Note: Descriptions are shown in the official language in which they were submitted.
CA 02425563 2003-04-11
base 1/1153 ff ' ' Boehringer Ingelheim Pharma KG
1
74337pct.205
New m-amino-phenylimino-imidazolidine derivatives
for treating urinary incontinence
The present invention relates to m-amino-phenylimino-imidazolidine derivatives
with
a new pattern of substituents at the phenyl ring and the use of m-amino-
phenylimino-
imidazolidine derivatives for the preparation of pharmaceutical compositions,
particularly for treating urinary incontinence, and processes for preparing
them.
Prior art
The compounds described within the scope of the present invention belong to
the
category of the m-amino-phenylimino-imidazolidines. Similar compounds are
known
from the prior art.
Thus, for example, EP-A-0 236 636 inter alia describes m-amino-phenylimino-
imidazolidine derivatives with a primary amino function in the 3 position and
their use
as haemostatic agents. Clonidine derivatives of this kind are also discussed
in EP-A-
0 070 084, EP-A-0081923, EP-A-0117102, EP-A-0 149 140, DE-A-2806811 or DE-A-
2854659.
Furthermore, US-A-4587257 and EP-A-0081924 disclose m-amino-phenylimino-
imidazolidine derivatives having a primary or acetylated amino function which
can be
used in ophthalmology. Biochem. Pharmacol. 32 (12) (1983) 1933 -1940 in
another
context discloses inter alia 2-(3-amino-2,6-dichlorophenylimino)-
imidazolidines. US-
A-4287201 for example describes 2-(3-diethylamino-2-methylphenylimino)-
imidazolidines for increasing egg production in hens. Among others, WO
95/19968
discloses aminophenyliminoimidazole derivatives which are used as alpha 2
agonists
in anaesthesia, in the treatment of pain, high blood pressure, hyperglycaemia
or as
soporifics.
WO 96/32939, to which reference is hereby made in its entirety, discloses
phenylimino-imidazoles, including those in which the phenyl ring has a primary
or
tertiary amine, an amide or imide in the meta position to the imino function.
The
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
2
compounds described therein count as alpha-1 L-agonists and can advantageously
be used in this capacity for treating urinary incontinence.
By incontinence is meant the involuntary release of urine, i.e. weakness of
the
bladder. The various forms of urinary incontinence include urge incontinence,
reflex
incontinence, overflow incontinence and stress or load incontinence. The most
common forms of urinary incontinence include load incontinence or stress
incontinence. These affect women in particular after more or less difficult
childbirth.
The reason for this is that pregnancy and childbirth can easily lead to
weakening of
the pelvic floor. Other causes of incontinence may be found, for example, in
damage
to the nerves of the pelvic floor, a congenitally short urinary tract or
damage to the
sphincter muscle.
The use of alpha-1 L-agonists in the treatment of urinary incontinence is
advantageous because they act selectively on the adrenoceptors of the bladder
and
thus exert a major influence on the tone of the ureter without significantly
affecting
the cardiovascular system.
In the prior art the possibility of using imidazole derivatives to treat
incontinence has
long been discussed. Surprisingly, there are opinions which indicate that many
imidazole derivatives can counteract weakness of the bladder, whereas other
authors
have observed an apparently directly opposite effect, namely that substances
of this
kind can relieve obstruction of the bladder. Still other authors, talking
about some of
the same substances, report that they would have no effect at all on bladder
function.
Thus, it is reported that alpha 2 agonists such as clonidine would have a
positive
effect on nocturnal incontinence (Urology, 43 (3) (1994) 324 - 327). On the
other
hand, in respect of clonidine itself, there is the contrary observation that
this
substance might even promote incontinence (Clin. BioLRes. 78 (1981 ) 101 -
103). A
similar observation is expressed in Jpn. J. Pharmacol. 58 (4) (1992) 339 -
346. The
authors find that clonidine does not have a distinct influence on bladder
function but
that phenyl-ethanol-amines such as phenylephrine, midodrine or ST 1059, which
are
similar to adrenaline, and are all alpha 1 agonists, do have such an effect.
EP-A-0
CA 02425563 2003-04-11
Case 1 /1153 ff ~ Boehringer Ingelheim Pharma KG
3
416 841 also deals with the influence of alpha agonists on bladder function.
