Note: Descriptions are shown in the official language in which they were submitted.
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
ISOLATED HUMAN G-PROTEIN COUPLED RECEPTORS, NUCLEIC ACID
MOLECULES ENCODING HUMAN GPCR PROTEINS, AND USES THEREOF
FIELD OF THE INVENTION
The present invention is in the field of G-Protein coupled receptors (GPCRs)
that are
related to the EGF-module-containing, mucin-like hormone receptor (EMR)
subfamily,
recombinant DNA molecules, and protein production. The present invention
specifically
provides a novel human EGF 1 splice form and nucleic acid molecules encoding
the novel splice
form, all of which are useful in the development of human therapeutics and
diagnostic
compositions and methods.
BACKGROUND OF THE INVENTION
G-protein coupled receptors
G-protein coupled receptors (GPCRs) constitute a major class of proteins
responsible for
transducing a signal within a cell. GPCRs have three structural domains: an
amino terminal
extracellular domain, a transmembrane domain containing seven transmembrane
segments, three
extracellular loops, and three intracellular loops, and a carboxy terminal
intracellular domain. Upon
binding of a ligand to an extracellular portion of a GPCR, a signal is
transduced within the cell that
results in a change in a biological or physiological property of the cell.
GPCRs, along with G-
proteins and effectors (intracellular enzymes and channels modulated by G-
proteins), are the
components of a modular signaling system that connects the state of
intracellular second
messengers to extracellular inputs.
GPCR genes and gene-products are potential causative agents of disease
(Spiegel et al., J.
Clin. Invest. 92:1119-1125 (1993); McKusick et al., J. Med. Genet. 30:1-26
(1993)). Specific
defects in the rhodopsin gene and the V2 vasopressin receptor gene have been
shown to cause
various forms of retinitis pigmentosum (Nathans et al., Annu. Rev. Genet.
26:403-424(1992)), and
nephrogenic diabetes insipidus (Holtzman et al., Hum. Mol. Genet. 2:1201-1204
(1993)). These
receptors are of critical importance to both the central nervous system and
peripheral physiological
processes. Evolutionary analyses suggest that the ancestor of these proteins
originally developed in
concert with complex body plans and nervous systems.
The GPCR protein superfamily can be divided into five families: Family I,
receptors
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
typified by rhodopsin and the (32-purinergic receptor and currently
represented by over 200 unique
members (Dohlinan et al., A~nu. Rev. Biochem. 60:653-688 (1991)); Family II,
the parathyroid
hormonelcalcitonin/secretin receptor family (Juppner et al., Science 254:1024-
1026 (1991); Lin et
al., Science 254:1022-1024 (1991)); Family III, the metabotropic glutamate
receptor family
(Nakanishi, Science 258 597:603 (1992)); Family IV, the cAMP receptor family,
important in the
chemotaxis and development of D. discoideum (Klein et al., Scie~zce 241:1467-
1472 (1988)); and
Family V, the fungal mating pheromone receptors such as STE2 (Kurjan, Aunu.
Rev. Biochem.
61:1097-1129 (1992)).
There are also a small number of other proteins that present seven putative
hydrophobic
segments and appear to be unrelated to GPCRs; they have not been shown to
couple to G-proteins.
Drosophila expresses a photoreceptor-specific protein, bride of sevenless
(boss), a seven-
transmembrane-segment protein that has been extensively studied and does not
show evidence of
being a GPCR (Hart et al., Proc. Natl. Acad. Sci. USA 90:5047-5051 (1993)).
The gene frizzled (fz)
in Drosophila is also thought to be a protein with seven transmembrane
segments. Like boss, fz has
not been shown to couple to G-proteins (Vinson et al., Nature 338:263-264
(1989)).
G proteins represent a family of heterotrimeric proteins composed of a, (3 and
y subunits,
that bind guanine nucleotides. These proteins are usually linked to cell
surface receptors, e.g.,
receptors containing seven transmembrane segments. Following ligand binding to
the GPCR, a
conformational change is transmitted to the G protein, which causes the a-
subunit to exchange a
bound GDP molecule for a GTP molecule and to dissociate from the [3y-subunits.
The GTP-bound
form of the a-subunit typically functions as an efFector-modulating moiety,
leading to the
production of second messengers, such as cAMP (e.g., by activation of adenyl
cyclase),
diacylglycerol or inositol phosphates. Greater than 20 different types of a-
subunits are known in
humans. These subunits associate with a smaller pool of (3 and y subunits.
Examples of
mammalian G proteins include Gi, Go, Gq, Gs and Gt. G proteins are described
extensively in
Lodish et al., Molecular Cell Biology, (Scientific American Books Inc., New
York, N.Y.,1995), the
contents of which are incorporated herein by reference. GPCRs, G proteins and
G protein-linked
effector and second messenger systems have been reviewed in The G-Protein
Li~zked Receptor Fact
Book, Watson et al., eds., Academic Press (1994).
Aminergic GPCRs
One family of the GPCRS, Family II, contains receptors for acetylcholine,
catecholamine, and indoleamine ligands (hereafter referred to as biogenic
amines). The biogenic
2
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
amine receptors (aminergic GPCRs) represent a large group of GPCRs that share
a common
evolutionary ancestor and which are present in both vertebrate (deuterostome),
and invertebrate
(protostome) lineages. This family of GPCRs includes, but is not limited to
the 5-HT-like, the
dopamine-like, the acetylcholine-like, the adrenaline-like and the melatonin-
like GPCRs.
Dopamine receptors
The understanding of the dopaminergic system relevance in brain function and
disease
developed several decades ago from three diverse observations following drug
treatments. These
were the observations that dopamine replacement therapy improved Parkinson's
disease symptoms,
depletion of dopamine and other catecholamines by reserpine caused depression
and antipsychotic
drugs blocked dopamine receptors. The fording that the dopamine receptor
binding affinities of
typical antipsychotic drugs correlate with their clinical potency led to the
dopamine overactivity
hypothesis of schizophrenia (Snyder, S.H., Am JPsychiatry 133, 197-202 (1976);
Seeman, P. and
Lee, T., Science 188, 1217-9 (1975)). Today, dopamine receptors are crucial
targets in the
pharmacological therapy of schizophrenia, Parkinson's disease, Tourette's
syndrome, tardive
dyskinesia and Huntington's disease. The dopaminergic system includes the
nigrostriatal,
mesocorticolimbic and tuberoinfundibular pathways. The nigrostriatal pathway
is part of the striatal
motor system and its degeneration leads to Parkinson's disease; the
mesocorticolimbic pathway
plays a key role in reinforcement and in emotional expression and is the
desired site of action of
antipsychotic drugs; the tuberoinfundibular pathways regulates prolactin
secretion from the
pituitary.
Dopamine receptors are members of the G protein coupled receptor superfamily,
a large
group proteins that share a seven helical membrane-spanning structure and
transduce signals
through coupling to heterotrimeric guanine nucleotide-binding regulatory
proteins (G proteins).
Dopamine receptors are classified into subfamilies: D1-like (D1 and DS) and D2-
like (D2, D3 and
D4) based on their different ligand binding profiles, signal transduction
properties, sequence
homologies and genomic organizations (Civelli, O., Bunzow, J.R. and Grandy,
D.K., Annu Rev
Pharmacol Toxicol 33, 281-307 (1993)). The D1-like receptors, Dl and D5,
stimulate cAMP
synthesis through coupling with Gs-like proteins and their genes do not
contain introns within their
protein coding regions. On the other hand, the D2-like receptors, D2, D3 and
D4, inhibit cAMf
synthesis through their interaction with Gi-like proteins and share a similar
genomic organization
which includes introns within their protein coding regions.
Serotonin receptors
3
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Serotonin (5-Hydroxytryptamine; 5-HT) was first isolated from blood serum,
where it was
shown to promote vasoconstriction (Rapport, M.M., Green, A.A. and Page, LH.,
JBiol Chem 176,
1243-1251 (1948). Interest on a possible relationship between 5-HT and
psychiatric disease was
spurred by the observations that hallucinogens such as LSD and psilocybin
inhibit the actions of 5-
HT on smooth muscle preparations (Gaddum, J.H. and Hameed, K.A., Br JPharmacol
9, 240-248
(1954)). This observation lead to the hypothesis that brain 5-HT activity
might be altered in
psychiatric disorders (Wooley, D.W. and Shaw, E., Proc Natl Acad Sci USA 40,
228-231 (1954);
Gaddum, J.H. and Picarelli, Z.P., Br JPharmacol 12, 323-328 (1957)). This
hypothesis was
strengthened by the introduction of tricyclic antidepressants and monoamine
oxidase inhibitors for
the treatment of major depression and the observation that those drugs
affected noradrenaline and 5-
HT metabolism. Today, drugs acting on the serotoninergic system have been
proved to be effective
in the pharmacotherapy of psychiatric diseases such as depression,
schizophrenia, obsessive-
compulsive disorder, panic disorder, generalized anxiety disorder and social
phobia as well as
migraine, vomiting induced by cancer chemotherapy and gastric motility
disorders.
Serotonin receptors represent a very large and diverse family of
neurotransmitter receptors.
To date thirteen 5-HT receptor proteins coupled to G proteins plus one ligand-
gated ion channel
receptor (5-HT3) have been described in mammals. This receptor diversity is
thought to reflect
serotonin's ancient origin as a neurotransmitter and a hormone as well as the
many different roles of
5-HT in mammals. The 5-HT receptors have been classified into seven
subfamilies or groups
according to theix different ligand-binding affinity profiles, molecular
structure and intracellular
transduction mechanisms (Hoyer, D. et al., Pharmacol. Rev. 46, 157-203
(1994)).
Adreneraic GPCRs
The adrenergic receptors comprise one of the largest and most extensively
characterized
families within the G-protein coupled receptor "superfamily". This superfamily
includes not only
adrenergic receptors, but also muscarinic, cholinergic, dopaminergic,
serotonergic, and
histaminergic receptors. Numerous peptide receptors include glucagon,
somatostatin, and
vasopressin receptors, as well as sensory receptors for vision (rhodopsin),
taste, and olfaction,
also belong to this growing family. Despite the diversity of signalling
molecules, G-protein
coupled receptors all possess a similar overall primary structure,
characterized by 7 putative
membrane-spanning .alpha. helices (Probst et al., 1992). In the most basic
sense, the adrenergic
receptors are the physiological sites of action of the catecholamines,
epinephrine and
norepinephrine. Adrenergic receptors were initially classified as either
.alpha. or .beta. by
4
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Ahlquist, who demonstrated that the order of potency for a series of agonists
to evoke a
physiological response was distinctly different at the 2 receptor subtypes
(Ahlquist, 1948).
Functionally, .alpha. adrenergic receptors were shown to control
vasoconstriction, pupil dilation
and uterine inhibition, while .beta. adrenergic receptors were implicated in
vasorelaxation,
myocardial stimulation and bronchodilation (Regan et al., 1990). Eventually,
pharmacologists
realized that these responses resulted from activation of several distinct
adrenergic receptor
subtypes. .beta. adrenergic receptors in the heart were defined as
.beta.<sub>l</sub>, while those in the
lung and vasculature were termed .beta.<sub>2</sub> (Lands et al., 1967).
.alpha. Adrenergic receptors, meanwhile, were first classified based on their
anatomical
location, as either pre or post-synaptic (.alpha.<sub>2</sub> and .alpha.<sub>l</sub>,
respectively) (Langer et
al., 1974). This classification scheme was confounded, however, by the
presence of .alpha.<sub>2</sub>
receptors in distinctly non-synaptic locations, such as platelets (Berthelsen
and Pettinger, 1977).
With the development of radioligand binding techniques, .alpha. adrenergic
receptors could be
distinguished pharmacologically based on their affinities for the antagonists
prazosin or
yohimbine (Stark, 1981). Definitive evidence for adrenergic receptor subtypes,
however, awaited
purification and molecular cloning of adrenergic receptor subtypes. In 1986,
the genes for the
hamster .beta.<sub>2</sub> (Dickson et al., 1986) and turkey .beta.<sub>l</sub> adrenergic
receptors (Yarden
et al., 1986) were cloned and sequenced. Hydropathy analysis revealed that
these proteins
contain 7 hydrophobic domains similar to rhodopsin, the receptor for light.
Since that time the
adrenergic receptor family has expanded to include 3 subtypes of .beta.
receptors (Emorine et al.,
1989), 3 subtypes of .alpha.<sub>l</sub> receptors (Schwinn et al., 1990), and 3
distinct types of
.beta.<sub>2</sub> receptors (Lomasney et al., 1990).
The cloning, sequencing and expression of alpha receptor subtypes from animal
tissues
has led to the subclassification of the alpha 1 receptors into alpha 1 d
(formerly known as alpha
1 a or 1 a/1 d), alpha 1 b and alpha 1 a (formerly known as alpha 1 c)
subtypes. Each alpha 1
receptor subtype exhibits its own pharmacologic and tissue specificities. The
designation "alpha
1 a" is the appellation recently approved by the IUPHAR Nomenclature Committee
for the
previously designated "alpha 1 c" cloned subtype as outlined in the 1995
Receptor and Ion
Channel Nomenclature Supplement (Watson and Girdlestone, 1995). The
designation alpha 1 a is
used throughout this application to refer to this subtype. At the same time,
the receptor formerly
designated alpha 1 a was renamed alpha 1 d. The new nomenclature is used
throughout this
application. Stable cell lines expressing these alpha 1 receptor subtypes are
referred to herein;
however, these cell lines were deposited with the American Type Culture
Collection (ATCC)
5
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
under the old nomenclature. For a review of the classification of alpha 1
adrenoceptor subtypes,
see, Martin C. Michel, et al., Naunyn-Schmiedeberg's Arch. Pharmacol. (1995)
352:1-10.
The differences in the alpha adrenergic receptor subtypes have relevance in
pathophysiologic conditions. Benign prostatic hyperplasia, also known as
benign prostatic
hypertrophy or BPH, is an illness typically affecting men over fifty years of
age, increasing in
severity with increasing age. The symptoms of the condition include, but are
not limited to,
increased difficulty in urination and sexual dysfunction. These symptoms are
induced by
enlargement, or hyperplasia, of the prostate gland. As the prostate increases
in size, it impinges
on free-flow of fluids through the male urethra. Concommitantly, the increased
noradrenergic
innervation of the enlarged prostate leads to an increased adrenergic tone of
the bladder neck and
urethra, further restricting the flow of urine through the urethra.
The .alpha.<sub>2</sub> receptors appear to have diverged rather early from either
.beta. or
.alpha.<sub>l</sub> receptors. The .alpha.<sub>2</sub> receptors have been broken down
into 3 molecularly
distinct subtypes termed .alpha.<sub>2</sub> C2, .alpha.<sub>2</sub> C4, and .alpha.<sub>2</sub>
C10 based on their
chromosomal location. These subtypes appear to correspond to the
pharmacologically defined
.alpha.<sub>2B</sub>, .alpha.<sub>2C</sub>, and .alpha.<sub>2A</sub> subtypes, respectively
(Bylund et al., 1992).
While all the receptors of the adrenergic type are recognized by epinephrine,
they are
pharmacologically distinct and are encoded by separate genes. These receptors
are generally
coupled to different second messenger pathways that are linked through G-
proteins. Among the
adrenergic receptors, .beta.<sub>l</sub> and .beta.<sub>2</sub> receptors activate the
adenylate cyclase,
.alpha.<sub>2</sub> receptors inhibit adenylate cyclase and .alpha.<sub>l</sub> receptors
activate
phospholipase C pathways, stimulating breakdown of polyphosphoinositides
(Ghung, F. Z. et al.,
J. Biol. Chem., 263:4052 (1988)). .alpha.<sub>l</sub> and .alpha.<sub>2</sub> adrenergic
receptors differ in
their cell activity for drugs.
Issued US patent that disclose the utility of members of this family of
proteins include,
but are not limited to, 6,063,785 Phthalimido arylpiperazines useful in the
treatment of benign
prostatic hyperplasia; 6,060,492 Selective .beta.3 adrenergic agonists;
6,057,350 Alpha la
adrenergic receptor antagonists; 6,046,192
Phenylethanolaminotetralincarboxamide derivatives;
6,046,183 Method of synergistic treatment for benign prostatic hyperplasia;
6,043,253 Fused
piperidine substituted arylsulfonamides as .beta.3-agonists; 6,043,224
Compositions and
methods for treatment of neurological disorders and neurodegenerative
diseases; 6,037,354
Alpha 1 a adrenergic receptor antagonists; 6,034,106 Oxadiazole
benzenesulfonamides as
selective .beta.<sub>3</sub> Agonist for the treatment of Diabetes and Obesity;
6,011,048 Thiazole
6
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
benzenesulfonamides as .beta.3 agonists for treatment of diabetes and obesity;
6,008,361
5,994,506 Adrenergic receptor; 5,994,294 Nitrosated and nitrosylated .alpha.-
adrenergic receptor
antagonist compounds, compositions and their uses; 5,990,128 .alpha.<sub>lC</sub>
specific
compounds to treat benign prostatic hyperplasia; 5,977,154 Selective .beta.3
adrenergic agonist;
S 5,977,115 Alpha la adrenergic receptor antagonists; 5,939,443 Selective
.beta.3 adrenergic
agonists; 5,932,538 Nitrosated and nitrosylated .alpha.-adrenergic receptor
antagonist
compounds, compositions and their uses; 5,922,722 Alpha la adrenergic receptor
antagonists 26
5,908,830 and 5,861,309 DNA endoding human alpha 1 adrenergic receptors.
Puriner~ic GPCRs
Purinoceptor P2Y1
P2 purinoceptors have been broadly classified as P2X receptors which are ATP-
gated
channels; P2Y receptors, a family of G protein-coupled receptors, and P2Z
receptors, which
mediate nonselective pores in mast cells. Numerous subtypes have been
identified for each of the
1 S P2 receptor classes. P2Y receptors are characterized by their selective
responsiveness towards ATP
and its analogs. Some respond also to UTP. Based on the recommendation for
nomenclature of P2
purinoceptors, the P2Y purinoceptors were numbered in the order of cloning.
P2Y1, P2Y2 and
P2Y3 have been cloned from a variety of species. P2Y1 responds to both ADP and
ATP. Analysis
of P2Y receptor subtype expression in human bone and 2 osteoblastic cell lines
by RT-PCR showed
that all known human P2Y receptor subtypes were expressed: P2Y1, P2Y2, P2Y4,
P2Y6, and P2Y7
(Maier et al. 1997). In contrast, analysis of brain-derived cell lines
suggested that a selective
expression of P2Y receptor subtypes occurs in brain tissue.
Leon et al. generated P2Yl-null mice to define the physiologic role of the
P2Y1 receptor. (J.
Clin. Invest. 104: 1731-1737(1999)) These mice were viable with no apparent
abnormalities
2S affecting their development, survival, reproduction, or morphology of
platelets, and the platelet
count in these animals was identical to that of wildtype mice. However,
platelets from P2Y1-
deficient mice were unable to aggregate in response to usual concentrations of
ADP and displayed
impaired aggregation to other agonists, while high concentrations of ADP
induced platelet
aggregation without shape change. In addition, ADP-induced inhibition of
adenylyl cyclase still
occurred, demonstrating the existence of an ADP receptor distinct from P2Y1.
P2Y1-null mice had
no spontaneous bleeding tendency but were resistant to thromboembolism induced
by intravenous
injection of ADP or collagen and adrenaline. Hence, the P2Yl receptor plays an
essential role in
7
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
thrombotic states and represents a potential target for antithrombotic drugs.
Somers et al. mapped
the P2RY1 gene between flanking markers D3S1279 and D3S1280 at a position 173
to 174 cM
from the most telomeric markers on the short arm of chromosome 3. (Genomics
44: 127-130
(1997)).
Purinocentor P2Y2
The chloride ion secretory pathway that is defective in cystic fibrosis (CF)
can be bypassed
by an alternative pathway for chloride ion transport that is activated by
extracellular nucleotides.
Accordingly, the P2 receptor that mediates this effect is a therapeutic target
for improving chloride
secretion in CF patients. Parr et al. reported the sequence and functional
expression of a cDNA
cloned from human airway epithelial cells that encodes a protein with
properties of a P2Y
nucleotide receptor. (Proc. Nat. Acad. Sci. 91: 3275-3279 (1994)) The human
P2RY2 gene was
mapped to chromosome 11q13.5-q14.1.
Purinoceptor P2RY4
The P2RY4 receptor appears to be activated specifically by UTP and UDP, but
not by ATP
and ADP. Activation of this uridine nucleotide receptor resulted in increased
inositol phosphate
formation and calcium mobilization. The UNR gene is located on chromosome
Xql3.
Purinoceptor P2Y6
Somers et al. mapped the P2RY6 gene to 11 q13.5, between polymorphic markers
D 11 S 1314 and D 115916, and P2RY2 maps within less than 4 cM of P2RY6.
(Genomics 44: 127-
130 (1997)) This was the first chromosomal clustering of this gene family to
be described.
Adenine and uridine nucleotides, in addition to their well established role in
intracellular
energy metabolism, phosphorylation, and nucleic acid synthesis, also are
important extracellular
signaling molecules. P2Y metabotropic receptors are GPCRs that mediate the
effects of
extracellular nucleotides to regulate a wide variety of physiological
processes. At least ten
subfamilies of P2Y receptors have been identified. These receptor subfamilies
differ greatly in their
sequences and in their nucleotide agonist selectivities and efficacies.
It has been demonstrated that the P2Yl receptors are strongly expressed in the
brain, but the
P2Y2, P2Y4 and P2Y6 receptors are also present. The localisation of one or
more of these subtypes
on neurons, on glia cells, on brain vasculature or on ventricle ependimal
cells was found by in situ
mRNA hybridisation and studies on those cells in culture. The P2Y1 receptors
are prominent on
neurons. The coupling of certain P2Y receptor subtypes to N-type Ca2+ channels
or to particular
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
K+ channels was also demonstrated.
It has also been demonstrated that several P2Y receptors mediate potent growth
stimulatory
effects on smooth muscle cells by stimulating intracellular pathways including
Gq-proteins, protein
kinase C and tyrosine phosphorylation, leading to increased immediate early
gene expression, cell
number, DNA and protein synthesis. It has been further demonstrated that P2Y
regulation plays a
mitogenic role in response to the development of artherosclerosis.
It has further been demonstrated that P2Y receptors play a critical role in
cystic fibrosis.
'The volume and composition of the liquid that lines the airway surface is
modulated by active
transport of ions across the airway epithelium. This in turn is regulated both
by autonomic agonists
acting on basolateral receptors and by agonists acting on luminal receptors.
Specifically,
extracellular nucleotides present in the airway surface liquid act on luminal
P2Y receptors to control
both Cl- secretion and Na+ absorption. Since nucleotides are released in a
regulated manner from
airway epithelial cells, it is likely that their control over airway ion
transport forms part of an
autocrine regulatory system localised to the luminal surface of airway
epithelia. In addition to this
physiological role, P2Y receptor agonists have the potential to be of crucial
benefit in the treatment
of CF, a disorder of epithelial ion transport. The airways of people with CF
have defective Cl-
secretion and abnormally high rates of Na+ absorption. Since P2Y receptor
agonists can regulate
both these ion transport pathways they have the potential to pharmacologically
bypass the ion
transport defects in CF.
EMR Proteins
The protein provided by the present invention is related to the EGF-module-
containing,
mucin-like hormone receptor (EMR) protein. The novel human protein, and
encoding gene,
provided by the present invention is likely a novel splice form of the EMRl
protein. Specifically,
the splice form of the present invention is missing exon 15, which results in
the loss of two
transmembrane domains compared with the art-known EMRl protein (Genbank
GI:4503565).
EMRl is a member of the family of hormone receptors with seven transmembrane
segments, including receptors for secretin, vasoactive intestinal peptide,
glucagon, parathyroid
hormone and parathyroid hormone-related peptides, calcitonin, growth hormone-
releasing factor,
corticotropin-releasing factor, pituitary adenylate cyclase activating
polypeptide, and gastric
inhibitory polypeptide. EMRl comprises three distinct domains: a C-terminal
domain that contains
the seven hydrophobic segments, an N-terminal domain that contains six EGF-
like modules, and an
intervening serine/threonine-rich domain (Baud et al., Gehomics 26 (2), 334-
344 (1995)). For a
9
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
further review of EMRl, see McKnight et al., Mammalian Genome 8: 946-947,1997.
GPCRs, particularly members of the EMR receptor subfamily, are a major target
for drug
action and development. Accordingly, it is valuable to the field of
pharmaceutical development to
identify and characterize previously unknown GPCRs. The present invention
advances the state of
the art by providing a previously unidentified human GPCR.
SUMMARY OF THE INVENTION
The present invention is based in part on the identification of nucleic acid
sequences that
encode amino acid sequences of a novel human EMRl splice form, allelic
variants thereof and
other mammalian orthologs thereof. These unique peptide sequences, and nucleic
acid
sequences that encode these peptides, can be used as models for the
development of human
therapeutic targets, aid in the identification of therapeutic proteins, and
serve as targets for the
development of human therapeutic agents.
The proteins of the present inventions are GPCRs that participate in signaling
pathways
mediated by the EMR subfamily in cells that express these proteins.
Experimental data as provided
in Figure 1 indicates expression in humans in neuroectodermal tumors, brain
glioblastomas, kidney
tumors, lymphocytes, fetal liver/spleen, sperm, and leukocytes. As used
herein, a "signaling
pathway" refers to the modulation (e.g., stimulation or inhibition) of a
cellular function/activity
upon the binding of a ligand to the GPCR protein. Examples of such functions
include mobilization
of intracellular molecules that participate in a signal transduction pathway,
e.g., phosphatidylinositol
4,5-bisphosphate (PIP2), inositol 1,4,5-triphosphate (IP3) and adenylate
cyclase; polarization of the
plasma membrane; production or secretion of molecules; alteration in the
structure of a cellular
component; cell proliferation, e.g., synthesis of DNA; cell migration; cell
differentiation; and cell
survival
The response mediated by the receptor protein depends on the type of cell it
is expressed on.
Some information regarding the types of cells that express other members of
the subfamily of
GPCRs of the present invention is already known in the art (see references
cited in Background and
information regarding closest homologous protein provided in Figure 2;
Experimental data as
provided in Figure 1 indicates expression in humans in neuroectodennal tumors,
brain
glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen, sperm, and
leukocytes. ). For
example, in some cells, binding of a ligand to the receptor protein may
stimulate an activity such as
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
release of compounds, gating of a channel, cellular adhesion, migration,
differentiation, etc.,
through phosphatidylinositol or cyclic AMP metabolism and turnover while in
other cells, the
binding of the ligand will produce a different result. Regardless of the
cellular activity/response
modulated by the particular GPCR of the present invention, a skilled artisan
will clearly know that
the receptor protein is a GPCR and interacts with G proteins to produce one or
more secondary
signals, in a variety of intracellular signal transduction pathways, e.g.,
through phosphatidylinositol
or cyclic AMP metabolism and turnover, in a cell thus participating in a
biological process in the
cells or tissues that express the GPCR. Experimental data as provided in
Figure 1 indicates that
GPCR proteins of the present invention are expressed in humans in
neuroectodermal tumors, brain
glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen tissue, and
sperm, as indicated by
virtual northern blot analysis. In addition, PCR-based tissue screening panels
indicate expression in
human leukocytes.
As used herein, "phosphatidylinositol turnover and metabolism" refers to the
molecules
involved in the turnover and metabolism of phosphatidylinositol 4,5-
bisphosphate (PIPS) as well as
to the activities of these molecules. PIPa is a phospholipid found in the
cytosolic leaflet of the
plasma membrane. Binding of ligand to the receptor activates, in some cells,
the plasma-membrane
enzyme phospholipase C that in turn can hydrolyze Plea to produce 1,2-
diacylglycerol (DAG) and
inositol 1,4,5-triphosphate (lP3). Once formed lP3 can diffuse to the
endoplasmic reticulum surface
where it can bind an 1P3 receptor, e.g., a calcium channel protein containing
an 1P3 binding site. lP3
binding can induce opening of the channel, allowing calcium ions to be
released into the cytoplasm.
lP3 can also be phosphorylated by a specific kinase to form inositol 1,3,4,5-
tetraphosphate (lP4), a
molecule that can cause calcium entry into the cytoplasm from the
extracellular medium. 1P3 and
IP4 can subsequently be hydrolyzed very rapidly to the inactive products
inositol 1,4-biphosphate
(IPa) and inositol 1,3,4-triphosphate, respectively. These inactive products
can be recycled by the
cell to synthesize PIP2. The other second messenger produced by the hydrolysis
of P1P2, namely
1,2-diacylglycerol (DAG), remains in the cell membrane where it can serve to
activate the enzyme,
protein kinase C. Protein kinase C is usually found soluble in the cytoplasm
of the cell, but upon an
increase in the intracellular calcium concentration, this enzyme can move to
the plasma membrane
where it can be activated by DAG. The activation of protein kinase C in
different cells results in
various cellular responses such as the phosphorylation of glycogen synthase,
or the phosphorylation
of various transcription factors, e.g., NF-kB. The language
"phosphatidylinositol activity", as used
herein, refers to an activity of PIP2 or one of its metabolites.
Another signaling pathway in which the receptor may participate is the CAMP
turnover
11
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
pathway. As used herein, "cyclic AMP turnover and metabolism" refers to the
molecules
involved in the turnover and metabolism of cyclic AMP (CAMP) as well as to the
activities of
these molecules. Cyclic AMP is a second messenger produced in response to
ligand-induced
stimulation of certain G protein coupled receptors. In the cAMP signaling
pathway, binding of a
ligand to a GPCR can lead to the activation of the enzyme adenyl cyclase,
which catalyzes the
synthesis of cAMP. The newly synthesized cAMP can in turn activate a cAMP-
dependent
protein kinase. This activated kinase can phosphorylate a voltage-gated
potassium channel
protein, or an associated protein, and lead to the inability of the potassium
channel to open
during an action potential. The inability of the potassium channel to open
results in a decrease in
the outward flow of potassium, which normally repolarizes the membrane of a
neuron, leading to
prolonged membrane depolarization.
By targeting an agent to modulate a GPCR, the signaling activity and
biological process
mediated by the receptor can be agonized or antagonized in specific cells and
tissues.
Experimental data as provided in Figure 1 indicates expression in humans in
neuroectodermal
tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen,
sperm, and
leukocytes. Such agonism and antagonism serves as a basis for modulating a
biological activity
in a therapeutic context (mammalian therapy) or toxic context (anti-cell
therapy, e.g. anti-cancer
agent).
