Note: Descriptions are shown in the official language in which they were submitted.
CA 02438409 2003-08-14
n
~ I
EPO
. ~ 19. M~rz 2003
tNTRAGASTRIC BALLOON ASSEMBLY
Field of the Invention .
The present invention refers to a kind of medical devices used to
reduce the food intake of persons suffering from overweight problems,
specifically persons with morbid obesity.
Backgrounds of the Invention
The exogenous obesity, also named as morbid obesity or other
names, generally means a weight increase in a significant proportion
according to height, age, etc., and that may be risky for life due to the:
problems caused by the overweight itself. Before this medical circumstance,
diverse medical treatments for weight reduction generally fail, therefore,
surgical procedures have been the only possibility of treatment with an actual
effectiveness, in most cases. .
The obesity problem is so significant in developed countries that it has
been considered as a public health problem, so the most important objective
of any therapeutic is trying to solve this ailment with more effective
treatments involving less complications, morbi-mortality and, cost.
During the sixties and seventies, surgical experiences trying to reduce
overweight were made and reported, by creating derivations that produce a
short intestine effect, usually having disastrous results, since morbi-
mortality
was so high that caused almost a total abandonment thereof, until surgical
2 5 devices appeared such as staplers, that arrived to our country at the end
of
the seventies, early eighties, starting a series of works published throughout
the world, mainly about gastroplasties and derivations with a plurality of
variations, results of which were good as to weight control and decrease
refer, but open surgery was needed, having therefore risks in surgery,
anesthesia, etc., in addition to high cost.
AMENDED SHED
.:;. ~ w. ..
' CA 02438409 2003-08-14
' I i '. ' ' t . , st
2
More recently, about 5 years ago, a new surgical technique appeared,
consisting in placing by laparoscopy an inflatable band encircling the
stomach on its upper part, by means of a subcutaneous valve that may be
controlled by radiology andlor endoscopy. Through this technique, a gastric
reduction and food intake decrease are produced, resulting in a significant
weight loss, practically the same as the one obtained by open surgery with
staplers, but with less morbi-mortality and the advantages of the laparoscopic
surgery, i.e., less pain and fast postoperative recovery. However, the most
important problem of this surgical technique is the cost increase, as it is
also.
an hospital, surgical and laparoscopic procedure, in addition to the band cost
and speciali2ed and experienced surgeons requirement.
At early eighties, the first reports on the use of intragastric balloons for
obesity treatment appeared, which partially fill the stomach to produce a
feeling of satiety, thus helping to reduce the food intake and to adopt new
eating habits, such as the balloon disclosed in European Patent No. 137 878
of Lloyd R. Garren et al. consisting in a flexible inflatable galloon which in
use
is free-floating and unattached. Since then and during the following.?0 years,
approximately, publications of scientific literature of several counties have
arisen about diverse experiences on the use of balloons made of different
materials. Most of those balloons reported failures or very tow ,success
percentages, due to many complications and troubles during the placing
procedure, handling and surveillance within the treatment period and
therefore, the method lost credibility and in many cases it was abandoned,
except for isolated experiences, reported in diverse publications. Currently,
Bioenterics Corporation manufactures and distributes an intragastric balloon
named BIB~ System, which is seldom used, because the said company
recommends it only as an alternative for limited reduction short programs or
in some cases, prior to a definitive surgery, for which it is recommended a
gastric capacity reduction surgery via tamparascopy by applying a gastric
3o band, produced by such company. .
AMi:NDFp SNEER'
~.~ . ~ n
~
' ' CA 02438409 2003-08-14
iy I
i
3
The most significant problems arising from the use of intragastric
balloons were, on the one hand, the manufacturing material, because the
elements employed at the beginning were easily destroyed by gastric juices,
so the balloon should be frequently replaced, however, nowadays, this issue
has been solved by the use of a resistant silicone material that is able to
stay
on several months inside the: patient. Other important difficulties are the
balloon rupture, which consequently causes its migration to the intestine,.
eventually causing an intestine obstruction requiring surgery, and also the
inflated balloon migration causing an esophagus or pylorus obstruction. Even
ZO more, the balloons available at the market, show a great technical
difficulty.
for endoscopic placing and the inflating maintenance or modification, once it
has been placed into the stomach.
There are several documents describing intragastric balloons with
improvements intended to overcome some of these inconveniences including .
the one described in the US Patent No. 5,259,399 of Alan Brown, consisting,:
in a balloon that is placed. at the stomach fundus by means of a
percutaneous gastrostomy and it is fixed up to the patient's:skin,~with a
rigid
or semi-rigid thick device, introducing the balloon there through which is
then
inflated and deflated with solutions and controlled by sensors placed on the.
