Note: Descriptions are shown in the official language in which they were submitted.
CA 02427851 2009-11-13
DESCRIPTION
POWER SUPPLY SYSTEM HAVING FUEL CELL UNITS WITH DIFFERENT
PROTON CONDUCTORS AND OPERATION METHOD THEREOF
TECHNICAL FIELD
The present invention relates to a new power supply
system including a hybrid structure of a humidified type
fuel cell and a low-grade humidified type fuel cell (non-
humidified type fuel cell), and to a method of operating
the power supply system.
BACKGROUND ART
A fuel cell is a device for allowing a power
generator to generate an electromotive force by supplying
hydrogen as a fuel gas and oxygen (air) thereto. In
general, a fuel cell has a structure that an electrolyte
film (proton conductive film) is held between gas
electrodes, wherein the fuel cell is operated to obtain a
desired electromotive force. Such a fuel cell is greatly
expected to be applied to electric cars and hybrid type
vehicles. In addition to the applications mounted on
vehicles such as cars, they are now being studied to be
applied to new applications different from those of
existing dry cells and chargeable batteries by making
1
CA 02427851 2003-05-02
effective use of an advantage of the fuel cell in terms
of easy reduction in weight and size.
By the way, the above-described fuel cell is mainly
classified, from the viewpoint of the kinds of
electrolytes used therefore, into a humidified type fuel
cell group and a low-grade humidified (or non-humidified)
type fuel cell group. Each of the humidified type fuel
cell and the low-grade humidified type fuel cell has a
disadvantage as well as an advantage. For example, the
humidified type fuel cell cannot obtain a good
startability unless moisture is contained in outside air
to some extent at the time of initial start-up because a
proton conductive function of an electrolyte film of the
fuel cell becomes effective only when moisture in outside
air and/or moisture generated by power generating
reaction are incorporated in the electrolyte film.
Meanwhile, the low-grade humidified type fuel cell (non-
humidified type fuel cell) can be started even in an
extremely low humidity atmosphere, for example, at a sub-
freezing temperature because the fuel cell uses an
electrolyte film having a proton conductive film
essentially requiring little moisture. The low-grade
humidified type fuel cell, however, is difficult to be
singly used for applications requiring a large output
2
CA 02427851 2003-05-02
because the electrolyte film of the fuel cell tends to be
poorer in electric conductivity than the electrolyte film,
having a conductive passage mainly composed of moisture,
of the humidified type fuel cell.
In view of the foregoing, the present invention has
been made, and an object of the present invention is to
provide a power supply system, which is capable of
obtaining a high output even in an extremely low
temperature and low humidity atmosphere, and which is
thereby suitable for portable equipment required to be
operable in a wide temperature range from a sub-freezing
temperature to a high temperature, and to provide a
method of operating the power supply system.
DISCLOSURE OF INVENTION
To achieve the above object, according to the
present invention, there is provided a power supply
system including a humidified type fuel cell unit using
as an electrolyte a humidified type proton conductor
having a proton conductive function in a humidified state,
and a low-grade humidified type fuel cell unit using as
an electrolyte a low-grade humidified proton conductor
developing a proton conductive function in a humidified
state lower than the above-mentioned humidified state.
3
CA 02427851 2003-05-02
Y T According to the present invention, there is also
provided a method of operating a power supply system
including a humidified type fuel cell unit using as an
electrolyte a humidified type proton conductor having a
proton conductive function in a humidified state, and a
low-grade humidified type fuel cell unit using as an
electrolyte a low-grade humidified proton conductor
developing a proton conductive function in a humidified
state lower than the above-mentioned humidified state.
The method includes the steps of operating at least the
low-grade humidified fuel cell unit at the time of the
initial start-up, and operating only the humidified type
fuel cell unit after the initial start-up.
