Note: Descriptions are shown in the official language in which they were submitted.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
TITLE: Method and Substance for Refrigerated Natural Gas Transport
FIELD OF THE INVENTION
This invention deals with the transport of natural gas in containers under
pressure,
at some level of refrigeration, and addresses the advantageous increase of gas
density at ranges of pressure and temperature which are amenable to relatively
inexpensive container and vehicle configurations using relatively conventional
materials and without need for excessive refrigeration or compression when
loading
or in transit. The invention is useful in both shipboard and other vehicular
refrigerated natural gas transport systems. The invention does not address
refrigerated pressurized natural gas pipelines.
BACKGROUND OF THE INVENTION
As is well known, natural gas defines a very broad range of gas compositions.
Methane is the largest component of produced natural gas; and usually accounts
for
at least 80% by volume of what is known as marketable natural gas. Other
components include, in declining volume percentages, ethane (3% - 10%),
propane
(0.5% - 3%), butane and C4 isomers (0.3% - 2%), pentane and C5 isomers (0.2% -
1%),
and hexane + and all C6+ isomers (less than 1%). Nitrogen and carbon dioxide
are
also commonly found in natural gas, in ranges of 0.1% to 10%.
Some gas fields have carbon dioxide contents of up to 30%. Common isomers
found
in natural gas are iso-butane and iso-pentane. Unsaturated hydrocarbons such
as
ethylene and propylene are not found in natural gas. Other contaminants
include
water and sulphur compounds, but these must typically be controlled to very
low
levels prior to sale of the marketable natural gas, regardless of the
transport system
used to get the produced gas from wellhead to market.
Secord and Clarke in US patents # 3,232,725 (1963) and # 3,298,805 (1965)
describe
the benefits of storage of gas at conditions of temperature and pressure which
occur
when the gas exists at a single dense phase fluid state, at pressures just
above the
phase transition pressure. This state is shown in the generic phase diagram
(taken
from patent # 3,232,725) attached hereto at Figure 12, and is shown as
occurring
within the dotted lines on the diagram.
The relation between pressure, volume and temperature of a gas can be
expressed by
the Ideal Gas Law, which is stated as PV = nRT where, using English units:
P = pressure of the gas in pounds per square inch absolute (psia)
V = volume of the gas in cubic feet (CF)
n = number of moles of the gas
R = the universal gas constant
T = temperature of the gas in degrees Rankin (degrees Fahrenheit plus 460)
The Ideal Gas Equation must be modified when dealing with hydrocarbon gases
under pressure, because of the intermolecular forces and the molecular shape.
To
correct for this, an added term, the compressibility factor z must be added to
the
Ideal Gas Equation such that PV=znRT. This z is a dimensionless factor that
reflects
the compressibility of the particular gas being measured, at the particular
conditions
of temperature and pressure.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
At or near atmospheric pressure, the z factor is sufficiently close to 1.0
that it can be
ignored for most gases, and the Ideal Gas Equation can be used without the
added z
term.
However, where pressures exceed a few hundred psia the z term can be much
lower
than 1 .0 so that it must be included in order for the Ideal Gas Equation to
give
correct results.
According to the van der Waal's theorem, the deviation of a natural gas from
the
Ideal Gas Law depends on how far the gas is from its critical temperature and
critical
pressure. Thus, the terms Tr and Pr (known as reduced temperature and reduced
pressure respectively) have been defined, where
Tr=T/Tc
Pr=P / Pc
Where,
T = the temperature of the gas in degrees R
Tc = the critical temperature of the gas in degrees R
P = the pressure of the gas in psia
PC = the critical pressure of the gas in psia
Critical pressures and critical temperatures for pure gases have been
calculated, and
are available in most handbooks. Where a mixture of gases of known composition
is
available, a "pseudo critical temperature" and "pseudo critical pressure"
which
apply to the mixture can be obtained by using the averages of the critical
temperatures and critical pressures of the pure gases in the mixture, weighted
according to the mole percentage of each pure gas present. The pseudo reduced
temperature and the pseudo reduced pressure can then be calculated using the
pseudo critical temperature and the pseudo- critical pressure respectively.
Once a pseudo reduced temperature and pseudo reduced pressure are known, the z
factor can be found by using standard charts. An example of one of these is
"Figure
23-3 Compressibility Factors for Natural Gas", by M. B. Stranding and D.L.
Katz
(1942), published in the Engineering Data Book, Gas Processors Suppliers
Association, 10th edition (Tulsa, Oklahoma, U. S. A.) 1987. (and a copy of
that chart is
attached hereto as Figure 13)
One aspect of the prior art is described in US patent # 6,217,626 "High
pressure
storage and transport of natural gas containing added C2 or C3, or ammonia,
hydrogen fluoride or carbon monoxide". That patent describes a method for
storing
and subsequently transporting gas by pipeline whereby adding the light
hydrocarbons of ethane and propane (or ammonia, hydrogen fluoride or carbon
monoxide) can increase the capacity of the pipeline or can reduce the
horsepower
required on a pipeline to propel such a gas mixture down the line. The primary
claim
is for creating a mixture by addition of propane of ethane where the product
of the z
factor (z) and the molecular weight (MW) for the new mixture reduces as
compared
to a mixture without the added ethane or propane, yet where there is no
presence of
liquids, only a single phase gas vapor.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
3
The benefit arises because of the gas pipeline flow equation. There are
several forms
of this equation, but they all have the following features in common:
Flow = constant 1 [((PI ~2 - P2~2) / (S * L *T * z))~ 0.5] * (D~2.5)
Where:
PI = starting pressure in a pipeline
P2 = ending pressure in a pipeline
S = specific gravity of the gas (which is equivalent to molecular weight)
L = length of the pipeline
T = temperature of the gas
z = compressibility factor of the gas
D = internal diameter of the pipeline
In this equation, the two factors that are altered by changing the gas
composition are
the specific gravity (or molecular weight) "S", and the z factor "z". Both of
these
appear in the denominator of the equation. Therefore, if the product of z and
MW or
"S" reduces, and all other factors remain constant, flow on the pipeline will
increase
at a similar pressure differential between the starting and ending points.
This is a
benefit in pipeline transmission, which can be described either as a capacity
gain or a
reduced horsepower requirement to propel a given volume down the pipeline.
The primary claim in the patent # 6,217,626 is adding C2 or C3 to natural gas
for a
reduction in the product of z and MW (or S), above a pressure of 1000 psig and
with
no discernible liquid formation. The benefits described under the patent
relate to
increased capacity or reduced horsepower on a pipeline.
The teachings under the patent describes a mixture in which the primary
barrier to
increasing benefits is the two-phase state created if too much NGL is added to
the
gas. This two-phase state leads to physical damage of the. pipeline equipment,
and
reduced flow, and must be avoided. Several of the subsequent claims limit the
amount of ethane to 35% and the amount of propane to 12% in order to avoid
this
two-phase state on the pipeline. Several of the claims state a minimum amount
of
added ethane and propane, again based on the benefits in pipeline application.
