Note: Descriptions are shown in the official language in which they were submitted.
CA 02438878 2003-08-20
Case 1/1190-Prio ~ ~'~
75057pri.204
Continuous process for preparing dihydropyrones
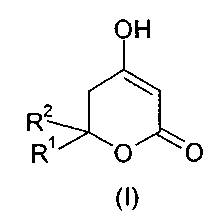
The invention relates to an improved process for preparing dihydropyrones of
s general formula I,
OH
R2
R' O O
wherein the groups R' and R2 may have the meanings given in the claims.
to
Background to the invention
Dihydropyrones are important as intermediate products in drug synthesis. In
is particular, 5,6-dihydro-4-hydroxy-6-phenethyl-6-propyl-2H-pyran-2-one is an
important intermediate product in the synthesis of tipranavir, an HIV protease
inhibitor. The compounds of formula I and processes for preparing them are
known from the prior art, e.g. frvm International Patent Application WO
98/19997
and from the "Journal of Medicinal Chemistry, 1998, Vol. 41, No. 18".
Recently, a
2o process for preparing a racemic mixture of 5,6-dihydro-4-hydroxy-6-
phenethyl-6-
propyl-2H-pyran-2-one has been described, which comprises in step a) reacting
a
dianion of methylacetoacetate with 1-phenyl-3-hexanone and in a subsequent
step
b) cyclising the resulting ~i-ketoester by alkaline hydrolysis followed by
acidification. This process is carried out discontinuously and achieves a 72%
yield.
The problem of the invention is therefore to provide a process which enables
dihydropyranones to be prepared with a high degree of purity and in a
significantly
improved yield compared with the prior art.
CA 02438878 2003-08-20
2
Detailed description of the invention
Surprisingly, it has been found that the compounds of formula I can be
obtained
with a high degree of purity and in a significantly improved yield if step a)
is carried
s out continuously in a microreactor.
The invention therefore relates to a process, suitable for use in the
laboratory and
on an industrial scale, for preparing a compound of general formula (I),
OH
R2
R' O O
to wherein
R' denotes a C~-C8-alkyl, Cs-Coo-aryl-C~-C4-alkyl or Cs-Cs-cycloalkyl-Ci-C4-
alkyl
group, and
R2 denotes a C~-C$-alkyl group,
is a) by reacting a ketone of formula (II)
O
R' R2
wherein R' and R2 are as hereinbefore defined,
with an acetoacetate in the presence of a strong base
and
2s b) by cyclising the resulting compound of formula (IV)
OH
O,Rs
R R2
O O
(IV)
CA 02438878 2003-08-20
3
wherein
R3 denotes a C~-C4-alkyl or benzyl group,
s wherein the ketone of formula II is continuously reacted with an
acetoacetate in
the form of its dianion in a microreactor.
The microreactors which are suitable for the process according to the
invention
are known, for example, from "Microreactors; Wolfgang Ehrfeld, Volker Hessel,
io Holger Lowe; Wiley-VCH;ISBN 3-527-29590-9; Chapter 3 Micromixers".
Microreactors which may be used in the process according to the invention
generally have a housing made of stainless steel, glass, titanium or metal
alloys
and an inlay or inlay structures of thermally oxidised silicon, copper,
aluminium,
nickel, silver, metal alloys, Foturan glass or metal-coated plastics, glass or
ceramic
is materials.
The educt currents may be mixed both turbulently as well as by laminar flow,
preferably by laminar flow. The preferred channel structures for laminar
mixing
generally include interdigital structures, star-shaped structures or
structures of a
2o worm-type mixer. Types of microreactor which may be used for the process
according to the invention may be obtained, for example, from the companies
Institut fur Mikrotechnik Mainz GmbH, Cellular Process Chemistry GmbH or
Mikroglas AG.
2s Particularly preferred according to the invention is a process wherein a
microreactor with an interdigital channel structure, most preferably a
microreactor
of the LIGA type (produced by Lithographie, Galvanoformung, Abformung
[lithography, electroforming and moulding]) with an interdigital channel
structure,
produced for example by the Institut fur Mikrotechnik Mainz GmbH, is used for
3o reaction step a).
Particularly preferred is a process wherein a current of educt A containing
the
compound of formula (II) and a current of educt B containing an acetoacetate
in
the form of a dianion are continuously mixed together in the mixing element of
a
3s microreacto~,and the liquid reaction mixture is conveyed into a capillary,
particularly a holding capillary.
