Note: Descriptions are shown in the official language in which they were submitted.
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
BLOOD TESTING DEVICE
CROSS-REFERENCE TO RELATED APPLICATIONS
The present Application claims the benefit of United States Provisional
Patent Application 60/281,957 titled "Unidirectional Self-sealing Blood
Testing
Apparatus," filed April 6, 2001; the contents of which are incorporated by
reference in this disclosure in their entirety.
BACKGROUND
The practice of medicine frequently involves testing blood for the
presence or amount of one or more than one constituent, such as glucose,
cardiac enzymes or red cells, or testing blood to determine the level of one
or
more than one parameter, such as pH. Such testing is often done while
performing an intravascular access procedure by allowing blood from the
distal end of an intravascular access apparatus to contact the end of a test
strip, and then inserting the test strip into an appropriate test strip
reader.
This might be done, for example, by allowing blood to leak out of a catheter
that has accessed the vascular structure, prior to attaching the intravenous
fluid administration tubing, onto the test strip. Another method of gaining
access to a patient's blood is to force the blood out of the vascular access
device onto the test strip.
Frequently, however, intravascular access procedures are performed by
emergency medical personnel or by paramedics under adverse environmental
conditions, such as inclement weather. Further, intravascular access
procedures are often performed by emergency medical personnel or by
paramedics in moving vehicles. These conditions increase the possibility of
inaccurate blood test results due to test strip contamination, and increase
the
risk of exposure of the emergency medical personnel or paramedics to
pathogens present in the blood being tested.
Therefore, it would be useful to have a device for testing blood
conditions that decreases the possibility of inaccurate blood test results due
to
test strip contamination. Further, it would be useful to have a device for
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
2
testing blood conditions that decreases the risk of exposure of healthcare
providers to pathogens present in the blood.
SUMMARY
In one embodiment, there is provided a blood testing device for testing
blood for the presence or for the amount of one or more than one constituent,
or for testing blood to determine the level of one or more than one parameter,
or for testing blood for a combination of the preceding.
The blood testing device comprises a proximal end of the blood testing
device and a distal end of the blood testing device; an intermediate portion
between the proximal end of the blood testing device and the distal end of the
blood testing device; and a pressure compensation system to regulate the
pressure in the intermediate portion; and the intermediate portion comprises
a proximal end of the intermediate portion comprising one or more than one
opening, a distal end of the intermediate portion and, between the proximal
end of the intermediate portion and the distal end of the intermediate
portion,
comprises a hollow, central chamber surrounded by an outer wall.
In one preferred embodiment, the pressure compensation system
comprises a distal portion between the proximal end of the blood testing
device and the distal end of the blood testing device, an inner membrane
separating the intermediate portion from the distal portion; and the distal
portion comprises a proximal end of the distal portion and a distal end of the
distal portion and, between the proximal end of the distal portion and the
distal end of the distal portion, comprises a hollow, central chamber
surrounded by an outer wall.
In another preferred embodiment, the pressure compensation system
comprises a pressure compensation valve, which can be a duck bill valve.
In one embodiment, the blood testing device further comprises a distal
end cap at the distal end of the blood testing device comprising a first
opening configured to receive a test strip. Embodiments are described using
an end cap that is a semi-permeable member, and a non-permeable member.
In another embodiment, the outer wall of the intermediate portion is
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
3
generally cylindrical.
In another embodiment, the outer wall of the distal portion is generally
cyl i nd rical.
In another preferred embodiment, the blood testing device further
comprises a proximal portion between the proximal end of the blood testing
device and the proximal end of the intermediate portion. In a further
embodiment, the proximal portion comprises a hollow, central chamber
surrounded by an outer wall, and the proximal portion is configured to mate
with a blood containing apparatus. In a further embodiment, the blood
containing apparatus is selected from the group consisting of a syringe and
the introducer part of an intravascular access apparatus. In a further
embodiment,
the outer wall of the proximal portion is generally cylindrical.
In another preferred embodiment, the hollow, central chamber of the
intermediate portion and the hollow, central chamber of the distal portion
comprise generally rectangular inferior parts that communicate with each
other and that are configured to accept a test strip. In a further
particularly
preferred embodiment, the hollow, central chamber of the distal portion
further comprises one or more than one ridge axially along the proximal to
distal axis.
In another embodiment, the outer wall of the intermediate portion is
substantially transparent.
In another embodiment, the distal end cap is an integral part of the
distal portion of the blood testing device.
In another embodiment, the distal end cap is removably affixed to the
distal portion of the blood testing device.
In another embodiment, the distal end cap further comprises one or
more than one second opening to allow the venting of air externally out of the
distal portion of the blood testing device.
