Note: Descriptions are shown in the official language in which they were submitted.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
1
ANTIMICROBIAL AGENT
The present invention relates to antimicrobial agents. More specifically, the
invention
relates to the antimicrobial activity of a series of anhydrofructose
derivatives.
Food degradation from various sources is recognized in the literature and
individual
chemicals are known which will inhibit one aspect or another of degradation
derived from
a single source. Degradation, and the loss of colour or flavour of freshly cut
plant parts
are known to be caused by oxidation, enzymes, microbes, and metal ions. For
example,
acidulants are known to prevent microbial degradation by maintaining a
relatively low pH
environment but their effectiveness is only temporary.
Liste~ia mo~cocytogenes is one example of an organism which can contaminate
certain
foodstuffs and which exhibits resistance to many physical and chemical
treatments.
Listeria monocytogenes is a gram-positive bacillus that causes serious
infection, mainly in
immunocompromised patients and newborn infants. Meningitis and bacteremia are
the
most frequent manifestations of listeriosis.
Bacillus ce~eus is another common cause of food poisoning. Two distinct
clinical
syndromes have been identified, the first having a short incubation period of
about 4
hours, the second having an incubation period of about 17 hours. B. cereus
food
poisoning is initiated when the spore forms survive cooking and the
contaminated food is
allowed to reach temperatures that permit germination of the spore and
elaboration of an
enterotoxin.
Salmonella, of which there are over two thousand different strains, is a
further cause of
food poisoning in humans. Salmonella is a genus of rod-shaped Gram-negative
Entey~obacte~iaceae that inhabit the intestine and cause infections such as
gastroenteritis
and typhoid. If invasive, they can cause enteric fevers (for example, typhoid
caused by
Salmonella typhi, or paratyphoid fever caused by Salmonella paratyphi). Other
strains of
Salmonella are associated with food poisoning (usually Salmonella Typhimurium,
Salmonella panama or Salmonella Enteritidis, the latter notorious for the
contamination
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
2
of poultry) and occasionally septicaemia in non-intestinal tissues.
It is well known in the art that Salmonella cannot propagate at pH values
below 4.5. As a
consequence, mildly acid products such as fine food and non-fermented meat
products are
especially susceptible to attack by Salmonella.
For meat products, nitrite is often used as a preservative. However, the
addition of nitrite
is restricted for toxological reasons (due to its acute toxicity, together
with the dangers
associated with nitrosamine formation). As a result, Salmonella is only
inhibited at
concentrations of nitrite beyond 1,000 ppm, which are far beyond legal limits.
Instead, it has been shown that combinations of nitrite and sorbic acid can
increase the
effectiveness against Salmonella [Inhibition of Salmonella by Sodium Nitrite
and
Potassium Sorbate in Franlcfiuters, Journal of Food Science, 47, 1982, p. 1615
ffJ.
Inhibition has been observed at concentrations beyond 50 ppm of nitrite
combined with
2600 ppm sorbic acid.
Other agents such as bacteriocins (Nisin) are unable to inhibit Salmonella in
food,
whereas benzoic acid is unsuitable because the inhibitory effect can only be
observed in
acid products. The inhibitory effect of phytogenic ingredients (or "natural
substances")
such as oil extracts from different spices, has also been tested, but again
the
concentrations required for achieving the inhibitory effect on Salmonella were
too high
and the sensorical influence on the food was too strong.
Thus, to date, the use of chemical substances has been severely limited
because on the
one hand they have to be safe from a toxicological view point, but on the
other hand they
must not influence the product sensorically.
The present invention seeks to alleviate the problems associated with prior
art chemical
substances and to provide new antimicrobial compositions based on
anhydrofructose
derivatives. In particular, the invention seeks to provide antimicrobial
agents that are
suitable for use in foodstuffs/feed.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
3
In a first aspect, the invention provides an antimicrobial composition
comprising a cyclic
compound having Formula I,
R3
R~ R4 R2/ Rs
I
wherein R1 and Ra are independently selected from -OH, =O, and -OC(O)R',
wherein R' is a
hydrocarbyl group; wherein R3 is selected from -OH, =O, a substituent
comprising an -OH
group and -OC(O)R', wherein R' is a H or a hydrocarbyl group; wherein R4 and
RS are each
independently selected from a hydrocarbyl group, H, OH, =O, and -OC(O)R',
wherein R' is
a H or a hydrocarbyl group or wherein R4 and RS represent a bond with an
adjacent atom on
the ring of the cyclic compound; and wherein said compound comprises at least
one ester
group.
A second aspect of the invention provides a process for preventing and/or
inhibiting the
growth of, and/or killing, microorganisms in a material, the process
comprising the step of
contacting the material with a cyclic compound having Formula I,
R3
R~ R4 2/ R5
R
I
wherein Rl and R2 are independently selected from -OH, =O, and -OC(O)R',
wherein R' is a
hydrocarbyl group; wherein R3 is selected from -OH, =O, a substituent
comprising an -OH
group and -OC(O)R', wherein R' is a H or a hydrocarbyl group; wherein R4 and
RS are each
independently selected from a hydrocarbyl group, H, OH, =O, and -OC(O)R',
wherein R' is
a H or a hydrocarbyl group or wherein R4 and RS represent a bond with an
adjacent atom on
the ring of the cyclic compound; and wherein said compound comprises at least
one ester
group.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
4
In a third aspect, the invention relates to the use of a compound having
Formula I,
R3
R~ R4 R2~ Rs
I
wherein R1 and Ra are independently selected from -OH, =O, and -OC(O)R',
wherein R' is a
hydrocarbyl group; wherein R3 is selected from -OH, =O, a substituent
comprising an -OH
group and -OC(O)R', wherein R' is a H or a hydrocarbyl group; wherein R4 and
RS are each
independently selected from a hydrocarbyl group, H, OH, =O, and -OC(O)R',
wherein R' is
a H or a hydrocarbyl group or wherein R4 and RS represent a bond with an
adjacent atom on
the ring of the cyclic compound; and wherein said compound comprises at least
one ester
group; for preventing and/or inhibiting the growth of, and/or killing,
microorganisms in a
material. _ _ . _ _ ~ .._ _ . _
It will be appreciated that by the term "ester group" it is meant a group of
the formula X-
C(O)O-Y wherein X and Y are hydrocarbyl groups.
