Note: Descriptions are shown in the official language in which they were submitted.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
HIGH POWER NICKEL-METAL HYDRIDE BATTERIES AND
HIGH POWER ALLOYS/ELECTRODES FOR USE THEREIN
Field of the Invention
The instant invention relates generally to nickel-metal hydride batteries and
more specifically to high power nickel-metal hydride batteries. The batteries
include
negative electrodes Which employ electrochemical hydrogen storage alloys with
enhanced high discharge rate capacities, thereby increasing the specific power
and
high rate capabilities of the batteries. The negative electrode
electrochemical
hydrogen storage alloys include means to dramatically alter the discharge
capacity
thereof. As will be discussed hereinbelow in the Detailed Description of the
invention, these means include tailoring the local chemical and structural
order of
the materials by adding transition metals having d orbitals. This alteration
results
in discharge capacity curves for non-misch metal hydrogen storage alloys which
are
provide high capacity even at high discharge rates. Because the instant
batteries
can provide both high energy density and high power, they are uniquely suited
for
application in new uses and areas to which batteries were previously not
apropos.
A specific example of such means is Pd which increases the electrochemical
hydrogen storage capacity at high discharge rates of high capacity non-misch
metal
alloys (misch metal alloys have inherently low hydrogen storage capacity.)
Virtually
the same results can be achieved by the proper balance of non-noble transition
metals. These alloys have great advantages over prior art alloys which makes
them
particularly useful for electric vehicles, hybrid electric vehicles and have
great utility
for power tools and other high drain rate applications even ultracapacitors.
Background of the Invention
Presently batteries which can deliver high power and yet be small and light
weight (i.e. have a high specific power) are in high demand. These types of
batteries are useful in application such as electric vehicle and hybrid
electric vehicle
propulsion for which lack of range has been a serious limitation. Also, in the
electric
vehicle industry, the high power capabilities allow for utilization of
regenerative
breaking to replenish the charge of the batteries. These high power batteries
are
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
2
also useful for other high drain rate applications such as power tools, as
starter
batteries for internal combustion engines and, because of the high
conductivity of
the metallic negative electrodes, as a replacement for power sources such as
ultracapacitors.
Advanced automotive battery development for vehicle propulsion has, in the
past, been directed primarily at the requirement of a true electric vehicle.
Utilizing
Ovshinsky's principles of disorder, local order, and chemical modification,
the
hallmark Energy Conversion Devices, Inc. ("ECD"), battery development teams at
ECD and Ovonic Battery Company ("OBC") have made great advances in nickel
metal hydride battery technology.
Initially Ovshinsky and his teams focused on metal hydride alloys that form
the negative electrode. As a result of their efforts, they were able to
greatly
increase the reversible hydrogen storage characteristics required for
efficient and
economical battery applications, and produce batteries capable of high density
energy storage, efficient reversibility, high electrical efficiency, efficient
bulk
hydrogen storage without structural changes or poisoning, long cycle fife, and
repeated deep discharge. The improved characteristics of these "Ovonic"
alloys,
as they are not called, results from tailoring the local chemical order and
hence the
local structural order by the incorporation of selected modifier element into
a host
matrix, as well as the use of non-equilibrium processing. Disordered metal
hydride
alloys have a substantially increased density of catalytically active sites
and storage
sites compared to single or ordered multi-phase crystalline materials. These
additional sites are responsible for improved efficiency of electrochemical
charging/discharging and an increase in electrical energy storage capacity.
The
nature and number of storage sites can even be designed independently of the
catalytically active sites which can themselves be increased no only by
individual
atoms but also be topology and chemistry. More specifically, these alloys are
tailored to allow bulk storage of the dissociated hydrogen atoms at binding
strengths
within the range of reversibility suitable for use in secondary battery
applications.
3o A complete description of the role of disorder in electrochemical alloys is
found in
U.S. Patent No. 4,623,597, the disclosure of which is incorporated herein by
reference.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
3
Some extremely efficient electrochemical hydrogen storage materials were
formulated, based on the disordered materials described above. These materials
reversibly form hydrides in order to storage hydrogen. The materials are
multiphase
materials, which may contain, but are not limited to, one or more phases with
C~4
and C,5 type crystal structures.
