Note: Descriptions are shown in the official language in which they were submitted.
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
ROBUST HIGHLY REFLECTIVE OPTICAL CONSTRUCTION
Field of the Invention
The present invention relates to optical constructions, and more
particularly to optical constructions having an optically transrnissive
substrate
material coated with a robust highly reflective optical layer.
Baclc~round of the Invention
Optical components such as waveguides are generally designed to confine
and direct the propagation of light waves for many applications. In
applications
that rely on the reflection and transmission of light, significant gains in
performance can be made when highly reflective materials are used in
combination with optically transmissive materials. For example, a step-index
fiber optic is composed of a thin strand of concentric layers of optically
transmissive materials- a central optical medium (i.e., the core) and a
surrounding
optical medium (i.e., the cladding), the latter having a lower index of
refraction.
Light is channeled through the core. During transmission, the light often
travels to
the boundary of the core and cladding, where it is reflected back towards the
core
1
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
by total internal reflection. However, total internal reflection is not total,
as some
of the light is Iost, for example, due to scatter induced by imperfections
within the
core or at the core/cladding boundary.
To reduce this loss, a reflective layer can be applied over the surface of the
cladding along the length of the fiber optic. The reflective layer
significantly
increases the amount of light directed back to the core and improves the
overall
light transmission through the fiber optic.
Ideally, the reflective layer used in optical components should possess a
high reflectance characteristic over a broad spectrum of Iight and over aII
incidence angles of reflectance. Silver is one metal known to possess a high
reflectance value. Silver has a reflectance of about 98% over the entire
visible
light spectrum at normal incidence. Silver also sustains a high reflectance of
about 96% for off normal light at near grazing incidence angles. In
comparison,
aluminum, a more commonly used reflective-layer material, possesses a
reflectance of about 93% at normal incidence. The reflectance of aluminum
drops
precipitously to 75% for Iight at grazing incidence angles.
Although silver possesses excellent optical characteristics, there are several
problems associated with the use of the reflective metal. Silver has a
tendency to
undesirably taxnish when exposed to the atmosphere, especially in the presence
of
2
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
corrosive gases and contaminants, including sulfur dioxide, hydrogen sulfide,
nitrogen dioxide, ozone, hydrogen chloride, chlorine, and organic acids. It is
l~nown that long-term performance of silver coatings is rarely, if ever,
guaranteed
by commercial coating facilities based on the aggressive nature of silver
tarmSlllllg
brought on by ordinary exposure to the environment, along with the laclc of
suitably available protective measures which have been successfully tested
under
corrosive conditions.
Further, silver's adherence to optically transmissive substrate materials,
including glass or polymeric materials such as polymethyl methacrylate, is
moderate at best. Polymethyl methacrylate is a low-cost acrylic resin
frequently
used in the fabrication of optical components.
For the foregoing reasons, there is a need for an optical construction
having a highly reflective coating that adheres favorably to a range of
optically
transmissive materials and that possesses improved resistance against
corrosion
and tarnishing to provide improved optically effective performance and longer
lasting operating life.
3
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Summary of the Invention
The present invention is generally directed to an optical construction for
optical components such as hollow and solid waveguides, solid and hollow light
pipes, fiber optics, prisms, microstructured sheets, curved mirrors
(ellipsoidal,
parabolic, etc.), piano mirrors, and other optics having topographic forms.
The
optical construction of the present invention is designed to maintain high
optical
performance and light transmission through the optical component in the
presence
of potentially corrosive substances including sulfur dioxide, hydrogen
sulfide,
nitrogen dioxide, ozone, hydrogen chloride, chlorine, organic acids and the
like,
which are present in the atmosphere at least in trace amounts.
The optical construction of the present invention is especially useful in
optical components where a highly reflective surface composed of a metal such
as
silver is desired. The optical construction is further adapted to provide
favorable
durability and preservation of the highly reflective surface in the optical
component without measurably degrading the total reflectance qualities of the
optical component.
In one aspect of the invention, the optical construction generally comprises
an optically transmissive substrate adapted for efficiently channeling light
therethrough with a highly reflective layer composed of a highly reflective
metal
4
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
deposited on the surface of the substrate, and bonded thereto. Overlying the
highly reflective metal layer and firmly adherently bonded thereto is a
protective
layer comprised of a parylene polymer film.
The parylene polymer protective layer as used in the present invention
serves to isolate the reflective layer from exposure to external elements such
as
ambient atmosphere, corrosive substances, salt, humidity and the like. Such
external elements can cause the destruction and degradation of the metal
reflective
layer over time through tarnishing, breakdown, delamination, or discoloration,
resulting in the loss of its reflectivity. The parylene polymer protective
layer
further improves the reflective layer's resistance to mechanical deformation
and
delasnination as indicated by a tape-pull test described hereinafter.