No
mention is made in US6,217,626 of adding any hydrocarbons heavier than
propane,
such as butane or pentane, and in fact, the teachings describe how these
heavier
hydrocarbons should be avoided, as they lead to premature development of the
two-
phase state. See page 6, "Thus C4 hydrocarbons are not additives contemplated
by
this invention." Furthermore, "The presence of more than 1% C4 hydrocarbons in
the
mixture is not preferred, however, as C4 hydrocarbons tend to liquefy easily
at
pressures between 1000 psia and 2200 psia and more than 1% C4 hydrocarbons
give
rise to increased danger that a liquid phase will separate out. C4
hydrocarbons also
have an unfavorable effect on the mixture's z factor at pressures under 900
psia so
care should be taken that, during transport through a pipeline, mixtures
according to
the invention that contain C4 hydrocarbons are not allowed to decompress to
less
than 900 psia and preferably not to less than 1000 psia.
The control mechanism proposed in the '626 invention to avoid the two-phase
state
is thus the type and amount of NGL added to the mixture. This is because, in a
pipeline, temperature and pressure are usually exogenous variables, not
subject to
any fine degree of control.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
4
Refrigeration is mentioned only once in '626, and in a negative sense. While
some of
the claims deal with mixtures down to a temperature of - 40 degrees F, the
following
statement appears on page 10 of the '626 patent: "Even more preferred
pressures are
1350-1750 psia (which gives good results without requiring vessels to
withstand
higher pressures) and particularly preferred temperatures are 35 to 120
degrees F.
(Which do not require undue refrigeration)". The benefits of the invention are
illustrated in the graphs attached to '626, which all terminate at a lower
temperature
limit of 30 to 35 degrees F. Even though the pipeline flow equation
illustrates that
pipelines are more efficient at colder temperatures (see the factor T in the
denominator), no analysis is provided at lower temperatures. This is primarily
because refrigeration is not practical in pipeline applications, as the pipe
temperature
should be above the freezing point of water, in order to prevent frost build
up on and
around the pipeline.
It is clear that the invention in patent # 6,217,626 is based on preparation
in storage of
a fluid with the stated desire of subsequent pipeline transport, and that no
refrigeration is contemplated, that the type and minimum amount of NGL added
is
limited by the benefits provided in pipeline transport, that the type and
maximum
amount of NGL added is limited by the two-phase problem which will occur on
the
contemplated pipeline transmission, and that the pressure regime is limited by
the
subsequent pipeline transmission. While the prior art implies benefits for
both
storage and pipeline transport, the storage aspect of the prior art is limited
to or by
pipeline applications, and does not contemplate storage in containers which
are
themselves later transported.
Another aspect of the prior art is contained within US patent # 5,315,054
"Liquid
Fuel Solutions of Methane and Light Hydrocarbons". This patent deals with a
method to store a liquid product where Liquified Natural Gas (LNG) is put into
an
insulated tank at a temperature of about - 265 degrees F. Both methane and NGL
are
introduced into the tank, the methane and LNG is dissolved in the NGL
hydrocarbon
solution (typically propane or butane), and the resulting mixture is stored as
a stable
liquid under moderate pressure. This invention does not contemplate storage as
a
single dense phase fluid, and it is also conditional upon LNG being present in
the
tank to begin with.
Another aspect of the prior art is described in US patents # 5,900,515 and
6,111,154
"High energy density storage of methane in light hydrocarbon solutions". This
invention is similar to the previous example #5,315,054 and is described as
the
"dissolution of gaseous methane into at least one light hydrocarbon into a
storage
tank" and "storage of the solution". In addition, the solution has to be
maintained at
a temperature above - 1 degree C. at a pressure above 8.0 Mpa comprise a
maximum
of 80% methane and have an energy density of at least 11,000 MJ/m.
Another aspect of the prior art is described in the previously referenced US
patent #
3,298,805 which describes storage of natural gas under pressure, without any
additives, at or near the phase transition pressure but at a temperature below
the
critical temperature of methane (-116.7 degrees F). This is a continuation of
US patent
# 3,232,725 which describes storing natural gas under pressure, again without
any
additives, at or near the phase transition pressure at a temperature 20
degrees (F)
below ambient temperature.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
Another aspect of the prior arts is described in US patent # 4,010,622 which
describes
adding hydrocarbons in the range of C5 - C20 sufficient to liquefy the gas at
ambient
pressure and store it as a liquid, which is given as an example with bearing
on the
formulae expressed above, but not of much relevance to this invention.
SUMMARY OF THIS INVENTION
For the storage of natural gas in a container under pressure, and the
subsequent
transport of the loaded storage container and gas, it is advantageous to
refrigerate
the natural gas below the ambient temperature, and to add to the natural gas
an
additive that is a natural gas liquid such as a C2, C3, C4 ,C5 or C6+
hydrocarbon
compound (including all isomers and both saturated and unsaturated
hydrocarbons), or carbon dioxide, or a mixture of such compounds.
Alternatively,
methane or a lean gas mixture can be removed from a natural gas mixture richer
in
indigenous NGL to achieve the same effect.
When combined with storage conditions at an optimal pressure and temperature,
the
addition of NGL will increase the net gas density (net referring here to the
gas's
density excluding the added NGL) above what the gas density would be at these
same conditions of temperature and pressure without the added NGL.
The increase in gas density leads to lower storage and transport costs.
The operating pressure range over which adding NGL to the gas provides
benefits
for storage and subsequent transport is between 75% and 150% of the phase
transition pressure (PTP) of the gas mixture, with the greatest benefit
occurring right
at and just above the phase transition pressure.
(The phase transition pressure is defined as that point at which a rising
pressure
causes the particular gas mixture to transition from a two-phase state to a
dense
single phase fluid, with no liquid/vapor separation within the container. This
point
is also commonly referred to as the bubble point line and/or the dew point
line. )
The temperature range over which adding NGL to the gas provides benefits for
storage and subsequent transport, when operating at or near the phase
transition
pressure, is -140 degrees F to +110 degrees F. As refrigeration on its own
provides
benefits in increased density and also has a synergistic effect on the benefit
provided
by adding NGL, refrigerating the gas to less than or equal to 30 degrees F is
another
aspect of this invention.
It has now been found that, for natural gas storage in a container, and
subsequent
transport of the loaded container and contained gas, for any typically
occurring
natural gas mixture, it is advantageous. to add to the natural gas an additive
that is
C2, C3, C4, C5 or C6+ or carbon dioxide, or a mixture of these compounds,
where the
resulting mixture is stored at a pressure between 75% and 150% of the phase
transition pressure of the gas mixture, and where the gas temperature is
between -
140 degrees F and +30 degrees F.