In contrast to the Ovonic alloys, the older alloys were "ordered" materials
that
had homogeneous chemistry, a uniform microstructure, and generally poor
electrochemical characteristics. In the early 1980's, as the degree of
modification
increased (that is as the number and amount of elemental modifiers increased),
their performance began to improve. Unbeknownst to the artisans of that era,
who
did all that was possible to keep the electrode materials uniform, the
improvement
in electrochemical performance was due as much to the compositional disorder
contributed by the modifiers as it was to the electrical and chemical
properties of the
electrode alloys.
Simply stated, in all metal-hydride alloys, as the degree of modification
increases, the role of the initially ordered base alloy is of minor importance
compared to the properties and disorder attributable to the particular
modifiers. In
addition, analysis of the present multiple component alloys available on the
market
and produced by a variety of manufacturers indicates that these alloys are
modified
following the guidelines established for Ovonic alloy systems. Thus, as stated
above, all highly modified alloys are disordered materials characterized by
multiple
components and multiple phases, i.e. Ovonic alloys.
As a result of this development of the negative electrode active materials,
the
Ovonic Nickel Metal Hydride (Ni-MH) battery has reached an advanced stage of
development for EVs. Ovshinksy's teams have been able to produce electric
vehicle batteries which are capable of propelling an electric vehicle to over
350
miles on a. single charge (Tour d' Sol 1996). the Ovonic Ni-MH battery has
demonstrated excellent energy density (up to about 90 Wh/Kg), long cycle life
(over
1000 cycles at 80% DOD), abuse tolerance, and rapid recharge capability (up to
60% in 15 minutes). Additionally, the Ovonic battery has demonstrated higher
power density than any other battery technology under test and evaluation for
use
as an EV stored energy source.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
4
While Ovshinsky and his teams have made great advances in batteries for
true electric vehicles, the Partnership for a New Generation of Vehicles
(PNGV), a
U.S. government-auto industry partnership initiated in 1996, has suggested
that
hybrid-electric vehicles (HEV's) could be the leading candidate to meet their
goals
of tripling auto fuel economy in the next decade. To realize this goal,
lightweight,
compact, high-power batteries would be required.
The use of a hybrid drive system offers critical advantages for both fuel
economy and ultra-low emissions. Fuel engines achieve maximum efficiency when
operating at constant rpm. Therefore, peak fuel efficiency can be achieved by
employing a constant rpm fuel engine to provide energy to a high-power energy
storage system that supplies peak power for acceleration and also recaptures
kinetic energy through the use of regenerative braking.
Similarly, the ability to use a small engine operating at maximum efficiency
and coupled with a pulse power energy storage system offers the best design
for
minimizing emissions associated with the use of a fuel engine. Therefore, a
key
enabling technology for HEV's is an energy storage system capable of providing
very high pulse power and accepting high regenerative braking currents are
very
high efficiency. The duty cycle of a pulse power application requires
exceptional
cycle life at low depths of discharge.
It is important to understand the different requirements for this energy
storage system compared to those for a pure electric vehicle. Range is the
critical
factor for a practical EV, making capacity and discharge rate (power) critical
evaluation parameters. Particularly in the HEV pulse power application, power
density is the overwhelming consideration. Excellent cycle life under low
depth
discharge is also critical. Energy density is important to minimize battery
weight and
space, but due to the smaller battery size this characteristic is less
critical than
power density. Ability for rapid recharge is also essential to allow efficient
regenerative braking, and charge/discharge efficiency is critical to maintain
battery
state of charge in the absence of external charging.
~ Given the fundamental differences in requirements between the EV and
those for an HEV application, those batteries currently optimized for use in
EV
applications will not be suitable for HEV without an increase in power
density.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
While the demonstrated performance of Ovonic EV batteries has been impressive,
these cell and battery designs have been optimized for use in pure EVs and
therefore do not meet the specific requirements for HEVs. Therefore, there is
a
need for high power batteries that have the peak power performance required by
5 HEVs coupled with the already demonstrated performance characteristics and
proven manufacturabifity of the Ovonic Ni-MH batteries.
Previously Ovshinsky, et al. have provided nickel-metal hydride batteries and
electrodes capable of increased power output and recharge rates that provide
sufficient power for EV and HEV applications by providing nickel-metal hydride
batteries having negative electrodes which were formed on porous metal
substrates
formed from copper, copper-plated nickel, or a copper-nickel alloy. These
highly
conductive substrates helped to improve the high power characteristics of the
nickel-metal hydride batteries.
