Note: Descriptions are shown in the official language in which they were submitted.
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
COMPOSITIONS COMPRISING HIGHER DIAMONDOIDS
AND PROCESSES FOR THEIR SEPARATION
BACKGROUND OF THE INVENTION
Field of the Invention
[0001] This invention is directed to isolated or enriched higher diamondoid
components
and to compositions comprising one or more higher diamondoid components. This
invention
is also directed to novel methods for the separation and isolation of higher
diamondoid
components into recoverable fractions from a feedstock containing one or more
lugher
diamondoid components.
References
[0002] The following publications and patents are cited in this application as
superscript
numbers:
to [0003] 1 Fort, Jr., et al., Adamantane: Consequences of the Diamondoid
Structure,
Chem. Rev., :277-300 (1964)
[0004] ' Sandia National Laboratories (2000), WoYld's FlYSt Diamond
Mic~omachines Created at Sandia, Press Release, (2/22/2000) www.Sandia.gov.
[0005] ' Lin, et al., Natural Occu~~eface of Tetf°amantarae
(Ca2Ha8),Pentamantane
15 (C26H3a) and Hexamantane (C3oH36) in a Deep Petroleum Reservoir; Fuel,
(10):1512-1521
(1995)
[0006] 4 Chen' et al., Isolation of High Purity Diamondoid F~actiofas and
Components,
U.S. Patent No. 5,414,189, issued May 9, 1995 '
[0007] ' Alexander, et al., Removal of Dianaoyadoid Compounds f~~m
2o Hydrocarbonaceous Factions, U.S. Patent No. 4,952,747, issued August 28,
1990
[0008] ° Alexander, et al., Purification ofHyd~ocanbonaceous Fractions,
U.S. Patent
No. 4,952,748, issued August 28, 1990
[0009] ' Alexander, et al., Removal of Diamondoid Compounds from
Hydf~ocarbonaceous Ff°actions, U.S. Patent No. 4,952,749, issued August
28, 1990
25 [00010] b Alexander, et al., Pu~ificatiora of HydYOCa~bonaceous Fractions,
U.S. Patent
No. 4,982,049, issued January 1, 1991
[00011] y Swanson, Method for Diamondoid Extraction Using a Solvent System,
U.S.
Patent No. 5,461,184, issued October 24, 1995
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
j00012] s° Partridge, et al., Shape-Selective Process fog
Concentnatizzg Diamondoid-
Corztainizzg Hydrocarbon Solvents, U.S. Patent No. 5,019,665, issued May 28,
1991
[00013] 11 Dahl, et al., Diamondoid Hydrocarbons as Indicators of Natural Oil
C>~acking, Nature, , 54-57 (1999).
[00014] 1z McI~ervey, Synthetic Approaches to Laz"ge Diamondoid Hydrocarbons,
Tetrahedron, 971-992 (1980).
[00015] 13 Wu, et al., High Viscosity Index Lubricant Fluid, U.S. Patent No.
5,306,851,
issued April 26, 1994.
[00016] 14 Chung et al., Recent Development in High-Ezzengy Density Liquid
Fuels,
to Energy and Fuels, 641-649 (1999).
[00017] 15 Balaban et al., Systematic Classification and Nomenclature
ofDiamond
Hydrocarbons-l, Tetrahedjron, 34,3599-3609.
[00018] All of the above publications and patents are herein incorporated by
reference in
their entirety to the same extent as if each individual publication or patent
was specifically
and individually indicated to be incorporated by reference in its entirety.
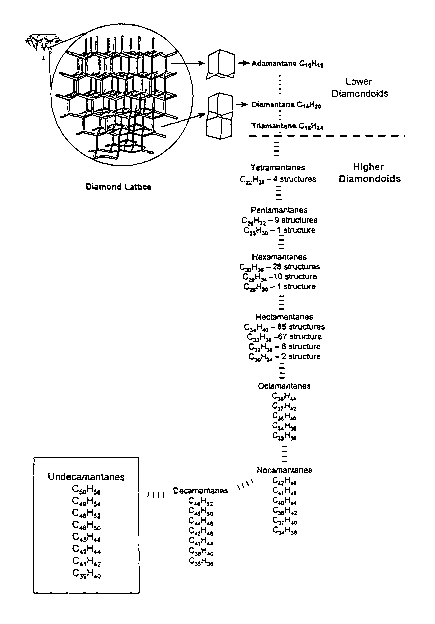
[00019] Diamondoids are hydrocarbon molecules possessing amazingly rigid
structures
that contain carbon atom frameworks that are superimposable on the diamond
crystal lattices
(see FIG. 1). Adamantane, a ten-carbon molecule, is the smallest member of the
diamondoid
series, consisting of one cage-shaped diamond crystal subunit. Adamantane is
commercially
available and is widely used as a chemical intermediate. It can be synthesized
and it can be
recovered from petroleum. Diamantane contains two face-fused diamond subunits
and
triamantane three. These three materials have been synthesized and isolated
from petroleum
and have received research attention. Adamantane, diamantane and triamantane
are
classified as "lower diamondoids". Tetramantane, pentamantanes, etc., have
characteristics
(including multiple isomers, chirality and, above tetramantane, multiple
molecular weight
forms) that differ from the lower diamondoids, and are classified as "higher
diamondoids".
While only one of the higher diamondoids has been synthesized, ideas
concerning their
structures and hypothetical properties have been set forth.
[00020] While adamantane, diamantane and triamantane show no isomers, it is
3o understood that there should be four different isomeric tetramantanes; four
different shapes
containing four diamond-cage subunits that can be superimposed on the diamond
crystal
lattice in different ways. Two of these isomers are enantiomers (mirror images
of each
other). Because the four tetramantanes each have ten faces to which the next
diamond-cage
2
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
unit could fuse, the number of pentamantanes increases over that of the
tetramantanes. The
number of possible isomers increases rapidly with each higher member of the
diamondoid
series. Also, because diamondoid crystal units can share more than a single
face in some
higher diamondoids, hydrogen to carbon ratios, i.e., the degree of
condensation, also shows
increasing variation, resulting in an increasing variety of molecular weights
for each
successive higher diamondoid family (FIG. 1). FIG. 2 is a table showing the
different series
of molecular weights calculated for higher diamondoids ranging from the
tetramantanes to
the undecamantanes.
[00021] Lower diamondoids are present in virtually every petroleum (oils and
gas
l0 condensates) as well as oil source-rock extracts.l l The natural
concentration of diamondoids
in petroleum varies by orders of magnitude. For instance, methyldiamantane
concentrations
in relatively low-maturity crude oils from the central valley of California,
are on the order of
a few parts per million (ppm). Low-maturity oils sourced from the Jurassic-age
Smackover
Formation, Gulf Coast, USA, have rnethyldiamantane concentrations of 20-30
ppm. Because
15 diamondoids show much greater stabilities than other petroleum
hydrocarbons, deeply-
buried petroleums, which have undergone substantial cracking as a result of
intense heat may
have methyldiamantane concentration in the thousands of ppms. It is not
understood how
higher diamondoids could be formed in natural systems, but it may involve a
process
requiring millions of years.
20 [00022] The higher diamondoids, which include the tetramantanes,
pentamantanes, etc.,
have received comparatively little attention. In fact, prior to the work of
inventors Dahl and
Carlson embodied in United States Patent Application Serial No. 60/262,842
filed January
19, 2001 and numerous subsequent filings, these compo~.mds were hypothetical
with only
one such compound having been synthesized and a few others tentatively
identified (but not
25 isolated). More specifically, McKervey, et al. reported the synthesis of
anti-tetramantane in
low yields using a laborious, multistep process.l2 Higher diamondoids cannot
be synthesized
by carbocation isoinerization methods useful for the lower diamondoids. Lin,
et al. have
suggested the existence of tetramantane, pentamantane and hexamantane in deep
petroleum
reservoirs from mass spectroscopy alone and without any attempt to isolate
materials.3 The
3o possible presence of tetramantane and pentamantane in pot material
recovered after a
distillation of a diamondoid-containing feedstock has been discussed by Chen,
et a1.4 Again,
they made no attempt to isolate materials from this pot material.
3
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00023] As additional background, it is pointed out that the present inventors
separately
isolated the higher diamondoid cyclohexamantane, the most condensed member of
the
hexamantane series, and have made that invention the subject of its own patent
application.
[00024] To summarize, the higher diamondoids have not been identified or
isolated or
otherwise provided with the following exceptions: iso-tetramantane -
synthesizedl2 and
unsubstituted cyclohexamantane - separately discovered by the present
inventors.
SUMMARY OF THE INVENTION
[00025] This invention provides higher diamondoids, as enriched or isolated
compounds.
l0 It also provides the individual higher diamondoid isomers (referred to as
"higher diamondoid
components" as enriched or isolated compounds for the first time. In addition,
this invention
provides processes with which the enriched and isolated higher diamondoids and
higher
diamondoid components can be obtained.
[00026] In accord with this invention we have isolated as crystals a variety
of previously
15 unavailable higher diamondoids including tetramantanes, pentamantanes,
hexamantanes,
heptamantanes, octamantanes, nonamantanes and even decamantane. The isolation
of the
higher molecular weight higher diamondoids is especially unexpected in light
of our finding
that the relative abundance of each diamondoid family (tetra vs. penta, etc.)
drops by a factor
of about 10 for each crystal sub-unit added to the structure. This means that
the decamantane
20 we have isolated, for example is about 10-6 times as prevalent in
feedstocks as any of the
tetramantanes, including the species which was synthesized in the prior art.
4
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
BRIEF DESCRIPTION OF THE DRAWINGS
[00027] FIG. 1 illustrates the cage-shaped structure of diamondoids and their
correlation
to diamonds. Specifically illustrated is the correlation of the structures of
diamondoids to
subunits of the diamond crystal lattice.
[00028] FIG. 2 is a table depicting the different molecular weights shown by
each higher
diamondoid series.
[00029] FIG. 3 illustrates the structure of the tetramantanes provided by this
invention.
[00030] FIG. 4 illustrates that the four tetramantanes have carbon frameworks
that
correlate with the diamond lattice and can be viewed into their 100 lattice
plane (FIG. 4A),
1o 110 lattice plane (FIG. 4B) and 111 diamond lattice plane (FIG. 4C).
[00031] FIG. 5 illustrates the structure of the pentamantanes provided by this
invention.
[00032] FIG's. 6A, 6B, 6C and 6D illustrate the structure of the hexamantanes
provided
by this invention.
[00033] FIG's. 7A, 7B and 7C illustrate the structure of the heptamantanes
provided by
this invention. Only one of each enantiomer is shown.
[00034] FIG. 8 illustrates the structure of the octamantanes provided by this
invention.
Only examples of the 500, 486, 472 and 432 molecular weight forms axe shown.
[00035] FIG. 9 illustrates the structure of the nonamantanes provided by this
invention.
Only examples of each molecular weight family are shown.
[00036] FIG. 10 illustrates the structure of the decamantanes provided by this
invention.
Only examples of each molecular weight family are shown.
[00037] FIG. 11 illustrates the structure of the undecamantanes provided by
this
invention. Only examples of each molecular weight family axe shown.
[00038] FIG. 12 gives a flow chart representing the various steps used in the
isolation of
higher diamondoid-containing fractions and individual higher diamondoid
components.
Note that the steps can in some cases be used in a different sequence and
possibly skipped as
discussed in the Examples.
5
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00039] FIG's. 13A and 13B are compilations of the GC/MS and HPLC properties
of
various higher diamondoids included in this application.
[00040] FIG. 14 shows the two-HPLC column strategy used to isolate individual
tetramantanes and pentamantanes.
[00041] FIG. 15 illustrates the size and shape of selected higher diamondoids
relative to
C6o (Buckminsterfullerene) and a representative carbon nanotube used in the
development of
molecular electronic devices. The carbon framework structures of the selected
diamondoids
can be found in FIG's 5, 6, 8, 9 and 10.
[00042] FIG. 16 illustrates the gas chromatogram of a gas condensate
feedstock; one of
l0 the original feedstocks used in the Examples (Feedstock A); showing minute
concentrations
of higher diamonds (not detectable on this scale).
[00043] FIG. 17 illustrates a high temperature simulated distillation profile
of Feedstock
B using the atmospheric distillation 650 °F + bottoms as feedstoclc.
This figure also
illustrates the targeted cut points (1-10) we used for higher diamondoid
isolations.
[00044] FIG's. 18A and 18B illustrate gas clmomatograms (FID) of distillate
fraction #6
(Table 3B, FIG. 18) of Feedstock B 650 °F + distillation bottoms, and
the resulting product
of pyrolytic processing. These figures show that nondiamondoid components have
been
destroyed by the pyrolytic processing and that higher diamondoids, especially
hexasnantanes,
have been concentrated and made available for isolation.
[00045] FIG's. 19 and 20 are charts illustrating elution sequences for a
variety of
individual higher diamondoids (hexamantanes) on two different HPLC
chromatography
colurmls: ODS and Hypercarb as discussed in Examples 1 and 7.
[00046] FIG's. 21A and 21B illustrate the preparative capillary gas
chromatographic data
for tetramantane isolations carried out in Examples 3 and 5. FIG. 21A shows
cuts made on
distillate fraction #33, Feedstock A. The bold face numbers refer to peaks of
the
tetramantanes. FIG. 21B shows peaks isolated and sent to the traps. The
circled numbered
peaks (2, 4, and 6) are the tetramantanes. It is noted that both enantiomers
of the optically-
active tetramantane are contained within one of these peaks.
6
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00047] FIG's. 22A, 22B and 22C illustrate photomicrographs of tetramantane
crystals
isolated from Feedstock A by preparative gas chromatography (FIG. 21). FIG.
22A was
isolated from trap fraction #2, FIG. 22B was isolated from trap fraction #4,
and FIG. 22C
was isolated from trap fraction #6. Because the two enantiomeric tetramantanes
have
identical GC retentions times in FIG. 21, one of the crystals contains both
enantiomers.
[00048] FIG. 23A illustrates the gas chromatogram of Feedstock B atmospheric
distillation hold up fraction, exemplified in Example 1, which was used as
feedstock in
pyrolytic processing. The hold up fraction is the material recovered from the
distillation
column after distillation of Feedstock B at approximately 650°F.
Tetramantanes #1 to #3 are
1 o shown.
[00049] FIG. 23B illustrates the gas chromatogram of the pyrolytic product
from the
starting material in FIG. 23A, i.e. the holdup fraction of Feedstock B
atmospheric distillation
650 °F + bottoms, showing the degradation of non-diamondoid components.
