Note: Descriptions are shown in the official language in which they were submitted.
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-1-
METAL AND ALLOY POWDERS AND POWDER FABRICATION
Field of the Invention
This invention relates to a method and an apparatus for
preparing metallic powders of well-defined particle sizes
and composition, and to metallic powders so produced. In a
further aspect the invention relates to powder fabrication
and the production of near net-shaped products.
Background to the Invention
Metallic powders have many applications and these
include:
(a) As feed materials for powder metallurgical techniques,
which offer the possibility of making near net-shaped
products rather than having to machine a component from a
large billet. In some cases 900 of the material is removed
during the machining process, and has to be recycled. A
method for making near-net shaped products may
advantageously reduce this wastage.
(b) Alloys; the use of metal powders in alloy preparation
results in rapid dissolution and minimal segregation within
an alloy.
(c) For their aesthetic properties; metal powders are used
often in metallic paints.
(d) As fuels in rockets.
(e) As fine powders for mixing as alloy constituents, for
example in making many high intensity magnetic phases.
There are a variety of conventional ways of making
metallic particles. These include crushing and grinding,
which are particularly energy-intensive processes as metals
inherently resist deformation, and for reactive metals the
grinding process needs to take place under inert conditions
to avoid oxidation. Metal powders can also be obtained by
the reduction of metal compounds such as oxides by hydrogen
but this is generally restricted to oxides that are less
stable than water vapour. To reduce the oxides of very
reactive metals would require reactants such as calcium and
the powders are then likely to be contaminated with calcium
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-2-
oxide. The injection of molten metal onto a spinning disc
results in fine particles of liquid being centrifugally
expelled from the disc as droplets that subsequently
solidify. Liquid metals can be atomised by impinging a high
velocity gas into a stream of molten metal. Metal powders
can be produced by shock-cooling metallic vapours. For some
metals with substantial solubility of hydrogen, it is
possible to form brittle hydride phases which can
subsequently be crushed or decrepitated into fine particles.
By heating at elevated temperatures, the hydrides simply
decompose to form metallic particles. Lastly,
electrochemical deposition of metal from a compound of the
metal dissolved in an aqueous or fused salt electrolyte can
result in a dendritic deposit that can easily be crushed to
a fine powder. Overall, these methods can give fine powders
but frequently the powders are highly oxidised and
contaminated with oxide products, and there is generally a
substantial range of particle sizes. This is a particular
problem when a metallic powder of a given particle size is
required, which typically necessitates sieving of the
product and rejection of a sizeable fraction. These
problems are exacerbated when alloy powders are required,
especially for those of the most reactive metals.
Metal oxide powders are much easier to obtain by
grinding, as oxides are typically highly brittle and crush
readily. Being oxides, they do not suffer from oxidation
during this process. Very fine oxide powders can also be
produced by precipitation from an aqueous or fused salt
solution. Alternatively, by reacting a volatile compound
with oxygen, it can be possible to form a fine oxide powder.
For example the reaction of titanium tetrachloride with
oxygen results in a very fine oxide powder. Frequently,
these particles are of a uniform size, but the problem
remains of producing fine metal powders.
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-3-
Summary of the Invention
In a first aspect, the invention provides a method and
an apparatus for producing a metallic powder, and a metallic
powder, as defined in the appended independent claims.
Preferred or advantageous features of the invention are set
out in dependent subclaims.
This aspect of the invention is based on the surprising
finding that powdered metal compounds such as metal oxide
powders may be treated electrochemically to yield metal
powders with a uniform structure and size. Thus, a method for
producing a metallic powder may advantageously be provided,
in which a precursor powder comprising a compound (M1X)
between a metal (M1) and a non-metal species (X) is treated by
electro-deoxidation. In this process, the precursor powder
forms a cathode contacting a melt comprising a fused salt
(MzY), under conditions such that the non-metal species
dissolves in the melt. This may advantageously form a porous
metallic sample which may be processed as required to form the
metallic powder.
