Note: Descriptions are shown in the official language in which they were submitted.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-1-
HYDROGEN SULPHIDE SCAVENGING METHOD
This invention relates to a high efficiency chemical
scavenging method for reducing hydrogen sulphide in
multiphase well streams, in particular where
conventional methods of removal such as the use of an
amine plant is not commercially viable.
Background
The presence of hydrogen sulphide in hydrocarbon fluids
is a well known problem in many oil and gas fields.
Hydrogen sulphide is an undesirable contaminant which
presents many environmental and safety hazards. It is
corrosive, malodorous, toxic if inhaled, a strong
irritant to eyes and mucous membranes and is associated
with the formation of acid rain. Accordingly it is
necessary to remove hydrogen sulphide from hydrocarbon
production, or at least reduce the levels of hydrogen
sulphide iri hydrocarbons during~the production, storage
or processing of hydrocarbon fluids to levels that
satisfy safety and product specification requirements.
Methods of removing or reducing hydrogen sulphide in
hydrocarbon production by treating the gas phase,
commonly termed hydrogen sulphide "removal" or
"scavenging" processes, are well known in the art. The
methods are generally described as being regenerative
(recoverable) or non-regenerative (non-recoverable).
One-known regenerative approach used in the oil
industry is to install contactor-based recoverable
scavenging systems which use suitable recoverable
chemical solvents such as alkanolaines to act as
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-2-
hydrogen sulphide absorption compounds which remove
hydrogen sulphides from the gas phase of separated
production streams.
Most commonly non-regenerative chemical scavengers are
injected into the gas phase. The non-regenerative
scavengers, such as triazines typically react with the
toxic hydrogen sulphide in-line to form a sulphur-
containing by-product. Such non-recoverable systems
are described for example in GB 22900542 A, EP-A-
0411745, US5354453, US6063346, US5674377, US5554349 and
US5744024.
Moreover, US6063346 describes the use, inter alia, of
formaldehyde to scavenge hydrogen sulphide non-
regeneratively from a hydrocarbon fluid that contains,
by weight of the fluid, between 5ppm and 200ppm of
hydrogen sulphide prior to treatment. However, it has,
up until the present invention, not been considered
feasible to remove hydrogen sulphide from hydrocarbon
fluids and, i~n particular, multiphase well streams and
other unprocessed streams containing higher
concentrations of hydrogen sulphide by non-regenerative
means.
Although contactor-based recoverable scavenging systems
are used to treat hydrocarbon fluids containing high
concentrations of hydrogen sulphide, these systems have
had limited application away from processing sites or
for relatively small fields with low production rates,
whether on-shore or off-shore. This is largely because
of the high capital and operating costs and the safety
issues that bringing such high levels of hydrogen
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
sulphide onto offshore platforms present. Furthermore,
it has been generally considered the non-recoverable
in-line methods would require the use of prohibitively
high amounts of chemical scavenger in order to reduce
the hydrogen sulphide levels sufficiently and would
promote the undesirable formation of large amounts of
reaction products. Accordingly, the development of
small offshore oil fields that contain such high levels
of hydrogen sulphide (250 ppm or more) has not been
considered to be economically viable or safe and thus
oil production form such fields is presently extremely
rate.
Accordingly, a need has been long felt in the industry
to develop a process which can successfully reduce
hydrogen sulphide concentrations to safe levels in
multiphase production containing high levels of
hydrogen sulphide, whilst overcoming problems such as
high cost, unacceptable safety hazards and unmanageable
solid by-product formation, that are associated with
developing sour oil reservoirs.
Svmmar~ of the Iaveatiox~.