In another preferred embodiment, the inner membrane is a
semipermeable member.
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
4
In another embodiment, the inner membrane comprises one or more
than one vent.
In another embodiment, the outer wall of the intermediate portion
comprises one or more than one vent.
In another embodiment, the inner membrane comprises an opening
configured to allow passage of a test strip from the distal portion of the
blood
testing device into the intermediate portion of the blood testing device.
In another embodiment, the intermediate portion is integrally affixed to
an introduces portion of an intravascular access apparatus. In a further
embodiment the intermediate portion is configured to affix to an introduces
portion of an intravascular access apparatus.
In another preferred embodiment, the distal end of the blood testing
device is integrally affixed to a blood collection apparatus. In a further
embodiment, the distal end of the blood testing device is configured to affix
to a blood collection apparatus. In one embodiment, the blood collection
apparatus may be a syringe.
In another preferred embodiment, the intermediate portion is either
integrally affixed or configured to affix to an introduces portion of an
intravascular access apparatus, and the distal end is either integrally
affixed or
configured to affix to a blood collection apparatus. In one embodiment, the
blood collection apparatus may be a syringe.
In another embodiment, the present invention is a blood testing
apparatus comprising a plurality of interconnected blood testing devices
according to the present invention.
In another embodiment, the present invention is a method for testing
blood for the presence or for the amount of one or more than one constituent,
or for testing blood to determine the level of one or more than one parameter,
or for testing blood for a combination of the preceding. The method
comprises the steps of, first, providing a blood testing device according to
the
present invention, a test strip comprising a proximal end and a distal end, a
test strip reader configured to accept the distal end of the test strip and a
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
blood containing apparatus. Next, the blood testing device is affixed to the
blood containing apparatus and blood present in the blood containing
apparatus is allowed to enter into the intermediate portion of the blood
testing device. Then, the distal end of the test strip is inserted into the
test
5 strip reader and the proximal end of the test strip is inserted into the
intermediate portion of the blood testing device. Next, an operator uses
information provided by the test strip reader to determine the presence or the
amount of one or more than one constituent of the blood, or to determine the
level of one or more than one parameter, or to determine a combination of the
preceding. In one embodiment, the blood testing apparatus is selected from
the group consisting of an intravascular access apparatus and a syringe. The
blood testing device and blood containing apparatus can be affixed integrally.
In another embodiment, the method further comprises selecting a
patient whose blood is to be tested and intravascularly accessing the
patient's
blood with the blood containing apparatus. In another embodiment, the
method further comprises the step of discarding the blood testing device.
FIGURES
These and other features, aspects and advantages of the present
invention will become better understood with regard to the following
description, appended claims, and accompanying figures where:
Figure 1 is a lateral perspective view of a blood testing device according
to one embodiment of the present invention;
Figure 2 is a cross-sectional, lateral perspective view of the blood
testing device shown in Figure 1 ;
Figure 3 is a lateral perspective view of a blood testing device shown in
Figure 1 attached to a standard intravascular access apparatus;
Figure 4 is a lateral perspective view of a semipermeable membrane
forming part of the blood testing device of Figure 1;
Figure 5 is a lateral perspective view of a blood testing device according
to another embodiment of the present invention;
Figure 6 is a lateral perspective view of a blood testing device according
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
6
to another embodiment of the present invention;
Figure 7 is a lateral perspective view of a blood testing device according
to another embodiment of the present invention;
Figure 8 is a lateral perspective view of a blood testing apparatus
comprising a plurality of interconnected blood testing devices according to
the
present invention;
Figure 9 is a cross-sectional, lateral perspective view of the blood
testing device shown in Figure 1 according to another embodiment of the
present invention;
Figure 1 OA is a cross-sectional view of a duck bill valve forming part of
the blood testing device of Figure 6, before insertion of a test strip;
Figure 1 OB is a cross-sectional view of a duck bill valve forming part of
the blood testing device of Figure 6, after insertion of a test strip;
Figure 1 1 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention;
Figure 12 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention;
Figure 13 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention;
Figure 14 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention;
Figure 15 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention;
Figure 16 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention; and
Figure 17 is a cross-section and cut-away lateral view of a blood testing
device according to another embodiment of the present invention.
DESCRIPTION
According to one embodiment of the present invention, there is
provided a blood testing device for testing blood for the presence or for the
amount of one or more than one constituent, such as glucose, cardiac
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
7
enzymes or red cells, or for testing blood to determine the level of one or
more than one parameter, such as pH, or for testing blood for a combination
of the preceding. The blood testing device decreases the possibility of
inaccurate blood test results due to test strip contamination, and decreases
the risk of exposing an operator to pathogens present in the blood.