It
describes how alpha 1 adrenoceptor-blocking substances could be used to treat
obstruction of the bladder. The observations according to US-A-4 226 773 also
point
in this direction. According to this specification, pyrazolyl-imino-imidazole
derivatives
can be used to promote the release of urine. Other alpha 1 adrenergic
imidazoles
such as thiophene-pyrroles, for example, may be used to treat urinary
incontinence
(EP-A-0 599 697).
These different observations from the prior art lead one to conclude that up
till now it
has been impossible to predict the influence of imidazole derivatives on
bladder
function.
Description of the invention
Compounds which may be used to treat urinary incontinence not only have to be
sufficiently effective but should also have as few side effects as possible.
In other
words, if possible they should act selectively on the bladder only.
Undesirable side
effects include, among other things, a negative effect on the cardiovascular
system.
For particularly effective treatment of urinary incontinence the
bioavailability of the
substances and their metabolism are also of particular importance. The
bioavailability
should be as great as possible and the metabolism should be such that the
substances are not broken down too rapidly on the one hand and no toxic
compounds or other compounds having undesirable pharmacological properties in
this context are formed on the other hand.
It is therefore an objective of the present invention to find new alpha-1 L-
agonists
from the category of the phenylimino-imidazolidines which are identical or
similar in
their activity to the compounds known from the prior art, which act
selectively on the
bladder without substantially affecting the cardiovascular system and have
enhanced
qualities in terms of their bioavailability or metabolism.
Surprisingly, it has been found that the m-amino-phenylimino-imidazolidines
according to the invention meet the objective of the present invention and are
therefore particularly suitable for treating urinary incontinence. For the use
of these
CA 02425563 2003-04-11
Case 1/1153 ff Boehringer Ingelheim Pharma KG
4
compounds in connection with urinary incontinence it is essential on the one
hand
that the amino group is a tertiary amine and on the other hand that the other
positions of the phenyl ring are substituted in a particular way.
Detailed description of the invention
According to the invention, the m-amino-phenylimino-2-imidazolidine
derivatives of
general formula I are used for the preparation of medicaments for the
treatment of
urinary incontinence:
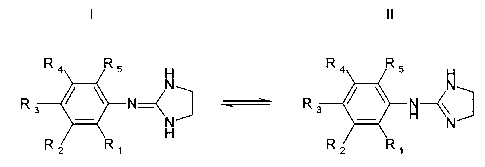
Formula I:
R4 R5 H
N
R3 ~ ~ N
N
H
R2 R~
wherein
R~ denotes F, CI, Br, CH2F, CF2H and/or CF3,
R2 denotes: NR6R~, where
R6 denotes Me, Et, Pr or iPr,
R~ denotes Me, Et or Pr,
R3, Ra, R5 independently of one another each denote H, Me, F, CI, Br,
CH2F, CF2H and/or CF3
and in the event that R4 denotes Me, F, CI, Br or CF3, then R~ additionally
also
denotes H or Me.
Me denotes Methyl, CF3 denotes trifluoromethyl, CH2F denotes fluoromethyl,
CF2H
denotes difluoromethyl, Et denotes ethyl, Pr denotes propyl, iPr denotes
isopropyl, H
denotes hydrogen, F denotes fluorine, CI denotes chlorine, Br denotes bromine
and
N represents nitrogen.
Case 1/1153 ff ' ~ Boehringer Ingelheim Pharma KG
Of these compounds with the hereinbefore-given structural formula, compounds
of
formula I are preferred wherein
R~ denotes F, CI, Br or CF3,
R2 denotes NRsR~, where
R6 denotes Me or Et,
R~ denotes Me or Et and
R3, R4, R5 independently of one another each denote H, F, CI, Br and/or CF3.
Also preferred are those compounds of formula I wherein R~ may additionally
also
denote H or Me if R4 is F, CI, Br or CFs.
Particularly preferred are compounds of formula I wherein
R~ denotes Me,
R2 denotes NR6R~, where
Rs denotes Me or Et, preferably Me,
R~ denotes Me or Et, preferably Me,
R3 denotes H, F, Br or CF3,
R4 denotes CI, Br or CFs,
R5 denotes H, Br or CFs
and/or if
R~ denotes CI, Br or CF3,
then
R4 denotes H.
Of these, the most preferred are compounds of formula I wherein
R~ denotes Me,
R2 denotes NR6R~, where
Rs denotes Me,
R~ denotes Me,
R3 denotes H, F, preferably H,
R4 denotes CI or Br,
R5 denotes H or Br
CA 02425563 2003-04-11
Case 1/1153 ff ' ' Boehringer Ingelheim Pharma KG
6
and/or if
R~ denotes CI or Br,
then
R4 denotes H.