Preferably, the material is a foodstuff or feed. Thus, in a preferred aspect,
the present
invention relates to antimicrobial substances that are suitable for use in
foodstuffs and/or
feed to inhibit food poisoning and spoiling bacteria contained therein.
In another preferred embodiment, the material is a home product, a body care
product or a
cosmetic product, for example, a body lotion.
By way of definition, the term "antimicrobial" refers to a substance that
kills or prevents or
inhibits the growth or reproduction of microorganisms. Antimicrobials are
generally
classified according to the type of microorganism they are effective against.
For example,
antibacterial substances are effective against bacteria, antifungal substances
are effective
against fungi, including yeast, and antiviral substances are effective against
viruses. Certain
antimicrobials can be used internally, for example antibiotic medications,
whereas other
antimicrobials are for external use only, such as antiseptics.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
S
As used herein, the term "hydrocarbyl group" means a group comprising at least
C and
H and may optionally comprise one or more other suitable substituents.
Examples of
such substituents may include halo-, alkoxy-, nitro-, hydroxy, carboxyl,
epoxy, acrylic,
hydrocarbon, N-acyl, or cyclic group etc. In addition to the possibility of
the
substituents being a cyclic group, a combination of substituents may form a
cyclic
group. If the hydrocarbyl group comprises more than one C then those carbons
need
not necessarily be linked to each other. For example, at least two of the
carbons may
be linked via a suitable element or group. Thus, the hydrocarbyl group may
contain
hetero atoms. Suitable hetero atoms will be apparent to those skilled in the
art and
include, for instance, sulphur, nitrogen and oxygen.
In a more preferred aspect, the cyclic compound of the invention is a compound
having
Formula II
R3
O
R5
R~ R4 R2
II
wherein Rl, R2, R3, R4, and RS are as defined hereinabove.
Preferably, the cyclic compound is a compound having Formula III
R3
O
R5
R~ R4 Rz
III
wherein Rl, R2, R3, R4, and RS are as defined hereinabove.
In one preferred embodiment, said cyclic compound is of Formula IV,
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
6
R3
R~ Rs
R \Rz
R R4
IV
wherein R1 and R2 are independently selected from -OH, =O, and -OC(O)R',
wherein R' is a
hydrocarbyl group; wherein R3 is selected from -OH, =O, a substituent
comprising an -OH
group and -OC(O)R', wherein R' is a H or a hydrocarbyl group; wherein R4 and
RS are each
independently selected from a hydrocarbyl group, H, OH, =O, and -OC(O)R',
wherein R' is
a H or a hydrocarbyl group or wherein R4 and RS represent a bond with an
adjacent atom on
the ring of the cyclic compound; wherein R6 and R' are each independently
selected from a
hydrocarbyl group, H, OH, =O, and -OC(O)R', wherein R' is a H or a hydrocarbyl
group or
wherein R4 and RS represent a bond with an adjacent atom on the ring of the
cyclic
compound; and wherein said compound comprises at least one ester group.
More preferably, said cyclic compound is of formula V,
R3
R~ O
s
R Rs
R~ Ra Rz
V
wherein R1, R2, R3, R4, R5, R6 and R' are as defined hereinabove.
Preferably, Rl is selected from -OH, =O, and -OC(O)R', wherein R' is a
hydrocarbyl group.
Preferably, R2 is selected from -OH, =O, and -OC(O)R', wherein R' is a
hydrocarbyl group.
Preferably, R3 is selected from a substituent comprising an -OH group and -
OC(O)R',
wherein R' is a H or a hydrocarbyl group.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
Even more preferably, R3 is -OC(O)R', wherein R' is a H or a hydrocarbyl
group.
Even more preferably, R3 is -OC(O)R', wherein R' is a hydrocarbyl group.
In one preferred embodiment, R3 is -OC(O)R', wherein R' is R" group.
Preferably, R' and/or R" is a branched or unbranched, substituted or
unsubstituted alkyl
group.
More preferably, R' and/or R" is (CH2)pCH3, wherein p is from 1 to 24.
Even more preferably, R' and/or R" is a C8 allcyl group.
In an another preferred embodiment, R' and/or R" is a C12 allcyl group.
In an another preferred embodiment, R' and/or R" is a C16 or a C1g alkyl
group.
In one preferred embodiment of the invention, R3 is of the formula -(CH2)n
OC(O)-
(CH2)pCH3, wherein n and p are each independently from 1 to 24.
More preferably, R3 is of the formula -(CH2)"-OC(O)-(CH2)~CH3, wherein n is
from 1 to
24, preferably from 1 to 20, preferably from 1 to 10, preferably from 1 to 5,
or preferably l,
2, or 3.
In an alternative preferred embodiment, R3 is of the formula -(CH2)"OC(O)-
(CHZ)11CH3,
wherein n is from 1 to 24, preferably from 1 to 20, preferably from 1 to 10,
preferably from
1 to 5, or preferably l, 2, or 3.
In one preferred embodiment, R4 is selected from a hydrocarbyl group, H, OH,
=O, and -
OC(O)R', wherein R' is a H or a hydrocarbyl group.
In a particularly preferred embodiment, R4 is selected from a hydrocarbyl
group, H, OH, and
=O.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
8
In one preferred embodiment, RS is selected from a hydrocarbyl group, H, OH,
=O, and -
OC(O)R', wherein R' is a H or a hydrocarbyl group.
In a particularly preferred embodiment, RS is selected from a hydrocarbyl
group, H, OH, and
=O.
In one preferred embodiment, R4 and RS represent a bond with an adjacent atom
on the ring
of the cyclic compound.
In one especially preferred embodiment, the compound is esterified
anhydrofructose wherein
at least one OH group of anhydrofructose is esterified to form a -OC(O)R"'
group, wherein
R"' is a hydrocarbyl group.