Optionally, the optical construction of the present invention can further
include an adhesion-promoting layer applied between the surface of the
substrate
and the reflective layer to strengthen the bond therebetween. The adhesion-
promoting layer as used in the present invention significantly improves the
adhesion between the $mctional reflective metal layer and the optically
transmissive substrate for improved resistance against delamination where the
reflective layer physically separates from the optically transmissive
substrate
resulting in degraded performance and reduction in reflectivity. Further, the
5
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
adhesion-promoting layer promotes uniformity and consistency in reflective
properties of the reflective layer along the substrate/reflective layer
interface.
In an alternative form of the invention, a waveguide structure such as a
fiber optic, comprising an optically transmissive glass or polymer material,
is
coated with an adhesion-promoting layer of the oxide form of a metal or
metalloid. A silver reflective layer is applied in contact with the adhesion-
promoting layer. A protective layer of a parylene polymer film is applied over
the
silver reflective layer to prevent the silver from losing its high reflective
luster or
from delaminating or degrading due to corrosive agents in the environment such
as ambient air. The preferred form of the invention forms a robust highly
reflective parylene/silver/metal-oxide/waveguide structure with improved
performance qualities including longer operating life.
Brief Description of the Drawings
Various embodiments of the invention are described in detail below with
reference to the drawings, in which like items are identified by the same
reference
designation, wherein:
Figure 1 is a cross sectional view of an optical construction having a
highly reflective layer illustrative of one embodiment of the present
invention;
6
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Figure 2 depicts a schematic diagram of a parylene vacuum evaporation
depositi<,n reactor system for depositing a parylene polymer film to make an
optical construction in accordance with the principles of the present
invention;
Figure 3 is a cross sectional view of an optical construction having a
highly reflective layer illustrative for a second embodiment of the present
invention;
Figure 4 is a cross sectional view of an optical construction having a
highly reflective layer illustrative for a third embodiment of the present
invention;
Figure 5 is a cross sectional view of a fiber optic waveguide comprising
the optical construction in accordance with the present invention;
Figure 6 is a graph plotting the silver corrosions rates for various samples
exposed in the presence of ambient air; and
Figure 7 is a graph plotting the silver corrosion rates for various samples
exposed in the presence of an ammonium sulfide solution.
7
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Description of the Invention and Preferred Embodiments
The present invention is generally directed to an optical construction and a
method of making such optical constructions. The optical construction of the
present invention includes a substrate, a highly reflective layer, an optional
adhesion-promoting layer in contact between the substrate and the reflective
layer,
and a protective layer comprising a parylene polymer film overlaying the
reflective layer. The optical construction of the present invention provides
favorable optical qualities with improved adherence of the reflective layer to
the
substrate and improved resistance to corrosion and tarnislung for a longer
operating life. The substrate material can be selected from the group
consisting of
glass and organic polymer-based materials such as polymethyl methacrylate
(PMMA), for example.
In the present invention, the parylene polymer film, useful as a protective
layer, has the following polymer repeat unit structure:
_C
n
8
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
where "n" indicates the number of repeating units in the structure. The
parylene
polymer coating may be exemplified in three forms or variations, with each
comprising varying degrees of chlorination. The three forms include parylene N
as shown above with no chlorine atoms, parylene C which is produced from the
same monomer as parylene N and is further modified by the substitution of a
chlorine atom for one of the aromatic hydrogens, and parylene D which is
produced from the same monomer as parylene N and is further modified by the
substitution of two chlorine atoms for two of the aromatic hydrogens.
With reference to Figure 1, there is depicted an optical construction
illustrative for one embodiment of the present invention. We note that the
thickness of the corresponding elements in the construction are not drawn to
scale,
and is shown for illustrating the general structure and relationships thereof.
The
optical construction denoted herein by reference numeral 10, can be applied
for
the fabrication of a range of optical components where a highly reflective
surface
composed of a metal such as silver, is desired.
The optical construction 10 generally comprises an optically transmissive
substrate 12 for efficiently transmitting and directing light therethrough, a
reflective layer 14 preferably composed of a highly reflective metal such as
silver
vapor-deposited on the surface of the optically transmissive substrate 12, and
a
protective layer 16 preferably composed of a parylene polymer film.
Preferably,
9
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
the surface of the substrate 12 is optically. smooth and substantially free
from
optical imperfections to provide the highest speculax reflectance. The surface
of
the substrate 12 can be optionally treated to promote adhesion with the
reflective
layer 14 including, but not limited to, plasma treatment as described in U.S.
Pat.