The compounds represented by formula I may be present in tautomeric
equilibrium
with the m-amino-anilino-2-imidazoline derivatives of formula II:
Formula II
R4 R5 H
R3 ~ ~ N \
H
N
R2 R~
in which the definitions of the groups R~, R2, R3, R4, R5, Rs and R~ are
identical to the
abovementioned compounds of formula I and all the preferences listed.
Therefore, the present invention also relates to the compounds which come
under
general formula II wherein the groups R~, R2, Rs, R4, R5, R6 and R~ fall
within the
scope of the definitions given under formula I. The same is true of the
preferred
ranges mentioned under formula I.
The compounds which fall within the scope of definitions of formulae I and II
are
equally preferred, but independently of one another.
With regard to the nomenclature used within the scope of the present
invention, it
should be pointed out that the term "phen-1'-yl-2-imidazolidine" denotes
compounds
having the following structural element:
CA 02425563 2003-04-11
CA 02425563 2003-04-11
base 1 /1153 ff ' ~ Boehringer Ingelheim Pharma KG
H
N
N
This means that the atoms of the imidazole ring are numbered 1, 2, 3, etc.,
with one
nitrogen atom being numbered 1 and the other nitrogen atom being numbered 3.
Consequently, the imino group is bound to the carbon atom, which is assigned
the
number 2. The atoms of the phenyl ring are numbered 1', 2', 3', etc., while
the
carbon atom of the phenyl ring which is bound to the imino group is designated
1'
throughout.
It is expressly mentioned that the corresponding tautomers according to
general
formula II are also included, even when they are not specifically mentioned.
The
same is true of all the m-amino-anilino-2-imidazolidine derivatives mentioned
in the
context of the present invention.
As representatives of all the compounds which come under general formula I or
formula II, some m-dialkylamino-phen-1 '-yl-2-imidazolidines are mentioned
hereinafter by way of example.
2'-Bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
1,
preferably in the form of the hydrochloride,
Me2N Me
H
H / \ N N
H ~~
N
CI Br
Gase 1/1153 ff ' Boehringer Ingelheim Pharma KG
3'-bromo-5'-diethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 2,
preferably in the form of the hydrochloride,
Et2N Me
H
H / \ N N
H--~~ ~
N
Br H
3'-bromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 3,
preferably in the form of the free base,
Me2N Me
H
N
H ~I
N
Br H
3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 4,
preferably in the form of the hydrochloride,
Me2N Me
H
H / \ N N
H~~ ~
N
CI H
2'-3'-dibromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 5,
preferably in the form of the free base
Me2N Me
H
N
Br Br
CA 02425563 2003-04-11
Case 1 /1153 ff ' Boehringer Ingelheim Pharma KG
9
~'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 6,
preferably in the form of the free base
Me2N CI
H
N
N
H H
4'-bromo-2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 7,
preferably in the form of the free base
Me2N CI
H
N
3r ~ \ H \
N
H H
2'-bromo-6'-chloro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 8,
Me2N CI
H
N
N
H Br
5'-bromo-2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 9,
2'-bromo-5'-dimethylamino-6'-fluoro-phen-1'-yl-2-iminoimidazolidine 10,
3'-chloro-6'-fluoro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 11,
3'-chloro-4'-fluoro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
12,
6'-chloro-3'-fluoro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 13,
4'-chloro-3'-fluoro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
14,
3'-6'-dichloro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 15,
3'-4'-dichloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 16,
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
3'-4'-difluoro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 17,
3'-6'-difluoro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 18,
5'-dimethylamino-6'-methyl-2'-trifluoromethyl-phen-1'-yl-2-iminoimidazolidine
19,
5'-dimethylamino-6'-methyl-3'-trifluoromethyl-phen-1'-yl-2-iminoimidazolidine
20.
Of these, the following are preferred:
2'-bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
1,
3'-bromo-5'-diethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 2,
3'-bromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 3,
3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 4,
2'-3'-dibromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 5,
2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 6,
4'-bromo-2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 7,
2'-bromo-6'-chloro-5'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 8,
5'-bromo-2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 9
and the pharmacologically acceptable salts thereof, particularly the
hydrochlorides.
The following are particularly preferred:
2'-bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
1,
3'-bromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 3,
3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 4,
2'-3'-dibromo-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 5 and
the
pharmacologically acceptable salts thereof, particularly the hydrochlorides.