However, once conductivity of the substrate was improved, it became
apparent that some of the negative alloy materials lacked the same high power
capabilities, particularly with regard to high capacity at high rate of
discharge. That
is, it was noticed that the alloys which contained higher amounts of hydride
forming
elements, such as Cr, Ti and Zr, and lower amounts of Ni had reduced
electrochemical hydrogen storage capacity at high discharge rates.
Relative thereto, U.S. Patent No. 4,699,856, to Heuts et a1. discloses misch
metal-nickel hydrogen storage alloys into which Ni, Pd, Pt, Ir, and/or Rh was
added
to improve the low temperature rate performance of these misch metal type
alloys.
However, there is no teaching or suggestion to use a catalyst of any type in
Ti-Zr-Ni
type alloys to improve the room temperature high discharge rate capacity
thereof.
This is particularly true because transition metal based alloys can inherently
store
a far greater amount of hydrogen than can their rare earth (misch metal)
counterparts. Therefore, the misch metal alloys could not afford to lose any
of their
hydrogen storage capacity. However, there remains a need in the art to develop
(transition metal) Ti-Zr-Ni negative alloy materials which retain their high
hydrogen
3o storage capacity, at even higher discharge rates.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
6
Summary of the Invention
One object of the instant invention is an electrochemical hydrogen storage
alloy including an effective amount of a catalytic transition metal to
substantially
increase the discharge capacity of the alloy at high discharge rates. This can
be
realized by an electrochemical hydrogen storage alloy comprising nickel,
titanium,
zirconium, vanadium and an effective amount of palladium to substantially
increase
the discharge capacity of the alloy at high discharge rates. An effective
amount of
palladium increases the discharge capacity of the alloy by at least 20% at a
discharge of rate of C over the same alloy without palladium.. The effective
amount
of palladium is generally from about 0.1 to 4 atomic percent of the alloy and
more
preferably 1 to 4 atomic percent. The alloys typically comprise, in atomic
percent,
0.1 to 60% Ti, 0.1 to 40% Zr, 0 to 60% V, 0.1 to 57% Ni, 0 to 56% Cr, and 0 to
20%
Mn and 0.1 to 4% Pd. Preferably the Ti, Zr, V, Ni, Cr, and Mn total at least
about
80 atomic percent of said alloy.
A second object of the instant invention is to achieve similar results without
the use of palladium by the proper combination of non-nobel metal transition
elements. Such a result has been achieved by an alloy containing in atomic
percentage, 15-19% Zr, 14-18% Ti, 8-12% V, 6-10% Cr, 16-20%~ Mn, and 28-33%
Ni.
2o Other objects of the present invention include negative electrodes formed
with the alloys and nickel-metal hydride batteries formed with the negative
electrodes.
Brief Description of the Drawing
The sole figure is a plot of discharge rate versus discharge capacity for
prior
art alloys and alloys of the instant invention.
Detailed Description of the Invention
Deficiencies of the basic prior art alloy materials are overcome by greatly
improving and expanding in a unique and fundamental way, both qualitatively
and
quantitatively, the characteristics of the hydrogen storage electrode by
providing
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
7
materials which are tailor-made to greatly increase the reversible hydrogen
storage
and release capacity at high discharge rates, a characteristic which is
required for
efficient and economical battery applications in high discharge rate
utilities.
The materials of the present invention have a greatly increased number of
catalytically active sites and a high number of storage sites when compared to
single phase crystalline materials and other prior art materials, which
improves the
electrochemical charging/discharging efficiencies and rates and provides for a
high
electrical energy storage capacity. The materials are tailored to allow bulk
storage
of the dissociated hydrogen atoms at binding strengths within the range of
reversibility suitable for use in secondary battery applications. Tailoring of
the local
structural and chemical environment of the stored hydrogen in the materials of
the
present invention is of great importance to achieve the desired
characteristics.
The improved characteristics of the anodes of the present invention are
accomplished by manipulating the local chemical environment and hence the
local
structural environment by the incorporation of selected modifier elements to
create
a desired material. The material has the desired electronic configurations
which
result in a large number of active catalytic and storage sites. The nature and
number of storage sites can be designed independently from the catalytically
active
sites. The desired material can be amorphous, polycrystalline (but lacking
long
range compositional order), or microcrystalline in structure or an intimate
mixture
of any combination of those phases. The ability to have a large number of
sites and
to simultaneously control the type of the active sites (i.e. catalytic or
storage) is also
unique to the anodes of the present invention. That is, the nature of the
sites is as
important as the number of sites.
