Note: Descriptions are shown in the official language in which they were submitted.
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
EVACUATED SORBENT ASSEMBLY
AND COOLING DEVICE
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to the mechanical arts. In particular, the
present
invention relates to a sorbent assembly fox use with sorbent-driven cooling
devices.
2. Discussion of the Related Art
There have been many attempts to manufacture an inexpensive, lightweight,
compact cooling device that employs an adsorbent to adsorb a liquid
refrigerant such as
water. In such a cooling device, there are typically two chambers, one housing
the
adsorbent and the other housing the liquid refrigerant, in thermal contact
with the medium
to be cooled. To achieve an effective cooling action, both the adsorbent
chamber and the
liquid refrigerant chamber must be evacuated. The adsorbent chamber, in
particular, must
have a substantial vacuum condition (evacuated to less than 8x10 mm Hg). When
communication is opened between the two chambers, some of the liquid
refrigerant is
caused to vaporize and flow into the adsorbent chamber, where the vapor is
adsorbed by
the adsorbent. The latent heat of vaporization causes heat to be removed from
the media
adjacent the liquid. The adsorption of the vapor causes additional liquid to
be vaporized,
thus further continuing the cooling process.
One particular application for which adsorbent-driven cooling devices have
been
considered is for the rapid chilling of a beverage. One such device is
described in U.S.
Pat. No. 4,928,495. This patent describes a self contained cooling device in
which a
cooling effect is produced by causing a liquid refrigerant to evaporate in a
chamber within
a beverage container and in the process absorb heat from its surroundings. The
resulting
refrigerant vapor is then adsorbed by an adsorbent housed in a chamber located
outside of
the beverage container. While this device may act to cool a beverage placed
within the
container, the difficulties and costs associated with manufacturing a beverage
container
with an external adsorbent chamber are a significant impediment to mass
production of
such containers. In addition, with this arrangement, the path in which the
vaporized liquid
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
must travel before it is adsorbed by the adsorbent is long, which prevents the
cooling
device, from adequately cooling the beverage within a commercially acceptable
amount of
time.
Another beverage cooling device is described in U.S. Patent No. 6,151,911.
This
patent describes a mechanism for cooling a contained beverage by use of an
absorption or
adsorption substrate in thermal contact with a phase change medium. It is a
drawback of
this cooling device in that it requires a cylindrical chamber with a lengthy
vapor pathway
to avoid liquid contact from the phase change medium with the adsorption or
adsorption
substrate.
Accordingly, it should be recognized that there remains a need for an
adsorbent
assembly and cooling device that is easy and inexpensive to manufacture, is
compact and
lightweight, and has a short vapor path while providing effective cooling
characteristics.
The present invention satisfies these and other needs.
SUMMARY OF THE INVENTION
The invention resides in an evacuated sorbent assembly and cooling device that
provide advantages over known adsorbent-driven cooling devices in that the
invention is
easy and inexpensive to manufacture. Also, the invention is compact and
lightweight, and
has a short vapor path. Additionally, the invention provides effective cooling
characteristics.
The present invention is embodied in an evacuated sorbent assembly for
coupling
to a liquid refrigerant reservoir and a cooling device comprised of at least
one sorbent
section, at least one liquid passageway section, and an actuator. The sorbent
section
contains a sorbent for a liquid refrigerant. The liquid passageway section is
adjacent the
sorbent section and defines a liquid passageway through a portion of the
evacuated sorbent
assembly or cooling device to the sorbent section. The liquid passageway
contains
wicking material of an amount sufficient to prevent the liquid refrigerant
from contacting
the sorbent. The actuator controls liquid communication between the liquid
passageway
section and the liquid refrigerant reservoir. In another embodiment, the
evacuated sorbent
assembly includes a vapor-permeable membrane that separates adjacent sorbent
and liquid
passageway sections whether or not the liquid passageway section contains
wicking
material.
2
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
Embodiments of the cooling device additionally include a liquid refrigerant
reservoir, adjacent the liquid passageway section, and a casing that surrounds
the sorbent
section, the liquid passageway section, the vapor-permeable membrane, the
liquid
refrigerant reservoir, and the actuator.