Most preferred is
3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 4 and the
pharmacologically acceptable salts thereof, particularly the hydrochloride.
Another aspect of the present invention relates to the compounds of the
abovementioned general formulae I and/or II and the pharmacologically
compatible
salts thereof with the groups R~ to R5 in all known definitions, as well as
pharmaceutical compositions which contain these compounds.
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
11
Within the framework of the present invention, the definition of all named
compounds
is not only to include the free bases but also the respective pharmaceutically-
acceptable acid addition salts.
Acids suitable for this purpose may be both inorganic and organic by nature.
Examples of suitable acids include:
Hydrochloric acid, hydrobromic acid, sulphuric acid, phosphoric acid, fumaric
acid,
citric acid, lactic acid, acetic acid, propionic acid, malic acid, succinic
acid, amino
acid, particularly gfutamic acid or aspartic acid, carbohydrate acids and
acids derived
from carbohydrate. Salts of this kind may be important for galenic
preparations both
for increasing the stability, especially the long-term stability of the
compounds and/or
for increasing the bioavailability. Hydrochloride salts are preferred, either
the
monohydrochlorides or dihydrochlorides, depending on the particular compound.
The
same is true of the preferred compounds.
As already described hereinbefore, within the framework of the present
invention the
abovementioned compounds are distinguished over the compounds known from the
prior art by their pharmacological properties, particularly with regard to
their
bioavailability andlor metabolism. It goes without saying that the most
preferred
compounds are those which have a high level of activity and bioavailability
and a low
metabolic breakdown. Another feature which is crucial when selecting
particularly
suitable compounds for treating urinary incontinence is the selectivity with
which the
compound in question acts on bladder function without seriously affecting
other
bodily functions, particularly the cardiovascular system.
Within the framework of the present invention, the named compounds and their
pharmacologically-compatible acid addition salts can be prepared in suitable
pharmaceutical formulations. This includes all formulations capable of being
used
medicinally. These are, for example, solutions, suspensions, aerosols,
powders,
tablets, coated tablets, suppositories, creams, etc.
The compounds according to the invention, the pharmacologically acceptable
acid
addition salts thereof andlor pharmaceutical preparations containing them may
be
CA 02425563 2003-04-11
Case 1 /1153 ff ' ' Boehringer Ingelheim Pharma KG
12
used medicinally for treating diseases, especially diseases of the bladder,
particularly
in urinary incontinence. The compounds according to the invention are most
preferably used for treating stress incontinence.
According to another aspect, the present invention relates to processes for
preparing
the abovementioned compounds, the pharmacologically acceptable acid addition
salts and/or pharmaceutical preparations thereof, and the use of the compound
described for preparing other pharmacologically active derivatives thereof.
Examples
1. Bioavailability
In order to determine bioavailability the test substances were administered
orally to a
group of 8 male rats which had been fasted. As a comparison, the test
substances
were administered intravenously to animals in an identical second group. 1 ml
blood
samples were taken from the animals of both groups at specified times after
administration (10 minutes, 30 minutes, 1 hour, 2 hours and 4 hours, and
additionally
after 6 hours in the case of the animals treated by oral route). The blood
samples
taken in each group were mixed together (8 ml). After further working up the
content
of the test substances in the blood for the particular time was determined
from the
plasma by HPLC (High Performance Liquid Chromatography) using standard
methods and compared for the two groups.
Results
compound bioavailability
4, hydrochloride11
5, dihydrochloride0.8%
CA 02425563 2003-04-11
Case 1 /1153 ff ' Boehringer Ingelheim Pharma KG
13
2. Metabolism
To determine the metabolism the enzyme CYP2D6 was allowed to act on the test
substances. After 30 minutes a check was made to see how much of the test
substance put in had been broken down by the enzyme.
The rate of breakdown under the effect of the enzyme HLM / 60 minutes is
tested
analogously.
compound % breakdown of substratebreakdown of substrate
after 30 minutes' after 60 minutes
incubation with CYP2D6'
incubation with HLM
1, hydrochloride40.5 32.1
3, base 33.9 17.0
4, hydrochloride36.5 17.1
5, dihydrochloride22.2 22.0
- -
6 base _ 7.4
7 base 46.4 ~ 55.8
3. Efficacy and selectivity
The efficacy and selectivity of the compounds is determined as follows:
compound activity in activity on humanselectivity
the dog in the
urethra dog
1, in the form of 109 100 0.92
the
hydrochloride
3, in the form of 87 72 0.76
the
base
4, in the form of 116 137 0.99
the
hydrochloride
5, in the form of 89 113 0.71
the
dihydrochloride
CA 02425563 2003-04-11
Case 1/1153 ff ~ Boehringer Ingelheim Pharma KG
14
The maximum contraction in the dog and the activity on the human urethra are
given
as percentages of contraction compared with noradrenalin.
