Note: Descriptions are shown in the official language in which they were submitted.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
ELECTRONIC TONGUE AS OZONE DETECTOR
The present invention relates to detectors of the type commonly referred to as
electronic
tongues, and in particular to an electronic tongue based on electrochemical
detection, for the
detection of the presence of ozone and measurement of its concentration in a
liquid sample.
Background of the Invention
The control of ozone levels in the ppm range is a very important tool i.a. for
the sterilization
of materials, e.g. preparations for medical use, equipment and apparatuses,
where ozone is
used for eliminating harmful and unwanted species. Ozone is a substance with
excellent
qualities to kill microbiological entities such as virus, bacteria, spores and
fungi. As ozone is
toxic to these entities already at low concentrations (ppm-range) it is
imperative to be able to
control and measure ozone on-line in real time. Such a method would be highly
valuable for
cleaning, disinfection and sterilization of various types of equipment and
processes, such as
medical devices, food and beverage processing equipment as well as in
agriculture and
breeding environments. The method could also be used for measuring the
oxidation of organic
material in the development and manufacturing of microelectronic products and
production
methods.
Ozone detectors according to prior art have been based on a number of
different methods.
Most methods require use of some kind of reagent, which means that either a
sample must be
withdrawn from the system in which the ozone is to be determined, or one has
to accept a
contamination of the system. The latter is unacceptable in e.g. sterilization
of water for
medical purposes. Spectroscopic methods would not cause such interferences,
but requires
fairly complex systems that are expensive. Also, they require the provision of
windows in the
light paths, where clogging can occur causing drift problems over time.
In WO 99/13325 there is disclosed an electronic tongue based on electrical
pulses according
to a pulse programme comprising a plurality of pulses in sequence and at
different
amplitudes, being applied to electrodes. The electrical pulses are i.a.
selected from voltage
pulses and current pulses. The obtained response signals are used as input to
a pattern
recognition program in a computer for interpretation and for outputting
results indicative of a
desired property of a sample, such as the concentration of an analyte, pH etc.
The analysis is
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
2
based on multivariate methods, such as PCA (Principal Component Analysis). A
brief account
of PCA is given in an article by F. Winquist et al in "An electronic tongue
based on
voltammetry", Analytica Chimica Acta, 357 (1997) 21-31. This article and the
WO
publication are both incorporated herein in their entirety by reference.
Summary of the Invention
The present inventors have now conceived a new application of an electronic
tongue of the
type discussed above, namely for detection of the presence of and the
measurement of the
concentration of ozone in a liquid sample.
The electronic tongue according to the invention is defined in claiml.
A system incorporating the inventive tongue is defined in claim 11.
The electronic tongue of the invention is based on voltammetry, and on a
specific selection of
metals) or metal alloys for the working electrode.
Advantages with the invention are i.a. the simplicity of the system, it is
long term stable. In
particular, it is possible to operate the system without a reference
electrode. Thereby any risk
of contamination of the system to be monitored with leaking electrolyte from a
reference
electrode is eliminated. Also, regular replacement of the reference electrode
is eliminated.
Such replacement would otherwise have to be done at regular intervals, and
adds further to
the overall cost.
Brief Description of the Drawings
The invention will be described in detain below with reference to the
drawings, in which
Fig. 1 shows a typical experimental setup for using the present invention;
Fig. 2 shows an embodiment of a sensor device incorporating the inventive
idea;
Fig. 3 shows a pulse sequence usable with the invention;
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
3
Fig. 4 is a PCA plot of a typical ozone measurement;
Fig. 5 shows correlation between measured and predicted concentration values
determined according to the invention;
Fig. 6a-d are PCA plots of measurements made with different single electrodes;
Fig. 7is a PCA plot of a measurement based on a four-electrode sensor with
different metals as electrodes;
Fig. 8 shows an alternative embodiment of a sensor according to the invention;
Fig. 9 shows still another embodiment of a sensor according to the invention;
Fig. 10 schematically illustrates an implementation of an inventive sensor in
a
sterilization equipment;
Fig. 11 is a schematic illustration of a LAPV stair case; and
Figs. 12-18 are graphs showing measurements with a number of electronic
tongues.
Detailed Description of Preferred Embodiments
For the purposes of the present invention the term "electronic tongue" shall
be taken to mean
a device comprising at least one sensing element, the response of which on
stimulus from a
sample is processed with multivariate methods. A "sensing element" can be any
one of a
plurality of devices, such as, but not limited to, electrodes at the surface
of which redox
reactions take place.
The invention will now be described with reference to one embodiment using a
voltammetric
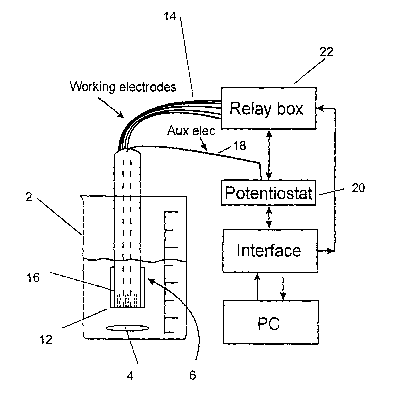
system, and a setup of this type is shown in Fig. 1. The setup includes a
sample reservoir 2
containing a sample, the ozone concentration of which is to be determined.
This reservoir can
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
4
be of a stationary type or designed as a flow cell, in the experiments
described below a
stationary cell with a magnetic stirrer 4 is used. A sensor device 6 is
immersed in the sample
liquid. The illustrated embodiment of the sensor device, shown in Fig 2,
comprises an
essentially rod shaped support structure ~, in which a plurality of metal
wires or metal pins 10
are imbedded, the ends of which are exposed. The exposed ends of the wires
form the
working electrodes 12 of the sensor device. The support structure is
preferably made of a
material that will ensure a very good sealing between the metal wires and the
material in
which they are embedded, in order to eliminate any interferences in the
measurements due to
liquid leaking in between the support material and the wire. A suitable
material is a dental
material, sold under the trade name KompositTM, FiltekTM 2250, obtainable from
3M Svenska
AB, Sweden. Of course any other material having the capability to provide
adequate sealing is
usable.