In addition to including a wicking material, other embodiments of the present
invention include: a heat-removing material, which may be a phase-changing
material, in
thermal contact with the sorbent; at least one liquid barrier between the heat-
removing
material and the sorbent; and at least one thermal spacer positioned between
the sorbent
section and the liquid passageway section. In some embodiments, the thermal
spacer is
interposed between the sorbent section and the vapor-permeable membrane. In
other
embodiments, the thermal spacer is interposed between the vapor-permeable
membrane
and the liquid passageway section. In another embodiment (FIG. 10) there is no
wicking
material, nor vapor permeable membrane, but rather an anisotropic insulation
material
hydrophobic on one side and hydrophilic on the other replaces the functions of
those
components. Furthermore, some embodiments include casings made from a flexible
material such as a metallicized plastic.
A feature of the present invention is that it is compact and lightweight. The
invention is designed to fit within a host container, i.e., a beverage
container. An
additional feature of the invention, related to its compact size, is the short
vapor path
between the liquid refrigerant reservoir and the sorbent. The vapor path is at
most several
millimeters.
Other features and advantages of the present invention will be set forth, in
part, in
the description which follows and the accompanying drawings, wherein the
preferred
embodiments of the present invention are described and shown, and in part will
become
apparent to those skilled in the art upon examination of the following
detailed description
taken in conjunction with the accompanying drawings, or may be learned by
practice of
the present invention. The advantages of the present invention may be realized
and
attained by means of the instrumentalities and combinations particularly
pointed out in the
appended claims.
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
DESCRIPTION OF THE DRAWINGS
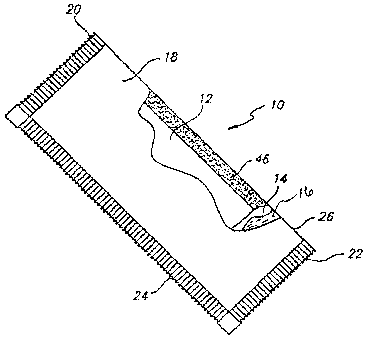
FIG. 1 is a top plan view, partially cut away, of a cooling device in
accordance
with the invention.
FIG. 2 is a sectional view of the cooling device of FIG. 1 showing details of
a
sorbent chamber and a liquid refrigerant reservoir.
FIG. 2B is a sectional view of a first alternative embodiment of a cooling
device in
accordance with the invention.
FIG. 3 is a plan view of a beverage container with the beverage and the
cooling
device of FIG. 1 shown in phantom.
FIG. 4 is a perspective view, partially cut away, of a second alternative
embodiment of a cooling device in accordance with the invention.
FIG. 5 is a sectional view of the cooling device of FIG. 4.
FIG. 6 is a sectional view of a third alternative embodiment of a cooling
device in
accordance with the invention.
FIG. 7 is a perspective view, partially cut away, of a fourth alternative
embodiment
of a cooling device in accordance with the invention.
FIG. 8 is a sectional view of a fifth alternative embodiment of a cooling
device in
accordance with the invention.
FIG. 9 is a sectional view of a sixth alternative embodiment of a cooling
device in
. accordance with the invention.
FIG. 10 is a perspective view of a beverage container pouch containing a
single
cooling device in accordance with the invention.
FIG. 11 is a cross sectional view of the front surface and the cooling device
of the
pouch shown in FIG. 10.
FIG. 12 is a perspective view of an alternative beverage container pouch
containing two cooling devices in accordance with the invention.
4
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
FIG. 13 is a cross sectional view of the front surface and the cooling device
of the
pouch shown in FIG. 12.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Detailed embodiments of the present invention are disclosed herein; however,
it is
to be understood that the disclosed embodiments are merely exemplary of the
invention,
which may be embodied in various forms. Therefore, specific structural and
functional
details disclosed herein are not to be interpreted as limiting, but merely as
a basis for
claims and as a representative basis for teaching one skilled in the art to
variously employ
the present invention in virtually any appropriately detailed structure.
Certain terminology will be used in the following specification for
convenience in
reference only and will not be limiting. For example, the word "absorption"
refers to the
occurrence of a substance (e.g., water vapor) penetrating the inner structure
of another (the
absorbent). Also, the word "adsorption" refers to the occurrence of a
substance (e.g.,
water vapor) being attracted and held onto the surface. of another (the
adsorbent). The
words "absorption" and "adsorption" include derivatives thereof. The word
"sorbent"
refers to a material that is either an absorbent and/or an adsorbent.
