Note: Descriptions are shown in the official language in which they were submitted.
WU UL/V5141 CA 02443638 2008-02-11
/ r~, l~ uavL~lvav0
TITLE
Process for Improved Yields of Higher Molecular Weight Olefins from Lower
Molecular Weight Olefins
10
FIELD OF INVENTION
This invention relates to a controlled process for improving the yields of
heavier olefins by using a substantially narrow range of lighter olefin-
containing
hydrocarbon feed stock which are fed into a reaction distillation column at a
predetermined point pind using varying arrangements of isomerizing and
disproportionating catalysts in relation to the point of feeding the narrow
range of
lighter ofefin-containing hydrocarbon feed stock. The controlled process
provides for
keeping the reaction mixture in a state of vapor / liquid phase equilibrium
for
separating the lighter products overhead and collecting the heavier reaction
products
in the bottoms by maintaining controlled pressure and temperature profiles In
relation
to the narrow range of lighter olefin-containing hydrocarbon feed stock being
used
and the desired range of heavier olefin-containing hydrocarbon products
desired as
product in the bottoms of the reaction distillation column. Further at least
one or
more zones for the purpose of vapor/liquid contacting are created in the
reaction
distillation column for improving the separation of lighter reaction olefin
products from
the heavier reaction olefin products and the olefin-containing hydrocarbon
feed stock
and for reducing the cost of the process.
BACKGROUND OF THE INVENTION
It is well known in the prior art to use metal catalysts to react/split and
recombine (disproportionate) hydrocarbon molecules, which contain olefrnic or
double bonds between the carbon atoms. This reaction of splitting and
recombining
the hydrocarbon molecules at the double bonds creates olefin hydrocarbon
molecules of varying size depending on the feed stock make up and where the
double bonds occurred on the feed stock molecules but it does not necessarily
give
an end product with a high commercial value either, as an end product.
1
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
For example some of the earlier prior art reacted propylene to make ethylene
and butene, or conversely make propylene from ethylene and butene in the
presence
of metal catalysts, which gave an olefin product but the product was not
greatly
different in value from the reactants. However, as these reactions are
reversible,
they will proceed, at most, to equilibrium, which limits the yield of the
desired
products. The prior art generally described only liquid phase reactions with
heterogeneous catalyst in fixed beds, fluidized beds or moving beds for
generally
controlling approach to equilibrium of olefin-containing reactant and product
mixtures.
The prior art also attempted to use other process variables like longer
residence time in such systems and higher temperatures to achieve better
approach
to equilibrium and to shift equilibrium to a more favorable to desired
products in
disproportionating reactions, but they generally led to increased
isomerization and
other by-product reactions which were undesirable in the desired product.
Some of the prior art taught improved selectivity and conversion of reactions
using 1- and 2-butene to ethylene, propylene, 2-pentene and 3-hexene by using
a
reactive distillation column, in the presence of a rhenium oxide as a
disproportionation catalyst. In this prior art the catalysts served as a
distillation
substrate to facilitate a phase transfer of some of the lighter products out
of the liquid
phase. In this particular system the conversion and yields went up but the
reaction
proceeded to ethylene and propylene as the light ends and only to 2-pentene, 3-
hexene as the heavy ends, which were of not much more value than the beginning
feed stock used to generate them.
The prior art is replete with teaching of methods and processes for
improving yields of medium-range olefins by reacting high carbon number
molecule olefins with a low carbon number molecule olefins by simultaneous
disproportionating and isomerizing of these olefins. In this process both the
high carbon number molecule olefins and low carbon number molecule olefins
are kept in a single liquid phase and the reaction process is allowed to reach
near equilibrium for the formation of midrange olefins, which are detergent-
range linear internal (CIo-C16) olefin from a feedstock of light (C4-Cs) and
heavy (C16-C2o+) alpha-olefins. Some variations of these prior art patents are
even used to produce commercial linear alcohols.
2
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
These prior art patents utilized the isomerization process, which distributed
the location of the double bond in the olefin molecules to make possible the
production of a wider range of olefins, which readily worked if one was
attempting to
generate a mid-range detergent grade olefin from light and heavy alpha and
internal
olefins. In these single liquid phase reactions a catalyst such as potassium,
cesium ,
or rubidium has been used to facilitate the isomerization of the double bonds
between the carbon molecules and to create a wider range of internal olefins
to be
reacted to form the mid-range olefins.
