Note: Descriptions are shown in the official language in which they were submitted.
CA 02441841 2003-09-22
TITLE OF THE INVENTION
PEROXIDE BLEACHING OF WOOD PULP
FIELD OF THE INVENTION
This invention relates to an improved hydrogen peroxide bleaching
process for mechanical or high yield pulps.
BACKGROUND OF THE INVENTION
There is continuous research in the pulp and paper industry aimed
at improving efficiencies in the various aspects of pulp and paper processes.
The
bleaching process is one aspect which has received ongoing attention.
The present invention is concerned with improvements in the process
for improving brightness in mechanical and ultra-high yield pulps, such as
groundwood pulp (GW), thermomechanical pulp (TMP), chemithermomechanical
pulp (CTMP), and alkaline peroxide mechanical pulp (APMP). The processes of
interest accomplish the bleaching utilizing hydrogen peroxide in an alkaline
environment.
The efficiency of such processes depends on various factors, a very
important one of which is the full utilization of the hydrogen peroxide. The
objective is to obtain the dual efficiency of the improved brightness and
reduced
chemical usage that comes from increased utilization.
It is known that chemicals present in the pulp slurry will result in
decomposition of a part of the hydrogen peroxide. This is particularly the
case in
the presence of transition metal ions. The hydrogen peroxide decomposition
obviously reduces potential bleaching power, but also affects brightness by
causing the formation of new chromophores. Manganese is known to be the most
harmful transition metal species in this regard.
It is common to utilize stabilizers and sequestering agents to reduce
the peroxide decomposition.
CA 02441841 2003-09-22
-2-
Notwithstanding the use of these methods, the peroxide
decomposition has been an ongoing problem.
Against this background the present invention provides a process in
which the peroxide decomposition is reduced and brightness enhanced relative
to
known processes. This process is carried out in the context of conventional
peroxide bleaching processes.
PRIOR ART
Typical state of the art processes are described in the following
references:
1. Presley, J.R. and Hill, R.T., Pulp Bleaching: Principles and Practice,
Edited by C.W. Dence and D.W. Reeve, Page 480. This is the so-called cascade
system for preparation of bleach liquor, in which magnesium sulfate and sodium
silicate are added to water and intimately mixed, followed by the addition of
caustic
soda and finally by the addition of hydrogen peroxide. The resulting liquor is
subsequently mixed with pulp.
2. Presley, J.R. and Hill, R.T., Pulp Bleaching: Principles and Practice,
Edited by C.W. Dence and D.W. Reeve, Page 481. This is the so-called in-line
system, where similar mixing and addition occurs but without the cascade
arrangement.
3. Ni, Y. et al., Proceedings, PAPTAC Annual Meeting, Montreal, 1999,
Page B183. This process provides a sequential addition of chemicals beginning
with the addition of hydrogen peroxide to a pulp slurry and the subsequent and
simultaneous addition of caustic soda and silicate stabilizer.
4. Vincent, A.H.D. et al, Magnesium Oxide Driven Peroxide Bleaching,
An economical and Environmentally Viable Process, APPITA Annual Conference
1997. This paper describes the use of magnesium oxide as an alkaline source in
peroxide bleaching.
5. Soteland, N., et. al., Use of Mg0 or Ca0 As The Only Alkaline
Source In Peroxide Bleaching of High Yield Pulps, pp. 231-236, 1988
International
Pulp Bleaching Conference, TAPPI Proceedings.
CA 02441841 2003-09-22
-3-
6. Griffiths, Paul, et. al., Magnesium Oxide as a Base for Peroxide
Bleaching of Radiata Pine TMP, pp. 50-54, Appita Vol. 47 No. 1, January 1994.
The following patents and published applications deal with bleaching
processes for mechanical or high yield pulp, but do not address the process of
the
present invention:
Canadian Patents 686,115; 820,190; 1,294,655; 1,310,797;
2,041,588; 2,070,556; Canadian published Application 2,278,399;
United States Patents 2,872,280; 3,023,140; 4,029,543; 4,731,161;
4,812,206; 4,915,785; 4,938,842; 5,118,389; United States published
Application
US 200110050153 A1;
Japanese Patent document 52-63402; and
Russian Patent document 1735463.