DESCRIPTION OF THE FIGURE SHEETS
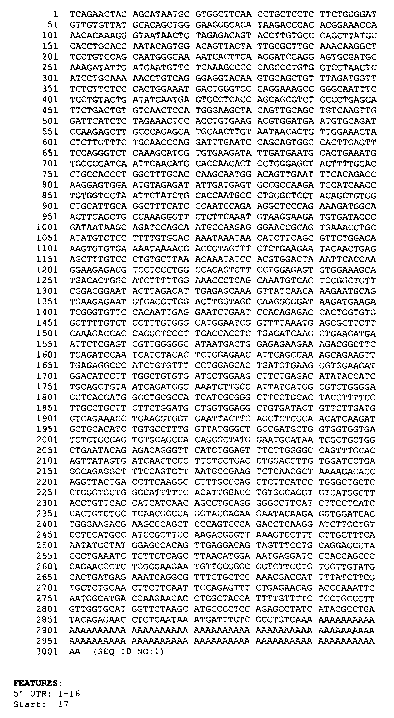
FIGURE 1 provides the nucleotide sequence of a cDNA molecule that encodes the
GPCR
of the present invention. (SEQ ID NO:1) In addition, structure and functional
information is
provided, such as ATG start, stop and tissue distribution, where available,
that allows one to
readily determine specific uses of inventions based on this molecular
sequence. Experimental
data as provided in Figure 1 indicates expression in humans in neuroectodermal
tumors, brain
glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen, sperm, and
leukocytes.
FIGURE 2 provides the predicted amino acid sequence of the GPCR of the present
invention. (SEQ ID N0:2) In addition structure and functional information such
as protein
family, function, and modification sites is provided where available, allowing
one to readily
determine specific uses of inventions based on this molecular sequence.
FIGURE 3 provides genomic sequences that span the gene encoding the GPCR
protein of
the present invention. (SEQ ID N0:3) In addition structure and functional
information, such as
12
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
intron/exon structure, promoter location, etc., is provided where available,
allowing one to
readily determine specific uses of inventions Based on this molecular
sequence.
DETAILED DESCRIPTION OF THE INVENTION
General Description
The present invention is based on the sequencing of the human genome. During
the
sequencing and assembly of the human genome, analysis of the sequence
information revealed
previously unidentified fragments of the human genome that encode peptides
that share
structural andlor sequence homology to protein/peptide/domains identified and
characterized
within the art as being a GPCR protein or part of a GPCR protein, that are
related to the EMR
subfamily. Utilizing these sequences, additional genomic sequences were
assembled and
transcript and/or cDNA sequences were isolated and characterized. Based on
this analysis, the
present invention provides amino acid sequences of a novel human EMRl splice
form, nucleic
acid sequences in the form of transcript sequences, cDNA sequences and/or
genomic sequences
that encode this EMRl splice form, nucleic acid variation (allelic
information), tissue
distribution of expression, and information about the closest art known
protein/peptide/domain
that has structural or sequence homology to the GPCR of the present invention.
In addition to being previously unknown, the peptides that are provided in the
present
invention are selected based on their ability to be used for the development
of commercially
important products and services. Specifically, the present peptides are
selected based on
homology and/or structural relatedness to known GPCR proteins of the EMR
subfamily and the
expression pattern observed. Experimental data as provided in Figure 1
indicates expression in
humans in neuroectodermal tumors, brain glioblastomas, kidney tumors,
lymphocytes, fetal
liver/spleen, sperm, and leukocytes. The art has clearly established the
commercial importance
of members of this family of proteins and proteins that have expression
patterns similar to that of
the present gene. Some of the more specific features of the peptides of the
present invention, and
the uses thereof, are described herein, particularly in the Background of the
Invention and in the
annotation provided in the Figures, and/or are known within the art for each
of the known EMR
family or subfamily of GPCR proteins.
Specific Embodiments
13
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Pe,~tide Molecules
The present invention provides nucleic acid sequences that encode protein
molecules that
have been identified as being members of the GPCR family of proteins and are
related to the
EMR subfamily (protein sequences are provided in Figure 2, transcript/cDNA
sequences are
provided in Figure 1 and genomic sequences are provided in Figure 3). The
peptide sequences
provided in Figure 2, as well as the obvious variants described herein,
particularly allelic variants
as identified herein and using the information in Figure 3, will be referred
herein as the GPCR
peptides of the present invention, GPCR peptides, or peptides/proteins of the
present invention.
The present invention provides isolated peptide and protein molecules that
consist of,
consist essentially of, or comprise the amino acid sequences of the GPCR
peptides disclosed in
Figure 2, (encoded by the nucleic acid molecule shown in Figure 1,
transcript/cDNA sequence,
or Figure 3, genomic sequence), as well as all obvious variants of these
peptides that are within
the art to make and use. Some of these variants are described in detail below.
As used herein, a peptide is said to be "isolated" or "purified" when it is
substantially free
of cellular material or free of chemical precursors or other chemicals. The
peptides of the present
invention can be purified to homogeneity or other degrees of purity. The level
of purification will
be based on the intended use. The critical feature is that the preparation
allows for the desired
function of the peptide, even if in the presence of considerable amounts of
other components (the
features of an isolated nucleic acid molecule is discussed below).
In some uses, "substantially free of cellular material" includes preparations
of the peptide
having less than about 30% (by dry weight) other proteins (i.e., contaminating
protein), less than
about 20% other proteins, less than about 10% other proteins, or less than
about 5% other proteins.
When the peptide is recombinantly produced, it can also be substantially free
of culture medium,
i.e., culture medium represents less than about 20% of the volume of the
protein preparation.
The language "substantially free of chemical precursors or other chemicals"
includes
preparations of the peptide in which it is separated from chemical precursors
or other chemicals that
are involved in its synthesis. In one embodiment, the language "substantially
free of chemical
precursors or other chemicals" includes preparations of the GPCR peptide
having less than about
30% (by dry weight) chemical precursors or other chemicals, less than about
20% chemical
precursors or other chemicals, less than about 10% chemical precursors or
other chemicals, or less
than about 5% chemical precursors or other chemicals.
The isolated GPCR peptide can be purified from cells that naturally express
it, purified from
cells that have been altered to express it (recombinant), or synthesized using
known protein
I4
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
synthesis methods. Experimental data as provided in Figure 1 indicates
expression in humans in
neuroectodermal tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal
liver/spleen,
sperm, and leukocytes. For example, a nucleic acid molecule encoding the GPCR
peptide is cloned
into an expression vector, the expression vector introduced into a host cell
and the protein expressed
in the host cell. The protein can then be isolated from the cells by an
appropriate purification
scheme using standard protein purification techniques. Many of these
techniques are described in
detail below.
Accordingly, the present invention provides proteins that consist of the amino
acid
sequences provided in Figure 2 (SEQ ID N0:2), for example, proteins encoded by
the
transcript/cDNA nucleic acid sequences shown in Figure 1 (SEQ ID NO:l) and the
genomic
sequences provided in Figure 3 (SEQ ID N0:3). The amino acid sequence of such
a protein is
provided in Figure 2. A protein consists of an amino acid sequence when the
amino acid sequence
is the final amino acid sequence of the protein.
The present invention further provides proteins that consist essentially of
the amino acid
1 S sequences provided in Figure 2 (SEQ ID N0:2), for example, proteins
encoded by the
transcript/cDNA nucleic acid sequences shown in Figure 1 (SEQ ID NO:1 ) and
the genomic
sequences provided in Figure 3 (SEQ ID N0:3). A protein consists essentially
of an amino acid
sequence when such an amino acid sequence is present with only a few
additional amino acid
residues, for example from about 1 to about 100 or so additional residues,
typically from 1 to about
20 additional residues in the final protein.
The present invention fiufiher provides proteins that comprise the amino acid
sequences
provided in Figure 2 (SEQ ID N0:2), for example, proteins encoded by the
transcript/cDNA nucleic
acid sequences shown in Figure 1 (SEQ ID NO:1) and the genomic sequences
provided in Figure 3
(SEQ ID N0:3). A protein comprises an amino acid sequence when the amino acid
sequence is at
least part of the final amino acid sequence of the protein. In such a fashion,
the protein can be only
the peptide or have additional amino acid molecules, such as amino acid
residues (contiguous
encoded sequence) that are naturally associated with it or heterologous amino
acid residues/peptide
sequences. Such a protein can have a few additional amino acid residues or can
comprise several
hundred or more additional amino acids. The preferred classes of proteins that
are comprised of the
GPCR peptides of the present invention are the naturally occurring mature
proteins. A brief
description of how various types of these proteins can be made/isolated is
provided below.
The GPCR peptides of the present invention can be attached to heterologous
sequences to
form chimeric or fusion proteins. Such chimeric and fusion proteins comprise a
GPCR peptide
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
operatively linked to a heterologous protein having an amino acid sequence not
substantially
homologous to the GPCR peptide. "Operatively linked" indicates that the GPCR
peptide and the
heterologous protein are fused in-frame. The heterologous protein can be fused
to the N-terminus
or C-terminus of the GPCR peptide.
In some uses, the fusion protein does not affect the activity of the GPCR
peptide per se. For
example, the fusion protein can include, but is not limited to, enzymatic
fusion proteins, for example
beta-galactosidase fusions, yeast two-hybrid GAL fusions, poly-His fusions,
MYC-tagged, HI-
tagged and Ig fusions. Such fusion proteins, particularly poly-His fusions,
can facilitate the
purification of recombinant GPCR peptide. In certain host cells (e.g.,
mammalian host cells),
expression and/or secretion of a protein can be increased by using a
heterologous signal sequence.
A chimeric or fusion protein can be produced by standard recombinant DNA
techniques.
For example, DNA fragments coding for the different protein sequences are
ligated together in-
frame in accordance with conventional techniques. In another embodiment, the
fusion gene can be
synthesized by conventional techniques including automated DNA synthesizers.
Alternatively, PCR
amplification of gene fragments can be carned out using anchor primers which
give rise to
complementary overhangs between two consecutive gene fragments which can
subsequently be
annealed and re-amplified to generate a chimeric gene sequence (see Ausubel et
crl., Current
Protocols in Molecular Biology, 1992). Moreover, many expression vectors are
commercially
available that already encode a fusion moiety (e.g., a GST protein). A GPCR
peptide-encoding
nucleic acid can be cloned into such an expression vector such that the fusion
moiety is linked in-
frame to the GPCR peptide.
As mentioned above, the present invention also provides and enables obvious
variants of the
amino acid sequence of the proteins of the present invention, such as
naturally occurring mature
forms of the peptide, allelic/sequence variants of the peptides, non-naturally
occurring
recombinantly derived variants of the peptides, and orthologs and paralogs of
the peptides. Such
variants can readily be generated using art-known techniques in the fields of
recombinant nucleic
acid technology and protein biochemistry. It is understood, however, that
variants exclude any
amino acid sequences disclosed prior to the invention.
Such variants can readily be identified/made using molecular techniques and
the sequence
information disclosed herein. Further, such variants can readily be
distinguished from other
peptides based on sequence and/or structural homology to the GPCR peptides of
the present
invention. The degree of homology/identity present will be based primarily on
whether the peptide
is a functional variant or non-functional variant, the amount of divergence
present in the paralog
16
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
family and the evolutionary distance between the orthologs.
To determine the percent identity of two amino acid sequences or two nucleic
acid
sequences, the sequences are aligned for optimal comparison purposes (e.g.,
gaps can be
introduced in one or both of a first and a second amino acid or nucleic acid
sequence for optimal
alignment and non-homologous sequences can be disregarded for comparison
purposes). In a
preferred embodiment, the length of a reference sequence aligned for
comparison purposes is at
least 30%, 40%, 50%, 60%, 70%, 80%, or 90% or more of the length of the
reference sequence.
The amino acid residues or nucleotides at corresponding amino acid positions
or nucleotide
positions are then compared. When a position in the first sequence is occupied
by the same
amino acid residue or nucleotide as the corresponding position in the second
sequence, then the
molecules are identical at that position (as used herein amino acid or nucleic
acid "identity" is
equivalent to amino acid or nucleic acid "homology"). The percent identity
between the two
sequences is a function of the number of identical positions shared by the
sequences, taking into
account the number of gaps, and the length of each gap, which need to be
introduced for optimal
alignment of the two sequences.
The comparison of sequences and determination of percent identity and
similarity
between two sequences can be accomplished using a mathematical algorithm.
(Computational
Molecular Biology, Lesk, A.M., ed., Oxford University Press, New York, 1988;
Biocomputing:
Informatics and Gehome Projects, Smith, D.W., ed., Academic Press, New
York,1993; Computer
Analysis of Sequence Data, Part 1, Griffin, A.M., and Griffin, H.G., eds.,
Humana Press, New
Jersey, 1994; Sequence Analysis in Molecular Biology, von Heinje, G., Academic
Press,1987; and
Sequence Analysis Primer, Gribskov, M. and Devereux, J., eds., M Stockton
Press, New York,
1991). In a preferred embodiment, the percent identity between two amino acid
sequences is
determined using the Needleman and Wunsch (J. Mol. Biol. (48):444-453 (1970))
algorithm
which has been incorporated into the GAP program in the GCG software package
(available at
http://www.gcg.com), using either a Blossom 62 matrix or a PAM250 matrix, and
a gap weight
of 16, 14, 12, 10, 8, 6, or 4 and a length weight of 1, 2, 3, 4, 5, or 6. In
yet another preferred
embodiment, the percent identity between two nucleotide sequences is
determined using the
GAP program in the GCG software package (Devereux, J., et al., Nucleic Acids
Res. 12(1):387
(1984)) (available at http://www.gcg.com), using a NWSgapdna.CMP matrix and a
gap weight of
40, 50, 60, 70, or 80 and a length weight of 1, 2, 3, 4, 5, or 6. In another
embodiment, the
percent identity between two amino acid or nucleotide sequences is determined
using the
algorithm of E. Meyers and W. Miller (CABIOS, 4:11-17 (1989)) which has been
incorporated
17
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
into the ALIGN program (version 2.0), using a PAM120 weight residue table, a
gap length
penalty of 12 and a gap penalty of 4.
The nucleic acid and protein sequences of the present invention can further be
used as a
"query sequence" to perform a search against sequence databases to, for
example, identify other
family members or related sequences. Such searches can be performed using the
NBLAST and
XBLAST programs (version 2.0) of Altschul, et al. (J. Mol. Biol. 215:403-10
(1990)). BLAST
nucleotide searches can be performed with the NBLAST program, score = 100,
wordlength =12
to obtain nucleotide sequences homologous to the nucleic acid molecules of the
invention.
BLAST protein searches can be performed with the XBLAST program, score = 50,
wordlength =
3 to obtain amino acid sequences homologous to the proteins of the invention.
To obtain gapped
alignments for comparison purposes, Gapped BLAST can be utilized as described
in Altschul et
al. (Nucleic Acids Res. 25(17):3389-3402 (1997)). When utilizing BLAST and
gapped BLAST
programs, the default parameters of the respective programs (e.g., XBLAST and
NBLAST) can
be used.
Full-length pre-processed forms, as well as mature processed forms, of
proteins that
comprise one of the peptides of the present invention can readily be
identified as having complete
sequence identity to one of the GPCR peptides of the present invention as well
as being encoded by
the same genetic locus as the GPCR peptide provided herein. The gene encoding
the novel
transporter protein of the present invention is located on a genome component
that has been mapped
to human chromosome 19 (as indicated in Figure 3), which is supported by
multiple lines of
evidence, such as STS and BAC map data.
Allelic variants of a GPCR peptide can readily be identified as being a human
protein
having a high degree (significant) of sequence homology/identity to at least a
portion of the GPCR
peptide as well as being encoded by the same genetic locus as the GPCR peptide
provided herein.
Genetic locus can readily be determined based on the genomic information
provided in Figure 3,
such as the genomic sequence mapped to the reference human. The gene encoding
the novel
transporter protein of the present invention is located on a genome component
that has been mapped
to human chromosome 19 (as indicated in Figure 3), which is supported by
multiple lines of
evidence, such as STS and BAC map data. As used herein, two proteins (or a
region of the
proteins) have significant homology when the amino acid sequences are
typically at least about
70-80%, 80-90%, and more typically at least about 90-95% or more homologous. A
significantly homologous amino acid sequence, according to the present
invention, will be
encoded by a nucleic acid sequence that will hybridize to a GPCR peptide
encoding nucleic acid
18
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
molecule under stringent conditions as more fully described below.
Paralogs of a GPCR peptide can readily be identified as having some degree of
significant
sequence homology/identity to at least a portion of the GPCR peptide, as being
encoded by a gene
from humans, and as having similar activity or function. Two proteins will
typically be considered
paralogs when the amino acid sequences are typically at least about 60% or
greater, and more
typically at least about 70% or greater homology through a given region or
domain. Such
paralogs will be encoded by a nucleic acid sequence that will hybridize to a
GPCR peptide
encoding nucleic acid molecule under moderate to stringent conditions as more
fully described
below.
Orthologs of a GPCR peptide can readily be identified as having some degree of
significant
sequence homology/identity to at least a portion of the GPCR peptide as well
as being encoded by a
gene from another organism. Preferred orthologs will be isolated from mammals,
preferably
primates, for the development of human therapeutic targets and agents. Such
orthologs will be
encoded by a nucleic acid sequence that will hybridize to a GPCR peptide
encoding nucleic acid
molecule under moderate to stringent conditions, as more fully described
below, depending on
the degree of relatedness of the two organisms yielding the proteins.
Non-naturally occurnng variants of the GPCR peptides of the present invention
can readily
be generated using recombinant techniques. Such variants include, but are not
limited to deletions,
additions and substitutions in the amino acid sequence of the GPCR peptide.
For example, one
class of substitutions are conserved amino acid substitution. Such
substitutions are those that
substitute a given amino acid in a GPCR peptide by another amino acid of like
characteristics.
Typically seen as conservative substitutions are the replacements, one for
another, among the
aliphatic amino acids Ala, Val, Leu, and Ile; interchange of the hydroxyl
residues Ser and Thr;
exchange of the acidic residues Asp and Glu; substitution between the amide
residues Asn and Gln;
exchange of the basic residues Lys and Arg; and replacements among the
aromatic residues Phe and
Tyr. Guidance concerning which amino acid changes are likely to be
phenotypically silent are
found in Bowie et al., Science 247:1306-1310 (1990).
Variant GPCR peptides can be fully functional or can lack function in one or
more
activities, e.g. ability to bind ligand, ability to bind G-protein, ability to
mediate signaling, etc.
Fully functional variants typically contain only conservative variation or
variation in non-critical
residues or in non-critical regions. Figure 2 provides the result of protein
analysis that identifies
critical domains/regions. Functional variants can also contain substitution of
similar amino acids
19
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
that result in no change or an insignificant change in function.
Alternatively, such substitutions may
positively or negatively affect function to some degree.
Non-functional variants typically contain one or more non-conservative amino
acid
substitutions, deletions, insertions, inversions, or truncation or a
substitution, insertion, inversion, or
deletion in a critical residue or critical region.
Amino acids that are essential for fiuiction can be identified by methods
known in the art,
such as site-directed mutagenesis or alanine-scanning mutagenesis (Cunningham
et al., Science
244:1081-1085 (1989)), particularly using the results provided in Figure 2.
The latter procedure
introduces single alanine mutations at every residue in the molecule. The
resulting mutant
molecules are then tested for biological activity such as ligand/effector
molecule binding or in
assays such as an in vitro proliferative activity. Sites that are critical for
ligand-receptor binding can
also be determined by structural analysis such as crystallization, nuclear
magnetic resonance or
photoaffinity labeling (Smith et al., J. Mol. Biol. 224:899-904 (1992); de Vos
et al. Science
255:306-312 (1992)).
The present invention further provides fragments of the GPCR peptides, in
addition to
proteins and peptides that comprise and consist of such fragments,
particularly those comprising the
residues identified in Figure 2. The fragments to which the invention
pertains, however, are not to
be construed as encompassing fragments that may be disclosed publicly prior to
the present
invention.
As used herein, a fragment comprises at least 8, 10, 12, 14, 16, or more
contiguous amino
acid residues from a GPCR peptide. Such fragments can be chosen based on the
ability to retain
one or more of the biological activities of the GPCR peptide or could be
chosen for the ability to
perform a function, e.g. ability to bind ligand or effector molecule or act as
an immunogen.
Particularly important fragments are biologically active fragments, peptides
which are, for example,
about 8 or more amino acids in length. Such fragments will typically comprise
a domain or motif of
the GPCR peptide, e.g., active site, a G-protein binding site, a transmembrane
domain or a ligand-
binding domain. Further, possible fragments include, but are not limited to,
domain or motif
containing fragments, soluble peptide fragments, and fragments containing
immunogenic structures.
Predicted domains and functional sites are readily identifiable by computer
programs well-known
and readily available to those of skill in the art (e.g., PROSITE analysis).
The results of one such
analysis are provided in Figure 2.
Polypeptides often contain amino acids other than the 20 amino acids commonly
referred to
as the 20 naturally occurring amino acids. Further, many amino acids,
including the terminal amino
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
acids, may be modified by natural processes, such as processing and other post-
translational
modifications, or by chemical modification techniques well known in the art.
Common
modifications that occur naturally in GPCR peptides are described in basic
texts, detailed
monographs, and the research literature, and they are well known to those of
skill in the art(some of
these features are identified in Figure 2).
Known modifications include, but are not limited to, acetylation, acylation,
ADP-
ribosylation, amidation, covalent attachment of flavin, covalent attachment of
a heme moiety,
covalent attachment of a nucleotide or nucleotide derivative, covalent
attachment of a lipid or lipid
derivative, covalent attachment of phosphotidylinositol, cross-linking,
cyclization, disulfide bond
formation, demethylation, formation of covalent crosslinks, formation of
cystine, formation of
pyroglutamate, formylation, gamma carboxylation, glycosylation, GPI anchor
formation,
hydroxylation, iodination, methylation, myristoylation, oxidation, proteolytic
processing,
phosphorylation, prenylation, racemization, selenoylation, sulfation, transfer-
RNA mediated
addition of amino acids to proteins such as arginylation, and ubiquitination.
Such modifications are well-known to those of skill in the art and have been
described in
great detail in the scientific literature. Several particularly common
modifications, glycosylation,
lipid attachment, sulfation, gamma-carboxylation of glutamic acid residues,
hydroxylation and
ADP-ribosylation, for instance, are described in most basic texts, such as
Proteins - Structure and
Molecular Properties, 2nd Ed., T.E. Creighton, W. H. Freeman and Company, New
York (1993).
Many detailed reviews are available on this subject, such as by Wold, F.,
Posttrahslational Covalent
' Modification ofProtei~s, B.C. Johnson, Ed., Academic Press, New York 1-12
(1983); Seifter et al.
(Meth. Euzymol. 182: 626-646 (1990)) and Rattan et al. (A~n. N. Y. Acad. Sci.
663:48-62 (1992)).
Accordingly, the GPCR peptides of the present invention also encompass
derivatives or
analogs in which a substituted amino acid residue is not one encoded by the
genetic code, in which
a substituent group is included, in which the mature GPCR peptide is fused
with another compound,
such as a compound to increase the half life of the GPCR peptide (for example,
polyethylene
glycol), or in which the additional amino acids are fused to the mature GPCR
peptide, such as a
leader or secretory sequence or a sequence for purification of the mature GPCR
peptide or a pro-
protein sequence.
Protein/Peptide Uses
The proteins of the present invention can be used in substantial and specific
assays
21
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
related to the functional information provided in the Figures and Back Ground
Section; to raise
antibodies or to elicit another immune response; as a reagent (including the
labeled reagent) in
assays designed to quantitatively determine levels of the protein (or its
binding partner or
receptor) in biological fluids; and as markers for tissues in which the
corresponding protein is
preferentially expressed (either constitutively or at a particular stage of
tissue differentiation or
development or in a disease state). Where the protein binds or potentially
binds to another
protein (such as, for example, in a receptor-ligand interaction), the protein
can be used to identify
the binding partner so as to develop a system to identify inhibitors of the
binding interaction.
Any or all of these research utilities are capable of being developed into
reagent grade or kit
format for commercialization as commercial products.
Methods for performing the uses listed above are well known to those skilled
in the art.
References disclosing such methods include "Molecular Cloning: A Laboratory
Manual", 2d ed.,
Cold Spring Harbor Laboratory Press, Sambrook, J., E. F. Fritsch and T.
Maniatis eds., 1989,
and "Methods in Enzymology: Guide to Molecular Cloning Techniques", Academic
Press,
Berger, S. L. and A. R. Kimmel eds., 1987.
The potential uses of the peptides of the present invention are based
primarily on the
source of the protein as well as the class/action of the protein. For example,
GPCRs isolated
from humans and their human/mammalian orthologs serve as targets for
identifying agents for
use in mammalian therapeutic applications, e.g. a human drug, particularly in
modulating a
biological or pathological response in a cell or tissue that expresses the
GPCR. Experimental
data as provided in Figure 1 indicates that GPCR proteins of the present
invention are expressed
in humans in neuroectodermal tumors, brain glioblastomas, kidney tumors,
lymphocytes, fetal
liver/spleen tissue, and sperm, as indicated by virtual northern blot
analysis. In addition, PCR-
based tissue screening panels indicate expression in human leukocytes.
Approximately 70% of
all pharmaceutical agents modulate the activity of a GPCR. A combination of
the invertebrate
and mammalian ortholog can be used in selective screening methods to find
agents specific for
invertebrates. The structural and functional information provided in the
Background and Figures
provide specific and substantial uses for the molecules of the present
invention, particularly in
combination with the expression information provided in Figure 1. Experimental
data as
provided in Figure 1 indicates expression in humans in neuroectodermal tumors,
brain
glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen, sperm, and
leukocytes. Such uses
can readily be determined using the information provided herein, that known in
the art and
routine experimentation.
22
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
The proteins of the present invention (including variants and fragments that
may have been
disclosed prior to the present invention) are useful for biological assays
related to GPCRs that are
related to members of the EMR subfamily. Such assays involve any of the known
GPCR functions
or activities or properties useful for diagnosis and treatment of GPCR-related
conditions that are
specific for the subfamily of GPCRs that the one of the present invention
belongs to, particularly in
cells and tissues that express this receptor. Experimental data as provided in
Figure 1 indicates that
GPCR proteins of the present invention are expressed in humans in
neuroectodermal tumors, brain
glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen tissue, and
sperm, as indicated by
virtual northern blot analysis. In addition, PCR-based tissue screening panels
indicate expression in
human leukocytes.
The proteins of the present invention are also useful in drug screening
assays, in cell-based
or cell-free systems. Cell-based systems can be native, i.e., cells that
normally express the receptor
protein, as a biopsy or expanded in cell culture. Experimental data as
provided in Figure 1 indicates
expression in humans in neuroectodermal tumors, brain glioblastomas, kidney
tumors, lymphocytes,
fetal liver/spleen, sperm, and leukocytes. In an alternate embodiment, cell-
based assays involve
recombinant host cells expressing the receptor protein.
The polypeptides can be used to identify compounds that modulate receptor
activity of the
protein in its natural state, or an altered form that causes a specific
disease or pathology associated
with the receptor. Both the GPCRs of the present invention and appropriate
variants and fragments
can be used in high-throughput screens to assay candidate compounds for the
ability to bind to the
receptor. These compounds can be further screened against a functional
receptor to determine the
effect of the compound on the receptor activity. Further, these compounds can
be tested in animal
or invertebrate systems to determine activity/efFectiveness. Compounds can be
identified that
activate (agonist) or inactivate (antagonist) the receptor to a desired
degree.
Further, the proteins of the present invention can be used to screen a
compound for the
ability to stimulate or inhibit interaction between the receptor protein and a
molecule that normally
interacts with the receptor protein, e.g. a ligand or a component of the
signal pathway that the
receptor protein normally interacts (for example, a G-protein or other
interactor involved in cAMP
or phosphatidylinositol turnover and/or adenylate cyclase, or phospholipase C
activation). Such
assays typically include the steps of combining the receptor protein with a
candidate compound
under conditions that allow the receptor protein, or fragment, to interact
with the target molecule,
and to detect the formation of a complex between the protein and the target or
to detect the
biochemical consequence of the interaction with the receptor protein and the
target, such as any of
23
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
the associated effects of signal transduction such as G-protein
phosphorylation, cAMP or
phosphatidylinositol turnover, and adenylate cyclase or phospholipase C
activation.
Candidate compounds include, for example, 1) peptides such as soluble
peptides, including
Ig-tailed fusion peptides and members of random peptide libraries (see, e.g.,
Lam et al., Nature
354:82-84 (1991); Houghten et al., Nature 354:84-86 (1991)) and combinatorial
chemistry-derived
molecular libraries made of D- and/or L- configuration amino acids; 2)
phosphopeptides (e.g.,
members of random and partially degenerate, directed phosphopeptide libraries,
see, e.g., Songyang
et al., Cell 72:767-778 (1993)); 3) antibodies (e.g., polyclonal, monoclonal,
humanized, anti-
idiotypic, chimeric, and single chain antibodies as well as Fab, F(ab')2, Fab
expression library
fragments, and epitope-binding fragments of antibodies); and 4) small organic
and inorganic
molecules (e.g., molecules obtained from combinatorial and natural product
libraries).
One candidate compound is a soluble fragment of the receptor that competes for
ligand
binding. Other candidate compounds include mutant receptors or appropriate
fragments containing
mutations that affect receptor function and thus compete for ligand.
Accordingly, a fragment that
competes for ligand, for example with a higher affinity, or a fragment that
binds ligand but does not
allow release, is encompassed by the invention.
'The invention fiu-ther includes other end point assays to identify compounds
that modulate
(stimulate or inhibit) receptor activity. The assays typically involve an
assay of events in the signal
transduction pathway that indicate receptor activity. Thus, a cellular process
such as proliferation,
the expression of genes that are up- or down-regulated in response to the
receptor protein dependent
signal cascade, can be assayed. In one embodiment, the regulatory region of
such genes can be
operably linked to a marker that is easily detectable, such as luciferase.
Any of the biological or biochenucal functions mediated by the receptor can be
used as an
endpoint assay. These include all of the biochemical or biochemical/biological
events described
herein, in the references cited herein, incorporated by reference for these
endpoint assay targets, and
other functions known to those of ordinary skill in the art or that can be
readily identified using the
information provided in the Figures, particularly Figure 2. Specifically, a
biological function of a
cell or tissues that expresses the receptor can be assayed. Experimental data
as provided in Figure 1
indicates that GPCR proteins of the present invention are expressed in humans
in neuroectodermal
tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal liver/spleen
tissue, and sperm, as
indicated by virtual northern blot analysis. In addition, PCR-based tissue
screening panels indicate
expression in human leukocytes.