Selectivity in the dog: percentage contraction on the femoral artery in the
dog at 10-5
M - percentage contraction on the carotid artery in the dog at 10-5 M.
4. Preparation
The compounds according to the invention may be prepared as described in WO
96/32939 on pages 11 to 16, to which reference is hereby expressly made. Some
of
the compounds according to the invention may be prepared, for example,
starting
from the compounds described therein, particularly the 2'-bromo-5'-
dimethylamino-6'-
methyl-phen-1'-yl-2-iminoimidazolidine described on page 16.
Preparation Examples
Compound 1:
2'-Bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1 '-yl-2-iminoimidazolidine
1
2 g of 2'-bromo-5'-dimethylamino-6'-methyl-phen-1-yl-2-iminoimidazolidine are
dissolved at 0°C in 6.7 ml of sulphuric acid and combined with 0.8 g of
1,3-dichloro-
5,5-dimethylhydantoin with stirring. The solution is heated to 55°C,
then after 3 days
diluted with ice, made alkaline with NH40H and extracted with ethyl acetate.
The
ethyl acetate extract is concentrated by evaporation under reduced pressure
and the
residue remaining is worked up by chromatography (silica gel, eluant:
CH2C121methanol/conc. NH40H (90/10/1 )).
1.1 g of 2'-bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1 '-yl-2-
iminoimidazolidine 1 are obtained as a white powder, melting point 239-
240°C.
' H NMR (250 MHz, CD30D, TMS = 0 ppm): 8 = 8.09 (1 H, s, aryl-H); 3.81 (4H, s,
imidazolidine-CH2-); 3.27 (6H, s, N(CH3)2); 2.51 (3H, s, aryl-CHs);
exchangeable H
under the solvent peak 4.88 ppm. MS m/z 331/3331335 (87/100/42).
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
Compound 3
3'-Bromo-5'-dimethylamino-6'-methyl-phen-1 '-yl-2-iminoimidazolidine 3
Step 1:
84 g of 2-methyl-3-nitroaniline are dissolved in 550 ml of THF. 53 ml of
acetic
anhydride are slowly added dropwise. The mixture is then stirred at
70°C. After about
1 hour THF is distilled off and the residue is combined with 250 ml of
petroleum
ether. The solid precipitate is filtered off and dried at 50°C.
106 g of N-acetyl-2-methyl-3-nitroaniline are obtained as a light brown
powder.
Step 2:
106 g of N-acetyl-2-methyl-3-nitroaniline are dissolved in 500 ml of
concentrated
sulphuric acid. 93 g of 1,3-dibromo-5,5-dimethylhydantoin are added at RT and
the
mixture is stirred for about 24 hours at RT with the exclusion of light and
for 2 hours
at 50°C. 2.5 I of ice water are added and the crystals precipitated are
filtered off,
washed with water and dried.
67 g of a mixture of N-acetyl-4-brom-2-methyl-3-nitroaniline and N-acetyl-4,5-
dibromo-2-methyl-3-nitroaniline are obtained as a white powder.
Step 3:
67 g of the above mixture and 300 ml of HCI (32%) are dissolved in a mixture
of 300
ml of methanol and 300 ml of THF and the solution is stirred at 90°C.
After about 2
hours the organic solvent is distilled off. The crystalline precipitate is
suction filtered
and washed with HCI (0.5 M) and water. The crystals are suspended in about 500
ml
of water. The aqueous phase is made alkaline with solid Na2COs and extracted
with
about 400 ml of ether. The organic phase is filtered and concentrated by
evaporation
under reduced pressure. The residue is recrystallised from hot ether.
18 g of 4-bromo-2-methyl-3-nitroaniline are obtained as orange-yellow
crystals. In
addition, about 32 g of a mixture of 4-bromo-2-methyl-3-nitroaniline and 4,5-
dibromo-
2-methyl-3-nitroaniline are obtained as orange-yellow crystals.