According to the present invention, the low-grade
humidified type fuel cell unit is operated at the time of
start-up, to compensate for a low output characteristic
of the humidified type fuel cell unit at the time of
start-up. The low-grade humidified type fuel cell unit,
particularly, the non-humidified type fuel cell unit uses
an electrolyte film having a proton conductive mechanism
essentially requiring little moisture, and as a
consequence, it can be started even in an extremely low
humidity atmosphere, for example, at a sub-freezing
temperature. At the same time, moisture generated in the
4
CA 02427851 2003-05-02
low-grade humidified type fuel cell is supplied to the
humidified type fuel cell unit, to promote the self-
humidification of the humidified type fuel cell. As a
result, the humidified type fuel cell unit can develop
its intrinsic high power generation ability, whereby the
power supply system can obtain a high output by the
humidified type fuel cell unit after the start-up.
BRIEF DESCRIPTION OF DRAWINGS
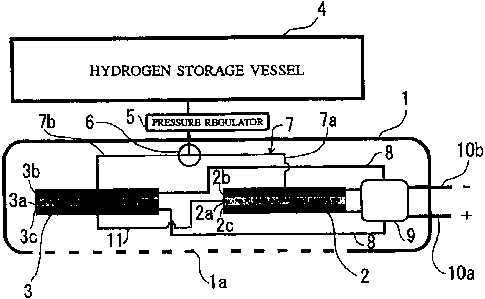
FIG. 1 is a typical view showing a configuration
example of a power supply system to which the present
invention is applied;
FIG. 2 is a typical view showing another
configuration example of the power supply system to which
the present invention is applied;
FIG. 3 is an exploded perspective view showing a
basic configuration example of a fuel cell;
FIG. 4 is a schematic sectional view showing a
configuration example of electrodes of the fuel cell;
FIG. 5 is a typical view showing various structural
examples of carbon clusters as base bodies of
electrolytes;
FIG. 6 is a typical view of further examples of
carbon clusters (partial fullerene structures);
CA 02427851 2003-05-02
FIGS. 7A and 7B are typical views showing further
examples of carbon clusters (tube-like carbonaceous
materials) ;
FIG. 8 is a typical view showing further examples
of carbon clusters (diamond structures);
FIG. 9 is a typical view showing further examples
of carbon cluster groups (in each of which clusters are
bonded to each other);
FIG. 10 is a characteristic diagram showing a low
temperature startability of a humidified type fuel cell;
FIG. 11 is a characteristic diagram showing a low
temperature startability of a non-humidified type fuel
cell; and
FIG. 12 is a characteristic diagram showing a low
temperature startability of a power supply system of the
present invention, which system includes a hybrid
structure of a humidified type fuel cell unit and a non-
humidified type fuel cell unit.
BEST MODE FOR CARRYING OUT THE INVENTION
Hereinafter, a power supply system and an operating
method thereof, to which the present invention is applied,
will be described in detail with reference to the
drawings.
6
CA 02427851 2003-05-02
A power supply system of the present invention is a
power generation module including a hybrid structure of a
humidified type fuel cell and a low-grade humidified type
(non-humidified type) fuel cell, and is configured, as
shown in FIG. 1, to have both a humidified type fuel cell
unit 2 and a low-grade humidified type fuel cell unit 3
accommodated in a housing 1.
The fuel cell unit 2 is composed of an electrolyte
2a, a fuel electrode 2b joined to one surface of the
electrolyte 2a, and an air electrode 2c joined to the
other surface of the electrolyte 2a, and similarly, the
fuel cell unit 3 is composed of an electrolyte 3a, a fuel
electrode 3b joined to one surface of the electrolyte 3a,
and an air electrode 3c joined to the other surface of
the electrolyte 3a. The housing 1 has, for example, in
its bottom surface, a number of air supply holes la to
allow air (oxygen) to be introduced in the housing 1
through the air supply holes la and then supplied to the
air electrodes 2c and 3c.
On the other hand, hydrogen is supplied as a fuel
to the fuel electrodes 2b and 3b. The power supply system
is provided with a hydrogen storage vessel 4 for storing
hydrogen, and is also provided with a hydrogen supply
line 7 for supplying hydrogen stored in the hydrogen
7
CA 02427851 2003-05-02
storage vessel 4 to the fuel electrodes 2b and 3b of the
humidified type fuel cell unit 2 and the low-grade
humidified type fuel cell unit 3 via a pressure regulator
and a flow passage switch 6. The hydrogen supply line 7
includes a hydrogen supply passage 7a connected to the
humidified type fuel cell unit 2 and a hydrogen supply
passage 7b connected to the low-grade humidified type
fuel cell unit 3, wherein the fuel cell unit to which
hydrogen is to be supplied (that is, the fuel cell unit
to be operated) can be selected by operating the flow
passage switch 6. Of course, hydrogen can be supplied
simultaneously to both the fuel cell units 2 and 3.