According to another embodiment of the present invention, there is
. provided a method for testing blood for the presence or for the amount of
one
or more than one constituent, such as glucose, cardiac enzymes or red cells,
or for testing blood to determine the level of one or more than one parameter,
such as pH, or for testing blood for a combination of the preceding. The
method comprises providing a testing means that decreases the possibility of
inaccurate blood test results due to test strip contamination, and decreases
the risk of exposing an operator to pathogens present in the blood. These
embodiments will be disclosed in greater detail in the following disclosure.
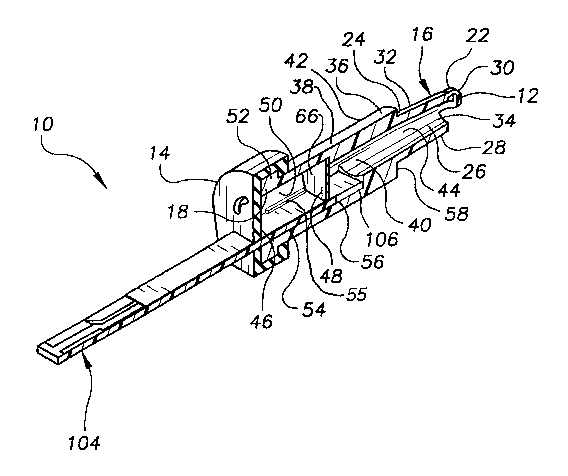
Referring now to Figure 1 , Figure 2 and Figure 3, there are shown,
respectively, a lateral perspective view of a blood testing device 10
according
to one embodiment of the present invention; a cross-sectional, lateral
perspective view of a blood testing device 10 shown in Figure 1 ; and a
lateral
perspective view of a blood testing device 10 shown in Figure 1 combined with
a intravascular access apparatus 100. As can be seen, in this embodiment the
blood testing device 10 comprises a proximal end 12 and a distal end 14.
Between the proximal end 12 and the distal end 14, the blood testing device
10 further comprises a proximal portion 16, a distal portion 18, and an
intermediate portion 20 between the proximal portion and the distal portion.
The proximal portion 16 comprises a proximal end 22 and a distal end
24 and, between the proximal end 22 and the distal end 24, further comprises
a hollow, central chamber 26 surrounded by an outer wall 28 having a long
axis in the proximal to distal direction. The outer wall 28 of the proximal
portion 16 is configured to mate with the distal end of a blood containing
apparatus, such as a syringe (not shown) or the introducer part 102 of an
intravascular access apparatus 100, as shown in Figure 3. Preferably, the
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
8
outer wall 28 of the proximal portion 16 is configured to mate with the distal
end of the introducer part of a standard intravascular access apparatus, such
as an Introcan~ SafetyT"' IV Catheter (B. Braun Medical Inc., Bethlehem, PA
US)
or other standard intravascular access apparatus, and, therefore, the outer
wall 28 of the proximal portion 16 will vary in configuration depending on the
intravascular access apparatus that will be used in conjunction with the blood
testing device 10 of the present invention, as will be understood by those
with
skill in the art with reference to this disclosure.
In a preferred embodiment, as shown in Figure 1 and Figure 2, the outer
wall 28 of the proximal portion 16 comprises a generally tapered proximal-
most part 30 and a generally cylindrical distal-most part 32. The proximal-
most part 30 of the outer wall 28 preferably further comprises one or more
than one opening 34 allowing the hollow, central chamber 26 to communicate
externally, or when combined with a blood containing apparatus, to
communicate with the blood containing part of the blood containing
apparatus. The one or more than one opening 34 is configured to allow the
passage of blood that is present in the blood containing apparatus into the
hollow, central chamber 26 of the proximal portion 16.
The intermediate portion 20 comprises a proximal end 36 and a distal
end 38 and, between the proximal end 36 and the distal end 38, comprises a
hollow, central chamber 40 surrounded by an outer wall 42 having a long axis
in the proximal to distal direction. Preferably, the outer wall 42 of the
intermediate portion 20 is generally cylindrical, though other configurations
are also suitable, as will be understood by those with skill in the art with
reference to this disclosure. The proximal end 36 of the intermediate portion
20 comprises one or more than one opening 44 allowing the hollow, central
chamber 40 of the intermediate portion 20 to communicate with the hollow,
central chamber 26 of the proximal portion 16.
The distal portion 18 comprises a proximal end 48 and a distal end 46
and, between the proximal end 48 and the distal end 46, comprises a hollow,
central chamber 50 surrounded by an outer wall 52 with a central axis in the
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
9
proximal to distal direction. Preferably, the outer wall 52 of the distal
portion
18 is generally cylindrical, though other configurations are also suitable, as
will be understood by those with skill in the art with reference to this
disclosure.