Surprisingly, the metal powders produced according to
embodiments of the invention have been found to have a uniform
microscopic structure, both in terms of the particle size of
the metal powder and the microstructure of individual
particles. In addition, it has been found that particles of
similar shapes may be produced. For example, the powders may
form a cube structure. The small, consistent particle sizes,
and the metal purity, produced by this method may be
particularly advantageous as the production of metal powders
by prior art methods has failed to produce high yields of such
materials; in prior art methods, sieving is generally required
to produce consistent particle sizes and entails very
significant wastage.
The term electro-deoxidation is used herein to describe
the process of removing the non-metal species (X) from a
compound in the solid state by contacting the compound with
the melt and applying a cathodic voltage to it such that the
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-4-
non-metal species, or anionic species, dissolves. In
electrochemistry, the term oxidation implies a change in
oxidation state and not necessarily a reaction with oxygen.
It should not, however, be inferred that electro-deoxidation
always involves a change in the oxidation states of both (or
all) of the components of the compound; this is believed to
depend on the nature of the compound, such as whether it is
primarily ionic or covalent. In addition, it should not be
inferred that electro-deoxidation can only be applied to an
oxide; any compound may be processed in this way. Other terms
to describe the electro-deoxidation process in particular
instances may be electro-decomposition, electro-reduction or
solid-state electrolysis.
In a preferred embodiment, the cathodic voltage applied
to the metal compound is less than the voltage for deposition
of cations from the fused salt at the cathode surface. This
may advantageously reduce contamination of the intermetallic
compound involving the cations. It is believed that this may
be achieved under the conditions of an embodiment in which the
decomposition potential of the salt, or electrolyte, is not
exceeded during electro-deoxidation, or electro-reduction, or
under the conditions of an embodiment providing a method for
producing a metallic powder by treating a powder of a metal
compound (M1X) by electrolysis in a fused salt M2Y or a
mixture of salts, under conditions whereby reaction, or
ionisation, of X rather than Mz deposition occurs at an
electrode surface, and X dissolves in the electrolyte MZY.
Further details of the electro-deoxidation process are
set out in International patent application number
PCT/GB99/01781, which is incorporated herein by reference in
its entirety.
In the method of invention, it is preferable that the
metal produced has a higher melting point than that of the
melt, or salt.
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-5-
Further, other metal compounds, such as metal oxides,
may be present and the electrolysis product may be an alloy
powder.
The method of the invention may advantageously give a
product which is of very uniform particle size and free of
oxygen or other contaminants.
In accordance with a preferred embodiment of the present
invention, it has been found that electrochemical reduction
of metal oxide powders, by cathodically ionising the oxygen
away from the oxide, results in agglomerates of pure metal
powder, the particle size of which depends upon the conditions
of pre-forming and sintering of the metal oxide powders and
the time and temperature of electro-deoxidation, or
electrolysis. Other electrolysis parameters such as voltage,
current and salt composition may also be varied to control the
metal powder morphology. Control of these parameters may
advantageously be applicable to precursor powders other than
oxides.
The metal compound or oxide should show at least some
electronic conductivity or be used in contact with a
conductor.
Metal alloy powders may advantageously be formed by
electro-deoxidation of precursor powders comprising a mixture
or solid solution of two or more metal compounds or one or
more metals or alloys with one or more metal compounds.
In a second aspect, the invention may advantageously
provide a method for forming a near net-shaped product. In
this method, a shaped precursor is formed from a powdered feed
material comprising a compound (M1X) between a metal (Ml) and
a non-metal species (X). The precursor is then treated by
electro-deoxidation, the precursor forming a cathode
contacting a melt comprising a fused salt (MzY) under
conditions such that the non-metal species dissolves in the
melt. The electro-deoxidation is carried out for a
sufficiently long time and/or at a sufficiently high
temperature to form interconnections between the metallic
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-6-
powder particles produced by the electro-deoxidation, in order
to produce the near net-shaped product strong enough for
further processing.
The advantages of the powder production aspect of the
invention described above may also be applicable to this
aspect of the invention. For example, carrying out electro
deoxidation at a cathodic potential less than the potential
for ration deposition from the melt may advantageously reduce
contamination of the near net-shaped product, and using a feed
material comprising a mixture or solid solution of two or more
metals may advantageously produce a near net-shaped product
of a desired alloy. The skilled person would readily
appreciate that other advantages described above may also be
applicable to near net-shaped product formation.