The evacuated sorbent assembly and cooling device is shown in the exemplary
drawings. With particular reference to FIGS. l, 2A and B, there is shown a
cooling device
10 housing an evacuated sorbent assembly 12 adjacent a liquid refrigerant
reservoir 14,
which contains a liquid refrigerant 16. The cooling device includes an
evacuable casing
18, with opposing ends 20 and 22, and opposing sides 24 and 26. The casing is
substantially impervious to air and moisture so as to provide the cooling
device with a
suitable shelf life (to allow for several years of storage/inactivation prior
to use). Useful
casing materials have an oxygen transmission rate (OTR) preferably less than
about 1 cm3
/m2/day, more preferably less than 0.1 cm3/m2/day, and most preferably less
than 0.01
cm3/m~/day. The vapor transmission rate of useful casing materials is
preferably less than
about 2 g/ma/day, more preferably less than 1 g/m2/day, and the most
preferably less than
about 0.1 g/m2/day.
The casing 18 is made from a flexible material such as a metallicized plastic
laminate or a metal foil plastic laminate. Suitable casing materials include
flexible films
such as those produced by the Rexam Corporation located in Bedford Park,
Illinois, and
5
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
Toyo Aluminum located in Osaka, Japan. The flexibility of the cooling device
allows it to
be deformed without losing its performance characteristics. For example, the
cooling
device may be curled and then placed within a beverage container without any
degradation
in its cooling abilities.
A sectional view of the cooling device 10 is shown in FIGS. 2A and B. Included
in the evacuated sorbent assembly 12 are a pair of sorbent sections 28 and 30
in which a
sorbent 32 is disposed. The sorbent preferably includes an absorbent material
dispersed
on, impregnated in, affixed to, or otherwise combined with the porous support
material.
The porous support material preferably has a high pore volume, and therefore a
high
surface area, to accommodate the absorption of large amounts of liquid
refrigerant 16, in
vapor form, by the sorbent. The pore volume is expressed in units of volume
per unit
mass. The porous support material has a pore volume of at least about 0.8
cc/g, more
preferably at least about 1 cclg, and even more preferably at least about 1.5
cclg.
In order to accommodate high absorption levels of liquid refrigerant 16, it is
also
important to control the average pore diameter and pore size distribution of
the porous
support material. The average pore diameter is preferably at least about 1
nanometer, and
typically in the range from about 1 to about 20 nanometers. The average pore
diameter
distribution is such that there are very few pores having a diameter of less
than about 0.5
nanometers. The porous support material can be selected from virtually any
material
having the above-identified properties. Preferred materials for the porous
support material
include activated carbon and silica.
The porous support material can come in a variety of shapes and sizes selected
for
a particular application. For example, in some embodiments, the porous support
material
is comprised of small activated carbon pellets having a size in the range of
from about 0.5
to 2 millimeters. In alternative embodiments, the porous support material is
silica pellets
having a size from about 0.25 to 0.5 millimeters. The size of the pellets can
be selected to
influence the rate at which the vapor from the liquid refrigerant 16 is
absorbed. Larger
pellets absorb liquid refrigerant vapor at a slower rate due to increased path
length.
It is preferred that the absorbent material have a pore volume that is at
least about
50 percent of the pore volume of the porous support material, and even more
preferably at
least about 66 percent of the pore volume of the porous support material. That
is, it is
6
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
preferred that if the pore volume of the porous support material is about 1.5
cc/g, then the
pore volume of the absorbent material is preferably no less than about 0.75
cc/g, more
preferably no less than about 1.0 cc/g.
When the liquid refrigerant 16 is water, the absorbent material is preferably
capable of absorbing at least about 100 percent of its weight in water, more
preferably at
least about 150 percent of its weight in water, and even more preferably at
least about 200
percent of its weight in water. The amount of water that can be absorbed will
also be
influenced by the relative humidity and temperature.
Any suitable absorbent material can be used. Representative absorbent
materials
include salts such as calcium chloride, lithium chloride, lithium bromide,
magnesium
chloride, calcium nitrate, and potassium fluoride. Other suitable absorbent
materials
include phosphorous pentoxide, magnesium perchlorate, barium oxide, calcium
oxide,
calcium sulfate, aluminum oxide, calcium bromide, barium perchlorate, and
copper
sulfate, zeolite 13x, zeolite Sa, silicalite, silica gel, alumina, carbon,
modified carbons and
the like. Furthermore, the absorbent material may also contain combinations of
two or
more of these materials.
