Note: Descriptions are shown in the official language in which they were submitted.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
1
SUSTAINED RELEASE DRUG DELIVERY DEVICES
WITH MULTIPLE AGENTS
FIELD. OF THE INVENTION
The present invention relates to an improved device and method for delivering
multiple
agents directly to the interior portions of the body of a mammalian organism,
such as to
the eye. The method includes administration of multiple agents effective in
obtaining a
desired diagnostic effect or local or systemic physiological or
pharmacological effect by
inserting in a'rles'iied location in the body of a mammalian organism a
sustained release
drug delivery device.
BACKGROUND
Over the years, various drugs have been developed to assist in the treatment
of a wide
variety of ailments and diseases. However, in many instances such drugs are
not capable
of being administered either orally or intravenously without the risk of
various
detrimental side effects.
CMV retinitis is a disease that is characterized by inflammation of the retina
caused by
infection with cytomegalovirus. CMV retinitis is one of the most common causes
of
sight-threatening infections among people with HIV. The symptoms include loss
of
visual acuity, blind spots, and the loss of peripheral vision. Left untreated,
CMV retinitis
can lead to blindness.
Intravenous ganciclovir (GCV) is effective in the treatment of CMV retinitis
in AIDS,
patients, but bone marrow toxicity limits its usefulness. Continuous
maintenance GCV
therapy is necessary to prevent progression or recrudescence of the disease,
but despite
maintenance tllerapy a significant number of patients experience a relapse
during
treatment. Additionally, there are other risks and problems associated with
systemic
GCV administration.
CA 02432203 2006-12-18
2
Intravitreal GCV injections administered once or twice weekly have resulted in
temporary remission of CMV retinitis in AIDS patients. Intravitreal GCV
injections may
provide a higher intraocular drag concentration than systemic therapy and
reduce the
incidence of neutropenia. However, current treatment of CMV retinitis in AIDS
patients
is clearly suboptimal. Ganciclovir is virustatic and thus disease inhibition
requires
maintenance drug administration.
A more detailed explanation of the use of intravenous of GCV and intravitreal
injections
of GCV can be found in U.S. Patent No. 5,902,598..
A discussion of the difficulties associated with the systemic therapy of
cyclosporine A in the treatment of uveitis can be found in U.S. Patent Nos.
5,773,019
and 6,001,386.
Accordingly, there exists a strong need for the elimination of the undesirable
physiological problems associated with GCV treatment of CMV retinitis, while
maintaining the advantageous properties of this treatment. Although delivering
the drug
locally with injections may m;n;mize the systemic toxicity of GCV, repeated
injection is
not a practical mode of administration.
Due to the risks that certain (hugs impose, researchers have developed systems
for
administering such drags to aid in the treatment of these ailments and
diseases. A
general discussion of drug delivery control systems is provided in Controlled
Drug
Delivery (Part 1), Xue Shen Wu, Ph.D. pp32, 33, 44-46, 63, 66, and 67
(Technomic
Publishing Co. Inc., 1996).
The systems have been designed largely to reduce and to control the release
rate of incorporated drugs. However, these systems fail to achieve the
advantages
claimed by the present invention.
For example, U.S. Patent No. 4,014,335 to Arnold, relates to various ocular
inserts that
act as a deposit or drug reservoir for slowly releasing a drug into the tear
film for
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
3
prolonged periods of time. These inserts are fabricated as a three-layer
laminate of
flexible polymeric materials that are biologically inert, non-allergenic, and
insoluble in
tear fluid. To initiate the therapeutic programs of these devices, the ocular
inserts are
placed in the cul-de-sac between the sclera of the eyeball and the eyelid for
administering
the drug to the eye. Multiple layer laminate systems can present a challenge
to
reproducibly manufacture and are more difficult to produce by large-scale or
commercial
manufacturing procedures.
The device of U.S. Patent No. 3,416,530 is manufactured with a plurality of
capillary
openings that cominunicate between the exterior of the device and the interior
chamber
generally defined from a polymeric membrane. While the capillary openings in
this
construction are effective for releasing certain drugs to the eye, they add
considerable
complexity to the mailufacture of the device because it is difficult to
cont'rol the size of
these openings in commercial manufacturing using various polymers.
U.S. Patent No. 3,618,604 describes a device that does not involve such
capillary
openings, but instead provides for the release of the drug by diffusion
through a
polymeric membrane. The device, as disclosed in a preferred embodiment,
comprises a
sealed container with the drug contained in an interior chainber. Nonetheless,
as
described in U.S. Patent No. 4,014,335, certain problems have been identified
with such
devices such as the difficult task of sealing the margins of the membrane to
form the
container. In addition, stresses and strains introduced into the membrane
walls from
deformation during manufacturing of those devices may cause the reservoir to
rupture
and leak.
The above described systems and devices are intended to provide sustained
release of
drugs effective in treating patients at a desired local or systemic level for
obtaining
certain physiological or pharmacological effects. However, there are many
disadvantages associated with their use, including the fact that it is often
difficult to
obtain the desired release rate of the drug.