Specific Embodiments and Best Mode of the Invention
Embodiments of the invention will now be described by
way of example, with reference to the drawings, in which;
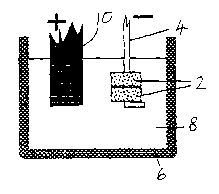
Figure 1 illustrates an apparatus for the electro
deoxidation of a metal oxide powder according to a first
embodiment of the invention;
Figure 2 illustrates an apparatus according to a second
embodiment of the invention;
Figure 3 is a photomicrograph of a titanium oxide
powder, as used as the starting material in Examples 1 and 2;
Figure 4 is a photomicrograph of a titanium powder
produced from the oxide of Figure 3 in Example 1;
Figure 5 is a photomicrograph of a titanium powder
produced from the oxide of Figure 3 in Example 2;
Figure 6 is a photomicrograph of a chromium powder as
produced in Example 3;
Figure 7 is a photomicrograph of an AlNi3 powder as
produced in Example 5;
Figure 8 is an XRD (X-ray diffraction) spectrum for the
powder of Figure 7, overlaid on a spectrum for a reference
sample of AlNi3;
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
Figure 9 is a photomicrograph of a niobium oxide powder,
as used as the starting material in Example 6;
Figure 10 is a photomicrograph of a niobium powder as
produced in Example 6 from the oxide powder of Figure 9;
Figure 11 is a schematic diagram of an apparatus for
electro-deox~dation as used in Example 6; and
Figure 12 is a plot of an XRD analysis of a niobium
powder produced as in Example 6.
.,
Figures 1 and 2 show pellets 2 of metal oxide in contact
with a cathode conductor. Each pellet is prepared by powder
processing techniques, such as pressing or slip-casting a
submicron or micron-sized powder (Figure 3) such as titanium
dioxide. The pellet may then be fired to give it structural
strength before being made the cathode in a cell in which a
crucible 6 contains a fused salt 8. In the embodiment, the
cell contains chloride salts, being either CaClz or BaCl2 or
their eutectic mixture with each other or with another
chloride salt such as NaCl.
In the embodiment of Figure 1, the pellets are annular
and are threaded onto a cathodic conductor in the form of a
Kanthal wire 4. The crucible is an inert crucible of graphite
or alumina. In the embodiment of Figure 2 the crucible 12 is
made of a conducting material such as titanium or graphite.
The pellets sink in the melt and contact the crucible, to
which the cathodic voltage is applied. The crucible itself
thus acts as a current collector.
In both embodiments the electrochemical process is the
same, as follows. On the application of current, the oxygen
ionises, dissolves in the salt and diffuses to a graphite
anode 10 where it is discharged. The oxygen is thereby
removed from the oxide, leaving the metal behind. The metal
product is a very fine powder of very uniform size, as shown
in Figure 4. It should be noted that the metal powder
produced has a much larger grain size than the initial grain
size of the oxide powder. By varying the temperature, the
time of electro-deoxidation (reduction), the voltage, the
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
_g_
current and/or the salt, it is possible to change and control
the size and morphology of the metallic powder.
The embodiment described above produces titanium metal
powder but it is possible to make alloy powders by the same
route simply by mixing the oxide powders together, and
preferably firing or sintering them to strengthen the pellet.
The pellet may also be fired so as to form a solid solution
of the oxides. It is preferable that the oxide powders are
not greater than microns in particle size and are finer than
the metal powder to be produced.
The electrolyte should consist of salts which are more
stable than the equivalent salts of the metal which is being
produced and, preferably, the salt should be as stable as
possible to remove the oxygen to as low as concentration as
possible. The choice of salt includes the chloride or other
halide salts of alkali and/or alkaline earth metals,
particularly barium, calcium, cesium, lithium, strontium and
yttrium.
