Note: Descriptions are shown in the official language in which they were submitted.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
METHOD TO ALTER SUGAR MOIETIES
Statement of Government Rights
This invention was made with a grant from the Government of the United
States of America (grants GM48562, GM35906, GM54346 and GM58196 from
the National Institutes of Health). The Government may have certain rights in
the invention.
Background of the Invention
Nature continues to be the inspiration for most pharmaceutical drug leads
and given the synthetic challenge posed by many complex secondary
metabolites, the emerging field of combinatorial biosynthesis has become a
rich
new source for modified non-natural scaffolds (I~atz et al., 1993; Hutchinson
et
al., 1995; Cameras et al., 1997; Jacobsen et al., 1997; Cane et al., 1998;
Marsden
et al., 1997; McDaniel et al., 1999). Yet, many naturally occurring bioactive
secondary metabolites, e.g., polyketides, possess unusual carbohydrate ligands
which serve as molecular recognition elements critical for biological activity
(Omura, 1984; Weymouth-Wilson, 1997). Without these essential sugar
attachments, the biological activities of most clinically important secondary
metabolites are either completely abolished or dramatically decreased.
Glycosyltransferases responsible for the final glycosylation of certain
secondary
metabolites show a high degree of promiscuity towards the nucleotide sugar
donor (Zhao et al., 1998a; Zhao et al., 1998b; Borisova et al., 1999; Weber et
al.,
1991; Decker et al., 1995, Sasaki et al., 1996; Solenberg et al., 1997;
Madduri et
al., 1998; Salah-Bey et al., 1998; Gaisser et al., 1998; Wohlert et al.,
1998).
These discoveries have opened the door to the possibility of manipulating the
corresponding biosynthetic pathways for modifying the crucial glycosylation
pattern of natural, or non-natural, secondary metabolite scaffolds in a
combinatorial fashion. To date, the genetic manipulation of the carbohydrate
appendage for any given metabolite has generally been lixriited to alterations
and/or knock-outs of the small subset of genes required to construct and
attach
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
that carbohydrate moiety (Madduri et al., 1998; Hutchinson, 1998; Wohlert et
al., 1998).
Thus, what is needed is a method to significantly modify or alter the
sugar appendage for a particular metabolite.
Summary of the Invention
The invention provides a method to alter the sugar structure diversity for
a particular metabolite via the recruitment and collaborative action of sugar
genes from a variety of sugar biosynthetic pathways to yield a metabolite
comprising a non-natural sugar, e.g., a novel glycosylated polyketide. This
alteration can be accomplished iu vivo through genetic engineering. For
example, the method of the invention provides a modified recombinant bacterial
host cell that is genetically engineered to produce novel polyketides having
non-
natural sugar structures. To prepare the modified recombinant host cell of the
invention, a sugar biosynthetic genes) from a heterologous (e.g., non-native
or
different) sugar biosynthetic pathway, or one that is modified in vitro and
encodes an enzyme having an activity or specificity that is different than the
native (wild type) enzyme, is introduced into a recombinant host cell that
produces a substrate for the enzymes) encoded by that genes) to yield a
modified recombinant host cell that produces a novel product, i.e., one not
produced by the corresponding recombinant host cell. Preferably, the product
from the modified recombinant host cell comprises a sugars) that is
significantly
different than the sugar on the naturally occurring product from the
corresponding wild type cell, e.g., the sugar on the modified product is not a
stereoisomer of the sugar on the naturally occurring product. Also preferably,
the recombinant host cell and the modified recombinant host cell are
genetically
modified so that at least one gene for sugar biosynthesis, for example, in a
sugar
biosynthetic gene cluster, in that cell is disrupted, e.g., via an insertion
or
deletion, resulting in the accumulation of an intermediate in the biosynthetic
pathway which is disrupted. The disruption may be in a nucleic acid sequence
2
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
present in the genome of the cell or present in an extrachromosomal element in
the cell. Thus, the invention is useful to generate libraries of polyketides
and
other sugar-containing molecules that are biologically active or can be
activated.
For example, if the product is an acetylated sugar, a deacetylase may be
employed to render the product biologically active. Moreover, the availability
of
such libraries can greatly decrease the time for drug discovery.
As described hereinbelow, a 4-ketohexose aminotransferase gene (calH)
from the calicheamicin pathway ofMicromonospora echinospora spp.
calicl2ensis was introduced into a mutant strain of Streptomyces venezuelae in
which the 4-dehydrase gene (desI) in the methymycin/pikromycin pathway was
deleted. Deletion of desI gene led to the accumulation of 4-keto-6-
deoxyglucose
intermediate which is the substrate of CaIH. Consequently, heterologous
expression of calH in this mutant resulted in the production~of two
methymycin/pikromycin-calicheamicin hybrids. These results not only reinforce
the indiscriminate nature of the corresponding glycosyltransferase (DesVII)
but
also clearly demonstrate the ability to engineer secondary metabolite
glycosylation through a rational selection of gene combinations. In addition,
the
results confirm that the calH gene codes for the TDP-6-deoxy-D-glycero-L-
tlareo-
4-hexulose 4-aminotransferase of the calicheamicin pathway.
As also described herein, a significant expansion of sugar structural
diversity can be achieved if various L-sugars are incorporated into
metabolites
such as macrolides. The heterologous expression of selected genes from the L-
dihydrostreptose pathway, for example, the stf M and strL genes of
Streptomyces
griseus that encode a 6-deoxy-4-hexulose 3,5-epimerase and a dihydrostreptose
synthase, respectively, was accomplished in a S. venezuelae mutant. Growth of
the engineered S. veraezuelae strain resulted in the accumulation of a set of
methymycin/pikromycin analogs, each carrying a L-rhamnose. Formation of
these new derivatives confirmed the relaxed substrate specificity of the
desosamine glycosyltransferase DesVII, and the feasibility of preparing novel
metabolites by reconstitution of a hybrid pathway. In addition, these results
provide evidence of the collaborative functions of StrM and StrL, and
3
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
established the close resemblance of the dihydrostreptose and apiose
biosynthetic
pathways.
Thus, the invention provides a modified recombinant bacterial host cell
comprising at least one nucleic acid segment which encodes at least one sugar
biosynthetic enzyme. Preferably, a nucleic acid segment of the invention does
not encode a glycosyltransferase or any other non-sugar biosynthetic sequences
such as polyketide synthase sequences. The modified recombinant host cell may
include more than one nucleic acid segment, each encoding a different enzyme,
or one nucleic acid segment encoding one or more enzymes. The modified
recombinant host cell also preferably comprises a disrupted nucleic acid
sequence, which corresponds to a nucleic acid sequence in a wild type host
cell
that encodes at least one sugar biosynthetic enzyme from a pathway that is
different than the pathway of the enzymes) encoded by the nucleic acid
segment. For example, the nondisrupted wild type nucleic acid sequence may
encode a dehydrase, a reductase, a TDP-sugar synthase, a TDP-sugar
dehydratase, an amino transferase, a N-methyltransferase, and/or a
tautomerase.
The disruption results in the accumulation of a substrates) for the enzymes)
encoded by the nucleic acid segment thus yielding a novel sugar. The modified
recombinant host cell also preferably produces a product having the novel
sugar
linked thereto, e:g., the native (endogenous) glycosyltransferase(s) transfers
the
novel sugar to another molecule, e.g., a polyketide such as an aglycone, to
yield a
novel product such as a macrolide. Alternatively, a nucleic acid molecule
encoding a glycosyltransferase having relaxed substrate specificity may also
be
introduced to the recombinant host cell so as to provide an enzyme which
attaches the novel sugar to another molecule in the modified recombinant host
cell.
Preferred cells for use in the invention include any cell which produces a
metabolite such as a polyketide, anticancer agent or antibiotic that has or
can be
modified to accommodate a sugar. Antibiotic-producing cells include but are
not limited to Actinoplanes, Actinomadura, Bacillus, Cephalosporium,
Micromonospora, Penicillium, Nocardia, and Streptomyces, which either
4
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
produce an antibiotic or contains genes which, if expressed, would produce an
antibiotic or other biologically active compound, e.g., any cell which
contains
the genes sno, stf; tyl, eat; srm, tat, act, gra, tcnz, mitlrranzc, elnz, sal,
rif, gfs, srf,
bac, dau, sty, dnf; sna, frera, avf; ole, of°d, ery, or any combination
thereof.
Examples of actinomycetes that naturally produce polyketides include but are
not
limited to Micromonospora rosaria, Micr-omonospora megalomicea,
Saccharopolyspo~°a erythraea, Streptomyces antibioticus,
Sts°eptomyces
albereticuli, Streptomyces ambofaciens, StreptonZyces avermitilis,
Sty°eptomyces
ff°adiae, Stf°eptomyces griseus, Streptomyces hydroscopicus,
Streptomyces
tsukulubaensis, Stf°eptomyces mycarofasciens, Streptomyces platenesis,
Sts°eptomyces violaceoniger, Streptonayces violaceoniger,
Streptonayees
that°motolef°ans, Sty°eptomyces rimosus,
Stf°eptonzyces peucetius, Str°eptonayces
coelicolor, Strepton2yces glaueescens, Streptomyces roseofulvus, Streptonayces
cinnamonensis, Sti°eptomyces curacoi, and Amycolatopsis
meditef°f°anei. Other
examples of polyketide-producing microorganisms that produce polyketides
naturally include various Actinomadura, Dactylospof°angium and Nocardia
strains. Preferred Sty°eptomyces spp. include but are not limited to
Streptonzyces
venezuelae (e.g., ATCC 15439, ATCC 1506, MCRL 0306, SC 2366 or 3629),
Streptomyces naf-bonensis (e.g., ATCC 19790), Streptomyees
eZtf°ocidicus,
Streptomyces zaomyceticus (MCRL 0405), Streptomyces
flavochf°omogens,
Streptomyces sp. AM400, Stf°eptomyces fellezcs, Streptotnyces
fradiae,
Stf°eptomyces argillaceus, Streptomyees olivaceus, Streptomyces
peucetius, and
Streptomyces gf°iseus.
Moreover, those same cells are a preferred source of the nucleic acid
segments of the invention. Thus, any cell which encodes a sugar biosynthetic
gene is a source for the nucleic acid segments of the invention. For example,
a
source for nucleic acid segments are cells Which produce a compound having a
sugar including but not limited to cells that produce streptomycin,
carbomycin,
tylosin, spiramycin, streptothricin, erythromycin, vancomycin, teicoplanin,
chloroer~mycin, methymycin, pikromycin, uramycin, granaticin, oleandomicin,
landomycin, tetracenomycin, doxorubicin, mithramycin, epirubicin, and
5
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
daunoribicin, or other sugar-containing compounds such as calicheamicin or
nystatin, are included within the scope of the nucleic acid segments for use
in the
practice of the invention.
In one embodiment of the invention, a recombinant host cell in which a
nucleic acid sequence encoding at least one of the enzymes in desosamine
biosynthesis is disrupted so as to alter desosamine synthesis, and is
augmented
with a nucleic acid segment which encodes a homolog of the enzyme encoded by
the nondisrupted form of the nucleic acid sequence, yielding a modified
recombinant host cell. In one embodiment, the modified recombinant host cell
does not have a disruption is dell and does not consist of a calH nucleic acid
segment. A "homolog" of a reference sugar biosynthetic enzyme is an enzyme
which can recognize the substrate of the reference biosynthetic enzyme and
catalyze a reaction. For example, TyIB is a homolog of DesI, CaIH is a homolog
of DesI, StrL and StrM together are a homolog of DesI, and TylM2 is a homolog
of DesVI. Preferred homologs catalyze a reaction that produces a product, such
an intermediate in sugar biosynthesis, that is different than the product of
the
reference enzyme. Homologs can be identified functionally using methods such
as those described herein. Generally, a homolog has at least about 28% amino
acid sequence identity to the reference enzyme.
Other methods to identify a nucleic acid segment for use in the invention
is by hybridization or computer assisted sequence alignments, e.g., using
default
settings. In one embodiment of the invention, the nucleic acid sequence of the
invention hybridizes under low, moderate or stringent hybridization conditions
to the nucleic acid segment of the invention. Low, moderate and stringent
hybridization conditions are well known to the art, see, for example sections
9.47-9.51 of Sambrook et al. (Molecular Cloning: A Laboratory Manual, Cold
Spring Harbor Laboratory, Cold Spring Harbor, NY (1989). For example,
stringent conditions are those that (1) employ low ionic strength and high
temperature for washing, for example, 0.015 M NaCI/0.0015 M sodium citrate
(SSC); 0.1% sodium lauryl sulfate (SDS) at 50°C, or (2) employ a
denaturing
agent such as formamide during hybridization, e.g., 50% formamide with 0.1%
6
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
bovine serum albumin/0.1 % Ficoll/0.1 % polyvinylpyrrolidone/50 mM sodium
phosphate buffer at pH 6.5 with 750 mM NaCl, 75 mM sodium citrate at
42°C.
Exemplary low stringency conditions include hybridization with a buffer
solution of 30 to 35% formamide, 1 M NaCI, 1% SDS (sodium dodecyl
sulphate) at 37°C, and a wash in 1X to 2X SSC (20X SSC = 3.0 M NaCI/0.3
M
trisodium, citrate) at 50 to 55°C. Exemplary moderate stringency
conditions
include hybridization in 40 to 45% formamide, 1.0 M NaCI, 1% SDS at
37°C,
and a wash in O.SX to 1X SSC at 50 to 60°C. Exemplary high stringency
conditions include hybridization in 50% formamide, 1 M NaCI, 1 % SDS at
37°C, and a wash in O.1X SSC at 60 to 65°C.
Also provided are methods of preparing the modified recombinant host
cells of the invention and methods of using them, e.g., to prepare
biologically
active products or products which can be modified to a biologically active
product.
The invention also provides an isolated and purified nucleic acid segment
comprising a nucleic acid sequence comprising a sugar (desosamine)
biosynthetic gene cluster, a biologically active variant or fragment thereof,
wherein the nucleic acid sequence is not derived from the efyC gene cluster of
Saccharopolyspora erytlaraea. The isolated nucleic acid segment comprising the
gene cluster preferably includes a nucleic acid sequence comprising SEQ ID
N0:3 (see PCT/LTS 99/14398, which is incorporated by reference herein), or a
fragment or variant thereof. The cluster was found to encode nine polypeptides
including DesI (e.g., SEQ ID N0:8 encoded by SEQ ID N0:7), DesII (e.g., SEQ
ID NO:10 encoded by SEQ ID N0:9), DesIII (e.g., SEQ ID NO:12 encoded by
SEQ ID NO:l 1), DesIV (e.g., SEQ ID N0:14 encoded by SEQ ID N0:13), DesV
(e.g., SEQ ID N0:16 encoded by SEQ ID NO:15), DesVI (e.g., SEQ ID NO:l 8
encoded by SEQ ID N0:17), DesVII (e.g., SEQ ID N0:20 encoded by SEQ ID
N0:19), DesVIII (e.g., SEQ ID N0:22 encoded by SEQ ID N0:21), and DesR
(e.g., SEQ ID N0:24 encoded by SEQ ID N0:23) (see Figure 1). It is also
preferred that the nucleic acid segment of the invention encoding DesR is not
derived from the efyB gene cluster of Sacclaaf°opolyspora e~ytlaraea or
the oleD
7
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
gene from Streptomyces antibioticus. Preferably, the nucleic acid segment
comprising the desosamine biosynthetic gene cluster hybridizes under moderate,
or more preferably stringent, hybridization conditions to SEQ ID N0:3, or a
fragment thereof.
The invention also provides a variant polypeptide having at least about
80%, more preferably at least about 90%, and even more preferably at least
about 95%, but Iess than 100%, contiguous amino acid sequence identity to the
polypeptide having an amino acid sequence comprising SEQ ID N0:8, SEQ ID
NO:10, SEQ ID N0:12, SEQ ID N0:14, SEQ ID N0:16, SEQ ID N0:18, SEQ
ID N0:20, SEQ ID N0:22, SEQ ID N0:24, or a fragment thereof. A preferred
variant polypeptide, or a subunit or fragment of a polypeptide, of the
invention
includes a variant or subunit polypeptide having at least about 1 %, more
preferably at least about 10%, and even more preferably at least about
50°/~, the
activity of the polypeptide having the amino acid sequence comprising SEQ ID
NO:B, SEQ ID NO:10, SEQ ID N0:12, SEQ ID N0:14, SEQ ID N0:16, SEQ ID
N0:18, SEQ ID N0:20, SEQ ID N0:22, or SEQ ID N0:24. Thus, for example,
the glycosyltransferase activity of a polypeptide of SEQ ID N0:20 can be
compared to a variant of SEQ ID N0:20 having at least one amino acid
substitution, insertion, or deletion relative to SEQ ID N0:20.
A variant nucleic acid sequence of the invention has at least about 80%,
more preferably at least about 90%, and even more preferably at least about
95%, but less than 100%, contiguous nucleic acid sequence identity to a
nucleic
acid sequence comprising SEQ ID N0:3, SEQ ID N0:7, SEQ ID N0:9, SEQ ID
NO:l l, SEQ ID N0:13, SEQ ID NO:15, SEQ ID N0:17, SEQ ID N0:19, SEQ
ID NO:21, SEQ ID N0:23, or a fragment thereof.
Also provided is an expression cassette comprising a nucleic acid
sequence comprising a desosamine biosynthetic gene cluster, a biologically
active variant or fragment thereof operably linked to a promoter functional in
a
host cell, as well as host cells comprising an expression cassette of the
invention.
Thus, the expression cassettes of the invention are useful to express
individual
genes within the cluster, e.g., the desR gene which encodes a glycosidase or
the
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
desVII gene which encodes a glycosyltransferase having relaxed substrate
specificity for polyketides and deoxysugars, i.e., the glycosyltransferase
processes sugar substrates other than TDP-desosamine. Thus, the desVII gene
can be employed in combinatorial biology approaches to synthesize a library of
macrolide compounds having various polyketide and deoxysugar structures.
Moreover, the expression of a glycosylase in a host cell which synthesizes a
macrolide antibiotic may be useful in a method to reduce toxicity of, e.g.,
inactivate, the antibiotic. For example, a host cell which produces the
antibiotic
is transformed with an expression cassette encoding the glycosyltransferase.
The
recombinant glycosyltransferase is expressed in an amount that reversibly
inactivates the antibiotic. To activate the antibiotic, the antibiotic,
preferably
the isolated antibiotic which is recovered from the host cell, is contacted
with an
appropriate native or recombinant glycosidase.
Preferably, the nucleic acid segment encoding desosamine in the
expression cassette of the invention is not derived form the efyC gene cluster
of
Sacclaa~°opolyspora efytlaf°aea. Preferred host cells are
prokaryotic cells,
although eukaryotic host cells are also envisioned. These host cells are
useful to
express desosamine, analogs or derivatives thereof as well as individual
polypeptides which can then be isolated from the host cell. Also provided is
an
expression cassette or host cell comprising antisense sequences from at least
a
portion of the desosamine biosynthetic gene cluster.
Another embodiment of the invention is a recombinant host cell, e.g., a
bacterial cell, in which at least a portion of a nucleic acid sequence
encoding
desosamine in the host chromosome is disrupted, e.g., deleted or interrupted
(e.g., by an insertion) with heterologous sequences, or substituted with a
variant
nucleic acid sequence of the invention, so as to alter, preferably so as to
result in
a decrease or lack of, desosamine synthesis andlor so as to result in the
synthesis
of an analog or derivative of desosamine. Preferably, the nucleic acid
sequence
which is disrupted is not derived from the eryC gene cluster of
Sacchaf°opolyspof-a e~ythraea. Thus, the recombinant host cell of the
invention
has at least one gene, i.e., desl, desll, deslll, deslV, desV, desT~I, desVII,
desVlll
9
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
or desR, which is disrupted. One embodiment of the invention includes a
recombinant host cell in which the deshl gene, which encodes an N-
methyltransferase, is disrupted, for example, by replacement with an
antibiotic
resistance gene. Preferably, such a host cell produces an aglycone having anN
acetylated aminodeoxy sugar, 10-deoxy-methylonide, a compound of formula
(7), a compound of formula (8), or a combination thereof. Thus, the deletion
or
disruption of the desPl gene may be useful in a method for preparing novel
sugars.
Another preferred embodiment of the invention is a recombinant
bacterial host cell in which the desR gene, which encodes a glycosidase such
as
(3-glucosidase, is disrupted. Preferably, the host cell synthesizes C-2' (3-
glucosylated macrolide antibiotics, for example, a compound of formula (13), a
compound of formula (14), or a combination thereof. Therefore, the invention
further provides a compound of formula (8), (9), (13) or (14). It will be
appreciated by those skilled in the art that each atom of the compounds of the
invention having a chiral center may exist in and be isolated in optically
active
and racemic forms. Some compounds may exhibit polymorphism. It is to be
understood that the present invention encompasses any racemic, optically
active,
polymorphic or stereoisomeric form, or mixtures thereof, of a compound of the
invention, which possess the useful properties described herein, it being well
known in the art how to prepare optically active forms (for example, by
resolution of the racemic form by recrystallization techniques, by synthesis
from
optically active starting materials, by chiral synthesis, or by
chromatographic
separation using a chiral stationary phase) and how to determine activity
using
the standard tests described herein, or using other similar tests which are
well
known in the art.
Also provided is a method for directing the biosynthesis of specific
glycosylation-modified polyketides by genetic manipulation of a polyketide-
producing microorganism. The method comprises introducing into a polyketide-
producing microorganism a DNA sequence encoding enzymes for sugar
biosynthesis, e.g., desosamine biosynthesis such as a DNA sequence comprising
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
SEQ ID N0:3, a variant or fragment thereof, so as to yield a microorganism
that
produces specific glycosylation-modified polyketides. Alternatively, an anti-
sense DNA sequence of the invention may be employed. Then the
glycosylation-modified polyketides are isolated from the microorganism. It is
preferred that the DNA sequence is modified so as to result in the
inactivation of
at least one enzymatic activity in sugar biosynthesis or in the attachment of
the
sugar to a polyketide.
The compounds (products) produced by the recombinant host cells and
modified recombinant host cells of the invention may be particularly useful as
biologically active agents, such as those useful to prepare a medicament for
the
treatment of a pathological condition or a symptom in a mammal, e.g., a human.
Thus, the products include pharmaceuticals such as chemotherapeutic agents,
immunosuppressants, agents to treat asthma, chronic obstructive pulmonary
disease as well as other diseases involving respiratory inflammation,
cholesterol-
lowering agents, or macrolide-based antibiotics which are active against a
variety
of organisms, e.g., bacteria, including mufti-drug-resistant pneumococci and
other respiratory pathogens, as well as viral and parasitic pathogens; or as
crop
protection agents (e.g., fungicides or insecticides). Methods employing these
compounds, e.g., to treat a mammal, bird or fish in need of such therapy, such
as
a patient having a bacterial, viral or parasitic infection, cancer,
respiratory
disease, or in need of immunosuppression, e.g., during cell, tissue or organ
transplantation, are also envisioned.
Brief Description of the Figures
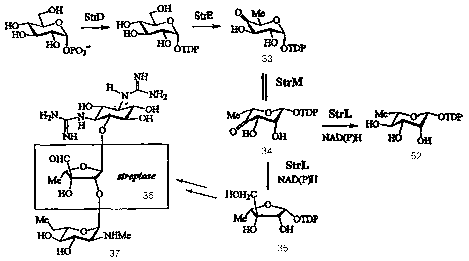
Figure 1. Schematic diagram of the desosamine biosynthetic pathway
and the enzymatic activity associated with each of the desosamine biosynthetic
polypeptides.
Figure 2. Schematic of the conversion of the inactive (diglycosylated)
form of methymycin and pikromycin to the active form of methymycin and
pikromycin.
Figure 3. Schematic diagram of the desosamine biosynthetic pathway.
11
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Figure 4. Pathway for the synthesis of a compound of formula 7 and 8 in
desVI- mutants of Sts°eptomyces.
Figure 5. Structure and biosynthesis of methymycin, pikromycin, and
related compounds in Streptomyces venezuelae ATCC 15439. Methymycin:
R~=OH, Rz H, neomethymycin: R~=H, RZ = OH; pikromycin: R3 OH,
narbomycin: R3 = H. Polyketide synthase components PikAI, PikAII, PikAIII,
PikAIV, and PikAV are represented by solid bars. Each circle represents an
enzymatic domain in the Pik PISS system. KS: [3-ketoacyl-ACP synthase, AT:
acyltransferase, ACP: acyl carrier protein, KR: (3-ketoacyl-ACP reductase, DH:
(3-hydroxyl-thioester dehydratase, ER: enoyl reductase, KS~: a IBS-like
domain,
I~R with a cross: nonfunctional I~R, TE: thioesterase domain, and TEII: type
II
thioesterase. Des represents all eight enzymes for desosamine biosynthesis and
transfer and PikC is the cytochrome P450 monooxygenase responsible for
hydroxylation at R~ , R2, and R3 positions (Xue et al., 1998).
Figure 6. Organization of the pik cluster in S. venezuelae. Each arrow
represents an open reading frame (ORF). The direction of transcription and
relative sizes of the ORFs deduced from nucleotide sequence are indicated. The
cluster is composed of four genetic loci: pikA, pikB (des), pikC, and pikR.
Cosmid clones are denoted as overlapping lines.
Figure 7. Conversion of YC-17 and narbomycin by PikC P450
hydroxylase.
Figure 8. Nucleotide sequence (SEQ ID N0:3) and inferred amino acid
sequence (SEQ ID N0:4) of the desosamine gene cluster.
Figure 9. Exemplary and preferred amino acid substitutions.
Figure 10. Pathway for desosamine biosynthesis.
Figure 11. Schematic of pathway leading to
methymycin/neomethymycin analogs 18 and 19.
Figure 12. Macrolide having D-quinovose.
Figure 13. Products produced by desl mutant.
Figure 14. Macrolides produced in a desl mutant which expresses CalH.
12
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Figure 15. Natural substrate for and product of CaIH, and structure of
calicheamicin.
Figure 16. Macrolides produced in a desI mutant which expresses StrL
and StrM.
Figure 17. Natural substrate for and product of StrL and StrM.
Figure 18. Substrate for and products of apiose synthase.
Figure 19. Scheme for desosamine biosynthesis and intermediates in des
mutants.
Figure 20. Alternative scheme for desosamine biosynthesis.
Detailed Description of the Invention
Definitions
As used herein, a "Type I polyketide synthas~' is a single polypeptide
with a single set of iteratively used active sites. This is in contrast to a
Type II
polyketide synthase which employs active sites on a series of polypeptides.
As used herein, a "module" is one of a series of repeated units in a
multifunctional protein, such as a Type I polyketide synthase or a fatty acid
synthase.
As used herein, a "premature termination product" is a product which is
produced by a recombinant multifunctional protein which is different than the
product produced by the non-recombinant multifunctional protein. In general,
the product produced by the recombinant multifunctional protein has fewer acyl
groups.
As used herein, a "recombinant" nucleic acid or protein (polypeptide)
molecule is a molecule where the nucleic acid molecule which encodes the
protein has been modified in vitro, so that its sequence is not naturally
occurring,
or corresponds to naturally occurring sequences that are not positioned as
they
would be positioned in a genome which has not been modified.
A "recombinant" host cell of the invention has been genetically
manipulated so as to alter, e.g., decrease or disrupt, or, alternatively,
increase, the
function or activity of at least one gene in a sugar biosynthetic pathway. The
13
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
manipulation may occur in an extrachromosomal genetic element which
comprises the at least one gene or in the genome of the cell. In contrast, a
"wild
type" or "nonrecombinant" cell has not been genetically manipulated. The
genetic manipulation in the recombinant cell preferably results in the absence
of
a product (compound) that is produced by the corresponding wild type cell or
the
production of a product that is not produced by the corresponding wild type
cell.
A "modified" recombinant host cell of the invention is a recombinant
host cell that has been genetically manipulated so as to express at least one
isolated nucleic acid segment, preferably in the form of an expression
cassette
which includes a promoter, that is introduced to the recombinant cell to form
the
modified recombinant host cell. The genetic manipulation in the modified
recombinant host cell preferably results in the production of a product
(compound) that is not produced by the corresponding recombinant host cell or
the corresponding wild type cell.
As used herein, a DNA that is "derived from" a gene or gene cluster is a
DNA that has been isolated and purified in vitro from genomic DNA, or
synthetically prepared on the basis of the sequence of genomic DNA.
As used herein, the 'pi7~' or 'piklmet" gene cluster includes sequences
encoding a polyketide synthase (pikA), desosamine biosynthetic enzymes (pikB,
also referred to as des), a cytochrome P450 (jaikC), regulatory factors (pikD)
and
enzymes for cellular self resistance (pikR).
As used herein, the terms "isolated and/or purified" refer to iT2 vitf-o
isolation of a DNA or polypeptide molecule from its natural cellular
environment, and from association with other components of the cell, such as
nucleic acid or polypeptide, so that is can be sequenced, replicated and/or
expressed. For example, "an isolated DNA molecule encoding an enzyme for
desosamine biosynthesis or a fragment thereof' is RNA or DNA containing
greater than 7, preferably 15, and more preferably 20 or more sequential
nucleotide bases that encode a biologically active polypeptide, fragment, or
variant thereof, that is complementary to the non-coding, or complementary to
the coding strand, of a RNA encoding at least one enzyme for desosamine
14
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
biosynthesis, or hybridizes to the RNA or DNA comprising the desosamine
biosynthetic gene cluster and remains stably bound under low, moderate or
preferably stringent conditions, as defined by methods well known to the art,
e.g., in Sambrook et al., 1989.
An "antibiotic" as used herein is a substance produced by a
microorganism which, either naturally or with limited chemical modification,
will inhibit the growth of or kill another microorganism or eukaryotic cell.
An "antibiotic biosynthetic gene" is a nucleic acid, e.g., DNA, segment
or sequence that encodes an enzymatic activity which is necessary for an
enzymatic reaction in the process of converting primary metabolites into
antibiotics.
An "antibiotic biosynthetic pathway" includes the entire set of antibiotic
biosynthetic genes necessary for the process of converting primary metabolites
into antibiotics. These genes can be isolated by methods well known to the
art,
e.g., see U.S. Patent No. 4,935,340.
Antibiotic-producing organisms include any organism, including, but not
limited to, Actinoplanes, Actinomadura, Bacillus, Cephalosporium,
Micromonospora, Penicillium, Nocardia, and Streptomyces, which either
produces an antibiotic or contains genes which, if expressed, would produce an
antibiotic.
An antibiotic resistance-conferring gene is a DNA segment that encodes
an enzymatic or other activity which confers resistance to an antibiotic.
The term "polyketide" as used herein refers to a large and diverse class of
natural products, including but not limited to antibiotic, antifungal,
anticancer,
and anti-helminthic compounds. Polyketides include but are not limited to
macrolides, anthracyclines, angucyclins, avermectins, milbemycins,
tetracyclines, polyenes, polyethers, ansamycins and isochromanequinones and
the like. Polyketide antibiotics include, but are not limited to
anthracyclines and
macrolides of different types (polyenes and avermectins as well as classical
macrolides such as erythromycins). Macrolides are produced by, for example, S.
erytheus, S. aratibioticus, S. venezuelae, S. fradiae and S. narbonensis.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
The term "glycosylated" in the context of another molecule refers to a
molecule that contains one or more sugar residues.
The term "sugar" or "saccharide" refers to a polyhydroxylated atdehyde
or ketone. The polyhydroxylated atdehyde or ketone can optionally be linked to
lipids, peptides and/or proteins. Sugars may have additional substituents such
as
amino, sulfate or phosphate groups, in addition to the carbon-hydrogen-oxygen
core. A polymer consisting of two to ten saccharide units is termed an
oligosaccharide (OS), e.g., monosaccharides, disaccharides, e.g., sucrose, and
trisaccharides, and those consisting of more than ten saccharide units is
termed a
polysaccharide (PS). These monosaccharide building blocks can be linked in at
least 10 different ways, leading to an astronomical number of different
combinations and permutations. Sugars include, e.g., trioses, pentoses and
hexoses, ribose, glucose, as well as deoxy sugars such as fructose, rhamnose,
and
deoxyribose, and 6-, 2,6-, 3,6-, 4,6-, 2,3,6-deoxysugars, such as otivose,
otiose,
mycarose, rhodinose, mycinose, and other modified sugars (e.g., amino sugars
including mycaminose, desosamine, vancosamine and daunosamine). Additional
suitable sugars are disclosed, e.g., in D. Voet, Biochemistry, Wiley: New
York,
1990; L. Stryer, Biochemistry, (3rd Ed.), W.H. Freeman and Co.: New York,
1975; J. March, Advanced Organic ChemistrX, Reactions, Mechanisms and
Structure, (2nd Ed.), McGraw Hill: New York, 1977; F. Carey and R. Sundberg,
Advanced Organic Chemistry, Part B: Reactions and Synthesisy (2nd Ed.),
Plenum: New York, 1977; and references cited therein). Saccharide derivatives
can conveniently be prepared as described in International Patent Applications
Publication Numbers WO 96/34005 and 97/03995.
The term "glycosylation-modified" as it relates to a particular molecule
refers to a molecule having a changed glycosylation pattern or configuration
relative to that particular molecule's unmodified or native state.
The term'"polyketide-producing microorganism" as used herein includes
any microorganism that can produce a potyketide naturally or after being
suitably
engineered (i.e., genetically). Examples of actinomycetes that naturally
produce
polyketides include but are not limited fo Micromonospora t°osaria,
16
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Micromonospora megalornicea, Saccharopolyspora erytlaraea, Streptonayces
antibioticus, Sts°eptomyces albereticuli, Streptomyces anZbofaciens,
Streptomyces
avernaitilis, Streptomyces fr°adiae, Streptornyces gf°iseZts,
Streptomyces
laydroscopicus, Streptomyces tsuleulubaensis, Streptomyces mycarofascierZS,
Streptomyces plateraesis, Sts°eptonZyces violaceoniger,
Streptomyces
violaceoraiger, Sty°eptomyces thermotolef°ans, Streptonayces
f°imosus,
Stt°eptomyces peucetius, Streptomyces coelicolor, St~~eptomyces
glaucescens,
Streptomyces roseofulvus, Sts°eptomyces cinnamoraensis, Streptonayces
curacoi,
and An2ycolatopsis mediterranei. Other examples of polyketide-producing
microorganisms that produce polyketides naturally include various
Actinomadura, Dactylospof°angium and Nocardia strains.
The term "sugar biosynthesis genes" as used herein refers to nucleic acid
sequences or segments from organisms such as Micromonospora, Streptomyces
vefaezuelae, Stf'eptonayces f -adiae, Sts°eptomyces griseus,
Streptonayces peucetius,
Stf°eptomyces a~gillaceous, and Streptornyces olivaceZfs that
encode sugar
biosynthesis enzymes, and is intended to include sugar biosynthetic DNA from
other polyketide-producing microorganisms.
The term "sugar biosynthesis enzymes" as used herein refers to
polypeptides which are involved in the biosynthesis and/or attachment of
polyketide-associated sugars and their derivatives and intermediates.