Adjacent to each sorbent section 28 and 30 are liquid passageway sections 34
and
36, respectively, defining liquid passageways 38 and 40, respectively, through
at least a
portion of the evacuated adsorbent assembly 12. A pair of actuators 42 and 44
control the
flow of liquid refrigerant 16 from the liquid refrigerant reservoir 14 into
the liquid
passageway sections. In some embodiments, the actuators are mechanically
activated. In
other embodiments the actuators are pressure activated such that a change in
pressure
causes the actuators to open and permit communication between the liquid
refrigerant
reservoir and the liquid passageway sections.
In the embodiment shown in FIG. 2A, a wicking material 46 is placed within the
liquid passageway sections 34 and 36. The wicking material draws liquid
refrigerant 16
from the liquid refrigerant reservoir 14 and retains the liquid refrigerant
for subsequent
vaporization and adsorption by the sorbent 32. In addition, the wicking
material absorbs
any vaporized liquid refrigerant in the liquid passageway sections that re-
condenses before
reaching the sorbent. When the liquid refrigerant is water, suitable wicking
materials
7
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
include hydrophilic materials such as microporous metals, porous plastics
(polyethylene,
polypropylene), cellulose products, sintered heat pipe material, or glass
paper, and the like.
No more wicking material 46 is required than is necessary to draw all of the
liquid
refrigerant 16 to be adsorbed in the evacuated sorbent assembly 12. The
wicking material
has a pore size sufficient to permit capillary action (the drawing of all the
liquid refrigerant
from the liquid refrigerant reservoir 14) to occur within 60 seconds, and most
preferably,
within 10 seconds after actuation.
In the embodiments shown in Figs. 4 and 5, the wicking material 46 provides a
direct interface between the liquid refrigerant 16 and the sorbent 32. In
these
embodiments, the wicking material maintains and holds the liquid refrigerant
until it is
vaporized and later adsorbed by the sorbent. Sufficient wicking material is
used so that
non-vaporized liquid refrigerant does not directly contact the sorbent.
Also seen in the embodiment shown in FIG. 2A is a vapor-permeable membrane
48 separating sorbent sections 28 and 30 and adjacent liquid passageway
sections 34 and
36. The vapor-permeable membrane is semi-permeable such that only vaporized
liquid
refrigerant 16 may pass through it to be adsorbed by the sorbent 32. In some
embodiments, the vapor-permeable membrane is a substantially flat film that is
heat-
sealed or sealed by an adhesive so as to encase the sorbent and to prevent
liquid from
contacting the sorbent within the vapor-permeable membrane. Useful vapor-
permeable
membranes include semi-permeable films such as films available under the
trademark
TYVEK~ produced by the E.I. DuPont de Nemours; Wilmington, Delaware, and films
available under the trademark GORETEX~ produced by the R.L. Gore Company,
Newark, Delaware. In other embodiments of the present invention, the vapor-
permeable
membrane is not substantially flat, but is corrugated or otherwise shaped so
as to increase
surface area and thereby the rate at which vaporized liquid refrigerant passes
through the
membrane.
Alternatively, the vapor-permeable membrane 48 may be a hydrophobic coating
applied to one or both of the surfaces of the sorbent sections 28 and 30 and
the liquid
passageway sections 34 and 36 which are facing one another. Suitable
hydrophobic
coatings include those available under the trademark SCOTCHGARD~ produced by
3M,
St. Paul, Minnesota.
8
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
As there can be large temperature differences between the wicking material 46
and
the sorbent sections 28 and 30, in some embodiments thermal spacers 56 and 58
are
interposed between the sorbent sections and the vapor-permeable membranes 48
or
between the sorbent sections and the wicking material. The thermal spacers are
utilized to
insulate heat generated by the sorbent 32. Since the temperature between the
wicking
material and sorbent sections can vary from 5°C to 150°C, the
thermal spacers have a
thermal resistance (thermal conductivity at package conditions divided by
thickness)
preferably less than 100 W/maK, more preferably less than 50 W/m2K, and most
preferably less than 20 W/m2K. The materials utilized for the thermal spacers
can be
selected from a range of materials known to the art that provide su~cient
vapor
permeability such as fiberglass, plastic fibers, and plastic foams.