Outputs from the fuel cell units 2 and 3 are
inputted to an output control line 9 via output paths 8,
and are taken out from output terminals 10a and 10b. The
low-grade humidified type fuel cell unit 3 is provided
with an electrolyte film humidifying passage 11 for
introducing moisture generated in the fuel cell unit 3 to
the electrolyte 2a of the humidified type fuel cell unit
2.
Moisture generated in the fuel cell unit 3 is not
necessarily introduced directly to the electrolyte 2a of
the humidified type fuel cell unit 2, but may be
introduced indirectly to the electrolyte 2a via the
8
CA 02427851 2003-05-02
hydrogen supply passage or oxygen supply passage of the
humidified type fuel cell unit 2. FIG. 2 shows an example
that moisture is introduced to the electrolyte 2a via the
hydrogen supply passage 7a of the humidified type fuel
cell unit 2, wherein the electrolyte humidifying passage
11 is connected to the hydrogen supply passage 7a of the
humidified type fuel cell unit 2.
A basic configuration of the fuel cell and a
mechanism for generating an electromotive force from the
fuel cell will be described below. As shown in FIG. 3,
the fuel cell is configured such that a fuel electrode 21
in contact with hydrogen as a fuel gas is overlapped to
an air electrode 22 in contact with air (oxygen) with an
electrolyte 23 held therebetween, and such a sub-assembly
is sandwiched between both current collectors 24. The
current collector 24 is made from dense graphite which is
high in current collecting performance and is stable in
an oxidizing steam atmosphere. Horizontal grooves 24a to
which hydrogen is to be supplied are formed in a surface,
opposed to the fuel electrode 21, of the current
collector 24, and vertical grooves 24b to which air is to
be supplied are formed in a surface, opposed to the air
electrode 22, of the current collector 24.
As shown in FIG. 4, the fuel electrode 21 and the
9
CA 02427851 2003-05-02
ar 1 '
air electrode 22 are overlapped to each other with the
electrolyte 23 held therebetween. The fuel electrode 21
is composed of a gas diffusion electrode 21a and a
catalyst layer 21b, and similarly the air electrode 22 is
composed of a gas diffusion electrode 22a and a catalyst
layer 22b. Each of the gas diffusion electrodes 21a and
22a is made from a porous material, and each of the
catalyst layers 21b and 22b is made from a mixture of an
electrolyte and carbon particles which supports an
electrode catalyst such as platinum.
A plurality of the fuel cells, which are taken as
basic units, are stacked to each other in series into a
fuel cell stack. Such a fuel cell is capable of
outputting a specific voltage by operating the fuel.cells
as the basic units.
In the fuel cell having the above-described
configuration, when hydrogen gas is supplied in the
grooves 24a formed in the current collector 24 so as to
be in contact with the fuel electrode 21 and air (oxygen)
is supplied in the grooves 24b so as to be in contact
with the air electrode 22, a reaction expressed by the
following formula occurs on the fuel electrode 21 side.
H2 -' 2H+ + 2e
Further, a reaction expressed by the following formula
CA 02427851 2003-05-02
T r
occurs on the air electrode 22 side.
1/202 + 2H+ + 2e- H2O + reaction heat Q
As a whole, a reaction expressed by the following formula
occurs.
H2 + 1/202 - H2O ()
To be more specific, hydrogen is converted into
protons by release of electrons on the fuel electrode 21
side. The protons migrate to the air electrode 22 side
through the electrolyte 23, and react with electrons and
oxygen on the air electrode 22 side. An electromotive
force is generated on the basis of such an
electrochemical reaction.
The humidified type fuel cell unit 2 and the low-
grade humidified type fuel cell unit 3, whose basic
configuration is as described above, are different from
each other in terms of the kind of electrolyte used
therefor. Each of the humidified type fuel cell unit 2
and the low-grade humidified type fuel cell unit 3 will
be described below.