An Ag/AgCI (KCl 3M) electrode can be used as a reference electrode, however
other
conventional reference electrode well known to the skilled man are equally
well usable.
The measurement set up can be implemented in several ways. La. a standard
three-electrode
system can be employed, i.e. a working electrode, an auxiliary (counter)
electrode and a
reference electrode. Alternatively only a reference electrode and a working
electrode can be
used.
It should be noted however, that the invention works very well without the use
of any
reference electrode at all. Thus, in a preferred embodiment, a two-electrode
set up with a
working electrode and an auxiliary electrode is used. The potentials are
controlled
electronically andlor with software in the control unit (e.g. a potentiostat).
In the shown embodiment (Fig. 2) the sensor device comprises 6 working
electrodes 12, made
of different metals. However, the number of electrodes is not critical and
could range from
one single electrode up to several tens of electrodes or even more. The limit
is in principle
only set by the number of external connections to be made. It becomes
increasingly difficult if
several hundred electrodes are to be connected to external devices, although
it should not be
ruled out as a possibility. The metals from which the electrodes are made can
be selected from
one ore more members of the group consisting of Rh, Pt, Au, Os, Ru, Ni, Ti, Re
and alloys
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
thereof, or alloys thereof with other metals. Any metal that yields the
desired effect would of
course be applicable.
The metal wires extend throughout the support 8 and exits at the opposite end
where they are
connected to electrical leads 14. As an auxiliary electrode 16 (counter
electrode) a tube of
stainless steel encloses the rod shaped support structure in a tight fit. If
the apparatus or
system, in which the invention is implemented, is itself made of e.g.
stainless steel, the
apparatus housing could be used as a counter electrode. Other materials for
the auxiliary
electrode are of course conceivable, e.g. Pt, Au. An electrical lead 18 is
connected also to the
auxiliary electrode. The electrical leads from all electrodes are coupled to a
potentiostat 20.
The working electrodes are couple via a relay box 22 allowing each working
electrode to be
coupled separately in a two-electrode configuration (without reference), or
three-electrode
configuration (with reference).
Current and current transient responses are measured by a potentiostat MA 5410
(ISKRA,
Chemel AB, Lund, Sweden) connected via an interface. An electronic filter with
a time
constant of 0.3 seconds is applied to the potentiostat in order to smooth the
time transient
responses. A personal computer is used for controlling the system, e.g. the
timing of onset of
pulses, operation of the relay box, measuring current transient responses and
for the storage of
data. A computer program written in Labview~ 'National Instruments) is used to
define the
applied voltages on the electronic tongue, to control the sampling frequency
and to define the
data points to be stored in a data matrix.
For the experiments that will be discussed below, a measurement sequence was
composed
with two types of voltages and two electrochemical cleaning procedures applied
to the
electronic tongue. Of course it should be realized that this is only an
exemplary sequence, and
virtually any combination of pulses (amplitude, duration etc) can be used, so
long as a useful
result can be obtained. Regarding the shape of pulses, there are many options
available, e.g.
square/rectangular pulses (as in the example below), sawtooth, sine wave, etc.
Also, a four
electrode (Au, Ir, Pt, Rh) sensor device was used. The sequence used in the
experiments is as
follows (illustrated in Fig. 3):
A: Electrochemical cleaning
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
6
This procedure starts with a positive potential of 1.5 V applied to a working
electrode during
0.5 s. Then a negative potential of 2.1 V is applied during 0.5 s. Finally the
potential is set to
0 V during 2 s. This is repeated for all working electrodes.
B: Large Amplitude Pulse Voltammetry (LAPV)
The LAPV procedure starts with a potential of -2.1 V applied to a first
working electrode
during 0.5 s. The potential is then dropped to 0 V and maintained there for
0.5 s. Again a
negative potential, but 300mV higher than the first potential, is applied and
maintained for 0.5
s, whereupon the potential again is set to 0 V. This sequence is continued
until a final
maximum potential of +2.1 V is reached.
C: Electrochemical cleaning
The same procedure as in A is repeated.
D: Staircase voltammetry
A potential of -2.1 V is applied to the working electrode, this potential is
maintained for 0.5 s,
and is then increased by 300 mV in steps until the final maximum potential of
+2.1 V is
reached.
This whole sequence A-D is repeated for each working electrode in the sensor
device, i.e. four
in the illustrated embodiment, and is defined as one cycle.
The measurement consists in sampling current values from the response curve
generated as a
result of the potential pulse programme. The measurement sequence is divided
in 57 steps,
each step having a duration of 500 ms. Current values are sampled at a rate of
1000 Hz, and
thus each step generates 500 values, of which 19 are selected and stored in a
data matrix. The
selection of data points can be adapted to the specific case, and is not
critical to the method. It
is simply necessary to reduce the number of points to a reasonable number.
However, a
reasonable number can be very different from case to case. In certain cases
perhaps it is
sufficient with four points, in other circumstances of the order of 100 points
could be relevant.
Consequently, in the above example, for each electrode there will be 19 x 57 =
10S3 values
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
7
stored in the matrix, and totally for all four electrodes 4332 measurement
values are generated
and stored.
The data processing is done by multivariate analysis, in particular so-called
Principal
Component Analysis is used, and will be briefly discussed below, with
reference to Figs. 4-7.
Thus, for the example given above where four different working electrodes are
employed, a
measurement consists of performing one pulse sequence for each electrode,
which generates a
data matrix with 4332 values. This matrix can be looked upon as one point in a
4332-
dimensional space. Then, a new measurement is made, which generates a new
matrix of 4332
values, and finally a set of matrices representing a number of points in 4332-
dimensional
space has been generated. In Table 1 a full data sampling experiment of 147
measurements is
shown, and it will be discussed in some detail, and Fig 4 is a graphical
representation of the
data in Table 1.