CA 02438878 2003-08-20
4
s
Also particularly preferred is a process wherein the capillary is 0.1 to 10 m,
preferably 0.3 to 8 m, preferably 0.5 to 6 m, most preferably 0.8 to 4 m,
particularly
preferably about 1 m long and has an internal diameter of 0.05 to 5 mm,
preferably
0.1 to 4 mm, preferably 0.3 to 3 mm, particularly preferably about 1 mm.
Particularly preferred is a process wherein 1-phenyl-3-hexanone is used as the
compound of formula (II) in step a).
Also particularly preferred is a process wherein in step a) the acetoacetate
is used
to in the form of a dilithium, monolithium, monosodium or disodium salt.
Of particular importance is a process wherein the molar ratio of the compound
of
formula (III) to the compound of formula (II) used is 2:1 to 1:2, preferably
1:1 to
1:1.5, particularly preferably 1:1 to 1:1.2, most preferably about 1:1.
is
Also of particular importance is a process wherein the reaction in step a) is
carried
out at a temperature of -78 to +85 °C, preferably at -40 to +50
°C, preferably at -30
to +20 °C, more preferably at -25 to +10 °C, particularly
preferably at -20 to 0 °C,
most preferably at -15 to -5 °C, especially preferably at about -10
°C.
Also preferred is a process wherein the reaction in step a) is carried out at
an
overall flow rate, calculated by adding together the flow rates of the
compound of
formula II and the acetoacetate, of 1.5 to 5 ml/min, preferably at 1.8 to 4
ml/min,
particularly preferably at 2 to 3.5 ml/min, particularly preferably at about
2.5
2s ml/min.
Also particularly preferred is a process wherein the flow rate of the compound
of
formula (II) to that of the acetoacetate is in a ratio of 1:1 to 1:2,
preferably 1:1.1 to
1:1.8, particularly preferably 1:1.2 to 1:1.5, particularly preferably about
1:1.3.
To achieve these flow rates it is generally advantageous to use low-vibration
pumps, preferably rotary pumps, preferably ceramic rotary pumps or HPLC
pumps. The flow rates may be adapted to different types of reactors to obtain
the
optimum space/time yield.
l,
Also particularly preferred is a process wherein the reaction is carried out
in a
plurality of microreactors connected in series or in parallel.
CA 02438878 2003-08-20
s
The 5,6-dihydro-4-hydroxy-6-phenethyl-6-propyl-2H-pyran-2-one obtained
according to the invention is preferably used to prepare tipranavir.
In the present invention, the term "alkyl" denotes a straight-chain or
branched alkyl
s group with 1 to 8 carbon atoms, preferably 2 to 7 carbon atoms, preferably 3
to 6
carbon atoms. Methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec.
butyl,
tert.butyl, n-pentyl, isopentyl or neopentyl are particularly prefer-ed.
The term "aryl" denotes an aromatic hydrocarbon group with 6 to 10 carbon
to atoms, preferably phenyl or naphthyl, particularly preferably phenyl, which
may be
substituted by one or more alkyl groups.
Examples of cycloalkyl groups with 3 - 8 carbon atoms include cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl or cyclooctyl.
is
R' is preferably phenylmethyl, phenylethyl or phenylpropyl, most preferably 2-
phenylethyl,
R2 is preferably methyl, ethyl, n-propyl or n-butyl, most preferably n-propyl.
R3 is preferably methyl, ethyl, n-propyi or benzyl, most preferably ethyl.
Metal hydrides, metal organyls, metal amides, metal dialkylamides or metal
hexamethyldisilazanes are preferably used as strong bases.
Examples of metal cations include lithium, sodium, potassium, rubidium,
caesium,
2s magnesium, calcium, titanium, silicon, tin and lanthanoids, preferably
lithium or
sodium, most preferably lithium.
Particularly preferred bases are sodium hydride, lithium diethylamide, butyl
lithium,
lithium diisopropylamide, lithium hexamethyl disilazane, sodium hexamethyl
3o disilazane or potassium hexamethyl disilazane or combinations thereof. As a
rule,
2 or more equivalents of these bases are used, preferably 1.8 to 3.0,
particularly
1.9 to 2.5 equivalents. The acetoacetate is generally in the form of a dianion
of
formula III in the presence of these bases.