The humidified type fuel cell unit 2 will be first
described. The humidified type fuel cell unit 2 is a fuel
cell unit, which uses an electrolyte film substantially
developing the proton conductivity thereof only when the
electrolyte film is humidified with moisture in outside
11
r r
air or moisture generated during power generation, and
which is thereby capable of obtaining a high output by
humidification. The humidified type fuel cell unit 2,
however, has an inconvenience that since the electrolyte
develops the proton conductivity only when moisture in
outside air or moisture generated during power generation
is incorporated in the electrolyte, the humidified type
fuel cell unit 2 cannot obtain a good startability unless
moisture is contained in outside air to some extent. For
example, in an extremely low humidity atmosphere, since a
sufficient amount of moisture cannot be incorporated from
outside air into the humidified type fuel cell unit, the
humidified type fuel cell unit 2 can little obtain its
output. To cope with such an inconvenience, a method has
been proposed of previously humidifying a fuel gas
(hydrogen) to be supplied to a fuel cell by a humidifier;
however, such a method presents another problem that the
system for carrying out the method is enlarged and
complicated.
Examples of humidified type proton conductors
usable for the electrolyte film of the humidified type
fuel cell unit 2 include a fluorocarbon resin based ion
exchange film made from perfluoroalkyl sulfonate, a non-
ester structure type fullerene such as a butyl sulfonate
12
CA 02427851 2003-05-02
CA 02427851 2003-05-02
introduced fullerene, and a hydrocarbon based ion
exchange film. The fluorocarbon resin based ion exchange
film is a polymer electrolyte film made from a polymer
containing perfluoroalkyl chains having a high
hydrophobic property as main chains to which side chains
composed of perfluoroalkyl ether chains having a
hydrophilic property and sulfonic acid groups bonded
thereto are introduced. Such a fluorocarbon resin based
ion exchange film is commercially available from de Pont
de Nemours and Company under the trade name of "Nafion",
W. L. Gore & Associates, Inc. under the trade name of
"Gore-Select", Asahi Glass Company under the trade name
of "Flemion", and Asahi Kasei Corporation under the trade
name of "Aciplex". The non-ester structure type fullerene
is a fullerene as one kind of carbon clusters, to which
function groups each having a non-ester structure such as
butyl sulfonate are introduced. The carbon cluster is an
aggregated (collected) body formed by bonding or
aggregating carbon atoms of the number of several to
several hundreds. The aggregated (collected) body of
carbon atoms makes it possible to improve the proton
conductivity, and to ensure a sufficient film strength
while keeping the chemical property and hence to
facilitate film formation. The carbon cluster is not
13
CA 02427851 2003-05-02
particularly limited in terms of carbon-to-carbon bonding,
and further, it is not necessarily formed of 100% carbon
atoms, but may contain other atoms. Examples of such
carbon clusters include fullerenes represented by C60, C7o,
and C82.
On the other hand, the low-grade humidified type
fuel cell unit 3 uses as an electrolyte a low-grade
humidified type proton conductor developing a proton
conductive function in a humidified state lower than that
necessary for the humidified proton conductor used for
the above-described humidified type fuel cell unit 2. In
particular, the low-grade humidified type fuel cell unit
2 is preferable to be configured as a non-humidified type
fuel cell unit using as an electrolyte a non-humidified
proton conductor essentially requiring little moisture
and thereby developing a proton conductive function even
in a non-humidified state. Since the non-humidified type
fuel cell unit has the proton conductive mechanism
essentially requiring no moisture, it can be instantly
started in any dry atmosphere. The non-humidified type
fuel cell unit, however, is difficult to be singly used
for applications requiring a large output because the
electrolyte thereof is poorer in proton conductivity than
an electrolyte using a main conductive path composed of
14
CA 02427851 2003-05-02
moisture.