The framework for the active battery materials of the present invention is a
host matrix of one or more elements. The host elements are chosen in general
to
be hydride formers and can be lightweight elements. The host matrix element or
elements are modified by incorporating selected modifier elements, which may
or
may not be hydride formers. The modifiers also can be lightweight elements and
enhance the disorder of the materials, thus creating a greater number and
spectrum
of catafytically active and hydrogen storage sites. Multi-orbital modifiers,
for
example transition elements, provide a greatly increased number of storage
sites
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
due to various binding configurations available, thus resulting in an increase
in
energy density. The technique of modification to provide a non-equilibrium
material
having a high degree of disorder provides unique binding configurations,
orbital
overlap and hence a spectrum of binding sites. Due to the different degrees of
orbital overlap and the disordered structure, an insignificant amount of
structural
rearrangement occurs during charge/discharge cycles or rest periods
therebetween
resulting in long cycle and shelf life.
By appropriate selection of these elements and the relative amounts of the
selected elements, or by utilizing additional elements, the structural
configuration
1o may be selected or designed to provide the electronic/structural
configurations with
desirable electrochemical features. Thus, the materials may be chemically and
electronically tailored.
The hydrogen storage and other characteristics of the disordered materials
of the present invention can be controllably altered depending on the selected
host
matrix and modifier elements and their relative percentages to allow the
tailor-
making of the anode materials. The anodes are resistant to degradation by
poisoning due to the increased number of selectively designed storage and
catalytically active sites which also contribute to the long cycle life. Also,
some of
the sites designed into the material can bond with and deactivate poisonous
species
without effecting the active hydrogen sites. The materials thus formed have a
very
low self discharge and hence good shelf life.
The improved battery includes electrode materials having tailor-made local
chemical environments which are designed to yield high electrochemical
capacity
at high charging and discharging rates. The manipulation of the local chemical
environments of the materials is made possible by utilization of a chemically
modified alloy to create a greatly increased density of catalytically active
sites for
hydrogen dissociation and also of hydrogen storage sites.
The modifier material added to the hydrogen storage material may provide
a wide spectrum of binding, as for example, including covalent bonding, dative
or
coordinate bonding, interaction of the orbitals of the modifier material with
the alloy
or the like, and various combinations thereof. When the modifier material is
added,
it sees many different sites therein, including, among others, nearest
neighbor
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
9
relationships, element spacings, bond angles and strengths, charged and
localized
states in the spectrum of binding energies, microvoids, dangling bonds, one
pairs,
and the like. Dangling bonds and other such defect states can grab hydrogen
and
form a chemical bond which may not be easily reversible. The modifier material
can
seek out these sites and readily modify the electronic configuration of the
material
by forming desired electronic states in the energy spectrum of binding
energies
thereof (and which can also modify the localized states in the energy spectrum
of
binding energies) thereby reducing the chances of unwanted bonds with the
stored
hydrogen.
The addition of these modifiers primarily affects the localized states or the
electrically active centers in the energy spectrum of binding energies and the
electrical activation energy of the electronic configurations to a substantial
degree.
In this connection, the modifier interacts with the material to form
electronic states
or electrically active centers and much of this involves the interaction of
the orbitals
of the modifier with the material. Thus, the addition of the modifier can have
a
spectrum of effects from subtle to drastic upon the electronic configurations
of the
material.
A solid material can have a wide spectrum of localized states, including
bonding and nonbonding states, which are herein referred to as deviant or
defect
electronic configurations and which have an effect upon material. Such defect
electronic configurations can include substitutional impurities and vacancies,
interstitials, dislocations, and so forth, which occur principally in
crystalline solids
because of periodic restraints therein. In solid amorphous materials, three-
dimensional orbital relationships can occur which are generally prohibited in
crystalline materials by reason of the periodic constraints in the latter.
Other defect
electronic configurations, particularly in amorphous materials can include
microvoids and dangling bonds, dangling bond and nearest neighbor
interactions,
lone pairs, lone-pair/lone-pair interactions, lone pair and nearest neighbor
interactions, valance alternation pairs, dative or coordinate bonds, charge
compensation, polyvalency, lone-pair compensation, hybridization, three-center
bonding, pi bonding, and others, all of which operate toward affecting the
materials.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
The orbitals of the modifier material interact with the localized states or
electrically active centers in the material to modify the electronic
configurations
thereof. The transition metals having d orbitrals provide particularly fine
results
when used as the modifier material. The transition metal elements including,
for
5 example, nickel, tungsten, molybdenum, iron, vanadium, rhodium, zinc or
copper,
having d orbitals, and the rare earth elements having d and f orbitals may be
readily
added to the materials. The transition metals having d orbitals, which at
least
atomically are not full, are generally preferred since such d orbitals thereof
have a
greater spectrum of interaction possibilities with the material than the
elements
10 having sp orbitals. The lone-pairs and their interactions with their
nearest
neighbors form orbitals or defect electronic configurations and the added
modifier
material with the same in the material to alter the same and to form
electronic states
or electrically active centers therein, and much of this involves the
interaction of the
orbitals of the modifier with such defect electronic configurations.