Binding and/or activating compounds can also be screened by using chimeric
receptor
24
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
proteins in which the amino terminal extracellular domain, or parts thereof,
the entire
transmembrane domain or subregions, such as any of the seven transmembrane
segments or any of
the intracellular or extracellular loops and the carboxy terminal
intracellular domain, or parts
thereof, can be replaced by heterologous domains or subregions. For example, a
G-protein-binding
region can be used that interacts with a different G-protein then that which
is recognized by the
native receptor. Accordingly, a different set of signal transduction
components is available as an
end-point assay for activation. Alternatively, the entire transmembrane
portion or subregions (such
as transmembrane segments or intracellular or extracellular loops) can be
replaced with the entire
transmembrane portion or subregions specific to a host cell that is different
from the host cell from
which the amino terminal extracellular domain and/or the G-protein-binding
region are derived.
This allows for assays to be performed in other than the specific host cell
from which the receptor is
derived. Alternatively, the amino terminal extracellular domain (and/or other
ligand-binding
regions) could be replaced by a domain (and/or other binding region) binding a
different ligand,
thus, providing an assay for test compounds that interact with the
heterologous amino terminal
extracellular domain (or region) but still cause signal transduction. Finally,
activation can be
detected by a reporter gene containing an easily detectable coding region
operably linked to a
transcriptional regulatory sequence that is part of the native signal
transduction pathway.
The proteins of the present invention are also useful in competition binding
assays in
methods designed to discover compounds that interact with the receptor. Thus,
a compound is
exposed to a receptor polypeptide under conditions that allow the compound to
bind or to otherwise
interact with the polypeptide (Hodgson, Biotechnology, 1992, Sept 10(9);973-
80). Soluble
receptor polypeptide is also added to the mixture. If the test compound
interacts with the soluble
receptor polypeptide, it decreases the amount of complex formed or activity
from the receptor
target. This type of assay is particularly useful in cases in which compounds
are sought that interact
with specific regions of the receptor. Thus, the soluble polypeptide that
competes with the target
receptor region is designed to contain peptide sequences corresponding to the
region of interest.
To perform cell free drug screening assays, it is sometimes desirable to
immobilize either
the receptor protein, or fragment, or its target molecule to facilitate
separation of complexes from
uncomplexed forms of one or both of the proteins, as well as to accommodate
automation of the
assay.
Techniques for immobilizing proteins on matrices can be used in the drug
screening assays.
In one embodiment, a fusion protein can be provided which adds a domain that
allows the protein to
be bound to a matrix. For example, glutafihione-S-transferase fusion proteins
can be adsorbed onto
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
glutathione sepharose beads (Sigma Chemical, St. Louis, MO) or glutathione
derivatized microtitre
plates, which are then combined with the cell lysates (e.g., 35S-labeled) and
the candidate
compound, and the mixture incubated under conditions conducive to complex
formation (e.g., at
physiological conditions for salt and pIT). Following incubation, the beads
are washed to remove
any unbound label, and the matrix immobilized and radiolabel determined
directly, or in the
supernatant after the complexes are dissociated. Alternatively, the complexes
can be dissociated
from the matrix, separated by SDS-PAGE, and the level of receptor-binding
protein found in the
bead fraction quantitated from the gel using standard electrophoretic
techniques. For example,
either the polypeptide or its target molecule can be immobilized utilizing
conjugation of biotin and
streptavidin using techniques well known in the art. Alternatively, antibodies
reactive with the
protein but which do not interfere with binding of the protein to its target
molecule can be
derivatized to the wells of the plate, and the protein trapped in the wells by
antibody conjugation.
Preparations of a receptor-binding protein and a candidate compound are
incubated in the receptor
protein-presenting wells and the amount of complex trapped in the well can be
quantitated.
Methods for detecting such complexes, in addition to those described above for
the GST-
immobilized complexes, include immunodetection of complexes using antibodies
reactive with the
receptor protein target molecule, or which are reactive with receptor protein
and compete with the
target molecule, as well as enzyme-linked assays which rely on detecting an
enzymatic activity
associated with the target molecule.
Agents that modulate one of the GPCRs of the present invention can be
identified using one
or more of the above assays, alone or in combination. It is generally
preferable to use a cell-based
or cell free system first and then confrm activity in an animal or other model
system. Such model
systems are well known in the art and can readily be employed in this context.
Modulators of receptor protein activity identified according to these drug
screening assays
can be used to treat a subject with a disorder mediated by the receptor
pathway, by treating cells or
tissues that express the GPCR. Experimental data as provided in Figure 1
indicates expression in
humans in neuroectodermal tumors, brain glioblastomas, kidney tumors,
lymphocytes, fetal
liverlspleen, sperm, and leukocytes. These methods of treatment include the
steps of administering
a modulator of the GPCR's activity in a pharmaceutical composition to a
subject in need of such
treatment, the modulator being identified as described herein.
In yet another aspect of the invention, the GPCR proteins can be used as "bait
proteins"
in a two-hybrid assay or three-hybrid assay (see, e.g., IJ.S. Patent No.
5,283,317; Zervos et al.
(1993) Cell 72:223-232; Madura et al. (1993) J. Biol. Chem. 268:12046-12054;
Bartel et al.
26
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
(1993) Biotechniques 14:920-924; Iwabuchi et al. (1993) Or~cogeue 8:1693-1696;
and Brent
W094/10300), to identify other proteins, which bind to or interact with the
GPCR and are
involved in GPCR activity. Such GPCR-binding proteins are also likely to be
involved in the
propagation of signals by the GPCR proteins or GPCR targets as, for example,
downstream
elements of a GPCR-mediated signaling pathway. Alternatively, such GPCR-
binding proteins
are likely to be GPCR inhibitors.
The two-hybrid system is based on the modular nature of most transcription
factors,
which consist of separable DNA-binding and activation domains. Briefly, the
assay utilizes two
different DNA constructs. In one construct, the gene that codes for a GPCR
protein is fused to a
gene encoding the DNA binding domain of a known transcription factor (e.g.,
GAL-4). In the
other construct, a DNA sequence, from a library of DNA sequences, that encodes
an unidentified
protein ("prey" or "sample") is fused to a gene that codes for the activation
domain of the known
transcription factor. If the "bait" and the "prey" proteins are able to
interact, in vivo, forming a
GPCR-dependent complex, the DNA-binding and activation domains of the
transcription factor
are brought into close proximity. This proximity allows transcription of a
reporter gene (e.g.,
LacZ) which is operably linked to a transcriptional regulatory site responsive
to the transcription
factor. Expression of the reporter gene can be detected and cell colonies
containing the
functional transcription factor can be isolated and used to obtain the cloned
gene which encodes
the protein which interacts with the GPCR protein.
This invention further pertains to novel agents identified by the above-
described
screening assays. Accordingly, it is within the scope of this invention to
further use an agent
identified as described herein in an appropriate animal model. For example, an
agent identified
as described herein (e.g., a GPCR modulating agent, an antisense GPCR nucleic
acid molecule, a
GPCR-specific antibody, or a GPCR-binding partner) can be used in an animal or
other model to
determine the efficacy, toxicity, or side effects of treatment with such an
agent. Alternatively, an
' agent identified as described herein can be used in an animal or other model
to determine the
mechanism of action of such an agent. Furthermore, this invention pertains to
uses of novel
agents identified by the above-described screening assays for treatments as
described herein.
The GPCR proteins of the present invention are also useful to provide a target
for
diagnosing a disease or predisposition to disease mediated by the peptide.
Accordingly, the
invention provides methods for detecting the presence, or levels of, the
protein (or encoding
mRNA) in a cell, tissue, or organism. Experimental data as provided in Figure
1 indicates
expression in humans in neuroectodermal tumors, brain glioblastomas, kidney
tumors, lymphocytes,
27
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
fetal liver/spleen, sperm, and leukocytes. The method involves contacting a
biological sample with
a compound capable of interacting with the receptor protein such that the
interaction can be
detected. Such an assay can be provided in a single detection format or a
mufti-detection format
such as an antibody chip array.
One agent for detecting a protein in a sample is an antibody capable of
selectively binding to
protein. A biological sample includes tissues, cells and biological fluids
isolated from a subject, as
well as tissues, cells and fluids present within a subject.
The peptides of the present invention also provide targets for diagnosing
active protein
activity, disease, or predisposition to disease, in a patient having a variant
peptide, particularly
activities and conditions that are known for other members of the family of
proteins to which the
present one belongs. Thus, the peptide can be isolated from a biological
sample and assayed for the
presence of a genetic mutation that results in aberrant peptide. This includes
amino acid
substitution, deletion, insertion, rearrangement, (as the result of aberrant
splicing events), and
inappropriate post-translational modification. Analytic methods include
altered electrophoretic
mobility, altered tryptic peptide digest, altered receptor activity in cell-
based or cell-free assay,
alteration in ligand or antibody-binding pattern, altered isoelectric point,
direct amino acid
sequencing, and any other of the known assay techniques useful for detecting
mutations in a protein.
Such an assay can be provided in a single detection format or a mufti-
detection format such as an
antibody chip array.
In vitro techniques for detection of peptide include enzyme linked
immunosorbent assays
(ELISAs), Western blots, immunoprecipitations and immunofluorescence using a
detection reagent,
such as an antibody or protein binding agent. Alternatively, the peptide can
be detected ih vivo in a
subject by introducing into the subject a labeled anti-peptide antibody or
other types of detection
agent. For example, the antibody can be labeled with a radioactive marker
whose presence and
location in a subject can be detected by standard imaging techniques.
Particularly useful are
methods that detect the allelic variant of a peptide expressed in a subject
and methods which detect
fragments of a peptide in a sample.
The peptides are also useful in pharmacogenomic analysis. Pharmacogenomics
deal with
clinically significant hereditary variations in the response to drugs due to
altered drug disposition
and abnormal action in affected persons. See, e.g., Eichelbaum, M. (Clip. Exp.
Pharmacol. Physiol.
23(10-11):983-985 (1996)), and Linder, M.W. (Clin. Chem. 43(2):254-266
(1997)). The clinical
outcomes of these variations result in severe toxicity of therapeutic drugs in
certain individuals or
therapeutic failure of drugs in certain individuals as a result of individual
variation in metabolism.
28
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Thus, the genotype of the individual can determine the way a therapeutic
compound acts on the
body or the way the body metabolizes the compound. Further, the activity of
drug metabolizing
enzymes effects both the intensity and duration of drug action. Thus, the
pharmacogenomics of the
individual permit the selection of effective compounds and effective dosages
of such compounds for
prophylactic or therapeutic treatment based on the individual's genotype. The
discovery of genetic
polymorphisms in some drug metabolizing enzymes has explained why some
patients do not obtain
the expected drug effects, show an exaggerated drug effect, or experience
serious toxicity from
standard drug dosages. Polymorphisms can be expressed in the phenotype of the
extensive
metabolizer and the phenotype of the poor metabolizer. Accordingly, genetic
polymorphism may
lead to allelic protein variants of the receptor protein in which one or more
of the receptor functions
in one population is different from those in another population. The peptides
thus allow a target to
ascertain a genetic predisposition that can affect treatment modality. Thus,
in a ligand-based
treatment, polymorphism may give rise to amino terminal extracellular domains
and/or other ligand-
binding regions that are more or less active in ligand binding, and receptor
activation. Accordingly,
ligand dosage would necessarily be modified to maximize the therapeutic effect
within a given
population containing a polymorphism. As an alternative to genotyping,
specific polymorphic
peptides could be identified.
The peptides are also useful for treating a disorder characterized by an
absence of,
inappropriate, or unwanted expression of the protein. Experimental data as
provided in Figure 1
indicates expression in humans in neuroectodermal tumors, brain glioblastomas,
kidney tumors,
lymphocytes, fetal liver/spleen, sperm, and leukocytes. Accordingly, methods
for treatment include
the use of the GPCR protein or fragments.
Antibodies
The invention also provides antibodies that selectively bind to one of the
peptides of the
present invention, a protein comprising such a peptide, as well as variants
and fragments thereof.
As used herein, an antibody selectively binds,a target peptide when it binds
the target peptide and
does not significantly bind to unrelated proteins. An antibody is still
considered to selectively bind
a peptide even if it also binds to other proteins that are not substantially
homologous with the target
peptide so long as such proteins share homology with a fragment or domain of
the peptide target of
the antibody. In this case, it would be understood that antibody binding to
the peptide is still
selective despite some degree of cross-reactivity.
29
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
As used herein, an antibody is defined in terms consistent with that
recognized within the
art: they are multi-subunit proteins produced by a mammalian organism in
response to an antigen
challenge. The antibodies of the present invention include polyclonal
antibodies and monoclonal
antibodies, as well as fragments of such antibodies, including, but not
limited to, Fab or F(ab')2, and
Fv fragments.
Many methods are known for generating andlor identifying antibodies to a given
target
peptide. Several such methods are described by Harlow, Antibodies, Cold Spring
Harbor Press,
(1989).
In general, to generate antibodies, an isolated peptide is used as an
immunogen and is
administered to a mammalian organism, such as a rat, rabbit or mouse. The full-
length protein, an
antigenic peptide fragment or a fusion protein can be used. Particularly
important fragments are
those covering functional domains, such as the domains identified in Figure 2,
and domain of
sequence homology or divergence amongst the family, such as those that can
readily be identified
using protein alignment methods and as presented in the Figures.
Antibodies are preferably prepared from regions or discrete fragments of the
GPCR
proteins. Antibodies can be prepared from any region of the peptide as
described herein.
However, preferred regions will include those involved in function/activity
and/or
receptorlbinding partner interaction. Figure 2 can be used to identify
particularly important
regions while sequence alignment can be used to identify conserved and unique
sequence
fragments.
An antigenic fragment will typically comprise at Least 8 contiguous amino acid
residues.
The antigenic peptide can comprise, however, at least 10, 12, 14, 16 or more
amino acid residues.
Such fragments can be selected on a physical property, such as fragments
correspond to regions that
are Located on the surface of the protein, e.g., hydrophilic regions or can be
selected based on
sequence uniqueness (see Figure 2).
Detection on an antibody of the present invention can be facilitated by
coupling (i.e.,
physically linking) the antibody to a detectable substance. Examples of
detectable substances
include various enzymes, prosthetic groups, fluorescent materials, luminescent
materials,
bioluminescent materials, and radioactive materials. Examples of suitable
enzymes include
horseradish peroxidase, alkaline phosphatase, (3-galactosidase, or
acetylcholinesterase; examples of
suitable prosthetic group complexes include streptavidin/biotin and
avidin/biotin; examples of
suitable fluorescent materials include umbelliferone, fluorescein, fluorescein
isothiocyanate,
rhodamine, dichlorotriazinylamine fluorescein, dansyl chloride or
phycoerythrin; an example of a
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
luminescent material includes luminol; examples of bioluminescent materials
include luciferase,
luciferin, and aequorin, and examples of suitable radioactive material include
l2sl,131h ass or 3H.
Antibody Uses
The antibodies can be used to isolate one of the proteins of the present
invention by standard
techniques, such as amity chromatography or immunoprecipitation. The
antibodies can facilitate
the purification of the natural protein from cells and recombinantly produced
protein expressed in
host cells. In addition, such antibodies are useful to detect the presence of
one of the proteins of the
present invention in cells or tissues to deternline the pattern of expression
of the protein among
various tissues in an organism and over the course of normal development.
Experimental data as
provided in Figure 1 indicates that GPCR proteins of the present invention are
expressed in humans
in neuroectodermal tumors, brain glioblastomas, kidney tumors, lymphocytes,
fetal liver/spleen
tissue, and sperm, as indicated by virtual northern blot analysis. In
addition, PCR-based tissue
screening panels indicate expression in human leukocytes. Further, such
antibodies can be used to
detect protein in situ, ih vitro, or in a cell lysate or supernatant in order
to evaluate the abundance
and pattern of expression. Also, such antibodies can be used to assess
abnormal tissue distribution
or abnormal expression during development or progression of a biological
condition. Antibody
detection of circulating fragments of the full length protein can be used to
identify turnover.
Further, the antibodies can be used to assess expression in disease states
such as in active
stages of the disease or in an individual with a predisposition toward disease
related to the protein's
function. When a disorder is caused by an inappropriate tissue distribution,
developmental
expression, level of expression of the protein, or expressed/processed form,
the antibody can be
prepared against the normal protein. Experimental data as provided in Figure 1
indicates expression
in humans in neuroectodermal tumors, brain glioblastomas, kidney tumors,
lymphocytes, fetal
liver/spleen, sperm, and leukocytes. If a disorder is characterized by a
specific mutation in the
protein, antibodies specific for this mutant protein can be used to assay for
the presence of the
specific mutant protein.
The antibodies can also be used to assess normal and aberrant subcellular
localization of
cells in the various tissues in an organism. Experimental data as provided in
Figure 1 indicates
expression in humans in neuroectodermal tumors, brain glioblastomas, kidney
tumors, lymphocytes,
fetal liver/spleen, sperm, and leukocytes. The diagnostic uses can be applied,
not only in genetic
testing, but also in monitoring a treatment modality. Accordingly, where
treatment is ultimately
31
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
aimed at correcting expression level or the presence of aberrant sequence and
aberrant tissue
distribution or developmental expression, antibodies directed against the
protein or relevant
fragments can be used to monitor therapeutic efficacy.
Additionally, antibodies are useful in pharmacogenomic analysis. Thus,
antibodies prepared
against polymorphic proteins can be used to identify individuals that require
modified treatment
modalities. The antibodies are also useful as diagnostic tools as an
immunological marker for
aberrant protein analyzed by electrophoretic mobility, isoelectric point,
tryptic peptide digest, and
other physical assays known to those in the art.
The antibodies are also useful for tissue typing. Experimental data as
provided in Figure 1
indicates expression in humans in neuroectodermal tumors, brain glioblastomas,
kidney tumors,
lymphocytes, fetal liver/spleen, sperm, and leukocytes. Thus, where a specific
protein has been
correlated with expression in a specific tissue, antibodies that are specific
for this protein can be
used to identify a tissue type.
The antibodies are also useful for inhibiting protein function, for example,
blocking the
binding of the GPCR peptide to a binding partner such as a ligand. These uses
can also be applied
in a therapeutic context in which treatment involves inhibiting the protein's
function. An antibody
can be used, for example, to block binding, thus modulating (agonizing or
antagonizing) the
peptides activity. Antibodies can be prepared against specific fragments
containing sites required
for function or against intact protein that is associated with a cell or cell
membrane. See Figure 2 for
structural information relating to the proteins of the present invention.
The invention also encompasses kits for using antibodies to detect the
presence of a protein
in a biological sample. The kit can comprise antibodies such as a labeled or
labelable antibody and
a compound or agent for detecting protein in a biological sample; means for
determining the amount
of protein in the sample; means for comparing the amount of protein in the
sample with a standard;
and instructions for use. Such a kit can be supplied to detect a single
protein or epitope or can be
configured to detect one of a multitude of epitopes, such as in an antibody
detection array. Arrays
are described in detail below for nucleic acid arrays and similar methods have
been developed for
antibody arrays.
Nucleic Acid Molecules
The present invention further provides isolated nucleic acid molecules that
encode a GPCR
peptide or protein of the present invention (cDNA, transcript and genomic
sequence). Such nucleic
32
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
acid molecules will consist of, consist essentially of, or comprise a
nucleotide sequence that encodes
one of the GPCR peptides of the present invention, an allelic variant thereof,
or an ortholog or
paralog thereof.
As used herein, an "isolated" nucleic acid molecule is one that is separated
from other
nucleic acid present in the natural source of the nucleic acid. Preferably, an
"isolated" nucleic acid
is free of sequences which naturally flank the nucleic acid (i.e., sequences
located at the 5' and 3'
ends of the nucleic acid) in the genomic DNA of the organism from which the
nucleic acid is
derived. However, there can be some flanking nucleotide sequences, for example
up to about SKB,
4KB, 3KB, 2KB, or lI~B or less, particularly contiguous peptide encoding
sequences and peptide
encoding sequences within the same gene but separated by introns in the
genomic sequence. The
important point is that the nucleic acid is isolated from remote and
unimportant flanking sequences
such that it can be subjected to the specific manipulations described herein
such as recombinant
expression, preparation of probes and primers, and other uses specific to the
nucleic acid sequences.
Moreover, an "isolated" nucleic acid molecule, such as a transcript/cDNA
molecule, can be
substantially free of other cellular material, or culture medium when produced
by recombinant
techniques, or chemical precursors or other chemicals when chemically
synthesized. However, the
nucleic acid molecule can be fused to other coding or regulatory sequences and
still be considered
isolated.
For example, recombinant DNA molecules contained in a vector are considered
isolated.
Further examples of isolated DNA molecules include recombinant DNA molecules
maintained in
heterologous host cells or purified (partially or substantially) DNA molecules
in solution. Isolated
RNA molecules include in vivo or in vitro RNA transcripts of the isolated DNA
molecules of the
present invention. Isolated nucleic acid molecules according to the present
invention further include
such molecules produced synthetically.
Accordingly, the present invention provides nucleic acid molecules that
consist of the
nucleotide sequence shown in Figure 1 or 3 (SEQ ID NO:l, transcript sequence
and SEQ ID N0:3,
genomic sequence), or any nucleic acid molecule that encodes the protein
provided in Figure 2,
SEQ ID N0:2. A nucleic acid molecule consists of a nucleotide sequence when
the nucleotide
sequence is the complete nucleotide sequence of the nucleic acid molecule.
The present invention further provides nucleic acid molecules that consist
essentially of the
nucleotide sequence shown in Figure 1 or 3 (SEQ ID NO:1, transcript sequence
and SEQ ID N0:3,
genomic sequence), or any nucleic acid molecule that encodes the protein
provided in Figure 2,
SEQ ID N0:2. A nucleic acid molecule consists essentially of a nucleotide
sequence when such a
33
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
nucleotide sequence is present with only a few additional nucleic acid
residues in the final nucleic
acid molecule.
The present invention further provides nucleic acid molecules that comprise
the nucleotide
sequences shown in Figure 1 or 3 (SEQ ID NO:1, transcript sequence and SEQ ID
N0:3, genomic
sequence), or any nucleic acid molecule that encodes the protein provided in
Figure 2, SEQ ID
N0:2. A nucleic acid molecule comprises a nucleotide sequence when the
nucleotide sequence is at
least part of the final nucleotide sequence of the nucleic acid molecule. In
such a fashion, the
nucleic acid molecule can be only the nucleotide sequence or have additional
nucleic acid residues,
such as nucleic acid residues that are naturally associated with it or
heterologous nucleotide
sequences. Such a nucleic acid molecule can have a few additional nucleotides
or can comprises
several hundred or more additional nucleotides. A brief description of how
various types of these
nucleic acid molecules can be readily made/isolated is provided below.
In Figures 1 and 3, both coding and non-coding sequences are provided. Because
of the
source of the present invention, human genomic sequences (Figure 3) and
cDNA/transcript
sequences (Figure 1), the nucleic acid molecules in the Figures will contain
genomic intronic
sequences, 5' and 3' non-coding sequences, gene regulatory regions and non-
coding intergenic
sequences. In general such sequence features are either noted in Figures l and
3 or can readily
be identified using computational tools known in the art. As discussed below,
some of the non-
coding regions, particularly gene regulatory elements such as promoters, are
useful for a variety
of purposes, e.g. control of heterologous gene expression, target for
identifying gene activity
modulating compounds, and are particularly claimed as fragments of the genomic
sequence
provided herein.
The isolated nucleic acid molecules can encode the mature protein plus
additional amino or
carboxyl-terminal amino acids, or amino acids interior to the mature peptide
(when the mature form
has more than one peptide chain, for instance). Such sequences may play a role
in processing of a
protein from precursor to a mature form, facilitate protein trafficking,
prolong or shorten protein
half life or facilitate manipulation of a protein for assay or production,
among other things. As
generally is the case i~ situ, the additional amino acids may be processed
away from the mature
protein by cellular enzymes.
As mentioned above, the isolated nucleic acid molecules include, but are not
limited to, the
sequence encoding the GPCR peptide alone, the sequence encoding the mature
peptide and
additional coding sequences, such as a leader or secretory sequence (e.g., a
pre-pro or pro-protein
sequence), the sequence encoding the mature peptide, with or without the
additional coding
34
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
sequences, plus additional non-coding sequences, for example introns and non-
coding 5' and 3'
sequences such as transcribed but non-translated sequences that play a role in
transcription, mRNA
processing (including splicing and polyadenylation signals), ribosome binding
and stability of
mRNA. In addition, the nucleic acid molecule may be fused to a marker sequence
encoding, for
example, a peptide that facilitates purification.
Isolated nucleic acid molecules can be in the form of RNA, such as mRNA, or in
the form
DNA, including cDNA and genomic DNA obtained by cloning or produced by
chemical synthetic
techniques or by a combination thereof. The nucleic acid, especially DNA, can
be double-stranded
or single-stranded. Single-stranded nucleic acid can be the coding strand
(sense strand) or the non
coding strand (anti-sense strand).
The invention further provides nucleic acid molecules that encode fragments of
the peptides
of the present invention as well as nucleic acid molecules that encode obvious
variants of the GPCR
proteins of the present invention that are described above. Such nucleic acid
molecules may be
naturally occurring, such as allelic variants (same locus), paralogs
(different locus), and orthologs
(different organism), or may be constructed by recombinant DNA methods or by
chemical
synthesis. Such non-naturally occurring variants may be made by mutagenesis
techniques,
including those applied to nucleic acid molecules, cells, or organisms.
Accordingly, as discussed
above, the variants can contain nucleotide substitutions, deletions,
inversions and insertions.
Variation can occur in either or both the coding and non-coding regions. The
variations can
produce both conservative and non-conservative amino acid substitutions.
The present invention further provides non-coding fragments of the nucleic
acid molecules
provided in Figures 1 and 3. Preferred non-coding fragments include, but are
not limited to,
promoter sequences, enhancer sequences, gene modulating sequences and gene
termination
sequences. Such fragments are useful in controlling heterologous gene
expression and in
developing screens to identify gene-modulating agents. A promoter can readily
be identified as
being 5' to the ATG start site in the genomic sequence provided in Figure 3.
A fragment comprises a contiguous nucleotide sequence greater than 12 or more
nucleotides. Further, a fragment could at least 30, 40, 50, 100, 250 or 500
nucleotides in length.
The length of the fragment will be based on its intended use. For example, the
fragment can encode
epitope bearing regions of the peptide, or can be useful as DNA probes and
primers. Such
fragments can be isolated using the known nucleotide sequence to synthesize an
oligonucleotide
probe. A labeled probe can then be used to screen a cDNA library, genomic DNA
library, or
mRNA to isolate nucleic acid corresponding to the coding region. Further,
primers can be used in
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
PCR reactions to clone specific regions of gene.
A probe/primer typically comprises substantially a purified oligonucleotide or
oligonucleotide pair. The oligonucleotide typically comprises a region of
nucleotide sequence that
hybridizes under stringent conditions to at least about 12, 20, 25, 40, 50 or
more consecutive
nucleotides.
Orthologs, homologs, and allelic variants can be identified using methods well
known in the
art. As described in the Peptide Section, these variants comprise a nucleotide
sequence encoding a
peptide that is typically 60-70%, 70-80%, 80-90%, and more typically at least
about 90-95% or
more homologous to the nucleotide sequence shown in the Figure sheets or a
fragment of this
sequence. Such nucleic acid molecules can readily be identified as being able
to hybridize under
moderate to stringent conditions, to the nucleotide sequence shown in the
Figure sheets or a
fragment of the sequence. Allelic variants can readily be determined by
genetic locus of the
encoding gene. The gene encoding the novel transporter protein of the present
invention is located
on a genome component that has been mapped to human chromosome 19 (as
indicated in Figure 3),
which is supported by multiple lines of evidence, such as STS and BAC map
data.
As used herein, the term "hybridizes under stringent conditions" is intended
to describe
conditions for hybridization and washing under which nucleotide sequences
encoding a peptide at
least 60-70% homologous to each other typically remain hybridized to each
other. The conditions
can be such that sequences at least about 60%, at least about 70%, or at least
about 80% or more
homologous to each other typically remain hybridized to each other. Such
stringent conditions are
known to those skilled in the art and can be found in Cut~rent Protocols in
Molecular Biology, John
Wiley & Sons, N.Y. (1989), 6.3.1-6.3.6. One example of stringent hybridization
conditions are
hybridization in 6X sodium chloride/sodium citrate (SSC) at about 45C,
followed by one or more
washes in 0.2 X SSC, 0.1% SDS at 50-65C. Examples of moderate to low
stringency hybridization
conditions are well known in the art.
Nucleic Acid Molecule Uses
The nucleic acid molecules of the present invention are useful for probes,
primers, chemical
intermediates, and in biological assays. The nucleic acid molecules are useful
as a hybridization
probe for messenger RNA, transcript/cDNA and genomic DNA to isolate full-
length cDNA and
genomic clones encoding the peptide described in Figure 2 and to isolate cDNA
and genomic
36
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
clones that correspond to variants (alleles, orthologs, etc.) producing the
same or related peptides
shown in Figure 2.
The probe can correspond to any sequence along the entire length of the
nucleic acid
molecules provided in the Figures. Accordingly, it could be derived from 5'
noncoding regions, the
coding region, and 3' noncoding regions. However, as discussed, fragments are
not to be construed
as encompassing fragments disclosed prior to the present invention.
The nucleic acid molecules are also useful as primers for PCR to amplify any
given region
of a nucleic acid molecule and are useful to synthesize antisense molecules of
desired length and
sequence.
The nucleic acid molecules are also useful for constructing recombinant
vectors. Such
vectors include expression vectors that express a portion of, or all of, the
peptide sequences.
Vectors also include insertion vectors, used to integrate into another nucleic
acid molecule
sequence, such as into the cellular genome, to alter ih situ expression of a
gene and/or gene product.
For example, an endogenous coding sequence can be replaced via homologous
recombination with
all or part of the coding region containing one or moxe specifically
introduced mutations.
The nucleic acid molecules are also useful for expressing antigenic portions
of the proteins.
The nucleic acid molecules are also useful as probes for determining the
chromosomal
positions of the nucleic acid molecules by means of in situ hybridization
methods. The gene
encoding the novel transporter protein of the present invention is located on
a genome component
that has been mapped to human chromosome 19 (as indicated in Figure 3), which
is supported by
multiple lines of evidence, such as STS and BAC map data.
The nucleic acid molecules are also useful in making vectors containing the
gene regulatory
regions of the nucleic acid molecules of the present invention.
The nucleic acid molecules are also useful for designing ribozymes
corresponding to all, or
a part, of the mRNA produced from the nucleic acid molecules described herein.
The nucleic acid molecules are also useful for making vectors that express
part, or all, of the
peptides.