To obtain a salt with a lower melting point than that
2,0 given by a pure salt and/or to modify the interactions between
the cathode and the electrolyte, a mixture of salts can be
used, preferably the eutectic composition.
At the end of reduction, the reduced compact is
withdrawn from the molten salt. Some of the salt is contained
within the withdrawn pellet, however, and stops the powder
oxidising. The salt can simply be removed by washing in water
or an organic solvent such as ethanol. Generally, the pellets
are very brittle and can easily be crushed to reveal the metal
powder.
The following Examples illustrate the invention.
Example 1
Three pellets, 5 mm in diameter and 1 mm in thickness,
prepared by pressing moisturised 0.25 ~Cm titanium dioxide
powder (Figure 3) followed by drying and sintering at 950°C
in air for 2 hours, were placed in a titanium crucible filled
with molten calcium chloride at 950°C. The cell arrangement
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-9-
is as shown in Figure 2. A potential of 3 V was applied
between a graphite anode and the titanium crucible. After
hours, the electro-deoxidation was terminated, the salt
allowed to solidify and then dissolved in water to reveal a
5 black/metallic pellet which was then removed from the crucible
and dried. Examination under the scanning electron microscope
showed that the particulate structure of the pellet had been
transformed from 0.25 um particles of titanium dioxide to
12 ~,m particles of titanium (Figure 4) . The titanium particle
10 size was advantageously very uniform, being about 12~.m +/-3~m.
Within experimental errors, no oxygen was detected by energy
dispersive X-ray analysis.
It should be mentioned that in other experiments it was
observed that increasing the time of electrolysis would
increase the size of the particles and, at the same time,
interconnections between individual particles became
significantly stronger. This could eventually lead to the
production of strong metallic pellets which could not be
crushed to powders and which are therefore a form of near net-
shaped product. In addition, such strong pellets may be used
directly as a feed-stock for various fabrication techniques,
such as sintering. The microstructure in these strong pellets
is believed to be similar to that in the conventional ICroll
titanium sponge. The formation of the titanium pellets
depended also on the nature of the molten salts and other
experimental conditions such as the pre-forming conditions and
sintering of the pellet.
Example 2
The TiOz powder as in Example 1 was mixed with water to
form a slurry which was then slip cast into small pellets,
dried and sintered at 950°C in air for 2 hours. The sintered
pellets were about 8 mm in diameter and 2 mm in thickness.
A hole, 1.5 mm in diameter, was drilled in each of the
sintered TiOz pellets. Two of them were threaded onto a
Kanthal wire, 1.5 mm in diameter, and then inserted into a
molten eutectic mixture of calcium chloride and barium
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-10-
chloride at 950°C. An alumina crucible was used to
accommodate the salts and the cell arrangement is as shown in
Figure 1. A potential of 3.1 V was applied between a graphite
anode and the Kanthal wire. After about 20 hours, the
temperature was lowered to 700°C, the pellets on the Kanthal
wire were removed from the crucible, cooled in air and then
washed in water to reveal grey/metallic pellets. Examination
under the scanning electron microscope showed that the
particulate structure of each pellet had been transformed from
0 .25 ~.m particles of titanium dioxide to two types of titanium
particles, about 3 ,um and about 20~cm respectively (see
Figure 5).
As shown in Example 1 above, it is possible to produce
titanium powder of a more consistent particle size than this
by appropriate control of process parameters but it should be
noted that the particle size range produced in Example 2 may
advantageously be substantially more uniform than that
produced by prior art methods.
Example 3
A 1 ~.m powder of chromic oxide was mixed with water to
form a slurry which was slip cast into small samples, or
pellets, about 8.10 mm in diameter and 3~5 mm in thickness,
followed by drying and sintering at 950°C in air for 2 hours.