Preferably, R"' is a branched or unbranched, substituted or unsubstituted
allcyl group.
Even more preferably, R"' is (CH2)pCH3, wherein p is from 1 to 24,
More preferably still, R"' is a C8 alkyl group.
In an alternative preferred embodiment, R"' is a C12 alkyl group.
In another preferred embodiment, R"' is a C16 or a Cl8 alkyl group
In one preferred embodiment of the invention, the cyclic compound is of the
formula:
0
o~~
P
O
OH
OH
O
p = 1-24
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
9
In one preferred embodiment of the invention, cyclic compound is of the
formula:
0
o~
0
OH
O
OH ~
OH
p = 1-24
More preferably, the cyclic compound is selected from the following:
0 0
0 0
0 0
OH
OH OH OH
OH OH~
OH OH
More preferably, the cyclic compound is selected from the following:
0 0
0 0
0 0
OH OH
OH ~ OH
O O
Preferably, the compound of the invention is a derivative of Ascopyrone P,
Ascopyrone M,
Ascopyrone T, Ascopyrone Tl, Ascopyrone T2, Ascopyrone T3, and mixtures
thereof.
Even more preferably, the compound of the invention is selected from esterfied
Ascopyrone
P, esterfied Ascopyrone M, esterfied Ascopyrone T, esterfied Ascopyrone Tl,
esterfied
Ascopyrone T2, esterfied Ascopyrone T3, and mixtures thereof.
The structures of Ascopyrone P, Ascopyrone M, Ascopyrone T, Ascopyrone Tl,
Ascopyrone
TZ and Ascopyrone T3 are shown below.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
HOCHZ HOCH2 HOCHa
O~ 0
H01~~~ H 0 O
0 0 0
Ascopyrone N! Ascopyrone P Ascopyrone T
HOCHZ HOCHZ HOGH~
O ,L--- 0 0
OH HO ~ HO HO
OH QH HO OH O "~ OH
Ascopyrone T1 Ascopyrane TZ Ascopyrone T3
Ascopyrone is a known compound. In 1978 and 1981, a group of American
scientists
prepared Ascopyrone P by pyrolysis of amylopectin, amylose and cellulose at
the Wood
5 Chemistry laboratory in Montana, with the intention of using Ascopyrone P as
a starting
material for organic synthesis [Shafizadeh, F., Furneaux R.H., Stevenson,
T.T., and
Cochran, T.G., 1,5-Anhydro-4-deoxy-D-glycero-hex-1-en-3-ulose and other
pyrolysis
products of cellulose, Carbohydr. Res. 67(1978): 433-447; Stevenson, T.T.,
Stenkmap,
R.E., Jensen, L.H., Cochran, T.T., Shafizadeh, F., and Furneaux R.H., The
crystal
10 structure of 1,5-anhydro-4-deoxy-D-glycero-hex-1-en-3-ulose, Carbohydr.
Res.
90(1981): 319-325]. They characterized Ascopyrone P by, for example, 1H and
13C NMR,
and IR spectroscopy techniques. A 3-dimensional structure of Ascopyrone P was
provided.
The yield of Ascopyrone P obtained by pyrolysis was under 3% and complicated
separation
methods had to be used.
The natural occurrence of Ascopyrone P in some species of very scarcely
studied fungi
collected from the Alps has been taught [M.-A. Baute, G. Deffieux, J.
Vercauteren, R.
Baute, and Badoc A., Enzymatic activity degrading 1,4-a-glucans to Ascopyrones
P and
T in Pezizales ad Tubercles, Phytochemistry, 33 (1993): 41-45]. The occurrence
of
Ascopyrone P in fungi immediately prompted the hypothesis that Ascopyrone P
would act as
an antibiotic. However, Ascopyrone P did not function satisfactorily as an
antibiotic in the
disclosed tests.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
11
Ascopyrone P and Ascopyrone T can be produced enzymatically from 1,5-anhydro-D-
fructose using cell-free extract prepared from the fungi of the order
Pezizales, such as
Plicaria leioca~pa and Anthracobia melaloma, and the order of Tuberales, such
as, Tubet~
melanosporum. Ascopyrone Tl is the dehydrate form of Ascopyrone T, whereas
Ascopyrone T2 and T3 are the tautomeric monohydrate forms of Ascopyrone T.
Ascopyrone M can be produced from 1,5-anhydro-D-fructose by EDTA-sensitive
dehydratases isolated from the fungi Morels, such as Mo~chella vulgaris,
Gyromitres,
pezizes, such as Peziza echinospora.
Ascopyrone M, P and T can also be produced chemically by treating 1,5-anhydro-
D-
fructose with alkali under mild conditions [Studies on the degradation of some
pentoses
and of 1,5-anhydro-D-fructose, the product of the starch-degrading enzyme a-
1,4-glucan
lyase; Thesis, Ahmad, T., The Swedish University of Agricultural Sciences,
Sweden,
1995].
When the compound of the present invention is prepared by chemical means, it
may be
prepared in accordance with one of the following methods:
(1) Ascopyrone P may be produced by treating 1,5-anhydro-D-fructose with non-
aqueous
acid at elevated temperature, for example at 70 °C.
(2) Ascopyrones (for example, Ascopyrone P, T and M) may be produced from 1,5-
anhydro-D-fructose by alkaline treatment according to Ahmad, T., 1995.
The structures of all ascopyrones produced were confirmed by NMR techniques.
Preferably, the compound of the present invention is prepared by enzymatic
means as
disclosed in M.-A. Baute et al, [Phytochemistry, 33 (1993): 41-45). For
example
ascopyrones (such as, Ascopyrone P, T and M) may be produced from 1,5-anhydro-
D-
fructose using enzymatic methods as disclosed in M.-A. Baute et al.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
12
In a particularly preferred embodiment, the compound is selected from the
following:
CHZOH CHZOH CHaOH
O O O
OH OH
OH OH ~ OH/
O OH O O
CHZOH CH~OH CHZOH
0 O O
OH OH OH
OH OH OHM
HO OH OH 0
O
CH20H OAc 0
P
O 0 0
OH
OH/ ~ OH
O OH Ac0 O 0
p = 1,2....24
OH
O
O ~ \0H
or an esterified derivative thereof.