[00050] FIG.' S 24A and 24B compare the gas chromatograms of a tetramantane-
containing starting mixture injected into a Vydac ODS HPLC column, and HPLC
cut #6
enriched in a tetramantane component.
[00051] FIG. 25 illustrates a preparative ODS HPLC isolation of the holdup
fraction of
Feedstock B atmospheric distillation 650 °F + bottoms, showing
fractions taken at various
retention times and the elution order of the tetramantane components and the
location time of
2o fraction #12 used in subsequent isolations steps. FIG. 23 above, displays
the gas
chromatograph of this feedstock.
[00052] FIG. 26 illustrates the HPLC chromatogram of fraction 12 (FIG. 25) run
on
Hypercarb stationary phase with acetone mobile phase resulting in the
isolation of
tetramantane #2.
[00053] FIG's. 27A and 27B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of tetramantane #1 isolated by using two different HPLC columns.
[00054] FIG's. 28A and 28B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of tetramantane #2 isolated by using two different HPLC columns.
7
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00055] FIG's. 29A and 29B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of tetramantane #3 isolated by using two different HPLC columns.
[00056] FIG's. 30A and 30B show the GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methyltetramantane isolated using Hypercarb HPLC.
[00057] FIG's. 31A and 31B illustrate a preparative capillary gas
chromatographic data
for pentamantane isolations. FIG. 31A, shows the first column cut containing
one of the
pentamantanes from thermally treated Feedstock B. The material in that cut was
separated
on a second column. FIG. 31B, shows the second column peak sent to the trap.
Pentamantane #l, the first pentamantane to elute in GC/MS analysis, was
isolated in trap 6.
[00058] FIG's. 32A and 32B show the GC/MS total ion chromatogram and mass
spectrum of pentamantane #1 isolated by preparative capillary gas
chromatography.
[00059] FIG. 33A is a photomicrograph of pentamantane #1 crystals isolated
from
Feedstock B by preparative gas chromatography (FIG. 31 and 32). FIG. 33B
illustrates a
pentamantane co-crystal.
[00060] FIG. 34 illustrates the preparative HPLC Refractive Index trace (with
negative
polarity) of Feedstock B distillate cut pyrolysis product saturated
hydrocarbon fraction
showing HPLC fractions taken using octadecyl silane columns and acetone mobile
phase.
Pentamantanes are numbered in order of their elution on the GC/MS analyses.
[00061] FIG. 35 illustrates the chromatogram of ODS HPLC fraction 11 (FIG. 34)
run on
Hypercarb stationary phase with acetone mobile phase resulting in the
isolation of
pentamantane #1.
[00062] FIG's. 36A and 36B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #1 isolated using two different HPLC columns.
[00063] FIG's. 37A and 37B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #2 isolated using two different HPLC columns.
[00064] FIG's. 38A and 38B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #3 isolated using two different HPLC columns.
8
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00065] FIG's. 39A and 39B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #4 isolated using two different HPLC columns.
[00066] FIG's. 40A and 40B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #5 isolated using two different HPLC columns.
[00067] FIG's. 41A and 41B illustrate GClMS total ion chromatogram (TIC) and
mass
spectrum of pentamantane #6 isolated using two different HPLC columns.
[00068] FIG's. 42A and 42B illustrate the preparative capillary gas
chromatographic data
for hexamantane isolations. FIG. 42A, shows the first column cuts containing
two of the
hexamantanes from Feedstock B. FIG. 42B, shows the second column peaks
isolated and
l0 sent to the traps. From this procedure pure hexamantanes were isolated
(FIG.'s 43 and 44),
hexamantane #2, the second hexamantane to elute in our GC/MS assay, while
hexamantane
#8 is the eighth to elute.
[00069] FIG's. 43A and 43B illustrate the GC/MS total ion chromatogram and
mass
spectrum of a hexamantane #2 isolated by preparative capillary gas
chromatography.
[00070] FIG's. 44A and 44B illustrate the GC/MS total ion chromatogram and
mass
spectrum of a hexamantane #8 highly concentrated by preparative capillary gas
chromatography. A minor amount of a methylheptamantane (408 molecular weight)
is
present in this sample.
[00071] FIG. 45 illustrates a photomicrograph of hexamantane #2 crystals
isolated from
2o Feedstock B by preparative gas chromatography (FIG. 42 and 44).
[00072] FIG. 46 illustrates a photomicrograph of hexamantane #8 crystals
isolated from
Feedstock B by preparative gas chromatography (FIG. 145 and 147).
[00073] FIG's. 47A and 47B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane #8 in ODS HPLC fraction #39.
[00074] FIG's. 48A and 48B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane #10 in ODS HPLC fraction #48.
[00075] FIG's. 49A and 49B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane #6 in ODS HPLC fraction #63.
9
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00076] FIG's. SOA and SOB illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane # 2 greatly enriched in Hypercarb HPLC fraction #53.
[00077] FIG's. S 1A and S 1B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane # 13 isolated using two different HPLC columns.
[00078] FIG's. 52A and 52B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of hexamantane # 7 isolated using two different HPLC columns.
[00079] FIG's. 53A and 53B illustrate GC/MS reconstructed ion chromatogram mlz
382
and mass spectrum of a condensed "irregular" hexamantane (mol. wt. 382) in the
saturated
hydrocarbon fraction of the product of the pyrolytic processing of Feedstock B
distillation
1o fraction #6.
[00080] FIG's. 54A and 54B illustrate GC/MS reconstructed ion chromatogram m/z
382
and mass spectrum of an irregular hexamantane (mol. wt. 382) in the ODS HPLC
fraction
#36.
[0008I] FIG's. SSA and 55B illustrate GC/MS total ion chromatogram (TIC) and
mass
15 spectrum of a methylhexamantane (mol. wt. 410) isolated in ODS HPLC
fraction #55.
[00082] FIG. 56 illustrates GC/MS total ion chromatogram (TIC) of
cyolohexamantane
and methylcyclohexamantane-containing ODS HPLC combined fractions #23-26.
[00083] FIG's. 57A and 57B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methylcyclohexamantane #1 (mol. wt. 356) isolated using mufti-
column
20 stationary phase HPLC (ODS followed by Hypercarb).
[00084] FIG's. S8A and 58B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methylcyclohexamantane #2 (mol. wt. 356) isolated in high purity
using multi-
column stationary phase HPLC (ODS followed by Hypercarb).
[00085] FIG's. 59 and 60 show photomicrographs of crystals of
25 methylcyclohexamantane #1 and methylcyclohexamantane #2 isolated using two
different
HPLC columns.
[00086] FIG's. 61A and 61B illustrate the preparative capillary gas
chromatographic data
for heptamantane isolations. FIG. 61A, shows the first column cuts containing
two of the
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
heptamantanes from Feedstock B. FIG. 61B, shows the second column peaks
isolated and
sent to the traps. From this procedure pure heptamantane components were
isolated (FIG 8
and 9) , heptamantane #1, the first heptamantane to elute in our GC/MS assay,
and
heptamantane #2 which is the second to elute.
[00087] FIG's. 62A and 62B illustrate the GC/MS total ion chromatogram and
mass
spectrum of a heptamantane #1 isolated by preparative capillary gas
chromatography.
[00088] FIG's. 63A and 63B illustrate the GC/MS total ion chromatogram and
mass
spectrum of a heptamantane #2 highly concentrated by preparative capillary gas
chromatography.
[00089] FIG. 64 illustrates photomicrographs of heptamantane #1 crystals
isolated from
Feedstock B by preparative gas chromatography (FIG's. 61 and 62).
[00090] FIG. 65 illustrates a photomicrograph of heptamantane #2 crystals
isolated from
Feedstock B by preparative gas chromatography (FIG's. 61 and 63).
[00091] FIG's. 66A and 66B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane component #1 in ODS HPLC fraction #45.
[00092] FIG's. 67A and 67B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane component #2 in ODS HPLC fraction #41.
[00093] FIG's. 68A and 68B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane component #9 in ODS HPLC fraction #61.
[00094] FIG's. 69A and 69B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane component #10 in ODS HPLC fraction #87.
[00095] FIG's. 70A and 70B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane # 1 greatly enriched in Hypercarb HPLC fraction #55.
[00096] FIG's. 71A and 71B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of heptamantane #2 isolated using two different HPLC columns.
Heptamantane #2
was isolated from ODS HPLC fraction #41 (FIG. 67) using the Hypercarb HPLC
system.
11
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[00097] FIG. 72 illustrates GC/MS reconstructed ion chromatogram m/z 420
showing a
partially condensed heptamantane component (mol. wt. 420) in the ODS HPLC
fraction #61.
[00098] FIG. 73 illustrates the mass spectrum of the molecular weight 420
heptamantane
in FIG. 72.
[00099] FIG's. 74A and 74B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methylheptamantane component (mol. wt. 408) isolated in ODS HPLC
fraction #51.
[000100] FIG's. 75A and 75B illustrate the GC/MS total ion chromatogram and
mass
spectrum of octamantane #1 highly concentrated by high performance liquid
chromatography.
[000101] FIG. 76 illustrates a photomicrograph of octamantane #1 crystals
isolated from
Feedstock B by high performance liquid chromatography.
[000102] FIG's. 77A and 77B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of co-crystalline octamantane #3 and octamantane #5 (FIG. 78) grown
from ODS
HPLC fraction #63.
[000103] FIG's. 78A and 78B illustrate photomicrographs of co-crystalline
octamantane
#3 and #5, crystal B was dissolved in cyclohexane and analyzed by GC/1VIS
(FIG. 77).
[000104] FIG's. 79A and 79B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of octamantane #1 and octamantane #10 containing ODS HPLC fraction
#80.
[000105] FIG's. 80A and 80B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of an octamantane (molecular weight 500)-containing ODS HPLC fraction
#92.
[000106] FIG's. 81A and 81B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methyloctamantane (mol. wt. 460) in ODS HPLC fraction #94.
[000107] FIG's. 82A and 82B illustrate the GC/MS total ion chromatogram and
mass
spectrum of a nonamantane concentrated by high performance liquid
chromatography.
[000108] FIG's. 83A and 83B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a nonamantane concentrated using two different HPLC columns.
12
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000109] FIG's. 84A and 84B illustrate a photomicrograph of a nonamantane
crystal and a
mass spectra of the dissolved crystal.
[000110] FIG's. 85A and 85B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of a methylnonamantane (mol. wt. 512).
[000111] FIG's. 86A and 86B illustrate the GC/MS total ion chromatogram and
mass
spectrum of [ 1231241 (2)3], molecular weight 456, decamantane concentrated by
high
performance liquid chromatography.
[000112] FIG's. 87A and 87B illustrate GC/MS total ion chromatogram (TIC) and
mass
spectrum of [1231241(2)3], molecular weight 456, decamantane isolated using
two different
1o HPLC columns.
[000113] FIG's. 88A and 88B illustrate a photomicrograph of [1231241(2)3],
molecular
weight 456, decamantane crystal and a mass spectra of the dissolved crystal.
[000114] FIG's. 89A and 89B illustrate GC/MS selected ion chromatogram (TIC)
and
mass spectrum of a decamantane (mol. wt. 496).
15 [000115] FIG's. 90A and 90B illustrate GC/MS total ion chromatogram (TIC)
of two
methyldecamantanes (mol. wt. 470), and the mass spectrum of the one eluting at
18.84 min.
in the GC/MS analysis.
[000116] FIG's. 91A and 91B illustrate illustrates the GC/MS selective ion
chromatogram
(mlz 508) and mass spectrum of pyrolysis product of Feedstock B atmospheric
distillation
2o fraction #7 (Table 3) concentrating undecamantanes.
[000117] FIG's. 92A, 92B and 92C illustrate a GC/MS selected ion chromatogram
(m/z
508) and mass spectrum of an undecamantane component (mol. wt. 508) eluting at
21.07
min. and the mass spectrum of a methylundecasnantane component (mol. wt. 522)
eluting at
21.30 min.
25 [000118] FIG. 93 is a chart illustrating distillation cuts of a higher
diamondoid-containing
feedstock (Feedstock B, atmospheric distillation residue) showing cut
selections to favor the
enrichment of specific groups of higher diamondoids.
13
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000119] FIG. 94 shows the screw-like structures (right and left-handed) of
[12341]
hexamantane.
DETAILED DESCRIPTION OF THE INVENTION
[000120] This Detailed Description is presented in the following subsections:
[000121] Definitions
[000122] The Higher Diamondoids
[000123] Feedstocks
[000124] Isolation Processes
to [000125] Utility
[000126] Examples
Definitions
[000127] As used herein, the following terms have the following meanings.
[000128] The term "diamondoid" refers to substituted and unsubstituted caged
compounds
of the adamantine series including adamantine, diamantane, triasnantane,
tetramantane,
pentamantane, hexamantane, heptamantane, octamantane, nonamantane,
decamantane,
undecamantane, and the like and also including all isomers and stereoisomers
thereof.
Substituted diamondoids preferably comprise from 1 to 10 and more preferably 1
to 4 alkyl
substituents.
[000129] The teen "lower diamondoid components" or "adamantine, diamantane and
triamantane components" refers to any and/or all unsubstituted and substituted
derivatives of
adamantine, diamantane and triamantane.
[000130] The term "higher diamondoid components" refers to any and/or all
substituted
and unsubstituted diamondoids corresponding to tetramantanes and above
including
tetramantanes, pentamantanes, hexamantanes, heptamantanes, octamantanes,
nonamantanes,
14
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
decamantanes, undecamantanes, and the like including all isomers and
stereoisomers thereof.
Preferably, the higher diamondoids include substituted and unsubstituted
tetramantanes,
pentamantanes, hexamantanes, heptamantanes, octamantanes, nonamantanes,
decamantanes
and undecamantanes. FIG. 2 is a Table which shows representative higher
diamondoids
together with their molecular weights. The terms "diamondoid family",
"tetramantane
family" and the like are used to define a group of like "diamondoid
components", having the
same number of diamond crystal lattice cage units.
[000131] The term "tetramantane components" refer to any and/or all
substituted and
unsubstituted diamondoids corresponding to tetramantane.
to [000132] The term "pentamantane components" refer to any and/or all
substituted and
unsubstituted diamondoids corresponding to pentamantane.
[000133] The term "non-ionized diamondoid components" refers to higher
diamondoid
components which do not carry a charge such as a positive charge generated
during mass
spectral analysis wherein the phrase "higher diamondoid components" is as
defined herein.