The nucleic acid molecules are also useful fox constructing host cells
expressing a part, or
all, of the nucleic acid molecules and peptides.
The nucleic acid molecules are also useful for constructing transgenic animals
expressing
all, or a part, of the nucleic acid molecules and peptides.
The nucleic acid molecules are also useful as hybridization probes for
determining the
presence, level, form and distribution of nucleic acid expression.
Experimental data as provided in
37
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Figure 1 indicates that GPCR proteins of the present invention are expressed
in humans in
neuroectodermal tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal
liver/spleen tissue,
and sperm, as indicated by virtual northern blot analysis. In addition, PCR-
based tissue screening
panels indicate expression in human leukocytes. Accordingly, the probes can be
used to detect the
presence of, or to determine levels of, a specific nucleic acid molecule in
cells, tissues, and in
organisms. The nucleic acid whose level is determined can be DNA or RNA.
Accordingly, probes
corresponding to the peptides described herein can be used to assess
expression and/or gene copy
number in a given cell, tissue, or organism. These uses are relevant for
diagnosis of disorders
involving an increase or decrease in GPCR protein expression relative to
normal results.
Ih vitro techniques for detection of mRNA include Northern hybridizations and
iu situ
hybridizations. In vitro techniques for detecting DNA includes Southern
hybridizations and ih situ
hybridization.
Probes can be used as a part of a diagnostic test kit for identifying cells or
tissues that
express a GPCR protein, such as by measuring a level of a receptor-encoding
nucleic acid in a
sample of cells from a subject e.g., mRNA or genomic DNA, or determining if a
receptor gene has
been mutated. Experimental data as provided in Figure 1 indicates that GPCR
proteins of the
present invention are expressed in humans in neuroectodermal tumors, brain
glioblastomas, kidney
tumors, lymphocytes, fetal liver/spleen tissue, and sperm, as indicated by
virtual northern blot
analysis. In addition, PCR-based tissue screening panels indicate expression
in human leukocytes.
Nucleic acid expression assays are useful for drug screening to identify
compounds that
modulate GPCR nucleic acid expression.
The invention thus provides a method for identifying a compound that can be
used to treat a
disorder associated with nucleic acid expression of the GPCR gene,
particularly biological and
pathological processes that are mediated by the GPCR in cells and tissues that
express it.
Experimental data as provided in Figure 1 indicates expression in humans in
neuroectodermal
tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal liverlspleen,
sperm, and leukocytes.
The method typically includes assaying the ability of the compound to modulate
the expression of
the GPCR nucleic acid and thus identifying a compound that can be used to
treat a disorder
characterized by undesired GPCR nucleic acid expression. The assays can be
performed in cell-
based and cell-free systems. Cell-based assays include cells naturally
expressing the GPCR nucleic
acid or recombinant cells genetically engineered to express specific nucleic
acid sequences.
The assay for GPCR nucleic acid expression can involve direct assay of nucleic
acid levels,
such as mRNA levels, or on collateral compounds involved in the signal
pathway. Further, the
38
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
expression of genes that are up- or down-regulated in response to the GPCR
protein signal pathway
can also be assayed. In this embodiment the regulatory regions of these genes
can be operably
linked to a reporter gene such as luciferase.
Thus, modulators of GPCR gene expression can be identified in a method wherein
a cell is
contacted with a candidate compound and the expression of mRNA determined. The
level of
expression of GPCR mRNA in the presence of the candidate compound is compared
to the level of
expression of GPCR mRNA in the absence of the candidate compound. The
candidate compound
can then be identified as a modulator of nucleic acid expression based on this
comparison and be
used, for example to treat a disorder chaxacterized by aberrant nucleic acid
expression. When
expression of mRNA is statistically significantly greater in the presence of
the candidate compound
than in its absence, the candidate compound is identified as a stimulator of
nucleic acid expression.
When nucleic acid expression is statistically significantly less in the
presence of the candidate
compound than in its absence, the candidate compound is identified as an
inhibitor of nucleic acid
expression.
The invention fiu-kher provides methods of treatment, with the nucleic acid as
a taxget, using
a compound identified through drug screening as a gene modulator to modulate
GPCR nucleic acid
expression, particularly to modulate activities within a cell or tissue that
expresses the proteins.
Experimental data as provided in Figure 1 indicates that GPCR proteins of the
present invention are
expressed in humans in neuroectodermal tumors, brain glioblastomas, kidney
tumors, lymphocytes,
fetal liver/spleen tissue, and sperm, as indicated by virtual northern blot
analysis. In addition, PCR-
based tissue screening panels indicate expression in human leukocytes.
Modulation includes both
up-regulation (i.e. activation or agonization) or down-regulation (suppression
or antagonization) or
nucleic acid expression.
Alternatively, a modulator for GPCR nucleic acid expression can be a small
molecule or
drug identified using the screening assays described herein as long as the
drug or small molecule
inhibits the GPCR nucleic acid expression in the cells and tissues that
express the protein.
Experimental data as provided in Figure 1 indicates expression in humans in
neuroectodermal
tumors, brain glioblastomas, kidney tumors, lymphocytes, fetal liverlspleen,
sperm, and leukocytes.
The nucleic acid molecules are also useful for monitoring the effectiveness of
modulating
compounds on the expression or activity of the GPCR gene in clinical trials or
in a treatment
regimen. Thus, the gene expression pattern can serve as a barometer for the
continuing
effectiveness of treatment with the compound, particularly with compounds to
which a patient can
develop resistance. The gene expression pattern can also serve as a marker
indicative of a
39
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
physiological response of the afFected cells to the compound. Accordingly,
such monitoring would
allow either increased administration of the compound or the administration of
alternative
compounds to which the patient has not become resistant. Similarly, if the
level of nucleic acid
expression falls below a desirable level, administration of the compound could
be commensurately
decreased.
The nucleic acid molecules are also useful in diagnostic assays for
qualitative changes in
GPCR nucleic acid, and particularly in qualitative changes that lead to
pathology. The nucleic acid
molecules can be used to detect mutations in GPCR genes and gene expression
products such as
mRNA. The nucleic acid molecules can be used as hybridization probes to detect
naturally-
occurring genetic mutations in the GPCR gene and thereby to determine whether
a subject with the
mutation is at risk for a disorder caused by the mutation. Mutations include
deletion, addition, or
substitution of one or more nucleotides in the gene, chromosomal
rearrangement, such as inversion
or transposition, modification of genomic DNA, such as aberrant methylation
patterns or changes in
gene copy number, such as amplification. Detection of a mutated form of the
GPCR gene
associated with a dysfunction provides a diagnostic tool for an active disease
or susceptibility to
disease when the disease results from overexpression, underexpression, or
altered expression of a
GPCR protein.
Individuals carrying mutations in the GPCR gene can be detected at the nucleic
acid level by
a variety of techniques. The gene encoding the novel transporter protein of
the present invention is
located on a genome component that has been mapped to human chromosome 19 (as
indicated in
Figure 3), which is supported by multiple lines of evidence, such as STS and
BAC map data.
Genomic DNA can be analyzed directly or can be amplified by using PCR prior to
analysis. RNA
or cDNA can be used in the same way. In some uses, detection of the mutation
involves the use of
a probe/primer in a polymerase chain reaction (PCR) (see, e.g. U.S. Patent
Nos. 4,683,195 and
4,683,202), such as anchor PCR or RACE PCR, or, alternatively, in a ligation
chain reaction (LCR)
(see, e.g., Landegran et al., Science 241:1077-1080 (1988); and Nakazawa et
al., PNAS 91:360-364
(1994)), the latter of which can be particularly useful for detecting point
mutations in the gene (see
Abravaya et al., Nucleic Acids Res. 23:675-682 (1995)). This method can
include the steps of
collecting a sample of cells from a patient, isolating nucleic acid (e.g.,
genomic, mRNA or both)
from the cells of the sample, contacting the nucleic acid sample with one or
more primers which
specifically hybridize to a gene under conditions such that hybridization and
amplification of the
gene (if present) occurs, and detecting the presence or absence of an
amplification product, or
detecting the size of the amplification product and comparing the length to a
control sample.
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Deletions and insertions can be detected by a change in size of the amplified
product compared to
the normal genotype. Point mutations can be identified by hybridizing
amplified DNA to normal
RNA or antisense DNA sequences.
Alternatively, mutations in a GPCR gene can be directly identified, for
example, by
alterations in restriction enzyme digestion patterns determined by gel
electrophoresis.
Further, sequence-specific ribozymes (LJ.S. Patent No. 5,498,531) can be used
to score for
the presence of specific mutations by development or loss of a ribozyme
cleavage site. Perfectly
matched sequences can be distinguished from mismatched sequences by nuclease
cleavage
digestion assays or by differences in melting temperature.
Sequence changes at specific locations can also be assessed by nuclease
protection assays
such as RNase and S1 protection or the chemical cleavage method. Furthermore,
sequence
differences between a mutant GPCR gene and a wild-type gene can be determined
by direct DNA
sequencing. A variety of automated sequencing procedures can be utilized when
performing the
diagnostic assays (Naeve, C.W., (1995) Biotechniques 19:448), including
sequencing by mass
spectrometry (see, e.g., PCT International Publication No. WO 94/16101; Cohen
et al., Adv.
Chromatogr. 36:127-162 (1996); and GrifFm et al., Appl. Biochem. Biotechnol.
38:147-159 (1993)).
Other methods for detecting mutations in the gene include methods in which
protection
from cleavage agents is used to detect mismatched bases in RNA/RNA or RNAIDNA
duplexes
(Myers et al., Scienee 230:1242 (1985)); Cotton et al., PNAS 85:4397 (1988);
Saleeba et al., Meth.
Ev~~ymol. 217:286-295 (1992)), electrophoretic mobility of mutant and wild
type nucleic acid is
compared (Orita et al., PNAS 86:2766 (1989); Cotton et al., Mutat. Res.
285:125-144 (1993); and
Hayashi et al., Genet. Anal. Tech. Appl. 9:73-79 (1992)), and movement of
mutant or wild-type
fragments in polyacrylamide gels containing a gradient of denaturant is
assayed using denaturing
gradient gel electrophoresis (Myers et al., Nature 313:495 (1985)). Examples
of other techniques
for detecting point mutations include selective oligonucleotide hybridization,
selective
amplification, and selective primer extension.
The nucleic acid molecules are also useful for testing an individual for a
genotype that while
not necessarily causing the disease, nevertheless affects the treatment
modality. Thus, the nucleic
acid molecules can be used to study the relationship between an individual's
genotype and the
individual's response to a compound used for treatment (pharmacogenomic
relationship).
Accordingly, the nucleic acid molecules described herein can be used to assess
the mutation content
of the GPCR gene in an individual in order to select an appropriate compound
or dosage regimen
for treatment.
41
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Thus nucleic acid molecules displaying genetic variations that affect
treatment provide a
diagnostic target that can be used to tailor treatment in an individual.
Accordingly, the production
of recombinant cells and animals containing these polymorphisms allow
effective clinical design of
treatment compounds and dosage regimens.
The nucleic acid molecules are thus useful as antisense constructs to control
GPCR gene
expression in cells, tissues, and organisms. A DNA antisense nucleic acid
molecule is designed to
be complementary to a region of the gene involved in transcription, preventing
transcription and
hence production of GPCR protein. An antisense RNA or DNA nucleic acid
molecule would
hybridize to the mRNA and thus block translation of mRNA into GPCR protein.
Alternatively, a class of antisense molecules can be used to inactivate mRNA
in order to
decrease expression of GPCR nucleic acid. Accordingly, these molecules can
treat a disorder
characterized by abnormal or undesired GPCR nucleic acid expression. This
technique involves
cleavage by means of ribozymes containing nucleotide sequences complementary
to one or more
regions in the mRNA that attenuate the ability of the mRNA to be translated.
Possible regions
include coding regions and particularly coding regions corresponding to the
catalytic and other
functional activities of the GPCR protein, such as ligand binding.
The nucleic acid molecules also provide vectors for gene therapy in patients
containing cells
that are aberrant in GPCR gene expression. Thus, recombinant cells, which
include the patient's
cells that have been engineered ex vivo and returned to the patient, are
introduced into an individual
where the cells produce the desired GPCR protein to treat the individual.
The invention also encompasses kits for detecting the presence of a GPCR
nucleic acid in a
biological sample. Experimental data as provided in Figure 1 indicates that
GPCR proteins of the
present invention are expressed in humans in neuroectodermal tumors, brain
glioblastomas, kidney
tumors, lymphocytes, fetal liver/spleen tissue, and sperm, as indicated by
virtual northern blot
analysis. In addition, PCR-based tissue screening panels indicate expression
in human leukocytes.
For example, the kit can comprise reagents such as a labeled or labelable
nucleic acid or agent
capable of detecting GPCR nucleic acid in a biological sample; means for
determining the amount
of GPCR nucleic acid in the sample; and means for comparing the amount of GPCR
nucleic acid in
the sample with a standard. The compound or agent can be packaged in a
suitable container. The
kit can further comprise instructions for using the kit to detect GPCR protein
mRNA or DNA.
42
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Nucleic Acid Arrays
The present invention fixrrher provides nucleic acid detection kits, such as
arrays or
microarrays of nucleic acid molecules that are based on the sequence
information provided in
Figures 1 and 3 (SEQ ID NOS:1 and 3).
As used herein "Arrays" or "Microarrays" refers to an array of distinct
polynucleotides or
oligonucleotides synthesized on a substrate, such as paper, nylon or other
type of membrane,
filter, chip, glass slide, or any other suitable solid support. In one
embodiment, the microarray is
prepared and used according to the methods described in US Patent 5,837,832,
Chee et al., PCT
application W095/11995 (Chee et al.), Lockhart, D. J, et al. (1996; Nat.
Biotech. 14: 1675-1680)
and Schena, M. et al. (1996; Proc. Natl. Acad. Sci. 93: 10614-10619), all of
which are
incorporated herein in their entirety by reference. In other embodiments, such
arrays are
produced by the methods described by Brown et. al., US Patent No. 5,807,522.
The microarray or detection kit is preferably composed of a large number of
unique,
single-stranded nucleic acid sequences, usually either synthetic antisense
oligonucleotides or
fragments of cDNAs, fixed to a solid support. The oligonucleotides are
preferably about 6-60
nucleotides in length, more preferably 15-30 nucleotides in length, and most
preferably about 20-
nucleotides in length. For a certain type of microarray or detection kit, it
may be preferable to
use oligonucleotides that are only 7-20 nucleotides in length. The microarray
or detection kit
may contain oligonucleotides that cover the known 5', or 3', sequence,
sequential
20 oligonucleotides which cover the full length sequence; or unique
oligonucleotides selected from
particular areas along the length of the sequence. Polynucleotides used in the
microarray or
detection kit may be oligonucleotides that are specific to a gene or genes of
interest.
In order to produce oligonucleotides to a known sequence for a microarray or
detection
kit, the genes) of interest (or an ORF identified from the contigs of the
present invention) is
25 typically examined using a computer algorithm which starts at the 5' or at
the 3' end of the
nucleotide sequence. Typical algorithms will then identify oligomers of
defined length that are
unique to the gene, have a GC content within a range suitable for
hybridization, and lack
predicted secondary structure that may interfere with hybridization. In
certain situations it may
be appropriate to use pairs of oligonucleotides on a microarray or detection
kit. The "pairs" will
be identical, except for one nucleotide that preferably is located in the
center of the sequence.
The second oligonucleotide in the pair (mismatched by one) serves as a
control. The number of
oligonucleotide pairs may range from two to one million. The oligomers are
synthesized at
designated areas on a substrate using a light-directed chemical process. The
substrate may be
43
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
paper, nylon or other type of membrane, filter, chip, glass slide or any other
suitable solid
support.
In another aspect, an oligonucleotide may be synthesized on the surface of the
substrate
by using a chemical coupling procedure and an ink jet application apparatus,
as described in PCT
application W095/251116 (Baldeschweiler et al.) which is incorporated herein
in its entirety by
reference. In another aspect, a "gridded" array analogous to a dot (or slot)
blot may be used to
arrange and link cDNA fragments or oligonucleotides to the surface of a
substrate using a
vacuum system, thermal, UV, mechanical or chemical bonding procedures. An
array, such as
those described above, may be produced by hand or by using available devices
(slot blot or dot
blot apparatus), materials (any suitable solid support), and machines
(including robotic
instruments), and may contain 8, 24, 96, 384, 1536, 6144 or more
oligonucleotides, or any other
number between two and one million which lends itself to the efficient use of
commercially
available instrumentation.
In order to conduct sample analysis using a microarray or detection kit, the
RNA or DNA
from a biological sample is made into hybridization probes. The mRNA is
isolated, and cDNA is
produced and used as a template to make antisense RNA (aRNA). The aRNA is
amplified in the
presence of fluorescent nucleotides, and labeled probes are incubated with the
microarray or
detection kit so that the probe sequences hybridize to complementary
oligonucleotides of the
microarray or detection kit. Incubation conditions are adjusted so that
hybridization occurs with
precise complementary matches or with various degrees of less complementarity.
After removal
of nonhybridized probes, a scanner is used to determine the levels and
patterns of fluorescence.
The scanned images are examined to determine degree of complementarity and the
relative
abundance of each oligonucleotide sequence on the microarray or detection kit.
The biological
samples may be obtained from any bodily fluids (such as blood, urine, saliva,
phlegm, gastric
juices, etc.), cultured cells, biopsies, or other tissue preparations. A
detection system may be
used to measure the absence, presence, and amount of hybridization for all of
the distinct
sequences simultaneously. This data may be used for large scale correlation
studies on the
sequences, expression patterns, mutations, variants, or polymorphisms among
samples.
Using such arrays, the present invention provides methods to identify the
expression of
the GPCR proteins/peptides of the present invention. In detail, such methods
comprise
incubating a test sample with one or more nucleic acid molecules and assaying
for binding of the
nucleic acid molecule with components within the test sample. Such assays will
typically
involve arrays comprising many genes, at least one of which is a gene of the
present invention
44
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
and or alleles of the GPCR gene of the present invention.
Conditions for incubating a nucleic acid molecule with a test sample vary.
Incubation
conditions depend on the format employed in the assay, the detection methods
employed, and the
type and nature of the nucleic acid molecule used in the assay. One skilled in
the art will
recognize that any one of the commonly available hybridization, amplification
or array assay
formats can readily be adapted to employ the novel fragments of the Human
genome disclosed
herein. Examples of such assays can be found in Chard, T, An Introduction to
Radioimmunoassay and Related Techniques, Elsevier Science Publishers,
Amsterdam, The
Netherlands (1986); Bullock, G. R. et al., Techniques in Immunocytochemistry,
Academic
Press, Orlando, FL Vol. 1 (1 982), Vol. 2 (1983), Vol. 3 (1985); Tijssen, P.,
Practice and
Theory of Enzyme Immunoassays: Laboratory Techniques in Biochemistry and
Molecular
Biology, Elsevier Science Publishers, Amsterdam, The Netherlands (1985).
The test samples of the present invention include cells, protein or membrane
extracts of
cells. The test sample used in the above-described method will vary based on
the assay format,
nature of the detection method and the tissues, cells or extracts used as the
sample to be assayed.
Methods for preparing nucleic acid extracts or of cells are well known in the
art and can be
readily be adapted in order to obtain a sample that is compatible with the
system utilized.
In another embodiment of the present invention, kits axe provided which
contain the
necessary reagents to carry out the assays of the present invention.
Specifically, the invention provides a compartmentalized kit to receive, in
close
confinement, one or more containers which comprises: (a) a first container
comprising one of the
nucleic acid molecules that can bind to a fragment of the Human genome
disclosed herein; and
(b) one or more other containers comprising one or more of the following: wash
reagents,
reagents capable of detecting presence of a bound nucleic acid.
In detail, a compartmentalized kit includes any kit in which reagents are
contained in
separate containers. Such containers include small glass containers, plastic
containers, strips of
plastic, glass or paper, or arraying material such as silica. Such containers
allows one to
efficiently transfer reagents from one compartment to another compartment such
that the
samples and reagents are not cross-contaminated, and the agents or solutions
of each container
can be added in a quantitative fashion from one compartment to another. Such
containers will
include a container which will accept the test sample, a container which
contains the nucleic acid
probe, containers which contain wash reagents (such as phosphate buffered
saline, Tris-buffers,
etc.), and containers which contain the reagents used to detect the bound
probe. One skilled in
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
the art will readily recognize that the previously unidentified GPCR genes of
the present
invention can be routinely identified using the sequence information disclosed
herein can be
readily incorporated into one of the established kit formats which are well
known in the art,
particularly expression arrays.
Vectors/host cells
The invention also provides vectors containing the nucleic acid molecules
described herein.
The term "vector" refers to a vehicle, preferably a nucleic acid molecule,
which can transport the
nucleic acid molecules. When the vector is a nucleic acid molecule, the
nucleic acid molecules are
covalently linked to the vector nucleic acid. With this aspect of the
invention, the vector includes a
plasmid, single or double stranded phage, a single or double stranded RNA or
DNA viral vector, or
artificial chromosome, such as a BAC, PAC, YAC, OR MAC.
A vector can be maintained in the host cell as an extrachromosomal element
where it
replicates and produces additional copies of the nucleic acid molecules.
Alternatively, the vector
may integrate into the host cell genome and produce additional copies of the
nucleic acid molecules
when the host cell replicates.
The invention provides vectors for the maintenance (cloning vectors) or
vectors for
expression (expression vectors) of the nucleic acid molecules. The vectors can
function in
procaryotic or eukaryotic cells or in both (shuttle vectors).
Expression vectors contain cis-acting regulatory regions that are operably
linked in the
vector to the nucleic acid molecules such that transcription of the nucleic
acid molecules is allowed
in a host cell. The nucleic acid molecules can be introduced into the host
cell with a separate
nucleic acid molecule capable of affecting transcription. Thus, the second
nucleic acid molecule
may provide a trans-acting factor interacting with the cis-regulatory control
region to allow
transcription of the nucleic acid molecules from the vector. Alternatively, a
trans-acting factor may
be supplied by the host cell. Finally, a trans-acting factor can be produced
from the vector itself. It
is understood, however, that in some embodiments, transcription and/or
translation of the nucleic
acid molecules can occur in a cell-free system.
The regulatory sequence to which the nucleic acid molecules described herein
can be
operably linked include promoters for directing mRNA transcription. These
include, but are not
limited to, the left promoter from bacteriophage ~,, the lac, TRP, and TAC
promoters from E. coli,
the early and late promoters from SV40, the CMV immediate early promoter, the
adenovirus early
46
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
and late promoters, and retrovirus long-terminal repeats.
In addition to control regions that promote transcription, expression vectors
may also
include regions that modulate transcription, such as repressor binding sites
and enhancers.
Examples include the SV40 enhancer, the cytomegalovirus immediate early
enhancer, polyoma
enhancer, adenovirus enhancers, and retrovirus LTR enhancers.
In addition to containing sites for transcription initiation and control,
expression vectors can
also contain sequences necessary for transcription termination and, in the
transcribed region a
ribosome binding site for translation. Other regulatory control elements for
expression include
initiation and termination codons as well as polyadenylation signals. The
person of ordinary skill in
the art would be aware of the numerous regulatory sequences that are useful in
expression vectors.
Such regulatory sequences are described, for example, in Sambrook et al.,
Molecular Cloning: A
Laboratory Manual. 2nd. ed., Cold Spring Harbor Laboratory Press, Cold Spring
Harbor, NY,
(1989).
A variety of expression vectors can be used to express a nucleic acid
molecule. Such
vectors include chromosomal, episomal, and virus-derived vectors, for example
vectors derived
from bacterial plasmids, from bacteriophage, from yeast episomes, from yeast
chromosomal
elements, including yeast artificial chromosomes, from viruses such as
baculoviruses,
papovaviruses such as SV40, Vaccinia viruses, adenoviruses, poxviruses,
pseudorabies viruses, and
retroviruses. Vectors may also be derived from combinations of these sources
such as those derived
from plasmid and bacteriophage genetic elements, eg. cosmids and phagemids.
Appropriate
cloning and expression vectors for prokaryotic and eukaryotic hosts are
described in Sambrook et
al., Molecular Cloning.' A Laboratory Manual. 2nd. ed., Cold Spring Harbor
Laboratory Press, Cold
Spring Harbor, NY, (1989).
The regulatory sequence may provide constitutive expression in one or more
host cells (i.e.
tissue specific) or may provide for inducible expression in one or more cell
types such as by
temperature, nutrient additive, or exogenous factor such as a hormone or other
ligand. A variety of
vectors providing for constitutive and inducible expression in prokaryotic and
eukaryotic hosts are
well known to those of ordinary skill in the art.
The nucleic acid molecules can be inserted into the vector nucleic acid by
well-known
methodology. Generally, the DNA sequence that will ultimately be expressed is
joined to an
expression vector by cleaving the DNA sequence and the expression vector with
one or more
restriction enzymes and then ligating the fragments together. Procedures for
restriction enzyme
digestion and ligation are well known to those of ordinary skill in the art.
47
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
The vector containing the appropriate nucleic acid molecule can be introduced
into an
appropriate host cell for propagation or expression using well-known
techniques. Bacterial cells
include, but are not limited to, E. coli, Streptomyces, and Salmonella
typhimurium. Eukaryotic cells
include, but are not limited to, yeast, insect cells such as D~osophila,
animal cells such as COS and
CHO cells, and plant cells.
As described herein, it may be desirable to express the peptide as a fusion
protein.
Accordingly, the invention provides fusion vectors that allow for the
production of the peptides.
Fusion vectors can increase the expression of a recombinant protein, increase
the solubility of the
recombinant protein, and aid in the purification of the protein by acting for
example as a ligand for
affinity purification. A proteolytic cleavage site may be introduced at the
junction of the fusion
moiety so that the desired peptide can ultimately be separated from the fusion
moiety. Proteolytic
enzymes include, but are not limited to, factor Xa, thrombin, and
enterokinase. Typical fusion
expression vectors include pGEX (Smith et al., Gehe 67:31-40 (1988)), pMAL
(New England
Biolabs, Beverly, MA) and pRITS (Pharmacia, Piscataway, NJ) which fuse
glutathione S-
transferase (GST), maltose E binding protein, or protein A, respectively, to
the target recombinant
protein. Examples of suitable inducible non-fusion E. coli expression vectors
include pTrc (Amann
et al., Gene 69:301-315 (1988)) and pET 1 1d (Studier et al., Gene Expression
Technology: Methods
i~ Ehzymology 185:60-89 (1990)).
Recombinant protein expression can be maximized in a host bacteria by
providing a genetic
background wherein the host cell has an impaired capacity to proteolytically
cleave the recombinant
protein. (Gottesman, S., Gene Expression Technology: Methods in Ehzymology
185, Academic
Press, San Diego, California (1990) 119-128). Alternatively, the sequence of
the nucleic acid
molecule of interest can be altered to provide preferential codon usage for a
specific host cell, for
example E. coli. (Wada et al., Nucleic Acids Res. X0:2111-2118 (1992)).
The nucleic acid molecules can also be expressed by expression vectors that
are operative in
yeast. Examples of vectors for expression in yeast e.g., S cerevisiae include
pYepSecl (Baldari, et
al., EMBO J. 6:229-234 (1987)), pMFa (Kurjan et al., Cell 30:933-943(1982)),
pJRY88 (Schultz et
al., Gehe 54:113-123 (1987)), and pYES2 (Invitrogen Corporation, San Diego,
CA).
The nucleic acid molecules can also be expressed in insect cells using, for
example,
baculovirus expression vectors. Baculovirus vectors available for expression
of proteins in cultured
insect cells (e.g., Sf 9 cells) include the pAc series (Smith et al., Mol.
Cell Biol. 3:2156-2165
(1983)) and the pVL series (Lucklow et al., Virology 170:31-39 (1989)).
In certain embodiments of the invention, the nucleic acid molecules described
herein are
48
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
expressed in mammalian cells using mammalian expression vectors. Examples of
mammalian
expression vectors include pCDM8 (Seed, B. Nature 329:840(1987)) and pMT2PC
(I~aufinan et al.,
EMBO J. 6:187-195 (1987)).
The expression vectors listed herein are provided by way of example only of
the well-
known vectors available to those of ordinary skill in the art that would be
useful to express the
nucleic acid molecules. The person of ordinary skill in the art would be aware
of other vectors
suitable for maintenance propagation or expression of the nucleic acid
molecules described herein.
These are found for example in Sambrook, J., Fritsh, E. F., and Maniatis, T.
Molecular Cloning: A
Laboratory Manual. 2nd, ed., Cold Spring Harbor Laboratory, Cold Spring Harbor
Laboratory
Press, Cold Spring Harbor, NY, 1989.
The invention also encompasses vectors in which the nucleic acid sequences
described
herein are cloned into the vector in reverse orientation, but operably linked
to a regulatory sequence
that permits transcription of antisense RNA. Thus, an antisense transcript can
be produced to all, or
to a portion, of the nucleic acid molecule sequences described herein,
including both coding and
non-coding regions. Expression of this antisense RNA is subject to each of the
parameters
described above in relation to expression of the sense RNA (regulatory
sequences, constitutive or
inducible expression, tissue-specific expression).
The invention also relates to recombinant host cells containing the vectors
described herein.
Host cells therefore include prokaryotic cells, lower eukaryotic cells such as
yeast, other eukaryotic
cells such as insect cells, and higher eukaryotic cells such as mammalian
cells.
The recombinant host cells are prepared by introducing the vector constructs
described
herein into the cells by techniques readily available to the person of
ordinary skill in the art. These
include, but are not limited to, calcium phosphate transfection, DEAE-dextran-
mediated
transfection, cationic lipid-mediated transfection, electroporation,
transduction, infection,
lipofection, and other techniques such as those found in Sambrook, et al.
(Molecular Cloning: A
Laboratory Manual. 2nd, ed., Cold Spring Harbor Laboratory, Cold Spring Harbor
Laboratory
Press, Cold Spring Harbor, NY, 1989).
Host cells can contain more than one vector. Thus, different nucleotide
sequences can be
introduced on different vectors of the same cell. Similarly, the nucleic acid
molecules can be
introduced either alone or with other nucleic acid molecules that are not
related to the nucleic acid
molecules such as those providing traps-acting factors for expression vectors.
When more than one
vector is introduced into a cell, the vectors can be introduced independently,
co-introduced or joined
to the nucleic acid molecule vector.
49
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
In the case of bacteriophage and viral vectors, these can be introduced into
cells as packaged
or encapsulated virus by standard procedures for infection and transduction.
Viral vectors can be
replication-competent or replication-defective. In the case in which viral
replication is defective,
replication will occur in host cells providing functions that complement the
defects.