The hollow, central chamber 50 has a sufficient size and suitable shape
to receive a test strip 104 or other test apparatus inserted into the hollow,
central chamber 50 from the distal end 14 of the blood testing device 10
toward the proximal end 12. As used in this disclosure, the term "test strip"
is
understood to include other test apparatuses that are not in linear strip form
but are suitable for use with the blood testing device 10 of the present
invention. Therefore, the configuration of the hollow, central chamber 50 of
the distal portion 18 will depend upon the configuration of the test strip to
be
used, as will be understood by those with skill in the art with reference to
this
disclosure. Suitable test strips include chemical test strips, such as One
Touch~ FastTake~ (LifeScan, Inc., Milpitas, CA US) and electrochemical test
strips, such as Precision QID (MediSense, Bedford, MA US).
In a particularly preferred embodiment, as shown in Figure 1 , Figure 2
and Figure 3, the hollow, central chamber 50 of the distal portion 18, and the
hollow, central chamber 40 of the intermediate portion 20, comprises
generally rectangular (when viewed from an inferior direction), inferior part
54
and inferior part 56, respectively, as shown, that communicate with each other
and that are configured to accept a test strip 104. The proximal end 58 of the
outer wall of the inferior part 56 of the intermediate portion 20 is closed.
In another preferred embodiment, as shown in Figure 9, inferior part 54
of central chamber 50 of distal portionl 8 further comprises one or more than
one ridge 55 having a long axis in the proximal to distal direction. Ridge 55
acts a guide for test strip 104 in inferior part 54, and also helps to prevent
blood from gaining access to the superior space in hollow, central chamber
50. Ridge 55 may extend for part or all of the length of central chamber 50,
and may extend for part or all of the width of central chamber 50.
The outer wall 28 of the proximal portion 16, the outer wall 42 of the
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
intermediate portion 20 and the outer wall 52 of the distal portion 18
preferably comprise a plastic material such as polycarbonate, that does not
affect the constituents or parameters of blood being tested, though other
materials are suitable as will be understood by those with skill in the art
with
5 reference to this disclosure. The material used is preferably inexpensive,
as
the blood testing device 10 is intended to be discarded after a single use,
and
is capable of being manufactured into the proper shape according to standard
techniques known to those with skill in the art, such as by injection molding.
Further preferably, the material is either substantially transparent or at
least
10 sufficiently transparent to allow visual confirmation that blood is present
in
the hollow, central chamber 40 of the intermediate portion 20.
The distal end 14 of the blood testing device 10 further comprises a
distal end cap 60. In one embodiment (not shown), the distal end cap 60 is an
integral part of the distal portion 18 of the blood testing device 10, and of
the
same or of a different material as the outer wall 52 of the distal portion 18.
In
a preferred embodiment, as shown in Figure 1, Figure 2 and Figure 3, the
distal end cap 60 is a separate part, removable from the distal portion 18,
and
comprises a resilient material such as natural or synthetic rubber that, when
placed over the distal portion 18 of the blood testing device 10, tends to
remain attached to the distal portion 18 of the blood testing device 10 due to
the resilient nature of the material.
In one embodiment, the distal end cap 60 further comprises at least a
first opening 62 configured to snugly receive a test strip 104 inserted into
the
hollow, central chamber 50 of distal portion 18 of the blood testing device
10.
Therefore, the configuration of the first opening 62 in the distal end cap 60
will depend upon the configuration of the test strip to be used, as will be
understood by those with skill in the art with reference to this disclosure.
In a
preferred embodiment, the first opening 62 is generally slit-like, as shown in
Figure 1 , Figure 2 and Figure 3. The first opening 62 can comprise a simple
opening into the hollow, central chamber 50 of the distal portion 18, as
shown, or can comprise a valve, such as a duck bill valve 65 as shown in
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
Figure 1 OA and Figure 1 OB. In a particularly preferred embodiment, as shown
in Figure 1, Figure 2 and Figure 3, the distal portion 18 and the intermediate
portion 20 each comprises the generally rectangular, inferior part 54 and 56
and the long axis of the first opening 62 is a slit that is aligned
perpendicular
to the direction of insertion of the long axis of the test strip 104 into the
distal
portion 18.
The distal end cap 60 can comprise a semipermeable membrane, as
shown in Figure 1 1, that allows the selective passage of air out of the
distal
portion 18, while retaining the fluid and cellular components of blood within
the distal portion 18. The semipermeable membrane can comprise any
suitable material, such as Porex T3 #X-7744, (Porex Medical Products Group,
Fairburn, GA US) or Type TTTP Isopore Membrane (Millipore Corporation,
Bedford, MA US), though other material is also suitable as will be understood
by those with skill in the art with reference to this disclosure.