O- O
O~~
3s (III)
CA 02438878 2003-08-20
6
The process according to the invention is generally carried out in the
presence of
an inert diluent. Preferred diluents are non-polar organic solvents such as
e.g.
_ aliphatic or aromatic hydrocarbons, ethers or mixtures thereof. In a
particularly
s preferred embodiment, the diluent is selected from among dimethoxyethane,
diethylether, tert-butyl-methylether, tetrahydrofuran, n-hexane, cyclohexane,
toluene, xylene or a mixture of these solvents, particularly tetrahydrofuran
and
dimethoxyethane.
io In addition to the abovementioned diluents the reaction may also contain
one or
more amines such as, for example, diethylamine, diisopropylamine or
tetramethylethylenediamine.
The advantage of the process according to the invention is in the high purity
and
is unexpectedly high yield of dihydropyranone of more than 90 %, which results
from
the continuous microreactor process of step a). The compound of formula IV may
be further processed as a product of the microreactor process without further
purification.
2o The following examples serve to illustrate the process according to the
invention
still further. They are intended solely as examples of procedures without
restricting
the invention to their content.
Example 1: 5,6-dihydro-4-h~droxy-6-phenethyl-6-propyl-2H-pyran-2-one
2s
Step a)
A mixture I of 81.9 g of 1-phenyl-3-hexanone and 840 ml of tetrahydrofuran and
a
mixture II of 72.9 g of ethyl-acetoacetate, 117 ml of diethylarnine and 450 ml
of
n-butyllithium in n-hexane (2.5 molar) in 361 ml of tetrahydrofuran at -10
°C are
3o pumped towards each other into a microreactor made by the company Institut
fur
Mikrotechnik Mainz GmbH (of the Liga type with interdigital channel structure)
and
mixed together. The volume flow of mixture I is set to 1 ml/min and the volume
flow of mixture II is set to 1.1 ml/min. The solution of product is passed
through a
capillary (length 1 m, diameter 1 mm) and then taken up in saturated ammonium
ss chloride solutionlhydrochloric acid solution at a pH of 5 - 6.
CA 02438878 2003-08-20
7
Step b)
140 g of the crude f3-ketoester resulting from step a) are taken up in 200 ml
of
methanol at 5 to 10 °C. Solid potassium hydroxide is added at 5 to 10
°C with
stirring and then the mixture is stirred for about 15 hours at ambient
temperature.
s The methanol is distilled off and the residue is mixed with 500 ml of water.
It is
extracted twice with 200 ml of toluene. After the organic phase has been
separated off, another 400 ml of fresh toluene are added to the aqueous phase.
This is acidified with conc. sulphuric acid to pH 1.9. The aqueous phase is
separated off and the organic phase is extracted 3 times more with water. The
io organic phase is evaporated to dryness in vacuo (60 mbar) at 40 °C.
The crude
product is dissolved in 200 ml of toluene at 60 °C and then filtered.
200 ml of
n-octane are slowly added dropwise to the filtrate with stirring at 40
°C. It is
seeded with 5,6-dihydro-4-hydroxy-6-phenethyl-6-propyl-2H-pyran-2-one crystals
and stirred for about 15 hours at ambient temperature. 400 ml of n-octane are
is added dropwise to the resulting crystal mass which is then cooled to 0 - 5
°C. After
stirring for about 1 hour at 0 - 5°C the crystals are suction filtered,
washed with
n-octane and dried. The yield is 92 %.
Analogously to Example 1, 5,6-dihydro-4-hydroxy-6-phenethyl-6-propyl-2H-pyran
20 2-one was prepared by carrying out step a) in a microreactor under the
conditions
specified in the following Table::
CA 02438878 2003-08-20
ExampleTemperature[Volume flowVolume Yield of ketoester
No. C] I flow II [HPLC % area]
(1-phenyl-3-(Acetoacetate)
hexanone) [mUmin]
[ml/min]
2 -25 1 1 84.5
3 -25 1 1.1 87.9
4 -25 1 1.2 83.8
-25 1 1.3 86.4
6 -25 2 2.4 81.1
7 -20 1 1.2 87.2
8 -20 2 2.4 83.8
9 -10 1 1.2 86.5
-10 2 2.4 86.8
11 ~ 10 ~ 2 ~-_ 2 .4 82 .-1