CA 02432203 2006-12-18
4
The need for a better release system is especially significant in the
treatment of CMV
retinitis. Thus, there remains a long-felt need in the art for an improved
device for
providing sustained release of a drug to a patient to obtain a desired local
or systemic
physiological or pharmacological effect.
Prior to the development of the present invention, there was developed a drag
delivery
device that ameliorated many of the problems associated with sustained release
drug
delivery. The device, which is disclosed in U.S. Patent No. 5,378,475,
included a first coating essentially impermeable to the
passage of the effective agent and a second coating permeable to the passage
of the
effective agent. In the device, the first coating covered at least a portion
of the inner
core; however, at least a small portion of the inner core is not coated with
the first
coating layer. The second coating layer essentially completely covers the
first coating
layer and the uncoated portion of the inner core. The portion of the innercore
which is
not coated with the first coating layer allows passage of the agent into the
second coating
layer thus allowing controlled release.
While the devices described in U.S. Patent No. 5,378,475 solve many of the
aforementioned problems pertaining to drug delivery, the devices and the
method of
making the devices are not without some problems. In particular, polymers
suitable for
coating the inner core are frequently relatively soft and technical
difficulties can arise in
production of uniform films. This is especially true when attempting to coat
non-
spherical bod'ies with edges (such as a cylindrical shape). In such cases,
relatively thick
films must be applied to achieve uninterrupted and uniform coatings, which
adds
significant bulk to the device. Thus, the devices tend to be larger than
necessary as a
result of the thickness needed to seal the ends of the inner core. In addition
to adding
bulk, multiple layer devices are more difficult to manufacture reproducibly
and are more
difficult to produce by large-scale manufacturing procedures. Often devices
such as these
require manual assembly that is time consuming, limits available supply, and
adds
variability.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
U.S. Patent No. 5,902,598 also presents solutions to some of the problems
associated
with manufacturing small devices. The device in U.S. Patent No. 5,902,598
includes a
third permeable coating layer that essen.tially completely covers the device.
While the
third coating layer improves the structural integrity of the device and helps
to prevent
potential lealcage, some manufacturing difficulties can limit scaled up
manufacturing.
For example, consistent application of the outermost coating layer and
reproducibility in
manufacturing can be problems with designs which require manual assembly, a
significant number of steps in the assembly process, or outer dip coatings.
In addition, depending on the materials selected for the outermost coating
layer of the
devices in U.S. Patent Nos. 5,902,598 and 5,378,475, there may exist a need to
cure the
entire device including the agent. Depending on the amount of curing required
and the
agents used, in some applications this could result in undesirable degradation
of the
agent.
U.S. Patent No. 6,004,582 provides a multi-layered osmotic device for the
controlled
delivery of one or more agents. The device includes a compressed core with a
first
active, a semi-permeable membrane with a passageway surrounding the core, and
an
erodible polymer coat coinprising a second active.
The problem of device size is extremely important in the design of devices for
implantation into the limited anatomical spaces such as small organs like the
eye. Larger
devices require more complex surgery to both implant and remove. The increased
complexity can result in complication, longer healing or recovery periods, and
potential
side effects (e.g. increased chance of astigmatism). Furtller, the extra
polymer required
to achieve a uniform coating reduces the potential internal voluine of the
implant and
hence limits the amount of drug that can be delivered, potentially limiting
both efficacy
and duration.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
6
The risk of infection is also a significant concern when implants are placed
in the body,
especially in organs such as the eye. Infections after implantation can
possibly lead to
complications such as blindness.
It would, therefore, be desirable to have a structurally stable device that
can be
reproducibly manufactured and manufactured by commercial techniques. It would
also
be desirable to have a device that can provide combination treatment, such as
treating the
immediate post-surgical risk of infection as well as providing the desired
long term
sustained release of agent. As a result of all of the above, there remains a
long felt need
in the art for an improved device that can provide sustained release of a drug
and deliver
multiple agents to a mammalian organism to obtain desired local or systemic
physiological or pharmacological effects, especially for ocular use.
SUMMARY OF THE INVENTION
The sustained release drug delivery device according to the first embodiment
of the
present invention comprises:
a) a drug core comprising a therapeutically effective amount of at
least one first agent effective in obtaining a diagnostic effect or effective
in obtaining a
desired local or systemic physiological or pharmacological effect;
b) at least one unitary cup essentially impermeable to the passage of
said agent that surrounds and defines an internal compartment to accept said
drug core,
said unitary cup comprising an open top end with at least one recessed groove
around at
least some portion of said open top end of said unitary cup;
c) a permeable plug which is permeable to the passage of said agent,
said permeable plug is positioned at said open top end of said unitary cup
wherein said
groove interacts with said permeable plug holding it in position and closing
said open top
end, said permeable plug allowing passage of said agent out of said drug core,
through
said permeable plug, and out said open top end of said unitary cup; and
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
7
d) at least one second agent effective in obtaining a diagnostic effect
or effective in obtaining a desired local or systemic physiological or
pharmacological
effect.