Further the prior art has disclosed many processes using both an
isomerization and disproportionation catalysts in a single liquid phase,
elevated
temperatures, and elevated pressure to attempt to achieve a desired range of
products for a broad base of olefin-containing hydrocarbon feed stock, with
only
limited success due to limitation of equilibrium and a large range of olefin-
containing
hydrocarbons mixed together, which required further processing to separate the
narrow desired range from both the lighter and the heavier olefin-containing
hydrocarbon feed stocks and products.
The prior art further just used metal catalysts for disproportionating and
isomerizing either singularly or in admixtures, but made no distinction
between where
these were located relative to the input of these feed stocks or which
catalyst should
be the first for reaction with the feed stock. The objective in the prior art
was thus to
make the deepest possible internal olefin of both light and heavy species
before or
during the disproportionation including symmetrical internal olefins. This was
highly
desirable when the goal was to create a mid-range olefin-containing
hydrocarbon, but
not for the production of heavier olefin-containing hydrocarbons from lighter
olefin-
containing hydrocarbons, where specifically a formation of asymmetrical
olefins is
desired.
OBJECTS OF THIS INVENTION
It is the object of the invention of this process to create improved yields of
heavier olefins using a substantially narrow range of lighter olefin-
containing
hydrocarbon feed stock in a reaction distillation column containing metal
catalysts
and controlled temperature and pressure for reacting the narrow range of
lighter
olefin-containing hydrocarbon feed stock to form the improved yields of the
heavier
olefins. In the process of reacting the lighter olefin-containing hydrocarbon
feed stock
3
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
to form the improved product yields of the heavier olefins, the removal of the
lighter
olefin-containing hydrocarbons and other light hydrocarbons occurs.
An object of the process of this invention is to create the improved yields of
heavier olefins without using high temperatures and/or longer residence time
in the
systems of these processes, so as to limit the formation of unwanted by-
products
which are undesirable in the desired product and which may interfere with the
formation of the desired heavier olefins or reduce the yields thereof.
Yet a further object of the process of this invention is to shift the
equilibrium of the reaction toward the formation of heavier olefin-containing
hydrocarbon feed stock by reacting the lighter olefin-containing hydrocarbon
feed stock with the metal catalysts and then controlling the pressure and
temperature to allow the lightest unwanted olefins and other light products
produced by the reaction with the metal catalysts to go into vapor phase for
its
removal from the reaction distillation vessel overhead.
Also an object of this process invention is to allow even the lightest olefin-
containing hydrocarbon feed stocks such as 1- and 2-butene and propylene to be
reacted with metal catalysts in the controlled temperatures and pressures of
this
process for the creation of more valuable heavier olefin-containing
hydrocarbon
products such as C5 to C10, which have significantly greater monetary value
than the
products of 2-pentene and 3-hexene.
The object of the process of this invention further allows the creation of
heavier olefins from a narrow range of lighter olefin-containing hydrocarbon
feed
stock and then running the narrow range of heavier olefins created in another
step to
create yet heavier olefins.
A yet further object of this process invention is to utilize the isomerization
process to adjust the location of the olefinic double bond to a predominantly
asymmetrical location in the olefin molecules and then disproportionate the
carbon
molecules, which effectively cuts them at the double bond and recombines the
asymmetrical fragments with other olefin molecules which have been
disproportionated to create heavier olefin molecules and light olefin
molecules and
then isomerlzed those heavier olefin molecules and then disproportionate them
again. After all disproportionations in the process the lighter undesirable
olefin-
4
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
containing hydrocarbons are removed in the vapor phase leaving the heavier
olefins
to be isomerized again before the process continues in the steps to the
desired
heavier olefin product.
Also an object of this invention is the use of both isomerization and
disproportionation catalysts with olefin-containing hydrocarbon feed stocks
and
reaction products in a vapor and liquid phase in relative low temperatures and
pressures to achieve a desired range of heavier olefin end products.
Further it is an object of this invention to provide at least one vapor/liquid
contacting zone to facilitate the separation of the lighter olefin-containing
hydrocarbons and the collection of the heavier olefins either as desired
products or
for further reacting in the reactive distillation column.