The term "polyketide-associated sugar" refers to a sugar that is known to
attach to polyketides or that can be attached to polyketides.
The term "sugar derivative" refers to a sugar which is naturally
associated with a polyketide but which is altered relative to the unmodified
or
native state, including but not limited to N-3-a-desdimethyl D-desosamine.
The term "sugar intermediate" refers to an intermediate compo"und
produced in a sugar biosynthesis pathway.
As used herein, the term "derivative" means that a particular compound
(product) produced by a host cell of the invention or prepared in vitro using
polypeptides encoded by the nucleic acid molecules of the invention, is
modified
so that it comprises other moieties, e.g., peptide or polypeptide molecules,
such
17
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
as antibodies.or fragments thereof, nucleic acid molecules, sugars, lipids,
fats, a
detectable signal molecule such as a radioisotope, e.g., gamma emitters, small
chemicals, metals, salts, synthetic polymers, e.g., polylactide and
polyglycolide,
surfactants and glycosaminoglycans, which are covalently or non-covalently
attached or linked to the compound.
It will be appreciated by those skilled in the art that each atom of the
compounds of the invention having a chiral center may exist in and be isolated
in
optically active and racemic forms. Some compounds may exhibit
polymorphism. It is to be understood that the present invention encompasses
any
racemic, optically active, polymorphic or stereoisomeric form, or mixtures
thereof, of a compound of the invention, which possess the useful properties
described herein, it being well known in the art how to prepare optically
active
forms (for example, by resolution of the racemic form by recrystallization
techniques, by synthesis from optically
active starting materials, by chiral synthesis, or by chromatographic
separation
using a chiral stationary phase) and how to determine activity using the
standard
tests described herein, or using other similar tests which are well known in
the
art.
The term "sequence homology" or "sequence identity" means the
proportion of base matches between two nucleic acid sequences or the
proportion amino acid matches between two amino acid sequences. When
sequence homology is expressed as a percentage, e.g., 50%, the percentage
denotes the proportion of matches over the length of sequence that is compared
to some other sequence. Gaps (in either of the two sequences) are permitted to
maximize matching; gap lengths of 15 bases or less are usually used, 6 bases
or
less are preferred with 2 bases or less more preferred. When using
oligonucleotides as probes, the sequence homology between the target nucleic
acid and the oligonucleotide sequence is generally not less than 17 target
base
matches out of 20 possible oligonucleotide base pair matches (85%); preferably
not less than 9 matches out of 10 possible base pair matches (90%), and more
preferably not less than 19 matches out of 20 possible base pair matches
(95%).
18
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Two amino acid sequences are homologous if there is a partial or
complete identity between their sequences and/or have the same or similar
activity. For example, 85% homology means that 85% of the amino acids are
identical when the two sequences are aligned for maximum matching. Gaps (in
either of the two sequences being matched) are allowed in maximizing matching;
gap lengths of 5 or less are preferred with 2 or less being more preferred.
Alternatively and preferably, two protein sequences (or polypeptide sequences
derived from them of at least 30 amino acids in length) are homologous, as
this
term is used herein, if they have an alignment score of at more than 5 (in
standard deviation units) using the program ALIGN with the mutation data
matrix and a gap penalty of 6 or greater (Dayhoff, 1972). The two sequences or
parts thereof are more preferably homologous as used herein if their amino
acids
are greater than or equal to 29% identical.
The following terms are used to describe the sequence relationships
between two or more polynucleotides or polypeptides: "reference sequence",
"comparison window", "sequence identity", "percentage of sequence identity",
and "substantial identity". A "reference sequence" is a defined sequence used
as
a basis for a sequence comparison; a reference sequence may be a subset of a
larger sequence, for example, as a segment of a full-length cDNA or gene
sequence given in a sequence listing, or may comprise a complete cDNA or gene
sequence. Generally, a reference sequence is at Least 20 nucleotides in
length,
frequently at least 25 nucleotides in length, and often at least 50
nucleotides in
length. Since two polynucleotides may each (1) comprise a sequence (i.e., a
portion of the complete polynucleotide sequence) that is similar between the
two
polynucleotides, and (2) may further comprise a sequence that is divergent
between the two polynucleotides, sequence comparisons between two (or more)
polynucleotides are typically performed by comparing sequences of the two
polynucleotides over a "comparison window" to identify and compare local
regions of sequence similarity.
A "comparison window", as used herein, refers to a conceptual segment
of at least 20 contiguous nucleotides and wherein the portion of the
19
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
polynucleotide sequence in the comparison window may comprise additions or
deletions (i.e., gaps) of 20 percent or less as compared to the reference
sequence
(which does not comprise additions or deletions) for optimal alignment of the
two sequences. Optimal alignment of sequences for aligning a comparison
window may be conducted by the local homology algorithm of Smith and
Waterman (1981) by the homology alignment algorithm of Needleman and
Wunsch (1970), by the search for similarity method of Pearson and Lipman
(1988), by computerized implementations of these algorithms (GAP, BESTFIT,
FASTA, and TFASTA in the Wisconsin Genetics Software Package Release 7.0,
Genetics Computer Group, 575 Science Dr., Madison, Wis.), or by inspection,
and the best alignment (i.e., resulting in the highest percentage of homology
over
the comparison window) generated by the various methods is selected.
Preferably, default settings are employed to identify homologs using
computerized algorithms.
The term "sequence identity" means that two polynucleotide sequences
are identical (i.e., on a nucleotide-by-nucleotide basis) over the window of
comparison. The term "percentage of sequence identity' means that two
polynucleotide sequences are identical (i.e., on a nucleotide-by-nucleotide
basis)
over the window of comparison. The term "percentage of sequence identity" is
calculated by comparing two optimally aligned sequences over the window of
comparison, determining the number of positions at which the identical nucleic
acid base (e.g., A, T, C, G, U, or I) occurs in both sequences to yield the
number
of matched positions, dividing the number of matched positions by the total
number of positions in the window of comparison (i.e., the window size), and
multiplying the result by 100 to yield the percentage of sequence identity.
As applied to polypeptides, the term "substantial identity" or "homology"
means that two peptide sequences, when optimally aligned, such as by the
programs GAP or BESTFIT using default gap weights, share at least about 29
percent sequence identity, preferably at least about 35 percent sequence
identity
and/or have the same or similar activity, i.e., recognize one or more common
substrates) and thereby produce a product.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
In accordance with the present invention there is provided a modified
recombinant host cell, derived from a recombinant host cell, the genorne of
which is altered, optionally to disrupt sugar biosynthesis that occurs in the
corresponding wild type cell. The modified recombinant host cell is augmented
with a nucleic acid segment that encodes at least one sugar biosynthetic
enzyme
that is a homolog of an enzyme encoded by the wild type cell which is absent
or
present in a reduced amount in the recombinant host cell as a result of the
disruption. Thus, the modified recombinant host cell includes a least one
expression cassette comprising at least one isolated and purified nucleic acid
I O segment which encodes a sugar biosynthetic enzymes) that recognizes the
substrate of an enzymes) encoded by the wild type cell and which is not
expressed, or expressed in a reduced amount, in the recombinant cell. The
enzymes) encoded by the nucleic acid segment produces a substrate for another
sugar biosynthetic enzyme or for a glycosyltransferase.
The invention described herein can be used for the production of a
diverse range of novel compounds including glycosylated polyketides, e.g.,
antibiotics, through genetic redesign of sugar biosynthetic DNA such as that
found in Sty°eptonzyces spp. as well as other polyketide producing
organisms.
This gene allows for the selective production of particular compounds,
including
the production of novel compounds. For example, combinational biosynthetic-
based modification of compounds may be accomplished by selective activation
or disruption of specific genes within the sugar gene cluster and expressing
other
sugar biosynthetic genes into biosynthetic libraries which are assayed for a
wide
range of biological activities, to derive greater chemical diversity. A
further
example includes the introduction of biosynthetic genes) into a particular
host
cell so as to result in the production of a novel compound due to the activity
of
the biosynthetic genes) on other metabolites, intermediates or components of
the host cells.
The nucleic acid sequences and segments employed in the invention
include those that hybridize under low, moderate or stringent hybridization
conditions to the genes encoding sugar biosynthetic enzymes, such as those set
21
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
forth herein, and/or encode enzymes that have the same or similar activity. A
nucleic acid molecule, segment or sequence of the present invention can also
be
an RNA molecule, segment or sequence which corresponds to, is complementary
to or hybridizes under low, moderate, or stringent conditions to any of the
DNA
segments or sequences described herein. Thus, the invention includes nucleic
acid sequences and segments that encode a homolog of a particular sugar
biosynthetic enzyme, including a polypeptide that has at least one amino acid
substitution (Figure 9; Alberts et al., 1989), relative to a wild type
polypeptide,
e.g., the homolog may have at least 29% identity to the wild type polypeptide,
as
long as the homolog can recognize and catalyze a reaction with a substrate for
the wild type enzyme. The homolog may be a naturally occuring enzyme or one
that is prepared recombinantly.
Thus, mutations can be made to a native (wild type) nucleic acid segment
or sequence of the invention to yield a variant nucleic acid segment or
sequence,
and such variants may be used in place of the native segment or sequence, so
long as the variant encodes an enzymes) that functions with other molecules to
collectively catalyze the synthesis of an identifiable glycosylatedmolecule
such
as a glycosylated polyketide or macrolide. Such mutations can be made to the
native sequences using conventional techniques such as by preparing synthetic
oligonucleotides including the mutations and inserting the mutated sequence
into
the gene using restriction endonuclease digestion (see, e.g., Kunkel, 1985;
Geisselsoder et al., 1987). Alternatively, the mutations can be effected using
a
mismatched primer (generally 10-20 nucleotides in length) which hybridizes to
the native nucleotide segment or sequence, at a temperature below the melting
temperature of the mismatched duplex. The primer can be made specific by
keeping primer length and base composition within relatively narrow limits and
by keeping the mutant base centrally located (Zoller and Smith, 1983). Primer
extension is effected using DNA polymerase, the product cloned and clones
containing the mutated DNA, derived by segregation of the primer extended
strand, selected. Selection can be accomplished using the mutant primer as a
hybridization probe. The technique is also applicable for generating multiple
22
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
point mutations. See, e.g., Dalbie-McFarland et al. (192). PCR mutagenesis
will also find use for effecting the desired mutations.
Random mutagenesis of the nucleotide sequence can be accomplished by
several different techniques known in the art, such as by altering sequences
within restriction endonuclease sites, inserting an oligonucleotide linker
randomly into a plasmid, by irradiation with X-rays or ultraviolet light, by
incorporating incorrect nucleotides during in vitf~o DNA synthesis, by error-
prone PCR mutagenesis, by preparing synthetic mutants or by damaging plasmid
DNA in vita°o with chemicals. Chemical mutagens include, for example,
sodium
bisulfite, nitrous acid, hydroxylamine, agents which damage or remove bases
thereby preventing normal base-pairing such as hydrazine or formic acid,
analogues of nucleotide precursors such as nitrosoguanidine, 5-bromouracil, 2-
aminopurine, or acridine intercalating agents such as proflavine, acriflavine,
quinacrine, and the like. Generally, plasmid DNA or DNA fragments are treated
with chemicals, transformed into E. coli and propagated as a pool or library
of
mutant plasmids.
Large populations of random enzyme variants can be constructed ira vivo
using "recombination-enhanced mutagenesis." This method employs two or
more pools of, for example, 106 mutants each of the wild type encoding
nucleotide sequence that are generated using any convenient mutagenesis
technique and then inserted into cloning vectors.
The gene sequences can be inserted into one or more expression vectors,
using methods known to those of skill in the art. Expression vectors may
include
control sequences operably linked to the desired genes. Suitable expression
systems for use with the present invention include systems which function in
eukaryotic and prokaryotic host cells. Prokaryotic systems are preferred, and
in
particular, systems compatible with Sty eptomyces spp. are of particular
interest.
Control elements for use in such systems include promoters, optionally
containing operator sequences, and ribosome binding sites. Particularly useful
promoters include control sequences derived from the gene clusters of the
invention. However, other bacterial promoters, such as those derived from
sugar
23
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
metabolizing enzymes, such as galactose, lactose (lac) and maltose, will also
find
use in the expression cassettes encoding desosamine: Preferred promoters are
Streptomyces promoters, including but not limited to the ef-mE*, pi7~A and
tipA
promoters. Additional examples include promoter sequences derived from
biosynthetic enzymes such as tryptophan (trp), the ~3-lactamase (bla) promoter
system, bacteriophage lambda PL, and T5. In addition, synthetic promoters,
such as the tac promoter (LT.S. Pat. No. 4,551,433), which do not occur in
nature,
also function in bacterial host cells.
Other regulatory sequences may also be desirable which allow for
regulation of expression of the genes relative to the growth of the host cell.
Regulatory sequences are known to those of skill in the art, and examples
include those which cause the expression of a gene to be turned on or off in
response to a chemical or physical stimulus, including the presence of a
regulatory compound. Other types of regulatory elements may also be present in
the vector, for example, enhancer sequences.
Selectable markers can also be included in the recombinant expression
vectors. A variety of markers are known which are useful in selecting for
transformed cell lines and generally comprise a gene whose expression confers
a
selectable phenotype on transformed cells when the cells are grown in an
appropriate selective medium. Such markers include, for example, genes which
confer antibiotic resistance or sensitivity to the plasmid.
The various sequences or segments of interest can be cloned into one or
more recombinant vectors as individual cassettes, with separate control
elements,
or under the control of, e.g., a single promoter. The sequences or segments
can
include flanking restriction sites to allow for the easy deletion and
insertion of
other sequences or segments. The design of such unique restriction sites is
known to those of skill in the art and can be accomplished using the
techniques
described above, such as site-directed mutagenesis and PCR.
For sequences generated by random mutagenesis, the choice of vector
depends on the pool of mutant sequences, i.e., donor or recipient, with which
they are to be employed. Furthermore, the choice of vector determines the host
24
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
cell to be employed in subsequent steps of the claimed method. Any
transducible cloning vector can be used as a cloning vector for the donor pool
of
mutants. It is preferred, however, that phagemids, cosmids, or similar cloning
vectors be used for cloning the donor pool of mutant encoding nucleotide
sequences into the host cell. Phagemids and cosmids, for example, are
advantageous vectors due to the ability to insert and stably propagate therein
larger fragments of DNA than in M13 phage and ~, phage, respectively.
Phagemids which will find use in this method generally include hybrids between
plasmids and filamentous phage cloning vehicles. Cosmids which will fnd use
in this method generally include ~, phage-based vectors into which cos sites
have
been inserted. Recipient pool cloning vectors can be any suitable plasmid.
'The
cloning vectors into which pools of mutants are inserted may be identical or
may
be constructed to harbor and express different genetic markers (see, e.g.,
Sambrook et al., supra). The utility of employing such vectors having
different
marker genes may be exploited to facilitate a determination of successful
transduction.
Thus, for example, the cloning vector employed may be an E.
colilStreptomyces shuttle vector (see, for example, U.S. Patent Nos.
4,416,994,
4,343,906, 4,477,571, 4,362,816, and 4,340,674), a cosmid, a plasmid, an
artificial bacterial chromosome (see, e.g., Zhang and Wing, 1997; Schalkwyk et
al., 1995; and Monaco and Lavin, 1994), or a phagemid, and the host cell may
be
a bacterial cell such as E. coli, Perzicillium patulum, and Streptorrzyces
spp. such
as S. lividans, S. verzezuelae, or- S. laverzdulae, or a eukaryotic cell such
as fungi,
yeast or a plant cell, e.g., monocot and dicot cells, preferably cells that
are
regenerable.
Moreover, recombinant polypeptides having a particular activity may be
prepared via "gene-shuffling". See, for example, Crameri et al., 1998; Patten
et
al., 1997, U.S. Patent Nos. 5,837,458, 5,834,252, 5,830,727, 5,811,238,
5,605,793.
For phagemids, upon infection of the host cell which contains a
phagemid, single-stranded phagemid DNA is produced, packaged and extruded
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
from the cell in the form of a transducing phage in a manner similar to other
phage vectors. Thus, clonal amplification of mutant encoding nucleotide
sequences carried by phagemids is accomplished by propagating the phagemids
in a suitable host cell.
Following clonal amplification, the cloned donor pool of mutants is
infected with a helper phage to obtain a mixture of phage particles containing
either the helper phage genome or phagemids mutant alleles of the wild-type
encoding nucleotide sequence.
Infection, or transfection, of host cells with helper phage is generally
accomplished by methods well known in the art (see., e.g., Sambrook et al.,
supra; and Russell et al., 1986).
The helper phage may be any phage which can be used in combination
with the cloning phage to produce an infective transducing phage. For example,
if the cloning vector is a cosmid, the helper phage will necessarily be a ~,
phage.
Preferably, the cloning vector is a phagemid and the helper phage is a
filamentous phage, and preferably phage M13.
If desired after infecting the phagemid with helper phage and obtaining a
mixture of phage particles, the transducing phage can be separated from helper
phage based on size difference (Barnes et al., 1983), or other similarly
effective
technique.
The entire spectrum of cloned donor mutations can now be transduced
into clonally amplified recipient cells into which has been transduced or
transformed a pool of mutant encoding nucleotide sequences. Recipient cells
which rnay be employed in the method disclosed and claimed herein may be, for
example, E. coli, or other bacterial expression systems which are not
recombination deficient. A recombination deficient cell is a cell iri which
recombinatorial events is greatly reduced, such as rec mutants of E. coli
(see,
Clark et al., 1965).
These transductants can now be selected for the desired expressed protein
property or characteristic and, if necessary or desirable, amplified.
Optionally, if
the phagemids into which each pool of mutants is cloned are constructed to
26
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
express different genetic markers, as described above, transductants may be
selected by way of their expression of both donor and recipient plasmid
markers.
The recombinants generated by the above-described methods can then be
subjected to selection or screening by any appropriate method, for example,
enzymatic or other biological activity.
The above cycle of amplification, infection, transduction, and
recombination may be repeated any number of times using additional donor
pools cloned on phagemids. As above, the phagemids into which each pool of
mutants is cloned may be constructed to express a different marker gene. Each
cycle could increase the number of distinct mutants by up to a factor of 106.
Thus, if the probability of occurrence of an inter-allelic recombination event
in
any individual cell is f (a parameter that is actually a function of the
distance
between the recombining mutations), the transduced culture from two pools of
106 allelic mutants will express up to 10'z distinct mutants in a population
of
10'Zlf cells.
The invention will be further described by the following non-limiting
examples.
Example 1
Deletion of the desR Gene of the Desosamine Biosynthetic Gene Cluster
As some macrolides have more than one attached sugar moiety, the
assignment of sugar biosynthetic genes to the appropriate sugar biosynthetic
pathway can be quite difficult. Since methymycin (a compound of formula (1))
and neomethymycin (a compound of formula (2)) (Figure 1 ) (Donin et al., 1953;
Djerassi et al., 1956), two closely related macrolide antibiotics produced by
Sts°eptomyces venezuelae, contain desosamine as their sole sugar
component, the
organization of the sugar biosynthetic genes in the methymycinlneomethymycin
gene cluster may be less complicated. Thus, this system was chosen for the
study of the biosynthesis of desosamine, aN,N dimethylamino-3,4,6-
trideoxyhexose, which also exists in the erythromycin structure (Flinn et al.,
1954).
27
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
To study the formation of this unusual sugar, a DNA library was
constructed by partially digesting the genomic DNA ofS. veraezuelae (ATCC
15439) with Sau3A I into 35-40 kb fragments which were ligated into the
cosmid vector pNJl (Tuan et al., 1990). The recombinant DNA was packaged
into bacteriophage ~, which was used to transfect E. coli DHSa. The resulting
cosmid library was screened for desired clones using the tylAl and tylA2 genes
from the tylosin biosynthetic cluster as probes (Baltz et al., 1988; Merson-
Davies
et al., 1994). These two probes are specific for sugar biosynthetic genes
whose
products catalyze the first two steps universally followed by all unusual 6-
deoxyhexoses studied thus far. The initial reaction involves conversion of
glucose-1-phosphate to TDP-D-glucose by a-D-glucose-1-phosphate
thymidylyltransferase (TylAl) and subsequently, TDP-D-glucose is transformed
to TDP-4-keto-6-deoxy-D-glucose by TDP-D-glucose 4,6-dehydratase (TylA2).
Three cosmids were found to contain genes homologous to tylAl and tylA2.
Further analysis of these cosmids led to the identification of nine open
reading
frames (ORFs) downstream of the PKS genes (Figure 1). Based on sequence
similarities to other sugar biosynthetic genes, especially those derived form
the
erythromycin cluster (Gaisser et al., 1997; Summers et al., 1997), eight of
these
nine ORFs are believed to be involved in the biosynthesis of TDP-D-
desosamine. Interestingly, the ery cluster lacks homologs of the tylAl and
tylA2
genes that are responsible for the first two steps in desosamine pathway. It
is
possible that the erythromycin biosynthetic machinery may rely on a general
cellular pool of TDP-4-keto-6-deoxy-D-glucose for mycarose and desosamine
formation. Depicted in Figure 1 is a biosynthetic pathway for TDP-D-
desosamine.
Although eight of the nine ORFs have been assigned to desosamine
formation, the presence of desR, which shows strong sequence homology to (3-
glucosidases (as high as 39% identity and 46% similarity) (Castle et al.,
1998),
within the desosamine gene cluster is puzzling. To investigate the function of
DesR relative to the biosynthesis of methymycin/neomethymycin, a disruption
plasmid (pBL1005) derived from pKCl 139 (containing an apramycin resistance
28
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
marker) (Bierman et al., 1992) was constructed in which a 1.0 kb NcoIlXhoI
fragment of the desR gene was deleted and replaced by the thiostrepton
resistance (tsr) gene (1.1 kb) (Bibb et al., 1985) via blunt-end ligation.
This
plasmid was used to transform E. coli S17-1; which serves as the donor strain
to
introduce the pBL1005 construct through conjugal transfer into the Wild-type S
venezuelae (Bierman et al., 1992). The double crossover mutants in which
chromosomal desR had been replaced with the disrupted gene were selected
according to their thiostrepton-resistant and apramycin-sensitive
characteristics.
Southern blot hybridization analysis was used to confirm the gene replacement.
The desired mutant was first grown at 29°C in seed medium for 48
hours,
and then inoculated and grown in vegetative medium for another 48 hours (Cane
et al., 1993). After the fermentation broth was centrifuged at 10,000 g to
remove
cellular debris and mycelia, the supernatant was adjusted to pH 9.5 with
concentrated KOH, and extracted with an equivolume of chloroform (four
times). The organic layer was dried over sodium sulfate and evaporated to
dryness. The amber oil-like crude products were first subjected to flash
chromatography on silica gel using a gradient of 0-40% methanol in chloroform,
followed by HPLC purification on a C,$ column eluted isocratically with 45%
acetonitrile in 57 mM ammonium acetate (pH 6.7). In addition to methymycin (a
compound of formula (1)) and neomethymycin (a compound of formula (2)),
two new products were isolated. The yield of a compound of formula (13) and a
compound of formula (14) was each in the range of 5-10 mg/L of fermentation
broth. However, a compound of formula (1) and a compound of formula (2)
remained to be the major products. High-resolution FAB-MS revealed that both
compounds have identical molecular compositions that differ from
methymycin/neomethymycin by an extra hexose. The chemical nature of these
two new compounds were elucidated to be C-2' (3-glucosylated methymycin and
neomethymycin (a compound of formula (13) and formula (14), respectively) by
extensive spectral analysis.
The spectral data of (13): 'H NMR (acetone-d6) 8 6.56 (1H, d, J= 16.0,
9-H), 6.46 (1H, d, J= 16.0, 8-H), 4.67 (1H, dd, J= 10.8, 2.0, 11-H), 4.39 (1H,
d,
29
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
J= 7.5, 1'-H), 4.32 (1H, d, J= 8.0, 1'°-H), 3.99 (1H, dd, J= 11.5, 2.5,
6"-H),
3.72 (1H, dd, J= 11.5, 5.5, 6"-H), 3.56 (1H, m, 5'-H), 3.52 (1H, d, J=10.0, 3-
H), 3.37 (1H, t, J= 8.5, 3"-H), 3.33 (1H, m, 5"-H), 3.28 (1H, t, J= 8.5, 4"-
H),
3 .23 ( 1 H, dd, J = 10.5, 7.5, 2'-H), 3.15 ( 1 H, dd, J = 8.5, 8.0, 2 "-H), 3
.10 ( 1 H, m,
2-H), 2.75 (1H, 3'-H, buried under HZO peak), 2.42 (1H, m, 6-H), 2.28 (6H, s,
NMe2), 1.95 ( 1 H, m, 12-H), 1.9 ( 1 H, m, 5-H), 1.82 ( 1 H, m, 4'-H), 1.50 (
1 H, m,
12-H), 1.44 (3H, d, J= 7.0, 2-Me), 1.4 (1H, m, 5-H), 1.34 (3H, s, 10-Me), 1.3
(1H, m, 4-H), 1.25 (1H, m, 4'-H), 1.20 (3H, d, J= 6.0, 5'-Me), 1.15 (3H, d, J=
7.0, 6-Me), 0.95 (3H, d, J= 6.0, 4-Me), 0.86 (3H, t, J= 7.5, 12-Me). High-
resolution FAB-MS: calc for C3~H54N0~2 (M+H)+ 632.3646, found 632.3686.
Spectral data of (14): 'H NMR (acetone-d6) b 6.69 (1H, dd, J= 16.0, 5.5
Hz, 9-H), 6.5 5 ( 1 H, dd, J = 16.0, 1.3, 8-H), 4.71 ( 1 H, dd, J = 9.0, 2.0,
11-H),
4.37 (1H, d, J= 7.0, 1'-H), 4.31 (1H, d, J= 8.0, 1 "-H), 3.97 (1H, dd, J=
11.5,
2.5, 6"-H), 3.81 ( 1 H, dq, J = 9.0, 6.0, 12-H), 3.72 ( 1 H, dd, J= 11.5, 5.0,
6"-H),
3.56 (1H, m, 5'-H), 3.50 (1H, bd, J= 10.0, 3-H), 3.36 (1H, t, J= 8.5, 3 "-H),
3.32
( 1 H, m, 5 "-H), 3.30 ( 1 H, t, J = 8.5, 4"-H), 3.23 ( 1 H, dd, J = 10.2,
7.0, 2'-H),
3 .13, ( 1 H, dd, J = 8.5, 8.0, 2 "-H), 3 .09 ( 1 H, m, 2-H), 3 .08 ( 1 H, m,
10-H), 2.77
(1H, ddd, J= 12.5, 10.2, 4.5, 3'-H), 2.41 (1H, m, 6-H), 2.28 (6H, s, NMez),
1.89
( 1 H, t, J= 13.0, 5-H), 1.83 ( 1 H, ddd, J = 12.5, 4.5, 1.5, 4'-H), 1.41 (3H,
d, J =
7.0, 2-Me), 1.3 ( 1 H, m, 4-H), 1.25 ( 1 H, m, 5-H), 1.2 ( 1 H, m, 4'-H, 1.20
(3H, d, J
= 6.0, 5'-Me), 1.17 (6H, d, J= 7.0, 6-Me, 10-Me), 1.12 (3H, d, J= 6.0, 12-me),
0.96 (3H, d, J= 6.0, 4-Me). '3C NMR (acetone-d6) b 204.1 (C-7), 175.8 (C-1),
148.2 (C-9), 126.7 (C-8), 108.3 (C-1 "), 104.2 (C-1'), 85.1 (C-3), 83.0 (C-
2'),
78.2 (C-3 "), 78.1 (C-5 "), 76.6 (C-2 "), 76.4 (C-11 ), 71.8 (C-4"), 69.3 (C-
5'),
66.1 (C-12), 66.0 (C-3'), 63.7 (C-6"), 46.2 (C-6), 44.4 (C-2) , 40.8 (NMe2),
36.4
(C-10), 34.7 (C-5), 34.0 (C-4), 29.5 (C-4'), 21.5 (5'-Me), 21.5 (12-Me), 17.9
(6-
Me), 17.7 (4-Me), 17.2 (2-Me), 9.9 (10-Me). High-resolution FAB-MS: calc for
C31H54N~12 (M+H)+ 632.3646, found 632.3648.
The coupling constant (d, J= 8.0 Hz) of the anomeric hydrogen (1 "-H)
of the added glucose and the magnitude of the downfield shift (11.8 ppm) of C-
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
2' of desosamine are all consistent with the assigned C-2' ~i-configuration
(Sao
et al., 1978).
The antibiotic activity of a compound of formula (13) and (14) against
Stf-eptococcus pyogeraes was examined by separately applying 20 ~.L of each
sample (1.6 mM in MeOH) to sterilized filter paper discs which were placed
onto the surface of S. pyogettes grown on Mueller-Hinton agar plates
(Mangahas,
1996). After being grown overnight at 37°C, the plates of the controls
(a
compound of formula (1) and (2)) showed clearly visible inhibition zones. In
contrast, no such clearings were discernible around the discs of a compound of
formula (13) and (14). Evidently, (3-glucosylation at C-2' of desosamine in
methymycin/neomethymycin renders these antibiotics inactive.
It should be noted that similar phenomena involving inactivation of
macrolide antibiotics by glycosylation,'are known (Calmer et al., 1985; Kuo et
al., 1989; Sasaki et al., 1996). For example, it was found that when
erythromycin was given to Stf-epton2yces lividans, which contains a macrolide
glycosyltransferase (MgtA), the bacterium was able to defend itself by
glycosylating the drug (Cundliffe, 1992; Jenkins et al., 1991). Such a
macrolide
glycosyltransferase activity has been detected in 15 out of a total of 32
actinomycete strains producing various polyketide antibiotics (Sasaki et al.,
1996). Interestingly, the co-existence of a macrolide glycosyltransferase
(OleD)
capable of deactivating oleandomycin by glucosylation (Hernandez et al.,
1993),
and an extracellular (3-glucosidase capable of removing the added glucose from
the deactivated oleandomycin in Streptorrlyces antibioticus (Vilches et al.,
1992)
has led to the speculation of glycosylation as a possible self resistance
mechanism in S aratibioticus. Although the genes of the aforementioned
glycosyltransferases have been cloned in a few cases, such as mgtA of S.
livida~cs
and oleD of S. afatibioticus, the whereabouts of macrolide ~3-glycosidase
genes
remain obscure: Interestingly, the recently released efyBl sequence, which is
part of the erythromycin biosynthetic cluster, is highly homologous todesR
(55%
identity) (Gaisser et al., 1997).
31
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
The discovery of desR, a macrolide (3-glucosidase gene, within the
desosamine gene cluster is thus significant, and the accumulation of
deactivated
compounds of formula (13) and (14) after desR disruption provides direct
molecular evidence indicating that a similar self defense mechanism via
glycosylation/deglycosylation may also be operative inS. venezuelae. However,
because a significant amount of methymycin and neomethymycin also exist in
the fermentation broth of the mutant strain, glucosylation of desosamine may
not
be the primary self resistance mechanism in S. venezuelae. Indeed, an rRNA
methyltransferase gene found upstream from the PKS genes in this cluster may
confer the primary self resistance protection. Thus, these results are
consistent
with the fact that antibiotic producing organisms generally have more than one
defensive option (Cundliffe, 1989). In light of this observation, it is
conceivable
that methymycin/neomethymycin may be produced in part as the inert
diglycosides (a compound of formula (13) or (14)), and the macrolide X3-
glucosidase encoded by desR is responsible for transforming
methymycin/neomethymycin from their dormant state to their active form.
Supporting this idea, the translated desR gene has a leader sequence
characteristic of secretory proteins (yon Heijne, 1986; yon Heijne, 1989).
Thus,
DesR may be transported through the cell membrane and hydrolyze the modified
antibiotics extracellularly to activate them (Figure 2).
Summary
Inspired by the complex assembly and the enzymology of aminodeoxy
sugars that are frequently found as essential components of macrolide
antibiotics, the entire desosamine biosynthetic gene cluster from the
methymycin
and neomethymycin producing strain Streptoniyces veuezuelae was cloned,
sequenced, and mapped. Eight of the nine mapped genes were assigned to the
biosynthesis of TDP-D-desosamine based on sequence similarities to those
derived from the erythromycin cluster. The remaining gene, designated desR,
showed strong sequence homology to (3-glucosidases.
To investigate the function of the encoded protein (DesR), a disruption
mutant was constructed in which a NcoIlXIzoI fragment of the desR gene was
32
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
deleted and replaced by the thiostrepton resistance (tsr) gene. In addition to
methymycin and neomethymycin, two new products were isolated from the
fermentation of the mutant strain. These two new compounds, which are
biologically inactive, were found to be C-2' (3-glucosylated methymycin and
neomethymycin. Since the translated desR gene has a leader sequence
characteristic of secretory proteins, the DesR protein may be an extracellular
(3-
glucosidase capable of removing the added glucose from the modified
antibiotics
to activate them. Thus, the occurrence of desR within the desosamine gene
cluster and the accumulation of deactivated glucosylated
methymycin/neomethymycin upon disruption ofdesR provide strong molecular
evidence suggesting that a self resistance mechanism via glucosylation may be
operative in S. ve~zezuelae.
Thus, the desR gene can be used as a probe to identify homologs in other
antibiotic biosynthetic pathways. Deletion of the corresponding macrolide
glycosidase gene in other antibiotic biosynthetic pathways may lead to the
accumulation of the glycosylated products which may be used as prodrugs with
reduced cytotoxicity. Glycosylation also holds promise as a tool to regulate
and/or minimize the potential toxicity associated with new macrolide
antibiotics
produced by genetically engineered microorganisms. Moreover, the availability
of macrolide glycosidases, which can be used for the activation of newly
formed
antibiotics that have been deliberately deactivated by engineered
glycosyltransferases, may be useful in the development of novel antibiotics
using
the combinatorial biosynthetic approach (Hopwood et,al., 1990; Katz et al.,
1993; Hutchinson et al., 1995; Cameras et al., 1997; I~rramer et al., 1996;
Khosla
et al., 1996; Jacobsen et al., 1997; Marsden et al., 1990.
Example 2
Deletion of the desT~I Gene of the Desosamine Biosynthetic Gene Cluster
The emergence of pathogenic bacteria resistant to many commonly used
antibiotics poses a serious threat to human health and has been the impetus of
the
present resurgent search for new antimicrobial agents (Box et al., 1997;
Davies,
33
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
1996; Service, 1995). Since the first report on using genetic engineering
techniques to create "hybrid" polyketides (Hopwood et al., 1995), the
potential
of manipulating the genes governing the biosynthesis of secondary metabolites
to
create new bioactive compounds, especially macrolide antibiotics, has received
much attention (Kramer et al., 1996; Khosla et al., 1996). This class of
clinically
important drugs consists of two essential structural components: a polyketide
aglycone and the appended deoxy sugars (Omura, 1984). The aglycone is
synthesized via sequential condensations of acyl thioesters catalyzed by a
highly
organized mufti-enzyme complex, polyketide synthase (PKS) (Hopwood et al.,
1990; Katz, 1993; Hutchinson et al., 1995; Cameras et al., 1997). Recent
advances in the understanding of the polyketide biosynthesis have allowed
recombination of the PKS genes to construct an impressive array of novel
skeletons (Kramer et al., 1996; Khosla et al., 1996; Hopwood et al., 1990;
Katz,
1993; Hutchinson et al., 1995; Cameras et al., 1997; Epp et al., 1989; Donadio
et
al., 1993; Arisawa et al., 1994; Jacobsen et al., 1997; Marsden et al., 1998).