As shown in the alternative embodiment illustrated in FIG. 2B, an insulating
material 71 is placed between the sorbent sections 28 and 30 and adjacent
liquid
passageway sections 34 and 36 replacing the wicking material 46, thermal
spacers 56 and
58, and the vapor-permeable membrane 48 shown in the embodiment illustrated in
FIG.
2A. The insulating material 71 is chosen to inhibit thermal leakback from the
sorbent
sections 28 and 30 to the exterior of the device. Typically, the insulating
material has
thermal conductivity limits less than 0.05 W/mK, preferably less than about
0.035 W/mK,
and most preferably, less than about 0.025 W/mK. Preferably, the insulating
material 71
has a collapse strength sufficient to resist about one bar uniaxial load, and
limit the
shrinkage, due to evacuation, to less than about 20%, more preferably less
than about 5%,
and most preferably less than about 2%.
In some embodiments, an anisotropic insulating material containing both a
hydrophilic region 72 and a hydrophobic region 73 is preferred. Such an
insulating
material inhibits the passage of liquid refrigerant 16 into the sorbent
sections 28 and 30,
yet allows the vapor of the liquid refrigerant to pass into the sorbent
sections 28 and 30.
The hydrophilic region 72 of the insulating material has pores with a
relatively
large diameter, not less than 10 mm in diameter, on average. The large pores
of the
hydrophilic region 72 encourage the rapid flow of liquid refrigerant 16 into
the material.
The hydrophobic region 73 has pores of a relatively small diameter, typically
less than
about 2 mm in diameter, so that the un-vaporized liquid refrigerant 16 is
inhibited from
9
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
passing into the sorbent section 28 and 30, but rather only the vapor from the
liquid
refrigerant 16 is directed into the sorbent section 28 and 30.
The ratio of the thickness of the hydrophobic region 73 to hydrophilic region
72 is
a function of the choice of materials used to form those regions, the quantity
of liquid
refrigerant 16 in the device, and the desired performance criteria of the
device.
The insulating material 73 can be formed by laminating a hydrophilic material
such as cellulose, paper, non-woven or woven cloth formed from fibers of
glass, plastic,
ceramic or cellulose, to a hydrophobic material. .The hydrophobic material can
be made
by modifying a hydrophilic material with a hydrophobic agent, such as by
impregnating a
hydrophilic material with wax or adding a hexamethyldisiliazane or a
flourinated reactive
group to the hydrophilic material.
Alternatively, the insulating material can be formed by surface modification,
whereby a sheet of material (either hydrophilic or hydrophobic) is modified to
change the
surface on one side. In general, the surface of one side of a hydrophobic
material can be
made hydrophilic by exposure to thermal or plasma treatments or by
impregnation with
surfactants. The surface of a hydrophilic material can be made hydrophobic by
treatment
with hydrophobing agents or impregnation of wax-like material.
The evacuated sorbent assembly 12 can also contain a heat-removing material 50
in thermal contact with the sorbent sections 28 and 30. The heat-removing
material is
placed adjacent to the surface of the sorbent sections) opposite the vapor-
permeable
membrane 48. The heat-removing material is one of three types: (1) a material
that
undergoes a change of phase when heat is applied (phase-change material); (2)
a material
that has a heat capacity greater than the sorbent 32; or (3) a material that
undergoes an
endothermic reaction when brought in contact with a vaporized liquid
refrigerant 16. It
will be understood by the skilled artisan that the heat-removing material, for
use in a
particular application may vary depending on the sorbent utilized, the thermal
insulation,
if any, between the phase-change material, the liquid refrigerant, and the
desired cooling
rate.
Suitable heat-removing materials 50 include paraffin, naphthalene sulphur,
hydrated calcium chloride, bromocamphor, cetyl alcohol, cyanamide, eleudic
acid, lauric
acid, hydrated calcium silicate, sodium thiosulfate pentahydrate, disodium
phosphate,
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
hydrated sodium carbonate, hydrated calcium nitrate, neopentyl glycol,
hydrated inorganic
salts including Glauber's salt, inorganic salts encapsulated in paraffin,
hydrated potassium
and sodium sulfate, and hydrated sodium and magnesium acetate. The preferred
heat-
removing material is an inorganic salt that has been melted and re-solidified
to form a
monolith (thereby reducing the volume of the heat-removing material by
approximately
30%).