According to the present invention there is provided a
method for reducing the amount of hydrogen sulphide in
a multiphase hydrocarbon produced fluid prior to phase
separation and processing, the method comprising the
step of adding formaldehyde to the produced fluid,
which produced fluid had, prior to the addition of
formaldehyde, a concentration of hydrogen sulphide of
at least 250ppm by weight of the fluid.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-4-
It has surprisingly been found that formaldehyde can be
used successfully to reduce the hydrogen sulphide
content of produced fluid that initially contains such
high levels of hydrogen sulphide which can be 10,000
ppm or more in the gas phase and more particularly more
than 250 ppm by weight of the multiphase hydrocarbon
fluid overall. Such technology is highly advantageous
since it allows, for example, oil production from
fields containing reservoir fluids having a hydrogen
sulphide content that is too high to satisfy safety
requirements or for commercial acceptance, anal which
would otherwise be left undeveloped, in a manner which
is highly effective and provides significant safety
benefits at relatively low cost.
The method of the invention is particularly valuable
because of its effectiveness on the liquid phase of the
multiphase system. In general, the multiphase system
will be crude oil which typically comprises a liquid
phase and an associated gas phase and may contain water
as a liquid phase additional to an oil phase and/or as
part of an oil/water phase. It is any such system
which is referred to herein as "produced fluid".
The method thus has particular application in the oil
industry, for example where the hydrocarbon fluid is an
oil reservoir fluid, such as crude oil and its
associated gas, and where the oil well produced fluids
(crude oil having a liquid phase and associated gas
phase) flow through a sub-sea flowline.
Although the method of this invention can be used to
good advantage when the produced fluid flows through an
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-5-
on-shore pipeline, the method is particularly useful
for in-line scavenging of hydrogen sulphide from a
subsea well-produced sour crude oil containing very
high hydrogen sulphide levels and where the well is
tied back via a flow line to a host facility at which
there is no provision for H2S scavenging and/or where a
H2S removal facility is too expensive and/or
impractical to install. As a result of this method,
the hydrogen sulphide content of the crude oil that is
delivered to the platform is reduced to safe and
commercially~acceptable levels and reaction by-product
formation is manageable. This process thus
advantageously provides a low cost manner of developing
sour oil fields that would otherwise not be safe or
economically viable to develop or advantageously
provides a way of modifying existing processes of
handling sour hydrocarbon fluids, for example, within
oil fields already in production, such that the method
according to the present invention can replace and/or
supplement existing methods.
The method of this invention is particularly suitable
for reducing the HzS content of the produced fluid by
at least 950. The examples which follow how efficiency
of scavenging to such an extent can be obtained.
However, the method can also be used to achieve less
efficient scavenging for example by a minimum of say
200, for example 500, 700 or 90a. This lower
efficiency may be acceptable when the H2S-reduced
produced fluid is to be co-mingled with a sweet
produced fluid.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-6-
Detailed Description, of the Ir~.ver~.tion,
The invention relates to a process for scavenging
(reducing or removing) hydrogen sulphide from any
hydrocarbon fluid that contains high levels of hydrogen
sulphide. By "high" is meant that the hydrogen
sulphide is contained in the produced fluid in an
amount, by weight of at least 250ppm, preferably at
least 500ppm, more preferably at least 1000ppm and most
preferably at least 2000ppm. This method is
IO particularly useful for scavenging hydrogen sulphide
from a produced fluid containing, by weight from 500 to
5000ppm so that, for example, the costs of the method
are economically justifiable.
The formaldehyde is usually added as an aqueous
solution, in the form of formalin. The formalin
solution typically comprises 30 to 40o active
formaldehyde, commonly being 37a active, with 5 to 10%
methanol added as a stabiliser. Where reference is
made hereinafter to quantities of formaldehyde, these
are related to 37% active formalin. Obviously,
adjustments are to be made in respect of formalin
solutions of different concentrations. The amount of
methanol may be increased in order to increase the low
temperature stability of formaldehyde and to compensate
for possible loss via the delivery systems.
In carrying out the method of the present invention,
the chemical scavenger, formaldehyde, is added to the
produced fluid in a concentration sufficient to reduce
substantially the amount of hydrogen sulphide in the
.30 fluid. Typically the formaldehyde will be used in an
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
_7_
amount in excess of stoichiometric with respect to the
hydrogen sulphide in the multiphase production.