2 0 patient's body and a battery driven electric pump, placed out of the
patient's
body. This device has the disadvantage that the patient must suffer a probe
or thick catheter out of the abdomen skin and such probe or catheter has- to
be connected to the inflating or deflating equipment each. time any food is
ingested, resulting very uncomfortable for the patient.
On the other hand, US Patent No. 5,234,454 of Roger G. Bangs,
describes an intragastric balloon ptaced through a surgical gastrostomy
previously made (several weeks before), to place the balloon later, implying a-
prior surgery. In addition, the used probe is very thick, approximately 1 cm.
diameter or more, and it stays externally, which means that the patient must .
have a probe out of hislher body through a puncture made on the abdominal
Aii~if~DEL~ SHEET,
,~ ~~ , ~,
CA 02438409 2006-O1-13
wall, which, in addition to the inconveniences found in the Alan Brown US
Patent, causes constant pain and discomfort to the patient. Bangs' device
has two balloons, one to be inflated and to maintain the stomach placed
against the abdominal wall on the inside and a distal balloon, used to fill
the
stomach.
Therefore, there is a need for a mean to treat the morbid obesity,
allowing to reduce the surgical and postoperatory hazards, which also
substantially eliminates the patient's discomfort and decreases the treatment
costs.
Summary of the Invention
The object of the present invention concerns an intragastric balloon
assembly comprising:
a) a balloon made of an acid resistant distensible material;
b) a support consisting in a lengthened element, being used as a
guideline and tractor to introduce the balloon into the stomach through the
mount; and
c) an inflating catheter joined by one end to the balloon to be carried
jointly with the support during the procedure of introducing the assembly into
the
stomach;
characterized in that
d) the support is rigid, unitarily fixed to the balloon and has a diameter
that is larger at the end attached to the balloon and reduced towards the
opposite end, the rigid support being used as a support to fix or anchor the
balloon in a position practically fixed, the rigid support having a slot
extended
along all its length from the end attached to the balloon up to approximately
15 cm ahead;
e) a handle is disposed at the end of the rigid support opposite to the
attachment to the balloon;
CA 02438409 2006-O1-13
4a
f) the catheter is received along its length within the slot of the rigid
support, and the catheter being easily detachable from the rigid support;
g) the intragastric balloon assembly further comprises an inflating valve
to appropriately inflate or deflate the balloon that is joined to the
inflating
catheter and has two or three flanges for its fixation; and
h) the intragastric balloon assembly further comprises a fixing plate
having a first hole with a circular flange which adjusts and fixes the rigid
support,
a second hole, through which the inflating catheter is introduced and several
fixation holes to join the fixing plate by stitches to the abdominal tissue.
Another object of the present invention concerns the use of the
intragastric balloon assembly as described above for reducing the food intake
of
an overweight person.
The 'object of the present invention is therefore, to provide an
int~agastric balloon assembly that remains fixed, avoiding the baEloon
migration and the possibility of"digestive tube obstruction or blockade.
Another object of the invention is to cause less anesthesic-surgical
trauma to the patient during the intragastric balloon implantation.
It is also an object of the invention is to provids ;lie caerr~ents. to
control
the size of the inflated intragastric balloon at any time after placement.
Even more, the balloon assembly of the invention intends both to
avoid the discomfort caused by external elements and make less evident the
presence of the assembly's elements on the patient's body.
Likewise, by the present invention it is intended to decrease the
hospital 'costs, by making a short stay or ambulatory surgery, and
notwithstanding that the balloon and other assembly elements may have a
similar or~ lesser costs than current medical devices, the high- cost of a
laparoscopic surgery will be eliminated.
In 'addition, during the placing of the intragastric balloon of this
invention, minimum personnel will be required, such as endoscopist, a
surgeon; an assistant, a surgical nurse and anesthesia staff, reducing
likewise the total cost.
. .~ CA 02438409 2003-08-14
~ ~ t
Three years ago, a procedure to carry out no open surgery
gastrostomies was started as a surgical endoscopic procedure, thus making
easier the implantation of the gastrostomy probe by percutaneous puncture;
previously inflating the stomach and surveying it with a panendoscope. This
5 procedure may be carried out with local sedation and anesthesia, with less
anesthetic-surgical trauma, thinking thus in an efficient option for the
implantation of the intragastric balloon.
In order to comply with these objectives, the present invention
combines the three medical-surgical elements above-described, such as an
intragastric balloon, a valve for post-operatory inflating control, and the
percutaneous gastrostomy technique with endoscopic control, as well as a
new mean for the assembly traction and fixing, avoiding the balloon
migration.