The resulting mixture exhibits a higher net density (excluding the additive)
at a
lower pressure than would the base natural gas without the additive.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
Refrigerating the gas below ambient temperature increases the benefit of
adding
NGL.
The temperature, pressure, optimum amount and optimum type of additive depends
on the particular characteristics of the gas in trade. These characteristics
include the
economically achievable refrigeration temperature, the base gas composition,
the
type of trade, being a Recycle Trade (where the additive is re-cycled) or a
NGL
Delivery Trade (where the additive is delivered to market along with the gas),
the
economics of the transportation system utilizing this invention (e.g. Ship,
truck,
barge, other), and the phase transition pressure of the gas mixture. As higher
gas
density implies greater capacity in a volume-limited storage-and-transport
system,
and lower pressure leads to lower cost preparation and storage containment,
the
resulting unit transportation cost will reduce as a result of using the
invention.
BRIEF DESCRIPTION OF THE FIGURES:
FIGURE 1: Gross Density v. Pressure at -40 degrees F
FIGURE 2: Net Gas Density of CNG (at +60 and -40 degrees F) and FNG at Phase
Transition Pressure and -40 degrees F with 5% to 60% propane addition
FIGURE 3: Optimum Amount of Propane Blend at the Phase Transition Pressure and
-40 degrees F with 10% to 60% added propane
FIGURE 4: Optimum Amount of Butane Blend at Phase Transition Pressure and -40
degrees F with 5% to 25% added Butane
FIGURE 5: Net Gas Density of Ethane, Propane, Butane and Pentane Blends at
Phase
Transition Pressure and -40 degrees F
FIGURE 6: Effect of Temperature and NGL Addition on Net Gas Density
FIGURE 7(a): Optimum NGL Injection at -40 F (by component) storage at phase
transition pressure
FIGURE 7(b): Optimum NGL Injection at -40 F (by component) storage at phase
transition pressure
FIGURE 7(c): Optimum NGL Injection at -40 F (by component) storage at phase
transition pressure
FIGURE 8: Effect of Temperature on Phase Transition Pressure and Gas Density -
base gas plus 17.5% propane
FIGURE 9: Pressure with and without NGL addition vs. temperature
FIGURE 10: Gas Density with and without NGL addition vs. %age of Phase
Transition Pressure
FIGURE 11: Bulk Density (liquid + vapour) vs. Pressure - Base Gas plus 11%
butane
at -40 degrees F
FIGURE 12: A reproduction of a generic phase diagram from US3,232,725
FIGURE 13: Figure 23-3 Compressibility Factors for Natural Gas", by M.
B.,Stranding
and D.L. Katz (1942), published in the Engineering Data Book, Gas
Processors Suppliers Association, 10th edition (Tulsa, Oklahoma, U. S. A.)
1987
DETAILED DESCRIPTION OF THIS INVENTION
Gas storage economics are improved by increasing the gas density of the
natural gas
and minimizing the pressure of the storage system. When one is trying to
maximize
the gas density at some minimum pressure, one way that this is achieved is by
minimizing the compressibility factor z.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
7
When the compressibility factor z is read from the attached textbook figure #
23-3 at
Figure 13, two factors become apparent. The first is that the minimum z factor
occurs
with a gas that has a pseudo reduced temperature close to 1. This means that
the
actual gas temperature should be close to the pseudo critical temperature of
the
mixture. The second is that, if one can economically achieve a pseudo reduced
temperature of about 1.2 and a resulting z factor of about 0.5 through low
cost
refrigeration alone, changing the gas composition by adding NGL to reduce the
pseudo reduced temperature to close to 1 can reduce the z factor to about
0.25.
Thus, a 16% reduction in the pseudo reduced temperature can reduce the z
factor by
50% and increase the gas density by a factor of 200%. Adding NGL reduces the
pseudo reduced temperature. If the portion of added NGL is less than the
increase in
density, the base gas will show an increase in net density. In addition, as
the
inflection point of the z factor curve is at a lower pressure as the pseudo
reduced
temperature approaches 1, the system can show this increased density at a
lower
pressure as NGL is added, thus effecting more benefit.
The following example will illustrate this principle df increased density at
reduced
pressure with refrigeration to -40 degrees F:
Methane has a critical temperature of -116.7 degrees F (343.3 degrees R) and a
critical pressure of 667 Asia. The minimum temperature one can currently
achieve with low cost single cycle refrigeration plants based on propane is in
the order of - 40 degrees F (420 degrees R). The pseudo reduced temperature
of methane at - 40 degrees F is 1.223, that being 420 degrees R divided by
343.3
degrees R. From drawing # 23-3 at Figure 13, this implies that the minimum z
factor for methane would occur at a pseudo reduced pressure of about 2.676
(1785 psia). The z factor would be 0.553. The resulting gas density is 11.5
lb/CF , or an increase of 272 times over the gas density at standard
temperature and pressure (STP) of 0.0423 lb/CF. The gas density of methane
at 1785 psia and an ambient temperature of +60 degrees F (pseudo reduced
temperature of 1.515) would be 6.52 lb/CF with a z factor of 0.787. Thus,
refrigeration increases the methane density by a factor of 11.50 divided by
6.52
or 1.76 times.
N-Butane has a critical temperature of 305.5 degrees F (765.5 degrees R) and a
critical pressure of 548.8 psia. Adding 14% n-butane to 86% methane would
yield a pseudo critical temperature of the mix of - 57.6 degrees F (402.4
degrees R) and a pseudo critical pressure of 650.5 psia. The pseudo reduced
temperature of the mix at - 40 degrees F (420 degrees R), is equal to 1.044.
The
phase transition pressure of this mixture at - 40 degrees F is 1532 psia at a
pseudo reduced pressure of 2.36. At these conditions, the z factor of the mix
is
0.358 and the gas density is 20.84 lb/CF. The density of an 86% to 14% (by
mole volume) methane/butane mix at STP is 0.0578 lb/CF of which the 14%
injected butane represents 37.06% by weight, the methane representing the
remaining 62.94%. The net methane density is 62.94% of 20.84 lb/CF or 13.1
lb/CF The process of adding n-butane increases the net gas density by a factor
of 13.11 lb/CF divided by 11.50 lb/CF or 1.14, while the pressure reduces by
253 psia from 1785 psia to 1532 psia.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
8
Combining the two actions of refrigeration from + 60 degrees F to - 40 degrees
F and adding 14% n-butane increases the net gas density by a factor of 2.05,
from 6.52 lb/CF to 13.1 lb/CF while reducing the pressure by 14% from 1785
psia to 1532 psia.