In accordance with another embodiment of the=present invention is a sustained
release
drug delivery device comprising:
a) a drug core comprising at least one agent effective in obtaining a
diagnostic effect or effective in obtaining a desired local or systemic
physiological or
pharmacological effect;
b) at least one unitary cup essentially impermeable to the passage of
said agent that surrounds and defines an internal coinpartment to accept said
drug core,
said unitary cup comprising an open top end and at least one lip around at
least a portion
of said open top end of said unitary cup;
c) a permeable plug permeable to the passage of said agent
positioned at said open top end of said unitary cup wherein said lip interacts
with said
permeable plug holding it in position and closing said open top end, said
permeable plug
allowing passage of said agent out of said drug core, through said permeable
plug, and
out said open top end of said unitary cup; and
d) at least one second agent effective in obtaining a diagnostic effect
or effective in obtaining a desired local or systemic physiological or
pharmacological
effect.
This invention is also directed to a method for providing controlled and
sustained
administration of an agent effective in obtaining a desired local or systemic
physiological
or pharmacological effect comprising inserting in a desired location in the
body of a
mammalian organism sustained release drug delivery devices of the first and
second
embodiments of the present invention.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
8
BRIEF DESCRIPTION OF THE DR.AWINGS
The drawings, which are not drawn to scale, are set forth to illustrate
various
embodiments of the invention. The drawings are as follows:
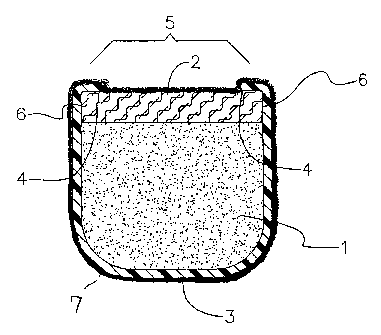
FIG. 1 of the present invention is an enlarged cross-sectional view down the
center of
one embodiment of the sustained release drug delivery device showing a unitary
cup
with a lip extending inward around some portion of the open end of the cup, a
permeable
plug, the cup and plug acting as a reservoir for the drug core, and an outer
coating of
agent.
FIG. 2 of the present invention is a.n enlarged cross-sectional view down the
center of
another embodiment of the sustained release drug delivery device showing a
unitary cup
with a recessed groove around some portion of the inside of the open end of
the cup, a
permeable plug, the cup and plug acting as a reservoir for the drug core.
FIG. 3 of the present invention is an enlarged top view of another embodiment
of the
sustained release drug delivery device showing a unitary cup with a plurality
of lips
extending inward around at least a portion of the open end of the cup, a
permeable plug,
the cup and plug acting as a reservoir for the drug core.
FIG. 4 of the present invention is an enlarged cross-sectional view down the
center of
another embodiment of the sustained release drug delivery device showing a
unitary cup
with a plurality of lips and an integral suture tab, a permeable plug, the cup
and plug
acting as a reservoir for the drug core.
FIG. 5 is an enlarged top view of the embodiment of a sustained release drug
delivery
device according to the present invention showing an lip extending outward
around only
a portion of the open end of the cup.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
9
FIG. 6 of the present invention is an enlarged cross-sectional view down the
center of
another embodiment of the sustained release drug delivery device showing a
unitary cup
with a plurality of grooves and an integral suture tab, an impermeable plug
with a
passageway, a permeable plug, the cup and plugs acting as a reservoir for the
drug core.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
DETAILED DESCRIPTION OF THE INVENTION
The inventors have unexpectedly discovered a sustained release drug delivery
device that
can be used provide combination treatment delivering multiple agents and
because of its
unitary cup and permeable plug design is structurally stabile and can be more
easily and
reproducibly manufactured than current designs that are known in the art.
In one preferred embodiment, the device includes an impermeable unitary cup
made of
silicone with an integral suture tab, the unitary cup acts as a reservoir for
a drug core
containing an agent such as fluocinolone acetonide. A hole through the
proximal end of
the suture tab enables a suture to be used for securing the device. The open
end of the
unitary cup has lips extending inwardly around a portion of the top open end
of said cup.
A permeable polymer solution of 10% polyvinyl alcohol (PVA) is filled in the
recess
above the drug core. The PVA solution is allowed to dry. The device is cured
for 60
minutes at 135-140 C. The PVA is sufficiently rigid to maintain its shape and
the
integrity of the device and thereby forming a permeable plug such that the
lips interact
with the plug holding it in position and closing the open top end. Together
the cup with
lips and the permeable plug act as a reservoir surrounding the drug core and
keeping it in
place. The entire device is coated with the antibiotic tobramyacin.
The expression "agent" as used herein broadly includes any compound,
composition of
inatter, or mixture thereof that can be delivered from the device to produce a
beneficial
and useful result.
The term "impermeable" refers to a material that is sufficiently impermeable
to
environmental fluids as well as ingredients contained within the delivery
device, such
that the migration of such fluids and ingredients into or out of the device
through the
impermeable material is so low as to have substantially no adverse impact on
the
function of the device.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
11
The term "permeable" refers to a material that is capable of being passed
through or
:= ~= ,
permeated. Permeating includes passing through openings, holes, pores, or
intersections.
The term "drug core" refers to any drug supply, drug depot, drug in
suspension, reservoir
or drug matrix. It includes one or more agents necessary to obtain the desired
diagnostic
effect or local or systemic physiological or pharmacological effect. It
includes any
excipients, suspending agents, or binders. Reference may be made to any
standard
pharmaceutical textbook such as Remington's Pharmaceutical Sciences. The drug
core
can be in liquid form, solid form, in dispersion, or any other form known in
the art.