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
16
Step 4:
5.8 g of paraformaldehyde and 15 ml of formic acid are dissolved in 15 ml of
DMF
and heated to 90-100°C. A solution of 18 g of 4-bromo-2-methyl-3-
nitroaniline in 15
ml of DMF is added dropwise within 10 minutes. After 2 hours the mixture is
added to
about 600 ml of ice water and the aqueous phase is extracted with 300 ml of
ether.
The organic phase is washed with Na2C03 (1 M), filtered and evaporated down.
The
product is purified by chromatography (silica gel, eluant: petroleum
ether/ethyl
acetate (40/60).
15 g of N,N-dimethyl-4-bromo-2-methyl-3-nitroaniline are obtained as a
brownish-
orange oil.
Step 5:
15 g of N,N-dimethyl-4-bromo-2-methyl-3-nitroaniline are dissolved in about
200 ml of
THF and hydrogenated at 20°C under 5 bars of hydrogen using Raney
nickel as
catalyst. 12 g of 2-bromo-5-dimethylamino-6-methylaniline are obtained as a
brown
oil.
Step 6:
2.4 g of 2-bromo-5-dimethylamino-6-methylaniline and 3.2 g of N-acetyl-2-
methylthioimidazolidinone are dissolved in 50 ml of ethanol at ambient
temperature
and the solution is refluxed. After about 6 hours the solvent is evaporated
and the oily
residue is crystallised from about 50 ml of ethyl acetate.
2.5 g of 3'-bromo-5'-dimethylamino~'-methyl-phen-1 '-yl-2-iminoimidazolidine 3
are obtained as a white powder, melting point 140-143°C.
'H NMR (250 MHz, CDC13, TMS = 0 ppm): 8 = 6,80 (2H, s, aryl-H); 4,39 (2H, s,
broad, NH); 3,49 (4H, s, imidazolidine-CH2-); 2,66 (6H, s, N(CH3)2); 2,10 (3H,
s, aryl-
CH3). MS m/z 297/299 (100/100).
CA 02425563 2003-04-11
Case 1/1153 ff ~ Boehringer Ingelheim Pharma KG
17
Compound 4
3'-Chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine 4
0.37 g of 2'-bromo-3'-chloro-5'-dimethylamino-6'-methyl-phen-1-yl-2-
iminoimidazolidine, 0.350 mg of zinc powder and 2 ml of HCI (10%) are
dissolved in
ml of ethanol and refluxed. After 1 hour the reaction mixture is filtered and
the
solvent is distilled off. The residue is treated with Na2C03 (1 M) while
cooling and the
product is extracted with ether. The ether extract is concentrated by
evaporation
under reduced pressure. The residue remaining is taken up in methanol and
acidified
with HCI.
0.2 g of 3'-chloro-5'-dimethylamino-6'-methyl-phen-1'-yl-2-iminoimidazolidine
4 (NCI)
are obtained as a (hygroscopic) white powder.
' H NMR (250 MHz, CD30D, TMS = 0 ppm): 8 = 7.93; 7.60 (2H, 2d (J = 1.8 Hz),
aryl-
H); 3.80 (4H, s, imidazolidine-CH2-); 3.31 (6H, s, N(CH3)2); 2.45 (3H, s, aryl-
CH3);
exchangeable H under the solvent peak 4.88 ppm. MS m/z 253/255 (100/43).
Compound 5
2',3'-Dibromo-5'-dimethylamino-6'-methyl-phen-1 '-yl-2-iminoimidazolidine 5
Steps 1, 2, 3 and 5 are analogous to those of the synthesis of compound 3.
St- ep 4:
3.6 g of a mixture of 4-bromo-2-methyl-3-nitroaniline and 4,5-dibromo-2-methyl-
3-
nitroaniline are reacted as in Example 3, Step 4. The product is purified by
chromatography (silica gel, eluant: cyclohexane/ethyl acetate (90/10).
1.9 g of N,N-dimethyl-4,5-dibromo-2-methyl-3-nitroaniline are obtained as a
yellow
powder.
CA 02425563 2003-04-11
Case 1/1153 ff ~ Boehringer Ingelheim Pharma KG
18
step 6:
0.9 g of 2,3-dibromo-5-dimethylamino-6-methylaniline and 0.4 g of N-acetyl-2-
imidazolidinone are dissolved at RT in 8 ml of POC13 and the solution is
stirred at
85°C. After about 8 hours the solvent is evaporated off and the residue
is combined
with 100 m I of water. The aqueous phase is extracted with ethyl acetate and
the
organic phase is made alkaline with Na2C03, filtered and evaporated down. The
product is purified by chromatography (silica gel, eluant: ethyl acetate).