The heat-removing material 50 removes some of the heat from the sorbent
sections
28 and 30 simply through the storage of sensible heat, because the heat-
removing material
heats up as the sorbent sections heat up, thereby removing heat from the
sorbent sections.
However, the most effective heat-removing material typically undergoes a
change of
phase. A large quantity of heat is absorbed in connection with a phase change
(i.e.,
change from a solid phase to a liquid phase, change from a solid phase to part
solid phase
and part liquid phase, or change from a liquid phase to a vapor phase). During
the phase
change, there is typically little change in the temperature of the heat-
removing material,
despite the relatively substantial amount of heat absorbed to effect the
change.
Another requirement of phase-change heat-removing material 50 is that it
change
phase at a temperature greater than the expected ambient temperature of the
material to be
cooled, but less than the temperature achieved by the sorbent sections 28 and
30 upon
absorption of a substantial fraction (i.e., one-third or one-quarter) of the
liquid refrigerant
16. For example, when the current invention is employed in a cooling device 10
for '
insertion into a typical beverage container, the phase change should take
place at a
temperature above about 30 °C, preferably above about 35 °C but
preferably below about
70 °C, and most preferably below about 60 °C.
When absorbing heat, a phase-change heat-removing material 50 may generate by-
products such as water, aqueous salt solutions, and organics. Therefore,
depending on the
particular heat-removing material utilized, in some embodiments it is
desirable to include
liquid barriers 52 and 54, such as polyethlene or polypropylene film,
interposed between
the sorbent sections 28 and 30, respectively, and the heat-removing material
to prevent any
by-products from contacting the sorbent 32 (and thereby decreasing its
effectiveness). The
liquid barriers are heat sealed or adhesively sealed to the heat-removing
material.
11
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
The liquid refrigerant reservoir 14 is positioned immediately adjacent one end
22
of the casing 18. This arrangement provides an advantage over prior art
sorbent chambers
that typically employ devices with long vapor paths which decrease the
effectiveness of
the vaporization of the liquid refrigerant 16. In addition, the short vapor
paths allow the
evacuated sorbent assembly 12 to operate at a much higher pressure level than
previous
sorbent assemblies.
In some embodiments, the liquid refrigerant reservoir 14 is a plastic 60,
typically
made of polyethylene, that is filled and heat sealed along its edges 62
enclosing the liquid
refrigerant 16. Weakened portions 64 and 66 of the plastic bag serve as
pressure sensitive
actuators 42 and 44.
The liquid refrigerant 16 stored in the liquid refrigerant reservoir 14 has a
high
vapor pressure at ambient temperature so that a reduction of pressure will
produce a high
vapor production rate. In addition, the liquid refrigerant has a high heat of
vaporization.
The vapor pressure of the liquid refrigerant at 20°C is typically at
least about 9 mm Hg,
preferably at least about 15 or 20 mm Hg. Suitable liquid refrigerants include
various
alcohols, such as methyl alcohol or ethyl alcohol; ketones or aldehydes such
as acetone
and acetaldehyde; and hydrofluorocarbons such as C318, 114, 21, 11, 114B2,
113, 112,
134A, 141B, and 245FA. The preferred liquid refrigerant is water because it is
plentiful
and does not pose any environmental problems while providing the desired
cooling
characteristics.
In some embodiments, the liquid refrigerant 16 is mixed with an effective
quantity
of a miscible nucleating agent (or a partial miscible nucleating agent) having
a greater
vapor pressure than the liquid refrigerant to promote ebullition so that the
liquid
refrigerant evaporates even more quickly and smoothly, while preventing the
liquid
refrigerant from super-cooling and thereby decreasing the adsorption rate in
the sorbent
32. Suitable nucleating agents include ethyl alcohol, acetone, methyl alcohol,
isopropyl
alcohol and isobutyl alcohol, all of which are miscible with water. For
example, a
combination of a nucleating agent with a compatible liquid might be a
combination of 5%
ethyl alcohol in water or 5% acetone in methyl alcohol. The nucleating agent
preferably
has a vapor pressure at 25°C of at least about 25 mm Hg, and, more
preferably, at least
about 35 mm Hg. Alternatively, a solid nucleating agent may be used, such as a
conventional boiling stone used in chemical laboratory applications.