Additionally preferably, and particularly when the distal end cap 60 does
not comprise a semipermeable membrane, the distal end cap 60 comprises a
vent of one or more than one second opening 64 to allow the venting of air
externally out of the distal portion 18 of the blood testing device 10. The
second opening 64 can be any suitable configuration, such as generally slit-
like, as shown in Figure 1, as will be understood by those with skill in the
art
with reference to this disclosure. Alternatively, or additionally, as shown in
Figure 1 3, the outer wall 52 of the distal portion 1 8 can comprise one or
more
than one vent 53.
In one preferred embodiment, the blood testing device 10 further
comprises an inner membrane 66 separating the distal end 38 of the hollow,
central chamber 40 of the intermediate portion 20 from the proximal end 48
of the hollow, central chamber 50 of the distal portion 18. In a preferred
embodiment, the inner membrane 66 is a semipermeable member. In another
embodiment, the inner membrane 66 is not permeable but comprises one or
more than one vent (not shown). Alternately, or additionally, as shown in
Figure 1 3 and Figure 1 5, the outer wall 42 of the intermediate portion 20
can
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
12
comprise one or more than one vent 43.
The inner membrane 66 comprises any suitable material, such as rubber
or Porex T3 #X-7744, (Porex Medical Products Group, Fairburn, GA US), and
can additionally be treated to be hydrophobic, as will be understood by those
with skill in the art with reference to this disclosure.
Referring now to Figure 4, there is shown a lateral perspective view of
one embodiment of an inner membrane 66 forming part of the embodiment of
the blood testing device 10 shown in Figure 1 , Figure 2 and Figure 3. The
inner membrane 66 allows the selective passage of air through the inner
membrane 66 between the distal end 38 of the hollow, central chamber 40 of
the intermediate portion 20 and the proximal end 48 of the hollow, central
chamber 50 of the distal portion 18, while preventing the passage of the fluid
and cellular components of blood through the inner membrane 66.
The inner membrane 66 is further configured to allow passage of the
test strip 104 from the distal portion 1 8 of the blood testing device 10 into
the intermediate portion 20 of the blood testing device 10. For example, as
shown in Figure 12, the inner membrane 66 can comprise a slit 67 aligned
with the first opening 62 in the distal end cap 60. In a further embodiment,
the inner membrane 66 can comprise a perforation (not shown) aligned with
the first opening 62 in the distal end cap 60. In that embodiment the
perforation can be constructed so that it is breached by the insertion of test
strip 104.
In a preferred embodiment, as shown in Figure 1, Figure 2 and Figure 3,
the intermediate portion 20 and the distal portion 18 each comprise the
generally rectangular, inferior part 54 and 56 and the first opening 62 is a
slit
that is aligned perpendicular to the direction of insertion of the long axis
of
the test strip 104 into the distal portion 18, and the inner membrane 66 has
an inferior extension 68 that further divides the inferior part 54 of the
distal
portion 18 from the inferior part 56 of the intermediate portion 20.
When a test strip is inserted through the distal end cap 60 and into the
hollow, central chamber 50 of the inferior part 54 of the distal portion 18,
the
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
13
inferior extension 68 of the inner membrane 66 is displaced proximally,
permitting the test strip to be further inserted into inferior part 56 of the
intermediate portion 20, and allowing blood in the hollow, central chamber 40
of the intermediate portion 20 to contact the proximal end 106 of the test
strip 104.
Referring now to Figure 5, Figure 14, and Figure 1 S, there is shown
lateral perspective views of a blood testing device 10 according to another
embodiment of the present invention. As can be seen, in these embodiments,
the blood testing device 10 is manufactured without a proximal portion 16
and with the intermediate portion 20 integrally affixed to the distal end of
the
introducer part 102 of a standard intravascular access apparatus 100. In
these embodiments, the blood testing device 10 and introducer part 102 of an
intravascular access apparatus 100 are inserted intravascularly and are
removed as a unit, as will be understood by those with skill in the art with
reference to this disclosure.
As will be understood by those with skill in the art with reference to this
disclosure, as the test strip is inserted through the distal end cap 60 and
into
the hollow, central chamber 50, the pressure within central chamber 50 will
increase. If no pressure compensation system has been included in the blood
testing device for amelioration of this pressure increase, depending upon the
nature of the material chosen for the inner membrane 66, a corresponding
increase in the pressure within the central chamber 40 of the intermediate
portion 20 may result. If no pressure compensation system has been included
for equalization of pressure within central chamber 40, a small amount of
blood may be expelled through the one or more than one opening 34 of the
proximal-most part 30 of outer wall 28 of proximal portion 16. This may be
undesirable to the health care worker inserting the test strip. As the test
strip
is further inserted into inferior part 56 of the intermediate portion 20, an
additional increase in pressure within central chamber 40 will occur,
increasing the likelihood of escape of blood through opening 34.