Table I
CycleConc.Temp.CycleConc. TempCycleConc. Temp.CycleConc. Temp.
Os C 03 C 03 C 03 C
~ppm) (ppm) ~ppm) ~ppm)
1 0 31 48 3.0-2.932 95 2.9-3.031 142 0.8-0.932
2 0 31 49 3.0-2.932 96 2.9-3.031 143 0.9 32
3 0 31 50 3.0-2.932 97 2.9-3.031 144 0.9 32
4 0 31 51 3.0-2.932 98 2.9-3.031 145 2.4-2.932
5 0 31 52 3.0-2.932 99 2.9-3.031 146 2.9-3.032
6 0 31 53 3.0-2.932 100 2.9-3.031 147 2.9-3.032
7 0 31 54 3.0-2.932 101 2.9-3.031 148
8 0 31 55 3.0-2.932 102 2.9-3.031 149
9 0 31 56 3.0-2.932 103 31 150
10 0 31 57 3.0-2.932 104 2.0 31 151
11 0 31 58 2.5-1.932 105 31 152
12 0 31 59 1.9-1.632 106 31 153
13 0.7-2.231 60 1.6-1.332 107 1.5 31 154
14 2.2-2.631 61 1.l-1.032 108 31 155
15 2.6-2.831 62 1.0-0.932 109 31 156
16 2.9 31 63 0.9-0.832 110 1.2 31 157
17 2.9 31 64 0.8-0.732 111 31 158
18 31 65 0.7 32 112 1.0 31 159
19 3.0-2.932 66 0.7-0.632 113 31 160
32 67 0.6-0.532 114 31 161
21 32 68 0.5 32 115 0.8 31 162
22 3.0-2.932 69 0.5 32 116 31 163
23 3.0-2.932 70 0.5-0.432 117 0.7 31 164
24 3.0-2.932 71 0.4 32 118 0.6 31 165
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
8
25 3.0-2.932 72 0.4-0.332 119 31 166
26 3.0-2.932 73 0.3 32 120 31 167
27 3.0-2.932 74 0.3 32 121 0.5 31 168
28 3.0-2.932 75 0.3-0.232 122 31 169
29 3.0-2.932 76 0.2 32 123 31 170
30 3.0-2.932 77 0.2 32 124 0.4 31 171
31 3.0-2.932 78 0.2-0.132 125 172
32 3.0-2.932 79 0.1 32 126 173
33 3.0-2.932 80 0.1-0 32 127 0.3 32 174
34 3.0-2.932 81 0.1-0 32 128 32 175
m
35 3.0-2.932 82 0 32 129 32 176
36 3.0-2.932 83 0 32 130 0.2 32 177
37 3.0-2.932 84 0 32 131 32 178
38 3.0-2.932 85 0 32 132 32 179
39 3.0-2.932 86 0 32 133 0.1 32 180
40 3.0-2.932 87 0 32 134 32 181
41 3.0-2.932 88 1.7-2.732 135 32 182
42 3.0-2.932 89 2.7-2.932 136 0.1 32 183
43 3.0-2.932 90 2.8-3.032 137 0.1-0 32 184
44 3.0-2.932 91 138 32 185
45 3.0-2.932 92 2.9-3.031 139 0.1-0 32 186
46 3.0-2.932 93 2.9-3.031 140 0 32 187
47 3.0-2.932 94 2.9-3.031 141 0 32 188
Table 1 can be regarded as representing 147 points in 4332-dimensional space.
Applying PCA
to the data involves fording the direction in this space where the variance in
the data is the
largest. This will be a vector, called the fist principal component PC1, in
the 4332-
dimensional space. Subsequently the largest variance in a direction orthogonal
to the first
principal component, which of course also is a vector, called the second
principal component
PC2 (further principal components can be calculated, until most observations
are explained).
A new matrix, as defined by the principal components, is then formed, and the
data set is
considerably reduced, depending on the significance of the principal
components. In many
cases the reduction will be only to two dimensions. Thus, the two vectors, PC1
and PC2,
define a two-dimensional plane which maximizes the variation in the original
observations.
The 147 points in 4332-dimensional space are now projected down onto the plane
spanned by
PC1 and PC2. Thereby the graph shown in Fig. 4 is generated.
During the sequence of measurements, the system is changed in terms of
concentration of
ozone, either by actively increasing the concentration with an ozone
generator, or letting the
concentration decay by decomposition of ozone over time. Table 1 clearly
illustrates the
changes. Thus, in cycles #1-12 the concentration was 0 ppm, in #13-18 it was
gradually
increased and maintained at approx. 3 ppm during cycles 22-57. Then the
concentration was
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
9
allowed to decay in cycles #58-81 down to 0 ppm during cycles #82-87. Again an
increase in
the concentration was performed in cycles #88- 91 up to approx. 3 ppm during
cycles #92-
102, followed by a decay (#103-139). An increase of the concentration was
brought about in
# 142-147.
As can be seen the measurements can be subdivided into groups relating to
different states of
the system, such as different concentrations, concentration decay periods,
etc. The
measurements on which the graph of Fig. 4 is based, are used to build a model
for the data
analysis with respect to the ozone concentration. When this model is applied
to a set of
measurements on a system with unknown ozone concentration, a prediction of the
concentrations can be made.
In order to validate that the model holds, a plot of predicted values vs.
known values is made.
Such a plot is shown in Fig. 5. As can be seen the correlation is very good.
In Figs. 6a-d a set of measurements represented by PCA plots, using the pulse
sequence A-D
above, on individual electrodes of four different metals (Au, Pt, Ir, Rh) is
shown, and will be
briefly discussed below.
As is clearly seen, there are qualitative differences between the experiments,
the most obvious
being that the graph representing Rh (Fig. 6d) has a significantly larger
variation in the Y
direction than the others. This variation can be used for modeling purposes
and in particular it
is applied to the determination of ozone concentration.