After sintering, no significant change was observed on the
colour (green) and size of the samples, but the mechanical
strength was enhanced significantly. Three of the sintered
a
samples were placed in a graphite crucible filled with molten
calcium chloride at 990°C as shown in Figure 1. Better
results have been obtained by adding NaCl to the melt to
reduce dissolution of chromic oxide in the melt. A potential
of 2.7 V was applied between a graphite anode and the graphite
crucible. After 15 hours, the electrolysis was terminated,
the salt allowed to solidify and then dissolved in water to
reveal the grey/metallic pellets. Examination under a
scanning electron microscope (Figure 6) revealed aggregates
of crystallites in two sizes in the reduced samples: the
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-11-
larger crystallites were 2050 ~,m in size while the smaller
ones were 5~8 ~.m. Energy dispersive X-ray analysis confirmed
both types of crystallites were pure chromium metal.
The particle size range produced in this Example may be
reduced through process parameter control but is significantly
narrower than the chromium particle size range produced by
prior art methods, typically by mechanical grinding.
Example 4
Powders of titanium dioxide (0.25 ~.m particle size),
alumina (0.25 Vim) and vanadium oxide (1 - 2 ~tm) were mixed in
a ratio such that the ratio of the metal elements was the same
as a desired alloy, being in this example the Ti-6A1-4V alloy.
The mixture was then made into a slurry with water and slip
cast into pellets, followed by drying and sintering at 950°C
for 2 hours in air. After sintering, the colour of the
pellets changed from light green to dark brown. The size of
the sintered pellets was about 8 mm in diameter and 6 mm in
thickness. After drilling a hole of 1.5 mm in diameter, one
of the sintered pellets was threaded onto a Kanthal wire, and
then inserted into a molten eutectic mixture of barium
chloride and calcium chloride at 950°C. An alumina crucible
was used to accommodate the molten salts and the cell
arrangement is shown in Figure 1. A potential of 3.1 V was
applied between a graphite anode and the Kanthal wire. After
20 hours, the temperature of the salt was allowed to cool to
700°C and then the electro-deoxidation terminated. The
pellets on the Kanthal wire were removed from the crucible,
cooled in air and then washed/leached in water to reveal the
grey/metallic pellets. Examination under the scanning
electron microscope showed that the particulate structure of
the pellet was similar to that shown in Figure 3 for titanium.
EDX analysis revealed no oxygen in the pellets and confirmed
the presence of titanium, aluminium and vanadium in individual
particles in the desired ratio, within experimental error.
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-12-
Example 5
Powders of A1303 and Ni0 were mixed in a molar ratio of
1:6, pressed into small cylindrical pellets (10 mm diameter,
510 mm height), and sintered in air at 980-1000°C for about
2 hours. After sintering, the grey-green colour of the
pellets became only slightly paler. Holes of 1.7 mm diameter
were drilled into the sintered pellets. Four of the sintered
pellets, weighing about 4 grams, were threaded onto a Kanthal
wire ( 1 . 0 mm diameter) to form an assembled cathode . Electro-
deoxidation was carried out between the assembled cathode and
a graphite anode in argon-protected molten CaClz at 950°C and
3.1 ST for about 18 hours, as shown in Figure 1. The pellets
were removed from the molten salt upon reduction, cooled first
in argon and then in air to room temperature. Water was used
to wash the reduced pellets which were then dried in aim,
showing a grey metallic colour. The surfaces and cross
sections of the reduced pellets consisted of nodular particles
of 2--20 microns in size (see Figure 7) and which contained Al
and Ni in the atomic ratio of 1:3. No oxygen was detected.
The pellets were then manually ground into powder in an agate
mortar. XRD (X-ray diffraction) was applied to the powder and
the spectrum showed an almost identical pattern to a standard
AlNi3 sample (see Figure 8).
Example 6
Nb205 powders used in experiments were 99.97 wt % and
99.99 wt % pure, with mean particle sizes of 4.03 ,um and
12.71 ,um, respectively. The powders were pressed into porous
compacts that were then strengthened by sintering. The
sintered pellets were attached onto a cathodic current
collector to form an assembled oxide cathode. The CaC1z2H20
and NaCl employed for the melt were analytical reagents. All
the chemicals were supplied by Aldrich Chemical. The
CaC122H20 was dehydrated in air at 373 K for 1 hour, heated up
slowly to 573 K and then was held at 573 K for 12 hours. The
dehydrated CaCl2 and dried NaCl were mixed thoroughly and the
mixture was then dried at 473 K before use. High-density
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-13-
graphite rods of 10 mm in diameter and 100 mm long were
purchased from Graphite Technologies and were used as the
anodes. A Kanthal° wire, 1.5 mm in diameter, was employed as
the cathodic current collector.
