Note: Descriptions are shown in the official language in which they were submitted.
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
HARD SURFACE CLEANING AND DISINFECTING COMPOSITIONS
The present invention relates to improvements in cleaning compositions. More
particularly the present invention is directed to improved cleaning
compositions which
find particular use in hard surface cleaning and disinfecting applications.
Background of the Invention
Compositions that both clean and disinfect hard surfaces are known in the art.
These compositions, in many instances, contain quaternary ammonium compounds
as
the active disinfectant, and nonionic surfactants as cleaning agents. Examples
of such
compositions may be found in United States Patent No. 5,454,984 to Graubart ef
al. and
United States Patent No. 5,522,942 to Graubart et al. These compositions are
generally
directed to a combination of quaternary ammonium compounds, a defined mixture
of
non-ionic surfactants, and a glycol ether solvent. In both Graubart et al.
patents, the
number of carbon atoms in those non-ionic surfactants which are ethoxylated
alcohols
range from 10 to 20 carbon atoms, preferably from 11 to 15 carbon atoms in the
alcohol
portion which is combined with the ethylene oxide.
Others have investigated the use of quaternary ammonium compounds with
ethoxylated alcohols of varying carbon chain lengths. For example, United
States
Patent No. 4,109,010 to Sias discloses a range of about 10 to 16 carbon atoms,
but
expresses no preference to the number of carbon atoms. United States Patent
No.
4,065,409 to Flanagan discloses a range of from about 10 to about 20 carbon
atoms,
preferably from about 12 to 16 carbon atoms. United States Patent No.
5,798,329 to
Taylor, although directed to a liquid laundry composition containing
quaternary
ammonium compounds, and not to a hard surface cleaner, discloses non-ionic
surfactants which include alcohol ethoxylates having 8 to 18 carbon atoms in
the alkyl
moiety with 10 to 14 carbon atoms being preferred. United States Patent No.
4,597,887
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
to Colodney is directed to a germicidal hard surface cleaning composition
comprising a
quaternary ammonium salt and an ethyleneoxylated nonionic detergent. The
nonionic
detergents include the condensation products of a higher alkanol containing
about 8 to
18 carbon atoms in a straight- or branched-chain configuration condensed with
about 5
to 30 moles of ethylene oxide. Mentioned therein are preferred examples of
these
detergents such as condensates of C9 -C,~ alkanol with 2.5 moles of ethylene
oxide,
condensates of C~2 -C13 alkanol with 6.5 moles of ethylene oxide and
condensates of Coo
-C~Z alkanol with about 60% by weight of ethylene oxide.
Notwithstanding any advantages which may be disclosed in the known art
formulations, there remains a real and continuing need in the art for improved
cleaning
and disinfecting compositions in general, and in specific such compositions
which
provide good santiziation or disinfection of hard surfaces together with good
cleaning but
at the same time using lower amounts of active ingredients in an aqueous
formulation.
The compositions of the invention are aqueous disinfecting and cleaning
compositions and concentrates thereof which are effective cleaning
compositions and
are efficacious as disinfecting compositions against gram positive and gram
negative
bacteria and are mild to the user of the compositions. That these results are
concurrently
achieved with a composition which includes a cationic surfactant having
germicidal
properties, for example, quaternary ammonium compounds, as its primary
germicidal
active agent is surprising, and indicates a synergistic effect not apparent
from the prior
art. These compositions also provide good cleaning and disinfecting properties
with low
amounts of active constituents, and according to certain preferred embodiments
the
inventive do not include organic solvents such as low molecular weight
alcohols, glycols
or glycol ethers, in significant amounts, i.e., amounts in excess of about 1 %
wt.
Summary of the Invention
The present invention provides an aqueous composition comprising (preferably
consisting essentially of):
at least one cationic surfactant having germicidal properties;
2
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
at least one non-ionic surfactant having from six to eleven carbon atoms in
the
non-polar hydrophobic portion of the surfactant;
optionally, up to about 5% wt. of one or more conventional additives selected
from coloring agents, fragrances and fragrance solubilizers, viscosity
modifying agents,
other non-short chain non-ionic surfactants, other antimicrobial/germicidal
agents, pH
adjusting agents and pH buffers including organic and inorganic salts, non-
aqueous
solvents, .optical brighteners, opacifying agents, hydrotropes, antifoaming
agents,
enzymes, anti-spotting agents, anti-oxidants, and anti-corrosion agents; and,
water to form 100% wt. of said composition.
Preferably, the at least one cationic surfactant having germicidal
properties~is
present in an amount.of from about 0.00.1 to about 10% wt. and the at least
one non-
ionic surfactant having from six to eleven carbon atoms in the non-
polar~hydrophobic
portion of the surfactant is present in an amount of from about 0.01 to about
10% wt.
Preferably, the non-ionic surfactant having from six to eleven carbon atoms in
the non-
polar hydrophobic portion of the surfactant is selected from C6 -C~~ linear
alcohol
ethoxylate having from about 1 to about 6 moles of ethylene oxide.
The aqueous composition of the present invention for use on hard surfaces can
20' be formulated as a ready to use composition which can be used as is by the
consumer
or as a concentrated composition which can be diluted in water by the consumer
prior to
use. In either instance, the composition provides disinfection of the hard
surface to
which it is applied and has good cleaning properties.
In a further aspect of the invention there is provided a process for cleaning
and/or
disinfecting surfaces in need of such treatment which includes contacting a
surface with
the compositions, in either ready to us or concentrate which is later diluted,
as taught
herein.
Brief description of the Drawings
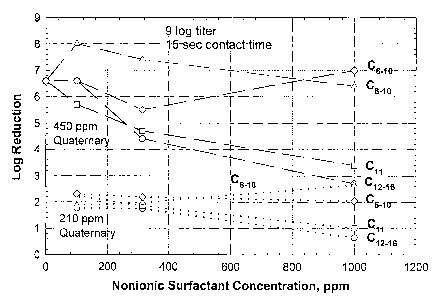
Figure 1 shows the effect of non-ionic surfactants on the efficacy of
benzylalleonium chloride quaternary versus Salmonella choleraesuis at two
concentrations (210 ppm, ~ ~ ~ ~ ~ ; 450 ppm, ---) of quaternary. 0 refers to
a C6_~o
3
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
non-ionic surfactant; O refers to C8_~o non-ionic surfactant; v refers to a
C~~ non-ionic
surfactant; and refers to a C,Z_16 non-ionic surfactant. When referring to a,
for
example, Cg_10 non-ionic surfactant, the C6_,o refers to the number of carbon
atoms in the
non-polar hydrophobic portion of the surfactant.
Detailed Description of the Invention
The present invention provides an aqueous composition comprising (preferably
consisting essentially of):
at least one cationic surfactant having germicidal properties;
at least one non-ionic surfactant having from six to eleven carbon atoms in
the
non-polar hydrophobic portion of the surfactant;
optionally, up to about 5% wt. of one or more conventional additives selected
from coloring agents, fragrances and fragrance solubilizers, viscosity
modifying agents,
other non-short chain non-ionic surfactants, other antimicrobial/germicidal
agents, pH
adjusting agents and pH buffers including organic and inorganic salts, non-
aqueous
solvents, optical brighteners, opacifying agents, hydrotropes, antifoaming
agents,
enzymes, anti-spotting agents, anti-oxidants, and anti-corrosion agents; and,
water to form 100% wt. of said composition.
Preferably, the at least one cationic surfactant having germicidal properties
is
present in an amount of from about 0.001 to about 10% wt. and the at least one
non-
ionic surfactant having from six to eleven carbon atoms in the non-polar
hydrophobic
portion of the surfactant is present in an amount of from about 0.01 to about
10% wt.