In a further experiment illustrated by Fig. 7, the graph contains data from
measurements of all
four electrodes. It can be seen that the electrode made of Rh is a major
contributor to the
curve.
If a model is made on the basis of "training data", and a sensor with four
different metals is
used for measurements, it turns out that although the contribution from the
less "ozone
specific" metals (Au, Ir, Pt in the example above) is small, it turns out that
the overall
performance of the four electrode sensor is better than a sensor with a single
electrode of Rh.
This better performance is reflected in a better correlation coefficient in a
corresponding PLS
plot. An explanation is that in the data reduction process inherent in PCA,
any "white nose" in
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
1J
the data does not contribute, but instead any significant information,
regardless of its
magnitude will have a positive contribution, and the final result will
therefore be improved.
In the measurements discussed above, the potential in the pulse sequence was
varied between
-2.1 V and +2.1 V. However, it is possible to select other intervals for the
measurements, and
it is possible that one can optimize the sweep interval. In particular it is
possible that it could
be sufficient to work in only the negative range, e.g. 0 to -3.0 V, since the
redox potentials for
the possible reactions involving ozone are on the negative side.
It has turned out that the conductivity is relatively important for the
quality of t he results, in
that the higher the conductivity is, the better the measurements will be.
Therefore it can be
desirable to measure the conductivity in order to be able to adjust it by
adding ionic species,
where the system so allows. For a closed in-line system it would mostly not be
possible, and
sometimes undesirable, in particular in systems for sterilization. For the
conductivity
measurements, two extra electrodes can be provided on the same support in the
vicinity of the
working electrodes of the electronic tongue.
The embodiment of the sensor device as discussed above is only one of many
configurations
possible for the working electrodes. Another way to make a device having a
plurality of
electrodes is schematically illustrated in Fig. 8, and is obtained by
providing a plate like
planar support member 24 of ceramic or other inert material, on which parallel
strips 26 of
different metals have been deposited. If one edge of the plate is immersed in
a medium
containing ozone such that a portion of each metal strip is in contact with
the medium, the
other end of each strip can be coupled to a potentiostat, in a similar way as
indicated above
for the rod shaped sensor device.
Still another design of a sensor device, schematically shown in Fig. 9, is to
integrate electrode
strips 2~ in the walls of a tubing segment 30 as part of a circulation conduit
for e.g. a
sterilization process. The metal strips could be inset in the wall of a
special tube segment and
having electrical through-connections 32 at least at one end of each metal
strip, in order to
provide for connection to suitable peripheral equipment, such as a
potentiostat.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
11
The skilled man could envisage several other variations and modifications of
the actual
arrangement and configuration of electrodes for a sensor device according to
the present
invention, all of which are intended to fall within the scope of the attached
claims.
A great advantage of the detector and measurement system according to the
present invention
is that it is suitable for on-line measurements of ozone in e.g. sterilization
or purification
equipment, where it is required that contamination is prevented. In Fig. 10 a
schematic
illustration of such an application is shown.
Thus, the illustrated system for purification comprises a treatment chamber
34, which can be a
chamber containing utensils, such as surgical instruments, to be sterilized,
or in itself can
comprise an apparatus, such as a dialysis apparatus or the like. A feed
conduit 36 having an
inlet is 38 sealingly comlected to the chamber. An outlet conduit 40
transports the used ozone-
containing gas or liquid to disposal. It could of course in certain
applications be recirculated
back to the feed conduit (not shown). Ozone sensors 42, 44 according to the
invention A
control unit 46 can be coupled so as to control the sensors and in response to
their outputs
determine when a desired degree of e.g. sterilization has been achieved, and
if desired, to
regulate the level of ozone in the feed.
Thus, as shown, the invention can be implemented as a detection system for
ozone, preferably
on-line or in-line in the circulation system for the liquid, the ozone
concentration of which it
is desirable to monitor. Such a system would be based on voltammetry and
comprises at least
one working electrode made of a material as indicated above under the
discussion of the
sensor device, and a counter electrode. The electrodes are coupled to a
programmable pulse
generator capable of applying a predetermined sequence of energizing pulses to
said working
electrode(s), one at a time. The system further comprises a recording device
for recording the
output from said working electrode generated in response to said applied pulse
sequence. A
sampling device is provided for sampling values of said output at
predetermined intervals, and
the sampled values are stored in a memory in a matrix. There is a processing
unit for
performing a multivariate analysis of said data matrix, and a display device
for displaying the
result of said multivariate analysis.
Below a number of examples of measurements with different electronic tongues
will be given
with reference to tables and graphs.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
12
Calibration curves
To study the drift in the built-in amperometric sensor in the ozone generator
13 calibration
experiments were performed during this work. Three (four for calibration curve
1) separate
measurements formed the basis of one calibration curve. A multipoint working
curve with 3
repetitions and a wavelength of 260 nm were chosen. The standard samples
consisted of
deionized water with the ozone concentrations 1, 1,5, 2, and 3 ppm. As
reference solution
deionized water was used. See Tables and graphs below for detailed
information.