Without the sugar components, however, these new compounds are usually
biologically impotent. Hence, if one plans to make new macrolide antibiotics
by
a combinatorial biosynthetic approach, two immediate challenges must be
overcome: assembling a repertoire of novel sugar structures and then having
the
capacity to couple these sugars to the structurally diverse macrolide
aglycones.
Unfortunately, knowledge of the formation of the unusual sugars in these
antibiotics remains limited (Liu et al., 1994; Kirschning et al., 1997;
Johnson et
al., 1998). Part of the reason for this comes from the fact that the sugar
genes are
generally scattered at both ends of the PKS genes. Such an organization within
the macrolide biosynthetic gene cluster makes it difficult to distinguish the
sugar
genes from those encoding regulatory proteins or aglycone modification enzymes
that are also interspersed in the same regions. The task can be made even more
formidable if the macrolides contain multiple sugar components. In view of the
"scattered" nature of the sugar biosynthetic genes, the antibiotic methymycin
(a
compound of formula (1) in Figure 1) and its co-metabolite, neomethymycin (a
compound of formula (2) in Figure 1 )), of Streptonzyces vesaezuelae present
34
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
themselves as an attractive system to study the formation of deoxy sugars
(Donin
et al., 1953; Djerassi et al., 1956). First, they carry D-desosamine (a
compound
of formula (3)) a prototypical aminodeoxy sugar that also exists in
erythromycin.
Second, since desosamine is the only sugar attached to the macrolactone of
formula (1) and (2), identification of the sugar biosynthetic genes within the
methymycin/neomethymycin gene cluster should be possible with much more
certainty.
A 10 kb stretch of DNA downstream from the
methymycin/neomethymycin gene cluster, which is about 60 kb in length, was
found to harbor the entire desosamine biosynthetic gene cluster (Figure 3).
Among the nine open reading frames (ORFs) mapped in this segment, eight are
likely to be involved in desosamine formation, while the remaining one, desR,
encodes a macrolide (3-glycosidase that may be involved in a self resistance
mechanism. Their identities, shown in Figure 3, are assigned based on sequence
similarities to other sugar biosynthetic genes (Gaisser et al., 1997; Summers
et
al., 1997). The proposed pathway is well founded on literature precedent and
mechanistic intuition for the construction of aminodeoxy sugars (Liu et al.,
1994;
Kirschning et al., 1997; Johnson et al., 1998).
To determine whether new methymycin/neomethymycin analogues
carrying modified sugars could be generated by altering the desosamine
biosynthetic genes, the deshl gene, which has been predicted to encode the N
methyltransferase, was chosen as a target (Gaisser et al., 1997; Summers et
al.,
1997). The deduced deshl product is most closely related to that of eryCTlI
from
the erythromycin producing strain Sacchaf-opolyspoz-a ezytlzf°aea (70%
identity),
and also strongly resembles the predicted products of z-dnzD from the
rhodomycin cluster of Stz°eptomyces pu~pur~ascens (Niemi et al., 1995),
sj~zX
from the spiromycin cluster of Stf-eptonzyces ambofaciens (Geistlich et al.,
1992),
and tylMl from the tylosin cluster of Streptomyces fi°adiae (Gandecha
et al.,
1997). All of these enzymes contain the consensus sequence LLDV(I)ACGTG
(SEQ ID N0:25) (Gaisser et al., 1997; Summers et al., 1997), near their N
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
terminus, which is part of the S-adenosylmethionine binding site (Ingrosso et
al.,
1989; Haydock et al., 1991 ).
The deletion of desVl should have little polar effect (Lin et al., 1984) on
the expression of other desosamine biosynthetic genes because the ORF (desR)
lying immediately downstream from desT~l is not directly involved in
desosamine formation, and those lying further downstream are transcribed in
the
opposite direction. Second, since N,N dimethylation is almost certainly the
last
step in the desosamine biosynthetic pathway (Liu et al., 1994; Kirschning et
al.,
1997; Johnson et al., 1998; Gaisser et al., 1997; Summers et al., 1997),
perturbing this step may lead to the accumulation of a compound of formula
(4),
which stands the best chance among all other intermediates of being recognized
by the glycosyltransferase (DesVII) for successful linkage to the macrolactone
of
formula (6) (Figure 2). Deletion and/or disruption of a single biosynthetic
gene
often affects the pathway at more than one specific step. In fact, disruption
of
eryCTjI, the desV~l equivalent in the erythromycin cluster, which has been
predicted to encode a similar N-methylase to make desosamine in erythromycin
(Gaisser et al., 1997; Summers et al., 1997), led to the accumulation of an
intermediate devoid of the entire desosamine moiety (Summers et al., 1997).
A plasmid pBL3001, in which desYl was replaced by the thiostrepton
gene (tsr) (Bibb et al., 1985), was constructed and introduced into wild type
S.
venezuelae by conjugal transfer using E. coli S 17-1 (Bierman et al., 1992).
Two
identical double crossover mutants, KdesVI-21 and KdesVI-22 with phenotypes
of thiostrepton resistance (ThioR) and apamycin sensitivity (Apms) were
obtained. Southern blot hybridization using tsr or a 1.1 kb IliracII fragment
from
the deshll region further confirmed that the desVl gene was indeed replaced by
tsf° on the chromosome of these mutants. The KdesVI-21 mutant was first
grown
at 29°C in seed medium (100 mL) for 48 hours, and then inoculated and
grown
in vegetative medium (3 L) for another 48 hours (Cane et al., 1993). The
fermentation broth was centrifuged to remove the cellular debris and mycelia,
and the supernatant was adjusted to pH 9.5 with concentrated KOH, followed by
extraction with chloroform. No methymycin or neomethymycin was found;
36
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
instead, the 10-deoxy-methynolide (6) (350 mg) (Lambalot et al., 1992) and two
new macrolides containing an N acetylated amino sugar, a compound of formula
(7) (20 mg) and a compound of formula (8) (15 mg), were isolated. Their
structures were determined by spectral analyses and high-resolution MS.
Spectral data of formula 7 are: 'H NMR (CDC13) S 6.62 (1H, d, J=16.0,
H-9), 6.22 (1H, d, J= 16.0, H-8), 5.75 (1 H, d, J= 7.5, N-H), 4.75 (1H, dd, J
10.8, 2.2, H-11), 4.28 (1H, d, J= 7.5, H-1'), 3.95 (1H, m, H-3'), 3.64 (1H, d,
J=
10.5, H-3), 3.56 (1H, m, H-5'), 3.16 (1H, dd, J= 10.0, 7.5, H-2'), 2.84 (1H,
dq, J
=10.5, 7.0, H-2), 2.55 (1H, m, H-6), 2.02 (3H, s, NAc), 1.95 (1H, m, H-12),
1.90 (1H, m, H-4'), 1.66 (1H, m, H-5), 1.50 (1H, m, H-12), 1.41 (3H, d, J=7.0,
2-Me), 1.40 (1H, m, H-5), 1.34 (3H, s, 10-Me), 1.25 (1H, m, H-4), 1.22 (1H, m,
H-4'), 1.21 (3H, d, J= 6.0, H-6'), 1.17 (3H, d, J= 7.0, 6-Me), 1.01 (3H, d, J=
6.5, 4-Me), 0.89 (3H, t, J= 7.2, 12-Me); '3C NMR (CDCl3) 8 204.3 (C-7), 175.1
(C-1), 171.8 (Me-C=O), 149.1 (C-9), 125.3 (C-8), 104.4 (C-1'), 85.4 (C-3),
76.3
(C-11), 75.4 (C-2'), 74.1 (C-10), 68.6 (C-5'), 51.9 (C-3'), 45.0 (C-6), 44.0
(C-2),
38.5 (C-4'), 33.8 (C-5), 33.3 (C-4), 23.1 (Me-C=O), 21.1 (C-12), 20.6 (C-6'),
19.2 (10-Me), 17.5 (6-Me), 17.2 (4-Me), 16.2 (2-Me), 10.6 (12-Me). High-
resolution FABMS: calc for CZSH~308N (M+H)''~ 484.2910, found 484.2903.
Spectral data of formula 8 are: 'H NMR (CDCl3) 8 6.76 (1H, dd, J=
16.0, 5.5, H-9), 6.44 (1H, dd, J= 16.0, 1.5, H-8), 5.50 (1H, d, J= 6.5, N-H),
4.80
(1H, dd, J= 9.0, 2.0, H-11), 4.28 (1H, d, J= 7.5, H-1'), 3.95 (1H, m, H-3'),
3.88
(1H, m, H-12), 3.62 (1H, d, J=11.0, H-3), 3.57 (1H, m, H-5'), 3.18 (1H, dd, J=
10.0, 7.5, H-2' ), 3.06 ( 1 H, m, H-10), 2.86 ( 1 H, dq, J = 11.0, 7.0, H-2),
2.54 ( 1 H,
m, H-6), 2.04 (3H, s, NAc), 1.98 ( 1 H, m, H-4'), 1.67 ( 1 H, m, H-5), 1.40 (
1 H, m,
H-5), 1.39 (3H, d, J= 7.0, 2-Me), 1.25 (1H, m, H-4), 1.22 (1H, m, H-4'), 1.22
(3H, d, J= 6.0, H-6'), 1.21 (3H, d, J= 6.0, 6-Me), 1.19 (3H, d, J= 7.0, 12-
Me),
1.16 (3H, d, J= 6.5, 10-Me), 1.01 (3H, d, J= 6.5, 4-Me); '3C NMR (CDCl3) 8
205.1 (C-7), 174.6 (C-1), 171.9 (Me-C=O), 147.2 (C-9), 126.2 (C-8), 104.4 (C-
1'), 85.3 (C-3), 75.7 (C-11), 75.4 (C-2'), 68.7 (C-5'), 66.4 (C-12), 52.0 (C-
3'),
45.1 (C-6), 43.8 (C-2), 38.6 (C-4'), 35.4 (C-10), 34.1 (C-5), 33.4 (C-4), 23.1
(Me-C=O), 21.0 (12-Me), 20.7 (C-6'), 17.7 (6-Me), 17.4 (4-Me), 16.1 (2-Me),
37
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
9.8 (10-Me). High-resolution FABMS: calc for CZSHa3DsN (M+H)+ 484.2910,
found 484.2892.
The fact that compounds of formula (7) and (8) bearing modified
desosamine are produced by the desvl deletion mutant is a thrilling discovery.
However, this result is also somewhat surprising since the sugar component in
the products is expected to be the aminodeoxy hexose (4). As illustrated in
Figure 4, it is possible that a compound of formula (7) and (8) are derived
from
the predicted compound of formula (9) and (10), respectively, by a post-
synthetic
nonspecific acetylation of the attached aminodeoxy sugar. It is also
conceivable
that N acetylation of (4) occurs first, followed by coupling of the resulting
sugar
(11) to the 10-deoxymethynolide (6). Nevertheless, the lack of N methylation
of
the sugar component in these new products provides convincing evidence
sustaining the assignment of desTll as the N methyltransferase gene. Most
significantly, the production of a compound of formula (7) and (8) by the
deslrl
deletion mutant attests to the fact that the glycosyltransferase (DesVII) in
methymycinlneomethymycin pathway is capable of recognizing and processing
sugar substrates other than TDP-desosamine (5).
Since both compounds of formula (7) and (8) are new compounds
synthesized in vivo by the S. venezuelae mutant strain, the observed N
acetylation might be a necessary step for self protection (Cundliffe, 1989).
In
view of these results, the potential toxicity associated with new macrolide
antibiotics produced by genetically engineered microorganisms can be
minimized and newly formed antibiotics that have been deactivated (either
deliberately or not) during production can be activated. Such an approach can
be
part of an overall strategy for the development of novel antibiotics using the
combinatorial biosynthetic approach. Indeed, purified compounds of formula (7)
and (8) are inactive against Sts~eptococcus pyogenes grown on Mueller-Hinton
agar plates (Mangahas, 1996), while the controls (a compound of formula (1)
and (2)) show clearly visible inhibition zones.
It should be pointed out that a few glycosyltransferases involved in the
biosynthesis of antibiotics have been shown to have relaxed specificity
towards
38
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
modified macrolactones (Jacobsen et al., 1997; Marsden et al., 1998; Weber et
al., 1991). However, a similar relaxed specificity toward sugar substrates has
only been reported for the daunorubicin glycosyltransferase, which is able to
recognize a modified daunosamine and catalyze its coupling to the aglycone, s-
rhodomycinone (Madduri et al., 1998). Thus, the fact that the
methymycin/neomethymycin glycosyltransferase can also tolerate structural
variants of its sugar substrate indicates that at least some
glycosyltransferases in
antibiotic biosynthetic pathways may be useful to create biologically active
hybrid natural products via genetic engineering.
Summary
The appended sugars in macrolide antibiotics are indispensable to the
biological activities of these clinically important drugs. Therefore, the
development of new antibiotics via a biological combinatorial approach
requires
detailed knowledge of the biosynthesis of these unusual sugars, as well as the
ability to manipulate the biosynthetic genes to create novel sugars that can
be
incorporated into the final macrolide structures. A targeted deletion of the
deslll
gene of Streptomyees venezuelae, which has been predicted to encode an N
methyltransferase based on sequence comparison, was prepared to determine
whether new methymycinlneomethymycin analogues bearing modified sugars
can be generated by altering the desosamine biosynthetic genes. Growth of the
S. venezuelae deletion mutant strain resulted in the accumulation of a
methymycin/neomethymycin analogue carrying anN acetylated aminodeoxy
sugar. Isolation and characterization of these derivatives not only provide
the
first direct evidence confirming the identity of desVl as the N
methyltransferase
gene, but also demonstrate the feasibility of preparing novel sugars by the
gene
deletion approach. Most significantly, the results also revealed that the
glycosyltransferase of methymycin/neomethymycin exhibits a relaxed specificity
towards its sugar substrates.
39
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Example 3
Cloning_and Sequencin~of the Met/Pik Biosynthetic Gene Cluster
Materials and Methods
Bacterial Strains and Media. E. coli DHSa was used as a cloning host.
E. coli LE392 was the host for a cosmid library derived from S. veuezuelae
genomic DNA. LB medium was used in E. coli propagation. Streptomyces
veraezuelae ATCC 15439 was obtained as a freeze-dried pellet from ATCC.
Media for vegetative growth and antibiotic production were used as described
(Lambalot et al., 1992). Briefly, SGGP liquid medium was for propagation ofS.
venezuelae mycelia. Sporulation agar (SPA) was used for production of S.
venezuelae spores. Methymycin production was conducted in either SCM or
vegetative medium and pikromycin production was performed in Suzuki
glucose-peptone medium.
Vectors, DNA Manipulation and Cosmid Library Construction. pUCl 19
was the routine cloning vector, and pNJl was the cosmid vector used for
genomic DNA library construction. Plasmid vectors for gene disruption were
either pGM160 (Muth et al., 1989) or pKCl 139 (Bierman et al., 1992). Plasmid,
cosmid, and genomic DNA preparation, restriction digestion, fragment
isolation,
and cloning were performed using standard procedures (Sambrook et al., 1989;
Hopwood et al., 1985). The cosmid library was made according to instructions
from the Packagene ~.-packaging system (Promega).
DNA Sequencin~and Anal.. An Exonuclease III (ExoIII) nested
deletion series combined with PCR-based double stranded DNA sequencing was
employed to sequence the pik cluster. The ExoIII procedure followed the Erase-
a-Base protocol (Stratagene) and DNA sequencing reactions were performed
using the Dye Primer Cycle Sequencing Ready Reaction Kit (Applied
Biosystems). The nucleotide sequences were read from an ABI PRISM 377
sequencer on both DNA strands. DNA and deduced protein sequence analyses
were performed using GeneWorks and GCG sequence analysis package. All
analyses were performed using the specific program default parameters.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Gene Disruption. A replicative plasmid-mediated homologous
recombination approach was developed to conduct gene disruption in S.
venezuelae. Plasmids for insertional inactivation were constructed by cloning
a
kanamycin resistance marker into target genes, and plasmid for gene
deletion/replacement was constructed by replacing the target gene with a
kanamycin or thiostrepton resistance gene in the plasmid. Disruption plasmids
were introduced into S. venezuelae by either PEG-mediated protoplast
transformation (Hopwood et al., 1985) or RK2-mediated conjugation (Bierman
et aL, 1992). Then, spores from individual transformants or transconjugants
were cultured on non-selective plates to induce recombination. The cycle was
repeated three times to enhance the opportunity for recombination. Double
crossovers yielding targeted gene disruption mutants were selected and
screened
using the appropriate combination of antibiotics and finally confirmed by
Southern hybridization.
Antibiotic Extraction and Anal.~is. Methymycin, pikromycin, and
related compounds were extracted following published procedures (Cane et al.,
1993). Thin layer chromatography (TLC) was routinely used to detect
methymycin, neomethymycin, narbomycin and pikromycin. Further purification
was conducted using flash column chromatography and HPLC, and the purified
compounds were analyzed by'H,'3C NMR spectroscopy and MS spectrometry.
Results
Cloning and Identification of the zaik Cluster. Heterologous hybridization
was used to identify genes for methymycin, neomethymycin, narbomycin and
pikromycin biosynthesis in S. venezuelae. Initial Southern blot hybridization
analysis using a type I PKS DNA probe revealed two multifunctional PISS
clusters of uncharacterized function in the genome. Since these four
antibiotics
are all comprised of an identical desosamine residue, a tylAl a-D-glucose-1-
phosphate thymidylyltransferase DNA probe (for
mycaminose/mycorose/mycinose biosynthesis in the tylosin pathway) (Merson-
Davies et al., 1994) was used to locate the corresponding biosynthetic gene
cluster(s). This analysis established that only one of the PISS pathways
41
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
contained a cluster of desosamine biosynthetic genes. Nine overlapping cosmid
clones were isolated spanning over 80 kilobases (kb) on the bacterial
chromosome that encompassed the entire gene cluster (pi7~) for methymycin,
neomethymycin, narbomycin and pikromycin biosynthesis (Figure 5). Through
subsequent gene disruption, the other PKS cluster (vep, devoid of linked
desosamine biosynthetic genes) was found to play no role in production of
methymycin, neomethymycin, narbomycin or pikromycin.
Nucleotide Sequence of the pik Cluster. The nucleotide sequence of the
pik cluster was completely determined and shown to contain 18 open reading
frames (ORFs) that span approximately 60 kb. Central to the cluster are four
large ORFs, pikAl, pikAll, pikAlII, and pilrAIV, encoding a multifunctional
PKS
(Figure 5). Analysis of the six modules comprising the pik PKS indicated that
it
would specify production of narbonolide, the 14-membered ring aglycone
precursor of narbomycin and pikromycin (Figure 5).
Initial analysis unveiled two significant architectural differences in the
pikA-encoded PKS. First, compared with eryA (Donadio et al., 1998) and oleA
(Swan et al., 1994), two PKS clusters that produce 14-membered ring macrolides
erythromycin and oleadomycin similar to pikromycin, the presence of separate
ORFs, pikAlII and pikAITl, encoding Pik module 5 and Pik module 6 (as
individual modules) as opposed to one bimodular protein as in eryAIII and
oIeAIII is striking. Secondly, the presence of a type II thioesterase
immediately
downstream of the type I PKS cluster is also unprecedented (Figure 5). These
two characteristics suggest that pikA may produce the 12-membered ring
macrolactone 10-deoxymethynolide as well. Indeed, the domain organization of
PikAI - AIII (module L-5) is consistent with the predicted biosynthesis of 10-
deoxymethynolide except for the absence of a TE function at the C-terminus of
Pik module 5 (PikAIII). The lack of a TE domain in PikAIII may be
compensated by the type II TE (encoded bypikAI~ immediately downstream of
pikAlh Consistent with the supposition that two distinct polyketide ring
systems are assembled from the pik PKS, two macrolide-lincosamide-
streptogramin B type resistant genes, pikRl and pikR2, are found upstream of
the
42
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
pik PKS (Figure 6), which presumably provide cellular self protection for S.
verzezuelae.
The genetic locus for desosamine biosynthesis and glycosyl transfer are
immediately downstream ofpikA. Seven genes, desl, desll, deslll, deslV, desTl
desVl, and desVIIl, are responsible for the biosynthesis of the deoxysugar,
and
the eighth gene, desYII, encodes a glycosyltransferase that apparently
catalyzes
transfer of desosamine onto the alternate (12- and 14-membered ring)
polyketide
aglycones. The existence of only one set of desosamine genes indicates that
DesVIII can accept both 10-deoxymethynolide and narbonolide as substrates
(Jacobsen et al., 1997). The largest ORF in the des locus, desR, encodes a (3-
glycosidase that is involved in a drug inactivation-reactivation cycle for
bacterial
self protection.
Just downstream of the des locus is a gene (pikC') encoding a cytochrome
P450 hydroxylase similar to eryF (Andersen et al., 1992), and eryK (Stassi et
al.,
1993), PikC, and a gene (pikD) encoding a putative regulator protein, PikD
(Figure 5). Interestingly, PikC is the only P450 hydroxylase identif ed in the
entire pik cluster, suggesting that the enzyme can accept both 12- and 14-
membered ring macrolide substrates and, more remarkably, it is active on both
C-10 and C-12 of the YC-17 (12-membered ring intermediate) to produce
methymycin and neomethymycin (Figure 7). PikD is a putative regulatory
protein similar to ORFH in the rapamycin gene cluster (Schwecke et al., 1995).
The combined functionality coded by the eighteen genes in thepik cluster
predicts biosynthesis of methymycin, neomethymycin, narbomycin and
pikromycin (Table 1 ). Flanking the pik cluster locus are genes presumably
involved in primary metabolism and genes that may be involved in both primary
and secondary metabolism. An S-adenosyl-methionine synthase gene is located
downstream of pikD that may help to provide the methyl group in desosamine
synthesis. A threonine dehydratase gene was identified upstream ofpilcRl that
may provide precursors for polyketide biosynthesis. It is not apparent that
any of
these genes are dedicated to antibiotic biosynthesis and they are not directly
linked to the pik cluster.
43
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Table 1. Deduced function of ORFs in the pik cluster
Polypeptide Amino Proposed function or sequence similarity
(ORF) detected
acids,
no.
PiIcAI 4,613 PKS
Loading module KSQ AT(P) ACP
Module 1 KS AT(P) KR ACP
Module 2 KS AT(A) DH KR ACP
PikAII 3,739 PKS
Module 3 KS AT(P) KR ACP
Module 4 KS AT(P) DH ER KR ACP
10PikAIII 1,562 PKS
Module 5 KS AT(P) KR ACP
PikAIV 1,346 PKS
Module 6 KS AT(P) ACP TE
PikAV 281 Thioesterase II (TEII)
15DesI 415 4-Dehydrase
DesII 485 Reductase
DesIII 292 a-D-Glucose-1-phosphate thymidylyltransferase
DesIV 337 TDP-glucose 4, 6-dehydratase
DesV 379 Transaminase
20DesVI 237 N,N-dimethyltransferase
DesVII 426 Glycosyl transferase
DesVIII 402 Tautomerase
DesR 809 ~3-Glucosidase (involved in resistance
mechanism)
PikC 418 P450 hydroxylase
25PikD 945? Putative regulator
PikRl 336 rRNA methyltransferase (mls resistance)
PikR2 288? rRNA methyltransferase (mls resistance)
AT(A), acyltransferase incorporating an acetate extender unit; AT(P),
30 acyltransferase incorporating a propionate extender unit. KR°, an
inactive KR.
Enzymes of uncertain function are denoted with a question mark.
44
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Table 2. Summary of mutational analyses of the pik cluster
Antibiotic production/
Type of Target Intermediate accumulation
Mutant mutation gene Met & neomethymycinPikromycin
AX903 Insertionpil~Al No/No No/No
LZ3001 Deletion/desVl No/10-deoxymethynolideNo/narbonolide
replacement
LZ4001 Deletion desV No/10-deoxymethynolideNo/narbonolide
replacement
AX905 Deletion/pikAh <5%lNo <S%/No
replacement
AX906 InsertionpikC No/YC-17 No/narbomycin
Mutational Analysis of the pik Cluster. Extensive disruption of genes in
the pik cluster were carried out to address the role of key enzymes in
antibiotic
production (Table 2). First, PikAI, the first putative enzyme involved in the
biosynthesis of 10-deoxymethynolide and narbonolide was inactivated by
insertional mutagenesis. The resulting mutant, AX903, produced neither
methymycin or neomethymycin, nor narbomycin or pikromycin, indicating that
pikA encodes a PKS required for both 12- and 14-membered ring macrolactone
formation.
Second, deletion of both desVl and desY abolished methymycin,
neomethyrnycin, narbomycin and pikromycin production, and the resulting
mutants, LZ3001 and LZ4001, accumulate 10-deoxymethynolide and
narbonolide in their culture broth, indicating that enzymes for desosamine
synthesis and transfer are also shared by the 12- and 14-membered ring
macrolides.
In order to understand the mechanism of polyketide chain termination at
PikAIII (PIKAIII (module 5) is presumed to' be the termination point in
constriction of 10-deoxyrnethynolide), the pik TEII gene, pikAY, was deleted.
The deletionlreplacement mutant, AX905, produces less than 5% of
methymycin, neomethymycin, and less than 5% of pikromycin compared to wild
type S. venezuelae. This abrogation in product formation occurs without
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
significant accumulation of the expected aglycone intermediates, suggesting
that
pilr TEII is involved in the termination of 12- as well as 14-membered ring
macrolides at PikAIII and PikAIV, respectively. Although the polar effects may
influence the observed phenotype in AX905, this has been ruled out after the
S consideration of mutant LZ3001, in which mutation in an enzyme downstream
of pikAV accumulated 10-deoxymethynolide and narbonolide. The fact that
mutant AX905 failed to accumulate these intermediates suggested that the
polyketide chains were not efficiently released from this PKS protein in the
absence of Pik TEII. Therefore, Pik TEII plays a crucial role in polyketide
chain
release and cyclization, and it presumably provides the mechanism for
alternative termination in pik polyketide biosynthesis.
Finally, disruption of pikC confirmed that PikC is the sole enzyme
catalyzing hydroxylation of both YC-17 (at C-10 and C-12) and narbomycin (at
C-12). The relaxed substrate specificity of PikC and its regional specificity
at C-
10 and C-12 provide another layer of metabolite diversity in thepik-encoded
biosynthetic system.
Discussion
The work described herein has established that methymycin,
neomethymycin, narbomycin and pikromycin biosynthesis is encoded by the pik
cluster in S. venezuelae. Three key enzymes as well as the unique architecture
of
the cluster enable this relatively compact system to produce multiple
macrolide
antibiotics. Foremost, the presence of pik module 5 and 6 as separate
proteins,
PiIcAIII and PikAIV, and the activity of pik TEII enable the bacterium to
terminate the polyketide chain at two different points of assembly, thereby
producing two macrolactones of different ring size. Second, DesVII, the
glycosyltransferase in the pik cluster, can accept both 12- and 14-membered
ring
macrolactones as substrates. Finally, PikC, the P450 hydroxylase, has a
remarkable substrate and regiochemical specificity that introduces another
layer
of diversity into the system.
It is interesting to consider that pikA evolved in a line analogous to eryA
and oleA since each of these PKSs specify the synthesis of 14-membered ring
46
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
macrolactones. Therefore, pik may have acquired the capacity to generate
methymycin when a mutation in the primordial pikAIII pikAIV linker region
caused splitting of Pik module 5 and 6 into two separate gene products. This
notion is raised by two features of the nucleotide sequence. First, the
intergenic
region between pikAIII and pikAIV which is 105 bp, may be the remanent of an
intramodular linker peptide of 35 amino acids. Moreover, the potential for
independently regulated expression of pikAIV is implied by the presence of a
100
nucleotide region at the 5' end of the gene that is relatively AT-rich (62% as
comparing 74% G+C content in coding region). Thus, as the mutation in an
original ORF encoding the bimodular multifunctional protein (PikAIII-PikAIV)
occurred, so too may have evolved a mechanism for regulated synthesis of the
new gene product (PikAIV).
The role of Pik TEII in alternative termination of polyketide chain
elongation intermediates provides a unique aspect of diversity generation in
natural product biosynthesis. Engineered polyketides of different chain length
are typically generated by moving the TE catalytic domain to alternate
positions
in a modular PKS (Comes et al., 1995). Repositioning of the TE domain
necessarily abolishes production of the original full-length polyketide so
only
one macrolide is produced each time. In contrast to the fixed-position TE
domain, the independent Pik TEII polypeptide presumably has the flexibility to
catalyze termination at different stages of polyketide assembly, therefore
enabling the system to produce multiple products of variant chain length.
Combinatorial biology technologies can now exploit this system for generating
molecular diversity through construction of novel PKS systems with TEIIs for
simultaneous production of several new molecules as opposed to the TE domains
alone that limit catalysis to a single termination step.
It is notewomhy that sequences similar to Pik TEII are found in almost all
known polyketide and non-ribosomal polypeptide biosynthetic systems
(Marahiel et al., 1997). Currently, the pik TEII is the first to be
characterized in
a modular PKS. However, recent work on a~TEII gene in the lipopeptide
surfactin biosynthetic cluster (Schneider et al., 1998) demonstrated that sYf
TEII
47
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
plays an important role in polypeptide chain release, and may suggest that s~f
TEII reacts at multiple stages in peptide assembly as well (Marahiel et al.,
1997).
The enzymes involved in post-polyketide assembly of 10-
deoxymethynolide and narbonolide are particularly intriguing, especially the
glycosyltransferase, DesVII, and P450 hydroxylase, PikC. Both have the
remarkable ability to accept substrates with significant structural
variability.
Moreover, disruption of desYl demonstrated that DesVII also tolerates
variations
in deoxysugar structure. Likewise, PikC has recently been shown to convert
YC-17 to methymycin/neomethymycin and narbomycin to pikromycin in vitro.
Targeted gene disruption of ORF1 abolished both pikromycin and
methymycin production, indicating that the single cluster is responsible for
biosynthesis of both antibiotics. Deletion of the TE2 gene substantially
reduced
methymycin and pikromycin production, which demonstrates that TE2, in
contrast to the position-fixed TEl domain, has the capacity to release
polylcetide
chain at different points during the assembly process, thereby producing
polyketides of different chain length.
The results described above were unexpected in that it was surprising that
one PKS cluster produces two macrolides which differ in the number of atoms in
their ring structure, that module 5 and module 6 of the PKS are in ORFs that
are
separated by a spacer region, that PikAIII lacked TE, that there was a Type II
thioesterase, that TEI domain was not separate, and that 2 resistance genes
were
identified which may be specific for either a 12- or 14-membered ring.
With eighteen genes spanning less than 60 kb of DNA capable of
producing four active macrolide antibiotics, the pik cluster represents the
least
complex yet most versatile modular PKS system so far investigated. This
simplicity provides the basis for a compelling expression system in which
novel
active ketoside products are engineered and produced with considerable
facility
for discovery of a diverse range of new biologically active compounds.
4S
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Summary
Complex polyketide synthesis follows a processive reaction mechanism,
and each module within a PKS harbors a string of three to six enzymatic
domains that catalyze reactions in nearly linear order as described in
particular
detail for the erythromycin-producing PKS (Katz, 1997; Khosla, 1997; Staunton
et al. 1997). The combined set of PKS modules and catalytic domains along
with genes that encode enzymes for post-polyketide tailoring (e.g., glycosyl
transferases, hydroxylases) typically limits a biosynthetic system to the
generation of a single polyketide product.
Combinatorial biology involves the genetic manipulation of multistep
biosynthetic pathways to create molecular diversity in natural products for
use in
novel drug discovery. PKSs represent one of the most amenable systems for
combinatorial technologies because of their inherent genetic organization and
ability to produce polyketide metabolites, a large group of natural products
generated by bacteria (primarily actinomycetes and myxobacteria) axed fungi
with diverse structures and biological activities. Complex polyketides are
produced by multifunctional PKSs involving a mechanism similar to long-chain
fatty acid synthesis in animals (Hopwood et al., 1990). Pioneering studies
(fortes et al., 1990; Donadio et al., 1991) on the erythromycin PKS in
Saccharopolyspora e~yth~aea revealed a modular organization. Characterization
of this multidomain protein system, followed by molecular analysis of
rapamycin (Aparicio et al., 1996), FK506 (Motamedi et al., 1997), soraphen A
(Schupp et al., 1995), niddamycin (Kakavas et al., 1997), and rifamycin
(August
et al., 1998) PKSs, demonstrated a co-lineax relationship between modular
structure of a multifunctional bacterial PKS and the structure of its
polyketide
product.
In a survey of microbial systems capable of generating unusual
metabolite structural variability, Streptomyces veyaezuelae ATCC 15439 is
notable in its ability to produce two distinct groups of macrolide
antibiotics.
Methymycin and neomethymycin are derived from the 12-membered ring
macrolactone 10-deoxymethynolide, while narbomycin and pikromycin are
49.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
derived from the 14-membered ring macrolactone, narbonolide. The cloning and
characterization of the biosynthetic gene cluster for these antibiotics
reveals the
key role of a type II thioesterase in forming a metabolic branch through which
polyketides of different chain length are generated by the pikromycin
multifunctional polyketide synthase (PKS). Immediately downstream of the
PKS genes (pikA) are a set of genes for desosamine (des) biosynthesis and
macrolide ring hydroxylation. The glycosyl transferase (encoded by desVII~ has
the remarkable ability to catalyze glycosylation of both the 12- and 14-
membered ring macrolactones. Moreover, thepikC-encoded P450 hydroxylase
provides yet another layer of structural variability by introducing
regiochemical
diversity into the macrolide ring systems.
Exam~,le 4
A desYDeletion Mutant Yields D-Quinovose
A mutant of S. vehezuelae (KdesV-41) was constructed that had the desV
gene disrupted (Zhao et al., J. Am. Chem. Soc., 120, 12159 (1998)). Since desY
encodes the 3-aminotransferase that catalyzes the conversion of the 3-keto
sugar
17 (Figure 11) to the corresponding amino sugar 4, deletion of this gene
should
prevent C-3 transamination, resulting in the accumulation of 17. It was
expected
that if the glycosyltransferase (DesVII) of this pathway is capable of
recognizing
and processing the keto sugar intermediate 17, the macrolide products)
produced by the KdesV-41 mutant should have an attached 3-keto sugar.
Surprisingly, the two products isolated were the methymycin/neomethymycin
analogues 18 and 19, each carrying a 4,6-dideoxyhexose (Figure 12). While this
result demonstrated a relaxed specificity for the glycosyltransferase toward
its
sugar substrate, it also indicated the existence of a pathway-independent
reductase in S. veraezuelae that can stereospecifically reduce the C-3 keto
group
of the sugar metabolite.