Preferably, the formaldehyde is added to the fluid in a
ratio by weight of formaldehyde to hydrogen sulphide in
the multiphase production of from 2:1 to 8:1. It has
been found that where a higher ratio of formaldehyde to
hydrogen sulphide then. stoichiometric is used, the fate
of hydrogen sulphide removal may be increased.
.Thus, in order.to optimise formaldehyde use by
minimising reaction by-product formation whilst
maximising the efficiency of hydrogen sulphide removal,
a weight ratio of formaldehyde to hydrogen sulphide of
from 2:1 to 6:1 is preferred and a ratio of between 2:1
to 4:1 is optimal.
Once the formaldehyde has been added to the multiphase
production by any conventional means, such as chemical
injection, the formaldehyde disperses through the
produced fluid substantially homogeneously by the
natural turbulence of the fluid flow. A mixing device
may be also be used to achieve thorough mixing if
desired.
Tn order to optimise the reduction of hydrogen sulphide
concentration in the produced fluid, the contact time
of the formaldehyde and hydrogen sulphide is preferably
at least 20 minutes. More preferably, the contact time
is from 30 to 60 minutes. For example, where the
weight ratio of formaldehyde added to hydrogen sulphide
to be removed is between 2:1 to 6:1, a contact time of
between 45 to 60 minutes has been found to achieve 95
to 99% removal of the hydrogen sulphide. Furthermore,
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
_g_
the temperature is preferably in the range 60 to 75 deg
C and, although no advantage in efficiency at higher
temperatures is seen, there is no detrimental effect at
up to 120 deg C. The efficiency and rate of reaction
is also pressure dependent and minimum pressure is
preferably 20 bar, more preferably 30 bar, and although
reaction continues at lower pressure, the scavenging
may not be to the same level.
In the treatment of produced fluid, the formaldehyde is
preferably added upstream at a point which provides an
appropriate residence time of the hydrogen sulphide and
formaldehyde in the production fluid. By the time the
produced fluid reaches the processing facility, the
hydrogen sulphide content of the fluid should generally
have been reduced to relatively safe and conventionally
treatable levels, such as between 0 to 600ppm by volume
in the gaseous phase.
In. situations where the contact time of the hydrogen
sulphide and formaldehyde is. restricted, for example
where the production well is close to the processing
facility the formaldehyde may be added into the
production tubing downhole as deep as may be necessary
to provide sufficient residence time to effect the
scavenging process.
.Any residual hydrogen sulphide that has not been
scavenged by the formaldehyde can be easily removed
from the gas phase by any conventional physical or
chemical method of reducing/removing hydrogen sulphide
from the separated gas phase containing low levels,
typically less than 600 ppm by volume. For example, a
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
_g_
chemical scavenger such as a triazine compound can be
added to the gaseous phase in the conventional manner.
A methyl triazine compound may be preferred due to its
efficiency in removing hydrogen sulphide from gaseous
hydrocarbon streams. The triazine can be added at a
ratio, by weight of triazine to hydrogen sulphide, of
between 15:1 to 6:1, most preferably between 8:1 to
13:1 and optimally at 10:1, to maximise the residual
hydrogen sulphide removal at minimum cost.