12
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
During manufacturing, the sorbent sections 28 and 30 are inserted into the
casing
18 along with the liquid refrigerant reservoir 14 prior to heat sealing the
casing.
Depending upon the embodiment, wicking material 46 is placed adjacent the
sorbent
sections and encased with a vapor-permeable membrane 48. Furthermore, in some
embodiments, the vapor-permeable membrane also encases a layer of heat-
removing
material 50 in thermal contact with the sorbent 32, liquid barriers 52 and 54
interposed
between the heat-removing material and the sorbent sections, respectively, and
thermal
spacers 56 and 58 interposed between the sorbent sections and the liquid
passageway
sections 34 and 36, respectively. Specifically, the thermal spacers may be
interposed
between the sorbent sections and the vapor-permeable membrane or between the
vapor-
permeable membrane and the liquid passageway sections. In other embodiments,
insulating material 71 is placed between the sorbent sections and liquid
passageway
sections 34 and 36. Next, the opposing ends 20 and 22 and at least one of the
opposing
sides 24 and 26 are heat sealed after evacuation to greater than 1 mm Hg. In
alternative
embodiments, the casing is sealed with an adhesive.
The method of use and operation of the evacuated sorbent assembly 12 and
cooling
device 10, constructed as described above, proceeds as follows. Initially, the
actuators 42
and 44 are actuated causing the liquid refrigerant 16 to flow into the liquid
passageways
38 and 40. In the embodiments of the invention where the liquid refrigerant
reservoir 14 is
a plastic bag 60 with weakened portions 64 and 66, external pressure is
applied to the
casing 18 and liquid refrigerant reservoir. The external pressure ruptures the
weakened
portions 64 and 66 and releases the liquid refrigerant into the liquid
passageways.
Liquid refrigerant 16, except for a small amount that is instantly vaporized,
is
introduced into the evacuated adsorbent assembly 12 from the liquid
refrigerant reservoir
14 via the liquid passageways 34 and 36. Depending upon the embodiment of the
invention, the liquid refrigerant collects in very thin layers among the
interstices of the
wicking material 46. The vaporized liquid refrigerant then passes through the
vapor-
permeable membrane 48, and enters the sorbent sections 28 and 30 where the
vaporized
liquid refrigerant is adsorbed by the sorbent 32. Alternatively, the liquid
refrigerant
collects in the hydrophilic region of the insulation material. The vaporized
refrigerant
then passes through the hydrophilic region 73 and absorbent sections 28 and
30. As the
sorbent adsorbs vaporized liquid refrigerant, the liquid refrigerant collected
within the
13
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
wicking material begins to vaporize and pass through the vapor-permeable
membrane into
the sorbent.
Vaporization of the liquid refrigerant 16 causes a cooling effect on the
outside of
the cooling device 10 which, as shown in FIG. 3 can be used to cool a beverage
80 in a
beverage container 82. Less than 1.5 grams of liquid refrigerant water per
fluid ounce of
beverage, less than 3 grams of sorbent 32 per fluid ounce of beverage, and
less than 5
cubic centimeters of sorbent 32 per fluid ounce of beverage is required to
cool the
beverage by 22 °C in preferably less than 10 minutes, more preferably
less than 5 minutes,
and most preferably less than 3 minutes after actuation. Also, the cooling
device occupies
less than 0.5 fluid ounces per fluid ounce of beverage.
Those skilled in the art will recognize that various modifications and
variations can
be made in the evacuated sorbent assembly 12 and cooling device 10 of the
invention and
in the construction and operation of the evacuated sorbent assembly and
cooling device
without departing from the scope or spirit of this invention. For example, the
evacuated
sorbent assembly may be used as part of a cooling device which may be wrapped
around
the outer circumference of a beverage container rather than being placed
therein. In
addition, the sorbent assembly need not be symmetrical, but rather, it can be
asymmetrical
and arranged, for example, such that the layer adjacent the casing 18 is the
sorbent section
28, with the next layer being the vapor-permeable membrane 48, and with the
final layer
being the wicking material 46.
Also, the sorbent assembly and cooling device can be arranged in a spherical
configuration as shown in FIGS. 4, 5, and 6. In the embodiment shown in FIG.