To explore the possibility of generating a mutant capable of synthesizing
new macrolides of this class containing an engineered sugar, the dell gene,
which has been proposed to encode the dehydrase responsible for the C-4
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
deoxygenation in the biosynthesis of desosamine, was altered with the
prediction
that it would lead to the incorporation of D-quinovose (22; Figure 13), also
known as 6-deoxy-D-glucose, into the final product(s). The rationale was based
on the following: (1) Desosamine biosynthesis will be "terminated" at the C-4
deoxygenation step due to desl deletion and, thus, should result in the
accumulation of 3-keto-6-deoxyhexose 16 (Figure 11). (2) By taking advantage
of the existence of a 3-ketohexose reductase in S. venezuelae, the sugar
intermediate 15 is expected to be reduced stereospecifically to D-quinovose
(22).
(3) The glycosyltransferase (DesVII), with its relaxed specificity toward the
sugar substrate, should catalyze the coupling of 22 to the macrolactones to
give
new macrolides 20 and 21 containing the engineered sugar D-quinovose (Figure
13).
A disruption plasmid, pDesI-K, derived from pKC1139 that contains an
apramycin resistant marker, was constructed in which desl was replaced by the
neomycin resistance gene, which also confers resistance to kanamycin. This
construct was then introduced into wild type S venezuelae by conjugal transfer
using Eschey~ichia coli S17-1 as the donor strain (Bierman et al., 1992).
Several
double crossover mutants were identified on the basis of their phenotypes of
kanamycin resistant (KanR) and apramycin sensitive (Aprs). One mutant, KdesI-
80, was selected and grown at 29°C in seed medium (100 rnL) for 48
hours and
then inoculated and grown in vegetative medium (5 L) for another 48 hours
(Cane et al., 1993). The fermentation broth was centrifuged to remove cellular
debris and mycelia, and the supernatant was adjusted to pH 9.5 with
concentrated potassium hydroxide solution. The resulting solution was
extracted
with chloroform, and the pooled organic extracts were dried over sodium
sulfate
and evaporated to dryness. The yellow oil was subjected to flash
chromatography on silica gel using a gradient of 0-12%. methanol in
chloroform,
and the isolated products were further purified by HPLC using a C18 column
eluted isocratically with 50% acetonitrile in water. As expected, no
methymycin
or neomethymycin was detected; instead, 10-deoxymethynolide 23 was found as
the major product (approximately 600 mg). Significant quantities of
SI
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
methynolide 24 (approximately 40 mg) and neomethynolide 25 (approximately 2
mg) were also isolated (Figure 13). A new macrolide 15 containing D-quinovose
(3.2 mg) was produced by this mutant. Its structure was fully established by
spectral analyses. Spectral data (Jvalues are in hertz) for 15: 'H NMR (CDCl3)
8 6.76 (1H, dd, J=16.0, 5.5, 9-H), 6.43 (1H, d, J=16.0, 8-H), 4.97 (1H, ddd, J
= 8.4, 5.9, 2.5, 11-H), 4.29 (1H, d, J= 8.0, 1'-H), 3.62 (1H, d, J= 10.5, 3-
H),
3.49 (1H, t, J= 9.0, 3'-H), 3.36 (1H, dd, J= 9.0, 8.0, 2'-H), 3.32 (1H, dq, J=
8.5, 5.5, 5'-H), 3.23 (1H, dd, J= 9.0, 8.5, 4'-H), 2.82 (1H, dq, J= 10.5, 7.0,
2-
H), 2.64 (1H, m, 10-H), 2.55 (1H, m, 6-H), 1.70 (1H, m, 12a-H), 1.66 (1H, bt,
J
= 12.5, 5b-H), 1.56 (1H, m, 12b-H), 1.40 (1H, dd, J= 12.5, 4.5, 5a-H), 1.35
(3H,
d, J= 7.0, 2-Me), 1.31 (3H, d, J= 5.5, 5'-Me), 1.24 (1H, bdd, J= 10.0, 4.5, 4-
H), 1.21 (3H, d, J= 7.0, 6-Me), 1.11 (3H, d, J= 6.5, 10-Me), 1.00 (3H, d, J=
7.0, 4-Me), 0.92 (3H, t, J= 7.5, 12-Me); I3C ~~MR (CDC13) 8 205.0 (C-7), 174.7
(C-1), 146.9 (C-9), 125.9 (C-8), 102.9 (C-1'), 85.4 (C-3), 76.5 (C-3'), 75.5
(C-
4'), 74.7 (C-2'), 73.9 (C-11), 71.6 (C-5'), 45.0 (C-6), 43.9 (C-2), 37.9 (C-
10),
34.1 (C-5), 33.4 (C-4), 25.2 (C-12), 17.7 (6-Me), 17.5 (5'-Me), 17.4 (4-Me),
16.2 (2-Me), 10.3 (12-Me), 9.6 (10-Me)high-resolution FAB-MS calculated for
Cz3H3808 (M + H)+ 443.2644, found 443.2661.
The fact that macrolide 15 containing D-quinovose is indeed produced by
the desl mutant is significant. First, the formation of quinovose as predicted
further corroborates the presence of a pathway-independent reductase in S.
venezuelae that reduces the 3-keto sugars. Interestingly, this reductase is
able to
act on the 4,6-dideoxy sugar 17 as well as the 6-deoxy sugar 16, suggesting
that
it is oblivious to the presence of a hydroxyl group at C-4. However, it is not
clear at this point whether the reduction occurs on the free sugar or after it
is
appended to the aglycone. Second, the retention of the 4-OH in quinovose as a
result of desl deletion provides strong evidence supporting the assigned role
of
dell to encode a C-4 dehydrase. Moreover, the results again show that the
glycosyltransferase (DesVII) of this pathway can recognize alternative sugar
substrates whose structures are considerably different from the original amino
sugar substrate desosamine. While the incorporation of quinovose is important,
52
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
another noteworthy, albeit unexpected, result was the fact that the aglycone
of
the isolated macrolide 15 was 10-deoxy-methynolide 23 instead of methynolide
24 and neomethynolide 25. It is possible that the cytochrome P450 hydroxylase
(PilcC), which catalyzes the hydroxylation of 10-deoxy-methynolide at either
its
C-10 or C-12 position (Xue et al., 1998), is sensitive to structural
variations in
the appended sugar. It could be argued that the presence of the 4-OH group in
the sugar moiety is somehow responsible for decreasing or preventing
hydroxylation of the macrolide.
Thus, the results demonstrate the feasibility of combining pathway-
dependent genetic manipulations and pathway-independent enzymatic reactions
to engineer a sugar of designed structure. It is conceivable that the pathway-
independent enzymes could also be used in concert with the natural
biosynthetic
machinery to generate further structural diversity, which can provide an array
of
random compounds.
Example 5
En ing eerin_~ybrid Macrolide
To alter the saccharide structure of a macrolide, the Streptomyces
venezuelae metlpik gene cluster was selected as the parent system and a gene
from the calicheamicin biosynthetic gene cluster (from Mic~omofzospo~a
eclaihospora spp. Calichehsis) as the foreign gene. The parent cluster encodes
the biosynthetic enzymes for methymycin, neomethymycin, pikromycin, and
narbomycin, of which all are macrolides containing desosamine as the sole
sugar
component for antibiotic activity (Xue et al., 1998; Zhao et al., 1998) Eight
open reading frames (desl deshll~ in this cluster have been assigned as genes
involved in desosamine biosynthesis (Figure 15). The antitumor agent
calicheamicin (26) contains four unique sugars crucial to tight DNA binding
(Ka
about 106-10$), one of which (29) is derived from 4-amino-4,6-dideoxyglucose
(28) and is part of the unusually restricted N O connection between sugars A
and
B (Figure 16) (Ding et al., 1991; Drak et al., 1991; Walker et al., 1991;
Ellestad
et al.; Borders et al., 1995). Compound 28 is anticipated to be derived from
the
53
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
corresponding 4-ketosugar 27 via a transamination reaction, and recent work
has
led to the assignment of a gene (calH) as encoding a C-4 aminotransferase
(Figure 16) (Albert et al.). Interestingly, the proposed substrate for CaIH,
27, is
also an intermediate in the desosamine pathway and is expected to exist in a
tautomerase (DesVIII)-mediated equilibrium with the substrate for DesI (Chen
et
al., 1999). Thus, it is conceivable that 27 might accumulate in a desl or
desVIlI
disruption/deletion S. ven.ezuelae mutant strain. Heterologous expression of
calH in this mutant may reconstitute a hybrid pathway towards new
methymycin/pikromycin derivatives which carry the 4-amino-4,6-dideoxy
glucose derived from 26.
To test this, the 1.2 kb calH gene was amplified by polymerase chain
reaction (PCR) from pJST1192~Pn~.oKb, a subclone containing a 7.0 kb KphI
fragment of cosmid 13a (Thorson et al., 1999). The amplified gene was cloned
into the EcoRIlXbaI sites of the expression vector pDHS617, which contains an
apramycin resistance marker. pDHS617 is derived from pOJ446 (Bierman et al.,
1992), and a promoter sequence from metlpik (Xue et al., 1998). The resulting
plasmid, pLZ-C242, was introduced by conjugal transfer using Esche~ichia coli
S 17-1 (Bierman et al., 1992) into a previously constructed S. venezuelae
mutant
(KdesI) (Borisova et al., 1999) in which dell was replaced by the neomycin
resistance gene that also confers resistance to kanamycin. The pLZ-C242
containing S. vehezuelae-KdesI colonies were identified on the basis of their
resistance to apramycin antibiotic (AprR). One of the engineered strains,
KdesI/calH-1, was first grown in 100 mL of seed medium at 29°C for
48 hours
and then inoculated and grown in vegetative medium (5 L) for another 48 hours
(Cane et al., 1993). The fermentation broth was centrifuged to remove the
cellular debris and mycelia, and the supernatant was adjusted to pH 9.5 with
concentrated KOH followed by chloroform extraction. The crude products (700
mg) were subjected to flash chromatography on silica gel using a gradient of 0-
20% methanol in chloroform. A major product, 10-deoxymethynolide, and a
mixture of two minor macrolide compounds were obtained. The two macrolides
were further purified by HPLC on a C1$ column using an isocratic mobile phase
54
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
of acetonitrile/HZO (1:1). They were later identified as 31 (11.0 mg) and 32
(1.5
mg) by spectral analyses. The spectral data of 31 is: 1H NMR (500 MHz,
CDC13) 8 6.75 (1H, dd, J=16.0, 5.5, 9-H), 6.44 (1H, dd, J=16.0, 1.2, 8-H),
5.34 (1H, d, J= 8.0, N-H), 4.96 (1H, m, 11-H), 4.27 (1H, d, J= 7.5, 1'-H),
3.66
(1H, dd, J= 9.5, 8.0, 4'-H), 3.60 (1H, d, J=10.5, 3-H), 3.50 (1H, t, J= 9.5,
3'-
H), 3 .4 ( 1 H, m, 5'-H), 3 .4 ( 1 H, m, 2'-H), 2. 84 ( 1 H, dq, J = 10.5, 7.
5, 2-H), 2.64
(1H, m, 10-H), 2.53 (1H, m, 6-H), 2.06 (3H, s, Me-C=O), 1.7 (1H, m, 12-H),
1.66 (1H, m, 5-H), 1.56 (1H, m, 12-H), 1.4 (1H, m, 5-H), 1.36 (3H, d, J= 7.5,
2-
Me), 1.25 (3H, d, J= 6.5, 5'-Me), 1.24 (1H, m, 4-H), 1.21 (3H, d, J= 7.5, 6-
Me),
1.10 (3H, d, J= 6.5, 10-Me), 0.99 (3H, d, J= 6.0, 4-Me), 0.91 (3H, t, J= 7.2,
12-
Me). 13C NMR (125 MHz, CDC13) 8 205.3 (C-7), 175.1 (C-1), 171.9 (Me-
C--O), 147.1 (C-9), 126.1 (C-8), 103.0 (C-1'), 85.8 (C-3), 75.8 (C-5'), 75.8
(C-
3'), 74.1 (C-11), 70.8 (C-2'), 57.6 (C-4'), 45.3 (C-6), 44.0 (C-2), 38.1 (C-
10),
34.2 (C-5), 33.6 (C-4), 25.4 (C-12), 23.7 (Me-C=O), 18.1 (C-6'), 17.9 (6-Me),
17.6 (4-Me), 16.4 (2-Me), 10.5 (12-Me), 9.8 (10-Me). High-resolution FAB-MS
calcd for CZSHøZNOB (M+H+) 484.2910, found 484.2903. The spectral data of 32
is: 'H NMR (500 MHz, CDC13) 8 6.69 (1H, dd, J=16.0, 6.0, 11-H), 6.09 (1H,
dd, J=16.0, 1.5, 10-H), 5.35 (1H, d, J= 8.5, N-H), 4.96 (1H, m, 13-H), 4.36
(1H, d, J= 7.5, 1'-H), 4.19 (1H, m, 5-H), 3.83 (1H, q, J= 6.5, 2-H), 3.68 (1H,
dt,
J= 10.0, 8.5, 4'-H), 3.52 (1H, t, J= 8.5, 3'-H), 3.50 (1H, m, 5'-H), 3.42 (1H,
t, J
= 7.5, 2'-H), 2.92 (1H, dq, J= 7.0, 5.0, 4-H), 2.81 (1H, m, 8-H), 2.73 (1H, m,
12-
H), 2.06 (3H, s, Me-C=O), 1. 8 ( 1 H, m, 6-H), 1.6 ( 1 H, m, 14-H), 1.5 5 ( 1
H, m, 7-
H), 1.37 (3H, d, J= 6.5, 2-Me), 1.32 (3H, d, J= 7.0, 4-Me), 1.3 (1H, m, H-14),
1.27 (3H, d, J= 6.5, 5'-Me), 1.25 (1H, m, 7-H), 1.12 (3H, d, J= 6.0, 8-Me),
1.11
(3H, d, J= 6.5, 12-Me), 1.07 (3H, d, J= 6.0, 6-Me), 0.91 (3H, t, J= 7.2, 14-
Me).
High-resolution FAB-MS calcd for Cz$H46NO9 (M+H+) 540.3172, found
540.3203.
The observed production of macrolides 31 and 32 by the KdesI/calH-1
has vast implications. First, the appended hexose (33), which indeed carries
the
predicted amino group at C-4, provides indisputable support for the caZH gene
assignment as encoding the TDP-6-deoxy-D-glyce~o-L-thYeo-4-hexulose 4-
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
aminotransferase of the calicheamicin pathway. Second, the successful
expression of the CalH protein in S. venezuelae by the newly constructed
expression vector highlights the potential of using this system to express
other
foreign genes in this strain, a prerequisite for developing more elaborate
combinatorial biosynthetic strategies. Moreover, this result also reveals that
the
glycosyltransferase (DesVII) of this pathway can recognize alternative sugar
substrates (such as 28) whose structures are considerably different from the
original amino sugar substrate, TDP-D-desosamine. While the sugar component
in the products is expected to be the aminodeoxy hexose 28, the 4-amino group
of the attached sugar component in 31 and 32 is N acetylated. It is not clear
at
this point whether the acetylation occurs on the free sugar or after it is
appended
to the aglycone. Since both 31 and 32 are new compounds synthesized in vivo
by the S. venezuelae mutant strain, the observed N acetylation might be a
necessary step for self protection (Cundliffe, 1989; Cundlife, 1992; McManus,
1997). Indeed, purified 31 and 32 are inactive against Streptococcus pyogehes
grown on Mueller-Hinton agar plates (Managahas, 1996), while the controls
(methymycin and pikromycin) show clearly visible inhibition zones.
Another noteworthy, albeit unexpected result was the fact that the
aglycone of the isolated macrolide 31 was 10-deoxymethynolide instead of
methymycin and neomethymycin analogues that are hydroxylated. Interestingly,
the aglycone of 32 was the 14-membered narbonolide that is also devoid of
hydroxylation. It is possible that the cytochrome P450 hydroxylase (PikC),
which catalyzes the hydroxylation of 10-deoxymethynolide and narbonolide
(Xue et al., 1998) is sensitive to structural variations on the appended
sugar.
Indeed, no aglycone hydroxylation was discernible when 31 and 32 were
incubated with purified PikC in vitro. A similar observation was also noted in
the case where desosamine was replaced by quinovose (Example 4). It could be
argued that the presence of a substituent (either hydroxyl or amino group) at
C-4
in the sugar moiety is responsible, at least in part, for decreasing or
preventing
hydroxylation of the macrolide.
56
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
In conclusion, the results show that non-natural secondary metabolite
glycosylation patterns can be engineered through a rational selection of
heterologous gene combinations. This demonstrated ability to engage foreign
enzymes in concert with the natural biosynthetic machinery offers a tremendous
potential to generate fiu-ther structural diversity. By extending the present
study,
the construction of diverse nucleotide sugar glycosylation precursor pools may
soon substantially enhance current novel drug discovery through combinatorial
biosynthesis efforts.
Example 6
En~ineerin~ybrid Su~ar.Biosynthetic Pathway
The 6-deoxy-4-hexulose 33 in the desosamine pathway has also been
suggested as a biosynthetic intermediate for TDP-z-dihydrostreptose (35), the
precursor of streptose (36) found in the antibiotic streptomycin (37) of
Streptom~ces g~iseus (Figure 16) (Ortmann et al., 1974; Wahl et al., 1975;
Maier
et al. 1975; Wahl et al. 1979). With the tentative assignment of genes in the
streptomycin cluster (Pisowotzki et al., 1991; Distler et al. 1992), a
biosynthetic
pathway for TDP-z-dihydrostreptose has been postulated. As illustrated in
Figure 16, the strM gene may encode a 3,5-epimerase responsible for the
conversion of 33 to 34, while the product of st~L gene is speculated to
catalyze
the ring contraction of 34 to give 35 (Pisowotzki et al., 1991; Distler et al.
1992).
Since the proposed substrate for StrM, 33, is also an intermediate in the
desosamine pathway, heterologous expression of StrM, StrL, or StrM/StrL in the
S. venezuelae desI-mutant in which 33 accumulates, may reconstitute hybrid
pathways toward new methymycin/pikromycin derivatives carrying an L-
pyranose or an z-furanose.
In these experiments, the st~M (0.8 kb) and stsL (1.0 kb) genes were
separately amplified by polymerase chain reaction (PCR) from the genomic
DNA of S. g~iseus. The amplified strM gene was cloned into the EcoRIlNsiI
sites of the expression vector pDHS702 (Xue et al., 2000), which contains a
thiostrepton resistance marker. The strL gene was cloned into the EcoRIlXbaI
57
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
sites of the vector pDHS617, which has an apramycin resistance marker. Each
plasmid was transformed into Eschericl2ia coli S 17-1 (Bierman et al., 1992)
and
then introduced separately by conjugal transfer into the previously
constructed
mutant S. venezuelae KdesI. The resulting strains, KdesI/strM and KdesI/strL,
were identified on the basis of their resistance to the corresponding
antibiotics.
Using the same strategy, the strL-containing plasmid was further engineered
into
the KdesI/strM mutant to produce the recombinant strain KdesI/strM/strL, which
confers resistance to both apramycin and thiostrepton. One such strain,
KdesI/strMlstrL-8, was chosen to grow in 150 mL of seed medium at
29°C for
48 hours, and then inoculated and grown in vegetative medium (6 L) for another
48 hours (Cane et al., 1993). The fermentation broth was centrifuged, and the
supernatant was extracted with chloroform. After concentration, the residual
yellow oil (1.5 g) was subjected to flash chromatography on silica gel using
10%
methanol in chloroform as eluent. The crude products were further purified by
HPLC on a C18 column eluted with a linear gradient of 0-50% acetoutrile in
water over 20 minutes to yield four new macrolide derivatives, 38 (31.1 mg),
39
(6.3 mg), 40 (3.0 mg), and 41 (3.9 mg).
Spectral analysis of these compounds revealed that 38-40 are 12-
membered macrolide derivatives, while 41 is a 14-membered macrolide.
Spectral data of 38: 1H NMR (500 MHz, acetone-d6, Jin hertz) 0.86 (3H, t, J=
7.5, 12-Me), 1.00 (3H, d, J= 6.5, 4-Me), 1.19 (3H, d, J=7.0, 6-Me), 1.20 (3H,
d,
J= 5.0, 5'-Me), 1.18-1.30 (1H, m, 4-H), 1.28 (3H, d, J= 6.5, 2-Me), 1.27-1.37
(1H, m, 5-H), 1.36 (3H, s, 10-Me), 1.50 (1H, ddq, J = 10.8, 14.3, 7.1, 12-H),
1.83 (1H, t, J=13.5, 5-H), 1.99 (1H, ddq, J= 2.0, 14.0, 7.3, 12-H), 2.44 -
2.54
(1H, m, 6-H), 2.84 (1H, dq, J--10.0, 6.8, 2-H), 3.42 (1H, t, J= 9.3, 4'-H),
3.49
(1H, d, J=10.5, 3-H), 3.58 (1H, dd, J= 3.5, 9.5, 3'-H), 3.70 (1H, dq, J= 9.3,
6.2, 5'-H), 3.94 (1H, brs, 2'-H), 4.70 (1H, dd, J= 2.0, 10.5, 11-H), 4.72 (1H,
brs,
1'-H), 6.53 (1H, d, J=16.0, 8-H), 6.57 (1H, d, J=16.0, 9-H);'3C NMR (126
MHz, acetone-db) 11.2 (C-13), 16.8 (2-Me), 17.4 (6-Me), 17.8 (4-Me), 17.9 (C-
6'), 19.3 (10-Me), 21.7 (C-12), 34.2 (C-4), 34.4 (C-5), 44.8 (C-2), 46.2 (C-
6),
70.0 (C-5'), 72.1 (C-2'), 72.5 (C-3'), 73.4 (C-4'), 74.4 (C-10), 77.2 (C-11),
89.0
58
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
(C-3), 104.4 (C-1'), 125.7 (C-8), 150.8 (C-9), 175.4 (C-1), 203.6 (C-7); high-
resolution FAB-MS calcd for C23H38O9Na (M + Na)+ 481.2414, found 481.2444.
Spectral data of 39: 1H NMR (500 MHz, acetone-d6, J in hertz) 1.00 (3H, d, J=
6.5, 4-Me), 1.12 (3H, d, J= 6.5, 12-Me), 1.17 (3H, d, J= 7.0, 10-Me), 1.199
(3H, d, J= 7.5, 6-Me), 1.200 (3H, d, J= 6.0, 5'-Me), 1.22-1.33 (1H, m, 4-H),
1.25 (3H, d, J= 7.0, 2-Me), 1.27-1.39 (1H, m, 5-H), 1.78 (1H, t, J= 13.3, 5-
H),
2.42-2. 51 ( 1 H, m, 6-H), 2.77-2. 85 ( 1 H, m, 2-H), 3 . 06-3 .13 ( 1 H, m,
10-H), 3 .42
(1H, t, J= 9.5, 4'-H), 3.48 (1H, d, J=10.5, 3-H), 3.58 (1H, brd, J= 9.0, 3'-
H),
3.70 (1H, dq, J= 9.4, 6.3, 5'-H), 3.79-3.87 (1H, m, 12-H), 3.93 (1H, brs, 2'-
H),
4.71 (1H, s, 1'-H), 4.75 (1H, dd, J= 2.0, 9.5, 11-H), 6.59 (1H, d, J- 15.5, 8-
H),
6.69 (1H, dd, J= 5.3, 15.8, 9-H); 13C NMR (126 MHz, acetone-d6) 9.9 (10-Me),
16.4 (2-Me), 17.5 (6-Me), 17.8 (4-Me), 17.9 (C-6'), 21.4 (13-Me), 34.2 (C-4),
34.5 (C-5), 36.3 (C-10), 44.5 (C-2), 46.1 (C-6), 66.1 (C-12), 70.0 (C-5'),
72.2
(C-2'), 72.5 (C-3'), 73.4 (C-4'), 76.8 (C-11), 89.1 (C-3), 104.4 (C-1'), 126.7
(C-
8), 148.2 (C-9), 175.0 (C-1), 203.8 (C-7); lugh-resolution FAB-MS calcd for
C23H38O9Na (M + Na)~ 481.2414, found 481.2426. Spectral data of 40: 'H NMR
(500 MHz, acetone-db, Jin hertz) 0.89 (3H, t, J= 7.3, 12-Me), 1.00 (3H, d, J=
7.0, 4-Me), 1.13 (3H, d, J= 7.0, 10-Me), 1.196 (3H, d, J= 7.0, 6-Me), 1.197
(3H, d, J= 6.0, 5'-Me), 1.17-1.27 (1H, m, 4-H), 1.25 (3H, d, J= 7.0, 2-Me),
1.30-1.37 (1H, m, 5-H), 1.57-1.73 (2H, m, 12-Hs), 1.79 (1H, t, J=12.8, 5-H),
2.42-2. 5 0 ( 1 H, m, 6-H), 2.69-2.74 ( 1 H, m, 10-H), 2.75-2. 8 3 ( 1 H, m, 2-
H), 3 .41
(1H, t, J= 9.3, 4'-H), 3.47 (1H, d, J--10.0, 3-H), 3.57 (1h, dd, J 3.0, 9.5,
3'-H),
3.70 (1H, dq, J--9.3, 6.3, 5'-H), 3.94 (1H, brs, 2'-H), 4.71 (1H, brs, 1'-H),
4.96
(1H, ddd, J= 2.3, 5.3, 9.3, 11-H), 6.58 (1H, dd, J= 1.3, 16.3, 8-H), 6.70 (1H,
dd,
J--5.3, 16.3, 9-H); '3C NMR (126 MHz, acetone-d6) 9.8 (10-Me), 10.8 (C-13),
16.7 (2-Me), 17.5 (6-Me), 17.8 (4-Me), 17.9 (C-6'), 25.9 (C-12), 34.3 (C-4),
34.5 (C-5), 38.9 (C-10), 44.7 (C-2), 46.0 (C-6), 70.0 (C-5'), 72.2 (C-2'),
72.5 (C-
3'), 73.5 (C-4'), 74.6 (C-11), 89.2 (C-3), 104.4 (C-1'), 126.5 (C-8), 147.8 (C-
9),
175.4 (C-1), 203.9 (C-7); high resolution FAB-MS calcd for Cz3H39O$ (M + H)+
443.2645, found 443.2620. Spectral data of 41: 1H NMR (500 MHz, acetone-d6,
Jin hertz) 0.89 (3H, t, J= 7.3, 16-Me), 1.01 (3H, d, J= 7.0, 6-Me), 1.11 (3H,
d,
59
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
J= 6.0, 12-Me), 1.13 (3H, d, J= 7.0, 8-Me), 1.16-1.19 (1H, m, 7-H), 1.22 (3H,
d, J= 6.5, 5'-Me), 1.277 (3H, d, J= 7.0, 4-Me), 1.284 (3H, d, J= 7.0, 2-Me),
1.49-1.74 (4H, m, 6-H, 7-H, 14-Hs), 2.69-2.78 (2H, m, 8-H, 12-H), 2.97-3.04
(1H, m, 4-H), 3.41 (1H, t, J= 9.5, 4'-H), 3.60 (1H, dd, J= 3.3, 9.3, 3'-H),
3.70
(1H, dq, J= 9.4, 6.3, 5'-H), 3.85 (1H, brs, 2'-H), 3.95 (1H, brs, 5-H), 4.08
(1H,
q, J= 7.0, 2-H), 4.80 (1H, brs, 1'-H), 4.91 (1H, dt, J= 9.3, 3.3, 13-H), 6.16
(1H,
brd, J= 15.5, 10-H), 6.69 (1H, dd, J= 5.0, 15.5, 11-H); high-resolution FAB-
MS calcd for C26H4309 .(M + H)+ 499.2907, found 499.2924.
Interestingly, the stereochemistry of the linkage between the aglycone
and the appended sugar in 38-41 was established to be (J1,2, = 0 Hz), which is
distinct from the glycosidic linkage (d, J1,2. = 6.5-7.5 Hz) found in the wild
type
structures. While these new compounds all carry an identical 6-deoxyhexose,
the NMR data could not distinguish whether the appended sugar is L-rhamnose
(42) or its enantiomer, 6-deoxy-D-mannose (43). In order to unambiguously
identify the newly incorporated sugar, 38 was treated with dimethoxypropane
followed by derivatization with (S) or (R)-MTAP chloride to generate the
corresponding Mosher's esters (44 and 45). Since the orientation of the phenyl
ring of MTAP is different in these two diastereomers, the protons adjacent to
MTAP will experience differential shielding depending on their spatial
relationship with respect to the anisotropic cone of the aryl group (Ohtani et
al.,
1991; Ferreiro et al., 1991). On the basis of this well-documented phenomenon,
the absolute stereochemistry of the chiral center (C-4') can be deduced from
the
difference in the chemical shifts measured as = ~g~_MTPA ester - (R)-MTPA
ester As
shown in the bottom of Figure 16, positive values were observed for 1'-H, 2'-
H,
3'-H, 4'-H, and the two methyl signals of the acetonide group, while negative
values were recorded for 5'-H and 5'-Me. These finding are indicative of an S
configuration at C-4', allowing the attached sugar in 38-41 to be assigned as -
L-
rhamnose.
With the identification of z-rhamnose (42) as the sugar component of
metabolites 38-41 produced by the engineered KdesI/strM/strL strain, the role
of
StrM as a 3,5-epimerase converting 33 to 34 is clearly confirmed. The
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
corresponding methymycin/pikromycin derivatives carrying a -linked D-
quinovose (47, 6-deoxy-D-glucose) were produced by the KdesI/strL strain
(Figure 16). These quinovose-containing macrolides were also found as
metabolites of the host strain, S. venezuelae KdesI and the KdesI/strM strain.
Since the substrate of StrL is expected to be 34, in the absence of StrM to
catalyze the necessary D-/L-conversion of 33 to provide 34, it is not
surprising
that both KdesI/strL and KdesI strains produce the same macrolide compounds
as observed. The fact that no new macrolide products were found in the broth
of
the KdesI/strM strain may be attributed to the instability of 34 ih vivo, or
the
inability of the glycosyltransferase DesVII to process 34 as a substrate.
Apparently, the host strain of S. venezuelae KdesI contains a pathway-
independent D-hexulose reductase that can reduce 33 to TDP-D-quinovose (46),
but lacks an z-hexulose reductase of its own to reduce 34. The StrM catalyzed
epimerization is expected to be reversible. Thus, in the presence of a D-
hexulose
reductase, the equilibrium between 33 and 34 in the KdesI/strM strain will be
shifted toward 33, which after reduction gives quinovose as observed in the
product. Since z-rhamnose is formed only in the St~L-containing strain, one
can
conclude that, in addition to its putative function as dihydrostreptose
synthase,
StrL could also serve as a sugar reductase capable of reducing an z-6-deoxy-4-
hexulose such as 34 to TDP-z-rhamnose (48).
It should be noted that the mechanism of the ring contraction step in the
dihydrostreptose pathway is remarkably similar to that proposed for the
biosynthesis of UDP-D-apiose (50), which is derived from UDP-D-glucuronic
acid (49) catalyzed by apiose synthase (Figure 18) (Kelleher et al., 1972;
Gebb et
al., 1975; Watson et al., 1975; Matern et al., 1977; Wahl et al., 1978). This
synthase has been assigned to have dual functions, possessing both 4-hexulose
reductase and ring-contraction activities, since UDP-D-xylose (51) is a
byproduct
of the catalysis of apiose synthase (Kelleher et al., 1972; Gebb et al., 1975;
Watson et al., 1975; Matern et al., 1977; Wahl et al., 1978). Thus, the fact
that
StrL resembles apiose synthase having hexulose reductase activity lends strong
credence for an analogous role of StrL as the catalyst for the ring
contraction
61
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
step in the dihydrostreptose pathway. The failure to detect the incorporation
of
35 into the macrolide structures may simply reflect the limitation of DesVII
to
accommodate a furanose in its active site.
The results described here present a rare example of a glycosyltransferase
that recognizes both D- and L-sugar as substrates (Wohlert et al., 1998). The
established versatility of this glycosyltransferase (DesVII) on substrate
selection
highlights its potential as a catalyst in the construction of new macrolides
carrying a broad range of modified sugars, a prerequisite for developing more
exquisite combinatorial biosynthetic strategies for new antibiotics. This work
once again demonstrates the feasibility of engineering secondary metabolite
glycosylation through a rational selection of gene combinations.
Example 7
Synthesis of TDP-4-amino-4,6 dideoxy-D-glucose by DesII
Carbohydrates are the focus of growing attention among biological
molecules in recent years due to the increased appreciation of their vital
roles in
many physiological processes (Weymouth-Wilson et al., 1997). As components
of many glycoconjugates, sugars, particularly the deoxysugars, contribute to a
diverse repertoire of biological activities. Since modifying the structure of
the
appended sugars holds promise for varying or enhancing the biological
activities
of the parent glycoconjugates, there is considerable and continuing effort to
explore how these unusual sugars axe made in the producing organisms (Hallis
et
al., 1999; He et al., 2000). Such striving has led to the discovery of several
elegant strategies evolved in nature for breaking the C-O bond of a hexose
sugar.
Thus, presently it can be concluded that a sequence of a,~i-dehydration
followed
by a hydride reduction is the mechanism for ~i-deoxygenation of a ketosugar
precursor (Draeger et al., 1999; Chen et al., 1999) whereas a collaborative
catalysis by a pyridoxamine 5'-phosphate (PMP)-dependent [2Fe-2S]-containing
enzyme (El) and an NADH-dependent iron-sulfur flavoprotein reductase (E3) is
require for a-deoxygenation of a ketosugar substrate (Thorson et al., 1993;
Johnson et al., 1996; Chang et al., 2000).
62
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
While the mechanisms of C-O bond cleavage at C-2, C-3, and C-6 of a
hexose have been fully established (Hallis et al., 1999; He et al., 2000),
little is
known about the mode of C-O bond scission at C-4 in making 4-deoxygenated
sugars. Genetic studies on the biosynthesis of D-desosamine (1 in Figure 19),
a
3-(dimethylamino)-3,4,6-trideoxyhexose found in a number of antibiotics,
resulted in the identification of the entire desosamine biosynthetic gene
cluster
from Streptomyces vefaezuelae (Scheme 1, Figure 19) (Zhao et al., 1998; Xue et
al., 1998), which produces methymycin (2), neomethymycin (3), pikromycin (4),
and narbomycin (5). From this, eight open reading frames (desl - desVII~
within this cluster are suggested to be involved in desosamine biosynthesis
including dell and desll that are assigned to be associated with the C-4
deoxygenation step (Zhao et al., 1998; and see Gaisser et al., 1997; Summers
et
al., 1997, which relate to the DesI/DesII equivalents in the erythromycin gene
cluster, i.e, EryCIV/EryCV). Since the translated sequence of desl shows high
homology to B6 dependent enzymes and is 24% identical to that of El, and the
translated desll sequence contains a conserved motif of C~XXCXXC (SEQ ID
NO:50) characteristic for a [4Fe-4S] center (Ruzicka et al., 2000), the C-4
deoxygenation has been postulated to follow a path similar to that catalyzed
by
El and E3 ~Zhao et al., 1998; and see Gaisser et al., 1997; Summers et al.,
1997.