BRIEF SUMMARY OF THE INVENTION
It has now been determined that improvement in the bleaching
process can be obtained if at least one stabilizer is added to the pulp slurry
prior
to the addition of the hydrogen peroxide, and the process is carried out
subject to
a specified pH limitation.
Thus, the invention provides a process for peroxide bleaching of
mechanical or high yield pulp, the process comprising adding to a pulp slurry
at
least one stabilizer for stabilizing transition metal ions in said slurry;
subsequently
adding hydrogen peroxide to said slurry at a preselected point; adding an
alkali
source to said slurry simultaneously with or subsequent to adding said at
least one
stabilizer, such that the pH of the resulting slurry does not exceed 11.5; and
subjecting said slurry to preselected conditions to complete said bleaching
process. The stabilizer is chosen from the group consisting of silicate, MgS04
or
other stabilizers. Other stabilizers may include DTPA or other sequestering
agents
or other stabilizers.
In a further embodiment, the process comprises the pretreatment
steps of adding a chelating agent to said slurry and subsequently removing
chelated transition metal ions from said slurry.
CA 02441841 2003-09-22
-4-
In a further embodiment, the process further comprises the
pretreatment steps of adding a chelating agent and a reducing agent to said
slurry
and subsequently removing chelated transition metal ions from said slurry
In a further embodiment there is provided a process for peroxide
bleaching of mechanical or high yield pulp, the process comprising adding to a
pulp slung sodium hydrosulfite and DTPA; subsequently removing from the slurry
chelated transition metal ions; adding to the slurry a stabilizer comprising
sodium
silicate andlor other stabilizer; subsequently adding hydrogen peroxide to
said
slurry at a preselected point in said process; adding an alkaline source to
said
slurry simultaneously with or subsequent to adding said at least one
stabilizer,
such that the pH of the slurry does not exceed 11.5; and subjecting said
slurry to
preselected conditions to complete said bleaching process.
BRIEF DESCRIPTION OF THE DRAWINGS
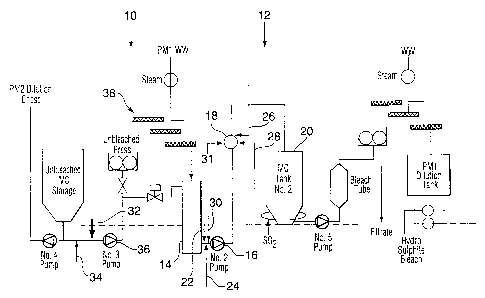
The figure illustrates in schematic form a process according to the
invention. While the invention will be described in conjunction with the
illustrated
embodiments, it will be understood that it is not intended to limit the
invention to
such embodiments. On the contrary, it is intended to cover all alternatives,
modifications and equivalents as may be included within the spirit and scope
of the
invention as defined by the appended claims.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the following description, similar features in the drawings have
been given similar reference numerals.
It is useful in understanding the invention to consider the problem
that arises in the peroxide bleaching process as a result of the naturally
occurring
transition metal ions in the wood. The particular transition metals present
and their
concentrations vary geographically and seasonally, but the problem caused by
the
transition metals will always be present.
CA 02441841 2003-09-22
-5-
As a fundamental part of process efficiency, there is a desire to
maximize peroxide utilization in the bleaching process. At the same time, it
is
desired to minimize the production of chemicals in the process which adversely
affect brightness by producing chromophores.
The invention therefore concerns the realization that if the transition
metal ions can be stabilized by the addition of stabilizers, such as silicate,
to a pulp
slurry prior to addition of hydrogen peroxide, the decomposition of peroxide
will be
greatly reduced and the overall efficiency of the process will be raised. It
is
advantageous to accomplish this in the cantext of the conventional peroxide
bleaching process, so that the parameters of that process utilized in a given
mill
need not be altered to any great extent.
As will be discussed below, one preferred embodiment of the
invention involves a pre-treatment process to remove some transition metal
ions.