It is also an object of this invention to adjust the type of catalysts and
where
that catalysts is located as the predetermined poinit for the first exposure
to the
lighter olefin-containing hydrocarbon feedstock depending on the degree of
symmetry or lack of symmetry of the olefin bonds on the lighter olefin-
containing
hydrocarbon feedstock which are being fed into the reactive distillation
column at that
predetermined point in the reactive distillation column.
Yet further and additional benefits and improvements of the process of this
invention will be appreciated by other skilled in the art and those advantages
and
benefits of the invention wiff become apparent to those skilled in the art
upon a
reading and understanding of the following detailed description and
diagrammatic
drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
The process of this invention may be practiced in certain physical forms and
arrangements and adjustment of the variable parts herein described, but
preferred
embodiments of which will be described in detail in the specification and
illustrated in
the accompanying diagrammatic drawings which will form a part thereof.
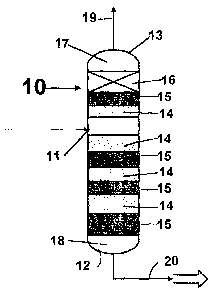
FIG. 1 is a diagrammatic drawing of a reactive distillation column used with
the process of this invention utilizing a narrow range of lighter olefin-
containing
5
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
hydrocarbon feed stock of C5 and higher carbon numbers being fed into the
reactive
distillation column at a predetermined point and with isomerizing catalyst
near the
predetermined feed point and disproportionating catalyst in place and in
alternating
arrangement with the isomerizing catalyst and with at least one vapor/liquid
zone
created at the top of the reactive distillation column for producing Cs
through Cl o
carbon number olefins.
FIG. 2 is a diagrammatic drawing of a reactive distillation column used with
the process of this invention utilizing a narrow range of lighter olefin-
containing
hydrocarbon feed stock of C6 through Cio and higher carbon numbers being fed
into
the reactive distillation column at a predetermined point and with isomerizing
catalyst
near the predetermined feed point and disproportionating catalyst in place and
in
alternating arrangement with the isomerizing catalyst and with at least one
vapor/liquid zone created at the top of the reactive distillation column for
producing
C10 and higher carbon number olefins.
FIG. 3 is a diagrammatic drawing of a reactive distillation column used with
the process utilizing a range of lighter olefin-containing hydrocarbon feed
stock of C3
to C4 carbon numbers being fed into the reactive distillation column at a
predetermined point and with disproportionating catalyst near the
predetermined
feed point and isomerizing catalyst in place and in alternating arrangement
with
disproportionating catalyst and with at least one vapor/liquid zone created at
the top
of the reactive distillation column for producing C5 to C10 carbon number
olefins.
FIG. 4 is a diagrammatic drawing of a series of reactive distillation columns
connected together for utilizing an initial range of lighter olefin-containing
hydrocarbon feed stock of C3 to C4 carbon numbers for producing Cio and higher
carbon number olefins by feeding the bottom products in stages from the first
to the
second and to the third reactive distillation column.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention relates to a process for improving the yields of heavier
olefins by using a substantially narrow range of lighter olefin-containing
hydrocarbon
feed stock, which is fed into a reaction distillation column, generally
referred to at
reference numeral 10. In at least one embodiment as shown in FIG 1, the
process of
this invention commences with the feeding of the substantially narrow range of
lighter
6
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
olefin-containing hydrocarbon feed stock from C6 and heavier carbon numbers
into
the reaction distillation column 10 at a predetermined point 11 in the
reaction
distillation column 10. Located near the predetermined point of feed 11 is an
isomerization catalyst 14 for isomerizing the olefin-containing hydrocarbon
feed stock
as it is passed into the reactive distillation column 10. As shown in FIG I
the
isomerization catalyst 14 would be located both near the predetermined feed
point 11
and directly above and below the predetermined feed point 11 for first
isomerizing the
olefin-containing hydrocarbon feed stock as completely as possible. Thus in
this
preferred embodiment the predetermined feed point 11 would be located on the
reactive distillation column for first directly feeding the olefin-containing
hydrocarbon
feedstock into the reactive distillation column 10 between the isomerization
catalysts
14. Isomerizing the olefin, as those skilled in the art will know, means that
the
double bonds between the carbon atoms, which characterize an olefin, are moved
from one pair of carbon atoms to another pair of carbon atoms with the purpose
of
creating predominantly an olefin molecule, which is not symmetrical, provided
the
olefin molecule has 5 or more carbonatoms. Once the olefin-containing
hydrocarbon
feed stock has been isomerized, it is then allowed to flow to a
disproportionation
catalyst 15 for disproportionating of the now isomerized olefin-containing
hydrocarbon feed stock. In this preferred embodiment a disproportionating
catalyst
15 would be located above and below the isomerization catalysts 14. This
arrangement of catalysts may be either in separate trays or as molecular
mixtures of
the catalysts which are created in admixture thereof. Disproportionating the
olefin, as
those skilled in the art will know, means that a splitting process occurs at
the point of
the olefinic bond on the olefin-containing hydrocarbon and a re-combining of
the split
parts with other split parts from other olefins which are being
disproportionated at the
same time to create both larger olefins and smaller olefins. In this preferred
embodiment by having the disproportionation catalyst 15 located above and
below
the isomerizing catalysts 14 the olefins are as soon as they are isomerized
move to
be disproportionated in the reaction distillation column, as shown in FIG 1.