In a preferred embodiment, the cyclic compound having formula I has an
antimicrobial
effect against gram positive bacteria and yeasts.
Preferably, the cyclic compound having formula I has an antimicrobial effect
against a
microorganism selected from Liste~ia, Salmonella, Bacillus, Saccharomyces,
Pseudomonas,
Clostridium, Lactobacillus, Brochoth~ix, Micrococcus, Yersiuia, Enterobacter
and
Zygosaccha~omyces, Staphylococcus, Escherichia.
Even more preferably, the cyclic compound having formula I has an
antimicrobial effect
against a microorganism selected from Listeria monocytoge~es, E. coli,
Staphylococcus
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
13
aureus, Listeria innocua, Salmonella Typhimurium, Salmonella sp., Bacillus
cereus,
Bacillus subtilis, Saccharomyces cerevisiae, Saccharomyces cerevisiae var.
paradoxus,
Saccharomyces carlsbergensis, Pseudomonas fluorescens, Clostridium sporogenes,
Lactobacillus sake, Brochothrix thermosphacta, Micrococcus luteus, Yersircia
enterocolitica,
Enterobacter aerogerces and Zygosaccharomyces bailiff.
Even more preferably, the cyclic compound having formula I has an
antimicrobial effect
against a micro-organism selected from Listeria monocytogehes, E. coli,
Bacillus cereus,
Saccharomyces cerevisiae, Saccharomyces carlsbergensis, Pseudomonas
fluorescer~s,
Clostridium sporogenes, Lactobacillus sake, Brochothrix thermosphacta and
Micrococcus
luteus.
In a highly preferred aspect a derivative of the compound of formula I is a
compound of
the formula
\/O O O
v ~O O
This compound (3,6-di-O-acetyl-1,5-anhydro-4-deoxy-D-glycero-hex-3-enopyranose-
2-
ulose) may be prepared in accordance with the teaching of Andersen et al.
(1998),
Structure of 1,5-anhydro-D-fructose: X-ray analysis of crystalline acetylated
dimeric
forms, J. Carbohydr. Chem. 17: 1027-1035.
The aspect of the present invention wherein the derivative of the compound of
formula I
is an ester is particularly preferred because the compound may be lipophilic
and/or may
have both hydrophobic and hydrophilic properties. When the compound has both
hydrophobic and hydrophilic properties the compound readily resides at a
water/oil
interface of an emulsion.
The residence of the compound at a water/oiI interface of an emulsion may
allow it to act
as an emulsifier. Thus the present invention may further provide compounds
having a
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
14
dual functional effect. The compounds may act both as an antimicrobial and as
an
emulsifier.
Many of the compounds of the present invention can be derived from 1,5-
anhydrofructose.
1,5-Anhydrofructose is monoketo sugar found in bacteria, red algae, fungi and
mammals.
In red algae and fungi 1,5-anhydrofructose is produced by the action of a-1,4-
glucan
lyase [EC 4.2.2.13] from floridean starch and glycogen, respectively.
When the compound of the present invention is prepared from 1,5-anhydro-D-
fructose,
preferably the 1,5-anhydro-D-fructose is prepared in accordance with GB-A-
2296717. In
other words, preferably the 1,5-anhydro-D-fructose is prepared by a method
comprising
treating an a-1,4-glucan with the enzyme a-1,4-glucan lyase characterised in
that enzyme is
used in substantially pure form.
Preferably, the cyclic compound of the invention comprises a five or a six
membered ring.
The compounds of the present invention comprise at least one ester group.
Thus, as used
herein the term "ester" includes mono-, di-, tri- and poly-esters.
In a preferred aspect the compound of formula I is a diester wherein the Rl
substituent is
an -OH group and wherein the ester linkages are formed from the -OH group of
the R4
substituent and from the -OH group of the R3 substituent.
As mentioned above, in a particularly preferred embodiment of the invention,
the
compound is 6-O-acyl-1,5-anhydro-D-fructose, as represented below.
0
~n~
0 0
OH
HO
O
The preparation of 6-O-acyl-1,5-anhydro-D-fructose may be addressed by a
chemical
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
approach or by an enzymatic approach, in accordance with the methods detailed
in WO
00/56745.
The chemical approach may comprise the following reaction to synthesise Cla
esters of
5 anhydrofructose:
0
off Jn~
0 o 0
OH -~ OH
HO HO
O O
The reaction is carried out with lauroyl chloride and pyridine. The acylation
sites were
assigned through derivatisation of NH2OR followed by separation and NMR of the
10 products. The products were found to be
50% 6-O-acyl-1,5-anhydro-D-fructose
11% 3-O-aryl-1,5-anhydro-D-fructose
A similar method may be used to prepare other ester derivatives of
anhydrofructose.
The enzymatic approach to prepare 6-O-acyl-1,5-anhydro-D-fructose may comprise
the
use of lipases and proteases. In aqueous solution lipases and proteases cleave
ester
linkages. Lipases are sugar specific and proteases fatty acid specific.
However, Synthesis
1990, 112-115 discloses that lipases and proteases in non-aqueous solution
offer a
reversal of activity, and form ester bonds. Thus lipases and proteases in non-
aqueous
solution may be used in the preparation of a compound in accordance with the
present
invention.
In accordance with J. Chem. Soc. Pef°kin Traps. I, 1995, 2203-2222
lipases were
screened to identify suitable lipases for the preparation of compounds in
accordance
with the present invention. Screening with pyridine identified Ca~dida
antarctica,
Pseudomo~as cepacia, Pseudomonas fluorescens, and hog pancreas. Screening with
tBuOH:pyridine 2:1 identified Candida a~tarctica, Caadida cylind~acea,
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
16
Pseudomonas cepacia, Pseudomonas fluorescens, hog pancreas.
