Note: Descriptions are shown in the official language in which they were submitted.
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
The title does not meet the requirements of PCT Rule 4.3. It is too long. The
new title is: METHOD
AND COMPOSITIONS FOR TREATING MIGRAINES
BACKGROUND OF THE INVENTION
Migraines are recurrent, often familial, symptom complexes
of periodic attacks of vascular headache. The condition is characterized by
intermittent attacks of headache, preceded by an aura in approximately
15% of patients. The headache is often accompanied by associated
symptoms, most commonly nausea, vomiting, photophobia and
phonophobia. Migraines affect approximately 17% of adult women and 6%
of adult men (Stewart et aZ., NeuroZogy, 1994, 44 (suppl. 4), 517-523).
Cyclooxygenase (COX), also known as prostaglandin H
synthase, is an enzyme implicated in the mediation of pain, fever and
inflammation. It catalyzes the oxidative conversion of arachidonic acid
into prostaglandin H2, a key intermediate in the biosynthetic pathway of
prostaglandins, prostacyclins and thromboxanes, which in turn mediate a
variety of physiological effects both beneficial and pathological.
Recently it was discovered that two COX isoforms exist:
COX-1, expressed constitutively in many tissues, and COX-2, an induced
isoform having elevated levels of expression in inflamed tissues. COX-1 is
thought to be involved in ongoing "housekeeping" functions, for example,
gastric cytoprotection, while COX-2 is implicated in the pathological
effects mentioned above.
Current cyclooxygenase inhibitors such as aspirin, ibuprofen
and indomethacin, used as non-steroidal anti-inflammatory drugs
(NSAIDs), inhibit both COX-1 and COX-2 and have associated side effects,
such as gastrotoxicity, which may be manifested as ulcer formation.
COX-2 selective inhibitors act as effective NSAIDs without substantial
gastrotoxic side effects. For purposes of this disclosure only, a COX-2
selective inhibitor is defined as a COX inhibitor having a selectivity for
the COX-2 isoform relative to the COX-1 isoform.
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
SUMMARY OF THE INVENTION
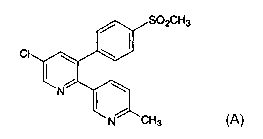
The present invention relates to a method for treating or
preventing migraines in a mammalian patient in need of such treatment
or prevention comprising administering to said patient a compound of
Formula A:
2CH3
A
or a pharmaceutically acceptable salt, hydrate or N-oxide thereof, in an
amount that is effective to treat or prevent migraines.
DETAILED DESCRIPTION
The present invention encompasses a method for treating or
preventing migraines in a mammalian patient in need of such treatment
or prevention comprising administering to said patient a compound of
Formula A:
$02CI-ig
A
in an amount that is effective to treat or prevent migraines.
The compound of Formula A, which has the generic name
etoricoxib, is a selective inhibitor of cyclooxygenase-2. Etoricoxib is
disclosed as Example 23 in U.S. No. 5,361,419, issued on January 19,
1999, which is hereby incorporated by reference in its entirety.
IV v~3
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
In an embodiment of the invention the compound of Formula
A is administered at a dose ranging from about 10 mg to about 200 mg. In
another embodiment of the invention the mammalian patient is human.
Another embodiment of the invention encompasses a method
for treating migraines in a mammalian patient in need of such treatment
comprising administering to said patient a compound of Formula A:
CI
A
or a pharmaceutically acceptable salt, hydrate or N-oxide thereof, in an
amount that is effective to treat migraines.
For purposes of this specification, treating migraines means
relieving both the headache and the consequent associated symptoms of
migraine. Treating migraines is synonymous with the acute treatment of
migraines.
Another embodiment of the invention encompasses a method
for preventing migraines in a mammalian patient in need of such
prevention comprising administering to said patient a compound of
Formula A:
CI
A
or a pharmaceutically acceptable salt, hydrate or N-oxide thereof, in an
amount that is effective to prevent migraines.