Further as shown in FIG. 1, at least in this embodiment, there are provided
alternating process steps of disproportionating and isomerizing of the olefin-
containing hydrocarbon feed stock after its initial feed into the
predetermined feed
point 11 of the reaction distillation column 10 and its first isomerization.
This
alternating of the process steps of disproportionating and isomerizing said
olefin-
containing hydrocarbon feed stock will continue depending on the size of the
reaction
distillation column 10, but will generally have as it last process step a
7
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
disproportionating step before reaching the bottoms 18 of the reaction
distillation
column 10.
As those skilled in the art will appreciate there are many catalysts and ways
to prepare them in a reaction distillation column 10, but in this preferred
embodiment,
the disproportionation catalysts are selected from the groups of molybdenum,
tungsten, cobalt, and rhenium metals and their oxides either individually or
as
combinations thereof and supported on porous substrates. For example in a
preferred embodiment the disproportionation catalyst selected from a group of
heavy
metals is used which contains tungsten or rhenium oxides on a porous alumina
or
silica-containing supports. The porous alumina or silica-containing support
used in
this embodiment is catalytic grade gamma-alumina or silica-alumina, but any
other
substrate which would be effective to make the catalysts available for
reaction with
the olefins may be used and not depart from the teachings of this invention.
Some of the conventional methods of preparing the disproportionation
catalyst mixture includes dry mixing, impregnation or co-precipitation. In one
of the
preferred embodiments a solution containing aqueous salts of rhenium or
rhenium
oxide and/or tungsten or tungsten oxide is prepared. Once prepared it is added
to an
alumina support which can be in the form of conventional dumped distillation
packing, such as, saddles, rings, spheres to enhance mass transfer and
reactive
surface during disproportionating and fractionation or separation to the
extent the
operating parameters are appropriate. After impregnations, the catalyst would
be
calcined at 300 degrees Centigrade to 700 degrees Centigrade in the flow of
air
and/or nitrogen to activate the catalyst. In one preferred embodiment the
disproportionation catalyst contained 5 to 20% by weight rhenium or 5 to 35%
tungsten.
Also as those skilled in the art will appreciate there are many catalysts and
ways to prepare isomerization catalyst for the reaction distillation column
10, but in
this preferred embodiment the isomerization catalysts are selected from the
groups
of alkali metals such as sodium, potassium, rubidium or cesium either
individually or
as combinations thereof and then supported on alumina support. For example,
carbonates, chelates, hydroxides, alkoxylates and other compounds can be used
as
the catalysts as long as they can be decomposed to leave some form of metal
oxides
on the surface for reaction with the olefins. In a preferred embodiment the
metals of
potassium carbonate and/or potassium carboxylates may be used, but after they
are
8
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
impregnated on a surface, they would be activated by being calcined at 400
degrees
Centigrade to 800 degrees Centigrade in the presence of air flow. In at least
one
embodiment the isomerization catalyst of an alkali metal on the alumnia
substrate
should be from 5 to 20% by weight.