Thus preferably the compound in accordance with the present invention is
prepared
with a lipase obtained from Candida antarctica, Pseudomonas cepacia,
Pseudomonas
fluorescens, hog pancreas, or Candida cylircdy°acea.
Preferably the compound in accordance with the present invention is prepared
with lipase
from Candida antarctica. Carcdida antarctica may be obtained from Novo Nodisk
A/S,
Denmark under the name Novozym 435.
The enzymatic approach was demonstrated by the enzymatic acylation of 1,5-
anhydro-D-
fructose with lauric acid to form 6-O-acyl-1,5-anhydro-D-fructose.
Lauric Solvent 3 t~ molecular TemperatureReaction Conversion
acid sieve. (oC) time
(mol/mol) (w/w) (h)
1 tert-BuOH- 40 24 21
1 tert-BuOH1 ( owd.) 40 24 56
1 tert-BuOH1 ( owd.) 40 72 62 %
1 acetone 1 ( owd.) 20 24 55
1 tert-BuOHS 45 24 56
1 tert-BuOH10 45 24 61
1 tert-BuOH20 45 24 66
3 tent-BuOH20 45 24 73
3 tent-BuOH20 ( owd.) 45 24 78
3 tert-BuOH20 ( owd.) 45 48 uantitative
3 acetone 20 ~ 20 ~ 72 quantitative
The chemical approach may comprise the quantitative conversion with lauric,
palmitic
and stearic acid of 1,5-anhydro-D-fructose to 6-O-acyl-1,5-anhydro-D-fructose
as
follows:
OH
O 3 eqv. RCOOH
CAL, 3A m.s.
OH -
HO
O
The reaction forms a composition comprising monomer ketone/dimer type 1/dimer
type 2
- 1:3:1. The mixture may be purified by chromatography on silica to give
approximately
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
17
70% yield.
The cyclic compound of the invention may be used alone, or in combination with
other
components, for example, one or more preservatives, one or more chelators
(such as
EDTA sodium salt, polyphosphate or citrate) and/or one or more antioxidants
(such as
ascorbate, isoascorbate, ascorbate palmitate, BHA or BHT).
By way of definition, in the broadest sense, the term "preservative" is
intended to encompass
all substances which inhibit the development of, or kill, micro-oganisms. In a
narrower
sense, it is generally understood that preservatives are used in
concentrations of 0.5 % or
less. Food additives which are allowed to be used as preservatives are listed
in the
Regulation No. 95/2/EG of the European Parliament and Council of 20 February
1995,
relating to food additives other than colouring agents and sweeteners.
Typical food preservatives permitted in the EU which are suitable for use in
combination
with the compounds of the invention include sorbic acid, benzoic acid, PHB
ester (p-
hydroxybenzoate), and sulphur dioxide. The mode of action of these
preservatives,
together with their range of effects are listed below.
Sorbic Acid (E200 to 203):
Mode of action: inhibits different enzymes in the cells of the microorganisms.
Range of effects: mainly against yeasts and moulds as well as catalase-
positive bacteria.
Catalase-negative bacteria as well as lactic acid bacteria and clostridia are
not inhibited.
Effective concentration: 500 - 3000 ppm.
Permitted maximum quantities in food: up to 2000 ppm in potato dough,
processed
cheese, packed bread, fine bakery products, emulsified sauces etc.
Benzoic Acid (E210 to 213):
Mode of action: inhibits exchange of oxygen through the cellular membrane and
affects
the enzymatic structure.
Range of effects: for acid products only, up to approx. pH 4.5; inhibits
yeasts and
moulds, restricted inhibition of bacteria (no, or only very little, inhibition
of lactic acid
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
18
bacteria and clostridia).
Permitted maximum quantities in food: 500 ppm in aspic, fruit preparations,
marmalades
etc.
PHB Ester (p-hydroxybenzoate) (E214 to 219)
Mode of action: damages the bacterial membrane because of the surface
activity,
poisonous to protoplasm because of protein denaturation.
Range of effects: mainly inhibits yeasts and fungi, but also Gram-positive
bacteria in a pH
range between 3.0 and 8Ø
Effective concentration: sensorical influence at concentrations beyond approx.
0.08 %.
Sulphur Dioxide (E220 to 224; E 226 to 227)
Mode of action: depends on pH to a great extent, in practice it is only
effective at acidic
pH values (< 4,0). Very complex mechanisms.
Range of effects: mainly antibacterial, above all against Gram-negative,
aerobic bacteria.
Effective concentrations: 250 - 500 ppm for inhibition of aerobic, Gram-
negative bacteria,
800 - 2000 ppm against Gram-positive bacteria, yeasts, and moulds.
Permitted maximum quantity in food products: max. 2000 ppm in dry fruits,
grape juice
concentrate for home production of wine, in some cases only max. quantities of
20 - 30
ppm are permitted.
For more specific applications, the compounds of the present invention may
also be used
in combination with the following preservatives: biphenyl, Biphenyl,
orthophenylphenol,
thiabendazol, raisin, natamycin, hexamethylentetramine, dimethyldicarbonate,
boric acid,
sodiumtetraborate, nitrite, propionic acid and propionate, and lysozyme. The
mode of
action of these preservatives, together with their range of effects and
specific uses are
listed below.
Biphenyl, Diphen~ (E 230)
Range of effects: Inhibition of moulds.
Substance for treatment of fruits: surface treatment of citrus fruits.
Permitted maximum quantity: 70 ppm
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
19
Orthophenylphenol (E 231 / E 232)
As with E230, limited to treatment of fruits as a surface treatment for citrus
fruits.
Thiabendazol (E 233)
Surface treatment of citrus fruits and bananas.
Nisin (E 234)
Mode of action: Disturbance of membrane functions.
Range of effects: Gram-positive bacteria, no influence on Gram-negative
bacteria.
Permitted maximum quantity in food products (EU): 3ppm in semolina pudding and
similar products, 12.5 ppm (= 12.5 IU/g) in ripened cheese and processed
cheese, 10 ppm
in clotted cream, 10 ppm in mascarpone.