0.7 g of N-acetyl 2',3'-dibromo-5'-dimethylamino~'-methyl-phenyl-2-
iminoimidazolidine are obtained as a white powder.
Step 7:
0.7 g of N-acetyl-2',3'-dibromo-5'-dimethylamino-6'-methyl-phenyl-2-
iminoimidazolidine are refluxed in 60 ml of methanol. After 7 hours the
solvent is
evaporated. The residue is dissolved in CH2C12 and acidified with HCI (4N) (pH
1 ).
The crystals precipitated are suction filtered, washed with ether and dried.
0.68 g of 2',3'-dibromo-5'-dimethylamino-s'-methyl-phen-1 '-yl-2-
iminoimidazolidine 5
are obtained as a white powder (melting point from 240°C,
decomposition).
'H NMR (250 MHz, CD30D, TMS = 0 ppm): 8 = 8.19 (1 H, s, aryl-H); 3.81 (4H, s,
imidazolidine-CH2-); 3.26 (6H, s, N(CH3)2); 2.48 (3H, s, aryl-CH3);
exchangeable H
under the solvent peak 4.89 ppm. MS m/z 375/377/379 (66/100/66).
Compound 6
2'-Chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine 6
Step 1:
25 g of N,N-dimethyl-3-nitroaniline are dissolved in 225 ml of DMF. 20 g of N-
chlorosuccinimide dissolved in 190 ml of DMF are slowly added dropwise and the
solution is stirred at RT. After 24 hours the solvent is evaporated. About 500
ml of
ice are added to the red residue. The suspension is extracted three times with
about
CA 02425563 2003-04-11
Case 1/1153 ff Boehringer Ingelheim Pharma KG
19
200 ml of diethylether. The ether phases are dried over Na2S04, filtered and
evaporated down.
30.4 g of N, N-dimethyl-2-chloro-3-nitroaniline are obtained as a red oil. The
crude
product is used further without any additional purification.
Step 2:
30.4 g of N,N-dimethyl-2-chloro-3-nitroaniline are dissolved in about 300 ml
of MeOH
and hydrogenated at 20°C and 5 bars of hydrogen using Raney nickel as
catalyst.
The solvent is evaporated down and the product is purified by chromatography
(silica
gel, eluant: petroleum ether/ethyl acetate (3/1 )).
12.8 g of 2-chloro-3-dimethylamino-aniline are obtained as a brown oil.
Step 3:
4.4 g of 2-chloro-3-dimethylamino-aniline, 3.35 g of N-acetylimidazolidine and
30.5 ml
of POC13 are mixed together and stirred at RT. After 12 hours the POC13 is
distilled off
and the residue is added to ice. The aqueous phase is made alkaline with a
concentrated NH3 solution and extracted twice with 200 ml of methylene
chloride.
The organic phases are dried, filtered and evaporated down. 6.2 g of 2'-chloro-
3'-
dimethylamino-phen-1'-yl-1-acetyl-2-iminoimidazolidine are obtained as a
yellow oil.
The crude product is refluxed in methanol. After 2 hours the solvent is
evaporated off.
The residue is dissolved in diethylether in an ultrasound bath. The yellow
crystals
precipitated are suction filtered, washed and dried.
2.2 g of 2'-chloro-3'-dimethylamino-phen-1'-yl-1-acetyl-2-iminoimidazolidine 6
are
obtained as a white powder, melting point 146-148°C.
'H NMR (250 MHz, CD30D, TMS = 0 ppm): ~ = 7.11 (1 H, t, J = 7.9 Hz, aryl-
H(5));
6.83; 6.74 (2H, 2dd, J = 7.9; 1.5 Hz, aryl-H(4/6)); 3.45 (4H, s, imidazolidine-
CH2-);
3.73 (6H, s, N(CHs)2). MS m/z 239/241 (100/46).
