Note: Descriptions are shown in the official language in which they were submitted.
CA 02437763 2006-09-07
1
TITLE OF THE INVENTION
ASSEMBLED IMPLANT INCLUDING MIXED-COMPOSITION SEGMENT
FIELD OF THE INVENTION
This invention relates to implants and methods for their preparation wherein
components of
the implant are assembled from constituent pieces to produce a complete
implant. An implant
according to this invention comprises two or more segments comprised of
mineralized, or
demineralized bone segments or a segment comprising both demineralized and
mineralized
regions juxtaposed to one another.
BACKGROUND OF THE INVENTION
In the field of medicine, there has been an increasing need to develop implant
materials for
correction of biological defects. Particularly in the field of orthopedic
medicine, there has
been the need to replace or correct bone, ligament and tendon defects or
injuries. As a result,
there have emerged a number of synthetic implant materials,
CA 02437763 2009-12-24
2
including but not limited to metallic implant materials and devices, devices
composed
in whole or in part from polymeric substances, as well as allograft,
autograft, and
xenograft implants. It is generally recognized that for implant materials to
be
acceptable, they must be pathogen-free, and must be biologically acceptable.
Generally, it is preferable if the implant materials may be remodeled over
time such
that autogenous bone replaces the implant materials. This goal is best
achieved by
utilizing autograft bone from a first site for implantation into a second
site. However,
use of autograft materials is attended by the significant disadvantage that a
second site
of morbidity must be created to harvest autograft for implantation into a
first diseased
to or injured site. As a result, allograft and xenograft implants have been
given increasing
attention in recent years. However, use of such materials has the disadvantage
that
human allograft materials are frequently low in availability and are high in
cost of
recovery, treatment and preparation for implantation. By contrast, while
xenograft
implant materials, such as bovine bone, may be of ready availability,
immunological
and disease transmission considerations imply significant constraints on the
ready use
of such materials.
In view of the foregoing considerations, it remains the case that there has
been a long
felt need for unlimited supplies of biologically acceptable implant materials
for repair
of bone and other defects or injuries. This invention provides a significant
advance in
the art, and largely meets this need, by providing materials and methods for
production
of essentially any form of implant from component parts to produce assembled
implants. In particular, the invention is directed to compositions, methods
and kits that
relate to an implant, in which at least one single segment is demineralized or
comprises
a combination of mineralized and demineralized regions. Among the advantages
of
this invention are the benefits in strength, structural support, and
flexibility, depending
on the particular implant and its use in a patient in need thereof.
In addition, reference is made herein to US Patent 5,899,939 to Boyce.
CA 02437763 2009-12-24
3
Finally, reference is made herein to US Patent 6,025,538 to Yaccarino.
The present invention advances the art beyond the references cited above by
disclosing
and claiming implants that comprise a combination of mineralized and
demineralized
regions provided in a single segment (discrete piece), which is
distinguishable from that
disclosed in U.S. Patent No. 6,200,347 (teaching homogenous demineralization
of a
single segment). The importance of demineralized bone in implants is described
in
U.S. Patents No. 6,090,998 and 6,652,592 and in PCT Publication No. WO
02/62405.
SUMMARY OF THE INVENTION
This invention provides a method for manufacture of autograft, allograft and
xenograft
implants which comprises assembling such implants from smaller pieces of graft
materials to form a larger graft implant product. Some pieces of such graft
materials
for assembly are demineralized, and are combined with other pieces of graft
materials
that are mineralized.
Accordingly, it is one object of this invention to provide a method for
assembly of
multiple bone implant shapes from smaller bone implant pieces.
Another object of this invention is to provide assembled bone implants.
Related to this
object is the object of assembling components of an assembled allograft in
such a way
as to compensate for disproportionate shrinkage among components during freeze
drying so as to still obtain precision interference fits.
3o Another object of this invention is to provide a method whereby otherwise
wasted
tissue may be used in the production of useful orthopedic implants.
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
4
Another object of this invention is to provide an implant having a combination
of at
least one region that is demineralized, juxtaposed to at least one region that
is
mineralized. Another object of this invention is to combine a segment of an
implant
having combination of mineralized and demineralized regions with other
segments that
are mineralized.
Further objects and advantages of this invention will be appreciated from a
review of
the complete disclosure and the appended claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Attached to this invention disclosure are a large number of sketches which
demonstrate
a wide variety of assembled implants which may be prepared and used according
to this
invention.
Figure 1 is a flow chart showing the formation of various sub-component parts
of an
assembled implant according to this invention, from which assembled implants
and a
kit comprising these parts may be formed according to the disclosure of this
invention.
Figure 2 provides a schematic of an assembled implant according to this
invention.
Figure 3 provides a schematic of an assembled implant according to this
invention.
Figures 4-7 provides a schematic of an assembled implant according to this
invention.
Figures 8-9 provides a schematic of an assembled implant according to this
invention.
Figures 10-14 provides a schematic of an assembled implant according to this
invention.
Figures 15-18 provides a schematic of an assembled implant according to this
invention.
Figure 19 provides a schematic of an assembled implant according to this
invention.
Figure 20 provides a schematic of an assembled implant according to this
invention.
Figure 21 provides a schematic of an assembled implant according to this
invention.
Figure 22 provides a schematic of an assembled implant according to this
invention.
Figure 23 shows the assembly of a dowel from component pieces.
Figure 24 shows the reinforcement of an implant using a cortical bone pin.
CA 02437763 2009-12-24
Figure 25 shows the reinforcement of an implant using a cortical bone pin and
a
cortical bone disc.
Figure 26 shows the reinforcement of cancellous bone implants using a
plurality of
cortical bone pins.
5 Figure 27 shows the formation of an assembled implant comprising soft and
hard
tissues.
Figure 28 shows a segment comprising a central mineralized region and
demineralized
regions.
Figure 29 shows the arrangement of the segment of Figure 28 positioned between
two
mineralized implant segments.
Figure 30 shows an alternative embodiment comprising more than one segment
having
mineralized and demineralized regions.
Figure 31 shows an embodiment of the subject assembled implant supported by a
scaffold.
Figure 32 shows an additional embodiment comprising segments fastened together
through a friction fit.
Figure 33 shows a two-segment assembled implant fastened together through a
friction
fit.