Also shown in FIG 1 of the reactive distillation column 10, a vapor/liquid
contacting zone 16 is located in the upper part of the reactive distillation
column 10
for providing a vapor/liquid contacting zone for separation of the lighter
reaction
products from the heavier olefin-containing hydrocarbon feed stock. This
vapor/liquid
contacting zone 16 may consist as shown in this embodiment of several stages
of
structured packing or trays located in the upper most stages of the upper part
17 of
the reaction distillation column 10. At this point the olefin-containing
hydrocarbon
feed stock has been both isomerized and disproportionated and olefin reaction
products have been produced which are both heavier, lighter and approximately
the
same size as the feed stock. The advantage of providing at least one
vapor/liquid
zone 16 is that it improves the separation or fractionation of the lighter
olefin reaction
products from the heavier olefin reaction products produced from the olefin-
containing hydrocarbon feedstock in the reaction distillation column 10. This
is
especially true at the top of the column, where reaction is inhibited by low
temperatures and the light species are flashed off thus preventing their
recombination with the other reactants. This removal of the light species thus
shifts
the equilibrium conversion of the feedstock toward heavier olefin-containing
hydrocarbons. These light olefin reaction products are then removed by the
overhead
line 19 located in the top 13 of the reaction distillation column 10.
The process variables of temperature and pressure used in the reaction
distillation column 10 to practice this invention will vary and depend on the
olefin-
containing hydrocarbon feedstock used, and the desired extent of reaction
required
to achieve the desired conversion and selectivity. In general, the temperature
range
will be between -50 degrees Fahrenheit to 200 degrees Fahrenheit at the top
13
where the lighter olefin reactant products are taken off by the overhead
streaml9. At
the bottom 12 in the reaction distillation column 10 where the heavier olefin
reactant
products are taken off by stream 20 from the bottoms 18 the temperature range
will
be between 200 degrees Fahrenheit to 600 degrees Fahrenheit. The pressures,
in general will range from -14.5 PSIG to 250 PSIG but will also be varied by
the
required process temperature to achieve the desired conversion and selectivity
of the
desired olefin product. These process variables will require those skilled in
the art
9
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
who practice this invention to do some experimentation within these variables
and
within the ranges set out herein to maximize their results because these
variables are
dependant on the olefin-containing hydrocarbon feedstock and the desired
product
ranges. How these process variables may be adjusted will become more clear to
those skilled in the art from the following examples herein set out and
disclosed.
In example 1, using the schematic drawing of FIG 1, a substantially narrow
range of lighter olefin-containing hydrocarbon feed stock composed of a
mixture,
which is substantially C5, C6, and heavier, was fed into the reactive
distillation column
10, at the predetermined feed point 11. It was isomerized and
disproportionated by
the isomerization catalyst 14 and the disproportionation catalyst 15
respectively and
then alternatively treated by the respective catalysts thereafter. The process
variables used in this example were:- 20 PSIG, + or-10 PSI and a temperature
of
40 degrees Fahrenheit , + or - 40 degrees Fahrenheit at the top 13. At the
bottoms 18 in the bottom 12 of the reaction distillation column 10 the
variables were
held at 400 degrees Fahrenheit + or -100 degrees Fahrenheit. Operated at
these
temperature and pressure variables the resultant product collected at the
bottoms 18
for removal as desired heavier olefins would be a composition in the wt% as
follows:
C6 3.1
C7 18.3
C8 61.7
C9 13.2
CIo 2.5
Cii 1.1
thus producing a heavier olefins of substantially from C6 through Clo.
In an example 2, using the schematic drawing of FIG 1, a substantially narrow
range of lighter olefin-containing hydrocarbon feed stock composed of a
mixture
which is substantially C5, C6, and heavier was fed into the reactive
distillation column
10, in FIG 1, at the predetermined feed point 11. It was immediately
isomerized and
disproportionated by the isomerization catalyst 14 and the disproportionation
catalyst
15 and then alternatively treated by the respective catalyst thereafter. The
process
variables used with this example were :- 20 PSIG + or - 10 PSI and
temperatures
of 40 degrees Fahrenheit + or - 40 degrees Fahrenheit at the top 13, while
the
bottom 12 of the reaction distillation column 10, and re-boiler temperature
were held
at 350 degrees Fahrenheit + or - 100 degrees Fahrenheit. Operated at these
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
temperature and pressure variables the resultant product collected at the
bottoms 18
for removal as desired heavier olefins would be a composition in the wt% as
follows:
C5 4.7
C6 38.5
C7 38.2
C8 18.6
thus producing a slightly different heavier olefins of substantially from C6
through C1o.