Preferably, the non-ionic surfactant having from six to eleven carbon atoms in
the non-
polar hydrophobic portion of the surfactant is selected from Cs -C~, linear
alcohol
ethoxylate having from about 1 to about 6 moles of ethylene oxide.
The aqueous composition of the present invention for use on hard surfaces can
be formulated as a ready to use composition which can be used as is by the
consumer
or as a concentrated composition which can be diluted in water by the consumer
prior to
4
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
use. In either instance, the composition provides disinfection of the hard
surface to
which it is applied and has good cleaning properties.
In a further aspect of the invention there is provided a process for cleaning
and/or
disinfecting surfaces in need of such treatment which includes contacting a
surface with
the compositions, in either ready to us or concentrate which later diluted, as
taught
herein.
The inventive compositions necessarily include at least one cationic
surfactant
having germicidal properties which provides a primary sanitizing benefit to
the
compositions.
Particularly preferred for use as the cationic surfactant which is found to
provide
a broad antibacterial or sanitizing function are well known, and useful
cationic
surfactants may be one or more of those described in, for example,
McCutcheon's
Detergents and Emulsifiers, North American Edition, 2000; Kirk Othmer,
Encyclopedia of
Chemical Technology, 4th Ed., Vol. 23, pp. 478-541, the contents of which are
herein
incorporated by reference.
Examples of preferred cationic surfactant compositions useful in the practice
of
the instant invention are those which provide a germicidal effect to the
concentrate
compositions, and especially preferred are quaternary ammonium compounds and
salts
thereof, which may be characterized by the general structural formula:
1
1
R2- ~ R3
-
Rq,
where at least one of R~, R2, R3 and R4 is an alkyl, aryl or alkylaryl
substituent of from 6
to 26 carbon atoms, and the entire cation portion of the molecule has a
molecular weight
of at least 165. The alkyl substituents may be long-chain alkyl, long-chain
alkoxyaryl,
long-chain alkylaryl, halogen-substituted long-chain alkylaryl, long-chain
5
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
alkylphenoxyalkyl, arylalkyl, etc. The remaining substituents on the nitrogen
atoms other
than the above mentioned alkyl substituents are hydrocarbons usually
containing no
more than 12 carbon atoms. The substituents R~, R2, R3 and R4 may be straight-
chained
or may be branched, but are preferably straight-chained, and may include one
or more
amide, ether or ester linkages. The counterion X may be any salt-forming anion
which
permits water solubility of the quaternary ammonium complex.
Exemplary quaternary ammonium salts within the above description include the
alkyl ammonium halides such as cetyl trimethyl ammonium bromide, alkyl aryl
ammonium halides such as octadecyl dimethyl benzyl ammonium bromide, N-alkyl
pryridinium halides such as N-cetyl pyridinium bromide, and the like. Other
suitable
types of quaternary ammonium salts include those in which the molecule
contains either
amide, ether or ester linkages such as octyl phenoxy ethoxy ethyl dimethyl
benzyl
ammonium chloride, N-(laurylcocoaminoformylmethyl)-pyridinium chloride, and
the like.
Other very effective types of quaternary ammonium compounds which are useful
as
germicides include those in which the hydrophobic radical is characterized by
a
substituted aromatic nucleus as in the case of lauryloxyphenyltrimethyl
ammonium
chloride, cetylaminophenyltrimethyl ammonium methosulfate,
dodecylphenyltrimethyl
ammonium methosulfate, dodecylphenyltrimethyl ammonium chloride, chlorinated
dodecylbenzyltrimethyl ammonium chloride, and the like.
Preferred quaternary ammonium compounds which act as germicides and which
are found to be useful in the practice of the present invention include those
which have
the structural formula:
i H3
CH3
wherein R2 and R3 are the same or different C8_C~2 alkyl, or R2 is C,2_~6
alkyl, C$
~8alkylethoxy, C$_~$alkylphenoxyethoxy and R3 is benzyl, and X is a halide,
for example
chloride, bromide or iodide, or is a methosulfate or saccharinate anion. The
alkyl groups
6
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
recited in R2 and R3 may be straight-chained or branched, but are preferably
substantially linear.
Particularly useful quaternary germicides include compositions which include a
single quaternary compound, as well as mixtures of two or more different
quaternary
compounds. Such useful quaternary compounds are available under the BARDAC~,
BARQUAT~, HYAMINE~, LONZABAC~, BTC~, and ONYXIDE~ trademarks, which are
more fully described in, for example, McCutcheon's Functional Materials (Vol.
2), North
American Edition, 1998, and the respective product literature from the
suppliers
identified below. For example, BARDAC~ 205M is described to be a liquid
containing
alkyl dimethyl benzyl ammonium chloride, octyl decyl dimethyl ammonium
chloride;
didecyl dimethyl ammonium chloride, and dioctyl dimethyl ammonium chloride
(50%
active) (also available as 80% active (BARDAC~ 208M)); described generally in
McCutcheon's as a combination of alkyl dimethyl benzyl ammonium chloride and
dialkyl
dimethyl ammonium chloride); BARDAC~ 2050 is described to be a combination of
octyl
decyl dimethyl ammonium chlorideldidecyl dimethyl ammonium chloride, and
dioctyl
dimethyl ammonium chloride (50% active) (also available as 80% active (BARDAC~
2080)); BARDAC~ 2250 is described to be didecyl dimethyl ammonium chloride
(50%
active); BARDAC~ LF (or BARDAC~ LF-80), described as being based on dioctyl
dimethyl ammonium chloride (BARQUAT~ MB-50, MX-50, OJ-50 (each 50% liquid) and
MB-80 or MX-80 (each 80% liquid) are each described as an alkyl dimethyl
benzyl
ammonium chloride; BARDAC~ 4250 and BARQUAT~ 42502 (each 50% active) or
BARQUAT~ 4280 and BARQUAT~ 42802 (each 80% active) are each described as
alkyl dimethyl benzyl ammonium chloride/alkyl dimethyl ethyl benzyl ammonium
chloride. Also, HYAMINE~ 1622, described as diisobutyl phenoxy ethoxy ethyl
dimethyl
benzyl ammonium chloride (available either as 100% actives or as a 50% actives
solution); HYAMINE~ 3500 (50% actives), described as alkyl dimethyl benzyl
ammonium chloride (also available as 80% active (HYAMIN~~ 3500-80); and
HYAMINE~ 2389 described as being based on methyldodecylbenzyl ammonium
chloride and/or methyldodecylxylene-bis-trimethyl ammonium chloride. (BARDAC~,
BARQUAT~ and HYAMINE~ are presently commercially available from Lonza, Inc.,
Fairlawn, NJ). BTC~ 50 NF (or BTCO 65 NF) is described to be alkyl dimethyl
benzyl
ammonium chloride (50% active); BTC~ 99 is described as didecyl dimethyl
ammonium
chloride (50% active); BTC~ 776 is described to be myristalkonium chloride
(50%
7
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
active); BTC~ 818 is described as being octyl decyl dimethyl ammonium
chloride,
didecyl dimethyl ammonium chloride, and dioctyl dimethyl ammonium chloride
(50%
active) (available also as 80% active (BTC~ 818-80%)); BTC~ 824 and BTC~ 835
are
each described as being of alkyl dimethyl benzyl ammonium chloride (each 50%
active);
BTC~ 885 is described as a combination of BTC~ 835 and BTC~ 818 (50% active)
(available also as 80% active (BTC~ 888)); BTC~ 1010 is described as didecyl
dimethyl
ammonium chloride (50% active) (also available as 80% active (BTC~ 1010-80));
BTC~
2125 (or BTC~ 2125 M) is described as alkyl dimethyl benzyl ammonium chloride
and
alkyl dimethyl ethylbenzyl ammonium chloride (each 50% active) (also available
as 80%
active (BTC~ 2125-80 or BTC~ 2125 M)); BTC~ 2565 is described as alkyl
dimethyl
benzyl ammonium chlorides (50% active) (also available as 80% active (BTC~
2568));
BTC~ 8248 (or BTCO 8358) is described as alkyl dimethyl benzyl ammonium
chloride
(80% active) (also available as 90% active (BTC~ 8249)); ONYXIDE~ 3300 is
described
as n-alkyl dimethyl benzyl ammonium saccharinate (95% active). (BTC~ and
ONYXIDE~ are presently commercially available from Stepan Company, Northfield,
IL).