Solid dose includes all conventional solid dose forms known in the art
including tablets
and pellets. Dispersions include all conventional forms lcnown in the art,
such as liquid
in liquid dispersions and solid in liquid dispersions.
The expression "passageway" as used herein includes an aperture, orifice, or
bore
sufficient to allow the agent to pass through. The passageway can be formed by
mechanical procedures such as erosion, laser, or molding; and chemical
procedures.
Referring to the drawing figures, like reference nunierals designate identical
or
corresponding elements throughout the several figures.
Turning now to the drawings in detail, which examples are not to be construed
as
limiting, one einbodiment of a device is indicated in Figure 1. While the
device shown
in Figure 1 is generally U like in shape, the cup can be any open container or
bowl of any
shape. Figure 1 is a cross sectional view of a drug delivery device in
accordance with the
present invention. Figure 1 includes an impermeable unitary cup 3 containing a
drug
core 1 comprising an agent, the cup 3 has lips 4 extending inward around the
open top
end 5 of the cup 3; and a permeable plug 2 formed of a material permeable to
the passage
of agent contained in the drug core 1. The permeable plug 2 is positioned in
the recess
between the top of the drug core 1 and below the lips 4 such that the lips 4
interact with
the permeable plug 2 holding it in position and closing the open top end 5 of
the cup 3.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
12
The lips 4 are the same impermeable material as the unitary cup 3 and protrude
inwardly
from the top open end 5 of the cup 3. The cup 3 and lips 4 are formed in a
single unitary
design to provide structural integrity to the device and facilitate
manufacturing and
handling. The lips 4 are designed to hold the plug 2 in place during use. They
can vary
in size or shape. The lips 4 of the present invention include nubs, tabs,
ridges, and any
other raised or protruding member.
The permeable plug 2 can be formed in the unitary cup by filling the penneable
material
in the device in one step, such as injecting a solution of PVA. The permeable
plug 2 can
be formed to various dimensional specifications which can be used to control
diffusion
properties to achieve a desired release rate. For example, changing the
ainount of the
permeable material filled into the cup can vary the thiclcness of the
permeable plug. The
same unitary cup and lips design can be used for implants with a variety of
release rates
making it possible to use a single manufacturing line or type of equipment.
Thus, the
present invention allows for ease of construction by more standard
manufacturing
techniques into devices with different release rates.
Together the cup 3 with lips 4 and the permeable plug 2 act as a reservoir
surrounding
the drug core 1 and keeping it in place. The agent diffuses out of the drug
core 1,
through the perineable plug 2, and out the open top end 5. The permeable plug
2 has
substantially the same radial extent as the cup 3, so that the only diffusion
pathway for
the agent(s) in the drug core is out of the plug 2 and not around the sides 6.
Glue or
other adhesion means can be employed to further bond the plug to the cup.
The entire device is coated with an additional agent 7, such as an antibiotic,
in order to
give an initial treatment or short term treatment with the additional agent.
This approach
may be especially useful when a device is first implanted into the mammalian
organism.
The antibiotic helps fight the post-surgical risk of infection. For example,
an infection
after surgical implantation of a sustained release device into the eye of a
mammalian
organism could possibly lead to complications such as blindness. Any portion
of the
devices in the present invention, such as the permeable plugs, the suture
tabs, the unitary
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
13
cups, or the drug core, can be coated with an additional agent as described
above. Any
method of coating known in the art may be used to coat the devices, or
portions of the
devices, of the present invention.
Coiubination treatment using multiple agents can be useful in treating or
diagnosing a
variety of conditions. The term of treatment or method of treatment does not
have to be
the same for each agent. It can be desirable to coat a low soluble agent with
a highly
soluble agent.
The invention further relates to a method for treating a mammalian organism to
obtain a
desired local or systemic physiological or pharinacological effect. The method
includes
administering the sustained release drug delivery device to the mammalian
organism and
allowing the agents effective in obtaining the desired local or systemic
physiological or
pharmacological to be delivered. The term "administering", as used herein,
means
positioning, inserting, injecting, implanting, or any other means for exposing
the device
to a mammalian organism. The route of administration depends on a variety of
factors
including type of response or treatment, type of agent, and the preferred site
of
administration. However, the preferred method is to insert the device into the
target
organ. In ocular applications, more preferably through a surgical procedure
followed by
suturing the device in place.
Figure 2 illustrates an enlarged cross sectional view down the center of a
sustained
release drug delivery device in accordance with the present invention. Figure
2 includes
an impermeable unitary cup 10 containing a drug core 1 coinprising an agent,
the cup 10
has a recessed groove 11 around the inside of the open top end 12 of the cup
10; and a
permeable plug 2 formed of a material permeable to the passage of agent
contained in the
drug core 1. The permeable plug 2 is formed with an additional agent in the
permeable
material such that the additional agent is also delivered to the organism in
need of
treatment. Additional agents can also be placed in the material of other parts
of the
sustained release drug delivery devices, such as in the suture tab. The
permeable plug 2
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
14
is positioned such that the groove 11 interacts with the permeable plug 2
holding it in
position and closing the open top end 12 of the cup 10.