In an example 3, using the schematic drawing of FIG 2, a substantially narrow
range of lighter olefin-containing hydrocarbon feed stock composed of a
mixture
which is substantially C5, through C-1o, and heavier was fed into the reactive
distillation column 10 at the feed point 11. It was immediately isomerized and
disproportionated by the isomerization catalyst 14 and the disproportionation
catalyst
and then alternatively treated by the respective catalyst therefore. The
process
15 variables used with this example were: 10 PSIG + or - 10 PSI and a
temperature of
40 degrees Fahrenheit + or - 40 degrees Fahrenheit at the top 13 while the
bottom 12 of the reaction distillation column 10, and a re-boiler temperature
were
held at 400 degrees Fahrenheit + or -100 degrees Fahrenheit. Operated at
these
temperature and pressure variables the resultant product collected at the
bottoms 18
for removal as desired heavier olefins would be a composition in the wt% as
follows:
C8 3.77
C9 20.16
Cio 34.97
Cii 25.1
C12 10.37
C13 3.87
C14 2.16
C1s 0.59
thus producing a slightly different heavier olefins of substantially from C1o
through
C2o.
In an example 4, using the schematic drawing of FIG 2, a substantially narrow
range of lighter olefin-containing hydrocarbon feed stock composed of a
mixture
which is substantially C5, through Cio, and heavier was feed into the reactive
distillation column 10, in FIG 2, at the predetermined feed point 11. It was
immediately isomerized and disproportionated by the isomerization catalyst 14
and
the disproportionation catalyst 15 and then alternatively treated by the
respective
11
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
cataiyst thereafter. The process variables used with this example were: at the
top
13 of the column generally 10 PSIG + or - 10 PSI and a temperature of 40
degrees
Fahrenheit + or - 40 degrees Fahrenheit while the bottom 12 of the reaction
distillation column 10, where a re-boiler would be located, would be 450
degrees
Fahrenheit + or -100 degrees Fahrenheit. Operated at these temperature and
pressure variables the resultant product collected at the bottoms 18 for
removal as
desired heavier olefins would be a composition in the wt% as follows:
C7 0.2
Cs 1.0
Cs 6.6
Cio 16.4
Cii 23.5
C12 21.4
C13 14.3
C14 8.3
C15 4.2
C16 2.0
C17 0.9
C18 0.4
thus producing a slightly different heavier olefins of substantially from
Clothrough
Czo. '
The yield in this process to heavy products (C6 and heavier) is thought to be
in the range of 20% to 80% by weight, more preferably 50 % to 75% by weight.
Most
preferabiy, the yield to heavy products will be about 70% by weight. As shown
above
the product distribution can be controlled or modified by varying temperatures
and
pressure variables in the reaction distillation column.
In at least another embodiment of this invention as shown in FIG 3, the
process of this invention commences with the feeding of a substantially narrow
range
of lighter olefin-containing hydrocarbon feed stock from C3 to C4, with C4
being
composed at least partially of 1- and 2-butene, into the reaction distillation
column 10
at a predetermined point 11 between the bottom 12 and the top 13 of the
reaction
distillation column 10. In this embodiment the resultant products are
ethylene,
propylene and some 2-butene which will be taken out by the overhead line or
stream
19 off the top 13 of the reaction distillation column 10 and C5 through Cio
which will
be taken from the bottoms 18 of the bottom 12 of the reaction distillation
column 10
12
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
by a line 20. In this embodiment a disproportionation catalyst 15 for
disproportionating the olefin-containing hydrocarbon feed stock as it is
passed into
the reactive distillation column 10, is provided and located near the
predetermined
point of feed 11. As shown in FIG 3 the disproportionating catalyst 15 would
be
located both near the predetermined feed point 11 and directly above and below
the
feed point 11 for first disproportionating the olefin-containing hydrocarbon
feed stock
as completely as possible. Thus in this preferred embodiment the predetermined
feed point 11 would be located on the reactive distillation column 10 for
first directly
feeding the olefin-containing hydrocarbon feedstock into the reactive
distillation
column 10 between the disproportionation catalyst 15. Once the olefin-
containing
hydrocarbon feed stock has been disproportionated, it is then allowed to flow
to an
isomerization catalyst 14 for isomerization of the now disproportionated
olefin-
containing hydrocarbon feed stock. In this preferred embodiment disclosed an
isomerization catalyst 14 would be located above and below the