As the critical temperature of methane is -116.7 degrees F, it is to be
expected that, as
the gas temperature approaches this value, and the pseudo reduced temperature
of
pure methane approaches 1.0, the benefit of reducing the z factor by adding
NGL
would be reduced or eliminated. Taken together with the fact that the added
NGL
takes up storage capacity of the blended mix, there is a lower temperature
limit
below which adding NGL will show no benefit.
Figure 13's textbook drawing # 23-3 shows that the beneficial effect of
reducing z
factor from reducing the critical temperature is much less at higher critical
temperatures. This is illustrated in drawing # 23-3 by calculating the
difference in z
factor between a critical temperature of 2.2 and 2.0 (the z factor goes from
0.96 to
0.94) and a critical temperature between 1.2 and 1.0 (the z factor goes from
0.52 to
0.25). Thus, there is an upper temperature limit, above which adding NGL will
show
no benefit. ' '
Were it not for the effect of the z factor, the NGL enriched gas would show a
lower
net density than the base gas, as it contains an exogenous component that must
be re-
cycled and does not contribute to the useable density. As this NGL enriched
gas is
much less compressible above the phase transition pressure, while the base gas
is
2S more compressible, there is an upper limit on pressure where the density of
the
refrigerated base gas would exceed the net density of the refrigerated NGL
enriched
gas.
There is also a lower limit on pressure where the density of the base gas
would
exceed the net density of the NGL enriched gas. This is because the NGL
enriched
gas immediately transforms into a two-phase state below the phase transition
pressure, and the density falls off dramatically with falling pressure. This
fall off in
density is caused by the vapor component of the two-phase state, which grows
rapidly as the pressure falls. While it is possible to remove the vapor to
maintain a
high density liquid within the container, this is accomplished by removing
methane,
and thus the net methane density falls dramatically below the phase transition
pressure. Thus, there is a lower pressure limit below which adding the NGL
would
show no benefit.
For preparation and storage of natural gas for long haul, ocean based, ship-
transport
applications, LNG is the only large-scale commercially viable technology
currently
available. With LNG, preparation is very costly, as it involves refrigerating
the gas to
-260 degrees F. However, once at this condition, transporting the natural gas
is
relatively low cost, as the density has increased 600 times over the density
of the gas
at STP and the storage is at or near atmospheric pressure.
This invention provides an alternative to LNG for ship-based applications.
With this
invention, natural gas can be mildly refrigerated to the economic temperature
limit
of low cost refrigeration systems and low cost, low carbon steel containment
systems,
NGL is added to the natural gas at the supply end, and the gas can be stored
at a
pressure which is at or near the phase transition pressure. In applications
where no
surplus NGL exists at the supply source, the added NGL is extracted at the
delivery
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
9
end and re-cycled back to the supply end in the same storage container for
adding to
the next shipment (Recycle Trade). For applications where surplus NGL exists
at the
supply end, or the combined blended mix is consumed in transit, none or only a
portion of the NGL needs to be re-cycled (NGL Delivery Trade).
The invention also provides an alternative to compressed natural gas (CNG) for
smaller scale applications such as cars, buses or rail. CNG operates at
ambient
temperature but at very high pressures of 3000 - 3600 psia. These high
pressures
require significant compression for preparation, and requires storage
containers to
handle almost three times the pressure of the invention described herein.
Achieving
similar density as CNG at one-third the pressure would provide benefits in
applications where the gas mixture was consumed to provide the fuel for
transport
(as in cars, buses and rail), as well as a transport mechanism for natural gas
in
overland applications where pipelines are not present or economical.
The benefit of refrigeration and adding NGL occurs over a large range of
temperature, pressure, NGL composition and NGL blending. The optimum type and
amount of added NGL is dependent on the base gas composition, the desired
conditions of temperature and pressure, whether the trade is a Recycle Trade
or an
NGL Delivery Trade and the economics of a specific trade.
With LNG, carbon dioxide must be removed, or else it would solidify in the
process
of refrigerating the gas to - 260 degrees F. With this invention, carbon
dioxide may be
left in the gas, and in fact, can have certain beneficial effects on the
system such that
it could be desirous to contain some carbon dioxide.
Due to the very lightweight nature of natural gas, (even LNG at 600 times the
density
gain over STP only has a specific gravity of about 0.4), gas carrying ship
transport
systems are primarily volume-limited systems, not weight-limited. For example,
an
LNG ship typically contains aluminum spheres with a 130 foot diameter, and
they
have 39 feet of draft. Thus, 70% of the ship is above the water line. The
extra weight
inherent in a ship utilizing this invention, caused by the weight of the re-
cycle NGL
and the steel container, would reduce this to about 55% above the water line,
still
quite acceptable in the shipping industry. This extra weight has minimal
economic
consequence, primarily related to additional fuel and power to go a given ship
transport speed. In a volume-limited gas transport system such as a ship, gas
density
is the key variable and is directly related to cargo capacity and unit cost.
The working temperature regime will be based on the economics of refrigerating
the
gas and storing it in containers. For illustrative purposes, all the following
examples
are based on a storage temperature of - 40 degrees F, unless otherwise noted.
This is
approximately the current lower limit of propane refrigeration, being based on
the
boiling point of propane at -44 degrees F.
The benefit of using this form of refrigeration is illustrated in the
following: The
refrigeration requirement of any gas storage system is very approximately
related to
the temperature change required. Thus, for LNG, a temperature drop of 320
degrees
F is required to go from + 60 degrees F to - 260 degrees F. With this system,
the
temperature drop is 100 degrees F, to go from + 60 degrees F to - 40 degrees
F. This
system requires about 1 /3 of the refrigeration of a comparable LNG system. In
order
to achieve a temperature of - 260 degrees F, LNG plants usually require 3
cycles of
refrigeration, involving propane, ethylene and methane as refrigerants
(referred to as
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
10
a "cascade cycle"). Each cycle involves inefficiency in the process, such that
the
overall efficiency of LNG refrigeration is about 60%. A single-cycle propane
refrigeration system has an efficiency of about 80%. This reduces the
refrigeration
requirement with the system of this invention even further, to about 1/4 of
that
5 required for LNG. The LNG refrigeration plant must be constructed of
cryogenic
materials and must remove all carbon dioxide from the base gas. The -40 degree
F
plant can be made of non-cryogenic material and the carbon dioxide may remain
in
the gas. The overall capital cost of the -40 degree F refrigeration plant is
therefore in
the range of 15% - 20% of a similarly sized LNG plant, and the fuel
consumption is
10 about 1 /4 of the LNG plant. An LNG plant will consume between 8% and 10%
of the
total product liquefied, while the -40 degree F plant will consume between 2%
and
2.5% of the total product refrigerated. As LNG liquefaction is a large portion
of the
overall cost of the LNG transport system, this savings translates into a large
economic advantage, which can help defray the potential extra cost of the
newer
style of non-LNG transport ships themselves.