Figure 34 shows an embodiment that comprises two segments that interlock
together in
a transverse cross-over configuration.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Currently, autograft, allograft and xenograft products are produced as solid,
continuous
materials. For example, bone dowels (see US Patent 5,814,084), Smith-Robinson
cervical
spine implants, iliac crest grafts, and the like are harvested and machined
from single,
continuous pieces of bone. The present invention provides methods for
manufacture of
autograft, allograft and xenograft implants by assembling such implants from
smaller pieces
of graft materials to form a larger graft implant product. As a result,
increased utilization of
valuable implant materials is achieved, thereby more effectively meeting the
ever-increasing
demands for graft implant materials. In addition, greater flexibility is
achieved in the types
and shapes of implant materials is achieved. Essentially, any implant piece
that may be
required may
CA 02437763 2009-12-24
6
be formed according to the present invention, and orthopedic surgeons may be
provided
with kits of assemblable parts which may be formed in the course of a surgical
procedure to precisely meet the needs of a given patient or procedure. In yet
another
aspect of this invention, existing graft products may be strengthened or
reinforced by
assembly of different types of graft materials into an assembled product. One
example
of such a reinforced product is a caneellous wedge, block, dowel or the like
into which
is inserted reinforcing pins of cortical bone. As a result, those skilled in
the art will
understand from this disclosure that different sections of tissue may be
assembled to
make a complete graft implant. Furthermore, this invention provides for the
product of
assembled implants comprising any one or combinations of allograft materials,
autograft materials, xenograft materials, synthetic materials, metallic
materials and the
like. Furthermore, the assembled implants or the component pieces which are
combined to form the assembled implant may be pre-treated or treated after
assembly
to incorporate any desired biologically active or inert materials. Thus, for
example, in
an assembled bone dowel implant according to this invention, the assembled
bone
dowel comprises segments of cortical bone pinned to each other by means of
cortical
bone pins. Prior to assembly or after assembly, the graft materials are
soaked, infused,
impregnated, coated or otherwise treated with bone morphogenetic proteins
(BMP's),
antibiotics, growth factors, nucleic acids, peptides, and the like.
It is also noted that the compositions and structures disclosed and claimed
herein may
be obtained from allograft, xenograft or autograft sources, and are comprised
of
cortical, cancellous, or cortico-cancellous types of bone tissue, or
combinations thereof.
As disclosed herein, the compositions and structures disclosed and claimed
herein are
comprised of mineralized bone, demineralized bone, or combinations thereof.
Also, a
preferred pre-treatment is to subject allograft and xenograft-sourced bone
material to
one of the cleansing processes described in PCT Publication No. WO 01/08715
and U.S.
Patent No. 6,482,584.
In essence, one method to reduce antigenicity (disclosed in WO 01 /08715 A 1)
is to treat bone material in hydrogen peroxide, or hydrogen peroxide
CA 02437763 2009-12-24
7
in combination with a detergent such as TritonTM X- 100 or Sodium Dodecyl
Sulfate
(SDS), or another chaotropic agent, such as urea, guanidinium hydrochloride,
TweenTM,
TNBP, and mixtures of these agents. This is followed by contacting with a
defatting
solvent, such as acetone, isopropanol, hexane, or combinations of these. The
primary
object is to remove the non-collagenous protein from bone graft materials, and
thereby
reduce antigenicity.
In another method, disclosed in US Patent No. 6,482,584, efficient cleaning
and
passivation (inactivation of pathogens) is achieved by sequential
depressurization and
pressurization of a chamber containing bone graft materials, where these
materials are
being exposed to cleaning/chaotropic solutions and solvents including those
described
above. This process has been found to improve penetration of the cleaning
solutions.
Thus, for the bone compositions and structures disclosed and claimed herein,
these pre-
treatments may be applied to clean and reduce antigenicity of the finished
materials.
It will be appreciated that variously shaped wafers, blocks, rings, washer-
shaped bone
pieces and the like may be affixed to each other in any secure and
biologically
acceptable manner. Preferably, the assembled pieces of bone are affixed to
each other
by means of pins, screws, rods, interference fit, threaded fits, key-way fit,
and the like
made from cortical bone. These fixation pieces are machined in a CNC lathe or
the like
to appropriate dimensions and are then threaded into mating holes tapped in
the pieces
to be assembled, or are pressed into drilled holes through adjacent pieces to
be
assembled by a pneumatic press or the like. In this fashion, very strong and
tightly
fitted pieces of implant materials maybe joined and implanted. The assembled
pieces
may first be machined to desired dimensions and shapes, prior to assembly, the
assembled implant may be machined, or both.
As noted above, the implant according to this invention may comprise an
assembled
cancellous block, dowel or the like, harvested from the iliac crest or another
suitable
3o site. As is known in the art, due to the wafer-like structure of cancellous
bone, such
grafts have low load-bearing characteristics. There exist reports in the
literature of
instances of extrusion, expulsion or collapse of iliac crest wedges, Cloward
Dowels,
and the like when utilized, for example, in spinal fusions. Nonetheless, use
of
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
8
cancellous bone is preferable over use of cortical bone implants, since
cancellous bone
is more osteoconductive than cortical bone. According to this invention, a
Cloward
Dowel, iliac crest wedge, or cancellous bone block, dowel or the like is
reinforced by
insertion therein of cortical bone pins. According to the method of this
invention,
cortical implants may also be reinforced by insertion therein of cortical bone
pins,
including when an assembled implant is prepared comprising different segments
of
cortical bone, cancellous bone or both. Insertion of the reinforcing pins
provides an
implant with multiple load-bearing pillars. The pins may be made to protrude
from the
surface of the implant to engage with inferior, superior or both surfaces of
bone
between which the implant is inserted. Thus, in a spinal implant, pin
protrusions may
be employed to created contact between the implant and the vertebral bodies,
thus
preventing extrusion and reinforcing a secure fit of the implant between
adjacent
vertebrae. We have, surprisingly, found that cortical pins of about 4.5 mm in
diameter
may each support a load of up about 2700 newtons (160 Mpa). Thus, according to
the
method of this invention, multiple pins may be inserted into an implant to
produce a
load-bearing capacity of known proportions (e.g. 10,000 newtons by insertion
of five
pins).
A further advantage of this invention is that it permits use of tissues that
are not
currently amenable to standard autograft, allograft or xenograft harvesting
and
processing procedures, such as ribs, metatarsal bone and the like. In
addition, useful
implant materials may be harvested and produced from otherwise un-useable
donor
tissues. In addition, due to the different nature of various segments of bone
that are
incorporated into the assembled, reinforced implants of this invention,
various shaping
methods aside from CNC lathe or other known procedures may be applied to
different
segments of the implant. Thus, a cancellous portion of bone implant may be
compression molded, and then affixed to other portions of cortical or
cancellous bone
machined according to different or similar principles. In addition, due to the
ability
provided by this invention to assemble implant pieces, implants of unusual
sizes and
dimensions may be prepared and machined. Thus, implants of 100 mm in size
could be
machined, for example, for corpectomies, when otherwise bone stock for
manufacture
of such implant dimensions would not be available.