15 [000134] The term "non-ionized tetramantane components" refers to
tetramantane
components which do not carry a charge such as a positive charge generated
during mass
spectral analysis.
[000135] The term "non-ionized pentamantane components and diamondoid
components
higher than pentamantane" refers to pentamantane components and higher
diamondoid
2o components larger than pentamantane which do not carry a charge such as a
positive charge
generated during mass spectral analysis.
[000136] The terms "selected higher diamondoid components" and the like refers
to one
or more substituted or unsubstituted higher diamondoids that are desired to be
isolated or
"enriched" in a product.
25 [000I37] The terms "nonselected higher diamondoid components" and the like
refer to
those higher diamondoids that are not "selected higher diamondoids".
[000138] The term "enriched" when used to describe the state of purity of one
or more
higher diamondoid components refers to such materials at least partially
separated from the
feedstock, and in the case of "enriched" individual higher diamondoid
components,
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
concentrated at least 25 and preferably at least 100 times the original
concentration exhibited
in the feedstock. Preferably "enriched" higher diamondoid or "enriched" higher
diamondoid
components make up at least 25%, especially at least 50% (i.e., 50-100%), more
preferably
at least 75% and yet more preferably at least 95% or even at least 99% by
weight of the
overall material in which they are present or in other words exhibit a weight
purity of at least
25%, 50%, 75%, 95% or 99% of such material.
[000139] The term "feedstock" or "hydrocarbonaceous feedstock" refers to
hydrocarbonaceous materials comprising recoverable amounts of higher
diaxnondoids.
Preferably, such feedstocks include oil, gas condensates, refinery streams,
oils derived from
l0 reservoir rocks, oil shale, tar sands, and source rocks, and the like. Such
components
typically, but not necessarily, comprise one or more lower diamondoid
components as well
as non-diamondoid components. The latter is typically characterized as
comprising
components having a boiling point both below and above the lowest boiling
point
tetramantane which boils at about 350°C at atmospheric pressure.
Typical feedstocks may
15 also contain impurities such as sediment, metals including nickel, vanadium
and other
inorganics. They may also contain heteromolecules containing sulfur, nitrogen
and the like.
All of these nondiamondoid materials are included in "nondiamondoid
components" as that
term is defined herein.
[000140] The term "nonselected materials" refers to the collection of
feedstock
2o components that are not "selected higher diamondoids" and include
"nondiamondoid
components", "lower diamondoids" and "nonselected higher diamondoid" as these
terms are
defined herein.
[000141] The term "remove" or "removing" refers to processes for removal of
non-
diamondoid components and/or lower diamondoid components and/or nonselected
higher
25 diamondoid components from the feedstock. Such processes include, by way of
example
only, size separation techniques, distillation, evaporation either under
normal or reduced
pressure, well head separators, sorption, chromatography, chemical extraction,
crystallization
and the like. For example, Chen, et a1.4 disclose distillation processes for
removing
adamantine, substituted adamantine, diamantane, substituted diamantane, and
triamantane
3o from a hydrocarbonaceous feedstock. Size separation techniques include
membrane
separations, molecular sieves, gel permeation, size exclusion chromatography
and the like.
16
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000142] The terms "distillation" or "distilling" refers to the fractionation
processes in
which materials are separated based on differences in vapor pressures, with
high vapor
pressure materials being taken overhead. Distillation can be carried out on
hydrocarbonaceous feedstocks and on fractions otherwise obtained during the
processing of
hydrocarbonaceous feedstocks. In this context, most commonly, distillations
are conducted
under vacuum but also could be at atmospheric or even elevated pressures.
[000143] The terms "fractionation" and "fractionating" refer to processes in
which
materials in a mixture are separated from each other such as by differential
solubility,
differential vapor pressure, differential chromatographic affinity and the
like.
to [000144] The terms "pyrolysis" and "thermal treating to pyrolyze" and the
like refer to
either atmospheric, reduced pressure or elevated pressure heating of the
feedstock or a
feedstock fraction to thermally degrade a portion of one or more components in
the
feedstock.
[000145] The term "non-diamondoid components of a feedstock" refers to
components of
15 the feedstock or a feedstock fraction which are not diamondoid in character
wherein the term
"diamondoid" is as defined herein.
[000146] The term "retained" refers to retention of at least a portion of the
higher
diamondoid components found in the recovered feedstock when compared to the
amount of
such diamondoids found in the original feedstock. In a preferred embodiment,
at least about
20 10 weight percent of the higher diamondoid components are retained in the
recovered
feedstock; more preferably, at least about 50 weight percent of the higher
diamondoid
components are retained in the recovered feedstock; and still more preferably,
at least about
90 weight percent of the higher diamondoid components are retained in the
recovered
feedstock; each based on the total amount of such diamondoids found in the
feedstock prior
25 to treatment.
[000147] The term "chromatography" refers to any of a number of well known
chromatographic techniques including, by way of example only, column or
gravity
chromatography (either normal or reverse phase), gas chromatography, high
performance
liquid chromatography, and the like.
17
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000148] The term "alkyl" refers to straight and branched chain saturated
aliphatic groups
typically having from 1 to 20 carbon atoms, more preferably 1 to 6 atoms
("lower allcyls"),
as well as cyclic saturated aliphatic groups typically having from 3 to 20
carbon atoms and
preferably from 3 to 6 carbon atoms ("lower alkyls" as well). The terms
"alkyl" and "lower
alkyl" are exemplified by groups such as methyl, ethyl, propyl, butyl,
isopropyl, isobutyl,
sec-butyl, t-butyl, n-heptyl, octyl, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl and the
like.
The Higher Diamondoids
[000149] As shown in FIG. 1, higher diamondoids are bridged-ring cycloalkanes
that have
to carbon-atom frameworks that can be superimposed on the diamond crystal
lattice (FIG's. 1
and 4). They are the tetramers, pentamers, hexamers, heptamers; octamers,
nonamers,
decamers, etc. of adamantine (tricyclo[3.3.1.13'7]decane) or CloHI6 in which
various
adamantine units are face-fused. The higher diamondoids can contain many alkyl
substituents. These compounds have extremely rigid structures and have the
highest stability
15 of any compound with their formula. There are four tetramantane structures
(FIG' S. 2 and
3); iso-tetramantane [1(2)3], ahti-tetramantane [121] and two enantiomers
ofskew-
tetramantane [123] (FIG. 3) with the more general bracketed nomenclature for
these
diamondoids in accordance to a convention by Balaban et al.ls There are ten
pentamantanes
(FIG. 5), nine have the molecular formula Cz6Hsa (molecular weight 344), and
among these
2o nine there are three pairs of enantiomers represented by: [12(1)3], [1234],
[1213] with the
non-enantiomeric pentamantanes represented by: [12(3)4], [1(2,3)4], [1212].
There also
exists a more strained pentamantane, [1231], represented by the molecular
formula C25H3o
(molecular weight 330). See FIG. 4. Hexamantanes exist with thirty-nine
different
structures (FIG. 6), twenty-eight having the molecular formula C3oH36
(molecular weight
25 396) and of these, six are aclural; ten more strained hexamantanes have the
molecular
formula Ca9H34 (molecular weight 382) and the remaining hexamantane [12312]
has the
molecular formula C26H3p (molecular weight 342), also called cyclohexamantane
because of
its highly condensed circular structure. Heptamantanes are postulated to exist
in one
hundred and sixty possible structures; with eighty-five having the molecular
formula C34H40
30 (molecular weight 448) (FIG. 7) and of these, seven are achiral, having no
enantiomers.
Only one of each of the two enantiomer structures of the chiral heptamantanes
is shown in
FIG. 7. Of the remaining heptamantanes, sixty-seven have the molecular formula
C33H38
18
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
(molecular weight 434), and six have the molecular formula C32H36 (molecular
weight 420).
These two heptamantane families have structures showing greater internal bond
strain, with
correspondingly lower stabilities and are not shown in FIG. 7. The remaining
two have the
molecular formula C3oH3~ (molecular weight 394) (FIG. 7). Octamantanes possess
eight of
the "diamond crystal cage units" and exist within five families of different
molecular weight
core structures (FIG. 2). Among the octamantanes, eighteen have the molecular
formula
C34H3g (molecular weight 446). FIG. 8 show, each of the 446 molecular weight
octamantane isomers. Other octamantanes have the molecular formula C3gH44
(molecular
weight 500). The remaining octamantane families, C37H4a (molecular weight
486), C36H4o
l0 (molecular weight 472) and C33H3s (molecular weight 432) show greater bond
strain and
correspondingly lower stability. Nonamantanes exist within six families of
different
molecular weights having the following molecular formulas: C42H48 (molecular
weight 552),
C41H46 (molecular weight 538), C4oH44 (molecular weight 524), C38H42
(molecular weight
498), C37H4o (molecular weight 484) and C34H36 (molecular weight 444).
Additionally,
decamantane exists within families of seven different molecular weights. Among
the
decamantanes, there is a single decamantane having the molecular formula
C3sH36
(molecular weight 456) which is structurally compact in relation to the other
decamantanes
and has low internal bond strain. The other decamantane families have the
molecular
formulas: C46Hs2 (molecular weight 604), C4sHso (molecular weight 590), C44H48
(molecular
2o weight 576), C4zHq6 (molecular weight 550), C41H44 (molecular weight 536)
and C38H4o
(molecular weight 496). Undecamantanes (FIG. 11) exist as molecular formulas
CsoHss
(molecular weight 656), C49Hs4 (molecular weight 642), C48Hs2 (molecular
weight 628),
C46H50 (molecular weight 602), C4sH48 (molecular weight 588), C4aH44
(molecular weight
548), C41H42 (molecular weight 534), C39H4o (molecular weight 508). More
preferred and
less preferred higher diamondoids (FIG. 2) are based on their internal bond
strain and
corresponding stabilities which is reflected by their relative concentrations
in the various
feedstocks.
Feedstocks
[000150] The higher diamondoids provided by this invention only exist in
dilute
3o concentrations in solution in petroleum feedstocks.
19
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000151] In the processes of this invention, a feedstock is selected such that
said feedstock
comprises recoverable amounts of one or more selected higher diamondoid
components.
Preferably, such feedstock comprises at least about 1 ppb of one or more
higher diamondoid
components, more preferably, at least about 25 ppb and still more preferably
at least about
100 ppb. It is understood, of course, that feedstocks having higher
concentrations of higher
diamondoid components facilitate recovery of these components.
[000152] Preferred feedstocks include, for example, natural gas condensates
and refinery
streams having high concentrations of higher diamondoids. With regard to the
latter, such
refinery streams include hydrocarbonaceous streams recoverable from cracking
processes,
l0 distillations, coking and the lilce. Particularly preferred feedstocks
include gas condensates
recovered from the Norphlet Formation in the Gulf of Mexico and from the LeDuc
Formation in Canada.
[000153] In one embodiment, the feedstocks used in the processes of this
invention
typically comprise non-diamondoid components having boiling points both below
and above
15 the lowest boiling point higher diamondoid component selected for recovery
as well as one
or more lower diamondoid components. These feedstocks will usually contain a
mixture of
higher diamondoids. Depending upon which higher diamondoids are selected, some
of these
higher diamondoids may have boiling points below the selected higher
diamondoid's boiling
point. Typically, the lowest boiling point higher diamondoid component
selected for
2o recovery will have a boiling point of greater than about 335°C. In
typical feedstocks, the
concentration of lower diamondoids to higher diamondoids is generally about
250:1 or
higher. Moreover as illustrated in FIG. 18, typical feedstocks comprising
higher diamondoid
components also comprise non-diamondoid components.
[000154] In such feedstocks, selected higher diamondoid components often
cannot be
25 effectively recovered directly from the feedstock because of their low
concentrations relative
to the nonselected components. Accordingly, the processes of this invention
may entail
removal of a sufficient amount of these contaminants from the feedstock under
conditions to
provide a treated feedstock from which the selected higher diamondoid
components can be
recovered.
20
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
Isolation Processes
[000155] The general isolation processes of higher diamondoids are shown in
FIG. I2.
[000156] In one embodiment, the removal of contaminants includes distillation
of the
feedstock to remove non-diamondoid components as well as lower diamondoid
components
and in some cases other nonselected higher diamondoids having boiling points
less than that
of the lowest boiling point higher diamondoid component selected for recovery.
[000157] In a particularly preferred embodiment, the feedstock is distilled to
provide cuts
above and below about 335°C, atmospheric equivalent boiling point and,
more preferably,
above and below about 345 °C atmospheric equivalent boiling point. In
either instance, the
to lower cuts, which are enriched in lower diamondoids and low boiling point
non-diamondoid
components are taken overhead and discarded and the higher boiling cut, which
is enriched
in higher diamondoids, is retained. It is understood, of course, that the
temperature for the
cut point during distillation is a function of pressure and that the above
temperatures are
referenced to atmospheric pressure. A reduced pressure will result in a lower
distillation
15 temperature to achieve the same cut point whereas an elevated pressure will
result in a higher
distillation temperature to achieve the same cut point. The correlation of
pressure/temperature from atmospheric distillation to either reduced pressure
or elevated
pressure distillation is well within the skill of the art.
[000158] Distillation can be operated to fractionate the feedstocks and
provide several cuts
2o in a temperature range of interest to provide the initial enrichment of the
selected higher
diamondoids or groups of selected higher diamondoids. The cuts, which are
enriched in one
or more selected diamondoids or a particular diamondoid component of interest,
are retained
and may require further purification. The following Table illustrates
representative
fractionation points that may be used to enrich various higher diamondoids in
overheads. In
25 practice it may be advantageous to make wider temperature range cuts which
would often
contain groups of higher diamondoids which could be separated together in
subsequent
separation steps.
21
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
Fractionation Points
Most Preferred Preferred
Lower Cut Higher Lower Cut Higher
Cut Cut
TemperatureTemperatureTemperatureTemperature
Higher Diamondoid(C) (C) (C) (C)
Tetramantanes349 382 330 400
Pentamantanes385 427 360 450
Cyclohexamantanes393 466 365 500
Hexamantanes 3i)3 466 365 500
Heptamantanes432 504 395 540
Octamantanes .154 527 420 560
Nonamantanes 463 549 425 590
Decamantanes 472 571 435 610
Undecamantanes499 588 455 62S
Useful
Lower Cut Higher
Cut
TemperatureTemperature
Higher Diamondoid(C) (C)
Tetramantanes300 430
Pentamantanes330 490
Cyclohexamantanes330 550
Hexamantanes 330 550
Heptamantanes350 600
Octamantanes 375 610
Nonamantanes 380 650
Decamantanes 390 660
Undecamantanes400 675
[000159] It shall be understood that substituted higher diamondoids may
accordingly shift
these preferred cut-point temperatures to higher temperatures due to the
addition of
substituent groups. Additional temperature refinements will allow for higher
purity cuts for
l0 the diamondoid of interest. FIG. 93 provides further illustrations of how
fractionation can
provide cuts enriched in individual or multiple higher diamondoid components.
