Note: Descriptions are shown in the official language in which they were submitted.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
1
Air Fresheners
The present invention relates to~ an air freshener and in
particular to substantially solid compositions for releasing
fragrance or perfume into the ambient atmosphere over a
prolonged period of time.
Water based gels of carrageenans have been used to carry a
fragrance which is slowly released into the atmosphere. A
drawback of these systems is that they carry a relatively low
amount of the fragrance, typically 5% by weight, and the product
will craze or crack over time as the fragrance is released and the
remaining material shrinks. Water based gels can also suffer from
syneresis.
There are many disclosures of gels based on a major
proportion of water, such as in GB-A-938039 which uses an
alginate gelling agent, EP-A-901794 which uses aluminium
stearate and a polymer such as a polyamide. Other disclosures
include GB-A-2 297 909 and WO 96/24389 which disclose an
aqueous system with less than 20 at % fragrance, and US-A-
4 891 388 discloses a free standing composition in which 5 to 25%
by weight of fragrance is held with a PVA (polyvinylalcohol)
polymer matrix in a water/ethanol solution. The "dispenser"
shrinks during use, i.e. as the fragrance evaporates.
WO 98/17243 describes a variety of uses for a novel ester
terminated polyamide including the formulation of fragrance
containing gels. The majority of gels disclosed use a major
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
2
proportion of polyamide mixed with a C13 - C14 iso paraffin and 25
by weight or less of fragrance. A composition having 80% fragrance
and 20°/ polyamide is disclosed, but we have found that this.
exhibits syneresis (vide hereinafter).
WO 96/19247 discloses a high fragrance containing self
supporting gel composition with a dibenzylidene alditol gelling
agent, and discusses the problems of syneresis in such systems and
non-uniform release of the fragrance.
US 5 780 527 describes a system in which a polymer is cross-
linked in the presence of a fragrance to form a three dimensional
network which contains the fragrance. These systems can contain
70 to 90°/ by weight of the fragrance. The preferred polymers are
polyolefins, particularly maleinised polybutadiene and maleinised
polyisoprene cross-linked with an ethoxylated molecule. Such
products are typically quite brittle and will shrink towards a
central point. This, again, provides an unsightly appearance as the
gel shrinks away from the sides of the container. . .
GB-A-2 298 841 discloses a gel housed in an inverted
container with a restricted neck or outlet. As the gel shrinks in the
region of the neck, it moves down to project out of the container,
into an airflow.
In US 5 060 858, a physical anchor is provided at a container
opening to anchor the gel to the edge of the opening. As the gel
shrinks, it is held across the container opening by the anchor.
In US-A-5 460 78'7 a card shaped fragrance is constructed of
fragrance and a thermoplastic and then exposed on both sides. The
fragrance occupies pockets formed within the continuous
thermoplastic matrix.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
3
WO 00/24434 describes a system in which the fragrance
releasing composition is carried in a narrow recess, apparently in
order to provide a more even release rate over the effective life of
the product. This is attractive, but the use of channels reduces the
surface area available to evaporation, and the product shrinks
away from the channel walls during use, marring the appearance of
the product. WO 00/24434 mentions the polyolefin systems
referred to above, but also mentions alginates, carrageenans, and a
variety of other polymers including polyamides, as the carrier
matrix.
In one aspect the present invention provides an air freshener
composition comprising a fragrance component, and a wax or a
polymer dissolved in the fragrance component. The amount of wax
or polymer dissolved in the fragrance component is sufficient to
form a gel like consistency. The composition is substantially self-
supporting.
The present invention also provides an air freshener
comprising a composition which is self-supporting and which
substantially retains its shape during the useful lifetime of the air
freshener.
The prior art gel compositions are typically housed in a
container with a restricted view. In part this is because the
compositions crack or craze as the fragrance evaporates, becoming
unsightly.
Another aspect of the present invention provides an air
freshener comprising a container which is open at one end, a
fragrance containing composition contained in the container and
having a surface exposed to the atmosphere at the one end,
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
4
wherein the exposed surface of the composition is substantially
uninterrupted.
By providing an uninterrupted exposed surface, a greater
surface area of the composition is open to atmosphere for a given
container size, enabling a greater rate of evaporation of the solvent.
Very preferably the composition will shrink away from the
exposed surface only, as the fragrance evaporates, and will stay in
contact with side walls of the container.