Engineering the
degree of localization provides a storage site for the hydrogen ion in which
it is most
comfortable.
The electrode materials of the present invention are designed to have
unusual electronic configurations, which result from the varying 3-dimensional
interactions of constituent atoms and their various orbitals. The disorder
comes
from compositional, positional and translational relationships of atoms that
are not
limited by crystalline symmetry in their freedom to interact. Selected
elements can
be utilized to further modify the disorder by their interaction with these
orbitals so
as to create the desired local chemical environments. These various
configurations
generate both a large number of catalytically active sites and hydrogen
storage
sites not only on the surface but throughout the bulk of the material. The
Internal
topology that is generated by these configurations also allows for selective
diffusion
of atoms and ions. The invention that we described makes these materials ideal
for
the specified use since one can independently control the type and number of
catalytically active and storage sites. All of the aforementioned properties
make not
only an important quantitative difference, but qualitatively change the
materials so
that, as shown by the results, unique new materials ensue.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
11
The superior battery of the invention has attained high density energy
stgorage, efficient reversibility, high electrical efficiency, bulk hydrogen
storage
without structural change or poisoning and hence long cycle life, deep
discharge
capability and a high rate capacity capability. This combination of battery
attributes
is unique to the present invention.
The materials of the present invention all have specially designed local order
far different than the highly ordered crystalline structures which provide the
single
phase materials such as used for many of the anodes of the prior art. The
types of
disordered structures which provide the local structural chemical environments
for
improved rate characteristics in accordance with the present invention include
multicomponent polycrystalline materials lacking long range compositional
order,
microcrystalline materials, amorphous materials having one or more phases of
multiphase materials containing both amorphous and crystalline phases or
mixtures
thereof.
An advantage of employing these materials is that with such materials
storage sites can be distributed throughout the bulk of the material. Also,
the
materials can be designed to have the desired porosity, which can further
increase
the storage capacity and charge/discharge rate. Two necessary considerations
for
atomic engineering of hydrogen storage sites are large spaces and regions of
low
electron density within the material. In a crystalline structure the storage
sites are
limited to a relatively few accidental occurring irregularities appearing on
the
surfaces of the material. In the instant material the locations of storage
sites are not
limited to just the surfaces of the material. In contrast to crystalline
structures, the
materials of the present invention have storage sites distributed throughout
the bulk
of the material. They provide a substantially increased surface area which
does not
depend merely on the presence of cracks, voids and grain boundaries. The
materials of the present invention have a greatly increased density of storage
and
catalytically active sites which provide a significant improvement of hydrogen
absorption and desorption in both amount of hydrogen stored and the efficiency
of
storage during charging. The catalytically active sites reduce the charging
and
discharging overvoltage and hence substantially the entire energy utilized
during
charging efficiently results in hydrogen stored in the bulk of the material.
The
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
12
density of storage sites is a major factor in enabling relatively high
hydrogen storage
capacity for electrochemical charging and discharging, opening up new usages
previously unavailable to electrochemical storage batteries.
An objective of the present invention is to improve the high power output from
a nickel-metal hydride (Ni-MH) rechargeable battery without sacrificing
storage
capacity. The instant invention accomplishes this by increasing the specific
capacity of the negative electrode alloy at high discharge rates. This can be
accomplished by adding palladium to the negative alloy. The palladium
contributes
catalytic sites to the material. Having these catalytic sites close to the
storage sites
facilitates the high rate capabilities of the material.
Generally, a Ni-MH battery includes at least one negative electrode and at
least one positive electrode. An electrode tab may be attached to each of the
negative and positive electrodes in order to electrically connect each of the
electrodes to the appropriate terminal of the Ni-MH battery (i.e., negative
electrode
to negative terminal and positive electrode to positive terminal).
Ni-MH batteries employ a negative electrode having an active material that
is capable of the reversible electrochemical storage of hydrogen. The negative
electrode also includes a porous metal substrate which holds the active
material.