As illustrated in Scheme 1 (Figure 19), the reaction may be initiated by a
tautomerization step presumably catalyzed by DesVIII to convert 6, a common
precursor for 6-deoxyhexoses, to 3-keto-6-deoxyhexose 7. DesI and DesII may
then effect the removal of 4-OH from 7 to give the 3-keto-4,6-dideoxyhexose
product (8) which has earlier been confirmed as the substrate of the next
enzyme
in the pathway, DesV (Zhao et al., 1998). This proposal is supported by the
fact
that 4-OH is retained in the appended sugar (D-quinovose, 9) of the modified
methymycin and pikromycin derivatives produced by the desl deleted mutant
(Borisova et al., 1999). To learn more about this C-O bond cleavage event,
targeted disruption of the desll gene and functional analyses of the DesI
enzyme
were conducted.
63
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
To confirm whether DesII is a part of the C-4 deoxygenation machinery,
a S. venezuelae mutant was generated in which the desll gene was replaced by
the kanamycin resistance gene through homologous recombination of a plasmid
containing the appropriate insert with the wild-type S. venezuelae chromosome
(Bierman et al., 1992). This mutant strain was isolated and used for
fermentation as previously described (Zhao et al., 1998; Cnae et al., 1993).
It
should be pointed out that El-catalyzed dehydration is a reversible reaction
with
equilibrium favoring the reverse direction (Weigel et al., 1992), and the
reduction by E3 is essential to drive the overall reaction to completion.
Hence, if
C-4 deoxygenation follows a path similar to E,/E3 catalysis and DesII is an E3-
equivalent, disruption of the desll gene is expected to give a mutant with a
phenotype that is identical to the desl mutant. Indeed, no wild-type
antibiotics
were found in the fermentation broth (6 L) of desll deleted mutant; instead,
two
macrolides containing an N acetylated 4-aminosugar, 11 (2.4 mg) and 12 (1 mg),
were obtained (see Zhao et al., 1998). Compounds 11 and 12 are likely derived
from the coupling of 14 and the respective aglycons, followed by N acetylation
(Scheme 1). However, it is also possible that N acetylation of 10 occurs prior
to
its coupling to the aglycons. Regardless of the sequence of the events, the
production of 11 and 12 clearly indicates that 10 must be accumulated in the
desll deleted mutant of S. veuezuelae.
The above results provide a hint that DesI is a 4-aminotransferase, and 4-
amination is the initial step of 4-deoxygenation. To verify the catalytic
function
of DesI, the dell gene was amplified by PCR and cloned into the pET-28b(+)
expression vector (Novagen) with a His6-tag at the N terminus. The produced
DesI protein was purified to near homogeneity by a Ni-NTA column (Qiagen)
followed by FPLC on a MonoQ column. As judged by SDS-PAGE, the subunit
Mr of DesI was estimated to be 45 kDa, which agrees well with the calculated
molecular mass of 45 765 Da (plus the His6 tag). Further analysis by size
exclusion chromatography revealed a Mr of 95.6 kDa for DesI. Therefore, DesI
exists as a homodimer in solution. The UV - vis spectnun of purified DesI is
64
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
transparent above 300 nm; however, that of the more concentrated sample shows
the presence of trace amount of PLP.
Interestingly, when the putative substrate, TDP-3-keto-deoxy-D-glucose
(7) was incubated with the purified DesI in the presence of z-glutamate, no
consumption of 7 and no new product were discernable by HPLC analysis. On
the contrary, when TDP-4-amino-4-keto-6-deoxy-D-glucose (6) was incubated
with DesI under identical conditions, consumption of 6 (retention time = 4.52
min) and the formation of a new product (retention = 3.87 min) were observed.
This new compound was purified by FPLC on a MonoQ column and
characterized as the TDP-4-amino-4,6-dideoxy-D-glucose (10). These results
firmly establish that DesI only recognizes 4-hexulose 6 as the substrate and
will
not processes 3-hexulose 7. These findings corroborate well with the desll
gene
disruption results. As a PLP-dependent 4-aminotransferase, a K~at value of
56.2 +
3.1 miri 1 and a KM value of 130 + 4 ~,M for the sugar substrate 6 were also
determined for DesI.
The fact that DesI, in the absence of DesII, catalyzes a transamination
reaction on 6 to generate a 4-aminosugar product 10 calls for the modification
of
the previously proposed biosynthetic pathway for TDP-D-desosamine (Scheme
1, Figure 19). Clearly, the tautomerization of 6 to 7 is no longer a necessary
step
in the desosamine pathway. Furthermore, this implies that the mechanism of C-
4 deoxygenation cannot be similar to that of the C-3 deoxygenation catalyzed
by
El/E3 (Hallis et al., 1999; He et al., 2000;Thorson et al., 1993; Johnson et
al.,
1996; Chang et al., 2000). Considering that DesIlDesII catalysis is initiated
by
the incorporation of a nitrogen functional group at C-4 (such as 13), a 1,2-
nitrogen shift from C-4 to C-3 to generate an aminal intermediate (such as 14)
may be the lcey step of C-4 deoxygenation. As illustrated in Scheme 2,
elimination of either a water or an ammonium molecule from C-3 of 14 will
generate the 3-keto-4,6-dideoxysugar product (8). There are enzymes capable of
promoting 1,2-amino shift. The two best studied examples are ethanolamine
ammonia lyase, and adenosylcobalamin (AdoCbl)-dependent enzyme that
catalyzes the degradation of ethanolamine to ammonia and apetaldehyde (Babior
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
et al., 1982; LoBrutto et al., 2001; Frey et al., 2000), and lysine 2,3-
aminomutase
which catalyzes the interconversion of z-lysine and z-~i-lysine via 1,2-
migration
of the amino group (Babior et al., 1982; LoBrutto et al., 2001; Frey et al.,
2000).
The latter enzyme from CZostYidium subte~minale SB4 contains an iron-sulfur
center and is PLP-as well as S-adenosylmethionine (SAM)-dependent. Both
reactions are believed to involve a putative 5'-deoxyadenosyl radical which is
generated by a reductive cleavage of SAM in lysine 2,3-aminomutase, or a
homolytic cleavage of the Co-C bond of adenosylcob(III)alamin in ethanolamine
ammonia lyase. This adenosyl radical then abstracts a hydrogen atom from the
substrate to initiate the isomerization. Since DesI is a PLP enzyme and DesII
has recently been identified as a member of radical SAM superfamily by
sequence analyses (Sofia et al., 2001), the DesI and DesII enzymes may work
together to catalyze a 1,2-amino migration analogous to that of lysine 2,3-
aminomutase (see Scheme 2, Figure 20) to achieve C-4 deoxygenation. (It is
also
possible that DesII may act alone by abstracting a 3-H~ directly from 10 to
generate a radical intermediate which, after deprotonation of OH, is converted
to
a ketyl equivalent. Subsequence ~3elimination of 4-amino group followerd by a
H' return and tautomerization can also afford 8).
There is no doubt that this study has furnished compelling evidence
indicating a new pathway for the biosynthesis of desosamine. These results
also
allow the postulation of a new mechanism for C-4 deoxygenation,. A
comparison of this new mechanism with that of C-3 deoxygenation clearly
shows that nature has evolved diverse and elaborate strategies to pursue the
removal of an a-OH from a ketohexose precursor in the biosynthesis unusual
sugars. Taken together, studies conducted on the biosynthesis of deoxyhexoses
have infused refreshing mechanistic insights into the general routes of
biological
deoxygenations. These findings are a good testament to the evolutionary
diversity of biological C-O bond cleavage events (Johnson et al., 1999).
66
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
References
Andersen, J. R., Hutchinson, C. R. J. Bacteriol., 174:725-735 (1992).
Aparicio, J. F., Molnar, L, Schwecke, T., Koni.g, A., Haydoclc, S. F.,
Khaw, L. E., Staunton, J., Leadlay, P. F. Gene, 169:9-16 (1996).
Arisawa, A., Kawamura, N., Takeda, K., Tsunekawa, H., Okamura, K.,
Okamoto, R. Appl. Environ. Microbiol., 60:2657-2660 (1994).
August, P. R., Tang, L., Yoon, Y. J., Ning, S., Muller, R., Yu, T. W.,
Taylor, M., Hoffinann, D., Kim, C. G., Zhang, X., Hutchinson, C. R. & Floss,
H.
G. Chem. Biol., 5:69-79 (1998).
Babior, B.M., In B12 Dolphin, D., Ed.; Wiley: New York, 1982; Vol. 2,
Chapter 10.
Baltz, R. H., Seno, E. T. Annu. Rev. Microbiol., 42:547-574 (1988).
Barnes et al. Methods Enzymol. 101:98-122 (1983).
Bibb, M. J., Bibb, M. J., Ward, J. M., Cohen, S. N. Mol. Gen. Genet.,
199:26-36 (1985).
Bierman, M., Logan, R., O'Brien, K., Seno, G., Nagaraja, R., Schoner, B.
E. Gene, 116:43-49 (1992).
Borisova, S.A., Zhao, L., Sherman, D.H., Liu, H.-w., Or_.~, 1, 133-
136 (1999).
Box, R. P. Clin. Infect. Dis., 24:5151 (1997).
Cane, D. E., Lambalot, R. H., Prabhakaran, P. C., Ott, W. R. J. Am.
Chem. Soc., 115:522-526 (1993).
Cane, D.E., Walsh, C.T., Khosla, C., Science, 282, 63-68 (1998).
Cameras, C. W., Pieper, R., Khosla, C. In Bioorganic Chemistry
Deoxysu~ars, Polyketides & Related Classes: Synthesis, Biosynthesis Enzymes,
Rohr, J. (ed.), Springer:Berlin, 85-126 (1997).
Castle, L. A., Smith, K. D., Momis, R. O. J. Bacteriol., 174:1478-1486
(1992).
Celiner, W. D., Nagel, A. A., Wadlow, J. W., Tatematsu, H., Ikenaga, S.,
Nakanishi, S. Abstracts of Papers of 24th Intersci. Conf. on Antimicrob.
Agents
Chemother., No. 1142, Washington, D. C. (1985).
67
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Chang et al., J. Am. Chem. Soc., 122:4239-4240 (2000).
Chen, H., Yeung, S.-M., Que, N.L.S., Miiller, T., Schmidt, R.R., Liu, H.-
w., J. Am. Chem. Soc., 121:8124(1999).
Clark et al. Proc. Natl. Acad. Sci. USA 53:451-459 (1965).
Cortes, J. Haydock, S. F., Roberts, G. A., Bevitt, D. J., Leadlay, P. F.
Nature, 348:176-8 (1990).
Cortes, J., Wiesmann, K. E., Roberts, G. A., Brown, M. J., Staunton, J.,
Leadlay, P. F. Science, 268:1487-9 (1995).
Crameri et al., Nature, 391, 288 (1998).
Cundliffe, E. C. Annu. Rev. Microbiol., 43:207-233 (1989).
Cundliffe, E. Antimicrob. Agents Chemother., 36:348-352 (1992).
Cundliffe, E., Self Protection Mechanisms in Antibiotic Producers;
Cundliffe, E., Ed., Wiley: Chichester, 199-214 (1992).
Dalbie-McFarland et al., Proc. Natl. Acad. Sci. USA 79:6409 (1982).
Davies, J. Nature, 383:219-220 (1996).
Dayhoff, M. 0., in Atlas of Protein Sequence and Structure, 1972,
volume 5, National Biomedical Research Foundation, pp. 101-110, and
Supplement 2 to this volume, pp. 1-10.
Decker, H., Haag, S., Udvarnoki, G., Rohr, J., Anew. Chem. Int. Ed.
(1995).
Denis, F., Brzezinski, R. Gene, 111:115-118 (1992).
Ding, W.-d., Ellestad, G.A., J. Am. Chem. Soc., 113, 6617 (1991).
Distler, J., Mansouri, K., Mayer, G., Stockmann, M., Piepersberg, W.,
Gene, 115, 105-111 (1992).
Djerassi, C., Zderic, J. A. J. Am. Chem. Soc., 78:6390-6395 (1956).
Donadio, S., McAlpine, J. B., Sheldon, P. J., Jackson, M., Katz, L. Proc.
Natl. Acad. Sci. U.S.A., 90:7119-7123 (1993).
Donadio, S., Staver, M. J., McAlpine, J. B., Swanson, S. J., Katz, L.
Science, 252:675-9 (1991).
Donadio, S., Katz, L. Gene, 111:51-60 (1992).
68
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Donin, M. N., Pagano, J., butcher, J. D., McKee, C. M. Antibiotics
Annu., 1:179-185 (1953-1954).
Draeger et al., J. Am. Chem. Soc., 121:2611-2612 (1999).
Drak, J., Iawasawa, S., Danishefsky, S., Crothers, D., Proc. Natl. Acad.
Sci., 88, 7464 (1991).
Ellestad, G.A., Ding, W.-D., Zein, N., Townsend, C.A., In
Calicheamicins: DNA-Cleaving Properties of Calicheamicin, Borders, D.B.,
Doyle, T.W., Eds.; Marcel Dekker: New York, 137-160 (1995).
Epp, J., Huber, M. L. B., Tuner, J. R., Goodson, T., Schoner, B. E. Gene,
85:293-301 (1989).
Ferreiro, M. J., Latypov, S. K., Quinoa, E., Riguera, R., J. O.r~. Chem.,
65, 2658-2666 (1991).
Flinn, E. H., Sigal, M. V., Jr., Wiley, P. F., Gerzon, K. J. Am. Chem.
Soc., 76:3121-3131 (1954).
Frey et al., Arch. Biochem. Bioph,~s., 382: 6-14 (2000).
Frey, P.A., In Comprehensive Chemistry of Natural Products; Barton, D.,
Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: New York, 1999; Vol. 5, pp.
205-223.
Gaisser, S., Bohm, G. A., Cortes, J., Leadlay, P. F. Mol. Gen. Genet.,
256:239-251 (1997).
Gaisser, S., Bohm, G.A., Doumith, M., Raynal, M.-C., Dhillon, N.,
Cortes, J., Leadlay, P.F., Mol. Gen. Genet., 258 78-88 (1998).
Gandecha, A. R., Large, S. L., Cundliffe, E. Gene, 184:197-203 (1997).
Gebb, C., Baron, D., Grisebach, H., Eur. J. Biochem., 54, 493-498
(1975).
Geisselsoder et al. BioTechniques 5:786 (1987).
Geistlich, M., Losick, R., Turner, J. R., Rao, R. N. Mol. Microbiol.,
6:2019-2029 (1992).
Gokhale, R.S., Hunziker, D., Cane, D.E., Khosla, C. Chem. Biol., 6:117-
125 (1999).
Hallis et al., Acc. Chem. Res., 3: 579-588 (1999).
69
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Haydock, S. F., Dowson, J. A., Dhillon, N., Roberts, G. A., Cortes, J.,
Leadlay, P. F. Mol. Gen. Genet., 230:120-128 (1991).
He et al., Chem. Rev., 100: 4615-4661 (2000).
Hernandez, C., Olano, C., Mendez, C., Salas; J. A. Gene, 134:139-140
(1993).
Hopwood, D. A., Sherman, D. H. Annu. Rev. Genet., 24:37-66 (1990).
Hopwood, D. A., Malpartida, F., Kieser, H. M., Ikeda, H., Duncan, J.,
Fujii, L, Rudd, B. A., Floss, H. G., Omura, S. Nature, 314:642-644 (1985).
Hopwood, D. A., Bibb, M. J., Chater, K. J., Kieser, T., Bruton, C. J.,
Kieser, H. M., Lydiate, D. J., Smith, C. P., Ward, J. M., Schrempf, H.,
Genetic
Manipulation of Streptomyces: A Laboratory Manual (The John Innes
Foundation) (1985).
Hori et al., Chem. Comm., 304 (1971).
Hutchinson, C. R., Fujii, I. Annu. Rev. Microbiol., 49:201-238 (1995).
Hutchinson, C. R., Curr. Op. Micro., 1, 319-329 (1998).
Ingrosso, D., Fowler, A. V., Bleibaum, J., Clarke, S. J. Biol. Chem.,
264:20130-20139 (1989).
Jacobsen, J. R., Hutchinson, C. R., Cane, D. E., Khosla, C. Science,
277:367-369 (1997).
Jenksins, G., Cundliffe, E. Gene, 108, 55-62 (1991).
Johnson et al., Biochemistry, 35:15846-15856 (1996).
Johnson et al., Ih The Biology-Claeynist~y Interface: A Tribute to Koji
Nakanishi; Cooper, R., Snyder, J.D., Eds.; Marcel Dekker: New York; 1999; pp
351-396.
Kakavas, S. J., Katz, L., Stassi, D. J. Bacteriol., 179:7515-22 (1997).
Kao, C.M., Luo, G.L., Katz, L., Cane, D.E., Khosla, C. J. Am. Chem.
Soc., 117:9105-9106 (1995).
Katz, L., Donadio, S. Annu. Rev. Microbiol., 47:875-912 (1993).
Katz, L., Chem. Rev., 97:2557-2575 (1997).
Kelleher, W. J., Baron, D., Ortmann, R., Grisebach, H., FEBS Lett., 22,
203-204 (1972).
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Khosla, C., Chem. Rev., 97:2577-2590 (1997).
Khosla, C., Zawada, R. J. Trends Biotechnol., 14:335-341 (1996).
Kirschning, A., Bechthold, A. F.-W., Rohr, J. In Bioorganic Chemistry
Deoxysugars, Polyketides & Related Classes: Synthesis, Biosynthesis, Enzymes,
Rohr, J. (ed.), Springer:Berlin 1-84 (1997).
Kirschning et al., Top. Curr. Chem., 188:1-84 (1997).
Kramer, P. J., Khosla, C. Annu. N.Y. Acad. Sci., 799:32-45 (1996).
Kunkel, T. A. Proc. Natl. Acad. Sci. USA 82:448 (1985).
Kuo, M.-S., Chirby, D. G., Argoudelis, A. D., Cialdella, J. L, Coats, J.
H., Marshall, V. P. Antimicrob. Agents Chemother., 33:2089-2091 (1989).
Lambalot, R. H., Cane, D. E. J. Antibiot., 45:1981-1982 (1992).
Lin, E. C. C., Goldstein, R., Syvanen, M. Bacteria, Plasmids, and
Phases, An Introduction to Molecular Biology, Harvard University
Press:Cambridge, p. 123 (1984).
, Liu, H.-w., Thorson, J. S. Annu. Rev. Microbiol., 48:223-256 (1994).
LoBrutto et al., Biochemistry" 40:9-14 (2001).
Lydiate, D.J., Malpartida, F., Hopwood, D.A. Gene, 35:223-235 (1985).
Madduri, K., Kennedy, J., Rivola, G., Inventi-Solari, A., Filippini, S.,
Zanuso, G., Colombo, A. L., Gewain, K. M., Occi, J. L., MacNeil, D. J.,
Hutchinson, C. R. Nature Biotech., 16:69-74 (1998).
Maier, S., Matern, U., Grisebach, H., FEBS Lett., 49, 317-319 (1975).
Mangahas, F. R. MS Thesis, University of Minnesota, 1996.
Marahiel, M. A., Stachelhaus, T., Mootz, H. D., Chem. Rev., 97:2651-
2673 (1997).
Marsden, A. F. A., Wilkinson, B., Cortes, J., Dunster, N. J., Staunton, J.,
Leadlay, P. F. Science, 279:199-201 (1998).
Matern, U., Grisebach, H., Eur. J. Biochem., 74, 303-312 (1977).
McDaniel, R., Thamchaipenet, A., Gustafsson, C., Fu, H., Betlach, M.,
Betlach, M., Ashley, G., Proc. Natl. Acad. Sci. USA, 96, 1846-1851 (1999).
McManus, M.C., Am. J. Health-Syst. Pharm., 54, 1420-1433 (1997).
Merson-Davies, L. A., Cundliffe, E. Mol. Microbiol., 13:349-355 (1994).
71
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Merson-Davies, L. A., Cundliffe, E. Mol. Microbiol., 13:347-355 (1994).
Monaco and Lavin, Trends in Biotech., 12, 280 (1994).
Motamedi, H., Cai, S. J., Shafiee, A., Elliston, K. O. Eur. J. Biochem.,
244:74-80 (1997).
Muller et al. Liebigs Ann., 1907-1914 (1997).
Muth, G., Nubhaumer, B., Wohlleben, W., Puhler, A. Mol. Gene. Genet.,
219:341-348 (1989).
Needleman and Wunsch J. Mol. Biol. 4~: 443 (1970).
Niemi, J., Mantsala, P. J. Bacteriol., 177:2942-2945 (1995).
Ohtani, L, Kusumi, T., Kashman, Y., Kakisawa, H., J. Am. Chem. Soc.,
113, 4092-4096 (1991).
Omura, S. (ed.) Macrolide Antibiotics, Chemis , , Biolo_y, and Practice,
Academic Press:New York (1984).
Omuras et al., J. Antibio., 29, 316 (1971).
Ortmann, P., Matern, U., Grisebach, H., Stadler, P., Sinnwell, V., Paulsen,
H., Eur. J. Biochem., 43, 265-271 (1974).
Patten et al., Curr. Op. Biotech., $, 724 (1997).
Pearson and Lipman Proc. Natl. Acad. Sci. (U.S.A.) 85: 2444 (1988).
Pisowotzki, K., Mansori, K., Piepersberg, M., Mol. Gen. Genet., 231, 113-
123 (1991).
Rangaswamy, V., Mitchell, R., Ullrich, M., Bender, C. J. Bacteriol.,
180:3330-3338 (1998).
Russell et al. Gene, 45:333-338 (1986).
Ruzicka et al., J. Bacteriol. 182:469-476 (2000).
Salah-Bey, K., Doumith, M., Michel, J.-M., Haydoclc, S., Cortes, J.,
Leadlay, P.F., Raynal, M.-C., Mol. Gen. Genet., 257, 542-553 (1998).
Sambrook, J., Fritsch, E. F., Maniatis, T. Molecular Cloning: A
Laboratory Manual (Cold Spring Harbor Laboratory Press), 2nd edition (1989).
Sasaki, J., Mizoue, K., Morimoto, S., Omura, S. J. Antibiotics, 49:1110-
1118 (1996).
Schalkwyk et al., Curr. Op. Biotech., 6, 37 (1995).
72
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Schneider, A., Marahiel, M. A., Arch. Microbiol., 169:404-410 (1998).
Schupp, T., Toupet, C., Cluzel, B., Neff, S., Hill, S., Beclc, J. J., Ligon,
J.
M., J. Bacteriol., 177:3673-9 (1995).
Schwecke, T., Aparicio, J. F., Molnar, L, Konig, A., Khaw, L. E.,
Haydock, S. F., Oliynyk, M., Caffrey, P., Cortes, J., Lester, J. B., et al.
Proc.
Natl. Acad. Sci. U.S.A., 92:7839-7843 (1995).
Seo, S., Tomita, Y., Tori, K., Yoshimura, Y. J. Am. Chem. Soc.,
100:3331-3339 (1978).
Service, R. F. Science, 270:724-727 (1995).
Smith and Waterman Adv. Appl. Math. 2: 482 (1981).
Sofia et al., Nucleic Acids Res., 29:1097-1106 (2001).
Solenberg, P.J., Matsushima, P., Stack, D.R., Wilkie, S.C., Thompson,
R.C., Baltz, R.H., Chem. Biol., 4, 195-202 (1997).
Stassi, D., Donadio, S., Staver, M. J., Katz, L. J. Bacteriol., 175:182-189
(1993).
Staunton, J., Caffrey, P., Aparicio, J.F., Roberts, G.A., Bethell, S.S.,
Leadlay, P.F. Nat. Struct. Biol., 3:188-192 (1996).
Staunton, J., Wilkinson, B., Chem. Rev., 97:2611-2629 (1997).
Summers, R. G., Donadio, S., Staver, M. J., Wendt-Pienkowslci, E.,
Hutchinson, C. R., Katz, L. Microbiology, 143:3251-3262 (1997).
Swan, D. G., Rodriguez, A. M., Vilches, C., Mendez, C., Salas, J. A.
Mol. Gen. Genet., 242:358-362 (1994).
Thorson, J.S., Shen, B., Whitwam, R.E., Liu, W., Li, Y., Ahlert, J.,
Bioor~. Chem., 27, 172-188 (1999).
Thorson et al., Am. Chem. Soc., 115:12177-12178 (1993). (b
Tuan, J. S., Weber, J. M., Staver, M. J., Leung, J. O., Donadio, S., Katz,
L. Gene, 90:21-29 (1990).
Vilches, C., Hernandez, C., Mendez, C., Salas, J. A. J. Bacteriol.,
174:161-165 (1992).
von Heijne, G. Nucleic Acids Res., 14:4683-4690 (1986).
von Heijne, G., Abrahmsen, L. FEBS Lett., 244:439-446 (1989).
73
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Wahl, P., Matern, U., Grisebach, H., Biochem. Biophys. Res. Commun.,
64, 1041-1045 (1975).
Wahl, H. P., Grisebach, H., Adv. Carboh~dr. Chem. Biochem., 35, 81-126
(1978).
Wahl, H. P., Grisebach, H., Biochem. Bio~h.~cta, 568, 243-252 (1979).
Walker, S., Yang, D., Kahne, D., Gange, D., J. Am. Chem. Soc., 113,
4716-4717 (1991).
Watson, R. R:, Orenstein, N. S., Adv. Carbohydr. Chem. Biochem., 31,
135-184 (1975).
Weber, J. M., Leung, J. O., Swanson, S. J., Idler, K. B., McAlpine, J. B.
Science, 252:114-117 (1991).
Weigel et al., Biochemistry, 31:2129-2139 (1992).
Weymouth-Wilson, A.C., Nat. Prod. Rep., 14, 99-110 (1997).
Wohlert, S.-E., Blanco, G., Lombo, F., Fernandez, E., Brana, A.F., Reich,
S., Udvarnoki, G., Mendez, C., Decker, H., Frevert, J., Salas, J.A., Rohr, J.,
J.
Am. Chem. Soc., 120, 10596-10601 (1998).
Xue, Y., Wilson, D., Zhao, L., Liu, H.-w., Sherman, D.H., Chem. Biol., 5,
661-667 (1998).
Xue, Y., Zhao, L., Liu, H.-w., Sherman, D.H. Proc. Natl. Acad. Sci.
U.S.A., 95: 12111-12116 (1998).
Xue, Y., Sherman, D. H., Nature, 403, 571-575 (2000).
Yu et al., J. Biol. Chem., 267:5868-5875 (1992).
Zhang and Wing, Plant Mol. Biol., 35, 115 (1997).
Zhao, L., Sherman, D.H., Liu, H.-w., J. Am. Chem. Soc., 120, 9374-9375
(1998).
Zhao, L., Sherman, D.H., Liu, H.-w., J. Am. Chem. Soc., 120, 10256-
10257 (1998).
Zhao, L., Que, N.L.S., Xue, Y., Sherman, D.H., Liu, H.-w., J. Am. Chem.
Soc., 120, 12159-12160 (1998).
Zhao et al., J. Am. Chem. Soc., 121:9881-9882 (1999).
Zoller and Smith, Methods Enzymol., 100:468 (1983).
74
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
The complete disclosure of all patents, patent documents and publications
cited herein are incorporated herein by reference as if individually
incorporated.
The foregoing detailed description and examples have been given for clarity of
understanding only. No unnecessary limitations are to be understood therefrom.
The invention is not limited to the exact details shown and described for
variations obvious to one skilled in the art will be included within the
invention
defined by the claims.
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
1
SEQUENCE LISTING
<110> Regents of the University of Minnesota
Sherman, D.H.
Liu, H.
Zhao, L.