The method further comprises the step of adding water
to the hydrocarbon fluid. This is likely to be
necessary with dry crude oil production before water
production has occurred. When water production has
occurred, water content of the multiphase system may
become sufficiently high for water addition to be
obviated. The presence of water, optionally an
addition, advantageously improves the efficiency of the
scavenging reaction and provides a carrier phase for
some of the reaction products. If water is added,
addition is preferably at a point substantially
upstream of the processing facility in order to enhance
the dispersion of some insoluble reaction products,
which may be the by-products of the hydrogen sulphide
and formaldehyde reaction. Ideally the water is added
at substantially the same time as the formaldehyde to
be sure that water is present from the start of the
formaldehyde/hydrogen sulphide reactions. It has been
found that the addition of water does not reduce the
efficiency of hydrogen. sulphide removal by the
formaldehyde in this method and has no effect on the
stoichiometry o the reaction, which requires 1 mol of
formaldehyde as such for 1 mol of hydrogen sulphide.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-10-
Dry crude oil is regarded as being "substantially free
of water", by which is meant here less than 2o by
volume of water is present in the produced fluid. It
has been found that formaldehyde treatment of dry crude
oil, that contains a high level of hydrogen sulphide,
particularly higher than 250ppm by weight of the fluid,
may produce insoluble by-products. Solids formation is
particularly important to avoid in offshore oil
processing systems to prevent blockages occurring and
thus to reduce downtime of the production process. The
extent of the formation of oil-soluble or oil and
water-insoluble products is dependent on the mercaptan
content of the produced fluid. Mercaptans are usually
present in significant quantities in sour crudes and
act as 'chain terminators' in the formaldehyde
scavenging reaction preventing the formation of
insoluble high molecular weight products and results in
formation of oil soluble by-products. However if the
mercaptan level is low or does not prevent formation of
insoluble products the presence of a water phase allows
dispersion of the water insoluble reaction products.
This positive addition of a water phase to such water-
free produced fluids, particularly, but not only, dry
crude oil, advantageously minimises the concentration.
of solids to a manageable amount. By "manageable
amount" is typically meant less than 30 mg of solids
formation per ml of the water phase.
The water used in the water addition step may be sea
water, modified sea water or fresh water depending on
availability and compatibility. The water is
preferably added in an amount such that the reduction
of the capacity of the lines for carrying production
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-11-
fluid is minimised, whilst the dispersion of insoluble
by-products is maximised. An amount of water of at
least 50, even as much as 500, but preferably 5o to
10%, by volume of the produced fluid, is added.
The method of the present invention has been found to
be particularly efficient in circumstances where the
C02 content of the produced fluid is high. For example
up to 140 mol% of the gas phase. Indeed in
circumstances where the produced fluid comprises a
gaseous phase containing carbon dioxide, the formation
of insoluble products is minimised whilst hydrogen
sulphide removal remains efficient.
For a better understanding of the invention, and to
show how the same may be carried into effect, reference
will now be made, by way of example, to the
accompanying drawings, in which:
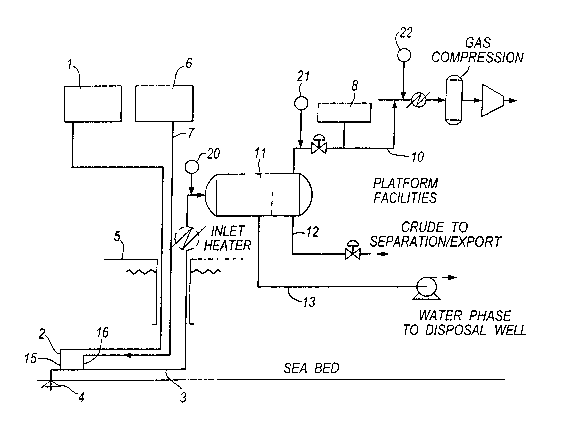
Figure 1 shows a schematic diagram showing the
application of the method of the present invention to
scavenge hydrogen sulphide from sour crude oil produced
via a subsea well; and
Figure 2 is a schematic diagram of a high pressure test
loop used in examples set out hereinafter and
With reference to Figure l, a typical application of
the method of the present invention is shown.
.A stabilised formaldehyde solution containing 37a
formaldehyde and 7% methanol is stored in the storage
tank 1. The tank 1 is connected by an injection or
umbilical line 2 to a valve injector (not shown) which
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-12-
is fitted into the wall of the flow line 3 immediately
down stream of a remote sub-sea wellhead 4. The valve
injector has a spray nozzle for atomising the
formaldehyde solution into the flowing stream of the
well produced crude oil flowing through the flowline 3
from the wellhead 4 to the platform 5.
Low sulphate sea water is supplied from facility 6
through an injection or umbilical line 7 to a valve
injector (not shown) which is fitted into the wall of
the flow line 3 immediately down stream of the
formaldehyde injection point 15.