A preferred embodiment of a pressure compensation system is as
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
14
shown in Figure 1, Figure 2, and Figure 4, in which inner membrane 66 is a
semipermeable member, comprising an inferior extension 68, and distal end
cap 60 comprises a resilient material such as natural or synthetic rubber, and
a vent of one or more than one second opening 64. As test strip 104 is
inserted into blood testing device 10, this embodiment of the pressure
compensation system allows pressure to equalize, resulting in little escape of
blood from the intermediate portion into the distal portion, and no escape of
blood from the blood testing device 10. Additionally preferably, central
chamber 50 of distal portion 18 comprises one or more than one ridge 55
reducing the amount of blood that enters central chamber 50.
Another embodiment of a pressure compensation system, as shown in
Figure 1 1, comprises a distal end cap 60 comprising a semipermeable
membrane that allows the selective passage of air out of the distal portion
18,
together with inner membrane 66 being a semipermeable member,
comprising an inferior extension 68. In this embodiment, second opening 64
is not required.
Another embodiment of a pressure compensation system comprises a
distal end cap 60 comprising an integral part of the distal portion 18 of the
blood testing device 10, and of the same or of a different material as the
outer
wall 52 of the distal portion 18, together with inner membrane 66 being a
semipermeable member, comprising an inferior extension 68. In this
embodiment, second opening 64 is located either on the distal end cap, or
one or more than one vent 53 is located on outer wall52.
Another embodiment of a pressure compensation system comprises an
inner membrane 66 which is not permeable but comprises one or more than
one vent (not shown). Alternately, or additionally, the outer wall 42 of the
intermediate portion 20 can comprise one or more than one vent 43. In this
embodiment, distal end cap 60 may optionally comprise (I) a resilient material
such as natural or synthetic rubber, and a vent of one or more than one
second opening 64 in the end cap, or the outer wall 52 of distal portion 18
can comprise one or more than one vent 53, (ii) a semipermeable membrane
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
that allows the selective passage of air out of the distal portion 18, or
(iii) an
integral part of the distal portion 18 of the blood testing device 10, and of
the
same or of a different material as the outer wall 52 of the distal portion 18,
and one or more than one second opening 64 in the end cap, or the outer wall
5 52 of distal portion 18 can comprise one or more than one vent 53.
It is also possible to ameliorate the pressure increase caused by the
insertion of the tests strip 104 through a pressure compensation valve.
Referring now to Figure 6, there is shown a lateral perspective view of a
blood
testing device 10 according to another embodiment of the present invention.
10 As can be seen, in this embodiment, the blood testing device 10 comprises a
distal end cap 60 where the first opening 62 comprises a duck bill valve 65,
shown in cross-section in Figure 1 OA before a test strip 104 is inserted, and
shown in cross-section in Figure 1 OB after a test strip 104 is inserted, the
sides of which adjoin and seal together when no test strip 104 is present and
15 which separate upon insertion of a test strip to permit insertion of the
test
strip into the hollow, central chamber 40 of the intermediate portion 20. In
this embodiment of a pressure compensation system, the equalization of
pressure is accomplished by the collapse of the structure of the duck bill
valve, as is shown in Figure l OB after the test strip 104 is inserted, and as
will
be understood by those with skill in the art with reference to this
disclosure.
Accordingly, in this embodiment opening 64 in end cap 60 and inner
membrane 66 are not needed.
Having thus described several embodiments of a pressure compensation
system, it will now be understood by those with skill in the art with
reference
to this disclosure that a pressure compensation system may be constructed
with various combinations of the described components, as further shown in
Figures 11 through 17.
Referring now to Figure 7 and Figure 17, there is shown a lateral
perspective view of a blood testing device 10 according to two other
embodiments of the present invention. As can be seen, in these
embodiments, the blood testing device 10 is manufactured integrally affixed
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
16
to the side of a syringe 108 by connecting either the intermediate portion 20
of a blood testing device 10, as disclosed above, directly to the side of the
syringe 108, as in Figure 7, or the inner membrane 66 directly to the side of
the syringe 108, as in Figure 1 7, the interior of syringe 108 thus becoming
the
intermediate portion 20. Other blood collection devices could be used, as will
be understood by those with skill in the art with reference to this
disclosure.