Examples of low-grade humidified type proton
conductors used for the electrolyte film of the low-grade
humidified type fuel cell unit 3, particularly, non-
humidified type proton conductors used for the
electrolyte of the non-humidified type fuel cell unit
include carbon clusters, to which function groups capable
of releasing protons are introduced, for example, an
ester structure type fullerene such as a sulfonic acid
introduced fullerene and an OH type fullerene, and also
include a compound mainly containing silicon oxide and
Bronsted acid, an acrylic acid based polymer having
phosphoric acid groups, a solid inorganic acid compound,
and an acid-base type hydrocarbon based ion exchange film
represented by a film made from a composite of a basic
polymer and an inorganic acid such as sulfuric acid or
phosphoric acid.
The ester structure type fullerene such as a
sulfonic acid introduced fullerene is a fullerene as one
kind of carbon clusters, to which an ester structure such
as sulfonic acid is introduced, and the OH type fullerene
is a fullerene to which OH groups are introduced. As
described above, the carbon cluster is an aggregated
(collected) body formed by bonding or aggregating carbon
CA 02427851 2003-05-02
atoms of the number'of several to several hundreds. The
aggregated (collected) body of carbon atoms makes it
possible to improve the proton conductivity, and to
ensure a sufficient film strength while keeping the
chemical property and hence to facilitate film formation.
The carbon cluster is not particularly limited in terms
of carbon-to-carbon bonding, and further, it is not
necessarily formed of 100% carbon atoms, but may contain
other atoms. Examples of such carbon clusters include
fullerenes represented by Cho, C70, and C82.
The proton conductor represented by the above-
described ester structure type fullerene or OH type
fullerene basically contains, as a main component, a
carbon cluster to which function groups capable of
releasing protons (H+) (proton releasable groups) are
introduced. In such a proton conductor, ion conductivity
is developed by migration of protons via the proton
releasable groups. Any type of carbon cluster is usable
as the main component of the proton conductor; however,
the carbon cluster used herein is required to have, after
the proton releasable groups are introduced thereto, the
ion conductivity larger than the electron conductivity.
The carbon cluster is generally an aggregated
(collected) body formed by bonding or aggregating carbon
16
CA 02427851 2003-05-02
atoms of the number of several to several hundreds. The
aggregated (collected) body of carbon atoms makes it
possible to improve the proton conductivity, and to
ensure a sufficient film strength while keeping the
chemical property and hence to facilitate film formation.
The carbon cluster is not particularly limited in terms
of carbon-to-carbon bonding, and further, it is not
necessarily formed of 100% carbon atoms, but may contain
other atoms. Examples of such carbon clusters include
fullerenes represented by Cho, C70, and C82, fullerenes in
each of which at least part of a fullerene structure has
an open end, and tube-like carbonaceous materials (so-
called carbon nanotubes). Since the SP2-bonded structure
of a fullerene or nanotube partially contains an element
of SP3-bonded structure, the fullerene or nanotube
generally has no electron conductivity and is thereby
preferable as the base body of the proton conductor.
FIG. 5 shows various carbon clusters formed by
aggregating a number of carbon atoms, for example,
spherical or spheroidal shaped carbon clusters, and
carbon clusters having closed plane structures similar to
the spherical or spheroidal structures. The above-
described fullerene pertains to those shown in FIG. 5.
FIG. 6 shows various carbon clusters in each of which
17
CA 02427851 2003-05-02
J
part of the spherical structure is lost, wherein an open
end is formed in the structure. Such a structure is
mainly generated as a sub-product during production of
fullerenes by arc discharge. FIGS. 7A and 7B show tube-
like carbon clusters. The tube-like carbon clusters are
typically classified into carbon nanotubes (CNTs) each
having a diameter of several nm or less, typically, 1 to
2 nm, and carbon nanofibers (CNFs) each having a diameter
of several nm or more and 1 /1 m at maximum. In particular,
the CNTs are further classified into single wall carbon
nanotubes (SWCNTs) (see FIG. 7A) composed of single layer
tubes and multi-wall carbon nanotubes (MWCNTs) (see FIG.
7B) composed of two or more layers overlapped
concentrically. FIG. 8 shows various carbon clusters
having a diamond structure in which most of carbon atoms
have a SP3-bond structure. FIG. 9 shows carbon cluster
groups, in each of which clusters are bonded to each
other. Such a carbon cluster group is also usable as the
base body of the proton conductor.