This comprises an enhanced chelation process preceding the bleaching process
itself.
Turning now to the drawing, the flow sheet can be divided as
between the pre-treatment section 10 and the bleach section 12. The process of
the present invention is not dependent on the presence of a pre-treatment
section
10 and can be said to begin at the tank 14. From tank 14 the unbleached pulp
slurry is pumped via pump 16 through T-mixer 18 to tank 20. The stabilizers
and
bleach chemicals are added between tanks 14 and 20. It is convenient to add
stabilizers via lines 22 and 24 at pump 16 to facilitate mixing at pump 16.
Alkali
can also be added via line 30 at pump 16 or farther downstream, via, for
example,
line 31 at mixer 18. Hydrogen peroxide and water are subsequently added
through
lines 26 and 28 to T-mixer 18.
Sodium silicate is the preferred stabilizer, it may be combined with
a small amount of magnesium sulfate, or DTPA or EDTA or their salts.
The alkali source can be chosen from those used in conventional
such processes including soda ash, magnesium oxide, magnesium hydroxide and
NaOH.
It is highly preferable that the inventive process described above be
preceded by a pre-treatment process, as is common in the art, for the removal
of
CA 02441841 2003-09-22
-6-
a significant proportion of the transition metal ions which are present in
varying
concentrations in the pulp.
It is further preferred that the pre-treatment process comprise a
reducing agent assisted chelation process. One such process is described in
Ni, Y. et. al., Pulp & Paper Canada, 100(10), 51-55 (1999).
Therefore, with reference to the drawing; the pre-treatment process
comprises adding at least one of a chelating agent at line 32 and preferably a
reducing agent at line 34. In the preferred case both the chelating agent and
the
reducing agent are used in the pretreatment process.
While known suitable chelating and reducing agents may be used
in the pre-treatment process, it is preferred that the chelating agent be DTPA
and
that the reducing agent be sodium hydrosulfite.
An advantage of these two additives is that conditions for their use
are similar, so that they can be added in a single step.
Subsequent to the addition of the additives, preferably downstream
of a pump 36, the pulp slurry is dewatered and washed at press and washers 38
to remove the chelated transition metal ions. The slurry is then transferred
into
tank 14 and hence into the bleach process.
With reference to the bleach process, the following are typical
parameters, where the alkali source is caustic soda:
~ Hydrogen peroxide charge 1 - 10%
~ Caustic soda charge 0.5 - 5%
~ Sodium silicate charge 1 - 5%
~ Magnesium sulfate charge 0 - 0.1
~ DTPA charge 0.05 - 0.5%
The following examples illustrate the invention.
Example 1.
Equivalent to 10 grams o.d. mill chelated TMP pulp from a mill in
Eastern Canada (60 ppm Mn, 53% ISO initial brightness) was treated in a
polyethylene bag with 2% Na2Si03, reagent grade, and 0.05% MgS04 for 1
CA 02441841 2003-09-22
7
minute; and then with addition of 3% H20z for about 5 minutes. A thorough
mixing
was provided after each reagent addition. Subsequently 1.5% NaOH was added
to the pulp in the polyethylene bag. The bag, along with its contents, was
placed
in a temperature bath at 60°C. The bleaching conditions were 120 min,
12% pulp
consistency.
After the completion of the required reaction time, a portion of the
filtrate was then taken to determine the residual H202 and ending pH. The
remaining pulp slurry was transferred from the bag to a beaker, further
diluted, and
neutralized with sulfuric acid to pH 5. The neutralized pulp slurry was
subsequently filtered and washed thoroughly with deionized water. A handsheet
was then made following TAPPI test method T272, air-dried and determined for
brightness.
The residual hydrogen peroxide was 0.65% on pulp, and the
brightness of the resulting pulp was 69.6% iSO.
The process described above is designated as the PM process.
Example 2.
The following is provided to illustrate that the residual hydrogen
peroxide is much less and the brightness of the resulting pulp is lower if the
same
TMP pulp was subjected to a conventional peroxide stage (P) under otherwise
the
same conditions.