A suitable composition can be prepared by dissolving a wax or
a polymer in a liquid fragrance, to form a gel.
Again it is preferable that the composition is substantially
self-supporting. In particular the container may be stood with the
exposed face of the composition substantially vertical.
Yet another aspect of the present invention provides an air
freshener comprising a gel-like or solid, fragrance containing,
composition which is exposed to the atmosphere on opposite
surfaces. The composition may be moulded to a substantially flat
shape and is surrounded by a peripheral container wall, leaving
opposed major surfaces exposed to the atmosphere. Preferably one
or both of the exposed surfaces is substantially uninterrupted.
Preferably, during evaporation of the fragrance, the
composition shrinks only in a direction perpendicular to the
exposed surface and so remains in contact with the peripheral wall.
A preferred composition is formed by dissolving a wax or a polymer
in a fragrance.
We have found that by appropriate selection of the fragrance
and the wax or polymer, it is possible to achieve a composition
having a high fragrance content, 50% by weight or more. Such a
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
high fragrance containing composition has improved performance
characteristics such as giving a greater impact in-use or
maintaining a more even release of fragrance over the product
lifetime. More particularly, the composition shrinks one
dimensionally as the fragrance evaporates, i.e. the product only
shrinks away from the exposed surfaces.
Thus, the composition may be shaped in a mould, and will
retain the shape during use, when the fragrance evaporates.
If the dissolved wax or polymer content or other gel forming
agent is too high, the available fragrance is reduced, and also the
product is likely to be harder or more brittle and so may have less
desirable shrinkage characteristics as the fragrance evaporates.
The gel forming agent to fragrance ratio can be optimised by trial
and error to meet the desired performance characteristics.
The composition preferably comprises more than about 50%
by weight of fragrance component, more preferably more than
about 70% by weight of fragrance component.
Very preferably the composition has from about 70°/ to
about 90°/ by weight of fragrance component, more preferably from
about 75% to about 85% by weight.
The fragrance component is a derivative of a liquid
hydrocarbon. It may be a discrete chemical but more typically will
be a complex mixture of volatile liquid ingredients of natural or
synthetic origin. The fragrance component may be presented in an
oily carrier liquid, typically 50% fragrance and 50% carrier. We
prefer a fragrance having a high fragrance content, i.e. little or no
oily carrier, as this maximises the fragrance available for
evaporation in use.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
6
The wax or polymer should be matched to the fragrance
component to achieve the desired degree of solubility in the
fragrance component. If there is a mismatch, the polymer may
form a cloudy solution or gel: although this is not necessarily
undesirable a clear product is usually preferred. Waxes tend to
give an opaque appearance both before and after mixing with a
fragrance. More importantly, a higher wax or polymer content, and
hence a reduced fragrance content, may be required to achieve a
sufficiently self supporting product.
Fragrance components with low polarity molecules are
generally preferred. The fragrance should also have a relatively
well defined working vapour pressure to provide the necessary
evaporation at ambient temperatures.
Functional groups on the polymer structure will also affect
the solubility of the polymer in the fragrance component.
Very preferably the, or the main, polymer component is a
polyamide polymer. A particularly preferred polyamide is supplied
as UNICLEAR 100 from Arizona Chemical Co., USA, which is solid
at room temperature. UNICLEAR 80V, which is from a vegetable
source and incorporates 20% mineral oil is also a preferred
polyamide. Such ester terminated polyamide polymers are
described in W098/17105,
Another preferred polymer is a styrene based polymer. such
as a styrene block copolymer
A wax, a high molecular weight hydrocarbon, may also be
used to form the gel.
Another formulation uses a stearate to form the gel.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
7
The composition may include a variety of additives as
commonly used in the art, including inert additives such as flowers
or beads for aesthetic purposes; soluble additives such as
colourants, or dispersed additives such as pearlescent particles,
glitters, metallic pigments or thermochromic pigments,
photochromic agents, optical brightener agents.
A composition or formulation in accordance with the
invention may be prepared by warming the wax or polymer and the
fragrance component with gentle mixing. At an elevated
temperature, typically about 65°C, the wax or polymer dissolves or
disperses in the fragrance component. The warm solution is
poured into containers or moulds. On cooling a single phase
anhydrous gel may be formed. Depending on the vapour pressure
characteristics of the fragrance, the mixing temperature should be
kept as low as possible to avoid driving off too much of the
fragrance components.