It would also be possible to use a pressure compensation valve in either
embodiment, and eliminate the inner membrane. In these embodiments, the
syringe 108 is used to draw blood from a blood source and the blood testing
device 10 is used to test the blood within the syringe 108 without having too
expel the blood from the syringe 108. The blood testing device 10 can also
be affixed to the distal end of the plunger of a syringe 1 O8 where the
plunger
has a central channel in communication with the intermediate portion 20 of
the blood testing device 10 (not shown).
In another embodiment, the present invention includes a blood testing
apparatus 1 10 comprising a plurality of interconnected blood testing devices
10 according to the present invention, to allow multiple test strips to be
read
from blood in the same blood containing apparatus 1 10. Referring now to
Figure 8, there is shown a lateral perspective view of such a blood testing
apparatus 1 10. As can be seen, in this embodiment, the blood testing
apparatus 1 10 comprises a proximal end 1 12 configured to mate with an
intravascular access apparatus 100, a distal end 1 14 configured to mate with
a
syringe 108, a hollow, central chamber 1 16 between the proximal end and the
distal end, and a plurality of blood testing devices 10 according to the
present
invention, each blood testing device 10 joined to the hollow, central chamber
1 16. In a preferred embodiment, the distal end 1 14 is configured to mate
with the proximal end of a standard flash plug or the proximal end of a
syringe 108 (as shown). This embodiment allows blood from one source to be
tested using a plurality of blood testing devices 10 according to the present
invention, as will be understood by those with skill in the art with reference
to
this disclosure.
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
17
According to another embodiment, referring now to Figure 1 1, blood
testing device 10 takes the place of a standard flash plug, the proximal end
12
of blood testing device 10 is configured to mate with the introducer part 102
of a intravascular access apparatus 100, as shown in Figure 1 1 , and
additionally optionally the distal end 14 of blood testing device 10 may be
configured to mate (not shown) integrally or removably with a blood
collection device such as syringe 108.
According to another embodiment of the present invention, there is
provided a method for testing blood for the presence or for the amount of
various constituents, such as glucose, cardiac enzymes or red cells, or for
testing blood to determine the level of various parameters, such as pH, or for
testing blood for a combination of the preceding. The method comprises
providing a blood testing device according to the present invention. The
method additionally comprises providing a test strip, a test strip reader and
a
blood containing apparatus, such as an intravascular access apparatus or a
syringe. The test strip can be any suitable test strip with a proximal end
configured to be inserted into the distal end cap of the blood testing device.
The test strip reader can be any suitable test strip reader configured to
accept
the distal end of the test strip and, after accepting the distal end, to
provide
the desired information on blood that has contacted the proximal end of the
test strip. The blood containing apparatus can be any intravascular access
apparatus suitable for accessing a vascular structure and suitable for joining
to the proximal end of a blood testing device according to the present
invention, or any other suitable blood containing apparatus as will be
understood by those with skill in the art with reference to this disclosure.
The blood testing device can be provided with or without the test strip
inserted through the distal end cap and into the distal portion and
intermediate portion of the blood testing device. If the blood testing device
is
provided with the test strip inserted through the distal end cap and into the
distal portion and intermediate portion of the blood testing device, then the
combined blood testing device and test strip can be provided with the distal
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
18
end of the test strip already inserted into the test strip reader. Further,
the
blood testing device, whether or not combined with the test strip or with the
test strip and test strip reader together, can be provided having its proximal
end already inserted into the distal end of the blood containing apparatus or
can be provided without its proximal end already inserted into the distal end
of the blood containing apparatus. As will be understood by those with skill
in
the art with reference to this disclosure, the blood containing apparatus,
blood
testing device, test strip and test strip reader are assembled together during
the method of the present invention, and can be assembled in various orders.
In a particularly preferred embodiment, the distal end of the test strip is
inserted into the test strip reader, and then the proximal end of the test
strip
is inserted into the distal end cap of the blood testing device and through or
around the inner membrane after blood has entered the intermediate portion
of the blood testing device.
Next, a patient is selected who is to have his or her blood tested. If the
blood containing apparatus is an intravascular access apparatus, the patient
is
prepped in a standard manner and intravascular access is begun using the
intravascular access apparatus according to standard techniques, as will be
understood by those with skill in the art with reference to this disclosure.
If
the proximal end of the blood testing device was not already inserted into the
distal end of the intravascular access apparatus before obtaining
intravascular
access, then the proximal end of the blood testing device is inserted into the
distal end of the intravascular access apparatus, such as by replacing the
flash
plug on the intravascular access apparatus with the proximal portion of the
blood testing device. Preferably, the proximal end of the blood testing device
is inserted into the distal end of the intravascular access apparatus before
insertion of the intravascular access apparatus. Alternately, a blood~testing
device is provided according to the present invention, without a proximal
portion and with the intermediate portion integrally affixed to the distal end
of
the introduces portion of a standard intravascular access apparatus.