[000160] It will be further understood that fractionation can be stopped
before a selected
higher diamondoid is taken overhead. In this case the higher diamondoid can be
isolated
from the fractionation bottoms.
22
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000161] Other processes for the removal of lower diamondoids, unselected
higher
diaanondoids, if any, and/or hydrocarbonaceous non-diamondoid components
include, by
way of example only, size separation techniques, evaporation either under
normal or reduced
pressure, crystallization, chromatography, well head separators, reduced
pressure and the
like. Removal processes can utilize the larger sizes of the higher diamondoids
to effect
separation of lower diamondoids therefrom. For example, size separation
techniques using
membranes will allow a feedstock retained in the membrane to selectively pass
lower
diamondoids across the membrane barrier provided that the pore size of the
membrane
barrier is selected to differentiate between compounds having the size of
higher diamondoid
l0 components as compared to lower diamondoid components. The pore size of
molecular
sieves such as zeolites and the like can also be used to effect size
separation.
[000162] In a preferred embodiment, the removal process provides for a treated
feedstock
having a ratio of lower diamondoid components to higher diamondoid components
of no
greater than 9:1; more preferably, no greater than 2:1; and even more
preferably, the ratio is
no greater than 1:1. Even more preferably, after removal of the lower
diamondoid
components) from the feedstock, at least about 10%, more preferably at least
50% and still
more preferably at least 90% of the higher diamondoid components are retained
in the
feedstock as compared to that amount found in the feedstock prior to the
removal.
[000163] When recovery of hexamantane and higher diamondoid components is
desired
2o and when the feedstock contains non-diamondoid contaminants, the feedstock
will also be
generally subj ected to pyrolysis to effect removal of at least a portion of
the
hydrocaxbonaceous non-diamondoid components from the feedstock. The pyrolysis
effectively concentrates the amount of higher diamondoids in the pyrolytically
treated
feedstock thereby rendering their recovery possible (FIG. 18).
[000164] Pyrolysis is effected by heating the feedstock under vacuum
conditions or in an
inert atmosphere, at a temperature of at least about 390°C and,
preferably, from about 400 to
about 550°C, more preferably from about 400 to about 450°C , and
especially 410 to 430°C;
for a period of time to effect pyrolysis of at Least a portion of the non-
diamondoid
components of the feedstock. The specific conditions employed are selected
such that
3o recoverable amounts of selected higher diamondoid components are retained
in the
feedstock. The selection of such conditions is well within the skill of the
art.
23
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000165] Preferably, pyrolysis is continued for a sufficient period and at a
sufficiently
high temperature to thermally degrade at least about 10% of the non-diamondoid
components (more preferably at least about 50% and even more preferably at
least about
90%) from the pyrolytically treated feedstock based on the total weight of the
non-
diamondoid components in the feedstock prior to pyrolysis.
[000166] In yet another preferred embodiment, after pyrolysis of the
feedstock, at least
about 10%, more preferably at least about 50%, and still more preferably at
least about 90%
of the higher diamondoid components are retained in the feedstock after
pyrolytic treatment
compared to that amount found in the feedstock prior to pyrolytic treatment.
l0 [000167] In a preferred embodiment, removal of lower diamondoids and low
boiling point
~hydrocarbonaceous non-diamondoid components from the feedstock precedes
pyrolytic
treatment. However, it is understood, that the order of these procedures can
be inverted such
that pyrolysis occurs prior to removal of lower diamondoids from the
feedstock.
[000168] The pyrolysis procedure, while a preferred embodiment, is not always
necessary.
15 Tlus arises because the concentration of higher diamondoids can be
sufficiently high in
certain feedstocks that the treated feedstock (after removal of the lower
diamondoid
components) can be used directly in purification techniques such as
chromatography,
crystallization, etc. to provide higher diamondoid components. However, when
the
concentration or purity of higher diamondoid components in the feedstock is
not at the level
2o to effect such a recovery, then a pyrolytic step should be employed.
[000169] Even when pyrolysis is employed, it is preferred to further purify
the recovered
feedstock using one or more purification techniques such as chromatography,
crystallization,
thermal diffusion techniques, zone refining, progressive recrystalization,
size separation and
the like. In a particularly preferred process, the recovered feedstock is
first subjected to
25 gravity column chromatography using silver nitrate impregnated silica gel
followed by
HPLC using two different columns of differing selectivities to isolate the
selected
diamondoids and crystallization to provide crystals of the highly concentrated
target higher
diamondoids. Where lugher diamondoid concentrations are not high enough for
crystallization to occur, further concentration by, for example, preparative
capillary gas
3o chromatography may be necessary.
24
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000170] Enantioselective (chiral) stationary phases have been applied in
chromatographic
methods to effectuate further separations. High performance liquid
chromatography methods
also offer the possibility of using chiral solvents or additives to achieve
resolution of
enantiomers.
[000171] For example, separation of enantioneric forms of the high diamondoids
can be
achieved using several approaches. One such approach is spontaneous
crystallization with
resolution and mechanical separation. This approach to enantiomer resolution
can be
enhanced by preparation of derivatives or by the use of additives, chiral
solvents, or various
types of seed crystals. Another resolution option is chemical separation under
kinetic or
l0 thermodynamic control. Other suitable processes for enantiomer resolution
include chiral
separations, which can be performed using a gas chromatographic (GC) see
"Chiral
Chromatography", T.E. Beesley, et. al, Wiley, Johnson & Sons, January 1998,
incorporated
herein by references, by high performance liquid chromatographic (HPLC) and by
supercritical fluid chromatographic (SFC) techniques, see Supercritical fluids
in
Chromatography and Extraction", R.M. Smith, Elsevier Science, December 1997,
incorporated herein by references.
Utility
[000172] The processes of this invention provide compositions enhanced in
higher
diamondoids. These higher diamondoids are useful in micro- and molecular-
electronics and
nanotechnology applications. In particular, the rigidity, strength, stability,
thermal
conductivity, variety of structural forms and multiple attachment sites shown
by these
molecules makes possible accurate construction of robust, durable, precision
devices with
nanometer dimensions. FIG. 15 shows the size and shapes of selected higher
diamondoids
relative to molecular components (Buckminsterfullerene and carbon nanotubes)
employed in
the development of molecular electronic devices.
[000173] The higher diamondoids are three-dimensional nanometer-sized units
showing
different diamond lattice arrangements. This translates into a great variety
of shapes and
sizes of these extremely rigid nanostructures, for example, [121(3)4]
hexamantane is "T"
shaped, [12134] is "L" shaped, and [1(2)3(1)2] is flat with four lobes.
[12(3,4)12]
3o heptamantane has a cross-shaped structure while [121234] is "L" shaped.
[12312]
hexamantane has a disc-like structure. [121321] heptamantane is disc-shaped
with one co-
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
planar lobe, while [1213(1)21] octamantane is disc-shaped with two, opposing,
co-planar
lobes. [1232(1)3] octamantane is wedge-shaped. [121(2)32(1)3] nonamatane has a
triangular
plate-like structure. [1231241(2)3] decamantane is a perfect octagon, while
[121231212]
decamantane is rectangular plate-like structure. [123(1,2)42143] undecamantane
is an
elongated pyramid. A variety of other shapes exist among the higher
diamondoids which
may serve in applications in nanotechnology and nano-structured materials
which depend
upon specific geometries. The carbon-framework structures of the tetramantanes
to the
undecamantanes are shown in FIG's 3 to 11.
[000174] Higher diamondoids also include a series of rod-like structures of
varying
to lengths. The tetramantane with the sequence "121" is the first member of
this rod-shaped
structural series, [ 1212] pentamantane is next, followed by [ 12121 ]
hexasnantane, and so on.
Each added diamond cage increases the length of the rod by about 0.3 nm, with
[1212]
pentamantane having a length of about 1.1 nm.
[000175] The [1(2)3] tetramantane begins a more compact series, a flat-topped,
pyramid-
15 like structure (FIG. 3). [1(2,3)4] pentamantane (FIG. 5) follows this
trend, being a perfect
tetrahedral pyramid.
[000176] Higher diamondoids also include screw-like structures of varying
lengths. The
first chiral diamondoid is the tetramantane with sequence 123. We have
specified the two
enantiomers of 123 tetramantane as A and B. Their structures can also be
implied by the
2o sequences 123 and 124 by a modification of the Balaban nomenclature. These
two
diamondoids have left (counter-clockwise), i.e., tetramantane A, and right
(clockwise)
(tetramantane B)-hand helix or screw-like structures, both representing a
partial-turn of the
helix. Unfortunately, the Balaban nomenclature does not provide a way of
specifying the left
and right helical forms, only demonstrating that there exists two forms. This
sequence
25 continues with the progression 1234 and 1243 (i.e., A and B) for
pentamantane (FIG. 5),
12341 and 12431 (again, A and B), for hexamantane (FIG. 6), and so on. The
hexamantane
members complete one full turn of a right and a left-handed helix for these
screw-shaped
nanostuctures (FIG 94).
[000177] These special structural characteristics set higher diamondoids apart
from acyclic
30 molecules, from condensed-ring systems and even from bridged-ring
counterparts. The great
stability, nanometer size, variable yet rigid geometry, well defined distances
for places of
26
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
attachment, nonplanar bridgeheads lead to their unique features. I~ue to the
rigidity,
specialized geometry, 3-dimensional shape and nanometer size of the higher
diamondoid
components, it is expected that molecular aggregates and building blocks
comprising them
will enable construction and synthesis of a unprecedented array of desirable
materials that
will find applications in molecular electronic computing devices, reduced-size
machines such
as molecular robots and self replicating manufacturing systems. Alternatively,
the higher
diamondoids may be used as novel materials of construction with special
chemicals, optical,
electric and thermal conductivity properties for coatings, film layering and
other applications
taking advantage of the diamond-like properties, etc. Novel uses of higher
diamondoid-
to containing materials in the field of microelectronics are disclosed.
Embodiments include,
but not limited to, thermally conductive films in integrated circuit
packaging, low-k
dielectric layers in integrated circuit multilevel interconnents, thermally
conductive adhesive
films, thermally conductive films in thermoelectric cooling devises,
passivation films for
integrated circuit devices (ICs), and field emission cathodes.
[000178] In addition, these higher diamondoids can also be used in a high
quality
lubricating fluid which exhibits a high Viscosity Index and a very low pour
point.l3 When so
employed, these fluids comprise a fluid of lubricating viscosity and from
about 0.1 to 10
weight percent diamondoids.
[000179] Still further, these higher diamondoids can be used as high density
fuels in the
manner described by Chung, et a1.14, incorporated herein by reference.
[000180] The following examples are offered to illustrate this invention and
are not to be
construed in any way as limiting the scope of this invention. Unless otherwise
stated, all
temperatures axe in degrees Celsius.
27
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLES
[000181] As used herein and in the Figures, the following abbreviations have
the
following meanings. Any abbreviation not defined below has its generally
accepted
meaning.
API = American Petroleum Institute
~
atm eqv - atmospheric equivalent
btms - bottoms
FOR Traps - end of run traps
1o fid = flame ionization detector
g - grams
GC = gas chromatography
GC/MS - gas chromatography/mass spectroscopy
h - hour
HPLC - high performance liquid chromatography
HYD RDG - hydrometer reading
L - liter
min = minute
mL = milliliters
2o mmol - millimols
N - normal
pA = pico amps
ppb = parts per billion
ppm= parts per million
RI - refractive index
SIM DIS - simulated distillation
ST = start
TIC = total ion current
TLC - thin layer chromatography
VLT - vapor line temperature
VOL PCT - volume percent
v/v = volume to volume
wt - weight
WT PCT - weight percent
Introduction
[000182] The steps used in the various Examples are shown schematically in
FIG. 12.
[000183] Example 1 describes a most universal route for isolating higher
diamondoids
components which can be applied to all feedstocks. This process uses HPLC
(Step 7, FIG.
12) as its final isolation step.
28
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000184] Example 2 describes a variation of the process of Example 1 in which
preparative gas chromatography (Step 7', FIG. 12) replaces HPLC as the final
isolation step.
[000185] Example 3 describes a variation of the process of Example 1 in which
the
pyrolysis (Step 5, FIG. 12) is omitted. As shown optionally in FIG. 12, the
liquid
chromatographic step (Step 6, FIG. 12) is also omitted. These variations
generally have
applicability only with selected feedstocks and generally when tetramantanes,
pentamantane
and cyclohexamantane are the target higher diamondoids.
[000186] Example 4 describes yet another process variation in which the final
products of
Examples 1 and 3 are subjected to preparative gas chromatography purification
to give
to further separation of higher diamondoid components (Step 8, FIG. 12).
[000187] Example 5 describes the enrichment and isolation of the tetramantane
components.
[000188] Example 6 describes the enrichment and isolation of the pentamantane
components.
[000189] Example 7 describes the enrichment and isolation of the hexamantane
components.
[000190] Example 8 describes the enrichment and isolation of the heptamantane
components.
[000191] Example 9 describes the enrichment and isolation of the octamantane
components.
[000192] Example 10 describes the enrichment and isolation of the nonamantane
components.
[000193] Example 11 describes the enrichment.and isolation of the decamantane
components.
[000194] Example 12 describes the enrichment and isolation of the
undecamantane
components.
29
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000195] It will be understood that it is possible to vary the order of the
various
distillation, chromatography and pyrolysis steps, although the order set forth
in Example 1
has given the best results.
EXAMPLE 1
[000196] This Example has seven steps (see Flow Chart in FIG. 12).
[000197] Step 1. Feedstock selection
[000198] Step 2. GCMG assay development
[000199] Step 3. Feedstock atmospheric distillation
l0 Step 4. Vacuum fractionation of atmospheric distillation residue
Step 5. Pyrolysis of isolated fractions
Step 6. Removal of aromatic and polar nondiamondoid components
[000203] Step 7. Multi-column HPLC isolation of higher diamondoids
a) First column of first selectivity to provide fractions enriched in specific
higher
diamondoids.
b) Second column of different selectivity to provide isolated higher
diamondoids.
[000204] This example is written in terms of isolating several hexamantanes.
As will be
shown in Examples 5-12 it can be easily adapted to isolate the other higher
diamondoids.