No. 5,982,546, the content of which is incorporated herein by reference to the
extent that there is no conflict.
The optically transmissive substrate used for fabricating optical
components such as fiber optic waveguides can be selected from a range of
materials depending, for example, on the application, the desired performance
characteristics, the cost, and the characteristics of the transmitted light.
The
optically transmissive substrate 12 can be composed of glass or polymer
material.
The polymer materials can include organic polymers such as polyhydrocarbons,
polyoxyhydrocarbons, polysulfohydrocarbons, and fluorocarbon and
fluorohydrocarbon materials, as well. Representative organic polymers include
polyesters such as poly(ethyleneterephthalate) and
poly(butyleneterephthalate),
polyacrylates and methacrylates such as poly(methyhnethacrylate) (PMMA),
poly(methacrylate), and poly(ethylacrylate), copolymers such as
poly(methylmethacrylate-co-ethylacrylate) and polycarbonates. Fluorocarbon
polymers such as TEFLON and the various fluorohydrocarbon polymers known in
the art can be used as well. More preferably, the polymer material is PMMA.
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Other polymers can be used as optically transmissive substrate materials,
particularly in applications where low birefringence is desired. Such polymers
include CR-39 allyl diglycol carbonate resin marlceted by PPG Industries of
Pittsburgh, Pennsylvania; OZ-1000 cycloaliphatic acrylic resin maxlceted by
Hitachi Chemical Co., Ltd. of Tolcyo, Japan; CALIBRE 1080 DVD polycarbonate
resin rnarketed by Dow Engineering Plastics of Midland, Michigan;
MAI~ROLON DP1-1265 polycarbonate resin marlceted by Bayer Corporation of
Pittsburgh, Pennsylvania; PLEXIGLAS VOD-100 acrylic molding resin marketed
by ATOFINA Chemicals, Inc. of Philadelplua, Pennsylvania, TOPAS cyclo-olefin
copolymer resin marketed by Ticona of Summit, New Jersey; ZEONEX cyclo-
olefin polymer resin marlceted by Nippon Zeon Co., Ltd of Tolcyo, Japan; and
the
like.
Although not a limitation to the application of this invention, the plastic or
polymer material can be clear, transparent, and optically transmissive. When
used
in context of plastic or polymer materials, the terms "clear", "traazsparent",
and
"optically transmissive" means a plastic or polymer that, in its configuration
of
use, exhibits transmission over a desired range of wavelengths. The polymer-
based substrates themselves axe commercially available or can be prepared by
various art-known processes and do not, in and of themselves, constitute an
aspect
of this invention. The polymer substrates can be formed into solid bodies,
sheets,
11
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
films, or coatings applied or laminated onto nonpolymeric surfaces such as
metal
and glass.
The reflective layer 14 of the optical construction 10 shown in Figure 1 is
preferably made up of one or more functional metals that possess high
reflectance
values such as silver, copper, gold, palladium, iridium, rhodium, combinations
in
the form of alloys thereof, and the life. Among these metals, copper, silver,
and
gold are preferred, with silver being the most preferred metal for the visible
range
of light. The reflective layer 14 comprising a metal or an alloy of metals,
can be
deposited onto the optically smooth surface of the optically transmissive
substrate
12 through conventionally lcnown deposition methods such as cathode
sputtering,
vacuum evaporation or vapor-phase deposition techniques for a thiclaless
ranging
from about 100 to 10,0001, preferably 500 to 3,0001, and more preferably from
about 1000 to 3,000 ~. Individual metals can be used, or a plurality of layers
of
different metals or layers of alloys of these metals can be used, if desired.
In another embodiment of the present invention, the reflective layer 14 of
the optical construction 10 is enclosed and sealed from ambient by the
protective
layer 16 for optimal protection against corrosion and tarnishing. The
protective
layer 16, in the form of a parylene polymer film, is vapor deposited on the
surface
of the reflective layer 14 distal from the optically transmissive substrate
12. The
12
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
parylene polymer protective layer 16, as applied, forms a continuously uniform
coating as will be further described.
The paiylene polymer film of the protective layer 16 can be composed of
parylene N, parylene C, parylene D, or combinations or mixtures thereof. The
parylene polymer film can be composed of an interpolymer of monomers of
parylene variants of varying mixture ratios. The thicl~ness of the parylene
polymer film of the protective layer 16 is preferably at least 0.0001 ", more
preferably in the range of from about 0.001 to 0.0001 ". We note that the
actual
thiclcness of and the mixture ratios of the variants in the parylene polymer
protective layer can be adjusted according to the application, requirements,
the
reflective layer metal used, the desired effect, the duration of effect, and
the types
of expected contaminant exposures and the like, and may be readily determined
by
orie slcilled in the art.