The conventional peroxide stage (designated as P process in the
subsequent discussion) was performed as follows:
Equivaientto 0.05% MgS04, 2.0% Na2Si03, 1.5% NaOH, and 3.0%
H202 were first added in that order to a beaker containing distilled water to
form
a mixture. This mixture was then added to a polyethylene bag which contains
equivalent to 10 grams o.d. of the same TMP pulp as in Example 1. The contents
were mixed thoroughly. Subsequently, the polyethylene bag, along with its
contents, was placed in a temperature bath at 60°C to start bleaching.
The
bleaching conditions were the same as those in Example 1. After the completion
of the required reaction time, samples were collected for residual H202,
ending pH
and brightness, in accordance with the procedures in Example 1.
CA 02441841 2003-09-22
_ 8 _
The residual hydrogen peroxide was 0.29% on pulp and the pulp
brightness was 68.7% ISO. These results are compared with those in Example 1
of 0.65% and 69.6% ISO, respectively, supporting that the peroxide bleaching
performance is improved by the process outlined in Example 1.
Example 3.
In this example, it will be shown that the PM process, described in
Example 1, can be varied and the improvement in bleaching performance overthe
P process can be maintained, even enhanced. In this case, DTPA was added as
part of the stabilizers, along with sodium silicate and magnesium sulfate.
These
stabilizers were mixed with the pulp slurry for 5 minutes. Subsequently, the
required amount of caustic soda was added. The same TMP pulp as that in
Example 1 was used. The procedures for the PM and P process were the same
as those in Example 1, except that 0.1 % DTPA solution was added to the pulp
slurry in a polyethylene bag, along with 2% Na2Si03 and 0.05% MgS04.
The residual hydrogen peroxide was 0.87% on pulp, and the
brightness of the resulting pulp is 70.8% ISO. Evidently, in comparison with
Example 2, the PM process given in this example leads to much improved
bleaching results.
Example 4.
In this example, it will be shown that the PM process, described in
Example 1, can be further varied, and the improvement in bleaching performance
over the P process can be maintained. In this case, the stabilizers, namely
sodium
silicate, magnesium sulfate, DTPA and other chemicals, needed for peroxide
bleaching, namely, caustic soda and hydrogen peroxide, are added to the pulp
slurry in various orders. Three more orders were conducted, namely:
A: 1St Sodium silicate and magnesium sulfate
2"d Caustic soda
3'° DTPA
H2~2
CA 02441841 2003-09-22
-9-
10
B: 1S' Sodium silicate and magnesium
sulfate
2"d Caustic soda
3~d H202
4'" DTPA
C: 1 St DTPA
2"d Sodium silicate and magnesium
sulfate
3' Caustic soda
4th Hz~2
Always a thorough mixing is provided before the next chemical is charged to
the
pulp. The key here, however, is that at least one of stabilizers, such as
sodium
silicate and DTPA, is charged to the pulp with sufficient mixing before the
addition
of hydrogen peroxide.
The same TMP pulp as that in Example 1 was used. The chemical
charges and other procedures were the same as those in Example 1. The results
are listed in Table 1.
Table 1.
Sequence A Sequence B Sequence C
Brightness 70.7 70.7 70.8
(% ISO)
Residual HZOZ
(% on pulp) 1.01 0.89 0.95
I n comparison with the P process, Example 2, one can conclude that
the bleaching performances of Sequences A, B, and C are much better.
Example 5.
in this example it will be shown that the benefit of the PM process, in
comparison with the P process, can still be achieved when applied to a TMP
pulp,
which was chelated in the Qy process, the so-called sodium hydrosulfite
assisted
chelation process.
Equivalent to 20 grams o.d. TMP pulp (brightness 54.3% ISO, 149
ppm Mn) was treated in a polyethylene bag with 0.2% DTPA (as active DTPA on
pulp) and 0.1 % sodium hydrosulfite (on pulp) under the conditions of 3% pulp
CA 02441841 2003-09-22
-10-
consistency, 60°C and 10 minutes. After the completion of the required
time the
pulp slurry was filtered and pressed to about 30% pulp consistency. The
chelated
pulp has a residual manganese content of about 34 ppm, which is then ready for
the bleaching experiments.