Vectors generally include selectable markers that enable the selection of the
subpopulation
of cells that contain the recombinant vector constructs. The marker can be
contained in the same
vector that contains the nucleic acid molecules described herein or may be on
a separate vector.
Markers include tetracycline or ampicillin-resistance genes for prokaryotic
host cells and
dihydrofolate reductase or neomycin resistance for eukaryotic host cells.
However, any marker that
provides selection for a phenotypic trait will be effective.
While the mature proteins can be produced in bacteria, yeast, mammalian cells,
and other
cells under the control of the appropriate regulatory sequences, cell- free
transcription and
translation systems can also be used to produce these proteins using RNA
derived from the DNA
constructs described herein.
Where secretion of the peptide is desired, which is difficult to achieve with
multi-
transmembrane domain containing proteins such as GPCRs, appropriate secretion
signals are
incorporated into the vector. The signal sequence can be endogenous to the
peptides or
heterologous to these peptides.
Where the peptide is not secreted into the medium, which is typically the case
with GPCRs,
the protein can be isolated from the host cell by standard disruption
procedures, including freeze
thaw, sonication, mechanical disruption, use of lysing agents and the like.
'The peptide can then be
recovered and purified by well-known purification methods including ammonium
sulfate
precipitation, acid extraction, anion or cationic exchange chromatography,
phosphocellulose
chromatography, hydrophobic-interaction chromatography, affinity
chromatography,
hydroxylapatite chromatography, lectin chromatography, or high performance
liquid
chromatography.
It is also understood that depending upon the host cell in recombinant
production of the
peptides described herein, the peptides can have various glycosylation
patterns, depending upon the
cell, or maybe non-glycosylated as when produced in bacteria. In addition, the
peptides may
include an initial modified methionine in some cases as a result of a host-
mediated process.
Uses of vectors and host cells
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
The recombinant host cells expressing the peptides described herein have a
variety of uses.
First, the cells are useful for producing a GPCR protein or peptide that can
be further purified to
produce desired amounts of GPCR protein or fragments. Thus, host cells
containing expression
vectors are useful for peptide production.
Host cells are also useful for conducting cell-based assays involving the GPCR
protein or
GPCR protein fragments, such as those described above as well as other formats
known in the art.
Thus, a recombinant host cell expressing a native GPCR protein is useful for
assaying compounds
that stimulate or inhibit GPCR protein function.
Host cells are also useful for identifying GPCR protein mutants in which these
functions are
affected. If the mutants naturally occur and give rise to a pathology, host
cells containing the
mutations are useful to assay compounds that have a desired effect on the
mutant GPCR protein (for
example, stimulating or inhibiting function) which may not be indicated by
their effect on the native
GPCR protein.
Genetically engineered host cells can be further used to produce non-human
transgenic
animals. A transgenic animal is preferably a mammal, for example a rodent,
such as a rat or mouse,
in which one or more of the cells of the animal include a transgene. A
transgene is exogenous DNA
which is integrated into the genome of a cell from which a transgenic animal
develops and which
remains in the genome of the mature animal in one or more cell types or
tissues of the transgenic
animal. These animals are useful for studying the function of a GPCR protein
and identifying and
evaluating modulators of GPCR protein activity. Other examples of transgenic
animals include
non-human primates, sheep, dogs, cows, goats, chickens, and amphibians.
A transgenic animal can be produced by introducing nucleic acid into the male
pronuclei of
a fertilized oocyte, e.g., by microinjection, retroviral infection, and
allowing the oocyte to develop
in a pseudopregnant female foster animal. Any of the GPCR protein nucleotide
sequences can be
introduced as a transgene into the genome of a non-human animal, such as a
mouse.
Any of the regulatory or other sequences useful in expression vectors can form
part of the
transgenic sequence. This includes intronic sequences and polyadenylation
signals, if not already
included. A tissue-specific regulatory sequences) can be operably linked to
the transgene to direct
expression of the GPCR protein to particular cells.
Methods for generating transgenic animals via embryo manipulation and
microinjection,
particularly animals such as mice, have become conventional in the art and are
described, for
example, in U.S. Patent Nos. 4,736,866 and 4,870,009, both by Leder et al.,
U.S. Patent No.
4,873,191 by Wagner et al. and in Hogan, B., Manipulating the Mouse Embryo,
(Cold Spring
51
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Harbor Laboratory Press, Cold Spring Harbor, N.Y., 1986). Similar methods are
used for
production of other transgenic animals. A transgenic founder animal can be
identified based upon
the presence of the transgene in its genome and/or expression of transgenic
mRNA in tissues or
cells of the animals. A transgenic founder animal can then be used to breed
additional animals
carrying the transgene. Moreover, transgenic animals carrying a transgene can
further be bred to
other transgenic animals carrying other transgenes. A transgenic animal also
includes animals in
which the entire animal or tissues in the animal have been produced using the
homologously
recombinant host cells described herein.
In another embodiment, transgenic non-human animals can be produced which
contain
selected systems that allow for regulated expression of the transgene. One
example of such a
system is the crelloxP recombinase system of bacteriophage P 1. For a
description of the crelloxP
recombinase system, see, e.g., Lakso et al. PNAS 89:6232-6236 (1992). Another
example of a
recombinase system is the FLP recombinase system of S cerevisiae (O'Gorman et
al. Science
251:1351-1355 (1991). If a crelloxP recombinase system is used to regulate
expression of the
transgene, animals containing transgenes encoding both the Cre recombinase and
a selected protein
is required. Such animals can be provided through the construction of "double"
transgenic animals,
e.g., by mating two transgenic animals, one containing a transgene encoding a
selected protein and
the other containing a transgene encoding a recombinase.
Clones of the non-human transgenic animals described herein can also be
produced
according to the methods described in Wilinut, I. et al. Nature 385:810-813
(1997) and PCT
International Publication Nos. WO 97/07668 and WO 97/07669. In brief, a cell,
e.g., a somatic cell,
from the transgenic animal can be isolated and induced to exit the growth
cycle and enter Go phase.
The quiescent cell can then be fused, e.g., through the use of electrical
pulses, to an enucleated
oocyte from an animal of the same species from which the quiescent cell is
isolated. The
reconstructed oocyte is then cultured such that it develops to morula or
blastocyst and then
transferred to pseudopregnant female foster animal. The offspring born of this
female foster animal
will be a clone of the animal from which the cell, e.g., the somatic cell, is
isolated.
Transgenic animals containing recombinant cells that express the peptides
described herein
are useful to conduct the assays described herein in an in vivo context.
Accordingly, the various
physiological factors that are present in vivo and that could effect ligand
binding, GPCR protein
activation, and signal transduction, may not be evident from ih vitro cell-
free or cell-based assays.
Accordingly, it is useful to provide non-human transgenic animals to assay in
vivo GPCR protein
function, including ligand interaction, the effect of specific mutant GPCR
proteins on GPCR protein
52
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
function and ligand interaction, and the effect of chimeric GPCR proteins. It
is also possible to
assess the effect of null mutations, that is mutations that substantially or
completely eliminate one or
more GPCR protein functions.
All publications and patents mentioned in the above specification are herein
incorporated
by reference. Various modifications and variations of the described method and
system of the
invention will be apparent to those skilled in the art without departing from
the scope and spirit
of the invention. Although the invention has been described in connection with
specific
preferred embodiments, it should be understood that the invention as claimed
should not be
unduly limited to such specific embodiments. Indeed, various modifications of
the above-
described modes for carrying out the invention which are obvious to those
skilled in the field of
molecular biology or related fields are intended to be within the scope of the
following claims.
53
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
SEQUENCE LISTING
<110> PE CORPORATION (NY)
<120> ISOLATED HUMAN G-PROTEIN COUPLED
RECEPTORS, NUCLEIC ACID MOLECULES ENCODING HUMAN GPCR
PROTEINS, AND USES THEREOF
<130> CL001140PCT
<140> TO BE ASSIGNED
<141> 2002-01-31
<150> 09/784,317
<151> 2002-02-16
<160> 4
<170> FastSEQ for Windows Version 4.0
<210> 1
<211> 3002
<212> DNA
<213> Homo sapiens
<400> 1
tcagaactac agcataatgc gtggcttcaa cctgctcctc ttctggggat gttgtgttat 60
gcacagctgg gaagggcaca taagacccac acggaaacca aacacaaagg gtaataactg 120
tagagacagt accttgtgcc cagcttatgc cacctgcacc aatacagtgg acagttacta 180
ttgcgcttgc aaacaaggct tcctgtccag caatgggcaa aatcacttca aggatccagg 240
agtgcgatgc aaagatattg atgaatgttc tcaaagcccc cagccctgtg gtcctaactc 300
atcctgcaaa aacctgtcag ggaggtacaa gtgcagctgt ttagatggtt tctcttctcc 360
cactggaaat gactgggtcc caggaaagcc gggcaatttc tcctgtactg atatcaatga 420
gtgcctcacc agcagcgtct gccctgagca ttctgactgt gtcaactcca tgggaagcta 480
cagttgcagc tgtcaagttg gattcatctc tagaaactcc acctgtgaag acgtggatga 540
atgtgcagat ccaagagctt gcccagagca tgcaacttgt aataacactg ttggaaacta 600
ctcttgtttc tgcaacccag gatttgaatc cagcagtggc cacttgagtt tccagggtct 660
caaagcatcg tgtgaagata ttgatgaatg cactgaaatg tgccccatca attcaacatg 720
caccaacact cctgggagct acttttgcac ctgccaccct ggctttgcac caagcaatgg 780
acagttgaat ttcacagacc aaggagtgga atgtagagat attgatgagt gccgccaaga 840
tccatcaacc tgtggtccta attctatctg caccaatgcc ctgggctcct acagctgtgg 900
ctgcattgca ggctttcatc ccaatccaga aggctcccag aaagatggca acttcagctg 960
ccaaagggtt ctcttcaaat gtaaggaaga tgtgataccc gataataagc agatccagca 1020
atgccaagag ggaaccgcag tgaaacctgc atatgtctcc ttttgtgcac aaataaataa 1080
catcttcagc gttctggaca aagtgtgtga aaataaaacg accgtagttt ctctgaagaa 1140
tacaactgag agctttgtcc ctgtgcttaa acaaatatcc acgtggacta aattcaccaa 1200
ggaagagacg tcctccctgg ccacagtctt cctggagagt gtggaaagca tgacactggc 1260
atctttttgg aaaccctcag caaatgtcac tccggctgtt cggacggaat acttagacat 1320
tgagagcaaa gttatcaaca aagaatgcag tgaagagaat gtgacgttgg acttggtagc 1380
caagggggat aagatgaaga tcgggtgttc cacaattgag gaatctgaat ccacagagac 1440
cactggtgtg gcttttgtct cctttgtggg catggaatcg gttttaaatg agcgcttctt 1500
caaagaccac caggctccct tgaccacctc tgagatcaag ctgaagatga attctcgagt 1560
cgttgggggc ataatgactg gagagaagaa agacggcttc tcagatccaa tcatctacac 1620
tctggagaac attcagccaa agcagaagtt tgagaggccc atctgtgttt cctggagcac 1680
tgatgtgaag ggtggaagat ggacatcctt tggctgtgtg atcctggaag cttctgagac 1740
atataccatc tgcagctgta atcagatggc aaatcttgcc attatcatgg cgtctgggga 1800
gctcacgatg ggctgcgcca tcatcgcggg cttcctgcac taccttttcc ttgcctgctt 1860
cttctggatg ctggtggagg ctgtgatact gttcttgatg gtcagaaacc tgaaggtggt 1920
gaattacttc agctctcgca acatcaagat gctgcacatc tgtgcctttg gttatgggct 1980
gccgatgctg gtggtggtga tctctgccag tgtgcagcca cagggctatg gaatgcataa 2040
tcgctgctgg ctgaatacag agacagggtt catctggagt ttcttggggc cagtttgcac 2100
agttatagtg atcaactccc ttctcctgac ctggaccttg tggatcctga ggcagaggct 2160
ttccagtgtt aatgccgaag tctcaacgct aaaagacaCC aggttactga ccttcaaggc.2220
ctttgcccag ctcttcatcc tgggctgctc ctgggtgctg ggcatttttc agattggacc 2280
tgtggcaggt gtcatggctt acctgttcac catcatcaac agcctgcagg gggccttcat 2340
cttcctcatc cactgtctgc tcaacggcca ggtacgagaa gaatacaaga ggtggatcac 2400
tgggaagacg aagcccagct cccagtccca gacctcaagg atcttgctgt cctccatgcc 2460
atccgcttcc aagacgggtt aaagtccttt cttgctttca aatatgctat ggagccacag 2520
ttgaggacag tagtttcctg caggagccta ccctgaaatc tcttctcagc ttaacatgga 2580
aatgaggatc ccaccagccc cagaaccctc tggggaagaa tgttgggggc ggtcttcctg 2640
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tggttgtatg cactgatgag aaatcaggcg tttctgctcc aaacgaccat tttatcttcg 2700
tgctctgcaa cttcttcaat tccagagttt ctgagaacag acccaaattc aatggcatga 2760
ccaagaacac ctggctacca ttttgttttc tcctgccctt gttggtgcat ggttctaagc 2820
atgcccctcc agagcctatc atacgcctga tacagagaac ctctcaataa atgatttgtc 2880
gcctgtcaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa 2940
aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa aaaaaaaaaa 3000
as 3002
<210> 2
<211> 821
<212> PRT
<213> Homo sapiens
<400> 2
Met Arg Gly Phe Asn Leu Leu Leu Phe Trp Gly Cys Cys Val Met His
1 5 10 15
Ser Trp Glu Gly His Ile Arg Pro Thr Arg Lys Pro Asn Thr Lys Gly
20 25 30
Asn Asn Cys Arg Asp Ser Thr Leu Cys Pro Ala Tyr Ala Thr Cys Thr
35 40 45
Asn Thr Val Asp Ser Tyr Tyr Cys Ala Cys Lys Gln Gly Phe Leu Ser
50 55 60
Ser Asn Gly Gln Asn His Phe Lys Asp Pro Gly Val Arg Cys Lys Asp
65 70 75 80
Ile Asp Glu Cys Ser Gln Ser Pro Gln Pro Cys Gly Pro Asn Ser Ser
85 90 95
Cys Lys Asn Leu Ser Gly Arg Tyr Lys Cys Ser Cys Leu Asp Gly Phe
100 105 210
Ser Ser Pro Thr Gly Asn Asp Trp Val Pro Gly Lys Pro Gly Asn Phe
115 120 125
Ser Cys Thr Asp Ile Asn Glu Cys Leu Thr Ser Ser Val Cys Pro Glu
130 135 140
His Ser Asp Cys Val Asn Ser Met Gly Ser Tyr Ser Cys Ser Cys Gln
145 150 155 160
Val Gly Phe Ile Ser Arg Asn Ser Thr Cys Glu Asp Val Asp Glu Cys
165 170 175
Ala Asp Pro Arg Ala Cys Pro Glu His Ala Thr Cys Asn Asn Thr Val
180 185 190
Gly Asn Tyr Ser Cys Phe Cys Asn Pro Gly Phe Glu Ser Ser Ser Gly
195 200 205
His Leu Ser Phe Gln Gly Leu Lys Ala Ser Cys Glu Asp Ile Asp Glu
210 215 220
Cys Thr Glu Met Cys Pro Ile Asn Ser Thr Cys Thr Asn Thr Pro Gly
225 230 235 ' 240
Ser Tyr Phe Cys Thr Cys His Pro Gly Phe Ala Pro Ser Asn Gly Gln
245 250 255
Leu Asn Phe Thr Asp Gln Gly Val Glu Cys Arg Asp Ile Asp Glu Cys
260 265 270
Arg Gln Asp Pro Ser Thr Cys Gly Pro Asn 5er Ile Cys Thr Asn Ala
275 280 285
Leu Gly Ser Tyr Ser Cys Gly Cys Ile Ala Gly Phe His Pro Asn Pro
290 295 300
Glu Gly Ser Gln Lys Asp Gly Asn Phe Ser Cys Gln Arg Val Leu Phe
305 310 315 320
Lys Cys Lys Glu Asp Val Ile Pro Asp Asn Lys Gln Ile Gln Gln Cys
325 330 335
Gln Glu Gly Thr Ala Val Lys Pro Ala Tyr Val Ser Phe Cys Ala Gln
340 345 350
Tle Asn Asn Ile Phe Ser Val Leu Asp Lys Val Cys Glu Asn Lys Thr
355 360 365
Thr Val Val Ser Leu Lys Asn Thr Thr Glu Ser .Phe Val Pro Val Leu
370 375 380
Lys Gln Ile Ser Thr Trp Thr Lys Phe Thr Lys Glu Glu Thr Ser Ser
385 390 395 400
Leu Ala Thr Val Phe Leu Glu Ser Val Glu Ser Met Thr Leu Ala Ser
405 410 415
Phe Trp Lys Pro Ser Ala Asn Val Thr Pro Ala Val Arg Thr Glu Tyr
420 425 430
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
Leu Asp Ile Glu Ser Lys Val Ile Asn Lys Glu Cys Ser Glu Glu Asn
435 440 445
Val Thr Leu Asp Leu Val Ala Lys Gly Asp Lys Met Lys Ile Gly Cys
450 455 460
Ser Thr I1e Glu Glu Ser Glu Ser Thr Glu Thr Thr Gly Val Ala Phe
465 470 475 480
Val Ser Phe Val Gly Met Glu Ser Val Leu Asn Glu Arg Phe Phe Lys
485 490 495
Asp His Gln Ala Pro Leu Thr Thr Ser Glu Ile Lys Leu Lys Met Asn
500 505 510
Ser Arg Val Val Gly Gly Ile Met Thr Gly Glu Lys Lys Asp Gly Phe
515 520 525
Ser Asp Pro Ile Ile Tyr Thr Leu Glu Asn Ile Gln Pro Lys Gln Lys
530 535 540
Phe Glu Arg Pro Ile Cys Val Ser Trp Ser Thr Asp Val Lys Gly Gly
545 550 555 560
Arg Trp Thr Ser Phe Gly Cys Val Ile Leu Glu Ala Ser Glu Thr Tyr
565 570 575
Thr Ile Cys Ser Cys Asn Gln Met Ala Asn Leu Ala Ile Ile Met Ala
580 585 590
Ser Gly G1u Leu Thr Met Gly Cys Ala Ile Ile Ala Gly Phe Leu His
595 600 605
Tyr Leu Phe Leu Ala Cys Phe Phe Trp Met Leu Val Glu Ala Val Ile
610 615 620
Leu Phe Leu Met Val Arg Asn Leu Lys Val Val Asn Tyr Phe Ser Ser
625 630 635 640
Arg Asn Ile Lys Met Leu His Ile Cys Ala Phe Gly Tyr Gly Leu Pro
645 650 655
Met Leu Val Val Val Ile Ser Ala Ser Val Gln Pro Gln G1y Tyr Gly
660 665 670
Met His Asn Arg Cys Trp Leu Asn Thr Glu Thr Gly Phe Ile Trp Ser
675 680 685
Phe Leu Gly Pro Val Cys Thr Val Ile Val Ile Asn Ser Leu Leu Leu
690 695 700
Thr Trp Thr Leu Trp Ile Leu Arg Gln Arg Leu Ser Ser Val Asn Ala
705 710 715 720
Glu Val Ser Thr Leu Lys Asp Thr Arg Leu Leu Thr Phe Lys Ala Phe
725 730 735
Ala Gln Leu Phe Ile Leu Gly Cys Ser Trp Val Leu Gly Ile Phe Gln
740 745 750
Ile Gly Pro Val Ala Gly Val Met Ala Tyr Leu Phe Thr Ile Ile Asn
755 760 765
Ser Leu Gln Gly Ala Phe Ile Phe Leu Ile His Cys Leu Leu Asn Gly
770 775 780
Gln Val Arg Glu Glu Tyr Lys Arg Trp Ile Thr Gly Lys Thr Lys Pro
785 790 795 800
Ser Ser Gln Ser Gln Thr Ser Arg Ile Leu Leu Ser Ser Met Pro Ser
805 810 815
Ala Ser Lys Thr Gly
820
<220> 3
<211> 62488
<212> DNA
<213> Homo sapiens
<220>
<221> misc_feature
<222> (1). .(62488)
<223> n = A,T,C or G
<400> 3
ggacccctcc tcctctcttc tccttctcct ccttcttcct tctttcttcc tttcttcgaa 60
taaacaattt tatgaaatat ctgtctatgc taggctgtgc tctgagtgct gagtattcag 120
agatgaacaa acacctaagg cccaaattct catgaaactc atagtctagt agtggaagac 180
agtttgcagt tacacaagca attaaacaag gtaacttacg atagtgatag ctgctgtttt 240
aaaaatgcag tgagctctgc tagatggata ccaggagcag agacaggttg ccctatctaa 300
3
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
ggatagtcag gagtggcttc tcagaggagg tgacatttga gctgagattt atttatttat 360
ttatttattt gttatttatt tatcttgaga cagagttttg ctcttgtcac ccaggctgga 420
gtgcggtggc gtgatctcag ctcactgcaa cctccacctc ctgggttcaa gcgattctcc 480
tatctcagcc tcccaggtag ctgggattac aggcgtgaga ccccacgccc ggcctgagct 540
gagatttgaa tgataaggtg taccagctgg agagttctgc atagagggaa acaggattac 600
aaacgttgtg aattgggaaa tgagctcaaa ccttgggcta ctaggtataa catttcaggt 660
acatttgccc aggaagaagg tctaaaagag gaagcgcggt gatctaaagg aaactgaaat 720
gcaaccggga ccatcctgag catcatctga gtctcacctc ccagtgttct cctccactag 780
ggcatttgtt gggacagggt taactgtaag gtcaggactc tgagacaagc gctgactcct 840
cctatttctc tccacagctg ctggctgaat acagagacag ggttcatctg gagtttcttg 900
gggccagttt gcacagttat agtggtaagc aaatactaca acagcctggc gaagtgtgtt 960
ctgaaggagg agcaaggaga cctgcgagat ctggaatttc cagggacgtg tgcagctgag 1020
agggtcactt attcccatca aaagttctcc tttccaggcc gggcgtggtg gctcacgcct 1080
gtaatcccag cacttttggg aggctgaggc gggtggatca cctgaggtca ggagttcgag 1140
aacagtctgg ccaacttggc gaaaccccat ctctgctaaa aatacaaaaa attagctggg 1200
tgtggtggcg agcacttgta atcccagcta cttaggaggc tgaggcagga gaatcgcttg 1260
aatccaggag acatggaggt tgcagtgagc caaattcatg ccactgtact ccagcctggg 1320
caacagagtg aaactccgtc tcaaaaaaat aaataaataa ataagttatc ggccaggttg 1380
cggtggctca tgcctgttat cccagcactt tgagaggccc aggatcactt gaggtcagga 1440
gttcaagacc agcctgacca acatggagaa accccatctc tactaaaaat acaaaattag 1500
ctgggtgtgt tggcacatgc cctgtaatcc cagctactca ggaggctgag gcaagagagg 1560
agaattgctt gaaccaggga ggcagaggtt gcggtgagcc gagattgcgc cattgcactc 1620
cagcctaggc aacaagagca aaactccatc tcaaaaaaaa aaaagttctc ctttccaatc 1680
cttcattctt gagatttcga agttagagag taatttcttt gacttgggaa ttttgttttt 1740
ccttctgaaa ggcaaatacc cctgggaggg accataattg tatccgtctt gtgcacaggt 1800
ccatgagtct ctctgaagca gtgtcattgt ctgggggtaa taatgcctga ggttcgttgc 1860
ctcatgccaa ggaaatcaag gacacagaca cacacaagga gtgagtttaa gagcagaggt 1920
ttaataggca aaagaaagag aaaggagact agctctctgt gtcttgcaag agagagggcc 1980
tcctgagtgg gacttctggc ccgcagcaaa gtgcaacaga ttttagactg gcttgagggg 2040
gcggtgtctg atttacatag ggcccaaaga ttggttggac ccagtgtgac atttacgtag 2100
agcgcgagga agctggccac ccaccctgat cttttattat gcaaatgggg tctttacttg 2160
gctggcacca tgttgtcttc tccttactgt ctacatggtt tacaaagaaa agggaagacg 2220
gagctgccat ttttttttct ttttttttag acggagtctc actctgtcac ccacgctgga 2280
gtgcagtggt gcaatctcgg ctcactgcaa cctctgcctc ctgggttcaa gcaattattc 2340
tgcctcagcc tcccgaatag ctgggattac aggcacgcac caccacaccc ggcaattttg 2400
tatttttaat agagatgggt tttcaccacg ttggccaagc tggtctcaaa ctcctgacct 2460
caggtagttt gcctgcctcg gcctccccaa gtgctgggat tacaggcata tgccactatg 2520
accagctaat ttttgtactt ttagtagaga tggggtttca ccacgttgac caggctccta 2580
acctcagtta atccacctgc ctcagcctcc caaagtgctg ggatttcaag cgtgagtctc 2640
tgcgcccagc cagagctgcc attttgaaca tgcctattcc ccaggtaacc gctttcctat 2700
tggcacaact gccagcattc acccatgcaa gcttctagct tgcctttcta tgtctatagc 2760
tcgattctac cggctgctct ccattagaaa agaaaatgat ttgggagctg cttttcatta 2820
aaaggaaaac cttaccgagg acttccttac cctcactatc tgcctaaata aatctttttt 2880
aaatcctatg tcatctccta ggcacagatg ttgaatgaaa gaaaaagtaa aaagaaatta 2940
ggaggaattg tgggggaagg agtgagatga cagagggaaa tggtggatcg aggcaaaggg 3000
aggccaggga atgttaaaca cccatctctt tgcctcccaa cctccatcca gacttgacag 3060
ccctgccaga tgatgattct tctgactctc actcatggtg gctcattccc ttatgtcttt 3120
tgtcatattt ttcgaggatt aaattttttt ttatttttaa tttttgtggg cacaaagtaa 3180
gtgtatatat ttatggggta tatgaaatat tttcatacag gcacagaata cataataatc 3240
acatcagagt caacgaggta tctgtcccct caagcatttg tcctttgtgt tacacacaat 3300
ccaattatgt tcttctggtt tttgttttgt tttattgttt ggtttatttt tatttttatt 3360
ttgagacaga gtcttgctct gtcacccagg ctgcagtgca atggcatgat ctcagctcat 3420
tgcagtctct gcctcctggg ttcaagcgat tctcctgcct cagcctcccg agtagctggg 3480
attacaggtg tgcgccacca ctcccggcta attttttgta tttttagtag agacgggatt 3540
tcgccatgtt ttccaggctg gtctcgaatt catgacctca ggtgacccac ccgcctcgac 3600
ctcccaaagt gctgggatta caggcatgag ccactgtgtc cagcctctcc tggttatttt 3660
taaatctaca atgcaattat tattaactat agtcaccctg ttttgctaac aaatactaga 3720
tcttattcat tctttctatt tttttgtacc cattaaccat cccctcttcc ctgttcccca 3780
cccacccctt cactatcctt cccagcttct ggtaactgct gttctactct ctagctccat 3840
gagttcacct gttttaaatg tttagctccc acaaaggaac tgagaacatg tgaaatgtgt 3900
ctttctgtgc ctggcttcat ttgtaatgtt tacttgactg atctaagtct tgggaatgct 3960
tgaaattata ttcctccaca gaggttttgt gctgatgtct atcagatttt ttttaatggg 4020
gggcatgtat taattttcag gggtcaccgt aataagctac tgcatgctgg gtggcttaga 4080
acaacagaga cttgttgttt cacagttctg gaggcttgaa gcctgaaatc aaggtattgg 4140
cagggccacg ctccccatga caacacgagg ggaggggtct gttccagacc cctctccaac 4200
aaattttccc atttgtaaga tcaccggcca ggtgcagtgg ctcatgcctg taatcccagc 4260
attttgggag gccgaggcgg gtggatcacc tgaagtcagg agttcgagac cagcctggcc 4320
aacatggcaa aaccctgtct ctactgaaaa atacaaaaaa tgagccaggc gtggtggcag 4380
4
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
gcaccaatag tcccagcctc gggaggctga ggcacaagaa taacttgacc cctggaggga 4440
gaggttgcag tgagccgaga taacatcact gcactccagc ctgggtgaca gagggagact 4500
gtctcaaaaa aaaagaaaaa aaaaaaggaa cactagtcat atttcagtca gattagggtc 4560
tgccctaatg agttcatctt aacttgatta tttctgtaaa gattctattt ccaaataagg 4620
tcttggggcg cctggcccca acagacagcc tcttaagctc gtaagaaaat aaaaacaaat 4680
cttcagaggc cataaattag acagcagtaa tgaaccagaa tgctgtagct gccctaaggg 4740
tatttcctaa aatcaagaat ctaaaacctt ggagattgaa tggtcatttg tggagttgga 4800
ggggcaagcc ctagggtctg aagaaggtgt agagtccacc aaataaaata aagaccccat 4860
tcttattgaa agagtggact agaaaataaa attctgggcc gggcgtggtg gtttatgcct 4920
gtaatcccag cactttggga ggctgaggta ggcggatcac gaggtcagga gatcgagacc 4980
atcctggcta acatggtgaa accccgtctc cactaaaaat acaaaaaatc agccaggcgt 5040
ggtggtgcac gcctgtagtc ccagctactc aggaggctga ggcaggagaa tggcgtgaac 5100
ctgggcggtg gagcttgcag tgagccgaga tggcaccact gccctccagc ctgggtgaca 5160
gagcaagact tggtctcaaa aaataaaata aaaataaata aataaataaa tttaaaagcc 5220
tatctgttgg ggttcctcca gcatttttgg tgttttgtag tgagagggat tttcagagtg 5280
agattcctaa aactcaaagt tgatcctgat gctcttctgt gtgaaactgt tctatagctt 5340
ctccattgca ctcagggtga tgtccactcc ctgagcctga cacctcagat ccttcactgc 5400
ctggccctac ccaacttccc tagggtccct ttctggcttt ttcctgcctg ctcggccctc 5460
tggctgtacc agccaacttt ctacttcctt gaaatgccaa gttctcccct ctagacctct 5520
ctcatggagg tggtggtgat gatgaacaaa ccatatgtgt gagatgctta gtacaatgcc 5580
aggcattgcc aagagtgcag taaatattag gtatgaacaa tcatggaaat aataattatg 5640
atgatgttgg ccagacatgg tggctcatgc ctgtaatccc agcactttgg gaggccaagg 5700
caggcagatc acttcgggtc aggagtccga gaccagcccg gccaacatgg tgaaaccccg 5760
tctctactaa aaatacaaaa tttagccagg tgtggtggcc cgcgcctgta atcccagcta 5820
ctcaggaggc tgaggcagga gaatcgcttg aacctgggag acagaggtta ctgtgagccg 5880
aggttgcgcc attgcactcc agcctgggag acaagagcga gactccgtct caataataat 5940
agtaatgcaa tgtttatctt acccattccc aaatcttcaa ggacattggg ctttcttgga 6000
agttctactc atctggaaaa aataattccc ctggggccgt gattctccac tggtgcagtt 6060
ttccctctaa ggggaagttg gcaaagtgtg aagacttttt tttttttttt tttgagacag 6120
agtttcgctc ttgttgccca ggctggagta caatggcatg atctctgctc attgaaagct 6180
ctgcctccca ggttcaagcg attctcctgc ctcagcctct gcggtagctg ggattacagg 6240
catgcgccac cacggccagc taattttgta tttttagtgg agatggggtt tccccatgtt 6300
ggtcaggctg gtctcgaact cctgacctca ggtgatccac ccaccttggc ctcccaatgt 6360
gctgggatta caggcgtgag ccaccgctcc cgaccagaag acatttttgg ttgttacaac 6420
tagagaggcg ggttctactg ccatttagtg cgtagaagcc agggatgctg gttaacatcc 6480
taccgtgcac aaggcagccc ccttctcccc gctgccccca cacaccaaga atgetctgac 6540
ccaaatgtca gtattgccgt gggtgaacag gccctgcgcc aggtgtatgt atatcttgct 6600
cagggatgct tttgagggag ggctttagaa gataataaac tcaagataat aaactccact 6660
caagttcgca gtctatgtcc taacgtactg gatgcacttt gggaaaatac acagagaccg 6720
ccccattgag aacaagcaaa ggctgtttat tccgagattt ctgtagtcca gtagtacgag 6780
agtcagctac catcatcact cgcgttggct cagactcaaa ggcaggcaga gaagtgggga 6840
aggtttatag aggaaaaaaa gagaaggcta caggtaagtc ctgcttggag gctgttgcac 6900
ggatgggcaa gctgtaggtg gctaactagg agcggggtat cctgtgtgat tggttagggg 6960
tacctattgg cttctacctg ttggtcctaa gttggaagta gggacaaaaa ttaaggaagc 7020
tgtcaattac taactaagtc ctgctgtctt ggttcaattg ctgcatggat cattctttgg 7080
ctttctggat tggttgctat aaatcgtggg tcagaattct tcttcttctt cttttttttt 7140
tttttttttt tgagacacag tcttgctctg ttgccaggct ggagtgcagt ggcacaatct 7200
cagctcactg caacctctgc ctcccaggtt caagtgattc tcatgcctca gcctcccaag 7260
tagctgggat tacaggtgtg caccaccaca cctgcctaat ttaatttttg tatttttttt 7320
ttttttttga gatggagtct cactctgttg ccaggctgga gtgcagtagc acaatctcag 7380
ctgtctgcaa cctctgcctc ccaggttcag gtgattcccc tgcctcagcc tcctgagtag 7440
ctgtgattac aggcatccac caccatgcat ggctaatttt tttttttttt ttgtatttta 7500
gtatagacag gtttcaccat gtttgccagg ctggtctcga attcctgacc tcaagagatc 7560
cgcccgcctc agcctcccaa agtgctggga ttacagacgt gagccaccac gcccagccca 7620
gagttctctt tttataggtg ttctggccct tgtctatttg tatatccagt ctctccatcc 7680
ttctgcttga ctttgcagat caactccctt ctcctgacct ggaccttgtg gatactgagg 7740
cagaggcttt ccagtgttaa tgccgaagtc tcaacgctaa aagacaccag gtaaagccct 7800
ctttcacctc cccctcctct tttaaaattt cctctttctt cttcccagaa aagttctttg 7860
agcacttctt gtgtgctggg tcctatgcct gagacagagg tgatgaggtc tgtttagcat 7920
atcacaccat ctagatctgg aggcttaaag tccagctcaa ctatttacga gctgtgtgac 7980
cttaggcaag ttgcgtaacc tctcagaagc ccatttgcct cacctacaga atggggatat 8040.