Record for measurements with spectophotometry
Calibration curve No. 1, 000829
Conc 03 1 2 3 4 MW Abs
(ppm) Abs Abs Abs Abs
1 0.0660.0590.06 0.0650.063
1.5 0.0910.0840.09 0.0870.088
2 0.1280.1130.1130.13 0.121
3 0.1860.1640.1640.1910.176
y = 0.059x + 0.0028
Correlation coefficient = 99.73%
Calibration curve No. 2, 000925
Conc 03 1 2 3 MW Abs
(ppm) Abs Abs Abs
1 0.0610.0640.06 0.063
1.5 0.0940.0870.0910.088
2 0.1120.1220.1180.121
3 0.1560.1800.1940.176
y = 0.057x + 0.0048
Correlation coefficient = 99.99%
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
13
Calibration curve No. 3, 001011
Conc 03 1 2 Abs 3 MW Abs
(ppm) Abs Abs
1 0.07 0.057 0.0590.062
1.5 0.1080.094 0.0990.1
2 0.1260.137 0.1360.133
3 0.2030.196 0.1890.196
y = 0.066x + 0.0017
Correlation coefficient = 99.90%
Calibration curve No. 4, 001108
Conc 03 1 2 3 MW Abs
(ppm) Abs Abs Abs
1 0.0680.0680.0630.066
1.5 0.1020.1020.0960.1
2 0.1250.1260.1240.125
3 0.1810.1950.1810.186
y = 0.059x + 0.0087
Correlation coefficient = 99.92%
Calibration curve No. 5, 001206
Conc 03 1 2 3 MW Abs
(ppm) Abs Abs Abs
1 0.0710.0640.0760.070
1.5 0.0880.0910.0910.09
2 0.1140.1230.1240.120
3 0.1660.1680.1800.171
y=0.051x+0.017
Correlation coefficient = 99.83%
A mearurement sequence (see Figure 11) was composed (Labview from National
Instruments) of two types of voltages and two electrochemical cleaning
procedures applied to
the electronic tongue in the following order:
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
I4
1) Electrochemical cleaning procedure
The electrochemical cleaning procedure of the electrode starts with a positive
voltage of 1.5 V
during 0.5 s. Then a negative potential of 1.5 V is applied for the same time.
Thereafter the
voltage Is set to 0 V for 2 s.
2) LAP V
The LAPV starts with a potential of -2.1 V, then the voltage is set to 0 V.
Then the potential is
increased by 300 mV and the sequence starts all over again. This continues
until the voltage
reaches a final maximum potential of +2.1 V.
3) Electrochemical cleanin~procedure
See I) above.
4) Staircase
The voltage starts at -2.1 V and is then increased by 300 mV until the final
maximum
potential is reached.
The measurement sequence is applied first to the gold wire, followed by the
wires of iridum,
platinum, and rhodium, which define a cycle. The measurement sequence was
divided in 57
steps, each with a step time of 500 ms. Current values are sampled with a
sample frequency of
1000 Hz. Each step generates 500 sample values (keys) of which nineteen are
stored in the
data matrix. On each working electrode 19 x 57 = 1083 values are stored in the
data matrix.
From all four working electrodes, 4 x 1083 = 4332 measurement values are
generated. The
applied potentials, the sampling frequency and the data points that are chosen
can be seen in
the table below.
Configureation for electronic tongue measurement
No. Cycles: 200
Time between cycles: 0 min
No. Propes: 4
SamplelStep: 495
Aq Rate: 1000 samplesls
No. Steps: 57
Step time: 500 ms
No.I~eys:l9
Data point/row: 4332
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
Output Out ut dataKeys
data
1.500 1.500 25
-1.500 0.000 50
0.000 1.800 75
0.000 0.000 100
0.000 2.100 125
0.000 -1.500 150
-2.100 0.000 175
0.000 0.000 200
-1.900 0.000 225
0.000 0.000 250
-1.500 -2.100 275
0.000 -1.800 300
-1.200 -1.500 325
0.000 -1.200 350
-0.900 -0.900 375
0.000 -0.600 400
-0.600 -0.300 425
0.000 0.000 450
-0.300 0.000 475
0.000 0.300
0.000 0.600
0,300 0.900
0,000 1.200
0.600 1.500
0.000 1.800
0.900 2.100
1.200 0.000
0.000 0.000
During the experiments the ozone concentration was manually varied from 0-3
ppm in the six
5 opening experiments. Thereafter an automatic program for changing the ozone
concentration
was used. The ozone concentration and the corresponding temperature were
recorded
manually respectively automatically for each cycle during the measurements.
In the six opening experiments the impact of a cold respectively warm ozone
generator, water
10 quality, old respectively new packing and conductivity were studied. See
the table below for
experiment data. For more detailed experiment data see the tables below, and
Figs 12-18.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
16
Record for measurement with the electronic tongue 000919 (Fib 12)
New deionized water (just before start), packing ring nr 1 and a cold ozone
generator are used
for experiment 1.
Cycle Conc Temp CycleConc Temp Cycle Conc Temp
03 03 03
(ppm) C (ppm) C (ppm) C
1 0 20 35 1.0-0.9 25 69
2 0 20 36 0.9-1.0 25 70
3 0 20 37 0.9-1.0 25 71
4 0 21 38 0.9-1.0 25 72*
0 21 39* 0.9-1.0 25 73 2.9-3.0 31
6 0 21 40 1.9-2.0 25 74 2.9-3.0 31
7 0 21 41 2.0-1.9 25 75 2.9-3.0 31
8 0 21 42 1.9-2.0 25 76 2.9-3.0 31
9 0 21 43 2.0 25 77 2.9-3.0 31.5
0 22 44 2.0-1.9 25 78 2.9-3.0 32
11 0 22 45 1.9 25 79 3.0-2.9 32
12 0 22 46 2.0-1.9 25 80 3.0-2.9 32
13 0 22 47 2.0-1.9 25 81 3.0-2.9 32
14 0 22 48 2.0-1.9 25 82 2.9-3.0 32
0 22 49 1.9-2.0 25 83 3.0-2.9 32
16 0 23 50 1.9-2.0 25 84 2.9-3.0 32
17 0 23 51 2.0 25.5 85 2.9-3.0 32
18* 0 23 52 1.9-2.0 26 86 2.9 32
19 1.4-1.7 23 53 1.9-2.0 26 87 2.9 32
1.7-1.6 23 54 1.9-2.0 26 88 2.9-3.0 32
21 1.6-1.4 23 55 1.9-2.0 26 89 2.9-3.0 32
22 1.2-1.1 23 56 1.9-2.0 26 90* 2.9 32
23 1.1-1.0 23.5 57 1.9-2.0 26 91 2.1-1.8 32
24 1.0-1.1 24 58 92 1.8-1.5 32
1.0-0.9 24 59 93 1.5-1.4 32
26 0.9 24 60 94 1.0-0.9 32
27 0.9-1.0 24 61 95 0.9-0.8 32
28 1.0 24 62 96 0.8-0.7 32
29 1.0 24 63 97 0.5-0.4 32
1.0 24 64 98 0.4-0.3 32
31 1.0 24 65 99 0.3-0.2 32
32 1.0-0.9 24.5 66 100 0.1-0 32
33 1.0 25 67 101 0 32
~ 34 1.0-1.1 25 68 ~ ~ 102
~ ~ ~ ~ 0 ~ 32
* The ozone concentration is changed manually after indicated cycle.