The negative electrode may be formed by pressing the active material (in
powdered
form) into the porous metal substrate. After the powdered active material is
pressed
into the porous metal substrate, the negative electrode may be sintered.
Upon application of an electrical potential across a Ni-MH battery, the active
negative electrode material is charged by the electrochemical absorption of
hydrogen and the electrochemical generation of hydroxyl ions. The
electrochemical
reaction at the negative electrode is as follows:
charge
M + H20 + e- ~--~ M_H + OH-
Discharge
The negative electrode reactions are reversible. Upon discharge, the stored
hydrogen is released to form a water molecule and evolve an electron.
The active material of the negative electrode is a hydrogen storage material.
The hydrogen storage material can be any Ti-V-Ni based active material to
which
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
13
Pd has been added to increase the high rate capacity of the material. There
are Ti-
V-Zr-Ni alloys which may also be modified with palladium as taught by the
instant
invention and used for the hydrogen storage alloy of the negative electrode.
One
family of materials are those described in U.S. Patent No. 4,728,586 ("the
'586
Patent"), the disclosure of which is incorporated by reference. The '586
Patent
discloses a specific sub-class of these Ti-V-Ni-Zr alloys comprising Ti, V,
Zr, Ni, and
a fifth component, Cr. The '586 Patent mentions the possibility of additives
and
modifiers beyond the Ti, V, Zr, Ni, and Cr components of the alloys, and
generally
discusses specific additives and modifiers, the amounts and interactions of
the
modifiers, and the particular benefits that could be expected from them.
In general, the alloys of the instant invention comprise, in atomic percent,
0.1
to 60% Ti, 0.1 to 40% Zr, 0 to 60% V, 0.1 to 57% Cr, and 0 to 20% Mn along
with
an effective amount palladium. The palladium is sufficient to significantly
increase
the discharge capacity of the alloy at high discharge rates. Significantly as
used
herein will refer to at least a 20% increase in the discharge capacity at a
discharge
rate of C over the same alloy with no palladium addition. Preferably, the Ti,
Zr, V,
Ni, Cr, and Mn total at least about 80 atomic percent of the alloy. The
palladium
ranges from about 0.1 to 4 atomic percent. Most preferably the palladium
ranges
from 1 to 4 atomic percent.
In another, separate, embodiment the instant inventors have developed an
alloy which has similar high rate capacities to those containing palladium,
but which
contains no noble metals. The alloy in it's broadest terms comprises in atomic
percentage, 15-19% Zr, 14-18% Ti, 8-12% V, 6-10% Cr, 16-20°I°
Mn, and 28-33%
Ni. A specific example of this alloy has been designated as Zr439 and has a
nominal composition of 17% Zr, 16.5% Ti, 10% V, 8% Cr, 18% Mn, and 30.5% Ni.
Table 1 shows the compositions and high rate capacities of negative
electrode alloys of the instant invention which contain palladium and
comparitive
examples. As can be seen, the comparative alloys which do not contain
palladium
have very poor high rate capacities. As the palladium content increases beyond
about 0.1 atomic percent, the capacity improves, up to about 4 atomic percent.
Beyond 4 atomic percent, no increased effect is observed.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
14
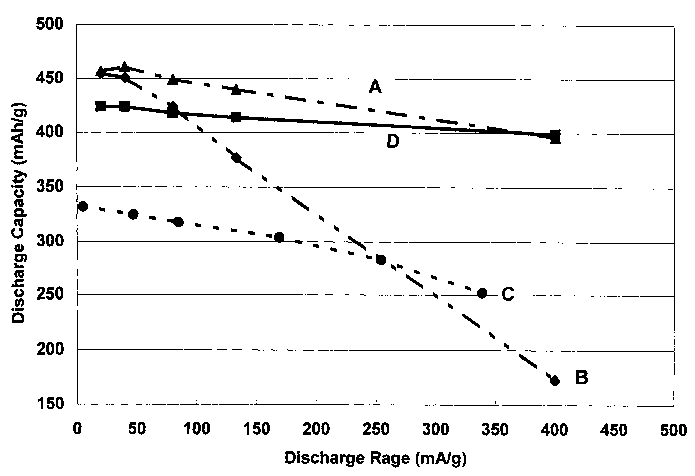
The effect of palladium, and the capabilities of the inventive alloy which
does
not contain palladium, can be seen in Figure 1, which is a plot of discharge
rate
versus discharge capacity for various comparative alloys and an alloys of the
instant
invention. The discharge rate verses discharge capacity of Alloy Zr212 of
Table 1
is shown as curve A (symbol 1) and the discharge rate verses discharge
capacity
of Alloy Zr151 (which is exactly the same as Zr212 but lacks the palladium of
the
instant invention) of Table 1 is shown as curve B (symbol ~). It is clear from
a
perusal of curves A and B that the capacity of the alloy without palladium
drops off
significantly as the rate of discharge is increased and by the time a 1 C
discharge
rate is achieved, the capacity of Zr151 alloy has dropped more than 75% from
it's
low rate capacity, while the alloy of the present invention having a palladium
content
of about 1 atomic percent suffers only a 10 percent loss. Incredibly this
palladium
modified material provides about 400 mAh/g at a discharge rate of C.