The parylene polymer film can be optionally processed using suitable
annealir~b or heat-treatment techniques to improve the chemical resistance and
durability of the coating as will be described: The term "annealing" or "heat-
treating" as used herein refers to any processes for treating a substance or
material
with heat followed by cooling to modify or alter the structural properties of
the
treated substance or material.
13
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
In accordance with the present invention, the parylene polymer film is
applied through a coating process using conventionally lcnov~m vapor phase
deposition or vacuum evaporation deposition techniques. It is understood that
the
present invention can utilize any suitable commercially available method for
applying parylene polymer on a surface as lcnown by one skilled in the art.
As an illustrative example, one process for applying a parylene polymer
coating is described in U.S. Pat. No. 3,342,754, the disclosure of which is
hereby
incorporated by reference in its entirety to the extent that no conflict
exists. It is
understood that the invention is not limited to the use of this process.
With reference to Figure 2, a general schematic diagram of a basic
parylene vacuum evaporation deposition reactor system 40 for carrying out the
vacuum evaporation deposition process described in U.S. Pat. No. 3,342,754, is
shown. As noted above, there are many known systems and processes known in
the art for applying a polymer film on a substrate. The following description
of
system 40 provides an illustration of the process that may be used for coating
a
substrate with a parylene polymer layer. The system 40 can be constructed
using
commercially available components and parts as known by those skilled in the
art.
With further reference to Figure 2, the system 40 comprises a vaporization
chamber 42, a cracking chamber 44, a deposition chamber 46, and a vacuum pmnp
14
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
48. The vacuum pump 48 operates to evacuate the air from the interior of the
system 40. The vaporization chamber 42 is adapted to heat a sample of the di-p-
xylylene dimer under vacuum at an elevated temperature sufficient to vaporize
the
diner. Under vacuum conditions, the vaporized diner radiates in all directions
within the chamber 42.
The vaporized diner proceeds to the cracking chamber 44 where the
dimmer is heated to a temperature of less than 700°C, preferably
between 450°C
and 700°C, and more preferably at about 680°C for a sufficient
time at a pressure
such that the vapor pressure is below 1.0 mm Hg, to form a parylene diradical
monomer of parylene.
The paiylene diradical monomer proceeds to the deposition chamber 46
where the diradical monomer condenses and polymerizes at a temperature of less
than 200°C, preferably below the ceiling condensation temperature of
the parylene
diradical monomer, and more preferably at room temperature on the cooler
surface
of the reflective metal-coated optically transmissive substrate. The
condensation
of the diradical monomer yields a tough, linear, non-fluorescent polymer. The
vacuum pump 48 is connected to the system 40 to ensure that the process is
carried out in an evacuated atmosphere for optimal processing.
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
ine vacuum evaporation technique of depositing parylene polymer
provides several advantages. The first is that the room temperature deposition
process enables a range of substrates to be coated with parylene polymer
films.
The second is the formation of a highly conforming and uniformly continuous
coating on substrates with complex shapes. The third is the capability to form
very thin coating layers while remaining continuous and uniform for precise
coating control.
With particular reference to Figures 1 and 2, the overall process of malting
the optical construction of the present invention will now be described. In a
preferred form of the optical construction 10, the construction is formed by
vapor
depositing a silver layer 14 onto the optically smooth surface of a PMMA-based
optically transmissive substrate 12. The reflective metal-coated optically
transmissive substrate is placed into the deposition chamber 46 of the reactor
40,
and suitably positioned for exposing the outer surface of the reflective
silver metal
14 to the parylene diradical monomer flow. The parylene vacuum evaporation
process produces a parylene polymer protective layer 16 of sufficient
thickness on
the surface of the silver metal layer 14. The thickness of the deposited
parylene
polymer protective layer 16 can be determined while in the deposition chamber
46
using any one of various optical methods known in the art. Alternatively, the
thickness of the parylene polymer protective layer 16 can be determined after
the
article is removed from the deposition chamber 46.
16
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
'fhe above deposition process can be repeated at least once using the same
or a different parylene variant (i.e., parylene N, parylene C, parylene D,
and/or
mixtures thereof) to produce a multilaminate parylene polymer coating on the
surface of the reflective silver layer 14 as will be further described
hereinafter.
The deposition chamber 46 is sealed from ambient air and the atmosphere of the
chamber 46 is evacuated with the vacuum pump 48. Alternatively, the atmosphere
in the deposition chamber 46 can be substituted at ambient pressure with an
inert
gas such as helium, argon or nitrogen.