Together the cup 10 with the groove 11 and the permeable plug 2 act as a
reservoir
surrounding the drug core 1 and keeping it in place. The agent diffuses out of
the drug
core 1, through the permeable plug 2, and out the open top end 12. The
additional agent
diffuses out of the permeable plug 2 and out the open top end 12. The
permeable plug 2
has substantially the same radial extent as the groove 11, so that the only
diffusion
pathway for the agent contained within the drug core 1 is out of the plug 2
and not
around the sides 6. Glue or other adliesion means can be employed to further
bond the
plug to the cup.
Figure 3 is an enlarged top view of another exemplary embodiment of a
sustained release
drug delivery device of the present invention. The view in Figure 3 is the top
of a
unitary cup comprising a plurality of lips 15 extending inwardly around the
open top end
of the cup. The permeable plug 2 is held in place by the lips 15 extending
inwardly
around the top open end of the cup.
Figure 4 is a enlarged cross sectional view of a drug delivery device in
accordance with
the present invention. Figure 4 includes an impermeable unitary cup 23
containing a
drug core 1 comprising an agent, the cup 23 has lips 24, 25 extending inward
around the
open top end 20 of the cup 23; and a permeable plug 2 formed of a material
permeable to
the passage of agent contained in the drug core 1. The permeable plug 2 is
positioned in
the recess between the top of the drug core 1 and the second lip 24 such that
the lips 24,
25 interact with the permeable plug 2 holding it in position and closing the
open top end
20 of the cup 23.
The cup 23 further comprises an integral suture tab 21 with a hole 22 through
the
proximal end through which a suture can be placed to anchor the device to a
structure of
the organism requiring treatment. The suture tab 21 can be coated with an
agent. The
proximal end of the suture tab is at the point of attachment, i.e. the point
where the suture
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
is attached. The preferred point of attachment is at the end of the suture tab
opposite the
cup.
The location of the suture and the structure the device is sutured to can be
any that meet
with current surgical techniques known in the art, such as the sclera of the
eye.
Depending upon the location of administration, the devices of the current
invention may
not require suturing in position.
Making the cup and suture tab in a single unitary design provides structural
integrity to
the device, and facilitates manufacturing and handling as one integral
structure. In
addition, by eliminating the assembly step of attaching the suture tab onto
the cup, the
single unitary design decreases variability in the size and shape of the
device.
Providing a suture hole 22 at the proximal end of the suture tab of the device
enables the
surgeon to attach the device without additional steps. Providing the suture
hole reduces
the possibility of tearing the tab while passing the needle through during
surgery. Some
materials, such as cured polyvinyl alcohol, are also very difficult to create
a suture hole
in once the device is assembled without causing cracks or breaks in the suture
tab.
The devices of the present invention may comprise a plurality of lips. These
lips can be
on the same vertical plane, as illustrated in Figure 3, or on a different
vertical plane, as
illustrated in Figure 4. The device may also be formed with any combination of
lips in
different vertical planes suitable to hold the permeable plug in place. For
example, a
single lip may be placed in the top vertical plane (position 24 in Figure 4)
and a plurality
of lips, as in Figure 3, at a lower vertical plane (position 25 in Figure 4)
positioned above
the drug core to facilitate holding the permeable plug in place. The funetion
of the lips is
to hold the permeable plug in place and prevent failure of the structural
integrity of the
device.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
16
The devices of the present invention that employ recessed grooves to secure
the
permeable plug in place may also have a plurality of grooves in the same or
different
vertical planes as described above.
Figure 5 is an enlarged top view of another exemplary embodiment of a
sustained release
drug delivery device of the present invention. The view in Figure 5 is the top
of a
unitary cup comprising a single lip 30. The permeable plug 2 is held in place
by the lip
30 extending inwardly around the top open end of the cup. The single lip can
extend
around the entire diameter of the top open end of the cup or extend around
some portion,
as illustrated in Figure 5.
Figure 6 illustrates an enlarged cross sectional view down the center of a
sustained
release drug delivery device in accordance with the present invention. Figure
6 includes
an impermeable unitary cup 35 containing a drug core 1 comprising at least one
agent,
the cup 35 has a plurality of grooves 38,39 around the inside of the open top
end 40 of
the cup 35; an impermeable plug 36 with a passageway 37, and a permeable plug
2
formed of a material permeable to the passage of agent contained in the drug
core 1. The
passageway 37 can also be filled with an additional agent. The impermeable
plug 36 is
positioned such that the groove 39 interacts with the impermeable plug 36
holding it in
position. The permeable plug 2 is positioned such that the groove 38 interacts
with the
permeable plug 2 holding it in position and closing the open top end 40 of the
cup 35.
Glue or other adhesion means can be employed to further bond the plugs to each
other or
the cup.
The impermeable plug of the embodiment in Figure 6, can interact with a
groove, as
illustrated, or be the same radial extent as the cup. An expanded recess
groove could
retain the impermeable plug and still provide an anchor groove for the
permeable plug.