Polymeric quaternary ammonium salts based on these monomeric structures are
also
considered desirable for the present invention. One example is POLYQUAT~,
described as being a 2-butenyldimethyl ammonium chloride polymer.
The cationic surfactant having germicidal properties may be present in any
effective amount, but generally need not be present in amounts in excess of
about 10%
wt. based on the total weight of the composition. The preferred germicidal
cationic
surfactants) may be present in the liquid disinfectant compositions in amounts
of from
about 0.001 % by weight to up to about 10% by weight, preferably about 0.01 -
8% by
weight, most preferably in amount of between 0.5-6 % by weight. It is
particularly
advantageous that the preferred germicidal cationic surfactants) are present
in amounts
bf at least 200 parts per million (ppm), preferably in amounts of 200 - 700
ppm, more
preferably in amounts of from 200 - 500 ppm.
A further constituent in the compositions of the present invention is a short
chain
non-ionic surfactant. The short chain non-ionic surfactant is one which has
from six to
eleven carbon atoms in the non-polar hydrophobic portion of the surfactant.
8
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Examples of short chain non-ionic surfactants include linear alcohol
ethoxylates.
The linear alcohol ethoxylates which may be employed in the present invention
are
generally the C6 -C~~ straight-chain alcohols which are ethoxylated with from
about 3 to
about 6 moles of ethylene oxide. Their derivation is well known in the art.
Examples
include Alfonic~ 810-4.5, which is described in product literature from Condea
Vista as
having an average molecular weight of 356, an ethylene oxide content of about
4.85
moles (about 60 wt.%), and an HLB of about 12; Alfonic~ 810-2, which is
described in
product literature from Condea Vista as having an average molecular weight of
242, an
ethylene oxide content of about 2.1 moles (about 40 wt.%), and an HLB of about
12; and
Alfonic~ 610-3.5, which is described in product literature from Condea Vista
as having
an average molecular weight of 276, an ethylene oxide content of about 3.1
moles
(about 50 wt.%), and an HLB of 10. Product literature from Condea Vista also
identifies
that the numbers in the alcohol ethoxylate name designate the carbon chain
length
(numbers before the hyphen) and the average moles of ethylene oxide (numbers
after
the hyphen) in the product.
Other examples of ethoxylated alcohols include the Neodol~ 91 series non-ionic
surfactants available from Shell Chemical Company which are described as C9-
C~~
ethoxylated alcohols. The Neodol~ 91 series non-ionic surfactants of interest
include
Neodol 91-2.5, Neodol 91-6, and Neodol 91-8. Neodol 91-2.5 has been described
as
having about 2.5 ethoxy groups per molecule; Neodol 91-6 has been described as
having about 6 ethoxy groups per molecule; and Neodol 91-8 has been described
as
having about 8 ethoxy groups per molecule.
Further examples of ethoxylated alcohols include the Rhodasurf~ DA series non-
ionic surfactants available from Rhodia which are described to be branched
isodecyl
alcohol ethoxylates. Rhodasurf DA-530 has been described as having 4 moles of
ethoxylation and an HLB of 10.5; Rhodasurf DA-630 has been described as having
6
moles of ethoxylation with an HLB of 12.5; and Rhodasurf DA-639 is a 90%
solution of
DA-630.
Further examples of short chain non-ionic surfactants contemplated include Ce-
C,o alkyl polyglycosides which are available under the Glucopon trademark from
Cognis.
9
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
These surfactants include Glucopon 225, 225DK, 225CS, and 225CSUP, described
as
Ca-C,o alkyl polysaccharide ether.
Another example of a short chain non-ionic surfactant includes a C9/C~~-
oxoalcohol polyglycol ether with 7 moles of ethylene oxide, available as
Genapol UD-
079 from Clariant.
The short chain non-ionic surfactant may be present in any effective amount,
but
generally need not be present in amounts in excess of about 10% wt. based on
the total
weight of the composition. The short chain non-ionic surfactant is preferably
present in
the liquid disinfectant compositions in amounts of from about 0.01 % by weight
to up to
about 10% by weight, preferably about 0.01 -5% by weight, and most preferably
in
amount of between 0.5 - 3 % by weight.
Other conventional optional additives, although not particularly elucidated
herein
may also be included in the present inventive compositions in order to provide
esthetic
or other beneficial properties thereto. Exemplary optional conventional
additives include
but are not limited to: other non-short chain non-ionic surfactants; other
antimicrobial/germicidal agents, pH adjusting agents and pH buffers including
organic
and inorganic salts; non-aqueous solvents, fragrances, fragrance solubilizers,
optical
brighteners, coloring agents such as dyes and pigments, opacifying agents,
hydrotropes,
antifoaming agents, viscosity modifying agents such as thickeners, enzymes,
anti-
spotting agents, anti-oxidants, anti-corrosion agents as well as others not
specifically
elucidated here. These should be present in minor amounts, preferably in total
comprise
less than about 5% by weight of the compositions, and desirably less than
about 3% wt.
The optional ingredients chosen should be compatible with the compositions to
which
they are added and the compatibility can be easily determined by one of
ordinary skill in
the art.
Examples of other non-short chain non-ionic surfactants which may be present
in
minor amounts in the inventive compositions can include alkoxy block
copolymers, and
in particular, compounds based on ethoxy/propoxy block copolymers. Polymeric
alkylene oxide block copolymers include non-ionic surfactants in which the
major portion
of the molecule is made up of block polymeric C2-C4 alkylene oxides. Such non-
short
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
chain non ionic surfactants, while preferably built up from an alkylene oxide
chain
starting group, and can have as a starting nucleus almost any active hydrogen
containing group including, without limitation, amides, phenols, thiols and
secondary
alcohols. When used herein, non-short chain non-ionic surfactants mean those
surfactants which have greater than eleven carbon atoms in the non-polar
hydrophobic
portion of the surfactant.
One example are those non-short chain nonionic surfactants which contain
alkylene oxide blocks and which may be represented by the formula (A):
HO-(EO)x(PO)y(EO)~-H (A)
where EO represents ethylene oxide,
PO represents propylene oxide,
y equals at least 15,
(EO)X+y equals 20 to 50% of the total weight of said compounds, and,
the total molecular weight is preferably in the range of about 2000 to 15,000.
Another example are those non-short chain nonionic surfactants which can be
represented by the formula (B):
R-(EO,PO)a(EO,PO)b-H (B)
wherein R is an alkyl, aryl'or aralkyl group, where the R group contains 1 to
20 carbon
atoms, the weight percent of EO is within the range of 0 to 45% in one of the
blocks a, b,
and within the range of 60 to 100% in the other of the blocks a, b, and the
total number
of moles of combined EO and PO is in the range of 6 to 125 moles, with 1 to 50
moles in
the PO rich block and 5 to 100 moles in the EO rich block.
Further non-short chain nonionic surfactants which in general are encompassed
by Formula B include butoxy derivatives of propylene oxide/ethylene oxide
block
polymers having molecular weights within the range of about 2000-5000.
11
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Another example are those non-short chain nonionic surfactants which contain
polymeric butoxy (BO) groups and which can be represented by formula (C) as
follows:
RO-(BO)n(EO)x-H (C)
wherein R is an alkyl group containing I to 20 carbon atoms,
n is about 5-15 and x is about 5-15.