CA 02437763 2003-08-07
WO 02/064180 PCT/USO1/27683
9
In view of the present disclosure, it will be appreciated that this invention
provides a
wide variety of assembled implants and implant parts: dowel shaped implants
comprising assembled dowel segments, between about two to about ten segments,
pinned together by one or more cortical bone pins. The assembled segments may
closely abut each other or may be spread apart from each other. Such implants
may be
prepared by harvesting discs of cortical bone, drilling and optionally tapping
holes
therein, and inserting shafts of cortical pins therethrough, or therein,
optionally by
threading portions thereof for torquing into optionally tapped holes. The thus
produced
dowels may be tapered or have parallel sides. In addition, dowels which are
harvested
as a cross-section across the intramedullary canal of a long bone, as in US
Patent 5,
814,084, which might otherwise not pass production specifications, due to
penetration
of one outside wall into the intramedullary canal, may be completed by
insertion
therein of a cortical pin. Likewise, where a sidewall is otherwise considered
to be too
narrow, a "doughnut" of bone may be affixed to the sidewall by means of a
cortical pin.
A longer dowel may be prepared by affixing two dowels to each other. A
posterior
longitudinal interbody fusion implant (PLIF) may be machined from a single
piece of
cortical bone, or be assembled from two pieces of bone which are affixed to
each other
by means of a cortical pin. A bone screw may also be prepared according to the
method of this invention by affixing multiple pieces of cortical bone to each
other with
a cortical bone pin, and then machining a thread on the exterior of the
assembled bone
pieces. It will further be appreciated from this disclosure that different
portions of the
assembled implant may be demineralized, partially or fully, to achieve a level
of
elasticity or compressibility not otherwise present in cortical or cancellous
bone.
Specific embodiments of assembled implants having a combination of
demineralized
and mineralized regions, present or assembled into a single discrete piece
(i.e., a
segment), are shown to possess superior properties. Different portions of bone
may
also be retained on a shaft by means of a cotter-pin type device.
According to one embodiment, a segment is mineralized allograft bone, and this
region
may be intimately contacted on two sides by two regions of partially
demineralized
allograft bone. Demineralized regions of a single segment may be formed
according to
conventional methods, such as by dipping a portion of a segment of mineralized
allograft bone in a demineralizing acidic solution to demineralize that
portion while
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
leaving the adjacent portion mineralized. Alternately, a segment of a larger
assembled
implant comprising both mineralized and demineralized regions may be formed
and
later joined together (such as by biocompatible adhesives, bone pastes, tongue
and
groove, etc.) into a structure that is or can be divided (such as cut
transversely) into a
5 number of segments and subsequently assembled. The term "demineralized" is
well
known in the art, and for the purposes of this invention is defined to be the
removal of
minerals, such as by dissolution in acid, from a material such as bone.
As used herein, a "mixed-composition segment" is defined to describe a segment
of an
10 allograft implant that is comprised of two or more regions having different
characteristics and/or properties. For example, a mixed-composition segment
can
comprise a region comprising demineralized bone or mineralized bone attached
to
another region comprising a synthetic material. Also, it is noted that
"demineralized,"
when not preceded by either "partially" or "fully," is taken to include,
subject to the
specific context, both partially and fully demineralized. Also, when referring
to a
particular mixed-composition segment, the segment may be described as a
"demineralized bone segment comprising a region of mineralized bone," and this
is
taken to mean a segment that has at least one region of mineralized bone and
at least
one region of demineralized bone.
In addition to assembled implants, instruments may be conveniently prepared
according
to the methods of this invention which may be utilized for insertion of other
implants.
In one embodiment of this invention, therefore, an implant driver is produced
wherein
the driving mechanism itself is formed from assembled cortical pins which
protrude
into mating recesses in an implant device. The instrument may be torqued to
adequate
loads to induce implantation of spinal implants and the like.
In developing the various embodiments of the present invention, one technical
issue of
merit is the need to develop a process whereby donor tissue, whether hard or
soft tissue,
allograft or xenograft tissue, may be treated in such a fashion as to
eliminate the
possibility of cross contamination between tissue segments obtained from
different
sources. While it is possible to practice the present invention to advantage
using tissue
obtained from a single screened donor, the real economies of scale and
commercially
CA 02437763 2009-12-24
11
viable application of the present technology is best realized by
implementation of an
efficient and reliable tissue decontamination process. Ideally, the process is
one which
permits multiple segments of soft or hard tissue to be treated simultaneously
so that a
stock of materials for assemblage of implants according to the present
invention is
facilitated. Accordingly, on preferred method for treatment of tissue,
disclosed in PCT
publication WO 00/29037. Accordingly, in this aspect of the invention, a
process is
claimed whereby an assembled allograft or xenograft tissue implant is prepared
by
treating the tissue in a closed container in which different cleaning
solutions are
contacted with the implant segments, either before or after assembly and
machining
into the final implant form, either in the presence or absence of sonication,
with rapid
oscillation of pressure in the closed container, to achieve deep cleaning and
interpenetration of cleaning solvents into the interstices of porous implants
or tissues.
Solutions including, but not limited to detergent solutions, peroxide
solutions and the
like are used in such procedure, and terminal sterilization with gamma
irradiation,
gaseous sterilants known in the art or other terminal sterilization procedures
known in
the art are employed to ensure safe implantation of the assembled implants
according
to this invention.
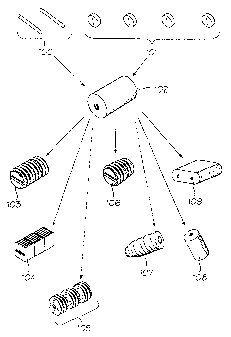
Referring now to figure 1, there is shown a flow-chart representing various
elements
that may be processed and assembled according to this invention. Cortical bone
pins
100 are used to assemble a series of bone discs 101 into a pre-part 102 which
is then
machined into a series of final products: Threaded dowels, 103; small blocks
104;
unique shapes, 105 such as a "wedding- cake" like shape wherein discs bearing
threads
are spaced apart from each other leaving voids 105' into which additional
materials
may be inserted, with the discs retained in fixed relation to each other by
means of the
through pins 100; tapered dowels 106; screws 107; smooth cylinders 108; or
large
blocks 109. From this figure, it will be appreciated that a central concept
relevant to the
present invention is the ability to machine smaller parts of tissue,
specifically bone
tissue, such as cortical bone, cancellous bone, cortical-cancellous bone,
portions of
which may be demineralized (see, for example, US Patent 6,090,998), and
assemble
these portions of tissue
CA 02437763 2009-12-24
12
using, preferably, cortical bone pins. The assembled tissue pieces may be
machined
prior to assembly, and then, upon assembly, a complete implant is ready for
implantation. Alternatively, the tissue pieces may first be assembled, and the
assembled pieces may then be machined into any desired final form. The order
of
assembly and machining will be determined by the specific forms of implant
required
for a particular application. In figure 1, a series of pre-machined tissue
forms are
disclosed, which may conveniently be included in a kit for use as needed by an
orthopedic surgeon. Thus, for example, where a particular implant of specific
dimensions is required, the surgeon is able to select pre-shaped implant
segments to fill
a particular geometric space and shape in the spine of an implant recipient.