-3-
IV
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
For purposes of this specification, prevention of migraines
means reducing the severity, the frequency or both the severity and
frequency of migraine attacks. Preventing migraines is synonymous with
migraine prophylaxis or the chronic treatment of migraines.
For purposes of this specification, migraine is meant to
include migraine without aura, migraine with aura, migraine with typical
aura, migraine with prolonged aura, familial hemiplegic migraine, basilar
migraine, migraine aura without headache, migraine with acute onset
aura, ophthalmoplegic migraine, retinal migraine, childhood periodic
syndromes that may be precursors to or associated with migraine, benign
paroxysmal vertigo of childhood, alternating hemiplegia of childhood,
status migrainosus and migrainous infarction. Reference is made to the
following: Headache Classification Committee of the International
Headache Society: Classification ad diagnostic criteria for headache
disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(suppl
7):1-96, which is hereby incorporated by reference in its entirety.
Etoricoxib has a shorter time to maximum concentration and
longer half life as compared to traditional NSAIDs such as naproxen and
will therefore have greater efficacy in the acute treatment of migraine.
Etoricoxib is also better suited.than traditional NSAIDs for chronic
administration.
For purpose of this specification, an amount that is effective
to treat or prevent migraines is that amount that will relieve the subject
being treated of the symptoms of the migraine attack and the specific dose
level and frequency of dosage may vary and will depend upon a variety of
factors including the activity of the specific compounds used in
combination, the metabolic stability and length of action of the
compounds, the age, body weight, general health, sex diet, mode and time
of administration, rate of excretion, the severity of the particular condition
and the host undergoing therapy. However, dosage levels of etoricoxib on
the order of about 0.01 mg/kg to about 100 mg/kg of body weight per day,
typically about 0.1 to about 10 mg/kg, more particularly about 0.2 to about
5 mg/kg and especially about 0.14 to about 3 mg/kg per day are useful in
the novel method of treatment. For the treatment of a migraine attack,
-4-
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
the active ingredient may be administered orally, topically, parenterally,
by inhalation, spray, rectally or intravaginally in formulations containing
pharmaceutically acceptable carriers.
The term parenteral as used herein includes subcutaneous
injections, intravenous, intramuscular, intracisternal injection or infusion
techniques.
Etoricoxib may be in a form suitable for oral use, for
example, tablets, troches, lozenges, aqueous or oily suspensions,
dispersible powders or granules, emulsions, hard or soft capsules,
solutions, syrups and elixirs. Compositions intended for oral use may be
prepared according to any method known to the art for the manufacture of
pharmaceutical compositions and typically such compositions contain one
or more agents selected from the group consisting of sweetening agents,
flavoring agents, coloring agents and preservatives in order to provide
pharmaceutically elegant and palatable preparations. These excipients
may be for example, diluents such as lactose, calcium carbonate, sodium
carbonate, calcium phosphate or sodium phosphate; granulating and
disintegrating agents, for example, corn starch or alginic acid; binding
agents, for example starch, gelatin or acacia, and lubricating agents, for
example, magnesium stearate, stearic acid or talc.
The tablets may be uncoated or they may be coated. Coating
can be included to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a longer
period. For example, a time delay material such as glyceryl monostearate
or glyceryl distearate may be employed. They may also be coated by the
technique described in the U.S. Patent 4,256,108; 4,166,452; and
4,265,874 to form osmotic therapeutic tablets for control release.
Formulations for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert solid
diluent, for example, calcium carbonate, calcium phosphate or kaolin, or
as soft gelatin capsules wherein the active ingredient is mixed with water
or miscible solvents such as propylene glycol, PEGS and ethanol, or an oil
medium, for example peanut oil, liquid paraffin or olive oil.