Another example are those non-short chain nonionic surfactants may be
represented by the following formula (D):
HO-(EO)x(BO)n(EO)y-H (D)
wherein n is about 5-15, preferably about 15,
x is about 5-15, preferably about 15, and
y is about 5-15, preferably about 15.
Another example are those non-short chain nonionic surfactants include
ethoxylated derivatives of propoxylated ethylene diamine, which may be
represented by
the following formula:
H(EO)y(PO)x S (PO)x(EO)yH
CH2-CH2 (E)
H EO PO ~ ~ PO EO H
( )y( )x ( )x( )y
where (E0) represents ethoxy,
(PO) represents propoxy,
the amount of (PO),~ is such as to provide a molecular weight prior to
ethoxylation of
about 300 to 7500, and the amount of (EO)y is such as to provide about 20% to
90% of
the total weight of said compound.
12
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
If such other non-short chain non-ionic surfactants are to be included in
minor
amounts, a preferred other non-short chain non-ionic surfactant is represented
by
formula (A) above; specific examples of which include those materials
presently
commercially available under the tradename "Pluronic~", and in particular the
Pluronic~
F series, Pluronic~ L series, Pluronic~ P series, as well as in the Pluronic~
R series,
each of which are generally described to be block copolymers of propylene
oxide and
ethylene oxide. Generally those of the Pluronic~ L series and the Pluronic~ R
series are
preferred as these are supplied in liquid form by the manufacturer and are
readily
formulated into the present inventive compositions. These are also available
in a wide
range of HLB values, and those having HLB values in the range of 1.0-23.0 may
be
used, although those with intermediate HLB values such as from about 12.0-18.0
are
found to be particularly advantageous. These materials are presently
commercially
available from BASF AG (Ludwigshafen, Germany) as well as from BASF Corp. (Mt.
Olive Township, New Jersey).
Other examples of non-short chain non-ionic surfactants which may be included
in minor amounts, based on a polymeric ethoxy/propoxy units, include those
presently
commercially available in the PolyTergent~ E, and PolyTergent~ P series of
materials
from BASF Corp. (Mt. Olive, NJ). These are described to be nonionic
surfactants based
on ethoxy/propoxy block copolymers, conveniently available in a liquid form
from its
supplier.
Other examples of non-short chain non-ionic surfactants which may be included
in minor amounts include alkoxylated alcohols wherein the non-polar portion of
the
surfactant contains greater than eleven carbon atoms . These include the
condensation
products of a higher alcohol (e.g., an alkanol containing about 12 to 18
carbon atoms in
a straight or branched chain configuration) condensed with about 2 to 30 moles
of
ethylene oxide, for example, lauryl or myristyl alcohol condensed. with about
16 moles of
ethylene oxide, tridecanol condensed with about 6 to moles of ethylene oxide,
myristyl
alcohol condensed with about 10 moles of ethylene oxide per mole of myristyl
alcohol.
Some examples of the foregoing non-short chain non-ionic surfactants include
certain Neodol~ ethoxylates (Shell Chemical Co., Houston Tex) such as C~a-~3
alkanol
condensed with 6.5 moles ethyaene oxide (Neodol~ 23-E.5), C~~_13 alkanol
condensed
13
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
with 7 moles ethylene oxide Neodol~ 23-7); C~2_~5 alkanol condensed with 7
moles of
ethylene oxide (Neodol~ 25-7), C~Z_15 alkanol condensed with 9 moles ethylene
oxide
(Neodol~ 25-9), 02_15 alkanol condensed with 12 moles ethylene oxide (Neodol~
25-
12), 04_15 alkanol condensed with 13 moles ethylene oxide (Neodol~ 45-13), and
the
like.
Other possible non-short chain non-ionic surfactants which can be used in
minor
amounts in the inventive compositions are those presently marketed under the
Genapol~ tradename. Particularly useful are those in the Genapol~ "26-L"
series which
include for example: 02_16 linear alcohols condensed with 1 mole of ethylene
oxide
(Genapol~ 26-L-3); C~2_~6 linear alcohols condensed with 1.6 moles of ethylene
oxide
(Genapol~ 26-L-1.6); 02_16 linear,alcohols condensed with 2 moles of ethylene
oxide
(Genapol~ 26-L-2); 02_16 linear alcohols condensed with 3 moles of ethylene
oxide
(Genapol~ 26-L-3); C~2_~6 linear alcohols condensed with 5 moles of ethylene
oxide
(Genapol~ 26-L-5); as well as 02_16 linear alcohols condensed with varying
amounts of
ethylene oxide to provide specific cloud points of the surfactant (i.e.,
Genapol~ 26-L-60,
Genapol~ 26-L-60N, and Genapol~ 26-L-98N). These materials are commercially
available from a variety of sources, including Clariant Corp. (Charlotte,
N.C.).
Other possible non-short chain non-ionic surfactants which can be used in
minor
amounts in the inventive compositions include those based on alcohol and
ethylene
oxide condensates of a secondary aliphatic alcohol. These alcohols contain 12
to 18
carbon atoms in a straight or branched chain configuration and are condensed
with 5 to
moles of an alkylene oxide, especially ethylene oxide. Examples of
commercially
25 available nonionic detergents of the foregoing type are C~1-15 secondary
alkanols
condensed with either 9 ethylene oxides (Tergitol~ 15-S-9) or 7 ethylene
oxides
(Tergitol~ 15-S-7) marketed by Union Carbide Corp. (Danbury Conn.). It is to
be
understood that these nonionic alkoxylated secondary alcohol surfactant
compounds
may be used singly or in mixtures of two or more such compounds.
Other possible non-short chain non-ionic surfactants which can be used-in-
minor
amounts in the inventive compositions include certain alkoxylated linear
aliphatic alcohol
surfactants which are believed to be the condensation products of a C$_~o
hydrophilic
moiety with alkylene oxides, especially polyethylene oxide and or
polypropylene oxide
14
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
moieties. Such alkoxylated linear alcohol surfactants are presently
commercially
available under the tradename PolyTergent~ (Olin Chemical Co., Stamford
Conn.). Of
these particularly useful are those which are marketed as PolyTergent~ SL-22,
PolyTergent~ SL-42, PolyTergent~ SL-62 and PolyTergent~ SL-29, of which
PolyTergent~ SL-62 is particularly advantageous. PolyTergent~ SL-92 is
described as
being a moderately foaming, biodegradable alkoxylated linear alcohol
surfactant having
on average 8 moles of oxyethylene groups per molecule. These alkoxylated
linear
alcohol surtactants provide good detersive action in the removal of many types
of fats
and greases such as are frequently found in soils on hard surfaces, as well as
providing
a further solubilizing effects and may be included in the concentrate
compositions
according to the present invention with advantage. The preferred alkoxylated
linear
alcohol surfactants should also exhibit low levels of ocular irritation in the
concentrate
compositions.
The inventive compositions optionally but desirably can include in minor
amounts
a builder. Such include water soluble inorganic builders which can be used
alone, in
admixture with other water soluble inorganic builders, as well as in
conjunction with one
or more organic alkaline sequestrant builder salts. Exemplary builders include
alkali
metal carbonates, phosphates, polyphosphates and silicates. More specific
examples
include sodium tripolyphosphate, sodium carbonate, sodium bicarbonate, sodium
borates, potassium carbonate, sodium polyphosphate, potassium pyrophosphate,
potassium tripolyphosphate, and sodium hexametaphosphate. Further exemplary
builders also include organic alkaline sequestrant builder salts such as
alkali metal
polycarboxylates including water-soluble citrates such as sodium and potassium
citrate,
sodium and potassium tartarate, sodium and potassium
ethylenediaminetetraacetate,
sodium and potassium N-(2-hydroxyethyl)-ethylene diamine triacetates, sodium
and
potassium nitrilotriacetates, as well as sodium and potassium tartrate mono-
and di-
succinates. Also useful are gluconate or glucoheptonate salts particularly
sodium
gluconate and sodium glucoheptonate. Particularly advantageously used are di-,
tri- and
tetra-sodium salts of -ethylenediaminetetraacetic acid, especially tetrasodium
salts
thereof. As noted, these organic builder salts may be used individually, as a
combination
of two or more organic builder salts, as well as in conjunction with one or
more
detergency builders, including those indicated above. It is also to be
appreciated that
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
many of these constituents which are useful as builders often also provide a
beneficial
pH adjusting effect.