25
For these reasons, manufacturing LNG as a mechanism to create the
refrigeration
required by this invention is not a very efficient method. Lower cost
refrigeration
systems exist, and are well known to those skilled in the art.
Heating the gas for delivery at the market end also shows a benefit with this
system
over LNG. This system consumes about 1/3 to 1/2 the energy as LNG. Thus, an
LNG
re-gasification plant consumes between 1.5% and 2% of the product as fuel,
while
this system consumes 0.5% to 1% of the product as fuel.
(The Clearstone Thermodynamics Programs developed by Clearstone Engineering
Ltd is used
as the source for all thermodynamic calculations included herein. )
Once a temperature regime is chosen, and a gas mixture is prepared by adding
NGL
to the base gas, the optimum storage pressure is that point at which, with
rising
pressure, the gas transitions from a two-phase state to a dense single phase
fluid
state. This is because, in a two-phase state, the mixture separates into a
vapor state
and a liquid state. As the density of the vapor phase would be very low, the
bulk
density of the overall two-phase state would be low. Increasing the pressure
to
achieve the dense single phase fluid state eliminates this loss of bulk
density. This
phenomenon is illustrated by Figure # 1 - Gross Density vs. Pressure C~3 minus
40
degrees F.
In Figure # 1 and the following figures, a Base gas is assumed to have the
following
composition:
Methane 89.5
Ethane 7.5
Propane 3.0
- The heat content is 1112 BTU/CF
- The critical temperature is - 91.5 degrees F
- The critical pressure is 668.5 psia.
- The density is 0.0473 lb/CF at 14.696 psia and 60 degrees F (STP)
Three gas mixtures are prepared by adding NGL to the Base gas:
- 35.0% ethane and 65.0% of the Base gas
-17.5% propane and 82.5% of the Base gas
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
11
11
-11 .0% n-butane and 89.0% of the Base gas
Figure #1 illustrates the bulk (gross) density of the mixtures at - 40 degrees
F. The
density increases dramatically with pressure for all three mixtures up to a
level of
about 21 lb/CF (pounds per cubic foot), at which point there is almost no
further
increase in density with rising pressure. This point corresponds to the phase
transition point between a two-phase state and a single dense phase fluid
state for
each of the mixtures. Above this phase transition point the gas is almost non-
compressible, such that there is minimal benefit of increased density,with
increases
in pressure beyond this point. The optimum storage pressure is therefore that
point
at which the phase transition between the two-phase state and the single dense
phase
fluid state occurs.
Note that the phase transition occurs at very different pressures, depending
on the
particular NGL chosen for the blend. The lower the carbon number of the NGL
additive (for example, butane has a carbon number of 4) the lower is the
pressure at
which the phase transition occurs.
This chart illustrates the wide range of choice in choosing the optimum
additive for
any particular trade, even after the temperature is chosen. Deciding on the
type and
quantity of added NGL is complex and depends on the economics of the
particular
trade.
For any particular NGL blend composition, deciding on the quantity of additive
is
relatively straightforward within a narrow range. For any chosen temperature,
with
storage at the phase transition pressure, any gas mixture will show increasing
net
density by adding additional NGL up to a sharp inflection point Above this
inflection point, even though the gross density continues to increase as
additional
NGL is added, the net density begins to reduce, along with a reducing phase
transition pressure. The added NGL is taking up a larger and larger portion of
the
increase in gross density, leaving less room for the net gas.
In Recycle Trades, the net density is the key variable, such that this sharp
inflection
point will define the optimum quantity of added NGL. This feature is
illustrated in
Figures # 2, 3, 4 and 5.
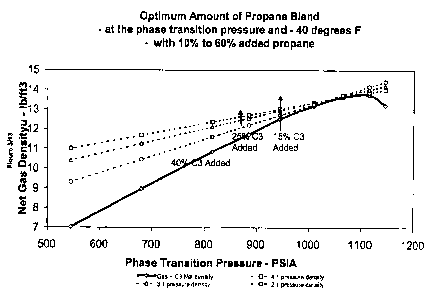
Figure # 2 shows the effect on net and gross gas density of varying levels of
propane
addition to the base gas, between 5% and 60% propane, as well as the density
of the
base gas mixture at both +60 degrees F and - 40 degrees F without any NGL
additive.
While the gross density continues to increase with larger levels of propane
addition,
the net density reaches an inflection point at between 15% and 25% propane
addition
and a pressure of about 1100 psia. Above this amount of blended propane, the
net
density begins to reduce, along with a reduction in the phase transition
pressure. As
density is a surrogate for capacity, while pressure is a surrogate for cost,
the
minimum unit system cost in $/MCF will require a relationship between pressure
and density to develop the optimum blend, as is apparent from the figures.
This cost/benefit relationship is shown in Figure # 3, where a relationship of
3:1 is
assumed to apply between the cost of pressure and the benefit of density in a
re-cycle
ship-based transport system. That is, an increase of 30% in net density
increases
capacity by 30%, while an increase in pressure of 30% increases cost by 10%.
With
this economic relationship, Figure # 3 shows that the optimum amount of added
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
12
12
propane is in the range of 15 - 25%. A similar result would occur with a 2:1
pressure:density relationship as well as a 4:1 relationship, which are also
shown in
Figure # 3.
Figure # 4 shows this same characteristic: for butane, where an optimum amount
of
added butane is in the 10 -15% range. Again, it shows that the sharp
inflection point
is not that sensitive to the economic relationship between pressure and
density.
Figure # 5 shows the same relationship for all four light NGL hydrocarbons,
being
ethane, propane, n-butane and n-pentane. Figures # 2 - 5 show that picking the
inflection point and therefore the quantity of a particular NGL additive is
fairly
straightforward within a narrow range.
Choosing the type of NGL for blending is sensitive to the economic
relationship
between pressure and density and also the characteristics of the trade. There
will be
discrete pressure barriers that carry added cost implications, such as
increasing the
pressure beyond 1440 psia and the consequential requirements for more
expensive
ANSI 900 valves and fittings. The base gas will also contain some level of
NGL, and
the ~NGL recovery mechanism at the delivery end of a re-cycle trade will
likely be
indiscriminate between recovering indigenous NGL and added NGL.. This implies
that the NGL recovery mechanism will also influence the optimum type of NGL
additive.
Figure # 6 illustrates the net density at the inflection point and the phase
transition
pressure for the NGL hydrocarbons ethane, propane, n-butane and n-pentane. It
also
illustrates the effect that combining two hydrocarbons in a mixed NGL blend
(such
as 50% /50% propane and butane by mole volume) will have on the net density.
It
also illustrates the net density of the base gas as compressed natural gas
(CNG) at +
60 degrees F and - 40 degrees F so that the relative contribution to
increasing density
can be more readily separated into the temperature effect and the NGL additive
effect.