Natamycin~Pimaricin) (E235)
Mode of action: specifically attacks cell membrane, where - in general - an
interaction
with sterines occurs which increases the permeability of the membrane.
Range of effects: Moulds and yeasts, not effective against bacteria. Usual
dosage rates
are below approx. 50 mg / 1. Maximum level is 1 mg/dma on the surface, with a
maximum penetration of S mm.
Applications: surface treatment of hard, semi-hard and semi-soft cheese and of
dried,
cured sausages.
Hexamethylentetramine (E 239)
Hexamethylentetramine is formed by adding ammonia to formaldehyde in an
aqueous
solution. The microbicidal effect is due to the formaldehyde.
Permitted only for Provolone cheese (25 ppm residual quantity).
Dimethyldicarbonate (E 242)
Permitted only for non-alcoholic drinks, non-alcoholic wine, and liquid
concentrate.
Boric Acid, Sodiumtetraborate (E284 / E 285)
Permitted only for caviar.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
Nitrite (E 249 and E 250)
Permitted in the form of nitrite curing salt for treatment of meat products
("red products").
For cured and dried meat products which are not heat treated and for other
cured meat
products an addition of 150 ppm has been fixed as a guideline. These
concentrations do
5 not show a preservative effect. They are mainly added for their
technological properties
(formation of colour, taste) as well as for their antioxidant effects.
Propionic Acid and Propionate (E 280, E 281, E 282, and E 283)
Mode of action: similar to sorbic acid, pH < 4.5 is optimal.
10 Accumulation in the cell leads to inhibition of enzymes.
Range of inhibition: moulds are inhibited at an pH of 5.5 by concentrations of
125 to
12500 ppm, for inhibition of bacteria higher concentrations are necessary (>
16000 ppm).
Application: Sliced and packaged bread.
Permitted maximum quantity: 3000 ppm.
Lysozyme (E 1105)
Permitted only for ripened cheese.
Permitted maximum quantity: quantum satis.
Studies by the applicant of the inhibitive effects of the present compounds
have been
tested in a medium (Elliker broth) with an almost neutral pH (pH 6.8) and have
been
shown to be efFective against both Gram-positive and Gram-negative bacteria.
As many
of the preservatives described above show an inhibitory effect mainly at low
pH, the use
of the compounds of the present invention clearly broadens the potential range
of
applications.
In principle, the use of substances for chemical preservation depends on the
following
factors:
(a) Toxicological harmlessness
~ the effects of the substance when applied acutely, subchronically, and for a
long
term period.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
21
~ Testing of acute toxicity (LDsn), cinetics and metabolism, pharmacological
effects, genotoxicity, etc.
(b) Technological / food chemical aspects:
~ Solubility in water: as growth takes place in the aqueous phase, a
preservative has
to be water-soluble
~ Reaction with food ingredients, problem of off flavours (sensory acceptance)
~ Interferences with food ingredients (e.g. destruction of vitamin Bl by
sulphuric
acid)
The antimicrobial effectiveness of chemical substances in food and feed
products is thus
determined by a range of different factors. Among others, the composition of
the
population of micro-organisms, the composition of the food product
(ingredients, pH,
water activity, content of salt, etc.), the packaging, time-temperature-
conditions, etc. are
key factors that influence the inhibitory activities of the antimicrobial
agent.
The invention will now be described only by way of example, and with reference
to the
accompanying figures, wherein:
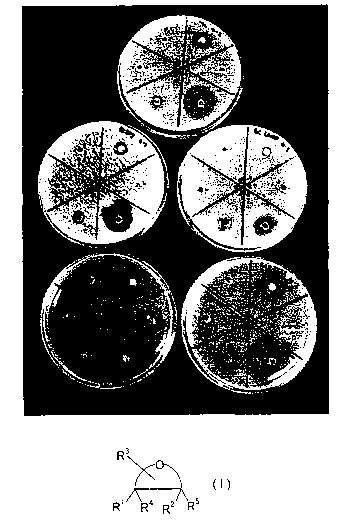
Figure 1 shows a photograph of well diffusion tests on M. luteus (top plate),
B. tarsus
(middle two plates), and Cl. Sporogenes (bottom two plates) treated with the
following:
Upper right segment: 3 % C8 anhydrofructose ester;
Middle right segment: 0.3 % C8 anhydrofructose ester;
Lower right segment: 3 % C12 anhydrofructose ester;
Lower left segment: 0.3 % C12 anhydrofructose ester;
Middle left segment: equivalent methanol control at 25 % methanol;
Upper left segment: equivalent methanol control at 2.5 % methanol.
Figure 2 shows a photograph of a well diffusion test on M. luteus treated with
the
following:
Segment 1: 3 % C8 anhydrofructose ester;
Segment 2: 0.3 % C8 anhydrofructose ester;
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
22
Segment 3: 3 % C12 anhydrofructose ester;
Segment 4: 0.3 % C12 anhydrofructose ester;
Segment 5: equivalent methanol control at 25 % methanol;
Segment 6: equivalent methanol control at 2.5 % methanol.
EXAMPLES
CHEMICAL SYNTHESIS
The compounds of the invention were prepared, characterised and purified in
accordance
with the general methods disclosed in WO 00/56745.
MATERIALS AND METHODS
TEST STRAINS
All microorganisms were taken from storage at -80 °C. Most organisms
were tested as
vegetative cell suspensions from overnight broth culture. Bacillus and
Clostridium
species were tested as endospore suspensions prepared earlier and stored at 4
°C.
For broth cultures and Bioscreen testing most bacteria were grown in Brain
Heart
Infusion (BHI, Oxoid, pH 7.4). Lactobacillus sake A10 was grown in de Man,
Rogosa,
Sharpe medium (MRS, Oaoid). Yeasts were grown in Sabouraud Liquid medium (SLM,
Oxoid). Most bacteria were cultured at 30 °C. Lactic acid bacteria were
grown on solid
medium in enriched COZ atmosphere. Clostridium species were grown in
Reinforced
Clostridia) Medium (RCM) at 37 °C anaerobically. Brvchothrix
thermosphacta and
yeasts were grown at 25 °C.