Methyl triazine is stored in the storage tank 8. The
tank 8 is connected by an injection or umbilical line 9
to a valve injector (not shown) which is fitted into
the wall of the on-platform line 10.
Line 10 carries the gaseous phase of the production on
the platform after separation of the fluid stream. Line
12 carries the liquid phase and line 13 carries the
aqueous phase.
In operation, well-produced dry crude oil having a
liquid phase and a gaseous phase and having a hydrogen
sulphide concentration of 2000ppm by weight of the
fluid, flows as a liquid and gaseous stream along a 10
km sub-sea flowline 3 from the wellhead 4 to the
platform 5. The residence time of the crude oil in the
flow line 3 from wellhead 4 to platform 5 is
approximately 1 hour.. The dry crude oil contains 40molo
of carbon dioxide in the gaseous phase. The pumps of
the storage tank 1 and facility 6, pump formaldehyde
solution and water into the flow line 3 at points 15
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-13-
and 16 respectively. The water is injected into the
flow line, at substantially the same point as the
formaldehyde injection, in an amount of 5o by volume of
the crude oil.
The flowing gaseous phase is analysed from time to time
for example (at points 20, 21 and 22) in the
conventional manner to determine the hydrogen sulphide
content of the gaseous phase. The flow of formaldehyde
is adjusted in the conventional manner to add an amount
that is sufficient to reduce the crude oil hydrogen
sulphide concentration to less than 600ppm by volume in
the gaseous phase, at the point where the crude oil is
brought onto the platforms. This concentration is
measured at point 20. Typically, where the residence
time is approximately one hour and the temperature
within the flowline is around 65°C and average pressure
is 30 bar, approximately 2 to 3 litres of the
formaldehyde solution per kg of hydrogen sulphide to be
scavenged is sufficient to reduce the hydrogen sulphide
concentration to 100ppm by volume in the gaseous phase,
at the point where the crude oil is brought onto the
platform. However, the ratios of formaldehyde added to
hydrogen sulphide to be removed depends on the
temperature and residence time.
The crude oil is delivered to the platform 5 and fed
into a separator 11, which separates the gaseous
hydrocarbon phase, liquid hydrocarbon phase and aqueous
phase into separate lines 10, 12 and 13 on the
platform. The aqueous phase, containing some
formaldehyde/hydrogen sulphide reaction by-products is
delivered by flow line 13 to a disposal well. The
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-14-
liquid hydrocarbon phase containing less than lOppm
hydrogen sulphide by weight of the liquid and some oil
soluble reaction product is delivered by line 12 for
export or further processing. The gaseous phase
containing 100ppm by volume of hydrogen sulphide in the
gaseous phase is delivered by line 10 for further
scavenging treatment. For this purpose, methyl
triazine, stored in tank 8, is injected into the line
10, at a weight ratio of methyl triazine to hydrogen
. sulphide of approximately 10:1, in the conventional
manner, in order to scavenge the residual hydrogen
sulphide not removed by the sub-sea formaldehyde
treatment.
By virtue of the above described process, the oil
reservoir crude oil fluid that, prior to formaldehyde
treatment contained hydrogen sulphide at a
concentration of approximately 2000ppm by weight of the
crude oil fluid, is delivered to the platform
containing, in the gaseous phase, a hydrogen sulphide
concentration of 100ppm by volume of the gaseous phase
and contains an insignificant amount of solid by-
products~ typically less than 30 mg of the solids per
ml of the aqueous phase. After the methyl triazine
scavenging treatment, the gaseous phase contains less
than lOppm by volume of hydrogen sulphide. The liquid
phase, in line 12 contains less than lOppm by weight of
liquid.
In order that the present invention may be more readily
understood, the following examples are given, by way of
illustration only. All examples and test data are based
on tests that were carried out either in a high
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-15-
pressure autoclave cell or a closed test loop as
illustrated in Figures 2a and 2b.