Step 1- Feedstock Selection
[000205] Suitable starting materials were obtained. These materials included a
gas
condensate, Feedstoclc A (FIG. 16), and a gas condensate containing petroleum
components,
Feedstock B. Although other condensates, petroleums, or refinery cuts and
products could
have been used, these two materials were chosen due to their high diamondoid
concentration,
approximately 0.3 weight percent higher diamondoids, as determined by GC and
GCIMS.
Both feedstocks were light colored and had API gravities between 19 and
20° API.
Step 2 - GC/MS Assay Development
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000206] Feedstock A was analyzed using gas chromatography/mass spectrometry
to
confirm the presence of target higher diamondoids and to provide gas
chromatographic
retention times for these target materials. This information is used to track
individual target
higher diamondoids through subsequent isolation procedures. FIG. 13A is a
table that lists
typical GC/MS assay information for the hexamantanes (GC retention times, mass
spectral
molecular ion (M+) and base peak). This table (FIG. 13A) also contains similar
GC/MS
assay information for other higher diamondoids. While relative GC retention
times are
approximately constant, non-referenced GC retentions vary with time. It is
recommended
that GC/MS assay values be routinely updated especially when GC retention time
drift is
l0 detected.
Step 3 - Feedstock Atmospheric Distillation
[000207] A sample of Feedstock B was distilled into a number of fractions
based on
boiling points to separate the lower boiling point components (nondiamondoids
and lower
diamondoids) and for further concentration and enrichment of particular higher
diamondoids
i5 in various fractions. The yields of atmospheric distillate fractions of two
separate samples of
Feedstock B are shown in Table 1, below and are contrasted to simulated
distillation yields.
As seen from Table 1, the simulated distillation data is in agreement with the
actual
distillation data. The simulated distillation data were used to plan
subsequent distillation
processes.
31
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
TABLE l: Yields of Atmospheric Distillation Fractions from Two Separate
Runs of Feedstock B
Cut (F) Sim Dis Feedstock B (RunDifference
Est.'d Yields 2)
(Wt Yields (Wt %)
To 349 8.0 7.6 0.4
349 to 491 57.0 57.7 -0.7
491 to 643 31.0 30.6 0.4
643 and hi 4.0 4.1 -0.1
her
Cut (F) Sim Dis Feedstock B (RunDifference
Est.'d Yields 1)
(Wt Yields (Wt %)
%
To 477 63.2 59.3 3.9
477 to 515 4.8 7.3 -2.5
S15 to 649 28.5 31.2 -2.7
649 and hi 3.5 2.1 1.4
her
Step 4 - Fractionation of Atmospheric Distillation Residue by Vacuum
Distillation
[000208] The resulting Feedstock B atmospheric residium from Step 3
(comprising 2-4
weight percent of the original feedstock) was distilled into fractions
containing higher
diamondoids as shown in FIG's. 17 and 93). The feed to this high temperature
distillation
l0 process was the atmospheric 650 °F + bottoms. Complete Feedstock B
distillation reports
are given in Tables 2A and 2B. Tables 3A and 3B illustrate the distillation
reports for
Feedstock B 650°F + distillation bottoms.
32
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
N ~ O ~ NO
O U N Q ~ O
O M ~ M
O
V oo M o~ o
H v M G1 l~ ~ ~ O 01O
p
O M ~ NO
N
g ~ 0 0
OU . o
00 ~ N O M O
In N O
E.., o 'c 'D C O o, o
U ' 0
~
Z ~ ~ t( M O d O
H
' w ~ o O N N M M
~ p M ~
i, W ~ 00 -I O O O 01 O
O ~ v-
Ov
~ O O v-Iri ri O O
~r
O
w ~ O
U r.~ O oo r.,vo vo 'r?
~
OW O v0
O
W
..r ~ ~ O
~
r.y ~ O ~ O ~ N O M O GMT M01
~
~ i~ A
~
d~
N
r, t
f1
H
a ~ A ~ w o c o
U, o N r,, O y 00 ~,C
~ O M N
~ E-~ ~ N 0 O
0 1
_ A
U
M ~ ~
~ M
~W
~
O
~ N M. d. ~M ~ Cl~
O O
W
U ~
-, N M ~ ~.. p
.,
W
33
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
o ° ~ ,-: W o U?
M N ~ ~ ~ H
H
a$
O O ~ O ~ O O O
0°0 0°0 ~ ° ~ 0°0
P~ ~C
''' ° a o N
M N ~ ~ O ~ N
x
o U' U' ~ o ~ ~ ~° ° N .,..,, ~c o d.
~~' O~ ~°n e~ ON ° °MO°°oo°,N
a
w
x~
0
w
a~~ ° ~a ~ ° °
~a
x ~°
°
A
O r-I N ~ ~ M .~
x
A ~ "~ ~ ~ ~ ~ o ~~ ~~~aA A
M M M ~ ~ M M ~
c~ ~ H
m p
a ~ ~~-' ~ U O ~ ~ ~ U A
0 0 °o, '.~ o 0
H a~ ~ ~ ~°n v°~ ,°,~ ''3 0 0
U w
W ~ N N M ~ M
O
'' 00 r1 00 ~ I~ M
O7 . 1/j 01 O ~ I~ M
F~ U ~ ~ O ~ W N M '~ V 'Md'
A
M N M
34
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
TABLE 3A: Vacuum Distillation Report for Feedstock B
Feedstock B - Atmospheric distillation resid 650°F + bottoms
Column Used: Sarnia Hi Vac
TEMP PRESSUREREFLUX CUT VOLUME WEIGHT API
ERATURE ml G GRAVITIES
DEGREESF
VAPOR POT TORR RATIO NO OBSERVED GO
F
VLT ATM 60 F HYD TEMP
EQV. RDG F
315 601.4350 5.000 START
OVERHEAD
344 636.8382 5.000 300 READING
342 644.9389 4.000 500 READING
344 656.3395 3.300 1 639 666.4 7.8 138.04.1
353 680.1411 2.500 400 READING
364 701.6430 2.100 2 646 G6G.9 9.4 138.05.G
333 736.0419 0.400 200 READING
336 751.9432 0.300 3 330 334.3 12.4 139.08.3
391 799.9468 0.500 4 173 167.7 19.0 139.014.5
411 851.6500 0.270 5 181 167.3 26.8 139.021.7
460 899.8538 0.360 G 181 167.1 27.0 139.021.9
484 950.3569 0.222 7 257 238.4 26.2 139.021.2
Shut
down
distillation
to
check
of
tem
erature
limits
with
customer.
(Drained
tra
material
5.3
rams)
472 935.7576 0.222 START
OVERHEAD
521 976.3595 0.340 8 91 85.4 23.7 139.018.9
527 999.9610 0.235 9 85 80.8 23.0 139.018.2
527 1025.6624 0.130 10 98 93.8 21.6 139.016.9
Drained rams
remainin (--4
tra rams
material of
of water)
16.5
MID END OF 20 17.8 (mathematically
AND RUN combined)
TRAPS
VOLUME 2701
DISTILLED
COLUMN 4 4.0 0.0 0.0 3.4
HOLDUP
BOTTOMS 593 621.8 11.0 214.03.4
RECOVERED 3298 3311.7
FEED 3298 3326.3 18.0 234.08.G
CHARGED
LOSS -5 14.6
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
~n o
a E"~~ lNpGV7O OC ~O0000N G v-I ~ M
o,osoN 'nv~oor vios~ y o, o '"'
P~I~ ~ ~V7V7V7l~N NN O ~ O H ~
O
O O O'V'M NL~t MN N ~ et ~ 'et
0 0 0 o o,~V;rroo,~ ~ ~ ~ d;
PIN N r-IV7N V7l~N NN O r1 O O~O~1
O
M ~' ~ r N
G Vo o oN V w,~ ~ ~o , ~ o o
'v'v , , -,
~ ~ e-HInInInl N NN O H O rl
~ ~ o0 0 oN ~ ~M ~ r ~
N N ~ ~ o O ~ O
O
inIri~l N NN
G
O
V1~ NN ~OetI~00NM 01 O\ O 01
WM N NO~M N\OO ~f7M 00 00 O M
0 0 00~10N10N1~ 0~10~10~0 O O O
W r1v-Ir-1b O ~O O OO v--1v-1 r1 rl
O O
~
O o
,.W .-i~ N I O~N O~NOy
~
y O~'N 00H N NN ~ ~~ M M 00 O~
O o W w
~
'-W D
c~n I~ 00 M
N O vC\\OOM r-1e-1l M 01 01
i-a M e1'Mt~0000N r-~i17OG O~ O N N
~O\OMv-1r-1r-1N 0100O~<t ~ N M ~M
O
M ~ ~ H
a ~ ~ ~ z
~
; <rO~Ml M e-~et~:~00 00 o0 ~ ~ON
~ ~Dv0t~ r r00 H
d
V c ~O~Oe~D~O~DM M OM O N l~ M ~tM A
' V~~D~OM~ ~ ~N 00000~'ct'~D ~ M OM
M
~ ~
H
~
~ H
~ON NO N OO ~OpN
O M O InO N O~ t~OO
~O ~
H
~ i ii i~ i i~
~ I i
C/W-I~ONN O NO O \D N
o v~o~ o ~o inr V
~D~Dl~l~0000OvO~01 ~ V~
<",q
o A U' ~ rn
a~ . W U
U ~",
f=
, O O W
~
U r-~N M'd'~ \Ol 0001~ ~ W H O~
r.~
36
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
TABLE 4: Elemental Composition of Feedstock S
Anal ses on ock
Feedst B
650+F
Resid
Measured Value
Nitrogen 0.991 wt%
Sulfur 0.863 wt%
Nickel 8.61 ppm
-.
Vanadium < 0.2 ppm
[000209] Table 4 illustrates the partial elemental composition of Feedstock B
atmospheric
distillation (650°F) residue including some of the identified
impurities. Table 4 displays the
weight percent nitrogen, sulfur, nickel and vanadium in Feedstoclc B
atmospheric distillation
residue. Subsequent steps remove these materials.
Step 5 - Pyrolysis of Isolated Fractions
[000210] A high-temperature reactor was used to pyrolyze and degrade a portion
of the
nondiamondoid components in various distillation fractions obtained in Step 4
(FIG. 12)
thereby enriclung the diamondoids in the residue. The pyrolysis process was
conducted at
l0 450 °C for 19.5 hours. The gas chromatogram (FID) of fraction #6
(Table 3B) is shown in
FIG. 18A. FIG. 18B is the chromatogram for the product of pyrolysis. A
comparison of
these chromatograms shows that pyrolysis has removed major nondiamondoid
hydrocarbons
and has significantly increased the higher diamondoid concentration,
especially the
hexamantanes. A 500 mL PARR~ reactor from PARR Instrument Company, Moline,
15 Illinois was used in this pyrolysis step.
Step 6 - Removal of Aromatic and Polar Nondiamondoid Components
[000211] The pyrolysate produced in Step 5 was passed through a silica-gel
gravity
chromatography column (using cyclohexane elution solvent) to remove polar
compounds
and asphaltenes (Step 6, FIG. 12). The use of a silver nitrate impregnated
silica gel (10
2o weight percent AgN03) provides cleaner diamondoid-contaiung fractions by
removing the
free aromatic and polar components. While it is not necessary to use this
chromatographic
aromatic separation method, it facilitates subsequent steps.
Step 7 - Multi-column HPLC Isolation of Higher Diamondoids
37
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000212] An excellent method for isolating high-purity higher diamondoids uses
two or
more HPLC columns of different selectivities in succession.
[000213] The first HPLC system consisted of two Whatman M20 10/50 ODS columns
operated in series using acetone as mobile phase at 5.00 mL/min. A series of
HPLC
fractions were taken (see FIG. 19). Fractions 36 and 37 were combined and
taken for further
purification on a second HPLC system. This combined fraction (36 and 37)
contained
hexamantanes #7, #11 and #13. (FIG. 19, also see FIG. 13B).
[000214] Further purification of this combined ODS HPLC fraction was achieved
using a
Hypercarb stationary phase HPLC column having a different selectivity in the
separation of
l0 various hexamantanes than the ODS column discussed above. FIG. 20 shows
elution times
of the individual hexamantanes on the Hypercarb HPLC column (with acetone as a
mobile
phase).
[000215] The differences in elution times and elution order of hexamantanes on
ODS and
Hypercarb HPLC columns are seen by comparing these two FIG's. 19 and 20. For
example,
15 Hexamantanes #11 and #13 elute together on the ODS HPLC system (FIG. 19)
but in
separate fractions (fractions 32 and 27, respectively) on the Hypercarb system
(FIG. 20).
[000216] The different elution orders and times of selected higher
diaxnondoids on these
two systems can be used to separate co-eluting higher diamondoids. It can also
be used to
remove impurities. Using this method on combined ODS HPLC fractions 36 & 37,
2o appropriate Hypercarb HPLC fractions were taken thus providing high-purity
hexamantane
#13 (FIG'S. 51A and S1B). Other ODS HPLC fractions and Hypercarb HPLC cut
points
could be used to isolate the remaining hexamantanes. This isolation strategy
is also
applicable to the other higher diamondoids although elution solvent
compositions can vary.
[000217] The ODS and Hypercarb columns can also be used in reverse order for
these
25 isolations. By using similar methodology as above, i.e. fractionating
hexamantane-
containing ODS fractions using the Hypercarb or other suitable column and
collecting at
corresponding elution times can lead to the isolation of the remaining
hexamantanes in high
purity. This is also true of the other higher diamondoids from tetramantanes
to
undecamantanes, including substituted forms.
38
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 2
[000218] Steps 1, 2, 3, 4, 5 and 6 of Example 1 were repeated (FIG. 12). The
following
variation of Step 7 was then carried out.
Step 7':
[000219] A two-column preparative capillary gas chromatograph was used to
isolate
hexamantanes from the product of Example 1, Step 6. The cut times for the
hexamantanes
were set for the first preparative capillary the GC column, methyl silicone DB
-1 equivalent,
using the retention times and patterns from GC/MS assay (Example 1, Step 2).
The results
are shown in FIG. 42A, two cuts identified as "peaks cut and sent to column
2", were taken
l0 which contains two of the hexamantane components from Feedstock B. The
preparative
capillary gas chromatograph used was manufactured by Gerstel, Zizc.,
Baltimore, Maryland,
USA.
[000220] The first column was used to concentrate the higher diamondoids, such
as
hexamantanes by taking cuts that were then sent to the second column (see FIG.