Numerous
permutations and combinations of implant pieces for assembly are possible,
based on
the pre-machined assemblable implant pieces included in such a kit, and those
skilled in
the art will appreciate that the skilled orthopedic surgeon will be able to
create implants
as needed when supplied with such a kit. Thus, a preferred kit includes discs
of bone,
cortical bone, cancellous bone, allograft or xenograft, also referred to
herein as
"washers" or "doughnuts" such that a center hole is provided for press-fitting
or
screwing on of the discs to a cortical bone or synthetic or metallic shaft or
pin. The
discs may be demineralized, mineralized, or partially demineralized. Also
desirable in
such a kit are plugs of cortical bone, cancellous bone, or cortical-cancellous
bone,
including at least one through hole, and optionally more than one such through
hole, for
insertion of pins therethrough. Ovals, squares, rectangles and irregular
shapes may also
be provided in certain kits for specific applications. It will further be
appreciated,
based on the present disclosure, that inclusion of a bone paste, such as that
disclosed in
WO 99/38543, may be beneficial for filling any voids that remain, and to
implant with the
assembled implant, osteogenic material, (i.e. osteoconductive material,
Osteoinductive
material, or both, as well as material that assists in adhering the implant to
the site of
implantation). Further, a molded implant may be combined with the assembled
implant of
this invention. A preferred molded implant for orthopedic applications is
disclosed in
PCT publication WO 00/54821.
It is noted that assembled allografts may be assembled at and distributed from
a central
location, or, as discussed above, assembled around the time of surgery to meet
a
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
13
specific requirement of a patient in need thereof. In many applications it is
desirable to
have a tight and accurate interference fit between cortical bone pins and the
holes in
bone pieces that are connected by the bone pin. The target range for such an
interference fit is 0.001 to 0.003 inches (e.g., the pin diameter is 0.001 to
0.003 inches
larger than the hole diameter, and is pressed fit into place). However, it has
been
learned that freeze-drying the pins and other bone pieces exerts a
disproportionate
shrinkage upon the pins compared to the hole diameters. That is, the pin
shrinks
slightly more than the hole. Uncorrected, this would result in a less
accurate, and less
acceptable, interference fit.
The following method has been adopted to solve this problem. A bone pin,
preferably
of cortical bone, of a desired diameter is vacuum dried for at least five
hours. This
drying is preferably at room temperature and at a negative pressure of
approximately
100 milliTorre. This pre-treatment results in a shrinkage of approximately 80
percent
of the total shrinkage that would occur in freeze drying. The pin diameter is
measured,
and a hole is made in the discs (or other shapes that are to be assembled)
using an
appropriately sized reamer. The target size for the hole is 0.002 to 0.0025
inches
smaller than the post-vacuum drying pin diameter. Preferably, prior to this
drilling the
discs or other shapes have been kept saturated with moisture to maintain a
consistent
size and subsequent shrinkage percent. After all holes are drilled, the pin(s)
and discs
or other shapes are assembled, and then freeze dried. The resulting assembled
allografts have been found to have interference fits in the desired target
range. This
method is applicable to the various embodiments described in this disclosure.
Alternatively, where segments are provided in a kit for assembly prior to
surgery, the
discs and pins are preferably freeze-dried as disassembled. After freeze-
drying, the
diameter of the pins is measured and the appropriate size hole is made in the
disc. This
allows the provision of multiple parts in a kit, wherein the parts can be
assembled
together such that the requisite friction is acheived to keep the parts
securely together.
With reference to figure 2, there is shown two machined bone pieces, T and Z
each of
which bear external threading X and holes Y into which pins A are inserted to
form the
assembled graft 200. As can be seen, the assembled graft 200 comprises a void,
201
into which osteogenic material may be inserted prior to or after implantation.
The pins
CA 02437763 2010-01-29
14
Y may be metal pins, but preferably are pins machined from cortical bone. This
enables the entire implant to remodel into autogenous tissue over time, such
as
vertebral bone, when the implant 200 is inserted into the intervertebral
space. The graft
200 is also shown with a groove, 202 in which a driver may be inserted to
provide
rotational torque for insertion of the implant. An instrument attachment hole,
203, is
also provided, to ensure that the implant remains securely on the head of the
driver
means in the process of surgical implantation. Naturally, those skilled in the
art will
appreciate that the segments Z and T may be brought into close abutment with
each
other, thereby eliminating the space 201. In that event, the length of the
pins A would
be modified to prevent unnecessary protrusion, although in some applications,
protrusion may be useful when driving the implant 200 into place. It will also
be
appreciated that the number of pins used, while represented as two in this
figure, may
be fewer or more in number, depending on the particular application, the
extent of
torsional or compressive loads, and the like anticipated to be experienced by
the
implant once in situ. In some applications, the insertion of reinforcing
cortical bone
pins establishes a pillar structure such that two or more cortical bone pins
are load-
bearing. This application allows the use of materials in the segments that do
not
initially bear a substantial load, that load being born by the cortical bone
pin pillars, and
these materials have the opportunity to reform into bone that will provide
subsequent
structural load-bearing.
Figure 3 shows an implant assembled from three principal segments F, D, and E,
which
are held together by pins 300. In this implant, the waffle-shaped structure of
implant
segment D is intended to represent the use of cancellous bone, which is
abutted on
either side by cortical bone, which forms segments F and E. The fully
assembled
implant is shown in figure 4, while figures 5, 6 and 7 show end-on views, and
cross
sectional views A-A and B-B, respectively. Those skilled in the art will
appreciate
from this disclosure that segment F, segment D, or segment E may be
demineralized
according to methods known in the art. Likewise, all of these segments may be
demineralized. Where a flexible implant is required, the implant may be
assembled,
and the entire implant may be demineralized. Where flexibility is important in
one
dimension and structural support is also required, one solution is to have one
or more
segments of an composite bone graft be a mixed-composition segment which
comprises
CA 02437763 2010-01-29
at least one mineralized region and at least one demineralized region
(described in
detail below).