Examples of non-aqueous solvents which can be used in minor amounts in the
inventive compositions include those which are at least partially water-
miscible such as
alcohols, (e.g., low molecular weight alcohols, such as, for example, ethanol,
propanol,
isopropanol, and the like), glycols (such as, for example, ethylene glycol,
propylene
glycol, hexylene glycol, and the like), water-miscible ethers (e.g. diethylene
glycol
diethylether, diethylene glycol dimethylether, propylene glycol
dimethylether), water-
miscible glycol ether (e.g. propylene glycol monomethylether, propylene glycol
mono
ethylether, propylene glycol monopropylether, propylene glycol monobutylether,
propylene glycol monohexyl ether, ethylene glycol monobutylether, dipropylene
glycol
monomethylether, dipropylene glycol monobutylether, diethyleneglycol
monobutylether),
lower esters of monoalkylethers of ethyleneglycol or propylene glycol (e.g.
propylene
glycol monomethyl ether acetate) all commercially available such as from Union
Carbide
(Danbury, CT), Dow Chemical Co. (Midland, MI) or Hoechst (Germany). Mixtures
of
several organic solvents can also be used.
Preferred non-aqueous solvents which can be used in minor amounts in the
inventive compositions are glycol ethers. Exemplary useful glycol ethers are
those
having the general structure Ra-O-Rb-OH, wherein Ra is an alkyl of 1 to 20
carbon
atoms, or an aryl of at least 6 carbon atoms, and Rb is an alkylene of 1 to 8
carbons or is
an ether or polyether containing from 2 to 20 carbon atoms. Exemplary glycol
ethers
include propylene glycol methyl ether, dipropylene glycol methyl ether,
tripropylene
glycol methyl ether, propylene glycol isobutyl ether, ethylene glycol methyl
ether,
ethylene glycol ethyl ether, ethylene glycol butyl ether, diethylene glycol
phenyl ether,
propylene glycol phenol ether, dipropylene glycol monobutyl ether and mixtures
thereof
Specific examples of more preferred glycol ether solvents include propylene
glycol
methyl ether, dipropylene glycol methyl ether, tripropylene glycol methyl
ether, propylene
glycol isobutyl ether, ethylene glycol methyl ether, ethylene glycol ethyl
ether, ethylene
glycol butyl ether, diethylene glycol phenyl ether, propylene glycol phenol
ether, and
mixtures thereof Particularly preferred solvents are described in the
Examples.
16
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Examples of other antimicrobial/germicidal agents which may be present in
minor
amounts in the inventive compositions of the present application include, in
addition to
the germicidal cationic surfactants mentioned above, pyrithiones especially
the zinc
complex (Zpt), Octopirox~, dimethyldimethyfol hydantoin (Glydant~)
methylchloroisothiazolinone/methylisothiazolinone (Kathon CG~), benzoic acid,
benzoyl
peroxide, salicylamides, picric acid, xylenol, pyrocatechol, pyrogallol,
phloroglucin,
sodium sulfite, sodium bisulfite, imidazolidinyl urea (Germall 115~),
diazolidinyl urea
(Germaill II~), benzyl alcohol, 2-bromo-2-nitropropane-1,3-diol (Bronopol~),
formalin
(formaldehyde), iodopropenyl butylcarbamate (Polyphase P100~),
chloroacetamide,
methanamine, methyldibromonitrile glutaronitrile (1,2-dibromo-2,4-
dicyanobutane or
Tektamer~), glutaraldehyde, 5-bromo-5-nitro-1,3-dioxane (Bronidox~), phenethyl
alcohol, o-phenylphenol/sodium o-phenylphenol, sodium hydroxymethylglycinate
(Suttocide AO), polymethoxy bicyclic oxazolidine (Nuosept C~), dimethoxane,
thimersal,
dichlorobenzyl alcohol, captan, chlorphenenesin, hexachlorophene,
tetrachlorophene,
2,3-dihydroxy-5,5'-dichlorodiphenyl sulfide, 2,2'-dihydroxy-3;3',5,5'-
tetrachlorodiphenyl
sulfide, 2,2'-dihydroxy-3,5',5,5',6,6'-hexachlorodiphenyl sulfide, and 3,3'-
dibromo-5,5'-
dichloro-2,2'-dihydroxydiphenylamine, dichlorophene, chlorbutanol, glyceryl
laurate,
halogenated diphenyl ethers, 2,4,4'-trichloro-2'-hydroxy-diphenyl ether
(Triclosan(~ or
TCS), 2,2'-dihydroxy-5,5'-dibromo-diphenyl ether, phenolic compounds, phenol,
2-methyl
phenol, 3-methyl phenol, 4-methyl phenol, 4-ethyl phenol, 2,4-dichlorophenol,
p-
nitrophenol,2,4-dimethyl phenol, 2,5-dimethyl phenol, 3,4-dimethyl phenol, 2,6-
dimethyl
phenol, 4-n-propyl phenol, 4-n-butyl phenol, 4-n-amyl phenol, 4-tert-amyl
phenol, 4-n-
hexyl phenol, 4-n-heptyl phenol, mono- and poly-alkyl and aromatic
halophenols, p-
chlorophenol, methyl p-chlorophenol, ethyl p-chlorophenol, n-propyl p-
chlorophenol, n-
butyl p-chlorophenol, n-amyl p-chlorophenol, sec-amyl p-chlorophenol, n-hexyl
p-
chlorophenol, cyclohexyl p-chlorophenol, n-heptyl p-chlorophenol, n-octyl p-
chlorophenol, o-chlorophenol, methyl o-chlorophenol, ethyl o-chlorophenol, n-
propyl o-
chlorophenol, n-butyl o-chlorophenol, n-amyl o-chlorophenol, tent-amyl o-
chlorophenol,
n-hexyl o-chlorophenol, n-heptyi o-chlorophenol, o-benzyl p-chlorophenol, o-
benzyl-m-
methyl p-chlorophenol, o-benzyl-m, m-dimethyl p-chlorophenol, o-phenylethyl p-
chlorophenol, o-phenylethyl-m-methyl p-chlorophenol, 3-methyl p-chlorophenol,
3,5-
dimethyl p-chlorophenol, 6-ethyl-3-methyl p-chlorophenol, 6-n-propyl-3-methyl
p-
chlorophenol, 6-iso-propyl-3-methyl p-chlorophenol, 2-ethyl-3,5-dimethyl p-
chlorophenol,
6-sec-butyl-3-methyl p-chlorophenol, 2-iso-propyl-3,5-dimethyl p-chlorophenol,
6-
17
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
diethylmethyl-3-methyl p-chlorophenol, 6-iso-propyl-2-ethyl-3-methyl p-
chlorophenol, 2-
sec-amyl-3,5-dimethyl p-chlorophenol, 2-diethylmethyl-3,5-dimethyl p-
chlorophenol, 6-
sec-octyl-3-methyl p-chlorophenol, o-benzylphenol, p-chloro-o-benzylphenol, 4-
phenolsulfonic acid cresols (o-, m-, p-), p-chloro-m-cresol, p-bromophenol,
methyl p-
bromophenol, ethyl p-bromophenol, n-propyl p-bromophenol, n-butyl p-
bromophenol, n-
amyl p-bromophenol, sec-amyl p-bromophenol, n-hexyl p-bromophenol, cyclohexyl
p-
bromophenol, o-bromophenol, tert-amyl o-bromophenol, n-hexyl o-bromophenol, n-
propyl-m,m-dimethyl o-bromophenol, 2-phenyl phenol, 4-chloro-2-methyl phenol,
4-
chloro-3-methyl phenol, 4-chloro-3,5-dimethyl phenol, 2,4-dichloro-3,5-
dimethylphenol,
3,4,5,6-terabromo-2-methylphenol, 5-methyl-2-pentylphenol, 4-isopropyl-3-
methylphenol, para-chloro-meta-xylenol, chlorothymol, phenoxyethanol,
phenoxyisopropanol, 5-chloro-2-hydroxydiphenylmethane, resorcinol and its
derivatives,
resorcinol, methyl resorcinol, ethyl resorcinol, n-propyl resorcinol, n-butyl
resorcinol, n-
amyl resorcinol, n-hexyl resorcinol, n-heptyl resorcinol, n-octyl resorcinol,
n-nonyl
resorcinol, phenyl resorcinol, benzyl resorcinol, phenylethyl resorcinol,
phenylpropyl
resorcinol, p-chlorobenzyl resorcinol, 5-chloro 2,4-dihydroxydiphenyl methane,
4'-chloro
2,4-dihydroxydiphenyl methane, 5-bromo 2,4-dihydroxydiphenyl methane, 4'-bromo
2,4-
dihydroxydiphenyl methane, bisphenolic compounds, 2,2'-methylene bis(4-
ch.lorophenol), 2,2'-methylene bis(3,4,6-trichlorophenol), 2,2'-methylene
bis(4-chloro-6-
bromophenol), bis(2-hydroxy-3,5-dichlorophenyl) sulphide, bis(2-hydroxy-5-
chlorobenzyl)sulphide, benzoic esters parabens such as methylparaben,
propylparaben,
butylparaben, ethylparaben, isopropylparaben, isobutylparaben, benzylparaben,
sodium
methylparaben, sodium propylparaben, halogenated carbanilides, 3,4,4'-
trichlorocarbanilides (Trichlocarban~ or TCC), 3-trifluoromethyl-4,4'-
dichlorocarbanilide,
and 3,3',4-trichlorocarbanilide.