Examples of the function groups capable of
releasing protons (H+) (proton releasable groups) to be
introduced in a carbon cluster include a function group
having -SO3H or -P0(OH)2r for example, a function group
expressed by -A-SO3H or -A-PO(OH)2 (where A is either of 0,
18
CA 02427851 2003-05-02
R, O-R, R-O, and O-R-O, and R is an alkylene portion
expressed by CXHy (15x520, and 25y540)) , and a function
group expressed by -A'-S03H or -A'-PO(OH)2 (where A' is
either of R', O-R', R'-O, R'-O-R", and O-R'-O, and each
of R' and R" is an alkylene fluoride portion expressed by
CXFyHZ (15x520, 15y540, and 0Sz539) .
The carbon cluster used herein may contain, in
addition to the above-described function group capable of
releasing protons, an electron attracting group such as a
nitro group, a carbonyl group, a carboxyl group, an
aldehyde group, an alkoxycarbonyl group, a sulfonic acid
group, a nitrile group, a halogenated alkyl group, or a
halogen atom (fluorine or chlorine atom), more concretely,
-NO2, -CN, -F, -Cl, -000H, -COOR , -CHO, -CPR , -CF3, or -
S03CF3 (here, R indicates an alkyl group) . The presence
of such an electron attracting group in combination with
the proton releasable function group is advantageous in
that protons are easy to be released from the proton
releasable function group due to an electron attracting
effect of the electron attracting group, and are thereby
easy to migrate via the function group.
The number of the above-described function groups
to be introduced in the carbon cluster used herein may be
suitably selected depending on the number of carbons
19
CA 02427851 2003-05-02
constituting the carbon cluster, and as a preferred
example, it may be set in a range of 5 or more. In the
case of using a fullerene, to allow the 7c-electron
characteristic of the fullerene to remain for obtaining
an effective electron attracting characteristic, the
number of the function groups is preferably set to a
value being a half or less of the number of carbons
constituting the fullerene.
The function groups capable of releasing protons
may be introduced in a carbon cluster by a manner of
synthesizing the carbon cluster by arc discharge using a
carbon based electrode, and subjecting the carbon cluster
to acid treatment (using sulfuric acid), followed by a
treatment such as hydrolysis, or subjecting the carbon
cluster to sulfonation, phosphonating or the like. With
this treatment, a carbon cluster derivative as a target
product (carbon cluster having function groups capable of
releasing protons) can be easily obtained.
For example, in the case of using an aggregated
body of a number of fullerene derivatives each of which
is obtained by introducing the above-described function
groups to a fullerene as a carbon cluster, the proton
conductivity of the fullerene derivatives in the form of
a bulk or aggregated body is directly dependent on
CA 02427851 2003-05-02
migration of protons released from a large amount of the
function (for example, OS03H groups) contained in
molecules, with a result that the proton conductivity of
the fullerene derivatives can be developed without the
need of incorporation of hydrogen or protons originating
from steam molecules in an external atmosphere, and
accordingly, without the need of supply of moisture from
external, particularly, absorption of moisture from
outside air. The fullerene derivates, therefore, can
develop the proton conductivity without any limitation to
atmosphere. Since a large amount of function groups can
be introduced in one fullerene molecule, the number
density of protons contributing to conduction per unit
volume of the conductor becomes very large. This is one
reason why the proton conductor of the present invention
develops an effective conductivity.
The fullerene as the base body of the derivative
molecule has, in particular, an electrophillic property,
which may be considered to greatly contribute to
promotion of ionization of hydrogen ions in the function
groups. The proton conduction may be considered to be due
greatly to the introduced groups, and in the case of the
fullerene derivative, the electric conduction via the
contour may occur by the electrophillic property of the
21
CA 02427851 2003-05-02
fullerene molecules. This is another reason why the
proton conductor of the present invention exhibits the
excellent proton conductivity.
Since most of the proton conductor is composed of
carbon atoms of fullerene, it is lightweight, less
altered, and relatively clean, and further it does not
contain any contamination material exerting adverse
effect to the proton conductivity. In addition, the
production cost of fullerenes is being rapidly lowered.
From the viewpoints of resource, environment, economy,
and the like, the fullerene is the most ideal carbon
based material.