<120> Method to alter sugar moieties
<130> 600.475W01
<150> US 60/238,185
<151> 2000-10-05
<160> 25
<170> FastSEQ for Windows Version 4.0
20<210> 1
<400> 1
000
25<210> 2
<400> 2
000
30<210> 3
<211> 13613
<212> DNA
<213> Streptomyces venezuelae
35<400>
3
ggatccggcg cttccaccccgcgccgaacagcgcggtgcggctggtctgcctgccgcacg 60
CCggCggCtC CgCCagCtaCttcttccgcttctcggaggagctgcacccctccgtcgagg 120
ccctgtcggt gcagtatccgggccgccaggaccggcgtgccgagccgtgtctggagagcg 180
tcgaggagct cgccgagcatgtggtcgcggccaccgaaccctggtggcaggagggccggc 240
40tggccttcttcgggcacagcctcggcgcctccgtcgccttcgagacggcccgcatcctgg 300
aacagcggca cggggtacggCCCgagggCCtgtacgtctccggtcggcgcgccccgtcgc 360
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
2
tggcgccggaccggctcgtccaccagctggacgaccgggcgttcctggccgagatccggc 420
ggctcagcggcaccgacgagcggttcctccaggacgacgagctgctgcggctggtgctgc 480
ccgcgctgcgcagcgactacaaggcggcggagaCgtaCCtgCaCCggCCgtCCgCCaagC 540
tCaCCtgCCCggtgatggccctggccggcgaccgtgacccgaaggcgccgctgaacgagg 600
5tggccgagtggcgtcggcacaccagcgggccgttctgcctccgggcgtactccggcggcc 660
acttctacctcaacgaccagtggcacgagatctgcaacgacatctccgaccacctgctcg 720
tcacccgcggcgcgcccgatgcccgcgtcgtgcagcccccgaccagccttatcgaaggag 780
cggcgaagagatggcagaacccacggtgaccgacgacctgacgggggccctcacgcagcc 840
CCCgCtgggCCgCc'~CCgtCCgcgcggtggccgaccgtgaactcggcacccacctcctgga 900
lOgacccgcggcatccactggatccacgccgcgaacggcgacccgtacgccaccgtgctgcg 960
cggccaggcggacgacccgtatcccgcgtacgagcgggtgcgtgcccgcggcgcgctctc 1020
CttCagCCCgacgggcagctgggtcaccgccgatcacgccctggcggcgagcatcctctg 1080
ctcgacggacttcggggtctccggcgccgacggcgtcccggtgccgcagcaggtcctctc 1140
gtacggggagggctgtccgctggagcgcgagcaggtgctgccggcggccggtgacgtgcc 1200
l5ggagggcgggcagcgtgccgtggtcgaggggatccaccgggagacgctggagggtctcgc 1260
gccggacccgtcggcgtcgtacgccttcgagctgctgggcggtttcgtccgcccggcggt 1320
gacggccgctgccgccgccgtgctgggtgttcccgcggaccggcgcgcggacttcgcgga 1380
tctgctggagcggctccggccgctgtccgacagcctgctggccccgcagtCCCtgCggaC 1440
ggtacgggcggcggacggcgcgctggccgagctcacggcgctgctcgccgattcggacga 1500
20ctcccccggggccctgctgtcggcgctcggggtcaccgcagccgtccagctcaccgggaa 1560
cgcggtgctcgcgctcctcgcgcatcccgagcagtggcgggagctgtgcgaccggcccgg 1620
gctcgcggcggccgcggtggaggagaccctccgctacgacccgccggtgcagctcgacgc 1680
ccgggtggtccgcggggagacggagctggcgggccggcggctgccggccggggcgcatgt 1740
cgtcgtcctgaccgccgcgaccggccgggacccggaggtcttcacggacccggagcgctt 1800
25cgacctcgcgcgccccgacgccgccgcgcacctcgcgctgcaccccgccggtccgtacgg 1860
cccggtggcgtccctggtccggcttcaggcggaggtcgcgctgcggaccctggccgggcg 1920
tttccccgggctgcggcaggcgggggacgtgctccgcccccgccgcgcgcctgtcggccg 1980
cgggccgctgagcgtcccggtcagcagctcctgagacaccggggccccggtccgcccggc 2040
cccccttcggacggaccggacggctcggaccacggggacggctcagaccgtcccgtgtgt 2100
30CCCCgtCCggCtCCCgtCCgCCCCatCCCgCCCCtCCdCCggcaaggaaggacacgacgc 2160
catgcgcgtcctgctgacctcgttcgcacatcacacgcactactacggcctggtgcccct 2220
ggcctgggc'gctgctcgccgccgggcacgaggtgcgggtcgccagccagcccgcgctcac 2280
ggacaccatcaccgggtccgggctcgccgcggtgccggtcggcaccgaccacctcatcca 2340
cgagtaccgggtgcggatggcgggcgagccgcgcccgaaccatccggcgatcgccttcga 2400
35cgaggcccgtcccgagccgctggactgggaccacgccctcggcatcgaggcgatcctcgc 2460
cccgtacttccatctgctcgccaacaacgactcgatggtcgacgacctcgtcgacttcgc 2520
ccggtcctggcagccggacctggtgctgtgggagccgacgacctacgcgggcgccgtcgc 2580
cgcccaggtcaccggtgccgcgcacgcccgggtcctgtgggggcccgacgtgatgggcag 2640
CgCCCgCCgCaagttCgtCgcgctgcgggaccggcagccgcccgagcaccgcgaggaccc 2700
40caccgcggagtggctgacgtggacgctcgaccggtacggcgcctccttcgaagaggagct 2760
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
3
gctcaccggccagttcacgatcgacccgaccccgccgagcctgcgcctcgacacgggcct2820
gccgaccgtcgggatgcgttatgttccgtacaacggcacgtcggtcgtgccggactggct2880
gagtgagccgcccgcgcggccccgggtctgCCtgaCCCtCggcgtctccgcgcgtgaggt2940
cctcggcggcgacggcgtctcgcagggcgacatcctggaggcgctcgccgacctcgacat3000
5cgagctcgtcgccacgctcgacgcgagtcagcgcgccgagatccgcaactacccgaagca3060
cacccggttcacggacttcgtgccgatgcacgcgctcctgccgagctgctcggcgatcat3120
ccaccacggcggggcgggcacctacgcgaccgccgtgatcaacgcggtgccgcaggtcat3180
gctcgccgagctgtgggacgcgccggtcaaggcgcgggccgtcgccgagcagggggcggg3240
gttcttcctgccgccggccgagCtC3CgCCgcaggccgtgcgggacgccgtcgtccgcat3300
lOcctcgacgacccctcggtcgCCdCCgCCgCgcaccggctgcgcgaggagaccttcggcga3360
ccccaccccggccgggatcgtccccgagctggagcggctcgccgcgcagcaccgccgccc3420
gccggccgacgcccggcactgagccgcacccctcgccccaggCCtCaCCCCtgtatCtgC3480
gccgggggacgcccccggcccaccctccgaaagaccgaaagcaggagcaccgtgtacgaa3540
gtcgaccacgccgacgtctacgacctcttctacctgggtcgcggcaaggactacgccgcc3600
l5gaggcctccgacatcgccgacctggtgcgctcccgtacccccgaggcctcctcgctcctg3660
gacgtggcctgcggtacgggcacgcatctggagcacttcaccaaggagttcggcgacacc3720
gccggcctggagctgtccgaggacatgctcacccacgcccgcaagcggctgcccgacgcc3780
acgctccaccagggcgacatgcgggacttccggctcggccggaagttctccgccgtggtc3840
agcatgttcagctccgtcggctacctgaagacgaccgaggaactcggcgcggccgtcgcc3900
20tcgttcgcggagcacctggagcccggtggcgtcgtcgtcgtcgagccgtggtggttcccg3960
gagaccttcgccgacggctgggtcagcgccgacgtcgtccgccgtgacgggcgcaccgtg4020
gcccgtgtctcgcactcggtgcgggaggggaacgcgacgcgcatggaggtccacttcacc4080
gtggccgacccgggcaagggcgtgcggcacttCtCCgaCgtCCatCtCatcaccctgttc47.40
caccaggccgagtacgaggccgcgttcacggccgccgggctgcgcgtcgagtacctggag4200
25ggcggcccgtcgggccgtggcctcttcgtcggcgtccccgcctgagcaccgcccaagacc4260
ccccggggcgggacgtcccgggtgcaccaagcaaagagagagaaacgaaccgtgacaggt4320
aagacccgaataccgcgtgtccgccgcggccgcaccacgcccagggccttcaccctggcc4380
gtcgtcggcaccctgctggcgggcaccaccgtggcggccgccgctcccggcgccgccgac4440
acggccaatgttcagtacacgagccgggcggcggagctcgtcgcccagatgacgctcgac4500
30gagaagatcagcttcgtccactgggcgctggaccccgaccggcagaacgtcggctacctt4560
cccggcgtgccgcgtctgggcatcccggagctgcgtgccgCCgaCggCCCgaacggcatc4620
cgcctggtggggcagaccgccaccgcgctgCCCgCgCCggtCgCCCtggCCagCdCCttC4680
gacgacaccatggccgacagctacggcaaggtcatgggccgcgacggtcgcgcgctcaac4740
caggacatggtcctgggcccgatgatgaacaacatccgggtgccgcacggcggccggaac4800
35tacgagaccttcagcgaggaccccctggtctcctcgcgcaccgcggtcgcccagatcaag4860
ggcatccagggtgcgggtctgatgaccacggccaagcacttcgcggccaacaaccaggag4920
aacaaccgcttctccgtgaacgccaatgtcgacgagcagacgctccgcgagatcgagttc4980
ccggcgttcgaggcgtcctccaaggccggcgcggcctccttcatgtgtgcctacaacggc5040
ctcaacgggaagccgtcctgcggcaacgacgagctcctcaacaacgtgctgcgcacgcag5100
40tggggcttccagggctgggtgatgtccgactggctcgccaccccgggcaccgacgccatc5160
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
4
accaagggcctcgaccaggagatgggcgtcgagCtCCCCggCgaCgtCCCgaagggcgag5220
ccctcgccgccggccaagttcttcggcgaggcgctgaagacggccgtcctgaacggcacg5280
gtccccgaggcggccgtgacgcggtcggcggagcggatcgtcggccagatggagaagttc5340
ggtctgctcctCgCCaCtCCggcgccgcggcccgagcgcgacaaggcgggtgcccaggcg5400
5gtgtcccgcaaggtcgccgagaacggcgcggtgctcctgcgcaacgagggccaggccctg5460
ccgctcgccggtgacgccggcaagagcatcgcggtcatcggcccgacggccgtcgacccc5520
aaggtcaccggcctgggcagCgCCCaCgtCgtcccggactcggcggcggcgccactcgac5580
accatcaaggcccgcgcgggtgcgggtgcgacggtgacgtacgagacgggtgaggagacc5640
ttcgggacgcagatcccggcggggaacctcagcccggcgttcaaccagggccaccagctc5700
lOgagccgggcaaggcgggggcgctgtacgacggcacgctgaccgtgcccgccgacggcgag5760
taccgcatcgcggtccgtgccaccggtggttacgccacggtgcagctcggcagccacacc5820
atcgaggccggtcaggtctacggcaaggtgagcagcccgctcctcaagctgaccaagggc5880
acgcacaagctcacgatctcgggcttcgcgatgagtgccaccccgctctccctggagctg5940
ggctgggtgacgccggcggcggccgacgcgacgatcgcgaaggccgtggagtcggcgcgg6000
l5aaggcccgtacggcggtcgtcttcgcctacgacgacggcaccgagggcgtcgaccgtccg6060
aacctgtcgctgccgggtacgcaggacaagctgatctcggctgtcgcggacgccaacccg6120
aacacgatcgtggtcctcaacaccggttcgtcggtgctgatgccgtggctgtccaagacc6180
cgcgcggtcctggacatgtggtacccgggccaggcgggcgccgaggccaccgccgcgctg6240
ctctacggtgacgtcaacccgagcggcaagctcacgcagagcttcccggccgccgagaac6300
20cagcacgcggtcgccggcgacccgacaagctacccgggcgtcgacaaccagcagacgtac6360
cgcgagggcatccacgtcgggtaccgctggttcgacaaggagaacgtcaagccgctgttc6420
ccgttcgggcacggcctgtcgtacacctcgttcacgcagagcgccccgaccgtcgtgcgt6480
acgtccacgggtggtctgaaggtcacggtcacggtccgcaacagcgggaagcgcgccggc6540
caggaggtcgtccaggcgtacctcggtgccagcccgaacgtgacggctccgcaggcgaag6600
25aagaagctcgtgggctacacgaaggtctcgctcgccgcgggcgaggcgaagacggtgacg6660
gtgaacgtcgaccgccgtcagctgcagaccggttcgtcctccgccgacctgcggggcagc6720
gccacggtcaacgtctggtgacgtgacgccgtgaaagcggcggtgcccgccacccgggag6780
ggtggcgggcaccgctttttcggcctgctgggtctaccggaccacctgactaggcctggt6840
CgaCCCgCtCggcccattcgcgcacggcgtcgatcacccgcagcgcctgcgggcgctcca6900
30ggtgcgggccgatcggcaggctgaggacctgccgcgcgaagctctcggcccgcgggagcg6960
agccttccggcggtgcctcgcccgcgtaggcgggcgagaggtgcacgggtaccgggtagt7020
gcgtgagggtgtcgatgccgcgggcgtcgaggtggctgcgcagctcgtcgcggcgctcgg7080
tgcgcacggtgaagaggtgccagaccgggtcggtgtcgggcgcggtcaccggcaggccga7140
tgccgggcagtccggcgagcccggagaggtactccgcggccagcgccgacctgcggccgt7200
35tccagctgtccaggtgggcgagccggatccgcagcacggcggcctgcatctcgtccaggc7260
gggagttggtgcccttcgtctcgtggctgtacttctgccgcgagccgtagttgcggagca7320
tccggagccgttcggcgagctcggggtcgccggtgacgacggcgccgccgtcgccgaagc7380
agccgaggttcttgcccgggtagaagctgaacgcggccaccgacgacccggcgccgatcc7440
gccggccccggtagcgggcgccgtgggcctgcgcggcgtcctcgacgatgtgcaggccgt7500
40gccggtccgcgagctcgcggagggcgtccatgtcggcggggtgcccgtagaggtggacgg7560
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
ggaggagcgcccgggtgcggggggtgatcgccttctcgacgagcagcgggtccagggtgg7620
ggtggtcctcgtgcggctcgacgggcacgggggtcgcgccggtggcggacaccgcgagcc7680
agctggcgatgtacgtgtgcgaggggacgatCaCCtCgtCCCCgggtCCgatgccgaggc7740
cgcggagggcgagctggagggcgtccatcccgctgttcacgccgacggcgtggtccgtct7800
5cgcagtacgcggcgaactccgcctcgaatccttcgagttcgggtccgaggaggtagcgcc7860
ccgagtcgaggacgcgggcgatcgcggcgtcggtctccgcgcggagctcctcgtaggcgg7920
ccttgaggtcgaggaaggggacgcggggggtctcggcgcggctgctcacgcggacacctc7980
cacggcggtggcgggcagctgcggggcggtcgccttgagcggctcccaccagccgcggtt8040
ctcccggtaccagcggacggtccgcgcgaggccgtccgcgaaggagacctgcgggcggta8100
lOgccgagctcgcgctcgatctcgccgccgtcgagggagtagcgcaggtcgtggcccttgcg8160
gtcggcgaccttccggaccgaggaccagtcggcgccgagcgagtccaggaggatgccggt8220
gagttcgcggttggtcagctccaggccgccgccgatgtggtagatctcgccggcccggcc8280
gcccgcgaggacgagcgcgatgccccggcagtggtcgtcggtgtgcacccactcgcggac8340
gttcgcgccgtcgccgtacagcgggagcgtcccgccgtcgaggaggttcgtcacgaagag8400
l5ggggatgagcttctcggggtgctggtacggcccgtagttgttgcagcagcgggtgatccg8460
tacgtcgaggccgtacgtccggtggtaggcgcgggcaacgaggtcggagccggccttgga8520
cgccgcgtagggcgagttgggctccagcgggctgctctcggtccaggagccggagtcgat8580
cgacccgtacacctcgtcggtggagacgtgcacgacccggccgacgccggcgtcgacggc8640
gcactggagcagcgtctgcgtgccctgcacgttggtctcggtgaacacggacgcgcccgc8700
20gatggagcggtccacgtggctctcggccgcgaagtggacgatggcgtccacgccgcgcag8760
ttcccgggcgaggaggccggcgtcgcggatgtcgccgtggacgaagcgcagtcgcgggtc8820
cgcgtccaccggggcgaggttggcgcggttgcccgcgtaggtgaggctgtccaggacgat8880
CdCCtCatCggCgggcaCgtcggggtacgccccggcgaggagctgccgcacgaagtgcga8940
gccgatgaagCCCgCa.CCtCCggtCdCCagaagccgcactgccgtcttcctttcggtcgc9000
25gctgtaggtcgcggtgtgggtcgcactgtcggtggcggtgcgggtcgcggtgtgggtcgc9060
actgtcggtggcgctgtcggtcgtgggaacgcgtcggccgcgaggtgccctcacggggct9120
ccctcgcggccggcgatctccatcagatagctgccgtactcggtgcgggagaggccttct9180
cccaggccgtgacaggcctcggcgtcgatgaagcccatgcggaaggcgatctcctcaagg9240
cccgcgatccagacgccctgCCgCtCCtCCaggaCCtggaCgtaCtgggCggcccgcagg9300
30agcgagtcgtgggtgccggtgtccagccaggcgaagccgcggcccaggttgacgagttcg9360
gCCCggCCCCgctccaggtagacgcggttgacgtcggtgatctccagctcgccgcgcggc9420
gagggccggatgttcttggcgatgtcgacgacgtcgttgtcgtagaggtagaggccggtg9480
acggcgaggttggagcgcggcttgacgggcttctcgacgaggtcggtcagCCggCCCgtC9540
gcgtccacctcggcgacgccgtaccgctcggggtccttgaccgggtagccgaagagcacg9600
35cagccgtcgaggcgcgcgatgctgtcccgcaggagcgtgtagaggccgggcccgtggaag9660
atgttgtcgcccaggatcagggcgcaggtgtcgtcgccgatgtgctcggctccgacgaga9720
agtgcgtccgcgattcctgcgggctctttctggaccgcatagtcgagttctattcccagg9780
tgcctgccgtttccgagaagcgactggaagagttcgatgtgctggggggtcgagatgatt9840
tgaatctcgcgaataccgccgagcatgagaaccgacagcggatagtagatcatcggtttg9900
40ttgtagaccggaagaatctgcttcgaaatgaccgaggtcgccggatgcagccgagttccg9960
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
6
ctcccgccggccaggactattcccttcattctcggaaactagcagcagggcgccggtgat10020
aacggtcggcgtggcgagttaggggggcgctaggggctgcgcagggggagtgtcaccacc10080
cctttggggggtgggaaaacaccgagggcccggccggacggccgggccctcaggtggggg10140
gatcgtgggggggggatcggggggatcggggcgggtgcgggtcagcgcaggaagccgcgg10200
SgCCrCCtCCCagCCgtCCgCggcgtcgcgctccagctggttcaggcgggcggtgacgacc10260
tgatcgaagccgtccatgaagtactcgtcgccgtcgacggCCgCCa.CCtCgCCgCCgCgC10320
tcgacgaagtccctgacgacctcggtgagggaggtgtcgggggtcacgcggcccgcgatg10380
tagcgggtcgcgccgtccaggtcggggaagccggcctcgcggtacaggtacacgtcgccg10440
aggagatcgacctgcaccgcgacctgcgggtgcgcggtgggccgcatggtggcgggcttg10500
l0atccgcagcagttcggcgtcggccccggtgcgcaggctgttcagggcgtagccgtagtcg10560
atgtggagtccgggggtgcgctcgcggacccgctcctcgaaggcgttgagggcctcctgg10620
agctcggcccgctcctcctgcggcagcttgccgtcgtcacggccgctgtagtcctcgcga10680
atgttgacgaagtcgatcgtcctgccctgcccggcgtcgttgaggtcggcgatgaagtcg10740
accaggtcgagcaggcgggaggcacggcccgggagcacgatgtaggcgaagccgaggttg10800
l5atcggcgactcgcgctcggcgcgcagctgctggaagcggcgcaggttctcgcggacgcgg10860
cggaaggcggccttcttgccggtggtctgctcgtactcctcgtcgttgaggccgtagagc10920
gaggtgcggatggcgtgcaggccccagaggccgggctggcgctccagggtgcgctcggtg10980
agcgcgaaggagttcgtgtagacggtgggccgcaggccgtggtcggtggcgtgcgcggcc11040
aggctcccgaggccggggttggtgagcggctccaggccgccggagaagtacatcgccgag11100
20gggttgcccgcgggtatctcgtcgatgaccgaccggaacatggcgttgccggcgtcgagg11160
gcggacgggtcgtagcgggcgccggtcacacggacgcagaagtggcagcggaacatgcag11220
gtcgggccggggtagaggccgacgctgtacgggaagacgggcttcctggcgagcgccgcg11280
tcgaagacgccgcgctgttcgagcgggagcagggtgttcttccagtacgccccggcgggg11340
ccggtctcgaccgcggtgcggagctccgggacctgcccgaacagggcgaggaggcgccgg11400
25aaggcgtcccggtcgacgcccaggtcgtggcgggcctcctccagcggggtgaaggggctg11460
ttgccgtagcgcacggcgagccggacgaggtggcgggcggtcgttccggcctcgtcgggc11520
ggcacgaggccgccggcggcgagggtctggccgacggcgtggaccgccgcccccagatcg11580
gctccggggtgcgcgcagcgttcggccggggcggtggcggaaagggcgggggcggtcatc11640
gggagcgtccaatcgtgggcgtggatgtctggggggccgcgagcggggcgggggccgtgt11700
30cgcggtggcgcgcggtcagttcgcggccgcgggtcgcgcagagacgcagcaggtcggcga11760
cccggcggatgtcgtcgtcgccgatggcggtgccggtcggcagggacagcacgcgcgcgg11820
cgaggcgttcggtgtgcggcagcggggcgtgcggctgcccgcggtacggctccagctcgt11880
ggcagcccggcgagaagtaggcgcgggtgtgcacgccttcggccttcaggacctccatga11940
cgaggtcgcggtggatgccggtggtggcctcgtcgatctcgacgatcacgtactggtggt12000
35tgttgaggccgtggcggtcgtggtcggcgacgaggacgccggggaggtccgcgaggtgct12060
cgcggtaggcggcgtggttgcgccggttccggtcgatgacctcgggaaacgcgtcgaggg12120
aggtgaggcccatggcggcggcggcctcgctcatcttggcgttggtcccgccggcggggc12180
tgccgccgggcaggtcgaagccgaagttgtggagggcgcggatccgggcggcgaggtcgg12240
cgtcgtcggtgacgacggcgccgccctcgaaggcgttgacggccttggtggcgtggaagc12300
40tgaagacctcggcgtcgccgaggctgccggcgggccggccgtcgaccgcgcagccgaggg12360
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
7
cgtgcgcggcgtcgaagtacagccgcaggccgtgctcgtcggcgaccttccgcagctggt12420
cggcggcgcaggggcggccccagaggtggacgccgacgacggccgaggtgcggggtgtga12480
ccgcggcggccacctggtccgggtcgaggttgccggtgtccgggtcgatgtcggcgaaga12540
ccggggtgaggccgatccagcgcagtgcgtgcggggtggcggcgaacgtcatcgacggca12600
5tgatcacttcgccggtgaggccggcggcgtgcgcgaggagctggagcccggccgtggcgt12660
tgcaggtggccacggcatgccggaccccggcgagcccggcgacgcgctcctcgaactcgc12720
ggacgagcgggccgccgttggacagccactggctgtcgagggcccggtcgagccgctcgt12780
acagcctggcgcggtcgatgcggttgggccgccccacgaggagcggctggtcgaaagcgg12840
cggggccgccgaagaatgcgaggtcggataaggcgcttttcacggatgttccctccgggc12900
lOCaCCgtCaCgaaatgattcgccgatccgggaatcccgaacgaggtcgccgogctccaccg12960
tgacgtacgacgagatggtcgattgtggtggtcgatttcggggggactctaatccgcgcg13020
gaacgggaccgacaagagcacgctatgcgctctcgatgtgcttcggatcacatccgcctc13080
cggggtattccatcggcggcccgaatgtgatgatccttgacaggatccgggaatcagccg13140
agccgccgggagggccggggCgCgCtCCgCggaagagtacgtgtgagaagtcccgttcct13200
l5cttcccgtttccgttccgcttccggcccggtctggagttctccgtgcgccgtacccagca13260
gggaacgaccgcttctcccccggtactcgacctcggggccctggggcaggatttcgcggc13320
cgatccgtatccgacgtacgcgagactgcgtgccgagggtccggcccaccgggtgcgcac13380
ccccgagggggacgaggtgtggctggtcgtcggctacgaccgggcgcgggCggtCCtCgC13440
cgatccccggttcagcaagactggcgcaactCCaCgaCtCCCCtgaCCgaagCCgaagCC13500
20gcgctcaaccacaacatgctgagttccgaacccgccgcggcacacccggctgcgccagct13560
ggtggcccgtgagttcaccatgcgccggtgcgagttgctgccgccccgggtcc 13613
<210> 4
<211> 3782
25<212> PRT
<213> Streptomyces venezuelae
<400> 4
Met Thr Asp Asp Leu Thr Gly Ala Leu Thr Gln Pro Pro Leu Gly Arg
30 1 5 10 15
Thr Val Arg Ala Val Ala Asp Arg Glu Leu Gly Thr His Leu Leu Glu
20 25 30
Thr Arg Gly Ile His Trp Ile His Ala Ala Asn Gly Asp Pro Tyr Ala
35 40 45
35Thr Val Leu Arg Gly Gln Ala Asp Asp Pro Tyr Pro Ala Tyr Glu Arg
50 55 60
Val Arg Ala Arg Gly Ala Leu Ser Phe Ser Pro Thr Gly Ser Trp Val
65 70 75 80
Thr Ala Asp His Ala Leu Ala Ala Ser Ile Leu Cys Ser Thr Asp Phe
40 85 90 95
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
8
Gly Val Ser Gly Ala Asp Gly Val Pro Val Pro Gln Gln Val Leu Ser
100 105 110
Tyr Gly Glu Gly Cys Pro Leu Glu Arg Glu Gln Val Leu Pro Ala Ala
115 120 125
5Gly Asp Val Pro Glu Gly Gly Gln Arg Ala Val Val Glu Gly Ile His
130 135 140
Arg Glu Thr Leu Glu Gly Leu Ala Pro Asp Pro Ser Ala Ser Tyr Ala
145 150 155 160
Phe Glu Leu Leu Gly Gly Phe Val Arg Pro Ala Val Thr Ala Ala Ala
165 170 175
Ala Ala Val Leu Gly Val Pro Ala Asp Arg Arg Ala Asp Phe Ala Asp
180 185 190
Leu Leu Glu Arg Leu Arg Pro Leu Ser Asp Ser Leu Leu Ala Pro Gln
195 200 205
l5Ser Leu Arg Thr Val Arg Ala Ala Asp Gly Ala Leu Ala Glu Leu Thr
210 215 220
Ala Leu Leu Ala Asp Ser Asp Asp Ser Pro Gly Ala Leu Leu Ser Ala
225 230 235 240
Leu Gly Val Thr A1a Ala Val Gln Leu Thr Gly Asn Ala Val Leu Ala
245 250 255
Leu Leu Ala His Pro Glu Gln Trp Arg Glu Leu Cys Asp Arg Pro Gly
260 265 270
Leu Ala Ala Ala Ala Val Glu Glu Thr Leu Arg Tyr Asp Pro Pro Val
275 280 285
25G1n Leu Asp Ala Arg Val Val Arg Gly Glu Thr Glu Leu Ala Gly Arg
290 295 300
Arg Leu Pro Ala Gly Ala His Val Val Val Leu Thr Ala Ala Thr Gly
305 310 315 320
Arg Asp Pro Glu Val Phe Thr Asp Pro Glu Arg Phe Asp Leu Ala Arg
325 330 335
Pro Asp Ala Ala Ala His Leu Ala Leu His Pro Ala Gly Pro Tyr Gly
340 345 350
Pro Val Ala Ser Leu Val Arg Leu Gln Ala Glu Val Ala Leu Arg Thr
355 360 365
35Leu Ala Gly Arg Phe Pro Gly Leu Arg Gln Ala Gly Asp Val Leu Arg
370 375 380
Pro Arg Arg Ala Pro Val Gly Arg Gly Pro Leu Ser Val Pro Val Ser
385 390 395 400
Ser Ser Met Arg Val Leu Leu Thr Ser Phe Ala His His Thr His Tyr
405 410 415
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
9
Tyr Gly Leu Val Pro Leu Ala Trp Ala Leu Leu Ala Ala Gly His Glu
420 425 ~ 430
Val Arg Val Ala Ser Gln Pro Ala Leu Thr Asp Thr Ile Thr Gly Ser
435 440 445
5Gly Leu Ala Ala Val Pro Val Gly Thr Asp His Leu Ile His Glu Tyr
450 455 460
Arg Val Arg Met Ala Gly Glu Pro Arg Pro Asn His Pro Ala Ile Ala
465 470 475 480
Phe Asp Glu Ala Arg Pro Glu Pro Leu Asp Trp Asp His Ala Leu Gly
485 490 495
Ile Glu Ala Ile Leu Ala Pro Tyr Phe His Leu Leu Ala Asn Asn Asp
500 505 510
Ser Met Val Asp Asp Leu Val Asp Phe Ala Arg Ser Trp Gln Pro Asp
515 520 525
l5Leu Val Leu Trp Glu Pro Thr Thr Tyr Ala Gly Ala Val Ala Ala Gln
530 535 540
Val Thr Gly Ala Ala His Ala Arg Val Leu Trp Gly Pro Asp Val Met
545 550 555 560
Gly Ser Ala Arg Arg Lys Phe Val Ala Leu Arg Asp Arg Gln Pro Pro
565 570 575
Glu His Arg Glu Asp Pro Thr Ala Glu Trp Leu Thr Trp Thr Leu Asp
580 585 590
Arg Tyr Gly Ala Ser Phe Glu Glu Glu Leu Leu Thr Gly Gln Phe Thr
595 600 605
25I1e Asp Pro Thr Pro Pro Ser Leu Arg Leu Asp Thr Gly Leu Pro Thr
610 615 620
Val Gly Met Arg Tyr Val Pro Tyr Asn Gly Thr Ser Val Val Pro Asp
625 630 ' 635 640
Trp Leu Ser Glu Pro Pro Ala Arg Pro Arg Val Cys Leu Thr Leu Gly
645 650 655
Val Ser Ala Arg Glu Val Leu Gly Gly Asp Gly Val Ser Gln Gly Asp
660 665 670
Ile Leu Glu Ala Leu Ala Asp Leu Asp Ile Glu Leu Val Ala Thr Leu
675 680 685
35Asp Ala Ser Gln Arg Ala Glu Ile Arg Asn Tyr Pro Lys His Thr Arg
690 695 700
Phe Thr Asp Phe Val Pro Met His Ala Leu Leu Pro Ser Cys Ser Ala
705 710 715 720
Ile Ile His His Gly Gly Ala Gly Thr Tyr Ala Thr Ala Val Ile Asn
725 730 735
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Ala Val Pro Gln Val Met Leu Ala Glu Leu Trp Asp Ala Pro Val Lys
740 745 750
Ala Arg Ala Val Ala Glu Gln Gly Ala Gly Phe Phe Leu Pro Pro Ala
755 760 765
5Glu Leu Thr Pro Gln Ala Val Arg Asp Ala Val Val Arg Ile Leu Asp
770 775 780
Asp Pro Ser Val Ala Thr Ala Ala His Arg Leu Arg Glu Glu Thr Phe
785 790 795 800
Gly Asp Pro Thr Pro Ala Gly Ile Val Pro Glu Leu Glu Arg Leu Ala
10 805 810 815
Ala Gln His Arg Arg Pro Pro Ala Asp Ala Arg His Met Tyr Glu Val
820 825 830
Asp His Ala Asp Val Tyr Asp Leu Phe Tyr Leu Gly Arg Gly Lys Asp
835 840 845
l5Tyr Ala Ala Glu Ala Ser Asp Ile Ala Asp Leu Val Arg Ser Arg Thr
850 855 860
Pro Glu Ala Ser Ser Leu Leu Asp Val Ala Cys Gly Thr Gly Thr His
865 870 875 880
Leu Glu His Phe Thr Lys Glu Phe Gly Asp Thr Ala Gly Leu Glu Leu
885 890 895
Ser Glu Asp Met Leu Thr His Ala Arg Lys Arg Leu Pro Asp Ala Thr
900 905 910
Leu His Gln Gly Asp Met Arg Asp Phe Arg Leu Gly Arg Lys Phe Ser
915 920 925
25A1a Val Val Ser Met Phe Ser Ser Val Gly Tyr Leu Lys Thr Thr Glu
930 935 940
Glu Leu Gly Ala Ala Val Ala Ser Phe Ala Glu His Leu Glu Pro Gly
945 950 955 960
Gly Val Val Val Val Glu Pro Trp Trp Phe Pro Glu Thr Phe Ala Asp
965 970 975
Gly Trp Val Ser Ala Asp Val Val Arg Arg Asp Gly Arg Thr Val Ala
980 985 990
Arg Val Ser His Ser Val Arg Glu Gly Asn Ala Thr Arg Met Glu Val
995 1000 1005
35His Phe Thr Val Ala Asp Pro Gly Lys Gly Val Arg His Phe Ser Asp
1010 1015 . 1020
Val His Leu Ile Thr Leu Phe His Gln Ala Glu Tyr Glu Ala Ala Phe
1025 1030 1035 1040
Thr Ala Ala Gly Leu Arg Val Glu Tyr Leu Glu Gly Gly Pro Ser Gly
1045 1050 1055
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
11
Arg Gly Leu Phe Val Gly Val Pro Ala Met Thr Gly Lys Thr Arg Ile
1060 1065 1070
Pro Arg Val Arg Arg Gly Arg Thr Thr Pro Arg Ala Phe Thr Leu Ala
1075 1080 1085
5Va1 Val Gly Thr Leu Leu Ala Gly Thr Thr Val Ala Ala Ala Ala Pro
1090 7.095 1100
Gly Ala Ala Asp Thr Ala Asn Val Gln Tyr Thr Ser Arg Ala Ala Glu
1105 1110 1115 1120
Leu Val Ala Gln Met Thr Leu Asp Glu Lys Ile Ser Phe Val His Trp
1125 1130 1135
Ala Leu Asp Pro Asp Arg Gln Asn Val Gly Tyr Leu Pro Gly Val Pro
1140 1145 1150
Arg Leu Gly Ile Pro Glu Leu Arg Ala Ala Asp Gly Pro Asn Gly Ile
1155 1160 1165
l5Arg Leu Val Gly Gln Thr Ala Thr Ala Leu Pro Ala Pro Val Ala Leu
1170 1175 1180
Ala Ser Thr Phe Asp Asp Thr Met Ala Asp Ser Tyr Gly Lys Val Met
1185 1190 1195 1200
Gly Arg Asp Gly Arg Ala Leu Asn Gln Asp Met Val Leu Gly Pro Met ,
1205 1210 1215
Met Asn Asn Ile Arg Val Pro His Gly Gly Arg Asn Tyr Glu Thr Phe
1220 1225 1230
Ser Glu Asp Pro Leu Val Ser Ser Arg Thr Ala Val Ala Gln Ile Lys
1235 1240 1245
25G1y Ile Gln Gly Ala Gly Leu Met Thr Thr Ala Lys His Phe Ala Ala
1250 1255 1260
Asn Asn Gln Glu Asn Asn Arg Phe Ser Val Asn Ala Asn Val Asp Glu
1265 1270 1275 1280
Gln Thr Leu Arg Glu Ile Glu Phe Pro Ala Phe Glu Ala Ser Ser Lys
1285 1290 1295
Ala Gly Ala Ala Ser Phe Met Cys Ala Tyr Asn Gly Leu Asn Gly Lys
1300 1305 1310
Pro Ser Cys Gly Asn Asp Glu Leu Leu Asn Asn Val Leu Arg Thr Gln
1315 1320 1325
35Trp Gly Phe Gln Gly Trp Val Met Ser Asp Trp Leu Ala Thr Pro Gly
1330 1335 1340
Thr Asp Ala Ile Thr Lys Gly Leu Asp Gln Glu Met Gly Val Glu Leu
1345 1350 1355 1360
Pro Gly Asp Val Pro Lys Gly Glu Pro Ser Pro Pro Ala Lys Phe Phe
1365 1370 1375
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
12
Gly Glu Ala Leu Lys Thr Ala Val Leu Asn Gly Thr Val Pro Glu Ala
1380 1385 1390
Ala Val Thr Arg Ser Ala Glu Arg Ile Val Gly Gln Met Glu Lys Phe
1395 1400 1405
5Gly Leu Leu Leu Ala Thr Pro Ala Pro Arg Pro Glu Arg Asp Lys Ala