Other antimicrobial/germicidal agents which may be present in minor amounts in
the inventive compositions of the present application also include the so-
called "natural"
antibacterial actives, referred to as natural oils. These actives derive their
names from
their natural occurrence in plants. Typical natural essential oil
antibacterial actives
include oils of anise, citrus, aniseed, roses, mint, camphor, lemon, orange,
rosemary,
wintergreen, thyme, lavender, cloves, hops, tea tree, citronella, wheat,
barley,
lemongrass, .cedar leaf, cedarwood, cinnamon, fleagrass, geranium, sandalwood,
violet,
cranberry, eucalyptus, vervain, peppermint, gum benzoin, basil, fennel, fir,
balsam,
18
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
menthol, ocmea origanum, hydastis carradensis, berberidaceae daceae, ratanhiae
and
curcuma longs. Also included in this class of natural essential oils are the
key chemical
components of the plant oils which have been found to provide the
antimicrobial benefit.
These chemicals include, but are not limited to anethol, catechole, camphene,
pinocarvone, cedrol, thymol, eugenol, eucalyptol, ferulic acid, farnesol,
hinokitiol,
tropolone, limonene, menthol, methyl salicylate, carvacol, terpineol,
verbenone,
berberine, ratanhiae extract, caryophellene oxide, citronellic acid, curcumin,
nerolidol
and geraniol. Additional antimicrobial/germicidal agents which may be present
in minor
amounts in the inventive compositions of the present application also include
antimicrobial metal salts. This class generally includes salts of metals in
groups 3b-7b, 8
and 3a-5a. Specifically are the salts of aluminum, zirconium, zinc, silver,
gold, copper,
lanthanum, tin, bismuth, selenium, strontium, scandium, yttrium, cerium,
praseodymiun,
neodymium, promethum, samarium, europium, gadolinium, terbium, dysprosium,
holmium, erbium, thulium, ytterbium, lutetium and mixtures thereof.
Additional other antimicrobial/germicidal agents which may be present in minor
amounts in the inventive compositions of the present application also include
oxidizers,
for example hydrogen peroxide, peracids and their salts; bleach based actives,
for
example, sodium hypochlorite; organic acids, for example, formic, citric,
glycolic, malefic,
malic, lactic, glutaric, succinic, benzoic; and aldehydes, for example,
glutaraldehyde,
succinaldehyde
The other optional additives which can be added in minor amounts, such as
coloring agents, fragrances and fragrance solubilizers, viscosity modifying
agents, pH
adjusting agents and pH buffers including organic and inorganic salts, optical
brighteners, opacifying agents, hydrotropes, antifoaming agents, enzymes, anti-
spotting
agents, anti-oxidants, and anti-corrosion agents are well known to those
skilled in the art
as is their use and selection.
As is noted above, the compositions according to the invention are aqueous in
nature. Water is added to the constituents in order to provide 100% by weight
of the
composition. The water may be tap water, but is preferably distilled and is
most
preferably deionized water. If the water is tap water, it is preferably
substantially free of
any undesirable impurities such as organics or inorganics, especially mineral
salts which
19
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
are present in hard water which may thus interfere with the operation of one
or more of
the constituents of the aqueous compositions according to the invention.
Preferably the
concentrate compositions comprise the least amount of water possible, if not
water-free.
Such materials described above are known to the art, including those described
in McCutcheon's Emulsifiers and Defiergents (Vol. 1), North American Edition,
2000;
ifirk-Othmer, Encyclopedia of Cf~emical Technology, 4th Ed., VoI. 23, the
contents of
which are herein incorporated by reference.
The compositions according to the invention are useful in the disinfecting
and/or
cleaning of surfaces, especially hard surfaces in need of such treatment.
These in
particular include surfaces wherein the presence of gram positive and/or gram
negative
bacteria are suspected. In accordance with the present inventive process,
cleaning
and/or disinfecting of such surfaces comprises the step of applying a stain
releasing and
a disinfecting effective amount of a composition as taught herein to such a
stained
surface. Afterwards, the compositions are optionally but desirably wiped,
scrubbed or
otherwise physically contacted with the hard surface, and further optionally,
may be
subsequently rinsed from such a cleaned and disinfected hard surface.
Such a hard surface cleaning and disinfecting composition according to the
invention is may be provided as a ready to use product which may be directly
applied to
a hard surface, but is desirably provided in a concentrated form intended to
be diluted in
water to form a cleaning composition therefrom.
By way of example, hard surfaces include surfaces composed of refractory
materials such as: glazed and unglazed tile, porcelain, ceramics as well as
stone
including marble, granite, and other stones surfaces; glass; metals; plastics
e.g.
polyester, vinyl; fiberglass, Formica, Corian~ and other hard surfaces known
to the
industry. Hard surfaces which are to be particularly denoted are lavatory
fixtures such as
shower stalls, bathtubs and bathing appliances (racks, shower doors, shower
bars)
toilets, bidets, wall and flooring surfaces especially those which include
refractory
materials and the like. Further hard surfaces which are to be denoted are
those
associated with kitchen environments and other environments associated with
food
preparation, including cabinets and countertop surtaces as well as walls and
floor
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
surfaces especially those which include refractory materials, plastics,
Formica,
Corian~ and stone.