Figure 8 shows an embodiment of this invention wherein rectangular bone
segments N
5 and G are assembled into implant 900, shown in figure 9. Features 901 and
902 which
comprises ridges, teeth, or other external features are machined into the
superior and
inferior faces of the implants in order to assist in retention of the implants
once placed
in situ.
10 Figures 10-14 show the assembly of elements J, H, and I into implant 1100,
shown
end-on, in cross-section A-A and B-B, in figures 12-14, respectively. As can
be seen,
bone element H is shown with a waffle-like structure, to represent that this
element
may be cancellous bone, demineralized bone, a polymer composite, such as poly-
L-
Lactic acid, polyglycolic acid, or the like. Features 1101 and 1102 represent
external
15 grooves or teeth machined into the superior and inferior surfaces of the
implant to assist
in retention of the implant once placed in situ.
Figures 15-18 show the assembly of elements M, K and L, each of which is a
substantially cubic bone element, using pins. Figure 17 is a top view, showing
cross section A-A, represented in figure 18, with the final assembled implant
1600
shown in figure 16.
Figure 19 shows a "Wedding-Cake" design of an implant 1900 assembled from
units
A-C, pinned together by pins a-c. Void area 1901 is available for filling with
osteogenic materials.
Figure 20 shows implant which is an assembled Cervical Smith Robinson implant
similar to
that shown in PCT publication WO 99/09914. This implant is fashioned from
a series of assembled bone pieces and machined into the desired final shape.
Figure 21 shows implant 2100 assembled from two cortical bone pieces and one
cancellous
bone piece, and pinned together. The implant has an anterior height H1
CA 02437763 2010-01-29
16
which is smaller than posterior height H2, which permits retention of correct
spinal
lordosis upon implantation, for example, in a posterior lumbar intervertebral
implant
fixation procedure. Superior and inferior features 2101, 2102 prevent
expulsion of the
implant once place in situ.
Figure 22 shows an implant assembled from a series of sub-implant pieces 2201.
The implant may contain cancellous bone 2202 segments, as well as cortical
bone 2203
segments and cortical bone pins 2204.
Figure 23 shows the formation of a tapered dowel 2300 by assembling "doughnut"
or
"disc" or "washer" shaped bone pieces 2301 on a cortical bone shaft 2302 by
using
washer pieces of differing diameter. This figure only shows two discs, but a
continuous
dowel is formed by using discs of a graded diameter between each end of the
cortical
bone shaft 2302. In figure 24, figure 24A shows a bone dowel in which one
sidewall of
a bone dowel 2400 such as that disclosed and claimed in US Patent 5,814,084,
hereby
incorporated by reference, is "out of specifications" due to being too narrow
or absent.
This is repaired in figure 24B according to this embodiment of the invention
by
incorporation of an allograft or xenograft cortical bone pin 2401, to form a
complete
bone dowel. In this manner, valuable biological material which might otherwise
be
unusable for a particular application may be salvaged for use by employing the
methodology of this invention.
In figure 25, a similar procedure for salvaging a dowel 2500 is shown whereby
a pin
2501 is driven through the center of the dowel 2500 to reinforce the dowel
longitudinally. Furthermore, where an endcap 2503 of the dowel is "out of
spec" for
being too narrow, the endcap is reinforced by press-fitting a cortical bone
disc 2502
onto the end of the pin 2501.
In figure 26, a series of cancellous bone implants 2600 are reinforced by
inclusion
therein of a series of cortical pins 100. Each cortical pin of a 2 mm diameter
has been
found to support approximately 2000 newtons of axial compressive load.
Accordingly,
cancellous bone implants of essentially any desired height and compressive
strength
may be assembled in this manner by affixing several layers of cancellous bone
with
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
17
cortical bone pins. Naturally, based on this disclosure, those skilled in the
art will
appreciate that other materials may be included in such a "sandwich" of bone
materials.
The cancellous bone may be soaked in a solution containing growth factors,
such as,
but not limited to, bone morphogenetic proteins, fibroblast growth factors,
platelet
derived growth factor, cartilage derived morphogenetic proteins, stem cells,
such as
mesenchymal stem cells, osteoprogenitor cells, antibiotics, antiinflammatory
compounds, anti-neoplastic compounds, nucleic acids, peptides, and the like.
Those
skilled in the art will also appreciate that layers of cortical bone may be
included, layers
of biocompatible synthetic polymers and the like may also be included in the
stacked
bone implant. Various shapes may also be built upon, using for example,
circles,
ellipses, squares, and the like, as necessary for a given application.
In a further aspect of the present invention, the assembled implant is driven
by cortical
pins to seat in an implant site, using a driver that engages cortical bone
pins with
purchase sites on the implant. Thus, for example, not meant to be limiting,
the driver
may comprise a handle with projecting cortical pins which engage with holes in
the
assembled allograft, thereby providing a site for torquing the implant into
position.
In a further embodiment according to this invention, assembled cortical bone
blocks, or
cortical cancellous bone blocks, or bone blocks comprised of a combination of
cortical
bone, cortico-cancellous bone, cancellous bone, and/or synthetic materials as
described
elsewhere herein, are assembled in combination with wedged or pinned soft
tissue, such
as tendon, ligament, skin, collagen sheets, or the like, to create grafts
similar to
naturally occurring tissue sites, such as the bone-tendon interface found at
the patella.
Such combination implants permit reconstruction of sites such as the Anterior
Cruciate
Ligament (ACL) or Posterior Cruciate Ligament (PCL). According to one
embodiment
of the invention, a ligament or tendon or skin or collagen sheet membrane is
pinned
between adjacent blocks of cortical bone. Accordingly, various implants, such
as
known bone-tendon-bone implants which are in short supply may be supplanted by
assemblage of an implant comprising assembled bone blocks, between which is
fixed a
ligamentous tissue, including but not limited to ligament, tendon,
demineralized bone,
and the like. Referring to figure 27, there is shown one example of this
embodiment of
the present invention in which an implant 2700 is assembled from a superior
bone
CA 02437763 2010-01-29
18
block 2701, an inferior bone block 2702 and a wedged flexible tissue, such as
a
ligament or tendon or portion of demineralized bone 2704, all of which are
pinned
together with cortical bone pins 2703 or other fixation means. The superior
bone
block, 2701, is comprised of three segments of bone pinned together by pin.
Naturally, those skilled in the art will appreciate, based on this disclosure,
that other
shapes of bone blocks, such as rounded bone blocks, and other types of
combinations of
soft and hard tissues may be assembled according to this disclosure. However,
the
example of such an implant 2700 maybe used instead of having to harvest a bone-
tendon-bone implant from cadaveric knees, which tissue is in short supply.