One half of the Qy treated pulp was then treated in a polyethylene
bag with 3% sodium silicate, 0.05% MgS04 for about 10 minutes. Subsequently,
2% H202 and 1.6% caustic soda were added to the pulp slurry in that order. A
thorough mixing was provided before the addition of each chemical. The other
bleaching conditions and procedures were the same as those in Example 1.
The residual hydrogen peroxide was 0.25% (on pulp) and the
brightness of the resulting pulp was 69.0% ISO.
The other half of the Qy treated pulp was subjected to a conventional
peroxide stage following the same procedure as those in Example 2 except that
the chemical charges were the same as those in the previous paragraph, i.e. 3%
Na2Si03, 0.05% MgS04, 1.6% NaOH and 2% H202 . The residual H202 was 0.05%
(on pulp) and the brightness of the resulting pulp was 65.8% ISO.
Example 6.
It will be shown that PM process, described in Example 1, can be
further varied and the improvement in bleaching performance over the P process
can be maintained. In this case, instead of reagent grade, a silicate solution
(37.56% solid, 8.90% Na20, 28.66% Si02, specific gravity @ 20°C, 1.394,
viscosity
@ 20°C, 177 cP), which is commercially available was used. The
stabilizers,
namely the silicate solution, and/or MgS04, were mixed with the required NaOH.
The above mixture was then added to the pulp.
A TMP pulp with initial brightness of 57.8% ISO and 48 ppm Mn
content was used. Equivalent to 10 grams o.d. of above pulp at a consistency
of
about 15%, was added to a polyethylene bag. A mixture prepared with 4% of the
above specified silicate solution (on o.d. pulp), 0.1 % MgS04 (on o.d. pulp)
and
2.8% NaOH (on o.d. pulp), was then admitted to the same polyethylene bag and
thoroughly mixed with the pulp fibres (the mixing time was about 1 min).
Subsequently, 3.5% Hz02 was added to the bag and mixed thoroughly with its
contents. The bag, along with its content, was placed in a temperature bath at
CA 02441841 2003-09-22
-11-
70°C. The bleaching conditions were: 120 min, 12% pulp consistency.
After the
completion of the required reaction, samples were collected for residual H202,
ending pH and brightness, in accordance with the procedures in Example 1. The
residual hydrogen peroxide was 1.28% and the pulp brightness was 72.5% ISO.
The same mill chelated TMP pulp, initial brightness of 57.8% ISO
and 48 ppm Mn content was subjected to a conventional peroxide stage (P) with
the same chemical charges as above (0.1 °,i° MgS04, 4% silicate
solution, 2.8%
NaOH, 3.5% H202, all based on o.d. pulp) under the same conditions
(70°C, 12%
pulp consistency). The above chemicals were added to a beaker containing
distilled water to form a mixture. The mixture was then added to a
polyethylene
bag which contains equivalent to 10 grams o.d. pulp. The subsequent procedures
were the same as those in Example 2.
The residual hydrogen peroxide was 0.28% and the pulp brightness
was 70.7%. The results are compared with above of 1.28% and the pulp
brightness of 72.5% ISO respectively, supporting that the peroxide bleaching
performance is improved by the PM process.
Example 7.
A TMP pulp (initial brightness of 50.4% ISO), directly obtained from
a 3-stage refiner process, was first subjected to a laboratory chelation
process,
which was performed under the conditions of 0.125% DTPA, 10% pulp
consistency, 50°C and 30 min. Then the pulp slurry was filtered and
pressed to
25% pulp consistency, and used for subsequent peroxide bleaching.
In the PM process, the above pressed pulp was diluted to about 12%
pulp consistency in a polyethylene bag with deionized water. The bag along
with
its content was pre-heated to 60°C. Subsequently, 3.0% industrial
silicates, 0.05%
MgS04 and 1.0% NaOH were added to the bag. A thorough mixing was provided
(its pH was 10.7). After about 1 min, 1.5% H202 was added to the pulp slurry
in the
bag (the pH was 10.2). The bleaching was allowed at 60°C for 2 hours at
10%
pulp consistency. The subsequent procedures were the same as those in
Example 1. The residual peroxide was 0.24%, the final pH was 7.5 and the
brightness of the resulting pulp was 63.1 % ISO.