taacattgct tattttaggt gccttacaaa gcatttagca cggttactag gaacagtgag 8100
tgtttcatat attacaactc ttaataaatt ttctaatttt tctctgtgca tacttatatg 8160
tgcacattta caagaaactg aatcatacta ttttgttttt tatggttttt tttctctcga 8220
gaatgtcctg atgatttctg acttcattag atattattct acaatatggt gtaaatttgt 8280
agcacattgt atttaagtca taccatattc atggacactt aatttgcttc tatgtgtttt 8340
tggttttggt ttgttctctg tataaacaat actgccttga gcacccttgt aggtaaatcc 8400
ttccacacca tggttattta attacaacac taaacatgaa actacccaaa tccaactgcc 8460
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tgaagagtag gttggactgt aatgctttta gaatgtgcgt cgtaaattga ttttcagaaa 8520
atttgtttcc gacggtgaga atacctattt acttgcaaca ttgtcaacac tggagtttat 8580
tgtttttgat gtattttctg ctgatttaat gcgtgaaata ttctatctca ttacttttgt 8640
tatacatatt ttatattagg gatttgggac ttccttaaat atttttacag gtcaattgtg 8700
tttcctaact tccccactca actactgttc attttaagaa acttcagcaa ccatcatcta 8760
ggttttattg tcagtaacct aaccccattc tttgcttctt ttttcttcgg tatccaatcc 8820
cttatccatt ttaaatatgt ttgaaaagtt agtacaaaag tcaaaaagga ggcctggtgt 8880
ggtggctcat gcctgtattc ccagcagttt gggaggctga ggcaggtgga tcacgaggtc 8940
aggagatcga gatcatcctg gctaacatgg tgaaaccctg tctttactaa aaatacaaaa 9000
aattagccgg gcatggtggt gggcgcctgt agtcccagct acttgggagg ctgaggcagg 9060
agaannnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9120
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9180
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9240
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9300
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9360
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9420
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9480
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9540
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9600
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9660
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9720
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9780
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9840
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 990o-
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn 9960
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10020
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10080
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10140
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10200
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10260
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10320
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10380
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10440
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10500
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10560
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10620
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10680
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10740
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10800
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10860
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10920
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
10980
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
11040
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
11100
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
11160
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnngg agtgcagtgg tgtgatcttg
11220
6
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
gctcattgca acctctgcct cctgggttca agcgattttc ctgcctcagc ctcccaagta
11280
gctgggatta caggcgcccg ccagcacacc cagataaatt ttgtattttt agtagagaca
11340
gggtttcatg atgttggcca ggttggtctc gaactcctga cctcaggtga tccacctgcc
11400
tcagcctccc aaagttctgg gattacaggt gtgagccacc acgcccagct ggtctatttt
11460
cattatgagg aaaccgaatg tcatagaggt taagtccagt gatttcagaa tgagccacag
11520
gaatcacccc actggaaaaa ttgcccatat catggatgac atattctctt ggcaatacat
11580
ttttaaacat aatgcagttt actatagttt taatgaaaaa gacagtatag gtatagaaca
11640
ttttagaaaa catttgtaag cattgaaaac aaataaaatt tcccattatc ccaccacata
11700
cagatcccag tgagtcatat ttttggatgc aagcacctta gatccagtgg cttaattaaa
11760
tagggaaatg attttccttg caagaagtcc agaattaggt gttgcgaata ttgctggtgt
11820
tgctggttct atgcttagtg gtgttgtaaa ggctctaaac taagaatctg ggttttcccc
11880
agttttccat tccaccatca acatgttgac attttattgt attttcatgg ttgcaatatg
11940
gttgccacag, ttccagacat catgactatg ttcaagtcaa gaagaagagg gatggacagg
12000
accagaaagc ccttctttca tgtctttgtc tttaacagta aatgaaagcc cagaaaactc
12060
tcagatttcc ctttacctaa ttggtcagaa gtgtatcatg tgaccactca tatgggcaaa
12120
aaattttgaa ccaacagctg tttggcttcc taaagtccag agtgggcgct gtcaatgctg
12180
aggaggttga gaatggcttt tgggttttcc aactctgcct gccacactag ggacaatcgc
12240
atttaacatt ttagtgttta tccttctagt ctttgttact gtatttttaa tacttctgtc
12300
cctgtagaca tgacccttgg aatcacgttt gtattaattc tgctgcagac tctgaggaaa
12360
ttcttttctc tctcacaggg ttaaagtcct ttcttgcttt caaatatgct atggagccac
12420
agttgaggac agtagtttcc tgcaggagcc taccctgaaa tctcttctca gcttaacatg
12480
gaaatgagga tcccaccagc cccagaaccc tctggggaag aatgttgggg gcggtcttcc
12540
tgtggttgta tgcactgatg agaaatcagg cgtttctgct ccaaacgacc attttatctt
12600
cgtgctctgc aacttcttca attccagagt ttctgagaac agacccaaat tcaatggcat
12660
gaccaagaac acctggctac cattttgttt tctcctgccc ttgttggtgc atggttctaa
12720
gcatgcccct ccagagccta tcatacgcct gatacagaga acctctcaat aaatgatttg
12780
tcgcctgtct gactgattta ccctaggata aagactcttt gtgattttaa agaaatggtc
12840
agtaaaactg taatgagagt cccagggaac caagacggca catagacaga taagctactt
12900
tggcacaaac acttaaaaat agttgtgttg gtaagtaggg ggatgtttga tgtagcagaa
12960
ttctagcact ccctggaggg ttgaagcact tggtgacaac aacctttgga aagtgctttg
13020
gtttcctgag gtccttcagg taaccaagac ctccctgttt tctagaaact ttctaatgac
13080
ttggctagga tttaggattt tctctatcac agttatcgct acctatcctt aatttttttt
13140
ttttttttga cagagtctcg gtctgttgcc caggccagag tgcagtggca tgatctcggc
13200
tcattgcaac ctctgcctcc cgggtgcaag tgattctcct gcctcagcct cccaagtagc
13260
7
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tggactgcag gcgtgcacca ccacgcctgg ctaatttttg tatttttagt agagatggag
13320
tttcagcatg ttggccaggc tgatcttgca ctcctgacct cagatgatcc acccgcctca
13380
ccctcccaaa gtgctgggat tacaggcatg agccactgcg cccggccacc atccttaaat
13440
tcttgagctt gggagagaag aagatgaagg tttaaatgca ggtattttca cttctatggg
13500
cactctaaaa cctcccaatc caaagcgtgg tccctggatc atcagcatca cctgagagct
13560
tgttagaaaa gcaaagaaga atctcagacc ccactccaga cctcctagat caggacttgc
13620
atgttaacat ccccagggga tgaatataac~catgggtgtc tgaaaagcac tattgtagaa
13680
cattcttctg tttgccatgc tgacagcaga gcttgtcttt aaagccagag agaaatgtct
13740
tcttcttgac ttggaccctt tgcatcttct cttagaataa cttccctgaa aactcaattg
13800
atgtcttctg tctctttgag ttgcagtgta gtcatcccct ctgctaggaa gcattctttg
13860
aatctcctac agtagaattt ggcattattt ttgtgtgtgc caaaaagctt catgctttct
13920
caccctgtat tatgatttct ctttttgttg ggtgtctcca acatagactg tgagcttcgt
13980
aagtacagag atttgcatta agtagcgtct caacaaatat gttgaatgaa tgaatgaatg
14040
aatgattaga atgattactc ttcattttga agacagggga actgaagctc agagaggtta
14100
agtgactatc ccaggatcac acagccacat aggtcgttga gctgggtttt gagctcttga
14160
ccataaagct ccttctccag atgccttgtt cccctttttc tcttcctttc gtgccaggaa
14220
tacttagttt agggttacag agactagaat aatatttact ctgagtcacc taagcagtta
14280
ggattcaatt agctcagtca tatcatgcac tggacaattt gttaacaata ttaaactcag
14340
ttgatggagg ctctgtcttt gttattcttg gcagcctaac ttgggaggtg ggacacatgt
14400
ttgtgaacaa gagttcatct atgataaatt agtcttcaac gcttggctta tccagagtgc
14460
tcaatacaga ggtgatagtt cttagagaga tggaagcaag agatggcact gctcctatcc
14520
atagccaatt gtcataggtc aatggcatca atgggcccaa taatggtctc catttagcca
14580
catccctttc ctgtaacttt gtaactctct ccttgtctga ctctggggtt ggacttgaga
1464Q
tttgctgcag ccagtggaag gttaggaaat gtgactggaa cgtaagtttc aaaatcgtat
14700
gtgtaatcct tcttaccagt tacacgatta catttgctca ccttgcttcc ttacccccac
14760
catgtgaaca tgcccaagcc cgcctgctgn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
14820
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
14880
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
14940
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
15000
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
15060
nnnnntagct ggaattacag gcatgagcca ccacacctgg ctaatttttg tatttttagt
15120
agagatgggg tttcactatg ttgcccaggc tggtctcaaa ctcctgatct taggtgatcc
15180
acccgtctca gcctcccaaa gtgctgggat tacaggcatg agccactgtg ctcggcctaa
15240
acctctgctc taaaagtaat tttttgtgtg tctgcatttt attttatttt attttatttt
15300
g
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
attttatttt attttatttt attttaagac agagtcccac tctgttgcct aggctggagt
15360
gcagtggcac gatctcggct cactgcagcc tccgcctcct gggttcaagc aattctctgc
15420
ctcagcctcc ggagtagctg ggattacagg tgcctaacac cataccacac tggctgattt
15480
tttgtatttt tagtaaagac ggggtttccc catcttggcc aggctggtct tgaactcctg
15540
acctcgtaat ccaccctcct cagtctccca aagtgctgag attacaggtg tgagccacgg
15600
tgcccggcca tgtctgtatt ttctatttcc ttggtgtgag gcaatgaacc tcgggtatta
15660
ccccagatgg atgacactgc ttcaatatct gccagtcact acactttgtt atcagtggga
15720
ttacatcaac tggagcccat tctatctgct tggaggccag gcctgaagat tcaaggggtt
15780
gacaccctct aggagcaagc ctcaagtcaa cccatgactg acagtaggtg ggatataaat
15840
acctcagctc cctcacccct cggttggaat aactgaagag tatgttccat gccgctcccc
7.5900
agtcatccct ggggaggtta agttccactt tctcaccatg taacttattt gaagacacac
15960
ccagtattgg ctctttgcct ttcctgattc actttctcct cccccagctg gtgctttctg
16020
gggccacctt cttgataaac tacttttgct agaatccttg tctgagggtc tgcccttgtg
16080
agaccctaag atgttaccag agagagttag gaagctggga atggggtgga agtgttgctg
16240
tataatcgtc agacagagtt gagatctgaa ggaaaataag atggttaaag ctcttgactc
16200
tggaatcaga gggtcctggg ttcaaatctc aagtctgaga gatcttggac aagttataac
16260
tcctctgcaa gtctcagttt ccattttgtt ggtgaataga gggagggagg ggaattccta
16320
ctttacaagg ttaacatgat aatgaaatta cactttgttg ccaatgtgcc tagcacactg
16380
cctggtacat gatggttgtg atggctgtct ttatacggga gactgggaag agaccctaaa
16440
ggctgagatt tgcttcactg gaggcaggaa gcggaagctc ctttattggg tgcaatgaaa
16500
gaaggtgttg agagatggga gatagatgtt gatgggaaag aaaaagggag agaccatggg
16560
aaggagagag agagagaatg atatacatta ggcagattaa cttctcttta ttgtgaagaa
16620
agggggaaat taggattttc taggctgtct tttagttgca gcaagtcagc aaaaagggct
16680
atgatgacca ggcagcattg agcaactctg actccgcctt cccctcttcg cacaccttct
16740
ggttactgaa aacccagcgt tagtagaaaa gtttcttttc tttgaatgac agaactacag
16800
cataatgcgt ggcttcaacc tgctcctctt ctggggtgag tgtgaggctg aatggggggc
16860
taggggaggc ctggattgga aatagacttc aggagaaatg ggagggccat ggatggtcca
16920
gccaagagat cataagtcca gactggatgc tgcaaacacc ttcattcagg gaacctttga
16980
caaaattcca tagctcaggg aagtccatct gttaggatag atttttctct atagtcattc
17040
atttgacctg agcaagtcct gtgtgtcagg tgctgttcta ggcactagag gatatagcag
17100
tgagaaaaaa agactaagtt cctgcactct tgaggctgat attctagtgg gtaggagcag
17260
acaggaaaga aggcacagat gatcatagcc cacttagcac tgctgtaact tctttaaaca
17220
tagagtgtat ccaattgtca caacatttct atgacattgg gtcattatta gtcctatttt
17280
gcaaatgaga aaactgaggc aaaagagata aaataatttt cccaaggcca cttagtcaat
17340
9
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
aaggggtaga ggcaggattt caagccagac agtctgactt cagagtctgt aagcttttcc
17400
actatactat gttgccccaa aaaataagta aacataggaa tttcaggtag tgataagtgc
17460
tatgaaagaa gttgttatag aatgtggtcg ggagttatgt tgaagggaga agtctggggt
17520
tgaggagggg cagttcgagc tgagtcctga aggatgagat agacccttcc tgtgctctta
17580
tttctctttc ttcaggtgtc agtttgtctc agccttgttt atcttctgtg tttctggttt
17640
ttcttctatg tcttgtgtgt tttggtcatt ggatcaaatt taagaatgga ggatattggg
17700
gacatattct aagtagcaag tatgggtttc ctctgctgtt gaagagtgga tctgaatacc
17760
ctattttacc aaggaggaaa ctgaggcaca aagagattaa ataacttcct caaggccacg
17820
caatcattaa ttggtgaagc cagattttta aacctaagca ctttgacttc agactttgta
17880
tgcctaaaca gcatgctgaa ttacctctaa aataattaat gaatgagtag aagaattttt
17940
gttagtaata atagatagaa tttgggagtt tagatctgtg ggacttgttg actggcaggc
18000
tacattagaa gaaataggtg gcctgtggac atcaaatgta ggcatgcaga gttgttattt
18060
tggggtgttg gcaatggtat taaccattaa cactccctat tctgccctga agataatcag
18120
caaagagttg tgataatgat agtgatgata atgatcatga tggtggtggt tataatagtg
18180
atgatgaaaa tggtgatgat gatggtgatg gtgatgatga tgatgatgat aatggtgatg
18240
atagtgataa tgaagatgat ggtgatgatg atggtgtgat aatgaccatg aagatggtga
18300
tgatggtgat gatgataatg gtgatgatag ggataatgat gatgatggtg gtgatgatgg
18360
tgtgataatg acgatgaaga tggtgatgtt ggtgattatg gtggtgatga tgatgatggt
18420
ggtgatggtg ataatggtga tggtgatgat gataatggtg atgatggtga taataatgat
18480
gatgatgatg gtgatgatga agatgatgat ggtgatgatg atagtgataa tgatggcgat
18540
aatgatgatg gtgcaggaga cctaaatgct gcagggctgc attgccttta aacactaaaa
18600
aatgggcagg gcgcagtggc tcacgcttgt aatcccagca ctttaggagg ctgaggcagg
18660
cagatcatga ggtcaggaga tcgagaccat cctggccaac atggagaaac cccgtttcta
18720
ctaaaaatgc aaaaattagc tgggcgttgg gtggtgcgtg cttgtaatcc cagctactcg
18780
ggaggctgag gcaggagaat agcttgaacc agggagttgg tggttgcagt gagcctagat
18840
cacgctactg cactccagcc tggtgacaga gtgagactcc atctcataaa aaaaaaaaaa
18900
aaaaaaaagg aaagaaaaac gaattgccct accctctcat gaaatgttaa tactacagat
18960
atactgtata cctatatatg tattttctat atatacatat gtatcttata cataaaagag
19020
taagcttaaa aagatctctt cctgagaacc aagtttgggt ccttagagga tatatcaccc
19080
cttttgagaa tgcatgcttt aatgtatgtt tttgaaaatc agctagaact ttttttttta
19140
gacagggtca cctaggctgg agcgcagtgg catgatcttg gctcactgca gtctctatct
19200
cctgggctca agcaatcctt ccacctcagc ctcctgagta gctggggcta caagttgtca
19260
ccaccaccca gctaatttaa aaaatttttt ttttgtagag acaaagtctc actgtgttgt
19320
ccaggccggt gtcaaactgc tggtctcaag tgatcctccc accttggcct cccgaagggc
19380
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tgggattata ggtgtgcacc acagtgctta gcctggaact atttaatcaa aaagaaaaag
19440
aagataaaga cagacaaaat gaaaagcaaa agcaaagtac agaaaaccag gaatggtcat
19500
tgttaagctt atgcaaaata gtgtatgttt gttccttaac taggtttgtc atctagcctc
19560
ttcaaaccta tttctgtggg gccaagccag gagtaatttt gcaggttgag cccaaaaggt
19620
tttttttttt tttttttttt ttggtggttc atggtgtttt tcttttcttt cttcacagga
19680
tgttgtgtta tgcacagctg ggaagggcac ataagaccca cacggaaacc aaacacaaag
19740
ggtaagttgg ccagagagaa tgcagatgcc tgaaggggta gagaaagttt tctccccggg
19800
gaaacatccc aggaagctga gcctcatcca gatgcttgct gggagctagt tagcggcaga
19860
gcaagggtta acatgcaagt ctcctgattc tcacttcagg ctctttcctt tgtgcttaga
19920
attgctgtgt gatctcagat aagtcacata acctctctga acttcagttt cccaagctgt
19980
gaaatacaga taacaatagg gatgttgtga aactttaatt caaagaggta gctttggaaa
20040
ccacctggta tgtagtatga gctttataaa tggtagtgta gtgagagtag ggatggaatt
20100
tactcaaact acaactcaat gtgcaaaaaa aaagtgactt ctttatgttt tgttaaatac
20160
tctcatctac ataaactctt gctggataaa ggaagaaaga cattgtcagt tcgtagttgt
20220
tgatatggtt tggctatgtc ctcacccaaa tctcacctta aattgtaaga atccccacgt
20280
gtcaagggcg gggccagttg gagataattg aatcatgggg gatggtttcc cccatactgt
20340
tctcgtggta gtgagtaagt ctcacgagat ctgatggttt tataaatggg agatccccgc
20400
acaagctctc ttgcctcctg ccatgtaagt aagacataat tttgctcctc atgattgtga
20460
agcctcccca gccatgtgca acagtgagtc aaattaaacc tcgttccctt ataaattacc
20520
tagtctcggg tatgtcttta ttagcagcat gaaaacagac taatacaact gttcccagtg
20580
tctctgtttc ctggttcttc tttgggcaac ttagcaactt ggtctatatt gtgttaacag
20640
ctcatctttc acattttttt tttttttttg agnnnnnnnn nnnnnnnnnn nnnnnnnnnn
20700
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
20760
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
20820
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
20880
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
20990
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21000
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21060
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21120
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21180
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21240
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21300
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21360
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21420
11
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21480
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21540
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21600
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21660
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21720
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21780
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21840
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21900
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
21960
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22020
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22080
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22140
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22200
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22260
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22320
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22380
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22440
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22500
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22560
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22620
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22680
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22740
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22800
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22860
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22920
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
22980
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23040
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23100
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23160
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23220
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn.nnnnnnnnnn
23280
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23340
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23400
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23460
12
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23520
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23580
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23640
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23700
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23760
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23820
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23880
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
23940
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24000
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24060
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24120
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24180
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24240
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24300
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24360
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24420
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24480
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24540
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24600
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24660
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24720
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24780
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24840
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24900
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
24960
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25020
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25080
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25140
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25200
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25260
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25320
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25380
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25440
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25500
13
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25560
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25620
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25680
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25740
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25800
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25860
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25920
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
25980
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26040
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26100
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26160
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26220
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26280
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26340
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26400
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26460
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26520
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26580
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26640
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26700
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
26760
nnnnnnnnnn nnnntgaagt gcatatggga tgggagatat tgttttaact tttttttttt
26820
ttttttttgc aaaatacagt cttctatcta gtataagtaa atttgtaact ccaattctct
26880
gtctagatat tgatgaatgt tctcaaagcc cccagccctg tggtcctaac tcatcctgca
26940
aaaacctgtc agggaggtac aagtgcagct gtttagatgg tttctcttct cccactggaa
27000
atgactgggt cccaggaaag ccgggcaatt tctcctgtac tggtaatgct ctcaggttcc
27060
cagggatggg tcttgggtgg atatctatca gtggggtgag ttcatgtatt tctgaactaa
27220
ggcacccaat ttcttatctg ctcaccctct tccactgctt ctcagatatc aatgagtgcc
27180
tcaccagcag ggtctgccct gagcattctg actgtgtcaa ctccatggga agctacagtt
27240
gcagctgtca agttggattc atctctagaa actccacctg tgaaggtatc catgaccatc
27300
tctttattat ttacctactt aattaattaa gggtcatctc actggaagac catatgagaa
27360
agggattttc tcatgtaggg ataagaaaat tggggctcag agaggtaaaa atatatagtt
27420
gcttatatct ttttctatcc ctttactttg agcctgtggg tgtctttaca tgctaggtag
27480
gtctcttgta gccagcacat ggttgggtct ttttaaaaaa ttttttatct ggtttaacaa
27540
14
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tctatatctt ttaagtggag cattaggcca tttctattca aggttaatat tgatatttga
27600
gggttttgtt cctgtcacag cattgctagc tagtattgct agcaaacatg atttccttcc
27660
ttccttcctt ccttccttcc ttccttcctt ccttccttcc ttcctacttc cccacccttc
27720
ctctctctga tcatgtaaat aattcctggt cactgtaaaa tttcatggat ggttgagttt
27780
cctcacaata tggtggctgg attccaagga taagcatccc aagagaacaa ggtggaagta
27840
catgatattt ttatgaccta gtgtcacttc caccatagtc acaagtatgc tcagattcaa
27900
gatggggacg taccttccat tgcttgacag aagaagtacc aaagtgaagc gcatatggga
27960
tgggagatat tgttttaact tttttttttt ggcaaaatac agtcttctat ctagtataag
28020
tgaatttgta actccaattc tctgtctaga tattgatgaa tgttctcaaa gcccccagcc
28080
ctgtggtcct aattcatcct gcaaaaacat gtcagggagg tacaagtgca gctgtttaga
28140
tggtttctct tctcccactg gaaatgactg gatcccagga aagccgggca atttctcctg
28200
tactggtgat gccctcagtt tcccagggat gggtcttgag tggatatcta tcagtggggt
28260
gagttcatgt atttctgaac tgaggcaccc aatttcttat ctgctcaccc tctttcactg
28320
ctttgcaaat atcagtgagt ccctcaccag cagcgtctgc cctgagcatt ctgactgtgt
28380
caactccatg ggaagctaca attgcagctg tcaagttgga ttcatctcta gaaactctat
28440
ctctttatta tttacctgct taattaatta agggtcatct cactggaaga ccatatgaga
28500
aagggatttt ctcatgtagg gatgagaaaa ttggggctca gagaggtaaa aatatatagt
28560
tgcctatata tttttctatc cctttacttt gggcctgtgg gtgtctttac atgttaggta
28620
ggtctcttgt aggcagcaca tggtttggtc ttttttacaa aaatttttat ctggtttaac
28680
aatctatatc ttttaagtgt agcattaggc catttctgtt caaggttaat attgatattt
28740
gagggttttg ttcctgtcac agtgttgctc gctacttgct ttgtgatctc agttgtgtaa
28800
ttgctttata ggatctgtga actatatgca aatattaagt ggaagagctg ggatttgaac
28860
tcctgctgag gacctactat aagtcaaaca atggtatcag aatataatgg ggtgcacttg
28920
tctccagacc gatattttcc ccaggggaca ttggcaacat ctggagatat ttttgattgt
28980
ctcaaccggt gatggtgggg agaggtgctg tgtatggcat ctgatgggaa gaggtcagag
29040
atgctgctaa acatcctata atgcaccgaa aggcccccac aaccaagaat aatctgaccc
29100
taaatgccaa tagtgctgag gtggaaaaac cttattctgg gctgggcgcg gtggctcacg
29260
cctgtaatac cagcactttg ggaggccaag gcggatggat cacttgaggt caggagttca
29220
agactagcct ggcgaacatg gtgaaacctt gtcactacta aaaatacaaa aaattagccg
29280
ggcatggtgg caggcacctg taatcccagc tactcgggag gctgaggcag gagaattgct
29340
tgaacctggg aggcggaggt tggagtgagc tgagattgtg ccattgcact ccagcctggg
29400
caacagagca agactctgtt tcaaaaaaaa aaaataccca acaacaagaa gaacaaaaca
29460
cacaaaacct attctgggga gattctaaag tctaaacttc agaacgttcc cctaaaaggt
29520
atttgaatta acagttgaaa gttatcttta atcagccggg cgcagtggct cctgcgtgta
29580
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
atctcagcac tttgggaggc caaggcaggc agatcatgag gtcaggagat ggagaccatc
29640
ctggctaaca aggtgaaacc ccgtctctac taaaaataca aaaaaaaata gctgggcctg
29700
gtggcacgtg cctgtaatcc cagctacttg agaggctgag gcaggagaat ggtgtgaacc
29760
caggaggcag agcttgcagt gagccgagat cacaccactg cgctccagcc tgggtgacag
29820
agcgagactc catctcaaaa aaaaaaaaaa gaaagttatc tttaatcttt tctctccctt
29880
cacattaaag cagtcatcac accttggaga atctacctgc taaatatgtt ttgagactgg
29940
gtgtggtggc tcacacctgt aataccagca cttagggagg ctgagatggg agaatcgctt
30000
aagaccaaga gtttgagacc agcctgggca acatagtgag accctgtctc tacaaaaaaa
30060
aaaaaaaatt agaaaattag ccaagtgtgg tggcatgtgc ctgtggtccc agctactcag
30120
gaggctgagg tgggaggatc acttgaaacc aggaggttga ggctgcagtg cactatgctt
30180
gcatcactgc attccagcct ggataacaga gcaagacctt gtctcaaaaa taaatatata
30240
aataaatatg tgttgaattc ctccactgtt ttccatcctt ggttcaggct tgatcatcac
30300
tgcagcagcc tcctctctag tcttgccttt ttcagtctta ttctctccaa tccatcctct
30360
acactgcagc cagaatgtcc tcaccaagag acaagagctc catcttcctg tgtctagtgc
30420
attgttattc tccacattca tttaagggat ggtgactcca ttgagaaagg caaggagtgg
30480
tcaagccctc attatatccc cctggcttag tttaagagat gtttgaaatc atctctgcct
30540
ttgctactcc atttgtagcg gtgagctatc actgggtaac aaattgtctt aaaaattagt
30600
gtctaaaaac aaccaccata tatttagcgc ttgattccat gagttgacat tttaagtggt
30660
ctattctagc cagatctttt ggtcccaact ggtcttgttc ttgtgtctgc agtcagctga
30720