Every third cycle (1, 4, 7 etc.) and the data points from the electrochemical
cleaning are
excluded from the data analysis.
10 Record for measurement with the electronic tongue 000920 (Fig 13)
New deionized water (just before warming up), packing ring nr 1 and a warm
ozone generator
are used for experiment 2.
Cycle Conc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03
(PPm) C (PPm) C (PPm) C
1 0 33 40 1.9-2.0 32 79 2.9-3.0 32
2 0 33 41 1.9-2.0 32 80 3.0 32
3 0 33 42 1.9-2.0 32 81 3.0-2.9 32
4 0 33 43 0.9-2.0 32 82 2.9-3.0 32
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
0 33 44 0.9-2.0 32 83 3.0-2.9 32
6 0 33 45 0.9-2.1 32 84 3.0-2.9 32
7 0 33 46 2.0 32 85 3.0-2.9 32
8 0 33 47 2.0-1.9 32 86 2.9-3.0 32
9 0 33 48 1.9-2.0 32 87 2.9-3.0 32
0 33 49 1.9-2.0 32 88 2.9-3.0 32
11 0 33 50 2.0 32 89 2.9-3.0 32
12 0 33 51 2.0-1.9 32 90 2.9-3.0 32
13 0 33 52 2.0-1.9 32 91 2.9-3.0 32
14 0 33 53 2.0 32 92 2.9-3.0
0 33 54 1.9-2.1 32 93 2.9-3.0 31
16 0 33 55 2.0 32 94 2.9-3.0 31
17 0 33 56 2.0 32 95 2.9-3.0 31
18* 0 33 57 2.0 32 96* 2.9-3.0 31
19 0.5-0.8 33 58 97 2.3-2.1 31
0.8-0.9 33 59 98 2.1-1.8 31
21 0.9-1.0 33 60 99 1.8-1.6 31
22 0.9-1.0 33 61 100 1.3-1.2 31
23 1.0-0.9 33 62 101 1,2-1.1 31
24 1.0-0.9 32.5 63 102 1.1-1.0 31
1.0-0.9 32 64 103 0.8 31
26 0.9-1.0 32 65 104 0.8-0.7 31
27 0.9-1.0 32 66 105 0.7-0.6 31
28 0.9-1.0 32 67 106 0.5 31
29 0.9-1.0 32 68 107 0.5-0.4 31
0.9-1.0 32 69 108 0.4 31
31 0.9-1,0 32 70 109 0.3 31
32 0.9-1.0 32 71 110 0.3-0.2 31
33 1.0-0.9 32 72 111 0.2 31
34 0.9-1.0 32 73 112 0.1 31
0.9-1.0 32 74 113 0.1 31
36 0.9-1.0 32 75 114 0.1 31
37 0.9-1.0 32 76 115 0-0.1 31
38 0.9-1.0 32 77 116 0 31
39* 0.9-1.0 32 78* 117 0 31
* The ozone concentration is changed manually after indicated cycle.
Every third cycle (1, 4, 7 etc.) and the data points from the electrochemical
cleaning are
excluded from the data analysis.
5
Record for measurement with the electronic tongue 000926
New milli-q water (just before warming up and before start), packing ring nr 1
and a warm
ozone generator are used for experiment 4.
Cycle Conc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03
~PPm) C ~PPm) C lPP C
m)
1 0 34 45 89 _
2 0 34 46 90
3 0 34 47 91
4 0 34 48 92
5 0 34 49 93 2.9-3.0 32
6 0 34 50 94 2.5-2,1 32
7 0 34 51 95 2.1-1.6 32
8 0 34 52 96 1.6-1.3 32
9 0 34 53 97 1.0-0.8 32
10 0 34 54 98 0.8-0.7 32
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
18
11 0 34 55 99 0.7-0.6 32
12* 0 34 56 32 100 0.5 32
13 0-0.5 33.5 57* 32 101 0.4 32
14 0.5-1.0 33 58 2.6 32 102 0.4-0.3 32
15 1.0-1.6 33 59 103 0.3-0.2 32
16 2.0-2.3 33 60 104 0.2-0.1 32
17 2.3 33 61 105 O.1 32
18 2.3-2.4 33 62 106 0.1-0 32
19 2.4-2.5 33 63 107 0 32
20 2.5 33 64 108 0 32
21 2.6 33 65 109 0 32
22 66 110 0 32
23 2.7 33 67 111 0 32
24 2.7 33 68 112 0 32
25 69 113 0 32
26 70 114* 0 32
27 71 115 0.7-0.9 32
28 72* 0 32 116 0.9-1.0 32
29 73 117 0,9-1.0 32
30 74 118 0.9-1.0 32
31 75 2.9 32 119 1.0 32
32 76 120* 1.0 32
33 77 121 1.0-1.8 32
34 78 122 1.8-2.0 32
35 79 123 1.9-2.0 32
36 80 124 1.9-2.0 32
37 81 125 1.9-2.0 32
38 82 126* 1.9-2.0 32
39 83 127 2.8-2.9 32
40 84 128 2.9-3.0 32
41 85 129 2.9-3.0 32
42 86 130 2.9-3.0 32
43 87 131 2.9-3.0 32
44 88 132 2.9-3.0 32
* The ozone concentration is changed manually after indicated cycle. Every
third cycle (1, 4,
7 etc.) and the data points from the electrochemical cleaning are excluded
from the data
analysis.