6, the
liquid refrigerant reservoir 16 is adjacent the length of the evacuated
sorbent assembly 12.
FIG. 7 shows another embodiment of the present invention where the evacuated
sorbent
assembly has a polygonal cross-section. In other embodiments, as shown in
FIGS. 8 and
9, two or more evacuated sorbent assemblies are adjacent to a single liquid
refrigerant
reservoir.
FIG. 10 shows a conventional beverage container pouch 80 constructed of a
plastic-lined, metallicized material, which is heat-sealable. The pouch has a
top end 82, a
bottom end 83 formed by panel 84, to create a base for the beverage container
pouch,
opposing side panels (one shown) 85, and opposing front and back panels, 90
and 92,
14
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
respectively. A single coolant device 94 is formed as part of, or affixed to
the exterior
surface of the front panel. In an alternative embodiment, the coolant device
may be
formed as part of, or affixed to the interior surface of the panel.
As best seen in FIG. 11, the coolant device 94 is affixed to the exterior
surface 95
of the front panel 92 of the pouch 80 by securing the exterior 95 of the
coolant device to
the exterior surface of the front panel 90 with the wicking material 46 facing
the pouch 80.
Adjacent to the interior surface of the affixed coolant device is the liquid
passageway 36
containing the wicking material 46. The cooling device casing material is
sealed 100 to a
portion of the wicking material 46 to form a cavity 102 housing the liquid
refrigerant 16
and the liquid refrigerant reservoir 14 including actuator 64. On the other
side of the
wicking material is a thermal spacer 56, followed by the sorbent material 28,
the liquid
barrier 52, and, finally, the heat-removing material 50. In practice, the user
squeezes the
portion of casing defining the liquid refrigerant cavity to actuate the
actuator 64 and
release the liquid refrigerant 16 into the liquid passageway. A weakened
region 103 in the
pouch (FIG. 10) forms an area adapted to be punctured by a plastic straw.
FIG. 12 shows a conventional beverage container pouch 80 constructed of a
plastic-lined, metallicized material, which is heat-sealable. The pouch has a
top end 82, a
bottom end 83, opposing side panels (one shown), 84, and opposing front and
back panels,
90 and 92, respectively. A brace of coolant devices 102 and 104 are formed as
part of, or
may be affixed to the front and back panels 90 and 92. In an alternative
embodiment, the
coolant devices are formed as part of, or may be affixed to the interior
surface of the
panels.
As shown in FIG. 13 for one of the coolant devices, the coolant device 102 is
formed as part of the pouch 80 by constructing a wall of the cooling device
from a portion
110 of the front panel 90. Adjacent to the interior surface of the wall 110 of
the wall of
the coolant device is a capillary membrane 112 that provides a liquid
passageway from the
liquid refrigerant reservoir 14 throughout the length of the sorbent assembly.
The cooling
device casing material is sealed 114 to a portion of the capillary membrane
112 material to
form a cavity 116 housing the liquid refrigerant reservoir 14 including
actuator 64. On the
other side of the capillary membrane, is an insulating material 71, having
hydrophilic and
CA 02426199 2003-04-17
WO 02/40929 PCT/USO1/51295
hydrophobic surfaces 72 and 73, respectively, followed by the sorbent material
28, the
liquid barrier 52, and finally, the heat removing material 50.
Embodiments of the cooling device decrease the temperature of a beverage in a
beverage container by at least about 12 °C and in some embodiments at
least 15 °C or
even 20 °C after actuation. In these embodiments, the liquid
refrigerant reservoir contains
less than 1.5 grams of liquid refrigerant per fluid ounce of beverage in the
container. In
some embodiments, the refrigerant liquid is water. Also, in some embodiments,
the
sorbent section has a mass of less than 3 grams of sorbent per fluid ounce of
beverage.
Depending upon the embodiment, the cooling device may decrease the beverage
temperature in 10 minutes, or only 5 minutes, or even only 3 minutes. In some
embodiments, the sorbent section occupies less than 5 cubic centimeters per
fluid ounce of
beverage, and the cooling device occupies less than 0.5 fluid ounces per fluid
ounce of a
beverage in a beverage container.
With such possibilities in mind, the invention is defined with reference to
the
following claims.
16