The electrolytic cell for electrolysis is schematically
shown in Figure 11. Two Farnell LS30-10 Autoranging Power
Supplies were used for conducting the electrolysis under
constant voltages. A first wire 50 for connecting the pellets
of Nb205 60 was led to the negative end of one power supply.
The stainless steel crucible 56 for holding the molten
electrolyte 58 was connected by a second wire 62 to the
negative end of the other power supply. Two positive ends of
the two power supplies were both connected to the graphite rod
anode 52. All electrical connections from the individual
electrodes to the power supplies were made by Kanthal°
wires 50, 62. The electrolyte temperature was measured using
a type K thermocouple in an alumina sheath 54. The cell was
placed in a vertical Inconel° reactor tube closed at one end.
The electrolytic cell was flushed with high purity argon
while it was heated to the required temperatures. When the
cell reached its electrolysis temperature, the graphite rod
anode was dipped into the molten electrolyte and the
pre-electrolysis was performed at Ua = 2.8-3.0 V and 1173 K
until no anode bubbles could be visually observed, usually for
a period of 12 hours. After completing the pre-electrolysis,
the oxide cathode was immersed in the melt. The electrolysis
was carried out under constant voltages (U1 and UZ) applied
respectively to the cell as shown in Figure 11. The applied
voltage (U1), along with the resulting currents, were
displayed and logged by a PC with Serial RS232 plus ADAMS
4017-8 Channel Analogue-to-Digital Convertor during the course
of electrolysis.
The samples as-reduced were removed quickly from the
melt under a flow of the argon at 873 K and quenched and
washed in cold water, followed by acid leaching, water rinse,
and acetone wash. The resulting porous pellets were made into
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-14-
powders by grinding manually. The obtained niobium metal
powders were then cleaned with acetone again and dried under
vacuum at'room temperature.
Morphology of the sintered or reduced pellets was
observed using a Jeol JSM-5800LV scanning electron microscope
(SEM) with an energy dispersive X-ray analysis (EDXA)
attachment. Concentrations of impurities were determined by
EDXA. Various phases present in the prepared powders were
examined by powder X-ray diffractometry (XRD) using a Philips
diffractometer PW1710 with Cu K«1 radiation. Contents of
oxygen were also determined by weighing the prepared niobium
metal powders before and after re-oxidation in air, where a
complete re-oxidation of the metal powders to the Nb205 was
confirmed by XRD analysis. A level of chlorine in the
off-gasses was monitored using a Drager QuadGard Chlorine
Detector.
The final product remaining at the cathode after
electrolysis was found to be metallic niobium, in the form of
porous pellets. Figures 9 and 10 show the typical
microstructures of cross sections of Nb205 pellets before (for
4.03 ~m particle size Nbz05) and after electro-deoxidation at
U1 = 3 . 1 V and 1123 K for 24 hours . It was interesting to
observe that after the reduction the form of the as-reduced
product was essentially a powder compact which had loosely
sintered together and also the particle sizes were enlarged
to some extent. The prepared niobium metal powder contained
2311 mass ppm oxygen.
A typical measured XRD (X-ray diffraction) pattern is
shown in Figure 12 for the niobium metal powders reduced at
1173 K for 48 hours, from which one can see that the powder
is pure niobium, free of any oxide phases.
Overall, the present experimental results provide
evidence that porous pellets of Nbz05 can be easily deoxidised
to the metallic niobium. The niobium metal powders prepared
are obviously acceptable for subsequent purification
treatments, such as high vacuum sintering at high
CA 02429024 2003-05-14
WO 02/40725 PCT/GBO1/05031
-15-
temperatures. Our experiments indicated that various ranges
of particle sizes of the niobium metal powders could be
readily prepared by a proper control of experimental
conditions and by changing the particle sizes of Nb205
powders.