Once vascular access is obtained and the blood testing device and the
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
19
intravascular access apparatus are combined, blood from the patient's
accessed vascular structure will pass through the one or more than one
opening in the proximal end of the proximal portion into the hollow, central
chamber of the proximal portion and, then, into the hollow, central chamber
of the intermediate portion, or will pass directly into the hollow, central
chamber of the intermediate portion if the blood testing device is integrally
affixed to the intravascular access apparatus. Air that is present in the
hollow,
central chamber of the proximal portion, if present, and hollow, central
chamber of the intermediate portion passes through the inner membrane into
the hollow, central chamber of the distal portion and then, externally. If not
already combined, the distal end of the test strip is inserted into the test
strip
reader and the proximal end of the test strip is inserted into the distal end
cap, through the distal portion and into the intermediate portion of the blood
testing device. The blood in the hollow, central chamber of the intermediate
portion will, then, contact the proximal end of the test strip.
Next, the distal end of the test strip is inserted into an appropriate test
strip reader, if not done previously. The operator uses information provided
by the test strip reader, such as a digital readout or visual indicator, to
determine the presence or the amount of one or more than one constituent of
the blood, such as glucose, cardiac enzymes or red cells, or to determine the
level of one or more than one parameter, such as pH, or to determine a
combination of the preceding.
In one embodiment, the operator withdraws the blood testing device
from the distal end of the intravascular access apparatus before using the
test
strip reader. In another embodiment, the blood testing device with an
inserted test strip is removed from the intravascular access apparatus and an
operator proceeds to establish intravascular access while the distal end of
the
test strip is being inserted into an appropriate testing apparatus, so that no
time is being lost in establishing intravascular access while blood testing is
being performed. In a preferred embodiment, the introducer part of the
intravascular access apparatus with the blood testing device still inserted
into
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
the distal end are removed together, and an operator proceeds to establish
intravascular access while the proximal end of the test strip is being
inserted
into distal end of the blood testing device, further increasing efficiency in
establishing intravascular access while blood testing is being performed. In a
5 particularly preferred embodiment, the blood testing device provided for use
in this method is the embodiment shown in Figure 5, and as will be
understood by those with skill in the art with reference to this disclosure,
removing the blood testing device necessarily removes the introducer part of
the intravascular access apparatus from the vascular structure as the blood
10 testing device is integrally affixed to the distal end of the introducer
part of
the intravascular access apparatus.
After the operator makes the appropriate determinations, the blood
testing device with the test strip still inserted is removed from the test
strip
reader and, preferably with the introducer portion if being used with an
15 introducer portion, is discarded in an appropriate manner as a biohazard.
As
can be appreciated by those with skill in the art with reference to this
disclosure, the present method decreases the possibility of inaccurate blood
test results due to test strip contamination, decreases the time necessary to
analyze blood being tested from standard methods, decreases the risk of
20 exposure of the operator to pathogens present in the blood, and simplifies
disposal of the equipment used in testing the blood.
According to another embodiment of the present invention, there is
provided a method for testing blood for the presence or for the amount of
various constituents, such as glucose, cardiac enzymes or red cells, or for
testing blood to determine the level of various parameters, such as pH, or for
testing blood for a combination of the preceding. The method comprises
providing a blood testing apparatus according to the present invention, where
the blood testing apparatus comprises a plurality of blood testing devices
according to the present invention joined to a common central chamber that is
configured at the proximal end to combine removably or to be affixed with a
blood containing apparatus, and to communicate with the blood containing
CA 02442962 2003-10-02
WO 02/080763 PCT/USO1/26666
2~
part of the blood containing apparatus. As will be understood by those with
skill in the art, with reference to this disclosure, the method corresponds to
the method disclosed above, except that blood test strips can be inserted into
the distal end caps of each blood testing device on the blood testing
apparatus, expanding the capacity to test the blood.
According to another embodiment of the present invention, there is
provided a method for testing blood for the presence or for the amount of
various constituents, such as glucose, cardiac enzymes or red cells, or for
testing blood to determine the level of various parameters, such as pH, or for
testing blood for a combination of the preceding. The method comprises
using a blood containing apparatus, such as a syringe, other than an
intravascular access apparatus. In this method, blood is drawn into the blood
containing apparatus other than by obtaining intravascular access directly
through the distal end of the blood containing apparatus, such as by attaching
the blood containing apparatus directly to an intravascular access apparatus
or
blood containing vial and drawing blood into the blood containing apparatus
from that source.
Although the present invention has been discussed in considerable
detail with reference to certain preferred embodiments, other embodiments
are possible. Therefore, the scope of the appended claims should not be
limited to the description of preferred embodiments contained in this
disclosure.