~;'e have discovered that by annealing the deposited parylene polymer
protective film in the protective layer at an elevated temperature for a
sufficient
time, and allowing them to cool, a substantially improved chemically resistant
parylene polymer barrier is formed. We have also discovered that the physical
barrier and mechanical properties of the parylene polymer coating are greatly
improved after the annealing thermal treatment. The annealing temperature can
be
at least 120°C, preferably from about 120°C to 220°C and
the annealing time may
range from about 1 hour to five (5) days. The annealing process can be carried
out
under suitable atmospheric conditions including, but not limited to vacuum,
inert
gas, and normal ambient atmosphere. The annealing conditions can be varied as
required by the thermal mass of the substrate, the maximum substrate
temperature
rating, and the like, as may be determined by those skilled in the art.
17
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
The parylene polymer film can be annealed immediately after the
completion of the parylene deposition process. The annealing process is
preferably conducted in a vacuum, or in the presence of at least one inert gas
such
as helium, argon, nitrogen, and the lilce, at atmospheric pressure. The
optimal
annealing conditions may differ slightly between each variant of the parylene
polymer. We further note that the annealing process may be utilized on each
palylene polymer protective layer individually as applied during the vapor
deposition process, or on the parylene polymer protective layer as a whole
after
applying more than one parylene polymer layer.
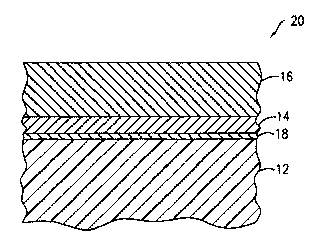
Ir: another embodiment of the present invention as shown in Figure 3, there
is provided an optical construction 20 which is not drawn to scale, comprising
an
optically transmissive substrate 12 as described above and a thin adhesion-
promoting layer 18 comprising the oxide form of at least one metal or
metalloid
that is applied to the substrate surface using conventional deposition
processes
such as vacuum evaporation, cathode sputtering, electron beam evaporation, and
the like. The adhesion-promoting layer 18 is applied to the substrate 12 prior
to
the application of the reflective layer 14. Details describing the use of
aluminum
oxides for enhancing the adhesion of silver to glass substrates, is found in
Hass et
al., Applied Optics, 14, 2639 (1975), the content of which is incorporated
herein
by reference.
18
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
The reflective layer 14 comprising a highly reflective metal such as silver
is deposited, using methods described above including electron beam
evaporation,
onto the surface of the adhesion-promoting layer 18 for a thickness sufficient
to
form an opaque, highly reflective surface at the interface between the
substrate 12
and the reflective layer 14. Finally, the surface of the reflective layer 14
is coated
with a protective layer 16 comprising a parylene polymer film preferably using
the
vacuum evaporation deposition or suitable process as described above.
As noted above, the adhesion-promoting layer 18 preferably comprises the
oxide form of at least one metal or metalloid that is sufficient to bond the
metal
atoms of the reflective layer 14 to the smooth surface of the optically
transmissive
substrate. 12. Preferably, the thickness of the adhesion-promoting layer 18
can
range from about 10 to 1000, and more preferably about 300 ~. The use and
application of metal- and metalloid-based oxides (collectively referred
hereinafter
as "metal oxides") as adhesion promoting materials between a metal and a
polymer substrate is further described in U.S. Pat. Nos. 5,589,280 and
5,902,634,
the pertinent teachings of both are incorporated herein by reference to the
extent
that there is no conflict.
For most applications, any of the adhesion-promoting materials selected
should be as nearly colorless as possible, at least in the amounts found
effective to
19
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
provide reliable adhesion. An adhesion-promoting material that imparts a
visually
detectable color to the substrate 12 under the desired illuminant not only
reduces
the efficiency of reflection by absorbing light passing to and fiom the
reflective
layer 14 but also changes the color value of the light rays directed at the
reflective
layer 14 through the substrate 12. We note that the adhesion-promoting
material,
in addition to promoting adhesion of the metallic reflective layer 14 to the
substrate 12, must resist corrosion to maintain its optical qualities. We
further
note that the selection of the materials for the adhesion-promoting layer must
also
take into account the effects of the relative expansion coefficients in order
to
preclude undesirable effects including delamination resulting from cyclic
temperature changes.
In one embodiment of the present invention, the adhesion-promoting layer
18 which is positioned between the optically transmissive substrate 12 and the
reflective layer 14, is composed of the oxide form of one or more metals
including, but not limited to, hafnium, zirconium, tantalum, titanium,
niobium,
silicon, tungsten, aluminum, vanadium, molybdenum, chromium, tin, antimony,
indium, zinc, bismuth, cadmium, nickel and the lilce.