As described above, the carbon cluster having the
proton releasable function groups can realize a structure
allowing protons to be easily dissociated and hopped
between sites due to a structural property that a spatial
density of the acidic function groups is high and an
electronic property of the carbon cluster as the base
body (for example, fullerene), and accordingly it can
realize good proton conduction even in a dry state.
On the other hand, examples of the compounds
(proton conductors) each mainly containing silicon oxide
and Bronsted acid include a proton conductor composed of
a compound mainly containing silicon oxide and Bronsted
22
CA 02427851 2003-05-02
acid and a thermoplastic elastomer (see Japanese Patent
Laid-open No. Hei 8-249923), a proton conductor composed
of a compound mainly containing silicon oxide and
Bronsted acid, and a polymer having sulfonic acid groups
as side chains (see Japanese Patent Laid-open No. Hei 10-
69817), and a proton conductor composed of a compound
mainly containing silicon oxide and Bronsted acid, and a
block copolymer produced by polymerizing conjugated diene
units and aromatic vinyl units (see Japanese Patent Laid-
open No. Hei 11-203936). Here, examples of the Bronsted
acid includes phosphoric acid and its derivative and
perchloric acid and its derivative. Silicon oxide has OH
groups as terminal groups on the surface, wherein protons
of the OH groups contribute to ion conduction. The
addition of Bronsted acid to silicon oxide is
advantageous in that Bronsted acid functions as a doner
of protons, to bond the OH groups as terminal groups on
the surface of silicon oxide at a high density. Protons
of such OH groups cause hopping migration, so that the
proton conductor develops a high proton conductivity even
in a dry atmosphere.
The acrylic acid based polymer having a phosphoric
acid group is a polymer obtained by polymerizing acrylic
monomers having phosphoric acid groups (which monomers
23
CA 02427851 2003-05-02
are typically commercially available from Unichemical
Mfg., Ltd. under the trade name of Phosmer) in a
relatively mild atmosphere. The acrylic acid polymer can
exhibit the proton conductivity at a high temperature
even if it contains no moisture because the electric
conductivity thereof is rapidly increased with an
increase in temperature. The reason for this may be
considered as follows. Namely, condensed or adsorbed
moisture in the phosphoric acid groups is less desorbed,
and consequently, in a high temperature region, the
molecules of the condensed or adsorbed moisture
contribute to proton conduction as the polymer
electrolyte becomes plasticized. The acrylic acid polymer
has another feature that the phosphoric acid group or
phosphonic acid group less causes any desorbing reaction
as compared with the sulfonic acid group, and thereby it
has a higher resistance against radicals.
Examples of the above-described solid inorganic
compounds include CsHSO4 and Rb3H(SeO4)2. Such a solid
inorganic compound develops the proton conductivity even
in a non-humidified state, and is stable even at a high
temperature of 25000 or more.
In the power supply system of the present invention,
it is most preferable to combine the above-described
24
CA 02427851 2003-05-02
humidified type fuel cell unit with the above-described
non-humidified type fuel cell. unit; however, the present
invention is not limited thereto but may be configured to
combine fuel cells relatively different from each other
in terms of the humidified state necessary for the fuel
cells. For example, with respect to the above-described
proton conductors, the degrees of the need of
humidification becomes lower in the order of the
fluorocarbon resin based ion exchange film, the non-ester
structure type fullerene, and the ester structure type
fullerene. As a result, there may be adopted a
combination of a fuel cell using the fluorocarbon resin
based ion exchange film as an electrolyte (equivalent to
the humidified type fuel cell) and a fuel cell using the
ester structure type fullerene as an electrolyte
(equivalent to the non-humidified type fuel cell), a
combination of a fuel cell using the non-ester structure
type fullerene as an electrolyte (equivalent to the
humidified type fuel cell) and a fuel cell using the
ester structure type fullerene as an electrolyte
(equivalent to the non-humidified type fuel cell), and a
combination of a fuel cell using the fluorocarbon resin
based ion exchange film as an electrolyte (equivalent to
the humidified type fuel cell) and a fuel cell using the
CA 02427851 2003-05-02
non-ester structure type fullerene as an electrolyte
(equivalent to the low-degree humidified fuel cell).