42B
illustrated for hexamantane #2 and #8). The second column, phenyl-methyl
silicone, a DB-
17 equivalent, further separated and purified the hexamantanes and then was
used to isolate
peaks of interest and retain them in individual traps (traps 1-6). GC trap
fraction 1 contained
crystals of hexamantane #2. GC trap fraction 3 contained crystals of
hexamantane #8.
Subsequent GC/MS analysis of trap #1 material (FIG. 43A and B) showed it to be
high
2o purity hexamantane #2 based upon the GC/MS assay of Step 2. Similarly, the
GC analysis
of trap #3 material (FIG. 44A and B) showed it to be primarily hexamantane #8.
Photomicrographs of hexamantane #2 and #8 crystals (analyzed in FIG's. 43 and
44) axe
shown in FIG's. 45 and 46. This procedure could be repeated to isolate the
other
hexamantanes. This is also true of the other higher diamondoids.
EXAMPLE 3
[000221] Steps 1, 2, 3; and 4 (FIG. 12) of Example 1 were repeated using
Feedstock A.
Feedstock A is especially low in nondiamondoids in the atmospheric residue
fraction
recovered in Step 4. The pyrolysis Step (5) of Example 1 may be omitted
especially when
the higher diamondoids being sought are tetramantanes, pentamantanes and
cyclohexamantane. In this case the fractions removed in Step 4 go directly to
Steps 6 and 7
39
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
in Example 1 or directly to Step 7 in Example 2 (FIG. 12). This process
variation can be
applied to lower-boiling tetramantane-containing fractions of Feedstock B as
well. However,
pyrolysis is highly desirable where significant nondiamondoid components are
present.
[000222] A fraction corresponding in cutpoint to fraction #1 of Step 4 (see
distillation
Table 3, Example 1 and FIG. 17) was taken from this feedstock. This fraction
was further
fractionated by preparative capillary gas chromatography similar to the
processing shown in
Step 7' of Example 2 (FIG. 12).
[000223] -A two-column preparative capillary gas chromatograph was then used
to isolate
the target tetramantanes from the distillate fraction cleaned-up by column
chromatography
l0 (Step 6, FIG. 12). Using the retention times and patterns from the GC/MS
assay (from Step
2 of Example 1), the cut times for the target diamondoids (e.g.,
tetramantanes) were set for
the first preparative capillary GC column, methyl silicone DB-1 equivalent.
The results are
shown on the top of FIG. 21 identified as cuts 1, 2 and 3.
[000224] The first column was used to concentrate the target diamondoids
(e.g.,
tetramantanes) by taking cuts that were then sent to the second column (phenyl-
methyl
silicone, a DB-17 equivalent) (see the bottom of FIG. 21). The second column
further
separated and purified the target diamondoids and then sent them into
individual traps (traps
1-6). GC traps 2, 4 and 6 contained the selected tetramantanes (FIG. 21).
[000225] The highly concentrated tetramantane higher diamondoids were then
allowed to
2o crystallize in the trap or dissolved and recrystallized from solution.
Under the microscope at
30X magnification, crystals of the tetramantanes were visible in preparative
GC traps 2, 4,
and 6 (see FIG. 22). Where concentrations were not high enough for
crystallization to
occur, further concentration by preparative GC was necessary. The process
would also work
to isolate other higher diamondoids from Feedstock A.
40
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 4: Preparative GC of HPLC Fractions
[000226] With the heptamantanes, octamantanes and higher diamondoids, etc., it
may be
desirable to further fractionate the HPLC products obtained in Example 1, Step
7. This can
be carried out using preparative capillary gas chromatography as described in
Example 2,
Step 7'.
[000227] The following higher diamondoid components were isolated and
crystallized: all
of the tetramantanes from both Feedstocks A and B, all pentamantanes (mol. wt.
344)
isolated from Feedstock B; two hexamantane crystals (mol. wt. 396) isolated
from Feedstock
B; and, two heptamantane crystals (mol. wt. 394) isolated from Feedstock B,
octamantane
1o crystal (mol. wt 446) isolated from Feedstock B. As well as a nonamantane
crystal (mol. wt.
498) and a decamantane crystal (mol. wt. 456) isolated from Feedstock B. The
other higher
diamondoid components could also be isolated using the procedures set forth in
these
examples.
EXAMPLE SA: Isolation of Tetramantanes
[000228] The general processes of Examples 1 and 2 were used to enrich and
isolate the
tetramantanes.
[000229] In this example, pyrolysis Step 5 (FIG. 12) was not employed and the
product of
Step 4 went directly to column chromatography (Step 6 of Example 1). The
column
2o chromatography product was then treated as follows:
[000230] The eluent from the column chromatography of Step 6 was analyzed by
GC/MS
to determine the approximate GC retention times of tetramantane isomers.
Individual
tetramantanes were assigned a number according to their elution order in the
GC/MS
analysis. This reference number was used to identify individual tetramantanes
in subsequent
steps. Note that enantiomeric pairs are not resolved in this analysis and so
these enantiomers
(racemic mixtuxes) were assigned a single number for these purposes. GC
retention times
vary with changing columns and GC conditions and new reference retention time
tables were
prepared as needed using this procedure. Below is a table used in Example SD
procedures
below.
41
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
Tetramantane
Reference # 1 2 3
GCMS Retention
Times in. 11.28 11.84 12.36
[000231] A two-column preparative capillary gas chromatograph was then used to
isolate
tetramantanes from the distillate fractions cleaned-up by column
chromatography. The
results are shown in FIG. 21, identified as cuts 1, 2 and 3.
[000232] The first column was used to concentrate the tetramantanes by taking
cuts that
were then sent to the second column (see FIG. 21). The second column, phenyl-
methyl
silicone a DB-17 equivalent, further separated and purified the tetramantanes
and then sent
them into individual vials (traps 1-6). GC trap fractions 2, 4 and 6 were
collected and further
processed.
[000233] The highly concentrated tetramantanes were then allowed to
crystallize from
solution. Under the microscope at 30X magnification, crystals were visible in
preparative
GC trap fractions 2, 4, and 6 (see FIG. 22). Where concentrations were not
high enough for
crystallization to occur, further concentration by preparative GC was
necessary. FIG.'s
22A, B and C illustrate photomicrographs of tetramantane crystals isolated
from Feedstock A
in trap, #2, #4 and #6 corresponding to tetramantane #1, #2, and #3
(respectively).
[000234] After obtaining crystals of suitable size, material could be sent for
structural
determination using X-ray diffraction. Enantiomeric tetramantanes can undergo
further
separations to resolve their two components as discussed previously.
2o E~~AMPLE 5B: Enrichment of Tetramantanes Using Pyrolysis.
[000235] This example shows that pyrolysis (Step 5, Example 1, FIG. 12) can be
useful in
the isolation of tetramantanes.
[000236] Prior to pyrolysis, nondiamondoid components are present (FIG. 23A)
in a
tetramantane-containing fraction (distillation hold-up fraction similar in
composition to Cut
1, FIG. 17). Pyrolysis degraded the nondiamondoid components to easily
removable gas and
coke-like solids. As shown in FIG. 23B, the nondiamondoid peaks axe gone after
pyrolysis.
42
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000237] Pyrolysis was conducted by heating the tetramantane-rich distillation
cut under
vacuum in the reactor at 450 °C for 20.4 hours.
EXAMPLE SC: Isolation of Tetramantanes Using a Single HPLC System.
Isolations of diamondoids using HPLC
[000238] In addition to the gas chromatographic and pyrolysis methods
described above,
HPLC was also shown to provide sufficient enrichments of the tetramantanes to
allow for
their crystallization. Suitable columns for use are well known to those
skilled in the art. In
some cases, reverse-phase HPLC with acetone as mobile phase can be used to
effect this
purification. A preparative HPLC run of a Feedstock A, gas condensate,
distillate fraction
corresponding in cut point to Cut #1 (FIG. 17) was performed and the HPLC
chromatogram
recorded. Nine fractions where taken during the run. The HPLC columns used
were two
25cm x lOmm LD. Vydac octadecyl silane ODS columns operated in series (Vydac
columns
are manufactured by The Separations Group, Inc., CA, USA). A 20 microliter
sample of a
solution of the tetramantane-containing fraction at a concentration of 55
mg/mL was injected
into the columns. The columns were set-up using acetone at 2.00 ml/min as a
mobile phase
carver.
[000239] FIG. 24 (A,B) compares the gas chromatogram of the starting material
(FIG.
24A) and HPLC fraction #6. HPLC Fraction #6 is significantly enriched in
tetramantane
FIG. 24B compared to the starting material (FIG. 24B compared to the starting
material
(FIG. 24A)). Tetramantane #2 in HPLC Fraction #6 is approaching a
concentration
sufficient to bring about its crystallization.
EXAMPLE SD: Isolation of Individual Tetramantane Isomers by HPLC Using
Multiple Columns with Different Selectivities
[000240] As shown in Example SC, tetramantanes can be isolated using HPLC
methods.
In this example, HPLC columns of different selectivities were used to isolate
single
tetramantane isomers. FIG. 25 shows a preparative separation of the
tetrasnaiitanes using an
43
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
octadecyl silane (ODS) HPLC column with acetone as a mobile phase. The
distillation
product used as starting material in Example 5B was the feedstock.
Specifically, preparative
HPLC fractionation of the holdup fraction from Feedstock B atmospheric
distillation taken at
about 650°F were performed. The first column consisted of two Whatman
M20 10/50 (x2)
ODS columns operated in series using acetone at 5.00 ml/min as mobile phase
(@590 psi),
0.500 ml injection containing 56 mg/ml of the holdup fraction in acetone. The
resulting
chromatogram is shown on FIG. 25. Tetramantane #1 elutes first, tetramantane
#3 elutes
second and tetramantane #2 elutes last on the HPLC system (FIG. 25). The
detector used
was a differential refractometer. From this run, fraction 12 (FIG. 25) was
taken for further
purification.
[000241] Further purification of fraction 12 was achieved using Hypercarb-S
HPLC
colurmls which have a different specificity than the ODS column above, to
isolate
tetramantane #2 (FIG. 26). Two Hypercarb-S columns (manufactured by Thermo
Hypersil,
Penn, USA), 4.6mm LD. x 250 mm, operated in series using acetone at 1.00
mL/min as
is mobile phase (@180 psi), 50 microliter injection of 4 mg/ml in acetone also
using a
differential refractometer. Tetramantane #3 elutes first, tetramantane #1
elutes second and
tetramantane #2 elutes last on this Hypercarb HPLC system (FIG. 14).
Tetramantane #2 was
cut from this HPLC run (FIG. 26) and its purity illustrated in FIG.'s 28A and
B. Hypercarb
HPLC runs on ODS HPLC cut led to isolation of all the tetramantanes
(enantiomers are
2o separatable by chiral HPLC methods).
[000242] FIG. 27A shows the GC/MS total ion chromatogram (TIC) of an HPLC
fraction
containing tetramantane #1; and below it, FIG. 27B shows its mass spectrum.
FIG. 29A
shows the GC/MS total ion chromatogram (TIC) of an HPLC fraction containing
isolated
tetramantane #3; and below FIG. 29B shows the mass spectrum.
EXAMPLE SE: Isolation of Substituted Tetramantanes
[000243] Alkyltetramantanes can be purified using methodologies described for
nonalkylated tetramantanes given in Examples 5A to 5D. FIG. 30 shows an
isolated
monomethylated tetramantane with molecular weight of 306 yielding a mass
spectrometric
molecular ion of m/z 306, and shows a mass spectrometric loss of the methyl
group giving
the m/z 291 mass spectrometric fragment ion (indicative of a tetramantane
moiety). This
44
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
alkylated compound was isolated by Hypercarb HPLC and shows a retention time
of 11.46
minutes in our GC/MS system (FIG. 30). It may be necessary to use additional
HPLC
separations or preparative GC (as is Examples 3 and 4) to isolate some
alkyltetramantanes.
EXAMPLE 6A: Isolation of Pentamantanes by Preparative Gas Chromatography
[000244] Steps 1-4 of Example 1 (FIG. 12) were repeated. In Step 5, 5.2 g. of
Feedstock
B 650°F + bottoms distillation cut 5 (Table 3, FIG. 18) was pyrolyzed
under vacuum at
450°C for 16.7 hours. This product was then treated in accord with
Example 1 Step 6.
[000245] The eluent from the column chromatography (Step 6) was analyzed by
GC/MS
to to determine the GC retention times of pentamantane isomers. Individual
pentamantane
components with molecular weight 344 were assigned a number according to their
elution
order on this GC/MS analysis.
[000246] The two-column preparative capillary gas chromatograph was then used
to
isolate pentamantanes from the product of Step 6 above. An exemplary result is
shown for
15 pentamantane #1 in FIG. 31. The pentamantane #1-containing GC peak on the
first column
is identified as "peak cut and sent to column 2" in FIG. 31A.
[000247] The first column was used to concentrate the pentamantane by taking a
cut that
was then sent to the second column. The second column, phenyl-methyl silicone,
a DB-17
equivalent, fiuther separated the pentamantane #1 from other materials. The
material in the
20 peak of interest identified as "peak sent to trap" was sent to GC trap
fraction 6 where crystals
of pentamantane #1 accumulated (see FIG. 31B). GCMS analysis of trap #6
material (FIG.
32) showed it to be pentamantane #1 (in the pentamantane reference GCMS
retention time
system set-up for this preparative GC procedure, the first eluting
pentamantane (#1) showed
a retention time of 16.233 min. FIG's. 32A and B show the high purity of
pentamantane #1
25 removed from GC trap 6. This procedure could be repeated to isolate the
four other
pentamantanes and three enantiomeric pairs which could be separated using
chiral HPLC or
other resolution techniques.
[000248] The highly concentrated pentamantanes crystallize either directly in
the trap or
from solution. Under the microscope at 30X magnification, crystals of
pentamantane #1
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
were visible in preparative GC trap 6 (see FIG. 33A). These crystals were
perfectly clear
and showed high refractive index. Crystals of pentamantane #1 had never
existed before this
isolation. Where concentrations are not high enough for crystallization to
occur, ftirther
concentration by preparative GC may be necessary. FIG. 33B is a
photomicrograph of two
pentamantanes that co-crystallized in a preparative GC trap.
[000249] After obtaining crystals of suitable size, non-enantiomeric
pentamantane
materials could be sent for structural determination using X-ray diffraction.
Enantiomeric
pentamantanes can undergo further separations to resolve their two components.