Generally, the method for producing the adhesion-promoting Iayer 18 is to
deposit the metal oxide via cathode sputter deposition, electron beam
evaporation
deposition or any suitable process for depositing metal oxides. The metal
oxides
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
are preferably deposited in the oxidized mode, which may be achieved for
example by sputtering in the presence of an excess of oxygen so that the metal
is
fully oxidized, to attain the desired adhesion promotion.
Since some of the metals considered here for the adhesion-promoting layer
18 exhibit substantial absorption in their metal state (i.e., >3% absorption
at
thiclcnesses less than 20 A), it is advantageous to deposit them as oxides.
Similarly, it may also be advantageous to up-oxidize the metal layers fully or
partially after their deposition.
Referring to Figure 4, an optical construction is depicted for a tlurd
embodiment of the invention. The optical construction denoted herein as
reference numeral 30 is similar to the optical construction 20 of Figure 3
previously described above. We again note that the thickness of the
corresponding elements in the construction are not drawn to scale, and is
shown
for illustrating the general structure and relationships thereof. In the
present
embodiment, the optical construction 30 includes a protective layer 16 that is
composed of a multilaminate structure with each layer being composed of a
distinct parylene polymer selected fiom the group consisting of parylene N,
parylene C, parylene D and combinations or mixtures thereof. The multilaminate
form of the protective layer 16 provides benefits of each parylene variant
and/or
21
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
mixtures of parylene variants for improved compatability with the reflective
metal
layer, chemical resistance and the life.
The protective layer 16 includes first parylene film 17 composed of a first
parylene variant or mixtures of parylene variants. The first parylene film 17
is
deposited on the reflective layer 14 using one of the suitable deposition
methods
described above. The protective layer 16 further includes a second paxylene
film
19 composed of a second parylene variant or mixtures of parylene variants
overlaying the surface of the first parylene film 17 distally from the
reflective
layer 14. The actual thickness of each parylene variant layer can be adjusted
according to the application, requirements, the reflective layer metal, the
desired
effect, the duration of effect, and the types of expected contaminant
exposures and
the lilce, and may be readily determined by one skilled in the art.
In one embodiment, the first parylene film 17 is composed of parylene C,
and the second paxylene film 19 is composed of parylene D. We have determined
from experimental results that when paxylene C was deposited as a protective
layer directly on the silver reflective layer, the change in silver
reflectance at the
parylene/silver interface, was observed to be within the noise of the
experimental
data. The findings indicated that there is little or no reactivity between
parylene C
and silver.
22
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
We have further determined from experimental results that when parylene
D was deposited on the silver layer as a protective layer, the silver
reflectance at
the parylene/silver interface, was measurably diminished or degraded. Since
parylene D is known to possess an average chlorine content of two chlorine
atoms
per monomer unit, we theorize that the presence of unbonded or trapped
chlorine
in the parylene polymer film may be reacting with the silver. Although the
findings indicated that there may be some reactivity between parylene D and
silver, parylene D is a suitable candidate for use as part of the protective
layer.
Parylene D is lmown to have a lower gas permeability value than parylene C for
better exposure protection of the silver reflective layer. The silver/paxylene
C/parylene D laminate combination provides an effective protective layer,
which
possesses the low reactivity with silver of parylene C, and the low gas
permeability of parylene D.
In yet another embodiment, the transitioning of the deposition of parylene
films from one parylene variant to another, can be made gradually to form a
transitional interlayer (not shown) between the first and second parylene
polymer
layers. As the deposition of the parylene variants transitions, the vapor flow
of the
first parylene polymer is gradually reduced while the vapor flow of the second
parylene polymer is ramped up in proportion to the corresponding reduction of
the
first parylene polymer vapor flow. Tlus action produces a graded interface
between the pure parylene polymer layers and forms an interpolymer with
23
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
improved adhesion therebetween. We note that the resulting parylene polymer
layer can be annealed or heat-treated as desired to modify the properties of
layer as
described above.
It is understood that the actual tluclcness of the interlayer can be adjusted
according to the application, requirements, the desired effect, the duration
of
effect, and the types of expected contaminant exposures and the like, and may
be
readily determined by one skilled in the art.
Referring to Figure 5, a fiber optic waveguide is depicted for one
illustrative embodiment of the present invention. The fiber optic waveguide
denoted generally by reference numeral 50, generally comprises an elongated
cylindrical body having concentric layers of glass for channeling light
therethrough. The fiber optic waveguide 50 of Figure 5 comprises a core 52
composed of an optically transmissive glass or polymer material, a cladding 54
composed of an optically transmissive glass or polymer material with a lower
refractive index than the core 52, a reflective layer 58 with an optional
adhesive-
promoting layer 56 interposed between the reflective layer 58 and the cladding
54,
and a parylene polymer protective layer 60 overlaying the reflective layer 56.