Bioscreen testing
An automated Microbiology Reader Bioscreen C was used to measure growth curves
of
the strains in the presence and absence of test samples. The Bioscreen C
measures the
development of turbidity (i.e. growth) kinetically by vertical photometry in
200 wells of a
honeycomb microtitre plate, simultaneously. The system consists of a Bioscreen
C
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
23
analyser, which is an incubator and measurement unit, integrated with a PC,
software
(BioLink v 5.30), printer and a 'Honeycomb 2' cuvette multiwell plate. Growth
curve
data can be analysed within the BioLink software or exported to programs such
as Excel.
Protocol
To a 14 mg sample was added 50 ~,l of 100% methanol. 66.7 ~,1 of IMS was then
added
(industrial methylated spirit, 96% ethanol) to make a 12% (w/v) solution.
For the test, this solution was then diluted 1 in 4 in sterile distilled
water. This was
necessary because the level of alcohol in the sample would otherwise be
inhibitory to the
test micro-organism. This made a final solution of 3% (w/v).
The test sample could not be filter sterilised because too much would have
been lost, and
only ca. 470 ~1 was available. The sample had been handled aseptically and it
was hoped
that it was sterile. For the same reason the pH of the sample was not
measured.
The sample was then tested at 0.3% concentration in the Bioscreen. However it
was
immediately realised that this may be problematic because the AF-ester 1 test
sample was
milky-white and turbid. Unfortunately, when this was added to the Bioscreen
wells, the
initial turbidity was too high for any microbial growth to be discerned.
Therefore to
ascertain if any inhibition had occurred, viable counts were taken of the
inoculum, and
then after 24 h incubation in the Bioscreen at 30 °C, by sampling
directly from the
Bioscreen plate. Inhibition could then be assessed by comparison with the
final numbers
achieved in the control wells that contained 2.5% alcohol.
Results of Bioscreen BS021100
AF ester 1 = C8 ester of anhydrofructose (structure shown in claim 32 - LHS).
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
24
Table 1
Count after
Test strain Initial count24 h at 30
C (cfulml)
(cfu/ml)
2.5% alcohol 0.3% AF-ester
control 1
B. tarsus 204 1 x 10 1.3 x 10 2.4 x 10
L. mo~ocytoge~es S23 1.2 x 10 1.1 x 10 < 10
Lb.sakeAlO <10 1.9x10 <10
E.coliSlS 5.3x10 1.1x10 3.7x10
Ps. uorescehs 3756 3.6 x 10 1.1 x 10 2.3 x 10
S. cerevisiae 9763 3.6 x 10 2.0 x 10 2 x 10
S carlsber ensis 6418 5.2 x 10 9.7 x 10 1 x 10
Conclusions
The results in Table 1 show that AF-ester 1 was inhibitory towards all the
micro-
organisms tested. The order of inhibitory activity was as follows: Gram
positives > yeasts
> Gram negatives. The sample was particularly effective against L.
mohocytogenes, but
was also very effective against Bacillus. There was evidence of tidal activity
towards L.
monocytogenes, and possibly the yeasts.
Anhydrofructose ester 1: Cidal test
A preliminary tidal experiment was undertaken with the sample that had earlier
been
tested in Bioscreen with viable count confirmation. This had shown good
activity. For the
tidal experiment the chosen test organism was L. monocytogenes 523, because
this had
shown the greatest sensitivity in the growth inhibition testing.
Protocol
Aliquot 3 x 890 ml 10 mM HEPES buffer, pH 7. To the control test was added 100
ml
water, to the other control test was added 100 ml equivalent alcohol control
and to the test
sample was added 100 ml AF ester 1. To all tests were added 10 ml of an
overnight
culture. The samples were left at ambient temperature for 2 h. A viable count
was carried
out. Note: the AF ester 1 precipitated out during the test.
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
2S
Results
Tests Viable count (cfu/ml)
Control/water 3.1 x 108
Control/alcohol 2.0 x 10~
Test/ AF ester 2.4 x 10'
1
From the results it was concluded that AF ester 1 does not have any cidal
activity.
Testing of new samples:
Anhydrofructose ester C8 (AFCB) = C8 ester of anhydrofructose (structure shown
in
claim 32 - LHS)
Anhydrofructose ester C12 (AFC12) = C12 ester of anhydrofructose (structure
shown in
claim 32 - RHS)
Glucose ester C8 (GC8) - control
Glucose ester C 12 (GC 12) - control
AF esters were dissolved in water by either heating at 70 °C for 10 -
15 min, or 100 °C
for 5-10 minutes. Both methods were unsuccessful, and the esters were
eventually tested
as 0.5 % (w/v) solutions in 50:50 methanol/water that had been heated. AFC8
did not
dissolve, but the others were better.
Results:
No zones observed for equivalent methanol controls.
Table 2
Test strain Well
diffusion
zone
(mm)
tested
against
0.5%
(wt/vol)
extracts
AF C8 AF C12 Glucose C8 Glucose
C12
B. cereus 204 0 6.82 0 0
Cl. s oro eves Cam 3.90 11.30 0 +/- (3.50)
den
L. monocyto eyes S23 0 0 0 0
Lb. sake A10 0 0 0 0
Br, thermos hacta CRA78830 7.50 0 0
Micrococcus luteus 0 8.95 0 0
E. coli S 15 0 0 0 0
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
26
Ps. uorescens 327 0 0 0 0
S. cerevisiae ATCC 0 +/- (10.000 0
9763
S. carlsber ensis 0 0 0 0
CRA6413
Results of Bioscreen run BS191200
Table 3
Strain Level Final
tested -
min
OD
for
18
or
24
h
rowth
at
30
C
(Control: % (wt/vol)AFC8 AFC1 GC8 GC12 Control:
average 2 Equivalent
OD without Methanol level
methanol)
B. cereus 0.05 0 0 0.242 0 0.194
204
(0.80) 0.025* 0.168 0 0.785 0.561 0.619
B. cereus 0.05 0 0 0.882 0.437 0.75
Carapden (0.72)0.025* 0.8 0 0.777 0.641 0.707
L. 0.05 0 0 0.242 0.19 0.27
nZOnocytogenes0.025 0.027 0 0.514 0.379 0.537
S23
0.66
Lb. sake A10 0.05 0 0 0.245 0.392 0.123
(0.90) 0.025 0.535 0.376 0.81 0.785 0.477
E. coli S15 0.05 0,573 0.478 0.654 0.745 0.716
(0.95) 0.025 0.753 0.703 0.818 0.875 0.851
E. coli CRA1090.05 0 0 0.285 0.149 0.187
(0.86) 0.025 0.697 0.738 0.789 0.759 0.84
Ps. fluorescens0.05 0 0 0 0 0
_
3756 0.025 0.068 0.192 0.82 0.953 0.973
1.2
Ps. , fluorescens0.05 0 0 0 0 0
327 0.025 0.12 0.178 0.21 0.354 0.219
(0.37
*AF C12 showed total inhibition of Bc204 and Bc Carapden at a minimum level
tested of
0.0125%.
