Note: Descriptions are shown in the official language in which they were submitted.
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
1
A MEDICAL STENT WITH A VALVE AND
RELATED METHODS OF MANUFACTURING
DESCRIPTION OF THE INVENTION
Field of the Invention
[001] The present invention relates to medical stents and methods of
manufacturing the same. In particular, the present invention relates to
medical stents with valves for preventing harmful gastric acid reflux in a
patient.
Background of the Invention
[002] Medical stents are generally flexible, tubular, expandable
bodies formed of a plurality of interconnecting wires. The stents are used in
a
wide variety of medical applications, such as treatment of esophageal
diseases or reinforcing constricted blood vessels or urinary tracts. The stent
is usually placed into a constricted portion of a patient's body using a
delivery
system, e.g. a catheter.
[003] When a medical stent is used for treatment of an esophageal
disease, such as esophageal tumor or stricture, the stent is placed at the
lesion within the esophagus to maintain the esophageal lumen open. If the
tumor or stricture is located near the junction between the stomach and the
esophagus, the esophageal stent is often implanted across the lower
esophageal sphincter (i.e. the ring-like muscle that constricts and relaxes
the
esophagus as required by normal physiological functions). However, the
implantation of a stent across the normally-closed esophageal sphincter may
hold the sphincter open unintentionally and cause harmful gastric acid reflux
from the stomach into the esophagus.
[004] In order to reduce the gastric acid reflux, it has been proposed
to use an anti-reflux valve with an esophageal stent. An example of
esophageal stent with an anti-reflux valve is disclosed by Kocher et al.
("Esophageal Stent with Antireflux Valve for Tumors Involving the Cardia:
Work in Progress," JVIR 1998; 9:1007-1010). The anti-reflux valve of Kocher
et al. is made of a pliable, soft polyurethane sleeve attached to the lower
end
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
2
of the stent. However, there are several problems associated with this type of
stent. For example, the sleeve must be long enough to prevent the reflux and
act as a barrier wall to defeat capillary flow of acid up the bore of the
device.
Since the sleeve must be long, greater deployment force and more complex
delivery catheter designs are required. Typically, the length of the sleeve
ranges from about 50 to 120 mm and requires extra length on the delivery
system to envelope it in the "folded" condition prior to deployment. In
addition, the sleeve may become twisted, tangled, or kinked, which may
inhibit the passage of food into the stomach. The sleeve also may become
reversed and pushed up into the esophagus during vomiting. In that case, it
may be difficult for the sleeve to return to its properly functioning
position.
SUMMARY OF THE INVENTION
[005] To overcome the drawbacks of the prior art and in accordance
with the purposes of the invention, as embodied and broadly described
herein, one aspect of the invention provides an esophageal medical stent
having a rigid but elastic valve formed, preferably, near a distal end portion
of
the stent. The valve is normally closed but configured to open in response to
a predetermined condition. For an esophageal stent, the predetermined
condition may be a pressure difference between the upstream and the
downstream of the valve. The normally closed valve then allows easy
opening of the valve when the pressure difference exceeds a predetermined
threshold value. For instance, a passage of food from the esophagus into the
stomach causes the pressure difference across the valve large enough to
open the valve and, upon completion of the food passage, the valve returns to
its normal-closed state to prevent the reflux. A reverse backflow.due to, for
example, vomiting, which causes a large pressure difference, may also be
permitted by configuring the valve with an appropriate threshold value for the
reverse backflow.
[006] According to another aspect of the present invention, the
esophageal stent of the present invention may also be used for treatment of
the gastroesophageal reflux disease (GERD). GERD is a frequent backflow
of harmful gastric acid from the stomach into the esophagus. When the lower
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
3
esophageal sphincter inadvertently relaxes at inappropriate times, e.g. after
meals, it allows acid and food particles to reflux back into the esophagus.
Although most of the reflux contents return back to the stomach, the
remaining gastric acid reflux irritates the wall of the esophagus and produces
discomfort or pain known as heartburn. GERD, however, is a medical
condition when such reflux is frequent or severe enough to cause more
significant problems. In order to treat GERD, a stent having an anti-reflux
valve of the present invention can be placed in the lower esophagus to
prevent the harmful gastric acid reflux.
[007] Another aspect of the present invention, therefore, is to provide
a method of manufacturing a medical stent having an valve. The method
includes: providing a generally tubular body formed of braided wires and
having a proximal end portion and a distal end portion; extending the braided
wires near the distal end portion; and deforming the extended wires to form
the valve, wherein the valve is configured to be normally closed and to be
open in response to a predetermined condition. The valve is formed basket-
shaped and at least a portion of the valve and/or at least a portion of the
tubular body are provided with a suitable covering material.