1o EXAMPLE 6B: Isolation of Pentamantanes by HPLC
[000250] Steps 1-6 of Example 6A were repeated. GCIMS assay reference numbers
and
retention times for the 344 molecular pentamantanes were as follows:
Pentamantane
Reference 1 2 3 4 5 6
#
GCMS
Retention 13.68 15.26 15.31 15.72 15.85 16.06
Times* (min.)
*(HP-SMS, 0.25 micron film, 0.25 mm LD. x 30 m, helium carrier gas)
[000251] Pentamantane #1-containing ODS HPLC fractions indicated in FIG. 34
were
15 ftu-ther purified using Hypercarb HPLC (FIG. 35) to isolate pentamantane
#l. FIG. 14 shows
how ODS HPLC and Hypercarb HPLC can be used together to isolate the remaining
pentamantanes. The ODS and Hypercarb columns can also be used in reverse order
for this
isolation. FIG. 36 shows the GC/MS total ion chromatogram (TIC) of the
isolated
pentamantane #1. The lower half of FIG. 36 illustrates the mass spectrum of
the
2o pentamantane #1 GC/MS peak. As indicated in FIG's. 14 and 34, the various
remaining
ODS HPLC fractions contain other pentamantanes. By using similar methodology
as above,
i.e. fractionating pentamantane containing ODS fractions using the Hypercarb
(as indicated
in FIG. 14) or another suitable column, and collecting at corresponding
elution times, leads
to the isolation of the remaining pentamantanes in high purity as shown in
FIG's 37-41.
25 Specifically, FIG. 37 illustrates GC/MS total ion chromatogram (TIC) and
mass spectrum of
pentamantane #2 isolated using two different HPLC columns; FIG. 38 illustrates
GC/MS
total ion chromatogram (TIC) and mass spectrum of pentamantane #3 isolated
using two
46
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
different HPLC columns; FIG. 39 illustrates GC/MS total ion chromatogram (TIC)
and mass
spectrum of pentamantane #4 isolated using two different HPLC columns; FIG. 40
illustrates
GC/MS total ion chromatogram (TIC) and mass spectrum of pentamantane #5
isolated using
two different HPLC columns; and FIG. 41 illustrates GC/MS total ion
chromatogram (TIC)
and mass spectrum of pentamantane #6 isolated using two different HPLC
columns. The
enantiomeric pentamantanes are not resolved in GS/MS and therefore, these
enantiomeric
pairs are referenced witlun a single number. These enantiomers can be
separated by chiral
separation methods. In addition, as previously noted, there is a condensed
isomer of
pentamantane having a molecular weight of 330 which is more sterically
strained and this
appears in significantly lower concentrations. This pentamantane component has
been
observed in GC/MS analyses of distillation cut 5 pyrolysis product cleaned up
using Step 6
of Example 1 (FIG. 12). This pentamantane component eluted at 14.4 minutes in
the
analysis of Example 1, Step 4 and could be isolated using procedures in this
Example.
EXAMPLE 6C: Purification of Substituted Pentamantane
[000252] Substituted pentamantanes are present in Feedstocks A and B.
Substituted
pentamantanes can be enriched from these feedstocks and purified using
methodologies
described for nonalkylated pentamantanes in Examples 1-4. The monomethylated
pentamantane enriched in this instance has a molecular weight of 358 (yielding
a mass
spectrometric molecular ion of m/z 358, and shows a mass spectrometric loss of
the methyl
group giving the m/z 343 mass spectrometric fragment ion indicative of a
pentamantane
moiety). This alkylated compound was enriched in ODS HPLC fraction #31 and
could be
further purified to form a crystal by an additional HPLC separation, or
alternatively by a
preparative GC procedure (as is Example 3).
EXAMPLE 7A: Isolation of Hexamantane Components
[000253] The purpose of this example is to demonstrate procedures which can be
used for
the enrichment and isolation of the thirty-nine hexamantane components. The
process of
Example 1 was repeated with the following changes. In Step 5, 34.4 g, of
Feedstock B 650°F
bottoms distillation cut #6 (Table 3, FIG. 18) was pyrolyzed under vacuum at
450°C for 17.3
hr.
47
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
[000254] The eluent from the column chromatography (Step 6) was analyzed by
GC/MS
to determine the GC retention times of hexamantanes. Individual hexamantane
components
with molecular weight 396 were assigned a number according to their elution
order on this
GC/MS assay. These hexamantanes were the most abundant and selected for
convenience.
Similar assays could be prepared for the other molecular weights. Hexaxnantane
elution
times ran between 17.88 min. (hexamantane #1) and 19.51 min. (hexamantane #7)
in this
GC/MS assay. Retention times vary with changing GC columns and conditions
requiring
remeasurement of retention times. FIG. 13A lists another GC/MS assay result
for the
hexamantane components,
[000255] A two-column preparative capillary gas chromatograph was used to
isolate
hexamantanes from the distillate fractions cleaned-up by column
chromatography. The cut
times for the hexamantanes were set for the first preparative capillary GC
column, methyl
silicone DB -1 equivalent, using the retention times and patterns from GC/MS
assay. The
results are shown in FIG. 42A, identified as "peak cut and sent to column 2"
which contains
two of the hexamantane fractions.
[000256] The first column was used to concentrate the hexamantanes by taking
cuts that
were then sent to the second column (see FIG. 42 illustrated for hexamantane
#2 and #8).
The second column, phenyl-methyl silicone a DB-17 equivalent, further
separated and
purified the hexamantanes and then was used to isolate peaks of interest and
retain them into
individual traps (traps 1-6). GC trap fraction 1 was collected and further
processed for the
separation of hexamantane #2. GC trap fraction 3 was collected and further
processed for
the separation of hexamantane #8. Subsequent GC/MS analysis of trap #1
material (FIG.
43) showed it to be hexamantane #2 based upon the earlier run GC/MS assay.
Similarly, the
GC analysis of trap #3 material (FIG. 44) showed it to be primarily
hexamantane #8. This
procedure could be repeated to isolate the other hexamantanes.
[000257] The highly concentrated hexamantanes were then allowed to crystallize
either
directly in the trap or from solution. Under the microscope at 30X
magnification, crystals
were visible in preparative GC trap fraction 1 (see FIG. 45). These crystals
were perfectly
clear and showed high refractive index. Crystals of hexamantane #2 had never
existed
before this isolation. Where concentrations are not high enough for
crystallization to occur,
48
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
further concentration by preparative GC may be necessary. FIG. 46 is a
photomicrograph of
hexamantane #8 that crystallized in preparative GC trap 3. Crystals of
hexamantane #8 had
never existed before this isolation.
[000258] After obtaining crystals of suitable size, non-enantiomeric
hexamantane
components could be sent for structural determination using X-ray diffraction.
Enantiomeric
hexamantanes must undergo further separations to resolve the two components.
EXAMPLE 7B: Isolation of Hexamantanes Using a Single HPLC System.
to [000259] The HPLC coluzr~ns used were two SOcm x 20mm LD. Whatman octadecyl
silane (ODS) columns operated in series (Whatman columns are manufactured by
Whatman
Inc., USA). A 500 microliter sample of a solution of the cut 6 pyrolysis
product saturated
hydrocarbon fraction (54 mg), the product of Example 1, Step 6, was injected
into the
columns. The columns were setup using acetone at 5.00 ml/min as the mobile
phase. Some
15 of the HPLC fractions reached the purity necessary for individual
hexamantanes to
crystallize as shown for Hexamantane # 8 in ODS HPLC fraction # 39 (FIG. 47),
Hexamantane # 10 in ODS HPLC fraction # 48 (FIG. 48) and Hexamantane # 6 in
ODS
HPLC fraction # 63 (FIG. 49). Alternatively a Hypercarb column (manufactured
by Thermo
Hypersil, Perm, USA) or other suitable column could be used to purify
hexamantanes to
20 concentrations necessary for them to crystallize. A preparative Hypercarb
HPLC run of
Feedstock B distillate cut~6 pyrolysis product saturated hydrocarbon fraction
was performed
and the HPLC chromatogram recorded using a differential refractometer.
Fractions (e.g.,
FIG. 50) where taken during the run and showed that most hexamantanes display
different
elution times (verified by GC/MS analysis) from one another on the Hypercarb
HPLC
25 system (FIG. 20).
49
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 7C: Isolation of Hexamantanes Using Multiple HPLC Columns of
Differing Selectivities
[000260] Hypercarb HPLC fractions were taken to obtain high purity hexamantane
#13
demonstrated in FIG. 51. Other ODS HPLC fractions and Hypercarb HPLC cut
points could
be used to isolate the remaining hexamantanes. The ODS and Hypercarb columns
can also
be used in reverse order for this isolation. FIG. 52 shows the GC/MS total ion
chromatogram (TIC) of the hexamantane #7 containing Hypercarb HPLC fraction.
The
lower half of FIG. 52 illustrates the mass spectrum of the GC/MS peak,
demonstrating the
high purity of the isolated hexamantane #7.
[000261] The various remaining ODS HPLC fractions (FIG. 19) containing other
hexamantanes could be separated in the same way. By using similar methodology
as above,
i.e. fractionating hexamantane-containing ODS fractions using the Hypercarb or
other
suitable column and collecting at corresponding elution times can lead to the
isolation of the
remaining hexamantanes in high purity. This is also true of the hexamantanes
with
molecular weights of 382, "irregular" hexamantanes, that are in much lower
abundance in
our feedstocks than hexamantanes showing molecular weight of 396. FIG.'s 53
and 54
present reconstructed ion chromatograms for m/z 382 showing hexamantanes
running at
18.30 min. and 18.07 min., respectively. FIG.'s 53 and 54 also show the
corresponding mass
spectra for these 18.30 min. and 18.07 min. peaks, demonstrating the presence
of
2o hexamantanes with a 382 molecular weight in the saturated hydrocarbon
fraction from the
product of pyrolytic processing of Feedstock B distillation fraction #6. The
382 molecular
weight hexamantanes show internal bond strain, lower stability, and
correspondingly lower
concentrations than the 396 molecular weight hexamantanes, making the 382
molecular
weight hexamantanes the less preferred hexamantanes.
[000262] The enantiomeric hexamantanes are not resolved in GS/MS and
therefore, these
enantiomeric pairs are referenced within a single number. These enantiomers
can be isolated
by chiral separation methods.
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 7D: Isolation of Substituted Hexamantanes
[000263] Substituted hexamantanes including alkylhexamantanes also are present
in
Feedstock A and B. These natural substituted hexamantanes have uses similar to
the
unsubstituted hexamantanes, can act as useful intermediates in various
hexamantane
applications (e.g., polymer production) and can be de-alkylated to yield the
corresponding
underivatized hexamantane. Accordingly, methods for the isolation of
individual substituted
hexamantane were devised and exemplified by the isolation of alkyl substituted
components.
Substituted hexamantanes, including alkylhexamantanes, can be isolated in high
purity using
a single HPLC separation of appropriate distillation cuts as exemplified by
FIG. 55. FIG. 55
l0 shows that fraction #55 from an ODS HPLC separation of the saturated
hydrocarbon fraction
from Feedstock B, distillation fraction 6 pyrolysis contains a methylated
hexamantane in
high purity. Monomethylated hexamantanes have a molecular weight of 410
(yielding a
mass spectrometric molecular ion of m/z 410, and show a mass spectrometric
loss of the
methyl group giving the m/z 395 fragment ion (FIG. 55B)). Isolation of
substituted '
hexamantane components by HPLC may require multiple columns with different
selectivities. For example, the ODS and Hypercarb HPLC columns were run in
succession
to isolate the methylcyclohexamantane components (methyl-substituted mol.
weight 342
hexamantane) from distillation cut 6-pyrolysis product saturated hydrocarbon
fraction. From
the first ODS HPLC run, fractions #23-26 were combined and taken for further
purification
on a second HPLC system. This combined fraction (FIG. 56) contained a mixture
of
hexamantane (mol. weight 342 referred to as cyclohexamantane), eluting on our
GC/MS
system at 12.31 minutes as well as three methylcyclohexamantanes (#1-3)
eluting at 12.56,
12.72 and 13.03 minutes, respectively. Further purification of this mixture
(i.e. combined
ODS HPLC fractions #23-26) was aclueved using a Hypercarb stationary phase
HPLC
column. A 50 microliter sample of approximately 1 mg of this combined fraction
in acetone
was injected into the Hypercarb column, 10 mm LD. x 250 mm, operated using
acetone at
3.00 mL/min as mobile phase (@480 psi) using a differential refractometer
detector. In this
Hypercarb system methylcyclohexamantane #1 elutes primarily in fractions 18-22
and
methylcyclohexamantane #2 elutes primarily in fractions 23-25.
Methylcyclohexamantane
#1 and #2 where isolated in sufficient purity to form crystals. A GC/MS total
ion
chromatogram and mass spectrum of these compounds is illustrated in FIG.'s 57
and 59 and
illustrated as crystals in photomicrographs in FIG's. 59 and 60. FIG. 59
illustrates a
methylcyclohexamantane crystal precipitated from Hypercarb HPLC fractions #19-
21 and
51
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
FIG. 60 illustrates a methylcyclohexamantane crystal precipitated from
Hypercarb HPLC
fractions #23.
[000264] Enantiomeric pairs must undergo further separations to resolve the
two
components. After obtaining crystals of suitable size, non-enantiomeric
allcylhexamantanes
can be sent for structural determination by x-ray crystallography.
EXAMPLE 8A: Isolation of Heptamantane Components
[000265] The eluent from the column chromatography (Step 6, FIG. 12) was
analyzed by
GC/MS to determine the GC retention times of heptamantanes. Individual
heptamantane
to components with molecular weight 394 and 448 were assigned a number
according to their
elution order on our GC/MS assay (see FIG. 13A for representative assay
values). Molecular
weight 448 heptamantanes, the most abundant heptamantane family, were selected
for
convenience in this Example. Similar assays could be prepared for the other
molecular
weight heptamantanes.
15 [000266] A two-column preparative capillary gas chromatograph was then used
to isolate
heptamantanes from the distillate fractions cleaned-up by column
chromatography. The cut
times for the heptamantanes were set for the first preparative capillary GC
column, methyl
silicone DB -1 equivalent, using the retention times and patterns from GC/MS
assay (from
Step 2 above, FIG. 12). An exemplary result is shown in the top of FIG. 61,
identified as
20 "peak cut and sent to column 2" which contains two of the heptamantanes
from Feedstock B.
[000267] The first column was used to concentrate the heptamantanes by taking
cuts that
were then sent to the second column (see FIG. 61 illustrated for heptamantanes
#1 and #2).