The hard surface cleaner composition provided according to the invention can
be
also be provided as a ready to use product in a manually operated spray
dispensing
container. Such a typical container is generally made of synthetic polymer
plastic
material such as polyethylene, polypropylene, polyvinyl chloride or the like
and includes
spray nozzle, a dip tube and associated pump dispensing parts and is thus
ideally suited
for use in a consumer "spray and wipe" application. In such an application,
the consumer
generally applies an effective amount of the cleaning composition using the
pump and
within a few moments thereafter, wipes off the treated area with a rag, towel,
or sponge,
usually a disposable paper towel or sponge. In certain applications, however,
especially
where undesirable stain deposits are heavy, the cleaning composition according
to the
invention may be left on the stained area until it has effectively loosened
the stain
deposits after which it may then be wiped off, rinsed off, or otherwise
removed. For
particularly heavy deposits of such undesired stains, multiple applications
may also be
used.
In a yet a further embodiment, the compositions according to the invention may
be formulated so that it may be useful in conjunction with a "aerosol" type
product
wherein it is discharged from a pressurized aerosol container. If the
inventive
compositions are used in an aerosol type product, it is preferred that
corrosion resistant
aerosol containers such as coated or lined aerosol containers be used. Such
are
preferred as they are known to be resistant to the effects of basic
formulations. Known
art propellants such as liquid propellants as well as propellants of the non-
liquid form,
i.e., pressurized gases, including carbon dioxide, air, nitrogen, hydrocarbons
as well as
others may be used. Also, while satisfactory for use, fluorocarbons may be
used as a
propellant but for environmental and regulatory reasons their use is
preferably avoided.
In such an embodiment, the cleaning composition is dispensed by activating the
release
nozzle of said aerosol type container onto the stain and/or stain area, and in
accordance
with a manner as above-described a stain is treated and removed.
Whereas the present invention is intended to be used in the types of liquid
forms
described, the compositions according to the invention are desirably diluted
with a
21
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
further amount of water to form a cleaning and disinfecting solution
therefrom. In such a
proposed diluted cleaning solution, the greater the proportion of water added
to form
said cleaning dilution, the greater may be the reduction of the rate and/or
efficacy of the
thus formed cleaning solution in the cleaning of a hard surface, as well as a
reduction in
disinfectant efficacy. Accordingly, longer residence times upon the stain to
effect their
loosening and/or the usage of greater amounts may be necessitated. Conversely,
nothing in the specification shall be also understood to limit the forming of
a "super-
concentrated" cleaning composition based upon the composition described above.
Such
a super-concentrated composition is essentially the same as the compositions
described
above except in that they include a lesser amount of water.
While the cleaning compositions are most beneficial for use in their form,
i.e.,
their form as described above, they may also be diluted to form a cleaning
composition
therefrom. Such cleaning compositions may be easily prepared by diluting
measured
amounts of a concentrated composition which is an object of the present
invention in
further amounts of water by the consumer or other end user in certain weight
ratios of
composition: water, and optionally, agitating the same to ensure even
distribution of the
composition in the water. The concentrate compositions according to the
invention may
be used without further dilution, but may also be used with a further aqueous
dilution,
i.e., in concentrate composition: water concentrations of 1:0, to extremely
dilute dilutions
such as 1:1000. When subjected to further aqueous dilution, such a dilution is
preferably
a weight or volume ratio proportion of from 1:10-1:128, and most desirably is
about 1:64.
The actual dilution selected is in part determinable by the degree and amount
of dirt and
grime to be removed from a surface(s), the amount of mechanical force imparted
to
remove the same, as well as the observed efficacy of a particular dilution.
Generally
better results and faster removal is to be expected at lower relative
dilutions of the
composition and the water.
The concentrated compositions of the present invention are ideally
formulations
wherein the amounts of components, excluding water, are concentrated five,
ten, fifteen,
twenty or even twenty-five fold such that when the concentrated compositions
are diluted
in water for further use, the amounts of cationic surtactant and short chain
non-ionic are
present in the diluted form in the amounts set forth hereinbelow.
22
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
The concentrated compositions of the present invention can be supplied in
liquid
form in bottles which are then added to the desired amount of water.
Additionally, the
concentrated compositions can be supplied in the form of sachets having a
water
dissolvable film covering, for example, polyvinyl alcohol, such that when the
sachet is
placed in a quantity of water, the polyvinyl alcohol dissolves, releasing the
concentrated
composition into the water, forming a dilutable composition. Preferably, the
polyvinyl
alcohol sachet is soluble in water of varying temperatures so to be useful in
both cold
water and warm water diiutions.
Figure 1 shows the effects of nonionic surfactants on antimicrobial efficacy
of
quaternary ammonium compound against Salmonella choleraesuis. For solutions
containing 450 ppm quaternary ammonium compound, short chain non-Tonics with
alkyl
chain lengths of 10 or shorter did not reduce antimicrobial efficacy of
quaternary
ammonium compound against Salmonella choleraesuis at short chain non-ionic
concentrations as high as 1000 ppm. This compares to a drop in quaternary
ammonium
compound efficacy of almost 4 logs at 1000 ppm of a C~2_~6 ethoxylated alcohol
non-
ionic. For the C~~ nonionic, a smaller decrease of 3 logs in quaternary
ammonium
compound efiFicacy is observed. At a quaternary ammonium compound
concentration of
210 ppm, the same trend is observed as short chain non-ionic surfactants with
alkyl
chain lengths of 10 or shorter do not have any effect on quaternary ammonium
compound antimicrobial efficacy. The C~~ nonionic results in a smaller
decrease, while
the C~2_,s ethoxylated alcohol non-ionic cause a larger decrease.
The data contained in Figure 1 demonstrate that ethoxylated alcohol nonionic
surfactants with alkyl chain lengths of 10 or less have minimal impact, and
those with
chain lengths of 11 have reduced impact on the antimicrobiaf efficacy of
quaternary
ammonium compounds. This compares to a greater impact on the antimicrobial
efficacy
of quaternary ammonium compound when used with a C~2 and longer chain non-
ionic.
Representative short chain non-Tonics evaluated in Figure 1 are Alfonic 610-
3.5
[Cs-C1o] from Condea Vista (0); Alfonic 810-4.5 [C$-C,o] from Condea Vista
(d); Genapoi
UD-79 [C~~] from Clariant (D); and Genapol 26-L-50 (C~2'C~6] from Clariant (
). The
quaternary ammonium compound evaluated was BTC-8358 (alley( dimethyl ben~yl
ammonium chloride (80% active) from Stepan).
23
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Samples for Figure 1 were evaluated for antimicrobial activity using the
Biomek~
2000 Laboratory Automation Workstation together with the BioWorks Operating
System
(available from Beckman Coulter Inc., Fullerton, CA). The organism tested was
Salmonella choleraesuis at an in test average concentration of 1.4 - 2.0 x 109
organisms/20 microliters (9log titer). The Biomek method measures the recovery
of
bacteria in suspension. One part of organism suspension (Salmonella
choleraesuis) is
added to 9 parts of each sample in an appropriate container. Deionized water
(DI H20)
was used a control. The organism and sample are then mixed thoroughly for 15
seconds. Serial tenfold dilutions are carried out in a neutralizing broth. The
diluted
samples are then incubated for 24-48 hours at 35-37°C. Thereafter,
surviving organisms
are quantified and log reduction, as a measurement of organism survivors are
calculated
as follows:
Log Reduction = (Log Survivors/DI H2O Control)-(Log Survivors/Sample)
The following examples below illustrate exemplary and preferred formulations
of
compositions according to the instant invention. It is to be understood that
these
examples are presented by means of illustration only and that further useful
formulations
fall within the scope of this invention and the claims may be readily produced
by one
skilled in the art and not deviate from the scope and spirit of the invention.
Throughout this specification and in the accompanying claims, weight percents
of
any constituent are to be understood as the weight percent of the active
portion of the
referenced constituent, unless otherwise indicated.