The impermeable plug can also be utilized in this mamzer in the unitary cup
design that
comprises lip(s). Due to elastic nature of some polymers, such as silicone,
the same
result could be achieved by essentially molding the impermeable plug as part
of the
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
17
unitary cup and stretching the passageway wide enough to insert the tablet or
filling in a
liquid or powder drug core through the passageway.
Multiple agents can be delivered in a variety of ways using the sustained
release drug
delivery devices of the present invention. Multiple agents can be combined in
the same
drug core or the device can have multiple reservoirs. For example, the core
can be
achieved by placing two tablets with agents in a single reservoir, placing a
tablet in the
reservoir than filling in a liquid with additional agent, tableting two agents
together,
placing two liquid agents in the reservoir, or coating a tablet containing an
agent with an
additional agent. These exainples were not meant to be limiting and combining
two or
more agents in the core can be achieved by any method currently known in the
art.
The sustained release drug delivery devices of the present invention can also
be formed
with two reservoirs, as described herein, to deliver multiple agents. The
reservoirs can
be placed side by side or back to back. The device can be formed as a unitary
device
providing structural stability and ease of manufacture.
In combination with the examples above, a variety of inethods may also be
utilized to
provide adhesion of the components of the sustained release drug delivery
device, such
as adhesion of the permeable plug to the unitary cup portion of the device.
These
methods include the use of adhesives, polymers such as PVA, or any other
procedure
known in the art to provide adhesion at the points of contact between the
permeable plug
and the unitary cup. The sealant can be permeable or impermeable to the agent
or agents
in the device depending upon the method and location of application. If the
adhesive is
permeable to the beneficial agent, such as in the case of a permeable polymer,
it could be
applied on top of the drug core or directly to the permeable plug. The methods
to
improve adhesion will vary depending on the materials that the components are
manufactured from.
The above-described methods of adhesion may also be utilized to provide
adhesion of
the impermeable plug to the unitary cup or permeable plug. For example,
impermeable
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
18
adhesives could be applied to only the edges of the impermeable plug and
because the
adhesive is present only on the edges, it improves the bond between the
impermeable
plug and the device without interfering with diffusion through the
passageway(s) and the
permeable plug. If the adhesive is permeable to the beneficial agent, such as
in the case
of a permeable polymer, it could be applied on top of the drug core or
directly to the
impermeable plug before the impermeable plug is put into place.
The following classes of agents could be incorporated into the devices of the
present
invention: anesthetics and pain killing agents such as lidocaine and related
compounds
and benzodiazepam and related compounds; anti-cancer agents such as 5-
fluorouracil,
adriamycin and related compounds; anti-fungal agents such as fluconazole and
related
compounds; anti-viral agents such as trisodium phosphomonoformate,
trifluorothymidine, acyclovir, ganciclovir, DDI and AZT; cell
transport/mobilit~
impendin agents such as colchicine, vincristine, cytochalasin B and related
compounds;
antiglaucoma drugs such as beta-blockers: timolol, betaxolol, atenalol, etc;
antih,ypertensives; decon es~ tants such as phenylephrine, naphazoline, and
tetrahydrazoline; immunological response modifiers such as muramyl dipeptide
and
related compounds; peptides and proteins such as cyclosporin, insulin, growth
hormones,
insulin related growth factor, heat shock proteins and related compounds;
steroidal
compounds such as dexamethasone, prednisolone and related compounds; low
solubility
steroids such as fluocinolone acetonide and related compounds; carbonic
anhydrize
inhibitors; diagnostic agents; antiapoptosis agents; gene therapy agents;
sequestering
agents; reductants such as glutathione; antipermeability agents; antisense
compounds;
antiproliferative a ents; antibody conjugates; antidepressants; bloodflow
enhancers;
antiastlunatic drugs; antiparasiticagents; non-steroidal anti inflammatory
agents such as
ibuprofen; nutrients and vitamins: enzyme inhibitors; antioxidants;
anticataract drugs;
aldose reductase inhibitors; cytoprotectants; c okines, cytokine inhibitors,
and c, okin
protectants; uv blockers; mast cell stabilizers; and anti neovascular agents
such as
antiangiogenic agents like matrix metalloprotease inhibitors.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
19
Examples of such agents also include neuroprotectants such as nimodipine and
related
compounds; antibiotics such as tetracycline, chlortetracycline, bacitracin,
neomycin,
polymyxin, gramicidin, oxytetracycline, chloramphenicol, gentamycin, and
erythromycin; antiinfectives; aiitibacterials such as sulfonamides,
sulfacetamide,
sulfamethizole,sulfisoxazole; nitrofurazone, and sodium propionate;
antiallergenics such
as antazoline, methapyriline, chlorpheniramine, pyrilamine and
prophenpyridamine; anti-
inflammatories such as hydrocortisone, hydrocortisone acetate, dexamethasone
21-
phosphate, fluocinolone, medrysone, metllylprednisolone, prednisolone 21 -
phosphate,
prednisolone acetate, fluoromethalone, betainethasone and triminolone; miotics
and anti-
cholinesterase such as pilocarpine, eserine salicylate, carbachol, di-
isopropyl
fluorophosphate, phospholine iodine, and deinecarium bromide; mydriatics such
as
atropine sulfate, cyclopentolate, homatropine, scopolamine, tropicamide,
eucatropine,
and hydroxyamphetamine; sympathomimetics such as epinephrine; and prodrugs
such as
those described in Design of Prodrugs, edited by Hans Bundgaard, Elsevier
Scientific
Publishing Co., Amsterdam, 1985. In addition to the above agents, other agents
suitable
for treating, managing, or diagnosing conditions in a mammalian organism may
be
placed in the inner core and administered using the sustained release drug
delivery
devices of the current invention. Once again, reference may be made to any
standard
pharmaceutical textbook such as Remington's Pharmaceutical Sciences for the
identity of
other agents.