The second column, phenyl-methyl silicone a DB-17 equivalent, further
separated and
purified the heptamantane components and then was used to isolate peaks of
interest and
25 retain them in individual vials (traps 1-6). GC trap fraction 2 was
collected and further
processed for the separation of heptamantane #1. GC trap fraction 4 was
collected and
further processed for the separation of heptamantane #2. Subsequent GC/MS
analysis of trap
#2 material (FIG. 62) showed it to be heptamantane #1 based upon the earlier
run GC/MS
assay of step 4. Similarly, the GC analysis of trap #4 material (FIG. 63)
showed it to be
52
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
heptamantane #2. This procedure could be repeated to isolate the other
heptamantane
components.
[000268] The highly concentrated heptamantanes were then allowed to
crystallize either
directly in the trap or from solution. Under the microscope at 30X
magnification, crystals
were visible in preparative GC trap fraction 2 (see FIG. 64). These crystals
were perfectly
clear and showed high refractive index. Crystals of heptamantane component #1
had never
existed before this isolation. Where concentrations are not high enough for
crystallization to
occur; further concentration by preparative GC may be necessary. FIG. 65 is a
photomicrograph of heptamantane component #2 that crystallized in preparative
GC trap 4.
l0 Crystals of heptamantane component #2 had never existed before this
isolation.
[000269] After obtaining crystals of suitable size, heptamantane materials
could be sent
for structural determination using X-ray diffraction. Enantiomeric
heptamantanes can
undergo further separations to resolve their two components.
EXAMPLE 8B: Purification of Single Heptamantane Isomers
[000270] HPLC was also shown to provide sufficient enrichments of some
heptamantanes
to allow for their crystallization.
[000271] The HPLC columns used were the same as those given in the other
examples
(ODS and Hypercarb). A 500 microliter sample of a solution of the cut 7
pyrolysis product
2o saturated hydrocarbon fraction (product of Step 6, FIG. 12) was injected
into the ODS
columns. Pyrolysis of Cut 7 used 25.8 g. heated at 450°C for 16 hrs.
Some of the ODS
HPLC fractions reached the purity necessary for individual heptamantanes to
crystallize as
shown for heptamantane # 1 in ODS HPLC fraction # 45 (FIG. 66). Others, such
as
heptamantane # 2 in ODS HPLC fraction # 41 (FIG. 67), heptamantane # 9 in ODS
HPLC
fraction # 61 (FIG. 68), and heptamantane #10 in ODS HPLC fraction # 87 (FIG.
69), may
require further separation on HPLC systems with different selectivities.
Running the ODS
fractions (FIG. 13B) on a Hypercarb column resulted in the purity necessary
for individual
heptamantane components to crystallize as shown for heptamantane component # 1
in
Hypercarb HPLC fraction # 55 (FIG. 70) and heptamantane #2 (FIG. 71). The
higher
3o diamondoids in various HPLC fractions could be separated using further
chromatographic
53
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
techniques including preparative gas chromatography and additional HPLC runs
using
columns of different selectivity as outlined below. Additionally other
techniques known in
the crystallization art could be utilized including but not limited to
fractional sublimation,
progressive recrystalization or zone refining could be used to purify the
heptamantanes.
[000272] By using similar methodology as above, i.e. fractionating
heptamantane-
containing ODS fractions using the Hypercarb or other suitable columns and
collecting at
corresponding elution times can lead to the isolation of the remaining
heptamantanes. This is
also true of the heptamantanes with molecular weights of 420 and 434, that are
in much
lower abundance in our feedstocks than heptamantane components showing
molecular
to weights of 394 and 448. A heptamantane component of molecular weight 420
shows up in
ODS HPLC fraction #61 (FIG. 73A) with a very strong molecular ion in the mass
spectrum
(in this case m/z 420, FIG. 73B) for the m/z 420 component running at 16.71
min. The mass
spectrum, with its prominent molecular ion and low number and abundance of
fragments is
characteristic of a diamondoid component.
EXAMPLE 8C: Isolation of Substituted Heptamantanes
[000273] Substituted heptamantanes including alkylheptamantanes also are
present in
Feedstock A and B. Alkylheptamantanes can be purified by removal of
nondiamondoid
impurities from feedstocks using pyrolysis as shown above. Certain
alkylheptamantanes
survive pyrolysis processing, as do the heptamantane components previously
identified.
Substituted heptamantanes including alkylheptamantanes can be isolated in high
purity using
a single HPLC separation as exemplified by FIG. 74. Monomethylated
heptamantanes have
a molecular weight of 408 (yielding a mass spectrometric molecular ion of m/z
408, and
show a mass spectrometric loss of the methyl group giving the m/z 393 mass
spectrometric
fragment ion indicative of a heptamantane moiety (FIG. 74B).
EXAMPLE 9A: Isolation of Octamantane Components
[000274] An octamantane-enriched fraction from Step 6 was subjected to reverse-
phase
HPLC. In some cases, reverse-phase HPLC with acetone as mobile phase can be
used to
effect this purification. A preparative ODS HPLC run of Feedstock B distillate
cut 7
54
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
pyrolysis product saturated hydrocarbon fraction (used in Example 8A) was
performed and
the HPLC chromatogram recorded using a differential refractometer. HPLC
fractions were
analyzed by GC/MS to determine octamantane HPLC elution times and monitor
purity (see
FIG. 13A for representative assay values). The HPLC columns used were the same
ODS and
Hypercarb systems used in previous examples. A 500 microliter sample of an
acetone
solution of the cut 7 pyrolysis product saturated hydrocarbon fraction (25 mg)
was injected
into the ODS columns. While using this HPLC system, some octamantanes reached
purity
needed for individual octamantanes to crystallize. For example, FIG. 75
illustrates a GC/MS
total ion chromatogram and mass spectra of an HPLC fraction in which
octamantane #1 has
l0 been purified to the point where it formed crystals (see FIG. 76). HPLC
Fraction 63 yielded
octamantane #3 and #5 together (FIG. 77), which co-crystallized from the
fraction (FIG. 78).
[000275] For isolation in high purity of other octamantane components (for
example
FIG.'S 79 and 80), multiple columns can be employed, e.g. Hypercarb.
EXAMPLE 9B: Isolation of Substituted Octamantane Components
[000276] Alkyloctamantanes can be purified using methodologies described for
non-
alkylated octamantanes given in Examples 1 and 3. FIG. 81 (A/B) shows that ODS
HPLC
fraction 94 contains a methylated octamantane in high purity. Monomethylated
octamantanes have a molecular weight of 460 (yielding a mass spectrometric
molecular ion
of m/z 460, and show a mass spectrometric loss of the methyl group giving the
mlz 445 mass
spectrometric fragment ion indicative of an octamantane moiety (FIG. 81B).
Also, where
more than one alkyloctamantane is present in an ODS or Hypercarb HPLC
fraction, an
additional HPLC separation of that fraction or preparative GC procedure (as in
Example 3)
can yield high purity alkyloctamantanes.
EXAMPLE 10A: Isolation of Nonamantane Components
[000277] A preparative ODS HPLC run of Feedstock B distillate cut 7 pyrolysis
product
saturated hydrocarbon fraction was performed (material described in Example
8A) and the
HPLC fractions were analyzed by GC/MS to determine nonamantane HPLC elution
times
(FIG. 82) and monitor purity. A 500 microliter sample of an acetone solution
of the cut 7
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
pyrolysis product saturated hydrocarbon fraction (25 mg) was injected into the
columns. The
columns were set-up using acetone at 5.00 ml/min as a mobile phase carrier.
[000278] For isolation of nonamantane components in lugh purity, multiple HPLC
columns can be employed. To illustrate this methodology, HPLC columns of
different
selectivities ODS and Hypercarb (as described in previous examples) were used
in
succession to isolate a single nonamantane. From the ODS HPLC run, the
nonamantane
containing fractions 84-88 (FIG. 13B) were combined for further purification
on a Hypercarb
HPLC system.
[000279] We injected a 50 microliter sample of approximately 1 mg of ODS HPLC
combined fraction (84-88) in methylene chloride onto two Hypercarb columns,
two 4.6 mm
LD. x 200 mm, operated in series using methylene chloride at 1.30 mL/min as
mobile phase.
[000280] FIG. 83 shows the GC/MS total ion chromatogram (TIC) of the
concentrated
nonamantane containing Hypercarb HPLC fraction. The lower half of FIG. 83
illustrates the
mass spectrum of the GC/MS peak. Nonamantane was isolated by a third HPLC run
using
the same Hypercarb stationary phase column but with a solvent consisting of
methylene
chloride/acetone (70:30 volume percent operating at 1.00 ml/min). The
resulting isolated
nonamantane crystal and corresponding mass spectrum are shown in FIG. 84.
[000281] By using a similar methodology as above, i.e. fractionating
nonamantane
containing ODS HPLC fractions using columns with different selectivities, such
as the
2o Hypercarb or other suitable columns, we isolated a molecular weight 498
nonamantane in
high purity. This method could be repeated to isolate the nonamantanes with
molecular
weights of 552, and the nonamantanes of molecular weights 538, 484 and 444,
which
respectively are in lower abundance in our feedstocks. Note that enantiomeric
nonamantanes
are not resolved in GS/MS, however these enantiomers can be isolated by chiral
separation
methods.
56
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 10B: Isolation of Substituted Nonamantanes
[000282] Substituted rionamantanes also are present in Feedstock A and B.
Alkylnonamantanes can be purified using methodologies described for non-
alkylated
nonamantanes. FIG. 85(AB) shows methylated nonamantane in a pyrolysis product
of
distillate fraction #7. One type of monomethylated nonamantane has a molecular
weight of
512 (yielding a mass spectrometric molecular ion of m/z 512, and show a mass
spectrometric
loss of the methyl group giving the m/z 497 mass spectrometric fragment ion
indicative of a
nonamantane moiety (FIG. 85B). More than one alkylnonamantane is present and
these
could be isolated using ODS or Hypercarb columns, an additional HPLC
separation, or by
1o preparative GC to yield high purity alkylnonarnantanes.
EXAMPLE 11A: Isolation of Decamantane Components
[000283] A preparative ODS HPLC run of Feedstock B distillate cut 7 pyrolysis
product
saturated hydrocarbon fraction was performed and HPLC fractions were analyzed
by GC/MS
to determine decamantane HPLC elution times (FIG. 86) and monitor purity. The
HPLC
columns used were two SOcm x 20mm LD. Whatman octadecyl silane (ODS) columns
operated in series. A 500 microliter sample of an acetone solution of the cut
7 pyrolysis
product saturated hydrocarbon fraction (25 mg) was injected into the columns.
The columns
were set-up using acetone at 5.00 ml/min as a mobile phase carrier.
2o [000284] For isolation of decamantane components in high purity, multiple
HPLC
columns can be employed. To illustrate this methodology, HPLC columns of
different
selectivities were used in succession to isolate a single decamantane. The
first HPLC system
consisted of the same ODS columns described previously. From this HPLC run,
the
decamantane containing fractions 74-83 were combined for further purification
on a second
HPLC system. Five such runs were completed and all decamantane containing
fractions
from the runs were combined. This combined fraction contained a molecular
weight 456
decamantane and various impurities.
[000285] To purify the combined HPLC fractions 74-83 from the ODS HPLC
separation,
we injected a 50 microliter sample of approximately 1 mg of ODS HPLC combined
fraction
in acetonelmethylene chloride (70:30 volume percent) onto two Hypercarb
columns, 4.6 mm
57
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
LD. x 200 mm, operated in series using acetone/methylene chloride (70:30
volume percent)
at 1.00 mL/min as mobile phase (@480 psi).
[000286] FIG. 87 shows the GC/MS total ion chromatogram (TIC) of the
concentrated
decamantane-containing Hypercarb HPLC fraction eluting at 18.55 minutes. The
lower half
of FIG. 87 illustrates the mass spectrum of the GC/MS peak with a prominent
peak at m/z
456. The resulting [1231241(2)3] molecular weight 456 decamantane crystal and
mass
spectrum are shown in FIG. 88. The 456 decamantane elutes before pentamantane
#3 on the
Hypercarb HPLC system due to its compact, low-surface area structure (FIG.
10). This
property of 456 molecular weight decamantane makes possible its isolation in
very high
1o purity.
[000287] By using a similar methodology as above, i.e. fractionating
decamantane-
containing ODS HPLC fractions using columns with different selectivities, such
as the
Hypercarb or other suitable columns, we isolated a molecular weight 456
decamantane in
high purity. This method could be repeated to isolate the decamantanes with
molecular
weights of 496 (shown in FIG. 89 in the saturated fraction of the pyrolysis
product of
distillate fraction #7) as well as molecular weights 550 or 604, and the
decamantanes of
molecular weights 536, 576 and 590, which respectively are in lower abundance
in our
feedstocks. Note that enantiomeric decamantanes are not resolved in GS/MS,
however these
enantiomers can be isolated by chiral separation methods.
EXAMPLE 11B: Isolation of Substituted Decamantanes
[000288] Substituted decamantanes also are present in Feedstock A and B.
Alkyldecamantanes can be purified using methodologies described for non-
alkylated
decamantanes. FIG. 90 shows that saturated fraction of the pyrolysis product
of distillate
fraction #7 contains methylated decamantanes. One type of monomethylated
decamantane
has a molecular weight of 470 (yielding a mass spectrometric molecular ion of
m/z 470).
Also, where more than one allcyldecamantane is present in an ODS or Hypercarb
HPLC
fraction, an additional HPLC separation of that fraction or an alternative
preparative GC
procedure can yield high purity alkyldecamantanes.
58
CA 02435132 2003-07-15
WO 02/057202 PCT/US02/00505
EXAMPLE 12: Isolation of Undecamantane Components
[000289] For isolation of undecamantane components in high purity, multiple
HPLC
columns can be employed. This methodology was demonstrated using decamantane
with
HPLC columns of different selectivities used in succession to isolate a single
decamantane
(Example 11). An appropriate starting material, Feedstock B, distillation cut
7 pyrolysis
product is shown to contain undecamantanes (FIG. 91).
[000290] The concentrated undecamantane from ODS HPLC fraction 100+ (FIG. 13B)
is
shown in FIG. 92. This fraction could be purified on a Hypercarb HPLC using a
system
(similar to that explained in Example 11) to isolate undecamantane. This
method could be
l0 repeated to isolate the undecamantanes with molecular weights of 656 and/or
602, as well as
molecular weights 642,628, 588, 548 or 534 which respectively are anticipated
to be in lower
abundance in our feedstocks.
59