ccttggctgg gcttttgtct tgttgcctct gccaccttag gtgtgagcct catccccatg
30780
gttgggaatg gatcacccca ctctgtccat cttccagccc aggtgaaaag ggagaagaag
30840
tgtagagtat gctctttctt ttcaagggca tgaccagatg gtgcacacat cacttctatt
30900
tattctcctt agccagattt tcaccatgta gctacaccta gctgcaagag aagctgggaa
30960
tgcagtcctc agctgtattg ttacattatt acaaggagga gggaatgggt ctcacatgtc
31020
tggggctcat ctgggatgat tcatttctct cccatttggt cttttgttct tcattaggct
31080
agtcaggggc ttgttcatat ggcagagatc agaggcccaa tagaatgaac agaagccaac
31140
aggtctcttg aggcccaggt ttggaattag cacctcatta cttcactgtc ttctattggt
31200
cagtgcaagt cgtagggcca gctgtattta aggggtagag aaacagactc cacctcttaa
31260
tgcaaggtat ggtaaagtca catctcttac aatctacatc ttggggtgta aatacagaga
31320
gaagtaaagg actatggcta cttttccaat ttctgataat tatgttttga aactatttgc
31380
cacccttctg cttacatgct attggctaga atttgctctc atggccatgc ctactgaaag
31440
aaggaactgg gaaatatgtc ccactgtgtg cctgggaaga aggggggaac atggatattg
31500
gggagcatca gccctgtctg ctgaaaatca acactcagag tgcatctcct atttgtagtc
31560
tctactcctt gctttctcat ttaccttgac ctgggttttc tcttccactc ttcagacgtg
31620
16
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
aatgaatgtg cagatccaag agcttgccca gagcatgcaa cttgtaataa cactgttgga
31680
aactactctt gtttctgcaa cccaggattt gaatccagca gtggccactt gagtttccag
31740
ggtctcaaag catcgtgtga aggtaggtgg gggtgtcttc tgagaagtca ggtccaagtc
31800
tgtttgaaaa gacagcagtg aggggattag ggtgggtctt ggttgagttt gagactttca
31860
tctgcaaatc tgaagccaat aatcattcat tcactcatta aagaaatacc tcttgagcac
31920
ttacagtata cgtggcacta ttctaggcac tggggataga gcagtaaata acatctccaa
31980
ataacatccc actggggctt agggcttcca tataggaatt tggaggaggg gcaccattca
32040
gtccatagca cccatgaaca aacagcttgt aagtgacaga gccaggctat gaacccagaa
32100
tgtccaatgt tctcaacccc tccactgtgt gcctcttttt gtttgtttgt ttgtttgttt
32160
tttgtttttt tgggtttttt ttgttttttg gttttgtttt gagatggagt cttgctctgt
32220
tgcccaggct ggagtgcagt ggtacaatct cggctcactg caacctcccc ctcctgggtt
32280
caagcaattc tcctgcctca gcctcccaag tagatgggat tacaggcatg tggcaccatg
32340
tccagctaat ttttgtattt ttagtagaga caaggtttca ccgtgttggc caggctggtc
32400
tcaaactcct gaccccaggt aatctgcctg cctcggcttc tcaaagcgtt tggattacag
32460
gcatgagcca ctgcgcccag cctgtttttt gtttttgaga cggagtcttg gtctgtcgcc
32520
caggctggag tgcagtggct tgatctcagc ttactgccac ctctgcctcc caggttcaag
32580
caattctttt gcctcagcct cttgagtagc tgggattaca ggtgtgtgcc accatgccca
32640
gctaattttt tattcgggac aggtaagccc caaagtgggg tttagctcac aagggttctt
32700
ggctttgccc aggagagaat tcaagggcaa gccagaggta gaagaaaaca gctttattga
32760
agaggcagtg ttgcagttct gtaagtgtta cagctcctgc agagcagggc tacttcatag
32820
gcagagagca gcagctcagg gcagttttgc agtcatactt atacctactt ttaataatat
32880
gcagattaac aggtggttta tgcaaaaatt tctagggaag gggtagtaac ttttgggtca
32940
ttgagtcatt gccatagaaa ggggcagtaa ctccccaata ttgccatggc aatggtaaac
33000
tgacctggca cactggtggg catgtcctat ggaaagctgc ttccactctg tccttgtttt
33060
agctagtcct caattttgtc cagtgtctga gtccctcatc tggagttgag tcctgtttcc
33120
tgcctcatta ctagcttagc gtaatagaaa tgtatcactc tcataataat tcaatatgga
33180
tatacctggc tggtgggcag cttttctcca catggtgata aaggagttca atctctctcc
33240
atcttttggt ttttg.cattc cttagaggct tggtgttcct ggtttagcca gttgaggtga
33300
aagagggagg agagaagtca cacccacttc ttgaccacct tggccctgaa gtgacatata
33360
tcatggctct catattccat gagtgaaaag tagtcacatg acctcaccta gatgcagagg
33420
gttctaggaa atgtagtccc tggctgggac accattttct agtgaaacat gactccatat
33480
tttgcaaagg gaagcatgat tttgttggct cattggccaa cttggctcca cacccattct
33540
gtaccccaca gatattgatg aatgcactga aatgtgcccc atcaattcaa catgcaccaa
33600
cactcctggg agctactttt gcacctgcca ccctggcttt gcaccaagca atggacagtt
33660
17
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
gaatttcaca gaccaaggag tggaatgtag aggtgagcag agagtttgat ggacaatcca
33720
gaaaagacat ttctctttgc tcttctgggt ttcttgatat tctccagttc tttgcagata
33780
ttgatgagtg ccgccaagat ccatcaacct gtggtcctaa ttctatctgc accaatgccc
33840
tgggctccta cagctgtggc tgcattgcag gctttcatcc caatccagaa ggctcccaga
33900
aagatggcaa cttcagctgc caaagtaata atctctttgt atgtcttggc aatggaatct
33960
gtttctggct ttggttggaa aattctcatt ctacccaacc tcgggatctt cctcttagtg
34020
ttgcctcatc cacatattct gtttcttttt cttcttcatc catttactct ttcaacaaat
34080
gatttttgga gtacctatta attaattagt taaacaggac cctgcagtag gcactgaaga
34140
tttggtcctg aatatcatag gcatgcttct tgtcctcttg tagcttatag tctagggaag
34200
gagccagaac acacacacac ccaattattt aattataact atgaatgaaa caacaacaaa
34260
aaagtgtgct tttttttttt tttttcaccc aggctggagt acagtggcag gatcttggct
34320
cactgcaagc tccacctccc gggttcacgc cattcttctg cctcagcctc ctgagtagct
34380
gggactacag gcgcccgcca ccacgcctgg ctaatatttt tgtattttta gtagagacgg
34440
tgtttcacca tgttagccag gatggtctcg atctactgac ctcgtgatca gcccgcctcg
34500
gcctcccaaa gtgctgggat tacaggcatg agccactgcg cctggccaaa acatagtgtg
34560
ctttgaagtg gctaatagga tattgattat gctccaagca aacaacattt aagcagaaac
34620
ctgaaggagg agaaggactt ctctagagga agaatggaga tcatgctgtt ctgggttata
34680
gcagatgcaa aggtcctgag gctcacacca aatgaggaca aggggaagtg gcagtagaca
34740
tatctgatga agtggacaga ggtcagattc tgcagggtct tggtggcttg tgaagaattt
34800
tgaaagggct ggatgcagtg acatacctat aaacaccact ttgggaggcc aaggtgagag
34860
gatcacctga ggccaggaat tcaagaccag cctgggcaac attgcaagac tccgtctcca
34920
caaaaaataa tgataaaaaa ttagccaggt gtagtagcat gggcttgtag tcctagctac
34980
tcaggaggct gaggtgggag gatcgcttga gcctgggagg ttgaggctgc agtgagctgt
35040
gctcatgcca ctgcactcaa gcctgggtga cagagtgaga ccctgtcttt tgtttttttt
35100
tctttttttt ttttttatgg agtctcactc tgtcgcccag actggagtgc aggggcgcga
35160
tctcagctca ccgcaacctc cacctcccag gttcaaatga ttcttctgcc tcagcctgcc
35220
gagtagctag gattacaggt gcccatcacc acacccagct aatttttgta tttttagtag
35280
agatggggtt tcaccatgtt ggccaagctg ttcttgaact cgtgatcttg tgatcctccc
35340
acctcagcct ctcaaagtgc tgggattaca ggtgtgagcc accgtgcccg gccaaccttt
35400
cttttaaaaa agaatttcag agtcctttcc cctcccccca agaataatgg ggaaccatga
35460
aaggtttaaa actgttaaat tatgtattat ttattacatt aaaaatatgt gtaacttatt
35520
ggaggcaatg aagtataaca agcaaatgaa tatatctgat gtatttccaa atataataaa
35580
gataatatca tcaatgtcgc tggttctcct atatgtttct tctccactct atctccttgt
35640
atcctcctta gacagccact tggaattttg tgtttattat ttccctactt aaaaaaatat
35700
1g
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tttcccgtgg tatgtacccc taaacaatat ctgcctagta ttgcttgtgt ttggagcttt
35760
gtaaaaatga tatcatacta catatgacta tagatgtatg tcttttatat gtatgacttc
35820
tctaatttgc tctgtttcac tcaatatcat gcctcttaga gtgtaagctg tgcagggaca
35880
taatattgag tgagaagtca cacatataaa agacatacat ccacaatcat atgcatgatt
35940
gcctctaaga ggcatgatat tgagtgaaac cgagcaaatt agaaaattgt gtgttgtagt
36000
aaacacgtcc ttcatgttgt agtgtgtagt tgtggtttgt tcattttccc ccttgtgtat
36060
tagggtaata gatggatttt aagtcgggca cgggaggaca tgaaatcaga cttgaatttt
36120
catcttattt tgctgaggtt gaagtttggt ttggttgctg ttgttttctt cctaggggtt
36180
ctcttcaaat gtaaggaaga tgtgataccc gataataagc agatccagca atgccaagag
36240
ggaaccgcag tgaaacctgc atatgcaagt attttttaag gttcctagtt tttgaggttt
3&300
tcttagaagc catttaggta gaatgttgtt tttgcagttc taacaaaggc aaaagtaata
36360
gtggccaaaa ccagacagca gtttattttt ctcctctgta acctgctgag tgtaagctgt
36420
gcagagctca taccatggct ccattatgct ctgccagcat caacacaaga ctcccacttt
36480
gtggcccaag gtggcggctc cagctcctgc cctcacatct gcattccagc ttgtgggaag
36540
gggagaagag gaagtgaaaa gcacggtcct tctctttaag catatgaccc agcaccactt
36600
ctgcttgcac ctgattgggt agaaattagt cacatggtcc cacctagctg caagggaagc
36660
tgggaagaga gaatgtttgc ctatctaaaa cttgaggatt ctcttaataa aagaacggga
36720
gaatatattg gagtcttcct gccagtgaag gtgttgatgc tgcaactgag taatgttggt
36780
gtgggggtgt tacacagcca gccaggccaa aaggaatttg gacatcctga ctgtctaatg
36840
aagtcattac aatgagtaaa gactccatga cctctttaga aatcaatgtc actattctaa
36900
atatgagctt gtaaattggc tatgggcttc ccacatgcct tggaccactg tttttctttt
36960
ttaattgtgg tgaggtacac ataacataaa attcaccact ttaactattt taaagtgtgc
37020
aattcagtgg cacttagacc attcacaatg ttatgcaatc atcacctcaa tctagttcta
37080
gaaatttttt tttttttttg agatggagtc ttgctctgtt gcccaggctg gagtgcagtg
37140
gggtgatctt ggctcactgc aacctccgcc tcctgggttc aagcaattct tcctgcctca
37200
gcctcccaaa tagctgggac tacaggcacc caccaccatg cctggctaat ttttgtgttt
37260
ttagtagaga cggggttttg ccatgttggc cagactggtc tcaaactcct gaccttgagt
37320
gatccacctg cctcagcctc ccaatgttct gggattacag gtgtgagcac cgtgctcagc
37380
caagttctgg aatattttta tcactaagca gtcactcccc attctcccct cccactagcc
37440
catggcaaac actaatcagc tttctgtctc tgtggatttg tctattcttg acatttcaca
37500
gaaatggaat catgcaatgt ttgctccttt gtgtctggct tctttcactt agcatgatat
37560
tttcaaggtt ctttcatgct gtagtacata tcagagaact ccattccttt ttatggctga
37620
ataatattcc acatggacca ctgttagttc atacatttcc tcttctttta atatcactgg
37680
ttgggaacag gccccccaaa atctggccat aaactggccc caaaactggc cataaacaaa
37740
19
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
atctctgcag cactgtgaca tgttcatgat ggccataatg cccacactgg aaggttgtgg
37800
gtttaccgga atgagggcaa ggaacacctg cccagcccag ggcggaaaaa tcgcttaaag
37860
gcgttcttaa accacaaaca atagcatgag ccatctgtgc cttaaggaca tgctcctgct
37920
gcagataact agccagaccc atccctttat ttcggcccat cccttcgttt cccataaggg
37980
atacttttag ttaatctaat atctatagaa acaatgctta ttactggctt gctgttaata
38040
aatacgtggg taaacctcgt tcaaggctct cagctctgaa ggctgtgaga cccctgattt
38100
cccacttcac acctctatat tcctgtgtgt gtgtctataa tttctctagt gccgctggct
38160
tagggtgtcc ccgattgagc tggtttcggc aatcactaag tagttttgct atttgtttat
38220
gtttgctttt tttattcatg gaaggaaaag gtgatgtgat tctaattctg atcaagtaat
38280
gagttttaat ttccctgaga attctagaga ctcaggaatt cagagccacc tcatatgatg
38340
ggctttatgg gctaatttgt agattacatg cttgggggaa tacatcgatt catgctcaca
38400
aatgctcttt tttttttttt ctgggacgca ggtctccttt tgtgCacaaa taaataacat
38460
cttcagcgtt ctggacaaag tgtgtgaaaa taaaacgacc gtagtttctc tgaaggtaac
38520
gattgggtct tttaaattgt gttttgagtt tcaaacatct tgggcacact ttgggtgcag
38580
aaagatgtct ttatggctgg gcgtggtggc tcacacccat aatcccagca ctttgggaga
38640
ttgagtcaga tgaatcactt gaggtcagga gttcaagacc agcctagcca acatgatgaa
38700
accctgtctc tactacaaat acaaaactta gccgggcatg gtggcaggtg cccatagtac
38760
cagctactca ggaggctgaa gcaggacgat tgcttgaacc cgggaggtgg aggttgtggt
38820
gagccaagat agcaccactg cacttcagcc ttggcaacag actgagactc catctcaaaa
38880
aaaaaaaaag aaagaaagaa atatctcttt ataatgaatg gattgtgaat taatatttaa
38940
ttttttatag ctgtaagatt tgaaaaatta gcatacagta aaattacctt tttacttgta
39000
tagtttgatg agttttctca cctgaataga cttgttgaac taccactata attaggatgc
39060
agaacagttt aattatctcc aaacattccc ttgggcttct cctttatagt cagatactcc
39120
ctccaccact aaactctata aatccctgat ctggtctctg tcaccagagt tttgtctttt
39180
gaagagtgtt atataaatga aaccacatag tatgtggtct tttgagactg gcttactttg
39240
actcagcata gtgcatttga gatgcctgga tagtgtgtat ataaacaatg ccatttactc
39300
ctcctcctcc ttcttccttc tttcttctgt taatgagatt cagcacattc tcattgctgt
39360
atataaccat caccattatc catctcgaga actttctcat ctttctcatc ttttcagggg
39420
gacagaattg gttggtggtt ttttttgttt gtttgttttg ttttgtttgt ttgtttgttt
39480
tgagatgaag tcttgctctg ttgcccaggc tagagtgcaa tggtgcgatc tcggctcacc
39540
gcaaactcca cctcccaggt tcaagcgatt ctcctgcctc agcctcccga gtagttggga
39600
ttacaggcat gcgccaccat gcctggctaa ttttgtattt ttagtagaga cggggtttct
39660
ccatgttgat caggctggtc tcaaactccc gacctcaggc gatccacctg cctcggcctc
39720
ccaaagtgct gggattagag gtgtgagcca ccaagtctgg ccaaaagcta gtttttctta
39780
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
agctttaagt tgaactcatt gatattgtca ttttctttat aaatctggtt atttttgtat
39840
gaaaataaga ataacacttt tacttataat tgtattaatt ttaaattaat acatttattt
39900
ttattcataa agtatacatt catccctagg gttttcagat taaattttga taaatgatta
39960
atcctattta caaacttagt tcaatttcct cgaatgtttt atactatgtt tataaatatt
40020
ccaattcccc cctcccgctc cacccaaatt tcaagtacct ggtgaagagg atttacaaat
40080
ctaataggca aagtcacttg ggtcctgagc gactcataaa tccaatcgat cgaatctata
40140
aatataataa ttcagattct tttagacatt caatatggtt tataaatagc cacgttctct
40200
taaattgttc atattaaaat aacaagtcta attgcttttt gcaataaaat tatgtggttt
40260
attggttagc ttctctaatg tgtcaaactg ttccaactct tttttttttt tttttttgag
40320
atggagtctt gctctgttgc ccaggctgca gtgcaatggc gcaatattga ctcactgcaa
40380
gctctgcctc ccgggttcat gccattctcc tgcctcagcc tcccaagtag ctgggactac
40440
aggcgcccac caccaagcgt gggtaatatt ttgtattttt aatagagacg gggtttcacc
40500
atgttggcca ggctggtctc gaactcctga cctcaggtga tccacccacc tcagcctccc
40560
aaagtgctgg gattacaggc ataagcccca cacccagccc caactcttta aataggagat
40620
gtttctagat tatgctaaag atgtcaaaaa cttctaaatt taacacaaat cctactaagg
40680
atgtttagtt tgcctaaaat tttctaactt tcctgaggat ataaaataac ttccctagct
40740
aagaagaata aatttaacat atcagagggg cgggatttga agagattttg ccagttgtcg
40800
aactgagtac tatgtttcct ggagatggtc ctagttgaga tgtttctgct atgtcagtgg
40860
ggaggggagt cccacaccca tgccttttgg ggattacaga gctcagggct tcctgtttct
40920
cttggacaaa tatctctcaa atgtcccgct taccccgtgt ttattgaaat cctttttttc
40980
cttttatcat ctaatttgga tagatagaaa cttgtattgc tgctcaaatt tcagtttaat
41040
ttcctctacc atctgactgg atttaattca tttaatgcat tttcctgcat ttattaggtt
41100
ctggattcct tttagttttt tttttttttt tttttgcttg ggtcagagtc acatgtgata
41160
gtcaatttat tgataataat caataataag gggaatcata tgaatattac tattactatt
41220
gttgttattg tttttcttac cttaatccta ttcctcaaac acatccagtg ttaagaggta
41280
gataagtatc ttcccctatc tttttacctt gtaagcatgt acacacatac acacatatac
41340
atacatacac atacaaataa acacacacac atatacacac atgcgcacac acatacacat
41400
acatacaaat acatacacgt atacacacgt gtacacacac atatacacct cccccacatg
41460
cacacacatg cacacacagt atgtacacac atacacacat atacatatgc atatacacat
41520
ataacataca tacacatgca cacatacaca tacaaatgca tacacacaca ccccacacac
41580
atttacacac atacacacac cccacacaca tttacacaca tacaccccac acacatttac
41640
acacatacac acatgtatac acacatctat gcatgtaccc atacacacat acagacatac
41700
acagacatct acacacagat acacacatac acatccacac acatatacac actcctgaca
41760
cacatacaca catatataca tacaggcata tacaaatgta cacacataca caaacacaca
41820
21
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tatatacaca cacgtataca tacatataca caaacacaca tacatacata ggcatacata
41880
gacatacaca gagatataca cacatgcaca cgcatgtaca caacccaaca catacacaca
41940
tatacacaca cccacatata taagcacaca cacacacatg cacacacagt gcagtcattt
42000
tattgagcac ttactatgag aggtttagga cagtgattaa tgattcagcc tctgctgcca
42060
gactgccaag gttcaaatcg tggttcttcc acctgctaga tatgagtcct tgtttctcct
42120
ttcaaaaatc aaaataatca taaatagcct ctacttcata gatatcttct acaaattaaa
42180
caagttaata tataaaagac atttagagca atacctggct cgtaataagt gtgtgctatt
42240
tttatttcta tatgccagaa cataaatacc ataatataaa attttaaatt aaaccaattg
42300
tttctaaacc aaatgcaata ctatatacat gatactctgt aatttgcttt agtgaacagt
42360
ggctcagaga cccttttgct tgttaatgcc tataggtata ctgcaatatt cattttaaca
42420
gctgtatggc tgttatttga tttcatcttc atttattgac ccagtctgta gggcatttag
42480
gctttccaca aactatcctg agatgaacat cactggacaa gatccttgca caacttttaa
42540
gtatttctga aaagattaat tccaggaagg ggaattgctg tgtcaaaggg tatatgcaat
42600
ttacatggct ataggtacaa acttactgca ttgcataaag gttacatcaa tttgtctttc
42660
ttgcttgctt tcttgctttc ttttgacaag atctcactct gtcacccaag ctgggagtgc
42720
agtgggagtg atcatagctc actgcagcct tgaactccag ggctcaagag atcctcccac
42780
ttcagcctcc tgagtcgctg ggactagcgt catgcaccac cgtgcctgct cttttttaat
42840
ttgtttgtac agatggggtc tcgttatgtt gtccaggctg gtcttgaatt cctgaacttc
42900
aatttgtatt tctttttttt tcttttttca gacagcctct ctcactgtcg cccaggctgg
42960
agtgcagtgg tgcgatctca gctcactgca agctctgctt cctgggttca agtgattctc
43020
ctgcctcagc ttcccaagta gctaggatta ctggcacgtg ccaccatgcc tggctaaatt
43080
tttttgtatt tttagtagag acagggtttc actatgttcg ccaggctggt ctcaaactgc
43140
tgacctcatg ggaaagtcct cagcctccca aagtgctggg cttacaagtg tgagccactg
43200
cacccggcct gaatttgtat ttctgatggg gcttcttgaa tcagaaaaag gagcccgagt
43260
ggatgcccct tttaattctc ctttggatgg actcccagcc tattcttaat cagaaaaggg
43320
acagctgtta tttcattaat gagagagaga gagagaatga acaaacacat ctttccttgc
43380
agaatacaac tgagagcttt gtccctgtgc ttaaacaaat atccacgtgg actaaattca
43440
ccaaggaaga gacgtcctcc ctggccacag tcttcctgga gagtgtggaa agcatgacac
43500
tggcatcttt ttggaaaccc tcagcaaata tcactccggc tgttcggacg gaatacttag
43560
gtaggagaca ccctttgtgg caaggttaca cctaggggat gattttgaaa gttgactttg
43620
gcaatgggat attccctggg tttcccaccc attctttccc caactgttta aaaactttga
43680
attcacagca aagcaagtct aatcatatta acaacatttt ccattcactg agtgcttggc
43740
aaaggccagg cactgtgcta agaccatatc attaatttat tctacaaata tttgctgatt
43800
agctgctatg tgctgggtta aggtatgaca gtcataggct gtgctcaagt acttcctgtt
43860
22
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tgccctgctg ggcccatggt agaattgtac ttcctggcta ctgtgtggtt aggtgggatt
43920
tggggagtag ctcaggccaa taaatatggg aatagagtag ttgcaggtat gagactttcc
43980
agaactttcc ctctgcccat atgaataaca gtgcttcaga tcacagctgc tccttctgcc
44040
tgggtcccag aataaagaca atatagaaca tggcctcaag tcaatttaca atagacccaa
44100
agcattaatg agatgtaaac acttgttatt ttttaatcca ctaagactta gggttgtttg
44160
ttactgctgc aagactgatc ctttcctgac tgatacatac ttgatagata actacttggg
44220
aatagtggtt gctgtagatc ttttacatta gcaccccact cctggtagta gttccagtat
44280
cattcacata aacctacgaa gttcctacct aatggatgcc cagacggcca agaggtggaa
44340
ataaagtatt tggtggactc caaccagtag tgaacaaaat tagcctcatg aatctcatat
44400
tttagtgagt tgagtcagat aataaacaaa cagacatctg gtataccaaa tagaggtagc
44460
actattgaga aaaagtacag caggctaggg aggttagaga gggttgggat tacagggaga
44520
gttgctattt tatatagcaa gatcagagta agtctcattg gggcagggat gtcagggaag
44580
agagccacgt aaatatctgc agaaagagca agtgcaggaa tacctggaga attcaaggaa
44640
caatgaggtg cacagtgtgg ctgaagtggc attgaagatg gtgagagcag taggagaaag
44700
gaagggagat ggaaatgtag ataaatgtag ggagttaagg tttttttttt tcttgagaca
44760
ggttctcact ccgtcaccta ggctggagtg cagtggtgtg atcatagctc actacaggtt
44820
tgcactccca ggctcaagtg atcctcctgc ctcagcctcc tgagtagctg ggaactatag
44880
gtgtgcacca ccatgcttgt ctagttttta aaatttttgg tagaaatgag gtcttgctat
44940
gttgcccacg ctggtcttga acttctgagc tcaagtgatc ctccttctcg gcctcccaaa
45000
gtactaggat tacaggcctg aaccactgta ctcagccaag attttatttg aagatgaaag
45060
gacattggaa aatttgggaa caggtatggt attatctggc ttatatttta caaggatact
45120
tcttgttcca ttatctagtt tattttcatg aagggccagt gggttaggtc ctgcaatgct
45180
tgtcatgata gagatgagaa attggaggca cagagaggtt aagtcaatgc gcaaatttag
45240
gcagctagtg cgtggcagag ctggacctcc cagttaccac catctgacta cagaacctga
45300
gctcttgtgt accatgctag gcttgcccca atcccttact atttcttttg gttttagaaa
45360
acttatataa ggccgggggc agtggctcac acctgtaatc ctagcacttt gggaggccga
45420
ggcaggtgga tcacaaggtc aggagatcga gaccatcctg gctaacacag tgaaacccca
45480
tctctactaa aaatacaaaa aattagctgg gtgtggtggt aggcacctgc aatcccagct
45540
acttggaagg ctgaggcagg agaatggcgt gaacccggga ggcagagctt gcagtgagcc
45600
gagatcatgc cactgcacac cagcctaagt gacagagtga gactccgtct caaaaaaaaa
45660
aaaaaaaaaa aaaaaaaact tagagagaaa atcatgactg ttgccttcca tcactgttga
45720
ctttgacccc atctttccct gatgcacccc ctaggagatg agagttcact tctaaggctc
45780
cagctgggtt actcatttat ttggtgctat ggtcccccta tcattcatca tgggcctcaa
45840
gatctttcct atgatgaatt gaactttcta ttcttttcct ttgctcgcca tgatggaggt
45900
23
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
gtaccttttt ttggacagaa accaaattca ggactgactt tctgggtgac ctcatacgaa
45960
ctctcccttt ctctttttag acattgagag caaagttatc aacaaagaat gcagtgaaga
46020
gaatgtgacg ttggacttgg tagccaaggg ggataagatg aagatcgggt gttccacaat
46080
tgaggaatct gaatccacag gtacaggtcc tctcctgaga atgcaggtta tggtgtattc
46140
catcaataag tatttatcga tccctttcta ggtgccaaga ccagtgccag atagttgaga
46200
accaatgagg gtagaaatgg tccatgctct cagagagttt tccatgtgct agggaagtca
46260
aacattaaat aaccttataa tactggagta ctgagacctt cactggccta tatgatatgt
46320
gctggtcaca ttctgctgtt gagcagttga aatttgtata agccaggttg agaggtactg
46380
taaacctaaa ataaacactg tatttcgaag ctttgttgca caaaaagaag gattaaaacg
46440
tctcactcat cttttttata tttaaaataa tttaaaaata aaaaatattt aaatttttaa
46500
aatatgtaat atttaaattt ttaaaatatg caatatttaa aatatatctt taaattttaa
46560
aatatcacat ttatatgttg aaatgataat atttggctta tactgagtta atatattatt
46620
aaaattaatt ttatcttttt ttacttttaa aaaatgtgta ctagaaaatt taaaattaca
46680
tatgtggttc atactgtatt tctgttggac agtgctaatc tgtgtcaggg atggcaaact
46740
ttttctgtag agggctcgtt agtaactatt ttaagcattg caattaatac agcctctatc
46800
acaatgattt aactaccatt ctagtgcaaa agcatccata gacaatatga atatgaatgt
46860
gcctgggcat gtttcaataa aactttattt aaaaaaagta ggtgacaacc agattggccc
46920
ataggccaca gttttccagc ccctgatctg atctatgcat acgattaatg ctatgattaa
46980
tactttaatg taaaagcaca gtgtgcaata tggaagagct atttaagcga agtcctgagg
47040
aataggtaga gaaagcagga gagagtgtgt tacaggaagt ataagctatg gctctgtgtt
47100
gaaaaaaagc ttggtatggt catggaacta aaagtagagt gggctggagc atcaggaatg
47160
tgggggagag agggaaaaga attgaaggtc gctgggcgtg gtggctcaca cctgtaatcc
47220
caacactttg ggaggtcgag gtgggtggat cacctgaggt tgggagttcg agaccagcct
47280
gaccaatatg gtgaaatctc atctctacta aaaatataaa gaatgagcct ggtgtggtgg
47340
cgggtgcctg taatcccagc tactcaggag gctgaggtag gagaattgct tgaacctggg
47400
aggtggaggc tgcagtgagc tgagatcgcg ccactgcact ccagcctggg tgacagaatg
47460
agactccatc tcaaaaaaaa aaaaaaaaaa gaattgaggg tgttgcatcc cagtttatta
47520
catttggact ttctctgttg ggcacagaaa gccttggaaa gttctgtata cagcaggatg
47580
atctgattat gtcttccaga tagatgtctt tttagaaata aatcctctgg ctgtgatatg
47640
ggcaatccat tggagaggtg aggctagggg aaggcaagga catttgtgag gaagccagtg
47700
gtctgggaga gagatgtgag gcttggtcta aggctaatta gaagggagaa gtggggagaa
47760
gacaggttca aggcacatta aggaggagga atctgttttg cggggagaag aattcaagga
47820
tgactctcca ggttccagat caggtaatat cagttattga agtaggagat gcagataaaa
47880
gaagatgaga gagccaggca tggtggctca cgcctgtaat cccagcactt tgggaggcca
47940
24
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
aggcgggcag atcacctgag gtccagagtt caagaccagc ctggccaaca tggtgaaacc
48000
ccgtctccac taaaaataca aaaaattagc tgggtgtggt ctcgggtgcc tgtaatccca
48060
gctactcggg aggctgaggc aggagaatcg cttgaacccg gaaggcagac gttgcagtga
48120
gccgagaacc tgccattgca ctccagcatg ggcaacagag caagactctg tctcaaaaaa
48180
aaaagaagat gggagaggaa ttgctcaatt tatgatgtct ggggggacat ccaaggggca
48240
gatgaagcct gggggttggt actgtctggt tgggacagga agcggcatag gacgtatccc
48300
tggagaccgt ccatatttaa aggggacact ccccaaagag actgaaacag aataaccata
48360
gatgtgggag aacttatttc ccactgagtt gcattgtccc ttaaaaacac aagttcaaat
48420
ttatttttaa atgtagccaa gacttctcca ggtaagagca ctgggaggtc tgttttgttt
48480
tgttttcttt ttttgtcacc caggctggag tgcagtggcg ggatcttggc tcattgcaac
48540
ctccgcctcc caggttcaag cgattgtcgt acctcggcct cccgagtagc tgggactaca
48600
ggcacctgcc accacgcctg gctagttttt ttattgttag tatagacagg atttcaccat
48660
attggccagg ttggcctgga actcctgacc tcaag.tgatc cacttgcctt ggcctcccaa
48720
agtgctggga ttacaggcgt gagccactct gcccagctgt ggaagttttt attttgtttt
48780
gttttgaaat ggagtcttgc tctgtcacta ggctggagtg cagtggtgcg atctcggctc
48840
actgcaatct ccacctaccg ggttcaagcg attcccctgc ctcagactcc caagtagctg
48900
gagctacagg tgcgcatcac cacgcccggc taattttttt gtatttttag tagaaacggg
48960
gtttcaccat gttggtcagg atggtctcga tctcctgacc tcgtgatcca cccgccttgg
49020
tctcccaaag tgctggatta taggcgtgag ccaccatgcc, cggccagagg tttttttttt
49080
ttaatggttc tagaagcccg ctggttattt taatctcaga gcagcacggt gaggtgcatg
49140
actctctctc tctctctctc tctccccctc cctccctctg tgtgtgtgtg tgtgtgtgtg
49200
tgtgtgtgtg tgtgtgttgg gtcttctata ataacaattg agcaaacgat tccctccttt
49260
ttttcatttg gggaaacctg cagagaccac tggtgtggct tttgtctcct ttgtgggcat
49320
ggaatcggtt ttaaatgagc gcttcttcaa agaccaccag gctcccttga ccacctctga
49380
gatcaagctg aagatgaatt ctcgagtcgt tgggggcata atgactggag agaagaaaga
49440
cggcttctca gatccaatca tctacactct ggagaacatt caggtttgtg aagaggtctc
49500
tactgagatt cttgtctacc tgttgggaac tcctcgtctc tcgattgcct taactctcat
49560
tttttacggg aagctattga ggccggtaag cttccacaat ttccagcaat tcaagccaat
49620
taacagaagc taccgctaga ggaatgaggt ctcccactgt caccaaacag aactaggtcc
49680
atttgcccat gtgcaacaga aagccaaaca ccgaagcacc gggtttttgt agagagagaa
49740
gtttattgca aggctgccaa gcaaggagac aagagtctgg ctcaaatctg tcttcccaag
49800
ctgggaggct ggggcaggtt ttatagtcgg tgggtaatga actgcaatct gattgcatct
49860
tacaatgaag tgatgcaggg aggtgtgatc tgattggatc cggccatggg gtgttgccag
49920
gtcttgatct gattggatcc tggatcctgc tatgtggtgg ttgcttcttt tttttttttt
49980
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
agtctcactc tgtcacccag gctggagtgc agtggcacaa tttcaagtca ttgcaagctc
50040
cacctcccag gttcacgcca gtctcctgcc tcagcctcct gagtagctag gattacaggc
50100
gccccctacc atgaccggct aattttttgt atttttactg cagacggggt ttcaccatgt
50160
tagccaggat ggtcttgatc tcctgacctc gtgatccgcc cgcttcggcc tcccaaagtg
50220
ctgggattac aggcatgaac caccatgccc gctttttttt tttttttttt tttttttttg
50280
agatggagtt tcactcttgt tgcccaggct ggagttcaat ggtgcaacct tggctcactg
50340
caaccctcac ctcctcgggt aaagccattc tcccgagtag ctgggattac gggtgcccac
50400
cacatgccca gctaactttt ttgtatttag tagagacgga gtttcaccgt gttgctcagg
50460
ttggtctgga actcctgagc ttaggtgatc cacccgcctc agcctcctga agtgctggga
50520
ttacaggcat gagccaccgt gcccaccctt ctaggataca ttcttaaagc atgttgttct
50580
taaactgtta ctaaaaatat ttctctgctt acttggtttt tatgattttt tctcccactg
50640
cactatgatc tctttttggg ggaagaactg ttttctcatg tctcctgtaa acagcacaga
50700
tcaaggcatg taataggtgt tcaataaaaa cacgttgaat gaggctgggt gtggtggctc
50760
ttgcctgtaa tcccaacact ttgggaggtc tatgcaggag gatcacttga ggtcaggagt
50820
tcgagaccag cctggccaat atggagaaac cccatcttta ctaaaaatac aaaaattagc
50880
tggatatggt gacacacacc tgtaatccca gaactttggg aggcccgagg caggtgaatc
50940
acttgacatc aggagttcaa tactggtctg gccaacatgg taaaaccccg tctctactaa
51000
aagtacaaaa attagctagg catggtggcg ggcacctgta atcccagcta cttgggaggc
51060
tgaggcagaa gaatcgcttg aaccccggag gcagaggttg cagcgagccg agactgcacc
51120
actgcactcc agcctgggca acagagtgag actctgtcac acacacacac acaaaccaca
51180
cattcaatga ataagtgaat aatgagtgac tatgcagaag gctttattat ggatttgatg
51240
aaaggaggag ttttcttcta gcaacagcaa tgtgagctgc cctatcagat tggccctcac
51300
tgcataatga aataccagca tttttcccca ttccctcaga acataccctg tgtattcttg
51360
ttcccagtga ctcttgagga tggtcacatt taatccattt gatggggatt cccagaagac
51420
ctttgttttt ttgttttttt gttttttttt agccaaagca gaagtttgag aggcccatct
51480
gtgtttcctg gagcactgat gtgaagggtg gaagatggac atcctttggc tgtgtgatcc
51540
tggaagcttc tgagacatat accatctgca gctgtaatca gatggcaaat cttgccgtta
51600
tcatggcgtc tggggagctc acggtcagta ctgatgattt gttccctgag gcagagtatc
51660
tgcctccaaa tccaatggga aaatattgat taaacatctg ttgtgtgtct cccatggggt
51720
tgggtgttgt gggatggagt cacggggaca gaaggggtag gtgatatgcc tttgcccctg
51780
cagaatttac agtctggttg ggaggccatg aaactcaaat atacaatgag aaataatgac .