Ethane blending implies an 830 psia system with a net density of 10.8 lb/CF.
Propane
blending implies a 10$8 psia system with a net density of 13.7 lb/CF. N-Butane
blending implies a 1305 psia system, with a net density of 15.0 lb/CF. N-
Pentane
blending implies a 1500 psia system with a net density of 15.8 lb/CF. N-
Pentane
blending takes the pressure regime beyond ANSI 600 limit and into the ANSI 900
range. The gross heat content of all of these optimum mixtures is within a
range of
1330 -1380 BTU/CF.
For the n-butane blend, the density increases from 5.5 lb/CF for the base gas
at +60
degrees F and 1305 psia, to 11.5 lb/CF through the action of refrigerating the
gas to
-40 degrees F, an increase to 210% of the base gas. Adding 11% butane
increases the
net density to 15.04 lb/CF an increase to 273% of the base gas. At -40 degrees
F and
1305 psia, with the addition of 11% n-butane, the net density (excludes the
added
butane) of an 1112 BTU/CF natural gas is 318 times the density of the base gas
at
STP. The gross density (includes the added butane) is 445 times the density of
the
base gas at STP.
In Figure # 6, blends containing two adjacent hydrocarbons fall between the
pure
blends, in a fashion related to the average carbon number of the NGL blend. In
fact,
blends of several NGL hydrocarbons are seen to act in a similar fashion as a
pure
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
13
blend, based on the average carbon number. The 11% pure butane blend has a net
density of 15.04 lb/CF at a transition pressure of 1305 psia. A 14% blend of a
50% /
50% (by mole volume) propane/pentane additive has a net density of 14.93 lb/CF
at
a transition pressure of 1294 Asia very similar to the pure butane case. A
12.5% blend
of a 25% /50% /25% propane/butane/pentane additive has a net density of 15.01
lb/CF at a transition pressure of 1298 psia also similar to the pure butane
case. Thus,
an NGL (additive) blend with a similar carbon number as butane, operating at
the
inflection point and the phase transition pressure, will behave similar to
pure butane.
This similarity also occurs if the components are isomers of the normal NGL,
such as
with iso-butane and normal butane, however both the net density and transition
pressure are lower with isomers. An 11% blend of iso-butane has a net density
of
14.42 lb/CF at a transition pressure of 1241 Asia. The net density is 4.1%
lower than
with n-butane, while the transition pressure is 4.9% lower. At a 3:1
pressure:density
economic relationship, the system prefers n-butane over iso-butane, however
the
difference is not that great so as to warrant any specific treatment of the
isomers.
The same outcome occurs with blends of small amounts of heavier NGL, even up
to
decane or C10H22. A blend of 17.5% propane and 82.5% base gas has a net
density of
13.75 lb/CF at a transition pressure of 1088 psia. A blend that includes 3%
octane
(C8H18) and 97% of this propane/base gas mixture has a net base gas density of
14.12 lb/CF at a transition pressure of 1239 psia. This is between the values
for a pure
propane and a pure butane additive. A blend that includes 3% decane and 97% of
the
propane/base gas mixture has a gross density of 25.74 lb/ft3 and a net base
gas
density of 14.15 lb/CF at a transition pressure of 1333 psia.
The very heavy NGL components will still vaporize into a gas state at the
phase
transition pressure, so long as they are present in small quantities. This is
an
important feature for production from gas-condensate or rich gas reservoirs,
where
the liquids condense out of the gas as the pressure is lowered in the
production
process. If the decane were viewed as cargo, the net density is actually 18.35
lb/CF as
compared to 14.15 lb/CF if the decane is recycled. On a 3000 MMCF ship, a 3%
decane content translates into 131,000 Bbl of decane or about 40 Bbl per MMCF.
This
implies that rich gas reservoirs can potentially be produced directly into the
system,
without the need for extensive dual gas/liquids handling systems in the
production
process.
For preparation of vehicular fuels, this implies that the combining of natural
gas,
NGL and gasoline type heavy hydrocarbons, in some proportionate amount, can be
used to create a very dense fuel in the dense single phase fluid state, which
can have
other desirable characteristics, such as octane or cetane number.
Figures # 7 (a, b, c) illustrate the choices for the optimum type of additive.
For this
particular illustration, the temperature is - 40 degrees F and the added NGL
is
assumed to be re-cycled. Figure # 7(a) shows the optimum at a 4:1
pressure:density
economic relationship. Figure # 7(b) shows this at a 3:1 relationship. Figure
# 7(c)
shows this at a 2: 1 relationship. The optimum occurs in a range of pressures
from
about 1100 psia to about 1450 psia, and a range of carbon counts of 3
(propane) and
4.5 (50% /50% butane/pentane). The basic pressure/density curve is fairly
close to a
3:1 ratio over this range of carbon counts, such that choosing any of these
mixtures
would be very close to optimum.
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
14
14
By reference to the very first example given in the above, that being an 86% /
14%
methane/butane mixture, the phase transition pressure was 1532 psia. By
reference
to the above 89% base gas / 11% butane mixture, the phase transition pressure
is
1305 psia. The reason for this difference is that the base gas contains some
NGL
components, 7.5% ethane and 3% propane.
Whether the NGL is indigenous to the base gas or is added through the use of
this
invention, the resulting physical parameters will be identical. Therefore, the
11
butane addition case (and a related carbon number of 4) should be placed in
the
context of an NGL component in the mixture that is actually 6.7% ethane, 2.7%
propane and 11% butane. The average carbon number of the entire NGL component
is actually 3.21. Thus, a 1305 psia phase transition pressure occurs with a
mixture
that has an average NGL carbon number (both indigenous and added) of about
3.2.
Using the 7.5% pentane case on the base gas, a phase transition pressure
occurs at
1500 psia for a mixture with an average carbon number of 3.8. The earlier
example of
an 86 %/14 %methane / butane mixture has an average carbon number of the total
NGL of 4, therefore the phase transition pressure is higher, at 1532 psia.
In a Re-cycle Trade, the base gas will likely contain some NGL that will be
recovered
along with the added NGL, through a fractionation system at the delivery end,
for
re-cycle back to the supply end. This incremental NGL must be offloaded from
the
transport vehicle at some point in time, or else the NGL content would grow
over
time and the net density would reduce. In this fashion, regardless of the
starting
NGL additive, over time, the re-cycle NGL will approximate the composition of
the
NGL contained in the base gas only, as produced from the fractionation system.
In
this fashion, the fractionation system can be used to tune the recovery so
that the
optimum mixture is recycled (rather than having to be offloaded elsewhere).
Recovery of propane plus is relatively low cost, while ethane recovery is
relatively
high cost. In addition, finding markets for the recovered NGL (assuming that
incremental NGL is recovered on each cycle and must be disposed of) would be
much more difficult if the NGL contained ethane due to its limited market
potential.