Example 1
This example demonstrates the optimum contact time
required for formaldehyde to effectively reduce the
concentration of hydrogen sulphide in a dry crude oil
stream containing, prior to the addition of
formaldehyde, a hydrogen sulphide concentration of
8,500ppm by volume in the gaseous phase as measured at
atmospheric conditions. The dry crude oil stream was
treated in a high pressure autoclave cell formaldehyde
solution at a ratio by weight of formaldehyde to
hydrogen sulphide of 4:1 and at a temperature of 75°C
and 60 Bar pressure. The results are set out in Table 1
below. The data was generated from a high pressure
autoclave.
Table 1
Time (minutes) ppm H2S by volume
in gas phase
0 6000
5 min 5500
10 min 3500
15 min 3000
min 2000
min 1300
45 min 1100
60 min 700
75 min 600
90 min 600
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-16-
As can be seen from the results, significant hydrogen
sulphide removal is achieved, about 90 o removal being
achieved after 60 minutes contact time while subject to
vigorous mixing.
Example 2
This example compares the effectiveness of two
scavengers, formaldehyde and triazine, in reducing
hydrogen sulphide from a crude oil stream containing,
prior to the addition of scavenger, hydrogen sulphide
at a concentration of 8,500ppm by volume in the gaseous
phase of the crude oil. The crude oil stream was
treated in a line using the scavengers at a ratio by
weight of scavenger to hydrogen sulphide as shown in
Table 2 and at a temperature of 65°C. The results are
set out in Table 2 below. The data was generated in a
closed test loop as shown in Figure 2 of the
accompanying drawings and which was constructed and
operated as follows.
A test loop 100 having a volume of 340 litres was
constructed from a 70.5m length of 7.6cm diameter
stainless steel pipe and incorporated a vertical
separator vessel 31 having a volume of 197 litres and a
centrifugal circulation pump 32. A gas by-pass line 33
was extended from the top of the vessel 31 to a 28mm
flow restriction orifice 34 located in the pipework
between flanges approximately 5m downstream of the pump
32 and acting as a venturi to produce a vacuum when oil
was circulated. This sucked the gas from the top of
the separator vessel 31 into the loop 100 so creating a
gas circulation when a gas by-pass valve 35 was opened.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-17-
The venturi 34, allowed gas circulation to be
restricted when the by-pass valve 35 was closed.
The venturi 34 acted to ensure a constant flow rate by
balancing the outlet from pump 32 with the fluid level
in the separator vessel l, which was maintained at an
approximate level of 700-800mm by adjusting vessel
outlet valve 36.
The test loop incorporated also three in-line mixer
units 37a, 37b, 37c spaced around the loop at
approximately 0.7m, 29.5 m and 48.8m from the
restriction orifice at 36. These were removable to
determine the effect of different mixing regimes.
Temperature control was provided by heat tracing the
loop and separator vessel and insulation of the system
insulated.
Additional features of the test loop are injector 38
for scavenger, sampling valves 39a, 39b and 39c and
vent stack 40 and flushing valve 41.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-18-
Table 2
Efficiency of Formaldehyde and Triazine
HZS removal Efficiency
Time
(Minutes) Formaldehyde
applied at 6:1 Triazine applied at
12 :1
30 I 99.8 I 37.5
45 I 99.9 I 70.0
60 I 99.9 I 75.0
As can be seen from the results, formaldehyde is
significantly more efficient than triazine, even at a
lower ratio, at scavenging hydrogen sulphide from such
sour crude oil as was used for testing.
Example 3
This example demonstrates the effect of adding water to
the dry crude oil on the scavenging efficiency of
formaldehyde when treating the crude oil stream of
Example 1 under the same conditions as Example 1. The
results are set out in Table 3 below. The data was
generated from a high pressure autoclave.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-19-
Table 3
Efficiency of Formaldehyde under dry and 10o water cut
Time H2S removal % HAS o removal
(minutes) efficiency with dry efficiency with
oil water in oil
8.33 10.00
41.67 60.00
50.00 80.00
66.67 87.00
78.33 96.80
45 81.67 98.00
60 88.33 98.60
75 90.00 99.00
90 90.00 99.00
5 As can be seen from the results, the addition of water
makes a significant improvement on the scavenging
efficiency of formaldehyde with high pressure.