Another variation of this embodiment is to construct a bone-tendon-bone type
of
implant that is comprised of at least one block made from substantially
synthetic
materials, attached to a tendon-like section of an allograft, autograft or
xenograft
sourced ligament, tendon, skin or collagen. Still another variation is to
construct a
bone-tendon-bone type of implant that is comprised of a synthetic tendon-like
material,
attached to a block at one or both ends, where the block is comprised of
allograft,
autograft or xenograft bone, and the block is a single piece or a multi-
segment
assembled bone graft. Examples of synthetic materials, not meant to be
limiting, are
biocompatible materials selected from the group consisting of nylon,
polycarbonate,
polypropylene, polyacetal, polyethylene oxide and its copolymers,
polyvinylpyrolidone, polyacrylates, polyesters, polysulfone, polylactide,
poly(L-
lactide) (PLLA), poly(D,L-lactide) (PLA), poly(glycolide) (PGA), poly(L-
lactide-co-
D,L-Lactide) (PLLA/PLA), poly(L-lactide-co-glycolide) (PLA/PGA),
poly(glocolide-
co-trimethylene carbonate) (PGA/PTMC), polydioxanone (PDS), polycaprolactone
(PCL), polyhydroxybutyrate (PHBT), poly(phosphazenes), poly(D,L-lactide-co-
caprolactone) (PLA/PCL), poly(glycolide-co-caprolactone) (PGA/PCL),
poly(phosphase ester), polyanhydrides, polyvinyl alcohol, and hydrophilic
polyurethanes. These materials, some of which are bioaborbable, can be used in
combination with one another to form the synthetic section of the graft.
Another aspect of the invention is an allograft segment wherein at least one
region is
mineralized, and at least one region is demineralized. For example, Figure 28
depicts
an allograft unit, 2800, that has a central mineralized region, 2801, with two
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
19
demineralized regions, 2802 and 2803, one to either side of the mineralized
region.
Two holes, 2804, pass from the top, 2805, to the bottom, 2806, of the
allograft segment,
2800. As described above, these holes, 2804, are used for assembly of this
segment
with other segments, as by passing pins, dowels, or other attachment means
through the
holes to connect two or more allograft segments in a line.
One method of producing this segment is to start with a fully mineralized
piece of
allograft of the shape depicted in Figure 28. One side, such as that
represented in
Figure 28 as 2802, is subjected to an appropriate acid demineralizing regime
(such as
described earlier in this application) until it is to a desired level of
demineralization for
the purpose of the allograft segment, 2800. Then the opposing side, 2803, is
similarly
subjected to said regime. The regions exposed to the demineralizing regime are
immersed in suitable solutions to remove acids and other components that may
be
toxic, inflammatory, or inhibitive of cellular infiltration. The middle
mineralized
region does not contact the acid solution of the regime. The resultant segment
is
referred to as a mixed-composition segment ("MCS"). As described below, this
may
be combined with other segments to form an assembled allograft.
The demineralizing regime is varied depending on the desired results. In one
example,
a central demineralized area is produced by blocking the outside surfaces
(sides and top
and bottom surfaces near the sides) of a cylinder of bone, allowing acid
solution
exposure only to a central circular area. In this and in other exposure
regimes, a
transition zone of demineralization may exist between the target area (subject
to
demineralization) and the blocked area (designed to remain mineralized), in
which the
degree of mineralization changes from the exposed demineralized region to the
non-
exposed mineralized region. The extent of the transition zone can vary, and
can be
adjusted to some extent by the demineralization regime to better meet a
particular
application for the implant.
An alternative means of producing an allograft segment such as 2800 is to
prepare one
or more demineralized regions and assemble them with one or more mineralized
regions. The assembly would be secured together by means previously described.
This
CA 02437763 2010-01-29
is referred to as an assembled mixed-composition segment ("AMCS"), which may
be
further combined with other segments to form a larger assembled allograft.
It is noted that the degree of demineralization spans a broad range, with
increased
5 exposure to acid (whether by time, acidity or solution, frequency of change-
out of
solutions, or any combination) resulting in a more demineralized, more
flexible
material. Thus, an implant or implant region may be partially demineralized,
wherein
some minerals remain and there is a range of flexibility. Alternately, an
implant or
implant region may be fully demineralized, wherein the minerals are basically
removed
10 and there is a maximum flexibility. As noted, during the demineralization
of one
region of a MCS, a transition zone may occur between the region being
demineralized
and an adjacent region of mineralized bone material.
Thus, an allograft segment, whether formed by either of the means described
above for
15 Figure 28, may be comprised of one or more fully mineralized regions in
combination
with one or more partially demineralized regions, or with one or more fully
demineralized regions, or with a combination of partially and fully
demineralized
regions. The arrangement in Figure 28 is not meant to be limiting, but merely
illustrative of the concept of forming or assembling two or more regions or
two or more
20 types of allografts (mineralized, partially demineralized, fully
demineralized) into a
single allograft segment. Thus, a wide variety of geometric arrangements may
be made
or assembled.
An allograft segment as described above can be combined with other allograft
segments
as exemplified in Figure 29. Figure 29 shows a first segment, 2901, that is
fully
mineralized, and a second segment, 2903, that is also fully mineralized.
Positioned
between these segments is a mixed allograft segment, 2902, such as described
above in
Figure 28. Two pins, 2904, are used to secure the three segments together.
Once
assembled, this allograft assembly can be used in a patient in need of a
degree of
flexibility in the A-A dimension. Such flexibility is provided largely by the
flexibility
of the partially or fully demineralized side regions of the mixed-composition
allograft
segment, 2902. Additional flexibility may be provided by the flexibility of
the pins,
2904, and the spacing between the segments, 2905.
CA 02437763 2010-01-29
21
This flexibility is advantageous post-operatively by reducing potential areas
of high
compression between an allograft implant and adjacent autologous bone
structures.
Another potential advantage for certain procedures and implants, the region(s)
of
demineralized or partially demineralized may remodel more rapidly and/or more
strongly than the region(s) of mineralized bone. The mineralized bone
region(s),
however, provide structural support to transfer load during the remodeling of
the
demineralized or partially demineralized region(s).
Also, as described in U.S. patent 6,090,998 and its daughter applications,
demineralized
or partially demineralized areas of an implant may provide flexibility that is
used to
simulate joint flexibility.