-5-
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
Aqueous suspensions contain the active material in
admixture with excipients suitable for the manufacture of aqueous
suspensions. Such excipients are suspending agents, for example sodium
carboxymethylcellulose, methylcellulose, hydroxy-propylmethycellulose,
sodium alginate, polyvinyl-pyrrolidone, tragacanth and acacia; dispersing
or wetting agents may be a naturally-occurring phosphatide, for example
lecithin, or condensation products of an alkylene oxide with fatty acids, for
example polyoxyethylene stearate, or condensation products of ethylene
oxide with long chain aliphatic alcohols, for example
heptadecaethyleneoxycetanol, or condensation products of ethylene oxide
with partial esters derived from fatty acids and a hexitol such as
polyoxyethylene sorbitol monooleate, or condensation products of ethylene
oxide with partial esters derived from fatty acids and hexitol anhydrides,
for example polyethylene sorbitan monooleate. The aqueous suspensions
may also contain one or more preservatives, for example ethyl or n-propyl
p-hydroxybenzoate, one or more coloring agents, one or more flavoring
agents, and one or more sweetening agents, such as sucrose, saccharin or
aspartame.
Oily suspensions may be formulated by suspending the active
ingredient in a vegetable oil, for example arachis oil, olive oil, sesame oil
or coconut oil, or in mineral oil such as liquid paraffin. The oily
suspensions may contain a thickening agent, for example beeswax, hard
paraffin or cetyl alcohol. Sweetening agents such as those set forth above,
and flavoring agents may be added to provide a palatable oral
preparation. These compositions may be preserved by the addition of an
anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation of
an aqueous suspension by the addition of water provide the active
ingredient in admixture with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending agents are exemplified by those already mentioned
above. Additional excipients, for example sweetening, flavoring and
coloring agents, may also be present.
-6-
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
The pharmaceutical compositions may also be in the form of
oil-in-water emulsions. The oily phase may be a vegetable oil, for example
olive oil or arachis oil, or a mineral oil, for example liquid paraffin or
mixtures of these. Suitable emulsifying agents may be naturally-
occurring phosphatides, for example soy bean, lecithin, and esters or
partial esters derived from fatty acids and hexitol anhydrides, for example
sorbitan monooleate, and condensation products of the said partial esters
with ethylene oxide, for example polyoxy-ethylene sorbitan monooleate.
The emulsions may also contain sweetening and flavoring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such
formulations may also contain demulcents, preservatives, flavorants and
coloring agents.
The pharmaceutical compositions may be in the form of a
sterile injectable aqueous or oleaginous suspension. This suspension may
be formulated according to the known art using those suitable dispersing
or wetting agents and suspending agents which have been mentioned
above.
Injectable compositions are typically in the form of sterile
solutions or suspensions, which include the active ingredient in a
parenterally acceptable diluent. Among these are sterile water, dextrose
5% in water (D5W), Ringer's solution and isotonic saline, as well as
mixtures thereof. Cosolvents such as ethanol, propylene glycol or
polyethylene glycols may also be used. Sterile, injectable oil is
occasionally employed as a solvent or suspending medium in
intramuscular preparations. A representative example is peanut oil. In
addition, fatty acids such as oleic acid, preservatives, buffers and local
anesthetics find use in the preparation of intramuscular injectables.
The combination of active ingredients may also be
administered rectally or intravaginally as suppositories. These can be
prepared by mixing the drug with a suitable non-irritating excipient
which is solid at ordinary room temperature but molten at normal or
elevated body temperature. Examples of such materials include cocoa
butter and polyethylene glycols.
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
For topical use, creams, ointments, gels, solutions,
suspensions and the like containing the compound are employed. (For
purposes of this application, topical application includes mouth washes
and gargles, as well as transdermal applications.) Topical formulations
are comprised of a pharmaceutical carrier, which may include, e.g.,
cosolvents, emulsifiers, penetration enhancers, preservatives or
emollients.
The active ingredient is combined with the carrier to produce
the dosage form. For example, a formulation intended for oral
administration may contain from as low as about 0.7 mg of etoricoxib to as
high as about 7 g of etoricoxib per dose, compounded with an appropriate
and convenient amount of carrier material which may vary from about 5
to about 95 percent of the total composition. A preferred pharmaceutical
composition contains from about 10 mg to about 200 mg of etoricoxib or a
salt thereof.