Controls for this run were based on the final - OD for 18 or 24 h growth at 30
°C for
growth in equivalent methanol levels. Inhibition by the AF esters was judged
by whether
I O the number was lower than the number derived for the methanol control.
CONCLUSIONS
~ The well diffusion results showed that 0.5% AFC8 and AFC12 both had anti-
clostridial activity, and AFC 12 had activity against Bacillus, Brochothrix,
1 S Micrococcus and perhaps yeasts, but not L. monocytogenes, or gram
negatives (GN).
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
27
~ Bioscreen results were from tests with 0.05% samples.
~ Bioscreen confirmed the order of activity was as follows: AFC12 > AFCB.
Bioscreen
also confirmed activity against Bacillus, but activity was also observed
against L.
monocytogenes, and Lb sake, as well as some activity against gram negatives
(GN).
Description of Bioscreen analysis: BS040101
0.3% AF ester was made up in 2.5% methanol. Serial dilutions were made. The
following
concentrations were tested: 0.3, 0.15, 0.075, 0.038 and 0%. APP was made up in
water.
The samples were analysed after 24 h at 30 °C (Table 4).
Table 4
Strain AF ester C8 AF ester C12
Bc 204 Total inhibition Total inhibition at
at 0.15% 0.038%
Inhibition to 0.075%
Lm 523 Total inhibition Total inhibition at
at 0.15% 0.038%
Inhibition to 0.038%
Lbs A 10 Total inhibition Total inhibition at
at 0.3 % 0.15%
Inhibition to 0.15% Inhibition to 0.038%
Ec 515 No inhibition at No inhibition at 0.3%
0.3%
Psf 3756 Inhibition to 0.3% Inhibition to 0.3%
Viable counts from BS040101
Inhibition was judged by whether the final count in the presence of either AF
ester was
lower than the final count in 2.5 % methanol (control). The results are shown
in Table 5.
Table 5
Final viable
count after
24 h (cfu/ml)
Strain 0.3% AFE C8 0.3% AFE 2.5% methanol
C12 control
Bc204 1x10 1.5x10 1x10
LmS23 2.5x10 1.5x10 2.9x10
Lbs A10 < 10 < 10 3.7 x10
EcSlS 3.5x10 1.7x10 2.3x10
Psf 3756 1.7 x 10 2.7 x 10 3.2 x 10
Sce 9763 9.4 x 10 7.4 x 10 4.5 x 10
Sca 6413 3.3 x 10 nd 1.4 x 10'"
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
28
Well diffusion testing
The results for M. luteus, B. tarsus 204, B. tarsus Carapden, Cl. sporogenes
1.221, and
Cl. sporogenes Carapden are illustrated in Figures 1 and 2 and Table 6. None
of the
methanol control tests gave any diffusion zones. Code for the wells: 1 = 3%
AFECB, 2 =
0.3% AFECB, 3 = 3% AFEC12, 4 = 0.3% AFEC12, 5 = equivalent methanol control at
25% methanol, 6 = equivalent methanol control at 2.5% methanol.
Table 6
Test strain Well diffusion
zone
(mm)
tested
against
0.3%
and 3%
(wt/vol)
extracts
AFE C8 AFE C8 AFE C12 AFE C12
3% 0.3% 3% 0.3%
B. tarsus 204 2.29 0 7.75 1.27
B tarsus Cam den < 0.5 0 3.23 < 0.5
Cl s oro eves 1.221 6.00 0 16.83 5.06
Cl. s oro eves Cam 6.15 5.05 14.15 6.15
den
L. monocyto eves S23 1.62 0 2.26 +/-
L. monocyto eves 272 2.36 0 +/- 8.82 0
Lb. sake A10 +/- 0 +/- (2.8)+/-
Br. thermos hacta 0.98 0 7.55 +/-
CR.A7883
Micrococcus luteus 3.95 0 9.42 2.33
E. coli S15 0 0 0 0
Ps. uorescens 327 0 0 0 0
Ps fluorescens 3756 0 0 0 0
S. cerevisiae ATCC E E + E
9763
S carlsber ensis CRA6413E E + E
CODE (for yeasts): E = enhanced growth; + = zone of inhibition observed.
Further testing of APP
Well diffusion zones at 3% vs CL sporogenes Carapden (4.72) and Br.
thermosphacta
7883 (2.96)
CA 02423139 2003-03-24
WO 02/26061 PCT/GBO1/04330
29
Viable counts of BS040101
Table 7
Strain Viable count
in 0.3 %
APP
Bc 204 7.0 x 10
Lm S23 5.4 x 10
Lbs A10 1.9 x 10
EcSlS 6.2x10
Psf 3756 < 10
Sce 9763 3.1 x 10
Sca 1.1 x 10
Various modifications and variations of the described methods and system of
the
invention will be apparent to those skilled in the art without departing from
the scope and
spirit of the invention. Modifications of the described modes for carrying out
the
invention which are obvious to those skilled in the relevant are, or related
fields, are thus
intended to fall within the scope of the following claims.