As most gas contains declining amounts of C3, C4, C5 and higher, an optimum
blend
of a carbon count of 3.5 - 4 can be achieved by recovering enough propane to
offset
the effect of heavier hydrocarbons in the final blend. Thus, if a carbon count
of 4 was
desired for the recycle NGL, and the base gas contained 4% propane, 2% butane
and
1% pentane, the fractionation system would be tuned to recover 25% of the
propane
and all of the C4 +. Controlling the level of propane recovery in a
fractionation
system is relatively straightforward and well understood by those skilled in
the art.
It is possible that the delivered gas could be too high in heat content or
WOBBE
index (equal to the square root of the heat content divided by the specific
gravity of
the gas) to be integrated into the downstream delivery systems. In such
situations,
additional NGL recovery (propane in the above example) could be required at
the
fractionation plant, to deliver a gas with lower heat content, and this could
result in a
less than optimum NGL additive. In such a situation, the presence of carbon
dioxide
in the gas could have beneficial effects as it preferentially ends up in the
delivered
gas off the fractionation tower and it reduces the heat content and WOBBE
index of
the delivered gas.
The impact of the presence of carbon dioxide on net density of the gas mixture
also
shows certain advantages as illustrated in the following: A blend of 82.5%
base gas
and 17.5% propane has a net density of 13.75 lb/CF at 1088 psia. Blending 98%
of this
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
15
mixture with 2% carbon dioxide reduces the net density to 13.53 lb/CF but also
reduces the transition pressure to 1072 psia. Thus, a 1.6% reduction in net
density
yields a 1.5% reduction in pressure. While not sufficient on its own to
justify the 3:1
pressure:density economic relationship, together with the reduction in
delivered gas
5 heat content, it may in some circumstances be preferable to a system with no
carbon
dioxide.
Carbon dioxide also can be used to increase the net density of methane in much
larger blending ratio applications where large volumes of carbon dioxide exist
in the
10 base gas. Adding 10% carbon dioxide to pure methane in a 90% methane and
10%
carbon dioxide mixture has a net density (excluding the added carbon dioxide)
of
7.37 lb/CF at a transition pressure of 1246 psia. Pure methane would have a
density
of 7.33 lb/CF at these conditions. Thus, the two are the same. A 50% / 50%
methane
/ carbon dioxide mixture has a net density of methane of 9.19 lb/CF at a
transition
15 pressure of 1053 psia. Pure methane has a density of 5.72 lb/CF at these
conditions.
Adding the carbon dioxide increases the net density of the methane to 160% of
what
it would otherwise be. A 60% /40% methane / carbon dioxide mixture has a net
density of methane of 8.28 lb/CF at a transition pressure of 975 psia. Pure
methane
would have a density of 5.12 lb/CF at these conditions. This represents an
increase in
net density of 162% of what it would otherwise be. This feature would be of
most
economic benefit for systems where large volumes of carbon dioxide exist in
the base
gas, and where removal at the source would be expensive, and particularly if
uses
could be found for the carbon dioxide along the same trade route as the
natural gas.
Unsaturated hydrocarbons such as propylene provide similar benefits as the
saturated hydrocarbon of the same carbon number. For example, the base gas
enriched with 17.5% propane has a net density of 13.75 lb/CF at a transition
pressure
of 1088 psia. Substituting propylene for propane in the mixture has almost no
effect
on the values. The net density is 13.74 lb/CF at a transition pressure of 1085
psia.
In an NGL Delivery Trade, the NGL additive will likely be based on the
available
supply of NGL, together with the available supply of base gas. In a system
where the
fuel is consumed during transit, the NGL additive could be a function of fuel
specification, such as octane rating for automobiles. The above optimization
calculations for net density will not be applicable, as the system will work
over a
wide range of conditions to handle the total volume of both gas and NGL to
achieve
the maximum bulk or gross density of the mixture at the lowest cost. Any
amount of
added NGL in such a system provides a benefit to the gross density of the
mixture. If
insufficient free NGL exists to achieve the desired composition, a portion of
the NGL
can be recycled to increase the density of the mixture.
Figure # 8 illustrates how the system capacity and pressure improves with
lower
temperatures than - 40 degrees F. At lower temperatures, the economics of the
system improve, as the net density increases and the phase transition pressure
reduces. This is shown for the propane addition mixture, but would be similar
for all
mixtures. For each 5% reduction in temperature from 420 degrees R, the net
density
increases by about 10% and the phase transition pressure reduces by about 15%.
However, reducing the temperature will also increase the density of the base
gas
without any NGL addition. As methane has a critical temperature of - 116.7
degrees
F, as the temperature approaches this limit, the benefits of NGL addition
reduce. It is
possible to achieve the same density for the base gas without NGL addition as
is
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
16
16
achieved with the NGL addition, by operating the system without the NGL
addition
at a higher pressure than for the NGL enriched gas. One of the key economic
aspects
of the technology relates to how much of a pressure reduction is realized
through the
addition of NGL as compared to storing the base gas for transport at a similar
temperature without NGL addition. This pressure saving is shown in Figure # 9.
Figure # 9 illustrates the pressure saving at different temperatures, for two
gas
compositions. The 1112 BTU/CF rich gas is shown (comparing it to a mixture
containing 89% rich gas and 11% n-butane), along with a 1018 BTU/CF lean gas
having a composition of 99% methane and 1% ethane (comparing it to a mixture
containing 86% lean gas and 14% n-butane). The saving on pressure maximizes at
about 420 psia and - 40 degrees F for the rich gas, and at about 550 psia and -
80
degrees F for the lean gas. The area where there is a saving on pressure for
the rich
gas occurs between -120 degrees F and +100 degrees F, while the range for lean
gas is
slightly larger, from - 140 degrees F to +110 degrees F. This graph defines
the
temperature range over which the invention adds economic value.
Even though the invention is beneficial at temperatures above +30 degrees F,
it is
unlikely that a storage system embodying the invention will operate at higher
temperatures than + 30 degrees F. The large increase in net density and large
reduction in phase transition pressure for small reductions in temperature
imply that
storage systems operating with some form of refrigeration will be the most
obvious
application for the invention. For this reason, the scope of the monopoly
claimed in
this disclosure of the invention is limited to gas temperatures below +30
degrees F,
implying the need for refrigeration.
Figure # 10 is used in defining the pressure range over which the invention
adds
value. For the 11% n-butane enriched base gas and - 4 0 degrees F, the net
density at
the phase transition pressure of 1305 psia is 15.04 lb/CF. Base gas without
NGL
addition would have to be stored at 1723 psia and - 40 degrees F to achieve
the same
density, a pressure saving of 418 psia. As the butane-enriched gas is almost
non
compressible above the phase transition pressure, while the base gas is still
quite
compressible, the net density of the two compositions becomes the same at
about
2000 psia. The savings on pressure reduces from 418 psia at the phase
transition
pressure to less than 50 psia above 150% of the phase transition pressure.