[008] Another aspect of the present invention is to provide a method
of manufacturing a medical stent having an elastomeric valve. The method
includes: providing a generally tubular body; positioning a fixture proximate
to
a portion of the tubular body; applying an elastomeric material onto the
fixture;
and removing the fixture to form the elastomeric valve, wherein the
elastomeric valve is configured to be normally closed and to be open in
response to a predetermined condition.
[009] In yet another aspect of the present invention, a method of
manufacturing a medical stent having a gasket valve includes: providing a
generally tubular body; and attaching an elastomeric gasket valve integral to
a
portion of the tubular body, wherein the integral gasket valve is configured
to
be normally closed and to be open in response to a predetermined condition.
[010] In still another aspect of the present invention, a medical stent
comprises: a generally tubular body formed of braided wires and having a
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
4
proximal end portion and a distal end portion; and a normally closed valve
formed from the braided wires extended from the distal end portion, wherein
the valve is configured to open in response to a predetermined condition. The
valve is a basket-shaped spring valve, and at least a portion of the tubular
body and/or the valve is provided with a suitable covering material.
[011] In still another aspect of the present invention, a medical stent
comprises: a generally tubular body having a proximal end portion and a distal
end portion; and a normally closed valve made of an elastomeric material and
formed integral to the distal portion of the tubular body, wherein the
elastomeric valve is configured to open in response to a predetermined
condition. The elastomeric valve is basket-shaped, and at least a portion of
the tubular body and/or the valve is provided with a suitable covering
material.
[012] Additional objects and advantages of the invention will be set
forth in part in the description which follows, and in part will be obvious
from
the description, or may be learned by practice of the invention. The objects
and advantages of the invention will be realized and attained by means of the
elements and combinations particularly pointed out in the appended claims.
[013] It is to be understood that both the foregoing general
description and the following detailed description are exemplary and
explanatory only and are not restrictive of the invention, as claimed.
BRIEF DESCRIPTION OF THE DRAWINGS
[014] The accompanying drawings, which are incorporated in and
constitute a part of this specification, illustrate the various embodiments of
the
invention and, together with the description, serve to explain its advantages
and principles.
[015] In the drawings:
[016] FIGS. 1A and 1 B are perspective views of embodiments of
medical stents with valves, according to the present invention;
[017] FIG. 2 is a schematic diagram showing a method of
manufacturing the embodiment shown in FIG. 1A, according to an
embodiment of the present invention;
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
[018] FIGS. 3A-D are side and cross-sectional views of various
embodiments of medical stents with valves, according to the present
invention, showing the open and closed states of the valves;
[019] FIG. 4 is a schematic diagram showing a method of
manufacturing the embodiment shown in FIG. 1 B, according to an
embodiment of the present invention;
[020] FIG. 5 is side and cross-sectional views of embodiments of
medical stents with valves, according to the present invention, showing the
open and closed states of the valves; and
[021] FIG. 6 is side and cross-sectional views of another
embodiment of a medical stent with a valve, according to the present
invention, showing the open and closed states of the valve.
DESCRIPTION OF THE EMBODIMENTS
[022] Reference will now be made in detail to the present
embodiments of the invention, examples of which are illustrated in the
accompanying drawings. Wherever possible, the same reference numbers
will be used throughout the drawings to refer to the same or like parts.
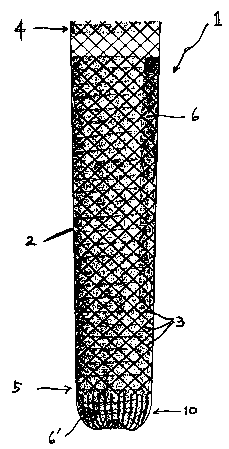
[023] Referring to FIG. 1A, a stent 1 according to an embodiment of
the present invention includes a self-expanding tubular body 2 having a
proximal end portion 4 and a distal end portion 5. The tubular body 2 is
formed by braiding or knitting a plurality of flexible wires 3 or filaments to
provide sufficient radial expansion force. The wires 3 or filaments can be
made of metal, polymeric materials, composites thereof, or other suitable
materials known in the art which exhibit sufficient elasticity, such as a
memory
material like nickel titanium alloy (i.e. nitinol). The tubular bodies 2 in
the
exemplary figures are of similar shape, i.e. funnel-like shape having the
cross-
sectional area in the proximal end portion being greater than the one in the
distal end portion. The shape of the tubular bodies 2 in the exemplary figures
are, however, not meant to limit or narrow the scope of the present invention.
One of ordinary skill in the art would recognize that other types and shapes
of
tubular bodies known in the art also may be used in the practice of the
present invention.
