meter. The flow through the entrance and exit gaps out of
the
chamber, M1, resulting from the induced pressure gradient was 0.00094
kg/second/meter.
Ml was calculated using Equation 1. The flow resulting from the evaporation of
the
toluene, M2, was 0.00061 kg/second/meter. The net flow into the gap resulting
from the
motion of the material through the chamber, M3, was 0. There was no additional
dilution
streams M5. The total net average gas phase velocity through the gap was
calculated from
Equations 1, 3, and 4 <v> = 0.123 m/sec.
Table 1
Example M4 M3 M2 M1 M5 H w <v> ~p v
Kg/sec/mkg/sec/mKg/sec/mKg/sec/mkg/sec/mCm cm m/secpa m/sec
2. Air 7.43 0 0.022 0.71 6.7 10.276.22.9 -5 1
Convection
Drying
System
3. Inert 1.48 0 0.03 -0.12 1.57 2.5476.22 2.5 1
Convection
Drying
System
4. Exhaust0.00660 =0 =0.00660 0.3230.50.086=-0.00450.015
Port
5. Drying 0.001550 0.000610.000940 0.3215 0.123=-0.0090.0254
System
From the above disclosure of the general principles of the present invention
and the
preceding detailed description, those skilled in this art will readily
comprehend the various
-2Q
CA 02423125 2003-03-19
WO 02/25193 PCT/USO1/42247
modifications to which the present invention is susceptible. Therefore, the
scope of the
invention should be limited only by the following claims and equivalents
thereof.
-2 p