1410 1415 1420
Gly Ala Gln Ala Val Ser Arg Lys Val Ala Glu Asn Gly Ala Val Leu
1425 1430 1435 1440
Leu Arg Asn Glu Gly Gln Ala Leu Pro Leu Ala Gly Asp Ala Gly Lys
1445 1450 1455
Ser Ile Ala Val Ile Gly Pro Thr Ala Val Asp Pro Lys Val Thr Gly
1460 1465 1470
Leu Gly Ser Ala His Val Val Pro Asp Ser Ala Ala Ala Pro Leu Asp
1475 1480 1485
lSThr Tle Lys Ala Arg Ala Gly Ala Gly Ala Thr Val Thr Tyr Glu Thr
1490 1495 1500
Gly Glu Glu Thr Phe Gly Thr Gln Ile Pro Ala Gly Asn Leu Ser Pro
1505 1510 1515 1520
Ala Phe Asn Gln Gly His Gln Leu Glu Pro Gly Lys Ala Gly Ala Leu
1525 1530 1535
Tyr Asp Gly Thr Leu Thr Val Pro Ala Asp Gly Glu Tyr Arg Ile Ala
1540 1545 1550
Val Arg Ala Thr Gly Gly Tyr Ala Thr Val Gln Leu Gly Ser His Thr
1555 1560 1565
25I1e Glu A1a Gly Gln Val Tyr Gly Lys Val Ser Ser Pro Leu Leu Lys
1570 1575 1580
Leu Thr Lys Gly Thr His Lys Leu Thr Ile Ser Gly Phe Ala Met Ser
1585 1590 1595 1600
Ala Thr Pro Leu Ser Leu Glu Leu Gly Trp Val Thr Pro Ala Ala Ala
1605 1610 1615
Asp Ala Thr Ile Ala Lys Ala Val Glu Ser Ala Arg Lys Ala Arg Thr
1620 1625 1630
Ala Val Val Phe Ala Tyr Asp Asp Gly Thr Glu Gly Val Asp Arg Pro
1635 1640 1645
35Asn Leu Ser Leu Pro Gly Thr Gln Asp Lys Leu Ile Ser Ala Val Ala
1650 1655 1660
Asp Ala Asn Pro Asn Thr Ile Val Val Leu Asn Thr Gly Ser Ser Val
1665 1670 1675 1680
Leu Met Pro Trp Leu Ser Lys Thr Arg Ala Val Leu Asp Met Trp Tyr
1685 1690 1695
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
13
Pro Gly Gln Ala Gly Ala Glu Ala Thr A1a Ala Leu Leu Tyr Gly Asp
1700 1705 1710
Val Asn Pro Ser Gly Lys Leu Thr Gln Ser Phe Pro Ala Ala Glu Asn
1715 1720 1725
5Gln His Ala Val Ala Gly Asp Pro Thr Ser Tyr Pro Gly Val Asp Asn
1730 1735 1740
Gln Gln Thr Tyr Arg Glu Gly Ile His Val Gly Tyr Arg Trp Phe Asp
1745 1750 1755 1760
Lys Glu Asn Val Lys Pro Leu Phe Pro Phe Gly His Gly Leu Ser Tyr
1765 1770 1775
Thr Ser Phe Thr Gln Ser Ala Pro Thr Val Val Arg Thr Ser Thr Gly
1780 1785 1790
Gly Leu Lys Val Thr Val Thr Val Arg Asn Ser Gly Lys Arg Ala Gly
1795 1800 1805
l5Gln Glu Val Val Gln Ala Tyr Leu Gly Ala Ser Pro Asn Val Thr Ala
1810 1815 1820
Pro Gln Ala Lys Lys Lys Leu Val Gly Tyr Thr Lys Val Ser Leu Ala
1825 1830 1835 1840
Ala Gly Glu Ala Lys Thr Val Thr Val Asn Val Asp Arg Arg Gln Leu
1845 1850 1855
Gln Thr Gly Ser Ser Ser Ala Asp Leu Arg Gly Ser Ala Thr Val Asn
1860 1865 1870
Val Trp Met Ser Ser Arg Ala Glu Thr Pro Arg Val Pro Phe Leu Asp
1875 1880 1885
25Leu Lys Ala Ala Tyr Glu Glu Leu Arg Ala Glu Thr Asp Ala Ala Ile
1890 1895 1900
Ala Arg Val Leu Asp °Ser Gly Arg Tyr Leu Leu Gly Pro Glu Leu Glu
1905 1910 1915 1920
Gly Phe Glu Ala Glu Phe Ala Ala Tyr Cys Glu Thr Asp His Ala Val
1925 1930 1935
Gly Val Asn Ser Gly Met Asp Ala Leu Gln Leu Ala Leu Arg Gly Leu
1940 1945 1950
Gly Ile Gly Pro Gly Asp Glu Val Ile Val Pro Ser His Thr Tyr Ile
1955 1960 1965
35A1a Ser Trp Leu Ala Val Ser Ala Thr Gly Ala Thr Pro Val Pro Val
1970 1975 1980
Glu Pro His Glu Asp His Pro Thr Leu Asp Pro Leu Leu Val Glu Lys
1985 1990 1995 2000
Ala,Ile Thr Pro Arg Thr Arg A1a Leu Leu Pro Val His Leu Tyr Gly
2005 2010 2015
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
14
His Pro Ala Asp Met Asp Ala Leu Arg Glu Leu Ala Asp Arg His Gly
2020 2025 2030
Leu His Ile Val Glu Asp Ala Ala Gln Ala His Gly Ala Arg Tyr Arg
2035 2040 2045
5Gly Arg Arg Ile Gly Ala Gly Ser Ser Val Ala Ala Phe Ser Phe Tyr
2050 2055 2060
Pro Gly Lys Asn Leu Gly Cys Phe Gly Asp Gly Gly Ala Val Val Thr
2065 2070 2075 2080
Gly Asp Pro Glu Leu Ala Glu Arg Leu Arg Met Leu Arg Asn Tyr Gly
2085 . 2090 2095
Ser Arg Gln Lys Tyr Ser His Glu Thr Lys Gly Thr Asn Ser Arg Leu
2100 2105 2110
Asp Glu Met Gln Ala Ala Val Leu Arg Ile Arg Leu Ala His Leu Asp
2115 2120 2125
l5Ser Trp Asn Gly Arg Arg Ser Ala Leu Ala Ala Glu Tyr Leu Ser Gly
2130 2135 2140
Leu Ala Gly Leu Pro Gly Ile Gly Leu Pro Val Thr Ala Pro Asp Thr
2145 2150 2155 2160
Asp Pro Val Trp His Leu Phe Thr Val Arg Thr Glu Arg Arg Asp Glu
2165 2170 2175
Leu Arg Ser His Leu Asp Ala Arg G1y Ile Asp Thr Leu Thr His Tyr
2180 2185 2190
Pro Val Pro Val His Leu Ser Pro Ala Tyr Ala Gly Glu Ala Pro Pro
2195 2200 2205
25G1u Gly Ser Leu Pro Arg Ala Glu Ser Phe Ala Arg Gln Val Leu Ser
2210 2215 2220
Leu Pro Ile Gly Pro His Leu Glu Arg Pro Gln Ala Leu Arg Val Ile
2225 2230 2235 2240
Asp Ala Val Arg Glu Trp Ala Glu Arg Val Asp Gln Ala Met Arg Leu
2245 2250 2255
Leu Val Thr Gly Gly Ala Gly Phe I1e Gly Ser His Phe Val Arg Gln
2260 2265 2270
Leu Leu Ala Gly Ala Tyr Pro Asp Val Pro Ala Asp Glu Val Ile Val
2275 2280 2285
35Leu Asp Ser Leu Thr Tyr Ala Gly Asn Arg Ala Asn Leu Ala Pro Val
2290 2295 2300
Asp Ala Asp Pro Arg Leu Arg Phe Val His Gly Asp Ile Arg Asp Ala
2305 2310 2315 2320
Gly Leu Leu Ala Arg Glu Leu Arg Gly Val Asp Ala Ile Val His Phe
2325 2330 2335
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Ala Ala Glu Ser His Val Asp Arg Ser Ile Ala Gly Ala Ser Val Phe
2340 2345 2350
Thr Glu Thr Asn Val Gln Gly Thr Gln Thr Leu Leu Gln Cys Ala Val
2355 2360 2365
5Asp Ala Gly Val Gly Arg Val Val His Val Ser Thr Asp Glu Val Tyr
2370 2375 2380
Gly Ser Ile Asp Ser Gly Ser Trp Thr Glu Ser Ser Pro Leu Glu Pro
2385 2390 2395 2400
Asn Ser Pro Tyr Ala Ala Ser Lys Ala Gly Ser Asp Leu Val Ala Arg
10 2405 2410 2415
Ala Tyr His Arg Thr Tyr Gly Leu Asp Val Arg Ile Thr Arg Cys Cys
2420 2425 2430
Asn Asn Tyr Gly Pro Tyr Gln His Pro Glu Lys Leu Ile Pro Leu Phe
2435 2440 2445
15Va1 Thr Asn Leu Leu Asp Gly Gly Thr Leu Pro Leu Tyr Gly Asp Gly
2450 2455 2460
Ala Asn Val, Arg Glu Trp Val His Thr Asp Asp His Cys Arg Gly Ile
2465 2470 2475 2480
Ala Leu Val Leu Ala Gly Gly Arg Ala Gly Glu Ile Tyr His Ile Gly
2485 2490 2495
Gly Gly Leu Glu Leu Thr Asn Arg Glu Leu Thr Gly Ile Leu Leu Asp
2500 2505 2510
Ser Leu Gly Ala Asp Trp Ser Ser Val Arg Lys Val Ala Asp Arg Lys
2515 2520 2525
25G1y His Asp Leu Arg Tyr Ser Leu Asp Gly Gly Glu Ile Glu Arg Glu
2530 2535 2540
Leu Gly Tyr Arg Pro Gln Val Ser Phe Ala Asp Gly Leu Ala Arg Thr
2545 2550 2555 2560
Val Arg Trp Tyr Arg Glu Asn Arg Gly Trp Trp Glu Pro Leu Lys Ala
2565 2570 2575
Thr Ala Pro Gln Leu Pro A1a Thr Ala Val Glu Val Ser Ala Met Lys
2580 2585 2590
Gly Ile Val Leu Ala Gly Gly Ser Gly Thr Arg Leu His Pro Ala Thr
2595 2600 2605
35Ser Val Ile Ser Lys Gln I1e Leu Pro Val Tyr Asn Lys Pro Met Ile
2610 2615 2620
Tyr Tyr Pro Leu Ser Val Leu Met Leu Gly Gly Ile Arg Glu Ile Gln
2625 2630 2635 2640
Ile Ile Ser Thr Pro Gln His Ile Glu Leu Phe Gln Ser Leu Leu Gly
2645 2650 2655
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
16
Asn Gly Arg His Leu Gly Ile Glu Leu Asp Tyr Ala Val Gln Lys Glu
2660 2665 2670
Pro Ala Gly Ile Ala Asp Ala Leu Leu Val Gly Ala Glu His Ile Gly
2675 2680 2685
5Asp Asp Thr Cys Ala Leu Ile Leu Gly Asp Asn Ile Phe His Gly Pro
2690 2695 2700
Gly Leu Tyr Thr Leu Leu Arg Asp Ser Ile Ala Arg Leu Asp Gly Cys
2705 2710 2715 2720
Val Leu Phe Gly Tyr Pro Val Lys Asp Pro Glu Arg Tyr Gly Val Ala
2725 2730 2735
Glu Val Asp Ala Thr Gly Arg Leu Thr Asp Leu Val Glu Lys Pro Val IL
2740 2745 2750
Lys Pro Arg Ser Asn Leu Ala Val Thr Gly Leu Tyr Leu Tyr Asp Asn
2755 2760 2765
l5Asp Val Val Asp Ile Ala Lys Asn Ile Arg Pro Ser Pro Arg Gly Glu
2770 2775 2780
Leu Glu Ile Thr Asp Val Asn Arg Val Tyr Leu Glu Arg Gly Arg Ala
2785 2790 2795 2800
Glu Leu Val Asn Leu Gly Arg Gly Phe Ala Trp Leu Asp Thr Gly Thr
2805 2810 2815
His Asp Ser Leu Leu Arg Ala Ala Gln Tyr Val Gln Val Leu Glu Glu
2820 2825 2830
Arg Gln Gly Val Trp Ile Ala Gly Leu Glu Glu Ile Ala Phe Arg Met
2835 2840 2845
25G1y Phe Ile Asp Ala Glu Ala Cys His Gly Leu Gly Glu Gly Leu Ser
2850 2855 2860
Arg Thr Glu Tyr Gly Ser Tyr Leu Met Glu Ile Ala Gly Arg Glu Gly
2865 2870 2875 2880
Ala Pro Met Thr Ala Pro Ala Leu Ser Ala Thr Ala Pro Ala Glu Arg
2885 2890 2895
Cys Ala His Pro Gly Ala Asp Leu Gly Ala Ala Val His Ala Val Gly
2900 2905 2910
Gln Thr Leu Ala Ala Gly Gly Leu Val Pro Pro Asp Glu Ala Gly Thr
2915 2920 2925
35Thr Ala Arg His Leu Val Arg Leu Ala Val Arg Tyr Gly Asn Ser Pro
2930 2935 2940
Phe Thr Pro Leu Glu Glu Ala Arg His Asp Leu Gly Val Asp Arg Asp
2945 2950 2955 2960
Ala Phe Arg Arg Leu Leu Ala Leu Phe Gly Gln Val Pro Glu Leu Arg
2965 2970 2975
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
17
Thr Ala Val Glu Thr Gly Pro Ala Gly Ala Tyr Trp Lys Asn Thr Leu
2980 2985 2990
Leu Pro Leu Glu Gln Arg Gly Val Phe Asp Ala Ala Leu Ala Arg Lys
2995 3000 3005
5Pro Val Phe Pro Tyr Ser Val Gly Leu Tyr Pro Gly Pro Thr Cys Met
3010 ' 3015 3020
Phe Arg Cys His Phe Cys Val Arg Val Thr Gly Ala Arg Tyr Asp Pro
3025 3030 3035 3040
Ser Ala Leu Asp Ala Gly Asn Ala Met Phe Arg Ser Val Ile Asp Glu
3045 3050 3055
Ile Pro Ala Gly Asn Pro Ser Ala Met Tyr Phe Ser Gly Gly Leu Glu
3060 3065 3070
Pro Leu Thr Asn Pro Gly Leu Gly Ser Leu Ala Ala His Ala Thr Asp
3075 3080 3085
l5His Gly Leu Arg Pro Thr Val Tyr Thr Asn Ser Phe Ala Leu Thr Glu
3090 3095 3100
Arg Thr Leu Glu Arg Gln Pro Gly Leu Trp Gly Leu His Ala Ile Arg
3105 3110 3115 3120
Thr Ser Leu Tyr Gly Leu Asn Asp Glu Glu Tyr Glu Gln Thr Thr Gly
3125 3130 3135
Lys Lys Ala Ala Phe Arg Arg Val Arg Glu Asn Leu Arg Arg Phe Gln
3140 3145 3150
Gln Leu Arg Ala Glu Arg Glu Ser Pro Ile Asn Leu Gly Phe Ala Tyr
3155 3160 3165
25I1e Val Leu Pro Gly Arg Ala Ser Arg Leu Leu Asp Leu Val Asp Phe
3170 3175 3180
Ile Ala Asp Leu Asn Asp Ala Gly Gln Gly Arg Thr Ile Asp Phe Val
3185 3190 3195 3200
' Asn Ile Arg Glu Asp Tyr Ser Gly Arg Asp Asp Gly Lys Leu Pro Gln
3205 3210 3215
Glu Glu Arg Ala Glu Leu Gln Glu Ala Leu Asn Ala Phe Glu Glu Arg
3220 3225 3230
Val Arg Glu Arg Thr Pro Gly Leu His Ile Asp Tyr Gly Tyr Ala Leu
3235 3240 3245
35Asn Ser Leu Arg Thr Gly Ala Asp Ala Glu Leu Leu Arg Ile Lys Pro
3250 3255 3260
Ala Thr Met Arg Pro Thr Ala His Pro Gln Val Ala Val Gln Val Asp
3265 3270 3275 3280
Leu Leu Gly Asp Val Tyr Leu Tyr Arg Glu Ala Gly Phe Pro Asp Leu
3285 3290 3295
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
18
Asp Gly Ala Thr Arg Tyr Ile Ala Gly Arg Val Thr Pro Asp Thr Ser
3300 3305 3310
Leu Thr Glu Val Val Arg Asp Phe Val Glu Arg Gly Gly Glu Val Ala
3315 3320 3325
5Ala Val Asp Gly Asp Glu Tyr Phe Met Asp Gly Phe Asp Gln Val Val
3330 3335 3340
Thr Ala Arg Leu Asn Gln Leu Glu Arg Asp Ala Ala Asp Gly Trp Glu
3345 3350 3355 3360
Glu Ala Arg Gly Phe Leu Arg Met Lys Ser Ala Leu Ser Asp Leu Ala
3365 3370 3375
Phe Phe Gly Gly Pro Ala Ala Phe Asp Gln Pro Leu Leu Val Gly Arg
3380 3385 3390
Pro Asn Arg Ile Asp Arg Ala Arg Leu Tyr Glu Arg Leu Asp Arg Ala
3395 3400 3405
l5Leu Asp Ser Gln Trp Leu Ser Asn Gly Gly Pro Leu Val Arg Glu Phe
3410 3415 3420
Glu Glu Arg Val Ala Gly Leu Ala Gly Val Arg His Ala Val Ala Thr
3425 3430 3435 3440
Cys Asn Ala Thr Ala Gly Leu Gln Leu Leu Ala His Ala Ala Gly Leu
3445 3450 3455
Thr Gly Glu Val Ile Met Pro Ser Met Thr Phe Ala Ala Thr Pro His
3460 3465 3470
Ala Leu Arg Trp Ile Gly Leu Thr Pro Val Phe Ala Asp Tle Asp Pro
3475 3480 3485
25Asp Thr Ghy Asn Leu Asp Pro Asp Gln Val Ala Ala Ala Val Thr Pro
3490 3495 3500
Arg Thr Ser Ala Val Val Gly Val His Leu Trp Gly Arg Pro Cys Ala
3505 3510 3515 3520
Ala Asp Gln Leu Arg Lys Val Ala Asp Glu His Gly Leu Arg Leu Tyr
3525 3530 3535
Phe Asp Ala Ala His Ala Leu Gly Cys Ala Val Asp Gly Arg Pro Ala
3540 3545 3550
Gly Ser Leu Gly Asp Ala Glu Val Phe Ser Phe His Ala Thr Lys Ala
3555 3560 3565
35Va1 Asn Ala Phe Glu Gly Gly Ala Val Val Thr Asp Asp Ala Asp Leu
3570 3575 3580
Ala Ala Arg Ile Arg Ala Leu His Asn Phe Gly Phe Asp Leu Pro Gly
3585 3590 3595 3600
Gly Ser Pro Ala Gly Gly Thr Asn Ala Lys Met Ser Glu Ala Ala Ala
3605 3610 3615
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
19
Ala Met Gly Leu Thr Ser Leu Asp Ala Phe Pro Glu Val Ile Asp Arg
3620 3625 3630
Asn Arg Arg Asn His Ala Ala Tyr Arg Glu His Leu Ala Asp Leu Pro
3635 3640 3645
5Gly Val Leu Val Ala Asp His Asp Arg His Gly Leu Asn Asn His Gln
3650 3655 3660
Tyr Val Ile Val Glu Ile Asp Glu Ala Thr Thr Gly Ile His Arg Asp
3665 3670 3675 3680
Leu Val Met Glu Val Leu Lys Ala Glu Gly Val His Thr Arg Ala Tyr
3685 3690 3695
Phe Ser Pro Gly Cys His Glu Leu Glu Pro Tyr Arg Gly Gln Pro His
3700 3705 3710
Ala Pro Leu Pro His Thr Glu Arg Leu Ala Ala Arg Val Leu Ser Leu
3715 3720 3725
l5Pro Thr Gly Thr Ala Ile Gly Asp Asp Asp Ile Arg Arg Val Ala Asp
3730 3735 3740
Leu Leu Arg Leu Cys Ala Thr Arg Gly Arg Glu Leu Thr Ala Arg His
3745 3750 3755 3760
Arg Asp Thr Ala Pro Ala Pro Leu Ala Ala Pro Gln Thr Ser Thr Pro
3765 3770 3775
Thr Ile Gly Arg Ser Arg
3780
<210> 5
<400> 5
000
<210> 6
<400> 6
000
<210> 7
35<211> 1248
<212> DNA
<213> Streptomyces venezuelae
<400> 7
40gtgaaaagcg ccttatccga cctcgcattc ttcggcggcc ccgccgcttt cgaccagccg 60
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
CtCCtCgtggggCggCCCaaCCgCatCgaCCgCgCCaggCtgtacgagcggctcgaccgg 120
gccctcgacagccagtggctgtccaacggcggcccgctcgtccgcgagttcgaggagcgc 180
gtcgccgggctcgccggggtccggcatgccgtggCCaCCtgCaaCgCCaCggccgggctc 240
cagctcctcgCgC3CgCCgCCggCC'tCaCCggcgaagtgatcatgccgtcgatgacgttc 300
SgCCgCCaCCCCgCaCgCaCtgCgCtggatCggCCtCaCCCCggtCttCgCCgaCatCgaC 360
ccggacaccggcaacctcgacccggaccaggtggccgccgCggtC3CdCCCCgCaCCtCg 420
gccgtcgtcggcgtccacctctggggccgcccctgcgccgccgaccagctgcggaaggtc 480
gccgacgagcacggcctgcggctgtacttcgaCgCCgCgCaCgCCCtCggctgcgcggtc 540
gacggccggcccgccggcagcctcggcgacgccgaggtcttcagcttccacgccaccaag 600
l0gccgtcaacgccttcgagggcggcgccgtcgtcaccgacgacgccgacctcgccgccegg 660
atCCgCgCCCtCCdCaaCttcggcttcgacCtgCCCggCggcagccccgccggcgggacc 720
aacgccaagatgagcgaggccgccgccgccatgggcctcaCCtCCCtCgacgcgtttccc 780
gaggtcatcgaccggaaccggcgcaaccacgccgcctaccgcgagcacctcgcggacctc 840
CCCggCgtCCtCgtCgCCgaCC3CgaCCgCCaCggCCtCaaCaaCCdCCagtaCgtgatC 900
l5gtcgagatcgacgaggccaccaccggcatccaccgcgacctcgtcatggaggtcctgaag 960
gccgaaggcgtgcacacccgcgcctacttctcgccgggctgccacgagctggagccgtac 1020
CgCgggCagCCgCaCgCCCCgCtgCCgCdCaCCgaaCgCCtcgccgcgcgcgtgctgtcc 1080
ctgccgaccggcaccgccatcggcgacgacgacatccgccgggtcgccgacctgctgcgt 1140
CtCtgCgCgaCCCgCggCCgcgaactgaccgcgcgccaccgcgacacggcccccgccccg 1200
20ctcgcggccccccagacatccacgcccacgattggacgctcccgatga 1248
<210> 8
<211> 415
25<212> PRT
<213> Streptomyces venezuelae
<400> 8
Met Lys Ser Ala Leu Ser Asp Leu Ala Phe Phe Gly Gly Pro Ala Ala
3 0 1 5 . 10 15
Phe Asp Gln Pro Leu Leu Val Gly Arg Pro Asn Arg Ile Asp Arg Ala
20 25 30
Arg Leu Tyr Glu Arg Leu Asp Arg Ala Leu Asp Ser Gln Trp Leu Ser
35 40 45
35Asn Gly Gly Pro Leu Val Arg Glu Phe Glu Glu Arg Val Ala Gly Leu
50 55 60
Ala Gly Val Arg His Ala Val Ala Thr Cys Asn Ala Thr Ala Gly Leu
65 70 75 80
Gln Leu Leu Ala His Ala Ala Gly Leu Thr Gly Glu Val Ile Met Pro
40 85 90 95
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
21
Ser Met Thr Phe Ala Ala Thr Pro His Ala Leu Arg Trp Ile Gly Leu
100 105 110
Thr Pro Val Phe Ala Asp Ile Asp Pro Asp Thr Gly Asn Leu Asp Pro
115 120 125
5Asp Gln Val Ala Ala Ala Val Thr Pro Arg Thr Ser Ala Val Val Gly
130 135 140
Val His Leu Trp Gly Arg Pro Cys Ala Ala Asp Gln Leu Arg Lys Val
145 150 155 160
Ala Asp Glu His Gly Leu Arg Leu Tyr Phe Asp Ala Ala His Ala Leu
165 170 175
Gly Cys Ala Val Asp Gly Arg Pro Ala Gly Ser Leu Gly Asp Ala Glu
180 185 190
Val Phe Ser Phe His Ala Thr Lys Ala Val Asn Ala Phe Glu Gly Gly
195 200 205
l5Ala Val Val Thr Asp Asp Ala Asp Leu Ala Ala Arg Ile Arg Ala Leu
210 215 220
His Asn Phe Gly Phe Asp Leu Pro Gly Gly Ser Pro Ala Gly Gly Thr
225 230 235 240
Asn Ala Lys Met Ser Glu Ala Ala Ala Ala Met Gly Leu Thr Ser Leu
245 250 255
Asp Ala Phe Pro Glu Val Ile Asp Arg Asn Arg Arg Asn His Ala Ala
260 265 270
Tyr Arg Glu His Leu Ala Asp Leu Pro Gly Val Leu Val Ala Asp His
275 280 285
25Asp Arg His Gly Leu Asn Asn His Gln Tyr Val Ile Val Glu Ile Asp
290 295 300
Glu Ala Thr Thr Gly Ile His Arg Asp Leu Val Met Glu Val Leu Lys
305 310 315 320
Ala Glu Gly Val His Thr Arg Ala Tyr Phe Ser Pro Gly Cys His Glu
325 330 335
Leu Glu Pro Tyr Arg Gly Gln Pro His Ala Pro Leu Pro His Thr Glu
340 345 350
Arg Leu Ala Ala Arg Val Leu Ser Leu Pro Thr Gly Thr Ala Ile Gly
355 360 365
35Asp Asp Asp Ile Arg Arg Val Ala Asp Leu Leu Arg Leu Cys Ala Thr
370 375 380
Arg Gly Arg Glu Leu Thr Ala Arg His Arg Asp Thr Ala Pro Ala Pro
385 390 395 400
Leu Ala Ala Pro Gln Thr Ser Thr Pro Thr Ile Gly Arg Ser Arg
405 410 415
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
22
<210> 9
<211> 1458
<212> DNA
<213> Streptomyces venezuelae
<400> 9
atgaCCgCCCCCgCCCtttCCgCCa.CCgCCCCggCCgaaCgctgcgcgcaccccggagcc 60
gatctgggggcggcggtccacgccgtcggccagaccctcgccgccggcggcctcgtgccg 120
cccgacgaggccggaacgaccgcccgccacctcgtccggctcgccgtgcgctacggcaac 180
l0agccccttcaccccgctggaggaggcccgccacgacctgggcgtcgaccgggacgccttc 240
CggCgCCtCCtcgccctgttcgggcaggtcccggagctccgcaccgcggtcgagaccggc 300
cccgccggggcgtactggaagaacaccctgctcccgctcgaacagcgcggcgtcttcgac 360
gcggcgctcgccaggaagcccgtcttcccgtacagcgtcggcctctaccccggcccgacc 420
tgCatgttCCgctgccacttctgcgtccgtgtgaccggcgCCCgCtaCgaCCCgtCCg'CC480
l5ctcgacgccggcaacgccatgttccggtcggtcatcgacgagatacccgcgggCaaCCCC 540
tcggcgatgtacttctccggcggcctggagCCgCtCa.CCaaCCCCggCCtcgggagcctg 600
gccgcgcacgCC3CCgaCCaCggCCtgCggcccaccgtctaCdCgaaCtCCttCgCgCtC 660
dCCgagCgCaCCCtggagCgCCagCCCggCCtCtggggCCtgcacgccatCCgCaCCtCg 720
ctctacggcctcaacgacgaggagtacgagcagaccaccggcaagaaggccgccttccgc 780
20CgCgtCCgCgagaacctgcgccgcttccagcagctgcgcgccgagcgcgagtcgccgatc 840
aaCCtCggCttCgCCtaCatcgtgctcccgggccgtgcctcccgcctgctcgacctggtc 900
gacttcatcgccgacctcaacgacgccgggcagggcaggacgatcgacttcgtcaacatt 960
cgcgaggactacagcggccgtgacgacggc.aagctgccgcaggaggagcgggccgagctc 1020
caggaggccctcaacgccttcgaggagcgggtccgcgagcgcacccccggactccacatc 1080
25gactacggctaCgCCCtgaaCagCCtgCgCaCCggggCCgacgccgaactgctgcggatc 1140
aagcccgccaccatgcggcccaccgcgcacccgcaggtcgcggtgcaggtcgatctcctc 1200
ggcgacgtgtacctgtaccgcgaggccggcttccccgacctggacggcgcgacccgctac 1260
atcgcgggccgcgtgacccccgacacctccctcaccgaggtcgtcagggacttcgtcgag 1320
cgcggcggcgaggtggcggccgtcgacggcgacgagtacttcatggacggcttcgatcag 1380
30gtcgtcaccgcccgcctgaaccagctggagcgcgacgccgcggacggctgggaggaggcc 1440
cgcggcttcctgcgctga 1458
<210> 10
<211> 485
35<212> PRT
<213> Streptomyces venezuelae
<400> 10
Met Thr Ala Pro Ala Leu Ser Ala Thr Ala Pro Ala Glu Arg Cys Ala
4D 1 5 10 15
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
23
His Pro Gly Ala Asp Leu Gly Ala Ala Val His Ala Val Gly Gln Thr
20 25 30
Leu Ala Ala Gly Gly Leu Val Pro Pro Asp Glu Ala Gly Thr Thr Ala
35 40 45
5Arg His Leu Val Arg Leu Ala Val Arg Tyr Gly Asn Ser Pro Phe Thr
50 55 60
Pro Leu Glu Glu Ala Arg His Asp Leu Gly Val Asp Arg Asp Ala Phe
65 70 75 80
Arg Arg Leu Leu Ala Leu Phe Gly Gln Val Pro Glu Leu Arg Thr Ala
85 90 95
Val Glu Thr Gly Pro Ala Gly Ala Tyr Trp Lys Asn Thr Leu Leu Pro
100 105 110
Leu Glu Gln Arg Gly Val Phe Asp Ala Ala Leu Ala Arg Lys Pro Val
115 120 125
l5Phe Pro Tyr Ser Val Gly Leu Tyr Pro Gly Pro Thr Cys Met Phe Arg
130 135 140
Cys His Phe Cys Val Arg Val Thr Gly Ala Arg Tyr Asp Pro Ser Ala
145 150 155 160
Leu Asp Ala Gly Asn Ala Met Phe Arg Ser Val Ile Asp Glu Ile Pro
165 170 175
Ala Gly Asn Pro Ser Ala Met Tyr Phe Ser Gly Gly Leu Glu Pro Leu
180 185 190
Thr Asn Pro Gly Leu Gly Ser Leu Ala Ala His Ala Thr Asp His Gly
195 200 205
25Leu Arg Pro Thr Val Tyr Thr Asn Ser Phe Ala Leu Thr Glu Arg Thr
210 215 220
Leu Glu Arg Gln Pro Gly Leu Trp Gly Leu His Ala Ile Arg Thr Ser
225 230 235 240
Leu Tyr Gly Leu Asn Asp Glu Glu Tyr Glu Gln Thr Thr Gly Lys Lys
245 250 255
Ala Ala Phe Arg Arg Val Arg Glu Asn Leu Arg Arg Phe Gln Gln Leu
260 265 270
Arg Ala Glu Arg Glu Ser Pro Ile Asn Leu Gly Phe Ala Tyr Ile Val
275 280 285
35Leu Pro Gly Arg Ala Ser Arg Leu Leu Asp Leu Val Asp Phe Ile Ala
290 295 300
Asp Leu Asn Asp Ala Gly Gln Gly Arg Thr Ile Asp Phe Val Asn Ile
305 310 315 320
Arg Glu Asp Tyr Ser Gly Arg Asp Asp Gly Lys Leu Pro Gln Glu Glu
325 330 335
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
24
Arg Ala Glu Leu Gln Glu Ala Leu Asn Ala Phe Glu Glu Arg Val Arg
340 345 350
Glu Arg Thr Pro Gly Leu His Ile Asp Tyr Gly Tyr Ala Leu Asn Ser
355 360 365
5Leu Arg Thr Gly Ala Asp Ala Glu Leu Leu Arg Ile Lys Pro Ala Thr
370 375 380
Met Arg Pro Thr Ala His Pro Gln Val Ala Val Gln Val Asp Leu Leu
385 390 395 400
Gly Asp Val Tyr Leu Tyr Arg Glu Ala Gly Phe Pro Asp Leu Asp Gly
405 410 415
Ala Thr Arg Tyr Ile Ala Gly Arg Val Thr Pro Asp Thr Ser Leu Thr
420 425 430
Glu Val Val Arg Asp Phe Val Glu Arg Gly Gly Glu Val Ala Ala Val
435 440 445
l5Asp Gly Asp Glu Tyr Phe Met Asp Gly Phe Asp Gln Val Val Thr Ala
450 455 460
Arg Leu Asn Gln Leu Glu Arg Asp Ala Ala Asp Gly Trp Glu Glu Ala
465 470 475 480
Arg Gly Phe Leu Arg
485
<210> 11
<211> 879
<212> DNA
25<213> Streptomyces venezuelae
<400> 11
atgaagggaa tagtcctggccggcgggagcggaactcggctgcatccggcgacctcggtc 60
atttcgaagc agattcttccggtctacaacaaaccgatgatctactatccgctgtcggtt 120
30ctcatgctcggcggtattcgcgagattcaaatcatctcgaccccccagcacatcgaactc 180
ttccagtcgc ttctcggaaacggcaggcacctgggaatagaactcgactatgcggtccag 240
aaagagcccg caggaatcgcggacgcacttctcgtcggagccgagcacatcggcgacgac 300
aCCtgCgCCC tgatcctgggcgacaacatcttccacgggcccggcctctacacgctcctg 360
cgggacagca tcgcgcgcctcgacggctgcgtgctcttcggctacccggtcaaggacccc 420
35gagcggtacggcgtcgccgaggtggacgcgacgggccggctgaccgacctcgtcgagaag 480
CCCgtCaagC cgcgctccaacctcgccgtcaCCggCCtCtaCCtCtaCgacaacgacgtc 540
gtcgacatcg ccaagaacatccggccctcgccgcgcggcgagctggagatCaCCgaCgtC 600
aaccgcgtct acctggagcggggccgggccgaactcgtcaacctgggccgcggcttcgcc 660
tggctggaca ccggcacccacgactcgctcctgcgggccgcccagtacgtccaggtcctg 720
40gaggagcggcagggcgtctggatcgcgggccttgaggagatcgccttccgcatgggcttc 780
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
atcgacgccg aggcctgtca cggcctggga gaaggcctct cccgcaccga gtacggcagc 840
tatctgatgg agatcgccgg ccgcgaggga gccccgtga 879
5<210> 12
<211> 292
<212> PRT
<213> Streptomyces venezuelae
10<400> 12
Met Lys Gly Ile Val Leu Ala Gly Gly Ser Gly Thr Arg Leu His Pro
1 5 10 15
Ala Thr Ser Val Ile Ser Lys Gln Ile Leu Pro Val Tyr Asn Lys Pro
20 25 30
l5Met Ile Tyr Tyr Pro Leu Ser Val Leu Met Leu Gly Gly Ile Arg Glu
40 45
Ile Gln Ile Ile Ser Thr Pro Gln His Ile Glu Leu Phe Gln Ser Leu
50 55 60
Leu Gly Asn Gly Arg His Leu Gly Ile Glu Leu Asp Tyr Ala Val Gln
2065 70 75 80
Lys Glu Pro Ala Gly Ile Ala Asp Ala Leu Leu Val Gly Ala Glu His
85 90 95
Ile Gly Asp Asp Thr Cys Ala Leu Ile Leu Gly Asp Asn Ile Phe His
100 105 110
25G1y Pro Gly Leu Tyr Thr Leu Leu Arg Asp Ser Ile Ala Arg Leu Asp
115 120 125
Gly Cys Val Leu Phe Gly Tyr Pro Val Lys Asp Pro Glu Arg Tyr Gly
130 135 140
Val Ala Glu Val Asp Ala Thr Gly Arg Leu Thr Asp Leu Val Glu Lys
30145 150 155 160
Pro Val Lys Pro Arg Ser Asn Leu Ala Val Thr Gly Leu Tyr Leu Tyr
165 170 175
Asp Asn Asp Val Val Asp Ile Ala Lys Asn Ile Arg Pro Ser Pro Arg
180 185 190
35G1y Glu Leu Glu Ile Thr Asp Val Asn Arg Val Tyr Leu Glu Arg Gly
195 200 205
Arg Ala Glu Leu Val Asn Leu Gly Arg Gly Phe Ala Trp Leu Asp Thr
210 215 220
Gly Thr His Asp Ser Leu Leu Arg Ala Ala Gln Tyr Val Gln Val Leu
40225 230 235 240
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
26
Glu Glu Arg Gln Gly Val Trp Ile Ala Gly Leu Glu Glu Ile Ala Phe
245 250 255
Arg Met Gly Phe Ile Asp Ala Glu Ala Cys His Gly Leu Gly Glu Gly
260 265 270
5Leu Ser Arg Thr Glu Tyr Gly Ser Tyr Leu Met Glu Ile Ala Gly Arg
275 280 285
Glu Gly Ala Pro
290
10<210> 13