EXAMPLE FORMULATIONS
Preparation of Example Formulations:
Exemplary formulations illustrating certain preferred embodiments of the
inventive compositions and described in more detail in Table 1 below were
formulated
generally in accordance with the following protocol. The indicated weight
percentages
24
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
are of the named constituent "as supplied" from its respective supplier. For
the dye, the
percent actives are shown in parenthesis for the particular formulation.
Into a suitably sized vessel, a measured amount of water was provided after
which the constituents were added in no specific or uniform sequence, which
indicated
that the order of addition of the constituents was not critical. All of the
constituents were
supplied at room temperature, and any remaining amount of water was added
thereafter.
Certain of the nonionic surfactants if gels at room temperature were first
preheated to
render them pourable liquids prior to addition and mixing. Mixing of the
constituents was
achieved by the use of a mechanical stirrer with a small diameter propeller at
the end of
its rotating shaft. Mixing, which generally lasted from 5 minutes to 120
minutes was
maintained until the particular exemplary formulation appeared to be
homogeneous. The
exemplary compositions were readily pourable, and retained well mixed
characteristics
(i.e., stable mixtures) upon standing for extend periods. The compositions of
the
example formulations are listed on Tables 1 and 2. Examples of concentrated
compositions which are suitable for dilution within the scope of this
invention are shown
in Tables 1 and 2. The various components used in Tables 1 and 2 are
identified in
Table 3.
25
Table 1
Com onents Com 1 Ex1 Ex2 Ex3 Ex4 Ex5 Ex6
BTC 1010 1.580 6. 6.000 6.000
000
BTC 8_3_58 1.482 0.960 1.482 _
Alfonic 810-4..5 1.5001.500 1.500 2.500 2.500 2.500
Gena 0126-L-602.730
Gluco on 3.636
325
Pluronic 1.818 1.500 1.500
PE6400
Na4 EDTA 0.227
Borax 0.091
~ NazC03 0.2000.200 0.200 0.250 0.250 0.250
~
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
NaHCOs 0.1000.100 0.100 0.250 0.250 0.250
NaOH 0.864
KOH 0.389 0.389 0.389
Fra rance 0.182 0.182 0.450 0.350 0.450
D a 0.182 0.182 0.450 0.006 0.450
3% 3% 1 % 1
D.I. Water q.s. q.s. q.s. q.s. q.s. q.s. q.s.
~
Table 2
Components Ex7 Ex8 Ex9 Ex10
BTC 1010 39.5
BTC 8358 37.05 24.0 37.05
Alfonic 810-4.537.5 37.5 37.5 37.5
Na2C03 5.0 5.0 5.0 5.0
NaHCOs 2.5 2.5 2.5 2.5
NaOH 9.0 9.0 9.0 5.4
Fra rance 7.5 7.5 7.5 4.55
D a 0.1 0.1 0.1 4.55 3%
D.I. Water .s. .s. .s. .s.
Table 3
Com onent Descri tion
BTC 1010 didec I dimeth I ammonium chloride 50%
active Ste an
BTC 8358 alk I dimeth I bent I ammonium chloride
80% active Ste an
Alfonio 810-4.5C$ -C,o straight-chain alcohols ethoxylated
with about 4.85
moles of eth lene oxide Condea Vista
Genapol 26-L-60C12-16 primary alcohol ethoxylate having
cloud point of --60 C
1.0 wt. % in water . Clariant
Gluco on 325 C9." alk I of I coside. Co nis
Pluronic PEE400EO/PO block copolymer BASF AG (also available
as Pluronic
L64
Na4 EDTA Tetrasodium eth lenediaminetetraacetate
Borax ~ Hydrated sodium borate
26
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Na2C03 Sodium carbonate
NaHC03 Sodium bicarbonate
NaOH Sodium h droxide 25% solution
KOH Potassium h droxide 45% solution
Fra rance Pro rieta blend
D a Pro rieta blend
D.I. Water Deionized water
Antimicrobial efficacy of Ex1 and Ex2 were evaluated generally in accordance
with the standardized AOAC Use-Dilution test method based on AOAC Official
Methods
of Analysis Procedures 955.14 "Testing disinfectants against Salmonella
Choleraesusis," and Procedure 955.15 "Testing disinfectants against
Staphylococcus
Aureus" (15th Edition, 1990, pages 135-137, Use Dilution Methods). The results
reported on Table 4 indicate the proportion of the number of carriers within
which the
organism remained alive after 10 minutes of exposure at 20°C. over the
total number of
carriers used in testing. Samples of Ex1 and Ex2 were diluted 1 part to 40
parts water
prior to testing. Both examples show disinfectant activity.
Table 4
Ex1 Ex2
Salmonella choleraesuis0/30 0/30
Staphylococcus aureus1/30 0/30
The eye toxicity of inventive formulations which are the subject of the
present
application were evaluated using the Draize eye test. The response measured
was the
time in days needed to produce a zero Draize score. Analysis of the results
showed a
significant nonionic type by quaternary ammonium compound level interaction,
thus
indicating the effect of increasing quaternary ammonium compound level on eye
toxicity
was different when short chain non-ionic surfactants were present in the
formulation than
when non-short chain non ionic surfactants were present, at equal weight
percents.
Specifically, the average effect of raising the quaternary ammonium compound
level
from its 'low' level to its 'high' level when a non-short chain non ionic
surfactant was
present was a 3.1X increase in the time required to reach zero Draize score
versus only
a 1.4X increase when a short chain ion ionic~surfactant was present.
27
CA 02423862 2003-03-26
WO 02/26268 PCT/GBO1/04323
Cleaning evaluation of formulation Ex3 was compared to Comp1 in accordance
with the testing protocol outlined according to ASTM D4488 A2 Test Method,
which
evaluated the efficacy of the cleaning compositions on masonite wallboard
samples
painted with wall paint. The soil applied was a greasy soil sample containing
vegetable
oil, food shortening and animal fat. The sponge (water dampened) of a Gardner
Abrasion Tester apparatus was squirted with a 15 gram sample of a tested
cleaning
composition, and the apparatus was cycled 10 times. The evaluation of cleaning
compositions was "paired" with one side of each of the test samples treated
with a
composition according to the invention, and the other side of the same sample
treated
with a comparative example's composition, thus allowing a "side-by-side"
comparison to
be made. Each of these tests were duplicated ort 20 wallboard tiles arid the
results
statistically analyzed. The cleaning efficacy of the tested compositions was
evaluated
utilizing a Minolta Chroma Meter CF-110, with Data Processor DP-100, which
evaluated
spectrophotomic characteristics of the sample.
Also, cleaning evaluation of formulation Ex3 was compared to Comp1 in
accordance with the testing protocol outlined according to ASTM D-4488-89
Annex A5
for particulate soil, which evaluated the efficacy of the cleaning
compositions on vinyl tile
samples. The soil applied was a particulate soil sample coritaining natural
humus,
paraffin oil, used crankcase motor oil, Portland cement, silica, lampblack
carbon, iron
oxide, bandy black clay, stearic acid, and oleic acid. Each of the soiled test
vinyl tile
samples (two samples) were placed into the apparatus and the center of each
tile was
wetted with a 20 ml sample of a test formulation and allowed to stand for 1
minute.
When approximately 30 seconds had elapsed, a further 50 ml sample was applied
to the
sponge (water damped, then wrung to remove excess water) of a Gardner Abrasion
Tester apparatus. The apparatus was then cycled 10 times, which provided 20
strokes
of the sponge across the face of each of the vinyl test tiles. The reflectance
values of
the cleaned samples were evaluated utilizing a Minolta Chromameter CF-110,
with Data
Processor DP-100, which evaluated spectrophotomic characteristics of the
sample.
In testing the cleaning efficacy of Ex3 to Comp1 under ASTM D-4488 Annex A2
and D-4488-89 Annex A5, Ex3 was at parity or better than Comp1 when tested
either full
strength or diluted at 1:16 or 1:32 (Ex3 or Comp1:water).
28