51840
cctaagtagg gccaggcact ggagcaatca cagatcttac ttaatgagca catacagtgt
51900
gctgggctct ttaagttaag tggttttaga cccatttcac agaatgggta aagtggcccg
51960
tctagtcttg aagctagaaa ttggagcagg tggaattcaa atctggggaa gtctctttcc
52020
26
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
actaacacat gcaaatgggt caagaagaaa tgctgtaaga attcagaggt gttggccggg
52080
tgtggtggct catgcctgtc atccgagcac tttgggaggc tgaggcgggc ggatcatgag
52140
gtcaagcgat cgagaccatc ctggccaaca tggtgaaacc cccgtctcta ctaagaatat
52200
aaaaattagc tgggcatggt ggtgcatgcc tgttgtccca gctacttggg aggctgaggc
52260
aggagaatcg cttgaacctg ggaggcagag gttgcactga gctgagatgg caccactgca
52320
ttccagcctg gcaacagagt gagactctgt ctcacacaca cacacacaca cacacacaca
52380
cacacacaca cacacaaaca aaaagaattc tgaggtgttt ccaagagagc cgatgataga
52440
tcatgaggaa acaatatgaa ataagccttg gtaaatgagt agatttggaa gagaggaagg
52500
acatcctcat tagtgttgta gaaagtatcc tttcacctgg ctcggtggct cacgcctgta
52560
atgccagcac ttcgggaggc cgaggtgggc agatcacaag gtcaggagat cgagaccatc
52620
ctggctaaca cggtgaaacc ccatctctac taaaaataca aaaaattagc cgggcgtggt
52680
ggcgggcacc tgcagtccca gctactcggg aggctgaggc gggagaatgg cgtgaacccg
52740
ggaggcaggg cttgcagtga gctgagatca tgccactgta ctccagcctg ggtgacagaa
52800
ccagactccg tctcaaaaag aaagaaagta tcctttccta tcatggtaga tccaaggttt
52860
aattccctac cagagaggcc ataagtaagt gccatttcga ggaccctgtg gcttaggatt
52920
taagagaact ggatccagaa caaaactgac ttagtttcaa gtcctgtctc taccactttc
52980
tagctgtgtt ttcttggaaa agtcacttaa cttctctgca ctgagcccag ctctctcatc
53040
tcaaaaatga gaacaacaat aacaaaaaaa tactgataac tagtatttat atagtatgct
53100
tctggcacag ttgtacttga aatgttttca tttaatccta aaacaactct ataaggcaat
53160
tgccattaat attctctttt caacagatga ggaaactgag ggcacagagc agttaagtgt
53220
cttgtccaaa atcacacaac tagaaattgg ccagtttttt tctttctttt tctttttctt
53280
ttttctagac tgagtttcac tcttgttgcc caggctggag tgcagaggtg tgatctcagc
53340
tcactgcaac ctctgcctcc caggttcaac caattctcct gcctcagcct cccatgtagc
53400
tgggattaca ggtgcccgcc accatgcctg gctaatattt tttgtttgtt tgtttagtag
53460
agataggatt tcgccatgtt ggccaggctg gtttcaagct tctgagctca ggtgatccac
53520
ccgccccagc ctcccataag tgctgggatt acaggcatga gccactgcgc ccagccaatg
53580
gtcagttttc ttaaccactt tgtgacaata tcatagagag gtgctcataa gatggtgcct
53640
ggcttatctt taacccacag aaaccactca gagacattta aaattaattt taaaattaat
53700
tatgaataaa tggcatagaa gaaggactta gcttgtcacg ttccactcct gaagttgaag
53760
cattcctgaa tgtctgcatt atcagttggg attcagtaca ggaagcaaaa accaccccag
53820
gtatttcaag cagaaaggca tttaacacag ggaattgggt.gctcacaaaa atctctggga
53880
ggggatggag gagttgaagt cagggggctg tcacttcaat tttggcttca aggtcatccc
53940
ccaatgcagc tgtaactcag aggtcagaaa ttgctgctgt cattttgcca ccccactcca
54000
ccatgaaatt ggggactgga cagttgaaat gtggaattag gctcactcct gtctgccgaa
54060
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
agctgatatt tgcctccctt ctgcattcca aaaccttcac agatgtatct cactggagaa
54120
cttaaattac atccaggacc ttagttccaa aggattctgg gagatgtagt ttgtggacat
54180
caagccccag taatacatgg agtaacgcat aggacaggac tgggaatagg tgctggtgga
54240
tgccagtgga ttgcaaagga taatgaagga cagtatctag ttcactctga gtgttagagg
54300
gagaaaattc ccccaagcta attttgcccg agccagggtt ttagataact cttcttcccg
54360
cctgggggga ttttgatccc attctcaggc caactctgga attccttcct gacagatgga
54420
cttttccttg tacatcatta gccatgtagg cattatcatc tccttggtgt gcctcgtctt
54480
ggccatcgcc acctttctgc tgtgtcgctc catccgaaat cacaacacct acctccacct
54540
gcacctctgc gtgtgtctcc tcttggcgaa gactctcttc ctcgccggta tacacaagac
54600
tgacaacaag gtctacatcg ctcgggctgt gtccccacca agccccatct tctccccacc
54660
tgcctcagct cattcctagc caactccctc tatccctgtt cctacctcag ggcctttgca
54720
tatgctgtgc cctctgccta taacactcac ttcccagctt cttacctgct tgactccttg
54780
tgacagctct cagtttaaac ttatgtccct gatcgtgtta tgaattatag tggttcctcc
54840
tattacttgt ttttgtttgt ttgtttgttt gtttttgata ctgagtttcg ctcttgttgc
54900
ccaggctgga gtgcaatggc acgatcttgg ctcactgcaa cccctgcctc ttggttcaag
54960
caattctcct gcctcagcct cctgagaagc tgggaataca ggcatgagac actatgccca
55020
gctaattttt ttgtattttt agtagagacg gggtttcacc attttggcca ggctagtcct
55080
gaactcctga cctcaggtga tccacccgcc ttggtctccc aaagtgctgg gattataggc
55140
ttcagccacc acgcacggcc cctcctgtta ctttctaaac cagaggttct ctccctgggt
55200
gctttttatc cccaggggag acttggcaat gtctggggac atttttcctt gtcacaactg
55260
gagcagcgtg ctactgctct ctggtgggta aaggttaggg atactgctaa gtatcttgtc
55320
gtgcccaagg cagccctcca cggcagagaa tgaccctgca ataatgttac agatgtcgat
55380
aatgctacag tttagaatct ctacttccct ttatttttct tttctttttt taaatttttt
55440
atttttttag tttttttgag acagggtctt gctatgtcac ccaggctaga gtgcagtggc
55500
cgaatcacag ctccttatag cctcccaggc tcaagcgatc ctcccacgtt tgcctcccaa
55560
gtacctggga ccacaggcct gtgccaccat gtctggctaa tttttgtatt tttgtataga
55620
gaggggggtt tcatcatgtt gcccagtctg gtctcaaact cctgggcttg agcgatctgc
55680
ctgcctcagc cttccaaagt gctgggatta caagcatgag ccaccacacc cagccagtaa
55740
aactttattt acaaggcagg cagccagcct atggggtcta gtttgctgaa cccgcctgtt
55800
ttcaaccatt ccctaaagta gggtgatcga caatcagttt tcctaaaact cttcctgttt
55860
tagaactgaa agtcatgcgt cttgggaaat ctcaggtcca gacaaaccag gaccatgggt
55920
ctccttacca agaagtgctc ctccctgtgg tcaagaacca ctgcacctct ctataaatgt
55980
ttgtgagatg aatgagtgag ctaggtttgg ggaatttcca gccgaggagt ctctctcttc
56040
ctttcttcct ttcgatttct ctctggggtg gaggattctg atgcgcatgc ttctcccctc
56100
28
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
cagatgggct gcgccatcat cgcgggcttc ctgcactacc ttttccttgc ctgcttcttc
56160
tggatgctgg tggaggctgt gatactgttc ttgatggtca gaaacctgaa ggtggtgaat
56220
tacttcagct ctcgcaacat caagatgctg cacatctgtg cctttggtta tgggctgccg
56280
atgctggtgg tggtgatctc tgccagtgtg cagccacagg gctatggaat gcataatcgg
56340
tgagtgacat cctctctctt cctgaagacc ctgctgccag ggcttatggt gataatgaca
56400
tgagcaacaa gaagagtaac aacagtagca gtagttgtgg ctaccattta tcgagctctt
56460
ctattatgta ctattggttt caggaccacc atgtacagca gcatgggttg tgcactgcac
56520
aaatccagga tcactactat ttgaggttta tgttccagga agagaacatc tggtttacct
56580
aacttgatta ttttcctttt cttgcgtgta tgacatgggg ggtggtgggg gtgaaagtgg
56640
gaaaggaata cttctgcatg gtggtgtgcg cctgtaatcc cagctactcg ggaggccgaa
56700
gcagaagaat cacctgaacc cgggaggcgg aggctgcagt gagatgagat ggcatcattg
56760
cactccagcc tgggggacaa gagcgagact tcatcctcag aaaaaagggg ggggaatatt
56820
ggggtgctgt tattgaacat gacggaatgg ttgttgtgtt gtaaaagcag ctaatgtaca
56880
ctagagacat cttgcagtga gccaagatca cgccactgca ctccagcctg ggtgacagta
56940
gtgacactcc atctccaaaa aaaaaaaaaa gacaaaaagg taaaacaaag ttgatacata
57000
cacatactca tagcagaaaa tgtaagcaat aagtttcaca tcagatgaaa ttcaccattt
57060
taaccatttt aaagcgtgta actcggtggc ttttcgtaca ttcacagtgt tgggcagtga
57120
ctgccactat ctagtggcaa atccttgttt tttttttttt ttgagacaga gtctctctct
57180
cgcccaggct agagggcatt ggcacggtct cgactcactg caacctccgc ctcccaggtt
57240
caagcgattc tcctgcctca gcctcctggg tagctgggat tacaggcatc tgccaccatg
57300
cccggctaat ttttctattt ttagtagaga cgggttttca ccatgttagc caggctgggc
57360
ttgaactcct gtcctcaggt gatccaccag ccttggcctc ccaagtgctg ggattacaag
57420
cacccaccac cacacccagc taatttttgt atttttagta gagatgggtt ttcaccatgt
57480
tggccaggct ggtctcgaac ttctgtcctc aggtgacccg cccacctcgg cctcccaaag
57540
tgctgggatt acaggcttga gccaccgcac ccagcctgat tgcatttaaa tgtctatagg
57600
atttgatttt gcaacaccta ggcttggaga ccccacccta catttgtcca cacctaggtg
57660
gcaaattgga gagtggccaa ctcagaccat tcctggaagt gtggcctgca aggacaatag
57720
ccacctccca gagccttaca tgctgtacat cttctcccca ggttactgac cttcaaggcc
57780
tttgcccagc tcttcatcct gggctgctcc tgggtgctgg gcatttttca gattggacct
57840
gtggcaggtg tcatggctta cctgttcacc atcatcaaca gcctgcaggg ggccttcatc
57900
ttcctcatcc actgtctgct caacggccag gtgtgtagct gctgccctcc ccatccccct
57960
cctcccatca ccctctaccc ccttccccac cggatccctc cacccaacat ccctgtcact
58020
ggaccattta cctgcatctg gatgatgtgt ctccttctcc aggtacgaga agaatacaag
58080
aggtggatca ctgggaagac gaagcccagc tcccagtccc agacctcaag gatcttgctg
58140
29
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
tcctccatgc catccgcttc caagacggtg agagactgca tgctccctgc aggtgctggt
58200
cgagggaggt gccggcctct tggtgacact cagctctgcc acgtgttggg tactgaatgg
58260
gagctgaggg tgcccaccct agttagctca tggatgaagt gtggagttga agactgggga
58320
tttatatgta tatattagca gacacacaca cattatctat ctctctattt aaataaaatt
58380
tcaagaataa gacatatata atttataaca gatttacata gatacatttg tatatgaatt
58440
atgtaacata ttaaaaataa tacatattta tatgttatgt ttataaaata catttatata
58500
cacttattat atacatataa tgtataatat tatatacata taaatgtaag atgtaagttt
58560
aaatatagat accggctggg cgcagtggct cacacctgta atcccaacac tttgggaggc
58620
caaggtgggc agattacctg aggccaggag ttcgagacca acctggccaa catggcaaaa
58680
cccctgtctc tattaaaaat acaaaattag ccgggcatgg tggcaggtgt ctgtaatccc
58740
agctactcat gaggctgagg caggagaatt gcttgaaccc aagaggcgga ggttgcagtg
58800
agcagagatt gtgccattgc actctagcct gggcaaaaag agcgaaactc cgtctcaaaa
58860
aaaaattaat taaataaata tagatatctt cacatgtata tttatataga gaaaattttt
58920
atgtattatg gataacattt atatatgtag attttcagga atattgaaat attggaatga
58980
atatgcaaat ggaggcaaag ctgggtttcc cactaaaatg tcttattttt ttcaacaatg
59040
tattccacgc ccctaaaatg ctgtctagca cacagtaagt tcttagtgaa tattttctga
59.100
gtaaataann nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59160
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59220
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59280
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59340
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59400
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59460
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59520
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59580
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59640
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59700
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59760
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59820
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59880
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
59940
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60000
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60060
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60120
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60180
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60240
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60300
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60360
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60420
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60480
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60540
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60600
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60660
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60720
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60780
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60840
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60900
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
60960
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn
61020
nnnnnnnnnn nnnnnnnnnn nnnnnnnnnn nntgcagcct cactctaatg ctctaatctt
61080
gtctaaactt ttctttatac actgcctagg taataactgt agagacagta ccttgtgccc
61140
agcttatgcc acctgcacca atacagtgga cagttactat tgcgcttgca aacaaggctt
61200
cctgtccagc aatgggcaaa atcacttcaa ggatccagga gtgcgatgca aaggtgagtt
61260
catgtcccct caaaccatcc agcatttggt aggaaaatca agtcaaagct ggctgggtat
61320
tgaatgtagg tactccccca ccccccattt ttttttaaat ctggtttaac tatatatttt
61380
aagtggagta ttaggtcatt tctgttcaag gttaatattg atatgtgggg gtgttgttac
61440
tgtcacagca ttgctagcta gtgttgctag caaacatgat tttcttcctt ccttccttcc
61500
ttccttgcta cttccccacc cttcctctct ttgatcatgt aaataattcc tggtcactgt
61560
aaaatttcat ggatggttga gtttcctcac aatatggtgg ctggattcca aggataagca
61620
tcccaagaga acaaggtaga actacatgat atttttatga cctagtgtca cttccaccat
61680
agtcacaagt atgctcagat tcaagatggg gacatacctt ccattgcttg acagaagaag
61740
taccaaagtg aagcgcatat gggatgggag atattgtttt aacttttttt tttggcaaaa
61800
tacagtcttc tatctagtat aagtgaattt gtaactccaa ttctctgtct agatattgat
61860 -
gaatgttctc aaagccccca gccctgtggt cctaactcat cctgcaaaaa cctgtcaggg
61920
aggtacaagt gcagctgttt agatggtttc tcttctccca ctggaaatga ctgggtccca
61980
ggaaagccgg gcaatttctc ctgtactggt aatgctctca ggttcccagg gatgggtctt
62040
gggtggatat ctatcagtgg ggtgagttca tgtatttctg aactaaggca cccaatttct
62100
tatctgctca ccctcttcca ctgcttctca gatatcaatg agtgcctcac cagcagggtc
62160
tgccctgagc attctgactg tgtcaactcc atgggaagct acagttgcag ctgtcaagtt
62220
31
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
ggattcatct ctagaaactc cacctgtgaa ggtatccatg accatctctt tattatttac
62280
ctacttaatt aattaagggt catctcactg gaagaccata tgagaaaggg attttctcat
62340
gtagggataa gaaaattggg gctcagagag gtaaaaatat atagttgctt atatcttttt
62400
ctatcccttt actttgagcc tgtgggtgtc tttacatgct aggtaggtct cttgtagcca
62460
gcacatggtt gggtcttttt aaaaaatt
62488
<210> 4
<211> 886
<212> PRT
<213> Homo sapiens
<400> 4
Met Arg Gly Phe Asn Leu Leu Leu Phe Trp Gly Cys Cys Val Met His
Z 5 10 15
Ser Trp Glu Gly His Ile Arg Pro Thr Arg Lys Pro Asn Thr Lys Gly
20 25 30
Asn Asn Cys Arg Asp Ser Thr Leu Cys Pro Ala Tyr Ala Thr Cys Thr
35 40 45
Asn Thr Val Asp Ser Tyr Tyr Cys Thr Cys Lys Gln Gly Phe Leu Ser
50 55 60
Ser Asn Gly Gln Asn His Phe Lys Asp Pro Gly Val Arg Cys Lys Asp
65 70 75 80
Ile Asp Glu Cys Ser Gln Ser Pro Gln Pro Cys Gly Pro Asn Ser Ser
85 90 95
Cys Lys Asn Leu Ser Gly Arg Tyr Lys Cys Ser Cys Leu Asp Gly Phe
100 105 110
Ser Ser Pro Thr Gly Asn Asp Trp Val Pro Gly Lys Pro Gly Asn Phe
115 120 125
Ser Cys Thr Asp Ile Asn Glu Cys Leu Thr Ser Arg Val Cys Pro Glu
130 135 140
His Ser Asp Cys Val Asn Ser Met Gly Ser Tyr Ser Cys Ser Cys Gln
145 150 155 160
Val Gly Phe Ile Ser Arg Asn Ser Thr Cys Glu Asp Val Asn Glu Cys
165 170 175
Ala Asp Pro Arg Ala Cys Pro Glu His Ala Thr Cys Asn Asn Thr Val
180 185 190
Gly Asn Tyr Ser Cys Phe Cys Asn Pro Gly Phe Glu Ser Ser Ser Gly
195 200 205
His Leu Ser Cys Gln Gly Leu Lys Ala Ser Cys Glu Asp Ile Asp Glu
210 215 220
Cys Thr Glu Met Cys Pro Ile Asn Ser Thr Cys Thr Asn Thr Pro Gly
225 230 235 240
Ser Tyr Phe Cys Thr Cys His Pro Gly Phe Ala Pro Ser Ser Gly Gln
245 250 255
Leu Asn Phe Thr Asp Gln Gly Va2 Glu Cys Arg Asp Ile Asp Glu Cys
260 265 270
Arg Gln Asp Pro Ser Thr Cys Gly Pro Asn Ser Ile Cys Thr Asn Ala
275 280 285
Leu Gly Ser Tyr Ser Cys GIy Cys Ile Val Gly Phe His Pro Asn Pro
290 295 300
Glu Gly Ser Gln Lys Asp Gly Asn Phe Ser Cys Gln Arg Val Leu Phe
305 310 315 320
Lys Cys Lys Glu Asp Val Ile Pro Asp Asn Lys Gln Ile Gln Gln Cys
325 330 335
Gln Glu Gly Thr Ala Val Lys Pro Ala Tyr Val Ser Phe Cys Ala Gln
340 345 350
Ile Asn Asn Ile Phe Ser Val Leu Asp Lys Val Cys Glu Asn Lys Thr
355 360 365
Thr Val Val Ser Leu Lys Asn Thr Thr Glu Sex Phe Val Pro Val Leu
370 375 380
Lys Gln Ile Ser Met Trp Thr Lys Phe Thr Lys Glu Glu Thr Ser Ser
385 390 395 400
Leu Ala Thr Val Phe Leu Glu Ser Val Glu Ser Met Thr Leu Ala Ser
32
CA 02438521 2003-08-15
WO 02/066644 PCT/US02/02627
405 410 415
Phe Trp Lys Pro Ser Ala Asn Val Thr Pro Ala Val Arg Ala Glu Tyr
420 425 430
Leu Asp Ile Glu Ser Lys Val Ile Asn Lys G1u Cys Ser Glu Glu Asn
435 440 445
Val Thr Leu Asp Leu Val Ala Lys Gly Asp Lys Met Lys Ile Gly Cys
450 455 460
Ser Thr Ile Glu Glu Ser Glu Ser Thr Glu Thr Thr Gly Val Ala Phe
465 470 475 480
Val Ser Phe Val Gly Met Glu Ser Val Leu Asn Glu Arg Phe Phe Gln
485 490 495
Asp His Gln Ala Pro Leu Thr Thr Ser Glu Ile Lys Leu Lys Met Asn
500 505 510
Ser Arg Val Val Gly Gly Ile Met Thr Gly Glu Lys Lys Asp Gly Phe
515 520 525
Ser Asp Pro Ile Ile Tyr Thr Leu Glu Asn Val Gln Pro Lys Gln Lys
530 535 540
Phe Glu Arg Pro Ile Cys Val Ser Trp Ser Thr Asp Val Lys Gly Gly
545 550 555 . 560
Arg Trp Thr Ser Phe Gly Cys Val Tle Leu Glu Ala Ser Glu Thr Tyr
565 . 570 575
Thr Ile Cys Ser Cys Asn Gln Met Ala Asn Leu Ala Val Ile Met Ala
580 585 590
Ser Gly Glu Leu Thr Met Asp Phe Ser Leu Tyr Ile Ile Ser His Val
595 600 605
Gly Ile Tle Ile Ser Leu Val Cys Leu Val Leu Ala Ile Ala Thr Phe
610 615 620
Leu Leu Cys Arg Ser Ile Arg Asn His Asn Thr Tyr Leu His Leu His
625 630 635 640
Leu Cys Val Cys Leu Leu Leu Ala Lys Thr Leu Phe Leu Ala Gly Ile
645 650 655
His Lys Thr Asp Asn Lys Thr Gly Cys Ala Ile Ile Ala Gly Phe Leu
660 665 670
His Tyr Leu Phe Leu Ala Cys Phe Phe Trp Met Leu Val Glu Ala Val
675 680 685
Ile Leu Phe Leu Met Val Arg Asn Leu Lys Val Val Asn Tyr Phe Ser
690 695 700
Ser Arg Asn Ile Lys Met Leu His Ile Cys Ala Phe Gly Tyr Gly Leu
705 710 715 720
Pro Met Leu Val Val Val Ile Ser Ala Ser Val Gln Pro Gln Gly Tyr
725 730 735
Gly Met His Asn Arg Cys Trp Leu Asn Thr Glu Thr Gly Phe Ile Trp
740 745 750
Ser Phe Leu Gly Pro Val Cys Thr Val Ile Val Ile Asn Ser Leu Leu
755 760 765
Leu Thr Trp Thr Leu Trp Ile Leu Arg Gln Arg Leu Ser Ser Val Asn
770 775 780
Ala Glu Val Ser Thr Leu Lys Asp Thr Arg Leu Leu Thr Phe Lys Ala
785 790 795 800
Phe Ala Gln Leu Phe Ile Leu Gly Cys Ser Trp Val Leu Gly Ile Phe
805 810 815
Gln Ile Gly Pro Val Ala Gly Val Met Ala Tyr Leu Phe Thr Ile Tle
820 825 830
Asn Ser Leu Gln Gly Ala Phe Ile Phe Leu Ile His Cys Leu Leu Asn
835 890 845
Gly Gln Val Arg Glu Glu Tyr Lys Arg Trp Ile Thr Gly Lys Thr Lys
850 855 860
Pro Ser Ser Gln Ser Gln Thr Ser Arg Ile Leu Leu Ser Ser Met Pro
865 870 ~ 875 880
Ser Ala Ser Lys Thr Gly
885
33