In further comparison, misch metal-nickel alloys, due to their large nickel
contents, suffer much less of a drop in capacity that Ti-Zr-NI type alloys.
For
example, the discharge rate versus discharge capacity for a commercially
available
misch metal-nickel electrochemical hydrogen storage alloys one is plotted as C
(symbol ~). The commercial misch metal alloy (curve C), has a composition of
(in
atomic %) La 10.5%, Ce 4.3%, Pr 0.5%, Nd 1.4%, Ni 60%, Co 12.7%, Mn 5.9%, and
AI 4.7%. Clearly, the low discharge rate capacity is much lower than the low
rate
capacity of Ti-Zr-Ni alloys, but it can also be seen that the drop in capacity
for the
misch metal-nickel alloy is much less than the drop for Ti-Zr-Ni type alloys.
Therefore, there is much less of a need in the misch metal-nickel alloys to
use
palladium to increase the high rate capabilities of these alloys.
Finally, the alternative embodiment of the instant invention, Zr439, without
palladium, is plotted in Figure 1 as curve D (symbol 1). As can be seen, the
instant
embodiment has a low rate capacity nearly that of the alloys with palladium
(i.e.
Zr212), and a very small loss is capacity such that at high rates, the
capacity of the
alloy is nearly identical with Zr212 containing palladium.
The negative electrode may be formed by pressing active hydrogen storage
material into a porous metal substrate. The conductivity of the negative
electrode
can be increased by increasing the conductivity of the negative electrode's
porous
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
metal substrate. Generally, the porous metal substrate includes, but is not
limited
to, mesh, grid, matte, foil, foam, plate, and expanded metal. Preferably, the
porous
metal substrate used for the negative electrode is a mesh, grid, or expanded
metal.
The porous metal substrate may be formed from nickel, copper, copper-plated
5 nickel, or a copper-nickel alloy. As used herein, "copper" refers to either
pure
copper or an alloy of copper, and "nickel" refers to either pure nickel or an
alloy of
nickel.
At the operating conditions of the metal hydride negative electrode, a copper
substrate material is protected from corrosion. However, to increase battery
10 reliability and further protect the negative electrode from the harsh
chemical
environment within the battery, the porous metal substrate formed from the
aforementioned materials of copper, copper-plated nickel, or a copper-nickel
alloy
may still be additionally plated with a material which is electrically
conductive yet
resistant to corrosion in the battery environment. An example of a material
that can
15 be used to plate the porous metal substrate includes, but is not limited
to, nickel.
Using copper to form the porous metal substrate of the negative electrode
has several important advantages. Copper is an excellent electrical conductor.
Hence, its use as a substrate material decreases the resistance of the
negative
electrode. This decreases the amount of battery power wasted due to internal
2o dissipation, and thereby provides a Ni-MH battery having increased output
power.
Copper is also a malleable metal. Malleability is very important because of
the expansion and contraction of the negative electrodes during charge and
discharge cycling of a Ni-MH battery. The increased pliability of the
substrate helps
prevent electrode breakage as a result of the expansion and contraction,
thereby
resulting in improved battery reliability.
Increased substrate malleability also allows the substrate to more reliably
hold the active hydrogen storage material that is compressed on the substrate
surface. This lessens the need to sinster the negative electrodes after the
active
material has been compressed onto the substrate, thereby simplifying and
reducing
3o the cost of the electrode manufacturing process.
The conductivity of the negative electrode can also be increased by copper-
plating the negative electrode after the active metal hydride material has
been
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
16
compressed (and possibly sintered) onto the substrate. The copper-plating may
be
patterned or unpatterned. As well as increasing electrode conductivity, the
copper-
plating provides an additional means of ensuring that the active material
remains
adhered to the substrate.
