Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent: | (11) CA 2427963 |
|---|---|
| (54) English Title: | DRUG DELIVERY DEVICE |
| (54) French Title: | DISPOSITIF D'ADMINISTRATION DE MEDICAMENTS |
| Status: | Expired and beyond the Period of Reversal |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | LAVERY, DE BILLY, LLP |
| (74) Associate agent: | |
| (45) Issued: | 2008-07-22 |
| (22) Filed Date: | 2003-05-06 |
| (41) Open to Public Inspection: | 2004-11-06 |
| Examination requested: | 2003-05-06 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | No |
|---|
| (30) Application Priority Data: | None |
|---|
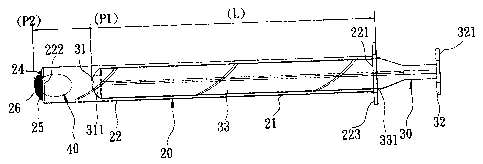
A drug delivery device includes an insert tube and an elongate plunger. The insert tube has a tubular wall that confines a passage and that has drug entrance and release ends. The drug release end has first and second radial parts, and is provided with a flexible grating that includes sets of first and second flexible strips. The first and second flexible strips extend from the first and second radial parts of the drug release end, respectively. The elongate plunger has a drug pushing end and an operating end, is slidably movable in the passage, and has a length sufficient to enable the drug pushing end to push a drug in the passage to move out of the passage and spread apart the first and second flexible strips for delivering the drug into a body cavity.
L'invention porte sur un dispositif d'administration de médicaments comprenant un tube d'administration et un piston allongé. Le tube d'administration possède une paroi tubulaire créant un passage et il est pourvu d'une extrémité où le médicament est admis dans le tube et d'une extrémité où le médicament est administré au patient. L'extrémité d'administration du médicament possède deux éléments radiaux et est pourvu d'un grillage souple composé de deux ensembles de bandes souples. Les première et deuxième bandes souples se prolongent respectivement à partir des premier et deuxième éléments radiaux de l'extrémité du tube où l'administration du médicament se fait. Le piston allongé possède une extrémité servant à pousser le médicament et une extrémité destinée à être manipulée par l'utilisateur, il est conçu pour glisser dans le passage et il possède une longueur suffisante pour permettre à son extrémité destinée à pousser le médicament de pousser le médicament dans le passage pour l'en faire sortir et le faire passer dans les deux jeux de bandes souples en vue de son administration dans une cavité corporelle.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Time Limit for Reversal Expired | 2022-03-01 |
| Letter Sent | 2021-05-06 |
| Letter Sent | 2021-03-01 |
| Letter Sent | 2020-08-31 |
| Inactive: COVID 19 - Deadline extended | 2020-08-19 |
| Inactive: COVID 19 - Deadline extended | 2020-08-06 |
| Inactive: COVID 19 - Deadline extended | 2020-07-16 |
| Inactive: COVID 19 - Deadline extended | 2020-07-02 |
| Inactive: COVID 19 - Deadline extended | 2020-06-10 |
| Inactive: COVID 19 - Deadline extended | 2020-05-28 |
| Inactive: COVID 19 - Deadline extended | 2020-05-14 |
| Inactive: COVID 19 - Deadline extended | 2020-04-28 |
| Common Representative Appointed | 2019-10-30 |
| Common Representative Appointed | 2019-10-30 |
| Revocation of Agent Request | 2018-09-14 |
| Appointment of Agent Request | 2018-09-14 |
| Inactive: Agents merged | 2018-09-01 |
| Inactive: Agents merged | 2018-08-30 |
| Inactive: Late MF processed | 2010-10-29 |
| Letter Sent | 2010-05-06 |
| Grant by Issuance | 2008-07-22 |
| Inactive: Cover page published | 2008-07-21 |
| Pre-grant | 2008-04-10 |
| Inactive: Final fee received | 2008-04-10 |
| Letter Sent | 2008-02-25 |
| Notice of Allowance is Issued | 2008-02-25 |
| Notice of Allowance is Issued | 2008-02-25 |
| Inactive: IPC removed | 2008-02-11 |
| Inactive: Approved for allowance (AFA) | 2007-12-11 |
| Small Entity Declaration Request Received | 2007-10-04 |
| Small Entity Declaration Determined Compliant | 2007-10-04 |
| Amendment Received - Voluntary Amendment | 2007-08-27 |
| Inactive: S.30(2) Rules - Examiner requisition | 2007-02-28 |
| Amendment Received - Voluntary Amendment | 2006-12-14 |
| Inactive: S.30(2) Rules - Examiner requisition | 2006-06-29 |
| Inactive: IPC from MCD | 2006-03-12 |
| Application Published (Open to Public Inspection) | 2004-11-06 |
| Inactive: Cover page published | 2004-11-05 |
| Inactive: IPC assigned | 2003-06-23 |
| Inactive: First IPC assigned | 2003-06-23 |
| Inactive: Filing certificate - RFE (English) | 2003-06-06 |
| Filing Requirements Determined Compliant | 2003-06-06 |
| Letter Sent | 2003-06-06 |
| Application Received - Regular National | 2003-06-06 |
| Request for Examination Requirements Determined Compliant | 2003-05-06 |
| All Requirements for Examination Determined Compliant | 2003-05-06 |
There is no abandonment history.
The last payment was received on 2008-04-10
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Please refer to the CIPO Patent Fees web page to see all current fee amounts.
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| DEBORAH HUANG |
| Past Owners on Record |
|---|
| None |