Example 4
10 This example demonstrates the effect of scavenging at
relatively low pressure of 20 bar under dry and with
loo water cut and the effect on the scavenging
efficiency of formaldehyde when treating the crude oil
under other conditions similar to Examples 1 and 3. The
15 data were generated from a high pressure autoclave.
The results are set out in Table 4 below:
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-20-
Table 4
Efficiency of Formaldehyde at 75°C under dry and 100
water cut conditions and at 60 Bar and 20 Bar pressures
and 75°C
Time (minutes)H2S removal HZS removalH2S removalHaS removal
% % % efficiencyefficiency
Efficiency efficiency with dry with
with with oil 10% water
dry oil @ 10% water @ 20 bar in oil
60 Bar in @ 20 bar
oil @ 60
bar
5 8.33 10.00 7.14 20.00
41.67 60.00 14.29 53.33
50.00 80.00 21.43 60.00
66.67 87.00 28.57 60.00
78.33 96.80 21.43 66.67
45 81.67 98.00 21.43 73.33
60 88.33 98.60 28.57 76.00
75 90.00 99.00 28.57 80.67
90 90.00 99.00 28.57 80.00
1
As can be seen from the results, the addition of water
make a significant improvement to the scavenging
process at lower pressure.
10 Example 5
This example demonstrates the effect of the presence of
carbon dioxide in the gaseous phase on the scavenging
efficiency of formaldehyde when treating the crude oil
under similar condition in terms of high H2S in the gas
15 phase (about 8000 - 10000 ppm), namely same chemical
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-21-
ratio of H2S scavenger, 65 C and 60. bar pressure. By
varying CO~ level in the mixture, it was demonstrated
that the efficiency would not be affected by the
different concentrations in the gas phase. The data
were generated using a high pressure autoclave. The
results are set out in Table 5 below.
Table 5
Efficiency of Formaldehyde when working under different
carbon dioxide contents and with loo water
Time ( minutes)HzS removalHAS removalHaS removalHaS removal
% % efficiency% efFciencyefficiency
efFciency with 30% with 20% without
with COZ in CO~ in any
40% COZ gas gas CO~ in.
in phase phase gas
gas phase phase
5 min 60.00 53.33 46.67 50.00
10 min 85.00 66.67 60.00 76.00
min 89.00 80.00 71.67 88.00
min 93.00 83.33 80.00 92.00
min 98.00 88.33 85.00 96.00
45 min 96.75 85.71 84.38 94.00
60 min 99.60 95.67 94.00 97.20
As can be seen from the results, the presence of carbon
dioxide does not markedly affect the scavenging
efficiency of formaldehyde.
Example 6
This example demonstrates the effect of temperature on
the scavenging efficiency of formaldehyde when treating
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-22-
the crude oil mixture under similar conditions to
earlier examples at 60 bar pressure, using 4:1
scavenging ratio, with 10a water by volume having been
added to the dry crude oil. The data were generated
using a high pressure autoclave. The results are set
out in Table 6 below.
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-23-
Table 6
Efficiency of Formaldehyde at different temperatures
and loo water cut
Time HaS removalHZS removalH2S H2S HZS
(minutes) % efficiency% efficiencyremoval removal removal
@ 45 C @ 65 C % % efficiency
efficiencyefficiency@ 120
@ 75 C @ 85 C C
5 35.71 40.00 10.00 57.45 50.00
35.71 50.00 60.00 78.72 71.43
64.29 50.00 80.00 81.91 87.14
71.43 66.00 87.00 92.55 97.14
73.57 72.00 96.80 98.72 98.86
45 78.57 79.00 98.00 97.87 99.50
60 82.86 85.00 98.60 98.5 i 99.43
75 85.00 96.00 99.00 98.51 99.29
90 85.71 98.60 1 99.00 98.72 99.29
( -
As can be 'seen from the results, the scavenging
efficiency of formaldehyde increases with temperature,
with an optimum scavenging efficiency being attained at
10 75°C.