Control
CA 02441841 2003-09-22
- 12-
In the control run, the same pressed pulp (25% pulp consistency)
was diluted to about 12% pulp consistency and pre-heated to 60°C.
Subsequently,
3.0% industrial silicates, 0.05% MgS04, 1.0°/~ NaOH and 1.5% H202 was
mixed in
a beaker, and then the mixture was transferred to the heated pulp slurry,
which
was in a polyethylene bag (the pH was 10.3). The bleaching conditions and the
subsequent procedure were the same as above. The residual peroxide was
0.04%, the final pH was 7.6 and the brightness of the resulting pulp was 61.7%
ISO.
It is evident that the PM process has higher pulp brightness and
higher residual peroxide, in comparison with the control.
Example 8.
A mill chelated SGW (initial brightness of 63.1 % ISO) was used for
the comparison of the PM process and the conventional peroxide process.
In the PM process, 1.5% NaOH, 3.9% industrial silicates were mixed
with pulp slurry at about 12% pulp consistency and 60°C (the pH was
11.0). After
about 1 min., 1.9% Hz02 was added (the pH was 10.4). The bleaching was carried
out at a 10% pulp consistency, 60°C for 70 min. The subsequent
procedures were
the same as those in Example 1. The residual peroxide was 0.74%, the end pH
was 8.7 and the brightness of the resulting pulp was 73.9% ISO.
Control
In the control, 3.9% industrial silicates, 1.5% NaOH and 1.9% H202
were mixed in a beaker first, the mixture was then added to the pulp slurry at
about
12% pulp consistency and 60°C (the pH was 10.7). The bleaching
conditions and
subsequent procedures were the same as the PM process. The residual peroxide
was 0.35%, the end pH was 8.7 and the brightness of the resulting pulp was
73.0%
ISO.
Again, the above results support the conclusion that the performance
of the PM process is superior to that of the control.
Example 9.
The same SGW pulp was subjected to peroxide bleaching under the
PM and conventional peroxide process at higher peroxide charge (3.9%) and
sodium hydroxide charge (2.0%). Other conditions and procedures were kept the
CA 02441841 2003-09-22
-13-
same. The results are listed in the following table. Once again, the PM
process
produces bleached pulps with higher brightness, yet at a lower peroxide
consumption.
Control, the P ProcessThe P Process
pH after the addition Not applicable 11.3
of
NaOH and silicates
pH after the addition Not applicable 10.5
of I
HO
pH after the addition 10.6 Not applicable
of
mixture of silicates,
NaOH
and H O
End H 8.7 8.7
Residual H O % 0.90 1.75
Bri htness % ISO) 76.5 ~ 77.3
Example 70.
A mill chelated pulp with initial brightness of 55% ISO was used. In
the PM process, 1.2% industrial silicates, 0.08% MgS04 and 1.0% NaOH were
added to the pre-heated pulp at 20% pulp consistency and 80°C (the pH
was
11.0). After about 1 min, additional 1 % NaOH (the total NaOH charge was 2%)
and 2% H202 were added to the pulp (the pH was 11.0). The bleaching was
carried out at 18% pulp consistency, 80°C and 2.5 hours. The subsequent
procedures were the same as those in Example 1. The residual peroxide was
0.32%, the end pH was 7.9 and the brightness of the resulting pulp was 75.6%
ISO.
Control
In the control,1.2% industrial silicates, 0.08% MgS04, 2% NaOH and
2% H2O2 were mixed in a beaker. The mixture was then added to the pre-heated
pulp at 20% pulp consistency and 80°C (the pH was 10.9). The bleaching
conditions and the subsequent procedures were the same as above. The residual
peroxide was 0.19%, the end pH was 7.8 arid the brightness of the resulting
pulps
CA 02441841 2003-09-22
-14-
was 74.7% ISO. The above example again shows that a higher brightness is
achieved for the PM process than the control under otherwise the same
conditions.