It is further noted that the present invention provides for fabrication of
implants having
specific, even complex, patterns of flexibility or "shock-absorbing"
characteristics
based on the use of MCS and/or AMCS positioned at specific orientations to
other
segments of an assembled allograft and to the structure in the patient in whom
the
implant is implanted. One example of this is depicted in Figure 30. An
assembled
allograft comprises two MCS, 3001 and 3002, which are oriented approximately
60 (and approximately 120, from a second aspect) degrees apart in relation to
one
another. The first MCS, 3001, permits shock absorption in the plane defined by
A-A,
and the second MCS, 3002, permits shock absorption in the plane defined by B-
B. This
allows for complex shock absorption/flexibility patterns. MCSs 3001 and 3002
are
attached by a single pin connector (not shown) passing through hole 3003. The
assembled allograft may include additional segments that are not MCS or AMCS,
in
combination with MCS or AMCS. Variations in design and construction will
result
from the specific requirements for an implant and the particular skill in the
art as to a
design or assembly means. Such variations are within the scope of the
invention
disclosed and claimed herein.
3o Regarding the assembly of an AMCS, one line of construction is to surround
and/or
support the separately prepared regions that are assembled together to form a
segment
with synthetic scaffolding. For instance, three regions, two demineralized
with one
mineralized region between (such as in Figure 28) may additionally comprise a
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
22
processed collagen sheet that is rolled around the assembled three regions.
Also, rigid
or semi-rigid synthetic structures may be used as noted above. The
supplemental
materials are to provide additional strength and lessen the bonding strength
required on
the surfaces between regions of the AMCS.
Another aspect of the invention is the use of synthetic segments and/or
scaffolding in
conjunction with an assembled allograft, where the assembled allograft is
comprised of
any combination of one or more segments each of. mineralized bone; partially
demineralized bone; fully demineralized bone; or MCS or AMCS of these
materials.
One or more segments of assembled implants as described herein may be
substituted by
a synthetic segment. In addition, synthetic materials can be in the form of
various
scaffolding used in conjunction with one or more of the assembled segments.
The
synthetic segment or scaffolding may be comprised of various materials,
including, but
not limited to stainless steel, titanium, cobalt chromium-molybdenum alloy,
and a
plastic of one or more members selected from the group consisting of nylon,
polycarbonate, polypropylene, polyacetal, polyethylene oxide and its
copolymers,
polyvinylpyrolidone, polyacrylates, polyesters, polysulfone, polylactide, and
a
combination of one or more bioabsorbable polymers.
In particular, biodegradable polymers suitable for use in the present
invention include:
poly(L-lactide) (PLLA), poly(D,L-lactide) (PLA), poly(glycolide) (PGA), poly(L-
lactide-co-D,L-Lactide) (PLLAIPLA), poly(L-lactide-co-glycolide) (PLA/PGA),
poly(glocolide-co-trimethylene carbonate) (PGA/PTMC), polydioxanone (PDS),
polycaprolactone (PCL), polyhydroxybutyrate (PHBT), poly(phosphazenes),
poly(D,L-
lactide-co-caprolactone) (PLA/PCL), poly(glycolide-co-caprolactone) (PGA/PCL),
poly(phosphase ester) and polyanhydrides. Other suitable materials, depending
on a
particular application, include hydrogels, gelatins, collagens, proteins,
sodium alginate,
karaya gum, guar gum, agar, algin, carrageenans, pectins, xanthan, starch
based gums,
hydroxyalkyl and ethyl ethers of cellulose, sodium carboxymethyl cellulose,
polyvinyl
alcohol, and hydrophilic polyurethanes.
For example, a synthetic sheet may be used to wrap around a MCS or AMCS to
support
bone growth. Alternately, synthetic scaffolding may be rods or bars or the
like, which
CA 02437763 2003-08-07
WO 02/064180 PCT/USO1/27683
23
pass through in-line holes in the respective segments. Alternately, synthetic
scaffolding
may be in the form of a frame that surrounds or encompasses the bulk of each
segment,
or the bulk of the demineralized segments, MCS, or AMCS that have flexible
regions
requiring structural support in the particular application in a patient in
need thereof.
This is employed, for instance, to add structural integrity to or around one
or more
segments, at least one of which has a high percentage of demineralized bone,
or is
otherwise in need of such additional structural support. Examples of synthetic
scaffolding designs, which are not meant to be limiting, are provided in
Figure 31.
Another aspect of the invention is an assembled graft implant that is formed
from at
least three segments that interlock along abutting edges with one another. The
shape of
each segment is such that upon final assembly the major plane of each segment
is non-
coplanar in relation to the other segments, e.g., the segments do not lie
parallel to one
another. For example, Figure 32 shows a four-piece assembled graft implant,
3200,
forming a roughly circular shape. This is made up of segments 3201, 3202,
3203, and
3204. Each segment has a male edge, 3205, and a female edge, 3206, which are
designed to mate with an adjoining edge. One male edge slides into a female
edge of
an adjacent segment, and this process continues for other edges to complete a
desired
assembly. When the joints of the edges interlock, as shown in Figure 32, the
joints
hold the segments together.
In a preferred embodiment, assembling three or more segments results in the
formation
of a central channel. A central channel, 3207, is shown in Figure 32. A
central channel
can be filled with osteogenic material, or may serve other purposes.
The interlocking edges are of shapes known by those skilled in the art to
provide an
interlocking joint. Examples, not meant to be limiting, of mateable joint
designs (e.g.,
shapes where one part fits into or around the other) include ball and socket
(as shown),
tongue and groove, and mortise and tenon, such as a dovetail joint.
Also, where a portion of the body of the recipient has a need to remain intact
(unsevered) yet there is a need to surround that portion with a structural
support or to
provide a protective barrier, segments of the present invention may be used,
where the
CA 02437763 2010-01-29
24
edges do not truly interlock, as defined above, but have sufficient tolerance
to permit
the direct insertion of the male edge into the female edge, at once along the
edges,
rather than sliding from one end. This facilitates the assembly around the
portion in
need of structural support or protection. Optionally, one or more bands of
resilient
material are wrapped around the assembled structure to increase rigidity,
and/or other
means known in the art can be used to increase the bonding at the interlocking
junctions (synthetic adhesives, bone paste, screws).
Another interlocking embodiment is two arcuate shaped segments, each having
two
edges of opposing interlocking edges. The edges are interlocked to form a
circular or
truncated circular shape, preferably with a central channel within. When the
arcuate
shape is a semicircle, the assembled graft is a circular. Examples, not meant
to be
limiting, are shown in Figure 33, wherein segment 3300 is interlocked with
segment
thereby forming a channel 3320. The two embodiments shown comprise different
interlocking configurations 3330.