<211> 1014
<212> DNA
<2l3> Streptomyces venezuelae
15<400>
13
gtgcggcttc tggtgaccggaggtgcgggcttcatcggctCc~CdcttCgtgCggCagCtC 60
ctcgccgggg cgtaccccgacgtgcccgccgatgaggtgatcgtcctggacagcctcacc 120
tacgcgggca accgcgccaacctcgccccggtggacgcggacccgcgactgcgcttcgtc 180
cacggcgaca tccgcgacgccggcctcctcgcccgggaactgcgcggcgtggacgccatc 240
20gtccacttcgcggccgagagccacgtggaccgctccatcgcgggcgcgtccgtgttcacc 300
gagaccaacg tgcagggcacgcagacgctgctccagtgcgccgtcgacgccggcgtcggc 360
cgggtcgtgc acgtctccaccgacgaggtgtacgggtcgatcgactccggCtCCtggaCC 420
gagagcagcc cgctggagcccaactcgccctacgcggcgtccaaggccggCtcCgaCCtC 480
gttgcccgcg cctaccaccggacgtacggcctcgacgtacggatcacccgctgctgcaac 540
25aactacgggccgtaccagcaccccgagaagctcatccccctcttcgtgacgaacctcctc 600
gacggcggga cgctcccgctgtacggcgacggcgcgaacgtccgcgagtgggtgcacacc 660
gacgaccact gccggggcatcgcgctcgtcctcgcgggcggccgggccggcgagatctac 720
cacatcggcg gcggcctggagctgaccaaccgcgaactcaccggcatcctcctggactcg 780
ctcggcgccg actggtcctcggtccggaaggtcgccgaccgcaagggccacgacctgcgc 840
30tactccctcgacggcggcgagatcgagcgcgagctcggctaccgcccgcaggtctccttc 900
gcggacggcc tcgcgcggaccgtccgctggtaccgggagaaccgcggctggtgggagccg 960
ctcaaggcga ccgccccgcagctgcccgccaccgccgtggaggtgtccgcgtga 1014
35<210> 14
<211> 337
<212> PRT
<213> Streptomyces venezuelae
40<400> 14
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
27
Met Arg Leu Leu Val Thr Gly Gly Ala Gly Phe Ile Gly Ser His Phe
1 5 10 15
Val Arg Gln Leu Leu Ala Gly Ala Tyr Pro Asp Val Pro Ala Asp Glu
20 25 30
5Val Ile Val Leu Asp Ser Leu Thr Tyr Ala Gly Asn Arg Ala Asn Leu
35 40 45
Ala Pro Val Asp Ala Asp Pro Arg Leu Arg Phe Val His Gly Asp Ile
50 55 60
Arg Asp Ala Gly Leu Leu Ala Arg Glu Leu Arg Gly Val Asp Ala Ile
1065 70 75 80
Val His Phe Ala Ala Glu Ser His Val Asp Arg Ser Ile Ala Gly Ala
85 90 95
Ser Val Phe Thr Glu Thr Asn Val Gln Gly Thr Gln Thr Leu Leu Gln
100 105 110
l5Cys Ala Val Asp Ala Gly Val Gly Arg Val Val His Val Ser Thr Asp
115 120 125
Glu Val Tyr Gly Ser Ile Asp Ser Gly Ser Trp Thr Glu Ser Ser Pro
130 135 140
Leu Glu Pro Asn Ser Pro Tyr Ala Ala Ser Lys Ala Gly Ser Asp Leu
20145 150 155 160
Val Ala Arg Ala Tyr His Arg Thr Tyr Gly Leu Asp Val Arg Ile Thr
165 170 175
Arg Cys Cys Asn Asn Tyr Gly Pro Tyr Gln His Pro Glu Lys Leu Ile
180 185 190
25Pro Leu Phe Val Thr Asn Leu Leu Asp Gly Gly Thr Leu Pro Leu Tyr
195 200 205
Gly Asp Gly Ala Asn Val Arg G1u Trp Val His Thr Asp Asp His Cys
210 215 220
Arg Gly Ile Ala Leu Val Leu Ala Gly Gly Arg Ala Gly Glu Ile Tyr
30225 230 235 240
His Ile Gly Gly Gly Leu Glu Leu Thr Asn Arg Glu Leu Thr Gly Ile
245 250 255
Leu Leu Asp Ser Leu Gly Ala Asp Trp Ser Ser Val Arg Lys Val Ala
260 265 270
35Asp Arg Lys Gly His Asp Leu Arg Tyr Ser Leu Asp Gly Gly Glu Ile
275 280 285
Glu Arg Glu Leu Gly Tyr Arg Pro Gln Val Ser Phe Ala Asp Gly Leu
290 ' 295 300
Ala Arg Thr Val Arg Trp Tyr Arg Glu Asn Arg Gly Trp Trp Glu Pro
40305 310 315 320
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
28
Leu Lys Ala Thr Ala Pro Gln Leu Pro Ala Thr Ala Val Glu Val Ser
325 330 335
Ala
<210> 15
<211> 1140
<212> DNA
<213> Streptomyces venezuelae
<400> 15
gtgagcagcc gcgccgagaccccccgcgtccccttcctcgacctcaaggccgcctacgag 60
gagctccgcg cggagaccgacgccgcgatcgcccgcgtcctcgactcggggcgctacctc 120
CtCggaCCCg aactcgaaggattcgaggcggagttcgccgcgtactgcgagaCggaCCaC 180
l5gccgtcggcgtgaacagcgggatggacgccctccagctcgccctccgcggcctcggcatc 240
ggacccgggg acgaggtgatcgtcccctcgcacacgtacatcgccagctggctcgcggtg 300
tCCgCCdCCg gCgCgaCCCCCgtgCCCgtCgagccgcacgaggaccaccccaccctggac 360
ccgctgctcg tcgagaaggcgatCaCCCCCCgCaCCCgggCgCtCCtCCCCgtCC3CCtC 420
tacgggcacc ccgccgacatggacgccctccgcgagctcgcggaccggcacggcctgcac 480
20atcgtcgaggacgccgcgcaggcccacggcgcccgctaccggggccggcggatcggcgcc 540
gggtcgtcgg tggccgcgttcagcttctacccgggcaagaacctcggctgcttcggcgac 600
ggcggcgccg tcgtcaccggcgaccccgagctcgccgaacggctccggatgctccgcaac 660
tacggctcgc ggcagaagtacagccacgagacgaagggcaccaactcccgcctggacgag 720
atgcaggccg ccgtgctgcggatccggctcgcccacctggacagctggaacggccgcagg 780
25tcggcgctggccgcggagtacctctccgggctcgccggactgcccggcatcggcctgccg 840
gtgaccgcgc ccgacaccgacccggtctggCa.CCtCttCaCCgtgCgCaCCgagCgCCgC 900
gacgagctgc gcagccacctCgaCgCCCgCggcatcgacaCCCtCaCgCactacccggta 960
cccgtgcacc tctcgcccgcctacgcgggcgaggcaccgccggaaggctcgctcccgcgg 1020
gccgagagct tcgcgcggcaggtcctcagcctgccgatcggcccgcacctggagcgcccg 1080
30caggcgctgcgggtgatcgacgccgtgcgcgaatgggccgagcgggtcgaccaggcctag 1140
<210> 16
<211> 379
35<212> PRT
<213> Streptomyces venezuelae
<400> 16
Met Ser Ser Arg Ala Glu Thr Pro Arg Val Pro Phe Leu-Asp Leu Lys
40 1 5 10 15
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
29
Ala Ala Tyr Glu Glu Leu Arg Ala Glu Thr Asp Ala Ala Ile Ala Arg
20 25 30
Val Leu Asp Ser Gly Arg Tyr Leu Leu Gly Pro Glu Leu Glu Gly Phe
35 40 45
5Glu Ala Glu Phe Ala Ala Tyr Cys Glu Thr Asp His Ala Val Gly Val
50 55 60
Asn Ser Gly Met Asp Ala Leu Gln Leu Ala Leu Arg Gly Leu Gly Ile
65 70 75 80
Gly Pro Gly Asp Glu Val Ile Val Pro Ser His Thr Tyr Ile Ala Ser
85 90 95
Trp Leu Ala Val Ser Ala Thr Gly Ala Thr Pro Val Pro Val Glu Pro
100 105 110
His Glu Asp His Pro Thr Leu Asp Pro Leu Leu Val Glu Lys Ala Ile
115 120 125
l5Thr Pro Arg Thr Arg Ala Leu Leu Pro Val His Leu Tyr Gly His Pro
130 135 140
Ala Asp Met Asp Ala Leu Arg Glu Leu Ala Asp Arg His Gly Leu His
145 150 155 160
Ile Val Glu Asp Ala Ala Gln Ala His Gly Ala Arg Tyr Arg Gly Arg
165 170 175
Arg Ile Gly Ala Gly Ser Ser Val Ala Ala Phe Ser Phe Tyr Pro Gly
180 185 190
Lys Asn Leu Gly Cys Phe Gly Asp Gly Gly Ala Val Val Thr Gly Asp
195 200 ~ 205
25Pro Glu Leu Ala Glu Arg Leu Arg Met Leu Arg Asn Tyr Gly Ser Arg
210 215 220
Gln Lys Tyr Ser His Glu Thr Lys Gly Thr Asn Ser Arg Leu Asp Glu
225 230 235 240
Met Gln Ala Ala Val Leu Arg Ile Arg Leu Ala His Leu Asp Ser Trp
245 250 255
Asn Gly Arg Arg Ser Ala Leu Ala Ala Glu Tyr Leu 5er Gly Leu Ala
260 265 270
Gly Leu Pro Gly Ile Gly Leu Pro Val Thr Ala Pro Asp Thr Asp Pro
275 280 285
35Va1 Trp His Leu Phe Thr Val Arg Thr Glu Arg Arg Asp Glu Leu Arg
290 295 300
Ser His Leu Asp Ala Arg Gly Ile Asp Thr Leu Thr His Tyr Pro Val
305 310 315 320
Pro Val His Leu Ser Pro Ala Tyr Ala Gly Glu Ala Pro Pro Glu Gly
325 330 335
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Ser Leu Pro Arg Ala Glu Ser Phe Ala Arg Gln Val Leu Ser Leu Pro
340 345 350
Ile Gly Pro His Leu Glu Arg Pro Gln Ala Leu Arg Val Ile Asp Ala
355 360 365
5Val Arg Glu Trp Ala Glu Arg Val Asp Gln Ala
370 375
<210> 17
<211> 714
10<212> DNA
<213> Streptomyces venezuelae
<400> 17
gtgtacgaagtcgaccacgccgacgtctacgacctcttctacctgggtcgcggcaaggac 60
l5tacgccgccgaggcctccgacatcgccgacctggtgcgctCCCgtaCCCCCgaggCCtCC 120
tcgctcctggacgtggcctgcggtacgggcacgcatctggagcacttcaccaaggagttc 180
ggcgacaccgccggcctggagctgtccgaggacatgctcacccacgcccgcaagcggctg 240
cccgacgccacgctccaccagggcgacatgcgggacttccggctcggccggaagttctcc 300
gccgtggtcagcatgttcagctccgtcggctacctgaagacgaccgaggaactcggcgcg 360
20gccgtcgcctcgttcgcggagcacctggagcccggtggcgtcgtcgtcgtcgagccgtgg 420
tggttcccggagaccttcgccgacggctgggtcagcgccgacgtcgtccgccgtgacggg 480
cgcaccgtggcccgtgtctcgcactcggtgcgggaggggaacgcgacgcgcatggaggtc 540
cacttcaccgtggccgacccgggcaagggcgtgcggcacttctccgacgtccatctcatc 600
accctgttccaccaggccgagtacgaggccgcgttcacggccgccgggctgcgcgtcgag 660
25tacctggagggcggcccgtcgggccgtggcctcttcgtcggcgtccccgcctga 714
<210> 18
<211> 237
<212> PRT
30<213> Streptomyces venezuelae
<400> 18
Met Tyr Glu Val Asp His Ala Asp Val Tyr Asp Leu Phe Tyr Leu Gly
1 5 10 ' 15
35Arg Gly Lys Asp Tyr Ala Ala Glu Ala Ser Asp Ile Ala Asp Leu Val
20 25 30
Arg Ser Arg Thr Pro Glu Ala Ser Ser Leu Leu Asp Val Ala Cys Gly
40 45
Thr Gly Thr His Leu Glu His Phe Thr Lys Glu Phe Gly Asp Thr Ala
50 55 60
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
31
Gly Leu Glu Leu Ser Glu Asp Met Leu Thr His Ala Arg Lys Arg Leu
65 70 75 80
Pro Asp Ala Thr Leu His Gln Gly Asp Met Arg Asp Phe Arg Leu Gly
85 90 95
5Arg Lys Phe Ser Ala Val Val Ser Met Phe Sex Ser Val Gly Tyr Leu
100 105 110
Lys Thr Thr Glu Glu Leu Gly Ala Ala Val Ala Ser Phe Ala Glu His
115 120 125
Leu Glu Pro Gly Gly Val Val Val Val Glu Pro Trp Trp Phe Pro Glu
130 135 140
Thr Phe Ala Asp Gly Trp Val Ser Ala Asp Val Val Arg Arg Asp Gly
145 150 155 160
Arg Thr Val Ala Arg Val Ser His Ser Val Arg Glu Gly Asn Ala Thr
165 170 175
l5Arg Met Glu Val His Phe Thr Val Ala Asp Pro Gly Lys Gly Val Arg
180 185 190
His Phe Ser Asp Val His Leu Ile Thr Leu Phe His Gln Ala Glu Tyr
195 200 205
Glu Ala Ala Phe Thr Ala Ala Gly Leu Arg Val Glu Tyr Leu Glu Gly
210 215 220
Gly Pro Ser Gly Arg Gly Leu Phe Val Gly Val Pro Ala
225 230 235
<210> 19
25<211> 1282
<212> DNA
<213> Streptomyces venezuelae
<400> 19
30atgcgcgtcctgctgacctcgttcgcacatcacacgcactactacggcctggtgcccctg 60
gcctgggcgctgctcgccgccgggcacgaggtgcgggtcgccagccagcccgcgctcacg 120
gacaccatcaccgggtccgggctcgccgcggtgccggtcggcaccgaccacctcatccac 180
gagtaccgggtgcggatggcgggcgagccgcgcccgaaccatccggcgatcgccttcgac 240
gaggcccgtcccgagccgctggaetgggaccacgccctcggcatcgaggcgatcctcgcc 300
35ccgtacttccatctgctcgccaacaacgactcgatggtcgacgacctcgtcgacttcgcc 360
cggtcctggcagccggacctggtgctgtgggagccgacgacctacgcgggcgccgtcgcc 420
gcccaggtcaccggtgccgcgcacgcccgggtcctgtgggggcccgacgtgatgggcagc 480
gcccgccgcaagttcgtcgcgctgcgggaccggcagccgcccgagcaccgcgaggacccc 540
accgcggagtggctgacgtggacgctcgaccggtacggcgcctccttcgaagaggagctg 600
40ctcaccggccagttcacgatCgaCCCgaCCCCgCCgagCCtgcgcctcgacacgggcctg 660
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
32
ccgaccgtcg ggatgcgtta tgttccgtac aacggcacgt cggtcgtgcc gga~tggctg 720
agtgagccgcccgcgcggccccgggtctgcctgaccctcggcgtctccgcgcgtgaggtc780
ctcggcggcgacggcgtctcgcagggcgacatcctggaggCgCtCgCCgaCCtCgaCatC840
gagctcgtcgccacgctcgacgcgagtcagcgcgccgagatccgcaactacccgaagcac900
5acccggttcacggacttcgtgccgatgcacgCgCtCCtgCCgagCtgCtCggcgatcatc960
caccacggcggggcgggcacctacgcgaccgccgtgatcaacgcggtgccgcaggtcatg1020
ctcgccgagctgtgggacgcgccggtcaaggcgcgggccgtcgccgagcagggggcgggg1080
ttcttcctgccgccggccgagctcacgccgcaggccgtgcgggacgccgtcgtccgcatc1140
ctcgacgacccctcggtcgccaccgccgcgcaccggctgcgcgaggagaccttcggcgac1200
lOcccaccccggccgggatcgtccccgagctggagcggctcgCCgCgCagCaCCgCCgCCCg1260
CCggCCgaCgcccggcactga 1281
<210> 20
<211> 426
15<212> PRT
<213> Streptomyces venezuelae
<400> 20
Met Arg Val Leu Leu Thr Ser Phe Ala His His Thr His Tyr Tyr G~.y
20 1 5 10 15
Leu Val Pro Leu Ala Trp Ala Leu Leu Ala Ala Gly His Glu Val Arg
20 25 30
Val Ala Ser Gln Pro Ala Leu Thr Asp Thr Ile Thr Gly Ser Gly Leu
35 40 45
25A1a Ala Val Pro Val Gly Thr Asp His Leu Ile His Glu Tyr Arg Val
50 55 60
Arg Met Ala Gly Glu Pro Arg Pro Asn His Pro Ala Ile Ala Phe Asp
65 70 75 80
Glu Ala Arg Pro Glu Pro Leu Asp Trp Asp His Ala Leu Gly Ile Glu
30 85 90 95
Ala Ile Leu Ala Pro Tyr Phe His Leu Leu Ala Asn Asn Asp Ser Met
100 105 110
Val Asp Asp Leu Val Asp Phe Ala Arg Ser Trp Gln Pro Asp Leu Val
115 120 125
35Leu Trp Glu Pro Thr Thr Tyr Ala Gly Ala Val Ala Ala Gln Val Thr
130 135 140
Gly Ala Ala His Ala Arg Val Leu Trp Gly Pro Asp Val Met Gly Ser
145 150 155 160
Ala Arg Arg Lys Phe Val Ala Leu Arg Asp Arg Gln Pro Pro Glu His
40 165 ~ 170 175
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
33
Arg Glu Asp Pro Thr Ala Glu Trp Leu Thr Trp Thr Leu Asp Arg Tyr
180 185 190
Gly Ala Ser Phe Glu Glu Glu Leu Leu Thr Gly Gln Phe Thr Ile Asp
195 200 205
5Pro Thr Pro Pro Ser Leu Arg Leu Asp Thr Gly Leu Pro Thr Val Gly
210 215 220
Met Arg Tyr Val Pro Tyr Asn Gly Thr Ser Val Val Pro Asp Trp Leu
225 230 235 240
Ser Glu Pro Pro Ala Arg Pro Arg Val Cys Leu Thr Leu Gly Val Ser
245 250 255
Ala Arg Glu Val Leu Gly Gly Asp Gly Val Ser Gln Gly Asp Ile Leu
260 265 270
Glu Ala Leu Ala Asp Leu Asp Ile Glu Leu Val Ala Thr Leu Asp Ala
275 280 285
l5Ser Gln Arg Ala Glu Ile Arg Asn Tyr Pro Lys His Thr Arg Phe Thr
290 295 300
Asp Phe Val Pro Met His Ala Leu Leu Pro Ser Cys Ser Ala Ile Ile
305 310 315 320
His His Gly Gly Ala Gly Thr Tyr Ala Thr Ala Val Ile Asn Ala Val
325 330 335
Pro Gln Val Met Leu Ala Glu Leu Trp Asp Ala Pro Val Lys Ala Arg
340 345 350
Ala Val Ala Glu Gln Gly Ala Gly Phe Phe Leu Pro Pro Ala Glu Leu
355 360 365
25Thr Pro Gln Ala Val Arg Asp Ala Val Val Arg Ile Leu Asp Asp Pro
370 375 380
Ser Val Ala Thr Ala Ala His Arg Leu Arg Glu Glu Thr Phe Gly Asp
385 390 395 400
Pro Thr Pro Ala Gly Ile Val Pro Glu Leu Glu Arg Leu Ala Ala Gln
405 410 415
His Arg Arg Pro Pro Ala Asp Ala Arg His
420 425
<210> 21
35<211> 1209
<212> DNA
<213> Streptomyces venezuelae
<400> 21
40gtgaccgacg acctgacggg ggccctcacg cagcccccgc tgggccgcac cgtccgcgcg 60
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
34
gtggccgaccgtgaactcggcacccacctcctggagacccgcggcatccactggatccac 120
gccgcgaacggcgacccgtacgccaccgtgctgcgcggccaggcggacgacccgtatccc 180
gcgtacgagcgggtgcgtgcccgcggcgcgCtCtCCttCagcccgacgggcagctgggtc 240
aCCgCCgatCaCgCCCtggCggcgagcatcctctgctcgacggacttcggggtctccggc 300
5gccgacggcgtcccggtgccgcagcaggtcctctcgtacggggagggctgtccgctggag 360
cgcgagcaggtgctgccggcggccggtgacgtgccggagggcgggcagcgtgccgtggtc 420
gaggggatccaccgggagacgctggagggtctcgcgccggacccgtcggcgtcgtacgcc 480
ttcgagctgctgggcggtttcgtccgcccggcggtgacgg.ccgctgccgccgccgtgctg 540
ggtgttcccgcggaccggcgcgcggacttcgcggatctgctggagcggctccggccgctg 600
l0tccgacagcctgctggccccgcagtccctgcggacggtacgggcggcggacggcgcgctg 660
gccgagctcacggcgctgctcgccgattcggacgactcccccggggccctgctgtcggcg 720
ctcggggtcaccgcagccgtccagctcaccgggaacgcggtgctcgcgctcctcgcgcat 780
cccgagcagtggcgggagctgtgcgaccggcccgggctcgcggcggccgcggtggaggag 840
accctccgctacgacccgccggtgcagctcgacgcccgggtggtccgcggggagacggag 900
l5ctggcgggccggcggctgccggccggggcgcatgtcgtcgtcctgaccgccgcgaccggc 960
cgggacccggaggtcttcacggacccggagcgcttcgacctCgCgCgCCCCgaCgCCgCC 1020
gcgcacctcgcgctgcaccccgccggtccgtacggcccggtggcgtccctggtccggctt 1080
caggcggaggtcgcgctgcggaccctggccgggcgtttccccgggctgcggcaggcgggg 1140
gacgtgctccgcccccgccgcgcgcctgtcggccgcgggccgctgagcgtcccggtcagc 1200
20agctcctga 1209
<210> 22
<211> 402
25<212> PRT
<213> Streptomyces venezuelae
<400> 22
Met Thr Asp Asp Leu Thr Gly Ala Leu Thr Gln Pro Pro Leu Gly Arg
30 1 5 10 15
Thr Val Arg Ala Val Ala Asp Arg Glu Leu Gly Thr His Leu Leu Glu
20 25 30
Thr Arg Gly Ile His Trp Ile His Ala Ala Asn Gly Asp Pro Tyr Ala
35 40 45
35Thr Val Leu Arg Gly Gln Ala Asp Asp Pro Tyr Pro Ala Tyr Glu Arg
50 55 60
Val Arg Ala Arg Gly Ala Leu Ser Phe Ser Pro Thr Gly Ser Trp Val
65 70 75 80
Thr Ala Asp His Ala Leu Ala Ala Ser Ile Leu Cys Ser Thr Asp Phe
40 85 90 95
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
Gly Val Ser Gly Ala Asp Gly Val Pro Val Pro Gln Gln Val Leu Ser
100 105 110
Tyr Gly Glu Gly Cys Pro Leu Glu Arg Glu Gln Val Leu Pro Ala Ala
115 120 125
5Gly Asp Val Pro Glu Gly Gly Gln Arg Ala Val Val Glu Gly Ile His
130 135 140
Arg Glu Thr Leu Glu Gly Leu Ala Pro Asp Pro Ser Ala Ser Tyr Ala
145 150 155 160
Phe Glu Leu Leu Gly Gly Phe Val Arg Pro Ala Val Thr Ala Ala Ala
10 165 170 175
Ala Ala Val Leu Gly Val Pro Ala Asp Arg Arg Ala Asp Phe Ala Asp
180 185 190
Leu Leu Glu Arg Leu Arg Pro Leu Ser Asp Ser Leu Leu Ala Pro Gln
195 200 205
l5Ser Leu Arg Thr Val Arg Ala Ala Asp Gly Ala Leu Ala Glu Leu Thr
210 215 220
Ala Leu Leu Ala Asp Ser Asp Asp Ser Pro Gly Ala Leu Leu Ser Ala
225 230 235 240
Leu Gly Val Thr Ala Ala Val Gln Leu Thr Gly Asn Ala Val Leu Ala
20 245 250 255
Leu Leu Ala His Pro Glu Gln Trp Arg Glu Leu Cys Asp Arg Pro Gly
260 265 270
Leu Ala Ala Ala Ala Val Glu Glu Thr Leu Arg Tyr Asp Pro Pro Val
275 280 285
25G1n Leu Asp Ala Arg Val Val Arg Gly Glu Thr Glu Leu Ala Gly Arg
290 295 300
Arg Leu Pro Ala Gly Ala His Val Val Val Leu Thr Ala Ala Thr Gly
305 310 315 320
Arg Asp Pro Glu Val Phe Thr Asp Pro Glu Arg Phe Asp Leu Ala Arg
30 325 330 335
Pro Asp Ala Ala Ala His Leu Ala Leu His Pro Ala Gly Pro Tyr Gly
340 345 350
Pro Val Ala Ser Leu Val Arg Leu Gln Ala Glu Val Ala Leu Arg Thr
355 360 365
35Leu Ala Gly Arg Phe Pro Gly Leu Arg Gln Ala Gly Asp Val Leu Arg
370 375 380
Pro Arg Arg Ala Pro Val Gly Arg Gly Pro Leu Ser Val Pro Val Ser
385 390 395 400
Ser Ser
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
36
<210> 23
<211> 2430
<212> DNA
<213> Streptomyces
venezuelae
<400> 23
gtgacaggtaagacccgaataCCgCgtgtCCgCCgCggCCgCaCCaCgCCCagggCCttC 60
accctggccgtcgtcggcaccctgctggcgggcaccaccgtggcggccgccgctcccggc 120
gccgccgacacggccaatgttcagtacacgagccgggcggcggagctcgtcgcccagatg 180
l0acgctcgacgagaagatcagcttcgtccactgggcgctggaccccgaccggcagaacgtc 240
ggctaccttcccggcgtgccgcgtctgggcatcccggagctgcgtgccgccgacggcccg 300
aacggcatccgcctggtggggcagaccgccaccgcgctgcccgcgccggtCgCCCtggCC 360
agcaccttcgacgacaccatggccgacagctacggcaaggtcatgggccgcgacggtcgc 420
gcgctcaaccaggacatggtcctgggcccgatgatgaacaacatccgggtgccgcacggc 480
l5ggccggaactacgagaccttcagcgaggaccccctggtctCCtCgCgCaCCgCggtCgCC 540
cagatcaagggcatccagggtgcgggtctgatgaccacggccaagcacttcgcggccaac 600
aaccaggagaacaaccgcttctccgtgaacgccaatgtcgacgagcagacgctccgcgag 660
atcgagttcccggcgttcgaggcgtcctccaaggccggcgcggcctccttcatgtgtgcc 720
tacaacggcctcaacgggaagccgtcctgcggcaacgacgagctcctcaacaacgtgctg 780
20cgcacgcagtggggcttccagggctgggtgatgtccgactggctcgccaccccgggcacc 840
gacgccatcaccaagggcctcgaccaggagatgggcgtcgagetccccggcgacgtcccg 900
aagggcgagccctcgccgccggccaagttcttcggcgaggcgctgaagacggccgtcctg 960
aacggcacggtccccgaggcggccgtgacgcggtcggcggagcggatcgtcggccagatg 1020
gagaagttcggtctgctcctcgccactccggcgccgcggcccgagcgcgacaaggcgggt 1080
25gcccaggcggtgtcccgcaaggtcgccgagaacggcgcggtgctcctgcgcaacgagggc 1140
caggccctgccgctcgccggtgacgccggcaagagcatcgcggtcatcggcccgacggcc 1200
gtcgaccccaaggtcaccggcctgggcagcgCCCaCgtCgtCCCggaCtCggcggcggcg 1260
ccactcgacaccatcaaggcccgcgcgggtgcgggtgcgacggtgacgtacgagacgggt 1320
gaggagaccttcgggacgcagatcccggcggggaacctcagcccggcgttcaaccagggc 1380
30caccagctcgagccgggcaaggcgggggcgCtgtaCgaCggCaCgCtgaCCgtgCCCgCC 1440
gacggcgagtaccgcatcgcggtccgtgccaccggtggttacgccacggtgcagctcggc 1500
agccacaccatcgaggccggtcaggtctacggcaaggtgagcagcccgctcctcaagctg 1560
accaagggcacgcacaagctcacgatctcgggcttcgcgatgagtgCCa.CCCCgCtCtCC 1620
ctggagctgggctgggtgacgccggcggcggccgacgcgacgatcgcgaaggccgtggag 1680
35tcggcgcggaaggcccgtacggcggtcgtcttcgcctacgacgacggcaccgagggcgtc 1740
gaccgtccgaacctgtcgctgccgggtacgcaggacaagctgatctcggctgtcgcggac 1800
gccaacccgaacacgatcgtggtcctcaacaccggttcgtcggtgctgatgccgtggctg 1860
tccaagacccgcgcggtcctggacatgtggtacccgggccaggcgggcgccgaggccacc 1920
gccgcgctgctctacggtgacgtcaacccgagcggcaagctcacgcagagcttcccggcc 1980
4Ogccgagaaccagcacgcggtcgccggcgacccgacaagctacccgggcgtcgacaaccag 2040
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
37
cagacgtaccgcgagggcatccacgtcgggtaccgctggttcgacaaggagaacgtcaag2100
ccgctgttcccgttcgggcacggcctgtcgtacacctcgttcacgcagagCgCCCCgaCC2160
gtcgtgcgtacgtccacgggtggtctgaaggtcacggtcacggtccgcaacagcgggaag2220
cgcgccggccaggaggtcgtccaggcgtacctcggtgccagcccgaacgtgacggctccg2280
5caggcgaagaagaagctcgtgggctacacgaaggtctcgctcgccgcgggcgaggcgaag2340
acggtgacggtgaacgtcgaccgccgtcagCtgCagaCCggttCgtCCtCCgCCgaCCtg2400
cggggcagcgccacggtcaacgtctggtga 2430
<210> 24
10<211> 809
<212> PRT
<213> Streptomyces venezuelae
<400> 24
l5Met Thr Gly Lys Thr Arg Ile Pro Arg Val Arg Arg Gly Arg Thr Thr
1 5 10 15
Pro Arg Ala Phe Thr Leu Ala Val Val Gly Thr Leu Leu Ala Gly Thr
20 25 30
Thr Val Ala Ala Ala Ala Pro Gly Ala Ala Asp Thr Ala Asn Val Gln
20 35 40 45
Tyr Thr Ser Arg Ala Ala Glu Leu Val Ala Gln Met Thr Leu Asp Glu
50 55 60
Lys Ile Ser Phe Val His Trp Ala Leu Asp Pro Asp Arg Gln Asn Val
65 70 75 80
25G1y Tyr Leu Pro Gly Val Pro Arg Leu Gly Ile Pro Glu Leu Arg Ala
85 90 95
Ala Asp Gly Pro Asn Gly Ile Arg Leu Val Gly Gln Thr Ala Thr Ala
100 105 110
Leu Pro Ala Pro Val Ala Leu Ala Ser Thr Phe Asp Asp Thr Met Ala
30 115 120 125
Asp Ser Tyr GIy Lys Val Met Gly Arg Asp Gly Arg Ala Leu Asn Gln
130 135 140
Asp Met Val Leu Gly Pro Met Met Asn Asn Ile Arg Val Pro His Gly
145 150 155 160
35G1y Arg Asn Tyr Glu Thr Phe Ser Glu Asp Pro Leu Val Sex Ser Arg
165 170 175
Thr Ala Val Ala Gln Ile Lys Gly Ile Gln Gly Ala Gly Leu Met Thr
180 185 190
Thr Ala Lys His Phe Ala Ala Asn Asn Gln Glu Asn Asn Arg Phe Ser
40 195 200 205
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
38
Val Asn Ala Asn Val Asp Glu Gln Thr Leu Arg Glu Ile Glu Phe Pro
210 215 220
Ala Phe Glu Ala Ser Ser Lys Ala Gly Ala Ala Ser Phe Met Cys Ala
225 230 235 240
5Tyr Asn Gly Leu Asn Gly Lys Pro Ser Cys Gly Asn Asp Glu Leu Leu
245 250 255
Asn Asn Val Leu Arg Thr Gln Trp Gly Phe Gln Gly Trp Val Met Ser
260 265 270
Asp Trp Leu Ala Thr Pro Gly Thr Asp Ala Ile Thr Lys Gly Leu Asp
275 . 280 285
Gln Glu Met Gly Val Glu Leu Pro Gly Asp Val Pro Lys Gly Glu Pro
290 295 300
Ser Pro Pro Ala Lys Phe Phe Gly Glu Ala Leu Lys Thr Ala Val Leu
305 310 315 320
l5Asn Gly Thr Val Pro Glu Ala Ala Val Thr Arg Ser Ala Glu Arg Ile
325 330 335
Val Gly Gln Met Glu Lys Phe Gly Leu Leu Leu Ala Thr Pro Ala Pro
340 345 350
Arg Pro Glu Arg Asp Lys Ala Gly Ala Gln Ala Val Ser Arg Lys Val
355 360 365
Ala Glu Asn Gly Ala Val Leu Leu Arg Asn Glu Gly Gln Ala Leu Pro
370 375 380
Leu Ala Gly Asp Ala Gly Lys Ser Ile Ala Val Ile Gly Pro Thr Ala
385 390 395 400
25Va1 Asp Pro Lys Val Thr Gly Leu Gly Ser Ala His Val Val Pro Asp
405 410 415
Ser Ala Ala Ala Pro Leu Asp Thr Ile Lys Ala Arg Ala Gly Ala Gly
420 425 430
Ala Thr Val Thr Tyr Glu Thr Gly Glu Glu Thr Phe Gly Thr Gln Ile
435 440 445
Pro Ala Gly Asn Leu Ser Pro Ala Phe Asn Gln Gly His Gln Leu Glu
450 455 460
Pro Gly Lys Ala Gly Ala Leu Tyr Asp Gly Thr Leu Thr Val Pro Ala
465 470 475 480
35Asp Gly Glu Tyr Arg Ile Ala Val Arg Ala Thr Gly Gly Tyr Ala Thr
485 490 495
Val Gln Leu Gly Ser His Thr Ile Glu Ala Gly Gln Val Tyr Gly Lys
500 505 510
Val Ser Ser Pro Leu Leu Lys Leu Thr Lys Gly Thr His Lys Leu Thr
515 520 525
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
39
Ile Ser Gly Phe Ala Met Ser Ala Thr Pro Leu Ser Leu Glu Leu Gly
530 535 540
Trp Val Thr Pro Ala Ala Ala Asp Ala Thr Ile Ala Lys Ala Val Glu
545 550 555 560
5Ser Ala Arg Lys Ala Arg Thr Ala Val Val Phe Ala Tyr Asp Asp Gly
565 570 575
Thr Glu Gly Val Asp Arg Pro Asn Leu Ser Leu Pro Gly Thr Gln Asp
580 585 590
Lys Leu Ile Ser Ala Val Ala Asp Ala Asn Pro Asn Thr Ile Val Val
595 600 605
Leu Asn Thr Gly Ser Ser Val Leu Met Pro Trp Leu Ser Lys Thr Arg
610 615 620
Ala Val Leu Asp Met Trp Tyr Pro Gly Gln Ala Gly Ala Glu Ala Thr
625 630 635 640
l5Ala Ala Leu Leu Tyr Gly Asp Val Asn Pro Ser Gly Lys Leu Thr Gln
645 650 655
Ser Phe Pro Ala Ala Glu Asn Gln His Ala Val Ala Gly Asp Pro Thr
660 665 670
Ser Tyr Pro Gly Val Asp Asn Gln Gln Thr Tyr Arg Glu Gly Ile His
675 680 685
Val Gly Tyr Arg Trp Phe Asp Lys Glu Asn Val Lys Pro Leu Phe Pro
690 695 700
Phe Gly His Gly Leu Ser Tyr Thr Ser Phe Thr Gln Ser Ala Pro Thr
705 710 71,5 720
25Va1 Val Arg Thr Ser Thr Gly Gly Leu Lys Val Thr Val Thr Val Arg
725 730 735
Asn Ser Gly Lys Arg Ala Gly Gln Glu Val Val Gln Ala Tyr Leu Gly
740 745 750
Ala Ser Pro Asn Val Thr Ala Pro Gln Ala Lys Lys Lys Leu Val Gly
755 760 765
Tyr Thr Lys Val Ser Leu Ala Ala Gly Glu Ala Lys Thr Val Thr Val
770 775 780
Asn Val Asp Arg Arg Gln Leu Gln Thr Gly Ser Ser Ser Ala Asp Leu
785 790 795 ~ 800
35Arg Gly Ser Ala Thr Val Asn Val Trp
805
<210> 25
<211> 9
40<212> PRT
CA 02424567 2003-04-02
WO 02/29035 PCT/USO1/31255
<213> Artificial Sequence
<220>
<223> A Consensus sequence.
5
<221> SITE
<222> (4) . . . (4)
<223> Xaa is V or I.
10<400> 25
Leu Leu Asp Xaa Ala Cys Gly Thr Gly
1 5