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
6
[024] At least a portion of the tubular body 2 is provided with a strong
covering 6 made of an elastic material such as polyurethane, silicone,
polytetrafluoroethylene (i.e. teflon), or other suitable material exhibiting
sufficient strength characteristics. The covering 6 functions primarily as a
barrier to resist tumor or other tissue ingrowth.
[025] Near the distal end portion of the tubular body 2, a basket-
shaped valve 10 is formed integral to the tubular body 2. The valve 10 is
normally closed to prevent acid reflux, but configured to open for the passage
of food from the esophagus into the stomach. The formation of the valve 10
can be carried out before or after heat treatment, or other final
manufacturing
steps, of the stent 1.
[026] A method of forming the valve 10 according to an embodiment
of the present invention is illustrated in FIG. 2. In this method, the braided
wires 3 are extended from the distal end portion 5 of the tubular body 2 to
form distal wires 8. The extended distal wires 8 are then deformed into a
basket-shaped valve 10. Deforming the distal wires 8 is performed, for
example, by curling the distal wires 8 inwards as shown in FIG. 2. Each of the
distal wires 8 is wound around and then released from a cylindrical member 9
so that the distal wires 8 form a basket-shaped spring valve 10, by curling
toward the center 12 of the circle 14 formed by the tubular body 2 (See FIG.
3A). FIG. 3A shows the side and distal end views of the embodiment of FIG.
1A. The distal end views include the open state (left-hand side) and closed
state (right-hand side) of the valve. The lower end view figures of FIG. 3A
show that the curled wires may also be curled sideways.
[027] According to another embodiment of the present invention,
shown in FIGS. 3B and 3C, the valves 10a, 10b are formed by straightening
the distal wires 8 and bending each of the distal wires 8 inwards at a
predetermined location 11, 11' of the distal wires 8.
[028] According to still another embodiment of the present invention,
shown in FIG. 3D, the valve 10c is formed by curling each of the distal wires
8
outward with the middle portion 13 of each of the distal wires 8 converged
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
7
toward the center 12 of the circle 14 formed by the tubular body 2 such that
each of the bent distal wires 8 forms a U-shaped wire.
[029] The valves 10, 10a, 10b, 10c also may be covered on at least
a portion of the valve 10, 10a, 10b, 10c with an elastic covering material to
function as a barrier to the reflux. The covering material is selected from a
group of polyurethane, silicone, and polytetrafluoroethylene (i.e. teflon), or
other suitable materials exhibiting similar characteristics. Preferably, the
same material used to cover the tubular portion 2 is used for the valve
covering material. The covering 6' of the valves 10, 10a, 10b, 10c may be
loose or pleated in order to not inhibit opening and closing of the valve 10,
10a, 10b, 10c.
[030] The right-hand side of FIGS. 3A-3D show cross-sectional
views of the valves 10, 10a, 10b, 10c looking down from the cross-sectional
plane A-A', which illustrate the open and closed states of the valves 10, 10a,
10b, 10c. The valves 10, 10a, 10b, 10c are normally closed but are
configured to open in response to a predetermined condition. The
predetermined condition may be a pressure difference between the upstream
and the downstream of the valves 10, 10a, 10b, 10c, so that the valves can
open while the pressure difference exceeds a certain threshold value, e.g.
one induced by a passage of food through into the stomach. The adjustment
of the threshold value may be performed, for example, by adjusting the
number of distal wires 8 used to form the valves 10, 10a, 10b, 10c. The
number of distal wires 8 can be tailored to create a certain amount of spring
force that allows the food to pass though the stent 1 into the stomach and
also
resists the backward reflux pressure. The threshold value also may be
adjusted by changing the material of the stent and wires, or the covering.
While the valves 10, 10a, 10b, 10c can be configured to be one-way valves, it
may be beneficial to allow occasional reverse opening caused by, for
example, vomiting, by configuring the valve (e.g. adjusting the spring force
required to open the valve) with an appropriate threshold value for a reverse
opening.
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
[031] The length of the valve ranges from 5 to 50 mm, preferably
from 10 to 30 mm. The length of the valve is relatively short compared to
other types of anti-reflux valves. Therefore, the valve of the present
invention
requires relatively lower deployment force and, thereby, uses less complex
delivery systems.
[032] FIG. 1 B shows a stent 1' having an elastomeric valve 20
according to still another embodiment of the present invention. In this
embodiment, a basket-shaped elastomeric valve 20 made of an elastomeric
material is formed integral to the distal end portion of the tubular body 2.
Although the tubular body 2 is formed of knitted or braided wires in the
exemplary figure, one skilled in the art would recognize that other types of
tubular bodies known in the art may also be utilized in the present invention.