A method of operating the above-described power
supply system will be described below. First, the low
temperature startability of the humidified type fuel cell
will be described below. In an extremely low humidity
atmosphere, the humidified type fuel cell cannot receive
moisture in an amount sufficient for power generation
from outside air, thereby failing to obtain any output.
FIG. 10 is a characteristic diagram showing the low
temperature startability of the humidified type fuel cell.
In this example, the output (current density) after 120
sec remains as 0 mA/cm2. It is to be noted that the low
temperature startability of the fuel cell was obtained by
measuring the current density at a low voltage (0.7 V)
under a condition with a temperature of -10 C. This
applies in the following examples.
With respect to the low temperature startability of
the non-humidified type fuel cell, as shown in FIG. 11,
the non-humidified type fuel cell can instantly be
started even in any dry atmosphere. In this example, it
can obtain an output of about 20 mA/cm2 immediately after
start-up. The output, however, is then little changed and
kept at a low level even after an elapse of 120 sec. The
26
CA 02427851 2003-05-02
reason why the output is somewhat increased by-power
generation is that the conductivity of the electrolyte is
increased by heat generation in the power generation unit,
and that depending on the material of the proton
conductor, the humidification due to moisture generated
by reaction contributes to the increase in conductivity.
The power supply system of the present invention
has the hybrid structure of the humidified type fuel cell
portion 2 and the low-grade humidified (or non-
humidified) type fuel cell unit 3. Accordingly, by
selectively operating both the fuel cell units 2 and 3,
it is possible to make effective use the advantages of
both the fuel cell units 2 and 3, and hence to maximize
the abilities of both the fuel cell units 2 and 3.
To be more specific, at the time of initial start-
up, hydrogen is supplied to both the humidified type fuel
cell unit 2 and the low-grade humidified type fuel cell
unit 3, to operate both the fuel cell units 2 and 3. At
this time, a low temperature startability can be obtained
to some extent due to contribution of the output of the
low-degree humidified type fuel cell unit 3. It is to be
noted that the operation of the power supply system is
not limited thereto. For example, only the low-degree
humidified type fuel cell unit 3 may be operated at the
27
CA 02427851 2003-05-02
time of initial start-up.
After start-up, self-humidification is promoted by
moisture generated in the low-grade humidified type fuel
cell unit 3, to increase the output of the humidified
type fuel cell unit 2. The moisture generated in the low-
grade humidified type fuel cell unit 3 passes through the
electrolyte film humidifying passage 11, to be supplied
to the electrolyte 2a of the humidified type fuel cell
unit 2, thereby promoting the self-humidification. As the
output of the humidified type fuel cell unit 2 is
increased by the self-humidification, the output of the
module is switched to the humidified type fuel cell unit
2 side by actuation of the output control line 9. At the
same time, the hydrogen gas, which has been supplied to
both the low-grade humidified type fuel cell unit 3 and
the humidified type fuel cell unit 2 at the start-up, is
switched to be supplied to only the humidified type fuel
cell unit 2. After that, only the humidified type fuel
cell unit 2 is operated.
Since the output of the low-grade humidified type
fuel cell unit 3 at the start-up is thus smoothly
switched to the output of the humidified type fuel cell
unit 2 after start-up, a high output can be obtained from
initial start-up even in an extremely low humidity
28
CA 02427851 2003-05-02
atmosphere. FIG. 12 shows a low temperature startability
of the power supply system of the present invention
including a hybrid structure of the humidified type fuel
cell unit 2 and the low-grade humidified (non-humidified)
type fuel cell unit 3. As is apparent from this graph, an
output of about 20 mA/cm2 can be obtained immediately
after start-up, being rapidly increased, and is kept at a
high value.
As is apparent from the above description,
according to the present invention, a high output can be
obtained even in an extremely low temperature and low
humidity atmosphere. Accordingly, it is possible to
provide a power supply system most suitable to be applied
to a power supply for portable equipment required to be
operated in a wide temperature range from a low
temperature, for example, a below-freezing temperature to
a high temperature, and to provide a method of operating
the power supply unit.
29