The compositions are adapted to be poured into a container
with an open surface. The container can then be placed with the
open surface vertical, and the composition adheres to the inner
surface of the container, without falling out as the mixture shrinks
on evaporation of the fragrance.
Preferably the composition is substantially transparent. A
label or the like may be provided on the container and visible
through the air freshener composition.
The mould may be shaped to allow release of the cooled solid
composition to provide a free standing product.
The invention will be further described by way of example.
All amounts are °/ by weight of the total composition.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
8
EXAMPLE 1.
A lemon fragrance product was produced by warming UNICLEAR
100 (20°/ by weight), Solvent Yellow 93 colorant (0.01%) (Clariant
Sandoplast Yellow 3G) and Orange Turpene fragrance (balance %)
to 65°C and mixing gently until a clear liquid was formed. The.
liquid was then poured into glass moulds and allowed to cool.
The cooled product had the following characteristics:
1. Shrinkage, due to fragrance evaporation, was one
dimensional, i.e. the moulded product when exposed on one surface
only became thinner with evaporation.
2. There was extended, slow release of fragrance, over a period
of more than two weeks.
3: Transparency was maintained through the effective life of
the product - the polymer did not precipitate out.
4. The composition adhered well to the sides of the mould, even
when inverted.
EXAMPLE 2
The following composition was prepared as in Example 1.
W/W
UIVICLEAR 100 50
~ Solvent Red 27 0.01
~ Solvent Blue 35 O.OI
French Lavender Oil Balance
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
9
* Clariant Fat-Red 5B02 and Clariant Fat Blue B01.
The cooled product had properties similar to those described
in Example 1.
EXAMPLES 3 TO 7
The following compositions were prepared as for Example 1.
The percentage loss (evaporation) of the fragrance was then
measured
Component/Ex 3 4 5 6 7
UNICLEAR 100 25 25 25 25 25
Fragrance 75
PF30551
" PF30549 ~5
" PF30550 75
" PF30552 75
" pF30548 75
Dye q.s q.s q.s q.s q.s
All fragrances were supplied by Phoenix Fragrance.
UNICLEAR 100 was supplied by Arizona Chemical Co, USA.
A blend of 80% Polyamide, 20% Mineral Oil, such as
UNICLEAR 80V was also used. It is thought that the blending of
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
the polyamide with the mineral oil, in UNICLEAR 80V, may help
to promote dissolution of the polymer in the fragrance. The
polymer is a hard waxy polymer melting at 90°C, but it will 'melt'
at about 65°C in the presence of the fragrance.
8 gm of the warm polymer/fragrance mix was poured into a glass
mould having an exposed upper surface of 16cm2, giving a depth of
about 5mm. The weight loss equates to the amount of volatile
fragrance components which evaporate. This was measured over
time, and is presented in Figure 1.
It can be seen that after an initial period of one or two days, the
rate of evaporation of fragrance is substantially linear for an
extended period of time. This provides fox a product having
substantially even performance for an extended period, four weeks
or more.
Comparative Example 8
By way of comparison a similar test was performed on a
commercial product, HAZE CRYSTAL ATR EXOTIC FRUITS
manufactured by Reckitt Benckiser which is believed to made in
accordance with WO 00/24434. This shows a steeper initial
fragrance loss, but then a much lower rate of fragrance evaporation
after about 10 days.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
11
EXAMPLE 9
Perfume PF30551, 65% by weight, was mixed with KRATON 1652
(ex Shell Chemicals) 65°/ by weight and heated to 100°C. The
KRATON polymer dissolved in the perfume and the mix was then
poured into a shallow mould and allowed to cool to a gel. The gel
was not as solid as Examples 1 to 7, but good fragrance release
characteristics were obtained.
It will be appreciated that a fragrance with a flash point above the
mixing temperature should be chosen.
KRATON is a styrenic block co-polymer. Such polymers are
produced by polymerising styrene and then sequentially reacting
with butadiene or isoprene to produce linear A-B-A, radial (A-B)n or
di-block (A-B) polymers as required.
EXAMPLES 10 to 15
Waxes were also dissolved in a fragrance composition to produce a
solid gel formulation. An opaque product is formed with a hard gel.