Any pharmaceutically acceptable form of such a compound may be employed in the
practice of the present invention, i.e., the free base or a pharmaceutically
acceptable salt
or ester thereof. Pharmaceutically acceptable salts, for instance, include
sulfate, lactate,
acetate, stearate, hydrochloride, tartrate, maleate and the like.
A large number of polymers can be used to construct the devices of the present
invention. The only requirements are that they are inert, non-immunogenic and
of the
desired permeability. Materials that may be suitable for fabricating the
device include
naturally occurring or synthetic materials that are biologically compatible
with body
fluids and body tissues, and essentially insoluble in the body fluids with
which the
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
material will come in contact. The use of rapidly dissolving materials or
materials highly
soluble in body fluids are to be avoided since dissolution of the wall would
affect the
constancy of the drug release, as well as the capability of the device to
remain in place
for a prolonged period of time.
Naturally occurring or synthetic materials that are biologically compatible
with body
fluids and eye tissues and essentially insoluble in body fluids which the
material will
come in contact include, but are not limited to, glass, metal, ceramics,
polyvinyl acetate,
cross-linked polyvinyl alcohol, cross-linked polyvinyl butyrate, ethylene
ethylacrylate
copolymer, polyethyl hexylacrylate, polyvinyl chloride, polyvinyl acetals,
plasiticized
ethylene vinylacetate copolymer, polyvinyl alcohol, polyvinyl acetate,
ethylene
vinylchloride copolymer, polyvinyl esters, polyvinylbutyrate, polyvinylfonnal,
polyamides, polymethylmethacrylate, polybutylmethacrylate, plasticized
polyvinyl
chloride, plasticized nylon, plasticized soft nylon, plasticized polyetliylene
terephthalate,
natural rubber, polyisoprene, polyisobutylene, polybutadiene, polyethylene,
polytetrafluoroethylene, polyvinylidene chloride, polyacrylonitrile, cross-
linked
polyvinylpyrrolidone, polytrifluorochloroethylene, chlorinated polyethylene,
poly(1,4'-
isopropylidene diphenylene carbonate), vinylidene chloride, acrylonitrile
copolymer,
vinyl chloride-diethyl fumerale copolymer, butadiene/styrene copolymers,
silicone
rubbers, especially the medical grade polydimethylsiloxanes, ethylene-
propylene rubber,
silicone-carbonate copolymers, vinylidene chloride-vinyl chloride copolymer,
vinyl
chloride-acrylonitrile copolymer and vinylidene chloride-acrylonitride
copolymer.
Additionally, the permeable member of the present invention can also be formed
of any
porous or semi-porous materials which will allow diffusion of the agent out of
said drug
core through said cup open end. If the prefabricated plug is made entirely
from the
permeable material, the material must be sufficiently rigid under the
conditions of use to
hold its shape and seal the top open end of the unitary cup. Such materials
include, but
are not limited to hydroxyapatite, porous polyethylene, calcium sulfate,
zeolites, rock,
porous ceramics, sinter, sintered metal, and porous metals.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
21
The device can be formulated in any convenient shape. For example, the device
can be
of any geometric shape dimensionally suitable for insertion in the eye. Thus,
the device
can be ellipsoid, rectangular, round, etc. The shape of the cup in the present
can be
optimized to provide a minimum profile for insertion.
The dimensions of the device can vary with the size of the device, the size of
the core or
reservoir, and the membrane that surrounds the core or reservoir. The targeted
disease
state, type of mammalian organism, location of administration, and agents or
agent
adininistered are among the factors which would effect the desired size of the
sustained
release drug delivery device.
The device according to the present invention may be made in a variety of
ways. For
example, if the unitary cup is going to be made entirely of polymer, then the
polymer can
be injection molded or die cast into a desired shape and size. The permeable
plug can
also be formed by any conventional means depending on the materials selected.
For
example, the permeable plug can be formed by injecting, pouring, adding drop
wise, or
molding the permeable material. Depending on the permeable material chosen, it
may be
required to dry and/or be cured to form the plug. The agent can be filled into
the
reservoir by any conventional means such as drop-wise, syringe, or pipette.
The agent
can also be made as a solid dose form such as a tablet or pellet and placed
into the
unitary cup. For example, a standard size tablet could be used with varying
compositions.
The preceding descriptions of how to make the device of the present invention
is merely
illustrative and should not be considered as limiting the scope of the
invention in any
way. In particular, the methods of making the device depend on the identity of
the agent.