The
fiber optic 50 includes the optical construction of the present invention
where the
cladding 54 establishes the optically transparent substrate. The fiber optic
50 can
be fabricated from any commercially available fiber optic waveguide while
using
24
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
the above-described techniques for applying the reflective layer, the optional
adhesion-promoting layer, and parylene polymer layer, all onto the surface of
the
cladding 54.
5~ EXAMPLE 1
Experimental Tests
We obtained samples of optical quality polymethyl methylaciylate
(PMMA) substrates with a reflective index of 1.49 for testing. An aluminum
oxide coating was evaporatively applied to one set of samples using
conventional
electron beam evaporation deposition process to form an adhesion-promoting
layer. The aluminwn oxide source having a purity of 99.999 %, was obtained
fiom Cerac of Milwaukee, WI. The aluminum oxide was deposited using a flow
of 21.8 % OZ/Ar at a total pressure of 2x10 Torr. The deposition rate was set
at
approximately 1 1~ per second to produce a final tluclcness of about 3001.
A layer of silver metal was applied to the surface of each sample substrate
using a conventional electron beam evaporation deposition process. The silver
metal source having a purity of 99.999 %, was obtained from Cerac. The silver
layer was applied at a thickness of 1,000 1~ at a deposition rate of from
about 1.2
to 7.3 ~ per second. The average deposition rate was about 3 1~ per second.
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Parylene D and C were each obtained from Paratronix, Inc. of Attleboro,
MA. The parylene polymers were applied to the samples using chemical
deposition processes resulting in a coating of about 0.0005". The degree of
protection the parylene polymer layer provided was measured by the changes in
reflectance of the silver layer through the substrate. Reflectance
measurements
were made using a MacBeth Color-Eye 7000 spectrometer with a spectral range of
from about 360 to 750 mn. Measurements at the interface were made through the
PMMA substrate and will include any absorption due to the PMMA or
interference effects from the first surface reflectance.
Accelerated silver tarnishing was induced by placing the sample in a
sealed 200 mm diameter Pyrex glass desiccator containing normal ambient air
and
a evaporation dish holding 2 cc of ammonium sulfide (20 % aqueous solution) in
18 cc of deionized water. The ammonium sulfide was obtained from Strem
Chemicals of Newburyport, MA. The samples of substrates were positioned 4 cm
above the solution with the silver layer side exposed to the solution. The
silver
reflectance was measured as a function of the exposure time in the desiccator
chamber. The ammonium sulfide solution generated hydrogen sulfide as the
primary corrosion agent. We had observed that elemental sulfur had deposited
on
the desiccator walls after long exposure times. Ammonium sulfide solution is
known to be one of the most aggressive tarnishing agent of silver. See, Dar-
Yuan
Song et al., Applied Optics 24 (8), 1164 (1985).
26
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Ambient Air Results
In order to estimate the rate of silver corrosion in ambient air for an
unprotected sample, the reflectance of a silver coated PMMA sample was
measured periodically when exposed to the ambient air of the laboratory. The
change in reflectance of the silver surface and the silver/PMMA interface as
measured through the optically transmissive PMMA substrate was recorded for
each sample. The reflectance was measured using Iight with a wavelength of
about 550 nm extending over a period of about 70 days. The points were plotted
and Iinear regression analysis was executed to generate a graph depicted in
Figure
6.
With reference to Figure 6, the graph shows that the ambient air exposure
resulted in tarnish rates of about 6.3 x 10-z %/day for the silver surface,
and about
2.2 x 10-2 %/day for the silver/PMMA interface. We believe that the lower
tarnish
rate at the interface as compared to the silver surface can be explained in
that the
diffusion of corrosion agents through the silver layer, or less lilcely,
through the
much thicker PMMA substrate was slower. Included in the graph are reflectance
measurements for samples (control) that had been stored in 3M Corrosion
Control
Absorber Paper (CPAP), an anticorrosion paper product of Minnesota Mining and
Manufacturing Co. of St. Paul, Minnesota. The anticorrosion paper is designed
to
27
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
prevent tarnishing from the presence of air contaminants that cause oxidation
and
corrosion. When the corrosive elements were removed from the air by the
anticorrosion paper, both the silver surface and the silver/PMMA interface
showed
no measurable change in reflectance. The change of reflectance was less than 3
x
10-4 %/day over the 70 day measurement period. Comparing the two sets of data,
we can conclude that the changes in silver reflectance were produced by air
corrosion alone, and there appeared to be no perceptible interaction of the
silver
mirror with the PMMA substrate at the interface.