CA 02425563 2003-04-11
Case 1/1153 ff ' Boehringer Ingelheim Pharma KG
Compound 7
4'-Bromo-2'-chloro-3'-dimethylamino-phen-1 '-yl-2-iminoimidazolidine 7
1 g of 2'-chloro-3'-dimethyfamino-phen-1'-yl-1-acetyl-2-iminoimidazofidine is
dissolved in 10 ml of DMF and the mixture is cooled to 0°C. A solution
of 0.75 g of N-
bromosuccimide in 4.5 ml of DMF is slowly added dropwise and the resulting
mixture
is stirred at 0°C. After 5 hours 60 ml of water are added. The aqueous
phase is
extracted three times with 50 ml of ethyl ether. The organic phases are dried,
filtered
and evaporated down. The product is purified by chromatography (silica gel,
eluant:
CH2C12/MeOH/ k. NH40H (90/10/1 )).
0.14 g of 4'-bromo-2'-chloro-3'-dimethylamino-phen-1'-yl-2-iminoimidazolidine
7 are
obtained as a yellow powder, melting point 150-155°C.
' H NMR (400 MHz, DMSO-d6, TMS = 0 ppm): s = 7.23; 6.54 (2H, 2d, J = 8.6 Hz, 2
aryl-H); 5.77 (2H, s, broad, NH); 3.28 (4H, s, imidazolidine-CH2-); 2.58 (6H,
s,
N(CH3)2). MS m/z 317/319/321 (85/100/36).
Compound 8
2'-Bromo-6'-chloro-5'-dimethylamino-phen-1 '-yl-2-iminoimidazolidine 8
Steps 1 and 2 are carried out as described for compound 6.
Step 3:
12.8 g of 2-chloro-3-dimethylamino-aniline are dissolved in 180 ml of DMF and
stirred
at 0°C. A solution of 13.4 g of N-bromosuccinimide in 80 ml of DMF is
slowly added
dropwise, so that the temperature does not rise above 2°C. The mixture
is stirred for
5 hours at 0°C and then at RT. After 48 hours 700 ml of ice-water are
added. The
white precipitate is filtered off and the aqueous phase is extracted three
times with
200 ml of diethyl ether. The organic phases are dried, filtered and evaporated
down.
17.5 g of 2-bromo-6-chloro-5-dimethylamino-aniline are obtained as a brown oil
and
used further without any additional purification.
CA 02425563 2003-04-11
Case 111153 ff ' ' CA 02425563 2003-04-llBOehrlnger Ingelheim Pharma KG
21
Step 4:
6.6 g of KSCN are dissolved in 220 ml of acetone at 10°C. 8 ml of
benzoyl chloride
are added. The solution is refluxed for 10 minutes and then cooled to
10°C. A
solution of 17.5 g of 2-bromo-6-chloro-5-dimethylamino-aniline in 150 ml of
acetone
is slowly added dropwise. The mixture is refluxed. After 3 hours, 500 ml of
ice water
are added and the aqueous phase is extracted three times with 100 ml of ethyl
acetate. The organic phases are dried, filtered and evaporated down. The
residue is
dissolved in 150 ml of ethanol and refluxed with 39 ml of aqueous KOH (50%).
After
2 hours the solvent is distilled off.
15 g of N-thioamido-2-bromo-6-chloro-5-dimethylamino-aniline are obtained and
used further without any additional purification.
Step 5:
15 g of N-thioamido-2-bromo-6-chloro-5-dimethylamino-aniline and 4.6 ml of
methyl
iodide are stirred into 280 ml of methanol under reflux. After 2 hours the
solvent is
evaporated off. 18.9 g of an orange-coloured oil are obtained which is
refluxed with
3.1 ml of ethylenediamine in 190 ml of acetonitrile. After 18 hours the
solvent is
distilled off. The residue is combined with 110 ml of HCI (1 M) and extracted
with
ethyl ether. The organic phase is dried, filtered and evaporated down. The
product is
purified by chromatography (silica gel, eluant: CH2C12/MeOH/ k. NH40H (90/10/1
)).
2.5 g of 2'-bromo-6'-chloro-5'-dimethylamino-phen-1 '-yl-2-iminoimidazolidine
8
are obtained as a pink powder, melting point 142-145°C.
H NMR (250 MHz, CDC13, TMS = 0 ppm): 8 = 7.30 (1 H, d, J = 9.2 Hz, aryl-H(5));
6.74 (1 H, d, J = 9.2 Hz, aryl-H(4)); 3.98 (2H, s, broad, NH); 3.53 (4H, s,
imidazolidine-CH2-); 2.87 (6H, s, N(CH3)2). MS m/z 318/3191321 (87/100/34).
Other compounds of the type described within the scope of this invention may
be
prepared analogously to the Examples provided above.