Referring to Figure 34, another interlocking embodiment of an assembled
allograft is
shown as 3400, whose final cross-sectional shape is a `tee-' or `cross'. The
embodiment comprises at two individual segments 3401 and 3402 that comprise a
slot
longitudinally defined thereon. Thus, the segments comprise a body portion
3406
and a slotted portion 3407. When the segments are assembled they form a bone
block
by interlocking pieces 3401 and 3402 together. As shown, the assembled implant
presents four fins, 3400a-d, that radiate from a center point. The preferred
length
of the assembled allograft, 3400, is approximately 2.5 mm, and the preferred
diameter
may range from approximately 2.0 to 12.0 mm. This assembled allograft is used
for
various applications where bone blocks are used. Preferably, embodiment 3400
is used
in conjunction with bone-tendon-bone grafts. When used in bone-tendon or bone-
tendon bone applications, preferably two separate flexible bands (natural or
synthetic)
are looped over the top of the embodiment 3400 wherein one band contacts fins
3400a
and c, and the second band contacts fins 3400b and d. When the bone block 3400
is
positioned into a channel, such as a bone tunnel formed in a patient, the two
bands are
compressed against the fins 3400a-d and thereby secured into place.
Alternatively, the
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
ends of the fins can comprise teeth or are otherwise irregular to further
prevent slippage
of the bands.
The interlocking segments described above may be made of cortical bone,
cancellous
5 bone, or a combination of cortical and cancellous bone. The segments may be
of
allograft or xenograft material, and preferably is treated to reduce
antigenicity. In
accordance with the requirements of the application, the interlocking segments
are
mineralized, demineralized, mixed-composition, synthetic, or a combination of
these.
Synthetic materials, such as those described above, may also be used in
forming a
10 segment, and alternately, in contributing to the connection of the segments
in addition
to the interlocking edges.
Based on the present disclosure, those skilled in the art will further
appreciate that the
cortical bone pins disclosed herein may have features defined thereon for
various
15 applications. For example, not meant to be limiting, the shafts may contain
stops, such
that other pieces of bone inserted thereon can only travel a certain distance
down the
shaft before encountering the stop. The shaft may also contain through holes,
to permit
insertion of cotter pins or the like. Furthermore, the cortical bone shaft may
be
demineralized, mineralized, or partially demineralized. In one specific
embodiment,
20 the end of the cortical shaft contains a tapped cannulation a short
distance into the
longitudinal end of the shaft. In this way, a screw may be driven into the
cannulation to
retain elements inserted over the shaft in association with the shaft. To
accommodate
the screw, the screw end bearing the cannulation may be partially
demineralized, such
that upon insertion of the retention screw, the shaft end does not shatter,
but expands to
25 accommodate the increasing diameter of the screw as it is driven into the
shaft.
Naturally, in certain applications, it may be desirable for the cortical pins
to be
cannulated throughout the longitudinal length thereof. However, care should be
taken
that this does not unduly weaken the overall compressive or torsional strength
of the
assembled implant. This may be addressed by including pins that are not
cannulated,
along with pins that are cannulated. The cannulated pins may be used in
combination
with sutures or the like, in order to hold an implant in a specific
orientation, until fusion
with adjacent bone has proceeded to a sufficient extent for the implant to
become stable
without the sutures.
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
26
It will be appreciated from the present disclosure that implants that have
classically
been fabricated from metals may be fabricated by assembling bone pieces. In
addition,
a benefit of the assembled graft according to this invention is that the
components of
the assembled graft can be derived from various anatomical structures, thus
circumventing limitations normally resulting from having to obtain a graft
from a
particular anatomical source of a particular donor. Not only can the
components be
sourced from different anatomies, but also different donors may yield various
components for assembly into a unitary implant. The end result is maximization
of the
gift of donation and the preservation of precious tissue resources. As noted
above,
being able to pool tissues from different sources depends, to some significant
extent, on
the ability to treat portions of tissue harvested from different anatomies or
donors so as
to prevent any contamination of a recipient with pathological or antigenic
agents. A
further benefit of the present invention is that different implants with
height or width
limitations due to the anatomical structures from which the implant has been
derived
may be pinned together to form implants of essentially any desired dimensions.
In this
fashion, an inventory of building blocks in combination with the appropriate
assembly
pins, threaded or unthreaded, is useful to provide implants of essentially any
dimensions in the course of given surgical procedure. According to this
embodiment of
the invention, for example, a cervical Smith-Robinson (CSR) of any desired
height
may be produced by attaching two or more existing CSR implants together with
cortical bone pins. This is accomplished preferably using two machined CSR's
of
known height such that when added together, the desired overall height is
achieved.
The two CSR's are stacked and drill holes are machined through the CSR bodies,
following which the cortical bone pins are press-fit through the thus machined
holes.
Preferably, the diameter of the pins is slightly greater than the diameter of
the drilled
holes, such that a tight press-fit is achieved.
From the present disclosure, it will further be appreciated that implants
according to
this invention may be assembled in the operating room by a surgeon, using pre-
formed
implant pieces, from a kit. It will further be appreciated that the assembled
implant
pieces may be adhered to each other using any of a number of biologically
acceptable
glues, pastes and the like. In one such embodiment, the assembled implant
pieces are
assembled using a polymethyl-methacrylate glue, a cyanoacrylate glue, or any
other
CA 02437763 2003-08-07
WO 02/064180 PCT/US01/27683
27
adhesive known in the art, so long as the use of such an adhesive is confirmed
to be
non-toxic. It will further be appreciated that in forming the assembled grafts
according
to the present invention, it is acceptable, although not required, for
interlocking features
to be included on abutting faces of implant segments to be assembled together.
Where
such features are included, it is preferred for the adjacent features to be
complementary,
such that a protrusion on a first surface is met by a compatible indentation
in the
abutting surface. Such abutting features assist to provide torsional and
structural
strength to the assembled implant, and to relieve a measure of stress on the
cortical
bone pins used to assemble the implant.
According to US patent 6,025,538, an elaborate system is disclosed for
ensuring that a
bore is provided in mating surfaces of a composite implant such that the bore
is
angularly aligned with respect to mating surfaces so as to be oblique to the
plane of
each mating surface. This is not required according to the present invention.
According to US patent 5,899,939, layers of bone are juxtaposed, but no
mechanical
fixation of the various layers to each other is provided for, such as the
cortical bone
pins disclosed herein.
Having generally described this invention, including the methods of
manufacture and
use thereof, including the best mode thereof, those skilled in the art will
appreciate that
a large number of variations on the principles described herein may be
accomplished.
Thus, the specifics of this description and the attached drawings should not
be
interpreted to limit the scope of this invention to the specifics thereof.
Rather, the
scope of this invention should be evaluated with reference to the claims
appended
hereto.