Some are subject to cracking as the perfume evaporates but good
performance with prolonged perfume release over several weeks is
obtained.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
12
Ex. Wax % Wax / Observations
Perfume?
Hydrogenated 50 50 Brittle and
Castor Oil1 cracks on
shrinka a
11 Polyglyceryl-3 28 72 Brittle and
Beeswax2 cracks on
shrinka a
12 Beeswax3 43 5'7 No crackin
13 Microcrystalline 18.5 81.5 No cracking
Wax4
14 Paraffin Waxy 35 65 No crackin
Carnauba Wax6 46.4 53.4 No crackin
1. Liowax PM80 from Miracema-Nuodex
2. Cera Bellina from Jan Dekker
3. White Beeswax BP from Poth Hille
4. Microcrystalline Wax 3749 from Poth Hille
5. Paraffin Wax I25/I30 from Astor Stag
6. Carnauba Wax from Stanley Black
7. Orange Turpenes
Example 16
A stearate based formulation was prepared as follows.
5.0°/ sodium stearate, 5.0°/ water, 10.0°/ ethanol and
80.0°/
fragrance were mixed at 80°C until homogenous. The.mixture wa.s
then passed into a mould and allowed to cool
The cooled formulation adhered to a glass mould.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
13
Comparative Example 17
A mixture of 20% UNTCLEAR 100 V and 80% d-Iimonene
fragrance was warmed to about 65°C and mixed to form a
homogenous mixture. The cooled mixture formed a self-supporting
block but exhibited syneresis at room temperature: fragrance oozed
or leaked from the Iower region of the block. The cooled block was
also brittle.
Figure i. is a plot of weight loss o~-i~r time for example 3 to 7 above;
Figure 2 shows a plan view of an air freshener product containing a
composition in accordance with the invention;
Figure 3 is a cross-section on line III-III of Figure 2;
Figure 4 is similar to Figure 3, but showing the product part used;
Figure 5 shows a plan view of a prior art product after use;
Figure 6 shows an air freshener product forming a second
embodiment of the invention;
Figure 7 shows an air freshener product forming a third
embodiment of the invention;
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
14
Figure 8 shows a perspective view partly cut away of a fourth
embodiment of the invention.
Figure 9 is a plan view of a fifth embodiment of the invention,
similar to the embodiment of Figure 8,
Figure 10 is a cross-section along lines X-X of Figure 9,
Figure 11 is a cross-section along lines XI-XI of Figure 9,
Figure 12 illustrates a modification of the embodiment of Figures 9
to 11.
Figure 13 shows a perspective view of yet another embodiment of
the invention, and
Figure 14 shows a container of the embodiment of Figure 13.
Figure 15 shows yet another embodiment of the invention.
Figure 2 shows a plan view of an air freshener comprising a
composition 2 prepared in accordance with Example 3 above, and
contained in a glass mould 4. The composition 2 fills channels 3
provided in a major surface 5 of the mould 4. As seen in Figure 3,
the channels 3 are initially filled with the composition 2. The
cross-section of Figure 4 shows the product after exposure to the
ambient atmosphere for about three weeks. Fragrance has
evaporated, causing apparent shrinkage of the remaining
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
composition into the channels 3. It can be seen that the
composition shrinks one-dimensionally, that is it continues to fill
the channels 3 across their full width. Hence the plan view is still
as seen in Figure 2. If the product is left until substantially all the
fragrance is evaporated, the polymer component will remain in the
bottom of the channels 3.
Figure 5 shows a plan view of the composition of comparative
example ~ after about three weeks. It can be seen that the
remaining composition has shrunk away from the sides of the
channels 3, forming gaps 6 and giving a quite different visual
appearance, which is uncontrolled during the life of the product.
Figure 6 shows a second embodiment of the invention. A shape 10,
in this case a rabbit shape about 5 cm high and 1.5 cm thick is
formed by casting the molten composition of one of Examples 1 to 7
or 9 to 16 in a mould. The mould may be coated with a release
agent to allow release of the shape, but the release agent should
not contaminate the surface of the finished product, or otherwise
inhibit evaporation of the fragrance component. The gel is self-
supporting. In practice the shape may be supplied on a base 12 to
protect any supporting surface from the oils etc. in the composition.
Components having little or no skin hazards are preferred because
of the likelihood of handling by the user.