[033] The elastomeric valve 20 functions similarly to the valve
illustrated in FIG. 1 B. The valve 20 is normally closed to prevent acid
reflux
but openable for the passage of food from the esophagus into the stomach.
At least a portion of the tubular body 2 is provided with strong covering 6
made of an elastic material such as polyurethane, silicone, or
polytetrafluoroethylene (i.e. teflon). The valve 20 may be provided with a
small opening 21 at the bottom of the valve 20, and the opening 21 may have
pleats or cuts 22 to facilitate easy opening of the valve 20. The purpose of
the opening 21 is to allow the flow of gas from the stomach up through the
esophagus so as to allow a patient to burp and relieve gas pressure in the
stomach. However, the opening 21 is small enough to prevent the passage of
significant amount of liquid or solid. The diameter of the opening 21
generally
ranges from 1 to 10 mm, preferably around 3 mm.
[034] A method of forming the elastomeric valve 20 is schematically
illustrated in FIG. 4. A mold or fixture 15 for a shape of the elastomeric
valve
20, preferably basket-shaped, is attached to the distal end portion 5 of the
tubular body 2. The fixture 15 is preferably inserted from the proximal end
portion 4 with an elongated handle 16. On the surface of the fixture 15, a
protrusion 19 is formed to provide a small opening 21 in the elastomeric
basket valve 20. Once the fixture 15 is fixed to the tubular body 2, the
fixture
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
9
15 is coated with an elastomeric material 18, preferably the same material
used for covering the tubular body 2 of the stent. The coating can be
performed, for example, by spraying 17 or dip-covering (not shown) the
elastomeric material 18 onto the surface of the fixture 15. Preferably, this
step is performed simultaneously with a step of covering the tubular portion 2
with the covering material 6. After the coating, the elastomeric material 18
coated on the surface of the fixture 15 is cured and the fixture 15 is removed
from the stent 1'. The elastomeric valve 20 having the shape of the fixture 15
is thereby formed. Although the exemplary embodiment illustrates the valve
20 attached at the distal end portion, one skilled in the art would recognize
that the valve can be formed anywhere in the tubular body 2 between the
proximal end portion 4 and the distal end portion 5.
[035] While the elastomeric valve 20 does not have the structural
reinforcement of the wires 3, the valve 20 is relatively rigid and, at the
same
time, sufficiently elastic to allow it to stretch open while food passes
through
the stent 1' into the stomach and to spring back and close to prevent the
reflux. The valve 20 may be provided with pleats or slits 22 to facilitate
opening of the valve 20. FIG. 5 is the side and cross-sectional views of
embodiments of medical stents with valves. The upper and lower figures on
the right-hand side of in FIG. 5 show the open and closed states of the valve
20 having the opening 21 with and without slits 22, respectively. While the
slits 22 facilitate the opening of the valve 20, the valve 20 either with or
without the slits 22 on the opening 21 is capable of functioning in the manner
described above.
[036] The rigidity and elasticity of the elastomeric valve 20 can be
tailored by carefully selecting characteristic parameters, such as the type of
elastomeric material 18, coating thickness, number of slits 22, size of hole
21,
treatment processes, etc.
[037] FIG. 6 shows a stent 1 " with an integral gasket valve 30
according to still another embodiment of the present invention. The term
"gasket valve" is defined as a flat piece of "gasket" material (e.g., a
polymer or
CA 02429255 2003-05-16
WO 03/030782 PCT/US02/29831
elastomer) made to function as a valve by providing pleats or cuts in it. In
this
embodiment, a basket-shaped gasket valve 30 made of an elastomeric
material is integrally adhered, sewed, or injection-molded to the distal end
portion 5 of the tubular body 2. The integral gasket valve 30 is made of,
preferably, the same material 6 used for the tubular portion 2, such as
polyurethane, silicone, polytetrafluoroethylene (i.e. teflon), or other
suitable
material exhibiting similar characteristics.
[038] A flexible gasket valve used in a typical vascular introducer
sheath for interventional radiology may be used as the integral gasket valve
30. The integral gasket valve 30 also functions similarly as the basket valves
illustrated in FIGS. 1A and 1B, i.e. normally closed to prevent acid reflux
but
opened for passage of food from the esophagus into the stomach. A small
opening 21 is provided at the center of the gasket valve 30 and may be
provided with pleats or cuts 22 to facilitate easy opening of the valve 30.
[039] Other embodiments of the invention will be apparent to those
skilled in the art from consideration of the specification and practice of the
invention disclosed herein. It is intended that the specification and examples
be considered as exemplary only, with a true scope and spirit of the invention
being indicated by the following claims.