The negative electrode described herein is applicable to all Ni-MH batteries
including, but not limited to, prismatic Ni-MH batteries and cylindrical jelly-
rolled Ni-
MH batteries.
The Ni-MH batteries employ at least one positive electrode having active
material formed from nickel hydroxide. The positive electrode also includes a
porous metal substrate which holds the active material, the positive electrode
may
be formed by pressing the active positive electrode material (in powdered
form) into
a porous metal substrate. One or more electrode tabs may be .attached to the
positive electrode to electrically connect the positive electrode to the
positive
battery terminal.
The reactions that take place at the positive electrode are as follows:
charge
Ni(OH)Z + OH- ~-? Ni00H + H20 + a
Discharge
The nickel hydroxide positive electrode is described in U.S. Patent No.
5,344,728
and 5,348,822 (which describe stabilized disordered positive electrode
materials)
and U.S. Patent No. 5,569,563 and U.S. Patent No. 5,567,549 the disclosures of
which are incorporated by reference.
The conductivity of the positive electrode may be increased by increasing the
conductivity of the positive electrode's porous metal substrate. The porous
metal
substrate of the positive electrode includes, but is not limited to, mesh,
grid, matte,
foil, foam, plate, and expanded metal. Preferably, the porous metal substrate
is
foam. Disclosed herein, is a positive electrode comprising a porous metal
substrate
that is formed from copper, copper-plated nickel, or a copper-nickel alloy.
Forming
the substrate from one or more of these materials increases the conductivity
of the
positive electrodes of the battery. This decreases the amount of power wasted
due
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
17
to internal power dissipation, and thereby increases the power output of the
Ni-MH
battery.
To protect the porous metal substrate of the positive electrode from the harsh
battery environment, the porous metal substrate may be plated with a material
which
is electrically conductive yet resistant to corrosion in the battery
environment.
Preferably, the porous metal substrate may be plated with nickel.
The conductivity of each positive electrode may be increased by increasing
the conductivity of each positive electrode's active material (i.e., the
nickel
hydroxide mixture). This can be done is several ways. One way of making the
active material more conductive is to add nickel-plated copper powder to the
active
material. Another way is to add copper powder to the active material and then
nickel plate the positive electrode to protect the electrode from the harsh
battery
environment. Yet another way of making the active material more conductive is
to
add carbon powder, copper-plated carbon powder, or nickel-plated carbon powder
to the active material.
The positive electrodes disclosed herein, are applicable for all Ni-MH
batteries including, but not limited to, prismatic Ni-MH batteries and
cylindrical jelly-
rolled Ni-MH batteries.
Batteries employing the negative alloy of the instant invention provide high
power. The inventors believe that employing the negative electrode alloy of
the
present invention, state-of-the-art high power batteries could deliver 1000-
2000
Watts/Kg. This specific power, along with high storage capacity, provides
batteries
which rival ultracapacitors, and opens up additional markets for the nickel-
metal
hydride battery, such as starter batteries, hybrid electric vehicles and
ultracapacitors.
While the invention has been described in connection with preferred
embodiments and procedures, it is to be understood that it is not intended to
limit
the invention to the preferred embodiments and procedures. On the contrary, it
is
intended to cover all alternatives, modifications and equivalence which may be
included within the spirit and scope of the invention as defined by the claims
appended hereinafter.
CA 02423529 2003-02-05
WO 02/14567 PCT/USO1/25620
18
Table 1
AI I At. At. At. At. At. At. At. H i g h Rate
oy % % % % % % % ( 1 C )
Number Zr Ti V Cr Mn Ni Pd Ca acit mAH/
Zr212 25.0 8.5 8.0 20.0 13.0 24.5 1.0 398
Zr216 25.0 8.5 12.0 20.0 9.0 23.5 2.0 407
Zr217 25.0 8.5 12.0 20.0 9.0 21.5 4.0 401
Zr232 25.0 8.5 8.0 20.0 13.0 25.0 0.5 341
Zr233 25.0 8.5 8.0 20.0 13.0 25.3 0.2 177
Zr234 25.0 8.5 8.0 20.0 13.0 25.4 0.1 92
Zr151 25.0 8.5 8.0 20.0 13.0 25.3 - 125
*
*Zr151 was made using 99.9% pure elements, therefore it's results are higher
than expected for commercially produced alloys.