Record for measurement with the electronic tongue 000927 (Fig. 15)
New milli-q water (just before warming up and before start), packing ring nr 1
and a warm
ozone generator are used for experiment 5. Conductivity measurements are
performed as well.
Cycle Conc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03 C
(ppm) C (ppm) C (ppm)
1 0 31 43 0.2 32 88
2 0 31 44 0.2 32 89
3 0 31 45 0.2 32 90
Cond 1.6 46 0.2 32 91
S
4 0 31 47 0.1 32 92
0 31 48 0.1 32 93
6* 0 31 49 0.1 32 94
7 0.3-1.731 50 95
8 1.7-2.331 51 0 32 96
9 2.3-2.631 52 97
2.7-2.831 53 98
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
19
11 2.8-2.9 31 54 99
12 2.9 31 55 100
Cond 4.5 S 56 101
13 2.9-3.0 31 57 102
14 2.9-3.0 31 58 103
15 2.9-3.0 31 59 0 32 104
16 2.9-3.0 31 60 105
17 2.9-3.0 31 Cond 7.6 S 106
18 2.9-3.0 31 61 0 32 107
Cond 7.3 S 62 108* 33
19 2.9-3.0 31 63* 109 33
20 2.9-3.0 31 64 110 1.9-1.4 32
21* 2.9-3.0 31 65 2.3-2.8 32 111 1.4-1.2 32
22 2.4-1,7 31 66 2.8-2.9 32 Cond 18.4
S
23 1.7-1.4 31 67 112 1.0-0.9 32
24 1.4-1.1 31 68 113 0.9-0.8 32
25 1.0-0.9 31 69 114 0.8-0.7 32
26 0.9-0.8 31 70 115 0.7 32
27 0.8 31 71 116 0.7 32
Cond 9.1 S 72 117 0.7 32
28 0.7-0.6 31 73 118 0.6 32
29 0.7-0.6 31 74 119 0,6 32
30 0.6 31 75 120* 0.6 32
31 0.6 31 76 121 0.5 32
32 0.6-0.5 31 77 122 0.5 32
33 0.5 31 78 123 0.5 31
34 0.5 31 79 124 0.4 31
35 0.5-0.4 31 80 125 0.4 31
36 0.4 31 81 126 0.4 31
37 0.4 31 82 127 0.3 31
38 0.4 31 83 128 0.3 31
39 0.4-0.3 31 84 129 0.3 31
40 0.3 32 85 130 0.3-0.2 31
41 0.3 32 86 131 0.2
42 0.3 32 87 132 0.2
Cycle Conc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03 C
(ppm) C (ppm) C (ppm)
133 0.2 32 140 0.1 32 146 2.8-2.9 31
134 0.2-0.1 32 141* 0.1 32 147 2.9-3.0 31
135 0.2-0.1 32 142 0.6-0.9 32 148 31
136 16.9 143 0.9-1.0 32 149 31
~S
137 0.1 32 144* 1.0 32 150 1.8-1.6 31
138 0.1 32 Cond 15.6
S
139 0.1 32 145 2.3-2.8 31
*The ozone concentration is changed manually after indicated cycle.
Cond = conductivity
Every third cycle (1, 4, 7 etc.) and the data points from the electrochemical
cleaning are
excluded from the data analysis.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
Record for measurement with the electronic tongue 001002 (Fig. 16)
New milli-q water (just before warming up and before start), packing ring nr 2
and a warm
ozone generator are used for experiment 6. Conductivity measurements axe
performed as well.
Cycle Conc Temp CycleConc Temp Cycle Conc Temp
03 03 03 C
~PPm) C ~PPm) C ~PPm)
1 0 32 44 0.4-0.3 32 88 1.2-2.6 26
2 0 32 45 0.4-0.3 32 89 2.6-3.0 26
3 0 32 46 0.3 32 90 2.9-3.0 26
Cond 2.4 S 47 0.3 32 91 2.9-3.0 26
4 0 32 48 0.2-0.3 32 92 2.9-3.0 26
5 0 32 49 0.2 32 93 26
6* 0 32 50 0.2 32 94
7 0.3-1.2 32 51 0.2-0.1 32 95
8 1.2-2.3 32 Cond 7.9 S 96
9 2.3-2.7 32 52 0.1 32 97
10 2.8-2.9 32 53 0.1 32 98 2.9-3.0 26
11 2.9 32 54 99
12 2.9-3.0 32 55 100
Cond 3.9 S 56 101
13 2.9-3.0 32 57 102 2.9-3.0 26
14 2.9-3.0 32 58 Cond 8.2 S
15 2.9-3.0 32 59 103
16 2.9-3.0 32 60 104
17 2.9-3.0 32 61 105 26
18 2.9-3.0 32 62 106
19 5.8 S 32 63* 107 2.9-3.0 26
20 2.9-3.0 32 64 108
21* 2.9-3.0 32 65 109 2.9-3.0 26
22 32 66 110 26
23 1.8-1.5 32 67 Cond 9.4 S
24 1.5-1.3 32 68 111 2.9-3.0 26
1.2-1.1 32 69 112 2.9-3.0 26
26 1.0-1.1 32 70 113
27 1.0 32 71 114* 2.9-3.0 26
Cond 7.9 S 72 115 26
28 0.9 32 73 116 26
29 0.9 32 74 117 26
0.8 32 75 118 26
31 0.8-0.7 32 76 119 1.7-1.6 26
32 0.7 32 77 120 1.6-1.5 26
33 0.7 32 78 121 26
34 0.6 32 79 122 26
0.6 32 80 123 26
36 0.6 32 81 124 26
37 0.6-0.5 32 82 125
38 0.5 32 83 126 1.2 26
39 0.5 32 84 127
0.5-0.4 32 8S 128
41 0.4 32 86 129 1.0
42 0.4 32 87* 130
43 0.4 32 Cond 5.1 S 131 0.9 26
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
21
Cycle Conc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03 C
(ppm) C (ppm) C (ppm)
132 137 142
133 138 0.7 25 143 0.5 25
134 0.8 139 144
135 140 145 0.4 25
136 0.7 25 141 0.6 25 146 0.4 25
*The ozone concentration is changed manually after indicated cycle.