Ammonium sulfide
To test the ability of parylene coatings to inhibit the tarnish of silver,
several silver coated PMMA samples were prepared in the manner as described
above. The PMMA samples were encapsulated with films of both C and D
variants of parylene. The parylene polymer coated PMMA samples were obtained
from Paratronix. The film thickness of the parylene coatings was measured to
be
on average of about 0.00043 of an inch.
Using the test procedure described above, the effectiveness of parylenes
coatings C and D were evaluated. Changes in silver reflectance as a function
of
exposure time in the corrosion chamber were measured and the results are shown
in Figure 7. Referring to Figure 7, the samples were each exposed to ammonium
28
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
sulfide solutions. The corrosion rates were determined from data analyses
using
linear least-square fits. The corresponding corrosion rates for exposure to
ambient
air and ammonium sulfide are listed below in Table 1.
Table 1
Silver Tarnish Rates Determined fiom Reflectance Measurements at 550 nm
Protective Silver Corroding Agent
Film Tarnish
Rate (%/day
at 550
nm)
Ag SurfaceAg/PMMA Interface
None 0.063 0.022 Air
None 7.1 x 104 5.3 x 103 Ammonium sulfide
Parylene 4.9 0.50 Ammonium sulfide
C
Parylene 0.33 0.17 Anunonium sulfide
D
Comparing the tarnish rates through the parylene C and D films, we had
observed that the tarnish rate for the parylene C was fifteen times higher
than the
rate for parylene D. Comparing the tarnish rates for parylene protected
samples to
the unprotected silver samples, we had observed that the tarnish rate was
reduced
by a factor of 6.9 x 10-5 for the parylene C coating and a factor of 4.6 x 10-
~ for the
parylene D coating. Assuming that similar corrosion agents are responsible for
the ambient air tarnish results, the above tarnish reduction factors can be
used to
estimate a tarnish rate for parylene polymer protected silver in normal
atmospheric
air. Applying the tarnish reduction factors to the ambient air data results in
an
estimated air tarnish rate of about 4.3 x10- %/day for a parylene C protected
silver
film and an estimated air tarnish rate of about 2.9 x 10-' %/day for parylene
D.
29
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Based on this analysis, either of the parylene variants would protect silver
for 50
years with less than a 0.1 % change in reflectance.
The measured tarnish rates at the silver/PMMA interface listed in Table 1
are at all times lower that those from the silver surface. This result is
expected
since there is the added requirement for the corrosion gases to diffuse
through the
silver layer to reach the silver/PMMA interface.
Silver Adhesion
Parylene C and D films were deposited directly onto PMMA to test the
adhesion of these films. Several samples of each variant were tested with
SCOTCH tape marketed by Minnesota Mining and Manufacturing. Co., and were
observed to be adherent to the substrate with no instances of the parylene
film
removal by the tape pulls.
Although silver appears to be compatible with PMMA when in direct
contact, the adhesion to this material is marginal. SCOTCH tape tests of
silver
coatings on PMMA consistently removed all of the silver film. Encapsulation of
the silver coated PMMA substrates with parylene, as would be done for the
final
silver coated waveguide structure, does improve the robustness of the silver
coating.
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Due to the high tensile strengths of the parylene films, silver films on
PMMA that have been coated with either parylene C or parylene D will usually
pass the SCOTCH tape test without any film delamination. However, in some
instances blisters can be seen in the film after the pull test indicating
areas where
the silver film has detached from the PMMA substrate. The parylene film,
however, remains intact and well-bonded to the underlying silver film. These
failures confirmed the need to improve the silver/PMMA interfacial bond.
As detailed previously, metal- or metalloid-oxides are lcnown to enhance
the adhesion of silver to glass substrates. Alumina was chosen since it is
also an
excellent candidate for the silver coated waveguide application due to its
high
transparency throughout the visible spectrum. In order to test alumina as an
adhesion layer for silver on PMMA, a 300 Angstrom-thiclc layer was deposited
on
PMMA prior to deposition of the silver mirror. SCOTCH tape tests indicate that
the alumina interfacial layer improves the silver adhesion. Approximately 80%
of
the tape pulls resulted in no loss of silver film with 20% of the pulls
removing a
portion of the silver mirror from the PMMA substrate. Once alumina-bonded
silver films were overcoated with parylene C, no removal or delamination of
the
silver mirror from the substrate was observed from tape test pulls.
31
CA 02436584 2003-07-28
WO 02/060686 PCT/US02/02559
Although various embodiments of the invention have been shown and
described, they axe not meant to be limiting. Those of slcill in the art may
recognize various modifications to these embodiments, which modifications are
meant to be covered by the spirit and scope of the appended claims.
32