The devices may be surgically implanted at or near the site of action. This is
the case for
devices of the present invention used in treatment of ocular conditions,
primary tumors,
rheumatic and arthritic conditions, and chronic pain. The devices may also be
implanted
subcutaneously, intramusclarly, intraarterially, or intraperitoneally. This is
the case
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
22
when devices are to give sustained systematic levels and avoid premature
metabolism.
In addition, such devices may be administered orally.
Once in place, the device functions as a drug reservoir gradually releasing
drug to the
organ such as the eye and surrounding tissue. This device is particularly
useful for
treating ocular conditions such as glaucoma, proliferative vitreoretimopathy,
diabetic
retinopathy, uveitis, and keratitis. The device is also particularly useful as
an ocular
device in treating mammalian organisms suffering from cytomegalovirus
retinitis
wherein the device is surgically implanted within the vitreous of the eye.
As would be readily understood by one skilled in the art, the preferred
amounts,
materials, and dimensions depend on the method of administration, the
effective agent
used, the polymers used, the desired release rate and the like. Likewise,
actual release
rates and release duration depend on a variety of factors in addition to the
above, such as
the disease state being treated, the age and condition of the patient, the
route of
administration, as well as other factors which would be readily apparent to
those skilled
in the art. All of the forgoing U.S. Patents and other publications are
expressly
incorporated by reference herein in each of their entities.
Thus, the devices of the present invention provide many important advantages
over
previously known sustained release drug delivery devices. The unitary cup and
plug
design of the present invention provide an improved device that maintains its
physical
and chemical integrity in both the environments of use and in the presence of
agent
during the controlled and continuous dispensing of agent over a prolonged
period of
time.
p'orming the permeable plug in the unitary cup enables superior interaction
with the lips
or grooves of the unitary cup thereby locking the permeable plug in place. The
resulting
device has superior structural stability under the conditions of use.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
23
Because of the structural integrity of the present design, the need for
coatings and
multiple layers can be eliminated. For transport of agent out of the device
and into the
target area, it is only necessary that the permeable layer cover the portions
of the device
not covered with the impermeable layer.
The unitary cup design and the use of plugs of the present invention result in
a device
that is more easily and reproducibly manufactured then current designs known
in the art.
Manufacturing with the single unitary cup and plugs minimizes the number of
steps and
decreases potential variability in assembly. The present design also allows
for
mechanized manufacture. Eliminating manual assembly greatly decreases the
potential
variability in the finished product.
Another advantage of the devices of the present invention is the ease of
construction by
more standard manufacturing techniques into devices with different release
rates. The
passageway in the impermeable plug allows for release of the agent. A single
standard
cup size can be used for multiple dosage configurations by varying the size or
number of
passageways in the impermeable plug, or by not using the impermeable plug at
all. The
permeable plug can also be made to various dimensional specifications that can
be used
to control diffusion properties to achieve a desired release rate. Thus, the
same unitary
cup can be used for implants with different release rates making it possible
to use a
single manufacturing line or type of equipment.
In addition, the use of a single unitary cup and permeable plug to form the
container or
drug reservoir of the present design provides more consistent and improved
sealing
capacity over the devices in the prior art. This permits the therapeutic
program to be
precisely controlled and the release of drug to be constant and predicted with
accuracy.
The ease of making the devices in the present invention minimizes stresses,
strains, and
deformations of the devices during manufacture which may cause the reservoir
to rupture
and leak. The leaking of agent can result in harm to the patient and is a
significant
concern in the manufacture of implantable devices.
CA 02432203 2003-06-19
WO 02/053128 PCT/US01/49536
24
The following specific examples demonstrate sustained release drug delivery
device
designs of the present invention. However, it is to be understood that these
examples are
for illustrative purposes only and do not purport to be wholly definitive as
to the
conditions and scope.
EXAMPLE 1
A device according to the present invention is prepared. The unitary cup is
made of
silicone and has eight inwardly extending lips around the top open end of the
cup. The
unitary cup has an integral suture tab with a hole at the end of the tab
opposite the cup.
The drug core is formed as a pellet composed of a 2.5 mg core of fluocinolone
acetonide
and inserted into the cup. A 10% PVA solution is injected into the unitary cup
filling the
recess between the drug core and the lips. The PVA is allowed to dry. The
device is
cured at 135-140 C for 50 minutes. The lips act to hold the perineable plug in
place.
The entire device is coated with tobramyacin.
EXAMPLE 2
The device of exainple 1 above is placed in a vial with 2.0 mL of a release
media of 0.1
Sodium Acetate, pH 4.2. The vial is maintained in a 37 C bath for 24 hours.
After 24
hours, the vial is inverted to ensure homogeneity and the device is removed to
a new vial
with fresh media. This process is repeated for each day. The media is tested
to
deterinine the amount of the agents and verifies that they are being released
from the
device. From the data that is collected, the release rate of the device can be
determined.
From the foregoing description, one of ordinary skill in the art can easily
ascertain the
essential characteristics of the instant invention, and without departing from
the spirit
and scope thereof, can malce various changes and/or modifications of the
inventions to
adapt it to various usages and conditions. As such, these changes and/or
modifications
are properly, equitably, and intended to be, within the full range of
equivalence of the
following claims.