Cond = conductivity
Every third cycle (1, 4, 7 etc.) and the data points from the electrochemical
cleaning are
excluded from the data analysis.
Record for measurement with the electronic tongue 001013 (Fig. 17)
New milli-q water (just before start), packing ring nr 2 and a cold ozone
generator are
used for experiment 7.
CycleConc Temp Cycle Conc Temp Cycle Conc Temp
03 03 03
(ppm) C (ppm) C (ppm) C
2 0.04 22.6 71 2.97 31.8 135 1.02 32.8
3 0.04 22.6 72 2.93 31.8 137 1.00 32.8
5 0.04 22.6 75 2.98 31.8 138 0.99 32.8
6 0.04 22.6 77 2.97 31.8 140 0.97 32.8
8 0.04 22.6 89 0.35 32.8 141 0.98 32.8
9 0.05 22.6 90 0.29 32.8 143 0.98 32.8
11 0.04 23.6 92 0.13 32.8 146 1.97 32.8
14 2.90 23.6 93 0.10 32.8 147 1.97 32.8
17 2.99 23.6 95 0.03 32.8 149 2.02 32.8
18 3.01 23.6 96 0.03 32.8 150 2.02 32.8
23 3.00 24.6 98 0.03 32.8 152 1.99 32.8
24 2.94 24.6 99 0.03 32.8 155 2.00 32.8
26 2.99 2.4.6 104 3.01 32.8 156 2.02 32.8
27 3.03 24.6 105 3.01 32.8 158 1.97 32.8
32 2.98 25.7 107 2.97 32.8 159 1.97 32.8
33 2.97 25.7 108 2.98 32.8 161 1.98 32.8
47 0.73 26.7 110 3.00 32.8 162 2.00 32.8
48 0.67 26.7 111 2.98 32.8 164 1.97 32.8
50 0.52 26.7 113 2.99 32.8 165 2.00 32.8
51 0.47 26.7 114 2.99 32.8 168 3.02 32.8
53 0.34 26.7 116 2.96 32.8 170 2.99 32.8
54 0.29 26.7 117 2.96 32.8 171 2.99 32.8
59 2.99 30.8 119 2.95 32.8 174 2.96 32.8
60 3.00 30.8 120 2.96 32.8 176 3.01 32.8
62 2.96 30.8 128 1.00 32.8 177 2.98 32.8
63 2.97 30.8 129 0.97 32.8 179 3.02 32.8
66 2.97 31.8 131 0.96 32.8 183 3.02 33.6
68 2.97 31.8 132 0.97 32.8 185 2.98 33.9
69 2.91 31.8 134 0.98 32.8 186 2.97 33.9
The ozone concentration is changed automatically, see the table below.
Every third cycle (1, 4, 7 etc.) (not included in the table above), the cycles
with an ozone
concentration that differs more than 0,1 ppm (not included in the table above)
and data points
from the electrochemical cleaning are excluded from the data analysis.
CA 02431836 2003-06-12
WO 02/052254 PCT/SE01/02848
22
Automatic program for the ozone generator:
Conc Time
03 min
(PPm)
0 30
3 60
0 60
3 60
0 60
3 60
1 60
2 60
3 60
Record for measurement with the electronic tongue 001014 (Fig. 18)
New milli-q water (just before start), packing ring nr 2 and a cold ozone
generator are used
for experiment 8.
CycleConc Temp Cycle Conc Temp Cycle Conc Temp
03 C 03 03
~PPm) (PPm) C (PPm) C
3 0.02 21.3 74 2.92 30.8 149 1.99 32.8
0.03 20.5 77 2.98 31.8 150 1.99 32.8
6 0.05 21.5 78 3.02 31.8 152 2.00 32.8
8 0.05 21.5 80 3.00 31.8 153 2.02 32.8
9 0.05 21.5 92 0.70 31.8 155 2.01 32.8
11 0.06 21.5 96 0.41 31.8 158 1.97 32.8
12 0.07 21.5 98 0.32 31.8 159 1.98 32.8
42 1.47 25.7 99 0.25 31.8 161 2.00 32.8
45 1.21 25.7 101 0.19 31.8 162 2.01 32.8
47 1.08 25.7 111 3.00 32.8 164 1.99 32.8
48 0.99 25.7 113 2.98 32.8 165 2.00 32.8
50 0.90 25.7 114 3.00 32.8 167 2.00 32.8
51 0.81 25.7 116 2.95 32.8 168 1.99 32.8
53 0.72 25.7 117 2.98 32.8 173 3.03 32.8
54 0.64 25.7 119 2.96 32.8 176 3.00 32.8
56 0.56 26.2 120 3.00 32.8 179 2.95 32.8
57 0.48 26.7 122 2.95 32.8 180 2.97 32.8
62 2.99 26.7 123 2.96 32.8 182 2.97 32.8
63 2.90 26.7 135 0.98 32.8 183 2.98 32.8
66 2.90 26.7 137 1.01 32.8 185 3.03 32.8
69 2.95 30.8 140 0.99 32.8 188 2.96 32.8
71 _2.98 30.8 143 0.98 32.8
72 ~ 2.96 30.8 144 1.00 32.8
The ozone concentration is changed automatically.
Every third cycle (1, 4, 7 etc.) (not included in the table above), the cycles
with an ozone
concentration that differs more than 0,1 ppm (not included in the table above)
and data points
from the electrochemical cleaning are excluded from the data analysis.