Therefore, above 150% of the phase transition pressure, the invention no
longer adds
significant value. Conversely, the net density of the butane-enriched gas
drops off
dramatically below the phase transition pressure, also shown in Figure # 10.
At a
pressure of about 1000 psia, or 75% of the phase transition pressure, the
pressure
savings again falls below 50 psia, and the invention no longer adds
significant value.
Thus, the invention adds value between 75% and 150% of the phase transition
pressure.
While the actual values will be somewhat different for different compositions,
similar features will be seen with all of the various blending compounds
discussed
herein.
In a transport system, this pressure saving will manifest itself in at least
the following
identifiable benefits:
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
17
17
- A smaller wall thickness for the container of a specific capacity, assumed
in
almost all cases be made of steel. This means less cost and weight and more
competitive purchase options as more steel mills can manufacture the thinner
walled steel container.
- Greater container diameter, as mills are usually limited by the wall
thickness
for a given diameter. This means fewer containers for a given capacity and
this reduces the installation and manifold cost to connect the containers.
- Reduced ANSI rating for the valves and fittings. Typically, systems using
this invention will use ANSI 600 valves and fittings (1440 psia) while CNG
and higher pressured systems would use much higher and more costly ANSI
rated fittings.
- Less weight means reduced fuel used to operate the transport system at a
given speed.
- Lower pressure means a reduced compression requirement to prepare the
gas for delivery to the container.
- Specifically for ships, less weight in the container means a higher ship
height
given the stability characteristics of the ship. This means more cargo.
- Specifically for ships, less weight means a lower ship draft, resulting in
the
ability to enter more ports.
Figure # 11 shows the shape of the decompression curve of the RNG system as
the
gas is unloaded at a delivery point. This can be used to provide additional
benefits
from the invention. This curve is non-linear and is shown for the 11% n-butane
case.
The bulk density of the single dense phase fluid mixture at 1305 psia is 21.06
lb/CF
The bulk density of the same mixture in a two-phase state at 650 psia is 5.47
lb/CF At
350 psia, the bulk density of the same mixture in a two-phase state is 2.41
lb/CF.
Thus, 75% of the cargo can be unloaded at 50% of the pressure reduction and
89% of
the cargo can be unloaded at 73% of the pressure reduction, assuming that a
proportionate amount of liquid and vapor is unloaded at the same time.
As gas delivery systems located close to market areas typically operate at
pressures
in the 350 - 650 psia range, this can minimize the amount of compression
required to
unload the gas from the ship once the pressure on the ship falls below the
market
delivery pressure.
It is also fairly typical that gas production is available at higher
pressures, close to the
1305 psia storage pressure. In this fashion, it can be seen that this system
preserves
useful pressure and minimizes the amount of power required to change the gas
pressure purely for the purpose of transport.
Compressed natural gas systems use a lot of power to compress gas for storage,
and
then most of the useful pressure is discarded when delivered into the market.
LNG
discards the pressure when delivered into storage, and then must rebuild the
pressure when delivering into the market. This system can be designed to
operate at
a pressure between the receipt pressure and the delivery pressure, thus
discarding or
wasting little pressure in the process of preparation for transport, loading
and
unloading.
The concept of methane or lean gas extraction to achieve the same results as
the
above is illustrated as follows:
CA 02443200 2003-10-10
WO 02/063205 PCT/CA02/00151
18
As it has particular application to gas which is produced from gas-condensate
reservoirs or from gas that is produced in association with oil, a gas
analysis was used
from a gas - condensate reservoir in Peru. The raw gas contains 1294 BTU/CF
with about 1.7% of the gas composed of C7+. On production of 1017.8
MMCFD, it is assumed that the 23,027 BPD of C7+ is extracted as oil, leaving
1000 MMCFD of gas at 1199.5 BTU/CF. If this gas is refrigerated to -70
degrees F, and put into a flash tank at 888 psia, a two-phase separation
occurs.
The vapor contains 50% mole volume or 500 MMCFD at a heat content of
1057.8 BTU/CF. While the vapor is mostly methane, there are small amounts
of ethane and propane, thus the invention refers to removal of methane or a
lean gas. The liquid contains 50% mole volume or 500 MMCFD at 1340.9
BTU/CF. The liquid off the flash tank can be pumped up to 1178 psia, and
then warmed up to - 40 degrees F by heat exchanging with inlet gas, where it
flashes into a vapor state. The phase transition pressure of this mixture is
1178
Asia at - 40 degrees F and the density is 21.25 lb/CF. This dense single phase
fluid can now be delivered to a ship and delivered to market without need of
an NGL re-cycle. The C3 - C6 component of this mixture represents 41,917
BPD of NGL that need not be re-cycled. The vapor off the flash tank can either
be delivered back to the reservoir for injection for pressure maintenance, or
can be delivered to an LNG plant for liquefaction and delivery to market. If
one assumes that the vapor is required for pressure maintenance, the cold can
be recovered by heat exchanging with the inlet gas. There is additionally a
benefit in reducing the heat content of the injected gas into a reservoir for
pressure maintenance. Assuming a reservoir condition of 150 degrees F and
2130 psia, the Z factor of the 1199.5 BTU/CF raw gas is 0.801-with a density
of
8.13 lb/CF The Z factor of the 1057.8 BTU/CF gas is 0.859 with a density of
6.59 lb/CF. Thus, a mass of lean gas equal to only 81% of the rich gas is
required to preserve the same pressure, allowing for greater sales of gas
during this pressure maintenance phase of the reservoir life. If one assumes
that the residual gas can be sold as LNG, the cold vapor continues to go
through additional refrigeration to become LNG. There is an overall system
benefit in delivering a lean gas to the LNG plant, and the rich gas to the
system described by this invention. The benefit of this system is that an
additional large amount of mass can be delivered to market for the same cost,
as the NGL is not re-cycled. The benefit on LNG arises because the
liquefaction temperature of NGL is much higher than methane, for example
ethane liquefies at - 127 degrees F, while propane liquefies at - 44 degrees
F.
Essentially, all the extra work done to refrigerate the NGL component of the
gas to the - 260 degree F temperature is wasted, and could show better value
refrigerating additional methane. In addition, there is an issue with LNG
transport of rollover, which tends to limit the amount of NGL in the system.
Typically, the NGL component of LNG is separated at the source using
fractionation and transported to market using LPG carriers.
The foregoing has illustrated certain specific embodiments of the invention,
but other
embodiments will be evident to those skilled in the art. Therefore it is
intended that
the scope of the invention not be limited by the embodiments described, but
rather
by the scope of the appended claims.