Example 7
This example demonstrates the scavenging efficiency of
formaldehyde under different ratios by weight of
15 formaldehyde to hydrogen sulphide when treating the
crude oil stream of Example 1 under the same conditions
as Example 1 except that 50% water by volume was added
to the dry crude oil. The data were generated using a
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-24-
high pressure autoclave. The results are set out in
Table 7 below.
Table 7
Efficiency of Formaldehyde at different scavenging
ratios and with 50o by volume water
o HZS removal Efficiency at HCHO . HZO
ratio of
Time
(Minutes) 02:01 04:01 06:01
30 I 91.2 I 98.75 I 80.0
45 ~ 98.0 ~ 99.0 ~ 96.0
60 ~ 98.8 ~ 99.65 ~ 99.0
As can be seen from the results, a significant hydrogen
sulphide removal efficiency is achieved at a 4:1 ratio.
Example 8
This example demonstrates the effect.of different
mixing rates on the scavenging efficiency of
formaldehyde when treating a dry crude oil mixture
under similar conditions to earlier examples at 75°C,
60 Bar, 4'1 scavenging ratio, with loo water by volume
added to the dry crude oil. The data were generated
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-25-
using a high pressure autoclave. The data were
generated using a high pressure autoclave. The results
are set out in Table 8 below.
Table 8
Efficiency of Formaldehyde under different mixing rates
and 10o water cut
Time H2S removal H2S removal HaS removal
(minutes? % % efficiency
efficiency efficiency C 200 rpm
C 800 rpm C 400 rpm
5 10.00 20.00 8.33
60.00 30.00 25.00
80.00 60.00 29.17
87.00 74.00 25.00
96.80 87.00 25.00
45 98.00 95.00 33.33
60 98.60 96.00 66.67
75 99.00 98.20 70.83
90 99.00 99.20 ~ 71.67
As can be seen from the results, the scavenging
10 efficiency of formaldehyde improves with increase in
the mixing rate since the mass transfer will improve.
Example 9
This example demonstrates the effect of pressure on the
15 scavenging efficiency of formaldehyde at different
degrees of mixing of formaldehyde with dry crude oil
and loo water added. Testing was carried out using the
test loop:~of Figure 2. Different mixing regimes were
achieved by controlling the flow of the gas phase
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-26-
through the dedicated separator and into the
circulating liquid. The gas flow into the main oil
loop was controlled by the valve in the gas line. The
results are set out in Table 9 below.
Table 9
o Efficiency of Formaldehyde under dry and 10o water
cut conditions
oH2S removal % Efficiency
Time 10% 10% Dry oil 10% Dry oil 10%
water watex @ 60 water @ 20 water
in in Bar in Bar in
oil @ oil @ / gas oil @ / gas oil @
60 60 line 20 line 20
Bar / Bar / valve Bar / valve Bar /
gas gas closed gas closed gas
Iine valveline line line
open valve valve valve
closed closed open
5 25.00 14.29 25.00 25.00 42.86 10.00
10 50.00 42.86 55.00 52.50 71.43 60.00
75.00 72.86 82.50 82.50 90.00 76.00
85.00 84.29 86.25 85.00 90.00 83.00
95.00 90.00 90.00 90.00 92.86 89.00
45 97.75 95.43 95.00 95.25 94.57 95.00
60 99.25 98.29 96.75 97.50 96.29 97.40
As can be seen from the results, whatever the levels of
mixing between the gas and the liquid phases, whether
15 using dry or wet oil, and when working at relatively
low pressure or high pressure, the efficiency of
scavenging is within 95o after one hour in the test
loop. This is an indication of the efficiency of the
mass transfer of the chemical scavenger process and the
z0 flexibility of the process in achieving optimum
CA 02432071 2003-06-16
WO 02/48284 PCT/GBO1/05499
-27-
scavenging under different flow conditions in
multiphase loops.