Therefore, a silicone balloon has been designed, having a fixed
2 5 inflated catheter and an attachment consisting in a firmer silicone bar at
the
entrance of the balloon, to be used as tension support and to fix it to the
aponeurosis, by means of a plate adjustable according to the needs and that
may be fixed with suture points or metallic staples to the aponeurosis. Upon
fixing these elements, the support is cut and the catheter is connected to an
inflating valve, which is subcutaneously placed and attached to the
aponeurosis through points or staples.
This way, elements external to the abdomen skin are avoided, such as
probes or thick trocars, uncomfortable for the patient and that are difficult
to
control, as the invention assembly remains fixed to the abdominal
aponeurosis and the inflating and deflating valve remains subcutaneously.
Likewise, the use of other elements such as an additional balloon is avoided,
as the air inflated balloon itself is used to make traction and place the .
stomach against the abdominal wall, and this way, it is allowed to reduce the
diameter of the catheter or the inflating and fixing system.
A4~Ei~D~D SH~~1
f
~ ' ' CA 02438409 2003-08-14
~~ '
6
It is important to note that by this technique, post operatory
annoyances are minimized and the only drugs required are antibiotics as
prophylaxis, and pain-relievers to control any discomfort. . Surveillance is
taken at the doctor's office once a month and a radiolog ical control to
verify
and modify the balloon inflating wiH take place only in special occasions.
Brief Description of Drawings:
Figure 1 shows the intragastric balloon assembly of the present
invention;
Figure 2 shows an embodiment of the intragastric balloon assembly of
the present invention;
Figure 3 shows a flat upper view of the fixing plate of the present
invention;
Figure 4 shows a flat lateral view of the fixing plate of the present
invention;
Figures 5 to 10 are schemes showing the surgical technique tc~ implant
the intragastric balloon assembly of this invention;
Figure 11 is a scheme showing how the intragastric balloon assembly
of this invention is implanted.
Detailed Description of the Invention
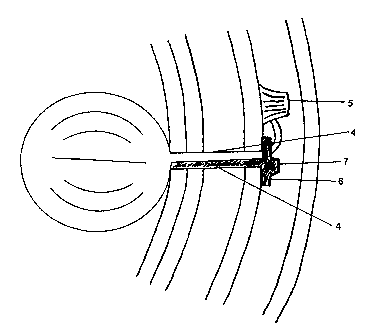
The intragastric balloon of the invention, shown in Figure 1, consists in
the balloon 1, preferably made of distensible elastic silicone having a
capacity between 200 to 500 c.c., approximately, and may have a circular
form (as shown in Figure 1) or a bilobed or kidney form (shown in Figure 2).
Said balloon 1 is provided with a rigid support 2, used as guideline and
support thereof, consisting in a lengthened element such as a solid tube
preferably made of silicone, unitary fixed to the balloon 1 and having a
diameter such that it is larger at the end joined to the balloon .1 and it
becomes smaller towards the opposite end where it is located a handle 3 to
AM ~ l~ a~D ~H~E'~,
".. ,..,
s.
CA 02438409 2003-08-14
"~
7
hold in place. Likewise, such rigid support 2 has a groove (not shown)
extended through its length from the end attached to the balloon 1 up to
approximately 15 cm ahead, disposed to receive the i nflating catheter 4,
which is attached to support 2 and may be easily detached from it. Said
inflating catheter 4 has a diameter of approximately 1.5 to 2.0 mm and a
length of approximately 20 cm.
Likewise, the invention contemplates providing an inflating valve . 5
which is a rigid plastic or silicone safety valve having a 2 cm diameter and 1
cm height, approximately, which, is easily available in the market and has two
or three fixing flanges. Said inflating valve 5 is joined to the inflating
catheter
and fixed to the aponeurosis.
In addition, the balloon assembly of the invention, includes one f<xfng
plate 6, shown in detail in Figure 3, which is a plate of ap proximately 1 to
1:5
mm wide and is preferably made of high hardness inert silicone or plastic ,
shown in a rectangle shape with rounded comers, but this may be different,
either circular, ovoid, etc. and its diameter is a Iaout 4~ to 5 cm.,
approximately. Said fixing plate 6, has a first hole 7 having a circular
flange 8,
which is adjusted and fixed to the rigid support 2 and a .second hole 9 to
pass the inflating catheter 4 there through, and also including several fixing
holes 10, preferably being disposed 4 of those holes.
Likewise, a trocar 11 is used to make a puncture on the abdominal
and gastric wall, in order to form a light for the traction , with the help of
a
suture through the abdominal wall, of the rigid support 2 with the catheter 4
of
the balloon assembly of the invention.
In order to make clear the way the elements of tha intragastric balloon.:.
assembly of the invention work, as well as its advantages, the surgical .
technique for its implantation is described as follows:
The balloon implanting must be made at the operating room under
conventional asepsis and antisepsis measures as any surgical procedure-.