Figure 7 shows a third embodiment of the invention, simple cube
shape 20, formed with a composition according to one of Examples
1 to 7 or 9 to 16. The initial cube has a side of about 10 cm, and a
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
16
fragrance content of about 75%. After loss of the fragrance, the
cube will have shrunk to about 25% of its volume, i.e. about 6 cm on
a side.
Figure 8 shows a perspective view of a fourth embodiment of the
invention. A glass container 30 has a bottom wall 32 which is flat
and a peripheral wall 34. The container has an internal depth of
about 6 mm. A label 36, which may carry a logo, flower design 42,
etc., is stuck to the inner surface 38 of bottom wall 32. A
transparent composition prepared in accordance with one of
Examples I to 7 is poured into the container 30 while molten. The
composition cools to a clear gel 40 which adheres well to the inner
surface 44 of the side wall 34 and the label 36. (If preferred, the
label could be provided on the underside of wall 32 and be visible
through the wall). As the fragrance evaporates, the gel becomes
thinner, but does not shrink away from the side wall 34, and hence
continues to provide an attractive appearance covering the label 36.
Printing 42 on the surface of label 36 can be seen through the geI
40.
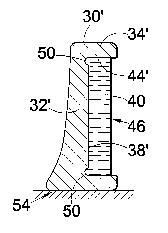
Referring to Figure 9, a glass container 30' is provided with a
peripheral groove 50 at the junction between the inner wall surface
44' and the bottom wall 32'. This forms an anchor which helps to
maintain the gel 40 flat against the surface 38' of the bottom wall
32' of the container 30'. The wall 32' is thicker towards one bottom
side 54, to provide a wider edge on which the container 32' can
stand so that the exposed gel surface 46 is vertical. In Figure 12,
grooves 56 are provided in the form of a pattern in suxface 38' of
bottom wall 32'. Again these provide an anchor, but they will also
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
17
provide an attractive effect. The increased depth of the
transparent or translucent gel at the groove 56 reduces light
transmission and results in a darker area, a pattern being formed
at the groove.
It will be understood that the degree of transparency or
translucence of the gel can be adjusted, for example by adjusting
the amount of colourant used. Examples 9 to 16 can also be used
with Figures 8 to 12, except that the gel is opaque.
Referring to Figure 13, another embodiment of the invention
comprises a metal container 60 which is in the shape of a
Christmas tree and, as seen in Figure 14, forms only a peripheral
wall 62, the major front and back surfaces being open. The
container 60 is filled with a composition 64 prepared in accordance
with one of Examples 1 to 7 and 9 to 16 and which is substantially
self supporting and adheres well to the wall 62. The composition
64 is exposed on front and back major surfaces 66, 68. In use, as
the fragrance evaporates, the composition decreases in thickness T,
between the major surfaces, but remains in contact with the
peripheral wall 62.
This arrangement may also be used with prior art
compositions such as described in WO 00/24434. The arrangement
provides the benefit of enhanced fragrance evaporation, because
two major surfaces of the air freshener composition axe exposed to
the atmosphere. However, with the prior art compositions some
shrinkage from the peripheral wall 12 is to be expected, with the
composition eventually shrinking to a husk at the lower region of
the container.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
18
An exemplary prior art composition is as follows.
Ingredient / w/w
Carrageenan 2.00
Monopropylene Glycol2.00
Biocide 0.1
Fragrance 3.0
Fragrance Emulsifier1.0
Colourant q.s
Soft Water balance
The carrageenan is mixed with the water and heated to 70°C.
The glycol and dye are then added. The fragrance and emulsifier
are pre-mixed and then added to the main mix. Sodium stearate
may also be added to improve the fragrance solvency. The
composition is then poured into the container and left to cool and
set. It will be appreciated that the container is laid flat on a
surface to support the composition while it cools to the setting
temperature.
In the embodiment of Figure 15, a glass spiral 70 was dipped
into the composition of Example 15 above, with the composition at
a temperature of about 60°C. The glass spiral was removed and a
coating 72 of the composition remained on the spiral. The spiral
was then re-dipped, to a total of three dips, to leave a coating of
about 2 to 3 mm thickness on the glass spiral. The spiral has a
hook shape 74 at its upper end for it to be hung by a thread, for
example.
CA 02438873 2003-08-21
WO 02/066084 PCT/GB02/00760
19
The embodiment of Figure 15 may also be prepared using one
of the other exemplary compositions.