Etoricoxib may also be administered in combination with
other agents for the treatment or prevention of migraines. Such
administration may either be in unit dosage form or concomitantly. All
conventional anti-migraine agents are used in conjunction with the
etoricoxib at conventional doses that are determined by the skilled
clinician. These compounds are known and normal daily dosages are well
established. Typically, the individual daily dosages for these
combinations may range from about one-fifth of the minimally
recommended clinical dosages to the maximum recommended levels fox
the entities when they are given alone. Precise dosages are left to the
discretion of the physician
Thus, in further aspects, the invention encompasses
pharmaceutical compositions for treating or preventing migraines
comprising etoricoxib and one or more agents selected from the group
consisting of: rofecoxib, indomethacin, sulindac, etodolac, mefenamic acid,
meclofenamic acid, flufenamic acid, tolfenamic acid, etofenamic acid,
tolmetin, ketorolac, diclofenac, ibuprofen, naproxen, fenoprofen,
ketoprofen, oxaprozin, piroxicam, meloxicam, tenoxicam, lornoxicam,
cinnoxicam, sudoxicam,tenoxicam, phenylbutazone, oxyphenbutazone,
_g_
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
apazone, azapropazone, nimesulide, diflunisal, nabumetone, aspirin,
sodium salicylate, choline, magnesium trisalicylate, salsalate, diflunisal,
salicylsalicyclic acid, sulfasalazine olsalazine, ergotamine, ergonovine,
ergonovine, mesylates, ergometrine, methylergonovine, methylsergide,
metergoline, ergoloid mesylate, dihydroergotamine, dihydroergocornine,
dihydroergocristine, dihydroergocryptine, dihydro-a-ergocryptine, dihydro-
[3-ergocryptine, ergotoxine, ergocornine, ergocristine, ergocryptine, a-
ergocryptine, [3-ergocryptine, ergosine, ergostine, bromocriptine,
amitriptyline, methysergide, propranolol, valproate, verapamil,
metoclopramide and prochlorperazine, in combination with a
pharmaceutically acceptable carrier. In a preferred embodiment, the
invention encompasses a pharmaceutical composition comprising
etoricoxib and metoclopramide, in combination with a pharmaceutically
acceptable carrier.
In another aspect, the invention encompasses a method for
treating or preventing migraines in a mammalian patient in need of such
treatment or prevention comprising administering to said patient a
compound of Formula A:
S02CH3
CI
A
or a pharmaceutically salt, hydrate or N-oxide thereof, in combination
with one or more agents selected from the group consisting of rofecoxib,
indomethacin, sulindac, etodolac, mefenamic acid, meclofenamic acid,
flufenamic acid, tolfenamic acid, etofenamic acid, tolmetin, ketorolac,
diclofenac, ibuprofen, naproxen, fenoprofen, ketoprofen, oxaprozin,
piroxicam, meloxicam, tenoxicam, lornoxicam, cinnoxicam, sudoxicam,
tenoxicam, phenylbutazone, oxyphenbutazone, apazone, azapropazone,
nimesulide, diflunisal, nabumetone, aspirin, sodium salicylate, choline,
-9-
CA 02445502 2003-10-27
WO 02/089798 PCT/US02/13750
magnesium trisalicylate, salsalate, diflunisal, salicylsalicyclic acid,
sulfasalazine olsalazine, ergotamine, ergonovine, ergonovine, mesylates,
ergometrine, methylergonovine, methylsergide, metergoline, ergoloid
mesylate, dihydroergotamine, dihydroergocornine, dihydroergocristine,
dihydroergocryptine, dihydro-a-ergocryptine, dihydro-[3-ergocryptine,
ergotoxine, ergocornine, ergocristine, ergocryptine, a-ergocryptine, (3-
ergocryptine, ergosine, ergostine, bromocriptine, amitriptyline,
methysergide, propranolol, valproate, verapamil, metoclopramide and
prochlorperazine, in amounts that are effective to treat or prevent
migraines. A preferred agent is metoclopramide.
-10-