Example 11.
Another CTMP Maple pulp with initial brightness of 54% was used.
Here, Mg(OH)2, instead of NaOH, was the alkali source. Also, no silicates were
added, and DTPA was used as the peroxide stabilizer. In the PM process, 1
Mg(OH)2 slurry and 0.1 % DTPA were added to the pre-heated pulp at 20% pulp
consistency and 80°C (the pH was 8.12). After about 1 min, 2% Hz02 was
added
to the pulp (the pH was 7.8). The bleaching conditions were 17% pulp
consistency, 80°C, 2.5 hours. The subsequent procedures were the same
as
those in Example 1. The residual peroxide was 0.66%, end pH was 6.9 and the
brightness of the resulting pulp was 70.1 % ISO.
Control
In the control process 0.1 % DTPA, 1 % Mg(OH)2 slurry and 2% HZOZ
were added to the pre-heated pulp at 20% pulp consistency and 80°C (the
pH was
7.9). The bleaching conditions and subsequent procedures were the same as
those above. The residual peroxide was 0.58%, the end pH was 7.2 and the
brightness of resulting pulps was 69.4%. The above results show that the
benefit
of the PM process is still apparent even Mg(OH)2 instead of NaOH, was used as
the
alkali source during peroxide bleaching.
The initial pH in this series of L_xamples (7 to 11 ) did not exceed 11.0
for conventional peroxide bleaching conditions. A fairly extreme example went
to
11.3, and it is contemplated that the initial pH would never exceed 11.5.
Furthermore, the pH at the end of the bleaching process was not
more than about 8.7. This would normally not exceed 9.0 and, in an extreme
case,
should not exceed 9.5.
CA 02441841 2003-09-22
-15-
Example 72.
A Stoneground wood pulp (SG'~1I) with an initial brightness of 57.6%
ISO was used.
In the PM process, 1 % Mg(OH)2 and 0.3% DTPA were mixed with the
pulp first. After 30 seconds to 1 min, 2.73% of hydrogen peroxide was then
added
to the pulp.
In the P process, the bleach liquor consisting of 1% Mg(OH)2, 0.3%
DTPA and 2.73% H202 was prepared first in a beaker. This mixture was then
added to the pulp.
The bleaching conditions of the P and PM processes were the same:
7.5% pulp consistency, 240 min. 75°C. The results are given in Table 1.
One can find that the PM process produced the pulp with a higher
brightness (73.0 versus 71.2% ISO) at a higher residual peroxide (1.3 versus
1.0%) than the P process.
Table 1.
Description Brightness Residual HZOZ
(% ISO) (%)
P 71.2 1.0
PM 73.0 1.3
Example 73.
Another SGW pulp with an initial brightness of 62.9% ISO was
tested, and a comparison is made between the P and PM processes of the
magnesium hydroxide based peroxide procE;ss.
In the PM process, 1.5% Na2SiO3 and 1 % Mg(OH)2 were mixed with
the pulp first. After 1 min, 3.4% hydrogen peroxide was then added to the
pulp.
In the P process, the bleach liquor, consisting of 1.5% Na2Si03, 1%
Mg(OH)2 and 3.4% H202 was prepared first in a beaker. This mixture was then
added to the pulp.
CA 02441841 2003-09-22
-16-
The bleaching conditions of the P and PM processes were the same:
25% pulp consistency, 52°C, 150 min. The results are given in Table 2.
Table 2..
Description Brightness Residual H202
(% ISO) (%)
P 80.6 1.9
P~ 81.6 2.1
Again Table 2 shows that, in comparison with the P process, the PM
process produces much better bleaching results.
Thus, it is apparent that there has been provided in accordance with
the invention a peroxide bleaching of wood pulp that fully satisfies the
objects,
aims and advantages set forth above. While the invention has been described in
conjunction with specific embodiments thereof, it is evident that many
alternatives,
modifications and variations will be apparent to those skilled in the art in
light of the
foregoing description. Accordingly, it is intended to embrace all such
alternatives,
modifications and variations as fall within the spirit and broad scope of the
invention.