The anesthesia must be made under anesthesiologist control and
AM~:NO~D Sl~~
' ~ CA 02438409 2003-08-14
surveillance using either general anesthesia or only sedation and a local
pain-reliever in the incision zone.
Likewise, an endoscopist must be present to start the procedure and
to make a permanent surveillance of the balloon placing and inflating,
The patient must be in a ventral decubitus position. Antisepsis of the
abdomen is carried out and sterile fields are placed limiting the upper
hemiabdomen. Local anesthesia is applied using diluted xylocaine without
epinephrine on the puncture place. This site will be located after the step
described below, which starts the procedure.
As shown in Figure 5, a flexible endoscope 14, preferably a central
view endoscope, is introduced through the mouth a nd the stomach is
identified by air inflation thereof, then the bottom of the -gastric .body is
pressured with the endoscope against the major curvature, to identify the
puncture site by locating the light through the abdominal wall:
Once the puncture site has been identified, a small incision is made
with a 2 mm knife, introducing there through a puncture trocar 19, as shown
in Figure 6, piercing al! the layers of the abdominal wall up to puncturing
the
stomach and watching this operation through the endoscope 14.
Upon placing the trocar 11 inside the stomach on the place where the
balloon 1 is to be placed and fixed, the pointed mandrin is removed and a
resistant suture 15 either prolene or mersilene , number zero.' or 1, is
introduced through trocar 11, and it is taken by the endoscopist with a clamp
and then the endoscope and the suture are removed through the mouth.
Once the suture has been removed through the mouth, the suture is
2 5 tied' to the thin end of the rigid support 2 of the balloon assembly, over
the
handle 3, placed for such purpose, as shown in Figure 7.
By making traction of suture 15 through the abdominal wall and with
endoscopist assistance, the balloon assembly 1 is inserted along with the
rigid support 2 which has the inflating catheter 4 attached, up to the
stomach,
and then the percutaneous trocar 11 is removed and by making a firm
Ag~,~tEt~DED SHE~i
~ CA 02438409 2003-08-14
I
t . '
9
traction of the rigid support 2 of balloon 1, it is placed in an appropriate
position into the stomach (see Figure 8).
At this moment, a 5-7 cm surgical incision is carried out, preferably in
a vertical or horizontal way, at the place where the rigid support 2 of the..
balloon 1 was removed, by such incision the skin and the cellular tissue are
cut up to the aponeurosis, dissecting the aponeurosis surface, enabling it to
place the fixing plate at this site. Later, the inflating catheter 4 is
separated
from the rigid support 2 and the solid tip thereof is cut to start inflation
(see.
Figure 9).
~ After balloon 1 has been inflated with air, it rnay be mixed with
physiologic serum, then, traction of rigid support 2 is made far the balloon 1
is perfectly attached to the gastric wall which in turns is .attached to the
abdominal wall at the peritoneal . layer, avoiding this way any possibility of
a .
gastric content leakage towards abdominal cavity.
Further, fixing plate 6 is placed, as shown in Figure 10, by inserting
rigid support 2 info the major hole 7, having an approxirr3ate 5 mm diameter,
: .
said base having a 3-4 mm height flange, the rigid support 2 being stitched
thereon, said suture may be a 1 or 2 zeros prolene, and by using this kind:of
sutures the fxing plate 6 may be also fixed to the abdomen anterior
aponeurosis at the holes 10 stitches. The inflating catheter is introduced
through the adjoining hole 9, .this catheter is placed without fixation, as
sustained traction is made by the rigid support 2, which is compact and soft,
enabling to perforate it and tie it, by stitching it on the flange of fixing
plate 6. :.
The rest of rigid support 2 is cut with a knife or scissors, close to the
level of the flange of fixing plate.6, once it has been stitched.
The inflating catheter 4 is cut in such a way that it is placed on the .
inflating valve 5, which is placed next to said incision, perforating the
cellular.
tissue at a distance of 5-6 cm from outside and it is set in place, if
necessary, ;, .
by making other small incision only to place and fix the valve to the . .
At~~~b~~ S~~E~
CA 02438409 2003-08-14
" , . , , '
Y
aponeurosis using stitches or staples, all the elements being arranged as
shown in Figure 11. .
To finish the surgery, the subcutaneous cellular tissue and the skin are
closed and the endoscope is removed, verifying the correct position and
5 inflating of the balloon inside the stomach.
It will be evident for the skilled in the art that many modifications or
variations may be made to the invention herein described without separating
from spirit and scope of the same, therefore, it will be understood that what
has been described and shown is merely illustrative of the invention and not
10 restrictive thereto. .
A ~.1~~ID~D SHEET