disproportionation
catalyst 15 for isomerizing the reaction product from the disproportionation
catalyst
15. The disproportionating catalyst 15 is located for first reacting the
olefin-containing
feedstock in this embodiment for at least the reason that the feedstock of C3
and C4,
with C4 being composed of 1- and 2- butenes, which can only be isomerizied to
predominantly 2-butene and that yields only 2-butene when it is
disproportionated by
the disproportionation catalyst 15. After the feed stock is first
disproportionated by
the disproportionation catalyst 15, some of the resultant reactant products
have a
molecular size and symmetry for which isomerization by the isomerization
catalyst 14
has a purpose and the other reactant products are smaller molecules which are
then
removed into the vapor phase by separation or fractionation of these lighter
olefin
and other light reaction products from the heavier olefin reaction products in
the
reaction distillation column 10. In the embodiment of FIG. 3, there is then
provided
alternation of the process steps of disproportionating and isomerizing after
this initial
stage of disproportionation, wherein disproportionation and isomerization
process
steps are continued in the reaction distillation column 10 and finally ending
in a
disproportionation process step before the resultant products of C5 through
Clo will
be delivered into the bottoms 18 at the bottom 12 of the reaction distillation
column
10.
The process variables using the schematic drawing shown in FIG 3 but with
an example 5 using the narrow range of lighter olefin-containing hydrocarbon
feed
stock made from C3 and C4 olefins and the process variables being at the top
13 of
the reactive distillation column 10 generally in the 100 PSIG, + or -80 PSI
and a
13
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
temperature of 100 0 Degrees Fahrenheit, + or - 500 degrees Fahrenheit while
the
bottom 12 of the reaction distillation column 10, where a re-boiler would be
located,
would be 300 degrees Fahrenheit + or - 100 degrees Fahrenheit. Operated at
these temperature and pressure variables the resultant product collected at
the
bottoms 18 for removal as desired heavier olefins would be a composition in
the wt%
as follows:
C4 8.15
C5 46.21
C6 26.92
C7 13.31
Cs 1.69
thus producing a heavier olefin of substantially from C5 through C-lo.
As those skilled in art will appreciate these process methods above disclosed
could be practiced all in one reaction distillation column or in a series of
columns as
shown in FIG 4 to take a lighter olefin-containing hydrocarbon from C3 to C4
to
heavier olefin-containing hydrocarbon of substantially C14 and heavier product
without
departing from the teachings of this invention. Obviously if all the process
methods
were combined into one reaction distillation column the variables for each
stage
would have to be maintained and the look of the reaction distillation columns
10, as
shown would have a different structure, but the process methods would be the
same
equivalent processes. In FIG 4 a serial process using multiple columns is
shown
with the first stage being generally shown at reference number 21, which is
generally
the process of Fig 3, the second stage is generally shown at reference number
22,
which is generally the process of FIG 1, and the third stage is generally
shown at
reference number 23, which is generally the process of FIG 2.
Also as those skilled in the art should appreciate these process methods
above disclosed while run with linear olefins, they could also have been run
with
branched chain olefin-containing hydrocarbons or as a mixed process with both
linear and branched chain olefins. In the case were a specific percent mixture
of
branched chain olefins with the linear olefin would be desired, then the
feedstock
mixture of the branched chain olefin-containing hydrocarbon feedstock would be
adjusted with the linear olefin-containing hydrocarbon feedstock to achieve
the
desired percentage of the branched olefin in the resultant products produced
by the
process methods of this invention.
14
CA 02443638 2003-10-09
WO 02/081417 PCT/US02/10806
While the preferred embodiments of the intention of this process and their
operational use have been described for the improved yields of heavier olefins
from
substantially narrow range of lighter olefin-containing hydrocarbon feed stock
in a
reactive distillation column , it will be appreciated that other embodiments
and
process variables may be used without departing from the spirit of the
invention of
this process here in claimed.
H: WlyDocu me ntsVWrm-AbazWA-Pat-Spec-PCT.doc
Ver 29 Jan 2002
20
30
15